Abstract
Background
Tuberculosis is one of the leading causes of mortality worldwide from an infectious disease. This review aimed to investigate the association between prior cytomegalovirus infection and tuberculosis disease.
Methods
Six bibliographic databases were searched from their respective inception to 31 December 2021. Data were pooled using random-effects meta-analysis.
Results
Of 5476 identified articles, 15 satisfied the inclusion criteria with a total sample size of 38 618 patients. Pooled findings showed that individuals with cytomegalovirus infection had a higher risk of tuberculosis disease compared to those not infected with cytomegalovirus (odds ratio [OR], 3.20; 95% confidence interval [CI], 2.18–4.70). Age was the only covariate that exerted a significant effect on the result of the association. Meta-analysis of risk estimates reported in individual studies showed a marked and significant correlation of cytomegalovirus infection with active tuberculosis (adjusted hazard ratio, 2.92; 95% CI, 1.34–4.51; adjusted OR, 1.14; 95% CI, .71–1.57). A clear dose-response relation was inferred between the levels of cytomegalovirus antibodies and the risks of tuberculosis events (OR for high levels of cytomegalovirus antibodies, 4.07; OR for medium levels of cytomegalovirus antibodies, 3.58).
Conclusions
The results suggest an elevated risk of tuberculosis disease among individuals with a prior cytomegalovirus infection.
Keywords: tuberculosis, mycobacterium, cytomegalovirus, latent infection, association
This study shows that among individuals with cytomegalovirus infection, there is an increased risk of tuberculosis disease, particularly those with high levels of cytomegalovirus antibodies. The finding suggests screening and treatment for latent tuberculosis among this high-risk population.
Cytomegalovirus, a member of the herpesvirus family, is a ubiquitous cause of infections and diseases globally that is transmitted predominantly through contact with another person’s body fluids or congenitally from mother to infant [1, 2]. It is a pathogen that leads to a disease of disparity, with higher incidence, prevalence, and severity among socioeconomically and geographically deprived populations, plausibly due to limited health care access and quality [3]. For example, in less-developed regions of the world, primary cytomegalovirus infection occurs during childhood, with a seroprevalence rate of more than 90% by the age of adolescence [4]. The infection typically occurs later in high-income industrialized countries, with an estimated prevalence of 60%–70% at the age of 60 years [3]. Notwithstanding, a study among young people in the United Kingdom reveals that nearly a quarter of individuals aged 11–24 years are infected with cytomegalovirus and the seroprevalence modestly increases with age [5].
In immunocompetent individuals, cytomegalovirus can exhibit a range of clinical manifestations in the digestive system such as colitis, esophagitis, and gastroenteritis. It also leads to ophthalmic (retinitis), hepatic (hepatitis), pulmonary (pneumonia and pneumonitis), and cardiovascular (vascular thrombosis and myocarditis) conditions [6]. Severe clinical manifestations of cytomegalovirus infection were thought to be rare in apparently immunocompetent individuals and are more widely documented in individuals who are immunocompromised such as patients with advanced human immunodeficiency virus (HIV) or organ transplantation [7]. Recent evidence suggests that individuals who experience a prior cytomegalovirus infection have a higher risk of developing tuberculosis disease [8]. With nearly a third of the world's population having latent tuberculosis infection, a better understanding of this correlation is essential as it will help elucidate the pathogenesis of tuberculosis and prevent the likelihood of progression to active disease [9]. More importantly, the provision of clinical interventions, including vaccination, screening, and pharmacologic treatment of latent tuberculosis infection, to those at risk can reduce tuberculosis incidence, improve quality of life, and meet the goal of tuberculosis elimination [10].
It has been postulated that cytomegalovirus establishes a lifelong persistence in the body, which may be activated by stimuli such as immunosuppression and inflammation, causing proliferation of antigen-specific CD8+ and CD4+ T cells and natural killer (NK) cells specific for cytomegalovirus. The resulting effects such as lower interferon-γ (IFN-γ) secretion, KLRC2 gene deletion, and pulmonary fibrosis drive progression to tuberculosis disease. Findings gleaned from human and animal studies have revealed high levels of type-1 IFNs produced after cytomegalovirus infection perturbate responses of proinflammatory cytokines and eicosanoids, T cells, and macrophages, inducing necrotic cell death and culminating in Mycobacterium tuberculosis evasion and subsequent cellular infection. M. tuberculosis interferes with the cellular machinery by increasing or suppressing cytokine synthesis and early secreted antigenic target protein of 6 kDa (ESAT-6) of M. tuberculosis, resulting in the downregulation of macrophage antigen expression via major histocompatibility complex class-1 molecules. Furthermore, cytomegalovirus-derived interleukin-10 (IL-10) homolog lowers immune protection against M. tuberculosis, and the cytomegalovirus-elicited immune activation and enhanced type-I IFNs production could elevate the potential risk and severity for tuberculosis disease [11].
To achieve the World Health Organization End Tuberculosis objectives of reducing tuberculosis incidence by 90% and mortality by 95% by 2035, it is of paramount importance that predisposing factors to tuberculosis disease are better understood [12]. To date, no comprehensive review is available on the impact of cytomegalovirus infection on tuberculosis disease. We conducted this systematic review and meta-analysis to illuminate the evidence from epidemiological studies concerning the association between cytomegalovirus infection and tuberculosis disease progression.
METHODS
Search Strategy and Screening Process
We conducted electronic searches on 6 databases (MEDLINE, Cochrane CENTRAL, AMED, EMBASE, Global Health, and PsychINFO) to identify articles that assessed the epidemiological association between cytomegalovirus infection and tuberculosis disease published from database inception until 31 December 2021. No language restriction was applied. The list of keywords and literature search strings is shown in the Supplementary Material.
Titles and abstracts were screened by 1 reviewer (K.P.K.), and all potentially relevant full texts were screened and evaluated by 2 reviewers independently (K.P.K. and B.C.). Any discrepancies were solved by discussion and adjudication with a third reviewer (S.W.H.L.). The bibliographic references of the included articles were manually searched to identify any additional relevant studies.
Studies of any design were eligible for inclusion, except studies not conducted on humans, case reports, and opinion-based papers. We applied the following inclusion criteria: (1) patients with tuberculosis disease diagnosed by medical imaging or microbiological assessment; (2) documented a positive test for cytomegalovirus infection; and (3) evaluated a causal link between cytomegalovirus infection and tuberculosis disease. After initial screening by title and abstract, full texts of relevant articles were obtained. Two reviewers independently assessed all full texts against the study eligibility and any differences were resolved through consensus.
Data Extraction
Data were extracted independently by 2 authors using a standardized data abstraction form. Information extracted included: first author, year of publication, sample size, number of cases, age range, effect estimates, coexisting medical conditions, laboratory results, chest radiography cavitation, and tuberculosis treatment (if any). All data were independently checked by a third reviewer and disagreements resolved by consensus. The risk of bias in each included cohort study was classed as low, moderate, or high based on the ROBINS-I tool [13]. For case-control studies, these were assessed using the Clarity Group at McMaster University [14] and cross-sectional studies using the tool developed by the Agency for Healthcare Research and Quality [15].
Statistical Analysis
We summarized data narratively and presented them in this systematic review. Where multiple studies reported the same outcome, we pooled estimates using random-effects models to generate the odds ratio (OR). When studies reported multiple follow-up periods, we used the longest follow-up time. Heterogeneity between studies was evaluated using I2 statistics and funnel plots were assessed visually for publication bias. All analyses were performed using RevMan version 5.3 (Cochrane Collaboration) and Stata version 16.0 (StataCorp).
RESULTS
The literature search captured 5476 articles. After initial screening, 113 studies were eligible for full-text review and 15 studies were included (Figure 1).
Figure 1.
PRISMA 2020 flow diagram for new systematic review, which included searches of databases, registers, and other sources. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Study Characteristics
The studies encompassed 2 prospective cohort studies [16, 17], 2 retrospective cohort studies [18, 19], 8 cross-sectional studies [20–27], and 3 case-control studies [8, 28, 29], describing cytomegalovirus infection to subsequent risk of tuberculosis disease (Table 1). Seven studies reported a dose-response relationship between cytomegalovirus load values or cytomegalovirus antibody levels and risk of active tuberculosis [16, 20–23, 27, 28]. The studies were carried out from 1990 to 2021 with sample sizes ranging from 65 to 30 433 participants. Of these, 6 were undertaken in countries with a high burden of multidrug-resistant tuberculosis [8, 16, 20, 24, 27, 29], 6 in high tuberculosis burden countries [19, 21–23, 26, 28], 1 in an intermediate tuberculosis burden country [18], and 2 in low tuberculosis burden countries [17, 25]. All studies reported significant association between cytomegalovirus infection and the development of tuberculosis disease, except for 1 study [18].
Table 1.
Summary of Included Studies
| Study (Year) Country | Study Population and Characteristics | Male Sex (%) | Follow-up Period | Cytomegalovirus Detection Method | Tuberculosis Assessment Method | Main Findings |
|---|---|---|---|---|---|---|
| Case-control studies | ||||||
| Fletcher et al (2016) South Africa [29] | 53 infants diagnosed with tuberculosis and 205 healthy matched controls (age range, 4–6 m) | … | 3 y | Interferon-γ enzyme-linked immunospot assay | Culture, GeneXpert MTB/RIF, acid-fast-positive smears, QuantiFERON-TB Gold In-tube test, tuberculin skin test, and radiography | Activated HLA-DR+ CD4+ T cells were associated with increased tuberculosis disease risk (OR, 1.83; 95% CI, 1.25–2.68; P = .002) A similar risk pattern was also demonstrated in the cohort study of adolescents (OR = 1.39; 95% CI, 1.07–1.80; P = .014) There was a positive correlation between T-cell activation and cytomegalovirus interferon-γ enzyme-linked immunospot response (R = 0.301, P < .0001) Immunologic assessment showed that on day 0 and day 28 following Bacille Calmette- Guérin vaccination, cytomegalovirus response was not significantly associated with tuberculosis disease risk (day 0 OR, 0.87; 95% CI, .54–1.41; P = .582; day 28 OR, 1.08; 95% CI; .72–1.61; P = .723) |
| Müller et al (2019) South Africa [8] | 49 infants who developed tuberculosis disease and 129 healthy matched controls (age range, 4–6 m) | 51.0% | 3 y | Interferon-γ enzyme-linked immunospot assay | Chest radiography, tuberculin skin test, QuantiFERON-TB Gold In-tube test, gastric lavage and sputum smear microscopy, GeneXpert MTB/RIF, MGIT, and PCR testing | Prior or subclinical infection with cytomegalovirus, measured by T-cell responses, was correlated with increased risk of tuberculosis disease over the following 3 y of life (OR, 2.22; 95% CI, 1.02–4.83; P = .043) Cytomegalovirus-positive infants developed tuberculosis disease earlier in follow-up than negative infants (log rank Mantel-Cox, P = .037) |
| Stockdale et al (2020) Uganda [28] | 281 cytomegalovirus seropositive individuals (mean age, 34.3 y, range, 2.75–56.5 y) | 38.8% | 10 y | IgG enzyme-linked immunosorbent assay | Sputum smear and culture | Individuals with medium cytomegalovirus IgG levels had 2.8 times higher risk of progression to active tuberculosis (OR, 2.801; 99% CI, .908–8.638; P = .055), whereas those with high cytomegalovirus IgG levels were 3.4 times more likely to have tuberculosis compared with low cytomegalovirus IgG levels (OR, 3.446; 99% CI, 1.072–11.074; P = .007) The magnitude of cytomegalovirus infection, as quantified by IgG levels, was associated with risk of tuberculosis disease in a dose-dependent manner (P = .006) |
| Cross-sectional studies | ||||||
| Amran et al (2016) Australia [25] | 112 patients with pulmonary nontuberculous mycobacterial disease (median age, 69 y; range, 37–98 y) and 117 healthy controls (median age, 61 y; range, 52–84 y) | … | No follow-up | Cytomegalovirus lysate, glycoprotein B, immediate early-1 antigen, EBV, total IgG, and sTNFR1 antibodies assays | Chest radiograph, chest high-resolution computed tomography scan, sputum specimens | Plasma levels of cytomegalovirus lysate, glycoprotein B, and immediate early-1 antigen were significantly higher in patients with pulmonary nontuberculous mycobacterial disease compared to controls, with significant correlations among the patients (r, 0.44–0.57; P < .0001) and controls (r, 0.57–0.81; P < .0001) The patients also exhibited significantly higher levels of EBV antibody, total IgG levels, and sTNFR1 levels than controls |
| Dube et al (2016) South Africa [24] | 214 children hospitalized with suspected pulmonary tuberculosis (median age, 36 m; IQR, 19–66 m) | 49.1% | No follow-up | PCR assay | Culture and GeneXpert MTB/RIF | 34 children (15.9%) had definite tuberculosis, 86 (40.2%) had unconfirmed tuberculosis, and 94 (43.9%) were deemed unlikely to have tuberculosis disease 14 children (6.5%) had cytomegalovirus detected in their nasopharyngeal samples Cytomegalovirus was a dominant microbial profile associated with definite tuberculosis cases |
| Ledru et al (1995) Burkina Faso [22] | 171 adults (mean age, 36 ± 11 y) | 66.7% | No follow-up | IgG enzyme-linked immunosorbent assay | Sputum smear and culture | 97.5% of tuberculosis patients without HIV infection showed high cytomegalovirus IgG antibodies titers compared to healthy controls (P < .03) Likewise, tuberculosis patients with and without HIV showed higher levels of cytomegalovirus IgG titers than healthy controls (37.5% and 25.0% vs 3.5%, P < .002 and P < .02, respectively) |
| Nagu et al (2017) Tanzania [26] | 234 adult patients with pulmonary tuberculosis (median age, 36 y) | 66.2% | No follow-up | Interferon-γ enzyme-linked immunospot assay | Sputum smear microscopy | Peripheral blood mononuclear cells from patients who survived following tuberculosis treatment completion had significantly stronger interferon-γ responses to cytomegalovirus (P = .035) and Mycobacterium tuberculosis antigen ESAT-6 (P = .043) at the time point of diagnosis in comparison to patients who succumbed to the disease during treatment |
| Olaleye et al (1990) Nigeria [20] | 161 tuberculosis patients, 89 patients other than tuberculosis, and 110 healthy blood donors of all ages | 63.6% | No follow-up | IgG enzyme-linked immunosorbent assay | Radiologically and bacteriologically confirmed tuberculosis | Tuberculosis patients had the highest prevalence of cytomegalovirus infection (87.6%) compared to nontuberculosis patients (50.6%) and healthy voluntary blood donors (54.5%) (P < .01) When titers of 1:8 and higher were considered as marked cytomegalovirus antibodies, a significantly higher proportion of tuberculosis patients had high cytomegalovirus antibody levels compared with all others (P < .05) |
| Sirenko et al (2003) Russia [27] | 65 children and adolescents with pulmonary tuberculosis | … | No follow-up | … | … | Children and adolescents infected with cytomegalovirus had a 3-fold higher risk of developing pulmonary tuberculosis (66.2% vs 21.0%), with severity of disease increasing with plasma levels of antigens and antibodies to cytomegalovirus |
| Stockdale et al (2018) Uganda [21] | 2174 individuals in the general population (mean age, 22.7 y; range, 0.08–100.75 y) | 49.8% | No follow-up | IgG enzyme-linked immunosorbent assay | Sputum smear microscopy | The mean cytomegalovirus IgG OD concentration among tuberculosis patients was 0.34 OD greater (99% CI, .15–.53; P < .001) compared to those without active tuberculosis After adjustment for age, sex, and HIV infection status, active tuberculosis disease remained significantly correlated with elevated cytomegalovirus IgG antibodies of 0.19 OD (99% CI, .01–.37; P = .006) Most active tuberculosis patients showed high cytomegalovirus IgG levels (59.3%), 33.3% had medium IgG levels, 7.4% had low IgG levels, and none were cytomegalovirus seronegative |
| Stockdale et al (2019) Uganda [23] | 2189 individuals in the general population (mean age, 23.4 y; range, 30 d–100 y) | 50.0% | No follow-up | IgG enzyme-linked immunosorbent assay | Sputum smear microscopy | 27 active tuberculosis cases (1.4%) were documented among the cytomegalovirus-seropositive population Active tuberculosis disease was not associated with differences in any mycobacterial antibody levels after adjusting for age, sex, HIV, and cytomegalovirus antibodies Individuals with higher cytomegalovirus exposure had lower levels of mycobacterial antibodies (Ag85A IgG, PPD IgG, and LAM IgG) but no corresponding change in total IgG levels |
| Prospective cohort studies | ||||||
| Gupta et al (2019) United Kingdom [17] | 30 433 renal transplant recipients (median age, 44 y; IQR, 36–55 y) | 61.5% | Median, 5.1 y; IQR, 2.2–8.7 y | IgG enzyme-linked immunosorbent assay | Culture-confirmed tuberculosis and clinically diagnosed with radiological or histological evidence of tuberculosis | Cytomegalovirus seropositivity was independently associated with tuberculosis incidence among transplant recipients, with an incidence rate of 49.5 per 100 000 (95% CI, 35.2–69.6) and a subdistribution hazard ratio of 2.42 (95% CI, 1.03–5.68; P = .043) |
| Martinez et al (2021) South Africa [16] | 963 infants (median gestational age, 39 wk; IQR, 38–40 wk) | 52.5% | Median, 6.9 y; IQR, 6.0–7.8 y | PCR assay | Tuberculin skin test, chest radiography, GeneXpert MTB/RIF, and sputum smear and culture | 48% (95% CI, 20.5%–69.3%) of tuberculosis disease in children older than 1 y had a cytomegalovirus infection before 1 y of age The risk of microbiologically confirmed tuberculosis disease was higher among children acquiring cytomegalovirus infection before age 3 mo (adjusted HR, 3.2; 95% CI, 1.0–10.6; P = .048), 6 mo (adjusted HR, 3.9; 95% CI, 1.2–13.0; P = .027), 12 mo (adjusted HR, 4.4; 95% CI, 1.2–16.3; P = .027), and 24 mo (adjusted HR, 6.1; 95% CI, 1.3–27.9; P = .020) Infants with a high cytomegalovirus load had a greater risk of tuberculosis disease compared to those with cytomegalovirus-negative results, irrespective of the timing of cytomegalovirus infection |
| Retrospective cohort studies | ||||||
| Kim et al (2021) South Korea [18] | 717 liver transplant recipients (median age, 54 y; mean, 53.70 ± 9.12 y) | 79.1% | Median, 1354 d; range, 16–3904 d | … | Chest X-ray, chest computed tomography, QuantiFERON-TB Gold In-tube test, and T-SPOT.TB assay | Univariate analysis showed that cytomegalovirus infection was not a risk factor for developing active tuberculosis (HR, 1.44; 95% CI, .61–3.41; P = .412) |
| Viana et al (2019) Brazil [19] | 152 kidney or combined kidney-pancreas transplant recipients diagnosed with tuberculosis (mean age, 42.0 ± 13.6 y) | 69.0% | Median, 1989 d; IQR, 932–3632 d | … | Chest radiography, acid-fast smear, culture, and histology | The overall incidence of tuberculosis in transplant patients was 1.33% (152/11 453) 17 patients (11.2%) had an episode of cytomegalovirus infection or disease occurring within the year before tuberculosis diagnosis Multivariate Cox regression analysis demonstrated that cytomegalovirus infection was independently associated with death (HR, 2.79; 95% CI, 1.15–6.73; P = .02) |
Abbreviations: Ag85A, antigen 85A; CD4+, cluster of differentiation antigen 4 positive; CI, confidence interval; EBV, Epstein-Barr virus; ESAT-6, early secreted antigenic target of 6 kDa; HIV, human immunodeficiency virus; HLA-DR+, human leukocyte antigen-DR isotype positive; HR, hazard ratio; IgG, immunoglobulin G; IQR, interquartile range; LAM, lipoarabinomannan; MGIT, mycobacteria growth indicator tube; MTB/RIF, mycobacterium tuberculosis and rifampicin resistance; OD, optical density; OR, odds ratio; PCR, polymerase chain reaction; PPD, purified protein derivative; QuantiFERON-TB Gold, whole-blood test to detect Mycobacterium tuberculosis infection; sTNFR1, soluble tumour necrosis factor receptor 1; T-SPOT.TB, single visit tuberculosis blood test; Tcells, thymus-derived lymphocytes.
The risk of bias was rated as low for the follow-up cohort studies (Supplementary Figure 1) and case-control studies (Supplementary Figure 2). However, the cross-sectional studies were deemed to have a low to unclear risk of bias because of the absence of adequate information to assess the individual domains with certainty (Supplementary Figure 3). Inspection of the funnel plot showed a small degree of asymmetry, suggesting the presence of publication bias among studies with lower precision due to small study effects (Supplementary Figure 4).
Risk of Developing Tuberculosis
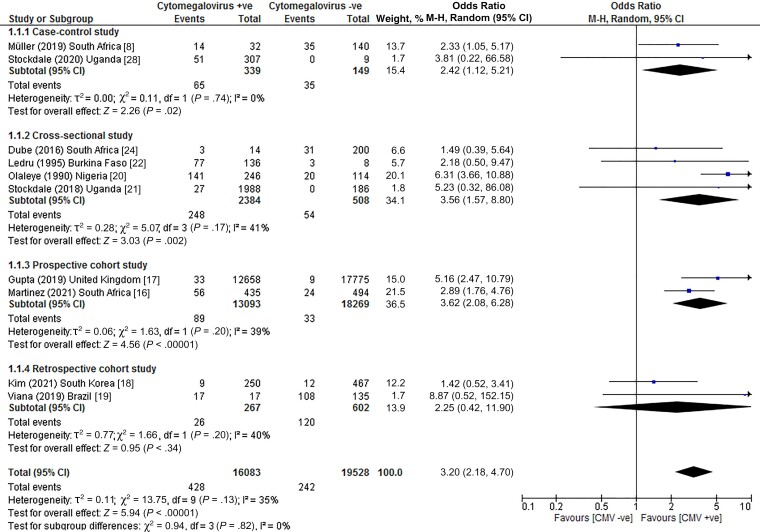
Pooled results across the 10 case-control, cross-sectional, and cohort studies showed that individuals with a cytomegalovirus infection had an increased risk of developing tuberculosis disease (OR, 3.20; 95% confidence interval [CI], 2.18–4.70; I2, 35%; P < .00001) compared to cytomegalovirus-negative counterparts (Figure 2). When a risk ratio was computed for the meta-analysis, we found that the correlation between infection with cytomegalovirus and tuberculosis disease remained significant (risk ratio [RR], 2.16; 95% CI, 1.08–4.31; I2, 94%; P < .00001; Supplementary Figure 5).
Figure 2.
Risk of tuberculosis disease among individuals with CMV infection. Abbreviations: CI, confidence interval; CMV, cytomegalovirus; M-H: Mantel-Haenszel method.
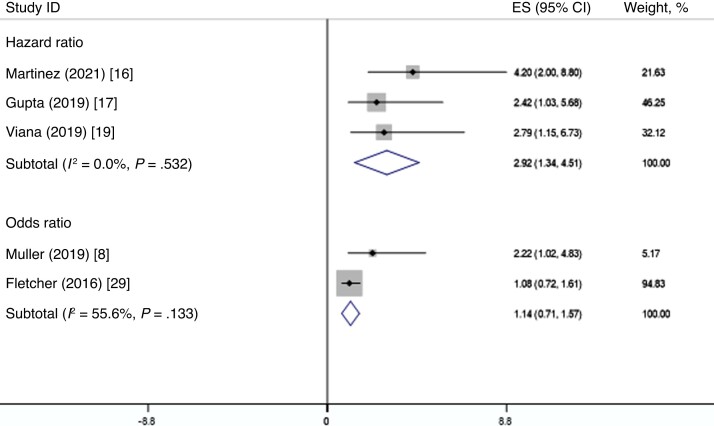
Five studies also adjusted for confounding factors when examining the association between the risk of cytomegalovirus infection and subsequent tuberculosis disease [8, 16, 17, 19, 29]. Confounders adjusted included sex [8, 16, 19], age [8, 17], ethnicity [8, 17], and HIV infection [16, 17]. The pooled effect measures in studies that adjusted for confounding depicted a highly significant relationship between cytomegalovirus infection and tuberculosis disease (adjusted hazard ratio [HR], 2.92; 95% CI, 1.34–4.51; adjusted OR, 1.14; 95% CI, .71–1.57; Figure 3). Pooling of studies that measured antibody titer levels showed that higher antibody levels for cytomegalovirus were associated with increased risks of incident tuberculosis disease, indicating a positive and significant dose-response relationship (OR for high levels of cytomegalovirus antibodies, 4.07, 99% CI, −.70 to 8.84; OR for medium levels of cytomegalovirus antibodies, 3.58, 99% CI, 2.18–4.97; Figure 4).
Figure 3.
Random-effects meta-analysis of the correlations between cytomegalovirus infection and tuberculosis disease. Abbreviations: CI, confidence interval; ES, effect size.
Figure 4.
Random-effects meta-analysis of the dose-response relationships between cytomegalovirus infection and tuberculosis disease. Abbreviations: CI, confidence interval; ES, effect size.
The potential impacts of confounding or mediating variables on the causal relationship were further assessed by stratifying the studies based upon study design, national burden of tuberculosis, age, risk of bias in studies, diagnosis of cytomegalovirus infection, study continent, and finally multiple adjusted estimates. Statistically significant subgroup findings on increased risk of tuberculosis disease were observed in high multidrug-resistant tuberculosis burden settings (OR, 3.22; P < .0001), high tuberculosis burden settings (OR, 3.32; P = .03), low tuberculosis burden setting (OR, 5.16; P < .0001; Supplementary Figure 6), infants who were enrolled for follow-up at birth or at 4–6 months old (OR, 2.72; P < .00001), general population of all age groups (OR, 5.81; P < .00001; Supplementary Figure 7), studies with low risk of bias (OR, 2.80; P < .00001), studies with unclear risk of bias (OR, 4.65; P = .001; Supplementary Figure 8), detection of cytomegalovirus infection by polymerase chain reaction (OR, 2.67; P < .0001), immunoglobulin G immunoassay (OR, 5.38; P < .00001), and IFN-γ release assay (OR, 2.33; P = .04; Supplementary Figure 9), Africa (OR, 3.30; P < .00001), and European continent (OR, 5.16; P < .0001; Supplementary Figure 10). Heterogeneity among studies was diminished sizably with stratified analysis by age and study continent. In particular, the major confounder of the association between cytomegalovirus infection and tuberculosis disease was age (χ2 = 11.60, df = 3, P = .009; Supplementary Figure 7).
DISCUSSION
This study, to the best of our knowledge, is the first systematic literature search to synthesize epidemiological evidence regarding the relationship between cytomegalovirus infection and tuberculosis disease. It provides a strong, sufficient, and consistent evidence base to highlight that cytomegalovirus infection is correlated with an increased risk of tuberculosis disease. Overall, the identified studies have a low risk of bias and are predominantly conducted on the African continent, which has been estimated to be harboring one of the highest rates of tuberculosis incidence and mortality in the world [30]. Whilst limited by inadequate geographical coverage in countries of substantial economic losses such as south Asia and east Asia [31], our study recognizes an apparent burden of tuberculosis disease among vulnerable groups, including immunocompromised patients, young children, and geographically marginalized communities. Infection with cytomegalovirus is common across the globe and results in possible severe sequelae [32]. Despite tremendous infection burden and multiple health outcomes across the life course following cytomegalovirus infection [1], vaccines and immune globulins are still investigational and have not yet been proven efficacious for clinical use to protect against the illness and transmission [33]. A number of potential cytomegalovirus vaccines are currently under development, including those targeted for the prevention of congenital infection and posttransplant infection [34]. In a phase 3, randomized, placebo-controlled trial, a novel DNA-based cytomegalovirus vaccine was found to be inefficacious in reducing overall mortality, end-organ disease, and viraemia by 1 year posttransplant [35]. At present, the best recommended preventive strategies are good hygiene, frequent hand washing, and avoiding contact with others’ body fluids [2].
Our meta-analysis depicts modest heterogeneity across studies, which is justified by broadly similar geographical region and burden for tuberculosis, in spite of different study designs. We deployed an odds ratio to measure the strength of association on the basis of integrating the quantitative findings from case-control studies and rare occurrence of outcome events in several studies (typically <10%). This relative measure may lead to an overestimation of risk. In addition, our subgroup analysis demonstrated that the pooled odds ratio for studies with low risk of bias was substantially lower than that for studies with unclear risk of bias. Studies rated as low risk of bias imply that the results are generally valid and represent the true exposure effect, whereas studies with unclear risk of bias have missing information and this may invalidate the results owing to possible errors in the research design, conduct, analysis, or reporting [15]. The 2 studies categorized as unclear risk of bias were conducted before the year 1995 [20, 22] and the results might not be reflective of the current epidemiological profiles of tuberculosis disease, in addition to suboptimal health care system, treatment, and public health measures over the past period of time [36].
It is notable that many areas with high endemic tuberculosis bear huge, persisting public health challenges, for instance, limited health care workers’ availability and capacity, scarce resources, conventional models of tuberculosis care delivery, and poor health literacy and health-seeking behavior in the communities [37]. Although most of the included studies utilized a multivariate analysis to adjust for potential confounding variables, we cannot rule out the possibility that residual confounding, such as socioeconomic status, cultural conditions, genetic susceptibility to tuberculosis infection, and M. tuberculosis exposure frequency and intensity, could be responsible for the risk increment identified. Nonetheless, we consider that such unmeasured confounding is fairly unlikely because similar patterns of tuberculosis risk have been identified across the diverse study populations in all countries studied. Future research may provide clarification and identify the degree of causality, particularly using large, prospective, multiyear epidemiologic studies with appropriate adjustment for known confounding factors. Of note, people with a lower socioeconomic status have been associated with a higher prevalence of cytomegalovirus infection and tuberculosis [38]. Taken together, this suggests that public health interventions aimed at poverty reduction and social protection could play a vital role in reducing the global burden of cytomegalovirus infection and tuberculosis disease, and help towards achieving the End Tuberculosis Strategy.
Our study possesses several strengths. First, it represents a comprehensive review of available epidemiological evidence about the significance of cytomegalovirus in the development of tuberculosis disease. We have deployed a rigorous meta-analysis approach to assess the dose-response relationship, strengthening support for our inferences that cytomegalovirus-specific immune responses have a role in modifying subsequent risk of tuberculosis disease. There are also sufficient data to construct a robust estimate of the rate of tuberculosis disease in those with cytomegalovirus infection. However, our findings warrant cautious interpretation in view of some possible limitations. We did not examine the evidence from laboratory-based pathological studies that might provide additional insights into the interpretation of the observed association as this granular detail lay beyond the scope of an epidemiological review. The small sample sizes from studies in several countries may introduce potential sources of bias in our analysis and interpretation of results.
As noted by the relatively small number of studies identified in this review, more high-quality research is warranted to bridge the gaps in the body of epidemiological evidence relating to long-term clinical implications following cytomegalovirus infection, both to prevent tuberculosis and minimize the risk of cytomegalovirus reactivation, for improving patient outcomes across the life course. When a more valid and consolidated evidence base is generated, we recommend the development of guidelines for clinical and population health action.
Cytomegalovirus is recognized to cause disruptions to systemic cytokine production and responses by CD8+ and γ-delta T cells and macrophages [39], thereby leading to an elevated risk of primary tuberculosis infection or progression to tuberculosis disease [40]. In light of the sparsity of clinical data, much still remains unknown about the mechanisms responsible for the increased susceptibility to tuberculosis disease [16]. However, an accumulating evidence base indicates an epidemiological and immunological correlation between cytomegalovirus infection and progression to tuberculosis disease [11], and findings from in vivo experiments show a dose-dependent relationship between cytomegalovirus-associated immunosenescence and subsequently impaired responses to heterologous infections [41]. Large, population-based, prospective, cohort studies are necessary to further decipher the tuberculosis pathogenesis and risk of infection among cytomegalovirus-infected individuals.
As the clinical community progresses towards attaining the End Tuberculosis Strategy vision with the goal of reducing tuberculosis incidence, mortality, and eliminating catastrophic costs for tuberculosis-affected households by 2030 [42], our findings draw attention to the increased risk of tuberculosis disease in people with existing cytomegalovirus infection, as determined by the presence of serum antibodies to cytomegalovirus, viral load values, or T-cell responses, is a key parameter to take into account in future tuberculosis control programs. Apart from preemptive therapy and antiviral prophylaxis with valganciclovir for the prevention of cytomegalovirus disease in high-risk patients [43], the strong association between cytomegalovirus infection and progression to active tuberculosis in our study suggests the importance of prioritizing latent tuberculosis screening for individuals with documented cytomegalovirus positivity and the need for preventive therapy to reduce the risk of active tuberculosis among this at-risk population [44]. Approximately 5%–15% of people with latent tuberculosis infection will develop active tuberculosis in their lifetime [45]. The effectiveness and cost-effectiveness of treatment for latent tuberculosis infection is limited by factors such as the large pool of the population, drug availability, and an insufficiently robust care cascade. Such treatment is therefore likely to be most effective when targeted at individuals with a higher risk of developing tuberculosis disease, such as people with diabetes, those who are immunocompromised, or those who are symptomatic. Introducing the risk factor of cytomegalovirus seropositivity into the implementation of risk-stratified medicine in tuberculosis control measures would improve the efficiency and effectiveness of tuberculosis health care and prevention. Findings gleaned from a recent modeling study showed that in settings of high tuberculosis incidence, efforts for prevention and prompt detection of recurrent tuberculosis offer novel opportunities for tuberculosis control [46]. In this context, if secondary preventive therapy is introduced to cytomegalovirus-positive individuals to complement existing tuberculosis control efforts, the burden of tuberculosis would be dramatically reduced. Current pharmacological regimens for the treatment of latent tuberculosis infection comprise isoniazid, rifapentine, and rifampicin [47]. Thus far, there is no evidence that the regimens are any less efficacious among cytomegalovirus-positive patients.
On top of that, attention can also be focused on interventions that have the potential to address cytomegalovirus infection, for instance, optimization and interconnection of current programs to deliver a culturally tailored, multicomponent implementation strategy for tuberculosis and cytomegalovirus control and outcome, parenting practices or sociobehavioral nudges to reduce or at least delay cytomegalovirus transmission in a lifetime, and drug therapies, as well as novel vaccines to prevent cytomegalovirus or tuberculosis disease [11]. Hygienic precautions and behavioral modifications remain the mainstay of mitigation strategies for preventing primary cytomegalovirus infection or reinfection with a new strain in low- and middle-income countries, where issues of safety, access, and cost preclude the use of valganciclovir [1]. It is noteworthy that infections that are classically considered to be relatively low risk but extremely common have profound clinical sequelae on human health. These include reduction in humoral immunity after measles infection, which increases future infection risks by other pathogens [48], Epstein-Barr virus infection, which increases the risk of subsequent multiple sclerosis [49], and coronavirus disease 2019 (COVID-19) infection causing significant end-organ dysfunction [50]. As such, the association between cytomegalovirus infection and tuberculosis disease should not necessarily be assumed to be benign. The important and unforeseen consequences of cytomegalovirus infection further underline the need for development of a broadly protective vaccine against the infection.
CONCLUSIONS
Current evidence from pooled quantitative analyses depicts a significant increase in the risk of active tuberculosis disease associated with cytomegalovirus infection. The consistent findings with narrative results of this review suggest that priority consideration should be given to cytomegalovirus-positive individuals in community-based screening and treatment for latent tuberculosis to address the global burden of tuberculosis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Kok Pim Kua, Pharmacy Unit, Puchong Health Clinic, Petaling District Health Office, Ministry of Health Malaysia, Puchong, Malaysia.
Bunchai Chongmelaxme, Department of Social and Administrative Pharmacy, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand.
Shaun Wen Huey Lee, School of Pharmacy, Monash University, Subang Jaya, Malaysia; Asian Centre for Evidence Synthesis in Population, Implementation, and Clinical Outcomes, Health and Well-being Cluster, Global Asia in the 21st Century Platform, Monash University, Subang Jaya, Malaysia; Gerontechnology Laboratory, Global Asia in the 21st Century Platform, Monash University, Subang Jaya, Malaysia; Faculty of Health and Medical Sciences, Taylor's University, Subang Jaya, Malaysia; Center for Global Health, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Notes
Financial support . No financial support was received for this work.
References
- 1. Ssentongo P, Hehnly C, Birungi P, et al. Congenital cytomegalovirus infection burden and epidemiologic risk factors in countries with universal screening: a systematic review and meta-analysis. JAMA Netw Open 2021; 4:e2120736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torpy JM, Burke AE, Glass RM. Cytomegalovirus. JAMA 2010; 303:1440. [DOI] [PubMed] [Google Scholar]
- 3. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20:202–13. [DOI] [PubMed] [Google Scholar]
- 4. Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis 2014; 22:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winter JR, Taylor GS, Thomas OG, Jackson C, Lewis JEA, Stagg HR. Factors associated with cytomegalovirus serostatus in young people in England: a cross-sectional study. BMC Infect Dis 2020; 20:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lancini D, Faddy HM, Flower R, Hogan C. Cytomegalovirus disease in immunocompetent adults. Med J Aust 2014; 201:578–80. [DOI] [PubMed] [Google Scholar]
- 7. Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J 2008; 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Müller J, Tanner R, Matsumiya M, et al. Cytomegalovirus infection is a risk factor for tuberculosis disease in infants. JCI Insight 2019; 4:e130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong YJ, Lee SWH. Prevalence of latent tuberculosis among refugee children in Malaysia. ERJ Open Res 2020; 6:00254-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong YJ, Noordin NM, Keshavjee S, Lee SWH. Impact of latent tuberculosis infection on health and wellbeing: A systematic review and meta-analysis. Eur Respir Rev 2021; 30:200260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olbrich L, Stockdale L, Basu Roy R, et al. Understanding the interaction between cytomegalovirus and tuberculosis in children: the way forward. PLoS Pathog 2021; 17:e1010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacNeil A, Glaziou P, Sismanidis C, Date A, Maloney S, Floyd K. Global epidemiology of tuberculosis and progress toward meeting global targets—worldwide, 2018. MMWR Morb Mortal Wkly Rep 2020; 69:281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benford D, Halldorsson T, Jeger MJ, et al. The principles and methods behind EFSA's guidance on uncertainty analysis in scientific assessment. EFSA J 2018; 16:e05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viswanathan M, Ansari MT, Berkman ND, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Updated 8 March 2012. In: Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality (US), 2008. https://www.ncbi.nlm.nih.gov/books/NBK91433/. Accessed 5 January 2022. [PubMed] [Google Scholar]
- 16. Martinez L, Nicol MP, Wedderburn CJ, et al. Cytomegalovirus acquisition in infancy and the risk of tuberculosis disease in childhood: a longitudinal birth cohort study in Cape Town, South Africa. Lancet Glob Health 2021; 9:e1740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta RK, Rosenberg G, Eriksen J, et al. Tuberculosis following renal transplantation in England, Wales and Northern Ireland: A national registry-based cohort study. Eur Respir J 2019; 54:1802245. [DOI] [PubMed] [Google Scholar]
- 18. Kim SH, Oh S, Nham E, et al. Risk groups of developing active tuberculosis in liver transplant recipients in a tuberculosis endemic area: risk stratification by chest image and interferon gamma release assay. Int J Infect Dis 2021; 113:359–66. [DOI] [PubMed] [Google Scholar]
- 19. Viana LA, Cristelli MP, Santos DW, et al. Influence of epidemiology, immunosuppressive regimens, clinical presentation, and treatment on kidney transplant outcomes of patients diagnosed with tuberculosis: A retrospective cohort analysis. Am J Transplant 2019; 19:1421–31. [DOI] [PubMed] [Google Scholar]
- 20. Olaleye OD, Omilabu SA, Baba SS. Cytomegalovirus infection among tuberculosis patients in a chest hospital in Nigeria. Comp Immunol Microbiol Infect Dis 1990; 13:101–6. [DOI] [PubMed] [Google Scholar]
- 21. Stockdale L, Nash S, Nalwoga A, et al. Human cytomegalovirus epidemiology and relationship to tuberculosis and cardiovascular disease risk factors in a rural Ugandan cohort. PLoS One 2018; 13:e0192086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledru E, Diagbouga S, Ledru S, et al. A study of toxoplasma and cytomegalovirus serology in tuberculosis and in HIV-infected patients in Burkina Faso. Acta Trop 1995; 59:149–54. [DOI] [PubMed] [Google Scholar]
- 23. Stockdale L, Nash S, Nalwoga A, et al. HIV, HCMV and mycobacterial antibody levels: a cross-sectional study in a rural Ugandan cohort. Trop Med Int Health 2019; 24:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dube FS, Kaba M, Robberts FJ, et al. Respiratory microbes present in the nasopharynx of children hospitalised with suspected pulmonary tuberculosis in Cape Town, South Africa. BMC Infect Dis 2016; 16:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amran FS, Kim K, Lim A, et al. Is pulmonary non-tuberculous mycobacterial disease linked with a high burden of latent cytomegalovirus? J Clin Immunol 2016; 36:113–6. [DOI] [PubMed] [Google Scholar]
- 26. Nagu T, Aboud S, Rao M, et al. Strong anti-Epstein Barr virus (EBV) or cytomegalovirus (CMV) cellular immune responses predict survival and a favourable response to anti-tuberculosis therapy. Int J Infect Dis 2017; 56:136–9. [DOI] [PubMed] [Google Scholar]
- 27. Sirenko IA, Shmat'ko SA, Smelianskaia MV, Peremot SD, Marchenko OI, Podoprigora NM. Impact of cytomegalovirus infection on the course of tuberculosis in children and adolescents. Probl Tuberk Bolezn Legk 2003; (8):7–9. [PubMed] [Google Scholar]
- 28. Stockdale L, Nash S, Farmer R, et al. Cytomegalovirus antibody responses associated with increased risk of tuberculosis disease in Ugandan adults. J Infect Dis 2020; 221:1127–34. [DOI] [PubMed] [Google Scholar]
- 29. Fletcher HA, Snowden MA, Landry B, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 2016; 7:11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ledesma JR, Ma J, Vongpradith A, et al. Global, regional, and national sex differences in the global burden of tuberculosis by HIV status, 1990–2019: results from the Global Burden of Disease Study 2019. Lancet Infect Dis 2022; 22:222–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Silva S, Arinaminpathy N, Atun R, Goosby E, Reid M. Economic impact of tuberculosis mortality in 120 countries and the cost of not achieving the Sustainable Development Goals tuberculosis targets: a full-income analysis. Lancet Glob Health 2021; 9:e1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta M, Shorman M. Cytomegalovirus. Updated 11 August 2021. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK459185/. Accessed 21 April 2022. [Google Scholar]
- 33. Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med 2021; 385:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plotkin SA, Wang D, Oualim A, et al. The status of vaccine development against the human cytomegalovirus. J Infect Dis 2020; 221:S113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ljungman P, Bermudez A, Logan AC, et al. A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients. EClinicalMedicine 2021; 33:100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamburg MA, Frieden TR. Tuberculosis transmission in the 1990s. N Engl J Med 1994; 330:1750–1. [DOI] [PubMed] [Google Scholar]
- 37. Burton DC, Flannery B, Onyango B, et al. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: A community-based house-to-house survey. J Health Popul Nutr 2011; 29:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stebbins RC, Noppert GA, Aiello AE, Cordoba E, Ward JB, Feinstein L. Persistent socioeconomic and racial and ethnic disparities in pathogen burden in the United States, 1999–2014. Epidemiol Infect 2019; 147:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Imlay H, Limaye AP. Current understanding of cytomegalovirus reactivation in critical illness. J Infect Dis 2020; 221:S94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cobelens F, Nagelkerke N, Fletcher H. The convergent epidemiology of tuberculosis and human cytomegalovirus infection. F1000Res 2018; 7:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Redeker A, Remmerswaal EBM, van der Gracht ETI, et al. The contribution of cytomegalovirus infection to immune senescence is set by the infectious dose. Front Immunol 2018; 8:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verguet S, Riumallo-Herl C, Gomez GB, et al. Catastrophic costs potentially averted by tuberculosis control in India and South Africa: a modelling study. Lancet Glob Health 2017; 5:e1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh N, Winston DJ, Razonable RR, et al. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors: a randomized clinical trial. JAMA 2020; 323:1378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saunders MJ, Evans CA. Ending tuberculosis through prevention. N Engl J Med 2019; 380:1073–4. [DOI] [PubMed] [Google Scholar]
- 45. Rustage K, Lobe J, Hayward SE, et al. Initiation and completion of treatment for latent tuberculosis infection in migrants globally: a systematic review and meta-analysis. Lancet Infect Dis 2021; 21:1701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marx FM, Yaesoubi R, Menzies NA, et al. Tuberculosis control interventions targeted to previously treated people in a high-incidence setting: a modelling study. Lancet Global Health 2018; 6:e426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mushtaq A. New guidelines for latent tuberculosis. Lancet Infect Dis 2020; 20:414. [DOI] [PubMed] [Google Scholar]
- 48. Mina MJ, Kula T, Leng Y, et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 2019; 366:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375:296–301. [DOI] [PubMed] [Google Scholar]
- 50. del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020; 324:1723–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.