Abstract
Apoptosis of CD4+ T lymphocytes, induced by contact between human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (gp120) and its receptors, could contribute to the cell depletion observed in HIV-infected individuals. CXCR4 appears to play an important role in gp120-induced cell death, but the mechanisms involved in this apoptotic process remain poorly understood. To get insight into the signal transduction pathways connecting CXCR4 to apoptosis following gp120 binding, we used different cell lines expressing wild-type CXCR4 and a truncated form of CD4 that binds gp120 but lacks the ability to transduce signals. The present study demonstrates that (i) the interaction of cell-associated gp120 with CXCR4-expressing target cells triggers a rapid dissipation of the mitochondrial transmembrane potential resulting in the cytosolic release of cytochrome c from the mitochondria to cytosol, concurrent with activation of caspase-9 and -3; (ii) this apoptotic process is independent of Fas signaling; and (iii) cooperation with a CD4 signal is not required. In addition, following coculture with cells expressing gp120, a Fas-independent apoptosis involving mitochondria and caspase activation is also observed in primary umbilical cord blood CD4+ T lymphocytes expressing high levels of CXCR4. Thus, this gp120-mediated apoptotic pathway may contribute to CD4+ T-cell depletion in AIDS.
Human immunodeficiency virus type 1 (HIV-1) infected patient evolution toward AIDS is characterized by a progressive drop in the number of CD4+ T lymphocytes, and virus-induced apoptosis has been proposed as a possible mechanism of HIV pathogenicity (17, 37, 42). Recent studies have demonstrated that CXCR4 triggers programmed cell death upon binding to the HIV-1 envelope glycoprotein gp120 (8, 9, 11, 26, 27). Although features of anti-CD4- and anti-CXCR4-induced T cell apoptosis have been described (8), few characteristics of cell death triggered upon gp120 binding to CXCR4 have been demonstrated. Fas signaling-mediated apoptosis may contribute to functional T lymphocyte defects and cell depletion observed in HIV-induced disease (2–4, 12, 29, 30, 43, 67), but involvement of this death receptor is still controversial (8, 19, 44, 46). In addition, direct implication of caspases in gp120-mediated apoptosis of CXCR4+ cells is a subject of debate. Berndt and collaborators described no involvement of known caspases in cross-linked recombinant gp120- and anti-CXCR4-induced apoptosis of human peripheral blood lymphocytes (8) and Vlahakis et al. reported that CXCR4-dependent cell death is caspase independent on the basis of caspase inhibitors (65). However, caspase-3 is cleaved in primary T lymphocytes (15) and endothelial cells (61) following binding of HIV-1 envelope glycoproteins. The manner in which gp120 is presented, the manner in which the cell population is analyzed, and the nature of the receptor directly involved in this cell death could be responsible for the discrepancies between these reports. We previously found indirect evidence for caspase involvement in this cascade, as the specific interaction of CXCR4 with cell-associated gp120 resulted in an apoptosis which was blocked by DEVD, a caspase-3 inhibitor, but not by YVAD, a caspase-1 inhibitor (9). We have therefore further investigated the role played by the Fas receptor, caspases as well as known upstream and downstream caspase-signaling elements in CXCR4-gp120-induced apoptosis.
The caspase family of cysteine proteases regulates the execution of the apoptotic cell death program (16, 55, 60). Caspases are synthesized as inactive proenzymes that are processed in cells undergoing apoptosis by self-proteolysis and/or cleavage by another protease. Caspase-3, a key effector caspase (58), can be activated by several activated initiator caspases such as caspase-9, whose activation is achieved within an apoptosome that consists of a large caspase-activating complex formed by apoptotic protease-activating factor 1, cytochrome c, and dATP (22, 38, 57, 72).
A large body of evidence now emerges that mitochondria play a central role in programmed cell death (23, 32). Several different events occur at the level of mitochondrial apoptosis, including loss of the inner mitochondrial transmembrane potential (ΔΨm), resulting in an uncoupling of oxidative phosphorylation, generation of superoxide free radicals, dumping of matrix-associated calcium into the cytosol, and apoptotic protein release (cytochrome c and apoptosis-inducing factor) (28). Cytochrome c release and mitochondrial membrane depolarization have both been proposed as early irreversible events in the initiation of the cell death program even if the relationship between these two phenomena is currently not clear. One hypothesis is that opening of the permeability transition pore (PTP), a complex composed of several polypeptides at the membrane of mitochondria, causes a dissipation of the ΔΨm (7, 31, 33, 69, 71), leading to the mechanical disruption of the outer mitochondrial membrane and consequently cytochrome c release (23, 33).
The aim of the present work was to analyze the cascade of events leading to apoptosis after gp120 binding to CXCR4. To specifically study the role of this coreceptor in the absence of a CD4 signal, which may also contribute to apoptosis after HIV envelope glycoprotein contact (8, 15), cell lines expressing only the external part of the CD4 molecule were generated. This domain is needed to allow subsequent gp120 binding to CXCR4 (35, 54). Using two complementary cellular models, we demonstrate that gp120-induced apoptosis of human embryonic kidney (HEK) cells or a T-cell line expressing CXCR4 and a truncated form of CD4, incapable of transducing a signal on its own, occurs independently of Fas activation. Importantly though, mitochondrial depolarization, cytochrome c release, activation of caspase-9 and -3, and DNA damage are successively triggered.
To confirm that this gp120-dependent apoptotic cascade occurs in primary T cells following HIV infection, we analyzed apoptosis of umbilical cord (UC) blood CD4+ T cells after coculture with HEK cells stably expressing gp120 molecules. Of note, these primary UC CD4+ T cells are truly naive and thus constitute a homogeneous population of cells expressing a high number of CXCR4 molecules. gp120-mediated death in these cells is inhibited by the CXCR4 ligand and involves mitochondrial transmembrane depolarization and caspase-3 activation. In agreement with the data obtained in cell lines, this apoptosis occurred independently of Fas-mediated signaling.
MATERIALS AND METHODS
Antibodies and reagents.
Anti-FasL antibody was purchased from TEBU (Le Perray en Yvelines, France). The anti-CD4 monoclonal antibody (MAb) (BL4) was kindly provided by M. Hirn (Beckman-Coulter, Villepinte, France). The anti-CXCR4 MAb (MAB173) and human SDF-1 were purchased from R&D Systems Europe, Ltd. (Abington, United Kingdom). Fluorescein isothiocyanate (FITC)-labeled Fab′2 goat anti-human, anti-rabbit, and anti-mouse immunoglobulins (Ig) were purchased from Immunotech (Beckman-Coulter). Anti-Fas MAbs (clones CH11 and ZB4) were purchased from Euromedex (Souffelweyersheim, France). Polyclonal anti-gp120 human antibodies were kindly provided by J. P. Vendrell (Hopital Lapeyronie, Montpellier, France). Anti-human Hsp60 antibody and the caspase-3 inhibitor z-DEVD.fmk were purchased from Merck Eurolab (Fontenay sous Bois, France). Annexin-V-FITC and antibodies against caspase-3 and -9 and cytochrome c were purchased from Becton Dickinson (Le Pont de Claix, France). Mito Tracker Orange CMTM Ros and the anti-human cytochrome oxidase subunit II MAb (12CA-F12) were purchased from Interchim (INTERBIOtech, Montlucon, France). Peroxidase-coupled goat anti-rabbit and anti-mouse Igs, carbamoyl cyanide m-chlorophenylhydrazone (mClCCP), 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)], and dexamethasone were purchased from Sigma-Aldrich (L'Isle D'Abeau Chesnes, France). IL-4 was purchased from PreproTech EC Ltd. (London, United Kingdom).
Cells.
The HEK-293 cell line was stably transfected with the T-tropic HIV-1 defective pBRUΔgag construct, an expression vector containing the HIV-1 LAI genome (formerly LAV/HIV-1 Bru strain, provided by L. Montagnier [Institut Pasteur, Paris, France] [66]) deleted of the gag gene segment PstI-ApaI). This vector allows T-tropic HIV-1 cell surface envelope expression (HEK.gp120 clone). The CEM T-cell line was provided by the American Type Culture Collection (Manassas, Va.). The A2.01/CD4.403 (A2.01 expressing a mutant form of CD4 truncated at position 403) cell clone has been previously described (6) and was provided by D. R. Littman (New York Medical College, New York, N.Y.). The 8.E5 cell line, a CEM-derived T-cell line containing a single integrated copy of HIV-1, provided by F. Barré-Sinoussi (Institut Pasteur), was cultured in RPMI 1640 medium supplemented with a 1% penicillin-streptomycin antibiotic mixture, 1% Glutamax, and 10% fetal calf serum (Life Technologies, Cergy Pontoise, France) to a density of 5 × 105 cells/ml in a 5% CO2 atmosphere. The HEK-293 cell lines stably expressing gp120 molecules, a truncated form of CD4 lacking the intracytoplasmic domain (CD4.403) or the CD4.403 and CXCR4 molecules (9) were maintained in Dulbecco's modified Eagle medium supplemented with 1% penicillin-streptomycin antibiotics, 1% Glutamax, and 10% FCS.
Mononuclear cells were isolated by Ficoll-Hypaque gradient from umbilical cord blood samples obtained from full-term deliveries. CD4+ T cells were purified by negative selection using the CD4+ Rosette separation technique (Stem Cell Technologies, Neylan, France). UC cells were cultured in complete RPMI 1640 medium containing IL4 (10 ng/ml). Vlahakis and colleagues demonstrated that IL-4 results in an upregulation of CXCR4 surface expression without modification of CD4 surface expression and that IL-4-treated cells remain susceptible to Fas-mediated apoptosis (65).
Flow cytometry.
Cells (105) were incubated for 1 h at 4°C with 50 μl of phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin (BSA) (PBS-BSA) or PBS-BSA supplemented with the appropriate MAb at concentrations necessary for saturation of cell-surface molecules. After washing three times with PBS-BSA, bound MAb was revealed by addition of 50 μl of a 1/100 dilution of FITC-conjugated secondary Ig. After a 30-min incubation, cells were washed with PBS-BSA and fluorescence intensity at 543 nm was measured on an EPICS XL4-C cytofluorometer (Beckman-Coulter). Flow cytometry analysis of FasL expression and caspase-3 activation were performed after cell permeabilization. Briefly, cells were fixed and permeabilized by addition of 20 μg of lysolecithin/ml in 1% paraformaldehyde for 2 min at room temperature, followed by incubation in absolute methanol on ice for 15 min and then in a 0.1% solution of NP-40 for 5 min on ice. After one wash, staining was performed as previously described.
Assessment of ΔΨm.
Mitochondrial transmembrane potential was measured by means of DiOC6(3) (40 nM in PBS). Cells (5 × 105) were incubated with DiOC6 for 15 min at 37°C, followed by analysis on a cytofluorometer (excitation, 488 nm; emission, 525 nm). Control experiments were performed in the presence of 5 μM carbamoyl cyanide mClCCP, an uncoupling agent that abolishes the ΔΨm, for 15 min at 37°C.
Cytochrome c measurements.
Mitochondrial and S-100 fractions were prepared from 40 × 106 HEK/CD4.403 and HEK/CD4.403/CXCR4 cells that had been cocultured with CEM or 8.E5 cells or from 40 × 106 A2.01/CD4.403 cells cocultured with HEK or HEK.gp120 cell lines by differential centrifugation in buffer containing 250 mM sucrose as previously described (67). Protein samples (25 μg) were loaded on sodium dodecyl sulfate (SDS)-prosieve 50 polyacrylamide gels, subjected to electrophoresis, and then transferred to polyvinylidene difluoride membranes (Millipore, St. Quentin en Yvelines, France). Western blottings were performed as described below.
Western blots.
Cells were washed twice in PBS and lysed in 50 mM Tris-HCl (pH 8)–1% Triton X-100–100 mM NaCl–1 mM MgCl2–2 mM Benzamidine, 2 μg of leupeptin/ml and 150 μM phenylmethylsulfonyl fluoride. Cell lysates were electrophoresed in SDS–10% polyacrylamide gel electrophoresis and blotted to polyvinylidene difluoride membranes. Membranes were then blocked in Tris-buffered saline–5% BSA–0.05% Tween 20 for 1 h at 20°C. Blots were incubated overnight at 4°C with the primary antibody in the blocking buffer. After three washes with TBS-Tween, the blots were incubated for 1 h at 20°C with peroxidase-coupled antiserum diluted 1/5,000 in TBS–5% milk–Tween. After further washes, the immune complexes were revealed by enhanced chemiluminescence (NEN) and autoradiographed.
Detection of apoptosis.
Detection of HIV-1-induced apoptosis of the adherent transfected HEK cells was monitored by nuclear chromatin condensation as previously described (9). Briefly, HEK/CD4.403 and HEK/CD4.403/CXCR4 cells were cocultured with 8.E5 (gp120+) or CEM (control) cells on slides in 24-well plates. After extensive washing with complete medium to eliminate the T cells in suspension, adherent cells were fixed in 3.7% paraformaldehyde in PBS (pH 7.4) containing 0.1% Triton X-100 for 15 min at 20°C. After two washes with PBS, cells were incubated with Hoechst solution (dye, Hoechst 33258; Sigma) at 0.2 μg/ml for 30 min at 20°C and examined by epifluorescence using a Leica microscope (Leica DMRB). Apoptosis of the A2.01/CD4.403 cell line and UC CD4+ T cells was studied after coculture with either the HEK.gp120 cell line or control untransfected HEK cells, by flow cytometry analysis using annexin-V-FITC as previously described (36). Briefly, suspension cells were washed once with PBS, carefully resuspended in 100 μl of binding buffer (100 mM HEPES [pH 7.4], 140 mM NaCl, 5 mM CaCl2); 2.5 μl of annexin-V-FITC and 2.5 μl of propidium iodide were then added. After incubation for 20 min in the dark at 20°C, cells were analyzed on an EPICS XL4-C cytofluorometer.
Immunofluorescence studies.
After coculture with CEM or 8.E5 cells, HEK/CD4.403 and HEK/CD4.403/CXCR4 cells were extensively washed and loaded with 250 nM MitoTracker Orange CMTM Ros for 45 min in complete culture medium. After two washes with PBS, adherent cells were fixed in 3.7% paraformaldehyde in PBS for 10 min at 20°C, permeabilized with PBS containing 0.2% Triton X-100 for 2 min, and then incubated with a 1 μg/ml solution of anti-caspase-3 MAb for 1 h at 4°C. After washing, cells were incubated with FITC-labeled goat anti-mouse Ig diluted 100-fold for 1 h at 4°C and washed and nuclei were stained with Hoechst solution as described above.
Statistical analysis.
Variance analysis was performed after arcsine transformation of the data (70): ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001.
RESULTS
Cellular models.
Two complementary cellular models were used in this study to directly analyze the cascade of events triggered after gp120 binding to CXCR4. They are based on coculture of transfected HEK adherent cells with a suspension T-cell line; one clone presents gp120 while the other expresses CXCR4 and a truncated form of CD4 (CD4.403) capable of binding to gp120 but unable to transduce a signal on its own. These cellular models allow apoptosis to be studied without cell separation. Apoptosis of an HEK cell line stably transfected with plasmids coding for both CXCR4 and CD4.403 (HEK/CD4.403/CXCR4) was analyzed following coculture with the CEM T-cell line (8.E5) expressing T-tropic HIV-1 gp120 molecules. To analyze gp120-induced apoptosis of the lymphoblastoid A2.01/CD4.403 T-cell line that expresses endogeneous CXCR4 and transfected CD4.403 molecules, we constructed an HEK clone stably expressing gp120 from an X4 isolate of HIV-1 (LAI strain), named HEK.gp120. The two cell populations (effector and target cells) present very different forward and side scatter characteristics on FACS, allowing the target cells to be specifically analyzed after selection. Furthermore, we counterstained target cells with an antibody directed against the adenovirus A1A antigen which is specifically expressed in HEK cells to verify that the selected population was not contaminated by effector cells (10).
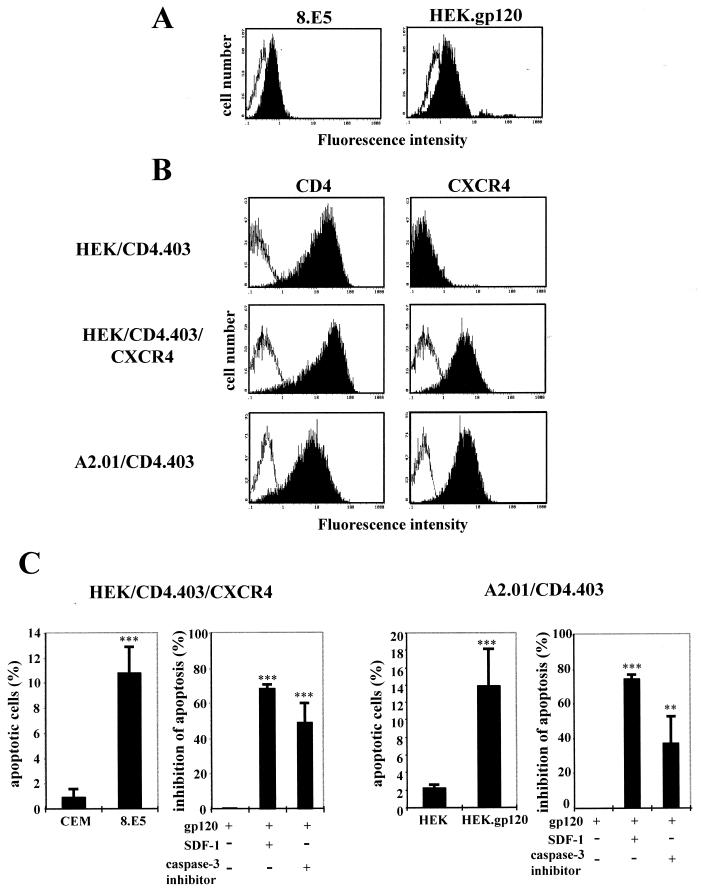
Expression of HIV-1 gp120 molecules at the surface of the 8.E5 and HEK.gp120 clones, as well as expression of CXCR4 and CD4.403 molecules on the HEK/CD4.403, HEK/CD4.403/CXCR4, and A2.01/CD4.403 cell lines, are shown in Fig. 1A and B, respectively.
FIG. 1.
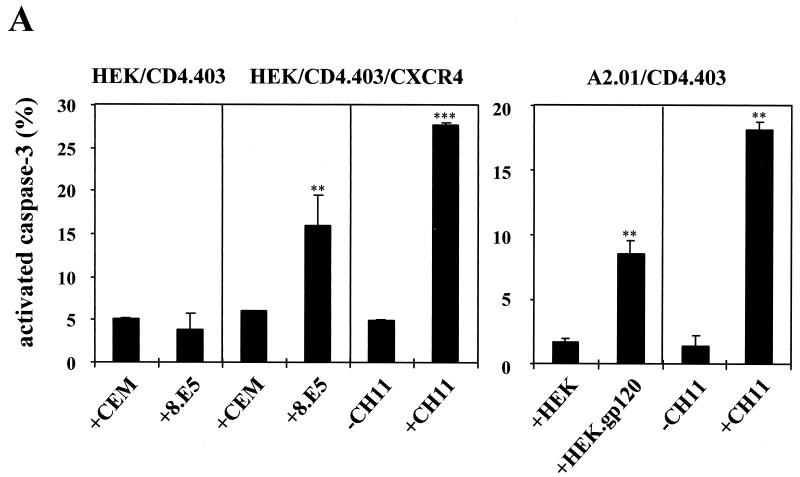
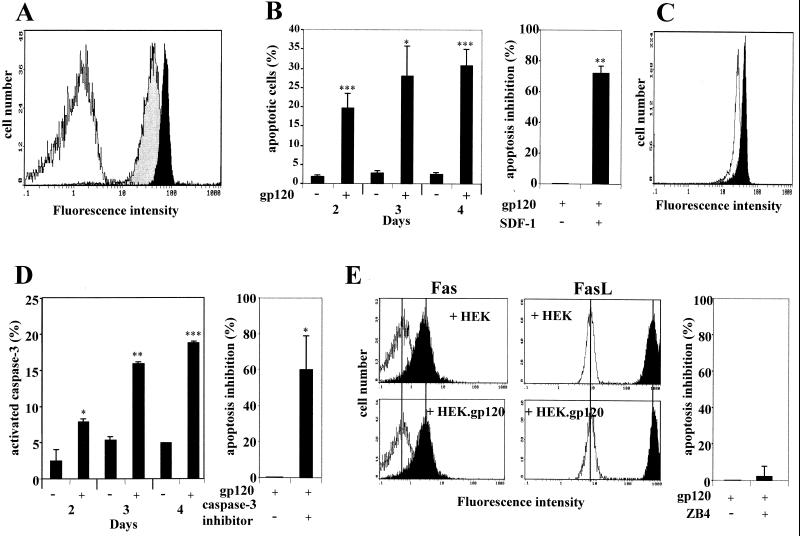
Description of the two cellular model systems. (A) Cell surface expression of gp120 at the surface of 8.E5 cells and the stable gp120-transfected HEK cell line (black histograms) are compared to the parental CEM and HEK lines (white histograms), as detected by flow cytometry. Cells were incubated with medium containing anti-gp120 human polyclonal antibodies and bound Ab was detected with a secondary FITC-labeled goat anti-human Ig. (B) Expression of mutated CD4 and CXCR4 molecules at the surface of the HEK/CD4.403 and HEK/CD4.403/CXCR4 clones and the A2.01/CD4.403 T-cell line. Cells were incubated with medium alone (white histograms) or medium containing the anti-CD4 (left) or anti-CXCR4 (right) MAbs at 10 μg/ml (black histograms). Bound MAb was detected with a FITC-labeled goat anti-mouse Ig. The fluorescence intensity was recorded in the log mode on an EPICS XL4 cytofluorometer. (C) Apoptosis of the HEK/CD4.403/CXCR4 and A2.01/CD4.403 cell lines cocultured with cells expressing gp120 (8.E5 and HEK.gp120) in the presence or absence of SDF-1 (500 ng/ml) or the caspase-3 inhibitor (50 μM).
Previously it was demonstrated that the expression of HIV-1 gp120 at the surface of 8.E5 cells triggers apoptosis of the HEK/CD4.403/CXCR4 clone (9). Under the same conditions, cell surface gp120 did not induce apoptosis of the HEK/CD4.403 cell line that does not express CXCR4. The apoptosis of HEK/CD4.403/CXCR4 cells was specifically triggered by the interaction with gp120 molecules on the 8.E5 clone since no apoptosis was observed upon coculture with the parental CEM line. Moreover, this cell death was inhibited by SDF-1. We found that gp120-induced cell death involves activation of the caspase cascade and is inhibited by a peptide inhibitor of the effector caspase-3 (Fig. 1C, left). These data demonstrate that CXCR4 transfected in HEK cells is able to transduce an apoptotic signal triggered by the gp120 epitopes uncovered after CD4 contact.
It has previously been demonstrated that the A2.01/CD4.403 cell line undergoes apoptosis after HIV-1 infection (24). This T-cell line, derived from CEM cells, was previously used to analyze Fas-mediated apoptosis (19, 39, 41, 49, 53, 59, 63). Furthermore, CEM and Jurkat cells are equally sensitive to agonistic anti-Fas MAbs (20, 21), and the Jurkat cell line is one of the most common T-cell lines used in Fas-dependent apoptosis analysis. Thus, the CEM cellular system is adapted to studying Fas-mediated apoptosis. Apoptosis of A2.01/CD4.403 cells following coculture with the HEK.gp120 clone was strongly inhibited by SDF-1, indicating that CXCR4 is involved in this process. Moreover, the process was partially inhibited by the caspase-3 inhibitor DEVD (Fig. 1C, right). These two model systems are thus suitable for analysis of the role of CXCR4, independently of CD4 signaling, in gp120-induced apoptosis.
Fas is not involved in gp120-induced apoptosis of CXCR4+ cells.
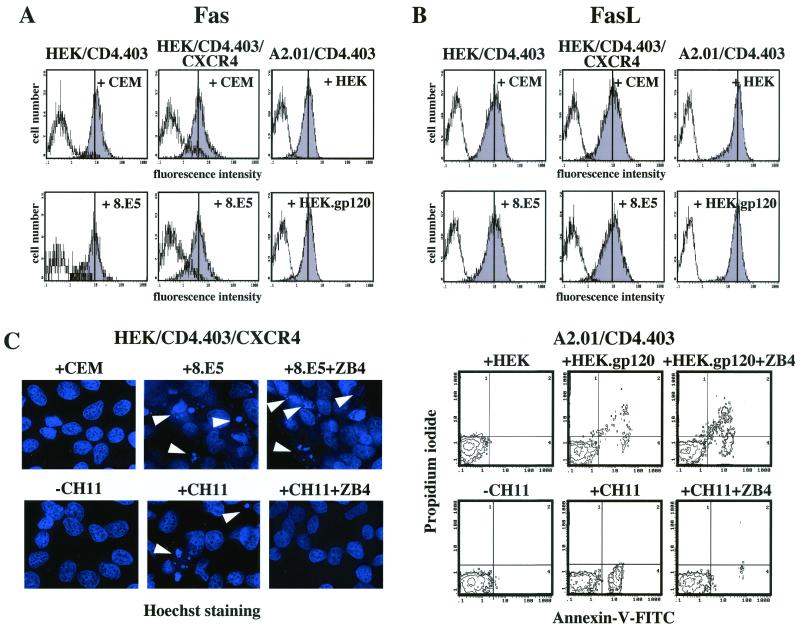
Although considerable controversy exists, the increased level of CD4+ T-cell apoptosis in HIV-infected persons might be due to an aberrant upregulation of death receptors, especially the Fas receptor (2–4, 12, 29, 30, 43, 67). Since HEK/CD4.403/CXCR4 and A2.01/CD4.403 cells undergo apoptosis following gp120 binding, we analyzed the expression of Fas and FasL molecules at the surface of these clones after 1, 4, 16, and 24 h and 2 and 3 days of coculture with cells expressing or not expressing gp120. We also determined the level of FasL expression by intracellular immunostaining because extracellular FasL staining protocols seem to be less reproducible, possibly due to the reported intracellular FasL protein storage not accessible to surface staining procedures (64). We did not observe any change in the surface expression of either Fas (Fig. 2A) or FasL (data not shown) during the 3-day coculture with gp120− or gp120+ cells. Furthermore, intracellular FasL levels did not increase following contact with gp120 (Fig. 2B). However, HEK/CD4.403/CXCR4 as well as A2.01/CD4.403 cells expressed a functional Fas receptor as demonstrated by the finding that cross-linking of Fas with the anti-Fas antibody CH11 induced a marked apoptosis of these cells (Fig. 2C and D). After calibration of the assay, CH11 MAb concentration and incubation times for further experiments were chosen to give percentages of apoptotic cells similar to those obtained in gp120-induced apoptosis. Although CH11-induced apoptosis was completely inhibited by the blocking anti-Fas ZB4 MAb at 5 μg/ml, this antibody did not protect HEK/CD4.403/CXCR4 or A2.01/CD4.403 cells from gp120-induced apoptosis (Fig. 2C and D). This strongly suggests that the Fas death receptor is not involved in this cell death program.
FIG. 2.
The death receptor Fas is not involved in gp120-induced apoptosis of CXCR4+ cells. Expression of extracellular Fas (A) and intracellular FasL (B) in HEK transfected cells and the A2.01/CD4.403 cell line after coculture for 3 days with 8.E5 or CEM cells and HEK.gp120 or HEK cell lines, respectively. These unpermeabilized or permeabilized cells were incubated with medium alone (white histograms) or medium containing anti-Fas and anti-FasL antibodies (grey histograms), respectively. (C) On the left, representative photographs of apoptotic HEK/CD4.403/CXCR4 cells (Hoechst staining) after coculture with CEM or 8.E5 cells for 3 days in the presence or absence of the anti-Fas ZB4 MAb inhibitor or after treatment with the anti-Fas MAb CH11 (1 μg/ml) for 6 h. Apoptotic cells are indicated by arrowheads. On the right, representative data from three independent flow cytometry experiments demonstrating annexin-V and propidium iodide labeling of A2.01/CD4.403 cells cocultured for 3 days with HEK or HEK.gp120 cells in the presence or absence of the anti-Fas ZB4 MAb inhibitor or the anti-Fas CH11 MAb (1 μg/ml) for 6 h. (D) Percentage of apoptotic HEK/CD4.403/CXCR4 (condensed chromatin) and A2.01/CD4.403 (annexin-V positive/propidium iodide negative) cells after coculture with gp120 negative or positive cells for 3 days in the presence or absence of the anti-Fas ZB4 MAb inhibitor or the anti-Fas CH11 MAb (1 μg/ml) for 6 h. Data shown reflect means ± standard deviations from at least three replicates. Statistical analysis was performed as described in Materials and Methods.
Mitochondrial depolarization and cytochrome c release occur during gp120-induced CXCR4-dependent apoptosis.
To determine the effect of gp120-CXCR4 binding on mitochondrial function, HEK/CD4.403 and HEK/CD4.403/CXCR4 cell lines were cocultured with CEM or 8.E5 cells and A2.01/CD4.403 cells were cocultured with HEK or HEK.gp120 cells. The mitochondrial transmembrane potential (ΔΨm) was then measured using the cationic dye DiOC6, a fluorochrome which is incorporated into cells depending upon their ΔΨm (52, 69). Following a 1-day coculture of HEK/CD4.403/CXCR4 cells and A2.01/CD4.403 cells with 8.E5 cells (Fig. 3A) and the HEK.gp120 clone (Fig. 3B), respectively, a reduced uptake of DiOC6 was detectable. As controls, mClCCP, an agent which uncouples oxidative phosphorylation thereby abolishing ΔΨm, and dexamethasone, an apoptotic agent which has previously been shown to induce a rapid reduction of ΔΨm in T cells (69), were used. Indeed, a 15-min exposure to mClCCP (5 μM) completely inhibited DiOC6 staining, confirming that the dye uptake was driven by ΔΨm and did not involve significant binding to other cellular components (Fig. 3A and B). Similarly, dexamethasone treatment of HEK/CD4.403/CXCR4 and A2.01/CD4.403 cells (100 μM for 12 h) reduced the incorporation of the fluorochrome DiOC6, indicating that this compound also acts on the mitochondrial function of HEK cells.
FIG. 3.
Mitochondrial depolarization occurs during gp120-induced apoptosis. (A) Following a 1-day coculture of HEK/CD4.403 and HEK/CD4.403/CXCR4 cells with CEM (black histograms) or 8.E5 (white histograms) cells, the former cells were stained with DiOC6 (40 nM, 15 min, 37°C), and ΔΨm was analyzed by flow cytometry. HEK/CD4.403/CXCR4 cells treated with the uncoupling reagent mClCCP (5 μM, 15 min) and dexamethazone (100 μM, overnight) were used as controls. Results of data from one of five representative experiments are shown. (B) The same experiments were performed following coculture of the A2.01/CD4.403 cell line with HEK (black histogram) or HEK.gp120 (white histogram) cells. Controls were identical to those described above for panel A.
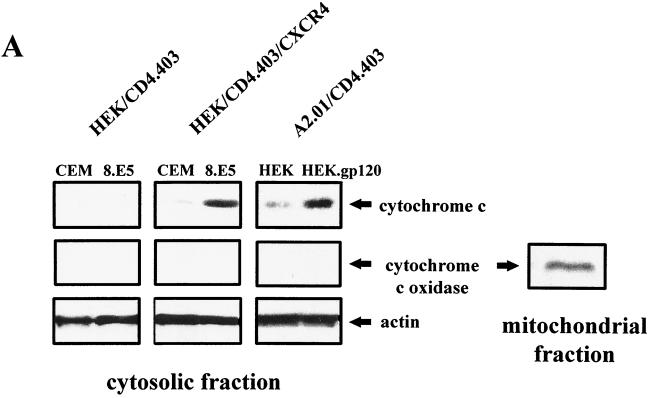
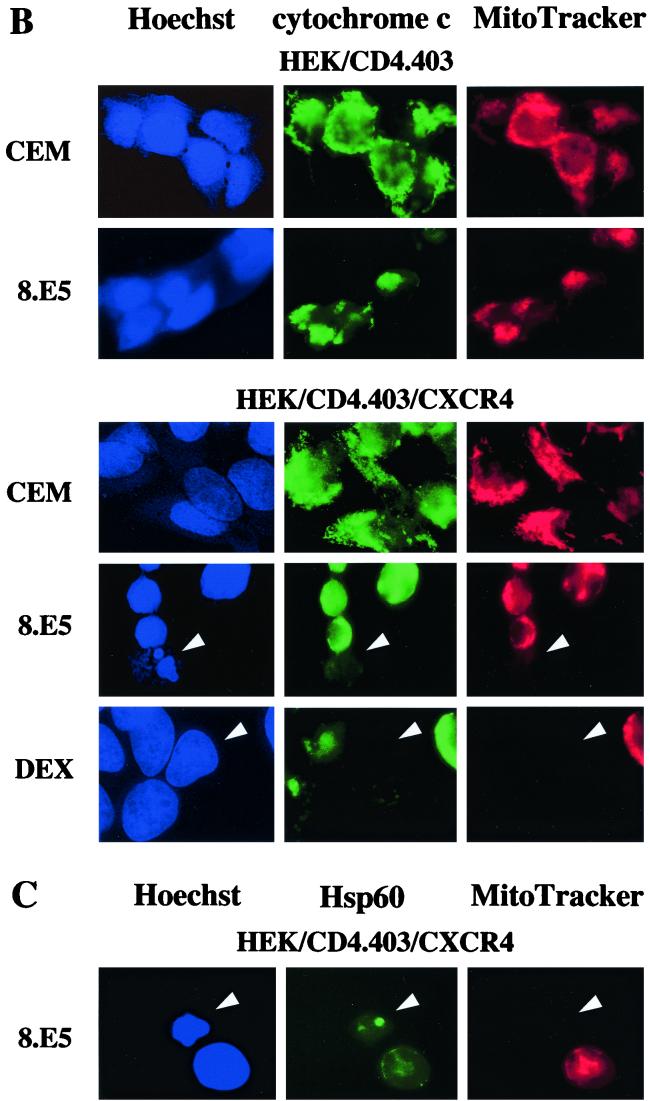
Cytochrome c release was assessed by immunoblotting analysis of the mitochondrial and S-100 fractions from HEK/CD4.403 and HEK/CD4.403/CXCR4 cells after contact with CEM or 8.E5 cells and from A2.01/CD4.403 cells after coculture with HEK or HEK.gp120 cells. An increase in the amount of cytochrome c in the cytosolic fraction was observed only in the CXCR4+ cell lines after a 1-day coculture with gp120+ cells (Fig. 4A), in parallel with a concomitant decrease in the mitochondrial fraction (data not shown). Cytochrome c release was not found in CXCR4+ cell lines cocultured with cells that do not express gp120 molecules, indicating that cytochrome c translocation from mitochondria to cytosol is specifically induced by cell-surface-expressed gp120 binding to CXCR4. We also verified that the S-100 fractions were not contaminated by mitochondria using the anti-cytochrome oxidase subunit II antibody (Fig. 4A); cytochrome oxidase was present in mitochondrial fractions but not in cytosolic fractions. To confirm the cytochrome c translocation after gp120 binding to CXCR4, CXCR4− and CXCR4+ HEK lines were cocultured with CEM or 8.E5 cells and then fixed and stained in order to simultaneously monitor nucleus morphology, cytochrome c localization, and the presence of intact mitochondria (high ΔΨm). Only the HEK/CD4.403/CXCR4 cell line cocultured with 8.E5 cells underwent cytochrome c translocation and demonstrated condensed chromatin and mitochondrial depolarization. Representative photographs are shown in Fig. 4B. Dexamethasone was used as a control of mitochondrial transmembrane depolarization. Physical damage to the mitochondria was ruled out in cells undergoing mitochondrial depolarization as the presence of the mitochondria-associated Hsp60 protein was detected (Fig. 4C). It is worth noting that we frequently observed cells with a low ΔΨm in the absence of cytochrome c release in the cytosol and apoptotic nuclei (data not shown). This suggests that mitochondrial potential depolarization may occur first, followed by cytochrome c release and DNA damage.
FIG. 4.
gp120-induced cytochrome c release from cells expressing CXCR4. (A) HEK/CD4.403 and HEK/CD4.403/CXCR4 cell lines were cocultured for 1 day with CEM or 8.E5 cells, and A2.01/CD4.403 cells were cocultured with HEK or HEK.gp120 cell lines. HEK/CD4.403, HEK/CD4.403/CXCR4 and A2.01/CD4.403 cells were dounced, and S100 cytosol was prepared as described in Materials and Methods. Cytosolic fractions (25 μg) were run on an SDS-polyacrylamide gel and Western blotted with an anti-cytochrome c antibody that recognizes a denaturated form of this molecule or an anti-cytochrome oxidase subunit II antibody (12CA-F12). Protein loading was controlled using an anti-actin antibody. Data of three independent experiments are shown. (B) Transfected HEK cell lines were cocultured for 3 days with 8.E5 or CEM cells and then triple stained with Hoechst to detect chromatin condensation (blue, left), an anti-cytochrome c antibody detected with FITC-conjugated secondary antibody (green, center) to detect the presence of cytochrome c in mitochondria, and ΔΨm-sensitive dye MitoTracker Orange to visualize mitochondrial polarization (red, right). Cells treated with dexamethasone (50 μM) were used as a positive control of mitochondrial depolarization. (C) Mitochondrion damage was controlled by triple staining with Hoechst (blue, left), an anti-Hsp60 antibody detected with a FITC-conjugated secondary antibody (green, center), and MitoTracker Orange (red, right).
Caspase-3 and -9 are activated in CXCR4+ cells after coculture with cells expressing gp120.
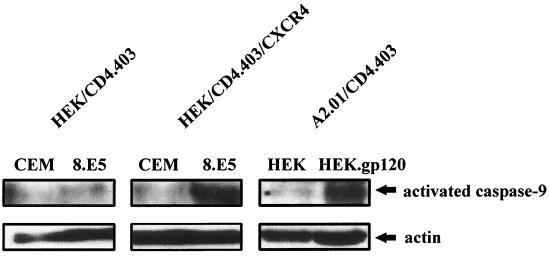
As caspase-9 is known to be critical for cytochrome c-dependent apoptosis, we analyzed its activation in our cellular models. After coculture of HEK/CD4.403 and HEK/CD4.403/CXCR4 cells with CEM or 8.E5 cells and of A2.01/CD4.403 cells with HEK or HEK.gp120 cells, we analyzed caspase-9 processing in the cytosolic fraction by immunoblotting using an antibody that recognizes the precursor (procaspase-9) and the p37 activated subunit form of caspase-9. A strong increase in activated caspase-9 was detected only in cells that express CXCR4 after 2 days of coculture with gp120+ cells (Fig. 5) and was associated with a decrease in procaspase-9 (data not shown). Thus, this caspase is activated after gp120 binding to CXCR4.
FIG. 5.
Caspase-9 is activated during the apoptotic process triggered by gp120 binding to CXCR4. HEK/CD4.403 and HEK/CD4.403/CXCR4 cell lines were cocultured for 2 days with CEM or 8.E5 cells, and A2.01/CD4.403 cells were cocultured with HEK or HEK.gp120 cell lines. The cytosolic fraction of these cells was then analyzed by immunoblotting for caspase-9 as described in Materials and Methods. Protein loading was controlled using an anti-actin antibody. Results representative of three independent experiments are shown.
Caspase-3 is expressed as a 32-kDa proenzyme which is activated by proteolytic cleavage into active 21- or 17-kDa forms. To determine whether viral gp120 induces the activation of caspase-3 in HEK/CD4.403, HEK/CD4.403/CXCR4, and A2.01/CD4.403 clones, and to compare the percentage of caspase-3-positive cells with the percentage of total apoptotic cells, cells were analyzed by flow cytometry by using an anti-active caspase-3 polyclonal antiserum that preferentially recognizes the activated form of caspase-3. The anti-Fas CH11 antibody was used as a positive control since Fas-mediated apoptosis involves the initial formation of a death-inducing signaling complex resulting in caspase-3 activation. Coculture of the CXCR4+ cell lines with gp120+ cells resulted in cleavage and activation of the caspase-3 (Fig. 6A) while no caspase-3 activation was found upon coculture of the CXCR4− cell line (HEK/CD4.403 cells). The level of caspase-3-positive cells is slightly higher than the level of cells visualized as apoptotic cells, indicating that caspase-3 plays a major role in gp120-mediated apoptosis. As expected, cells that did not express gp120 did not trigger caspase-3 cleavage in CXCR4+ cells (Fig. 6A).
FIG. 6.
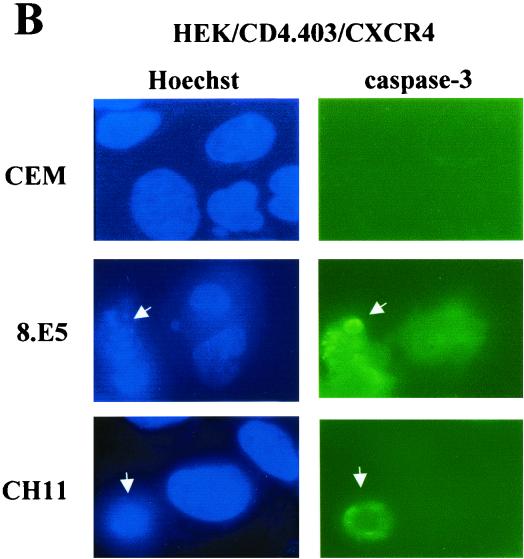
Induction of caspase-3 activation after gp120 binding to CXCR4. (A) Activation of caspase-3 was determined by flow cytometry after labeling of HEK/CD4.403 and HEK/CD4.403/CXCR4 cells cocultured with CEM or 8.E5 cells and A2.01/CD4.403 cells cocultured with HEK or HEK.gp120 cells with a phycoerythrin-conjugated anti-caspase-3 antibody that preferentially recognizes activated caspase-3. The anti-Fas CH11 MAb was used as a positive control; HEK/CD4.403/CXCR4 and A201/CD4.403 cells were incubated in the presence or absence of CH11 MAb (1 μg/ml). Results are the means ± standard deviations of three independent experiments. (B) Immunofluorescence detection of activated and cleaved caspase-3 and apoptotic nuclei in HEK transfected cells expressing CD4.403 and/or CXCR4 molecules after coculture for 3 days with CEM or 8.E5 cells or following treatment with the CH11 MAb. Apoptotic cells are indicated by arrowheads.
To assess caspase-3 activation, immunofluorescence studies were performed using CXCR4− and CXCR4+ HEK cell lines cocultured with CEM or 8.E5 cells for 3 days. Cells were then fixed and stained to simultaneously analyze nucleus morphology by Hoechst 33258 staining and caspase-3 activation by means of indirect labeling with an anti-cleaved caspase-3 MAb. The HEK indicator cells were cocultured with CEM or 8.E5 cells for 3 days in order to observe condensed chromatin that is not initially detectable. In HEK/CD4.403 cells cocultured with 8.E5 or CEM cells (data not shown), and in HEK/CD4.403/CXCR4 cells cocultured with CEM cells (Fig. 6B), there was no evidence of chromatin condensation. Activated caspase-3 was detected in HEK/CD4.403/CXCR4 cells only after coculture with 8.E5 cells (Fig. 6B), and a strong correlation was detectable between gp120-induced apoptosis evidenced by Hoechst staining and caspase-3 activation. The anti-Fas CH11 MAb shown as a control also triggered caspase-3 activation (Fig. 6B).
gp120 binding to UC CD4+ T cells induces CXCR4-dependent apoptosis involving mitochondrial depolarization and caspase-3 activation independently of Fas signaling.
To study gp120-induced apoptosis in a primary T-cell model, CD4+ T cells isolated from umbilical cord were used. The CD4+ population is composed almost entirely of naive T cells which express high levels of CXCR4 and are highly homogeneous with respect to CD4 and CXCR4 (Fig. 7A). Notably, expression of these two molecules was stable during the 4 days of coculture with HEK or HEK.gp120 cells in the presence of IL4.
FIG. 7.
gp120-induced apoptosis of UC CD4+ T cells. (A) Cell-surface expression of CD4 (black histogram) and CXCR4 (grey histogram) in purified UC CD4+ T cells, detected by flow cytometry as described in the Fig. 1 legend. (B) Percentage of apoptotic CD4+ cells cocultured with gp120+ or gp120− HEK cells for 2 to 4 days and (on the right) inhibition of apoptosis by SDF-1 (500 ng/ml). Results are from at least two independent experiments. (C) Mitochondrial depolarization of CD4+ T cells after coculture for 1 day with HEK (black histogram) or HEK.gp120 (white histogram) cells, analyzed by flow cytometry using DiOC6 as previously described. Data representative of five individual experiments are shown. (D) gp120-induced caspase-3 activation in CD4+ T cells after coculture with HEK.gp120 cells for 2 to 4 days and (on the right) inhibition of apoptosis by the caspase-3 inhibitor (50 μM). Results are the means ± standard deviations of two independent experiments. (E) The death receptor Fas is not involved in gp120-mediated UC CD4+ T-cell apoptosis. The expression of extracellular Fas and intracellular FasL was analyzed by flow cytometry after 4, 16, and 24 h of coculture with HEK or HEK.gp120 cells. Cells were incubated with medium alone (white histograms) or medium containing anti-Fas or anti-FasL (black histograms). Representative data from one of two independent experiments are shown. The percentage of inhibition of gp120-induced UC apoptosis by the anti-Fas antibody ZB4 is shown on the right. Error bar reflects means ± standard deviations from three replicates.
UC cell death was observed after coculture with HEK.gp120 cells, while UC cell apoptosis did not occur after coculture with HEK cells that did not express gp120. It is worth noting that UC cells or HEK and HEK.gp120 clones alone treated under the same experimental culture conditions did not undergo apoptosis (data not shown). gp120-induced apoptosis was strongly inhibited by SDF-1, demonstrating that CXCR4 is involved in this process (Fig. 7B). To further analyze the gp120-induced apoptotic signaling pathway activated in those primary CD4+ T cells, mitochondrial depolarization and caspase-3 activation were monitored under the same experimental conditions as those used with the CEM T-cell line. The purified UC CD4+ T cells showed a reduced uptake of DiOC6, detectable after 1 day of coculture with HEK.gp120 cells. No decrease in membrane polarization was detected after coculture with HEK cells (Fig. 7C). As the intrinsic apoptotic pathway involving mitochondria results in caspase-3 cleavage, we analyzed its activation in UC cells after coculture with HEK or HEK.gp120 cells. Direct caspase-3 activation was demonstrated in UC cells after coculture with HEK.gp120 cells, and a caspase-3 inhibitor induced a strong inhibition of gp120-induced apoptosis (Fig. 7D). The putative involvement of the Fas death receptor was then analyzed. No change in Fas or FasL expression was detected in UC cells during the coculture with HEK or HEK.gp120 cells (Fig. 7E). Under all conditions, these two proteins were expressed. Importantly, the Fas inhibitor ZB4 did not protect UC cells from gp120-induced apoptosis (Fig. 7E). Together, these results indicate that the intrinsic apoptotic pathway depending upon mitochondria is activated after gp120 binding to CXCR4 expressed on primary UC CD4+ T cells. Thus, the apoptotic pathway observed in cell lines is also actuated in primary CD4+ T cells expressing high levels of CXCR4.
DISCUSSION
Apoptosis has previously been observed following binding of gp120 to cells expressing the CD4 and CXCR4 molecules (9, 11, 15, 27). The aim of the present study was to further investigate the biological events occurring exclusively through CXCR4. The two major apoptotic pathways are the extrinsic (i.e., Fas/death receptor) and intrinsic (i.e., mitochondrial events) ones (1, 39, 50, 56, 62). Even if the Fas receptor can utilize mitochondria as part of an amplification mechanism, the Fas/FasL and mitochondrial pathways are capable of operating independently. The clearest demonstration comes from studies of caspase-9- and Apaf-1-deficient mice. Both types of modified mice display reduced apoptosis in response to many commonly used in vitro stimuli but not in response to Fas signaling (14, 25). In addition, the existence of separate pathways which induce apoptosis was demonstrated in CEM (39) and Jurkat cells (1, 62).
The Fas death receptor was shown to contribute to HIV-induced apoptosis of T cells. Upregulation of FasL expression, which occurs in monocytes after HIV-1 infection, has been proposed as a possible mechanism for bystander T-cell death (4, 48). Tat also enhances apoptosis via upregulation of FasL expression (40). Moreover, Fas levels were found to be higher in HIV-infected individuals (30). However, Fas involvement in HIV-induced apoptosis is controversial, and multiple mechanisms of CD4+ cell apoptosis are probably operative in HIV infection. Here, we use several cellular model systems, including a T-cell line and umbilical cord blood CD4+ T cells, to demonstrate that gp120-induced apoptosis through CXCR4 is not triggered by this death receptor. Specifically, the antagonist anti-Fas IgG MAb ZB4, which competes with CH11 for binding to the Fas receptor, did not inhibit gp120-induced apoptosis of cells expressing CXCR4 and a truncated form of CD4. Furthermore, we did not observe any upregulation of Fas or FasL in CXCR4+ cells after coculture with cells expressing gp120 molecules at their surface. This result agrees with those found in other cellular systems such as transfected T-cell lines (45), CD8+ T cells (26), and peripheral blood lymphocytes (8, 46), where HIV was shown to transduce a Fas-independent apoptotic signal. Furthermore, Badley and collaborators demonstrated that HIV-induced apoptosis was not associated with changes in Fas receptor expression but, rather, correlated with changes in activation marker profiles (5).
Interestingly, binding of cell-associated gp120 molecules to CXCR4 induced mitochondrial transmembrane depolarization and cytochrome c release from the mitochondria to the cytosol. No mitochondrial depolarization occurred in cells that lacked CXCR4 expression. Moreover, mitochondrial transmembrane depolarization and cytochrome c release were not induced in CXCR4+ cells by coculture with gp120− cell lines, indicating that these phenomena were specifically induced by cell surface-expressed gp120. This result agrees with those found in CD4+ T cells where cross-linked anti-CD4 and anti-CXCR4 antibodies induced a reduction of ΔΨm, but the authors did not determine the role of gp120 in this process (8). A very recent work on HIV-1 envelope glycoprotein-induced apoptosis in syncytia described a role for mitochondria and caspases (18). Although the present work does not provide insight into the mechanism of cytochrome c release from mitochondria to cytosol, the results are compatible with the sequence of events proposed in the PTP hypothesis: opening of the PTP could cause a dissipation of the inner mitochondrial transmembrane potential and an increase in the matrix volume that induces the mechanical disruption of the outer mitochondrial membrane, leading to cytochrome c release (51, 68). A further argument in favor of this hypothesis of sequential events resides in the observation that after coculture with 8.E5 cells, numerous HEK/CD4.403/CXCR4 cells had a decreased ΔΨm whereas cytochrome c was still present in the mitochondria and there were no morphological signs of apoptosis. Additional studies are needed to better understand the mechanisms by which cytochrome c is released from mitochondria after gp120 binding to CXCR4.
In the cytosol, cytochrome c, together with dATP, forms a complex with Apaf-1 that results in the cleavage of procaspase-9 and subsequent activation of downstream caspases (22, 38, 57, 72). Recently, a larger caspase-activating complex, named aposome, was found in the cytosol of apoptotic cells that includes processed caspase-9, -3, and -7 in addition to Apaf-1 (13). Furthermore, caspase-9 is described as a critical upstream activator of a caspase cascade in vivo and in some situations is essential for caspase-3 processing (25, 34). To assess the link between cytochrome c release and cell apoptosis, activation of procaspase-9 and -3 was analyzed. Decreased ΔΨm and release of cytochrome c occured in CXCR4+ cells after a 1-day coculture with cells expressing gp120, while caspases-9 and -3 cleavage was observed after 2 days of coculture. These data indicate an association between mitochondria function and the caspase activation pathway in gp120-induced apoptosis.
Although engagement of caspases in gp120-induced apoptosis has been controversial (8), caspase inhibitors were shown to suppress HIV envelope-mediated apoptosis, providing indirect evidence that gp120 may itself activate caspases (9, 47). Moreover, caspase-3 was shown to be activated by HIV envelope proteins in syncytia (18) and lymphocytes in a CD4 receptor-dependent manner (15). Our study confirmed that the effector caspase-3 plays a major role in gp120-induced apoptosis through CXCR4.
This work is the first demonstration that the apoptotic cascade composed of mitochondrial transmembrane depolarization, release of cytochrome c from the mitochondria to the cytosol, caspase-9 and -3 activation, and, finally, DNA damage is specifically activated by gp120 binding to CXCR4. This finding may enable us to better understand the progressive decline in the number of CD4+ T cells during AIDS and may explain the T-cell drop occurring during the later stages of disease that coincides with the emergence of X4 isolates.
ACKNOWLEDGMENTS
We are greatly indebted to C. Theillet and B. Orsetti for making their fluorescent microscope available to us. We thank N. Taylor for providing purified UC CD4+ T cells and for helpful scientific discussions and careful critical reading of the manuscript. We thank R. A. Hipskind for providing antibody to active caspase-3, R. Sabatier for statistical analysis, and B. Murphy and M. Piechaczyk for fruitful discussions.
This work was supported by institutional funds from the Centre National de la Recherche Scientifique (CNRS) and grants from the Agence Nationale de Recherches sur le SIDA (ANRS).
REFERENCES
- 1.Adachi S, Gottlieb R A, Babior B M. Lack of release of cytochrome C from mitochondria into cytosol early in the course of Fas-mediated apoptosis of Jurkat cells. J Biol Chem. 1998;273:19892–19894. doi: 10.1074/jbc.273.31.19892. [DOI] [PubMed] [Google Scholar]
- 2.Badley A D, Dockrell D, Paya C V. Apoptosis in AIDS. Adv Pharmacol. 1997;41:271–294. doi: 10.1016/s1054-3589(08)61062-5. [DOI] [PubMed] [Google Scholar]
- 3.Badley A D, Dockrell D, Simpson M, Schut R, Lynch D H, Leibson P, Paya C V. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badley A D, Parato K, Cameron D W, Kravcik S, Phenix B N, Ashby D, Kumar A, Lynch D H, Tschopp J, Angel J B. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ. 1999;6:420–432. doi: 10.1038/sj.cdd.4400509. [DOI] [PubMed] [Google Scholar]
- 6.Bedinger P, Moriarty A, VonBorstel R C, Donovan N J, Steinmer K S, Littman D R. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988;334:162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi P. The permeability transition pore. Control points of a cyclosporin A-sensitive mitochondrial channel involved in cell death. Biochim Biophys Acta. 1996;1275:5–9. doi: 10.1016/0005-2728(96)00041-2. [DOI] [PubMed] [Google Scholar]
- 8.Berndt C, Mopps B, Angermuller S, Gierschik P, Krammer P H. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc Natl Acad Sci USA. 1998;95:12556–12561. doi: 10.1073/pnas.95.21.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind R, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–344. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- 10.Biard-Piechaczyk M, Robert-Hebmann V, Roland J, Devaux C. ECEAR '99. Bologna, Italy: Nonduzzi Editore; 1999. Role played by the CD4/CXCR4 complex in HIV-1 gp120 env-induced signal transduction leading to apoptosis; pp. 3–11. [Google Scholar]
- 11.Blanco J, Jacotot E, Cabrera C, Cardona A, Clotet B, DeClereq E, Esté J A. The implication of the chemokine receptor CXCR4 in HIV-1 envelope protein-induced apoptosis is independent of the G protein-mediated signalling. AIDS. 1999;13:909–917. doi: 10.1097/00002030-199905280-00006. [DOI] [PubMed] [Google Scholar]
- 12.Bottarel F, Feito M J, Bragardo M, Bonissoni S, Buonfiglio D, DeFranco S, Malavasi F, Bensi T, Ramenghi U, Dianzani U. The cell death-inducing ability of glycoprotein 120 from different HIV strains correlates with their ability to induce CD4 lateral association with CD95 on CD4+ T cells. AIDS Res Hum Retrovir. 1999;15:1255–1263. doi: 10.1089/088922299310151. [DOI] [PubMed] [Google Scholar]
- 13.Cain K, Brown D G, Langlais C, Cohen G M. Caspase activation involves the formation of the aposome, a large (700 kDa) caspase-activating complex. J Biol Chem. 1999;274:22686–22692. doi: 10.1074/jbc.274.32.22686. [DOI] [PubMed] [Google Scholar]
- 14.Cecconi F, Alvarez-Bolado G, Meyer B I, Roth K A, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 15.Cicala C, Arthos J, Rubbert A, Selig S, Wildt K, Cohen O, Fauci A. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4+ T cells. Proc Natl Acad Sci USA. 2000;97:1178–1183. doi: 10.1073/pnas.97.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen G. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbeil J, Richman D D. Productive infection and subsequent interaction of CD4-gp120 at the cellular membrane is required for HIV-induced apoptosis of CD4+ T cells. J Gen Virol. 1995;76:681–690. doi: 10.1099/0022-1317-76-3-681. [DOI] [PubMed] [Google Scholar]
- 18.Ferri K F, Jacotot E, Blanco J, Este J A, Zamzami N, Susin S A, Xie Z, Brothers G, Reed J C, Penninger J M, Kroemer G. Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex. Role of mitochondria and caspases. J Exp Med. 2000;192:1081–1092. doi: 10.1084/jem.192.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi R T, Chen B K, Straus S E, Dale J K, Lenardo M J, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geley S, Hartmann B L, Kofler R. Ceramides induce a form of apoptosis in human acute lymphoblastic leukemia cells that is inhibited by Bcl-2, but not by CrmA. FEBS Lett. 1997;400:15–18. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb R A, Nordberg J, Skowronski E, Babior B M. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc Natl Acad Sci USA. 1996;93:654–658. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green D R. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 23.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 24.Guillerm C, Robert-Hebmann V, Hibner U, Hirn M, Devaux C. An anti-CD4 (CDR3-loop) monoclonal antibody inhibits human immunodeficiency virus type 1 envelope glycoprotein-induced apoptosis. Virology. 1998;248:254–263. doi: 10.1006/viro.1998.9265. [DOI] [PubMed] [Google Scholar]
- 25.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman S A, Lowe S W, Penninger J M, Mak T W. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 26.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 27.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch T, Marzo I, Kroemer G. Role of the mitochondrial permeability transition pore in apoptosis. Biosci Rep. 1997;17:67–76. doi: 10.1023/a:1027339418683. [DOI] [PubMed] [Google Scholar]
- 29.Katsikis P, Garcia-Ojeda M, Torres-Roca J, Tijoe I, Smith C, Herzenberg L, Herzenberg L. Interleukin-1β converting enzyme-like protease involvement in Fas-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L A, Herzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 32.Kroemer G, Reed J C. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G, Zamzami N, Susin S A. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 34.Kuida K, Haydar T F, Kuan C Y, Gu Y, Taya C, Karasuyama H, Su M S, Rakie P, Flavell R A. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 35.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassus P, Hibner U. Detection and quantification of apoptosis in transiently transfected adherent cells. Nucleic Acids Res. 1998;26:5233–5234. doi: 10.1093/nar/26.22.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retrovir. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Budihardjo I, Srinivasula S, Ahmed M, Alnemri E, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase 9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 39.Linsinger G, Wilhelm S, Wagner H, Hacker G. Uncouplers of oxidative phosphorylation can enhance a Fas death signal. Mol Cell Biol. 1999;19:3299–3311. doi: 10.1128/mcb.19.5.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li-Weber M, Laur O, Dern K, Krammer P H. T cell activation-induced and HIV tat-enhanced CD95(APO-1/Fas) ligand transcription involves NF-kappaB. Eur J Immunol. 2000;30:661–670. doi: 10.1002/1521-4141(200002)30:2<661::AID-IMMU661>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Longthorne V L, Williams G T. Caspase activity is required for commitment to Fas-mediated apoptosis. EMBO J. 1997;16:3805–3812. doi: 10.1093/emboj/16.13.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maldarelli F, Sato H, Berthold E, Orenstein J, Martin M A. Rapid induction of apoptosis by cell-to-cell transmission of human immunodeficiency virus type 1. J Virol. 1995;69:6457–6465. doi: 10.1128/jvi.69.10.6457-6465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Micoli K J, Pan G, Wu Y, Williams J P, Cook W J, McDonald J M. Requirement of calmodulin binding by HIV-1 gp160 for enhanced FAS-mediated apoptosis. J Biol Chem. 2000;275:1233–1240. doi: 10.1074/jbc.275.2.1233. [DOI] [PubMed] [Google Scholar]
- 44.Moutouh L, Estaquier J, Richman D D, Corbell J. Molecular and cellular analysis of human immunodeficiency virus-induced apoptosis in lymphoblastoid T-cell-line-expressing wild-type and mutated CD4 receptors. J Virol. 1998;72:8061–8072. doi: 10.1128/jvi.72.10.8061-8072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moutouh L, Richmann D D, Corbeil J. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. HIV-induced apoptosis requires the CD4 cytoplasmic tail and is not Fas-dependent in A2.01 cell line expressing wild type and mutants of the CD4 receptor; p. 283. [Google Scholar]
- 46.Noraz N, Gozlan J, Corbeil J, Brunner T, Spector S A. HIV-induced apoptosis of activated primary CD4+ T lymphocytes is not mediated by Fas-Fas ligand. AIDS. 1997;11:1671–1680. doi: 10.1097/00002030-199714000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Ohnimus H, Heinkelein M, Jassoy C. Apoptotic cell death upon contact of CD4+ T lymphocytes with HIV glycoprotein-expressing cells is mediated by caspases but bypasses CD95 (Fas/Apo-1) and TNF receptor 1. J Immunol. 1997;159:5246–5252. [PubMed] [Google Scholar]
- 48.Oyaizu N, Adachi Y, Hashimoto F, McCloskey T W, Hosaka N, Kayagaki N, Yagita H, Pahwa S. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis: a possible mechanism of bystander cell death in HIV infection. J Immunol. 1997;158:2456–2463. [PubMed] [Google Scholar]
- 49.Parlato S, Santini S M, Lapenta C, Spada M, Logozzi M, Rizza P, Proietti E, Belardelli F, Fais S. Primary HIV-1 infection of human CD4+ T cells passaged into SCID mice leads to selection of chronically infected cells through a massive fas-mediated autocrine suicide of uninfected cells. Cell Death Differ. 2000;7:37–47. doi: 10.1038/sj.cdd.4400586. [DOI] [PubMed] [Google Scholar]
- 50.Peter M F, Krammer P H. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 51.Petit P X, Goubern M, Diolez P, Susin S A, Zamzami N, Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998;426:111–116. doi: 10.1016/s0014-5793(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 52.Petit P X, O'Connor J E, Grunwald D, Brown S C. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem. 1990;194:389–397. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- 53.Richardson D S, Allen P D, Kelsey S M, Newland A C. Inhibition of FAS/FAS-ligand does not block chemotherapy-induced apoptosis in drug sensitive and resistant cells. Adv Exp Med Biol. 1999;457:259–266. doi: 10.1007/978-1-4615-4811-9_28. [DOI] [PubMed] [Google Scholar]
- 54.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 55.Salvesen G, Dixit V. Caspases: intracellular signalling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 56.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Alnemri E S. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 58.Stroh C, Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998;5:997–1000. doi: 10.1038/sj.cdd.4400451. [DOI] [PubMed] [Google Scholar]
- 59.Tang D, Lahti J M, Grenet J, Kidd V J. Cycloheximide-induced T-cell death is mediated by a Fas-associated death domain-dependent mechanism. J Biol Chem. 1999;274:7245–7252. doi: 10.1074/jbc.274.11.7245. [DOI] [PubMed] [Google Scholar]
- 60.Thornberry N. The caspase family of cysteine proteases. Br Med Bull. 1997;53:478–490. doi: 10.1093/oxfordjournals.bmb.a011625. [DOI] [PubMed] [Google Scholar]
- 61.Ullrich C K, Groopman J E, Ganju R K. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood. 2000;96:1438–1442. [PubMed] [Google Scholar]
- 62.Vier J, Linsinger G, Hacker G. Cytochrome c is dispensible for fas-induced caspase activation and apoptosis. Biochem Biophys Res Commun. 1999;261:71–78. doi: 10.1006/bbrc.1999.0942. [DOI] [PubMed] [Google Scholar]
- 63.Villunger A, Egle A, Kos M, Hartmann B L, Geley S, Kofler R, Greil R. Drug-induced apoptosis is associated with enhanced Fas (Apo-1/CD95) ligand expression but occurs independently of Fas (Apo-1/CD95) signaling in human T-acute lymphatic leukemia cells. Cancer Res. 1997;57:3331–3334. [PubMed] [Google Scholar]
- 64.Villunger A, Ghaffari-Tabrizi N, Tinhofer I, Krumbock N, Bauer B, Schneider T, Kasibhatla S, Greil R, Baier-Bitterlich G, Uberall F, Green D R, Baier G. Synergistic action of protein kinase C theta and calcineurin is sufficient for Fas ligand expression and induction of a crmA-sensitive apoptosis pathway in Jurkat T cells. Eur J Immunol. 1999;29:3549–3561. doi: 10.1002/(SICI)1521-4141(199911)29:11<3549::AID-IMMU3549>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 65.Vlahakis S R, Algeciras-Schimnich A, Bou G, Heppelmann C J, Villasis-Keever A, Collman R C, Paya C V. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J Clin Investig. 2001;107:207–215. doi: 10.1172/JCI11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wain-Hobson S, Sonigo P, Danos O, Cole S, Alizon M. Nucleotide sequence of the AIDS virus. LAV. Cell. 1985;40:9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- 67.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bel-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 68.Yang J C, Cortopassi G A. Induction of the mitochondrial permeability transition causes release of the apoptogenic factor cytochrome c. Free Radic Biol Med. 1998;24:624–631. doi: 10.1016/s0891-5849(97)00367-5. [DOI] [PubMed] [Google Scholar]
- 69.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zar J H. Biostatistical analysis. Upper Saddle River, N.J: Prentice-Hall; 1996. [Google Scholar]
- 71.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 72.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]