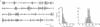Abstract
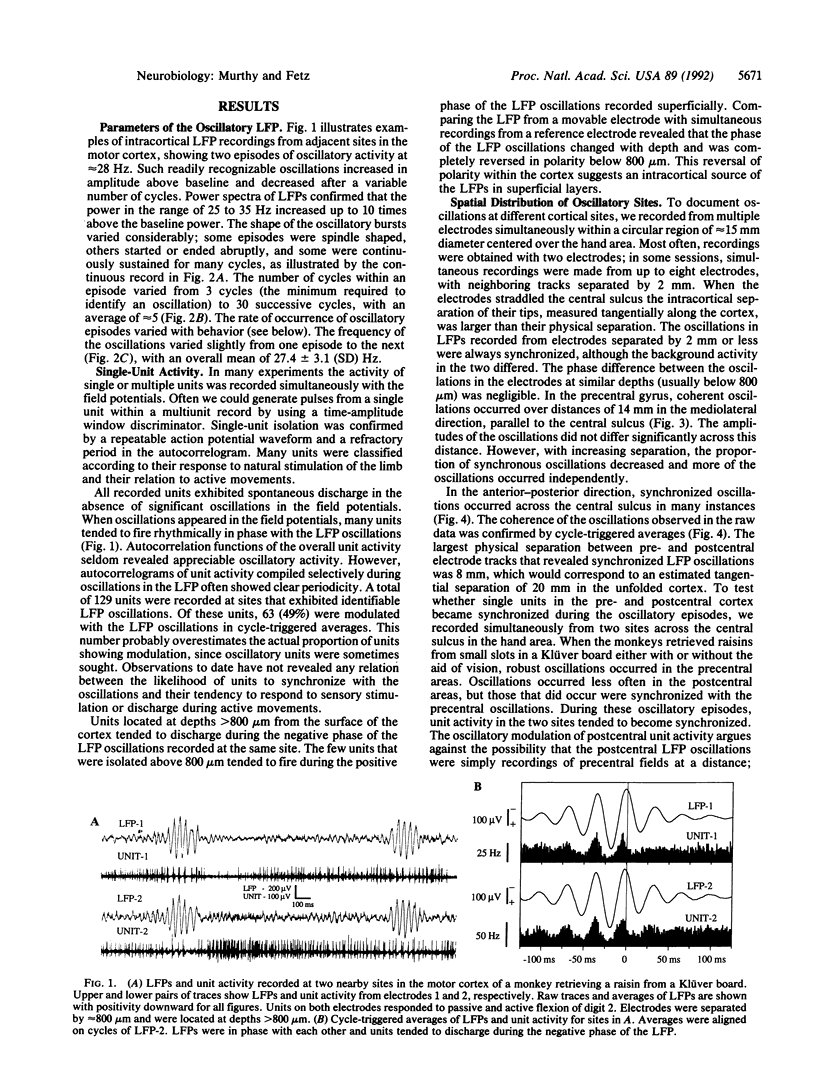
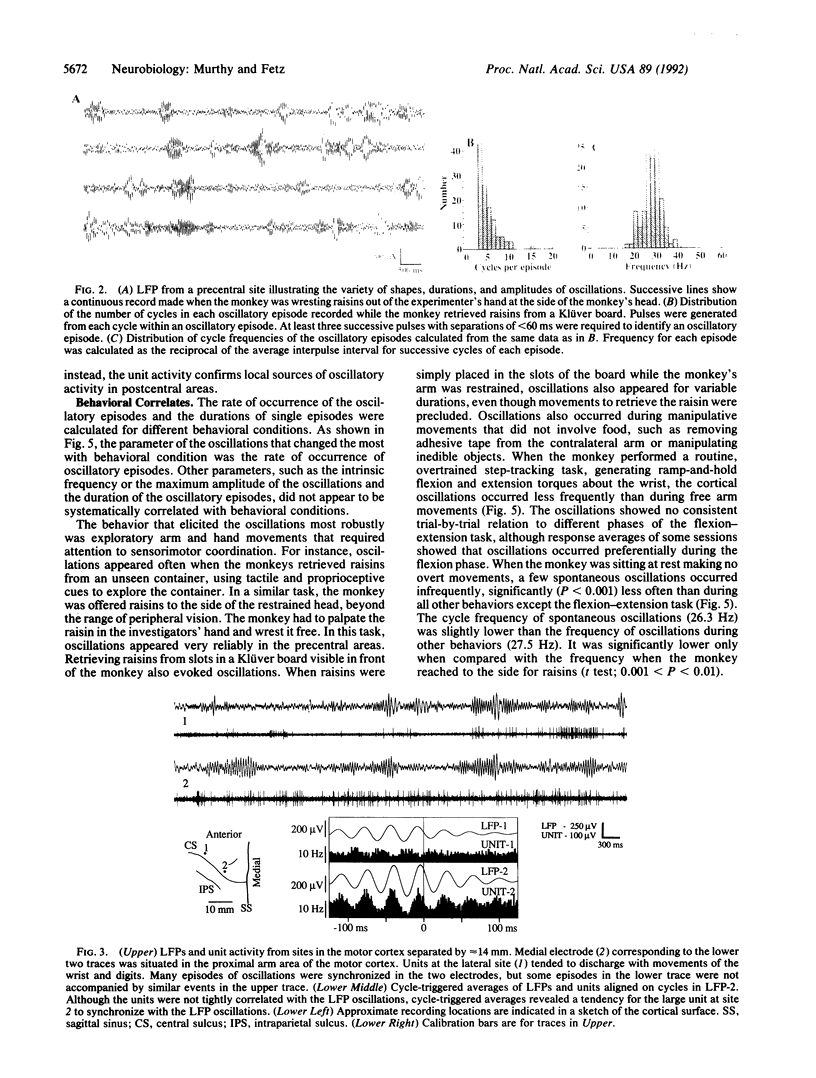
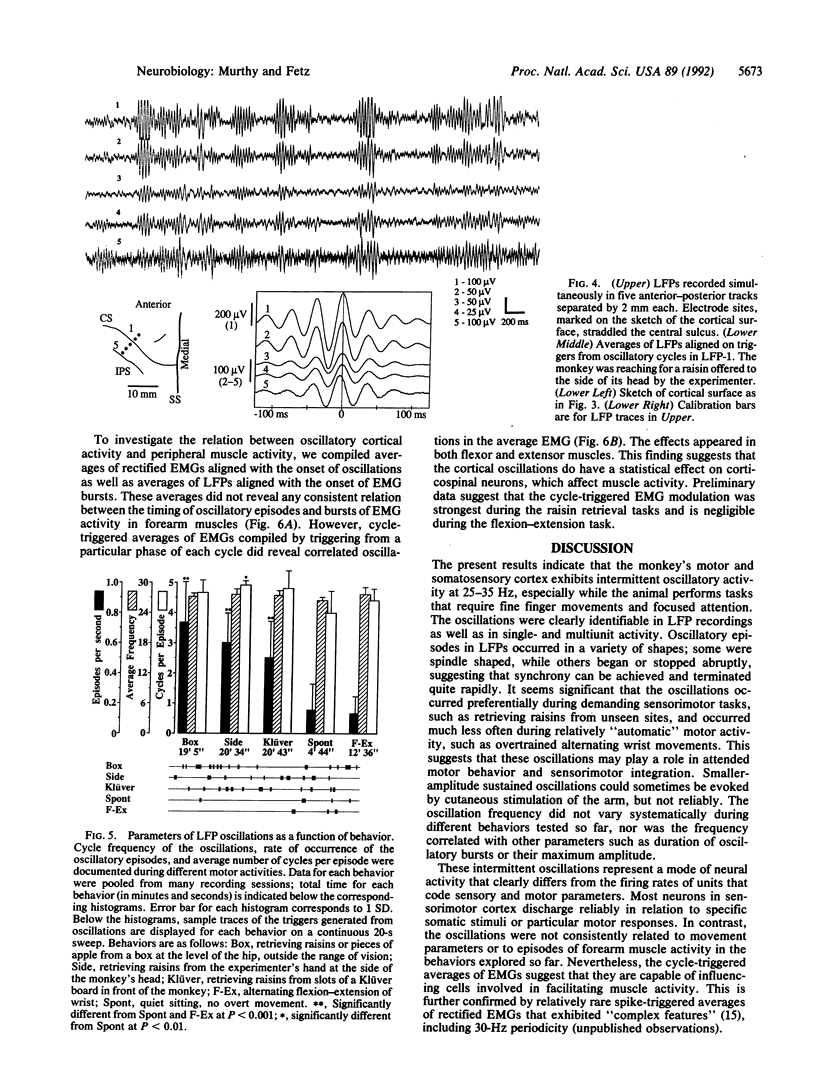
Synchronous 25- to 35-Hz oscillations were observed in local field potentials and unit activity in sensorimotor cortex of awake rhesus monkeys. The oscillatory episodes occurred often when the monkeys retrieved raisins from a Klüver board or from unseen locations using somatosensory feedback; they occurred less often during performance of repetitive wrist flexion and extension movements. The amplitude, duration, and frequency of oscillations were not directly related to movement parameters in behaviors studied so far. The occurrence of the oscillations was not consistently related to bursts of activity in forearm muscles, but cycle-triggered averages of electromyograms revealed synchronous modulation in flexor and extensor muscles. The phase of the oscillations changed continuously from the surface to the deeper layers of the cortex, reversing their polarity completely at depths exceeding 800 microns. The oscillations could become synchronized over a distance of 14 mm mediolaterally in precentral cortex. Coherent oscillations could also occur at pre- and postcentral sites separated by an estimated tangential intracortical distance of 20 mm. Activity of single units was commonly seen to burst in synchrony with field potential oscillations. These findings suggest that such oscillations may facilitate interactions between cells during exploratory and manipulative movements, requiring attention to sensorimotor integration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahissar E., Vaadia E. Oscillatory activity of single units in a somatosensory cortex of an awake monkey and their possible role in texture analysis. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8935–8939. doi: 10.1073/pnas.87.22.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander O., Douglas R. J., Martin K. A., Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer J. J., Montaron M. F., Vahnée J. M., Albert M. P., Rougeul A. Anatomical localization of cortical beta rhythms in cat. Neuroscience. 1987 Sep;22(3):863–869. doi: 10.1016/0306-4522(87)92965-4. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y., Connors B. W. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989 Nov;62(5):1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Engel A. K., Kreiter A. K., König P., Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6048–6052. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980 Oct;44(4):751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Freeman W. J. Spatial properties of an EEG event in the olfactory bulb and cortex. Electroencephalogr Clin Neurophysiol. 1978 May;44(5):586–605. doi: 10.1016/0013-4694(78)90126-8. [DOI] [PubMed] [Google Scholar]
- Freeman W. J., van Dijk B. W. Spatial patterns of visual cortical fast EEG during conditioned reflex in a rhesus monkey. Brain Res. 1987 Oct 6;422(2):267–276. doi: 10.1016/0006-8993(87)90933-4. [DOI] [PubMed] [Google Scholar]
- Galambos R., Makeig S., Talmachoff P. J. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. M., König P., Engel A. K., Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989 Mar 23;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Llinás R. R., Grace A. A., Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R., Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44(3):521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- Rougeul A., Bouyer J. J., Dedet L., Debray O. Fast somato-parietal rhythms during combined focal attention and immobility in baboon and squirrel monkey. Electroencephalogr Clin Neurophysiol. 1979 Mar;46(3):310–319. doi: 10.1016/0013-4694(79)90205-0. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H., Golomb D., Kleinfeld D. Global processing of visual stimuli in a neural network of coupled oscillators. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7200–7204. doi: 10.1073/pnas.87.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Gally J. A., Reeke G. N., Jr, Edelman G. M. Reentrant signaling among simulated neuronal groups leads to coherency in their oscillatory activity. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7265–7269. doi: 10.1073/pnas.86.18.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Paré D., Oakson G. Fast oscillations (20-40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Malsburg C., Schneider W. A neural cocktail-party processor. Biol Cybern. 1986;54(1):29–40. doi: 10.1007/BF00337113. [DOI] [PubMed] [Google Scholar]