We present evidence of astrovirus burden in vulnerable communities and suggestive protective immunity to infection, incentivizing ongoing vaccine development for viral gastroenteritis in young children.
Abstract
BACKGROUND AND OBJECTIVES:
Astroviruses are important drivers of viral gastroenteritis but remain understudied in community settings and low- and middle-income countries. We present data from 8 countries with high prevalence of diarrhea and undernutrition to describe astrovirus epidemiology and assess evidence for protective immunity among children 0 to 2 years of age.
METHODS:
We used 25 898 surveillance stools and 7077 diarrheal stools contributed by 2082 children for enteropathogen testing, and longitudinal statistical analysis to describe incidence, risk factors, and protective immunity.
RESULTS:
Thirty-five percent of children experienced astrovirus infections. Prevalence in diarrheal stools was 5.6%, and severity exceeded all enteropathogens except rotavirus. Incidence of infection and diarrhea were 2.12 and 0.88 episodes per 100 child-months, respectively. Children with astrovirus infection had 2.30 times the odds of experiencing diarrhea after adjustment for covariates (95% confidence interval [CI], 2.01–2.62; P < .001). Undernutrition was a risk factor: odds of infection and diarrhea were reduced by 10% and 13%, respectively, per increase in length-for-age z score (infection: odds ratio, 0.90 [95% CI, 0.85–0.96]; P < .001; diarrhea: odds ratio, 0.87 [95% CI, 0.79–0.96]; P = .006). Some evidence of protective immunity to infection was detected (hazard ratio, 0.84 [95% CI, 0.71–1.00], P = .052), although this was heterogeneous between sites and significant in India and Peru.
CONCLUSIONS:
Astrovirus is an overlooked cause of diarrhea among vulnerable children worldwide. With the evidence presented here, we highlight the need for future research as well as the potential for astrovirus to be a target for vaccine development.
What’s Known on This Subject:
Astroviruses commonly cause viral gastroenteritis among young children. Researchers traditionally focus on clinical outbreaks in hospital-based or high-income settings, and a more complete description of asymptomatic infection, disease burden, and immunogenicity in vulnerable communities is needed.
What This Study Adds:
We present the most comprehensive description of astrovirus epidemiology in young children to date. With our longitudinal, community-derived estimates in 8 low- to middle-income settings, we demonstrate high prevalence, clinical episodes of comparable severity to other enteropathogens, key risk factors, and evidence of protective immunity.
Astroviruses are a common cause of viral gastroenteritis across geographic and developmental settings.1–3 In temperate settings, infection is associated with seasonal outbreaks in hospitals, child care centers, cruise ships, and hotels. In tropical settings with poor water and sanitation infrastructure, astroviruses contribute to the burden of childhood diarrhea, which accounts for considerable loss of life and human potential in low- and middle-income countries (LMIC).
Human astroviruses are small, nonenveloped, single-stranded RNA viruses belonging to the Astroviridae family.2 They are estimated to account for 2% to 9% of acute, nonbacterial childhood diarrhea,2 with novel strains linked to central nervous system consequences (including meningitis and encephalitis) in children.2–7 Astrovirus gastroenteritis commonly manifests as an acute, self-limiting watery diarrhea, sometimes accompanied by vomiting, anorexia, and fever. Type 1 astroviruses are the most prevalent of 8 serotypes across epidemiologic contexts.8 Disease occurs mostly in young children and immunocompromised adults, and although symptoms in adults are rare, seroprevalence to common strains is high.8,9 This implies that infection confers some protection among humans, although evidence is conflicting.9 Severity of symptoms has been negatively correlated with the presence of antibodies to astrovirus.10,11 However, our understanding of the mechanisms of protective immunity remains incomplete, with the majority of evidence generated from high-income settings and a paucity of data on seroconversion and immunity in LMIC.
In studies of astrovirus epidemiology, researchers have generally focused on children exhibiting diarrhea or acute gastroenteritis, with few reporting longitudinal history of infection and symptoms. Lack of evidence from LMIC highlights an important gap in knowledge considering the undue burden of childhood diarrhea in these communities and the increasing appreciation of complex interactions between undernutrition, enteric infections with or without symptoms, and child growth and cognitive development. Multisite cohort studies in LMIC can help characterize the patterns of asymptomatic and symptomatic infection and protective immunity against astrovirus in community-based settings, where the majority of the consequences of childhood diarrhea occur. In this longitudinal study, we describe the epidemiology of astrovirus infection among infants 0 to 24 months of age in 8 geographically distinct sites with a high incidence of childhood diarrhea and undernutrition.
Methods
Study Population
The Etiology, Risk Factors, and Interactions of Enteric Infection and Malnutrition and the Consequences for Child Health and Development (MAL-ED) Study enrolled children in 8 countries: Bangladesh, India, Nepal, Pakistan, Brazil, Peru, Tanzania, and South Africa. Healthy infants were enrolled within 17 days of birth and were managed through 2 years of age; details of our study design are described elsewhere.12 All sites received ethical approval and written informed consent.
Data Collection
Sex, birthdate, and anthropometric data were recorded at enrollment, and data collection of anthropometrics was conducted monthly. Length-for-age z scores (LAZs) to assess growth faltering were calculated according to the World Health Organization’s Multicentre Growth Reference Study Group guidelines.13 Stool samples were collected monthly in the first year and quarterly thereafter for surveillance of enteric infections. Active surveillance of breastfeeding, diarrhea, and symptoms of other illnesses (including fever, vomiting, and respiratory problems) was conducted twice weekly in the home. Additional stool samples were obtained during diarrheal episodes, defined as ≥3 loose stools in a 24-hour period and separated by at least 2 diarrhea-free days. Children experiencing moderate to severe diarrhea were referred to local health services.
For incidence calculations, distinct infections were defined as astrovirus-positive surveillance stool samples separated by ≥1 negative sample from the previous infection. Distinct diarrheal episodes were defined as astrovirus-positive samples preceded by either an astrovirus-negative diarrheal stool sample or a 14-day lag from the previous astrovirus-positive diarrheal sample. Duration of exclusive breastfeeding (EBF) was defined as time from birth until introduction of clear liquids, solids, or milk formula. Diarrheal severity was calculated by using a community diarrheal assessment scale that with which scores out of 15 points were assigned according to presence and duration of fever, vomiting, anorexia, liquid stools, and maximum stool output within a 24-hour period.14
Microbiology
A diagnostic and quality assurance protocol was harmonized across all 8 sites for the detection of enteropathogens. The ProSpecT (Remel, obtained from Fisher Scientific, Pittsburgh, PA) enzyme-linked immunosorbent assay was used to detect astrovirus, rotavirus, and adenovirus within 1 week of stool collection and suspension in diluent. This assay employs a combination of genus-specific monoclonal and polyclonal antibodies and detects all described genotypes of astrovirus. Microbiologic methods of detection for additional enteropathogens are described in detail elsewhere.15
Statistical Analyses
Cumulative incidence and incidence rates were calculated by using Kaplan-Meier curves and survival analysis, allowing for multiple failures per child. Associations between astrovirus, diarrhea, and undernutrition were modeled by using linear regression with a generalized estimating equations approach, adjusting for within-child clustering. Adjustment for EBF was included in all analyses because of the well-described relationship between breastfeeding, immunity, and growth in early life. A Cox proportional hazards model was used to calculate hazard rates of infection and diarrheal episodes in exposed versus naïve children. Countries with at least 5 children who were experiencing at least 10 events (subsequent infections or diarrheal episodes) were selected for site-specific estimation of protective immunity. We calculated the adjusted population attributable fraction (PAF),5 with which differences in prevalence and strength of association with diarrheal symptoms are incorporated and LAZ, EBF, and age are adjusted for to estimate the astrovirus-specific burden of diarrhea.
Results
Astrovirus Epidemiology Across 8 Sites
Across 8 sites, 1036 boys and 1046 girls were managed between November 2009 and February 2012. These 2082 children contributed 7077 diarrheal samples and 25 868 surveillance stools through 24 months of age (Table 1).
TABLE 1.
Sample Population by Age and Site
| No. Children | Diarrheal Samples Contributed | Surveillance Stools Contributed | |
|---|---|---|---|
| Total No. children | 2082 | 7077 | 25 868 |
| No. children recruited at each site | |||
| Asia | |||
| Bangladesh | 260 | 1525 | 3331 |
| India | 243 | 615 | 3212 |
| Nepal | 238 | 860 | 3322 |
| Pakistan | 274 | 1793 | 3232 |
| Africa | |||
| South Africa | 288 | 172 | 3758 |
| Tanzania | 259 | 171 | 3448 |
| South America | |||
| Brazil | 221 | 98 | 2076 |
| Peru | 299 | 1843 | 3489 |
| No. children contributing samples within each age group, mo | |||
| 0–5 | 2071 | 1810 | 9154 |
| 6–11 | 1938 | 2273 | 9516 |
| 12–17 | 1814 | 1700 | 3001 |
| 18–24 | 1759 | 1294 | 4197 |
Children experienced 2.1 distinct astrovirus infections per 100 child-months across sites, with a peak of 3.1 detections at 6 to 11 months of age. There were marked differences between countries, with overall incidence of infection highest in Peru, India, and Bangladesh and lowest in Brazil and South Africa. Incidence rates and cumulative incidence of infection and diarrhea are shown in Table 2 and Fig 1, respectively. Across sites, 550 (26.4%) children experienced at least 1 astrovirus infection in the first year of life; by the end of follow-up, 732 (35.2%) had documented infections.
TABLE 2.
Incidence Rates of Astrovirus Infection Among 2082 Children Across 8 MAL-ED Sites by Age Group and Country
| Child-Months at Risk | Total Episodes With Astrovirus Detected | Incidence of Infection (95% CI) | Diarrheal Episodes | Incidence of Diarrhea (95% CI) | |
|---|---|---|---|---|---|
| Age group, mo | |||||
| 0–5 | 11 025.2 | 265 | 2.40 (2.13–2.71) | 75 | 0.68 (0.54–0.85) |
| 6–11 | 11 235.0 | 344 | 3.06 (2.76–3.40) | 127 | 1.13 (0.95–1.35) |
| 12–17 | 8391.6 | 177 | 2.11 (1.82–2.44) | 121 | 1.44 (1.21–1.72) |
| 18–24 | 13 443.2 | 150 | 1.11 (0.95–1.31) | 65 | 0.48 (0.38–0.62) |
| Country | |||||
| Asia | |||||
| Bangladesh | 5476.8 | 185 | 3.38 (2.92–3.90) | 80 | 1.46 (1.17–1.82) |
| India | 5535.4 | 92 | 3.67 (1.36–2.04) | 32 | 0.58 (0.41–0.82) |
| Nepal | 5497.0 | 117 | 2.13 (1.78–2.55) | 40 | 0.73 (0.53–0.99) |
| Pakistan | 6181.6 | 156 | 2.52 (2.16–2.95) | 74 | 1.20 (0.95–1.50) |
| Africa | |||||
| South Africa | 6001.35 | 50 | 0.83 (0.63–1.10) | 5 | 0.08 (0.03–0.20) |
| Tanzania | 5441.7 | 112 | 2.06 (1.71–2.48) | 12 | 0.22 (0.13–0.39) |
| South America | |||||
| Brazil | 4309.2 | 9 | 0.21 (0.11–0.40) | 4 | 0.09 (0.03–0.25) |
| Peru | 5651.8 | 215 | 3.80 (3.33–4.35) | 141 | 2.49 (2.11–2.94) |
| Total | 44 094.9 | 936 | 2.12 (1.99–2.26) | 388 | 0.88 (0.80–0.97) |
Incidence expressed as number of detections per 100 child-months. All detections, whether asymptomatic or diarrheal, were considered episodes for calculation of incidence rates for infection. Only episodes of diarrhea with astrovirus detected were included as events for calculation of incidence rates for diarrhea. New episodes were defined as separated from previous episodes by either astrovirus-negative stool samples or a period of ≥14 d from previous astrovirus-positive stools.
FIGURE 1.
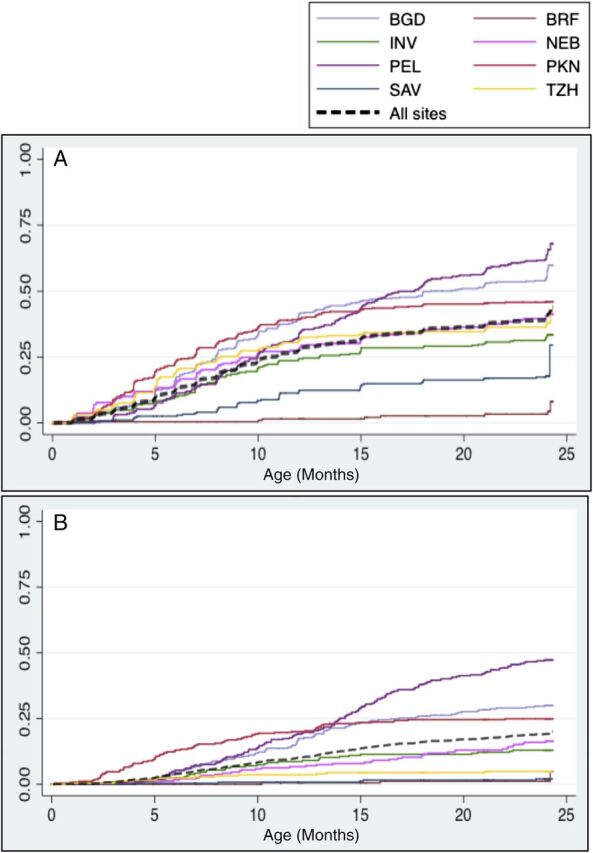
Cumulative incidence of astrovirus in (A) surveillance stools and (B) diarrheal samples in the MAL-ED cohort. Time to first infection and clinical episode of diarrhea is shown globally (black dotted line) and separately by site. BGD: Mirpur, Bangladesh; BRF: Fortaleza, Brazil; INV: Vellore, India; NEB: Bhaktapur, Nepal; PEL: Iquitos, Peru; PKN: Naushero Feroze, Pakistan; SAV: Limpopo, South Africa; TZH: Heydom, Tanzania.
Astrovirus detection in diarrheal stools exceeded that in surveillance stools across the full cohort: 5.6% (394) of diarrheal stools and 2.2% (573) of surveillance stools were positive for astrovirus infection, respectively. In contrast with asymptomatic astrovirus detection, prevalence during diarrhea increased with age in the first year of life, peaking at 12 to 14 months and declined thereafter, with the exception of a second peak from 21 to 24 months (Fig 2A). We observed heterogeneity in astrovirus infection and symptoms by country. Children in Peru exhibited the highest prevalence of astrovirus-positive diarrhea. Prevalence ratios of astrovirus infection in diarrhea relative to surveillance stools also differed by site (Fig 2B).
FIGURE 2.
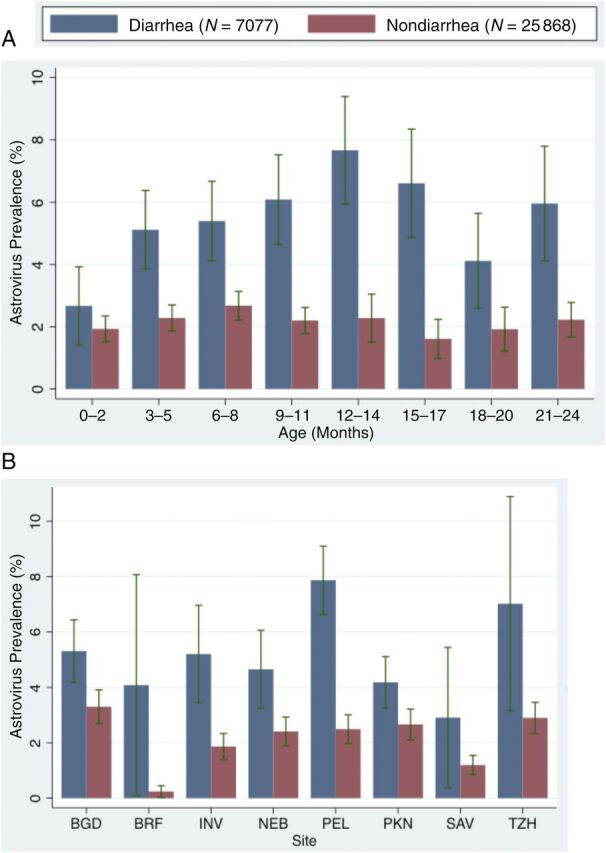
Astrovirus prevalence by (A) age and (B) site across 2082 children in the MAL-ED cohort. Surveillance stools are shown in red, and diarrheal samples are shown in blue. BGD: Mirpur, Bangladesh; BRF: Fortaleza, Brazil; INV: Vellore, India; NEB: Bhaktapur, Nepal; PEL: Iquitos, Peru; PKN: Naushero Feroze, Pakistan; SAV: Limpopo, South Africa; TZH: Heydom, Tanzania.
Mean severity of astrovirus-positive diarrhea was 3.15 points (95% confidence interval [CI], 2.90–3.40) out of a 15-point community diarrheal severity scale, compared with 2.89 (95% CI, 2.84–2.95) points for all-cause diarrhea, 3.92 (95% CI 3.66–4.18) for rotavirus, and 2.95 (95% CI, 2.83–3.06) for norovirus. Overall, 77% (744) of all astrovirus-positive diarrheal samples were coinfected with at least 1 other pathogen. Prevalence of common coinfections is shown in the full cohort in Table 3, and by site in Supplemental Table 6. The most common coinfection was with Campylobacter spp., which was present in 28.7% and 40.1% of all surveillance and diarrheal stools, respectively. Other prevalent coinfections with diarrheal symptoms included norovirus, enteroaggregative Escherichia coli, and Giardia. These findings were consistent across surveillance and diarrheal samples.
TABLE 3.
Astrovirus Coinfections in Surveillance and Diarrheal Stools
| Astrovirus-Positive Diarrhea Samples (N = 394), % (n) | Astrovirus-Positive Surveillance Stools (N = 573), % (n) | |
|---|---|---|
| Viruses | ||
| Rotavirus | 5.6 (22) | 3.7 (21) |
| Norovirus | 24.9 (98) | 26.3 (26)a |
| Adenovirus | 4.8 (19) | 3.8 (22) |
| Bacteria | ||
| Campylobacter | 40.1 (158) | 34.4 (197) |
| Shigella | 1.3 (5) | 0.7 (4) |
| EAEC | 28.2 (111) | 31.4 (180) |
| EPEC | 4.6 (18) | 3.8 (22) |
| ETEC | 11.9 (47) | 7.7 (44) |
| Aeromonas | 3.3 (13) | 1.9 (11) |
| Protozoa | ||
| Giardia | 17.5 (69) | 16.2 (93) |
| Cryptosporidium | 5.6 (22) | 3.8 (22) |
EAEC, enteroaggregative E coli; EPEC, enteropathogenic E coli; ETEC, enterotoxigenic E coli.
Norovirus testing was conducted on a subsample of children in the global cohort and thus only available for 99 of the 573 surveillance stools positive for astrovirus.
Evidence of Protective Immunity
Protective immunity was assessed by comparing the hazard of subsequent infections and diarrhea between children with and without previous exposure to infection. Models were adjusted for EBF, LAZ, and site. The results revealed a reduced incidence of infection among children with previous infection, although this was marginally nonsignificant (hazard ratio [HR], 0.84 [95% CI, 0.71–1.00]; P = .052). When the number of previous infections was categorized, the same trend was seen among children with 1 previous infection (HR, 0.84 [95% CI, 0.70–1.01]; P = .065) as well as those with ≥2 previous infections (HR, 0.84 [95% CI, 0.59–1.20]; P = .334), although neither was statistically significant. No relationship between previous infection and risk of subsequent diarrhea was detected in the full cohort (HR, 0.94 [95% CI, 0.74–1.20]; P = .613). When explored by modeling the hazard of infections and diarrheal episodes among children with previous astrovirus diarrhea, no protective immunity was detected. Children with exposure to astrovirus-positive diarrhea did not have a significantly reduced hazard of future infections (HR, 0.97 [95% CI, 0.77–1.21]; P = .766) nor future diarrheal episodes (HR, 1.01 [95% CI, 0.74–1.37]; P = .975) relative to children without. HRs for each model are shown in Table 4. We could not explore dose responses because of insufficient events across the full cohort.
TABLE 4.
Protection Associated With Astrovirus Across MAL-ED Sites
| Exposure | No. Previous Astrovirus Infections | HRs (95% CI) | |
|---|---|---|---|
| Subsequent Infections | Subsequent Diarrheal Episodes | ||
| Previous detection | None (reference) | ||
| Any | 0.84 (0.71–1.00) | 0.94 (0.74–1.20) | |
| P = .054 | P = .613 | ||
| No. previous detections | None (reference) | ||
| 1 | 0.84 (0.70–1.01) | 0.95 (0.73–1.23) | |
| .065 | .682 | ||
| 2+ | 0.84 (0.59–1.19) | 0.91 (0.58–1.42) | |
| P = .334 | P = .663 | ||
| Previous astrovirus diarrhea | None (reference) | ||
| Any | 0.97 (0.77–1.21) | 1.01 (0.74–1.36) | |
| P = .766 | P = .975 | ||
Immunity was also explored at each site separately. Brazil and South Africa were excluded from this portion of the analysis because the number of reinfections at these sites was insufficient (none in Brazil, 3 in South Africa). When modeling protection incurred by previous astrovirus diarrhea, only 3 sites met the inclusion criteria of 10 events: Bangladesh, Pakistan, and Peru (15, 11, and 25 subsequent diarrheal episodes at each site, respectively). Site-specific HRs are shown in Table 5. We found no evidence that differences in overall coinfection or coinfection by specific pathogens explained the observed heterogeneities in protective immunity by site (M.P.O., S.R., P.P.Y., et al, unpublished data).
TABLE 5.
Protection Associated With Astrovirus in MAL-ED by Site
| Site | Previous Astrovirus Exposure | HRs (95% CI) | |
|---|---|---|---|
| Subsequent Infection | Subsequent Diarrheal Episodes | ||
| Asia | |||
| Bangladesh | Any infection | 0.94 (0.65–1.36) | 1.22 (0.76–1.95) |
| n = 131 | P = .739 | P = .416 | |
| Diarrheal episode | 1.13 (0.67–1.92) | 1.63 (0.85–3.12) | |
| n = 65 | P = .654 | P = .139 | |
| India | Any infection | 0.50 (0.26–0.96) | 0.52 (0.15–1.80) |
| n = 81 | P = .037 | P = .302 | |
| Diarrheal episode | — | — | |
| n = 31 | |||
| Nepal | Any infection | 0.99 (0.59–1.66) | 1.12 (0.56–2.23) |
| n = 93 | P = .964 | P = .743 | |
| Diarrheal episode | — | — | |
| n = 37 | |||
| Pakistan | Any infection | 0.84 (0.56–1.25) | 1.11 (0.63, 1.96) |
| n = 120 | P = .386 | P = .727 | |
| Diarrheal episode | 0.79 (0.48–1.31) | 1.17 (0.64–2.13) | |
| n = 63 | P = .356 | P = .608 | |
| Africa | |||
| Tanzania | Any infection | 1.11 (0.70–1.78) | 2.74 (0.86–8.71) |
| n = 88 | P = .655 | P = .088 | |
| Diarrheal episode | — | — | |
| n = 12 | |||
| South America | |||
| Peru | Any infection | 0.54 (0.38–0.75) | 0.57 (0.38–0.87) |
| n = 163 | P < .001 | P = .009 | |
| Diarrheal episode | 0.74 (0.53–1.04) | 0.64 (0.41–1.00) | |
| n = 115 | P = .082 | P = .051 | |
South Africa and Brazil were excluded because of an insufficient number of subsequent infections (<10). Sites where there were sufficient infections but <10 subsequent diarrheal episodes are included, with (—) denoting the excluded components of the analyses.
Evidence for protective immunity was found in India and Peru. In India, previous infection was associated with a 50% decrease in the subsequent hazard of infection (HR, 0.50 [95% CI, 0.26–0.96]; P = .037), suggesting a reduced risk among children with past exposure. No evidence of protection against subsequent diarrheal episodes or of protection from previous astrovirus diarrhea was detected (Table 5). Children living in Peru had a 46% and 43% reduced risk of future infections and diarrheal episodes, respectively, on the basis of previous infection (infection: HR, 0.54 [95% CI, 0.38–0.75]; P ≤ .001; diarrhea: HR, 0.57 [95% CI, 0.38–0.87]; P = .009). A protective trend against future infections and symptoms was seen among children who experienced at least 1 clinical episode of astrovirus diarrhea, although this was not significant at the 95% level (infection: HR, 0.74 [95% CI, 0.53–1.04]; P = .082; diarrhea: HR, 0.64 [95% CI, 0.41–1.00]; P = .051).
On the basis of these findings, evidence of a dose response was assessed in Peru. Of the 163 children infected with astrovirus during follow-up, 39 experienced 1 reinfection, and 7 experienced ≥2 reinfections. Children with 1 previous infection had a 42% reduced incidence of infection (HR, 0.58 [95% CI, 0.40–0.84]; P = .004), whereas those with ≥2 previous exposures experienced a 63% reduction in subsequent infections (HR, 0.37 [95% CI, 0.17–0.81]; P = .013). There were insufficient instances of tertiary or quaternary infection (n = 7) to meet the inclusion threshold of 10 events. Nonetheless, this model produced the expected direction of effect, demonstrating a gradient of protection as the number of infections increased. The 7 children with ≥2 previous detections experienced an additional 20% reduction in the subsequent risk of future infections (P = .013) and diarrheal episodes (P = .032) relative to children with 1 previous detection.
Associations Between Astrovirus Infection, Diarrhea, and Undernutrition
Across sites, there was a strong relationship between astrovirus and diarrheal symptoms. After adjustment for age, EBF, and LAZ, odds of astrovirus detection were 2.30 times higher in diarrheal samples than in surveillance stools (95% CI, 2.01–2.62; P < .001). This association was statistically significant at all sites except South Africa, where only 5 episodes of astrovirus-positive diarrhea were reported among 285 children throughout follow-up (odds ratio [OR], 2.51 [95% CI, 0.96–6.54]; P = .061). Using estimates from the global cohort, we found that the adjusted PAF was 3.44%.
An additional analysis was run to investigate whether the association with symptoms was because of coinfection. After adjustment for the 4 most prevalent coinfections (Campylobacter spp., enteroaggregative E coli, norovirus, and Giardia), the strength of association between infection and diarrheal symptoms was attenuated but remained significant and was stronger for astrovirus than other pathogens considered (OR, 1.88 [95% CI, 1.57–2.34]; P < .001). When a binary indicator of any other coinfection was considered, the odds of astrovirus detection were 1.48 times higher in diarrheal stools relative to surveillance stools among children infected with only astrovirus (95% CI, 1.09–2.00; P = .012) and 2.61 times higher among children with coinfections (95% CI, 2.22–2.99; P < .001) relative to pathogen-negative stools.
Undernutrition was a risk factor for astrovirus. Odds of infection were diminished by a mean of 10% per increase in LAZ (OR, 0.90 [95% CI, 0.85–0.96]; P < .001), and odds of experiencing astrovirus-positive diarrhea decreased by 13% per increase in LAZ (OR, 0.87 [95% CI, 0.79–0.96]; P = .006) after accounting for EBF. However, when adjusted for site, no significant relationship was observed. At the site level, a significant reduction in astrovirus diarrhea with increasing LAZ was detected only in Nepal (OR, 0.68 [95% CI, 0.51–0.92]; P = .013). Increased odds of experiencing astrovirus diarrhea were observed with increasing LAZ in Tanzania (OR, 1.73 [95% CI, 1.05–2.88]; P = .032). There was no association between astrovirus and subsequent growth insults at 3, 6, or 9 months postinfection (3 months: P = .389; 6 months: P = .339; 9 months: P = .335), even after adjustment for EBF or site.
Discussion
Gastroenteritis is an important cause of morbidity and hospitalizations across developmental contexts and of childhood morbidity in LMIC. Etiologies include bacterial, protozoal, and viral agents16,17; among these, the astrovirus burden is poorly recognized. With the findings reported here and elsewhere, an impetus for greater attention to astrovirus as an important pathogen and potential vaccine target is provided.
Astrovirus was strongly associated with diarrhea of equal or greater severity than well-recognized enteropathogens, including norovirus, Shigella spp, and E coli spp.18,19 It has been noted that astroviruses are more frequently associated with symptoms than many other pathogens receiving research investments.1 The PAF of astrovirus is exceeded only by norovirus genogroup II, rotavirus, and Campylobacter spp. in the MAL-ED cohort.18 Here we report a PAF of 3.4%, which if applied to the estimated 1.731 billion annual diarrheal episodes20 suggests that astrovirus may account for up to 5.96 million cases each year. Astroviruses are estimated to account for 2.3% to 8.9% of children presenting with diarrhea in ambulatory, clinical, or hospital-based settings worldwide1,21–23 and 4% to 5% of diarrheal disease in community settings in Egypt and Mexico.24,25 In our community-based assessment, astrovirus was detected in 5.6% of study-defined diarrhea and up to 7.9% in Peru, where prevalence was highest. We also observed a high overall incidence of astrovirus diarrhea relative to reports from longitudinal community cohorts.24,25 The heterogeneity in infection and diarrhea between sites demonstrates that estimates vary considerably on the basis of geographic setting and across urban and rural contexts.
In observational studies, researchers have shown that infection is associated with antibody responses that coincide with reduction in disease severity.11,26 To our knowledge, in only 1 other study have researchers modeled protective immunity to astrovirus in a longitudinal cohort. Naficy et al25 conducted active surveillance for diarrhea in rural Egypt and modeled the hazard of clinical disease with and without previous exposure to diarrhea. They did not find any evidence of protection, although they did report possible homotypic immunity. Across 8 sites and with 2082 participants, we report suggestive evidence of immunity to infection (but not diarrheal disease) among children in the first 2 years of life. Lack of protection against symptomatic disease may be partially explained by coinfection; because astrovirus incidence was lower, there is a greater risk that clinical symptoms were driven by other pathogens in diarrheal samples. The inclusion of asymptomatic infection as an indicator of exposure may have allowed us to capture upstream evidence of immunity that a study in which researchers observed only clinical disease would not detect. Furthermore, we report vast epidemiologic heterogeneity by site. We found previous astrovirus exposure to be associated with a 40% to 50% reduction in the hazard of subsequent infection in Peru and India. In contrast, South Africa, Tanzania, and Brazil had 1 or fewer occurrences of reinfection and no diarrheal episodes reported after initial exposure. Whether this is a function of rapid acquisition of immunity25 or of low incidence of astrovirus cannot be inferred from this study. Nonetheless, we believe this work provides suggestive evidence for protective immunity to astrovirus among children living in poverty.
We note a lack of serotype-level data as a limitation, especially in light of observed disparities in evidence for protective immunity. That serotypes exhibit different epidemiology is well described,27 and their distribution is likely to differ between sites. Furthermore, it precludes this work from contributing to our understanding of homotypic and heterotypic immunity. Serotyping of astrovirus is an important recommendation for future work. Another limitation of this study is the diagnostic technique. Enzyme-linked immunosorbent assays are a popular method for detection of viruses in large samples and has comparable sensitivity to electron microscopy, but a greater proportion of positive samples can be identified by molecular diagnostics.28–31 However, assuming the decreased sensitivity is nondifferential between asymptomatic and diarrheal stools, this would translate into the reporting of conservative estimates here. Finally, high prevalence of coinfection makes it impossible to attribute episodes exclusively to astrovirus. However, we note that aside from Campylobacter spp., the most prevalent coinfections were with pathogens less frequently associated with diarrhea than astrovirus,32 and a strong statistical association between astrovirus infection and diarrheal diseases has been demonstrated after adjustment for coinfections, here and elsewhere.1
With 72.6% of detected infections occurring after 6 months of age, we suggest that astrovirus may be an appropriate vaccine target. This would not only be relevant to children living in poverty. The persistence of astrovirus in high-income settings suggests that, like norovirus, prevention of morbidity is unlikely to be achieved through water, sanitation, and hygiene improvements alone. Indeed, 65% to 90% of adults were reported to be seropositive in studies in the United States and Great Britain.8,33,34 A vaccine could therefore have applications in a range of populations.
Conclusions
This study is a significant contribution to our understanding of an important cause of pediatric gastroenteritis worldwide.3,30,35 Through the use of a large, multicountry birth cohort, we provide a description of the epidemiology of infection and clinical disease of a pathogen that, despite its contribution to the diarrheal burden, has received little attention to date. We also report evidence of protective immunity and a strong relationship between infection and diarrheal symptoms of considerable severity. We believe that these findings provide impetus for continued studies into the development of protective immunity to astrovirus in different settings to elucidate the potential public health merit of combination vaccines that would include astrovirus as well as rotavirus36 and to broaden protection against viral gastroenteritis in children.
Supplementary Material
Acknowledgments
We thank the staff and participants of the MAL-ED Network for their important contributions, patience, and tireless work.
Glossary
- CI
confidence interval
- HR
hazard ratio
- EBF
exclusive breastfeeding
- LAZ
length-for-age z score
- LMIC
low- and middle-income countries
- MAL-ED
The Etiology, Risk Factors, and Interactions of Enteric Infection and Malnutrition and the Consequences for Child Health and Development
- OR
odds ratio
- PAF
population attributable fraction
Footnotes
Ms Olortegui, Ms Yori, Ms Salas, Ms Trigoso, Dr Mondal, Dr Bodhidatta, Dr Samie, Mr Kabir, Dr Lima, Dr Babji, Dr Shrestha, Dr Mason, Mr Kalam, Dr Bessong, Dr Ahmed, Dr Mduma, Dr Bhutta, Dr Lima, Ms Ramdass, Dr Lang, Ms George, Dr Zaidi, Dr Kang, Dr Houpt and Dr Kosek conceptualized and designed the study; Ms Rouhani led the initial analysis, and she and Ms Olortegui contributed equally to the drafting and revision of the manuscript; Dr Platts-Mills and Dr Moulton provided critical review and contributions to the analysis and revision of the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the National Institutes of Health Fogarty International Center. Dr Margaret Kosek was supported in part by the the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases at the Johns Hopkins School of Medicine.
References
- 1.Herrmann JE, Taylor DN, Echeverria P, Blacklow NR. Astroviruses as a cause of gastroenteritis in children. N Engl J Med. 1991;324(25):1757–1760 [DOI] [PubMed] [Google Scholar]
- 2.Bosch A, Pintó RM, Guix S. Human astroviruses. Clin Microbiol Rev. 2014;27(4):1048–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnan T. Novel human astroviruses: challenges for developing countries. Virusdisease. 2014;25(2):208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appleton H, Higgins PG. Letter: viruses and gastroenteritis in infants. Lancet. 1975;1(7919):1297. [DOI] [PubMed] [Google Scholar]
- 5.Cordey S, Vu DL, Schibler M, et al. Astrovirus MLB2, a new gastroenteric virus associated with meningitis and disseminated infection. Emerg Infect Dis. 2016;22(5):846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quan PL, Wagner TA, Briese T, et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg Infect Dis. 2010;16(6):918–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naccache SN, Peggs KS, Mattes FM, et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clin Infect Dis. 2015;60(6):919–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbelo PD, Ching KH, Esper F, et al. Serological studies confirm the novel astrovirus HMOAstV-C as a highly prevalent human infectious agent. PLoS One. 2011;6(8):e22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guix S, Bosch A, Pintó RM. Human astrovirus diagnosis and typing: current and future prospects. Lett Appl Microbiol. 2005;41(2):103–105 [DOI] [PubMed] [Google Scholar]
- 10.Midthun K, Greenberg HB, Kurtz JB, Gary GW, Lin FY, Kapikian AZ. Characterization and seroepidemiology of a type 5 astrovirus associated with an outbreak of gastroenteritis in Marin County, California. J Clin Microbiol. 1993;31(4):955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtz JB, Lee TW, Craig JW, Reed SE. Astrovirus infection in volunteers. J Med Virol. 1979;3(3):221–230 [DOI] [PubMed] [Google Scholar]
- 12.MAL-ED Network Investigators . The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014;59(suppl 4):S193–S206 [DOI] [PubMed] [Google Scholar]
- 13.WHO Multicentre Growth Reference Study Group . WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85 [DOI] [PubMed] [Google Scholar]
- 14.Lee G, Peñataro Yori P, Paredes Olortegui M, et al. An instrument for the assessment of diarrhoeal severity based on a longitudinal community-based study. BMJ Open. 2014;4(6):e004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houpt E, Gratz J, Kosek M, et al. ; MAL-ED Network Investigators . Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis. 2014;59(suppl 4):S225–S232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerrant RL, Bobak DA. Bacterial and protozoal gastroenteritis. N Engl J Med. 1991;325(5):327–340 [DOI] [PubMed] [Google Scholar]
- 17.Clark B, McKendrick M. A review of viral gastroenteritis. Curr Opin Infect Dis. 2004;17(5):461–469 [DOI] [PubMed] [Google Scholar]
- 18.Platts-Mills JA, Babji S, Bodhidatta L, et al. ; MAL-ED Network Investigators . Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health. 2015;3(9):e564–e575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouhani S, Peñataro Yori P, Paredes Olortegui M, et al. ; Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) Network Investigators . Norovirus infection and acquired immunity in 8 countries: results from the MAL-ED study. Clin Infect Dis. 2016;62(10):1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz JR, Bartlett AV, Herrmann JE, Cáceres P, Blacklow NR, Cano F. Astrovirus-associated diarrhea among Guatemalan ambulatory rural children. J Clin Microbiol. 1992;30(5):1140–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotloff KL, Herrmann JE, Blacklow NR, et al. The frequency of astrovirus as a cause of diarrhea in Baltimore children. Pediatr Infect Dis J. 1992;11(7):587–589 [PubMed] [Google Scholar]
- 23.Steele AD, Basetse HR, Blacklow NR, Herrmann JE. Astrovirus infection in South Africa: a pilot study. Ann Trop Paediatr. 1998;18(4):315–319 [DOI] [PubMed] [Google Scholar]
- 24.Guerrero ML, Noel JS, Mitchell DK, et al. A prospective study of astrovirus diarrhea of infancy in Mexico City. Pediatr Infect Dis J. 1998;17(8):723–727 [DOI] [PubMed] [Google Scholar]
- 25.Naficy AB, Rao MR, Holmes JL, et al. Astrovirus diarrhea in Egyptian children. J Infect Dis. 2000;182(3):685–690 [DOI] [PubMed] [Google Scholar]
- 26.Koci MD. Immunity and resistance to astrovirus infection. Viral Immunol. 2005;18(1):11–16 [DOI] [PubMed] [Google Scholar]
- 27.Vu DL, Cordey S, Brito F, Kaiser L. Novel human astroviruses: novel human diseases? J Clin Virol. 2016;82:56–63 [DOI] [PubMed] [Google Scholar]
- 28.Cubitt WD, Mitchell DK, Carter MJ, Willcocks MM, Holzel H. Application of electronmicroscopy, enzyme immunoassay, and RT-PCR to monitor an outbreak of astrovirus type 1 in a paediatric bone marrow transplant unit. J Med Virol. 1999;57(3):313–321 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell DK, Monroe SS, Jiang X, Matson DO, Glass RI, Pickering LK. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse transcriptase-polymerase chain reaction. J Infect Dis. 1995;172(6):1437–1444 [DOI] [PubMed] [Google Scholar]
- 30.Glass RI, Noel J, Mitchell D, et al. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch Virol Suppl. 1996;12:287–300 [DOI] [PubMed] [Google Scholar]
- 31.Walter JE, Mitchell DK, Guerrero ML, et al. Molecular epidemiology of human astrovirus diarrhea among children from a periurban community of Mexico City. J Infect Dis. 2001;183(5):681–686 [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis [published correction appears in Lancet. 2015;385(9966):420 and Lancet. 2016;387(10037):2506]. Lancet. 2015;385(9966):430–440 [DOI] [PubMed] [Google Scholar]
- 33.Kriston S, Willcocks MM, Carter MJ, Cubitt WD. Seroprevalence of astrovirus types 1 and 6 in London, determined using recombinant virus antigen. Epidemiol Infect. 1996;117(1):159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtz JB, Lee TW. Human astrovirus serotypes. Lancet. 1984;2(8416):1405. [DOI] [PubMed] [Google Scholar]
- 35.Walter JE, Mitchell DK. Astrovirus infection in children. Curr Opin Infect Dis. 2003;16(3):247–253 [DOI] [PubMed] [Google Scholar]
- 36.Xia M, Wei C, Wang L, et al. Development and evaluation of two subunit vaccine candidates containing antigens of hepatitis E virus, rotavirus, and astrovirus. Sci Rep. 2016;6:25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


