Abstract
The Sydney Blood Bank Cohort is a group of patients with slowly progressive infection by a human immunodeficiency virus strain containing spontaneous deletions within the nef long terminal repeat region. In 1999, 18 years after the initial infection, one of the members (D36) developed AIDS. In this work, we used an ex vivo human lymphoid cell culture system to analyze two viral isolates obtained from this patient, one prior to the onset of AIDS in 1995 and one after disease progression in 1999. Both D36 isolates were less potent in depleting CD4+ T cells than a reference dualtropic, nef-bearing viral isolate. However, the 1999 isolate was measurably more cytotoxic to CD4+ T cells than the 1995 isolate. Interestingly, although both isolates were nearly equally potent in depleting CCR5+ CD4+ T cells, the cytotoxic effect of the 1999 isolate toward CCR5− CD4+ T cells was significantly higher. Furthermore, GHOST cell infection assays and blocking experiments with the CXCR4 inhibitor AMD3100 showed that the later D36 1999 isolate could infect both CCR5+ and CCR5− CXCR4+ cells efficiently, while infection by the 1995 isolate was nearly completely restricted to CCR5+ cells. Sequence analysis of the V1/V2 and V3 regions of the viral envelope protein gp120 revealed that the more efficient CXCR4 usage of the later isolate might be caused by an additional potential N-glycosylation site in the V1/V2 loop. In conclusion, these data show that an in vivo evolution of the tropism of this nef-deleted strain toward an X4 phenotype was associated with a higher cytopathic potential and progression to AIDS.
In human immunodeficiency virus (HIV)-infected individuals, both viral and host factors regulate viral replication, depletion of CD4+ T cells, and disease progression. Host factors include genetic determinants such as the expression of coreceptors (17, 24, 60) and the ability to establish an efficient immune response (reviewed in references 41 and 50). Furthermore, mutations in the HIV envelope protein Env that cause a change in coreceptor usage have been shown to influence disease progression (14, 37, 55, 56, 65).
In many but not all patients, disease progression coincides with broadened coreceptor usage. HIV strains isolated early after infection encode Env proteins that utilize CCR5 as a coreceptor to enter host cells (R5 viruses), whereas viruses isolated at later disease stages often represent Env variants that can utilize CXCR4 (X4 viruses) or both CCR5 and CXCR4 (R5X4 viruses) (reviewed in reference 5). In addition, viral accessory genes such as rev, tat, vif, vpr, and vpu (42, 66, 68) and nef (16, 40) have been implicated in disease development. In particular, Nef is a multifunctional protein (46) that enhances viral replication (43, 59) and infectivity (13), modulates apoptosis (26, 49, 67), and decreases the expression of CD4 (1), the major histocompatibility (MHC) class I (57), and CD28 (61). Long-term studies have shown that disease progression is markedly delayed in humans or rhesus macaques infected with HIV or simian immunodeficiency virus (SIV) strains, respectively, that carry deletions in the nef gene (15, 16, 36). Interestingly, spontaneous partial repair of the nef gene in vivo has been correlated with enhanced infectivity and disease progression (10, 52).
One of the best-examined patient groups of long-term nonprogressors and long-term survivors is the Sydney Blood Bank Cohort (SBBC) (16), which consists of one blood donor (D36) and eight transfusion recipients who were infected with an HIV-1 strain containing multiple spontaneous alterations in the viral genome. These alterations include deletions in the nef open reading frame and a part of the long terminal repeat (LTR) region as well as duplications and rearrangements within the LTR. Three of the cohort members remained asymptomatic for at least 14 years and are classified as long-term nonprogressors (8), two died of causes unrelated to HIV, and one member with systemic lupus erythematosus died of causes possibly related to HIV (38). Three members had declining CD4 counts and detectable viral loads and therefore are grouped as long-term survivors (8). One of these long-term survivors, the original blood donor D36, developed AIDS 18 years after infection with this nef-deleted HIV-1 strain and started highly active antiretroviral therapy in 1999 (38). Sequence analysis of virus recovered from D36 in 1999 revealed additional deletions in the nef LTR region, thereby reducing the possibility that restored Nef caused the onset of AIDS (8).
In this study, we sought to determine whether the clinical progression of patient D36 corresponds to changes in the virulence of HIV isolates from this patient and to identify possible mechanisms underlying such changes. With a modified version of ex vivo human lymphoid histoculture, we compared virus isolated from patient D36 prior to disease progression in 1995 (D36/95) and after progression but before therapy was initiated in 1999 (D36/99). We found that the later viral isolate was more cytotoxic than the earlier isolate and that this increased cytotoxicity was caused by more efficient CXCR4 usage and expanded target cell range.
MATERIALS AND METHODS
Preparation of viral stocks.
NL4-3 was a gift from Malcolm Martin via the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The molecular clone 49-5 was a gift from Bruce Chesebro. Infectious virus stocks were prepared by transfecting 293T cells with proviral DNA as described previously (2). The primary isolates D36/95, D36/99, 7/86, and 1/85 were expanded by infection of heterologous peripheral blood mononuclear cells (PBMC). Isolates 7/86 and 1/85 were gifts from Ruth Connor (14). The p24gag concentrations of viral stocks were assessed by enzyme-linked immunosorbent assay (ELISA) (NEN Life Sciences, Boston, Mass.).
Culture and infection of human lymphoid tissues ex vivo.
Human noninflammatory tonsil tissue removed during tonsillectomy (provided by the National Disease Research Interchange, Philadelphia, Pa., and the Kaiser hospitals in San Francisco, South San Francisco, and San Rafael) was prepared for lymphoid aggregate culture as previously described (23, 37). In brief, tonsil tissue was mechanically dispersed, and isolated cells were transferred to 96-well U-bottomed plates at a concentration of 107 cells per ml, 200 μl per well. Cells were allowed to aggregate at the bottom of the well and were not dispersed for the remainder of the culture period. Ex vivo human lymphoid cell cultures were inoculated within 24 h of preparation with HIV-1 at 80 50% tissue culture infective doses, as determined by terminal dilution of the virus stocks in quadruplicate on heterologous phytohemagglutin-activated PBMC as described previously (45).
Assessment of CD4+ T-cell depletion by FACS analysis.
At the indicated time points, cells from infected and uninfected lymphoid cell cultures were stained for cell surface markers CD3, CD4, CD8, and CCR5 as described previously (45, 55) with the following monoclonal antibodies: anti-CD3 (clone SK7, phycoerythrin [PE] conjugated), anti-CD4 (clone SK3, fluorescein isothiocyanate [FITC] conjugated), anti-CD8 (clone SK1, peridimin chlorophyll protein [PerCP] conjugated) (Becton Dickinson), and anti-CCR5 (clone 2D7, allophycocyanin conjugated) (PharMingen). Then, 10,000 lymphocytes positive for CD3 surface marker were counted by fluorescence-activated cell sorting (FACS), and the data were analyzed with Cellquest software (Becton Dickinson). To facilitate comparison among experiments, CD4+ T-cell depletion was assessed by measuring the ratio of CD4+ to CD8+ T cells. This value was normalized to the CD4/CD8 ratio of control (uninfected) samples.
Measurement of apoptosis.
At the indicated time points, cells from infected and uninfected lymphoid cell cultures were washed with phosphate-buffered saline-2% fetal bovine serum-2.5 mM CaCl2, stained for cell surface markers CD3, CD4, and either annexin V-phycoerythrin (Alexis) or 200 nM tetramethylrhodamine methyl ester (TMRM; Molecular Probes) for 30 min at room temperature, washed again, and subjected to flow cytometric analysis. To determine activation of caspase-3, cells were incubated with 10 μM PhiPhiLux-G1D2 (Alexis) for 1 h at 37°C, washed, stained for CD4 and CD3, and subjected to FACS analysis.
GHOST cell and HeLa infection assays.
GHOST cell assays were performed as reported previously (11). Briefly, 20,000 CXCR4+ CD4+ (GHOST-X4) or CCR5+ CD4+ GHOST (GHOST-R5) cells were plated in 12-well plates and infected at a multiplicity of infection (MOI) of 0.1. At 72 h after infection, infected cells were identified by flow cytometric analysis. CXCR4+or CCR5+ HeLa CD4 cells were pretreated for at least 12 h with the indicated concentrations of AMD3100 and inoculated with HIV at an MOI of 0.01. At 72 h after infection, the concentration of p24gag in the culture supernatant was assessed by anti-p24 ELISA.
Sequence analysis of the gp120 V1/V2 and V3 regions of D36/99.
CXCR4+ CCR5+ CD4+ HeLa cells were infected with D36/99 at an MOI of 0.5. At 12 days after infection, genomic DNA was isolated with the Qiagen DNeasy Tissue kit. The gp120 gene was PCR amplified with the following primers at an annealing temperature of 51°C: 5′ primer D36-5680, GTTGGTCACAGTCTATTATGG; 3′ primer D36-7089rev, TGGGTGCTACTCCTAATGGTT. Specific bands were purified from an agarose gel with the Qiagen gel extraction kit. PCR products were cloned into the pCR2.1 vector (Invitrogen). Inserts of two clones were sequenced with the M13 reverse sequencing primer (CAGGAAACAGCTATGAC) and the M13 sequencing (−20) primer (GTAAAACGACGGCCAGT).
Nucleotide sequence accession number.
The sequence of the D36/95 isolate is available under accession number AF042105.
RESULTS
Characterization of the virulence of D36/95 and D36/99.
First, the clinical isolates from patient D36 were tested for their potential to deplete CD4+ T cells in ex vivo human lymphoid cultures. The kinetics of HIV-induced CD4+ T-lymphocyte depletion was measured by staining cultures with antibodies to CD3, CD4, and CD8 and displayed as a ratio of CD4+ to CD8+ T cells as described previously (23, 37, 45, 54, 55). We compared the two D36 isolates with two other primary isolates from a different cohort. These isolates, called 7/86, an R5X4 strain, and 1/85, an R5 strain (14), are wild-type nef strains. As expected, the number of CD4+ T cells in 1/85-infected cultures decreased only slightly over time, since R5 strains can infect and deplete only the small subset of CCR5-expressing CD4+ T cells (28, 45, 54, 55). In contrast, the dualtropic virus 7/86 depleted CD4+ T cells markedly due to its expanded target cell range (Fig. 1A) (55).
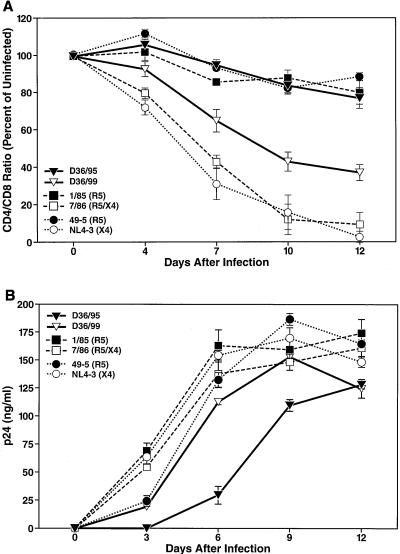
FIG. 1.
D36/99 isolate depletes CD4+ T cells more efficiently and replicates faster than D36/95 in ex vivo human lymphoid cell cultures. (A) CD4 depletion induced by infection with viral strains D36/99, D36/95, 7/86, 1/85, NL4-3, and 49-5 was assessed by FACS analysis at the indicated time points. Shown are the mean relative CD4/CD8 ratios (n = 3) with standard error of the mean (SEM) of a representative experiment from among experiments with six different donor tissues. (B) Viral replication was monitored in the same infections by assessing the accumulation of p24 in the culture medium at days 3, 6, 9, and 12 after infection with an anti-p24 ELISA. Shown are the mean values (n = 3) with SEM of a representative experiment from among experiments with six different donor tissues.
Overall, CD4 depletion was less severe in cultures infected with either of the two D36 isolates than in cultures infected with the R5X4 reference strain 7/86 (Fig. 1A). However, the later D36/99 isolate was measurably more cytopathic than the earlier D36/95 isolate, with more severe CD4+ T-cell depletion despite comparable inoculum size (Fig. 1A). The increased virulence of D36/99 was also reflected in faster replication kinetics. D36/99 replicated with a profile similar to that of the two reference strains, whereas replication of D36/95 was significantly delayed (Fig. 1B).
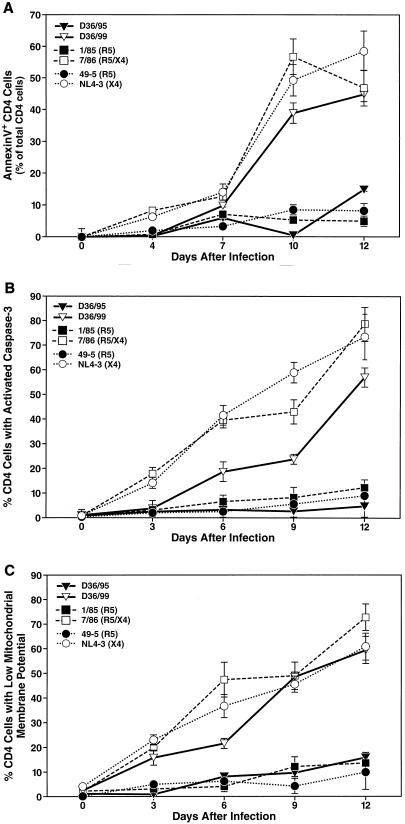
The elevated potential of D36/99 to deplete CD4+ T lymphocytes was paralleled by its ability to induce apoptosis in CD4+ T cells. Apoptosis was measured independently by several assays: annexin V binding to phosphatidylserine as a marker for the loss of cell membrane asymmetry (Fig. 2A); activation of caspase-3, a key enzyme of the apoptotic signal transduction pathway (Fig. 2B); and depolarization of the mitochondrial membrane as a marker of the mitochondrial branch of apoptotic signaling (Fig. 2C). To this end, cells were stained with antibodies to CD4 and CD3 along with annexin V-FITC, the membrane-permeable caspase-3 substrate PhiPhiLux-G1D2, or TMRM and subjected to FACS analysis (6, 29). Loss of TMRM binding to mitochondria is an indicator of the breakdown of the mitochondrial membrane potential (6). Examination by all three methods showed that cultures infected with D36/99 had much higher levels of apoptosis in CD4+ T cells than those infected with the earlier D36/95 isolate (Fig. 2). However, apoptosis in D36/99-infected cultures was still slightly lower than that observed in cultures infected with the wild-type strain 7/86.
FIG. 2.
D36/99 induces high levels of apoptosis among CD4+ T cells in human lymphoid cell cultures. Apoptosis in CD4+ T cells induced by infection with viral strains D36/99, D36/95, 7/86, 1/85, NL4-3, and 49-5 was measured at the indicated time points independently by annexin V binding (A), activation of caspase-3 (B), and depolarization of the mitochondrial membrane potential (C) by FACS analysis. Shown are the mean values (n = 3) with SEM of a representative experiment from among experiments with six different donor tissues.
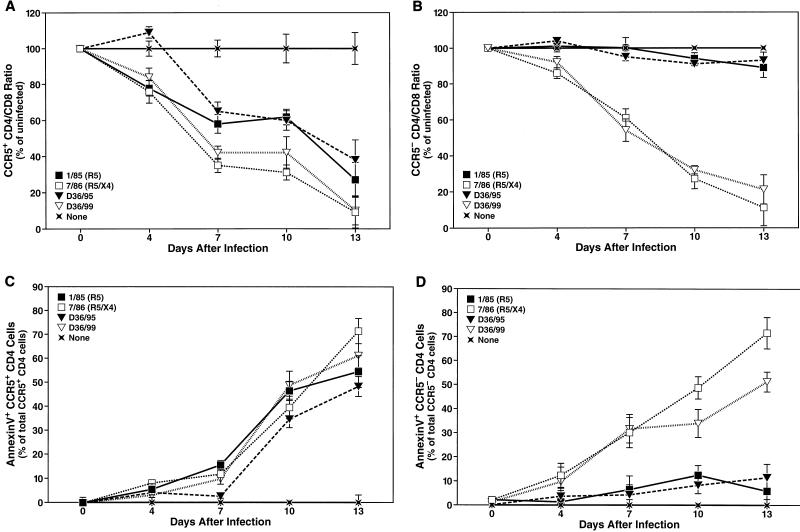
Depletion and apoptosis of CCR5+ and CCR5− CD4+ T cells.
Disease progression in HIV-infected patients has been shown to correlate with a change in coreceptor usage in many, though not all, individuals (14, 56, 65). Therefore, we addressed the question of whether the increased potential of D36/99 to induce apoptosis and hence deplete CD4+ T cells was caused by a broadened coreceptor usage. We used flow cytometry to distinguish CD4+ T cells into CCR5+ and CCR5− subsets as described previously (28, 37, 45, 55). Interestingly, D36/95 depleted the CCR5+ subset of CD4+ T cells to an extent comparable to that of both D36/99 and the R5X4 reference strain 7/86 (Fig. 3A). In contrast, while D36/99 and 7/86 also caused marked depletion of the CCR5− subset of CD4+ T cells, the earlier D36/95 isolate and the R5 reference strain 1/85 did not affect this subset significantly (Fig. 3B). Similarly, D36/95 and 1/85 induced apoptosis mainly in CCR5+ CD4+ T cells, whereas D36/99 and 7/86 promoted apoptosis equally in both the CCR5+ and CCR5− cellular subsets (Fig. 3C and D). Thus, the effects of D36/95 appeared to be restricted to CCR5+ CD4+ T cells, while cytopathic effects of the later D36/99 isolate were broadened to include both CCR5+ and CCR5− CD4+ T cells.
FIG. 3.
D36/99 induces depletion and apoptosis of both CCR5+ and CCR5− CD4+ T cells in human ex vivo lymphoid cell cultures. CD4 depletion induced by infection with viral strains D36/99, D36/95, 7/86, and 1/85 within the CCR5+ (A) and CCR5− (B) subsets of CD4+ T cells was analyzed at the indicated time points by multiparameter FACS analysis. Shown are the mean relative CD4/CD8 ratios (n = 3) with SEM of a representative experiment from among experiments with six different donor tissues. In the same infections, apoptosis was measured in the CCR5+ (C) and CCR5− (D) CD4+ T-cell subsets by annexin V binding. Shown are the mean values (n = 3) with SEM of a representative experiment from among experiments with six different donor tissues.
Coreceptor phenotype of the D36 isolates.
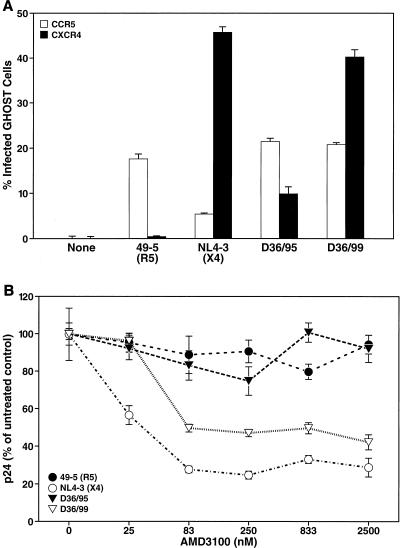
To define the coreceptor usage by the two D36 isolates more directly and quantitatively, GHOST cell infection assays were performed. CCR5-expressing GHOST (GHOST-R5) cells and CXCR4-expressing GHOST (GHOST-X4) cells were infected with D36/95 and D36/99 at an MOI of 0.1 (11). The prototypical strains 49-5 (R5) and NL4-3 (X4) were used as positive controls for CCR5 and CXCR4 usage, respectively (62). As expected, 49-5 exclusively infected GHOST-R5 cells, as indicated by LTR-mediated expression of the fluorescent marker, whereas NL4-3 infected GHOST-X4 cells (Fig. 4A). The residual infection of GHOST-R5 cells by the X4 strain NL4-3 was due to the endogenous low-level expression of CXCR4 in parental GHOST cells. Importantly, both D36/95 and D36/99 infected GHOST-R5 cells to an extent comparable to that of 49-5. In contrast, D36/95 infected GHOST-X4 cells but with a fourfold lower efficiency than D36/99. These results indicate that D36/99 has a significantly higher potential to infect CXCR4-expressing cells than D36/95 (Fig. 4A).
FIG. 4.
D36/99 can use CXCR4 as a coreceptor more efficiently than D36/95. (A) GHOST-R5 (open bars) and GHOST-X4 cells (solid bars) were infected with D36/99, D36/95, NL4-3, and 49-5 at an MOI of 0.1 and cultured for 72 h. Infected cells were identified by FACS analysis. Shown are the mean values (n = 3) with SEM of a representative experiment from among three independent experiments. (B) CXCR4+ CCR5+ HeLa CD4 cells were pretreated for 12 h with the indicated concentrations of AMD3100 and infected with D36/99, D36/95, NL4-3, and 49-5 at an MOI of 0.01. Viral replication was measured 72 h after infection by assessing the concentration of p24 in the culture medium. Shown are the mean values (n = 3) with SEM of a representative experiment from among three independent experiments.
We tested the differential CXCR4 utilization by these viruses further by analyzing their sensitivity to the CXCR4 antagonist AMD3100 (22, 53). Dose-response curves were determined with AMD3100 to inhibit infection of CXCR4+ CCR5+ HeLa CD4 cells (7, 33) by D36/95, D36/99, and the two reference viruses 49-5 (R5) and NL4-3 (X4). Infection by either 49-5 or D36/95 was not significantly inhibited by AMD3100, confirming their independence from CXCR4 (Fig. 4B). In contrast, infection by NL4-3 or D36/99 was inhibited effectively by AMD3100, with a 50% inhibitory concentration (IC50) of 38 nM and 85 nM, respectively (Fig. 4B). Notably, even at AMD3100 concentrations as high as 2,500 nM, infection with D36/99 could not be inhibited completely, while that by NL4-3 was nearly fully abolished (Fig. 4B). This suggests that D36/99 can use both CCR5 and CXCR4 as a coreceptor. Taken together, these results demonstrate that both D36 isolates can utilize CCR5 as a coreceptor and that D36/99 can also use CXCR4 efficiently.
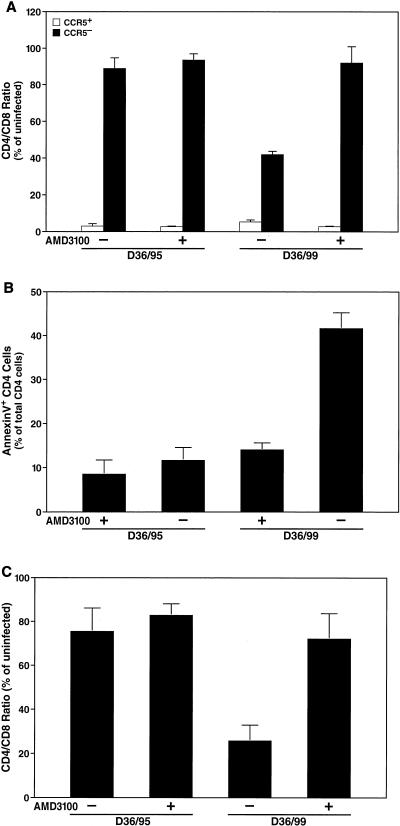
Next, we analyzed the coreceptor usage of the D36 isolates within the biologically relevant ex vivo lymphoid cell culture system. Lymphoid cultures were pretreated with AMD3100 (250 nM) and infected with equal doses of D36/95 or D36/99. Depletion and apoptosis of CD4+ T cells overall as well as specific depletion of the CCR5− and CCR5+ subsets of CD4+ T cells were determined 12 days after the infection. As expected, the modest depletion (Fig. 5A) and apoptosis (Fig. 5B) of CD4+ T cells induced by D36/95 were not significantly affected by AMD3100. In contrast, both CD4 depletion (Fig. 5A) and apoptosis (Fig. 5B) induced by D36/99 were strongly inhibited by AMD3100 (Fig. 5A and B). Furthermore, analysis of the CCR5− and CCR5+ subsets showed that AMD3100 prevented depletion of CCR5− CD4+ T cells by D36/99, but not depletion of CCR5+ CD4+ T cells (Fig. 5C). Again, depletion of the CCR5+ subset by D36/95 was unaffected by AMD3100 (Fig. 5C). These results confirm that D36/99 has a broadened coreceptor usage that allows it to infect both CCR5+ cells and CCR5−CXCR4+ CD4+ T cells.
FIG. 5.
D36/99 depletes and induces apoptosis in both the CCR5+ and CCR5− subsets of CD4+ T cells in human ex vivo lymphoid cultures. Human ex vivo lymphoid cell cultures were pretreated with AMD3100 (250 nM) for 12 h and infected with D36/95 and D36/99. AMD3100 was replenished in the cultures at each medium change. Depletion of total CD4+ T cells (A) and of the CCR5+ and CCR5− subsets of CD4+ T cells (C) as well as apoptosis of total CD4+ T cells (B) were measured by FACS analysis 12 days after infection. Shown are the mean values (n = 3) with SEM of a representative experiment from among experiments with four different donor tissues.
Sequence analysis of the gp120 V1/V2 and V3 regions of the D36 isolates.
Coreceptor usage is mostly determined by specific amino acid residues in the V1/V2 and V3 loops of the viral envelope protein gp120 (5, 9, 18, 25, 30, 31, 58). In particular, the high charge and low N-glycosylation status of the V3 loop as well as the high N-glycosylation status in V1/V2 have been linked to X4 usage (12, 18, 34, 39, 47, 63). Therefore, we compared the V1/V2 and V3 regions of the two different D36 isolates to analyze whether specific amino acid changes in these domains could be the molecular determinants for the broadened coreceptor usage of D36/99. We did not detect any amino acid mutations in the V3 loop that would be expected to alter its charge or glycosylation status (Fig. 6). However, we found that the later D36/99 isolate contains an additional potential N-glycosylation site at position 161 in the V1/V2 region (Fig. 6). Thus, it appears that the more efficient CXCR4 usage of the later D36/99 isolate may be attributable to an additional N-glycosylation site in the V1/V2 region.
FIG. 6.
V1/V2 loop of D36/99 contains an additional potential N-glycosylation site. The top panel shows the gp120 V1/V2 and the bottom panel shows the gp120 V3 loop sequence of both D36 isolates. Potential N-glycosylation sites are printed in bold, and changes between the D36/95 and the D36/99 isolates are highlighted by a box. Amino acid numbers are based on the D36/95 sequence.
DISCUSSION
Evidence from in vivo observations and ex vivo experiments has strongly pointed to a switch in coreceptor usage as an important factor in HIV disease progression. Although the precise mechanism remains unclear, such phenotypic and genotypic changes are thought to be the result of an evolutionary selection process (14, 56, 65). The present study reveals that the low level of replication in vivo of an HIV-1 strain with spontaneous deletions in the nef LTR region (Fig. 1B) (16) was sufficient to allow evolution of the viral env gene from a predominantly R5 phenotype toward a predominantly X4 phenotype. The more efficient CXCR4 usage of D36/99 appears to be caused by a single additional N-glycosylation site in the V1/V2 region of the viral envelope gp120. Moreover, such viral evolution seems to have been dictated by the same evolutionary processes that cause changes in typical wild-type nef HIV strains.
Importantly, the change in coreceptor usage was manifested by an increased potential of the virus to infect CCR5− CXCR4+ lymphocytes (Fig. 4) and to deplete and induce apoptosis in this T-cell subset of human lymphoid tissue (Fig. 1A, 3, and 5). Earlier studies had implicated a more promiscuous coreceptor usage and a wider target cell range as a key pathological mechanism underlying disease acceleration following emergence of X4 strains in patients infected with typical wild-type nef strains (14, 56, 65). The increased cytotoxicity of the D36 virus observed in this study strongly suggests that a similar evolution of the viral tropism is an important factor in the disease progression of patient D36 despite the absence of Nef. However, additional mechanisms not delineated here may be involved as well.
Recently, Kirchhoff and coworkers reported a case of a long-term nonprogressor infected with an HIV-1 strain with deletions in the nef gene that were found to be partially repaired (10). Moreover, the virus with this repaired Nef protein displayed augmented infectivity and increased downregulation of CD4 and of MHC class I molecule expression (10). Similarly, rhesus macaques infected with SIV strains with mutations of the nef start codon, as well as deletions and insertions in the coding sequence of nef, were able to express a truncated version of Nef with a reverted start codon after several months of infection (52). Virus isolates with these truncated Nef proteins were much more pathogenic than their parental strains and caused an AIDS-like disease in the macaques (52).
Interestingly, vaccination of one of four rhesus macaques with proviral DNA of an SIV strain with the same nef LTR deletion as in the SBBC (SIVSBBCΔ3) resulted in sustained SIV infection and AIDS (35, 48). PCR analysis showed that this animal had completely repaired the nef LTR deletion (35, 48). In contrast, the increased cytopathicity of the D36/99 isolate reported here was not caused by a reversion or partial repair of the nef gene. Sequence analysis showed that the deletions in the nef gene of the D36/99 isolate are even more extensive than that of D36/95 (8). Furthermore, immunoblot analysis of PBMC infected with D36/95 and D36/99 revealed that the two D36 isolates do not express a Nef protein that could be recognized by a polyclonal anti-Nef antibody (data not shown). However, a contribution of further genetic rearrangements in the nef LTR region to the enhanced cytotoxic potential of the later D36/99 isolate cannot be entirely excluded (8).
Live attenuated HIV strains like the one seen in the SBBC have been proposed as a vaccine to prevent infection with wild-type HIV (19). A key challenge in developing a safe, effective attenuated virus vaccine is that a strong immune response seems to depend on the degree of viral replication (51). However, even at a very low level, replication harbors the risk of evolution toward higher virulence. Several reports of spontaneous repair of deletions in the nef gene in vivo resulting in increased virulence and the onset of an AIDS-like disease (21, 52, 64) have challenged the safety of this vaccination concept. Therefore, vaccine candidates have been developed with multiple mutations in accessory genes (20, 27, 44). These second-generation vaccines are thought to be much safer but may still be able to elicit an effective immune protection (32, 44). However, even a triple-deleted SIV strain caused AIDS in neonatal macaques and in a small proportion of adult animals (3, 4). Moreover, the present study shows that the attenuation of a virus with deletions in the nef gene can be overcome not only by repair of the nef gene, but also by compensatory changes in other genes. In particular, the deletions in the D36 virus did not prevent it from evolving to higher virulence by broadening its coreceptor usage. Indeed, in contrast to nef and other accessory genes, env is an essential HIV gene that cannot be deleted to provide a higher level of protection without completely inhibiting replication. The high risk of using a live attenuated virus as a vaccine is further supported by the fact that two more members of the SBBC now have declining CD4 counts and detectable viral loads (8), although the underlying mechanisms in these two cases remain unknown.
In conclusion, we have shown that an HIV-1 strain with multiple deletions in the nef LTR region can still mutate to a higher level of virulence in vivo by widening its coreceptor usage and target cell range. This implies that the low level of replication of an attenuated virus is sufficient to allow substantial viral evolution and shows that the use of live attenuated nef-deleted viruses as a vaccine is not safe.
Acknowledgments
We thank the members of the surgical staff at Kaiser hospitals (San Rafael, San Francisco, and South San Francisco) for generous assistance in obtaining posttonsillectomy samples. We acknowledge the assistance of Heather Gravois, John Carroll, and Jack Hull in the preparation of the manuscript. We thank Peggy Chin, Valerie Stepps, and Katherine Kedzierska for technical assistance and Dan Eckstein, Becky Schweighardt, Oliver Keppler, and Jason Kreisberg for valuable discussions.
B.S. was supported by the Boehringer Ingelheim Fund, and P.J. was supported by the Biomedical Sciences Graduate program at the University of California—San Francisco (UCSF). This work was supported by NIH grants to M.A.G. (CA86814 and AI43695) and by the J. David Gladstone Institutes.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Atchison, R. E., J. Gosling, F. S. Monteclaro, C. Franci, L. Digilio, I. F. Charo, and M. A. Goldsmith. 1996. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 274:1924-1926. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 5.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 6.Bernardi, P., L. Scorrano, R. Colonna, V. Petronilli, and F. Di Lisa. 1999. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 264:687-701. [DOI] [PubMed] [Google Scholar]
- 7.Bestwick, R. K., S. L. Kozak, and D. Kabat. 1988. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc. Natl. Acad. Sci. USA 85:5404-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birch, M., J. C. Learmont, W. B. Dyer, N. J. Deacon, J. J. Zaunders, N. Saksena, A. L. Cunningham, J. Mills, and J. S. Sullivan. 2001. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC). J. Clin. Virol. 22:263-270. [DOI] [PubMed] [Google Scholar]
- 9.Cann, A. J., M. J. Churcher, M. Boyd, W. O'Brien, J. Q. Zhao, J. Zack, and I. S. Chen. 1992. The region of the envelope gene of human immunodeficiency virus type 1 responsible for determination of cell tropism. J. Virol. 66:305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carl, S., R. Daniels, A. J. Iafrate, P. Easterbrook, T. C. Greenough, J. Skowronski, and F. Kirchhoff. 2000. Partial “repair” of defective NEF genes in a long-term nonprogressor with human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:132-140. [DOI] [PubMed] [Google Scholar]
- 11.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho, M. W., M. K. Lee, M. C. Carney, J. F. Berson, R. W. Doms, and M. A. Martin. 1998. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J. Virol. 72:2509-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 16.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, A. Sonza, J. Learmont, J. S. Sullivan, A. Cunnigham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 17.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 18.De Jong, J. J., A. De Ronde, W. Keulen, M. Tersmette, and J. Goudsmit. 1992. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J. Virol. 66:6777-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desrosiers, R. C. 1992. HIV with multiple gene deletions as a live attenuated vaccine for AIDS. AIDS Res. Hum. Retrovir. 8:411-421. [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittmer, U., T. Nisslein, W. Bodemer, H. Petry, U. Sauermann, C. Stahl-Hennig, and G. Hunsmann. 1995. Cellular immune response of rhesus monkeys infected with a partially attenuated nef deletion mutant of the simian immunodeficiency virus. Virology 212:392-397. [DOI] [PubMed] [Google Scholar]
- 22.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 23.Eckstein, D. A., M. L. Penn, Y. D. Korin, D. D. Scripture-Adans, J. A. Zack, J. F. Kreisberg, M. Roederer, M. P. Sherman, P. S. Chin, and M. A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4+ T cells residing within human lymphoid tissues. Immunity 15:671-682. [DOI] [PubMed] [Google Scholar]
- 24.Fauci, A. S. 1996. Host factors and the pathogenesis of HIV-induced disease. Nature 384:529-534. [DOI] [PubMed] [Google Scholar]
- 25.Fouchier, R. A., M. Groenink, N. A. Kootstra, M. Tersmette, H. G. Huisman, F. Miedema, and H. Schuitemaker. 1992. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J. Virol. 66:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grivel, J. C., M. L. Penn, D. A. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith. 2000. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J. Virol. 74:5347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata, H., A. Takahashi, S. Kobayashi, S. Yonehara, H. Sawai, T. Okazaki, K. Yamamoto, and M. Sasada. 1998. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J. Exp. Med. 187:587-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman, T. L., and R. W. Doms. 1999. HIV-1 envelope determinants for cell tropism and chemokine receptor use. Mol. Membr. Biol. 16:57-65. [DOI] [PubMed] [Google Scholar]
- 31.Hwang, S. S., T. J. Boyle, H. K. Lyerly, and B. R. Cullen. 1991. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science 253:71-74. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, R. P., and R. C. Desrosiers. 1998. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 10:436-443. [DOI] [PubMed] [Google Scholar]
- 33.Kabat, D., S. L. Kozak, K. Wehrly, and B. Chesebro. 1994. Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J. Virol. 68:2570-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato, K., H. Sato, and Y. Takebe. 1999. Role of naturally occurring basic amino acid substitutions in the human immunodeficiency virus type 1 subtype E envelope V3 loop on viral coreceptor usage and cell tropism. J. Virol. 73:5520-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kent, S. J., C. J. Dale, S. Preiss, J. Mills, D. Campagna, and D. F. Purcell. 2001. Vaccination with attenuated simian immunodeficiency virus by DNA inoculation. J. Virol. 75:11930-11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 37.Kreisberg, J. F., D. Kwa, B. Schramm, V. Trautner, R. Connor, H. Schuitemaker, J. I. Mullins, A. B. van't Wout, and M. A. Goldsmith. 2001. Cytopathicity of human immunodeficiency virus type 1 primary isolates depends on coreceptor usage and not patient disease status. J. Virol. 75:8842-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 39.Li, Y., M. A. Rey-Cuille, and S. L. Hu. 2001. N-linked glycosylation in the V3 region of HIV type 1 surface antigen modulates coreceptor usage in viral infection. AIDS Res. Hum. Retrovir. 17:1473-1479. [DOI] [PubMed] [Google Scholar]
- 40.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 42.Michael, N. L., G. Chang, L. A. d'Arcy, P. K. Ehrenberg, R. Mariani, M. P. Busch, D. L. Birx, and D. H. Schwartz. 1995. Defective accessory genes in a human immunodeficiency virus type 1-infected long-term survivor lacking recoverable virus. J. Virol. 69:4228-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills, J., R. Desrosiers, E. Rud, and N. Almond. 2000. Live attenuated HIV vaccines: a proposal for further research and development. AIDS Res. Hum. Retrovir. 16:1453-1461. [DOI] [PubMed] [Google Scholar]
- 45.Penn, M. L., J. C. Grivel, B. Schramm, M. A. Goldsmith, and L. Margolis. 1999. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 96:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peter, F. 1998. HIV nef: the mother of all evil? Immunity 9:433-437. [DOI] [PubMed] [Google Scholar]
- 47.Pollakis, G., S. Kang, A. Kliphuis, M. I. Chalaby, J. Goudsmit, and W. A. Paxton. 2001. N-linked glycosylation of the HIV type-1 gp120 envelope glycoprotein as a major determinant of CCR5 and CXCR4 coreceptor utilization. J. Biol. Chem. 276:13433-13441. [DOI] [PubMed] [Google Scholar]
- 48.Purcell, D. F., P. U. Cameron, J. Mills, and S. Kent. 2001. Infectivity of wild-type and deleted proviral SIV DNA. Dev. Biol. 106:395-406. [PubMed] [Google Scholar]
- 49.Robichaud, G. A., and L. Poulin. 2000. HIV type 1 nef gene inhibits tumor necrosis factor alpha-induced apoptosis and promotes cell proliferation through the action of MAPK and JNK in human glial cells. AIDS Res. Hum. Retrovir. 16:1959-1965. [DOI] [PubMed] [Google Scholar]
- 50.Rowland-Jones, S., S. Pinheiro, and R. Kaul. 2001. New insights into host factors in HIV-1 pathogenesis. Cell 104:473-476. [DOI] [PubMed] [Google Scholar]
- 51.Ruprecht, R. M. 1999. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol. Rev. 170:135-149. [DOI] [PubMed] [Google Scholar]
- 52.Sawai, E. T., M. S. Hamza, M. Ye, K. E. S. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 74:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schramm, B., M. L. Penn, E. H. Palacios, R. M. Grant, F. Kirchhoff, and M. A. Goldsmith. 2000. Cytopathicity of human immunodeficiency virus type 2 (HIV-2) in human lymphoid tissue is coreceptor dependent and comparable to that of HIV-1. J. Virol. 74:9594-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schramm, B., M. L. Penn, R. F. Speck, S. Y. Chan, E. De Clercq, D. Schols, R. I. Connor, and M. A. Goldsmith. 2000. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J. Virol. 74:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 58.Speck, R. F., K. Wehrly, E. J. Platt, R. E. Atchison, I. F. Charo, D. Kabat, B. Chesebro, and M. A. Goldsmith. 1997. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J. Virol. 71:7136-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart, G. J., L. J. Ashton, R. A. Biti, R. A. Ffrench, B. H. Bennetts, N. R. Newcombe, E. M. Benson, A. Carr, D. A. Cooper, and J. M. Kaldor. 1997. Increased frequency of CCR-5 delta 32 heterozygotes among long-term non-progressors with HIV-1 infection. The Australian Long-Term Non-Progressor Study Group. AIDS 11:1833-1838. [DOI] [PubMed] [Google Scholar]
- 61.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toohey, K., K. Wehrly, J. Nishio, S. Perryman, and B. Chesebro. 1995. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology 213:70-79. [DOI] [PubMed] [Google Scholar]
- 63.Wang, W. K., M. Essex, and T. H. Lee. 1996. Single amino acid substitution in constant region 1 or 4 of gp120 causes the phenotype of a human immunodeficiency virus type 1 variant with mutations in hypervariable regions 1 and 2 to revert. J. Virol. 70:607-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whatmore, A. M., N. Cook, G. A. Hall, S. Sharpe, E. W. Rud, and M. P. Cranage. 1995. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J. Virol. 69:5117-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao, L., D. L. Rudolph, S. M. Owen, T. J. Spira, and R. B. Lal. 1998. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS 12:F137-F143. [DOI] [PubMed] [Google Scholar]
- 66.Yamada, T., and A. Iwamoto. 2000. Comparison of proviral accessory genes between long-term nonprogressors and progressors of human immunodeficiency virus type 1 infection. Arch. Virol. 145:1021-1027. [DOI] [PubMed] [Google Scholar]
- 67.Yoon, K., J. G. Jeong, and S. Kim. 2001. Stable expression of human immunodeficiency virus type 1 Nef confers resistance against Fas-mediated apoptosis. AIDS Res. Hum. Retrovir. 17:99-104. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, L., Y. Huang, H. Yuan, S. Tuttleton, and D. D. Ho. 1997. Genetic characterization of vif, vpr, and vpu sequences from long-term survivors of human immunodeficiency virus type 1 infection. Virology 228:340-349. [DOI] [PubMed] [Google Scholar]