Abstract
The mouse Murr1 gene contains an imprinted gene, U2af1-rs1, in its first intron. U2af1-rs1 shows paternal allele-specific expression and is transcribed in the direction opposite to that of the Murr1 gene. In contrast to a previous report of biallelic expression of Murr1 in neonatal mice, we have found that the maternal allele is expressed predominantly in the adult brain and also preferentially in other adult tissues. This maternal-predominant expression is not observed in embryonic and neonatal brains. In situ hybridization experiments that used the adult brain indicated that Murr1 gene was maternally expressed in neuronal cells in all regions of the brain. We analyzed the developmental change in the expression levels of both Murr1 and U2af1-rs1 in the brain and liver, and we propose that the maternal-predominant expression of Murr1 results from transcriptional interference of the gene by U2af1-rs1 through the Murr1 promoter region.
Genomic imprinting is a type of gene regulation wherein one of the two parental alleles is predominantly expressed according to parental origin (5). Nearly 131 imprinted genes have been identified in mammals (http://cancer.otago.ac.nz/IGC/Web/home.html). Many of the genes have essential functions in embryonic development, and thus abnormalities of imprinted genes are often associated with human diseases, including disorders affecting cell growth, development, and behavior (23). However, the mechanism of imprinting is still poorly understood. Some common features have been observed in the genome structure of the imprinted genes that have been reported to date. Most imprinted genes are found in clusters in specific chromosomal domains. Colocalization of the imprinted genes implies that some mechanisms related to genomic imprinting may function over a long chromosomal region. For example, approximately 10 of the known mouse imprinted genes map to a distal region of mouse chromosome 7 (3, 31; http://www.mgu.har.mrc.ac.uk/imprinting/imprinting.html). This region consists of two imprinted domains in terms of imprinting control. The imprinted genes in each domain are under the control of a region termed the imprinting control center (11, 39). Epigenetic modification of chromatin is considered to be a fundamental and important mechanism involved in the generation and maintenance of the imprinted states of imprinted genes (8). Epigenetic modifications, such as DNA methylation, modifications of histones, and the organization of nucleosomes, have been regarded as important because they have essential and general functions, not only for imprinted genes but also in the regulation of all genes (19, 26, 28, 45).
U2af1-rs1 is a mouse imprinted gene and is transcribed exclusively from the paternal allele in embryonic and neonatal mice (16, 17). This gene is intronless and located within the first intron of Murr1. It is transcribed in the direction opposite of that of the host gene in the proximal region A3.2 of mouse chromosome 11 (27). The presence of two maternal copies of this region resulted in prenatal growth retardation, while two paternal copies resulted in fetal overgrowth (6). The U2af1-rs1 gene was supposedly created by retrotransposition during mouse evolution after the divergence of mice and humans, as the human MURR1 gene does not contain the homologue of the U2af1-rs1 gene within its introns (reference 27 and unpublished data).
Imprinted genes are a suitable model system for the study of epigenetic control of gene expression because their allelic expressions are essentially controlled by this mechanism. We initiated the analysis of the Murr1 gene in order to investigate the imprinting mechanisms of the U2af1-rs1 locus. The U2af1-rs1 gene was the only gene reported to be imprinted in this chromosomal region at that time. It was necessary to know if there were other imprinted genes around the U2af1-rs1 locus because clustered imprinted genes are thought to be controlled by a common regulatory mechanism rather than being independently regulated (10, 30). Although Murr1 has been reported to be expressed biallelically in the neonatal mouse (27), we have analyzed Murr1 expression in the tissues of both adult and embryonic mice because allelic expression of some imprinted genes is tissue- and developmental stage specific (7, 15, 24, 38). In this study we have found that the expression of the Murr1 gene shows a moderate bias of expression toward the maternal allele in the adult tissues analyzed so far. Moreover, this gene showed a predominant expression of the maternal allele specifically in adult brain but not in brains of neonatal or embryonic mice. We proposed that this mechanism might come from transcriptional interference by antisense-oriented U2af1-rs1.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6), PWK mouse strains, and the F1 progeny obtained by reciprocal crosses between them were used as the sources of total RNAs and genomic DNAs. BPF1 progeny resulted from the cross of B6 females and PWK males. PBF1 progeny resulted from crosses of PWK females and B6 males.
Mice with two paternal copies of proximal chromosome 11, including the Murr1 locus (paternal duplication [PatDp] prox11), were produced by a standard protocol using the mouse reciprocal translocation T(7;11)40Ad and identified by using genetic markers (4).
Allelic expression of the Murr1 and U2af1-rs1 genes.
Total RNAs were isolated with ISOGEN (Nippon Gene) from various tissues of adult F1 progeny obtained by reciprocal crosses between C57BL/6 and PWK mice. Contaminating chromosomal DNA was removed by DNase I treatment. Reverse transcription was conducted with oligo(dT) primers according to the manufacturer's protocol of the RNA PCR kit (Takara). To exclude the problem of heteroduplex formation skewing the results of restriction endonuclease digestion, hot-stop reverse transcription-PCR (RT-PCR) was employed (40). Thirty cycles of PCR with primers MuF1 (5′-TGGAGGGTGGCAAGTCCCTG-3′) and UMUR6 (5′-GGTAACACCAGTGGGCAAAG-3′) (Fig. 1) were followed by one cycle of amplification with primer UMUR6 labeled with α-32P. The resulting 686-bp fragment was digested with BspHI and electrophoresed. The intensity of electrophoretic bands was measured by using BAS2000 (Fujifilm). For the analysis of U2af1-rs1 gene, a regular PCR with primers 5′-U2af1rs1 (5′-AGATAACCACGGATACCTGG-3′) and 3′-U2af1rs1 (5′-AGTACATAGGCCTGCCCATG-3′) was performed. The resulting 236-bp PCR product was digested with MspI and electrophoresed.
FIG. 1.
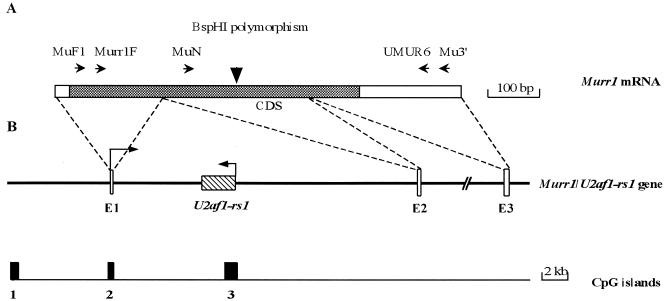
Structure of the mouse Murr1 gene. (A) The mRNA and coding sequence (CDS, shown as gray box) of Murr1. The arrows indicate the primers employed in the allelic expression analysis (MuF1 and UMUR6) and probe syntheses for Northern blot (MuN and UMUR6) and in situ hybridization (Murr1F and Mu3′). The BspHI site, which is polymorphic between the C57BL/6 and PWK strains, is shown above the mRNA. The site exists only in the C57BL/6 sequence. (B) The Murr1 gene consists of three exons and two introns with a length of about 83 kb. An imprinted gene, U2af1-rs1, is located within the first intron of the Murr1 gene. Three CGIs were detected in the region ranging from 10 kb upstream to 10 kb downstream of Murr1.
Quantification of mRNAs by real-time RT-PCR.
A real-time kinetic PCR method using the SYBR Green and Light-Cycler technique (Roche) was employed to quantify the amount of the Murr1 and U2af1-rs1 mRNAs in the brains and livers of different developmental stages (13.5-days-postcoitum [dpc] embryonic, newborn, 2-week-old, and 12-week-old mice). Total RNAs were isolated from the tissues of BPF1 and PBF1 mice by using ISOGEN (Nippon Gene). The RNAs were reverse transcribed by using oligo(dT) primer and then were used as templates in each real-time kinetic PCR containing a primer pair for Murr1, U2af1-rs1, or Gapdh (glyceraldehyde-3-phosphate dehydrogenase) mRNA. The primer pairs used were as follows. For Murr1, MU109 (5′-ACTCTATCCGGAAGTGCCAC-3′) and UMUR5 (5′-AGGCTTGCCATCGACTCTCC-3′) were used; for U2af1-rs1, U2F1 (5′-AGACCTACCAGCAGTTCTTG-3′) and U2R (5′-GGTCACTGGACAGAATTCAC-3′) were used; and for Gapdh, GAPDHR1 (5′-CAGTCTTCTGGGTGGCAGTG-3′) and GAPDHF (5′-GCCAAACGGGTCATCATCTC-3′) were used.
The kinetics of PCR amplification was monitored as the kinetics of enhancement of the fluorescence of SYBR Green (a dye specific for double-stranded DNA) in the reactions. The relative amounts of three mRNAs were obtained by determining the numbers of cycles required to obtain the same levels of fluorescence in each PCR. In order to evaluate the quantification of this assay and to condition the PCRs, pilot experiments were performed by using serially diluted cDNAs of these mRNAs as the templates according to the manufacturer's protocol.
In situ hybridization of Murr1 mRNAs in mouse brain.
The fresh cuts of brains were fixed immediately in paraformaldehyde after removal and sectioned after being embedded in paraffin. The product of RT-PCR with primers Murr1F (5′-TAGCGCAGAACGCCTTTCAC-3′) and Mu3′ (5′-ATCCTTGAAGACTTTCATGC-3′) (Fig. 1) was cloned into the pT7Blue T-vector (Takara) and used as the template for in vitro transcription of antisense and sense single-stranded RNA probes for Murr1. The antisense and sense RNA probes for U2af1-rs1 were prepared in the same manner from the RT-PCR product with primers U2F (5′-GGAAACACGACTTTCCCACG-3′) and U2AluI-R (5′-GCTGTCCTCTTCTCTTCCTC-3′). The digoxigenin-labeled antisense and sense probes were synthesized with T7 RNA polymerase and digoxigenin-UTP according to the manufacturer's protocol (RNA labeling and detection kit; Roche). The transcripts were used as probes at a concentration of 10 ng/μl in hybridization buffer. The hybridized probe was detected with alkaline phosphatase-coupled antibodies to digoxigenin according to the manufacturer's protocol (RNA labeling and detection kit; Roche).
Methylation analyses.
The DNA methylation status of the CpG islands (CGIs) was assessed by sodium bisulfite PCR and sequencing assay as described previously (46). Briefly, genomic DNA from the brain of a C57BL/6 mouse was extracted by standard phenol-chloroform extraction methods and treated with sodium bisulfite prior to PCR with each primer set. PCR products were cloned into pT7Blue T-vector, and each cloned PCR product was amplified by colony PCR using a Takara Ex Taq Kit (Takara Shuzo). The amplified products were subjected to sequencing reaction using BigDye (Applied Biosystems) and DYEnamic ET terminator kits (Amersham Pharmacia Biotech) after treatment with exonuclease I and shrimp alkaline phosphatase (both from Amersham Pharmacia Biotech).
Detection and quantification of the primary transcript of the Murr1 and U2af1-rs1 by strand-specific RT-PCR and semiquantitative RT-PCR.
DNase I-treated total RNAs (0.25 μg) isolated from the brain, liver, heart, or lung of adult mice using ISOGEN (Nippon Gene) were mixed with 5 pmol of either forward primers 1, 3, 4, and 5 or reverse primer 2 (see Fig. 6) in a final volume of 10 μl; the RNAs were then reverse transcribed for 30 min at 55°C, heat inactivated at 99°C for 5 min, and cooled on ice. Following the reverse transcription and denaturation, 33 PCR cycles with four different primer sets corresponding to the primers for reverse transcription were performed, respectively.
FIG. 6.
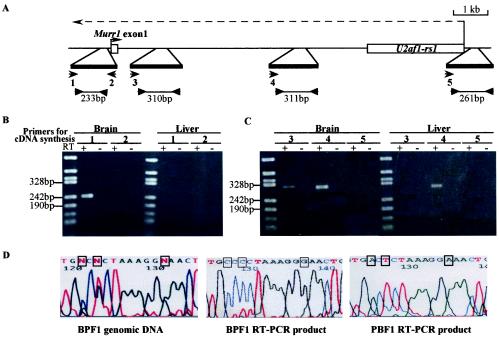
Strand-specific RT-PCR of the Murr1 gene. (A) Indicated are the locations of the primers for RT (1, 2, 3, 4 and 5) and the regions amplified by PCR in the Murr1/U2af1-rs1 region. (B) RT-PCR in the 300-bp region upstream of Murr1 with the total RNAs of adult brain and liver of an adult C57BL/6 mouse. A transcript with antisense orientation relative to Murr1 was detected in the brain but not in the liver. (C) Transcripts with antisense orientation were also investigated in the intronic region downstream and upstream of U2af1-rs1 with the total RNAs of adult brain and liver. A transcript near the exon 1 of Murr1 was detected in the brain but not in the liver, while the transcript near U2af1-rs1 was detected in both the brain and the liver. The antisense primary transcript was not detected in the region upstream of the U2af1-rs1 gene. All PCRs were performed with 33 amplification cycles. (D) The sequence analysis of the PCR products in the 300-bp region upstream of Murr1. The genomic DNA and the total RNAs were obtained from the brains of the adult F1 progeny from the crosses of B6 and PWK mice. The polymorphic bases are boxed in the sequences.
Semiquantitative RT-PCR was employed to detect the downstream primary transcript of the Murr1 and U2af1-rs1. The product of reverse transcription with either primer 1 or 3 as described above was subjected to 33 and 36 PCR cycles, respectively, the products of which were verified as linear ranges of the PCR with corresponding primers. After agarose electrophoresis and staining with ethidium bromide, the quantity of the PCR products was analyzed by National Institutes of Health Image software (version 1.62).
RESULTS
Murr1 contains U2af1-rs1 in its first intron.
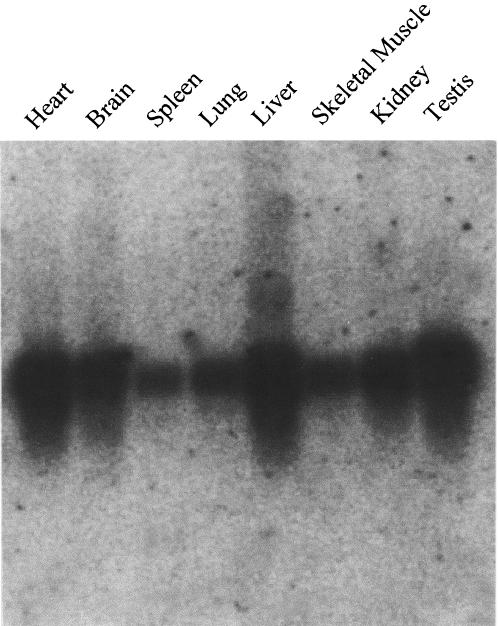
Murr1 spans 83 kb and consists of three exons and two introns. An imprinted gene, U2af1-rs1, which shows paternal allele-specific expression, is located within the first intron of Murr1 and transcribed in a direction opposite to that of the host gene (Fig. 1) (27). The genomic sequence, including that of the Murr1 gene, is available from DDBJ under the accession numbers AB089806 and NT_039515. This gene encodes a mRNA of approximately 1 kb and is expressed ubiquitously in adult tissues as shown by Northern blotting in Fig. 2. Cloning and sequencing of the cDNA revealed that Murr1 mRNA is 793 bases long, excluding the polyA tail, and encodes a protein of 188 amino acids. The transcriptional initiation site was determined by 5′ rapid amplification of cDNA ends analysis. Several of the largest clones had the same 5′ ends (data not shown). The cDNA sequence has been submitted to the DDBJ database under accession numbers D85430 (27) and AB104816. Although the function of the protein has not yet been analyzed in mice, the homologue in the dog was reported to be involved in copper metabolism (41, 42).
FIG. 2.
Expression of the Murr1 gene in mouse adult tissues. A premade mouse multiple tissue Northern blot (Clontech Co.) was probed with the PCR fragment synthesized with primers MuN and UMUR6 (Fig. 1). The Murr1 gene is expressed ubiquitously but is most abundant in the heart, liver, and testis.
The maternal allele is expressed preferentially in all tissues but predominantly in adult brain.
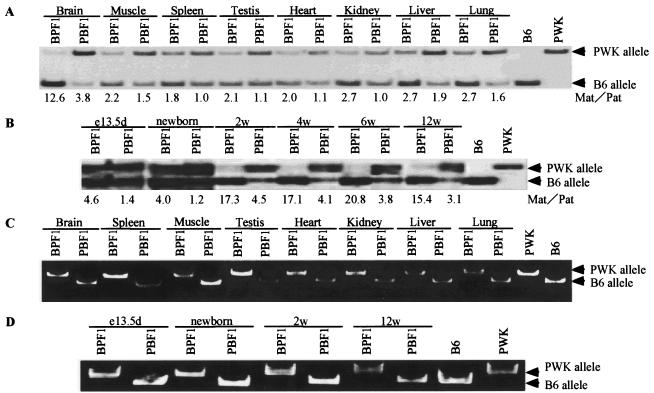
The existence of an imprinted gene within the Murr1 gene has led us to analyze the allelic expression of Murr1 because many imprinted genes are found to be organized as clusters (3, 10). The gene has been reported to show biallelic expression in the whole body of neonatal mice specimens (27). Tissues of adult mice were examined by using the F1 progeny of reciprocal crosses between C57BL/6 and PWK mice. We performed hot-stop RT-PCR followed by digestion with BspHI that could distinguish between the Murr1 alleles of each parental strain (Fig. 1). As seen in Fig. 3A, Murr1 showed preferential expression of the maternal allele in all the adult tissues examined. In the brain, predominant expression of the maternal allele was the most significant. In order to know whether this allelic expression also occurs in early developmental stages, we analyzed embryonic and neonatal brains (Fig. 3B). These brains express the Murr1 gene biallelically with moderate preference for the maternal allele, as seen in adult tissues other than the brain. The brain of a 2-week-old mouse showed predominant expression of the maternal allele to the same extent as that found in the brains of 6- and 12-week-old mice.
FIG. 3.
Analysis of allelic expression of Murr1 and U2af1-rs1. Total RNAs were prepared from various tissues of adult, newborn, and embryonic F1 progeny obtained from crosses between C57BL/6 and PWK mice. Allelic expression analyses of Murr1 in the tissues of adult mice (A) or in the brains of 13.5-dpc embryonic, newborn, 2-, 4-, 6-, and 12-week-old mice (B). The products of hot-stop RT-PCR were digested with BspHI, which digests only the product from the B6 allele (Fig. 1). The ratio of the intensity of maternal product to paternal product is indicated beneath each lane. Allelic expression analyses of U2af1-rs1 in the tissues of adult mice (C) or in the brains of 13.5-dpc embryonic, newborn, 2-week-old, and 12-week-old mice (D). The products of RT-PCR were digested with MspI, which digests only the product from the B6 allele.
Because there is a possibility that the paternal expression of U2af1-rs1 affects the allelic expression of its host gene, Murr1, its allelic expression was also examined in the same tissues. In previous reports, U2af1-rs1 was shown to be expressed exclusively from the paternal allele in the early embryo and whole neonate (16, 17). Our analysis showed that the U2af1-rs1 gene was expressed exclusively from the paternal allele in all the adult tissues and in the brains of mice in all the developmental stages so far examined (Fig. 3C and D).
Predominant maternal expression was observed in all regions of adult brain.
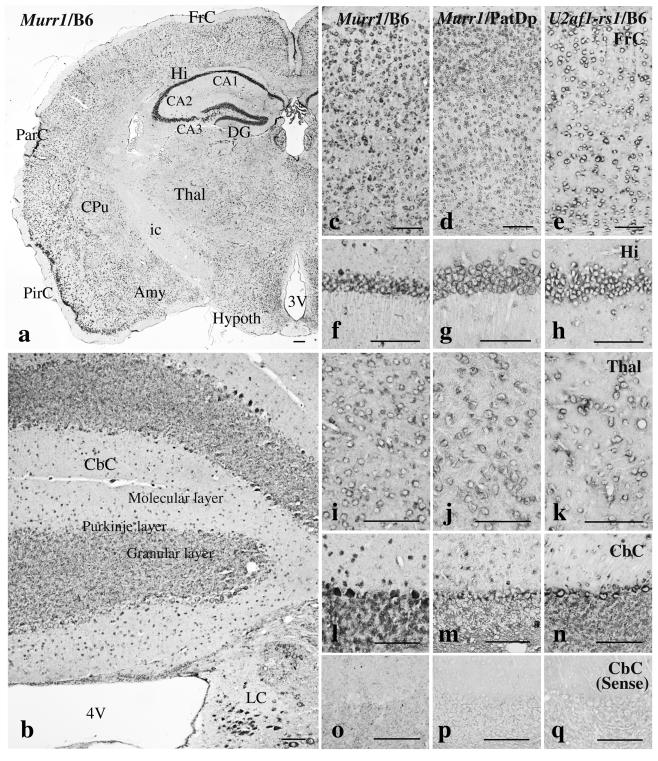
Maternal allele-predominant expression of the Murr1 gene in the adult brain may suggest a function of the gene in the development of the brain after birth. In order to gain insight into this function, we determined the regions expressing the gene by RNA in situ hybridization on the adult brains of C57BL/6 mice (Fig. 4a, b, c, f, i, and l). The expression of Murr1 was observed in all the regions of the cerebrum, cerebellum, and medulla oblongata. The hippocampus region of the cerebrum had the highest levels of expression. The expression was seen mainly in neuronal cells.
FIG. 4.
In situ hybridization of Murr1 and U2af1-rs1 mRNAs in mouse brain. RNA probes of Murr1 and U2af1-rs1 were hybridized on brains of C57BL/6 (B6) and PatDp(prox11) mice. Antisense probes (panels a through n) and sense probes as negative control (panels o through q) were used. Shown are coronal sections through the forebrain (a) and the cerebellum (b) of C57BL/6 mice hybridized with Murr1 probe; also shown are magnified images of the frontal cortex (c through e), hippocampus (f through h), thalamus (i through k), and cerebellar cortex (l through q) hybridized with the Murr1 probe on C57BL/6 brain (panels c, f, i, l, and o), Murr1 probe on PatDp(prox11) brain (panels d, g, j, m, and p), and U2af1-rs1 probe on C57BL/6 brain (panels e, h, k, n, and q) are shown. FrC, frontal cortex; Hi, hippocampus; Thal, thalamus; Hypoth, hypothalamus; CA1 to -3, fields CA1 to CA3 of Ammon's horn; DG, dentate gyrus; ParC, parietal cortex; PirC, piriform cortex; CPu, caudate putamen; Amy, amygdala; ic, internal capsule; CbC, cerebellar cortex; 3V, third ventricle. Scale bars, 100 μm.
Maternal allele-predominant expression of Murr1 raises two possibilities for the allelic expression of this gene in adult brain. First, Murr1 may be expressed predominantly from the maternal allele in all the regions of adult brain. Alternatively, the gene may be expressed exclusively from the maternal allele in some regions but biallelically in other regions. Ube3a is an imprinted gene with such a regional-imprinted expression (1). In situ hybridization was performed using adult mouse brain cells with two paternal copies of proximal chromosome 11, including the Murr1 locus [PatDp(prox11)]. Weak but detectable signals for Murr1 mRNA were observed in all the regions and were essentially similar to those of wild-type C57BL/6 brains (Fig. 4d, g, j, and m). There was no region lacking hybridization signal, and the expression pattern of Murr1 appeared to be normal in the PatDp(prox11) brain. This result indicated that the gene was expressed maternal allele predominantly in all the regions expressing the gene.
The CGIs associated with the Murr1 promoter region are not methylated.
Three CGIs have been identified in the region spanning from 10 kb upstream to 10 kb downstream of the Murr1 gene (Fig. 1). CGI1 is located approximately 8 kb upstream from the Murr1 gene, and CGI2 spans from the promoter region to the 5′ part of exon 1. CGI3 is the region spanning from the promoter to the body of the U2af1-rs1 gene. CGI3 has been reported to be methylated only on the maternal allele and has been thought to be involved in the paternal expression of U2af1-rs1 (17). These three CGIs were analyzed for methylation in the brain genome by a sodium bisulfite sequencing method (data not shown). One can expect that the CGIs 1 and/or 2 may be paternally methylated if differential methylation is involved in the maternal expression of Murr1. The CGI3 was found to be differentially methylated in the brain as reported previously. On the other hand, no methylation was found in the other two CGIs, 1 and 2. Nonmethylation of these CGIs suggests that differential methylation within the genome is not involved in the allelic expression of Murr1, in contrast to many of the imprinted genes.
The expression levels of Murr1 and U2af1-rs1 change inversely during brain development.
In the adult brain, U2af1-rs1 and Murr1 show reciprocal allelic expression. The U2af1-rs1 gene is expressed exclusively from the paternal allele, and the Murr1 gene is expressed predominantly from the maternal allele. This fact suggests that there may be some kinds of interaction, such as transcriptional interference, between allelic expressions of these genes. In order to investigate this possibility, the mRNA levels of these genes were quantified in the brain and liver during development by quantitative real-time RT-PCR (Fig. 5).
FIG. 5.
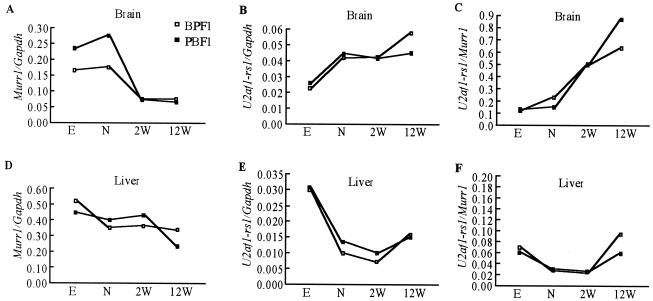
Change of the expression of Murr1 and U2af1-rs1 during development in the brain and liver. The expression of Murr1 and U2af1-rs1 was analyzed in the brains and the livers of BPF1 and PBF1 mice during development by quantitative real-time PCR. All the analyses were performed twice for each of the RNA samples. The relative amounts of Murr1 and U2af1-rs1 mRNAs were calculated by normalizing their values with the housekeeping gene Gapdh. Shown are expression levels of Murr1/Gapdh (A), U2af1-rs1/Gapdh (B), and Murr1/U2afi-rs1 (C) in the brains; also shown are expression levels of Murr1/Gapdh (D), U2af1-rs1/Gapdh (E), and Murr1/U2afi-rs1 (F) in the livers. E, 13.5-dpc embryo; N, newborn; 2W, 2-week-old mice; 12W, 12-week-old mice; □, BPF1; ▪, PBF1.
In the brain (Fig. 5A), Murr1 expression occurs at a relatively high level during the embryonic and neonatal periods. The expression levels decrease considerably between the neonatal stage and 2 weeks after birth, the period during which maternal allele-predominant expression of Murr1 becomes remarkable (Fig. 3B). This overall decrease from the embryo to adult stages is approximately fourfold. In contrast, the expression of U2af1-rs1 continues to increase during development (Fig. 5B). Overall, this increase is approximately two- to threefold. As a result, the ratio of expression of U2af1-rs1 versus that of Murr1 increased about 10-fold during development (Fig. 5C). The final level of U2af1-rs1 expression (0.05) is almost the same as that of Murr1 expression (0.06) in the adult brain.
On the other hand, the expression of both genes decreased simultaneously during development in the liver, in which the maternal allele-predominant expression of Murr1 does not occur (Fig. 5D and E). Relative levels of expression of U2af1-rs1 and Murr1 do not change significantly during development (Fig. 5F). The expression of Murr1 is more than 10-fold higher than that of U2af1-rs1 in both embryonic and adult periods.
Transcription from U2af1-rs1 may act through the promoter region of Murr1.
Antisense orientation of the U2af1-rs1 gene relative to Murr1 suggests the possibility that transcription originating from U2af1-rs1 promoter overlaps the promoter region of the Murr1 gene and interferes with Murr1 transcription. The 3′ end of a mature mRNA is created by the cleavage of the primary transcript. A primary transcript is synthesized beyond the end of the gene, sometimes far more than a distance of 1 kb (29). If this is because of the paternal expression of U2af1-rs1, this interference should occur only on the paternal allele and may cause the reduction of the paternal expression of Murr1. We employed strand-specific and semiquantitative RT-PCR to detect and quantify the transcripts in the region downstream of U2af1-rs1 up to the promoter region of Murr1 (Fig. 6).
In the analysis performed using adult brain RNA, the transcript was detected in the promoter region when primer 1 with sense orientation was used for cDNA synthesis. In contrast, no transcript was detected in this region when primer 2 with antisense orientation was used as the primer for cDNA synthesis (Fig. 6B). This result indicated that the Murr1 promoter region was transcribed in the antisense direction relative to the Murr1 gene. The sequencing of the PCR product revealed that the transcript was expressed exclusively from the paternal allele (Fig. 6D). On the other hand, when liver RNA was used for this analysis, no transcript was detected with either of the primers for cDNA synthesis using PCR with same number of amplification cycles (33 cycles) (Fig. 6B). An antisense transcript was, however, detected in the liver when PCR was done with 36 amplification cycles (data not shown). In the brain, transcripts with antisense orientation relative to Murr1 were also detected at two sites in the first intron downstream of U2af1-rs1. Only the transcript at the site close to U2af1-rs1 was detected in the liver, however. No transcript with antisense orientation was detected at the promoter region of U2af1-rs1 in either the brain or liver (Fig. 6C). These results strongly suggest that transcription from the U2af1-rs1 promoter to the upstream region of the Murr1 gene on the paternal chromosome occurs more abundantly in the brain than in the liver, although we have no direct evidence that these antisense transcripts are parts of one continuous transcript. This finding implies that the transcription of U2af1-rs1 interferes with the expression of the paternal allele of Murr1 in the adult brain. If this is the case, U2af1-rs1 should be expressed in the regions where Murr1 is expressed in the brain. This has been shown to be true by in situ hybridization, in which the expression of U2af1-rs1 observed in the regions was essentially the same as that of Murr1 expression in C57BL/6 brain (Fig. 4e, h, k, and n). We also detected the same antisense transcripts at the Murr1 promoter in lung and kidney cells. Semiquantitative RT-PCR indicated that the primary antisense transcript in the Murr1 promoter region was much more abundant in the adult brain than in the heart, lung, and liver (data not shown).
DISCUSSION
We have shown that the mouse Murr1 gene is imprinted in the adult brain. The gene is expressed predominantly from the maternal allele in the neurons of all regions of the adult brain but shows only a preferential maternal expression in the brain at earlier developmental stages. Some imprinted genes have been reported to show a change in allelic expression during development (7, 15, 24, 38). These are, however, genes that are imprinted only in the early stages of development. Murr1, therefore, is the first imprinted gene which shows allelic expression in later stages of development. Murr1 also has a moderate bias toward expression of the maternal allele in all other adult tissues analyzed so far.
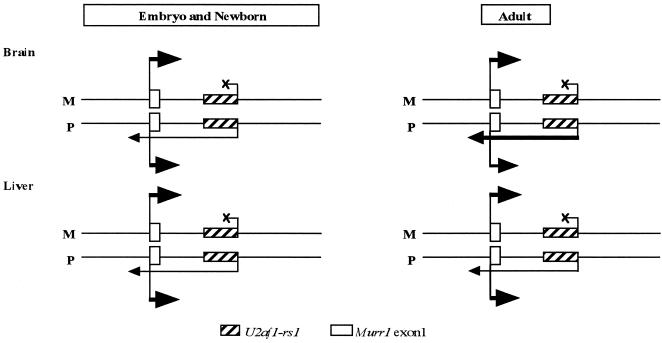
Our preliminary analysis indicates that MURR1, the human homologue of mouse Murr1, is expressed biallelically in the adult brain and contains no imprinted gene in the first intron (Zhang et al., unpublished data). This strongly suggests that the antisense gene, U2af1-rs1, causes the maternal expression of Murr1 in the mouse. The expressions of Murr1 and U2af1-rs1 show a reverse change during the development of the brain (Fig. 5A through C). Murr1 expression decreases during brain development, whereas U2af1-rs1 expression increases. Given the assumption that the mRNA levels reflect the transcriptional activities of these genes, these results indicate that relative transcriptional activity of U2af1-rs1 is increasing compared to that of Murr1 during brain development. On the other hand, analyses by strand-specific RT-PCR suggested that the transcription from the U2af1-rs1 promoter reaches to the promoter region of Murr1 on the paternal allele in adult brain, liver, kidney, and lung to a different extent (Fig. 6B and C and data not shown). A possible hypothesis is that the transcriptional interference of the U2af1-rs1 gene against Murr1 causes maternal allele-preferential or -predominant expression of the Murr1 gene (Fig. 7). In this hypothesis, we assume that the transcription from U2af1-rs1 gene may interfere with Murr1 transcription, possibly by its initiation on the paternal allele, and cause a reduction in Murr1 expression dependent on the balance of the transcriptional activities of both genes. Transcriptional interference has been reported in yeast, where two genes are closely spaced and transcribed in the same direction. The transcription of the downstream gene is affected when the upstream gene is expressed (13). A similar phenomenon has been reported in mammalian cells (14).
FIG. 7.
The expression-interference model between the Murr1 and U2af1-rs1 genes. The antisense transcription from the U2af1-rs1 gene (starting from the slashed square) is hypothesized to interfere with the Murr1 transcription (starting from the blank square) on the paternal allele and to cause the maternal allele-preferential or -predominant expression in the adult brain.
In the embryonic and neonatal brain, U2af1-rs1 expression is relatively low compared to Murr1 expression. We propose that during these developmental stages this low expression of U2af1-rs1 would moderately interfere with the transcription of Murr1 on the paternal chromosome, resulting in the maternal allele-preferential expression of Murr1. The expression of Murr1 decreases in the period between the neonatal and 2-week-old stages, a time when the maternal expression of Murr1 becomes noticeable (Fig. 3B). The expression of U2af1-rs1, on the other hand, continues to increase during development. This could enhance the interference with Murr1 transcription and result in maternal allele-predominant expression in the adult brain. During the development of the liver, in contrast to that of the brain, the relative level of expression of U2af1-rs1 compared with that of Murr1 does not change significantly (Fig. 5D through F). This would cause only a slight reduction of the paternal transcription of Murr1 and lead to the maternal allele-preferential expression as observed in most of the adult tissues and in the immature brain. Such a transcriptional interference should be reciprocal; that is, the Murr1 transcription may also interfere with the U2af1-rs1 transcription. Even if this is the case, the allelic expression of U2af1-rs1 should not be affected. Since U2af1-rs1 is expressed exclusively from the paternal allele, the gene still shows the exclusive paternal expression even when the transcriptional interference by Murr1 decreases the paternal expression of U2af1-rs1. In order to observe the interference with U2af1-rs1, the transcription of Murr1 should be stopped on the paternal chromosome by targeted deletion of the Murr1 promoter region.
Another possible explanation for the developmental stage-specific and tissue-specific imprinting is the usage of distinct promoters, such as those reported on the human imprinted gene IGF2. Among four promoters of this gene, the expression of the P1 promoter is biallelic, whereas those of the P2 to P4 promoters are paternal allele specific. In human liver, the P1 promoter and the P3 and P4 promoters are used primarily in the adult and fetal stages, respectively. This promoter switch causes fetal stage-specific imprinting of the gene in the human liver (43). We have obtained evidence for a single promoter of the Murr1 gene by 5′ rapid amplification of cDNA ends. However, performing other assays, such as S1 mapping or RNase protection, would be needed in order to confirm a single promoter of this gene and to exclude the alternative explanation.
Antisense RNAs have been found previously to be associated with imprinted genes (33). The representative pairs of imprinted gene with antisense RNA are Igf2r/Air, Ube3A/Ube3A-ATS, Kvlqt1/Lit1, and Nesp/Nespas. These antisense RNAs are very long noncoding RNAs (more than 100 kb), and their genes are imprinted oppositely to the partner gene (21, 25, 32, 44). They are suggested to have a role in establishing and/or maintaining imprinting of the partner genes. For example, the antisense RNA, Air, has been shown to function in cis to repress not only the partner, Igf2r, but also two other imprinted genes located upstream of Air (35). Premature disruption of Air transcription resulted in the loss of imprinting of all three genes. The imprinting of the upstream genes cannot be due to the sort of transcriptional interference discussed here. One hypothesis is that the Air RNA functions in cis to cause a repressed chromatin state in the Igf2r-imprinted cluster in a way similar to that of Xist RNA in X-chromosome inactivation (2). Although we cannot rule out the possibility that U2af1-rs1 RNA also harbors such a cis-acting function to form a repressed chromatin state in this locus, the transcriptional interference is more plausible because of the following reasons. First, U2af1-rs1 is expressed paternally in all the tissues, whereas adult brain is the only tissue in which Murr1 is imprinted. In contrast, the pairing of Igf2r and Air shows reciprocal imprinting in many adult tissues (18). Second, we have searched imprinted genes in 500-kb genomic region around Murr1, and no neighboring genes were imprinted (Zhang et al., unpublished data). Third, U2af1-rs1 RNA is a protein-coding mRNA and should diffuse out to the cytoplasm. Harboring cis-acting functions may be difficult in this kind of RNA.
In contrast to the clustering of many imprinted genes, the Murr1/U2af1-rs1 locus seems to be an isolated imprinted locus. U2af1-rs1 is thought to be a neomorphic gene caused by retrotransposition (27). This gene shows complete imprinted expression, whereas Murr1, containing U2af1-rs1, shows incomplete imprinting, that is, maternal-predominant or -preferential expression. The mouse Bc10/Nnat locus shares some features with the Murr1/U2af1-rs1 locus. Nnat is expressed exclusively from the paternal allele and is associated with a maternally and differentially methylated region, and it resides within the intron of a gene, Bc10, which is transcribed in the opposite direction (20). They have some differences, however. The imprinted gene Nnat contains introns, unlike U2af1-rs1. The host gene, Bc10, is expressed biallelically, although the analysis was done only in embryos. Moreover, a similar arrangement described as “a microimprinted domain” was reported recently for the imprinted genes Nap115 (chromosome 6) and Peg13 (chromosome 15) (36). As is true for the Nnat gene, the Nap115 and Peg13 genes are also expressed exclusively from the paternal allele and located within the introns of other biallelically expressed genes. In order to know whether these loci are isolated imprinted loci, one should analyze neighboring genes on their allelic expression. We are performing this line of analyses with the Murr1/U2af1-rs1 locus, and all the neighboring genes are not imprinted in 500-kb region around Murr1 as mentioned above. The studies on these isolated loci may be valuable for studies on imprinting mechanisms because of their simplicity and their imprinting mechanisms, which possibly differ from those of large imprinted domains.
DNA methylation can serve as a signal to mark the parental alleles. Allele-specific methylation patterns are a common feature of most imprinted genes (9, 12, 37). Such differentially methylated regions (DMRs) are usually located within CGIs, in contrast to the general observation that CGIs are unmethylated. Many of the DMRs have important functions in the allele-specific expression of the imprinted genes (22, 34). Contrary to this general association of DMRs with imprinted genes, the CGIs found in the Murr1 locus are not methylated on both alleles in brain cells, except for the maternally methylated CGI3 spanning from the promoter to the body of theU2af1-rs1 gene. The DMR in CGI3 is considered to be involved in the paternal expression of U2af1-rs1. This observation suggests that the allelic expression of Murr1 does not depend on differential DNA methylation of the genome. Similar results were observed for the Kip2/Lit1 subdomain of mouse chromosome 7F5 (46). There are seven imprinted genes in this region, and no DMR was found in three out of six genes carrying CGIs in the promoter. There was an imprinting control region (ICR) in this subdomain, and results suggested that this ICR governs the imprinted expression of those seven imprinted genes (11). Similarly, an ICR would also probably exist in the Murr1/U2af1-rs1 region. CGI3 is one such candidate. To clarify if this is the case, it would be necessary first to determine the span of the imprinting domain, including Murr1/U2af1-rs1, and then to search for an ICR in this domain.
Moderate preferential expression of the maternal allele is the general expression pattern of the Murr1 gene (Fig. 3A and B). This maternal preference is higher in tissues of BPF1 mice than in those of PBF1 mice. This difference between these F1 mice may be due to the disparity in promoter activities of Murr1 alleles of the mouse strains C57BL/6 and PWK. It can be assumed that the promoter of the C57BL/6 allele may have higher activity than that of the PWK allele. In the PBF1 mouse, in spite of a mechanism to express the maternal allele preferentially, the greater activity of the paternal allele makes the difference between the two alleles small. In BPF1, the C57BL/6 allele is maternal, and this situation enhances the allelic difference. We have, in fact, found some polymorphisms in the promoter region of the Murr1 gene between these two strains, although we have not analyzed whether these sequence variations affect the promoter activity.
The dog Murr1 homologue has recently been reported (42). Exon 2 of the canine MURR1 gene was found to be deleted in both alleles of a certain purebred dog population that suffered from copper toxicosis. Copper toxicosis is characterized by accumulation of copper in the liver and subsequently in the brain and other organs as a result of inefficient biliary excretion of copper. The presence of the mutation suggests that MURR1 is the disease-causing gene in copper toxicosis in dogs. Therefore, it is possible that the mouse Murr1 gene is also involved in copper metabolism. In order to analyze the function of the gene, it would be necessary to produce mice with a deletion of the gene.
Acknowledgments
We thank Sachiko Matsuhashi for technical assistance with in situ hybridization.
This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology in Japan.
REFERENCES
- 1.Albrecht, U., J. S. Sutcliffe, B. M. Cattanach, C. V. Beechey, D. Armstrong, G. Eichele, and A. L. Beaudet. 1997. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat. Genet. 17:75-78. [DOI] [PubMed] [Google Scholar]
- 2.Avner, P., and E. Heard. 2001. X-chromosome inactivation: counting, choice and initiation. Nat. Rev. Genet. 2:59-67. [DOI] [PubMed] [Google Scholar]
- 3.Barlow, D. P. 1995. Gametic imprinting in mammals. Science 270:1610-1613. [DOI] [PubMed] [Google Scholar]
- 4.Beechey, C. V. 1999. Imprinted genes and regions in mouse and human, p. 303-323. In R. Ohlsson (ed.), Genomic imprinting: an interdisciplinary approach. Results and problems in cell differentiation. Springer-Verlag, Heidelberg, Germany. [PubMed]
- 5.Cattanach, B. M., and M. Kirk. 1985. Differential activity of maternally and paternally derived chromosome regions in mice. Nature 315:496-498. [DOI] [PubMed] [Google Scholar]
- 6.Cattanach, B. M., H. Shibata, Y. Hayashizaki, K. M. Townsend, S. Ball, and C. V. Beechey. 1998. Association of a redefined proximal mouse chromosome 11 imprinting region and U2afbp-rs/U2af1-rs1 expression. Cytogenet. Cell Genet. 80:41-47. [DOI] [PubMed] [Google Scholar]
- 7.Dao, D., D. Frank, N. Qian, D. O'Keefe, R. J. Vosatka, C. P. Walsh, and B. Tycko. 1998. IMPT1, an imprinted gene similar to polyspecific transporter and multi-drug resistance genes. Hum. Mol. Genet. 7:597-608. [DOI] [PubMed] [Google Scholar]
- 8.Feil, R., and S. Khosla. 1999. Genomic imprinting in mammals: an interplay between chromatin and DNA methylation? Trends Genet. 15:431-435. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson-Smith, A. C., H. Sasaki, B. M. Cattanach, and M. A. Surani. 1993. Parental-origin-specific epigenetic modification of the mouse H19 gene. Nature 362:751-755. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson-Smith, A. C., and M. A. Surani. 2001. Imprinting and the epigenetic asymmetry between parental genomes. Science 293:1086-1089. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrik, G. V., P. D. Soloway, and M. J. Higgins. 2002. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat. Genet. 32:426-431. [DOI] [PubMed] [Google Scholar]
- 12.Glenn, C. C., S. Saitoh, M. T. Jong, M. M. Filbrandt, U. Surti, D. J. Driscoll, and R. D. Nicholls. 1996. Gene structure, DNA methylation, and imprinted expression of the human SNRPN gene. Am. J. Hum. Genet. 58:335-346. [PMC free article] [PubMed] [Google Scholar]
- 13.Greger, I. H., and N. J. Proudfoot. 1998. Poly(A) signals control both transcriptional termination and initiation between the tandem GAL10 and GAL7 genes of Saccharomyces cerevisiae. EMBO J. 17:4771-4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greger, I. H., F. Demarchi, M. Giacca, and N. J. Proudfoot. 1998. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res. 26:1294-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould, T. D., and K. Pfeifer. 1998. Imprinting of mouse Kvlqt1 is developmentally regulated. Hum. Mol. Genet. 7:483-487. [DOI] [PubMed] [Google Scholar]
- 16.Hatada, I., T. Sugama, and T. Mukai. 1993. A new imprinted gene cloned by a methylation-sensitive genome scanning method. Nucleic Acids Res. 21:5577-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatada, I., K. Kitagawa, T. Yamaoka, X. Wang, Y. Arai, K. Hashido, S. Ohishi, J. Masuda, J. Ogata, and T. Mukai. 1995. Allele-specific methylation and expression of an imprinted U2af1-rs1 (SP2) gene. Nucleic Acids Res. 23:36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu, J. F., K. A. Balaguru, R. D. Ivaturi, H. Oruganti, T. Li, B. T. Nguyen, T. H. Vu, and A. R. Hoffman. 1999. Lack of reciprocal genomic imprinting of sense and antisense RNA of mouse insulin-like growth factor II receptor in the central nervous system. Biochem. Biophys. Res. Commun. 257:604-608. [DOI] [PubMed] [Google Scholar]
- 19.Jaenisch, R., and A. Bird. 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33:245-254. [DOI] [PubMed] [Google Scholar]
- 20.John, R. M., S. A. Aparicio, J. F. Ainscough, K. L. Arney, S. Khosla, K. Hawker, K. J. Hilton, S. C. Barton, and M. A. Surani. 2001. Imprinted expression of neuronatin from modified BAC transgenes reveals regulation by distinct and distant enhancers. Dev. Biol. 236:387-399. [DOI] [PubMed] [Google Scholar]
- 21.Lee, M. P., M. R. DeBaun, K. Mitsuya, H. L. Galonek, S. Brandenburg, M. Oshimura, and A. P. Feinberg. 1999. Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc. Natl. Acad. Sci. USA 96:5203-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, E., C. Beard, and R. Jaenisch. 1993. Role for DNA methylation in genomic imprinting. Nature 366:362-365. [DOI] [PubMed] [Google Scholar]
- 23.Li, M., J. A. Squire, and R. Weksberg. 1998. Overgrowth syndromes and genomic imprinting: from mouse to man. Clin. Genet. 53:165-170. [PubMed] [Google Scholar]
- 24.Li, X., G. Adam, H. Cui, B. Sandstedt, R. Ohlsson, and T. J. Ekstrom. 1995. Expression, promoter usage and parental imprinting status of insulin-like growth factor II (IGF2) in human hepatoblastoma: uncoupling of IGF2 and H19 imprinting. Oncogene 11:221-229. [PubMed] [Google Scholar]
- 25.Lyle, R., D. Watanabe, D. te Vruchte, W. Lerchner, O. W. Smrzka, A. Wutz, J. Schageman, L. Hahner, C. Davies, and D. P. Barlow. 2000. The imprinted antisense RNA at the Igf2r locus overlaps but does not imprint Mas1. Nat. Genet. 25:19-21. [DOI] [PubMed] [Google Scholar]
- 26.Mukai, T., and M. Sekiguchi. 2002. Gene silencing in phenomena related to DNA repair. Oncogene 21:9033-9042. [DOI] [PubMed] [Google Scholar]
- 27.Nabetani, A., I. Hatada, H. Morisaki, M. Oshimura, and T. Mukai. 1997. Mouse U2af1-rs1 is a neomorphic imprinted gene. Mol. Cell. Biol. 17:789-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeifer, K. 2000. Mechanisms of genomic imprinting. Am. J. Hum. Genet. 67:777-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proudfoot, N. J., A. Furger, and M. J. Dye. 2002. Integrating mRNA processing with transcription. Cell 108:501-512. [DOI] [PubMed] [Google Scholar]
- 30.Reik, W., and E. R. Maher. 1997. Imprinting in clusters: lessons from Beckwith-Wiedemann syndrome. Trends Genet. 13:330-334. [DOI] [PubMed] [Google Scholar]
- 31.Reik, W., and J. Walter. 2001. Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2:21-32. [DOI] [PubMed] [Google Scholar]
- 32.Rougeulle, C., C. Cardoso, M. Fontes, L. Colleaux, and M. Lalande. 1998. An imprinted antisense RNA overlaps UBE3A and a second maternally expressed transcript. Nat. Genet. 19:15-16. [DOI] [PubMed] [Google Scholar]
- 33.Rougeulle, C., and E. Heard. 2002. Antisense RNA in imprinting: spreading silence through Air. Trends Genet. 18:434-437. [DOI] [PubMed] [Google Scholar]
- 34.Shemer, R., Y. Birger, A. D. Riggs, and A. Razin. 1997. Structure of the imprinted mouse Snrpn gene and establishment of its parental-specific methylation pattern Proc. Natl. Acad. Sci. USA 94:10267-10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sleutels, F., R. Zwart, and D. P. Barlow. 2002. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature 415:810-813. [DOI] [PubMed] [Google Scholar]
- 36.Smith, R. J., W. Dean, G. Konfortova, and G. Kelsey. 2003. Identification of novel imprinted genes in a genome-wide screen for maternal methylation. Genome Res. 13:558-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smrzka, O. W., I. Fae, R. Stoger, R. Kurzbauer, G. F. Fischer, T. Henn, A. Weith, and D. P. Barlow. 1995. Conservation of a maternal-specific methylation signal at the human IGF2R locus. Hum. Mol. Genet. 4:1945-1952. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi, T., A. E. Schofield, J. L. Scarlett, I. M. Morison, M. J. Sullivan, and A. E. Reeve. 1995. Altered specificity of IGF2 promoter imprinting during fetal development and onset of Wilms tumour. Oncogene 11:751-756. [PubMed] [Google Scholar]
- 39.Thorvaldsen, J. L., K. L. Duran, and M. S. Bartolomei. 1998. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 12:3693-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uejima, H., M. P. Lee, H. Cui, and A. P. Feinberg. 2000. Hot-stop PCR: a simple and general assay for linear quantitation of allele ratios. Nature 25:375-376. [DOI] [PubMed] [Google Scholar]
- 41.van de Sluis, B. J., M. Breen, M. Nanji, M. van Wolferen, P. de Jong, M. M. Binns, P. L. Pearson, J. Kuipers, J. Rothuizen, D. W. Cox, C. Wijmenga, and B. A. van Oost. 1999. Genetic mapping of the copper toxicosis locus in Bedlington terriers to dog chromosome 10, in a region syntenic to human chromosome region 2p13-16. Hum. Mol. Genet. 8:501-507. [DOI] [PubMed] [Google Scholar]
- 42.van de Sluis, B., J. Rothuizen, P. L. Pearson, B. A. van Oost, and C. Wijmenga. 2002. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum. Mol. Genet. 11:165-173. [DOI] [PubMed] [Google Scholar]
- 43.Vu, T. H., and A. R. Hoffman. 1994. Promoter-specific imprinting of the human insulin-like growth factor II gene. Nature 371:714-717. [DOI] [PubMed] [Google Scholar]
- 44.Wroe, S. F., G. Kelsey, J. A. Skinner, D. Bodle, S. T. Ball, C. V. Beechey, J. Peters, and C. M. Williamson. 2000. An imprinted transcript, antisense to Nesp, adds complexity to the cluster of imprinted genes at the mouse Gnas locus. Proc. Natl. Acad. Sci. USA 97:3342-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin, Z., C. D. Allis, and J. Wagstaff. 2001. Parent-specific complementary patterns of histone H3 lysine 9 and H3 lysine 4 methylation at the Prader-Willi syndrome imprinting center. Am. J. Hum. Genet. 69:1389-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yatsuki, H., K. Joh, K. Higashimoto, H. Soejima, Y. Arai, Y. Wang, I. Hatada, Y. Obata, H. Morisaki, Z. Zhang, T. Nakagawachi, Y. Satoh, and T. Mukai. 2002. Domain regulation of imprinting cluster in Kip2/Lit1 subdomain on mouse chromosome 7F4/F5: large-scale DNA methylation analysis reveals that DMR-Lit1 is a putative imprinting control region. Genome Res. 12:1860-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]