Abstract
Neutralizing antibody induction is a key feature of many effective vaccines and is the only immune response that has proven to be capable of completely blocking AIDS virus infection in animal models. Unfortunately, the extensive genetic variability and complex immune-evasion strategies of HIV-1 have thwarted all attempts to date at eliciting an effective neutralizing antibody response with candidate HIV-1 vaccine immunogens. Recent advances in our understanding of how these evasion strategies operate, coupled with growing progress in unravelling the structure and immunobiology of the viral envelope glycoproteins, are contributing to novel immunogen designs to overcome the many barriers to inducing protective antibodies against HIV-1.
Keywords: antiretroviral therapy, HIV-1, host cell fusion, immunogen, neutralizing antibody induction
Development of a safe, practical and effective HIV-1 vaccine is one of the highest priorities of the global scientific community [1,2]. Although antiretroviral therapy (ART) has dramatically prolonged the lives of HIV-1 infected patients, ART is not yet routinely available in developing countries, and the global rate of spread of HIV-1 continues unabated. If no effective AIDS vaccine is developed by 2010, the number of people infected world-wide with HIV-1 could exceed 60 million [3].
In spite of more than 20 years of research, the types of immune responses needed to protect an immunized individual from HIV-1 infection are not known. Its is known that CD8+ cytotoxic T-cell responses can control HIV-1 replication to varying degrees in acute HIV-1 infection (AHI) [4,5], and when induced by immunogens, can control viral set point after simian immunodeficiancy virus (SIV) or simian HIV (SHIV) challenges in non-human primates [4]. Strong proliferative CD4+ T-cell responses to HIV-1 proteins have been demonstrated to correlate well with immune control of HIV-1 viral load [5]. Therefore, it is highly desirable for an HIV-1 vaccine co induce robust CD4+ and CD8+ T-cell responses in order to control virus replication and reduce the likelihood of subsequent transmission [4–7].
The potential for complete (‘sterilizing’) immunity from HIV-1 infection may depend on the presence of pre-existing neutralizing antibodies. We know that neutralizing antibodies can prevent the acquisition of AIDS virus infection after intravenous, vaginal, rectal and oral virus challenge in nonhuman primates [8–12]. This level of protection is highly attractive for a vaccine against a virus, such as HIV-1, which integrates genetically and forms latent viral reservoirs soon after infection. However, the diversity of transmitted HIV-1 genetic variants and the relative resistance of the majority of HIV-1 primary isolates to most types of inducible neutralizing antibodies have posed major hurdles to current vaccine efforts. Furthermore, the few rare broadly reactive neutralizing monoclonal antibodies (mAbs) that have been isolated from HIV-1 infected patients represent species of antibodies that are not able to be routinely induced in animals or uninfected humans by HIV-1 Env immunogens [13,14].
The strategies for induction of neutralizing antibodies that have been successful for other infectious agent vaccines have thus far failed for HIV-1 vaccine development, including immunization with an outer envelope protein (glycoprotein [gp]120) [15,16], and immunization with a killed or inactivated virus [17,18]. Live attenuated SIVs have indeed induced substantial protection to SIV challenge, but attenuated SIV strains have reverted to wild type in vivo, and have proven to be unsafe in neonatal monkeys [19,20].
Important areas of vaccine research include understanding the host immune response to HIV-1 virions and HIV-1 infected cells at sites of mucosal transmission, and work on characterizing the transmitted virus. For example, recent work suggests that the variable loops of transmitted HIV-1 of certain Clades (A and C), but not others (B), may be shorter and the transmitted viruses more neutralization-sensitive than chronic HIV-1 strains [21–23]. Another example is the observation that broadly neutralizing immunoglobulin (Ig)A antibodies have been reported in the genitourinary tracts of seropositive [24], and highly exposed and uninfected subjects [25]. If these observations can be shown to be a correlate of protective immunity for mucosal transmission of HIV-1, it will be critical to translate these findings to strategies of how to induce protective IgA mucosal antibodies in humans. Furthermore, although IgG is the most common antibody type in cervicovaginal secretions, little attention has been paid to the specificity and regulation of mucosal IgG anti-HIV-1 immune responses.
A major conundrum for developers of HIV-1 vaccines has been that, in spite of the presence of epitopes on the HIV-1 envelope that are targets for broadly neutralizing HIV-1 human mAbs, these species of antibodies are not made following immunization with antigenic Env proteins, nor are they routinely produced after infection with HIV-1 [13,14]. This review discusses what may be two major reasons for the this failure: the lack of current immunogens that mirror the native envelope structures needed to induce neutralizing antibodies, and the likely tolerant state of the host to some conserved HIV-1 envelope epitopes.
Yang and colleagues have demonstrated that only one trimer per virion is needed to bind to host cells to mediate infection [26]. Therefore, to prevent virion infection of CD4+, CCR5+ host cells, all trimers on each virion must be bound by at least one neutralizing antibody molecule. For induction of antibody that will neutralize HIV-1, it is critical to learn how to induce high affinity, durable and broadly reactive, neutralizing antibodies that are present both systemically and at mucosal surfaces.
Finally, that there are rare human broadly neutralizing mAbs [13], that HIV-1 cellular responses can transiently and in some cases persistently control HIV-1 [4,5], and that high levels of passively administered mAbs can protect against chimeric SHIV challenges [8–11,27,28], all give hope that a successful AIDS vaccine can be made. Similarly, that a successful vaccine has been made against feline immunodeficiency virus (FIV) also provides some measure of hope for a successful HIV-1 vaccine [29]. However, for reasons that are poorly understood and require more study, the strategies that have been successful for protecting against FIV have not been successful for protecting against SIV or SHIVs in nonhuman primates.
This review summarizes the current status of efforts to understand why broadly neutralizing antibodies are so difficult to induce, discusses HIV-1 mechanisms of evasion of HIV-1 Env from protective anti-HIV-1 antibodies and outlines some novel vaccine design strategies that are currently being studied.
Status of neutralizing antibody responses that are induced with current HIV-1 immunogens
Current efforts to elicit effective antibody responses against HIV-1 are focused on the need to generate broadly neutralizing antibodies against a wide spectrum of primary isolates. This is an ambitious goal that has proven to be an enormous scientific challenge. Despite two decades of research and a wide range of immunogens tested in animal models and human clinical trials, little progress has been made. Many candidate Env immunogens have in fact generated high titers of neutralizing antibodies; however, with rare exceptions these antibodies neutralize a very small subset of viruses that are either matched to the vaccine strain or are unusually sensitive to neutralization [30,31]. Even after repeated boosting, desirable breadth of neutralization has not been achieved.
Most information on the immunogenicity of candidate HIV-1 Env vaccines in human clinical trials is based on monomeric, monovalent gp120 and gp160 from three T-cell line adapted (TCLA) strains of virus that were among the first strains isolated in the epidemic (e.g., IIIB, MN, SF2). These various TCLA Env immunogens have been delivered as either recombinant pox-viruses (e.g., vaccinia, canarypox), adjuvanted recombinant glycoprotein or a combination of the two. Subunit proteins alone have elicited high titers of neutralizing antibodies against TCLA strains, where a minimum of three to four inoculations generally achieves peak titers [32–36]. Peak responses with recombinant pox-virus vectors have been lower, but can increase dramatically after one or two subunit protein boosts [37–42], suggesting effective B-cell priming by the vectored immunogen. Similar secondary neutralizing antibody responses are observed post-AIDS virus infection in macaques that are previously immunized with recombinant Env-containing DNA and viral vectors [43–49]. Furthermore, a recent study in rabbits showed that priming with a recombinant vector followed by boosting with subunit Env protein can be superior to multiple inoculations with Env protein alone [50]. Another recent study found that priming with recombinant DNA and boosting with a recombinant adenovirus vector can generate neutralizing antibody responses that rival subunit protein boosting [51].
Vaccine-elicited serum neutralizing antibody responses against HIV-1 wane rapidly (~8-week half-life) and may be extremely difficult to maintain at adequate titers to achieve sterilizing immunity over extended periods of time without frequent boosting. Little is known regarding the half-life of induced mucosal anti-HIV-1 antibody responses. Memory B cells capable of rapid recall serum neutralizing antibody responses have persisted for at least 4 years in recipients of candidate HIV-1 Env-containing vaccines [52]. Few studies have addressed the fundamental role of B cell memory and antibody recall in the absence of sterilizing immunity [53,54]. Given the limited duration of serum neutralizing antibody titers, the high cost of Env protein production and the many logistic barriers associated with frequent boosting, especially in the developing world, effective priming with recombinant vectors may be the most practical and affordable means to deliver an appropriate immunogen for broadly neutralizing antibody induction. Furthermore, unlike subunit proteins, recombinant vectors offer the added advantage of eliciting virus-specific T-cell responses. Studies are needed to determine whether a rapid-recall neutralizing antibody response during acute infection can lead to better viremia control, improved CD4+ T cell preservation and, importantly, make it less likely that an infected individual will transmit virus. New strategies of immunization are also needed to induce protective antibody levels of sufficient strength and duration to prevent mucosal transmission of HIV-1.
The antibodies that current Env immunogens generate, although limited in neutralizing activity, might possess broadly cross-reactive binding activity against diverse primary isolates. This could be especially true in the case of antibodies that bind exposed epitopes on defective Env glycoprotein spikes [55]. Non-neutralizing antibodies have the potential to exhibit biological functions associated with Fc receptor engagement and/or complement activation that could influence the risk of transmission and either curb or exacerbate disease [56–58]. Recently, the efficacy of two bivalent gp120 vaccines formulated in alum (AIDSVAX B/B and AIDSVAX B/E) was tested in Phase III clinical trails [15,16,36]. Unfortunately, both vaccines failed to prevent the acquisition of infection, reduce plasma virus loads and delay disease progression [15,16]. These poor outcomes occured despite sustained high titers of neutralizing antibodies against the Clade B TCLA strain, MN, that was part of both vaccines [36]. As expected, serum samples from trial participants possessed little or no neutralizing activity against Clade B primary isolates as measured in a highly sensitive luciferase reporter gene assay (MONTEFIORI ET AL. UNPUBLISHED DATA), reinforcing the notion that neutralizing antibodies will need to target a broad spectrum of primary isolates to be effective. The overall clinical outcome of these Phase III trials further suggests that non-neutralizing antibodies were neither beneficial nor detrimental. One possible exception is a specific genetic polymorphism in FcγRIIIa that was found to be associated with an increased risk of infection in one of these trials [59]. As this was a small subset analysis with weak statistical power, additional work will be needed to establish whether non-neutralizing antibodies may pose a genuine risk to some vaccine recipients.
The emphasis on inducing broadly cross-reactive, neutralizing antibody responses has created a need for highly standardized and validated assays to properly evaluate these responses. Assays are needed that can identify improved immunogens and ultimately predict an effective vaccine as judged by the magnitude of neutralization against multiple strains of the virus. Just how many and which strains of virus will be needed to predict vaccine potency has been a matter of intense discussion. Paradoxically, the ability of any assay to predict vaccine potency will not be known until a vaccine that is at least partially effective becomes available. Furthermore, the same complex biological, immunological and genetic properties of the virus that pose difficult challenges for vaccine design pose similar challenges when deciding appropriate assay reference strains. Different sets of viruses have been used by different groups to assess vaccine-elicited neutralizing antibody responses, making it extremely difficult to compare responses and rank vaccine immunogens. Recent initiatives by the Division of AIDS, NIH and the Global HIV/AIDS Vaccine Enterprise [1,2] aim to change this scenario and have yielded guidelines [60] and initial standard reference strains [61] that promise to enhance the quality of HIV/AIDS vaccine research in the future. These guidelines and initial reference strains place a heavy emphasis on viruses from acute/early, sexually acquired infections with the rationale that such viruses are the major ‘transmitted’ strains that a vaccine will need to counter.
Antibody escape & evasion mechanisms of HIV-1
The outer coat protein of HIV-1 is a trimer comprised of three gp120 molecules that are non-covalently linked to a trimeric gp41 stalk anchored in the HIV-1 viral membrane (FIGURE 1). Upon binding of the gp120 to CD4, a dramatic set of conformation changes occurs that exposes the CD4-induced (CD4i) coreceptor binding site, and leads to exposure of the fusion domain of gp41 (FIGURE 1) [62]. The gp41 fusion domain inserts into the lipid bilayer of the host cell, and the ‘prefusion’ gp41 state that is a presumed linear form of gp41, is induced to change to a coiled-coil structure that pulls the viral and host membranes into close approximation, leading to fusion of the host and viral membranes (FIGURE 2) [63,64]. Therefore, a preventive vaccine must induce an immune response that can prevent viral attachment to host receptor and coreceptor or can prevent viral fusion.
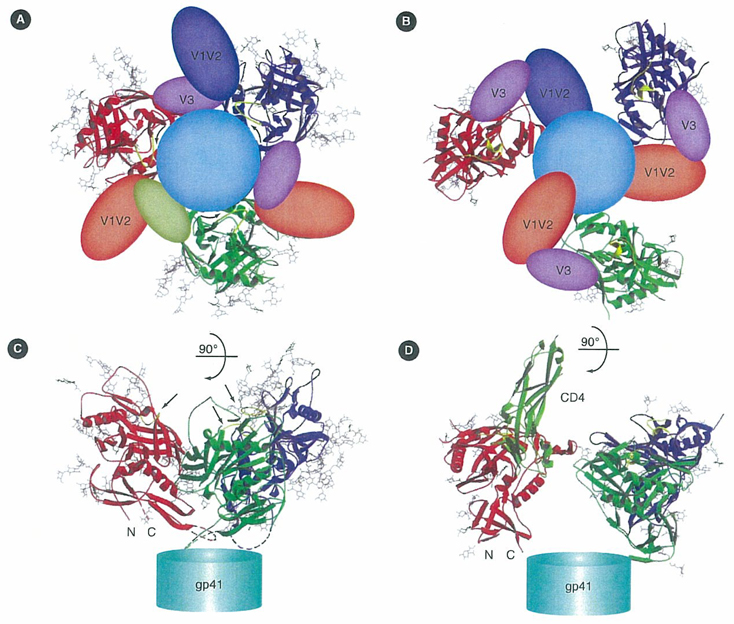
Figure 1. Proposed models for gp120/gp41 trimers in unliganded and CD4-bound conformations.
(A) A trimer in the unliganded conformation, viewed along the threefold axis from outside the virion towards gp41. The polypeptide chain backbones are in ribbon representation; N-linked glycans are stick models; deleted V1–V2 and V3 segments are transparent balloons. The three monomers are in red, green and blue, respectively; the sugars, in grey. Gp41 is shown as a circle in the rear. (B) The same view of a gp120/gp41 trimer as in panel A, but in the CD4-bound conformation, generated by superposing the CD4-bound HIV gp120 structure onto the unliganded SIV gp120 subunits in panel A, assuming that the three-strand, inner-domain-sheet remains roughly in place. Structural elements depicted as in panel A; CD4 omitted for clarity. (C) "Side" view of the same model as in panel A. The N and C termini of the gp120 core are labelled. Gp41 is shown as a cylinder at the bottom. Green arrows indicate CD4-binding loops. (D) Side view of the same model as in panel B. The first two domains of CD4 are shown in light green on only one gp120 monomer. N and C termini of the gp120 core are labelled. Gp41 is shown as a cylinder at the bottom.
gp: Glycoprotein; SIV: Simian immunodeficiency virus.
Reproduced with permission from [62] Nature Publishing Group® (2005).
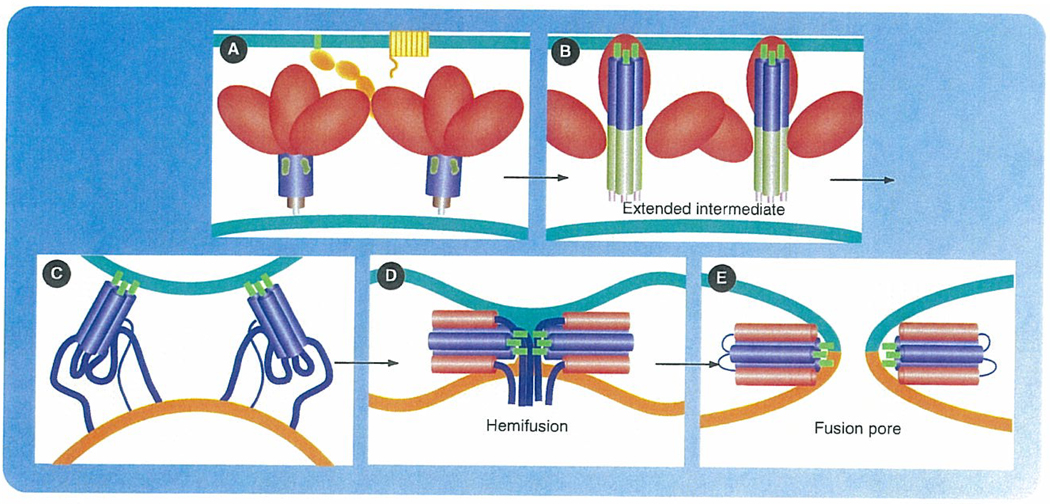
Figure 2. Model for HIV-1 host cell fusion.
Schematic representation of the ligand-triggered conformational changes in gp120/gp41 that promote membrane fusion. (A) Encounter between virus, with gp120/gp41 on its surface, and cell, with CD4 and coreceptor. The Env trimers are shown with gp120 (red), gp41 (blue) and the fusion peptide at the N-terminus of gp41 (green). CD4 and coreceptor (yellow), anchored in the cell membrane (green). Viral membrane in brown. (B) Binding of receptor and coreceptor triggers conformational change in gp120, leading to its release from gp41. Gp41 unfolds into an extended, intermediate conformation, so that the fusion peptides at one end insert into the target-cell membrane. Residues 25–75 (light blue) of gp41 form a central, three-chain coiled coil. The C-terminal part of the gp41 ectodomain (darker blue) may have a less ordered conformation. This intermediate has a relatively long lifetime (at least several minutes). (C) The C-terminal segment of the gp41 ectodomain (residues ~117–170) zips up into an outer-layer helix, bringing the transmembrane anchor of gp41 toward the fusion peptide. Two Env trimers are shown cooperating here, but a single trimer may be sufficient. The coming together of the fusion peptide at the N-terminus of gp41 and the transmembrane anchor toward its C-terminus draws the two membranes toward each other and ultimately promotes hemifusion. (D) A hemifusion stalk has formed. The apposed leaflets of the two bilayers have merged, but not the distal leaflets. If the fusion peptides insert only into the outer leaflets (as is the case for flavivirus and alphavirus fusion loops and probably for the influenza HA fusion peptide), then they can migrate into the hemifusion stalk, as shown here. However, direct evidence for this arrangement is lacking. (E) Formation of a complete fusion pore. The C-terminal residues of the gp41 ectodomain, which contain the 2F5 and 4E10 epitopes, snap into place, bringing the cytoplasmic domains of gp41 through the fusion pore and making the fusion step irreversible.
Reproduced with permission from Stephen Harrison, Boston Children’s Hospital and Harvard Medical School, Boston, MA, USA.
HIV-1 has adapted the gp120 portion of Env to escape immune recognition by a number of mechanisms, including glycan shielding [65], mutation of variable regions [66–69] and conformation masking [70].
The outer face of the gp120 envelope protein is an immunologically ‘silent face’ that is covered by N-linked glycans (FIGURE 3); up to 50% of the weight of gp120 is carbohydrate [62,65,71–73]. The number of glycosylation sites on gp120 remains approximately the same (~25 sites), but the sites shift or ‘evolve’ in position over time as neutralizing antibodies are generated - a phenomenon termed ‘evolving glycan shield’ [65]. A rare human mAb exists (2G12) that binds to Manα 1–2Man at the termini of both the D1 and D3 arms of a variety of oligomannose carbohydrates on the HIV-1 trimer and broadly neutralizes HIV-1 [71–73]. There are two potential reasons why HIV-1 virion carbohydrates are poorly immunogenic. First, as HIV-1 has no glycosylation machinery of its own, the sugars to which 2G12 bind, like other virion carbohydrates, are similar to host carbohydrates and are likely regarded as ‘self’ antigens [71]. A second reason for the poor immunogenicity of carbohydrates on HIV-1 is microheterogencity, that is a single protein sequence would be expected to express multiple carbohydrate forms leading to a dilution of an immune response [71].
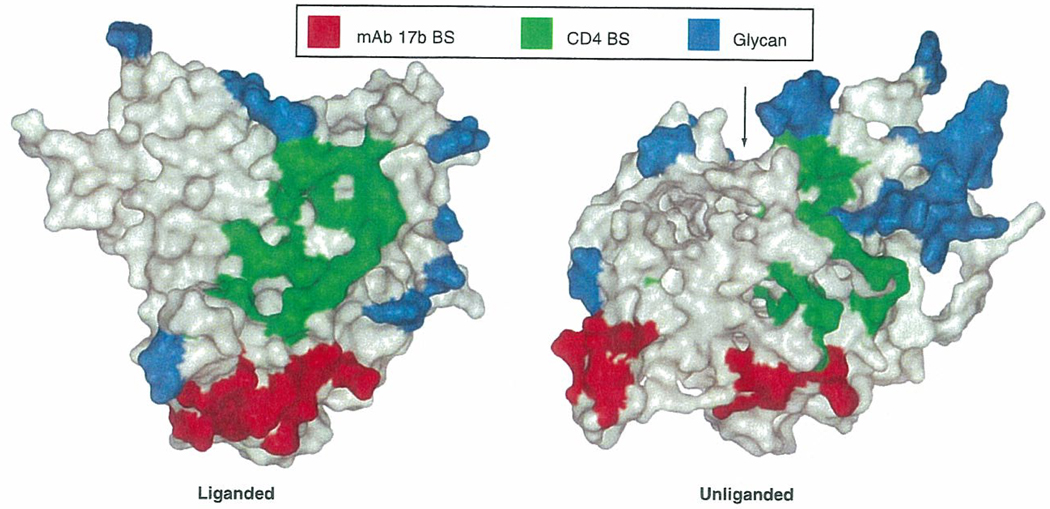
Figure 3. Carbohydrates on gp120, and the binding sites of CD4 and mAb 17b.
Molecular surface representations of gp120 core structures in liganded and unliganded states. Residues in direct contact with CD4 (green); residues contacting human mAb 17b (red); carbohydrate (blue). The arrow indicates the mouth of the envelope hydrophobic cavity.
BS; Binding sites; gp: Glycoprotein; mAb: Monoclonal antibody.
Reproduced with permission from [62] Nature Publishing Group® (2005).
The vulnerable exposed regions of gp120 include the five variable regions, V1–5 (FIGURES 1 & 2). A second potent mechanism of gp120 escape from antibody is both to modify the sequence of these variable region epitopes to which neutralizing antibodies bind, as well as altering the conformation of the variable loops to occlude neutralization epitopes [66–69,74–81].
The third variable loop (V3) was originally thought to be the main accessible Env neutralizing epitope and would be a successful target for a vaccine [79–84]. However, it was subsequently recognized that anti-V3 loop antibodies were highly strain specific [79,81–84]. As the extent of diversity of HIV-1 quasi-species became more apparent in the early 1990s, hope for a polyvalent V3 immunogen faded. Similarly, with the difference in neutralization sensitivity between T-cell line adapted (TCLA) HIV-1 and primary isolates that was observed with V3 antibodies [85,86], it was realized that the envelope structure of TCLA viruses was likely quite different than the native HIV-1 trimer of primary isolates [87]. Hope for a practical V3 immunogen has resurfaced with the work of Zolla-Pazner and colleagues who have shown similarity of the V3 loop with chemokine receptor structure, and human anti-V3 mAbs that can neutralize a subset of HIV-1 grown in PBMC [80,81].
With Korber, we have studied a panel of V3 peptides selected to represent a spectrum of potential higher order V3 motifs of Clades B [88] and C (HAYNES B, KORBER B, MONTEFIORI D, LIAO HX, UNPUBLISHED DATA). From these studies, we found that there were no V3 peptide motifs that when used as immunogens, induced discrete neutralization profiles for isolates in a panel of HIV-1 primary isolates [88]. Rather, there was only a spectrum of weak-to-strong inducers of the same Tier 1 (relatively easy to neutralize) HIV-1 strains. Of the 35 Clade B V3 peptides studied as immunogens, five V3 sequences, all clustered around the Clade B consensus, induced the broadest neutralizing antibodies. The optimal peptide (V3 62.19) with the sequence GPGRAFYTE at the tip of the V3 loop, generated antibodies that neutralized 30% of the Clade B primary isolates or Env pseudotyped viruses tested; however, many of the isolates not neutralized by ant-62.19 sera also had V3 sequences similar to 62.19 [88]. Zolla-Pazner and colleagues have produced a human mAb, 447–52D that was reported to neutralize approximately 40% of Clade B strains [80], but a subsequent study reported that this antibody rarely neutralizes typical (Tier 2) primary patient isolates [89]. Therefore, it appears that most HIV-1 Clade B strains either are in a conformation that obscures the V3 loop neutralizing determinants [87], or that induced antibodies do not bind the V3 loop sufficiently to neutralize Tier 2 (relatively more difficult to neutralize) HIV-1 [90].
In contrast to Clade B, Clade C V3 is far more conserved, and the most variable region near to the Clade C V3 are the approximate 30 aa immediately C-terminal to V3, called V3′ [91]. For Clade C isolates, none of the computer designed V3 peptide motifs induced significant neutralizing antibodies to Clade C (HAYNES B, KORBER B, MONTEFIORI HX, LIAO, UNPUBLISHED DATA). Interestingly, our best immunogen for induction of antibodies to Clade C primary HIV-1 strains is the year 2001, group-M consensus Env with shortened variable loops, termed CON-S, that generated antibodies that neutralized approximately 25% of Clade C isolates tested [92]. Most of the CON-S-induced neutralizing antibody for Tier 1 HIV-1 strains is against the CON-S V3 loop, but only approximately 40% of the CON-S oligomer-induced neutralizing activity for Tier 2 HIV-1 strains is against V3.
Krachmarov and colleagues have now made human mAbs against non-B V3s and found antibodies that neutralize a subset of non-B HIV-1 strains [90]. The structure of a V3-containing HIV-1 gp120 core has recently been published and provides evidence that the V3 loop functions as a molecular ‘hook’, not only for binding to coreceptor but also for modulating sub-unit associations within the viral spike of the HIV-1 envelope trimer [93].
It should be pointed out that because the V3 loop is highly immunogenic in animals and humans, it may serve as a ‘decoy’ epitope for diverting the immune system away from more vulnerable conserved HIV-1 Env epitopes [94,95]. In this regard, Herrera and colleagues have shown that much of the host reponse to HIV-1 is directed at shed gp120 in non-native forms, again suggesting that most induce anti-Env antibody in immunizations and infection is against non-native Env forms that might serve as a decoy to the immune system [96].
Taken together, the data to date suggest limitations of the V3 loop as an immunogen for HIV-1, mostly due to V3 inaccessibility on a large proportion of primary isolate Envs. However, one goal of vaccine development is to find antibody specificities that can bind to native Env trimers and induce the exposure of critical epitopes on the V3 loop. V3 immunogens that reflect conserved functional regions of the V3 loop would then become viable vaccine targets.
The V1, V2 and V4 Env variable loops can also serve as targets for neutralizing antibodies to HIV-1 Env [97–102]. Similar to the V3 loop antibody repertoire, many of the V1, V2 and V4 specifities of induced antibodies are strain specific. The V1 and V2 loops together are postulated to form a shield that covers the CCR5 binding site and in some circumstances, can cover the V3 loop (FIGURE 1A) [97,103]. Ren and colleagues inserted a foreign epitope into the envelope V4 region; antibodies against the foreign epitope neutralized these chimeric viruses in vitro [104]. Although crystal structures are not yet available for V1, V2 and V4, it can be anticipated that these loops will also exhibit both conserved and variable motifs as has been found for the more well studied V3 loop.
A third mechanism of gp120 evasion of neutralizing antibodies is conformational masking. Gp120-CD4 and CCR5 interactions invoke considerable conformational changes in gp120 (FIGURE 2). Kwong and colleagues demonstrated a correlation of the neutralization potency of antireceptor antibodies with thermodynamic analyses, suggesting a receptor-binding-site ‘conformational masking’ mechanism of neutralization escape [70]. This mechanism of escape is postulated to allow HIV-1 to maintain receptor binding and simultaneously to resist neutralization [70]. Although most CD4 binding site mAbs do not effectively neutralize HIV-1 primary isolates, one exception is the IgG1b12 mAb that was derived from a phage display library and mutated for high affinity binding to gp120 [105]. IgG1b12 also has some degree of polyreactivity to self antigens, and this trait, by predisposing these types of antibodies to B cell negative selection, may limit the production of IgG1b12-like antibodies [14,106]. Pantophlet and colleagues have presented elegant solutions to gp120 design that builds on the observations of conformational masking. Structurally designed mutants of gp120 with the CD4 binding site exposed, but held in stable forms to minimize the energy of binding were produced; however, these designs have yet to induce broadly reactive anti-CD4 binding site antibodies [107–109].
A potential highly conserved neutralization site on primary HIV-1 isolates is the coreceptor binding site that is induced following gp120-CD4 binding CD4i. CD4i antibodies are commonly made in HIV-1 infected patients [110], but most primary isolate HIV-1 strains are resistant to neutralization by CD4i antibodies due to physical occlusion at the virus—cell interface. Labrijn and colleagues have found that the human mAbs that recognize the CCR5 binding site cannot neutralize CCR5-utilizing viruses as whole immunoglobulin molecules, whereas they can neutralize these viruses as fab or sFv fragments [111]. Whole IgG molecule CD4i mAbs can only neutralize primary isolates in the presence of exogenously-added soluble CD4 [110,112]. Therefore, in order to be neutralizing, these antibodies must bind to susceptible virus prior to virion engagement of the cell surface. Only a small number of TCLA CXCR4 HIV-1 strains and CD4-independent strains on which the coreceptor binding domain of gp120 is constitutively exposed are susceptible to CD4i antibodies [111–113].
There are several epitopes of gp41 that are conserved targets for broadly neutralizing anti-gp41 antibodies. These sites are located in the membrane proximal external region (MPER) of gp41 (FIGURE 3), and are defined by the whole Ig mAbs 2F5 and 4E10 and the Fab phage display selected molecule (Z13) [114,115]. mAbs 2F5 and 4E10 were derived from transformed B cells from the blood of HIV-1+ patients. Both of the original 2F5 and 4E10 mAbs were IgG3 (they have now been engineered onto an IgG1 backbone), and both have long hydrophobic CDR3 regions [114–117]. The 2F5 epitope is represented by gp41 Env aa 662–668 (ELDKWAS) and the 4E10 epitope is represented by aa 671–676 (NWFDIT) [118–120]. Both epitopes are located just N-terminal to the insertion of gp41 into the viral membrane (FIGURE 3). Crystal structures of the 2F5 and 4E10 mAb Fabs bound to their nominal peptide epitopes have demonstrated that the peptides bind only to a small portion of the mAb Fab CDR3 regions, leaving large hydrophobic portions of the CDR3s to interact with the viral lipid membrane [119,120]. Both Ofek and colleagues [119] and Cardoso and colleagues [120] have suggested that the viral membrane is a component of the 2F5 and 4E10 epitopes, respectively.
Although HIV-1+ patients and immunized animals have been reported to occasionally make antibodies to both 2F5 and 4E10 peptide epitopes, 2F5 and 4E10-like neutralizing antibody responses are difficult to induce [121,122]. Bibollet-Rusche and colleagues have made HIV-1/HIV-2 chimeric Env pseudoviruses in which either the 2F5 or 4E10 epitope of HIV-1 is placed in the homologous region in the HIV-2 gp41 Env [122]. When these HIV-1/HIV-2 MPER chimeric Envs were used in a pseudovirus neutralization assay, Bibollet-Rusche and colleagues found that only 0.5% of people infected with HIV-1 of Clades A–H had 2F5-like neutralizing antibodies, and 0.9% had 4E10-like neutralizing antibodies [122]. By contrast, 92% of subjects had anti-CD4i gp120 antibodies [122]. Interestingly, when the authors made a chimeric HIV-2 containing a longer MPER insert that contained both the entire membrane proximal region, they found that approximately 30% of HIV-1 patients had antibodies that neutralized this HIV-2 chimeric strain, many with anti-MPER antibodies that reacted N-terminal to the 2F5 epitope [122].
One plausible notion for why the 2F5 and 4E10 epitopes are poorly immunogenic for induction of 2F5- and 4E10-like neutralizing antibodies is that immunogens containing the MPER 2F5 and 4E10 antigens, though they bind 2F5 and 4E10 mAbs, are not in a stable and correct conformation to induce MPER neutralizing antibodies. The observations suggest that the viral membrane is likely a major component of the 2F5 and 4E10 epitopes in infectious virions [119,120].
The 2F5 epitope is pan of the gp41 heptad repeat (HR)-2 sequence of the peptide fusion inhibitor, T-20, or Fuzeon [64,123]. mAb 2F5 inhibits the binding of T20 to the gp41 HR-1 region and, therefore, inhibits T20 drug anti-fusion activity [124] (T20 binding to HR-1 inhibits HIV-1 mediated fusion and is the drug mechanism of action). Similarly, humans treated with T20/Fuzeon make high levels of anti-T20 antibody that does not inhibit T20 efficacy as a fusion inhibitor [123]. Interestingly a mAb, D50, that binds to an epitope just N-terminal to the 2F5 epitope, also binds to T20, but does not inhibit T20 activity as a fusion inhibitor [124,125]. One possible explanation for the poor immunogenicity of the MPER neutralizing epitopes is that the conformation of the gp41 MPER region in most immunogens is not in the correct prefusion confirmation to induce 2F5 and 4E10-like antibodies, but rather is in a non-native conformation.
There is evidence that in HIV-1 infection the prefusion native MPER epitopes are only transiently expressed and are not presented for a sufficient length of time to host B cells to be immunogenic [126]. By contrast 2F5 mab binds to the gp41 HR-2 peptide, T20, and inhibits T20 activity in vitro (i.e., the 2F5 functional epitope for fusion inhibition is present on the T20 peptide) [124]. Therefore, either the functional conformation of the 2F5 MPER neutralizing epitope is present on the T20 peptide, or 2F5 binding to T20 prevents induction of the functional conformation needed for fusion inhibition.
A second contributing factor resulting in nonproduction of 2F5 and perhaps 4E10-like anti-MPER neutralizing antibodies, is that host B cells may be tolerized to the MPER neutralizing epitopes [107]. This would either be due to homologies in the gp41 MPER with self antigens [127], self recognition of the lipid components of the viral membrane [106], or cross-reactivity of the MPER with, as yet unknown environmental or commensural bacterial antigens. If tolerance proves to be a factor in limiting anti-MPER antibody production, antibodies made in the setting of breaks in tolerance could be expected to have some degree of polyspecificity for self antigens.
In this regard, the ability of 2F5, 4E10, IgG1b12 and 2G12 mAbs to react with a variety of autoantigens has been tested [102]. 2F5 and 4E10 mAbs were found to bind cardiolipin and other protein autoantigens, IgG1b12 bound dsDNA and other host proteins, although mAb 2G12 did not score positive in any of these assays [106]. The apparent affinity of 4E10 for cardiolipin was in the nM range, and 4E10 also bound to prothrombin and phosphatidylethanolamine, as well as had lupus anticoagulant activity (PTT prolongation) has been tested [106]. By contrast, the apparent affinity of mAb 2F5 for cardiolipin was in the micromolar range, and 2F5 did not react with other phospholipids tested in enzyme-linked immunosorbant assay (ELISA). One measure of cardiolipin auto-antibody potential for pathogenicity is reactivity of the auto-antibody with β-2-glycoproiein-1 in complex with cardiolipin [106]. Neither MPER mAb required fetal calf serum as a source for β-2-glycoprotein-1 to bind to cardiolipin [106].
Therefore, in addition to structural limitations of MPER immunogens, a second plausible reason why MPER broadly reactive neutralizing antibodies are rarely made may be that the 2F5 and 4E10 MPER epitopes, for reasons that are poorly understood, may only trigger polyreactive pools of B cells, such as B1, transitional-1 and marginal zone B cells, to make these types of antibodies [14,106,128]. Due to the possibility that the viral lipid bilayer comprises a component of both MPER neutralizing epitopes, 2F5 and 4E10-like antibodies may require polyreactivity to be able to bind to the MPER epitopes that are comprised of gp41 and the viral membrane. In this regard, we have recently found that 2F5 and 4E10 mAbs bind to their nominal gp41 epitopes anchored in liposomes in a two step conformational change model. This model suggests that these two mAbs initially bind and induce a conformational change in the lipid-gp41 antigen that exposes the full mAb binding site (ALAM M, HAYNES B, UNPUBLISHED DATA).
Under normal circumstances, these polyreactive B cells may be subjected to B cell downregulation control mechanisms such as anergy, receptor editing, and B cell deletion [14,129,130]. A corollary hypothesis is that patients with diseases associated with B cell tolerance defects such as systemic lupus erythematosus (SLE) may be able to make a more salutary B cell response when infected with HIV-1. It is of interest that the diagnosis of AIDS in SLE is rare, and the two diagnoses have been characterized as mutually exclusive, with only 32 patients with SLE and HIV-1 infection reported since the AIDS epidemic began [14]. Currently, a prospective study is ongoing, to determine the frequency of HIV-1 infection in patients with autoimmune disease, and when HIV-1+ SLE patients are found, to determine the types of neutralizing antibodies made. Lopalco and colleagues suggested that a subset of both long-term nonprogressors and exposed and uninfected subjects have antibodies against the CCR5 coreceptor, again suggesting control of HIV-1 associated with breaks in tolerance to self antigens [131].
Novel immunogen design strategies
Novel approaches to elicit broadly neutralizing antibodies fall into six major categories and differ from early prototypes by placing a greater emphasis on Env structure.
One approach is to preserve the native structure of functional Env trimers. The most direct way of accomplishing this has been with pseudovirions [132], virus-like particles [133–135] and chemically inactivated virus [136,137]. A major technical problem with all three approaches is that they elicit antibodies to cellular antigens that are present on the virus surface after budding. These xenogeneic anti-cell antibodies are toxic to cells at low serum dilutions [138], overshadow potential neutralizing antibodies by enhancing virus infectivity at higher scrum dilutions [139] and are not practical for vaccination. Extra care must be taken to remove anticell antibodies from serum samples in order to properly assess Env-specific neutralizing activity — a task that is not easily accomplished [140].
An alternative, but more difficult approach, to making native Env immunogens has been to stabilize the trimer by cross-linking both the cleaved and noncleaved forms of gp140. Two main approaches have been to either introduce intermolecular disulfide bonds that form stable trimers of cleaved gp120-gp41 [141] or to fuse a modified GCN4 transcription factor motif C-terminal to the gp41 coiled coil to form stable trimers of non-cleaved gp140 [142,143]. Both products are functional, immunogenic and appear to be superior to gp120 monomers [144,145], but neither approach has elicited broadly neutralizing antibodies. This may be, in part, due to incomplete non-native structure of the expressed envs to date. Nonstabilized gp140 oligomers were also recently tested, but were similarly limited in their ability to elicit antibodies that neutralize primary isolates [146].
A second category of immunogens are those that aim to expose cryptic epitopes by either removing N-linked glycans [147–150] or deleting one or more variable loops on gp120 [151–155]. Both approaches have been shown to increase the neutralization-sensitivity of the virus as evidence that neutralization epitopes are indeed uncovered. However, simply unmasking these epitopes as immunogens has not been sufficient to generate a potent neutralizing antibody response. A plausible explanation for this poor response is that the targeted epitopes remain hidden from antibodies on native viral particles.
A third category of immunogens are those that aim to stabilize intermediate epitopes that form during binding and fusion. One such approach utilized a fusion competent, cell-based immunogen that at first appeared to generate antibodies with broadly reactive neutralizing activity in mice [156], but this activity was later deemed to be an artifact [157]. Others are exploring gp120-CD4 complexes that trigger the formation of CD4i epitopes in and around the coreceptor-binding domain. Indeed, CD4i antibodies exhibit enhanced binding to these complexes compared with noncomplexed gp120 [158]. There have been reports that one of these gp120-CD4 immunogens can generate broadly neutralizing antibody responses in goats [159] and rhesus monkeys [160]. That these antibodies could be competed separately with both gp120 and sCD4 suggests unique epitopes are recognized [160]. A more recent study in guinea pigs confirmed the observation of broadly neutralizing antibody induction, but concluded that this activity was mostly associated with anti-CD4 antibodies and that although antibodies to CD4i epitopes were indeed generated, these latter antibodies were non-neutralizing [161]. Additional studies are needed in order to clarify the specificity of neutralizing antibodies generated by gp120-CD4 immunogens in small animals compared to primates. In an effort to circumvent CD4-specific antibody production, a separate study used mAb A32 in place of sCD4 to trigger CD4i epitope formation. A32 binds the CD4 binding site of gp120 and is known to enhance the exposure of CD4i epitopes [162]; however, when tested in guinea pigs, these gp120-A32 stabilized Env complexes were no better than gp120 as immunogens [163].
Immunogens that elicit antibodies to CD4i epitopes on gp120 would seem to be particularly attractive given recent evidence that this region is perhaps the most conserved neutralization target on the virus [110]. However, these antibodies only neutralize when either sCD4 is present to induce the epitope prior to virus-cell contact [164] or when Fab and scFv fragments are used in place of whole IgG molecules [111]. These latter findings strongly suggest that whole IgG molecules are physically occluded from their target epitopes at the virus-cell interface. The inability of CD4i antibodies to bind most HIV-1 variants prior to cell attachment severely limits their neutralizing capacity.
A fourth category of immunogens is based on structural analogs of conserved epitopes recognized by broadly neutralizing mAbs. Various presentations of the MPER of gp41 have been explored and found to generate peptide-reactive antibody responses, but these antibodies have not possessed neutralizing activity [165–169]. A structural analog of the 1b12 epitope also was found to be a poor immunogen [170]. To date, little work has been carried out by way of exploring structural analogs of neutralization epitopes on carbohydrate moieties of the virus, such as the epitope recognized by 2G12. The inability of 2G12 to neutralize most Clade C variants [171] would need to be overcome by identifying new carbohydrate epitopes that are targets for neutralizing a broader spectrum of genetic variants, and as well, need to be made immunogenic in man.
A fifth category of immunogens utilizes polyvalent Env and consensus and ancestral Envs to minimize the genetic and antigenic differences between vaccine strains and field isolates. Polyvalent Env aims to incorporate multiple epitope specificities that would encompass a wide and useful range of antigenic variation. Furthermore, some antibody specificities have potential to combine for synergistic effects on neutralization [172–178]. In other cases, focusing the B-cell response on minor variations in a single epitope has the potential to drive somatic mutations for increased antibody affinity, greater neutralization potency and possibly higher antibody titers [179–181]. Few studies have examined polyvalent Env as candidate HIV-1 immunogens and, although outperforming monovalent Env, substantial breadth has not been achieved with early formulations [182–184], Notably, these early studies examined a limited number of strains that were not preselected on the basis of their performance as monovalent immunogens. Efforts to improve this strategy might focus on the selection of appropriate strains and ways to enhance the magnitude of antibody-induction with better adjuvants [185].
Consensus and ancestral Env differs from polyvalent Env by focusing on single Env sequences [91,186]. Initial studies have shown that these immunogens are functional, immunogenic and generate a neutralizing antibody response; however, the limited breadth of neutralization has been disappointing [187,188]. Notably, a second generation consensus Env immunogen has shown greater potential for broadly neutralizing subtype B and C primary isolates [92], but nonetheless still needs improvement. Improvements in consensus Envs might be possible as a greater number of transmitted Env sequences become available, thereby increasing the likelihood of selecting the most commonly shared amino acid residues.
A sixth category of immunogens employs hyperglycosylation and other mutations to mask non-neutralizing ‘decoy’ epitopes with the hope that this will redirect the B cell response to key conserved elements of Env [189,190]. Early evidence suggests this approach does indeed refocus the antibody response, but it is not clear whether it will yield an improved response against conserved neutralization epitopes on HIV-1 Env [191,192].
The picture that is emerging from early studies of novel immunogens suggests that strategies to uncloak neutralization epitopes by removing N-linked glycans and deleting variable loops face the daunting challenge of targeting epitopes that remain cloaked on the real-world virus. Furthermore, it is becoming increasingly apparant that antibodies have little possibility of gaining access to many neutralizing epitopes at the virus-cell interface. Therefore, antibodies need to bind the virus before it engages a target cell in order to neutralize. Neutralization requires antibodies that are able to bind native Env trimers, but it is not clear that stabilized trimers will work as immunogens without further modification. Additional efforts to target conserved neutralization epitopes, such as those in the MPER of gp41 and perhaps elsewhere on the gp140 molecule, focus on breaking tolerance and preserving non-pathogenic autoreactive B cell clones. Other future prospects include various combinations of these new Env design concepts, some of which are in early stages of development [193,194].
Adjuvant design for HIV-1 vaccines
Concomitant with the field of HIV-1 vaccine development have come rapid advances in adjuvant design for understanding on a rational basis the optimal formulation of immunogens for induction of the right balance of effector memory and central memory T cells, and plasma cells and memory B cells. Although review of the field of vaccine adjuvant development is outside the scope of this review, it is critical to note that rapid advances in the field of dendritic cell biology, B cell immunoregulation and Toll-like receptor signaling have provided important new insights that the HIV-1 vaccine field needs to access for design of optimal HIV-1 vaccines [195–203].
Similarly, new knowledge of the role of CD4+, CD25+, FOX-P3+ regulatory T cells in both controlling HIV-1 and other vaccine responses [204] and the role of adjuvants in regulation both the production in the thymus and peripheral proliferation of regulatory T cells [PEACOCK J, HAYNES BF, SEMPOWSKI G, MANUSCRIPT SUBMITTED], have also enabled of design of new formulations of adjuvants and vaccines for induction and maintenance of the desired immune responses.
In particular, it will be important to identify B-cell pools that produce broadly reactive neutralizing antibodies [14], and to formulate Env immunogens with adjuvants that selectively activate these B-cell pools. Research targeted at understanding the differential signaling requirements of ‘innate’ and ‘adaptive’ B-cell triggering is critical for HIV-1 vaccine development.
Expert commentary
The field of antibody induction for HIV-1 vaccine development is at a turning point, with the recent remarkable insights made into the structures of liganded and unliganded HIV-1 Env, and insights into structures of how broadly neutralizing antibodies bind to Env epitopes and work to neutralize HIV-1. This elegant structural work should provide insights into how to design the most immunogenic envelope vaccine candidates. Insights into the B cell subsets capable of producing broadly neutralizing antibodies and their immunoregulatory controls should provide new and enabling insights into how to safely induce desired antibody specificities. Taken together with work on optimizing adjuvant formulation of vaccines, optimizing systemic and mucosal memory T and B cell-induction, and development of new vectors for systemic and mucosal immunogen delivery, we believe that a new generation of vaccines will be developed with real promise for controlling, or even preventing, HIV-1 infection.
Five-year view
The next few years will be an exciting time of discovery and new vaccine product development, based on the progress made by the field in the last 5 years. We anticipate proof of concept experiments in nonhuman primates for many of the postulates and strategies discussed in this review. The ultimate success of HIV vaccine development will rely on our understanding why broadly neutralizing antibodies cannot be made, and in learning how to safely induce protective anti-HIV-1 antibodies.
Key issues
A conundrum is why broadly neutralizing antibodies are not routinely made in acutely infected individuals and immunized subjects with current vaccines?
HIV-1 has evolved many immune evasion mechanisms to escape HIV-1 antibody responses that need to be breached in order for antibodies to be effective.
Immune evasion mechanisms include structural modifications and characteristics of HIV-1 Env, and epitopes of Env that appear to be silent due to self tolerance mechanisms.
Although we know the structures of unliganded and liganded HIV-1 Env monomers, we have yet to see a high resolution picture of a native transmitted HIV-1 Env trimer - a key and elusive achievement that many believe will help design an immunogen that will induce broad neutralizing antibodies.
A number of broadly reactive human neutralizing monoclonal Abs (mAbs) have been made that can neutralize many HIV-1 primary isolates and their structures in complex with their epitopes have been solved.
Novel rational immunogen designs are coming forth based on structural analysis of Env and mAbs as well as basic science discoveries of the past 5 years.
Little is known about the role of anti-HIV-1 antibody responses at mucosal sites and how to measure these responses.
Acknowledgements
The authors thank Joyce Lowery and Kim McClammy for their expert secretarial assistance, Hua-Xin Liao for assistance with graphic design and critical comments, and Bing Chen and Steve Harrison for the illustrations. This work was supported by the NIAID Center for HIV/AIDS Vaccine Immunology grant, AI06787501, and NIH grants AI52816, AI067134, AI51445, AI30034, and AI46705.
Contributor Information
Barton F Haynes, Box 3258, RP-1 Building, Building 107, Circuit Drive, Duke University Medical Center, Durham, NC 27710, USA, Tel: +1919 684 5279, Fax: +1 919 684 5230, hayne002@mc.duke.edu.
David C Montefiori, Department of Surgery, Box 2926, Duke University Medical Center, Durham, NC 27710, USA, Tel: +1 919 684 5278, Fax: +1 919 684 4288, monte@acpub.duke.edu.
References
- 1.Klausner R, Fauci A, Corey L, et al. Medicine. The need for a global vaccine enterprise. Science. 2003;5628:2036–2039. doi: 10.1126/science.1086916. [DOI] [PubMed] [Google Scholar]
- 2.Esparza J, Klausner RI The Coordinating Committee of the Global HIV/AIDS Vaccine Enterprise. The Global HIV/AIDS Vaccine Enterprise: Scientific Strategic Plan. 2005;2:e25. [Google Scholar]
- 3.Derived from Statistics in Global Summary of the AIDS Epidemic, ‘AIDS Epidemic Update’ UNAIDS. World Health Organization; 2005. Dec, [Google Scholar]
- 4.Letvin NL. Progress toward a HIV vaccine. Ann. Rev. Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi R, Walker B. Immunologic control of HIV-1. Ann. Rev. Med. 2002;53:149–172. doi: 10.1146/annurev.med.53.082901.104011. [DOI] [PubMed] [Google Scholar]
- 6.Mastro TD, Kitayaporn D. HIV type 1 transmission probabilities: estimates from epidemiologic studies. AIDS Res. Hum. Retrovir. 1998;14(Suppl. 3):S223–S227. [PubMed] [Google Scholar]
- 7.Quinn TC, Wawer JM, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 8.Shibata R, Igarashi T, Haigwood N, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 9.Mascola JR, Lewis GM, Stiegler G, et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 11.Parren WHI, Marx AP, Hessell AJ, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrantelli F, Rasmussen RA, Buckley KA, et al. Complete protection of neonatal macaques against oral challenge with pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. J. Infect. Dis. 2004;189:2167–2173. doi: 10.1086/420833. [DOI] [PubMed] [Google Scholar]
- 13.Burton D, Stanfield R, Wilson I. Antibody vs HIV in a clash of evolutionary titans. PNAS. 2005;102:14943–34948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes B, Moody A, Verkcozy L, Kelsoe G, Alam M. Polyspecificity and neutralization of HIV-1: an hypothesis. Human Antibodies. 2005;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 15.The rgp 120 HIV Vaccine Study Group. Placebo-controlled Phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2006;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert P, Ackers M, Berman P, et al. HIV-1 virologic and immunologic progression and initiation of antiretroviral therapy among HIV-1-infected subjects in a trial of the efficacy of recombinant glycoprotein 120 vaccine. J. Infect. Dis. 2005;192:974–983. doi: 10.1086/432734. [DOI] [PubMed] [Google Scholar]
- 17.Levine A, Groshen S, Allen J, et al. Initial studies on active immunization of HIV-infected subjects using a gp120-depleted HIV-1 immunogen: long-term follow-up. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996;11:351–364. doi: 10.1097/00042560-199604010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Lifson J, Rossio J, Piatak M, et al. Evaluation of the safety, immunogenicity, and protective efficacy of whole inactivated simian immunodeficiency virus (SIV) vaccines with conformationally and functionally intact envelope glycoproteins. AIDS Res. Hum. Retrovir. 2004;20:772–787. doi: 10.1089/0889222041524661. [DOI] [PubMed] [Google Scholar]
- 19.Whitney J, Ruprecht R. Live attenuated HIV vaccines: pitfalls and prospects. Curr. Opin. Infect. Dis. 2004;17:17–16. doi: 10.1097/00001432-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Koff W, Johnson P, Watkins D, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 2005;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 21.Derdeyn C, Decker J, Bibollet-Ruche F, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 22.Frost S, Liu Y, Pond S, Chappey C, et al. Characterization of human immunodeficiency virus type 1 (HIV-1) envelope variation and neutralizing antibody responses during transmission of HIV-1 subtype B. J. Virol. 2005;79:6523–6527. doi: 10.1128/JVI.79.10.6523-6527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chohan B, Lang D, Sagar M, et al. Selection of human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J. Virol. 2005;79:6528–6531. doi: 10.1128/JVI.79.10.6528-6531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfsen A, Iniguez P, Bouguyon E, Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial trancytosis of HIV-1. J. Immunol. 2001;166:6257–6265. doi: 10.4049/jimmunol.166.10.6257. [DOI] [PubMed] [Google Scholar]
- 25.Devito C, Hinkula J, Kaul R, et al. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 2002;30:413–420. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Kurteva S, Ren X, Lee S, Sodroski J. Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus Type 1. J. Virol. 2005;79:12132–12147. doi: 10.1128/JVI.79.19.12132-12147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascola J. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine. 2002;20:1922–1925. doi: 10.1016/s0264-410x(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 28.Mascola J. Defining the protective antibody response for HIV-1. Current Mol. Med. 2003;3:209–216. doi: 10.2174/1566524033479799. [DOI] [PubMed] [Google Scholar]
- 29.Kkusuhara H, Hohdatsu T, Okumura M, et al. Dual-subtype vaccine (Fel-O-Vax FIV) Protects cats against contact challenge with heterologous subtype B FIV infected cats. Vet. Microbiol. 2005;108:155–165. doi: 10.1016/j.vetmic.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Beddows S, Lister S, Cheingsong R, Bruck C, Weber J. Comparison of the antibody repertoire generated in healthy volunteers following immunization with a monomeric recombinant gp120 construct derived from a CCR5/CXCR4-using human immunodeficiency virus type 1 isolate with sera from naturally infected individuals. J. Virol. 1999;73:1740–1745. doi: 10.1128/jvi.73.2.1740-1745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bures RA, Gaitan T, Zhu C, et al. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by gp160 protein boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 32.Mascola JR, Snyder WS, Weislow OS, et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 33.Belshe RB, Graham SB, Keefer MC, et al. Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. JAMA. 1994;272:475–480. doi: 10.1001/jama.272.6.475. [DOI] [PubMed] [Google Scholar]
- 34.Gorse GJ, McElrath JM, Matthews TJ, et al. Modulation of immunologic responses to HIV-1MN recombinant gp160 vaccine by dose and schedule of administration. Vaccine. 1998;16:493–506. doi: 10.1016/s0264-410x(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 35.Evans TG, McElrath JM, Matthews T, et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine. 2001;19:2080–2091. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert PB, Peterson LM, Follman D, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a Phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 37.Goepfert P, Horton J, McElrath S, et al. High-dose recombinant canarypox vaccine expressing HIVI-1 protein in seronegative human subjects. J. Infect. Dis. 2005;192:1249–1259. doi: 10.1086/432915. [DOI] [PubMed] [Google Scholar]
- 38.Graham BS, Matthews JT, Belshe RB, et al. The NIAID AIDS Vaccine Clinical trials Network. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. J. Infect. Dis. 1993;167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 39.Evans TG, Keefer CM, Weinhold K, et al. A canarypox vaccine expressing multiple HIV-1 genes given alone or with SF-2 rgp120 elicits broad and durable CTL responses in seronegative volunteers. J. Infect. Dis. 1999;180:290–298. doi: 10.1086/314895. [DOI] [PubMed] [Google Scholar]
- 40.Belshe RB, Gorse JG, Mulligan MJ, et al. Induction of immune responses to HIV-1 canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Corey L, Mulligan M, Goepfert P, et al. Cellular and humoral immune responses to a canarypox vaccine containing human immunodeficiency virus type 1 Env, Gag, and Pro in combination with rgp120. J. Infect. Dis. 2001;183:563–570. doi: 10.1086/318523. [DOI] [PubMed] [Google Scholar]
- 42.Russell ND, Graham SB, Keefer M, et al. A qualifying Phase II study of an HIV-1 canarypox vaccine (vCP1452), alone and in combination with rgp120, fails to trigger a Phase III correlate of efficacy trial. J. Infect. Dis. 2006 doi: 10.1097/01.qai.0000248356.48501.ff. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ourmanov I, Bilska M, Hirsch VH, Montefiori DC. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol. 2000;74:2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckner C, Gines LG, Saunders CJ, et al. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology. 2004;320:167–180. doi: 10.1016/j.virol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Rose NF, Marx AP, Luckay A, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 46.Amara RR, Villinger F, Altman JD, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 47.Barouch DH, Santra S, Schmitz JE, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 48.Barouch DH, Santra S, Kuroda MJ, et al. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia Ankara (MVA) vaccination. J. Virol. 2001;75:5151–5158. doi: 10.1128/JVI.75.11.5151-5158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis NL, Caley IJ, Brown KW, et al. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 2000;74:371–378. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Arthos J, Lawrence MJ, van Ryk D, et al. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J. Virol. 2005;79:7933–7937. doi: 10.1128/JVI.79.12.7933-7937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mascola JR, Sambor A, Beaudry K, et al. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J. Virol. 2005;79:771–779. doi: 10.1128/JVI.79.2.771-779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans TG, Frey S, Israel H, et al. Long term memory B-cell responses in recipients of candidate human immunodeficiency virus type 1 vaccines. Vaccine. 2004;22:2626–2630. doi: 10.1016/j.vaccine.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Montefiori DC, Hill ST, Vo HTT, Walker DB, Rosenberg ES. Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection. J. Virol. 2001;75:10200–10207. doi: 10.1128/JVI.75.21.10200-10207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montefiori DC, Altfeld M, Lee PK, et al. Viremia control despite escape from a rapid and potent autologous neutralizing antibody response after treatment-cessation in an HIV-1-infected individual. J. Immunol. 2003;170:3906–3914. doi: 10.4049/jimmunol.170.7.3906. [DOI] [PubMed] [Google Scholar]
- 55.Moore PL, Crooks TE, Porter L, et al. The nature of non-functional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 2006;80(5):2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montefiori DC. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Sem. Immunopathol. 1997;18:371–390. doi: 10.1007/BF00813504. [DOI] [PubMed] [Google Scholar]
- 57.Forthal DN, Landucci G, Haubrich R. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J. Infect. Dis. 1999;180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 58.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landucci G, Phan T, Higa-Tanner R, Gilbert P, Forthal D. AIDS Vaccine 2005. Montreal, Quebec, Canada: 2005. Sep 6–9, Individuals homozygous for the V allele of FcγRIIIa may have increased risk of HIV infection following vaccination with recombinant gp120. Abstract #119. [Google Scholar]
- 60.Mascola JR, D'Souza P, Gilbert P, et al. Recommendations for the design and use of standard virus panels to assess the neutralizing antibody response elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen B, Vogah E, Gong H, Skehel J, Wiley D, Harrison S. Structure of an unliganded simian immunodeficiency virus gp120 Core. Nature. 2005;433:834–841. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- 63.Eckert D, Kim P. Mechanisms of viral membrane fusion and its inhibition. Ann. Rev. Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 64.Matthews T, Salgo M, Greenberg M, Chung J, Demasi R, Bolognesi D. Efuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat. Rev. Drug Discovery. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 65.Wei X, Decker J, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 66.Richman D, Wrin T, Little S, Petropoulos C. Rapid evolution of the neutralizing antibody response to HIV Type 1 infection. Proc. Natl. Acad. Sci. USA. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frost S, Wrin T, Smith D, et al. Neutralizing antibody responses drive the evolution of human immunodeficiency virus Type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. USA. 2005;201:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palker T, Matthews T, Langlois A, et al. Polyvalent human immunodeficiency virus synthetic immunogen comprised of envelope gp120 T helper cell sites and B cell neutralization epitopes. J. Immunol. 1989;142:3612–3619. [PubMed] [Google Scholar]
- 69.Korber B, MacInnes K, Smith R, Myers G. Mutational trends in V3 loop protein sequences observed in different genetic lineages of human immunodeficiency virus Type 1. J. Virol. 1994;68:6730–6744. doi: 10.1128/jvi.68.10.6730-6744.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwong P, Doyle M, Casper D, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 71.Scanlan CN, Pantophlet R, Wormald MR, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α 1–2 mannose residues on the outer face of gp120. J. Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scanlan CN, Pantophlet R, Wormald, et al. The carbohydrate epitope of the neutralizing anti-HIV-1 antibody 2G12. Adv. Exp. Med. Biol. 2003;535:205–218. doi: 10.1007/978-1-4615-0065-0_13. [DOI] [PubMed] [Google Scholar]
- 73.Calarese DA, Lee HK, Huang CY, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc. Natl. Acad. Sci. USA. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nara PL, Smit L, Dunlop N, et al. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type IIIb infection of chimpanzees. J. Virol. 1990;62:3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andeweg A, Boers P, Osterhaus A, Bosch M. Impact of natural sequences variation in the V2 region of the envelope protein of human immunodeficiency virus Type 1 on syncytium induction: a mutational analysis. J. Gen. Virol. 1995;76:1901–1907. doi: 10.1099/0022-1317-76-8-1901. [DOI] [PubMed] [Google Scholar]
- 76.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus Type 1 gp120 epitopes induced by receptor binding. J. Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labrosse B, Treboute C, Brelot A, Alizon M. Cooperation of the V1/V2 and V3 domains of human immunodeficiency virus Type 1 gp120 for Interaction with the CXCR4 Receptor. J. Virol. 2001;75:5457–5464. doi: 10.1128/JVI.75.12.5457-5464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okamoto Y, Shiosaki K, Eda Y, et al. Father-to-mother-to-infant transmission of HIV-1: clonally transmitted isolate of infant mutates more rapidly than that of the mother and rapidly loses reactivity with neutralizing antibody. Microbiol. Immunol. 1997;41:131–138. doi: 10.1111/j.1348-0421.1997.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 79.Palker T, Clark M, Langlois A, et al. Type-specific neutralization of the human immunodeficiency virus with antibodies to Env-coded synthetic peptides. Proc. Natl. Acad. Sci. USA. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorny M, Revesz K, Williams C, et al. The V3 loop is accessible on the surface of most human immunlodeficiency virus type 1 primary isolates and serves as a neutralization epitope. J. Virol. 2004;78:2394–2404. doi: 10.1128/JVI.78.5.2394-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zolla-Pazner S. Identifying epitopes of HIV-1 that induce protective antibodies. Nat. Rev. Immunol. 2004;4:199–210. doi: 10.1038/nri1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rusche J, Javaherian K, McDanal C, et al. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc. Natl. Acad. Sci. USA. 1988;85:3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Javaherian K, Langlois A, LaRosa G, et al. Broadly neutralizing antibodies elicited by the hyupervariable neutralizing determinant of HIV-1. Science. 1990;250:1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- 84.Looney D, Fisher A, Putney S, et al. Type-restricted neutralization of molecular clones of human immunodeficiency virus. Science. 1988;241:357–359. doi: 10.1126/science.3388046. [DOI] [PubMed] [Google Scholar]
- 85.Golding H, D’Souza M, Bradac J, Mathieson B, Fast P. Neutralization of HIV-1. AIDS Res. Hum. Retrovir. 1994;10:633–643. doi: 10.1089/aid.1994.10.633. [DOI] [PubMed] [Google Scholar]
- 86.Matthews T. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res. Hum. Retrovir. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 87.Bou-Habib DC, Roderiquez G, Oravecz T, Berman PW, Lusso P, Norcross MA. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haynes B, Ma B, Montefiori D, et al. Analysis of HIV-1 subtype B third variable region peptide motifs for induction of neutralizing antibodies against HIV-1 primary isolates. Virology. 2005;345:44–55. doi: 10.1016/j.virol.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 89.Binley J, Wrin T, Korber B, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krachmarov C, Pinter A, Honnen W, et al. Antibodies that are cross-reactive for human immunodeficiency virus type 1 Clade A and B v3 domains are common in patient sera from Cameroon, but their neutralization activity is usually restricted by epitope masking. J. Virol. 2005;79:780–790. doi: 10.1128/JVI.79.2.780-790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaschen B, Taylor KJ, Yusim K, et al. Diversity of considerations in HIV-1 vaccine selection. Science. 2003;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 92.Liao H-X, Sutherland LL, Xia S-M, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C primary viruses. Virology. 2006 doi: 10.1016/j.virol.2006.04.043. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang C, Tang M, Zhang M, et al. Structure of V3-containing HIV-1 gp120 Core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nara PL, Robey WG, Pyle SW, et al. Purified envelope glycoproteins from human immunodeficiency virus type 1 induce individuals type-specific neutralizing antibodies. J. Virol. 1988;62:2622–2628. doi: 10.1128/jvi.62.8.2622-2628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohler H, Gouldsmit J, Nara P. Clonal antibody dominance in HIB-1 infection: cause for a limited failing immune response. J. Acquir. Immun. Defic. Syndr. 2005;5:1158–1168. [PubMed] [Google Scholar]
- 96.Herrera C, Klasse P, Michael E, et al. The impact of envelope glycoprotein cleavage on the antigenicity, infectivity, and neutralization sensitivity of env-pseudotyped human immunodeficiency virus type 1 particles. Virology. 2005;338:154–172. doi: 10.1016/j.virol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Pinter A, Honnen W, D’AGostino P, Gorny M, Zolla-Pazner S, Kayman S. The C108g epitope in the V2 domain of gp120 functions as a potent neutralization target when introduced into envelope proteins derived from human immunodeficiency virus type 1 primary isolates. J. Virol. 2005;79:6909–6917. doi: 10.1128/JVI.79.11.6909-6917.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edinger A, Ahuja M, Sung T, et al. Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein. J. Virol. 2000;74:7922–7935. doi: 10.1128/jvi.74.17.7922-7935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pinter A, Honnen W, Kayman S, Trochev O, Wu Z. Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine. 1998;16:1803–1811. doi: 10.1016/s0264-410x(98)00182-0. [DOI] [PubMed] [Google Scholar]
- 100.Wu Z, Kayman S, Honnen W, et al. Characterization of neutralization epitopes in the V2 region of human immunodeficiency virus type 1 gp120: role of glycosylation in the correct folding of the V1/V2 domain. J. Virol. 1995;69:2271–2278. doi: 10.1128/jvi.69.4.2271-2278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skott P, Achour A, Norin M, Thorstensson R, Bjorling E. Characterization of neutralization sites in the second variable and fourth variable region in gp125 and a conserved region in gp36 of human immunodeficiency virus type 2. Viral Immunol. 1999;12:79–88. doi: 10.1089/vim.1999.12.79. [DOI] [PubMed] [Google Scholar]
- 102.Bolmstedt A, Sjolander S, Hansen J, et al. Influence of N-linked glycans in V4-V5 region of human immunodeficiency virus type 1 glycoprotein gp160 on induction of a virus-neutralizing humoral response. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996;12:213–220. doi: 10.1097/00042560-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 103.Pinter A, Honnen W, He Y, Gorney M, Zolla-Pazner S, Kayman S. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 2004;78:5205–5215. doi: 10.1128/JVI.78.10.5205-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ren X, Sodroski J, Yang X. An unrelated monoclonal antibody neutralizes human immunodeficiency virus type 1 by binding to an artificial epitope engineered in a functionally neutral region of the viral envelope glycoproteins. J. Virol. 2005;79:5616–5624. doi: 10.1128/JVI.79.9.5616-5624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burton D, Pyati R, Koduri S, et al. Efficient neutralization of primary isolates of HIV-1 by recombinant human monoclonal antibody. Science. 1994;266:1024. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 106.Haynes B, Fleming J, St Clair E, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 107.Pantophlet R, Burton D. Immunofocusing: antigen engineering to promote the induction of HIV-neutralizing antibodies. Trends Mol. Med. 2003;9:468–473. doi: 10.1016/j.molmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Pantophlet R, Wilson I, Burton D. Improved design of an antigen with enhanced specificity for the broadly HIV-neutralizing antibody b12. Prot. Eng. Design Selection. 2004;17:749–758. doi: 10.1093/protein/gzh085. [DOI] [PubMed] [Google Scholar]
- 109.Selvarjah S, Puffer B, Pantophlet R, Law M, Doms R, Burton D. Comparing antigenicity and immunogenicity of engineered gp120. J. Virol. 2005;79:12148–12163. doi: 10.1128/JVI.79.19.12148-12163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Decker J, Bibollet-Ruche F, Wei X, et al. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 2005;201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Labrijn A, Poignard P, Raja A, et al. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 2003;77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LaBranche C, Hoffman T, Romano J, et al. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J. Virol. 1999;73:10310–10319. doi: 10.1128/jvi.73.12.10310-10319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edwards T, Hoffman T, Baribaud F, et al. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 2001;75:5230–5239. doi: 10.1128/JVI.75.11.5230-5239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conley A, Kessler A, Boots L, et al. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc. Natl. Acad. Sci. USA. 1994;91:3348. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stiegler G, Kunert R, Purtscher M, et al. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 2001;17:1757. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 116.Roben P, Moore J, Thali M, Sodroski J, Barbas C, Burton D. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 1994;68:4821. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muster T, Srindl F, Purtscher M, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 1993;67:6642. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zwick MB, Labrijn AF, Wang M, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ofek G, Tang M, Sambor A, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus tpe 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 2004;78:10724. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cardoso R, Zwick M, Stanfield R, et al. Broadly neutralzing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 121.Burton D, Desrosiers R, Doms R, et al. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 2004;5:233. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 122.Bibollet-Ruche F, Li H, Decker M, et al. Detection of novel neutralizing antibody reactivities against the membrane proximal external region (MPER) of gp41 in HIV-1 infected humans. AIDS Vaccine (2005) Meeting; September 6–9, 2005; Montreal, Canada. 2005. Abstract 71. [Google Scholar]
- 123.Walmsley SK, Katlama HC, Nelson M, et al. Enfuvirtide (T-20) cross-reactive glycoprotein 41 antiobdy does not impair the efficacy or safety of enfuvirtide. J. Infect. Diseases. 2003;188:1827–1833. doi: 10.1086/379810. [DOI] [PubMed] [Google Scholar]
- 124.Zwick M, Jensen R, Church W, et al. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial studies in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J. Virol. 2005;79:1252. doi: 10.1128/JVI.79.2.1252-1261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Earl P, Broder C, Doms R, Moss B. Epitope map of human immunodeficiency virus gype 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 1997;71:2674. doi: 10.1128/jvi.71.4.2674-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Golding H, Zaitseva M, de Rosny E, et al. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 2002;76:6780–6790. doi: 10.1128/JVI.76.13.6780-6790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maksyutov AZ, Bachinskii AG, Bazhan SI, et al. Exclusion of HIV epitopes shared with human proteins is prerequisite for designing safer AIDS vaccines. J. Clin. Virol. 2004;1(Suppl.):S26–S38. doi: 10.1016/j.jcv.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 128.Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol. Rev. 2004;197:161–178. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- 129.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J. Exp. Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meffre E, Milili M, Blanco-Betancourt C, Antunes H, Nussenzweig MC, Schiff C. Immunoglobulin heavy chain expression shapes the B cell receptro repertor in human B cell development. J. Clin. Invest. 2001;108:879–886. doi: 10.1172/JCI13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lopalco L, Barassi C, Paolucci C, et al. Predictive value of anti-cell and anti-human immunodeficiency virus (HIV) humoral responses in HIV-1-exposed seronegative cohorts of European and Asian origin. J. Gen. Virol. 2005;86:339–348. doi: 10.1099/vir.0.80585-0. [DOI] [PubMed] [Google Scholar]
- 132.Montefiori DC, Safrit TJ, Lydy SL, et al. Induction of neutralizing antibodies and Gag-specific cellular immune responses to an R5 primary isolate of human immunodeficiency virus type 1 in vaccinated rhesus macaques. J. Virol. 2001;75:5939–5948. doi: 10.1128/JVI.75.13.5879-5890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berkower IM, Raymond J, Muller A, Spadaccini A. Aberseen, Assembly, structure, and antigenic properties of virus-like particles rich in HIV-1 envelope gp120. Virology. 2004;321:75–86. doi: 10.1016/j.virol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 134.Grundner C, Mirzabekov T, Sodroski J, Wyatt R. Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins. J. Virol. 2002;76:3511–3521. doi: 10.1128/JVI.76.7.3511-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sakaue G, Hiroi T, Nakagawa Y, et al. HIV mucosal vaccine: nasal immunization with gp160-encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. J. Immunol. 2003;170:495–502. doi: 10.4049/jimmunol.170.1.495. [DOI] [PubMed] [Google Scholar]
- 136.Arthur LO, Bess WJ, Chertova EN., Jr Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retrovir. 1998;14(Suppl. 3):S311–S319. [PubMed] [Google Scholar]
- 137.Rossio JL, Esser TM, Suryanarayana K. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Montefiori DC, Hirsch MV, Johnson PR. AIDS response. (Scientific Correspondence) Nature. 1991;354:439–440. [Google Scholar]
- 139.Langlois AJ, Weinhold JK, Matthews JT, Greenberg ML, Bolognesi DP. Detection of anti-human cell antibodies in sera from macaques immunized with whole inactivated virus. AIDS Res. Hum. Retrovir. 2005;8:1641–1652. doi: 10.1089/aid.1992.8.1641. [DOI] [PubMed] [Google Scholar]
- 140.Hammonds J, Chen X, Fouts T, DeVico A, Montefiori D, Spearman P. Induction of neutralizing antibodies against human immunodeficiency virus type 1 primary isolates by Gag-Env pseudovirion immunization. J. Virol. 1992;79:14804–14814. doi: 10.1128/JVI.79.23.14804-14814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sanders RW, Vesanen M, Schuelke N. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 2002;76:8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang X, Farzan M, Wyatt R, Sodroski J. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 2000;74:5716–5725. doi: 10.1128/jvi.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang X, Florin L, M Farzan P, et al. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 2000;74:4746–4654. doi: 10.1128/jvi.74.10.4746-4754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Beddows S, Schulke N, Kirschner M, et al. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 2005;79:8812–8827. doi: 10.1128/JVI.79.14.8812-8827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang X, Wyatt R, Sodroski J. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 2001;75:1165–1171. doi: 10.1128/JVI.75.3.1165-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim M, Qiao Z, Montefiori CD, Haynes BF, Reinherz LE, Liao H-X. Comparison of HIV type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res. Hum. Retrovir. 2005;21:58–67. doi: 10.1089/aid.2005.21.58. [DOI] [PubMed] [Google Scholar]
- 147.Bolmstedt A, Hinkula J, Rowcliffe E, Biller M, Wahren B, Olofsson S. Enhanced immunogenicity of a human immunodeficiency virus type 1 env DNA vaccine by manipulating N-glycosylation signals: Effects of elimination of the V3 N306 glycan. Vaccine. 2002;20:397–405. doi: 10.1016/s0264-410x(01)00358-9. [DOI] [PubMed] [Google Scholar]
- 148.Quinones-Kochs MI, Buonocore L, Rose JK. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 2002;76:4199–4211. doi: 10.1128/JVI.76.9.4199-4211.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reitter JN, Means ER, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 150.Mori K, Sugimoto C, Ohgimoro S, et al. Influence of glycosylation on the efficacy of an Env-based vaccine against simian immunodeficiency virus SIV mau239 in a macaque AIDS model. J. Virol. 2005;79:10386–10396. doi: 10.1128/JVI.79.16.10386-10396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barnett SW, Lu S, Sravastava I, et al. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 2001;75:5526–5540. doi: 10.1128/JVI.75.12.5526-5540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cherpelis S, Shrivastava I, Gettie A, Jin X, Ho DD, Barnett SW. DNA vaccination with the human immunodeficiency virus type 1 SF162EV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CDS+ T cell- depleted rhesus macaques. J. Virol. 2001;75:1547–1550. doi: 10.1128/JVI.75.3.1547-1550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gzyl J, Bolesta E, Wierzbicki A, et al. Effect of partial and complete variable loop deletions of the human immunodeficiency virus type 1 envelope glycoprotein on the breadth of gp160-specific immune responses. Virology. 2004;318:493–506. doi: 10.1016/j.virol.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 154.Kim YB, Han PD, Cao C, Cho MW. Immunogenicity and ability of variable loop-deleted human immunodeficiency virus type 1 envelope glycoproteins to elicit neutralizing antibodies. Virology. 2003;305:124–137. doi: 10.1006/viro.2002.1727. [DOI] [PubMed] [Google Scholar]
- 155.Srivastava IK, VanDorsten K, Vojtech L, Barnett WS, Stomatatos L. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J. Virol. 2003;77:2310–2320. doi: 10.1128/JVI.77.4.2310-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.LaCasse RA, Follis KE, Trahey M, Scarborough JD, Littman DR, Nunberg JH. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 2002;283(5570):1025. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 157.Nunberg JH. Retraction of "LaCasse RA, Follis EK, Trahey M, Scarborough DJ, Littman RD, Nunberg JH. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science 283, 357–362. Retraction in Science. 1999;296:1025. doi: 10.1126/science.283.5400.357. (2002) [DOI] [PubMed] [Google Scholar]
- 158.Fouts TR, Tuskan R, Godfrey K, et al. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J. Virol. 2000;74:11427–11436. doi: 10.1128/jvi.74.24.11427-11436.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.DeVico A, Silver A, Thornton MA, Sarngadharan GM, Pal R. Covalently crosslinked complexes of human immunodeficiency virus type 1 (HIV-1) gp120 and CD4 receptor elicit a neutralizing immune response that includes antibodies selective for primary virus isolates. Virology. 1996;218:258–263. doi: 10.1006/viro.1996.0188. [DOI] [PubMed] [Google Scholar]
- 160.Fouts T, Godfrey K, Bobb K, et al. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc. Natl. Acad. Sci. USA. 2002;99:11842–11847. doi: 10.1073/pnas.182412199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Varadarajan R, Sharma D, Chakraborty K, et al. Characterization of gp120 and its single-chain derivatives, gp120-CD4D12 and gp120-M9: Implications for targeting the CD4i epitope in human immunodeficiency virus vaccine design. J. Virol. 2005;79:1713–1723. doi: 10.1128/JVI.79.3.1713-1723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liao H-X, Munir Alam S, Mascola JR, et al. Immunogenicity of constrained monoclonal antibody A32-human immunodeficiency virus (HIV) Env gp120 complexes compared to that of recombinant HIV type 1 gp120 envelope glycoprotein. J. Virol. 2004;78:5270–5278. doi: 10.1128/JVI.78.10.5270-5278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sullivan N, Sun Y, Sattentau Q, et al. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Coëeffier E, Clément J-M, Cussac V, et al. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine. 2001;19:684–693. doi: 10.1016/s0264-410x(00)00267-x. [DOI] [PubMed] [Google Scholar]
- 166.Eckhart L, Raffelsberger W, Ferko B, et al. Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J. Gen. Virol. 1996;77:2001–2008. doi: 10.1099/0022-1317-77-9-2001. [DOI] [PubMed] [Google Scholar]
- 167.Joyce JG, Hurni MW, Bogusky MJ, et al. Enhancement of -helicity in the HIV-1 inhibitory peptide DP178 leads to an increased affinity for human monoclonal antibody 2F5 but does not elicit neutralizing responses in vitro: implications for vaccine design. J. Biol. Chem. 2002;277:45811–45820. doi: 10.1074/jbc.M205862200. [DOI] [PubMed] [Google Scholar]
- 168.Liang X, Munshi S, Shendure J, et al. Epitope insertion into variable loops of HIV-1 gp120 as a potential means to improve immunogenicity of viral envelope protein. Vaccine. 1999;17:2862–2872. doi: 10.1016/s0264-410x(99)00125-5. [DOI] [PubMed] [Google Scholar]
- 169.McGaughey GB, Citron M, Danzeisen RC, et al. HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemisty. 2003;42:3214–3223. doi: 10.1021/bi026952u. [DOI] [PubMed] [Google Scholar]
- 170.Zwick MB, Bonnycastle A, Menendez MB, et al. Identification and characterization of a peptide that specifically binds the human, broadly neutralizing anti-human immunodeficiency virus type 1 antibody b12. J. Virol. 2001;75:6692–6699. doi: 10.1128/JVI.75.14.6692-6699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bures K, Morris L, Williamson C, et al. Regional clustering of shared neutralization determinants on primary isolates of Clade C human immunodeficiency virus type 1 from South Africa. J. Virol. 2002;76:2233–2244. doi: 10.1128/jvi.76.5.2233-2244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McKeating JA, Cordell J, Dean JC, Balfe P. Synergistic interaction between ligands binding to the CD4 binding site and V3 domain of human immunodeficiency virus type 1 gp120. Virology. 1992;191:732–742. doi: 10.1016/0042-6822(92)90249-o. [DOI] [PubMed] [Google Scholar]
- 173.Tilley SA, Honnen JW, Racho EM, Chou T-C, Pinter A. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res. Hum. Retrovir. 1992;8:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 174.Potts BJ, Field GK, Wu Y, Posner M, Cavacini L, White-Scharf M. Synergistic inhibition of HIV-1 by CD4 binding domain reagents and V3-directed monoclonal antibodies. Virology. 1993;197:415–419. doi: 10.1006/viro.1993.1604. [DOI] [PubMed] [Google Scholar]
- 175.Montefiori DC, Graham SB, Zhou JT, et al. The NIH AIDS Vaccine Clinical Trials Network. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding region of gp120. J. Clin. Invest. 1993;92:840–847. doi: 10.1172/JCI116658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mascola JR, Louder KM, VanCott TC, et al. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zwick MB, Wang M, Poignard P, et al. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 2001;75:12198–12208. doi: 10.1128/JVI.75.24.12198-12208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu W, Smith-Franklin BA, Li P-L, et al. Potent neutralization of primary human immunodeficiency virus Clade C isolates with a synergistic combination of human monoclonal antibodies raised against Clade B. J. Hum. Virol. 2001;4:55–61. [PubMed] [Google Scholar]
- 179.Zhang M-Y, Shu Y, Rudolph D, et al. Improved breadth and potency of an HIV-1 neutralizing human single-chain antibody by random mutagenesis and sequential antigen panning. J. Mol. Biol. 2004;335:209–219. doi: 10.1016/j.jmb.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 180.Barbas CF, III, Hu D, Dunlop N, et al. In vitro evolution of a neutralizing human antibody to HIV-1 to enhance affinity and broaden strain cross-reactivity. Proc. Natl. Acad. Sci. USA. 1994;91:3809–3813. doi: 10.1073/pnas.91.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang WP, Green K, Pinz-Sweeney S, Briones TA, Burton RD, Barbas CF., III CDR walking mutagenesis for the affinity maturation of a potent human anti-HIV-1 antibody into the picomolar range. J. Mol. Biol. 1995;254:392–403. doi: 10.1006/jmbi.1995.0626. [DOI] [PubMed] [Google Scholar]
- 182.Cho MW, Kim BY, Lee MK, et al. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J. Virol. 2001;75:2224–2234. doi: 10.1128/JVI.75.5.2224-2234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rollman E, Hinkula J, Arteaga J, et al. Multi-subtype gp160 DNA immunization induces broadly neutralizing anti-HIV antibodies. Gen. Ther. 2004:1–9. doi: 10.1038/sj.gt.3302275. [DOI] [PubMed] [Google Scholar]
- 184.Pal R, Wang S, Kalyanaraman V, et al. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. AIDS Res. Hum. Retrovir. 2005;14(Suppl. 3):226–236. doi: 10.1111/j.1600-0684.2005.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhan X, Martin LN, Slobod KS, et al. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005;23:5306–5320. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 186.Nickle DC, Jensen AM, Gottlieb GS, et al. Consensus and ancestral state HIV vaccines. Science. 2003;299:1515–1518. doi: 10.1126/science.299.5612.1515c. [DOI] [PubMed] [Google Scholar]
- 187.Doria-Rose N, Learn HG, Rodrigo AG, et al. Human immunodeficiency virus Type 1 subtype B ancestral envelope protein is functional and elicits neutralizing antibodies in rabbits similar to those elicited by a native contemporary subtype B envelope. J. Virol. 2005;79:11214–11224. doi: 10.1128/JVI.79.17.11214-11224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao F, Weaver AE, Lu Z, et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group M consensus envelope glycoprotein. J. Virol. 2005;79:1154–1163. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pantophlet R, Wilson AI, Burton DR. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J. Virol. 2003;77:5889–5910. doi: 10.1128/JVI.77.10.5889-5901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nara PL, Garrity R. Deceptive imprinting: a cosmopolitan strategy for complicating vaccination. Vaccine. 1998;16:1780–1787. doi: 10.1016/s0264-410x(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 191.Garrity RR, Rimmelzwaan G, Minassian A, et al. Refocusing neutralizing antibody responses by targeted dampening of an immunodominant epitope. J. Immunol. 2003;77:5589–5910. [PubMed] [Google Scholar]
- 192.Selvarajah S, Puffer B, Pantophlet R, Law M, Doms R, Burton D. Evaluating antigenicity and immunogenicity of engineered gp120s. AIDS Vaccine 2005; September 6–9, 2005; Montreal, Quebec, Canada. 2005. Abstract #72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sanders RW, Schiffner L, Master A, et al. Variable-loop deleted variants of the human immunogenicity virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J. Virol. 2000;74:5091–5100. doi: 10.1128/jvi.74.11.5091-5100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Srivastava IK, Stamatatos L, Kan E. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J. Virol. 2003;77:11244–11259. doi: 10.1128/JVI.77.20.11244-11259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jiang ZH, Koganty RR. Synthetic vaccines: the role of adjuvants in immune targeting. Curr. Med. Chem. 2003;10:1423–1439. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 196.Kaisho T, Akira S. Regulation of dendritic cell function through toll-like receptors. Curr. Mol. Med. 2003;3:759–771. doi: 10.2174/1566524033479366. [DOI] [PubMed] [Google Scholar]
- 197.Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Sem. Immunol. 2004;16:17–22. doi: 10.1016/j.smim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 198.Baldridge JR, McGowan P, Evans JT, et al. Taking a toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and mono therapeutic agents. Expert Opin. Biol. Ther. 2004;4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 199.Kaisho T, Akira S. Pleiotropic function of Toll-like receptors. Microb. Infect. 2004;6:1388–1394. doi: 10.1016/j.micinf.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 200.Vollmer J. Progress in drug development of immunostimulatory CpG oligodeoxynucleotide ligands for TLR9. Expert Opin. Biol. Ther. 2005;5:673–682. doi: 10.1517/14712598.5.5.673. [DOI] [PubMed] [Google Scholar]
- 201.Gluck R, Burri KG, Metcalfe I. Adjuvant and antigen delivery properties of virosomes. Curr. Drug Deliv. 2005;2:395–400. doi: 10.2174/156720105774370302. [DOI] [PubMed] [Google Scholar]
- 202.Brown LE, Jackson DC. Lipid-based self-adjuvanting vaccines. Curr. Drug Deliv. 2005;2:383–393. doi: 10.2174/156720105774370258. [DOI] [PubMed] [Google Scholar]
- 203.Graham BS. New Approaches to Vaccine Adjuvants: Inhibiting the Inhibitor. PLoS Med. 2006;3:e57. doi: 10.1371/journal.pmed.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J. Immunol. 2005;175:7264–7273. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]