Abstract
Background
Interactions of peripheral microtubule tips with the cell cortex are of crucial importance for nuclear migration, spindle orientation, centrosome positioning and directional cell movement. Microtubule plus end binding proteins are thought to mediate interactions of microtubule tips with cortical actin and membrane proteins in a dynein-dependent manner. XMAP215-family proteins are main regulators of microtubule plus end dynamics but so far they have not been implicated in the interactions of microtubule tips with the cell cortex.
Results
Here we show that overexpression of an N-terminal fragment of DdCP224, the Dictyostelium XMAP215 homologue, caused a collapse of the radial microtubule cytoskeleton, whereby microtubules lost contact with the cell cortex and were dragged behind like a comet tail of an unusually motile centrosome. This phenotype was indistinguishable from mutants overexpressing fragments of the dynein heavy chain or intermediate chain. Moreover, it was accompanied by dispersal of the Golgi apparatus and reduced cortical localization of the dynein heavy chain indicating a disrupted dynein/dynactin interaction. The interference of DdCP224 with cortical dynein function is strongly supported by the observations that DdCP224 and its N-terminal fragment colocalize with dynein and coimmunoprecipitate with dynein and dynactin.
Conclusions
Our data show that XMAP215-like proteins are required for the interaction of microtubule plus ends with the cell cortex in interphase cells and strongly suggest that this function is mediated by dynein.
Background
Interactions of peripheral microtubule plus ends with the cell cortex are of crucial importance for nuclear migration, spindle orientation, centrosome positioning and directional cell movement. Cortical dynein and dynactin components play an important role in mediating such interactions, in cooperation with a microtubule plus end complex consisting of a growing number of microtubule-associated proteins [1,2]. Only little is known about a role of the XMAP215-family (named after their Xenopus representative) of microtubule-associated proteins in this process. The ubiquitous occurrence of these proteins in all kinds of organisms including plants suggests general and indispensable functions [3]. In addition to their role as promoters of microtubule elongation, further functions in microtubule growth and nucleation [4,5] and centrosome duplication [5,6] have been described. In most species, XMAP215-family proteins are elongated, monomeric molecules with a size of approximately 230 kDa [7]. By contrast, the yeast homologues (Stu2p in S. cerevisiae, dis1 and Alp14 in S. pombe) occur as dimers and are less than half as long as their counterparts in Dictyostelium and higher cells [8-10]. In budding yeast the major function of Stu2p is observed during mitosis where it regulates microtubule dynamics and is required for chromosome segregation [11-13]. Furthermore, Stu2p interacts with the cortical protein Kar9p [13] and genetic evidence, i.e. crossings of temperature sensitive stu2p mutants with kar9Δ or dynein (dhc1Δ) mutants, suggests that Stu2p plays a role in the Kar9p dependent pathway for spindle orientation [12]. However, until this work there was no evidence for a physical interaction of the long, monomeric members of the XMAP215-family with dynein or a Kar9p-like protein such as APC [14], and there were no data supporting a role in microtubule plus-end/cell cortex interactions in interphase cells.
Like XMAP215, its Dictyostelium homologue, DdCP224, is both a microtubule-associated protein and a genuine centrosomal component [6,15]. Furthermore, it was the first member of the XMAP215-family that was clearly localized at microtubule plus ends, both at kinetochores and microtubule tips near the cell cortex [6,16]. Overexpression of the N-terminal half of DdCP224 as a GFP-fusion protein caused a cytokinesis defect [6]. Since cleavage furrow positioning is determined by the pattern of interaction of astral microtubules with the cell cortex [17], both the cytokinesis defect of ΔC-GFP overexpressing mutants and the detection of DdCP224 at microtubule tips were in agreement with a novel role of DdCP224 in the crosstalk of microtubule tips with the cell cortex. Here we provide evidence for such a function of XMAP215-like proteins and suggest that it is mediated through the interaction with dynein.
Results
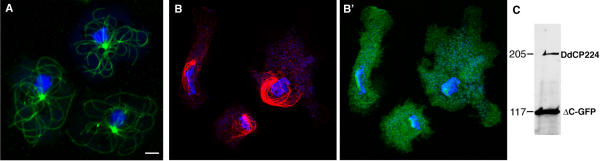
DdCP224-ΔC-GFP mutants overexpress a C-terminal fusion of the N-terminal 813 amino acids of DdCP224 with GFP. By contrast to the full-length protein, the DdCP224-ΔC-GFP fragment itself is unable to bind to microtubules or centrosomes neither in vivo nor in vitro [6]. In our previous study, we have not analyzed the effect of DdCP224-ΔC-GFP overexpression on microtubules. In this work we show that overexpression of DdCP224-ΔC-GFP has a profound effect on the arrangement of interphase microtubules. In wildtype or GFP-α-tubulin cells, all interphase microtubules emanate from the centrosome and are arranged in a radial array with their tips close to the cell cortex (Fig 1A). By contrast, in mutants overexpressing DdCP224-ΔC-GFP, these arrays were collapsed. Microtubules now frequently appeared bundled, were longer than usual and whorled around the nucleus (Fig 1B). Moreover, microtubule tips had lost contact with cortical regions. As calculated from Western blots, the DdCP224-ΔC-GFP fragment was overexpressed approximately 5-fold (Fig. 1C).
Figure 1.
Overexpression of DdCP224-ΔC-GFP causes a collapse of interphase microtubule arrays. (A, B, B') Confocal microscopy of GFP-α-tubulin cells (A; control) and DdCP224-ΔC-GFP cells showing brightest point projections of GFP fluorescence (A, B') or immunofluorescence staining (B) using the YL1/2 anti-tubulin antibody (Chemicon, Hofheim, Germany). Cells were fixed with methanol. DNA was stained with TOPRO3 (Molecular Probes, Hilversum, Netherlands) (blue). (C) Immunoblot of a cytosolic extract of DdCP224-ΔC-GFP cells stained with anti-DdCP-HindIII. This rabbit polyclonal antibody was raised against the recombinant His-tagged N-terminus of DdCP224 using the 5'-HindIII fragment of the DdCP224 coding sequence. As calculated by the ImageJ program, DdCP224-ΔC-GFP is overexpressed approximately 5-fold. Bar 2 μm.
In order to investigate the behavior of these unusual microtubule arrays in living cells, we have transformed the untagged DdCP-ΔC fragment into Dictyostelium cells expressing GFP-α-tubulin. Four-dimensional confocal microscopy revealed an extraordinary motility of the microtubule arrays. Microtubules often were arranged like a comet-tail attached to the centrosome. The centrosome itself circulated around rapidly and continuously, often close to the cell cortex (Fig. 2B,2D; see additional data file movie2.mov). In control cells, the centrosome always stayed close to the cell center and moved only short distances (Fig. 2A,2C; see additional data file movie1.mov). The phenotype upon overexpression of DdCP-ΔC was indistinguishable from Dictyostelium cells overexpressing the motor domain of the dynein heavy chain [18,19]. Interestingly, disruption of the dynein/dynactin interaction in Dictyostelium cells by overexpression of fragments of the dynein intermediate chain also resulted in this phenotype [20].
Figure 2.
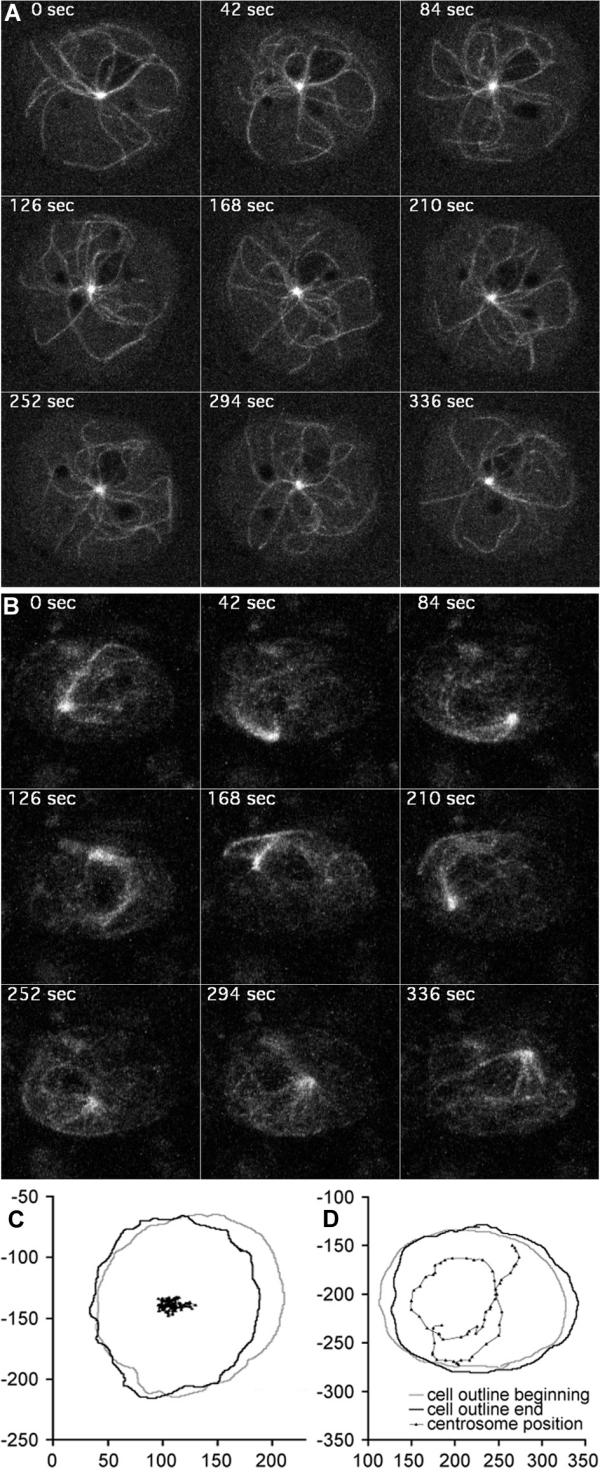
Microtubule and centrosome dynamics upon overexpression of DdCP224-ΔC. GFP-α-tubulin control cells (A, see additional data file movie1.mov) and GFP-α-tubulin cells overexpressing DdCP224-ΔC (B, see additional data file movie2.mov) were analyzed by confocal 4D-microscopy as described [5]. Each image represents a brightest point z-projection of 5 confocal slices with a distance of 1 μm each. The time is indicated in seconds. The movements of the centrosome shown in (C) for control cells and in (D) for DdCP224-ΔC/GFP-α-tubulin cells were calculated from 60 single images each using ImageJ.
The striking similarity of the DdCP-ΔC mutants to these dynein mutants strongly suggested that our phenotype can also be attributed to a defect of dynein function, e.g. due to a disruption of dynein/dynactin interaction. Since this interaction is also crucial for positioning of the Golgi apparatus, we analysed this issue in our DdCP224-ΔC-GFP mutants. Indeed, cells with disrupted microtubule arrays often showed complete dispersal of the Golgi apparatus (Fig. 3), suggesting that overexpression of the N-terminal DdCP224 fragment causes dissociation of dynein and dynactin. This interpretation is also supported by the observation that such cells usually showed reduced cortical localization of the dynein heavy chain compared to cells which have maintained a radial microtubule array (Fig. 4).
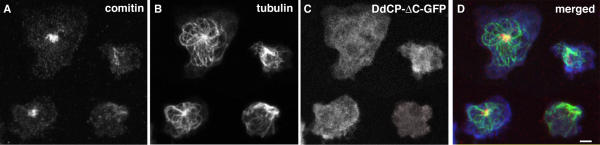
Figure 3.
Cells with disrupted microtubule arrays show Golgi dispersal. Confocal microscopy of DdCP224-ΔC-GFP cells showing brightest point projections of immunofluorescence stainings using the Golgi-specific anti-comitin antibody [34] (A) and the YL1/2 anti-tubulin antibody (B). GFP fluorescence is shown in (C). The merged image (D) shows the Golgi in red, microtubules in green and GFP fluorescence in blue. Cells were fixed with formaldehyde/acetone. The two cells exhibiting Golgi dispersal and disrupted microtubule arrays are marked by an arrow. Bar 2 μm.
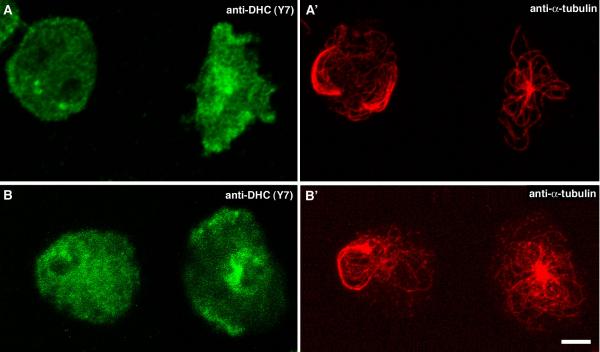
Figure 4.
Cells with disrupted microtubule arrays show reduced cortical localization of dynein. Confocal microscopy of DdCP224-ΔC-GFP cells showing brightest point projections of immunofluorescence stainings using anti-dynein-Y7 [19] (A, B) and and the YL1/2 anti-tubulin antibody (A', B'). In both examples, the left cell exhibits a disrupted microtubule array and is characterized by reduced cortical distribution of the dynein heavy chain compared to the right cell which shows normal microtubules. Cells were fixed with formaldehyde/acetone. Bar 2 μm.
The interference of DdCP224 with dynein function raised the question whether DdCP224 could interact with dynein. Although a major fraction of cytosolic DdCP224 occurs as a monomer [6], we could coimmunoprecipitate the dynein heavy and intermediate chains with DdCP224 from a cytosolic extract (Fig. 5A,5B). Vice versa, DdCP224 could be coimmunoprecipitated with both the dynein heavy and intermediate chains. Hence, a considerable fraction of DdCP224 occurs in cytosolic complexes with dynein and possibly other proteins. We cannot judge whether DdCP224 binds directly to dynein, since these proteins cannot be functionally expressed in E. coli for pull-down assays. The cytosolic complex consisting of DdCP224 and dynein may contain the Dictyostelium EB1 (DdEB1) [16] as well, since DdEB1 coprecipitates both with DdCP224 and dynein (Fig. 5C,5D).
Figure 5.
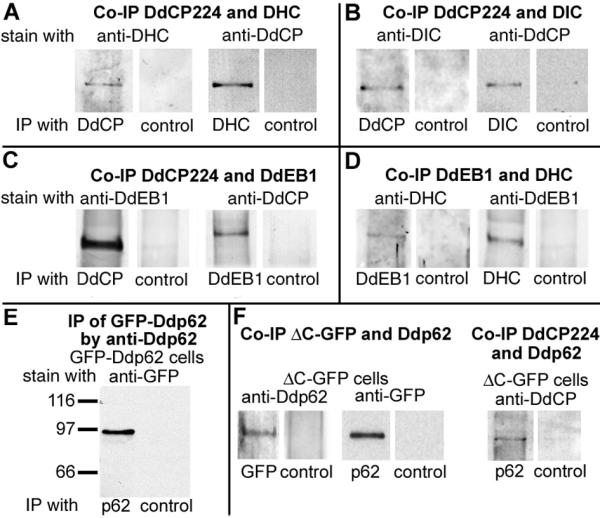
Coprecipitation of DdCP224 with dynein and DdEB1. Experiments were performed using cytosolic extracts from wildtype cells (strain AX2) (A-D), GFP-Ddp62 cells (E) and DdCP224-ΔC-GFP cells (F). The respective co-immunoprecipitation (Co-IP) experiment is given in bold letters on top of each subfigure. The respective antibodies used for immunoprecipitation and staining of the immunoblots are indicated. Abbreviations and antibodies: DHC, anti-dynein heavy chain Y7 [19]; DIC, anti-dynein intermediate chain [20]; DdCP, anti-DdCP224 mAb 2/165 [35] for immunoblot staining and anti-DdCP-HindIII for immunoprecipitation; DdEB1, anti-DdEB1 [16]; p62, anti-Ddp62; control, anti-rabbit or anti-rat (in case of DIC) preimmune serum.
DdCP224 does not only coprecipitate with dynein, it also colocalizes with the dynein heavy chain not only at the centrosome but also at the cell cortex at the leading edges and in pinocytic cups (Fig. 6A). The DdCP224-ΔC-GFP fragment also strongly colocalized with endogenous DdCP224 at these cortical regions (Fig. 6B). Visualization of the cortical localization of DdCP224 was overlooked in our earlier studies where the cells were fixed with methanol since it requires fixation with formaldehyde/acetone or glutaraldehyde.
Figure 6.
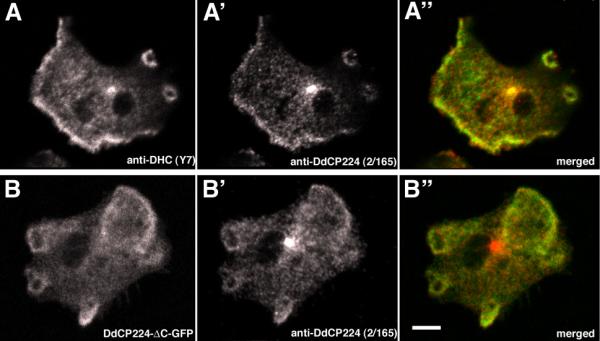
Colocalization of DdCP224, DdCP224-ΔC-GFP and dynein at the cell cortex. Confocal microscopy of DdCP224-ΔC-GFP cells showing brightest point projections of immunofluorescence stainings using anti-dynein-Y7 [19] (A) and anti-DdCP224 (2/165) (A', B'). GFP fluorescence is shown in (B). The merged images (A", B") shows endogenous DdCP224 in red and dynein (A) or DdCP-ΔC-GFP (B'), respectively, in green. Cells were fixed with formaldehyde/acetone. Bar 2 μm.
To elucidate how overexpression of the DdCP224-ΔC-GFP fragment could cause the mutant phenotype, we investigated its intermolecular interactions. Since the DdCP224-ΔC-GFP fragment did not coimmunoprecipitate with either dynein or DdEB1 (data not shown), we wondered whether it might interact with dynactin. Due to the lack of specific antibodies against dynactin components, we cloned the Dictyostelium homologue of dynactin-p62 (Ddp62) as a marker for dynactin and raised antibodies against the recombinant protein. These antibodies showed only weak staining of denatured Ddp62 in Western blots. However, they were capable of specific immunoprecipitation of a GFP-Ddp62 fusion protein from cytosolic extracts of Dictyostelium GFP-Ddp62 mutants (Fig. 5E). Hence, we concluded that these antibodies showed a higher avidity to native than to denatured Ddp62. Using these antibodies we could demonstrate by co-immunoprecipitation that Ddp62 binds to endogenous DdCP224 and to the cytosolic DdCP224-ΔC-GFP fragment (Fig. 5F).
Discussion
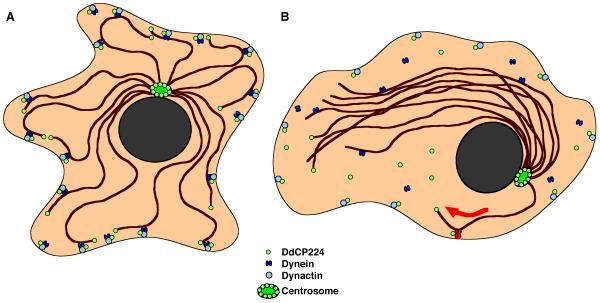
Centrosome movements and positioning are thought to be a result of balanced pulling forces that are transmitted through microtubules interacting with a cell cortex-associated motor protein. Dynein, which was recently localized to the cell cortex in Dictyostelium cells [21], provides such microtubule minus end-directed forces. These pulling forces are required for maintenance of the radial array of interphase microtubules and centrosome centering in wildtype cells (Fig. 7A). If most of the cortical dynein/dynactin complexes were dissociated or non-functional, cortical tethering of most microtubules would be lost. Yet, single microtubules remaining in contact with intact cortical dynein/dynactin complexes could rapidly be pulled to the cortex together with the centrosome and all the untethered microtubules that are dragged behind like a comet tail (Fig. 7B). An alternative explanation for this type of centrosome movement would be that the loss of minus-end directed forces could render microtubules more susceptible for pushing forces when they occasionally interact with cortically localized plus-end-directed motors [18]. However, the existence of such cortical plus-end directed motors remains to be shown, while the necessity of dynein and dynactin for centrosome positioning is undisputed, since it has also been proven in wound-healing experiments using fibroblast monolayers [22]. During the healing process, cells at the wound edge reorient their centrosomes toward the direction of migration [23]. Centrosome reorientation between the leading edge of the cell and the nucleus is blocked by inhibition of cytoplasmic dynein and dynactin and regulated through the small GTPase Cdc42 and PKCζ [24,25]. This suggests that cortical dynein/dynactin is required for capturing of microtubules extending into cortical regions of a freshly formed pseudopod.
Figure 7.
Model for the collapse of radial interphase microtubule arrays by disruption of cortical dynein/dynactin function. (A) Cortical dynein/dynactin in cooperation with DdCP224 provides the pulling force for maintenance of radial microtubule arrays. (B) Collapse of the radial microtubule array and altered centrosome positioning due to disruption of most cortical dynein/dynactin complexes and asymmetric pulling forces provided by only a few remaining functional cortical dynein/dynactin complexes (shown in red). This pathway may also involve further proteins which are not depicted in this model.
What could be the role of the cortical and the microtubule tip populations of DdCP224 [16] in microtubule/cortex interactions? At microtubule tips it could play a role in the capturing of microtubule plus ends at cortical sites, however, since DdCP224, like XMAP215, promotes microtubule growth [5], it is likely that the major function of tip-localized DdCP224 is the regulation of microtubule plus-end dynamics and the prevention of catastrophes induced by antagonistic Kin I-family kinesins [26]. By contrast, the cortical population of DdCP224 that colocalizes with dynein appears to be required for proper dynein function at the cortex. This is supported by two observations. First, DdCP224 binds to dynein/dynactin and second, overexpression of the DdCP224-ΔC-fragment disrupts cortical dynein function. Excess amounts of this DdCP224 fragment appear to interfere with the interaction between dynein and dynactin, since the characteristic collapse of the radial microtubule array was accompanied by reduced cortical dynein localization and by Golgi dispersal which is indicative for disrupted dynein/dynactin interaction [27]. The simplest explanation is that the DdCP224-ΔC-fragment sequesters Ddp62 and possibly other dynactin components in the cytosol which are then missing at the cell cortex where they are required for proper dynein function. The cytokinesis defect observed upon overexpression of the DdCP224-ΔC-GFP fragment [6] also agrees with an active role of DdCP224 in the interaction of microtubule tips with cortical sites, since these interactions are involved in cleavage furrow positioning [17].
The pathway of dynein/dynactin/DdCP224-dependent cortical interactions of interphase microtubules reported herein has to be distinguished from that of spindle orientation in mitotic yeast cells. In the latter case, Stu2p is involved in Kar9p-dependent capture of cytoplasmic MT plus-ends at the bud tip [12,13], a process that essentially requires yeast EB1 (Bim1p) at the MT tips [28,29]. Although Dictyostelium EB1 interacts with both DdCP224 and dynein, the process of MT/cortex interaction described here is clearly independent of DdEB1. DdEB1 null mutants showed only a defect in mitotic spindle formation, but neither a defect in microtubule organization or centrosome positioning, nor a cytokinesis defect [16].
Conclusions
Taken together, our results demonstrate for the first time that XMAP215-family proteins such as DdCP224 are involved in microtubule plus-end/cell cortex interactions and centrosome positioning in interphase cells and that this is mediated through an interaction of DdCP224 with dynein and dynactin.
Methods
Generation of the GFP-α-tubulin/DdCP224-ΔC mutant
The Dictyostelium vector for expression of the untagged N-terminal 813 amino acids of DdCP224 was constructed by deletion of the GFP sequence in pΔC-GFP [6]. It was then transformed into a Dictyostelium cell line expressing GFP-α-tubulin [16]. Cells were cultured as described earlier [6].
Cloning of Ddp62, protein expression and generation of polyclonal antibodies
The gene encoding the Dictyostelium homologue of the p62 subunit of dynactin (Ddp62; DictybaseID DDB0206421) was identified in the Dictyostelium genome project [30]. Its complete coding sequence (1647 bp) was amplified by PCR using an oligo dT-primed cDNA library [6] as a template. The Ddp62 cDNA was re-amplified using either BamHI and PstI linker primers for cloning into the pMALc2 (NEB, Frankfurt, Germany) or SalI and BamHI linker primers for cloning into pIS77, a vector obtained after replacement of the discoidin promoter of pDiscGFPSSEB2 [31] by the actin6 promoter. The former construct was used for protein expression in E. coli, the latter for expression of a GFP-Ddp62 fusion protein in Dictyostelium. The MBP-Ddp62 fusion protein expressed in E. coli was purified by affinity chromatography on amylose resin and used for custom immunization of two rabbits (Pineda Antikörperservice, Berlin, Germany). Both antisera showed the same characteristics.
Immunoprecipitation experiments
Immunoprecipitation was performed essentially as described previously [32]. In brief, 2 × 108 cells (80 ml) were lysed in 5 ml of lysis buffer (50 mM Hepes, 100 mM NaCl, 4 mM EGTA, 2 mM MgCl2, 10% sucrose, 0.3% NP40, 1 × protease inhibitor cocktail [33]). A cytosolic extract was obtained after centrifugation at 14.000 × g for 10 min at 4°C. After incubation of 0.6 ml of cytosolic extract with 10 μg of purified antibodies or 1.5 μl of antiserum for 1 h at 4°C, 20 μl of Protein G beads (50% slurry preincubated with 0.1% BSA in Tris-buffered saline) were added for a further incubation for 1 h at 4°C in a rotator. Beads were washed 4 times with lysis buffer, resuspended with 30 μl of SDS sample buffer (10% SDS, 125 mM Tris/HCl, pH = 6.8, 50 mM DTT, 5% glycerol) and subjected to electrophoresis and Western blotting as described previously [33].
Microscopy and image processing
Immunofluorescence microscopy and live cell observation was performed as described previously [5,33]. All microscopic images were acquired on a Zeiss Axiovert 200M/510META confocal microscope equipped with a 63x/1.4N.A. lens.
Authors' contributions
AH performed confocal microscopy of living cells and of most fixed immunofluorescence specimens. RG has cloned Ddp62, made protein purifications and carried out the immunoprecipitations. The manuscript was written by RG. AH has read and approved the final manuscript.
Supplementary Material
Microtubule and centrosome dynamics in GFP-α-tubulin control cells. Cells were viewed by confocal 4D-microscopy as described [5]. Each single image represents a brightest point z-projection of 5 confocal slices with a distance of 1 μm each. The time is indicated in seconds.
Microtubule and centrosome dynamics upon overexpression of DdCP224-ΔC. GFP-α-tubulin control overexpressing DdCP224-ΔC were viewed by confocal 4D-microscopy as described [5]. Each single image represents a brightest point z-projection of 5 confocal slices with a distance of 1 μm each. The time is indicated in seconds.
Acknowledgments
Acknowledgements
We are very grateful to Mike Koonce, Rex Chisholm and Michael Schleicher for providing antibodies against the dynein heavy chain, dynein intermediate chain and comitin, respectively. We also would like to thank Manfred Schliwa for his continuous support, Markus Rehberg and Alexandra Lepier for critical comments and Thi-Hieu Ho for expert technical assistance. Supported by the DFG (SFB413 and GR1642/2-1).
Contributor Information
Andrea Hestermann, Email: andrea.hestermann@lrz.uni-muenchen.de.
Ralph Gräf, Email: rgraef@lrz.uni-muenchen.de.
References
- Schuyler SC, Pellman D. Microtubule "plus-end-tracking proteins": The end is just the beginning. Cell. 2001;105:421–424. doi: 10.1016/S0092-8674(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Dujardin DL, Vallee RB. Dynein at the cortex. Curr Opin Cell Biol. 2002;14:44–49. doi: 10.1016/S0955-0674(01)00292-7. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Garcia MA, Toda T. Dis1/TOG universal microtubule adaptors - one MAP for all? J Cell Sci. 2001;114:3805–3812. doi: 10.1242/jcs.114.21.3805. [DOI] [PubMed] [Google Scholar]
- Popov A, Severin F, Karsenti E. XMAP215 Is Required for the Microtubule-Nucleating Activity of Centrosomes. Curr Biol. 2002;12:1326. doi: 10.1016/S0960-9822(02)01033-3. [DOI] [PubMed] [Google Scholar]
- Gräf R, Euteneuer U, Ho TH, Rehberg M. Regulated Expression of the Centrosomal Protein DdCP224 Affects Microtubule Dynamics and Reveals Mechanisms for the Control of Supernumerary Centrosome Number. Mol Biol Cell. 2003;14:4067–4074. doi: 10.1091/mbc.E03-04-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf R, Daunderer C, Schliwa M. Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J Cell Sci. 2000;113:1747–1758. doi: 10.1242/jcs.113.10.1747. [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Gard D, Tran PT, Erickson HP. XMAP215 is a long thin molecule that does not increase microtubule stiffness. J Cell Sci. 2001;114:3025–3033. doi: 10.1242/jcs.114.16.3025. [DOI] [PubMed] [Google Scholar]
- Wang PJ, Huffaker TC. Stu2p: A microtubule-binding protein that is an essential component of the yeast spindle pole body. J Cell Biol. 1997;139:1271–1280. doi: 10.1083/jcb.139.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima K, Kurooka H, Takeuchi M, Kinoshita K, Nakaseko Y, Yanagida M. p93dis1, which is required for sister chromatid separation, is a novel microtubule and spindle pole body-associating protein phosphorylated at the Cdc2 target sites. Genes Dev. 1995;9:1572–1585. doi: 10.1101/gad.9.13.1572. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Vardy L, Koonrugsa N, Toda T. Fission yeast ch-TOG/XMAP215 homologue Alp14 connects mitotic spindles with the kinetochore and is a component of the Mad2-dependent spindle checkpoint. EMBO J. 2001;20:3389–3401. doi: 10.1093/emboj/20.13.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breugel M, Drechsel D, Hyman A. Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer. J Cell Biol. 2003;161:359–369. doi: 10.1083/jcb.200211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosco KA, Pearson CG, Maddox PS, Wang PJ, Adams IR, Salmon ED, Bloom K, Huffaker TC. Control of microtubule dynamics by stu2p is essential for spindle orientation and metaphase chromosome alignment in yeast. Mol Biol Cell. 2001;12:2870–2880. doi: 10.1091/mbc.12.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Cheng SC, Rose MD. Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of cytoplasmic microtubules. Mol Biol Cell. 2000;11:2949–2959. doi: 10.1091/mbc.11.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. It's a kar9ochore to capture microtubules. Nat Cell Biol. 2000;2:E96–8. doi: 10.1038/35014089. [DOI] [PubMed] [Google Scholar]
- Popov AV, Pozniakovsky A, Arnal I, Antony C, Ashford AJ, Kinoshita K, Tournebize R, Hyman AA, Karsenti E. XMAP215 regulates microtubule dynamics through two distinct domains. EMBO J. 2001;20:397–410. doi: 10.1093/emboj/20.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg M, Gräf R. Dictyostelium EB1 Is a Genuine Centrosomal Component Required for Proper Spindle Formation. Mol Biol Cell. 2002;13:2301–2310. doi: 10.1091/mbc.E02-01-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neujahr R, Albrecht R, Kohler J, Matzner M, Schwartz JM, Westphal M, Gerisch G. Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J Cell Sci. 1998;111:1227–1240. doi: 10.1242/jcs.111.9.1227. [DOI] [PubMed] [Google Scholar]
- Koonce MP, Kohler J, Neujahr R, Schwartz JM, Tikhonenko I, Gerisch G. Dynein motor regulation stabilizes interphase microtubule arrays and determines centrosome position. EMBO J. 1999;18:6786–6792. doi: 10.1093/emboj/18.23.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce MP, Samso M. Overexpression of cytoplasmic dynein's globular head causes a collapse of the interphase microtubule network in Dictyostelium. Mol Biol Cell. 1996;7:935–948. doi: 10.1091/mbc.7.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Trivinos Lagos L, Gräf R, Chisholm RL. Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J Cell Biol. 1999;147:1261–1274. doi: 10.1083/jcb.147.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce MP, Khodjakov A. Dynamic microtubules in Dictyostelium. J Muscle Res Cell Motil. 2002;23:613–619. doi: 10.1023/A:1024446821701. [DOI] [PubMed] [Google Scholar]
- Dujardin DL, Barnhart LE, Stehman SA, Gomes ER, Gundersen GG, Vallee RB. A role for cytoplasmic dynein and LIS1 in directed cell movement. J Cell Biol. 2003;163:1205–1211. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M, Euteneuer U, Gräf R, Ueda M. Centrosomes, microtubules and cell migration. Biochem Soc Symp. 1999;65:223–231. [PubMed] [Google Scholar]
- Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol. 2001;11:1536–1541. doi: 10.1016/S0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Habermann B, Hyman AA. XMAP215: a key component of the dynamic microtubule cytoskeleton. Trends Cell Biol. 2002;12:267–273. doi: 10.1016/S0962-8924(02)02295-X. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa H, Usui T, Knop M, Schiebel E. Yeast Cdk1 translocates to the plus end of cytoplasmic microtubules to regulate bud cortex interactions. Embo J. 2003;22:438–449. doi: 10.1093/emboj/cdg063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E, Kusch J, Barral Y, Huffaker TC. Spindle orientation in Saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J Cell Biol. 2003;161:483–488. doi: 10.1083/jcb.200302030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger L, Noegel AA. Crawling into a new era-the Dictyostelium genome project. Embo J. 2003;22:1941–1946. doi: 10.1093/emboj/cdg214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunderer C, Gräf R. Molecular analysis of the cytosolic Dictyostelium gamma-tubulin complex. Eur J Cell Biol. 2002;81:175–184. doi: 10.1078/0171-9335-00241. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Using Antibodies - A Laboratory Manual. Cold Spring Harbour, New York, Cold Spring Harbour Laboratory Press; 1999. [Google Scholar]
- Gräf R, Euteneuer U, Ueda M, Schliwa M. Isolation of nucleation-competent centrosomes from Dictyostelium discoideum. Eur J Cell Biol. 1998;76:167–175. doi: 10.1016/S0171-9335(98)80031-9. [DOI] [PubMed] [Google Scholar]
- Weiner OH, Murphy J, Griffiths G, Schleicher M, Noegel AA. The actin-binding protein comitin (p24) is a component of the golgi apparatus. J Cell Biol. 1993;123:23–34. doi: 10.1083/jcb.123.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf R, Daunderer C, Schliwa M. Cell cycle-dependent localization of monoclonal antibodies raised against isolated Dictyostelium centrosomes. Biol Cell. 1999;91:471–477. doi: 10.1016/S0248-4900(99)80088-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microtubule and centrosome dynamics in GFP-α-tubulin control cells. Cells were viewed by confocal 4D-microscopy as described [5]. Each single image represents a brightest point z-projection of 5 confocal slices with a distance of 1 μm each. The time is indicated in seconds.
Microtubule and centrosome dynamics upon overexpression of DdCP224-ΔC. GFP-α-tubulin control overexpressing DdCP224-ΔC were viewed by confocal 4D-microscopy as described [5]. Each single image represents a brightest point z-projection of 5 confocal slices with a distance of 1 μm each. The time is indicated in seconds.