Abstract
The cottontail rabbit papillomavirus (CRPV)-rabbit model has been used in several studies for testing prophylactic and therapeutic papillomavirus vaccines. Earlier observations had shown that the CRPV nonstructural genes E1, E2, and E6 induced strong to partial protective immunity against CRPV infection. In this study, we found that CRPV E8 immunization eliminated virus-induced papillomas in EIII/JC inbred rabbits (100%) and provided partial protection (55%) against virus challenge in outbred New Zealand White rabbits. CRPV-E8 is a small open reading frame, coding for a 50-amino-acid protein, that is colinear with the CRPV E6 gene and has features similar to those of the bovine papillomavirus and human papillomavirus E5 genes. Papillomas that grew on E8-vaccinated outbred rabbits were significantly smaller than those on vector-vaccinated rabbits (P < 0.01; t test). Delayed-type hypersensitivity skin tests showed that some of the E8-vaccinated rabbits had positive responses to E8-specific peptides.
Papillomaviruses are small DNA tumor viruses that induce cutaneous and mucosal epithelial lesions. In humans, high-risk human papillomaviruses (HPVs), such as HPV type 16 (HPV-16) and HPV-18, are strongly associated with anogenital carcinomas, whereas low-risk papillomaviruses, such as HPV-6 and HPV-11, usually induce benign genital and laryngeal papillomas (49). Systematic studies of host immune reactions to papillomaviruses require effective animal models in which papillomas can be induced experimentally (35). Cottontail rabbit papillomavirus (CRPV) infection of domestic rabbits is an important model system for the study of papillomavirus host immune responses in immunocompetent hosts (1, 15, 24, 27, 38). CRPV-induced papillomas may spontaneously regress or progress to invasive carcinoma. These characteristics resemble those of HPV infections with high-risk HPV types.
Several studies have tested protective and therapeutic vaccines against papillomavirus infection based on products of viral structural and nonstructural genes (reviewed in references 12 and 35). Papillomavirus genomes contain two structural, or late, genes defined as the major (L1) and minor (L2) capsid genes and seven nonstructural, or early, genes (E1, E2, E4, E5, E6, E7, and E8) which regulate virus replication and have transforming activities. The two late genes and several early genes, including E1, E2, E6, and E7, have been tested as candidate vaccines in several animal papillomavirus models (16, 24, 30, 35, 43).
Earlier studies focused on immunization with papillomavirus proteins. Prophylactic immunity was induced by vaccination with recombinant virus-like particles in bovine papillomavirus (BPV)-infected cattle (26), canine oral papillomavirus-infected beagle dogs (44), and CRPV-infected rabbits (1, 7, 23). Immunization with the CRPV L1 or L2 capsid protein protected animals against infectious virus but not viral DNA challenge (31, 32). Immunization with CRPV E6 protein did not protect against virus challenge but increased the rate of tumor regression (29). Vaccination with E1 and E2 proteins simultaneously or individually triggered virus-induced papilloma regression, and combined immunizations with E1 and E2 showed increased protective immunity (39). These studies have shown that both early and late genes contain epitopes that can serve as targets for immune responses to papillomavirus virions and papillomavirus-infected cells. Cell-mediated immune responses have been demonstrated to be crucial in eradicating papillomavirus infections (12, 40).
Recently, intracutaneous gene gun delivery of plasmid DNA containing individual viral genes has been shown to trigger effective protective immunity to papillomavirus infections (10, 16, 41, 42, 46). Vaccination with recombinant expression vectors by this delivery route can induce potent and long-term humoral and cellular immune responses in animal systems (34, 36, 45, 47). In the CRPV-rabbit model, recombinant Listeria monocytogenes expressing CRPV E1 induced strong protection against CRPV viral DNA challenge and induced regression of papillomas (24). CRPV E6 gene vaccination provided partial protection against virus challenge but induced an increase in the regression of papillomas when given in combination with granulocyte-macrophage colony-stimulating factor (30, 43).
We have immunized rabbits with CRPV E1, E2, E6, and E7 genes delivered intramuscularly and detected cell-mediated immune responses. However, protection against virus infection was not achieved using this vaccine strategy (17). When the same DNA expression vectors were delivered intracutaneously using a gene gun delivery system, we observed both strong cell-mediated immunity and strong protection against virus infection (16). Interestingly, vaccinations with CRPV E7 alone did not induce any protective immunity (16, 29). Combinations of E1 and E2 genes delivered intracutaneously also induced strong protective immunity in both inbred EIII/JC and outbred New Zealand White (NZW) rabbits (18).
For this report, we expanded our studies on protective intracutaneous vaccination against CRPV to another viral gene, CRPV E8. CRPV E8 is a very small nonstructural gene which is colinear with CRPV E6. E8 encodes a hydrophobic polypeptide of 50 amino acids (aa) with features similar to those of the E5 genes of both BPV type 1 (BPV-1) (37) and most HPVs (9). There are only a limited number of studies that have tested whether these small hydrophobic proteins can be immunological targets in patients with HPV infections (D. Gill, J. Cason, and N. Punchard, abstract from the 660th Meeting of the Joint Congress with the British Society for Immunology, Harrogate, United Kingdom, May 1997; Biochem. Soc. Trans. 25:S281, 1997) and in mouse models containing tumor lines stably expressing HPV-16 E5 proteins (33). In an earlier in vitro study, CRPV E8 showed no transforming activity in NIH 3T3 cells but affected E6 transforming activity (19). Our studies have shown that CRPV E8 had weak transforming activities when stably expressed in two rodent fibroblast cell lines (14). To determine the role of E8 in papilloma formation, we generated a CRPV genome containing a mutated E8 (ATG→ACG) and tested this mutant genome by infection of domestic rabbit skin. The results showed that this E8 mutant, or knockout, CRPV genome induced small, slow-growing, but persistent papillomas. We also tested whether the E8 gene could be an immunological target in wild-type CRPV-induced rabbit papillomas. Our results demonstrated that E8 vaccination eliminated virus-induced papillomas in inbred EIII/JC rabbits and provided partial protection against virus challenge in outbred rabbits. Immunity to E8 was also measured using the in vivo delayed-type hypersensitivity (DTH) test with three synthesized peptides which spanned the N- and C-terminal regions of the E8 protein. Several rabbits vaccinated with E8 showed positive DTH responses to these E8 peptides. These data demonstrated that the E8 protein of CRPV is expressed in papillomas and can be an immunological target leading to the regression of papillomas.
MATERIALS AND METHODS
Establishment of E8 mutant CRPV and DNA challenge in rabbits.
A CRPV genome previously described as Hershey CRPV (H-CRPV) and initially obtained from a Kansas cottontail rabbit (27) was cloned into pUC19 at the SalI site. A SacII restriction site at bp 169 to 174 (A171→G; C174→ G; E6 aa 6 and 7) was engineered into this genome by site-directed mutagenesis (Stratagene, La Jolla, Calif.), following the manufacturer's instructions. We next subcloned the CRPV E6/E8 region from SacII to EcoRI (bp 169 to 1064) into a modified pUC19 vector from which we had deleted the vector EcoRI site. This construct was subjected to site-directed mutagenesis to alter the E8 ATG start codon to ACG, which did not alter the corresponding E6 amino acid at this position. The mutated E6/E8 fragment was isolated by standard molecular biology techniques and replaced into the H-CRPV backbone. The mutation was confirmed by DNA sequence analysis of the entire fragment (bp 169 to 1064) prior to and after reinsertion into the H-CRPV genome. Plasmids containing the CRPV E8 knockout genome and the wild-type (wt) CRPV genome (containing the SacII site) were purified using a Qiagen Maxiprep kit followed by cesium chloride density gradient centrifugation. The final concentration of the plasmids was adjusted to 200 μg/ml in 1× TE buffer. Before DNA challenge, the back skin of the rabbits was pretreated with a mixture of turpentine and acetone (50:50 [vol/vol]) a total of four times, once every other day, to make the skin hyperplastic (13, 28). Several sites on the back were then scarified with a scalpel blade and inoculated with plasmid containing the CRPV genome at a concentration of 10 μg per site.
E8 gene expression vector and gene gun microparticle preparation.
The CRPV E8 gene was amplified by PCR and cloned into the V1Jns expression vector (a generous gift of M. A. Liu, Merck & Co., West Point, Pa.) at the BglII site (17). This plasmid was identified as E8V1Jns. E8V1Jns and V1Jns (empty vector) plasmid DNAs were purified with the Qiagen Maxiprep kit and by cesium chloride density gradient ultracentrifugation and then precipitated onto 1.6-mm-diameter gold microparticles (Bio-Rad, Hercules, Calif.) at a ratio of 1 μg of DNA/0.5 mg of gold particles as described by the manufacturer.
Rabbit vaccination and challenge.
Outbred NZW rabbits were purchased from Covance Research Products Inc. (Denver, Pa.). EIII/JC inbred rabbits were maintained in the animal facility of the Pennsylvania State University College of Medicine. These rabbits were obtained from an inbred audiogenic line of rabbits originally developed at the National Institutes of Health and do not reject skin grafts from inbred cagemates (data not shown). All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University. Inner ear skin sites were shaved and swabbed with 70% ethanol, and then the sites were bombarded with DNA-gold particles by a gene gun at 400 lb/in2. The animals were immunized with E8V1Jns or V1Jns vector alone a total of three times at 3-week intervals. Each plasmid DNA was applied at a total dose of 20 μg per rabbit for each immunization. Two weeks after the final booster immunization, the rabbits were challenged with infectious CRPV (H-CRPV). All rabbits were challenged at four scarified dorsal sites with 100 μl of virus suspension diluted 1:100 in phosphate-buffered saline (PBS). Papilloma measurements began 3 weeks after virus challenge and continued weekly for 12 weeks.
Peptide synthesis.
Three peptides encoded by the CRPV E8 gene and one peptide encoded by the rabbit oral papillomavirus E5 gene were synthesized and purified by Genemed Co. (South San Francisco, Calif.). Peptide 1 (MGPAETALYC; aa 1 to 10) and peptide 2 (RKYLAGSCVVQFAEEDC; aa 35 to 50) were dissolved in conjugation solution (0.83 M sodium phosphate, 0.9 M NaCl, 0.1 M EDTA, pH 7.2) to make stock solutions (20 mg/ml) and then diluted in sterile PBS prior to injection. The control for peptides 1 and 2 was a peptide (SMGVLECTLGVWEC) encoded by the rabbit oral papillomavirus E5 gene (6); peptide 3 (YCLVLWIFIVTLLLL; aa 9 to 23) was dissolved in dimethyl sulfoxide to make a stock solution (3.6 mg/ml) and then diluted in sterile PBS prior to injection. The control for peptide 3 consisted of dimethyl sulfoxide dissolved in PBS.
Skin test.
The skin test procedure consisted of the intracutaneous injection of 0.03 ml of test solution (10 μg of peptide) into the outer ear skin (22). Pilot studies were conducted to optimize the concentrations and volumes of antigen preparations. Peptide 1, peptide 2, and their control peptide were injected at different sites on one ear, and peptide 3 and its control solution were injected into different sites on the second ear. Elizabethan collars were fitted to all rabbits after ear injections to prevent nonspecific inflammation caused by scratching (22). In order to avoid antigen interference, the test was conducted several weeks after the virus challenge. Ear swelling was monitored over 5 days and measured to the nearest 0.01 mm with a constant-tension thickness gauge. In addition, the intensity of erythema was documented.
Papilloma size determination and statistical analysis.
Papilloma size was determined by calculating the cubic root of the product of length × width × height of individual papillomas in millimeters to obtain a geometric mean diameter (GMD). Data were represented as the means (± standard errors of the mean [SEM]) of the GMDs for each test group. Statistical significance was determined by unpaired t test comparison.
RESULTS
E8 mutant CRPV genomes induce small, slow-growing, but persistent papillomas in NZW rabbits.
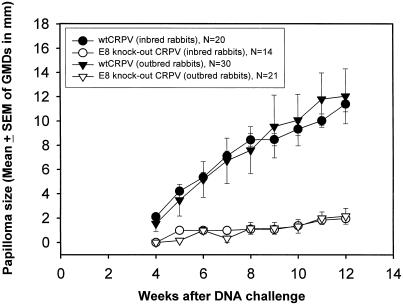
Previous studies in our laboratory demonstrated that CRPV-E8 has weak oncogenic activity, analogous to those of several HPV E5 genes (14). To explore the role of E8 in the CRPV virus life cycle, an E8 ATG mutation was engineered into the CRPV genome and tested for infectivity. Five of both outbred and EIII/JC inbred rabbits were challenged with the wt CRPV DNA containing the introduced SacII site, and another five rabbits were challenged with DNA from the CRPV-E8 knockout genome at six dorsal sites. Three weeks after challenge, papilloma sizes were monitored and GMDs were calculated. Papillomas that developed from the wt CRPV DNA were significantly larger than those established from the DNA of the CRPV-E8 knockout genome (P < 0.01) (Fig. 1). Papillomas that developed from the wt CRPV DNA containing the introduced SacII site grew at rates comparable to those from wt H-CRPV DNA (data not shown). We conclude that the CRPV E8 gene plays a role in papilloma growth but is not essential for the formation of papillomas.
FIG. 1.
Mean (± SEM) of papilloma size of E8 knockout and wild-type CRPV DNA (10 μg of DNA per site) on NZW and EIII/JC outbred and inbred rabbits. Groups of five inbred and outbred rabbits were challenged with E8 knockout CRPV, while a second set of five rabbits was challenged with wt CRPV which included the introduced SacII site (see Materials and Methods). Mean papilloma sizes were significantly different (P < 0.001; t test) when E8 knockout CRPV- and wt CRPV-inoculated sites were compared after week 5. N, number of papillomas for each group.
E8 immunization promotes papilloma regression in EIII/JC inbred rabbits.
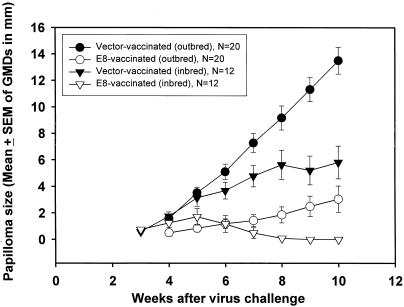
The results of the above-mentioned studies indicated that CRPV E8 plays a functional role in the CRPV life cycle. We next wished to determine whether the E8 protein could represent an immunological target within developing papillomas established from wt CRPV virion and viral DNA infections. To examine the protective effect of E8 immunization, six EIII/JC inbred rabbits were separated into two groups of three rabbits each and vaccinated with E8V1Jns or V1Jns vector, respectively. Two weeks after the final immunizations, the rabbits were challenged with H-CRPV virus at four scarified dorsal sites per animal. Three weeks later, papillomas appeared in both groups. However, all rabbits in the control group developed papillomas at all four challenge sites, and these papillomas persisted throughout the course of the experiment. Of the three E8-vaccinated rabbits, one had two papillomas, the second had three papillomas, and the third had four papillomas. Between weeks 4 and 8, all papillomas on the E8-vaccinated rabbits regressed (Fig. 2 and Table 1). Therefore, E8 vaccination can provide partial protection against virus challenge and trigger regression of papillomas in inbred rabbits.
FIG. 2.
Mean (± SEM) of papilloma size on outbred and inbred rabbits following genetic vaccinations with vector alone or E8 expression vector. All rabbits were challenged with CRPV (10−2 dilution of stock virus) at four sites on the back of each rabbit. Mean papilloma sizes were significantly different (P < 0.05; t test) when vector and E8 vaccinations for outbred and inbred rabbits were compared. N, number of papillomas for each group.
TABLE 1.
Papilloma formation on H-CRPV E8-vaccinated inbred EIII/JC and outbred NZW rabbits
| Rabbit type | Rabbit no. | Vaccine | Papilloma frequencya | Regres- sionb | Regression rate (%) |
|---|---|---|---|---|---|
| Inbred EIII/JCc | R1 | VIJns | 4/4 | 0/4 | 0 |
| R2 | VIJns | 4/4 | 0/4 | 0 | |
| R3 | VIJns | 4/4 | 0/4 | 0 | |
| Total | 12/12 | 0/12 | 0 | ||
| R4 | E8 VIJns | 2/4 | 2/2 | 100 | |
| R5 | E8 VIJns | 3/4 | 3/3 | 100 | |
| R6 | E8 VIJns | 4/4 | 4/4 | 100 | |
| Total | 9/12 | 9/9 | 100 | ||
| Outbred NZWc | R1 | VIJns | 4/4 | 0/4 | 0 |
| R2 | VIJns | 4/4 | 0/4 | 0 | |
| R3 | VIJns | 4/4 | 0/4 | 0 | |
| R4 | VIJns | 4/4 | 0/4 | 0 | |
| R5 | VIJns | 4/4 | 0/4 | 0 | |
| Total | 20/20 | 0/20 | 0 | ||
| R6 | E8 VIJns | 0/4 | |||
| R7 | E8 VIJns | 1/4 | 0/1 | 0 | |
| R8 | E8 VIJns | 3/4 | 1/3 | 33 | |
| R9 | E8 VIJns | 4/4 | 0/4 | 0 | |
| R10 | E8 VIJns | 3/4 | 1/3 | 33 | |
| Total | 11/20 | 2/11 | 18 |
Number of papillomas/number of challenge sites.
Number of regressed papillomas/number of sites with papillomas.
Mean papilloma sizes are shown in Fig. 2.
E8 immunization partially protects against CRPV virus challenge in outbred NZW rabbits.
Outbred rabbits were also immunized with CRPV E8 to determine whether protection could be achieved in outbred populations. One group of five outbred NZW rabbits was intracutaneously immunized with the CRPV E8 gene. A second group of five rabbits received vector alone as a control vaccine. The five control rabbits grew papillomas at all challenge sites, and these papillomas became large and persisted for the duration of the experiment. In contrast, E8-vaccinated rabbits showed strong protective immunity to CRPV infection (Fig. 2). One of the E8-vaccinated rabbits was completely protected against papilloma formation; three rabbits had either one or three tiny papillomas, of which two on two different rabbits regressed; and the remaining rabbit showed persistent papilloma growth (Fig. 2 and Table 1). The total sites protected against CRPV infection by E8 immunization in these rabbits were about 55% (9 of 20 challenge sites did not have papillomas [Table 1]). In addition, papilloma growth on vaccinated rabbits was significantly suppressed for all time points after week 5 compared to growth rates on control-vaccinated rabbits (P < 0.01; t test) (Fig. 2). These observations indicated that strong anti-papillomavirus immunity was induced in the E8-vaccinated rabbits but that it was insufficient to completely protect against infection and/or to eradicate developing papillomas on all outbred NZW rabbits.
E8 vaccination does not protect against E8 mutant (knockout) CRPV genomes.
We tested E8-vaccinated rabbits for protection against infection with E8 knockout CRPV DNA as an additional strategy to support our conclusion that specific immunity to E8 is responsible for the strong protections seen in the previous two experiments. One group of four inbred rabbits was intracutaneously immunized with the CRPV E8 gene. A second group of five rabbits received vector alone as a control vaccine. One week after the final booster immunizations, the back skin of the rabbits was challenged with E8 knockout CRPV DNA at six scarified dorsal sites for the E8-vaccinated rabbits and at four sites for rabbits immunized with vector alone. Small papillomas grew at approximately 70% of the challenge sites in both groups, and none of these papillomas regressed during the course of the experiment (Table 2). In addition, there was no significant difference between the mean papilloma sizes in these two groups (P > 0.05; unpaired t test) (data not shown). The results of these studies showed that E8-vaccinated rabbits were susceptible to infection by the E8 knockout CRPV DNA with no regressions of the lesions. We have demonstrated that rabbits vaccinated with E8 also generate strong responses to CRPV infections initiated with wt CRPV DNA (data not shown).
TABLE 2.
Papilloma formation on E8VIJns- and VIJns-vaccinated outbred NZW rabbits inoculated with E8 knockout CRPV DNA
| Rabbit no. | Vaccine | Papilloma frequencya | Regressionb | Papilloma appear- ance rate (%) |
|---|---|---|---|---|
| B0246 | E8 VIJns | 6/6 | 0/6 | 100 |
| B0247 | E8 VIJns | 2/6 | 0/2 | 33.3 |
| B0254 | E8 VIJns | 5/6 | 0/5 | 83.3 |
| B0255 | E8 VIJns | 4/6 | 0/4 | 66.7 |
| Total | 17/24 | 0/17 | 70.8 | |
| A0503 | VIJns | 2/4 | 0/2 | 50 |
| A0505 | VIJns | 2/4 | 0/2 | 50 |
| A0507 | VIJns | 4/4 | 0/4 | 100 |
| A0509 | VIJns | 4/4 | 0/4 | 100 |
| A0510 | VIJns | 2/4 | 0/2 | 50 |
| Total | 14/20 | 0/14 | 70 |
Number of papilloma-forming sites/number of challenge sites.
Number of regression sites/number of papilloma-forming sites.
DTH response.
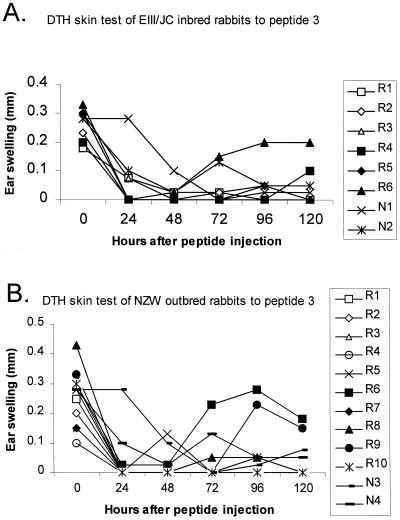
A second in vivo E8-specific cellular immune response was measured in rabbits by the DTH test. Three different peptides that covered the N- and C-terminal portions of CRPV E8 protein were synthesized and tested for DTH reactions in the ear skin as described in Materials and Methods. Inbred and outbred rabbits were tested at 9 and 3 months after virus challenge, respectively. Peptides 1 and 2 and their control peptide were injected intracutaneously into different sites of one ear, while peptide 3 and its control solution were injected into the second ear. Ear swellings were checked at 24-h intervals for a period of 120 h after peptide injection (Fig. 3).
FIG. 3.
DTH skin test reactions to peptide 3 (YCLVLWIFIVTLLLL) on various EIII/JC inbred rabbits (A) and NZW outbred rabbits (B). (A) One of three E8-immunized EIII/JC inbred rabbits (R4 to R6) showed positive skin swelling 96 and 120 h after peptide injection; no swelling was observed on vector-immunized rabbits (R1 to R3) or naïve rabbits (N1 and N2). (B) Two of five E8-immunized NZW outbred rabbits (R6 to R10) showed positive skin swelling 96 h after peptide injection; none of the vector-immunized (R1 to R5) or naïve (N3 and N4) rabbits showed swelling at this time point.
Among the E8-vaccinated inbred rabbits, one rabbit showed positive skin test reactions to all three E8 peptides. A second vaccinated rabbit had positive reactions to peptides 1 and 2, while the remaining rabbit was reactive to peptide 1. In contrast, none of the rabbits in the control group showed reactivity to any of these E8 peptides (Table 3).
TABLE 3.
Summary of DTH skin test results at 120 h after intracutaneous peptide injection into EIII/JC inbred rabbits
| Rabbit no.a | Vaccine | Resultb
|
||
|---|---|---|---|---|
| Peptide 1c | Peptide 2d | Peptide 3e | ||
| R1 | VIJns | − | − | − |
| R2 | VIJns | − | − | − |
| R3 | VIJns | − | − | − |
| R4 | E8 VIJns | − | − | + |
| R5 | E8 VIJns | + | + | − |
| R6 | E8 VIJns | + | + | + |
Rabbit numbers represent animals shown in Fig. 3.
−, negative; +, positive.
Peptide 1, MGPAETALYC; aa 1 to 10.
Peptide 2, RKYLAGSCVVQFAEEDC; aa 35 to 50.
Peptide 3, YCLVLWIFIVTLLLL; aa 9 to 23.
Among the E8-vaccinated outbred rabbits, one rabbit that was completely protected against CRPV infection showed positive DTH reactions to all three peptides. Erythema was seen in another of the E8-vaccinated rabbits, although no obvious swelling was observed. The rabbit that failed to show protection against papilloma growth also showed a positive skin test reaction to peptide 3 (Table 4). The remaining two rabbits in the E8 vaccination group had no DTH responses to the E8 peptides. These observations showed that strong skin test reactions were correlated with complete eradication of papillomas.
TABLE 4.
Summary ofDTH skin test results 96 h after intracutaneous peptide injection in NZW outbred rabbits
| Rabbit no.a | Vaccine | Resultsb
|
||
|---|---|---|---|---|
| Peptide 1c | Peptide 2d | Peptide 3e | ||
| R1 | VIJns | − | − | − |
| R2 | VIJns | − | − | − |
| R3 | VIJns | − | − | − |
| R4 | VIJns | − | − | − |
| R5 | VIJns | − | − | − |
| R6 | E8 VIJns | + | + | + |
| R7 | E8 VIJns | − | − | − |
| R8 | E8 VIJns | − | + | − |
| R9 | E8 VIJns | − | − | + |
| R10 | E8 VIJns | − | − | − |
Rabbit numbers represent animals shown in Fig. 3.
−, negative; +, positive.
Peptide 1, MGPAETALYC; aa 1 to 10.
Peptide 2, RKYLAGSCVVQFAEEDC; aa 35 to 50.
Peptide 3, YCLVLWIFIVTLLLL; aa 9 to 23.
DISCUSSION
The CRPV E8 protein is a small hydrophobic polypeptide of only 50 aa which has homology with BPV and HPV E5 gene products. The last two gene products bind to various cellular receptors, including platelet-derived growth factor beta receptor, resulting in constitutive activation of the receptor and cell growth transformation (8, 9, 25), and epidermal growth factor receptor (48). Little is known about the function of CRPV E8 protein in developing rabbit papillomas, and the protein has not been detected immunohistologically within lesions. Attempts to stably express E8 in mouse and rabbit cell lines led to levels of protein undetectable by conventional detection methods (14). In a previous study, CRPV-E8 failed to transform NIH 3T3 cells (19). Our studies demonstrated that E8 was a weak oncogene that could transform two rodent cell lines in the presence of platelet-derived growth factor (14). In the present study, we demonstrated that a CRPV genome containing an inactivated E8 gene (by an E8 ATG mutation) induced small, slow-growing, but predominantly persistent papillomas on rabbits. We have generated several hundred papillomas on more than 50 outbred NZW rabbits with this construct and have consistently seen a significant growth reduction of the papillomas compared to those from wt CRPV (Fig. 1 and data not shown). These initial studies demonstrated that CRPV E8 has functional activity in the CRPV life cycle. E8 appears to contribute to the growth rate of CRPV-induced papillomas, and this is consistent with a possible role of E8 as a stimulator of cell growth through receptor engagement.
Alternative and additional effects of the ATG-to-ACG mutation in the E8 gene need to be considered. For example, this position may include an unknown regulatory site that is modified by the T→C mutation and contributes to the reduction in the growth rate of the mutant CRPV genome. Secondly, E8 proteins may be produced from a spliced mRNA that initiates from an exon that is upstream in the upstream regulatory region and subsequently splices into the E8 gene downstream of the E8 ATG. However, if a spliced mRNA is the predominant mechanism for production of E8 in papillomas, then we would expect that the E8 ATG-to-ACG mutation would have a minimal impact on the CRPV genome and that papilloma growth rates would be similar to those initiated with the wt CRPV DNA. Finally, E8 may have an impact on other viral proteins in situ. For example, a previous study showed that in vitro E6 transforming activity was enhanced following E8 ATG mutation (19).
Based on the homology between CRPV E8 and papillomavirus E5 genes, and the detection of papillomavirus E5 proteins in the basal cells of papillomas (2, 4, 5, 9, 25), we hypothesized that CRPV E8 (and, by inference, HPV E5) could be an important immunological target for cell-mediated immunity against CRPV infections. Previous studies using CRPV E1, E2, E6, and E7 vaccinations indicated that gene gun delivery of expression plasmids via the intracutaneous route was the most effective vaccination strategy (17, 18). We therefore used gene gun delivery of CRPV E8 expression vectors in both inbred and outbred NZW rabbits to test whether the small hydrophobic E8 protein could also be an effective immunogen. CRPV E8 vaccination of inbred rabbits led to incomplete protection but subsequent regression of developing papillomas. Outbred rabbits showed partial protective immunity and induced regression of papillomas following CRPV E8 vaccinations. Vaccinations with the control vector led to persistently growing papillomas and no protection. These data indicated that vaccination with the E8 gene induced strong immune responses to CRPV infections and that the E8 protein is present within the developing papillomas.
Strong but incomplete protection against CRPV infection occurred in outbred NZW rabbits vaccinated with CRPV E8. Four of five E8-vaccinated outbred rabbits had at least partial protection against virus challenge, but the remaining rabbit showed no protection and developed persistent papillomas that were as large as those in the control group. Failure to protect all rabbits following immunizations with CRPV E1, E2, and E6 was also observed in other studies (18, 24, 39, 43). Lack of protective immunity in some rabbits may be a consequence of certain major histocompatibility complex genes in these rabbits failing to present strong T-cell epitopes from the viral proteins. This may be especially true for CRPV E8, which is a small protein of only 50 aa and thus has a reduced number of potential T-cell epitopes. In support of this hypothesis is the observation that all inbred rabbits showed protection and/or subsequent regression of papillomas whereas some of the outbred rabbits had partial protection with persistent papillomas. Further support for genetic polymorphism of the major histocompatibility complex contributing to failed immune responses to viral antigens has been described for some inbred strains of mice which fail to present any cytotoxic-T-lymphocyte (CTL) epitopes of HPV-16 E7 proteins (11).
In previous studies, we and others observed a poor correlation between in vitro peripheral blood mononuclear cell proliferative responses to antigen stimulation and protective immunity against viral infection in vivo (15-18, 39). In this study, we introduced DTH skin tests as an additional method to assess host immunity after E8 vaccination. DTH skin tests have been used to examine T-cell-mediated immunity to HPV antigens in human and mouse models (3, 20, 21). Höpfl and coworkers also used skin tests to assess viral immunity to CRPV virions in rabbits with CRPV infections (22). These data indicated that positive DTH responses to viral antigens correlated with strong immune responses and papilloma regression. Our results showed that several vaccinated rabbits had positive responses to the E8 test peptides, whereas there were no DTH responses in the control vaccinated rabbits. These responses, however, were relatively weak and reached a maximum 72 to 96 h after peptide injection. Studies of patients also demonstrated that DTH responses to HPV-16 E7 peptides arose within 2 to 6 days, reaching a maximum in 1 week (20). The slow response to the CRPV E8 peptides may have been due to the delay between immunizations and peptide challenge (3 and 9 months for outbred and inbred vaccinated rabbits, respectively). In addition, we tested only three peptides spanning the N- and C-terminal regions of E8, and these peptides may not encompass all epitopes present in E8. More recent DTH studies of E8-vaccinated rabbits included a fourth peptide (aa 24 to 36), and there were no responses to this peptide (data not shown).
We further tested the role of anti-E8 immunity in rabbits by challenging E8-vaccinated rabbits with the CRPV E8 knockout genome. These experiments were designed to assess the direct role of anti-E8 immunity in protection and regression of wt CRPV infections. An additional possibility is that effector cells with E8 reactivity help to locally recruit and nonspecifically activate effector cells specific to other (i.e., non-E8) viral proteins to enhance cell-mediated immune regression of the wt CRPV-induced papillomas. The results of these experiments indicated that E8-vaccinated rabbits grew persistent papillomas when challenged with DNA from the E8 knockout CRPV genome and supported earlier studies suggesting that specific anti-E8 responses can induce strong immunity to wt CRPV infections and that the E8 gene product is expressed in wt CRPV papillomas. We have additional unpublished studies showing that E8-vaccinated rabbits also show strong immunity to CRPV infections initiated with wt CRPV DNA (data not shown).
The immunological events of E8 vaccination leading to protection and/or regression of wt CRPV infections await elucidation. Potential mechanisms include activation of E8-specific CTL responses that can directly lyse CRPV-infected papilloma cells. In addition, there may be E8-specific TH cell responses which may subsequently recruit to the developing papillomas CTLs that recognize epitopes on other CRPV viral proteins, such as E1, E2, or E6, as discussed above. These studies with CRPV E8 vaccinations and CRPV E8 knockout genomes represent useful models to measure host immune responses to individual viral proteins within the context of a natural papillomavirus infection that expresses endogenous levels of all the viral proteins. The related 74-aa HPV-16 E5 protein can induce E5-specific CTLs in a mouse tumor model (33), further supporting a role for these small hydrophobic proteins as potential immune targets in HPV infections.
Acknowledgments
This study was supported by Public Health Service grant RO1 CA47622 from the National Cancer Institute, National Institutes of Health, and the Jake Gittlen Memorial Golf Tournament.
REFERENCES
- 1.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnett, S., N. Jareborg, and D. DiMaio. 1992. Localization of bovine papillomavirus type 1 E5 protein to transformed basal keratinocytes and permissive differentiated cells in fibropapilloma tissue. Proc. Natl. Acad. Sci. USA 89:5665-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers, M. A., S. N. Stacey, J. R. Arrand, and M. A. Stanley. 1994. Delayed-type hypersensitivity response to human papillomavirus type 16 E6 protein in a mouse model. J. Gen. Virol. 75:165-169. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S.-L., and P. Mounts. 1989. Detection by antibody probes of human papillomavirus type 6 E5 proteins in respiratory papillomata. J. Med. Virol. 29:273-283. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S. L., T. B. Hsieh, Y. P. Tsao, C. P. Han, and Y. F. Yang. 1996. Coincidental expression of E5a and c-jun in human papillomavirus type 6/11-infected condylomata. J. Gen. Virol. 77:1145-1149. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, N. D., N. M. Cladel, C. A. Reed, and R. Han. 2000. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection. Virology 269:451-461. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, N. D., C. A. Reed, N. M. Cladel, R. Han, and J. W. Kreider. 1996. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 70:960-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad, M., D. Goldstein, T. Andresson, and R. Schlegel. 1994. The E5 protein of HPV-6, but not HPV-16, associates efficiently with cellular growth factor receptors. Virology 200:796-803. [DOI] [PubMed] [Google Scholar]
- 9.DiMaio, D., and D. Mattoon. 2001. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene 20:7866-7873. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly, J. J., D. Martinez, K. U. Jansen, R. W. Ellis, D. L. Montgomery, and M. A. Liu. 1996. Protection against papillomavirus with a polynucleotide vaccine. J. Infect. Dis. 173:314.. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, L. A., M. Evander, R. W. Tindle, A. L. Bulloch, R. L. De Kluyver, G. J. P. Fernando, P. F. Lambert, and I. H. Frazer. 1997. Presentation of the HPV16E7 protein by skin grafts is insufficient to allow graft rejection in an E7-primed animal. Virology 235:94-103. [DOI] [PubMed] [Google Scholar]
- 12.Frazer, I. H. 1996. Immunology of papillomavirus infection. Curr. Opin. Immunol. 8:484-491. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald, W. F. 1942. Cell state as affecting susceptibility to a virus; enhanced effectiveness of the rabbit papilloma virus on hyperplastic epidermis. J. Exp. Med. 75:197-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, R., N. M. Cladel, C. A. Reed, and N. D. Christensen. 1998. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology 251:253-263. [DOI] [PubMed] [Google Scholar]
- 15.Han, R., N. M. Cladel, C. A. Reed, X. Peng, L. R. Budgeon, M. D. Pickel, and N. D. Christensen. 2000. DNA vaccination prevents and/or delays carcinoma development of papillomavirus-induced skin papillomas on rabbits. J. Virol. 74:9712-9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han, R., N. M. Cladel, C. A. Reed, X. Peng, and N. D. Christensen. 1999. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J. Virol. 73:7039-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han, R., C. A. Reed, N. M. Cladel, and N. D. Christensen. 1999. Intramuscular injection of plasmid DNA encoding cottontail rabbit papillomavirus E1, E2, E6 and E7 induces T cell-mediated but not humoral immune responses in rabbits. Vaccine 17:1558-1566. [DOI] [PubMed] [Google Scholar]
- 18.Han, R., C. A. Reed, N. M. Cladel, and N. D. Christensen. 2000. Immunization of rabbits with cottontail rabbit papillomavirus E1 and E2 genes: protective immunity induced by gene gun-mediated intracutaneous delivery but not by intramuscular injection. Vaccine 18:2937-2944. [DOI] [PubMed] [Google Scholar]
- 19.Harry, J. B., and F. O. Wettstein. 1996. Transforming properties of the cottontail rabbit papillomavirus oncoproteins LE6 and SE6 and of the E8 protein. J. Virol. 70:3355-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopfl, R., K. Heim, N. Christensen, K. Zumbach, U. Wieland, B. Volgger, A. Widschwendter, S. Haimbuchner, E. Muller-Holzner, M. Pawlita, H. Pfister, and P. Fritsch. 2000. Spontaneous regression of CIN and delayed-type hypersensitivity to HPV-16 oncoprotein E7. Lancet 356:1985-1986. [DOI] [PubMed] [Google Scholar]
- 21.Hopfl, R., M. Sandbichler, N. Sepp, K. Heim, E. Muller-Holzner, B. Wartusch, O. Dapunt, I. Jochmus-Kudielka, J. Ter Meulen, L. Gissmann, and P. Fritsch. 1991. Skin test for HPV type 16 proteins in cervical intraepithelial neoplasia. Lancet 337:373-374. [DOI] [PubMed] [Google Scholar]
- 22.Höpfl, R. M., N. D. Christensen, M. G. Angell, and J. W. Kreider. 1993. Skin test to assess immunity against cottontail rabbit papillomavirus antigens in rabbits with progressing papillomas or after papilloma regression. J. Investig. Dermatol. 101:227-231. [DOI] [PubMed] [Google Scholar]
- 23.Jansen, K. U., M. Rosolowsky, L. D. Schultz, H. Z. Markus, J. C. Cook, J. J. Donnelly, D. Martinez, R. W. Ellis, and A. R. Shaw. 1995. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine 13:1509-1512. [DOI] [PubMed] [Google Scholar]
- 24.Jensen, E. R., R. Selvakumar, H. Shen, R. Ahmed, F. O. Wettstein, and J. F. Miller. 1997. Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA. J. Virol. 71:8467-8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kell, B., R. J. Jewers, J. Cason, F. Pakarian, J. N. Kaye, and J. M. Best. 1994. Detection of E5 oncoprotein in human papillomavirus type 16-positive cervical scrapes using antibodies raised to synthetic peptides. J. Gen. Virol. 75:2451-2456. [DOI] [PubMed] [Google Scholar]
- 26.Kirnbauer, R., L. M. Chandrachud, B. W. O'Neil, E. R. Wagner, G. J. Grindlay, A. Armstrong, G. M. McGarvie, J. T. Schiller, D. R. Lowy, and M. S. Campo. 1996. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 219:37-44. [DOI] [PubMed] [Google Scholar]
- 27.Kreider, J. W., and G. L. Bartlett. 1981. The Shope papilloma-carcinoma complex of rabbits: a model system of neoplastic progression and spontaneous regression. Adv. Cancer Res. 35:81-110. [DOI] [PubMed] [Google Scholar]
- 28.Kreider, J. W., N. M. Cladel, S. D. Patrick, P. A. Welsh, S. L. DiAngelo, J. M. Bower, and N. D. Christensen. 1995. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. J. Virol. Methods 55:233-244. [DOI] [PubMed] [Google Scholar]
- 29.Lathe, R., M. P. Kieny, K. Dott, C. Gautier, P. Clertant, F. Cuzin, F. Breitburd, G. Orth, and G. Meneguzzi. 1989. Vaccination against polyoma- and papillomavirus-induced tumors using vaccinia recombinants expressing non-structural proteins, p. 166-176. In A. Mehens and R. E. Spier (ed.), Vaccines for sexually transmitted diseases. Butterworths, London, United Kingdom.
- 30.Leachman, S. A., R. E. Tigelaar, M. Shlyankevich, M. D. Slade, M. Irwin, E. Chang, T. C. Wu, W. Xiao, S. Pazhani, D. Zelterman, and J. L. Brandsma. 2000. Granulocyte-macrophage colony-stimulating factor priming plus papillomavirus E6 DNA vaccination: effects on papilloma formation and regression in the cottontail rabbit papillomavirus-rabbit model. J. Virol. 74:8700-8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, Y.-L., L. A. Borenstein, R. Ahmed, and F. O. Wettstein. 1993. Cottontail rabbit papillomavirus L1 protein-based vaccines: protection is achieved only with a full-length, nondenatured product. J. Virol. 67:4154-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, Y. L., L. A. Borenstein, R. Selvakumar, R. Ahmed, and F. O. Wettstein. 1992. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 187:612-619. [DOI] [PubMed] [Google Scholar]
- 33.Liu, D. W., Y. P. Tsao, C. H. Hsieh, J. T. Hsieh, J. T. Kung, C. L. Chiang, S. J. Huang, and S. L. Chen. 2000. Induction of CD8 T cells by vaccination with recombinant adenovirus expressing human papillomavirus type 16 E5 gene reduces tumor growth. J. Virol. 74:9083-9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonnell, W. M., and F. K. Askari. 1996. DNA vaccines. N. Engl. J. Med. 334:42-45. [DOI] [PubMed] [Google Scholar]
- 35.Nicholls, P. K., and M. A. Stanley. 2000. The immunology of animal papillomaviruses. Vet. Immunol. Immunopathol. 73:101-127. [DOI] [PubMed] [Google Scholar]
- 36.Pardoll, D. M. 1998. Cancer vaccines. Nat. Med. 4:525-531. [DOI] [PubMed] [Google Scholar]
- 37.Schapiro, F., J. Sparkowski, A. Adduci, F. Suprynowicz, R. Schlegel, and S. Grinstein. 2000. Golgi alkalinization by the papillomavirus E5 oncoprotein. J. Cell Biol. 148:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvakumar, R., R. Ahmed, and F. O. Wettstein. 1995. Tumor regression is associated with a specific immune response to the E2 protein of cottontail rabbit papillomavirus. Virology 208:298-302. [DOI] [PubMed] [Google Scholar]
- 39.Selvakumar, R., L. A. Borenstein, Y. L. Lin, R. Ahmed, and F. O. Wettstein. 1995. Immunization with nonstructural proteins E1 and E2 of cottontail rabbit papillomavirus stimulates regression of virus-induced papillomas. J. Virol. 69:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selvakumar, R., A. Schmitt, T. Iftner, R. Ahmed, and F. O. Wettstein. 1997. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and persistence of viral DNA after regression. J. Virol. 71:5540-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanley, M. A., R. A. Moore, P. K. Nicholls, E. B. Santos, L. Thomsen, N. Parry, S. Walcott, and G. Gough. 2001. Intra-epithelial vaccination with COPV L1 DNA by particle-mediated DNA delivery protects against mucosal challenge with infectious COPV in beagle dogs. Vaccine 19:2783-2792. [DOI] [PubMed] [Google Scholar]
- 42.Sundaram, P., R. E. Tigelaar, and J. L. Brandsma. 1997. Intracutaneous vaccination of rabbits with the cottontail rabbit papillomavirus (CRPV) L1 gene protects against virus challenge. Vaccine 15:664-671. [DOI] [PubMed] [Google Scholar]
- 43.Sundaram, P., R. E. Tigelaar, W. Xiao, and J. L. Brandsma. 1998. Intracutaneous vaccination of rabbits with the E6 gene of cottontail rabbit papillomavirus provides partial protection against virus challenge. Vaccine 16:613-623. [DOI] [PubMed] [Google Scholar]
- 44.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 46.Ulmer, J. B., J. C. Sadoff, and M. A. Liu. 1996. DNA vaccines. Curr. Opin. Immunol. 8:531-536. [DOI] [PubMed] [Google Scholar]
- 47.Xiao, W., and J. L. Brandsma. 1996. High efficiency, long-term clinical expression of cottontail rabbit papillomavirus (CRPV) DNA in rabbit skin following particle-mediated DNA transfer. Nucleic Acids Res. 24:2620-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, B., D. F. Spandau, and A. Roman. 2002. E5 protein of human papillomavirus type 16 protects human foreskin keratinocytes from UV B-irradiation-induced apoptosis. J. Virol. 76:220-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.zur Hausen, H. 2000. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 92:690-698. [DOI] [PubMed] [Google Scholar]