Abstract
The extraordinary sensitivity of CD8+ T cells to recognize antigen impinges to a large extent on the coreceptor CD8. While several studies have shown that the CD8β chain endows CD8 with efficient coreceptor function, the molecular basis for this is enigmatic. Here we report that cell-associated CD8αβ, but not CD8αα or soluble CD8αβ, substantially increases the avidity of T cell receptor (TCR)-ligand binding. To elucidate how the cytoplasmic and transmembrane portions of CD8β endow CD8 with efficient coreceptor function, we examined T1.4 T cell hybridomas transfected with various CD8β constructs. T1.4 hybridomas recognize a photoreactive Plasmodium berghei circumsporozoite (PbCS) peptide derivative (PbCS (4-azidobezoic acid [ABA])) in the context of H-2Kd, and permit assessment of TCR-ligand binding by TCR photoaffinity labeling. We find that the cytoplasmic portion of CD8β, mainly due to its palmitoylation, mediates partitioning of CD8 in lipid rafts, where it efficiently associates with p56lck. In addition, the cytoplasmic portion of CD8β mediates constitutive association of CD8 with TCR/CD3. The resulting TCR-CD8 adducts exhibit high affinity for major histocompatibility complex (MHC)-peptide. Importantly, because CD8αβ partitions in rafts, its interaction with TCR/CD3 promotes raft association of TCR/CD3. Engagement of these TCR/CD3-CD8/lck adducts by multimeric MHC-peptide induces activation of p56lck in rafts, which in turn phosphorylates CD3 and initiates T cell activation.
Keywords: T lymphocytes, cytotoxicity, phosphorylation, protein-tyrosine kinase, plasmon resonance
Introduction
The coreceptor CD8 plays a key role in the activation of mature CD8+ T cells. In the absence of CD8, or upon blocking of CD8, activation of CD8+ T cells requires considerably higher and longer TCR engagement (1–4). While CD8 on peripheral T cell consists of a disulfide-linked α and β chains, intestinal T cells, γδ T cells, and NK cells express homodimeric CD8αα (1, 2). Heterodimeric CD8αβ is a much more potent coreceptor than homodimeric CD8αα, but there is considerable controversy on what domains of CD8β and by what mechanism CD8β endows CD8 with efficient coreceptor function (1–8).
Using Kd-restricted CD8+ T cells, which recognize a photoreactive derivative of the Plasmodium berghei circumsporozoite (PbCS)* peptide 252–260 (SYIPSAEKI) (PbCS (4-azidobezoic acid [ABA])) and allow detection of TCR-ligand interactions by TCR photoaffinity labeling, we have shown that on cells CD8αβ, but not CD8αα, substantially strengthens TCR-ligand binding (4, 9–11). Similar findings have been obtained by staining of CD8+ T cells with fluorescent labeled MHC-peptide multimers (12). Thus, the extraordinary sensitivity of CD8+ CTL to recognize target cells expressing very few MHC-peptide complexes or low affinity MHC-peptide variants is attributed to, at least in part, the ability of CD8αβ to enhance the avidity of TCR-ligand binding.
Binding studies with soluble recombinant CD8 have shown that CD8αα and CD8αβ bind class I molecules with similar affinities (13, 14). By contrast, cell-associated CD8αβ seems to bind MHC class I molecules more avidly than CD8αα (3–5). According to one study, soluble CD8αβ enhances the binding of soluble TCR to MHC-peptide (13). However, another study indicated that soluble CD8αα has no effect on TCR-ligand binding, i.e., that binding of soluble CD8 and TCR to MHC-peptide are two independent events (15). Here we report that the extracellular portion of CD8αβ in soluble form does not strengthen the interaction of the T1 TCR with Kd-PbCS (ABA), indicating that the CD8-mediated increase in T1 TCR-ligand binding observed on cells (4, 10) is attributed to the transmembrane and/or cytoplasmic portions of CD8β.
The tail of CD8α is 30 residues long and contains two vicinal cysteines, which interact with the Src kinase p56lck (lck) by means of a zinc chelate complex (1, 2). The tail of CD8β contains 19 residues and seems to play an important role for the positive selection and activation of CD8+ T cells (5, 6–8, 16). We have shown previously that the CD8β tail is palmitoylated at a membrane-proximal cysteine and mediates CD8 partitioning in lipid rafts (6). Rafts, also called detergent-insoluble membranes (DIMs), are ordered microdomains, enriched in sphingolipids and cholesterol, which include molecules containing short saturated fatty acids, such as GPI-linked proteins, or on the inner membrane leaflet palmitoylated molecules, like CD8, lck, p59fyn (fyn), and the linker for activation of T cells (LAT) (6, 17–22). Other molecules are excluded from rafts, namely phosphatases (e.g., CD45), which makes rafts privileged sites for the induction of TCR signaling. Raft localization of CD8 increases its association with lck, which is important, because cross-linking-mediated lck activation in rafts is a crucial initial event in T cell activation (6, 20). The CD8β tail has also been implicated in association of CD8 with LAT (5, 23).
In this study we prepared T1 T cell hybridomas expressing CD8α and various CD8β variants. We show that on cells, the CD8β cytoplasmic portion mediates association of CD8 with TCR/CD3. The resulting TCR/CD3 adducts with CD8/lck are raft associated, exhibit increased avidity for MHC-peptide, and upon cross-linking, induce phosphorylation of lck and CD3 and mobilization of intracellular calcium
Materials and Methods
Antibodies and Cell Culture.
The following antibodies were obtained from American Type Culture Collection: anti-CD8α mAb 53.6.72, anti-CD8β mAb H35–17, anti-CD8β mAb 53.5.81, anti-TCRCβ mAb H57, anti-TCRCα mAb H28, and anti-CD3ζ mAb HAM146. Anti-phospho-tyrosine (pY) mAb 4G10 and anti-lck mAb 3A5 were from Upstate Biologicals. Anti-LAT, anti-lck, and anti-CD3ε polyclonal antibodies were from Santa Cruz Biotechnology, Inc. Transfected Chinese hamster ovary (CHO) cells were maintained in Glasgow minimum essential medium (Life Technologies) supplemented with 25 μM methionine sulfoximine and 5% dialyzed FCS, as described (14). T cell hybridomas were cultured in DMEM (Life Technologies) supplemented with 5% FCS (Life Technologies), penicillin/streptomycin/neomycin (PSN; Life Technologies), and 50 μM β-mercaptoethanol.
Soluble CD8αβ, T1 TCR, and Biotinlyated Kd-PbCS(ABA).
Soluble T1 TCR and CD8 were prepared essentially as described (14). In brief, the cDNA encoding the extracellular portions of the T1 TCR α and β chains were fused to the sequences encoding the basic and acidic units of a leucine zipper, respectively. The constructs were cloned into the pEE14GS vector using the EcoRI site and stably transfected into CHO-K1 cells with yields of ∼8 mg/l. sT1 TCR was purified on H28-Sepharose, using acid elution (pH 3.1). The extracellular portions of murine CD8α (residues 1–156) and CD8β (residues 1–146) were fused to basic and acidic leucine zipper, cloned into pEE14GS, and stably expressed in CHO-K1 cells, with yields of ∼10 mg/l. sCD8 was purified on H35–17 Sepharose, using acid elution (pH 3.1). Correct folding of the purified sCD8, was confirmed by ELISA using five different anti-CD8 monoclonal antibodies (anti-CD8α mAb 53.6.72, 19/178 and anti-CD8β mAb 53.5.8, KT112, H35–17). Monomeric covalent Kd-125”iodo-4-azidosalicylic acid (IASA)”-YIPSAEK (ABA) I complexes (∼2,000 Ci/mMol) were prepared as described (6, 9, 20). Biotinylated Kd-SYIPSAEK(ABA)I was obtained by refolding of Kd heavy chain and human β 2-microglobulin from Escherichia coli as described (20). The monomers were biotinylated, purified, and oligomerized by reaction with streptavidin (Molecular Probes) as described (6, 20).
CD8 Transfections of T1.4 Hybridomas.
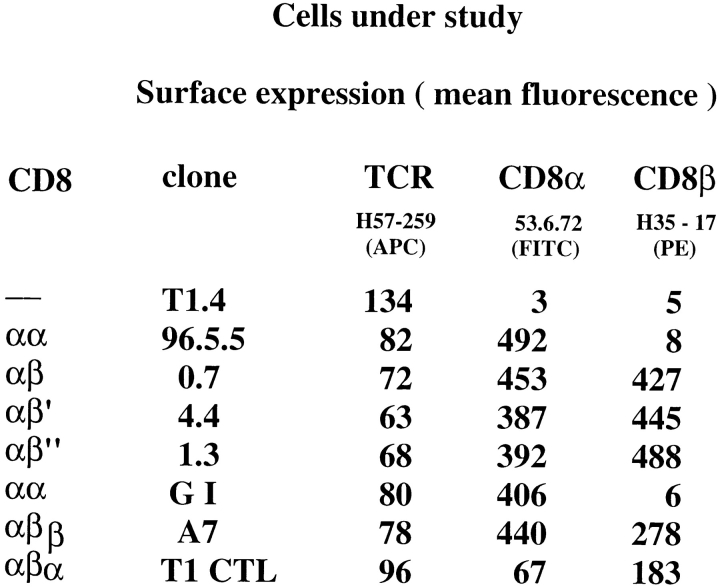
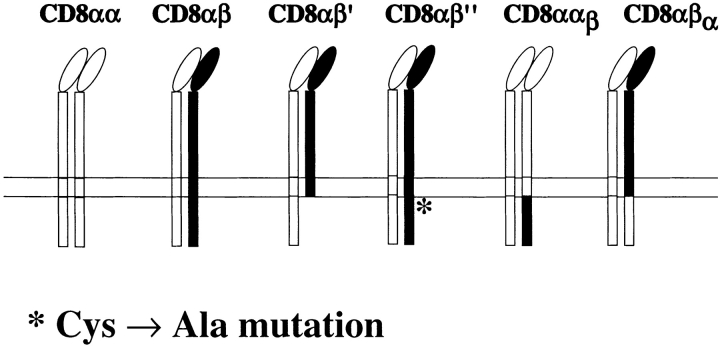
T1.4 T cell hybridomas and their CD8α transfectants have been described previously (6, 10). The CD8β cDNA, cloned in the pMI vector, which contains human tailless CD2 (24), was mutated at position 178 (Tyr → Stop) or 179 (Cys → Ala) resulting in CD8β′ and CD8β′′, respectively, using the Quick-Change Mutagenesis Kit (Stratagene). For the chimeric CD8α construct αβ the CD8α extracellular and transmembrane cDNA (residues 1–190) was fused with the cytoplasmic CD8β cDNA (residues 176–194). The cDNA of the chimeric CD8β construct βα was obtained by fusing the CD8β extracellular and transmembrane coding regions (residues 1–175) with the cytoplasmic CD8α cDNA (residues 191–220). The different cDNA were cloned in the pMI vector (24) and transfected into CD8β−, CD8α+ T1.4 hybridomas by retroviral gene transfer using the Phoenix packaging cell line (24). Phoenix cells were transfected using calcium phosphate precipitation. After 4 d the cells were sorted and then cloned for high CD2 expression using PE-labeled anti-CD2 mAb (BD PharMingen) and a FACStar™ (Becton Dickinson).
Calcium Measurements and TCR Photoaffinity Labeling.
P815 cells (106/ml) were pulsed or not with 1 μM of IASA-YIPSAEK(ABA)I or IASA-YISSAEK(ABA)I (P255S) and then UV irradiated at >350 nm as described (6, 25). T cell hybridomas (106/ml) were incubated with 5 μM Indo-1/AM (Sigma-Aldrich) at 37°C for 45 min, washed in DMEM, and incubated with P815 cells at an E/T ratio of 1/3 for 2 min. Calcium-dependent Indo-1 fluorescence was measured on a FACStar™ as described (25). TCR photoaffinity labeling of T1.4 hybridomas was performed as described (9–11, 20). In brief, hybridomas (107/ml) were incubated with Kd-125IASA-YIPSAEK(ABA)I for 2 h at 0–4°C. After UV-cross-linking at 312 ± 20 nm the cells were washed and lysed in RIPA buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, 1 mM PMSF, 10 μg/ml leupeptin, 10 μM pepstatin A, 5 mM benzamidine, 2 μg/ml aprotinin) for 30 min on ice. The detergent soluble fractions were immunoprecipitated with H57-Sepharose and the samples analyzed by SDS-PAGE (10%, reducing) and PhosphorImaging using a Fuji BAS1000 PhosphorImager.
Isolation of DIM.
T cell hybridomas (5 × 107) were lysed for 30 min on ice in MNE buffer (25 mM MES [pH 6.5], 150 mM NaCl, 5 mM EDTA) containing 1% Triton X-100 (Sigma-Aldrich). Alternatively, 0.5% Brij58 (Fluka) without EDTA was used (21). The lysates were homogenized with a Dounce homogenizer (10 strokes) and fractionated on sucrose density gradients as described (6). The gradients were fractionated from the top in 10 fractions. In some experiments the fractions 2–4 and 6–9 were pooled and referred to as DIM and detergent soluble membrane (M) fractions, respectively. Surface biotinylation was performed as described (6). For immunoprecipitation the fractions were incubated with Sepharose-conjugated anti-CD8α mAb 53.6.72, anti-CD8β mAb H35–17, or anti-TCR mAb H57 for 3 h at 4°C. The immunoprecipitates were resolved on SDS-PAGE (10%, reducing), Western blotted with streptavidin-horseradish peroxidase (HRP; GIBCO BRL) or anti-CD8α antiserum and revealed by enhanced chemoluminescence (ECL) as described (6, 20).
Coimmunoprecipitations.
To assess association of CD8 with lck, hybridomas (107) were lysed in lysis buffer (1 ml; 50 mM HEPES [pH 7.4], 150 mM NaCl, 1% Brij96 [Fluka], and protease inhibitors) for 60 min on ice. After centrifugation for 20 min at 12,000 g and 4°C, the supernatants were incubated for 3 h at 4°C with anti-CD8 mAb 53.6.72 or H35–17 conjugated to Sepharose 4B (6). After two washes with lysis buffer, the samples were analyzed by SDS-PAGE (10%, reducing) and Western blotting using anti-lck mAb 3A5. To detect association of CD8 with TCR/CD3, cells (2 × 107) were lysed likewise in 1 ml lysis buffer containing 0.3% NP-40 (Sigma-Aldrich) instead of Brij96. After one wash with lysis buffer, the immunoprecipitates were analyzed by SDS-PAGE (10%, reducing) and Western blotting using anti-CD8α antiserum.
Analysis of lck and CD3ζ Tyrosine Phosphorylation.
T1.4 hybridomas (5 × 107) were incubated in DMEM (5 ml), supplemented with 1% BSA and 10 mM HEPES (pH 7.4) with 50 nM of Kd −SYIPSAEK(ABA)I tetramer for 20 min at 26°C followed by 2 min at 37°C. The cells were lysed in 1 ml of MNE buffer containing 1% Triton X-100, protease inhibitors (see above), 1 mM Na3VO4, and 10 mM NaF for 30 min on ice. The DIM fractions were lysed in 1 ml RIPA buffer supplemented with protease and phosphatase inhibitors. The soluble fractions were incubated for 3 h at 4°C with anti-CD3ζ HAM146-Sepharose or Protein A Sepharose, preincubated with anti-lck antiserum. After two washes with lysis buffer, the immunoprecipitates were analyzed by SDS-PAGE and Western blotting with anti-pY mAb 4G10.
BIAcore Measurements.
Surface plasmon resonance (SPR) studies were performed on a BIAcore 2000 (BIAcore AB) as described previously (15, 26). In brief, streptavidin (Sigma-Aldrich) was covalently coupled to Research Grade CM5 sensor chips using the Amine Coupling Kit (BIAcore). Coupling levels ranged from 6,000 to 8,000 response units (RU). Biotinylated Kd-SYIPSAEK(ABA) or, as control, soluble human CD4 (sCD4), were immobilized via streptavidin. To eliminate aggregates, sCD8αβ and sT1 TCR were purified by gel-filtration on a Superdex S-200 column (Amersham Pharmacia Biotech) and used within 24 h. Experiments were performed at 25°C using HBS buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3.4 mM EDTA, and 0.005% Surfactant P20; BIAcore) and a flow-rate of 10 μl/ml.
Results
Soluble CD8αβ and T1 TCR Bind Independently to Kd-PbCS(ABA).
To assess whether the extracellular portion of CD8αβ affects TCR-ligand binding, we prepared soluble CD8αβ and T1 TCR. Purified soluble sT1 TCR was tested for ligand binding by TCR photoaffinity labeling with soluble Kd-125“IASA”-YIPSAEK(ABA)I (9–11). After UV irradiation it exhibited on reducing SDS-PAGE gels one labeled specie of 80–90 kD corresponding to the complex of sKd-PbCS (ABA) and one chain of the T1 TCR. This material was immunoprecipitated with anti-TCRCβ mAb H57 or anti-TCRCα mAb H28, confirming the heterodimeric nature of the sT1 TCR. To ascertain the biological activity of the soluble CD8αβ, it was tested as inhibitor in a cytolytic assay using PbCS(ABA) pulsed P815 cells as targets. Increasing concentrations of sCD8αβ inhibited the lysis of P815 cells by cloned PbCS(ABA)-specific S14 CTL, with a half maximal inhibition at 0.66 mg/ml (10 μM), and maximal inhibition of 80% at 2 mg/ml (data not shown), indicating that sCD8αβ competes with cell-associated CD8αβ.
SPR studies were performed to assess the interactions of sT1 TCR and sCD8αβ with immobilized Kd-PbCS(ABA). As shown in Fig. 1 A, sT1 αβ bound to Kd-PbCS(ABA) in a dose-dependent manner with an equilibrium constant KD of 4 ± 0.4 μM. No binding was detectable when HLA-A2-flu matrix peptide complexes or CD4 were used instead of Kd-PbCS(ABA) or sT1 TCR (data not shown). sCD8αβ bound also in a dose-dependent manner to immobilized Kd-PbCS(ABA) (Fig. 1 B), but with low affinity (KD 99 ± 10 μM), which is in agreement with published values (13, 14). To determine whether CD8αβ affects TCR-ligand binding, sT1 TCR was tested for binding to immobilized Kd-PbCS(ABA) in the presence or absence of 80 μM of sCD8αβ. As shown in Fig. 1 C, sCD8 did not increase the binding of sT1 TCR to Kd-PbCS(ABA). Consistent with this we found that sCD8αβ has no effect on TCR photoaffinity labeling on CD8− T1.4 cell hybridomas (data not shown). These findings demonstrate that sCD8αβ does not affect the interaction of T1 TCR with Kd-PbCS(ABA). This is in accordance with a study showing that CD8αα has no effect on TCR-ligand binding (15), but not with another study claiming that soluble CD8αβ does (13).
Figure 1.
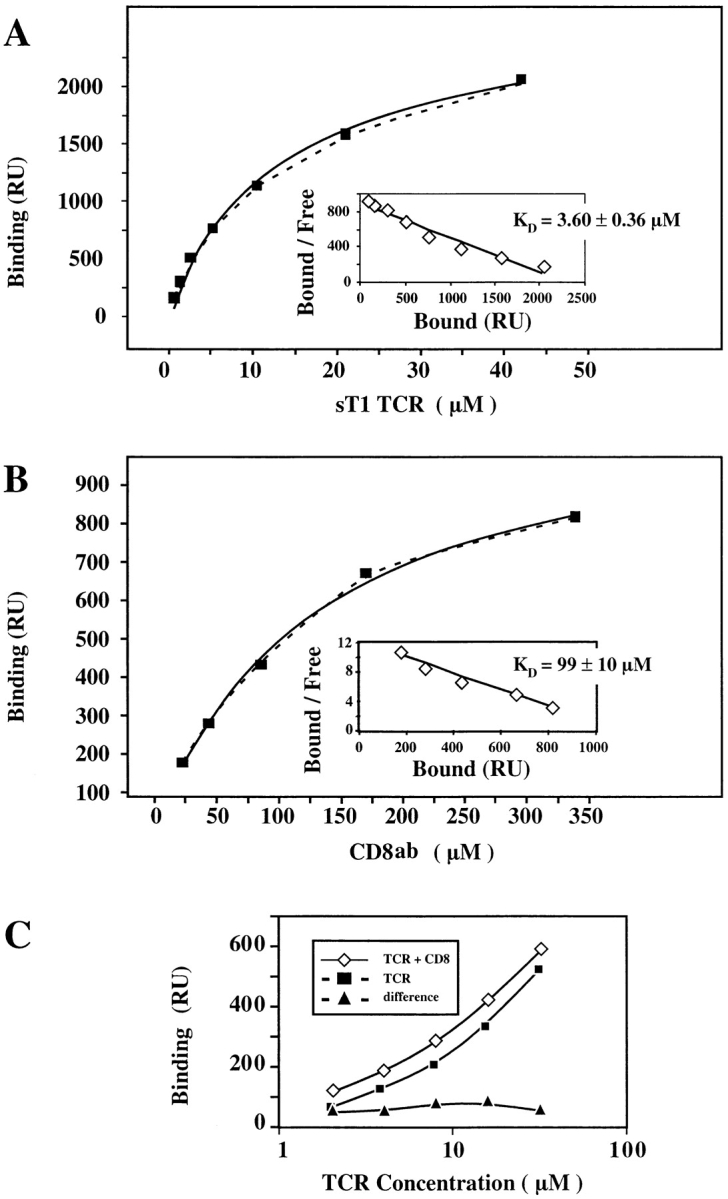
Soluble CD8αβ binds to Kd-PbCS(ABA) without affecting its interaction with T1 TCR. For affinity measurements sT1 TCR (A) and sCD8αβ (B) were injected for 30 s at the indicated concentrations over surfaces expressing Kd-SYIPSAEK(ABA)I (4,500 RU) or sCD4 (4,200 RU). Binding was calculated as the difference in the observed equilibrium response between the Kd-SYIPSAEK(ABA)I and the control sCD4 flow cells. The solid lines represent nonlinear fits of the Langmuir binding isotherm to the data, which yielded the indicated KD values. The maximal binding was 2,600 RU for sT1 TCR and 1,200 RU for sCD8αβ. The insets show Scatchard transformations of the same data. (C) The indicated concentrations of sT1 TCR were injected for 30 s over Kd-SYIPSAEK(ABA)I (2,000 RU) or CD4 (2,300 RU) coated sensor chips in the absence (▪) or presence (⋄) of added sCD8αβ (80 μM). The calculated difference in the responses observed with sT1 alone versus sT1 plus sCD8αβ are also shown (▴). The binding level observed with sCD8αβ alone at 80 μM is shown as dashed line, indicating that the effect of adding sCD8αβ on sT1 binding was never more than additive.
The CD8β Transmembrane and Cytoplasmic Portions Are Required for Efficient Intracellular Calcium Mobilization in CD8-transfected T1.4 Hybridomas.
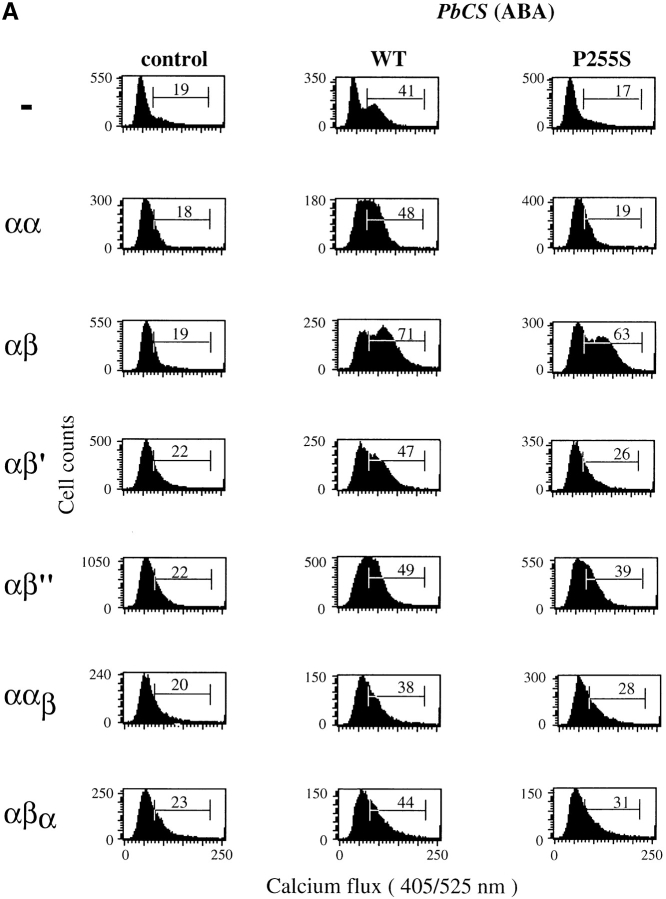
To evaluate the role of CD8β transmembrane and cytoplasmic portions for CD8 coreceptor function, we transfected CD8α+ T1.4 hybridomas (6, 10) with CD8β or the CD8β variants shown in Fig. 2. While the T1 CTL clone expresses only CD8αβ, the hybridomas express substantially more CD8α than CD8β, i.e., express homodimeric CD8αα and heterodimeric CD8αβ. The different hybridomas were tested for intracellular calcium mobilization upon incubation with P815 cells sensitized with IASA-YIPSAEK(ABA)I. CD8αβ cells exhibited higher calcium mobilization than the other cells (Fig. 3 A). More striking differences among the different cells emerged, when the peptide variant IASA-YISSAEK(ABA)I (P255S) was used, which is a weak agonist for T1 CTL (25). In this case, the CD8αβ+ cells displayed nearly the same calcium mobilization as observed for the wild-type peptide, whereas no change was observed in CD8− or CD8αα+ cells. Hybridomas expressing CD8αβ′ or CD8ααβ responded less efficiently than those expressing CD8αβ′′ or CD8αβα.
Figure 2.
TCR and CD8 expression of cells under study.
Figure 3.
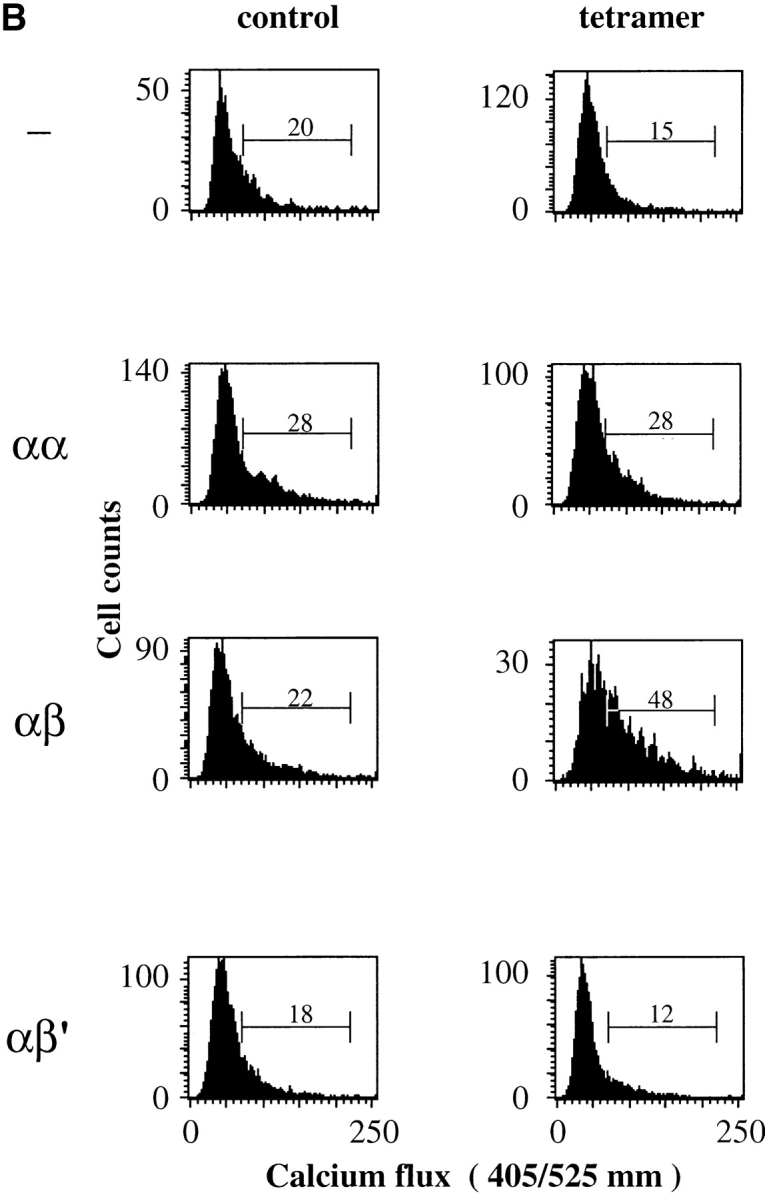
Efficient calcium mobilization of T1.4 hybridomas requires that they express CD8β transmembrane and cytoplasmic portions. (A) Indo-1–labeled hybridomas with the indicated CD8 expression were incubated with P815 cells either untreated (control) or previously sensitized with 1 μM of IASA-YIPSAEK(ABA)I (PbCS(ABA) wt) or IASA-YISSAEK(ABA)I (PbCS(ABA) P255S) at an E/T ratio of 1/3 for 3 min at 37°C and calcium-dependent Indo-1 fluorescence was measured by FACS®. (B) Alternatively the indicated Indo-1–labeled cells were incubated or not (control) with Kd-PbCS(ABA) tetramer (50 nM) and calcium flux was measured likewise after 2 min of incubation at 37°C. One out of three experiments is shown.
We also measured intracellular calcium mobilization of the indicated hybridomas after incubation with Kd-SYIPSAEK(ABA)I tetramer. As shown in Fig. 3 B, only cells expressing CD8αβ, but not those expressing no CD8, CD8αα, or CD8αβ′ exhibited significant increase in intracellular calcium upon incubation with tetramer. As assessed by staining with Cy5 labeled tetramer, binding was maximal on all cells under the indicated conditions (data not shown).
The CD8β Tail Is Essential for CD8 Partitioning in Rafts and Efficient Association with lck.
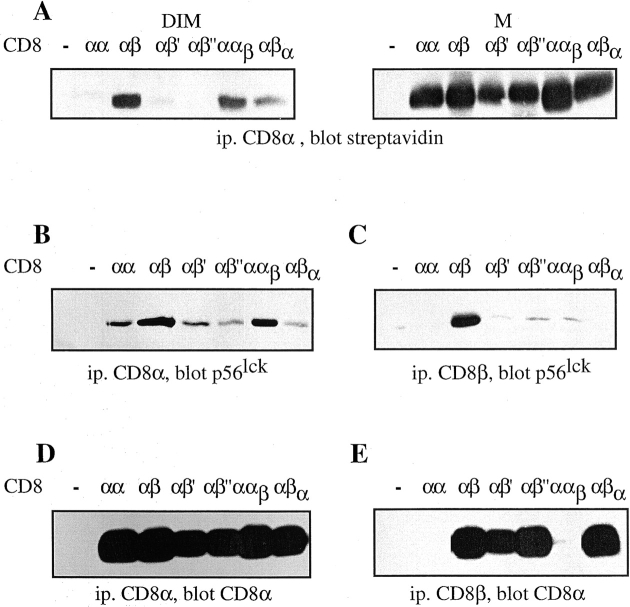
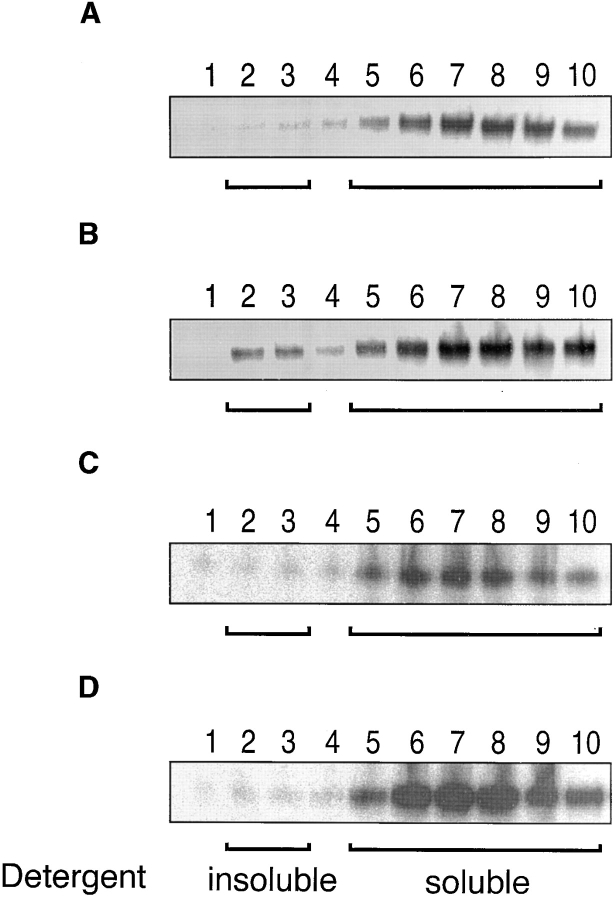
As CD8 association with lck seems to take place preferentially in rafts (6), we examined the partitioning of the different CD8 molecules in rafts. To this end the different cells were fractionated in Triton X-100 soluble (M) and insoluble (DIM) fractions and their CD8 content assessed. As shown in Fig. 4 A, CD8αβ and C8ααβ, efficiently partition in DIM, whereas CD8αβ′ and CD8αβ′′ did not. Thus, mutation of Cys179 of CD8β impaired CD8 association with rafts as dramatically as deletion of the CD8β tail. Interestingly CD8αβα was significantly more raft-associated as compared with CD8αβ′ and CD8αβ′′, indicating that the transmembrane portion of CD8β is also involved in the recruitment of CD8 to rafts. Essentially the same results were obtained, whether or not EDTA was present in the lysis buffer, i.e., whether or not CD8 could associate with lck (data not shown). These results show that raft-association of CD8 is essentially mediated by the CD8β tail and this mainly due to palmitoylation of its Cys 179.
Figure 4.
Palmitoylation of CD8β is essential for CD8 localization in rafts and efficient lck association. (A) T1.4 hybridomas with the indicated CD8 expression were surface-biotinylated, lysed in cold TX-100 (1%), and fractionated in M and DIM fractions. These were immunoprecipitated with anti-CD8α mAb 53.6.72. The samples were analyzed by SDS-PAGE and Western blotting with streptavidin. (B–E) T1.4 hybridomas with the indicated CD8 expression were lysed in Brij96 (1%) and the lysates immunoprecipitated with mAb 53.6.72 (B and D) or anti-CD8β mAB H35–17 (C and E). The immunoprecipitates were analyzed by SDS-PAGE and Western blotting with anti-lck mAb 3A5 (B and C) or anti-CD8α antiserum (D and E). Note that anti-CD8β mAb H35 is unable to precipitate CD8αα and CD8ααβ. One out of two experiments is shown.
We next examined the association of CD8 with lck by coimmunoprecipitation. For simplicity we used here total Brij96 lysates, rather than M and DIM fractions, as this detergent has been used previously to assess CD8 association with lck (6–8). As the CD8 transfectants under study express CD8αα and CD8αβ (Fig. 2), we performed immunoprecipitation with anti-CD8α mAb, which precipitates CD8αα and CD8αβ (Fig. 4 B), as well as with anti-CD8β mAb, which precipitates only CD8αβ (Fig. 4 C). We find that CD8αβ and CD8ααβ associate more efficiently with lck, compared with CD8αα, CD8αβ′, CD8αβ′′, and CD8αβα. The superior lck association with CD8αβ was especially visible in anti-CD8β immunoprecipitates. This is so, because these cells express substantially higher amounts of CD8αα than CD8αβ. Thus, even though the association of CD8αα with lck is weak, it is well visible due to its high expression. The amounts of CD8 in anti-CD8α or CD8β immunoprecipitates were comparable for the different hybridomas (Fig. 4, D and E). These results demonstrate that efficient association of CD8 with lck takes place in rafts.
As it has been reported that CD8 associates with LAT, in a manner similar as it associates with lck (5, 23), we Western blotted the same CD8 immunoprecipitates with anti-LAT antibody. Even though LAT was well detectable in total lysates, no LAT was found in the CD8 immunoprecipitates (data not shown).
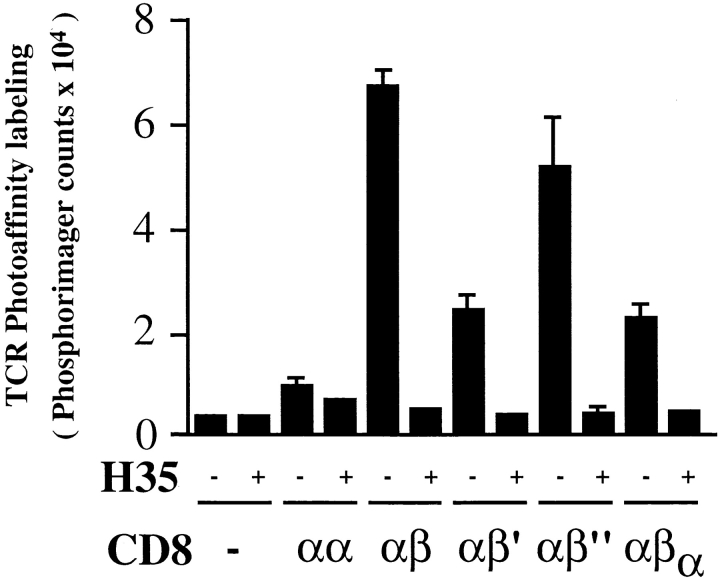
CD8β Tail Is Required for CD8-mediated Increase in TCR-ligand Binding.
To assess the ability of the various CD8 constructs to strengthen TCR-ligand binding, we TCR photoaffinity labeled the different cells with soluble, monomeric Kd-125”IASA”-YIPSAEK(ABA)I. As shown in Fig. 5, T1 TCR photoaffinity labeling was highest on CD8αβ+ cells. In the presence of anti-CD8β mAb H35–17, which blocks binding of CD8 to TCR-associated Kd-PbCS(ABA) (10), TCR photoaffinity labeling was reduced by 12-fold, to the same low levels observed on CD8− cells. CD8αα was quite unable to increase TCR-ligand binding, which is consistent with previous findings in a related system (4). Importantly, deletion of the cytoplasmic domain of CD8β (CD8αβ′) or substitution with the one of CD8α (CD8αβα) caused a significant (67%) reduction in TCR photoaffinity labeling, demonstrating that the CD8β tail is required for efficient CD8-mediated increase of TCR-ligand binding. By contrast, mutation of CD8β Cys 179 (CD8αβ′′) caused only a modest decrease in TCR photoaffinity labeling. As this variant poorly associates with lck (6; Fig. 4), it appears that CD8/lck supports TCR-ligand binding somewhat better than CD8αβ alone. It is noteworthy that CD8αβ′ and CD8αβα exhibit partially increased TCR photoaffinity labeling as compared with CD8αα, indicating that the extracellular and/or transmembrane regions of CD8β also play a role in enhancing TCR interactions with MHC-peptide.
Figure 5.
The cytoplasmic tail of CD8β is required for efficient CD8-mediated increase in TCR photoaffinity labeling. T1.4 hybridoma cells (5 × 106/ml) with the indicated CD8 expression were incubated for 2 h at 0–4°C with soluble monomeric Kd -125”IASA”-YIPSAEK(ABA)I (2–6 × 106 cpm/ml) in the absence or presence of anti-CD8β mAb H35–17 (10 μg/ml). After UV irradiation cells were lysed in RIPA buffer, TCR immunoprecipitated, and analyzed by SDS-PAGE and PhosphorImaging. Mean values and SD were calculated from six experiments.
The CD8β Tail Mediates Constitutive Association of CD8 with TCR/CD3.
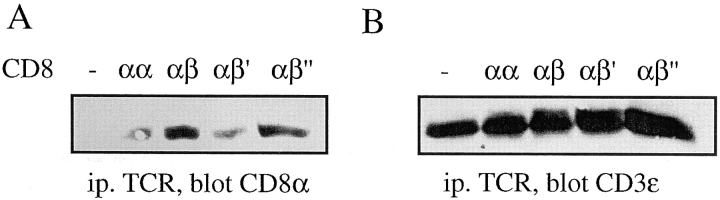
Our results so far indicate that CD8αβ increases the avidity of TCR-ligand binding on cells, but not in solution and that the tail of CD8β is needed for this effect (Figs. 1 and 5). We next examined whether this is so, because on cells CD8 associates with the TCR. To this end, we lysed hybridomas expressing no CD8, CD8αα, CD8αβ, CD8αβ′, or CD8αβ′′ in 0.3% NP-40 and immunoprecipitated TCR. As shown in Fig. 6 A, the amount of CD8α coimmunoprecipitated with TCR was substantially higher on CD8αβ+ or CD8αβ′′+ cells, than on CD8αα+ or CD8αβ′+ cells. These differences were not accounted for by variations of TCR or CD8 expression, as all cells expressed comparable levels of CD3ε, CD8α, and CD8β (Fig. 6, B and C).
Figure 6.
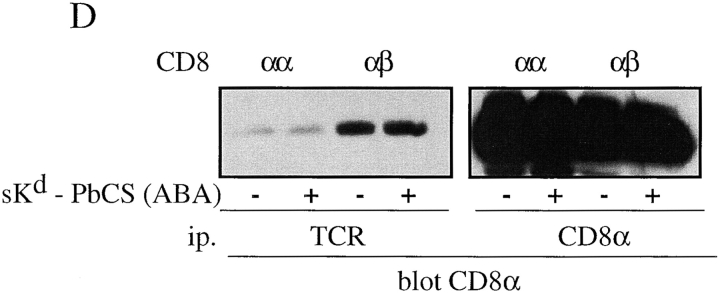
The CD8β tail mediates CD8 association with TCR/CD3. (A–C) T1.4 hybridomas with the indicated CD8 expression were lysed in 0.3% NP-40, and lysates immunoprecipitated with anti-TCR mAb H57 (A and B), anti-CD8α mAb 53.6.72 (C, left), or with anti-CD8β mAb H35–17 (C, right). The samples were analyzed by SDS-PAGE and Western blotting with anti-CD8α antiserum (A and C) or anti-CD3ε antiserum (B). (D) T1.4 T cell hybridomas expressing CD8αα or CD8αβ were incubated with monomeric Kd-SYIPSAEK(ABA)I complexes (1.16 μM) for 2 h at 0–4°C. After UV irradiation the cells were lysed in 0.3% NP-40. Lysates were immunoprecipitated with anti-TCR mAb H57 or anti-CD8α mAb 53.6.72, as indicated and the immunoprecipitates analyzed by SDS-PAGE and Western blotting with anti-CD8α antiserum.
To find out whether Kd-PbCS(ABA) complexes strengthen association of CD8 with TCR, CD8αβ+ cells were photo–cross-linked with soluble Kd-SYIPSAEK(ABA)I complexes and analyzed as described in the previous paragraph. No significant increase in CD8 association with TCR/CD3 was observed (Fig. 6 D), indicating that the interaction of CD8αβ with TCR/CD3 is constitutive and not induced by MHC-peptide. Moreover, the coimmunoprecipitation was not impaired by the SH2 peptide pYEEI (data not shown), which has been shown to disrupt activation-induced coupling of CD8 to TCR/CD3 via lck and ZAP-70 (27). Constitutive association of CD8 (and CD4) with TCR/CD3 has been reported previously and low concentrations of NP-40 or Triton X-100 have been recommended to preserve these interactions (28–30). Essentially the same findings where obtained when Brij78 was used, which unlike NP-40 and Triton X-100 solubilizes lipid rafts, indicating that raft integrity was not required for this interaction (data not shown).
CD8αβ Mediates Raft-association of TCR/CD3.
As the previous experiments indicated that CD8β mediates association of CD8 with lck in rafts (Fig. 4), as well as with TCR/CD3 (Fig. 6), we examined whether therefore CD8β induces raft-association of TCR/CD3. To this end we TCR photoaffinity labeled T1.4 T cell hybridomas that expressed CD8αβ, CD8αβ′, CD8αβ′ or no CD8 with soluble Kd-125”IASA”-YIPSAEK(ABA)I and fractionated their Brij58 lysates on sucrose density gradients. Brij58 was used rather than Triton X-100, because it better preserves weak molecular interaction of membrane proteins (21). This difference becomes especially apparent after long periods of incubation, such as fractionation on sucrose density gradients. As shown in Fig. 7, the DIM-containing light fractions exhibited substantial amounts of photoaffinity labeled TCR on CD8αβ+ cells, but not on cells expressing CD8αβ′, CD8αβ′′, or no CD8. Cells expressing CD8αβα also lacked significant amounts of photoaffinity labeled TCR in the light fractions (data not shown). The gradient fractions displayed the expected distributions of thy-1, which is a marker of rafts and CD45, which is excluded from rafts (data not shown, and references 17–21). These findings indicate that the association of TCR/CD3 with CD8αβ promotes association of TCR/CD3 with rafts, and that tail of CD8β is required for this.
Figure 7.
CD8αβ mediates raft association of TCR/CD3. T1.4 hybridomas (5 × 107) expressing no CD8 (A), CD8αβ (B), CD8αβ′ (C), or CD8αβ′′ (D) were photoaffinity labeled at 26°C with Kd -125”IASA”-YIPSAEK(ABA)I (0.5–1.5 × 108 cpm/7 ml). After UV irradiation the washed cells were lysed in 0.5% Brij58 and the lysates fractionated on sucrose density gradients. Fractions were collected from the top and immunoprecipitated with anti-TCRCβ mAb H57. The immunoprecipitates were analyzed by SDS-PAGE and PhosphorImaging.
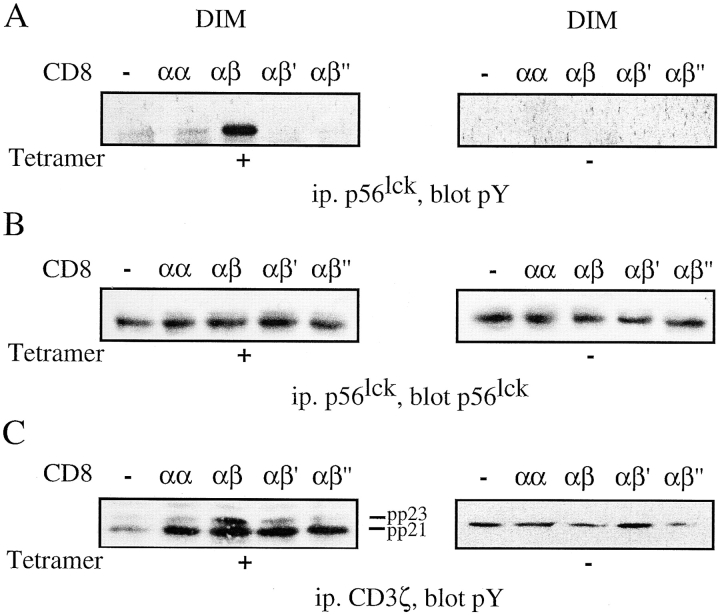
CD8αβ+ Hybridomas Exhibit Strong lck and CD3ζ Phosphorylation in Response to Kd-PbCS(ABA) Tetramer.
To correlate the intracellular calcium mobilization with activation of signaling molecules, we assessed lck phosphorylation in the different CD8 transfectants. The cells were stimulated with Kd-SYIPSAEK(ABA)I tetramer and lck tyrosine phosphorylation assessed in DIM fractions. CD8αβ cells displayed strong lck tyrosine phosphorylation upon incubation with Kd-SYIPSAEK(ABA)I tetramer, while cells expressing no CD8 or CD8αα, CD8αβ′, or CD8αβ′′ cells exhibited only background lck phosphorylation (Fig. 8, A and B). In accordance with this, CD3ζ tyrosine phosphorylation was highest in CD8αβ+ cells activated by Kd-SYIPSAEK(ABA)I tetramer, in particular of the pp23 hyperphosphorylated form (Fig. 8 C). In the M fractions the same pattern of tyrosine phosphorylated lck was discernible in the presence, but not absence of phosphatase inhibitors (data not shown).
Figure 8.
T1.4 cells expressing CD8αβ exhibit strong lck and CD3ζ tyrosine phosphorylation in DIM upon incubation with Kd-PbCS(ABA) tetramer. (A–C) T1.4 hybridomas with the indicated CD8 expression were incubated with or without Kd-SYIPSAEK(ABA)I tetramer (50 nM) for 3 min at 37°C. The cells were lysed in 1% TX-100 and fractionated in M and DIM. DIM fractions were immunoprecipitated with anti-lck (A and B) antiserum or anti-CD3ζ mAb HAM 146 (C). The samples were analyzed by SDS-PAGE and Western blotting with anti-pY mAb 4G10 (A and C), or anti-lck mAb 3A5 (B). A representative experiment out of three is shown.
Discussion
The extraordinary sensitivity of antigen recognition by CD8+ T cells, namely CTL, impinges to a high degree on the coreceptor CD8. As CD8αα is a much less efficient coreceptor, as compared with CD8αβ (1, 2; Fig. 3), we investigated the molecular basis by which CD8β endows CD8 with efficient coreceptor function. Binding studies with soluble recombinant proteins showed that TCR bind cognate MHC-peptide in general with low affinity (KD of 10−4 to 10−6 M) (31). Our experiments with soluble CD8αβ demonstrate that the extracellular portion of CD8αβ has no effect on TCR-ligand binding (Fig. 1, and unpublished results). By contrast, using TCR photoaffinity labeling, we find that on cells CD8αβ, but not CD8αα, increases the avidity of TCR-ligand binding by a factor of 10 or more (4, 9–11; Fig. 5). Taken together, this implies that the CD8-mediated increase in TCR-ligand binding observed on cells is attributed to the transmembrane and/or cytoplasmic portions of CD8β.
The observation that deletion of the tail of CD8β greatly reduces CD8-mediated increase in TCR-ligand binding on cells (Fig. 5), as well as of association of CD8 with TCR/CD3 (Fig. 6), indicates that these two effects are related. As CD8αβα and CD8αβ' still caused a modest increase in TCR-ligand binding (Fig. 5), it appears that the transmembrane portion of CD8β also contributes to the association of CD8 with TCR/CD3. Consistent with this is the observation that hybridomas expressing CD8ααβ, which lacks the transmembrane, but not the cytoplasmic portion of CD8β, recognizes antigen poorly, even though they exhibit good association of CD8 with lck (Figs. 3 A and 4).
It is not clear how the CD8β tail interacts with TCR/CD3 and to what extent the CD8 transmembrane portion is involved in this interaction. The CD8β and to a lesser degree the CD8α tail contain abundant positive charges (Fig. 9). By contrast the NH2 terminus of lck, which is membrane associated because it is palmitoylated (35, 36) and the membrane proximal cytoplasmic sequence of LAT are negatively charged and hence likely to undergo electrostatic interactions with the CD8 tails (Fig. 9). Remarkably, this LAT sequence becomes highly negatively charged upon activation-dependent phosphorylation of its tyrosines and serine/threonines (37–39) and thus is expected to undergo strong electrostatic interactions with CD8. Differences in LAT phosphorylation may explain why we were unable to detect association of LAT with CD8, as has been observed in other systems (5, 23). The membrane proximal cytoplasmic sequences of CD3γ, CD3δ, and CD3ζ, similarly become acidic upon phosphorylation of their serines/threonines.
Figure 9.
Membrane proximal cytoplasmic sequences of CD3, CD8, lck and LAT. The indicated sequences were taken from Swiss-Prot (http://www. expasy.ch/cgi-bin/sprotsearch-de). Cysteines labeled with an asterisk are known to be palmitoylated and hence are membrane integrated. The numbers of the displayed residues are indicated in parenthesis. Basic residues are shown in oval, acidic ones in boxes, tyrosines in diamonds, and serines and threonines in shaded gray.
Association of CD8 (and CD4) with TCR/CD3 has been previously observed in different systems (28–30). Of particular interest is a study showing that anti-CD8 immunoprecipitates contain a high proportion of CD3δ, suggesting that CD8 associates with TCR via CD3δ (28). This may explain why mice lacking CD3δ (32), or expressing a variant TCR which fails to associate with CD3δ (33), exhibit severely impaired activation and positive selection of CD8+ T cells. It is important to note that the association of CD8 with TCR/CD3 described here (Fig. 6) and in previous studies (28–30) is constitutive, i.e., is not, or little, induced by MHC-peptide.
Moreover, the tail of CD8β mediates raft localization of CD8 (6, 20; Fig. 4). The finding that point mutation of CD8β Cys 179 (CD8αβ′′) abolishes this (Fig. 4 A), demonstrates that this is attributed essentially to palmitoylation of the CD8β tail. As lck is myristoylated and dipalmitoylated, it also efficiently partitions in rafts (17–19, 35, 36). Thus, the observation that efficient association of CD8 with lck takes place in rafts (6; Fig. 4), is most likely explained by increased concentrations of both molecules in microdomains, which constitute only a small fraction of the cell membrane (17). By contrast, association of CD8 with TCR/CD3 and hence TCR-ligand binding were greatly impaired upon deletion of the CD8β tail, but hardly by mutation of CD8β Cys 179 (Figs. 5 and 6). This demonstrates that the tail of CD8β exerts two different functions: (a) it mediates raft localization of CD8 and hence efficient association of CD8 with lck, and (b) it mediates association of CD8 with TCR/CD3. Because CD8β exerts both functions at the same time, it promotes raft-association of TCR/CD3 (Fig. 7).
While TCR/CD3 per se do not partition significantly in rafts (19–22), this study indicates that they become raft-associated by interacting with raft-resident CD8-lck complexes. Small fractions of TCR/CD3 have been shown to be raft-associated in resting cells in a fractionation study (21) and by confocal microscopy (40). Although CD3 components seem to bind weakly to raft-associated Src kinases in resting cells, which may be important for coreceptor independent T cell activation (41), we show here that CD8 substantially strengthens raft-association of TCR/CD3 (Fig. 7). As association of TCR/CD3 with CD8/lck also enhances the avidity of TCR-ligand binding (20; Fig. 5), it appears that raft-associated TCR have an increased avidity for MHC-peptide, i.e., are those that are engaged first by MHC-peptide, particularly when these are scant or of low affinity. Thus, for the induction of TCR signaling this constitutive TCR/CD3 association with CD8/lck is important, as it provides a significant increase in TCR-ligand binding and at the same time raft association of engaged TCR/CD3 (Figs. 5–8).
Because phosphatases are excluded from rafts, they are privileged sites for the induction of TCR signaling (19–22). Indeed we find that tetrameric Kd-PbCS(ABA) complexes efficiently induce tyrosine phosphorylation of lck and CD3ζ in rafts on CD8αβ+ hybridoma, but much less so on hybridomas expressing no CD8, CD8αα, CD8αβ′, or CD8αβ′′ (Fig. 8). As lck is activated by cross-linking, even when Y505 is phosphorylated (6–8, 20, 42), our data propose that TCR signaling is induced by engagement of raft-associated TCR/CD3 adducts with CD8/lck by multimeric MHC-peptide complexes. While it is clear that once TCR signaling is induced, SH2-mediated interactions provide additional coupling of the TCR/CD3 with CD8, LAT, ZAP-70, and other molecules (1, 27, 34), the present study shows that CD8β significantly facilitates TCR signal induction, by increasing the avidity of TCR-ligand binding and by docking TCR/CD3 to rafts.
Acknowledgments
We thank Drs. M. Bevan for his generous gift of the pMI vector, H.-C. Chang for the plasmids encoding the leucine zippers and helpful advice, and J.-C. Cerottini for valuable discussions. We are grateful to Pierre Zaeck for help with cell sorting.
Footnotes
Abbreviations used in this paper: CHO, Chinese hamster ovary; DIM, detergent insoluble microdomains; LAT, linker for activation of T cells; lck, p56lck; M, detergent soluble membrane fraction; PbCS, Plasmodium berghei circumsporozoite; pY, phospho-tyrosine.
References
- 1.Janeway, C.A., Jr. 1992. The T cell receptor as a multicomponent signaling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu. Rev. Immunol. 10:645–674. [DOI] [PubMed] [Google Scholar]
- 2.Zamoyska, R. 1994. The CD8 coreceptor revisited: one chain good, two chains better. Immunity. 1:243–246. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler, C.J., J.-Y. Chen, T.A. Potter, and J.R. Potter. 1998. Mechanism of CD8β-mediated T cell response enhancement: interaction with MHC class I-β2-microglobulin and functional coupling to TCR/CD3. J. Immunol. 160:4199–4207. [PubMed] [Google Scholar]
- 4.Renard, V., P. Romero, E. Vivier, B. Malissen, and I.F. Luescher. 1996. CD8β increases CD8 coreceptor function and participation in TCR-ligand binding. J. Exp. Med. 184:2439–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosselut, R., S. Kubo, T. Guinter, J.L. Kopacz, J.D. Altman, L. Feigenbaum, and A. Singer. 2000. Role of CD8beta domains in CD8 coreceptor function: importance for MHC I binding, signaling, and positive selection of CD8+ T cells in the thymus. Immunity. 12:409–418. [DOI] [PubMed] [Google Scholar]
- 6.Arcaro, A., C. Gregoire, N. Boucheron, S. Stotz, E. Palmer, B. Malissen, and I.F. Luescher. 2000. Essential role of CD8 palmitoylation in CD8 coreceptor function. J. Immunol. 165:2068–2076. [DOI] [PubMed] [Google Scholar]
- 7.Irie, H.Y., K.S. Ravichandran, and S.J. Burakoff. 1995. CD8β chain influences CD8α chain-associated Lck kinase activity. J. Exp. Med. 181:1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irie, H.Y., M.S. Mong, A. Itano, M.E. Crooks, D.R. Littman, S.J. Burakoff, and E. Robey. 1998. The cytoplasmic domain of CD8β regulates Lck kinase activation and CD8 T cell development. J. Immunol. 161:183–191. [PubMed] [Google Scholar]
- 9.Luescher, I.F., J.C. Cerottini and P. Romero. 1994. Photoaffinity labeling of the T cell receptor on cloned cytotoxic T lymohocytes by covalent photoreactive ligand. J. Biol. Chem. 269:5547-5582. [PubMed] [Google Scholar]
- 10.Luescher, I.F., E. Vivier, A. Layer, J. Mahiou, F. Godeau, B. Malissen, and P. Romero. 1995. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 373:353–356. [DOI] [PubMed] [Google Scholar]
- 11.Luescher, I.F., F. Anjuere, M.C. Peitsch, C.V. Jongeneel, J.C. Cerottini, and P. Romero. 1995. Structural analysis of TCR-ligand interactions studied on H-2Kd-restricted cloned CTL specific for a photoreactive peptide derivative. Immunity. 3:51–63. [DOI] [PubMed] [Google Scholar]
- 12.Daniels, M.A., and S.C. Jameson. 2000. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med. 191:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia, K.C., C.A. Scott, A. Brunmark, F.R. Carbone, P.A. Petersen, I.A. Wilson, and L. Teyton. 1996. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 384:577–581. [DOI] [PubMed] [Google Scholar]
- 14.Kern, P., R.E. Hussey, R. Spoerl, E.L. Reinherz, and H.-C. Chang. 1999. Expression, purification, and functional analysis of murine ectodomain fragments of CD8αα and CD8αβ dimers. J. Biol. Chem. 274:27237. [DOI] [PubMed] [Google Scholar]
- 15.Wyer, J.R., B.E. Willcox, G.F. Goa, U.C. Gerth, S.J. Davis, J.I. Bell, A. van der Merwe, and B.K Jakobsen. 1999. T cell receptor and coreceptor CD8αα bind peptide-MHC independently and with distinct kinetics. Immunity. 10:219–225. [DOI] [PubMed] [Google Scholar]
- 16.Itano, A., D. Cado, F.K.M. Chan, and E. Robey. 1994. A role for the cytoplasmic tail of the β chain of CD8 in thymic selection. Immunity. 1:287–290. [DOI] [PubMed] [Google Scholar]
- 17.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Mol. Biol. 1:31–38. [DOI] [PubMed] [Google Scholar]
- 18.Melkonian, K.A., A.G. Ostermeyer, J.Z. Chen, M.G. Roth, and D.A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910–3917. [DOI] [PubMed] [Google Scholar]
- 19.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity. 8:723–732. [DOI] [PubMed] [Google Scholar]
- 20.Doucey, M.A., D.F. Legler, N. Boucheron, J.-C. Cerottini, C. Bron, and I.F. Luescher. 2001. CTL activation is induced by cross linking of TCR/MHC peptide-CD8/lck adducts in rafts. Eur. J. Immunol. 31:1561–1570. [DOI] [PubMed] [Google Scholar]
- 21.Montixi, C., C. Langlet, A.M. Bernard, J. Thimonier, C. Dubois, M.A. Wurbel, J.P. Chauvin, M. Pierres, and H.T. He. 1998. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, W., R.P. Trible, and L.E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 9:239–246. [DOI] [PubMed] [Google Scholar]
- 23.Bosselut, R., W. Zhang, J.M. Ashe, J.L. Kopacz, L.E. Samelson, and A. Singer. 1999. Association of the adaptor molecule LAT with CD4 and CD8 coreceptors identifies a new coreceptor function in T cell receptor signal transduction. J. Exp. Med. 190:1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deftos, M.L., Y.W. He, E.W. Ojala, and M.J. Bevan. 1998. Correlating notch signaling with thymocyte maturation. Immunity. 9:777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudrisier, D., B. Kessler, S. Valitutti, C. Horvath, J.-C. Cerottini, and I.F. Luescher. 1998. The efficiency of antigen recognition by CD8+ CTL clones is determined by the frequency of serial TCR engagement. J. Immunol. 161:553–562. [PubMed] [Google Scholar]
- 26.Willcox, B.E., G.F. Gao, J.R. Wyer, J.E. Ladbury, J.I. Bell, B.K. Jakobsen, and P.A. van der Merwe. 1999. TCR binding to peptide-MHC stabilizes a flexible recognition interface. Immunity. 10:357–365. [DOI] [PubMed] [Google Scholar]
- 27.Thome, M., V. Germain, J.P. DiSanto, and O. Acuto. 1996. The p56lck SH2 domain mediates recruitment of CD8/p56lck to the activated T cell receptor/CD3ζ complex. Eur. J. Immunol. 26:2093–2100. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, S., J. Kupsch, K. Eichmann, and M. Saizawa. 1992. Biochemical evidence of the physical association of the majority of CD3δ chains with the accessory/co-recptor molecules CD4 and CD8 on nonactivated T lymphocytes. Eur. J. Immunol. 22:2475–2479. [DOI] [PubMed] [Google Scholar]
- 29.Beyers, A.D., L.L. Spruty, and A.F. Williams. 1992. Molecular association between the T-lymphocyte antigen receptor complex and the surface antigens CD2, CD4, or CD8 and CD5. Proc. Natl. Acad. Sci. USA. 89:2945–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher, P.F., B. Fazekas de St. Groth, and J.A.A.P. Miller. 1989. CD4 and CD8 molecules can physically associate with the same T cell receptor. Proc. Natl. Acad. Sci. USA. 86:10044-10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fremont, D.H., W.A. Rees, and H. Kozono. 1996. Biophysical studies of T-cell receptors and their ligands. Curr. Opin. Immunol. 8:93–100. [DOI] [PubMed] [Google Scholar]
- 32.Delgado, P., E. Fernandez, V. Dave, D. Kappas, and B. Alarcon. 2000. CD3δ couples T-cell receptor signaling to ERK activation and thymocyte positive selection. Nature. 406:426–430. [DOI] [PubMed] [Google Scholar]
- 33.Guy, W., B. Hausmann, and E. Palmer. 2000. A motif in the T-cell receptor controls positive selection by modulating ERK activity. Nature. 406:422–426. [DOI] [PubMed] [Google Scholar]
- 34.Qian, D., and A. Weiss A. 1997. T cell antigen receptor signal transduction. Curr. Opin. Cell Biol. 9:205-212. [DOI] [PubMed] [Google Scholar]
- 35.Webb, Y., L. Hermida-Matsumoto, and M.D. Resh. 2000. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and poyunsaturated fatty acids. J. Biol. Chem. 275:261–270. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda, K., A. Kosugi, F. Hayashi, S. Saitoh, M. Nagafuku, Y. Mori, M. Ogata, and T. Hamaoka. 2000. Serine 6 of Lck tyrosine kinase: a critical site for Lck myristoylation, membrane localization, and function in T lymphocytes. J. Immunol. 165:3226–3231. [DOI] [PubMed] [Google Scholar]
- 37.Lin, J., A. Weiss, and T.S. Finco. 1999. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J. Biol. Chem. 274:28861–28864. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, W., and L.E. Samelson. 2000. The role of membrane-associated adaptors in T cell receptor signalling. Semin. Immunol. 12:35–41. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R.P. Trible, and L.E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 92:83–92. [DOI] [PubMed] [Google Scholar]
- 40.Janes, P.W., S.C. Ley, and A.I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147:447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van't Hof, W., and M.D. Resh. 1999. Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J. Cell Biol. 145:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi, H., and W.A. Hendrickson. 1996. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature. 384:484–489. [DOI] [PubMed] [Google Scholar]