Abstract
Objective
To analyze the changing pattern in tumor type and postoperative deaths at a national referral center for esophageal cancer in the Western world and to assess prognostic factors for long-term survival after resection.
Summary Background Data
During the past two decades, the epidemiology and treatment strategies of esophageal cancer have changed markedly in the Western world. The influence of these factors on postoperative deaths and long-term prognosis has not been adequately evaluated.
Methods
Between 1982 and 2000, 1,059 patients with primary esophageal squamous cell cancer or adenocarcinoma had resection with curative intention at a single center. Patient and tumor characteristics and details of the surgical procedure and outcome were documented during this period. Follow-up was available for 95.8% of the patients. Changing patterns in tumor type and postoperative deaths were analyzed. Prognostic factors for long-term survival were assessed by multivariate analysis.
Results
The prevalence of adenocarcinoma in patients with resected esophageal cancer increased markedly during the study period. The postoperative death rate decreased from about 10% before 1990 to less than 2% since 1994, coinciding with the introduction of a procedure-specific composite risk score and exclusion of high-risk patients from surgical resection. In addition to the well-established prognostic parameters, tumor cell type “adenocarcinoma” was identified as a favorable independent predictor of long-term survival after resection. The independent prognostic effect of tumor cell type persisted in the subgroups of patients with primary resection and patients with primary resection and R0 category.
Conclusion
Esophagectomy for esophageal cancer has become a safe procedure in experienced hands. Esophageal adenocarcinoma has a better long-term prognosis after resection than squamous cell carcinoma.
During recent years, the management and epidemiology of esophageal cancer have markedly changed in the Western world. Advances in perioperative management and standardization of the surgical technique have resulted in a substantial reduction in the number of postoperative deaths after esophagectomy in experienced centers. 1–3 There has been an alarming rise in the incidence of esophageal adenocarcinoma, while the incidence of squamous cell esophageal cancer appears to be stable. 4,5
Adenocarcinoma of the distal esophagus and squamous cell esophageal cancer are frequently treated as a single entity and no differentiation is made when reporting treatment results, 6–8 although there are marked differences in the pathogenesis, tumor location, tumor biology, and characteristics of these tumor entities. 9,10 This is because the prognostic impact of tumor type on the outcome of surgical treatment in patients with esophageal cancer has so far not been adequately addressed.
At the Department of Surgery of the Technische Universität München, adenocarcinoma and squamous cell carcinoma of the esophagus have been considered separate tumor entities since 1982. Clinical and treatment-related data as well as follow-up of more than 1,500 such patients were documented prospectively during the 18-year period. This database provided the unique opportunity to analyze the prognostic impact of tumor type and to assess the changing pattern in the use of multimodal concepts and postoperative deaths at a national referral center for esophageal cancer in the Western world.
METHODS
Patients
Between July 1982 and December 2000, 1,635 patients with primary malignant esophageal tumors were treated at the Chirurgische Klinik und Poliklinik, Klinikum rechts der Isar, Technische Universität München, Munich, Germany. Primary esophageal adenocarcinoma (n = 539) and squamous cell esophageal cancer (n = 1,013) represented the vast majority of these patients (1,552/1,635). The remainder were esophageal small carcinoma, undifferentiated carcinoma, neuroendocrine carcinoma, sarcoma, and other rare entities.
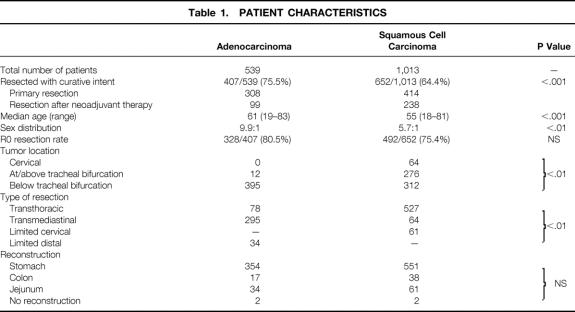
Of the 1,552 patients with primary esophageal adenocarcinoma or squamous cell cancer, 1,059 patients (407 with adenocarcinoma and 652 with squamous cell esophageal cancer) underwent resection with curative intent after exclusion of distant solid organ metastases. These 1,059 patients represent the study population. Patient and tumor characteristics as well as details of the surgical procedure were documented during the entire study period in these patients and are shown separately for adenocarcinoma and squamous cell carcinoma in Table 1.
Table 1. PATIENT CHARACTERISTICS
Staging and Selection of Therapeutic Approach
After histologic confirmation of the tumor, all patients were staged with standard imaging techniques to exclude distant solid organ metastases and to assess the resectability of the primary tumor. 11 During the initial phase, staging modalities included contrast radiography, computed tomography scan of the neck, chest, and abdomen, and percutaneous ultrasound. In patients with tumors at or above the level of the tracheal bifurcation, tracheobronchoscopy was performed to exclude invasion of the airways. 12 Since 1987 endoscopic ultrasound has also been routinely performed in all patients to assess the T category.
A standardized and previously validated detailed procedure-specific risk analysis has been performed systematically since 1994 to assess the physiologic reserve of the patients considered for esophagectomy. 13,14 A total composite score value of more than 21 constituted an exclusion criterion for esophagectomy or neoadjuvant protocols.
The selection of the therapeutic strategy was based on the resectability of the tumor and the patient’s physiologic status. 10 A primary surgical resection was performed when, based on pretherapeutic staging, a complete tumor resection (R0 resection according to the International Union against Cancer/American Joint Committee on Cancer Classification (UICC/AJCC) 15,16) could be anticipated with a high likelihood and the risk analysis indicated that the patient would tolerate an extensive surgical procedure. This was the case in 722 patients. If, based on pretherapeutic staging, a complete tumor resection appeared unlikely, the patient was included in neoadjuvant protocols provided his or her general status permitted aggressive treatment. 17 A total of 337 patients with locally advanced tumors underwent resection after neoadjuvant chemotherapy or combined radiochemotherapy within prospective phase 2 trials. The presence of enlarged lymph nodes at the lesser curvature of the stomach or along the celiac axis did not constitute a contraindication to a surgical or multimodal approach with curative intent. Primary nonsurgical treatment modalities with curative intent were used in patients with a poor general status. Patients with distant solid organ metastases on preoperative staging or esophagobronchial fistula received nonsurgical palliative treatment.
The preferred surgical approach was different for patients with adenocarcinoma and squamous cell carcinoma. The surgical procedure of choice for patients with adenocarcinoma of the distal esophagus was a radical transmediastinal subtotal esophagectomy and fundectomy with systematic lymphadenectomy in the lower posterior mediastinum and the upper abdominal compartment (n = 295). 10 A total of 78 patients with esophageal adenocarcinoma had an abdomino/right transthoracic en bloc esophagectomy with two-field lymphadenectomy 10 within a prospective study (n = 60), because of proximal tumor extent to or beyond the level of the tracheal bifurcation (n = 12) or because of extensive mediastinal lymphadenopathy (n = 6). Within a prospective study, a total of 34 patients with early adenocarcinoma of the distal esophagus (uT1N0 category on preoperative staging) had a limited transabdominal resection of the distal esophagus and esophagogastric junction with locoregional lymphadenectomy and interposition of a pedicled jejunal segment. 18
In patients with intrathoracic squamous cell esophageal cancer, an abdomino/right transthoracic en bloc esophagectomy with extended mediastinal lymphadenectomy and lymph node dissection of the upper abdominal compartment (two-field lymphadenectomy) 10 was the procedure of choice (n = 527). Sixty-four patients with squamous cell esophageal cancer had a radical transmediastinal subtotal esophagectomy and fundectomy with systematic lymphadenectomy in the lower posterior mediastinum and the upper abdominal compartment because of a compromised physiologic status and early tumors in the very distal esophagus. A total of 61 patients with cancer limited to the cervical esophagus had a sleeve resection of the cervical esophagus with interposition of a free jejunal transplant. This procedure was usually performed after neoadjuvant combined radiochemotherapy.
Reconstruction after transmediastinal or transthoracic esophagectomy was performed with a gastric pullup whenever possible (n = 905). Left or transverse colon interposition was performed if the stomach was not available for reconstruction (n = 55).
The selection of neoadjuvant treatment also differed between patients with adenocarcinoma and squamous cell carcinoma. In patients with adenocarcinoma, neoadjuvant treatment consisted of cisplatin-based polychemotherapy. 10,17 In patients with squamous cell esophageal cancer, combined radiotherapy and chemotherapy with 30 to 60 Gy and 5-fluorouracil with or without cisplatin or mitomycin was given within an series of phase 2 studies. 10,17
Histopathologic Assessment of the Removed Specimen and Lymph Nodes
All resected specimens were assessed according to the UICC/AJCC standards and classified or reclassified according to the 1997 guidelines 15,16 by an experienced pathologist. The pT1 tumors were subclassified as pT1a (limited to the mucosa) or pT1b (infiltration of the submucosa). All removed lymph nodes were counted and assessed separately for tumor involvement with standard histopathologic techniques.
Follow-Up
The survival status of all patients in the study population was ascertained between October and December 2000. Survival data were available for 1,014 of the 1,059 (95.8%) patients. Median follow-up of the surviving patients was 58 months (range 1–211).
Data Analysis
A two-tailed Fisher exact test was used to compare proportions. Mean and median values were compared by standard statistical tests as appropriate. Death within 30 days of the surgical resection and survival time were used as endpoints for assessing postoperative death and prognostic factors. Survival probabilities were calculated using the Kaplan-Meier method. 19 Comparison of survival rates was performed by log-rank analysis. Prognostic factors were assessed by multiple stepwise regression analysis using the Cox model. 20 Because of the large patient population, only variables with a significance level of more than 1% were included in the model. The entire statistical analysis was performed using the SPSS software package (Version 10.0.5; SPSS Inc, Chicago, IL).
RESULTS
The overall resection rate was 68.2% (1,059/1,552 patients) and was significantly greater in patients with adenocarcinoma (75.5%) than in patients with squamous cell carcinoma (64.4%) (P < .001). Patients with resected adenocarcinoma (median age 61 years, range 19–83) were significantly older than patients with squamous cell esophageal cancer (median 55 years, range 18–81, P < .01). There was also a higher preponderance of the male sex in patients with adenocarcinoma (9.9:1 vs. 5.7:1, P < .01). In contrast to squamous cell esophageal cancer, the vast majority of adenocarcinomas were in the distal esophagus (see Table 1).
Among the patients who underwent resection, the rate of adenocarcinoma steadily increased during the study period from less than 30.9% before 1987 to more than 47% since 1997 (Fig. 1). The use of neoadjuvant treatment modalities (preoperative chemotherapy or combined radiochemotherapy) also increased markedly during the study period (Fig. 2).
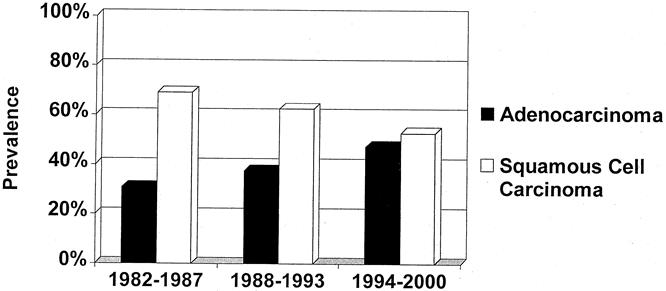
Figure 1. Increasing prevalence of adenocarcinoma among patients with resected esophageal cancer.
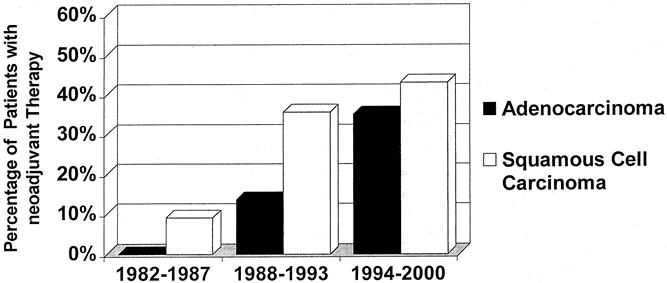
Figure 2. Increasing use of neoadjuvant chemotherapy or combined radiochemotherapy in patients who underwent resection for esophageal cancer during the study period.
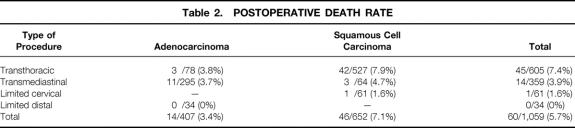
The overall postoperative death rates for the various procedures and tumor types during the study period are shown in Table 2. Overall, transthoracic en bloc esophagectomy was associated with a higher postoperative death rate than radical transmediastinal esophagectomy (P < .05). The overall postoperative death rate was lower when resection was performed for esophageal adenocarcinoma versus squamous cell esophageal cancer (P < .01). During the study period, the postoperative death rate markedly decreased from about 10% before 1990 to less than 2% since 1994 (Fig. 3). This coincided with the introduction of a procedure-specific composite risk score for exclusion of high-risk patients from surgical resection. 13,14
Table 2. POSTOPERATIVE DEATH RATE
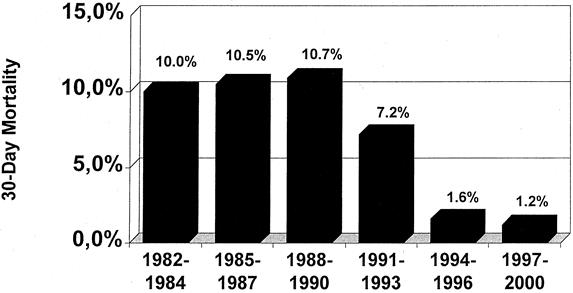
Figure 3. Decrease in the postoperative death rate after esophagectomy for esophageal cancer during the study period.
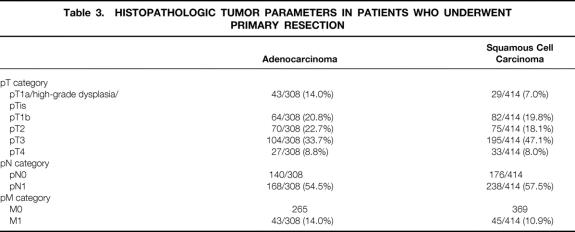
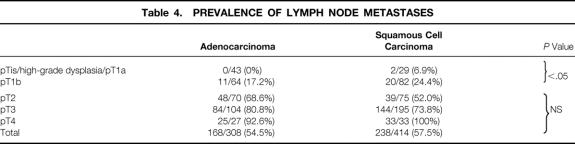
The distribution of the histopathologic tumor parameters of the patients who had a primary resection are shown separately for adenocarcinoma and squamous cell carcinoma in Table 3. There was a higher prevalence of patients with early tumors (pT1) in the adenocarcinoma group, whereas pT3 predominated in the squamous cell carcinoma group. The overall prevalence of patients with lymph node metastases was similar for both groups. For both adenocarcinoma and squamous cell carcinoma, the prevalence of patients with positive lymph nodes increased markedly with increasing pT category (Table 4). In patients with early tumors (high-grade dysplasia, pT1a, pT1b), the likelihood of lymph node metastases was, however, significantly less if the tumor cell type was of the adenocarcinoma type (P < .05).
Table 3. HISTOPATHOLOGIC TUMOR PARAMETERS IN PATIENTS WHO UNDERWENT PRIMARY RESECTION
Table 4. PREVALENCE OF LYMPH NODE METASTASES
A complete macroscopic and microscopic tumor resection (R0 resection) was achieved in 82.1% of patients with adenocarcinoma and 78.0% of patients with squamous cell carcinoma who had a primary resection (not significant). The relationship between the pT category and the R0 resection rate is shown in Table 5. The chance of complete tumor removal decreased markedly with increasing pT category in both tumor entities. However, although an R0 resection could be achieved in all patients with early adenocarcinoma (high-grade dysplasia, pT1a, pT1b), this was not the case in patients with squamous cell carcinoma. Reasons for incomplete tumor resection in these patients were invasion of lymphatic vessels or multicentric tumor growth at the oral resection margin.
Table 5. RATE OF R0 RESECTION IN RELATION TO pT CATEGORY
Multivariate analysis of predictors of long-term survival in the entire population of patients (n = 1,059) identified tumor cell type “adenocarcinoma” as an independent favorable prognostic parameter in addition to the well-established prognostic parameters R category, pT category, pN category, and M category (Table 6). The independent prognostic effect of tumor cell type was confirmed when multivariate analysis was performed in the subgroups of patients with primary resection (n = 722) and patients with primary resection and R0 category (n = 578).
Table 6. INDEPENDENT PROGNOSTIC FACTORS
The overall 5-year survival rate of patients with resected adenocarcinoma was 42.3% versus 30.3% for patients with resected squamous cell esophageal cancer (P < .01) (Fig. 4). In patients with primary resection and complete tumor removal (R0 category), the 5-year survival rate was 46.8% for those with adenocarcinoma and 37.4% for those with squamous cell carcinoma (P < .01) (Fig. 5). In patients with a primary resection, R0 category, and pN0 category, the 5-year survival rate was 72.9% for those with adenocarcinoma and 56.8% for those with squamous cell carcinoma (Fig. 6).
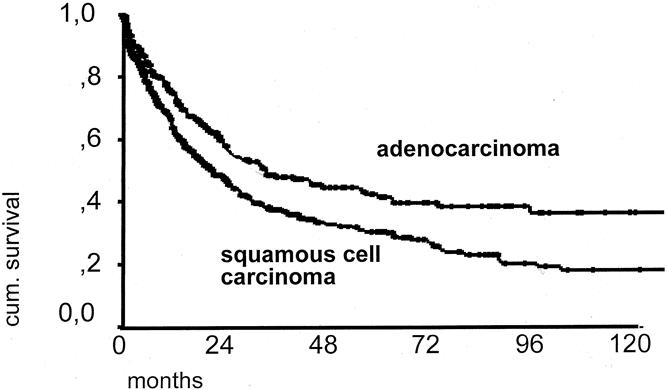
Figure 4. Overall 10-year survival curve of patients with resected adenocarcinoma (n = 407) versus patients with resected squamous cell carcinoma of the esophagus (n = 652).
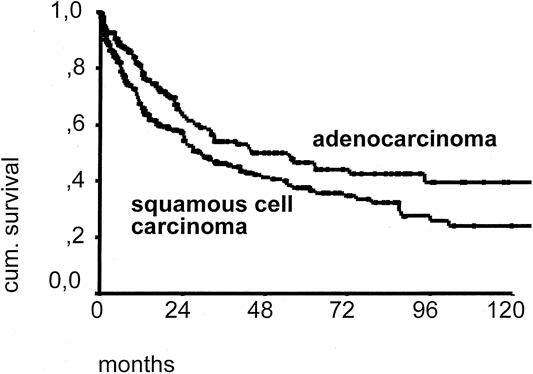
Figure 5. Ten-year survival curve of patients with adenocarcinoma (n = 253) versus patients with resected squamous cell carcinoma of the esophagus (n = 323). Only patients with primary resection and complete macroscopic and microscopic tumor removal (R0 resection) are included.
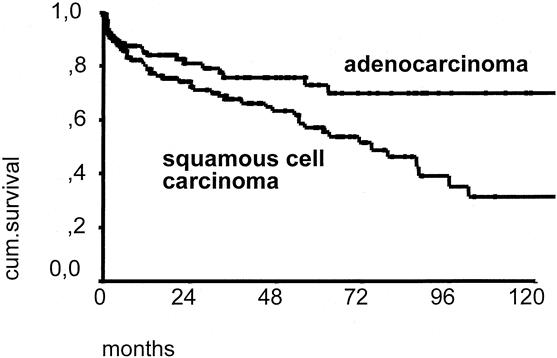
Figure 6. Ten-year survival curve of patients with adenocarcinoma (n = 137) versus patients with resected squamous cell carcinoma of the esophagus (n = 160). Only patients with primary resection, complete macroscopic and microscopic tumor removal (R0 category), and N0 category are included.
DISCUSSION
Adenocarcinoma of the esophagus, once considered a rare entity, continues to increase in prevalence and incidence in the Western world and now equals or outnumbers squamous cell esophageal cancer at many referral centers in the United States and Europe. 4,5 Whereas esophageal adenocarcinoma usually arises as a consequence of persistent gastroesophageal reflux from areas with specialized intestinal metaplasia in the distal esophagus (i.e., Barrett esophagus 21), squamous cell esophageal carcinoma is clearly related to alcohol and nicotine abuse. The present study is the first to show that esophageal adenocarcinoma and squamous cell carcinoma markedly differ in their prognosis after surgical resection. Compared with squamous cell esophageal cancer, long-term survival after surgical resection was significantly better in patients with adenocarcinoma independent of the classic histopathologic parameters (i.e., T, N, and R categories), surgical approach (i.e., transthoracic or transmediastinal), or neoadjuvant therapy and despite a markedly higher median age at the time of surgery. This establishes esophageal adenocarcinoma as a tumor entity separate from esophageal squamous cell carcinoma.
In agreement with other studies, 6–8,22–25 we identified a complete macroscopic and microscopic tumor resection (R0 resection) and the presence of lymph node metastases were also identified as independent prognostic factors in patients with resected esophageal carcinoma. To achieve a complete tumor resection, a transthoracic approach is favored by most surgeons in the Western world for the resection of both squamous cell cancer and adenocarcinoma of the esophagus. 6–8,22–24 The present study shows that in patients with adenocarcinoma of the distal esophagus, an R0 resection can also be achieved using a radical transmediastinal approach in the vast majority of patients. Further, little is known about the pattern of lymphatic spread and thus the optimal extent of lymphadenectomy in patients with distal esophageal adenocarcinoma. Whereas extended mediastinal lymphadenectomy via a transthoracic approach is widely accepted as standard in patients with squamous cell esophageal cancer, 26 the need for lymphadenectomy in the upper mediastinum, and consequently a transthoracic approach, in patients with esophageal adenocarcinoma is usually inferred from the experience with squamous cell esophageal cancer. Analysis revealed that lymphatic spread in patients with esophageal adenocarcinoma, in contrast to squamous cell esophageal cancer, is primarily directed toward the lower posterior mediastinum, the paracardial region, and along the lesser curvature of the stomach to the celiac axis. Skip metastases to the tracheal bifurcation or upper mediastinum occurred in only 6% of patients who did not have positive regional nodes (data presented at the European Surgical Association Meeting, April 2001, Berlin). This questions the need for a routine extended mediastinal lymphadenectomy, and thus a thoracotomy, in patients with adenocarcinoma of the distal esophagus because the primary area of lymphatic spread can usually be reached via a transabdominal approach and wide splitting of the esophageal hiatus. 10 In contrast to squamous cell esophageal cancer, a radical transmediastinal resection with systematic lymphadenectomy in the lower posterior mediastinum and upper abdominal compartment therefore appears to be the appropriate procedure for the vast majority of patients with adenocarcinoma of the distal esophagus in terms of achieving an R0 resection and performing an adequate lymphadenectomy. This is underlined by the impressive 5-year survival rate of about 80% in patients with adenocarcinoma of the distal esophagus staged as R0N0 after transmediastinal esophagectomy and the lower postoperative death rate of this procedure compared with a transthoracic approach.
The current study also shows a close relation between the depth of tumor penetration and the prevalence of lymph node metastases in both adenocarcinoma and squamous cell carcinoma. This has also been noted by others. 27 Of particular interest is that in contrast to squamous cell carcinoma, lymph node metastases were never present in patients with adenocarcinoma limited to the mucosa (high-grade dysplasia, pT1a) and were uncommon in patients with adenocarcinoma limited to the submucosa (pT1b). Occlusion of submucosal channels as a result of the inflammatory changes caused by the usually underlying chronic reflux disease in patients with adenocarcinoma of the distal esophagus may account for these differences. This concept is supported by the low rate of lymph node microinvolvement in patients with adenocarcinoma of the distal esophagus compared with patients with squamous cell esophageal cancer. 28 These observations provide the justification for a limited surgical approach to early adenocarcinoma of the distal esophagus. 18
Irrespective of the surgical approach, the postoperative death rate after esophageal resection has markedly decreased in experienced centers during the past decade. 1–3 This has been attributed to improvements in anesthesia, standardization and refinements of surgical techniques, and aggressive management of postoperative complications. Surgical volume and experience is another factor that has been linked to death after esophagectomy. 3,29,30 We found a marked decline in postoperative deaths from around 10% before 1992 to less than 2% since 1994. This coincided with the introduction of a procedure-specific composite risk score and strict exclusion of high-risk patients from surgical resection or multimodal treatment protocols. 13,14 A combination of patient selection, accumulating experience, standardization of surgical technique, and detailed attention to postoperative care therefore appears to be key to avoiding death after esophagectomy. These parameters can be provided only in centers with a high patient volume and a dedicated interest in the management of this disease.
The overall long-term survival rates after primary surgical resection of esophageal adenocarcinoma in the present series compare very favorably to the survival rates reported from other dedicated esophageal cancer centers in the Western world 24,31,32 and are clearly superior to the survival rates in the surgical treatment arm of a recent randomized multicenter trial comparing multimodal therapy with primary surgical resection. 33 Primary resection at an experienced center should therefore remain standard in all patients with potentially resectable esophageal adenocarcinoma, and neoadjuvant treatment should be reserved for those with locally advanced tumors.
In contrast, the survival rates for patients with resected squamous cell esophageal cancer are unsatisfactory, even for patients with RO and N0 category. This may be explained by the high prevalence of occult micrometastases in these patients. 34 Better survival rates after surgical resection for squamous cell esophageal cancer are reported by Japanese centers who practice a more radical approach toward lymphadenectomy, with extended three-field lymph node dissection. 22,23 The complications associated with three-field lymph node dissection, however, appear unacceptable for patients in the Western world. 35 Rather, more efforts are required to improve current multimodal treatment concepts and identify patient groups who will benefit from neoadjuvant or adjuvant treatment. 36,37
In summary, our study is the first to show that independent of the classic histopathologic parameters (i.e., the T, N, and R categories), esophageal adenocarcinoma has a more favorable prognosis after surgical resection than squamous cell esophageal cancer. In addition, the pattern of lymphatic metastases of esophageal adenocarcinoma appears to differ from that of squamous cell esophageal carcinoma. Together with the well-documented differences in the pathogenesis and associated risk factors, this establishes esophageal adenocarcinoma as a tumor entity separate from esophageal squamous cell carcinoma and justifies differentiated therapeutic concepts for these two tumor entities.
DISCUSSION
Dr. Murray F. Brennan (New York, New York): I would like to thank Professor Siewert for the kind invitation to discuss this paper and of course, for the privilege of receiving the manuscript. As many would appreciate, esophageal cancer is certainly not my forte, and I will leave the erudite questions to the more facile esophageal surgeons.
Dr. Siewert and his group, however, illustrate a difference between the way in which surgery is practiced in this country and in Germany. In the main, esophageal cancer is managed in this country by surgeons working in non-cardiac thoracic surgical units. A consequence of this is that the GE junction tumor, a rapidly increasing adenocarcinoma in the Western world, as Dr. Siewert emphasized, is often subject to two different approaches.
Dr. Siewert and his group, however, managing, as they do, both esophageal and gastric carcinoma, have been able to provide a more standardized approach, and certainly his contributions in defining GE junction adenocarcinoma types 1 through 3 and their behavior is a major contribution.
The present paper builds on his group’s experience with esophageal adeno and squamous carcinoma. The manuscript is extraordinarily replete with extensive demographic data that I recommend to you and clearly divides the approach between squamous cell and adenocarcinoma. In addition, they emphasize the marked increase in adenocarcinoma and, interestingly, define early stage adenocarcinoma, pathological T1A or 1B, as having a lesser prevalence of lymph node metastases than similar stage for squamous cell.
Dr. Siewert, is the reason for this known? Does this observation account for the better survival for patients with adenocarcinoma? It would appear not, in fact, as the five-year survival rate for adenocarcinoma in the pathologically N-zero R-zero group remains 15%, better than for squamous cell carcinoma.
I think we should recognize the privilege of having this presentation from one of the world leaders in this problem. Professor Siewert, thank you.
Presenter Dr. J. Rudiger Siewert (Munich, Germany): Thank you very much for your very kind remarks. You have stressed, and this is correct, that it is sometimes difficult to have a discussion on esophageal surgery between American surgeons and European surgeons, because in America general thoracic surgeons are interested in the field of esophageal disease and in Europe the GI tract surgeons are interested in esophageal surgery. So the access to this problem, not only the approach of the operation, is sometimes a little bit different.
May I answer one short question that you have touched? That is the delay in the beginning of LN. That needs an explanation. I can offer to you only a hypothesis. I have the feeling that as a result of the long-lasting inflammation as a consequence of reflux esophagitis the lymphatic channels in the submucosa are obliterated over time. Maybe this is an explanation for the late beginning of LN, but it is only a hypothesis. Dr. Tom R. DeMeester (Los Angeles, California): I thank the society for the opportunity to comment on Dr. Siewert’s presentation, and to thank him personally for a copy of the manuscript. The work certainly comes from a center with a large and extensive experience in esophageal surgery. Dr. Brennan’s previous comments have made that very clear, and emphasized the esteem with which the society holds its honorary member, Dr. Siewert.
The message of the paper is that esophageal adenocarcinoma has a better long-term prognosis than squamous cell carcinoma. This was derived from a study group of 1,059 patients, of whom 40% had adenocarcinoma and 50% had squamous cell carcinoma. All were operated upon with an attempt to cure.
When you read through the manuscript, you realize that this is not a homogeneous population. It differs statistically in a number of ways, as Dr. Siewert has pointed out. There is a difference in the prevalence of early disease between the two groups, particularly those with T1B stage of disease. The two groups differ in tumor location that is, above or below the carina, a factor that limits an en bloc procedure and, to a considerable extent, the completeness of a resection. Twenty-five percent of the patients with adenocarcinoma received neoadjuvant therapy, compared to 35% of the patients with squamous carcinoma. There was a difference in the operative approach, abdominal or thoracic, in the two groups. Over the study period, there was a change in the operative mortality that could affect the analysis. The prevalence of concomitant operative risk factors are different between the groups, such as smoking, alcohol use, and the existence of liver, lung, or cardiac disease.
Despite these variations, multivariate analysis did identify that cell type was an independent prognostic factor with a relative risk factor that was just behind complete resection, nodal metastases, and tumor depth. The analysis goes on to show that the relative risk of cell type became less as the population became more homogeneous, that is by including only those patients that had surgery alone and did not receive chemotherapy.
My question to you, Dr. Siewert, is this: Do you think that the statistical tool of multivariate analysis is strong enough to accurately analyze such a varied population and come up with a sound conclusion? An alternative approach would be to use a more selected patient population, that were similar to each other in extent of disease, therapy and comorbid conditions. This is what an epidemiologist would do in such an analysis; using a computer-matching program to make the other variables similar, and then ask the question whether adenocarcinoma has a better prognosis than squamous cell carcinoma. I personally believe that it does, but I am not sure if your analysis gives evidence to my belief.
Dr. J. Rudiger Siewert: Thank you for this very critical analysis of our data. In some points you are quite right, in some points not.
There is no difference in tumor stages. Both groups have comparable tumor stages, as I have shown to you. Of course, there is a difference in location. There is no difference in our resection rates. There is a difference in the type and frequence of neoadjuvant therapies. There is a difference in operative approach. And there is a difference in risk factors. That is absolutely correct.
The reason why we have done multivariate analysis is from the statistical point of view that a multivariate analysis, if it was done stepwise, excludes all the dependent prognostic factors. And it was done stepwise. It ends with the independent factors. Of course, I agree with you, at the end it remains a retrospective analysis. But the only way to bring such a retrospective analysis to a success is to do it with a multivariate analysis.
Dr. John Wong (Hong Kong, China): Dr. Way, members and guests of the Association, I thank Professor Siewert for sending me his manuscript for review. May I commend him for the excellent surgical results, and his thesis that adenocarcinoma cell type is a favorable, independent prognostic factor in esophageal cancer survival after resection.
As you can see in my slide, in our 1,000 or so resections for esophageal cancer in Hong Kong, we did not find any survival advantage for adenocarcinoma against squamous cancer. However, our population is different from those treated in Munich and perhaps the most striking difference is that more than half of our patients were resected for palliation i.e. not RO resections. But even with RO resections — we did not find a difference in the two cell types.
In the data presented by Professor Siewert, there are explanations other than cell type to account for the difference in survival. In my opinion, there are many differences in the two groups of patients, some of which have been shown to be a significant impact survival.
These are — to reiterate and emphasis:
1. The vast majority of adenocarcinoma is in the distal esophagus.
2. Incidences of adenocarcinoma have increased dramatically in the 18-year study period.
3. The operation for adenocarcinoma mostly did not involve a thoracotomy.
4. The operative mortality (30-day) is lower for adenocarcinoma.
5. Overall reduction in mortality rate may benefit adenocarcinoma (more than squamous cancer).
6. High prevalence of early tumor in adenocarcinoma (perhaps from surveillance progress for Barrett’s esophagus).
7. Early adenocarcinoma is less likely to metastasis than squamous carcinoma.
8. More complete RO resection was achieved for adenocarcinoma.
I have three questions for Professor Siewert:
1. Has subset analysis of some of these variables over different time periods been carried out to more categorically can establish that these variables are not relevant?
2. In the non-resected groups, has the same adenocarcinoma cell type advantage been found?
3. Has cellular or molecular mechanisms been investigated to account for the favorable outcome for adenocarcinoma, and if so can he share any results with us?
Once again, my congratulation on a fine paper and a stimulating concept. My thanks to Professor Siewert for the opportunity to review the manuscript.
Dr. J. Rudiger Siewert: Thank you very much for the very kind remarks.
I think there is a little misunderstanding. I never have said that the cell type alone makes a difference. I have said that the different population and the type of disease and the histological type all together make the difference. So it is very difficult to say that only the cell type is responsible for the different prognoses.
Coming to your question: I have no information about non-resected patients because these patients are going to the oncologists and they are not included in our database. I am sorry I cannot give you the answer you want to hear.
And of course it makes sense in the future to look for molecular markers to identify interesting sub-groups in Barrett and squamous cell cancer. That is the reason why we have just started in Germany the so-called German Barrett-CA project, which is sponsored by the German Research Foundation and is organized in Munich. We will do this research in the future and maybe we have a chance to come back in two or three years to present to you some new data about the molecular markers.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Correspondence: Prof. J. R. Siewert, Chairman and Director, Chirurgische Klinik und Poliklinik, Klinikum rechts der Isar der TU München, Ismaningerstr 22, D-81675 München, Germany.
E-mail: siewert@nt1.chir.med.tu-muenchen.de
Accepted for publication April 26, 2001.
References
- 1.Whooley BP, Law S, Murthy SC, et al. Analysis of reduced death and complication rates after esophageal resection. Ann Surg 2001; 233: 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999; 230: 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg 2000; 119: 1126–1132. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83: 2049–2053. [PubMed] [Google Scholar]
- 5.Daly JD, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000; 190: 562–573. [DOI] [PubMed] [Google Scholar]
- 6.Lerut T, DeDeyn P, Coosemanns W, et al. Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy. Ann Surg 1992; 216: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990; 77: 845–857. [DOI] [PubMed] [Google Scholar]
- 8.Hagen JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993; 106: 850–858. [PubMed] [Google Scholar]
- 9.Bollschweiler E, Schroder W, Holscher AH, Siewert JR. Preoperative risk analysis in patients with adenocarcinoma or squamous cell carcinoma of the oesophagus. Br J Surg 2000; 87: 1106–1110. [DOI] [PubMed] [Google Scholar]
- 10.Siewert JR, Stein HJ, Sendler A, et al. Esophageal cancer: clinical management. In: Kelsen DA, ed. Principles and practice of gastrointestinal oncology. Philadelphia: Lippincott Williams & Williams; 2001.
- 11.Stein HJ, Brücher BLDM, Sendler A, Siewert JR. Esophageal cancer: patient evaluation and pretreatment staging. Surg Oncol 2001 (in press). [DOI] [PubMed]
- 12.Riedel M, Stein HJ, Mounyam L, et al. Extensive sampling improves preoperative bronchoscopic assessment of airway invasion by supracarinal esophageal cancer: a prospective study in 166 patients. Chest 2001; 119: 1652–1660. [DOI] [PubMed] [Google Scholar]
- 13.Bartels H, Stein HJ, Siewert JR. Risk analysis in esophageal surgery. Recent Results Cancer Res 2000; 155: 89–96. [DOI] [PubMed] [Google Scholar]
- 14.Bartels H, Stein HJ, Siewert JR. Preoperative risk-analysis and postoperative mortality of osophagectomy for resectable oesophageal cancer. Br J Surg 1998; 85: 840–844. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Wittekind C, International Union Against Cancer (IUCC). TNM classification of malignant tumors, 5th ed. New York: John Wiley & Sons; 1997.
- 16.Fleming ID, American Joint Committee on Cancer Classification (AJCC). AJCC cancer Staging manual. Philadelphia: Lippincott; 1997.
- 17.Stein HJ, Sendler A, Fink U, Siewert JR. Multidisciplinary approach to esophageal and gastric cancer. Surg Clin North Am 2000; 80: 659–682. [DOI] [PubMed] [Google Scholar]
- 18.Stein HJ, Feith M, Mueller J, et al. Limited resection for early adenocarcinoma of the Barrett’s esophagus. Ann Surg 2000; 232: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–462. [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J Royal Stat Soc 1972; 34: 187–220. [Google Scholar]
- 21.DeMeester SR, DeMeester TR. Columnar mucosa and intestinal metaplasia of the esophagus: fifty years of controversy. Ann Surg 2000; 231: 303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994; 220: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous cell esophageal carcinoma during 15 consecutive years. Ann Surg 2000; 232: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collard JM. Exclusive radical surgery for esophageal adenocarcinoma. Cancer 2001; 91: 1098–1104. [PubMed] [Google Scholar]
- 25.Stein HJ, Feith M. Cancer of the esophagus. In: Gospodarowicz M, ed. Prognostic factors in cancer. New York: Wiley-Liss; 2001: 211–221.
- 26.Fumagalli U. Resective surgery for cancer of the thoracic esophagus: results of a consensus conference. Dis Esoph 1996; 9 (suppl): 30–38. [Google Scholar]
- 27.Rice TW, Zuccaro G, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 1998; 65: 787–792. [DOI] [PubMed] [Google Scholar]
- 28.Feith M, Werner M, Rosenberg R, et al. Lymph node ‘micrometastases’ and ‘microinvolvement’ in esophageal carcinoma. Onkologie 2000; 13: 330–333. [DOI] [PubMed] [Google Scholar]
- 29.Dudley RA, Johansen KL, Brand R, et al. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA 2000; 283: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 30.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 31.Lerut T, Coosemans W, Van Raemdonck D, et al. Surgical treatment of Barrett’s carcinoma. Correlations between morphologic findings and prognosis. J Thorac Cardiovasc Surg 1994; 107: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 32.Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 1999; 117: 16–23. [DOI] [PubMed] [Google Scholar]
- 33.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335: 462–467. [DOI] [PubMed] [Google Scholar]
- 34.Natsugoe S, Mueller J, Stein HJ, et al. Micrometastasis and tumor cell microinvolvement of lymph nodes esophageal squamous cell cancer: frequency, associated tumor charcteristics and impact on prognosis. Cancer 1998; 83: 858–866. [PubMed] [Google Scholar]
- 35.Siewert JR, Stein HJ. Lymphadenectomy for squamous cell esophageal cancer. Langenbecks Arch Surg 1999; 384: 141–148. [DOI] [PubMed] [Google Scholar]
- 36.Stein HJ, Fink U, Siewert JR. Who benefits from combined modality treatment of esophageal carcinoma? Dis Esoph 1994; 7: 156–161. [Google Scholar]
- 37.Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg 2001; 88: 338–356. [DOI] [PubMed] [Google Scholar]