Abstract
Human cytomegalovirus (HCMV), a member of the β-herpesvirus family, encodes four homologues of cellular G protein-coupled receptors (GPCRs). One of these, the protein product of HCMV open reading frame (ORF) UL33, has been identified in HCMV-infected cells and virus particles and shown to be heat-aggregatable and N-glycosylated. Another, the product of ORF US28, has been functionally characterized as a β-chemokine receptor. Here we report the use of RT-PCR, coupled in vitro transcription–translation, immunoprecipitation, and Western immunoassays to (i) show that RNA from the open reading frame US27 appears predominantly during the late phase of replication; (ii) identify the protein encoded by HCMV US27 in infected cells and enveloped virus particles; (iii) demonstrate that the US27-encoded protein is heterogeneously N-glycosylated and resolves as two species following treatment with peptide N-glycosidase F; and (iv) show that both the recombinant and deglycoylated infected cell US27 protein aggregate when heated in the presence of SDS prior to electrophoresis in polyacrylamide gels, a property which is abrogated with the addition of urea to sample buffer.
Keywords: Cytomegalovirus, Chemokine receptor, Signal transduction, Viral homologue, US28, UL33, UL78
1. Introduction
Human cytomegalovirus (HCMV), a widespread human pathogen (Griffiths and Walter, 2005; Lawson, 2005; Ross and Boppana, 2005), is a prototypic β-herpesvirus, having a long replicative cycle, a restricted host range, and a cytopathic effect resulting in pronounced cell rounding and swelling (Drago et al., 2000; Griffiths and Walter, 2005; Kano and Shiohara, 2000; Sampathkumar and Paya, 2000). Sequence analysis of its ≈ 230-kb genome (Chee et al., 1990a) revealed four open reading frames (ORFs), US27, US28, UL33, and UL78 (Attwood and Findlay, 1994a,b; Chee et al., 1990b), that encode potential homologues of cellular G protein-coupled receptors (GPCRs) (Lomize et al., 1999; Pierce et al., 2002; Pin et al., 2003; Schoneberg et al., 1999; Strange, 1999), a large group of cell surface-exposed signal transducing proteins (Pierce et al., 2002; Pin et al., 2003). GPCRs typically bind an extracellular ligand, and an ensuing conformational change in the receptor activates a neighboring intracellular heterotrimeric G protein (Gudermann et al., 1997; Morris and Malbon, 1999; Pierce et al., 2002; Pin et al., 2003), which in turn regulates the activity of other proteins, such as enzymes (e.g., adenylate cyclase) (Karnushina et al., 1983) and channels (e.g., sodium transporters) (Cardenas et al., 1997; Yatani, 1995). These proteins in turn regulate intracellular levels of second messengers, such as cyclic AMP and calcium (Sleight and Lieberman, 1995; Yatani, 1995), which may regulate the levels of transcription and translation (Polson et al., 2002; Raymond et al., 1990) or the activities of locomotion or granulation (especially in immune cells) (Handel and Lau, 2004; Horuk, 1994; Kim, 2004), all in metered responses to the original extracellular stimuli.
Many forms of signal transduction have been noted early in HCMV infection. These include activation of phosphatidylinositol-3-kinase, as a result of HCMV binding epidermal growth factor receptor at the cell surface (Evers et al., 2004; Wang et al., 2003), activation of src, via αvβ3 integrin binding by the virus (Evers et al., 2004; Wang et al., 2005), and activation of PLCγ (Evers et al., 2004; Johnson et al., 2001) and the ERK1/ERK2 paths (Evers et al., 2004; Widmann et al., 1999). Upregulation of the transcriptional activating factors NF-κB (Wang et al., 2003; Yurochko et al., 1995, 1997a) and Sp1 (Wang et al., 2003; Yurochko et al., 1997b) have also been noted in the absence of any HCMV protein production.
Fluxes in intracellular second messengers typically associated with G protein activation have also been measured early in HCMV infection. Intracellular levels of calcium (Albrecht et al., 1991; Fortunato et al., 2000), sodium (Fons et al., 1991; Fortunato et al., 2000; Nokta et al., 1988), cyclic AMP (Albrecht et al., 1991; Fortunato et al., 2000), inositol trisphosphate (Albrecht et al., 1991; Fortunato et al., 2000; Minisini et al., 2003; Valyi-Nagy et al., 1988), and arachidonic acid (AbuBakar et al., 1990a,b; Albrecht et al., 1991; Fortunato et al., 2000; Shibutani et al., 1997) have all been shown to change either immediately or very early after infection, suggesting that one or more of the virus-encoded GPCR homologues may also be responsible for the observed changes. Supporting such a relationship are data showing that the US28 open reading frame encodes a functional GPCR for β-chemokines (Gao and Murphy, 1994; Kuhn et al., 1995; Neote et al., 1993) and for the δ chemokine CX3CL1 (fractalkine) (Casarosa et al., 2005; Minisini et al., 2003; Rosenkilde et al., 2001), families of closely related immune modulators (Handel and Lau, 2004; Horuk, 1994; Murphy, 1996; Premack and Schall, 1996); many different chemokines can bind this receptor (Casarosa et al., 2005; Kuhn et al., 1995; Neote et al., 1993), which apparently couples to Giα, Ga12, and/or Gq11, resulting in a calcium flux upon activation (Bakker et al., 2004; Billstrom et al., 1998; Casarosa et al., 2001; Gao and Murphy, 1994; McLean et al., 2004; Melnychuk et al., 2004; Vieira et al., 1998; Waldhoer et al., 2002). Recent studies have also shown the receptor encoded by US28 to be responsible for partially activating the HCMV major immediate-early promoter (Boomker et al., 2006).
The similarities between the protein potentially encoded by US28 and that encoded by US27 have led to the hypothesis that the latter is also a functional chemokine receptor, yet no reports to date have proven this idea (Fraile-Ramos et al., 2002; Waldhoer et al., 2002). The conservation of genes for these GPCRs through the cytomegaloviruses (Beisser et al., 1998; Davis-Poynter et al., 1997; Davison et al., 2003; Oliveira and Shenk, 2001; Penfold et al., 2003; Sahagun-Ruiz et al., 2004) and even across other human beta- and gamma-herpesviruses, in which functional virus chemokine receptors have also been shown (Bais et al., 1998; Beisser et al., 2005; Geras-Raaka et al., 1998; Isegawa et al., 1998; Milne et al., 2000; Nakano et al., 2003; Paulsen et al., 2005; Polson et al., 2002; Tadagaki et al., 2005), speaks to the evolutionary necessity of these glycoproteins to the biology of these pathogens. Their true functions as virulence factors to enhance infection in vivo, though, are just beginning to be understood (Beisser et al., 1998, 1999; Davis-Poynter et al., 1997; Kaptein et al., 2003; Melnychuk et al., 2005; Streblow et al., 2005).
Additionally, the presence of at least three HCMV GPCRs, the chemokine receptor homologue encoded by US28 (Penfold et al., 2003), GCR33 (Margulies et al., 1996), and GCR27 [(Fraile-Ramos et al., 2002; Varnum et al., 2004) and reported here], in enveloped virus particles provides a mechanism for their incorporation into the plasma membrane during the process of virus entry, and a plausible explanation of how they could activate the observed signaling pathways so soon after infection, a phenomenon displayed by the GPCR homologue encoded by murine CMV M78 (Oliveira and Shenk, 2001). In the work described here, we corroborate reports that GCR27 is present in infected cell membranes (Fraile-Ramos et al., 2002) and virions (Varnum et al., 2004), extend those studies to demonstrate that GCR27 is also present in extracellular non-infectious enveloped particles and dense bodies, and show that GCR27 shares biochemical characteristics with HCMV GCR33.
2. Materials and methods
2.1. Plasmids
PCR amplification of US27 was done as described for UL33 (Margulies et al., 1996), using the HindIII B fragment of HCMV AD169 genomic DNA (provided by Marc Chee, Medical Research Council) and primers to the 5′ and 3′ end of the US27 ORF (Chee et al., 1990a). The resulting PCR product was cloned into the BamHI site of the vector pcDNA1 (Invitrogen, San Diego, CA) in the T7/CMV orientation to create BJM2. The insert in BJM2 was sequenced (Kraft et al., 1988; Sanger et al., 1977) and found to contain a single base change (C for T at base 867 of original PCR product) compared to the published sequence (Chee et al., 1990a,b). Because this change was not conservative (i.e., coding specificity was changed from isoleucine to threonine), it was corrected by replacing the Bsu36I–BspMI fragment of BJM2 with the corresponding fragment from the genomic HindIII B fragment. The corrected construct was called BJM2.1.
2.2. In vitro transcription–translation
Proteins were synthesized in vitro with a TNT rabbit reticulocyte kit (Promega, Madison, WI), using 1 μg of Qiagen column-purified (Qiagen, Chatsworth, CA) BJM2.1 (or appropriate control DNAs), T7 RNA polymerase, and 10 μCi [35S]-methionine (New England Nuclear, Boston, MA, #NEG-009A) per reaction, according to manufacturers’ protocol.
2.3. Antisera and immunoprecipitation
Anti-27 is a polyclonal antipeptide antiserum prepared by immunizing one female New Zealand White rabbit with a synthetic peptide corresponding to the seventeen carboxy-terminal amino acids of the predicted protein product of US27 (i.e., NH2-YDRKNAPMESGEEEFLL-CO2H), conjugated to keyhole limpet hemocyanin via an EDC (1-ethyl-3-(dimethylaminopropyl) carbodiimide) linkage (Imject; Pierce, Rockford, IL), all essentially as described previously for the Anti-33 antiserum (Margulies et al., 1996). Anti-C1 is a polyclonal antipeptide antiserum specific for the carboxyl end of the CMV assembly protein precursor; its preparation and characterization were described elsewhere (Schenk et al., 1991; Welch et al., 1991b). Generation of the Anti-33 antiserum has been described (Margulies et al., 1996).
Immunoprecipitations were performed by combining 7 μl of each in vitro transcription–translation reaction with 18 μl of IP wash buffer, consisting of 0.4% CHAPS (3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate; Sigma, St. Louis, MO) and 1 mM PMSF (phenylmethylsulfonyl fluoride; Sigma) in calcium and magnesium-free-PBS (CMF-PBS). One sample each of the diluted preparations was combined with 25 μL of Anti-27, and one of each was combined with 25 μL of an irrelevant antiserum (rabbit Anti-Neurothelin [Margulies, B.J., and Clements, J.E., unpublished data]). The samples were rocked overnight at 4 °C, combined with 50 μL Protein A-Sepharose beads (100 mg/mL in 0.02% NaN3 and CMF-PBS; Sigma, #P3391), and rocked another 3 h at 4 °C. The beads were washed four times with 50 μL IP wash buffer and once with CMF-PBS, and the final pellet was analyzed by SDS-PAGE and fluorography (Section 2.4).
2.4. Gel electrophoresis, Western immunoassay, fluorography, and autoradiography
Proteins were separated by electrophoresis in 12% polyacrylamide gels containing sodium dodecyl sulfate (SDS-PAGE), essentially as described by Laemmli (1970); specific modifications and optimizations, including not heating samples prior to electrophoresis, were implemented as before (Gibson, 1981; Margulies et al., 1996). Urea was added to some samples to a final concentration of 4 M before electrophoresis (see Section 3.9) (Soulie et al., 1996). Western immunoassays with the Anti-33 (Margulies et al., 1996), Anti-C1 antisera (Welch et al., 1993), and Anti-27 antisera (Section 2.3) were done by the general procedure of Towbin et al. (1979), with modifications as described previously (Margulies et al., 1996; Welch et al., 1993). Fluorograms were obtained from the processed blots with XAR-5 film (Eastman-Kodak, Rochester, NY) or Biomax MS film (Eastman-Kodak) and a calcium–tungstate intensifying screen at −80 °C (Laskey and Mills, 1977). Gels with [35S]-methionine-labeled in vitro-synthesized samples were processed for sodium salicylate-enhanced fluorography (Chamberlain, 1979) and an image was recorded similarly. The autoradiogram in Fig. 2B was obtained from direct exposure of Biomax MR film (Eastman-Kodak) at room temperature for 1 week.
Fig. 2.
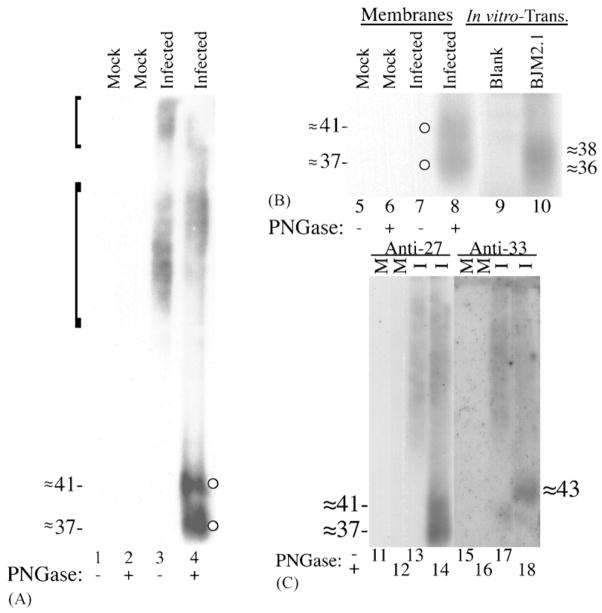
(A) HCMV-infected HFF cells express N-glycosylated GCR27. HFFs were infected with HCMV (infected; lanes 3 and 4) or not infected (mock; lanes 1 and 2). Total cell membranes were isolated 8 days after infection and incubated with or without PNGase F (±PNGase). Membrane protein (≈20 μg) was subjected to SDS-PAGE and analyzed by Western immunoassay with Anti-27. The brackets represent apparent molecular weight ranges of ≈ 80–130 and ≈ 150–180 kDa, respectively; molecular weights (in kDa) were estimated from molecular weight markers (See Blue™ Prestained Markers; Novex) included during SDS-PAGE that were electrotransfered to the Immobilon-P. Circles denote deglycosylated forms of GCR27. (B) Comparison of deglycosylated GCR27 and in vitro-synthesized pUS27. The same samples from lanes 1 to 4 were resolved in lanes 5–8, respectively, by 12% SDS-PAGE alongside TNT-derived pUS27 (lane 10) and a TNT reaction containing no DNA (lane 9). Western immunoassay was conducted with Anti-27. (C) Validating the specificity of Anti-27. The same samples from lanes 1 to 4 were resolved in duplicate, side-by-side, in lanes 11–14 and lanes 15–18, by 12% SDS-PAGE (“M” denotes mock-infected and “I” denotes HCMV-infected membrane samples). One-half of the gel was analyzed by a Western blot with Anti-27 (lanes 11–14); the other half was analyzed with Anti-33 (Margulies et al., 1996) (lanes 15–18). Molecular weights (in kDa) in parts B and C were estimated from pre-stained markers (Multi Mark™; Invitrogen) included during SDS-PAGE that were electrotransfered to the Immobilon-P. Images of the resulting fluorographs and autoradiogram were captured with Adobe Photoshop for Macintosh via Umax PowerLook II and Epson 1200 U scanners.
2.5. Cells and virus
Human foreskin fibroblast (HFF) cultures were prepared, maintained, and infected with the AD169 strain (ATCC VR-538) of human cytomegalovirus (HCMV) as described before (Gibson, 1981; Weiner and Gibson, 1983). The three types of extracellular enveloped virus particles, non-infectious enveloped particles (NIEPs), virions, and dense bodies were effectively purified, separated from one another, and concentrated by banding three times in potassium tartrate gradients as described (Irmiere and Gibson, 1983; Margulies et al., 1996; Talbot and Almeida, 1977). The inhibitory chemicals cycloheximide (CHX; supplied in DMSO and used at a final concentration of 50 μg/mL, Sigma, St. Louis, MO) (Jeang and Gibson, 1980) or phosphonoformic acid (PFA; 200 μg/mL, Sigma) (Welch et al., 1991a) were added to some cultures to inhibit translation or virus DNA replication, respectively (Honess and Roizman, 1974) (see also Section 2.7).
2.6. Membrane preparation and peptide N-glycosidase F (PNGase F) treatment
Membrane fractions were prepared from HCMV-infected and non-infected HFF 8 days after infection as described before (Margulies et al., 1996; Nathans et al., 1989; Parker et al., 1993; Sung et al., 1991). Membrane preparations and virus particle preparations were treated or not treated overnight with PNGase F (200 mU per sample) as outlined previously (Haltiwanger and Hart, 1993; Margulies et al., 1996; Tarentino and Plummer, 1994).
2.7. RNA isolation
Tissue culture plates (six-well) were seeded with HFFs and infected with HCMV AD169, clarified by low-speed centrifguation, the next day. One hour prior to infection, cells were treated with the corresponding levels of CHX, PFA (Section 2.5), or DMSO (at 0.05%); these treatments were left in the culture medium throughout the course of infection. Total RNA from the cells in a single well (approximately 106 cells) was isolated at 6, 24, and 168 h post-infection with the Qiagen RNEasy system, supplemented with an on-column RNAse-free DNAse step (Qiagen), all according to manufacturer’s protocols. Total RNA was isolated from extracellular virus particles (100 μL of clarified inoculum), which were first pretreated with DNAse-free RNAse (0.5 μg, Roche Diagnostics Corporation, Indianapolis, IN) at 37 °C for 1 h to remove any residual RNA outside any virus particles, via a QIAamp Viral RNA Mini Kit (Qiagen). All RNAs isolated from both cellular and virion sources were also subsequently treated with a second round of RNAse-free DNase (Amplification Grade DNAse I; Invitrogen) at 25 °C for 30 min to remove any remaining genomic DNA prior to RT-PCR. One-twentieth of each eluted RNA was used per amplification reaction.
2.8. Polymerase chain reaction (PCR) and reverse-transcription-PCR (RT-PCR)
Each RNA (Section 2.7) was amplified by PCR alone, using only Platinum Taq DNA polymerase (Invitrogen), or by RT-PCR, using a Superscript II One-Step RT-PCR kit (Invitrogen). Each RNA was tested for the presence of transcripts for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with the deoxyoligonucleotide primers 5′-ACCACCATGGAGAAGGCTGG-3′ and 5-CTC-AGTGTAGCCCAGGATGC-3′ (Ercolani et al., 1988), for transcripts from the US27 gene, with primers 5′-GCGTGCGA-AATAACAAGCGG-3′ and 5′-GCAACAGGCGAATACCC-TTAGG-3′ (Chee et al., 1990b), and for the RNA encoded by the HCMV UL109 gene, with the primers 5′-CGTTAC-GATGAACTGCGG-3′ and 5′-AGCTTATTGAGCGCAGCC-3′ (Bresnahan and Shenk, 2000) (all oligonucleotides synthesized by MWG Biotech, High Point, NC). Every PCR and RT-PCR reaction contained 1/2 volume of 2× reaction mix (Superscript II One-Step RT-PCR kit; contains buffer, MgCl2, and dNTPs; Invitrogen), 200 μM each primer, 5 U. recombinant RNasin (Promega, Madison, WI), and 1 μL of enzyme (either Platinum Taq alone or the Superscript II/Platinum Taq mix); these reaction conditions corresponded to manufacturer’s specifications for the One-Step RT-PCR kit. Both PCR and RT-PCR reactions for GAPDH were amplified under the same thermal cycling parameters: 50 °C for 30 min (the RT step), followed by 30 cycles of 94 °C for 15 s, the Tm of the primers (60 °C) for 30 s, and 72 °C for 1 min; PCR and RT-PCR amplification for US27 and UL109 was performed with the same times as those used for GAPDH in each step, except the initial reverse transcription was conducted at 52 °C (for US27) or 45 °C (for UL109), cycling was continued 30 or 60 times (as noted in Sections 3.6 and 3.7), and the Tm during each cycle was 62 °C (for US27) or 55 °C (for UL109). Approximately 50 ng of a green fluorescent protein-HCMV (AD169) recombinant bacterial artificial chromosome (Marschall et al., 2000) was also employed as a positive control for each set of reactions. PCR products were resolved on 2% agarose gels in SB buffer (Brody and Kern, 2004), stained with ethidium bromide, and images of the gels were captured on a Bio-Rad Molecular Imager Gel Doc XR system (Bio-Rad, Hercules, CA). Digital images were then compiled with Adobe Photoshop and Illustrator.
3. Results
3.1. In vitro transcription–translation and immunoprecipitation
These experiments were initiated by PCR amplifying (Scharf, 1990) the HCMV US27 ORF (Chee et al., 1990a,b) from a genomic fragment and cloning it into a vector, pcDNA1, to produce the plasmid BJM2.1, as described in Section 2.1. The protein encoded by BJM2.1 was then synthesized in a TNT coupled in vitro transcription–translation system, along with the luciferase protein, encoded on a plasmid included in the TNT kit as a positive control. Small portions of the reaction mixtures were subjected to SDS-PAGE in a 12% polyacrylamide gel.
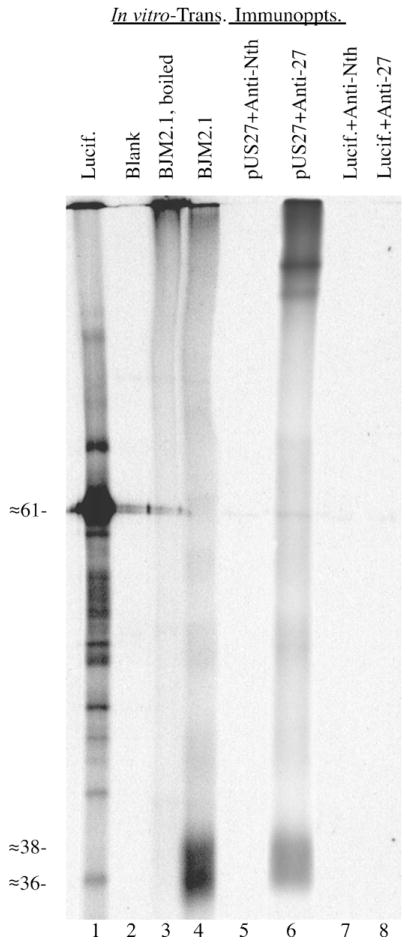
An image prepared from the resulting gel showed that the luciferase control plasmid predominantly gave rise to the expected ≈61-kDa luciferase protein (Fig. 1, lane 1) and US27 in BJM2.1 gave rise to a broad, ≈36–38-kDa band (pUS27) (Fig. 1, lane 4). Neither of these proteins was present in the control with no DNA added (Fig. 1, lane 2). The apparent size of this pUS27 band is smaller than expected from the predicted amino acid sequence of the US27 ORF (i.e., ≈41 kDa). When heated in solubilization buffer before SDS-PAGE, pUS27 aggregated and did not appreciably enter the resolving gel (Fig. 1, lane 3). The anomolously small size and heat sensitivity of pUS27 are attributed to strong irreversible intra- and inter-molecular bonds, resulting from non-covalent interactions between the proteins’ multiple hydrophobic transmembrane domains. Both the size discrepancy and heat sensitivity in SDS-PAGE are frequently observed with membrane-bound proteins (Maeshima, 1991; Sazuka et al., 2004; Schubert et al., 1992; Semenza et al., 1990), especially cellular (Keinanen et al., 1987) and viral (Chiou et al., 2002; Zhen et al., 2005) GPCRs, including pUL33 (Margulies et al., 1996).
Fig. 1.
In vitro transcription–translation and immunoprecipitation of pUS27. The plasmid BJM2.1 (1 μg; BJM2.1; lanes 3 and 4) was transcribed and translated in vitro in a 25 μL TNT coupled reaction. Reactions containing no DNA (Blank; lane 2) or DNA encoding luciferase (Lucif.; lane 1) were also included. A small sample (1.5 μL) was removed from each reaction, solubilized, boiled (lanes 1–3) or not heated (lane 4), and subjected to SDS-PAGE and fluorography. Immunoprecipitations of in vitro-translated luciferase and pUS27 were done with Anti-27 (+Anti-27; lanes 6 and 8) or Anti-Neurothelin (+Anti-Nth; lanes 5 and 7) antiserum, and analyzed in parallel. Samples in lanes 4–8 were not heated prior to SDS-PAGE. An image of the resulting fluorograph was captured with Adobe Photoshop for Macintosh via a Umax PowerLook II scanner.
In vitro-synthesized pUS27 was then used in an immuno-precipitation experiment to validate the reactivity of Anti-27, an antiserum prepared to identify the US27 protein in HCMV-infected cells (Section 2.3). Portions (7 μL) of the BJM2.1 and luciferase in vitro-translation reactions described above were subjected to immunoprecipitation with either Anti-27 or Anti-Neurothelin (irrelevant antiserum control), as described in Section 2.3. Anti-27 immunoprecipitated in vitro-synthesized pUS27 (Fig. 1, lane 6), but not luciferase (Fig. 1, lane 8), and neither pUS27 nor luciferase was immunoprecipitated by the irrelevant antiserum (Fig. 1, lanes 5 and 7). As was observed with immunoprecipitation of the in vitro-synthesized pUL33 with the Anti-33 antiserum (Margulies, 1996) and another GPCR (Rozell et al., 1998), immunoprecipitation with Anti-27 promoted a significant amount of aggregation of pUS27 (Fig. 1, lane 6, see top of lane).
3.2. The US27 gene product is present in HCMV-infected cell membranes and is N-glycosylated
The qualified Anti-27 serum was then used as a probe to identify the US27-encoded GPCR produced in HCMV-infected HFF cells; we called this protein GCR27 to differentiate it from the recombinant protein, pUS27 (Fig. 1), and for consistency with the designation GCR33, given the UL33-encoded protein in HCMV-infected cells (Margulies et al., 1996). Membrane fractions, prepared 8 days after infection with HCMV, were used as the starting material because earlier work showed that GCR33 was enriched and more easily identified there (Margulies et al., 1996). These membrane preparations from HCMV-infected and non-infected cells were subjected to SDS-PAGE in a 12% polyacrylamide gel, followed by Western immunoassay with the Anti-27 antiserum, as described in Sections 2.3–2.6.
An image prepared from the Western blot showed a broad, high-molecular-weight band of Anti-27-reactive material (≈ 80–180 kDa) in the membrane preparation from infected or cells (Fig. 2A, lane 3, left-margin brackets), but not in that from non-infected cells (Fig. 2A, lane 1). The high-molecular-weight material was also discernable as two discrete regions, from approximately 80 to 130 kDa and from about 150 to 180 kDa. The size of this material, which was larger than predicted based on the translated nucleotide sequence of the US27 ORF (Chee et al., 1990a,b), and its heterogeneity suggested that GCR27, like GCR33, is extensively N-glycosylated (Margulies et al., 1996). To test this hypothesis, equal portions of the same membrane preparations were treated with PNGase F (Tarentino and Plummer, 1994), to remove N-linked oligosaccharides, and analyzed in parallel. PNGase F treatment resulted in the appearance of two smaller bands (≈41 and ≈37 kDa, Fig. 2A, lane 4, circles) that were not present in either the nontreated, infected cell (Fig. 2A, lane 3) or the PNGase F-treated, non-infected (Fig. 2A, lane 2) preparations. Deglycosylation did not appear to be complete, though, as there was residual high-molecular-weight, Anti-27 reactive material still present after PNGase F treatment (Fig. 2A, lane 4). The finding that GCR27 was detected as a pair of bands following analysis of material treated with PNGase F is in contrast to findings with GCR33, which was detected as a single band in similar assays (Margulies et al., 1996); however, the deglycosylated GCR27 doublet (Fig. 2A, lane 4) is consistent with the two separate electrophoretic mobility groups of glycosylated GCR27 (Fig. 2A, lane 3) and with the appearance of two proteins from in vitro-translated BJM2.1 (Fig. 1, lane 4).
3.3. Direct comparison of in vitro-translated pUS27 and deglycosylated GCR27 by SDS-PAGE
The appearance of a protein doublet for both pUS27 (Fig. 1, lane 4) and GCR27 (Fig. 2A, lane 3) led us to examine the relationship between these polypeptides. Samples of deglycosylated GCR27 and in vitro-synthesized pUS27 were compared side-by-side on the same gel to see if the respective bands of each doublet co-migrated. In vitro-synthesized pUS27 was radiolabeled during synthesis with [35S]-methionine (Section 2.2) while deglycosylated GCR27 was detected by Western immunoassay with Anti-27 and [125I]-labeled protein A (Sections 2.3–2.6).
As was shown in Fig. 2A, no material was detected in non-infected cell membranes (Fig. 2B, lanes 5 and 6); HCMV-infected cell membranes that were not deglycosylated contained high-molecular-weight GCR27 that was too large to be displayed in the selected window for Fig. 2B, but is displayed for other preparations (Figs. 2A and C, 3A, 4C, and 5). Deglycosylated GCR27 appeared as a partially resolved doublet of approximately 37 and 41 kDa (Fig. 2B, lane 8), as previously observed (Fig. 2A, lane 4). An in vitro translation reaction containing no DNA did not give rise to any [35S]-labeled polypeptides that resolved in the selected range (Fig. 2B, lane 9), while pUS27 synthesized from BJM2.1 gave rise to a poorly resolved ≈36–38-kDa doublet (Fig. 2B, lane 10), as was previously observed (Fig. 1, lane 4). Therefore, although both deglycosylated GCR27 and pUS27 can be resolved as doublets in SDS-PAGE, their respective apparent molecular weights do not match exactly. This characteristic is common for membrane-bound proteins, in which recombinant polypeptide products are misfolded when compared to the bona fide polypeptides, and these misfolded species migrate anomalously in SDS-PAGE (Chiou et al., 2002; Keinanen et al., 1987; Maeshima, 1991; Margulies et al., 1996; Sazuka et al., 2004; Schubert et al., 1992; Semenza et al., 1990; Zhen et al., 2005).
Fig. 3.
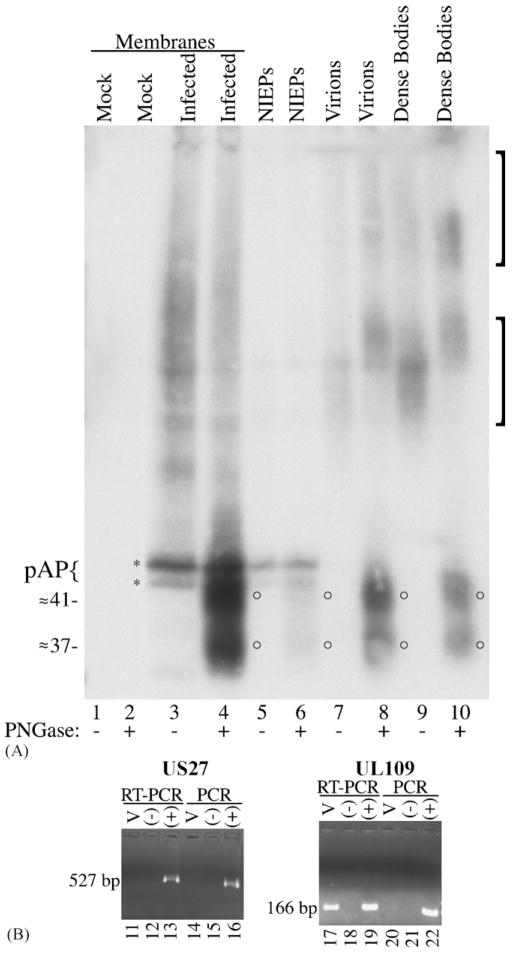
(A) N-Glycosylated GCR27 is present in HCMV extracellular enveloped particles. Cell membranes were prepared from non-infected (mock; lanes 1 and 2) or HCMV-infected (infected; lanes 3 and 4) HFF cells 8 days after infection and treated or not treated with PNGase F (±PNGase). HCMV enveloped extracellular virus particles, NIEPs (lanes 5 and 6), virions (lanes 7 and 8), and dense bodies (lanes 9 and 10), were purified from infected cells, treated or not treated with PNGase F (± PNGase), and subjected to SDS-PAGE, followed by Western immunoassay with a mixture of Anti-27 and Anti-C1, an antiserum specific for the CMV assembly protein precursor, pAP (Schenk et al., 1991; Welch et al., 1991b). Brackets denote two forms of glycosylated GCR27; circles denote deglycosylated forms of GCR27; brace and asterisks denote pAP (top) and pUL80.4, a slightly smaller protein also encoded by the HCMV UL80 nested genes (Casaday et al., 2004; Welch et al., 1991a). An image of the resulting fluorograph was captured with Adobe Photoshop for Macintosh via an Epson 1200 U scanner. (B) RT-PCR assay for UL27 and UL109 RNAs in virus particles. Clarified HCMV inoculum (100 μL) was treated with DNase-free RNase, then RNA was isolated from these samples (lanes 11, 14, 17, and 20) and subsequently treated with RNase-free DNase. Negative controls (lanes 12, 15, 18, and 21) contained no RNA; positive controls (lanes 13, 16, 19, and 22) contained approximately 50 ng of DNA from an HCMV (AD169) bacmid (Marschall et al., 2000), to show that RT-PCR and PCR did not fail because of poor enzyme cycling conditions. One-twentieth of the RNA isolated from virus inoculum was used for each experiment. Samples were amplified in the presence of an RT/Taq DNA polymerase mix (RT-PCR, lanes 11–13 and 17–19) or Platinum Taq DNA polymerase alone (PCR, lanes 14–16 and 20–22) for 60 cycles. PCR products were resolved on 2% agarose gels in SB buffer (Brody and Kern, 2004), stained with ethidium bromide, and photographed on a UV transilluminator as part of a Bio-Rad Molecular Imager Gel Doc XR system. Markers (1-kb markers or PCR markers, both from Promega) run in adjacent lanes allowed characterization of the 527-bp US27 PCR product and the 166-bp UL109 PCR product.
Fig. 4.
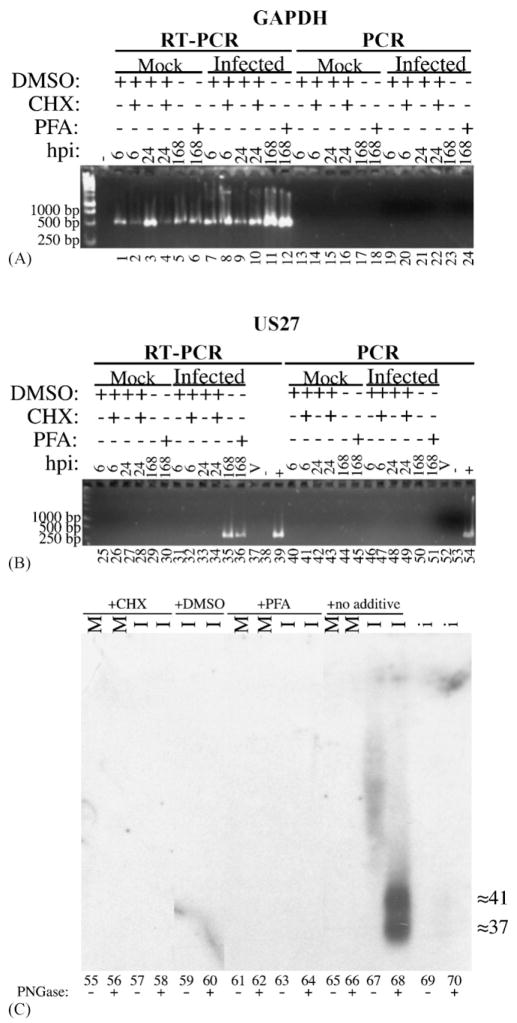
(A) Detection of RNA for the cellular housekeeping gene GAPDH. Total RNA was isolated from mock-infected (lanes 1–6 and 13–18) or HCMV-infected (lanes 7–12 and 19–22) human fibroblasts at 6 h (lanes 1, 2, 7, 8, 13, 14, 19, and 20), 24 h (lanes 3, 4, 9, 10, 15, 16, 21, and 22), and 168 h (lanes 5, 6, 11, 12, 17, 18, 23, and 24) after infection. These cells were treated 1 h prior to infection, then continuously until RNA was harvested, with either CHX in DMSO (lanes 2, 4, 8, 10, 14, 16, 20, and 22), DMSO alone (lanes 1, 3, 7, 9, 13, 15, 19, and 21), PFA (lanes 6, 12, 18, and 24), or no agent (lanes 5, 11, 17, and 23). A negative control (-) contained no RNA. Amplification was conducted in the presence of either an RT/Taq polymerase mix (RT-PCR; lanes 1–12) or only Platinum Taq DNA polymerase (PCR; lanes 13–24) for 30 cycles. One-twentieth of the RNA isolated from approximately 106 cells was used for each experiment. PCR products were resolved, stained, and imaged as in Fig. 3. Markers (1-kb markers, Promega) run in adjacent lanes allowed characterization of 1000-, 500-, and 250-base pair regions; sizes (bp) of marker DNAs are indicated beside each image. Primers were designed to produce an approximately 500-bp product from a target in the middle of the gene. (B) Time course of expression of the RNA produced from the viral US27 gene. One-Step RT-PCR and PCR were performed as in part A for 30 cycles. Primers were designed to produce a 527-bp product from the middle of US27. Virion RNA, isolated as described in Fig. 3B, was also included (V; lanes 37 and 52); negative controls (lanes 38 and 53) contained no RNA. A positive control (lanes 39 and 54) contained approximately 50 ng of DNA of an HCMV bacmid, as in Fig. 3. (C) Time course of expression of the GCR27 glycoprotein. Membranes were isolated from HCMV-infected (I; lanes 57, 58, 59, 60, 63, 64, 67, and 68) or mock-infected (M; lanes 55, 56, 61, 62, 65, and 66) cells treated with either CHX in DMSO (lanes 55–59), DMSO alone (DMSO; lanes 59 and 60), PFA (PFA; lanes 61–64), or no treatment (lanes 65–68) at either 24 h (for CHX and DMSO) or 168 h (for PFA and no additive) after infection. These membrane preparations were then either treated (lanes 56, 58, 60, 62, 64, 66, and 68) or not treated (lanes 55, 57, 59, 61, 63, 65, and 67) with PNGase F. A portion (50 μL) of the inoculum used for these experiments was similarly treated with PNGase F (i; lanes 69 and 70). Samples were resolved by SDS-PAGE, transferred to Immobilon-P, and analyzed by Western blot with the Anti-27 antiserum. Molecular weights (in kDa) were estimated from pre-stained markers (Multi Mark™; Invitrogen) included during SDS-PAGE that were electrotransfered to the Immobilon-P. An image of the resulting fluorograph was captured with Adobe Photoshop for Macintosh via an Epson 1200 U scanner.
Fig. 5.
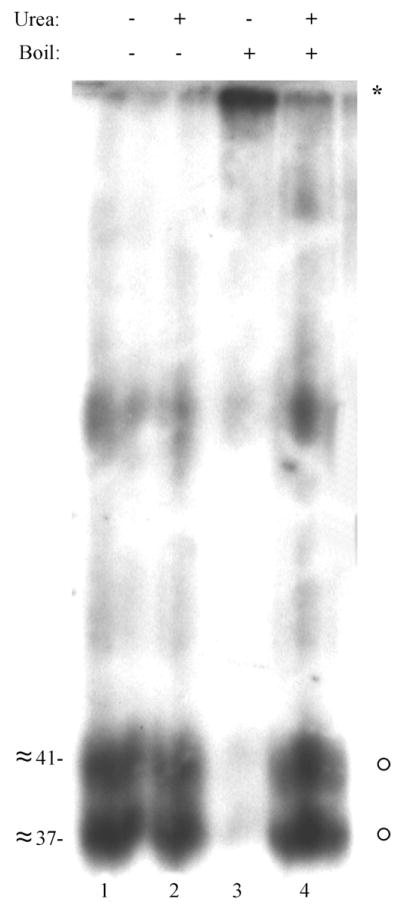
Urea abrogates the heat-induced aggregation of GCR27. A preparation of dense bodies was treated with PNGase F. Half was denatured in solubilizing buffer without urea, and either boiled (lane 3) or not boiled (lane 1). The other half of the preparation was denatured in solubilizing buffer containing 4 M urea, and either boiled (lane 4) or not boiled (lane 2). All samples were resolved by 12% SDS-PAGE and then analyzed by Western blot with Anti-27. Molecular weights (in kDa) were estimated from molecular weight markers (see Blue™ Prestained Markers; Novex) included during SDS-PAGE that were electrotransfered to the Immobilon-P. An image of the resulting fluorograph was captured with Adobe Photoshop for Macintosh via a Umax PowerLook II scanner.
3.4. Anti-27 detects GCR27 uniquely and specifically
To further prove the specificity of Anti-27, we compared the reactivity of this antiserum against a previously characterized antiserum, Anti-33, which reacts specifically with another HCMV GPCR encoded by UL33 (Margulies et al., 1996). A single gel (Fig. 2C) was run with mock-infected (Fig. 2C, lanes 11, 12, 15, and 16) and HCMV-infected (Fig. 2C, lanes 13, 14, 17, and 18) cell membranes that were either not treated (Fig. 2C, odd numbered lanes) or treated (Fig. 2C, even-numbered lanes) with PNGase F. One-half of the gel was processed for a Western blot with Anti-27 (Fig. 2C, lanes 11–14); the other was processed with Anti-33 (Fig. 2C, lanes 15–18). As shown previously, Anti-27 reacted with only HCMV-infected cell material (Fig. 1C, lanes 13 and 14), and deglycosylated GCR27 appeared as a doublet of closely spaced bands (Fig. 2C, lane 14). Anti-33 reacted with only infected cell material (Fig. 2C, lanes 17 and 18); however, deglycosylated GCR33 was specifically detected as a ≈43-kDa band (Fig. 2C, lane 18), a slightly higher apparent molecular weight than the upper band of deglycosylated GCR27 (Fig. 2C, lane 14). Therefore, the Anti-27 antiserum is not cross-reactive with GCR33, and the Anti-33 antiserum is not cross-reactive with GCR27.
3.5. Presence of GCR27 in HCMV enveloped virus particles
GCR27 was also detected in preparations of glycerol/tartrate gradient-purified (Talbot and Almeida, 1977) enveloped virus particles. Virions, non-infectious enveloped particles (NIEPs), and dense bodies, the three forms of extracellular enveloped particles produced by HCMV (Irmiere and Gibson, 1983), were treated with PNGase F or not treated as described (Margulies et al., 1996) and subjected to SDS-PAGE and Western immunoassay with the Anti-27 antiserum. The antiserum Anti-C1, specific for the capsid assembly protein precursor (pAP) that is present in NIEPs, but not virions or dense bodies (Irmiere and Gibson, 1983; Schenk et al., 1991; Welch et al., 1991b), was mixed with Anti-27 to demonstrate the resolution of these extracellular particles, to provide an internal size marker for the GCR27 bands, and to help visualize and distinguish the NIEP patterns. Cell membrane preparations were included as controls.
Results obtained from the cell membrane controls were essentially the same as before (i.e., Fig. 2A); Anti-27 reacted with high-molecular-weight, heterogeneous material in HCMV-infected cell membrane preparations (Fig. 3A, lane 3), and PNGase F treatment of these membranes resulted in the appearance of ≈37- and ≈41-kDa bands (Fig. 3A, lane 4, circles), neither of which was seen in the non-treated membrane preparation (Fig. 3A, lane 3) or in non-infected cells (Fig. 3A, lanes 1 and 2). The ≈45–50-kDa pAP doublet, detected by Anti-C1 in this experiment, is not known to be glycosylated, consistent with its unchanged size in the PNGase F-treated preparations (Fig. 3A, lanes 3–6). Furthermore, the similar intensity of the pAP band in lanes 4 versus 3, and 6 versus 5, indicates that the PNGase F treatment did not grossly alter the amount of material present.
Results similar to those seen with infected cell membranes were obtained with the NIEP (Fig. 3A, lanes 5 and 6), virion (Fig. 3A, lanes 7 and 8), and dense body (Fig. 3A, lanes 9 and 10) preparations. High-molecular-weight, Anti-27-reactive material was present in enveloped particles that were not treated with PNGase F (Fig. 3A, lanes 5, 7, and 9, brackets); this material resolved as a pair of glycosylated forms of GCR27 of approximately 80–130 and 150–180 kDa, consistent with the electrophoretic migration of glycosylated GCR27 found in infected cell membranes (Fig. 2A, lane 3). Bands of ≈37- and ≈41-kDa were observed in all three preparations of enveloped virus particles following PNGase F treatment (Fig. 3A, lanes 6, 8, and 10, circles). Characteristically only NIEPs contained detectable pAP (Fig. 3A, lanes 5 and 6). Consistent with the membrane preparations (Fig. 2A, lane 4 and Fig. 3A, lane 4), deglycosylation of GCR27 in particles was incomplete, as evidenced by residual higher-molecular-weight material that reacted with Anti-27 (Fig. 3A, lanes 8 and 10, in particular). The fact that GCR27 presumably contains seven membrane-spanning domains, is homologous to other GPCR proteins that are characteristically plasma membrane constituents, and is present in HCMV dense bodies, which are composed of only a viral envelope surrounding an aggregation of predominantly the lower matrix protein (Gibson, 1983; Irmiere and Gibson, 1983; Varnum et al., 2004), provides compelling evidence that this protein is an envelope constituent, like other herpesvirus GPCR homologues (Fraile-Ramos et al., 2002; Margulies et al., 1996; Oliveira and Shenk, 2001; Penfold et al., 2003).
3.6. US27 RNA is not present in HCMV virions
Several reports have described the presence of HCMV-specific RNAs in mature virions, usually associated with the tegument (Bresnahan and Shenk, 2000; Greijer et al., 2000; Sarcinella et al., 2004; Terhune et al., 2004). If the US27 RNA were also incorporated into virions, it would provide a means for GCR27 expression prior to de novo RNA synthesis, as part of the infectious material delivered by incoming particles. Therefore, to investigate this possibility, RNA was isolated from the clarified inoculum that was used for the previous experiments (Figs. 2 and 3A).
Using 30 cycles of RT-PCR and PCR, we were unable to detect the presence of RNA for UL109, previously shown to be incorporated into virions (Bresnahan and Shenk, 2000), in our HCMV inoculum (data not shown). Improving the sensitivity of the RT-PCR and PCR reactions by increasing to 60 cycles of amplification revealed the presence of the expected UL109 RNA in virions (Fig. 3B, lane 17). Under the same PCR conditions, and using the same starting material, RNA for US27 was never detected (Fig. 3B, lane 11), indicating either its absence from virions or an abundance below the limit of detection.
3.7. US27 is expressed as a γ1 RNA in the presence of cycloheximide or phosphonoformic acid
Previous reports showed that blocking HCMV infection with phosphonoformic acid prevented expression of the RNA for US27, placing this open reading frame into the true late gene (γ2) category (Honess and Roizman, 1974; Mocarski and Courcelle, 2001; Roizman and Pellett, 2001; Welch et al., 1991a). More recent work suggested that the US27 gene is transcribed at earlier times during infection (Chambers et al., 1999), so we attempted to resolve this discrepancy by using a comparatively more sensitive RT-PCR assay to investigate the gene’s time course of expression. HCMV-infected cells were blocked at various times after infection with either CHX, to prevent translation of immediate-early proteins and abate the transition to early stage infection (Fioretti et al., 1973; Honess and Roizman, 1974; Jeang and Gibson, 1980; Michelson-Fiske et al., 1977; Mocarski and Courcelle, 2001), or PFA, to diminish DNA synthesis and the complete transition to late stage infection (Honess and Roizman, 1974; Mocarski and Courcelle, 2001; Welch et al., 1991a). A parallel set of mock- and HCMV-infected cells were also treated with only 0.05% DMSO, the solvent for CHX, as a control for this solvent in the CHX-treated cells.
RT-PCR amplification of these samples (Fig. 4A) showed that RNA for the GAPDH housekeeping gene was present in every RNA sample recovered (Fig. 4A, lanes 1–12), as evidenced by the presence of an approximately 500-bp PCR product that was not present in RT-PCR reactions without RNA (Fig. 4A, lane marked with minus sign). To control for the possible presence of residual genomic DNA in the samples, the same RNAs were subjected to the same amplification conditions without a reverse transcriptase (i.e., with Platinum Taq DNA polymerase alone) (Fig. 4A, lanes 13–24); no PCR product was synthesized in any of these experiments. Hence there was either no DNA contamination present in the samples, to serve as a false template for amplification in the RT-PCR reactions (Fig. 4A, lanes 1–12), or the amount of DNA was below the limit of detection for the assay. In either instance, the lack of a PCR product, yet the qualitative presence of an RT-PCR product, for GAPDH showed that the RNA samples were intact and of good quality for the subsequent detection of US27 transcripts.
RT-PCR of these qualified RNAs under similar conditions (see Sections 2.7 and 2.8) to detect US27 transcripts resulted in amplification products of 527 base pairs (Fig. 4B, lanes 35 and 36) that were not present in samples without RNA (Fig. 4B, lane 38), or RNA from non-infected cells (Fig. 4B, lanes 25–30). These US27-derived PCR products were present in only those HCMV-infected cell samples from 168 h post-infection, whether treated with PFA (Fig. 4B, lane 36) or left untreated (Fig. 4B, lane 35), while the same RT-PCR product was not present in RNAs isolated from any other times post-infection and treated with either CHX (in DMSO) or DMSO alone (Fig. 4B, lanes 31–34). As with GAPDH, PCR amplification alone with only Platinum Taq DNA polymerase (Fig. 4B, lanes 40–51) generated no product, proving that the RT-PCR products in Fig. 4B, lanes 35 and 36 arose from only RNA.
Although strictly not the RNA expression pattern of a typical truly late gene (Honess and Roizman, 1974; Mocarski and Courcelle, 2001; Roizman and Pellett, 2001), the US27 gene may be characterized as γ1, “leaky” or “early-” late gene; that is, the US27 RNA is expressed regardless of the presence of PFA (Boriskin and Butcher, 2001; Honess and Roizman, 1974; Mocarski and Courcelle, 2001), yet is not transcribed in the presence of CHX (Honess and Roizman, 1974; Mocarski and Courcelle, 2001).
3.8. Temporal regulation of GCR27 expression
We also examined the temporal control of expression of the glycoprotein encoded by US27, GCR27. Cells were treated with either CHX, DMSO, or PFA, as above (Sections 2.5, 2.7 and 3.7); membrane preparations were collected and were either treated or not treated with PNGase F (Section 2.6), then subjected to SDS-PAGE and Western blot with the Anti-US27 antiserum (Section 2.4). A single aliquot (50 μL) of inoculum was also prepared in the same fashion, to assay for the presence of GCR27 as a component of entering virions.
The GCR27 glycoprotein was detected in only samples that were left untreated through 168 h after infection (Fig. 4C, lanes 67 and 68). As was shown previously (Figs. 2 and 3A), the heavily glycosylated forms of GCR27 (Fig. 4C, lane 67) could be resolved as two closely spaced products of about 37 and 41 kDa when samples were first treated with PNGase F (Fig. 4C, lane 68). No Anti-27 reactive material was found in mock-infected cell membrane preparations (Fig. 4C, lanes 55, 56, 61, 62, 64, and 65), and also none could be detected when HCMV-infected cells were treated with CHX for 24 h (Fig. 4C, lanes 57 and 58), DMSO for 24 h (Fig. 4C, lanes 59 and 60), or PFA for 168 h (Fig. 4C, lanes 63 and 64), perhaps because the amount produced in this last case was below our limit of detection. Although extremely light, a very small amount of deglycosylated GCR27 is present in the inoculum at approximately 37 and 41 kDa (Fig. 4C, lane 70), showing the extremely low quantities of GCR27 that arrive in the infected cell with virus particles. Therefore, although the US27 RNA is present as a γ1 transcript (Section 3.7), the glycoprotein expressed from that RNA appears to be expressed most abundantly with truly late kinetics, corroborating the first report describing US27 RNA expression (Welch et al., 1991a).
3.9. Aggregation of GCR27 by heating in the presence of SDS can be decreased with the addition of urea
To determine whether the heat-induced aggregation of GCR27 could be abrogated, samples of deglycosylated GCR27 were analyzed for their electrophoretic mobility once SDS was added and the samples were boiled. Dense bodies, enriched in GCR27, were isolated by positive density-negative viscosity gradients (Section 2.5) and deglycosylated with PNGase F (Section 2.6), then analyzed by SDS-PAGE and Western blot with Anti-27 (Section 2.4). As seen with pUS27 expressed in vitro from BJM2.1 (Fig. 1, lane 3), heating the samples containing SDS before electrophoresis resulted in significant aggregation of GCR27 (Fig. 5, lane 3, asterisk) that was not observed when the samples were not heated (Fig. 5, lane 1). Addition of urea to a final concentration of 4 M did not alter the mobility of the polypeptides when left unheated (Fig. 5, lane 2). Most importantly, adding urea significantly reduced the heat-induced aggregation of deglycosylated GCR27 (Fig. 5, lane 4, circles), which migrated with the same electrophoretic mobility as deglycosylated GCR27 that was not heated (Fig. 5, lanes 1 and 2). Therefore, as has been observed with other membrane proteins (Schubert et al., 1992; Soulie et al., 1996), addition of urea to SDS-PAGE sample buffer appears to diminish the non-denaturable intra- and inter-molecular interactions that cause aggregation of these polypeptides when heated with SDS.
4. Discussion
Our most significant findings are that the HCMV GPCR, GCR27, (i) is transcribed as a γ1, or “leaky late phase” RNA, but is synthesized predominantly as a γ2, or “true late phase” protein; (ii) does not have a transcript incorporated into the virion; (iii) is heterogeneously N-glycosylated; and (iv) is present in all three forms of extracellular enveloped virus particles.
Previous experiments showed that the US27 RNA was expressed either early (Chambers et al., 1999) or late (Bodaghi et al., 1998; Welch et al., 1991a), a finding potentially in line with the appearance of RNAs for the HCMV US28 (Zipeto et al., 1999) and MCMV M33 (Davis-Poynter et al., 1997) GPCR homologues at more than one time period after infection. However, our data corroborate previous studies that showed late expression for US27 (Welch et al., 1991a). The more sensitive RT-PCR assay used here enabled us to extend those observations to show that US27 is expressed as a γ1 transcript (Fig. 4). The appearance of US27 RNA as a γ1 transcript (Fig. 4) does not conflict with translational control of the mRNA, resulting in expression of the GCR27 glycoprotein as a γ2, true late, polypeptide (Fig. 4).
GCR27 shares similar biochemical properties (i.e., N-glycosylation, appearance in enveloped virus particles, and susceptibility to heat-induced aggregation) with another HCMV chemokine receptor homologue, GCR33 (Margulies, 1996; Margulies et al., 1996). However, there is one major difference between the two, in that GCR27 resolves as two species upon treatment with PNGase F. One explanation for this doublet of GCR27 could be incomplete deglycosylation by PNGase F, which would be consistent with the residual high-molecular-weight GCR27, but less compatible with the generally comparable amounts of the ≈37- and ≈41-kDa proteins in different preparations and different assays and the presence of two glycosylated forms of GCR27 when left untreated (Figs. 2A and C, 3A, and 4C). This potential explanation is also not easily reconciled with the production of two apparent counterpart proteins from the US27 ORF via rabbit reticulocyte lysates (Fig. 1), which are generally unable to N-glycosylate without pancreatic microsomes added to the reaction mix (Roberts and Lord, 1981; Walter and Blobel, 1983). Other explanations for the doublet include (i) differential type or degree of post-translational modification, e.g., phosphorylation (Roth et al., 1997) or palmitoylation (Ovchinnikov et al., 1988); (ii) strong intramolecular interactions between hydrophobic domains giving rise to electrophoretically different forms of the protein, which gains some credence from the observation that in vitro-synthesized pUS27, lacking most post-translational modifications (Roberts and Lord, 1981; Walter and Blobel, 1983), yielded a broad band that resolved into a doublet (Figs. 1 and 2B); or (iii) a difference in the length of the polypeptide backbone which did not occur at the carboxyl ends of pUS27 and GCR27 (Fig. 5; note that the presumed carboxyl ends of each polypeptide in each doublet are most likely identical because each pair reacts with the carboxy-terminal-directed Anti-27 antiserum), potentially due to alternate splicing at the 5′ end of the transcript, as observed with other chemokine receptors and their homologues (Davis-Poynter et al., 1997; Gerard and Gerard, 1994), or other differences in polypeptide expression due to differences in the 5′ end of the US27 mRNA. We presently cannot discriminate among these possibilities, although we are currently investigating them all because they may reflect novel means of gene expression not commonly observed in HCMV.
The finding that the US27 proteins identified in deglycosylated infected cell preparations (i.e., ≈37 and ≈41 kDa, Figs. 2–5) are larger than pUS27 synthesized in vitro (i.e., ≈36–38 kDa, Figs. 1 and 2B) was also true of the recombinant HCMV UL33 protein (Margulies et al., 1996). One explanation for this difference is mRNA splicing, increasing the coding region at the 5′ end of the message, as reported for the GPCR encoded by UL33 (Davis-Poynter et al., 1997; Margulies et al., 1996). Secondly, pUS27 may have an altered electrophoretic mobility caused by misfolding (e.g., non-denaturable intramolecular interactions). Alternatively, or in addition, the infected cell proteins may contain post-translational modifications not present on the in vitro-synthesized product, as suggested above for the appearance of the polypeptide doublets. It should be noted, though, that neither palmitoylation nor phosphorylation are large enough individually to account directly for the 1–3-kDa molecular weight discrepancy; nevertheless, such modifications are known to have unexpectedly large influences on electrophoretic mobility (Casaday et al., 2004).
As with GCR33 (Margulies et al., 1996), GCR27 was found in virions (Fig. 3A), an observation in line with previous studies (Varnum et al., 2004), and the other two types of extracellular enveloped virus particles, NIEPs and dense bodies (Fig. 3A). However, the aforementioned previously published experiments failed to detect GCR27 in dense bodies (Varnum et al., 2004); furthermore, those experiments did not identify the chemokine receptor homologues encoded by HCMV UL78 (Oliveira and Shenk, 2001) and US28 (Penfold et al., 2003) in the virion and GCR33 in dense bodies (Margulies et al., 1996), data that were previously established. Perhaps these glycoproteins were absent in the preparations of Varnum et al., because of alternative virus particle purification schemes (Varnum et al., 2004) which frequently result in altered contents of the purified material. The use of positive density-negative viscosity tartrate-glycerol gradients (Talbot and Almeida, 1977) has been documented as a stringent and effective method for purifying intact extracellular HCMV particles (Irmiere and Gibson, 1983).
Because dense bodies are composed primarily of lower matrix protein (pp65) and the viral envelope (Gibson, 1983; Irmiere and Gibson, 1983; Varnum et al., 2004), and because GPCRs are typically associated with a lipid bilayer (Fraser et al., 1994), GCR27 is likely to be a constituent of the virus envelope as posited previously (Margulies et al., 1996). Although not proven to transduce a G protein-mediated signal constitutively like its other CMV counterparts M33 and GCR28 (Casarosa et al., 2003; Gruijthuijsen et al., 2002; Minisini et al., 2003; Pleskoff et al., 2005; Waldhoer et al., 2002), the presence of GCR27 in the virion would allow for its deposition in the infected cell plasma membrane, along with the rest of the envelope, immediately upon infection of a target cell. It should be noted, however, that early signal transduction events are also initiated by interactions of incoming virions with cellular EGF receptors and αvβ3 integrins (Evers et al., 2004; Wang et al., 2003, 2005). The presence of GCR27 in the newly infected cell’s membrane, though, could thereby provide a means to initiate or contribute to the signal transduction events observed early during HCMV infections (AbuBakar et al., 1990a,b; Albrecht et al., 1991; Fons et al., 1991; Nokta et al., 1988; Shibutani et al., 1997; Valyi-Nagy et al., 1988), as suggested previously (Margulies et al., 1996; Oliveira and Shenk, 2001; Waldhoer et al., 2002; Welch et al., 1991a).
All of the functional viral GPCR homologues identified to date are encoded by members of the β and γ-herpesvirus groups or by poxviruses. GCR27 is likely to contribute to this same theme; although a ligand for GCR27 has not yet been identified (Waldhoer et al., 2002), characteristics in its amino acid sequence make it likely to be a member of the chemokine receptor sub-family of GPCRs (Horuk, 1994; Murphy, 1994; Murphy, 1996): four consensus N-glycosylation sites in its acidic amino terminal domain, a short second intracellular loop having a net basic charge, and a pair of conserved cysteines, one in the amino terminal domain and one in the third extracellular loop. Although the HCMV GPCRs encoded by US27, US28, and UL33 are dispensable for growth in cell culture (Bodaghi et al., 1998; Dunn et al., 2003; Margulies et al., 1996), it is thought that they may have important immunomodulatory functions during the course of natural infections (Margulies et al., 1996; Michelson, 2004; Murphy, 1996; Smit et al., 2003). Deleting the MCMV GPCR homologue M33 or rat CMV (RCMV) homologues encoded by R33 or R78 results in severely attenuated viruses in vivo that grow to wild-type titers in vitro (Beisser et al., 1998; Davis-Poynter et al., 1997; Kaptein et al., 2003), providing evidence that these glycoproteins play a significant role in pathogenesis in the whole infected organism. Discerning the enhancements in vivo provided by GCR27, though, may be difficult to determine.
Although MCMV and RCMV provide valuable model systems for studying viral protein function in the context of a natural host, these viruses both lack homologues of HCMV GCR27 and the protein encoded by HCMV US28 (i.e., GCR28) (Rawlinson et al., 1996; Vink et al., 2000). Therefore, other means of studying the biology of GCR27 and GCR28, as well as complementary ways to investigate GCR33, must be pursued to determine the biological significance and potential clinical relevance of these HCMV chemokine receptor homologues.
Acknowledgments
We thank Jenny Borchelt, Amy Loveland, Sara Meerdter, Nahir Romero, and Joseph Norris for excellent technical assistance. Marc Chee (Medical Research Council) provided the AD169 HindIII B fragment. This work was initiated by BJM as part of a thesis project in the Biochemistry, Cellular, and Molecular Biology graduate program at Johns Hopkins University School of Medicine (USPHS grant T32 GM07445), and was aided by grants from the Towson University Faculty Development program (to BJM), the REU and Bridges to the Baccalaureate program from the NIH to Towson University, and USPHS research grants AI13718 and AI227711 (to WG).
References
- AbuBakar S, Boldogh I, Albrecht T. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase A2 and protein kinase C. Biochem Biophys Res Commun. 1990a;166(2):953–959. doi: 10.1016/0006-291x(90)90903-z. [DOI] [PubMed] [Google Scholar]
- AbuBakar S, Boldogh I, Albrecht T. Human cytomegalovirus: stimulation of [3H] release from [3H]-arachidonic acid prelabelled cells. Arch Virol. 1990b;113:255–266. doi: 10.1007/BF01316678. [DOI] [PubMed] [Google Scholar]
- Albrecht T, Fons MP, Boldogh I, AbuBakar S, Deng CZ, Millinoff D. Metabolic and cellular effects of human cytomegalovirus infection. Transplant Proc. 1991;23(3):48S–55S. [PubMed] [Google Scholar]
- Attwood TK, Findlay JBC. Design of a discriminating fingerprint for G-protein-coupled receptors. Protein Eng. 1994a;6(2):167–176. doi: 10.1093/protein/6.2.167. [DOI] [PubMed] [Google Scholar]
- Attwood TK, Findlay JBC. Fingerprinting G-protein-coupled receptors. Protein Eng. 1994b;7(2):195–203. doi: 10.1093/protein/7.2.195. [DOI] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gerhengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature (Lond) 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Bakker RA, Casarosa P, Timmerman H, Smit MJ, Leurs R. Constitutively active Gq/11-coupled receptors enable signaling by co-expressed G(i/o)-coupled receptors. J Biol Chem. 2004;279(7):5152–5161. doi: 10.1074/jbc.M309200200. [DOI] [PubMed] [Google Scholar]
- Beisser PS, Grauls G, Bruggeman CA, Vink C. Deletion of the R78 G protein-coupled receptor gene from rat cytomegalovirus resutls in an attenuated, syncytium-inducing mutant virus. J Virol. 1999;73(9):7218–7230. doi: 10.1128/jvi.73.9.7218-7230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisser PS, Verzijl D, Gruijthuijsen YK, Beuken E, Smit MJ, Leurs R, Bruggeman CA, Vink C. The Epstein-Barr virus BILF1 gene encodes a G protein-coupled receptor that inhibits phosphorylation of RNA-dependent protein kinase. J Virol. 2005;79(1):441–449. doi: 10.1128/JVI.79.1.441-449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisser PS, Vink C, Van Dam JG, Grauls G, Vanherle SJV, Bruggeman CA. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J Virol. 1998;72(3):2352–2363. doi: 10.1128/jvi.72.3.2352-2363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstrom MA, Johnson GL, Avdi NJ, Worthen GS. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J Virol. 1998;72(7):5535–5544. doi: 10.1128/jvi.72.7.5535-5544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodaghi B, Jones TR, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier JL, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188(5):855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomker JM, The TH, de Leij LF, Harmsen MC. The human cytomegalovirus-encoded receptor US28 increases the activity of the major immediate-early promoter/enhancer. Virus Res. 2006;118(1–2):196–200. doi: 10.1016/j.virusres.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Boriskin YS, Butcher PD. Human cytomegalovirus UL21.5 gene is expressed as an “early–late” gene in cultured human fibroblasts. Acta Virol. 2001;45(3):185–189. [PubMed] [Google Scholar]
- Bresnahan WA, Shenk T. A subset of viral transcripts packaged within human cytomegalovirus particles. Science. 2000;288(5475):2373–2376. doi: 10.1126/science.288.5475.2373. [DOI] [PubMed] [Google Scholar]
- Brody JR, Kern SE. Sodium boric acid: a Tris-free, cooler conductive medium for DNA electrophoresis. Biotechniques. 2004;36(2):214–216. doi: 10.2144/04362BM02. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Cooper BY, Scroggs RS. 5HT4 receptors couple positively to tetrodotoxin-insensitive sodium channels in a subpopulation of capsaicin-sensitive rat sensory neurons. J Neurosci. 1997;17:7181–7189. doi: 10.1523/JNEUROSCI.17-19-07181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaday RJ, Bailey JR, Kalb SR, Brignole EJ, Loveland AN, Cotter RJ, Gibson W. Assembly protein precursor (pUL80.5 homolog) of simian cytomegalovirus is phosphorylated at a glycogen synthase kinase 3 site and its downstream “priming” site: phosphorylation affects interactions of protein with itself and with major capsid protein. J Virol. 2004;78(24):13501–13511. doi: 10.1128/JVI.78.24.13501-13511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276(2):1133–1137. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Gruijthuijsen YK, Michel D, Beisser PS, Holl J, Fitzsimons CP, Verzijl D, Bruggeman CA, Mertens T, Leurs R, Vink C, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. J Biol Chem. 2003;278(50):50010–50023. doi: 10.1074/jbc.M306530200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Waldhoer M, LiWang PJ, Vischer HF, Kledal T, Timmerman H, Schwartz TW, Smit MJ, Leurs R. CC and CX3C chemokines differentially interact with the N terminus of the human cytomegalovirus-encoded US28 receptor. J Biol Chem. 2005;280(5):3275–3285. doi: 10.1074/jbc.M407536200. [DOI] [PubMed] [Google Scholar]
- Chamberlain JP. Fluorographic detection of radioactivity in polyacrylamide gels with the water soluble fluor, sodium salicylate. Anal Biochem. 1979;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan JS, Bittner A, Frueh K, Jackson MR, Peterson PA, Erlander MG, Ghazal P. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J Virol. 1999;73(7):5757–5766. doi: 10.1128/jvi.73.7.5757-5766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Horsnell T, Hutchison CA, Kouzarides T, Martignetti JA, Preddie E, Satchwell SC, Tomlinson P, Weston KM, Barrell BG. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990a;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chee MS, Satchwell SC, Preddie E, Weston KM, Barrell BG. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature (Lond) 1990b;344:774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Chiou CJ, Poole LJ, Kim PS, Ciufo DM, Cannon JS, ap Rhys CM, Alcendor DJ, Zong JC, Ambinder RF, Hayward GS. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2002;76(7):3421–3439. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Poynter NJ, Lynch DM, Vally H, Shellam GR, Rawlinson WD, Barrell BG, Farrell HE. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J Virol. 1997;71(2):1521–1529. doi: 10.1128/jvi.71.2.1521-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ, Dolan A, Akter P, Addison C, Dargan DJ, Alcendor DJ, McGeoch DJ, Hayward GS. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J Gen Virol. 2003;84(Pt 1):17–28. doi: 10.1099/vir.0.18606-0. [DOI] [PubMed] [Google Scholar]
- Drago F, Aragone MG, Lugani C, Rebora A. Cytomegalovirus infection in normal and immunocompromised humans. A review Dermatology. 2000;200(3):189–195. doi: 10.1159/000018381. [DOI] [PubMed] [Google Scholar]
- Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, Zhu H, Liu F. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci USA. 2003;100(24):14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolani L, Florence B, Denaro M, Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988;263(30):15335–15341. [PubMed] [Google Scholar]
- Evers DL, Wang X, Huang ES. Cellular stress and signal transduction responses to human cytomegalovirus infection. Microbes Infect. 2004;6(12):1084–1093. doi: 10.1016/j.micinf.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Fioretti A, Furukawa T, Santoli D, Plotkin SA. Nonproductive infection of guinea pig cells with human cytomegalovirus. J Virol. 1973;11(6):998–1003. doi: 10.1128/jvi.11.6.998-1003.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fons M, Nokta M, Cerruti-Sola S, Albrecht T. Amiloride inhibition of human cytomegalovirus replication. Proc Soc Exp Biol Med. 1991;196:89–96. doi: 10.3181/00379727-196-43167. [DOI] [PubMed] [Google Scholar]
- Fortunato EA, McElroy AK, Sanchez I, Spector DH. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8(3):111–119. doi: 10.1016/s0966-842x(00)01699-1. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos A, Pelchen-Matthews A, Kledal TN, Browne H, Schwartz TW, Marsh M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic. 2002;3(3):218–232. doi: 10.1034/j.1600-0854.2002.030307.x. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Lee NH, Pellegrino SM, Kerlavage AR. Molecular properties of G-protein-coupled receptors. Prog Nucleic Acid Res Mol Biol. 1994;49:113–156. doi: 10.1016/s0079-6603(08)60049-5. [DOI] [PubMed] [Google Scholar]
- Gao JL, Murphy PM. Human cytomegalovirus open reading frame US28 encodes a functional β chemokine receptor. J Biol Chem. 1994;269(46):28539–28542. [PubMed] [Google Scholar]
- Gerard C, Gerard NP. C5a anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- Geras-Raaka E, Arvanitakis L, Bais C, Cesarman E, Mesri EA, Gershengorn MC. Inhibition of constitutive signaling of Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor by protein kinases in mammalian cell culture. J Exp Med. 1998;187(5):801–806. doi: 10.1084/jem.187.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981;111:516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983;128:391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Greijer AE, Dekkers CA, Middeldorp JM. Human cytomegalovirus virions differentially incorporate viral and host cell RNA during the assembly process. J Virol. 2000;74(19):9078–9082. doi: 10.1128/jvi.74.19.9078-9082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths PD, Walter S. Cytomegalovirus. Curr Opin Infect Dis. 2005;18(3):241–245. doi: 10.1097/01.qco.0000168385.39390.1b. [DOI] [PubMed] [Google Scholar]
- Gruijthuijsen YK, Casarosa P, Kaptein SJ, Broers JL, Leurs R, Bruggeman CA, Smit MJ, Vink C. The rat cytomegalovirus R33-encoded G protein-coupled receptor signals in a constitutive fashion. J Virol. 2002;76(3):1328–1338. doi: 10.1128/JVI.76.3.1328-1338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudermann T, Schoneberg T, Schultz G. Functional and structural complexity of signal transduction via G-protein-coupled receptors. Annu Rev Neurosci. 1997;20:399–427. doi: 10.1146/annurev.neuro.20.1.399. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Hart GW. Glycosyltransferases as tools in cell biological studies. Methods Mol Biol. 1993;14:175–187. doi: 10.1385/0-89603-226-4:175. [DOI] [PubMed] [Google Scholar]
- Handel TM, Lau EK. Chemokine structure and receptor interactions. Ernst Schering Res Found Workshop. 2004;(45):101–124. doi: 10.1007/978-3-662-05403-1_8. [DOI] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis. I Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R. Molecular properties of the chemokine receptor family. Trends Pharmacol Sci. 1994;15:159–165. doi: 10.1016/0165-6147(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Irmiere A, Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983;130:118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional β-chemokine receptor. J Virol. 1998;72(7):6104–6112. doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT, Gibson W. A cycloheximide-enhanced protein in cytomegalovirus-infected cells. Virology. 1980;107(2):362–374. doi: 10.1016/0042-6822(80)90304-9. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signalling. J Virol. 2001;75(13):6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y, Shiohara T. Current understanding of cytomegalovirus infection in immunocompetent individuals. J Dermatol Sci. 2000;22(3):196–204. doi: 10.1016/s0923-1811(99)00085-7. [DOI] [PubMed] [Google Scholar]
- Kaptein SJ, Beisser PS, Gruijthuijsen YK, Savelkouls KG, van Cleef KW, Beuken E, Grauls GE, Bruggeman CA, Vink C. The rat cytomegalovirus R78 G protein-coupled receptor gene is required for production of infectious virus in the spleen. J Gen Virol. 2003;84(Pt 9):2517–2530. doi: 10.1099/vir.0.19227-0. [DOI] [PubMed] [Google Scholar]
- Karnushina IL, Spatz M, Bembry J. Cerebral endothelial cell culture II. Adenylate cyclase response to prostaglandins and their interaction with the adrenergic system. Life Sci. 1983;32:1427–1435. doi: 10.1016/0024-3205(83)90907-4. [DOI] [PubMed] [Google Scholar]
- Keinanen KP, Kellokumpu S, Rajaniemi HJ. Visualization of the rat ovarian lutropin receptor by ligand blotting. Mol Cell Endocrinol. 1987;49(1):33–38. doi: 10.1016/0303-7207(87)90061-x. [DOI] [PubMed] [Google Scholar]
- Kim CH. Chemokine-chemokine receptor network in immune cell trafficking. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4(4):343–361. doi: 10.2174/1568008043339712. [DOI] [PubMed] [Google Scholar]
- Kraft R, Tardiff J, Krauter KS, Leinwand LA. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase™. Bio Tech. 1988;6(6):544–546. [PubMed] [Google Scholar]
- Kuhn DE, Beall CJ, Kolattukudy PE. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem Biophys Res Commun. 1995;211(1):325–330. doi: 10.1006/bbrc.1995.1814. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey RA, Mills AD. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977;82:314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lawson CA. Cytomegalovirus after kidney transplantation: a case review. Prog Transplant. 2005;15(2):157–160. doi: 10.1177/152692480501500208. [DOI] [PubMed] [Google Scholar]
- Lomize AL, Pogozheva ID, Mosberg HI. Structural organization of G-protein-coupled receptors. J Comput Aided Mol Des. 1999;13(4):325–353. doi: 10.1023/a:1008050821744. [DOI] [PubMed] [Google Scholar]
- Maeshima M. Characterization of the major integral protein of vacuolar membrane. Plant Physiol. 1991;98:1248–1254. doi: 10.1104/pp.98.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies BJ. PhD Thesis. The Johns Hopkins University School of Medicine; 1996. Studies on the G protein-coupled receptor homologues encoded by UL33, US27, and US28 of the human cytomegalovirus genome. [Google Scholar]
- Margulies BJ, Browne H, Gibson W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology. 1996;225:111–125. doi: 10.1006/viro.1996.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall M, Freitag M, Weiler S, Sorg G, Stamminger T. Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents. Antimicrob Agents Chemother. 2000;44(6):1588–1597. doi: 10.1128/aac.44.6.1588-1597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean KA, Holst PJ, Martini L, Schwartz TW, Rosenkilde MM. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology. 2004;325(2):241–251. doi: 10.1016/j.virol.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Melnychuk RM, Smith P, Kreklywich CN, Ruchti F, Vomaske J, Hall L, Loh L, Nelson JA, Orloff SL, Streblow DN. Mouse cytomegalovirus M33 is necessary and sufficient in virus-induced vascular smooth muscle cell migration. J Virol. 2005;79(16):10788–10795. doi: 10.1128/JVI.79.16.10788-10795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnychuk RM, Streblow DN, Smith PP, Hirsch AJ, Pancheva D, Nelson JA. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Galpha12. J Virol. 2004;78(15):8382–8391. doi: 10.1128/JVI.78.15.8382-8391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S. Consequences of human cytomegalovirus mimicry. Hum Immunol. 2004;65(5):465–475. doi: 10.1016/j.humimm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Michelson-Fiske S, Horodniceanu F, Guillon JC. Immediate early antigens in human cytomegalovirus infected cells. Nature. 1977;270(5638):615–617. doi: 10.1038/270615a0. [DOI] [PubMed] [Google Scholar]
- Milne RS, Mattick C, Nicholson L, Devaraj P, Alcami A, Gompels UA. RANTES binding and down-regulation by a novel human herpesvirus-6 beta chemokine receptor. J Immunol. 2000;164(5):2396–2404. doi: 10.4049/jimmunol.164.5.2396. [DOI] [PubMed] [Google Scholar]
- Minisini R, Tulone C, Luske A, Michel D, Mertens T, Gierschik P, Moepps B. Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28. J Virol. 2003;77(8):4489–4501. doi: 10.1128/JVI.77.8.4489-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Courcelle CT. Cytomegaloviruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 4. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2629–2673. [Google Scholar]
- Morris AJ, Malbon CC. Physiological regulation of G protein-linked signalling. Physiol Rev. 1999;79(4):1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- Murphy PM. Chemokine receptors: structure, function, and role in microbial pathogenesis. Cytokine Growth Factor Rev. 1996;7(1):47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- Nakano K, Tadagaki K, Isegawa Y, Aye MM, Zou P, Yamanishi K. Human herpesvirus 7 open reading frame U12 encodes a functional beta-chemokine receptor. J Virol. 2003;77(14):8108–8115. doi: 10.1128/JVI.77.14.8108-8115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J, Weitz CJ, Agarwal N, Nir I, Papermaster DS. Production of bovine rhodopsin by mammalian cell lines expressing cloned cDNA: spectrophotometry and subcellular localization. Vis Res. 1989;29(8):907–914. doi: 10.1016/0042-6989(89)90105-3. [DOI] [PubMed] [Google Scholar]
- Neote K, DiGregorio D, Mak JY, Horuk R, Schall TJ. Molecular cloning, functional expression, and signaling characteristics of a C–C chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- Nokta M, Fons MP, Eaton DC, Albrecht T. Cytomegalovirus: sodium entry and development of cytomegaly in human fibroblasts. Virology. 1988;164:411–419. doi: 10.1016/0042-6822(88)90554-5. [DOI] [PubMed] [Google Scholar]
- Oliveira SA, Shenk TE. Murine cytomegalovirus M78 protein, a G protein-coupled receptor homologue, is a constituent of the virion and facilitates accumulation of immediate-early viral mRNA. Proc Natl Acad Sci USA. 2001;98(6):3237–3242. doi: 10.1073/pnas.051629898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YA, Abdulaev NG, Bogachuk AS. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988;230(1):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Parker EM, Kameyama K, Higashijima T, Ross EM. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. J Biol Chem. 1993;266(1):519–527. [PubMed] [Google Scholar]
- Paulsen SJ, Rosenkilde MM, Eugen-Olsen J, Kledal TN. Epstein-Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. J Virol. 2005;79(1):536–546. doi: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold ME, Schmidt TL, Dairaghi DJ, Barry PA, Schall TJ. Characterization of the rhesus cytomegalovirus US28 locus. J Virol. 2003;77(19):10404–10413. doi: 10.1128/JVI.77.19.10404-10413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98(3):325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Pleskoff O, Casarosa P, Verneuil L, Ainoun F, Beisser P, Smit M, Leurs R, Schneider P, Michelson S, Ameisen JC. The human cytomegalovirus-encoded chemokine receptor US28 induces caspase-dependent apoptosis. FEBS J. 2005;272(16):4163–4177. doi: 10.1111/j.1742-4658.2005.04829.x. [DOI] [PubMed] [Google Scholar]
- Polson AG, Wang D, DeRisi J, Ganem D. Modulation of host gene expression by the constitutively active G protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2002;62(15):4525–4530. [PubMed] [Google Scholar]
- Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2(11):1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- Rawlinson WD, Farrell HE, Barrell BG. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70(12):8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Hnatowich M, Caron MG, Lefkowitz RJ. Structure-function relationships of G-protein-coupled receptors. In: Moss J, Vaughan M, editors. ADP-ribosylating Toxins and G Proteins: Insights into Signal Transduction. American Society for Microbiology; Washington: 1990. pp. 163–188. [Google Scholar]
- Roberts LM, Lord JM. The synthesis of Ricinus communis agglutinin, cotranslational and posttranslational modification of agglutinin polypeptides. Eur J Biochem. 1981;119(1):31–41. doi: 10.1111/j.1432-1033.1981.tb05573.x. [DOI] [PubMed] [Google Scholar]
- Roizman B, Pellett PE. The family Herpesviridae: a brief introduction. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 4. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2381–2397. [Google Scholar]
- Rosenkilde MM, Waldhoer M, Luttichau HR, Schwartz TW. Virally encoded 7TM receptors. Oncogene. 2001;20(13):1582–1593. doi: 10.1038/sj.onc.1204191. [DOI] [PubMed] [Google Scholar]
- Ross SA, Boppana SB. Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis. 2005;16(1):44–49. doi: 10.1053/j.spid.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Roth A, Kreienkamp HJ, Meyerhoff W, Richter D. Phosphorylation of four amino acid residues in the carboxyl terminus of the rat somatostatin receptor subtype 3 is crucial for its desensitization and internalisation. J Biol Chem. 1997;272(38):23769–23774. doi: 10.1074/jbc.272.38.23769. [DOI] [PubMed] [Google Scholar]
- Rozell TG, Davis DP, Chai Y, Segaloff DL. Association of gonadotropin receptor precursors with the protein folding chaperone calnexin. Endocrinology. 1998;139(4):1588–1593. doi: 10.1210/endo.139.4.5881. [DOI] [PubMed] [Google Scholar]
- Sahagun-Ruiz A, Sierra-Honigmann AM, Krause P, Murphy PM. Simian cytomegalovirus encodes five rapidly evolving chemokine receptor homologues. Virus Genes. 2004;28(1):71–83. doi: 10.1023/B:VIRU.0000012265.33168.b5. [DOI] [PubMed] [Google Scholar]
- Sampathkumar P, Paya CV. Management of cytomegalovirus infection after liver transplantation. Liver Transplant. 2000;6(2):144–156. doi: 10.1002/lt.500060220. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarcinella E, Brown M, Tellier R, Petric M, Mazzulli T. Detection of RNA in purified cytomegalovirus virions. Virus Res. 2004;104(2):129–137. doi: 10.1016/j.virusres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Sazuka T, Keta S, Shiratake K, Yamaki S, Shibata D. A proteomic approach to identification of transmembrane proteins and membrane-anchored proteins of Arabidopsis thaliana by peptide sequencing. DNA Res. 2004;11(2):101–113. doi: 10.1093/dnares/11.2.101. [DOI] [PubMed] [Google Scholar]
- Scharf SJ. Cloning with PCR. In: Innis MA, Gelfand DH, editors. PCR Protocols: A Guide to Methods and Applications. Academic Press, Inc; San Diego: 1990. pp. 84–91. [Google Scholar]
- Schenk P, Wood AS, Gibson W. The 45-kilodalton protein of cytomegalovirus (Colburn) B-capsids is an amino-terminal extension form of the assembly protein. J Virol. 1991;65(3):1525–1529. doi: 10.1128/jvi.65.3.1525-1529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoneberg T, Schultz G, Gudermann T. Structural basis of G protein-coupled receptor function. Mol Cell Endocrinol. 1999;151(1–2):181–193. doi: 10.1016/s0303-7207(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Schubert U, Schneider T, Henklein P, Hoffmann K, Berthold E, Hauser H, Pauli G, Porstmann T. Human-immunodeficiency-virus-type-1-encoded Vpu protein is phosphorylated by casein kinase II. Eur J Biochem. 1992;204(2):875–883. doi: 10.1111/j.1432-1033.1992.tb16707.x. [DOI] [PubMed] [Google Scholar]
- Semenza JC, Hardwick KG, Dean N, Pelham HRB. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990;61:1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Shibutani T, Johnson TM, Yu ZX, Ferrans VJ, Moss J, Epstein SE. Pertussis toxin-sensitive G proteins as mediators of the signal transduction pathways activated by cytomegalovirus infection of smooth muscle cells. J Clin Invest. 1997;100(8):2054–2061. doi: 10.1172/JCI119738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight RG, Lieberman MA. Signal transduction. In: Sperelakis N, editor. Cell Physiology Source Book. Academic Press, Inc; San Diego: 1995. pp. 117–127. [Google Scholar]
- Smit MJ, Vink C, Verzijl D, Casarosa P, Bruggeman CA, Leurs R. Virally encoded G protein-coupled receptors: targets for potentially innovative anti-viral drug development. Curr Drug Targets. 2003;4(5):431–441. doi: 10.2174/1389450033491000. [DOI] [PubMed] [Google Scholar]
- Soulie S, Moller JV, Falson P, le Maire M. Urea reduces the aggregation of membrane proteins on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1996;236(2):363–364. doi: 10.1006/abio.1996.0183. [DOI] [PubMed] [Google Scholar]
- Strange PG. G-protein coupled receptors: conformations and states. Biochem Pharmacol. 1999;58(7):1081–1088. doi: 10.1016/s0006-2952(99)00144-6. [DOI] [PubMed] [Google Scholar]
- Streblow DN, Kreklywich CN, Smith P, Soule JL, Meyer C, Yin M, Beisser P, Vink C, Nelson JA, Orloff SL. Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am J Transplant. 2005;5(3):436–442. doi: 10.1111/j.1600-6143.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- Sung CH, Schneider BG, Agarwal N, Papermaster DS, Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991;88:8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadagaki K, Nakano K, Yamanishi K. Human herpesvirus 7 open reading frames U12 and U51 encode functional beta-chemokine receptors. J Virol. 2005;79(11):7068–7076. doi: 10.1128/JVI.79.11.7068-7076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P, Almeida JD. Human cytomegalovirus: purification of enveloped particles and dense bodies. J Gen Virol. 1977;36(2):345–349. doi: 10.1099/0022-1317-36-2-345. [DOI] [PubMed] [Google Scholar]
- Tarentino AL, Plummer TH., Jr Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- Terhune SS, Schroer J, Shenk T. RNAs are packaged into human cytomegalovirus virions in proportion to their intracellular concentration. J Virol. 2004;78(19):10390–10398. doi: 10.1128/JVI.78.19.10390-10398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyi-Nagy T, Bandi Z, Boldogh I, Albrecht T. Hydrolysis of inositol lipids: an early signal of human cytomegalovirus infection. Arch Virol. 1988;101:199–207. doi: 10.1007/BF01311001. [DOI] [PubMed] [Google Scholar]
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG, 2nd, Rodland K, Wiley S, Britt W, Shenk T, Smith RD, Nelson JA. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J Virol. 2004;78(20):10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, Schall TJ, Corey L, Geballe AP. Functional analysis of the human cytomegalovirus US28 gene by insertional mutagenesis with the green fluorescent protein. J Virol. 1998;72(10):8158–8165. doi: 10.1128/jvi.72.10.8158-8165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink C, Beuken E, Bruggeman CA. Complete DNA sequence of the rat cytomegalovirus genome. J Virol. 2000;74(16):7656–7665. doi: 10.1128/jvi.74.16.7656-7665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Kledal TN, Farrell H, Schwartz TW. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol. 2002;76(16):8161–8168. doi: 10.1128/JVI.76.16.8161-8168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Huang DY, Huong SM, Huang ES. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11(5):515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424(6947):456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- Weiner D, Gibson W. Phosphorylation, maturational processing, and relatedness of strain Colburn matrix proteins. Virology. 1983;129:155–169. doi: 10.1016/0042-6822(83)90403-8. [DOI] [PubMed] [Google Scholar]
- Welch AR, McGregor LM, Gibson W. Cytomegalovirus homologs of cellular G protein-coupled receptor genes are transcribed. J Virol. 1991a;65(7):3915–3918. doi: 10.1128/jvi.65.7.3915-3918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AR, McNally LM, Gibson W. Cytomegalovirus assembly protein nested gene family: four 3′-coterminal transcripts encode four in-frame, overlapping proteins. J Virol. 1991b;65(8):4091–4100. doi: 10.1128/jvi.65.8.4091-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AR, McNally LM, Hall MRT, Gibson W. Herpesvirus proteinase: site-directed mutagenesis used to study maturational, release, and inactivation cleavage sites of precursor and to identify a possible catalytic site serine and histidine. J Virol. 1993;67:7360–7372. doi: 10.1128/jvi.67.12.7360-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Yatani A. Ion channels that are directly regulated by G proteins. In: Sperelakis N, editor. Cell Physiology Source Book. Academic Press, Inc; San Diego: 1995. pp. 378–385. [Google Scholar]
- Yurochko AD, Hwang ES, Rasmussen L, Keay S, Pereira L, Huang ES. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol. 1997a;71(7):5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko AD, Kowalik TF, Huong SM, Huang ES. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol. 1995;69(9):5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko AD, Mayo MW, Poma EE, Baldwin AS, Jr, Huang ES. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J Virol. 1997b;71(6):4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Z, Bradel-Tretheway BG, Sumagin S, Bidlack JM, Dewhurst S. The human herpsvirus 6 G protein-coupled receptor homolog U51 positively regulates virus replication and enhances cell-cell fusion in vitro. J Virol. 2005;79(18):11914–11924. doi: 10.1128/JVI.79.18.11914-11924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipeto D, Bodaghi B, Laurent L, Virelizier JL, Michelson S. Kinetics of transcription of human cytomegalovirus chemokine receptor US28 in different cell types. J Gen Virol. 1999;80(Pt 3):543–547. doi: 10.1099/0022-1317-80-3-543. [DOI] [PubMed] [Google Scholar]