Abstract
Purpose:
Airway protective deficits (swallowing and cough) greatly reduce health and quality of life and are a pervasive consequence of neurodegenerative movement disorders. Expiratory muscle strength training (EMST) and cough skill training (CST) are two treatment approaches to improve airway protection; however, many patients are unable to access these treatments. Telehealth may improve access to care, but it remains unknown whether these treatments are feasible and efficacious via telehealth. This study aimed to determine the practical feasibility and preliminary treatment effect of EMST and CST via telehealth.
Method:
Twenty participants with movement disorders completed 4 weeks of EMST and 2 weeks of CST, including two clinician-directed treatment sessions via telehealth and 3 days of home practice per week. Feasibility was calculated for each treatment. Practical feasibility was defined as completing treatment (EMST or CST) and obtaining the relevant outcome measures—a proxy of maximum expiratory pressure (pMEP) for EMST and peak expiratory flow rate (PEFR) for CST—within a 30-min session/period. Session factors that may have influenced feasibility were examined. Preliminary treatment effect was defined as changes in pMEP and PEFR.
Results:
Time taken to obtain pMEP and complete EMST was 17.48 min, and time taken to obtain PEFR and complete CST was 17.69 min. pMEP, single voluntary cough PEFR, and sequential voluntary cough PEFR increased from pre- to posttreatment.
Conclusions:
Findings suggest that the delivery of EMST and CST is feasible via telehealth and yield improvements to pMEP and PEFR. This has important implications for expanding service delivery of airway protective interventions and reducing health care disparities in people with neurodegenerative movement disorders.
Supplemental Material:
Airway protective deficits are a pervasive consequence of neurodegenerative movement disorders and fall on a continuum from disordered swallowing to disordered cough (Troche, Brandimore, Godoy, et al., 2014). In fact, swallowing and cough function have been shown to decline together in neurodegenerative populations (Ebihara et al., 2003, 2016; Pitts et al., 2008, 2010; Plowman, Watts, Robison, et al., 2016; Silverman et al., 2016; Tabor-Gray et al., 2020; Troche, Brandimore, Okun, et al., 2014; Troche et al., 2016) and may be attributed to motor impairments (e.g., strength, coordination), sensory impairments, or both (Clark et al., 2020; Vogel et al., 2015; Walshe, 2014). This results in multifactorial deficits across the continuum of airway protective function. Cough impairments often include increased cough motor and sensory thresholds (Tabor-Gray et al., 2020; Troche, Brandimore, Okun, et al., 2014), reduced perception of cough stimuli (Troche et al., 2016), reduced voluntary cough strength (Aiello et al., 2008; Ebihara et al., 2016; Hegland et al., 2014; Tabor-Gray, Gallestagui, et al., 2019), and peripheral weakness of the respiratory muscles (Aiello et al., 2008; Haas et al., 2004). Swallowing impairments include a wide range of deficits across oral and pharyngeal phase swallow function (Clark et al., 2020; Vogel et al., 2015; Walshe, 2014), with a high prevalence of silent aspiration, particularly in later disease stages (Bianchi et al., 2012; Kaneoka et al., 2018; Marik & Kaplan, 2003; Plowman, Watts, Robison, et al., 2016; Sohn et al., 2018; Troche, Brandimore, Okun, et al., 2014). This frequently results in decrements to quality of life such as reduced mealtime enjoyment and social isolation (Marik & Kaplan, 2003; Rogus-Pulia & Plowman, 2020; Rönnefarth et al., 2020; Takizawa et al., 2016), as well as significant health consequences including malnutrition, dehydration, and aspiration pneumonia (Bakheit, 2001; D'Amelio et al., 2006; Fall et al., 2003; Kaneoka et al., 2018; Marik & Kaplan, 2003; Ortega et al., 2017; Takizawa et al., 2016). Moreover, aspiration pneumonia is a leading cause of death in neurodegenerative diseases (Akbar et al., 2015; D'Amelio et al., 2006; Fall et al., 2003; Heemskerk & Roos, 2012; Nakashima et al., 2018). Given the significant and detrimental impact on both health and quality of life, addressing airway protective dysfunction in individuals with degenerative movement disorders is critical.
Traditional management of airway protective dysfunction in neurodegenerative populations with movement disorders has focused on compensatory strategies and functional adaptations (Bakheit, 2001; Clavé et al., 2006; Ortega et al., 2017; Rogus-Pulia & Plowman, 2020; Sura et al., 2012). Furthermore, these interventions are often initiated later in the disease course, once airway protection is already significantly compromised (Rogus-Pulia & Plowman, 2020; Walshe, 2014). However, a growing body of literature supports the efficacy and importance of exercise-based interventions to target airway protective dysfunction in neurodegenerative diseases (Argolo et al., 2013; Athukorala et al., 2014; Chiara et al., 2006; Malandraki et al., 2012, 2016; Miles et al., 2017; Pitts et al., 2009; Plowman et al., 2019; Reyes et al., 2015; Rogus-Pulia & Plowman, 2020; Sura et al., 2012; Troche et al., 2010; Tye et al., 2021), with an emphasis on the need for early intervention to maintain and/or improve function (Rogus-Pulia & Plowman, 2020). Two treatment approaches that have demonstrated preliminary efficacy to rehabilitate cough and swallowing in neurodegenerative diseases are expiratory muscle strength training (EMST; Chiara et al., 2006; Pitts et al., 2009; Plowman et al., 2019; Reyes et al., 2015, 2018, 2020; Troche et al., 2010; van de Wetering-van Dongen et al., 2020) and cough skill training (CST; Borders, Curtis, et al., 2021; Brandimore et al., 2017; Curtis et al., 2020). EMST has been utilized in Parkinson's disease (PD), multiple sclerosis (MS), Huntington disease, and amyotrophic lateral sclerosis (ALS; Chiara et al., 2006; Pitts et al., 2009; Plowman et al., 2019; Reyes et al., 2015; Troche et al., 2010) with positive treatment outcomes. Across these populations, EMST treatment has resulted in improved maximum expiratory pressure (MEP; Chiara et al., 2006; Kuo et al., 2017; Plowman, Watts, Tabor, et al., 2016; Plowman et al., 2019; Reyes et al., 2015), improved cough effectiveness (Chiara et al., 2006; Kim et al., 2009; Pitts et al., 2009; Reyes et al., 2015, 2018, 2020), and less frequent airway invasion during swallowing (Claus et al., 2021; Guillén-Solà et al., 2017; Moon et al., 2017; Pitts et al., 2009; Troche et al., 2010). CST has more recently been identified as an approach to specifically target cough effectiveness, with preliminary treatment outcomes that have demonstrated improved cough effectiveness in PD and progressive supranuclear palsy (Borders, Curtis, et al., 2021; Curtis et al., 2020). Positive treatment effects include increased single voluntary, sequential voluntary, and reflex cough peak expiratory flow rate (PEFR; Curtis et al., 2020), as well as increased inspiratory volume and compression phase duration (Borders, Curtis, et al., 2021), and decreased PEFR variability after training (Borders, Curtis, et al., 2021; Curtis et al., 2020).
Despite the benefits of airway protective interventions such as EMST and CST in neurodegenerative diseases, utilization of speech-language pathology services, which include management of airway protective dysfunction, is extremely low. In a study of Medicare beneficiaries with PD, as few as 14.6% accessed speech-language pathology services (Fullard et al., 2017). Geographic distance from specialist providers (Elson et al., 2018), impaired driving (Crizzle et al., 2012; Schneider & Biglan, 2017), and caregiver burden (Beck et al., 2017; Mosley et al., 2017) have been identified as barriers to accessing treatment. Telehealth has been shown to address many of these barriers, expand provision of services, and reduce widening disparities in health care delivery across many neurodegenerative populations (Dorsey et al., 2010, 2020; Haulman et al., 2020; O'Hara & Jackson, 2017; Schneider & Biglan, 2017; Turner et al., 2013; Weidner & Lowman, 2020). A recent survey of 1,342 persons with PD revealed a large increase in utilization of telehealth across allied health care during the coronavirus pandemic (Feeney et al., 2021). Furthermore, almost half of the survey respondents reported that they would like to continue using telehealth after the pandemic. Despite the increased uptake in telehealth, only 7.9% of respondents accessed telehealth speech-language pathology services during this time (Feeney et al., 2021). A clear finding from this survey was that there is an urgent need for expansion of speech-language pathology telehealth services (Feeney et al., 2021). However, in order to provide efficacious services, more research is needed to understand best practices for the delivery of airway protective management via telehealth in people with neurodegenerative movement disorders.
The feasibility and utility of managing dysphagia via telehealth more generally has been defined in the literature (Borders, Sevitz, et al., 2021; Burns et al., 2017, 2019; Cassel, 2016; Collins et al., 2017; Kantarcigil et al., 2016; Kantarcigil & Malandraki, 2017; Malandraki et al., 2013, 2021; Malandraki & Kantarcigil, 2017; Morrell et al., 2017; Nordio et al., 2018; Sharma et al., 2013; Wall et al., 2020; Ward et al., 2012a, 2012b; Ward & Burns, 2014). These studies have demonstrated that clinical swallowing evaluations are feasible, reliable, and valid when performed via telehealth (Burns et al., 2019; Morrell et al., 2017; Sharma et al., 2013; Ward et al., 2012a, 2012b). Emerging evidence also suggests that swallowing interventions can be implemented across patient populations via telehealth (Cassel, 2016; Collins et al., 2017; Constantinescu et al., 2021; Nordio et al., 2018; Shinn et al., 2019; Starmer et al., 2018; Wall et al., 2020). In the head and neck cancer population, studies have demonstrated feasibility of and adherence to dysphagia exercises such as the effortful swallow, Mendelsohn maneuver, effortful pitch glides, Masako tongue hold, and jaw stretches, with improved quality of life following treatment (Constantinescu et al., 2021; Starmer et al., 2018). One small case series identified that managing compensatory swallowing strategies (e.g., chin tuck, head turn) was feasible via telehealth for patients with neurogenic dysphagia following a cerebrovascular accident or traumatic brain injury (Cassel, 2016). Recently, it has been identified that utilizing an asynchronous telehealth model to provide swallowing exercises was equivalent to in-person service delivery in a large cohort of patients with diverse diagnoses, including those with neurologic disease (Bascuñana-Ambrós et al., 2021), and most recently, an application has been developed to track adherence of EMST when completed at home (Srp et al., 2021). However, to our knowledge, there have been no studies that have explored the feasibility of delivering EMST and CST via telehealth in patients with neurodegenerative movement disorders.
Therefore, the primary goal of this study was to determine the initial feasibility of EMST and CST via telehealth in patients with neurodegenerative movement disorders. Given the protocols and devices used for EMST and CST, we felt that the first step in assessing feasibility was to determine whether these treatments could practically be completed via telehealth in a typical treatment session. Practical feasibility explores the extent to which an intervention can be delivered under practical constraints (Bowen et al., 2009). Given that telehealth constrains the way in which clinicians can physically manipulate the devices and physically support patients, we utilized a practical feasibility lens and considered whether each treatment could be completed within 30 min—within the typical duration of a treatment session. Based on U.S. clinical practice standards, 30 min represents a typical treatment session duration. To further understand feasibility, we explored several session factors that may have influenced telehealth delivery to determine any future enhancements required to the telehealth model. Additionally, another important component of feasibility is limited-efficacy testing, in which outcomes are assessed in a limited and preliminary way (Bowen et al., 2009). To this end, we tested the preliminary treatment effect of EMST and CST via telehealth by examining the change in key treatment outcomes—proxy of MEP (pMEP) and PEFR.
Method
Participants
Twenty participants enrolled in the study and were consecutively recruited via physician referrals from the Columbia University Medical Center Movement Disorders Division of the Neurology Department. Inclusion criteria were as follows: (a) at least 18 years of age, (b) a diagnosis of a neurodegenerative disease by a movement disorders fellowship-trained neurologist, (c) Internet connection, and (d) a device (e.g., cellphone, tablet, laptop, desktop) with audio and video capability. Exclusion criteria included (a) history of other neurologic diagnoses unrelated to the primary diagnosis (e.g., stroke); (b) EMST-related exclusion criteria including smoking, respiratory conditions, heart disease, uncontrolled hypertension, recent surgeries, and any other head/neck conditions; (c) the inability to use videoconferencing software independently or with assistance from a caregiver; and (d) inability to follow commands for participation in behavioral therapy. There was no minimum Internet bandwidth required. Exclusion criteria were broad to allow for generalization about feasibility across diverse participant factors. This study was approved by the institutional review board from Teachers College, Columbia University, and electronic informed consent was obtained from all participants.
Information was obtained regarding diagnosis and disease duration from the referring neurologist. All participants completed the Mini-Mental State Examination (MMSE), which has shown acceptable reliability via telehealth (McEachern et al., 2008), at initial assessment to determine cognitive status. To allow for inclusion of participants with varying cognitive abilities, we did not exclude participants based on MMSE scores. We obtained these scores to provide an accurate description of cognitive functioning in our cohort of participants. It was recommended that a caregiver/facilitator be present during telehealth sessions; however, it was not required given that both treatments have been found to be safe and do not involve eating/drinking, and this would reduce clinical translation to participants who live independently.
Study Design
Participant Preparation
In an initial phone call before study initiation, prospective participants were asked whether they had a device with audio and video capability that they could use for treatment (e.g., their iPhone, iPad, or laptop). All prospective participants stated they had an appropriate device. Given recent findings to suggest that telehealth clinical assessment of dysphagia is feasible with varying devices and Internet speeds (Borders, Sevitz, et al., 2021), we did not standardize or track device use or Internet speed in this study. Participants were provided written instructions (via e-mail) on downloading the Zoom application (if they did not already have it) and were provided a secure, unique link for their treatment sessions. They were given written instructions (via e-mail) to click the link to enter their session and were given the clinician's contact information if they needed assistance. Of note, given the timing of this study (during the coronavirus pandemic), all participants were referred by their neurologist to participate in this study following a virtual neurology consult. Thus, all participants had experience with telehealth for at least one neurology appointment. Participants were instructed (via e-mail and at the start of the first session) to sit upright and position their camera to provide a direct view of their face and upper torso. Participants were asked not to move their camera angle or position throughout the session. EMST-150 (Aspire Products, LLC) and peak flow meter (Omron PF9940 PeakAir Peak Flow Meter) devices were sent to participants' homes. These devices were used to obtain key outcomes of pMEP and PEFR and complete training exercises. Participants received written instructions and a video tutorial explaining the devices and their uses, with additional training provided by the clinician in the first session. Ongoing support for device use was provided by the clinician, as necessary.
Clinician Preparation
All speech-language pathology clinicians received specialized training with an experienced researcher-clinician, which included a basic orientation to the telehealth platform (Zoom), session practicalities (e.g., recording the session, obtaining emergency contact information), and the treatment protocol. All study clinicians had experience using Zoom and administering the treatments. Clinicians were given the EMST-150 and peak flow meter devices to provide visual modeling to participants.
Treatment Design
Participants completed 4 weeks of EMST and 2 weeks of CST. Treatment consisted of two telehealth sessions and 3 days of independent home practice per week (for a total of five training days per week; see Figure 1). Participants were given exercise logs to track home practice exercises. Adherence was not a primary outcome of this study; however, for participants who returned exercise logs, adherence was calculated as the number of home exercise repetitions completed over the total number of home exercise repetitions prescribed. Fifteen participants began EMST and CST together, and five completed 2 weeks of EMST before beginning CST. An effort was made to schedule telehealth sessions during the “on” phase in medication cycle, when applicable (i.e., for participants with PD). pMEP and cough PEFR were the key treatment outcomes for EMST and CST, respectively, and were obtained before treatment initiation, at the start of each treatment week, and following treatment completion. pMEP and PEFR were used to set weekly EMST and CST training targets and to measure treatment effects. During telehealth sessions, participants completed EMST and CST training with specific feedback regarding task performance from the clinician and augmented by feedback from the device (i.e., audible burst of air during EMST, visual target during CST).
Figure 1.
Treatment protocol and devices. Participants completed expiratory muscle strength training and cough skill training treatment. pMEP = proxy of maximum expiratory pressure; PEFR = peak expiratory flow rate.
The sessions were delivered either by a certified speech-language pathologist or by a speech-language pathology master's student with 100% supervision from an experienced speech-language pathology clinician via Zoom, a videoconferencing platform (Zoom Video Communications Inc., 2016), recorded in real time, and stored to a secure server. At the conclusion of each session, the clinician documented whether a care partner was present and the percentage of the session that the care partner was present for. The clinician also documented any treatment- or assessment-related safety concerns. As an additional safety precaution, the treating clinician had the participants' emergency contact numbers and participants' local emergency response numbers available during the session.
EMST
pMEP
MEP is a measure used to quantify the force-generating capacity of the expiratory muscles. The gold standard is that MEP be obtained using a respiratory manometer (per the American Thoracic Society [ATS]/European Respiratory Society [ERS] guidelines); however, it is not financially feasible to send this equipment to patients' homes to obtain MEP with a manometer via telehealth. Therefore, a proxy measure of the strength of the expiratory muscles was obtained using the EMST-150 device (see Figure 1). This measure provided a measure of baseline function, a guide to set the EMST training level, and a means to track change over time. We refer to this measure as a proxy for MEP (i.e., pMEP). The EMST-150 device ranges from 30 to 150 cm H2O and contains a one-way spring-loaded valve that blocks airflow until a sufficient “threshold” pressure is produced to overcome the force and open the valve. To obtain pMEP, a slightly modified version of the “quarter turn” method outlined by Aspire products was used (EMST150, 2021). To begin, participants were instructed to sit comfortably in a chair and set their EMST-150 device to the lowest resistance level by turning the device to the lowest setting. Participants were then instructed to occlude the nose with nose clips, take a big breath in, make a tight seal around the mouthpiece, and blow forcefully into the device. Verbal instructions and a visual model were provided by the clinician. If the participant could generate sufficient force to successfully open the valve inside the device, they were instructed to increase the resistance by turning the device one full turn clockwise. This procedure was repeated until the participant could no longer blow with sufficient force to open the valve. At that point, the dial was turned back (i.e., counterclockwise) one half turn. If the participant was able to open the valve, they were instructed to increase the resistance by one quarter turn; if unable to open the valve, the participant was instructed to decrease the resistance by one quarter turn. pMEP was defined as the highest threshold at which the participant was able to open the valve of the EMST-150 device. The number of turns on the EMST-150 device was converted to pMEP values to reflect the amount of resistance in cm H2O. To determine this, the numbers on the EMST-150 device (i.e., 30, 60, 90, 120) were used as a guide, and to ensure reliability, the number of turns were counted and calculated as follows: lowest resistance = 30 cm H2O, one turn = 45 cm H2O, two turns = 60 cm H2O, three turns = 75 cm H2O, four turns = 90 cm H2O, five turns = 120 cm H2O, and six turns = 150 cm H2O.
EMST Exercises
EMST exercises were completed using the same EMST-150 device, and the resistance training level was set each week to approximately 75% of the participant's pMEP (Troche et al., 2010). To complete a training breath, participants were instructed to (a) put on nose clips, (b) take a big breath in, (c) wrap their lips tightly around the mouthpiece of the device, and (d) exhale quickly and forcefully until they heard the popping or whistling sound of the valve opening. Five sets of five successful trials were completed per session (totaling 25 breaths per session).
CST
Cough PEFR
Cough PEFR was obtained using a handheld analog peak flow meter (Silverman et al., 2014), with measurements ranging from 60 to 750 L/min (Omron PF9940 PeakAir Peak Flow Meter; see Figure 1) in 10-unit increments. If a participant's PEFR was between two lines, it was visually determined to which line it was closest, and that served as the PEFR. Participants were instructed to take a big breath and “cough strong” (single) or “cough as if something went down the wrong pipe” (sequential) into the device. Verbal instructions and a visual model were provided by the clinician. Three trials of single coughs followed by three trials of sequential coughs were completed. For each trial, PEFR was read by the participants and shown to the clinician for visual confirmation. If there was any disagreement, PEFR was determined by the clinician. In cases where the participant could not read the PEFR value and a care partner was not present, the clinician made a determination from visualization. PEFR was obtained at the beginning of each week, and training targets were set based on the maximum of the three PEFR trials for single and sequential coughs, respectively. However, for analysis, all three trials were included in multilevel statistical analyses.
CST Exercises
CST exercises were completed utilizing the same peak flow meter used to measure PEFR. A target was set at 25% above the participant's maximum baseline PEFR for single and sequential coughs. A small dial on the side measured the amount of air that entered the device and provided a measure of PEFR for each trial. Practice included single coughs to primarily target strength and sequential coughs to target coordination (Ebihara et al., 2003; Fontana & Lavorini, 2006; Hegland et al., 2013). Both strong and weak coughs were also incorporated into practice given literature to suggest that variable practice enhances motor learning (Levin & Demers, 2020; Muratori et al., 2013). Participants were instructed to use the color markings on the side of the peak flow meter to mark their single and sequential cough training targets: 25% above baseline for “strong” cough practice and 25% below baseline for “weak” cough practice. Participants were instructed to (a) take a big breath in, (b) wrap lips tightly around the mouthpiece of the device, and (c) cough into the device, aiming for your target: either a “strong” cough or a “weak” cough. Five sets of five coughs were completed (totaling 25 coughs per session). These sets were divided into two sets of single strong coughs, one set of single weak coughs, one set of sequential strong coughs, and one set of sequential weak coughs.
Outcome Measures
Practical Feasibility
The practical feasibility of EMST was defined as obtaining pMEP and completing EMST treatment within 30 min, and the practical feasibility of CST was defined as obtaining voluntary cough PEFR and completing CST treatment within 30 min. Time taken to obtain pMEP and PEFR was calculated from video recordings of the first session in which they were obtained (i.e., before treatment initiation). Time taken to complete each treatment (EMST and CST) was calculated from video recordings of the first treatment session in which all exercises were completed. We also calculated the time it took to complete exercises and obtain pMEP and PEFR after 2 weeks of treatment to see if efficiency improved. Time included task instructions and necessary cueing to complete the tasks.
Session Factors Influencing Feasibility
Based on extensive experience with in-person EMST and CST delivery and the challenges that typically occur when training patients to perform EMST correctly, we identified four key session factors that we thought may impact telehealth feasibility. These were (a) patient factors impacting trial success, (b) technology-related issues, (c) clinician-directed facilitatory supports, and (d) care-partner–directed facilitatory supports. To identify these key session factors, the same assessment and treatment sessions that were used to determine practical feasibility and treatment effect (at baseline, midtreatment, and posttreatment) were reviewed as a representative sample of sessions.
Preliminary Treatment Effect
EMST treatment effect was measured by pre–post treatment changes in pMEP, as measured before training, after 2 weeks of training, and after 4 weeks of training. CST treatment effect was measured by pre–post treatment changes in single and sequential voluntary cough PEFR, as measured pretraining and after 2 weeks of training.
Statistical Analysis
Statistical analyses were completed using R Version 4.0.1 (R Core Team, 2018). Two-way random effects (single measure, absolute agreement) intraclass correlation coefficients (ICCs) were computed for the primary feasibility outcome to determine intrarater and interrater reliability of the offline measurement of time to complete training. To determine treatment effect, pre–post treatment changes in pMEP were analyzed using paired-sample t tests—comparisons were made pre to post 2 weeks of training, pre to post 4 weeks of training, and post 2 weeks to post 4 weeks of training. Pre–post treatment changes in single and sequential voluntary cough PEFR were analyzed using linear multilevel models with participant as a random factor and time as a fixed factor. Cohen's d was used to determine t-test effect sizes, and Cohen's d with pooled variances was used for multilevel models (Westfall et al., 2014). To facilitate future meta-analyses, we report Pearson's r correlation coefficients for the treatment effect of pMEP and multilevel correlation coefficients for the treatment effect of PEFR. Holm–Bonferroni adjustments were used for multiple comparisons within each treatment outcome (i.e., three comparisons for pMEP, two comparisons for PEFR), and adjusted p values are reported. Statistical significance was set at p < .05.
Session Factors Influencing Telehealth Feasibility
A directed content analysis (Hsieh & Shannon, 2005) was used to identify session factors that may have influenced treatment feasibility. Following study completion, a rater unfamiliar with the participants reviewed videos of the same assessment and treatment sessions that were used to determine feasibility, documenting and categorizing all (a) patient factors impacting trial success, (b) technology-related issues, (c) clinician-directed facilitatory supports, and (d) care-partner–directed facilitatory supports. Within each of the four defined categories, a list of barriers/facilitations was compiled. The list was refined by the primary researcher who consolidated all items into clusters that represented the underlying barriers/facilitators documented and determined a code for each barrier/facilitator cluster. The number of participants for whom each “session factor” occurred is reported. To ensure rigor and trustworthiness, interrater reliability was assessed (Morse, 2015). A second rater who was unfamiliar with the study reviewed 20% of the sessions, following an identical process to Rater 1—documenting and categorizing barriers and facilitators. Interrater reliability between the two raters was calculated as percent absolute agreement of session factors listed.
Results
Twenty participants (four women, 16 men; aged 48–83 years; disease duration = 0.17–42 years) completed 4 weeks of EMST and 2 weeks of CST (see Table 1). Participant diagnoses included PD (n = 12), Lewy body dementia (n = 2), multiple system atrophy-cerebellar (n = 4), and cerebellar ataxia not otherwise specified (n = 2). MMSE scores ranged from 23 to 30, excluding one participant who could not complete the MMSE due to visual impairment. Eight participants had care partners who were present during the sessions. Whether care partners were present at each session and the percentage of the session for which they were present are documented in Table 2. Further information regarding the ways in which care partners assisted is detailed in Table 3. For all participants (n = 6) who returned exercise logs at the conclusion of treatment, adherence was above 90%. Due to missing video recordings secondary to researcher error, initial MEP duration could not be calculated for Participants 12 and 17, initial PEFR duration could not be calculated for Participant 17, and EMST and CST treatment duration from the first session could not be calculated for Participant 17. For these two participants, practical feasibility data regarding MEP, PEFR, EMST, and/or CST duration were obtained from a subsequent session and are reported descriptively. Participant 18 did not complete all treatment exercises. All participants were included in the preliminary treatment effect analyses. No safety concerns were documented during the assessment and treatment sessions.
Table 1.
Participant demographics.
| ID | Sex | Age | Primary diagnosis | Disease duration (years) | MMSE score/30 | Care partner |
|---|---|---|---|---|---|---|
| 1 | Male | 78 | PD | 9 | 30 | No |
| 2 | Female | 61 | PD | 11 | 16/22 a | Yes |
| 3 | Male | 81 | PD | 12 | 25 | Yes |
| 4 | Female | 74 | DLB | 2 | 23 | Yes |
| 5 | Male | 65 | PD | 8 | 29 | No |
| 6 | Male | 75 | PD | 4 | 26 | Yes |
| 7 | Male | 66 | PD | 0.17 | 24 | Yes |
| 8 | Male | 82 | DLB | 4 | 25 | Yes |
| 9 | Male | 60 | MSA-C | 1 | 30 | No |
| 10 | Male | 75 | PD | 10 | 26 | No |
| 11 | Male | 62 | MSA-C | 3 | 30 | No |
| 12 | Male | 76 | PD | 10 | 30 | No |
| 13 | Female | 67 | PD | 4 | 30 | No |
| 14 | Male | 73 | PD | 2 | 29 | No |
| 15 | Male | 79 | PD | 5 | 25 | No |
| 16 | Female | 49 | Idiopathic CA | 10 | 28 | No |
| 17 | Male | 56 | MSA-C | 4 | 30 | No |
| 18 | Male | 63 | MSA | 9 | 25 | Yes |
| 19 | Male | 48 | CA | 42 | 29 | No |
| 20 | Male | 83 | PD | 0.58 | 30 | Yes |
Note. MMSE = Mini-Mental State Examination; PD = Parkinson's disease; DLB = dementia with Lewy bodies; MSA-C = multiple system atrophy-cerebellar subtype; CA = cerebellar ataxia.
Unable to complete all components of MMSE due to visual impairment.
Table 2.
Caregiver presence during treatment sessions.
| ID | Caregiver present at the session (percentage of time present) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 | Session 7 | Session 8 | |
| 2 | Yes (100) | Yes (100) | Yes (100) | Yes (95) | Yes (90) | Yes (80) | Yes (80) | Yes (80) |
| 3 | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (40) | Yes (100) | Yes (5) |
| 4 | Yes (100) | Yes (60) | Yes (75) | Yes (67) | Yes (75) | Yes (75) | Yes (65) | Yes (60) |
| 6 | Yes (25) | Yes (5) | No | No | No | No | No | No |
| 7 | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (100) | No | Yes (100) | Yes (98) |
| 8 | Yes (100) | Yes (100) | Yes (95) | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (100) |
| 18 | Yes (100) | Yes (100) | Yes (90) | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (100) |
| 20 | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (100) | Yes (75) | Yes (100) |
Table 3.
Type of care-partner involvement at key assessment and treatment sessions for participants who had care partners.
| ID | Session | Care-partner assistance | Type of care-partner assistance |
|||||
|---|---|---|---|---|---|---|---|---|
| Rotating EMST device | Repeating instructions/providing verbal cues or encouragement | Holding participants' cheeks to obtain lip seal | Adjusting participant posture | Tracking data | Reading numbers on the peak flow meter | |||
| 2 | Pre | Yes | X | X | ||||
| Mid | Yes | X | ||||||
| Post | Yes | X | ||||||
| 3 | Pre | Yes | X | X | X | |||
| Mid | Yes | X | X | X | ||||
| Post | Yes | X | X | X | X | X | ||
| 4 | Pre | Yes | X | X | ||||
| Mid | Yes | X | X | X | ||||
| Post | Yes | X | X | X | ||||
| 6 | Pre | No | ||||||
| Mid | No | |||||||
| Post | No | |||||||
| 7 | Pre | No | ||||||
| Mid | Not present | |||||||
| Post | Not present | |||||||
| 8 | Pre | Yes | X | X | ||||
| Mid | Yes | X | X | X | ||||
| Post | Yes | X | X | X | ||||
| 18 | Pre | Yes | X | X | ||||
| Mid | Yes | X | ||||||
| Post | Yes | X | ||||||
| 20 | Pre | Yes | X | X | X | |||
| Mid | Yes | X | ||||||
| Post | Yes | X | X | |||||
Note. Pre includes obtaining pMEP and PEFR, initial setting of EMST/CST devices, and first training session. Mid includes obtaining pMEP and PEFR, resetting of devices, and training session. Post includes obtaining pMEP and PEFR. EMST = expiratory muscle strength training; CST = cough skill training; pMEP = proxy of maximum expiratory pressure; PEFR = peak expiratory flow rate.
Practical Feasibility
EMST
The average time taken to obtain pMEP pretreatment was 10.40 min (SD = 8.45), and the time taken to complete EMST exercises in the first treatment session was 7.08 min (SD = 3.04). For those who could not be included in the quantitative feasibility analysis due to missing video recordings, MEP durations from a later session were 5.06 min (Participant 12) and 9.66 min (Participant 17) and EMST duration was 6.58 min (Participant 17). Participant 18 did not complete all EMST exercises in any session.
Reliability. ICCs for the time taken to obtain MEP were .993 for intrarater reliability and .993 for interrater reliability. ICCs for the time taken to complete EMST were .992 for intrarater reliability and .996 for interrater reliability.
CST
At pretreatment, the time taken to obtain PEFR was an average of 4.79 min (SD = 2.43) and the time taken to complete CST exercises in the first treatment session was 12.90 min (SD = 5.69). For one participant who could not be included in the quantitative feasibility analysis due to a missing video recording, CST duration from a subsequent session was 7.23 min (Participant 17). Participant 18 did not complete all CST exercises in any session.
Reliability. ICCs for the time taken to obtain PEFR were .819 for intrarater reliability and .888 for interrater reliability. ICCs for the time taken to complete CST were .957 for intrarater reliability and .977 for interrater reliability.
Preliminary Treatment Effect
EMST
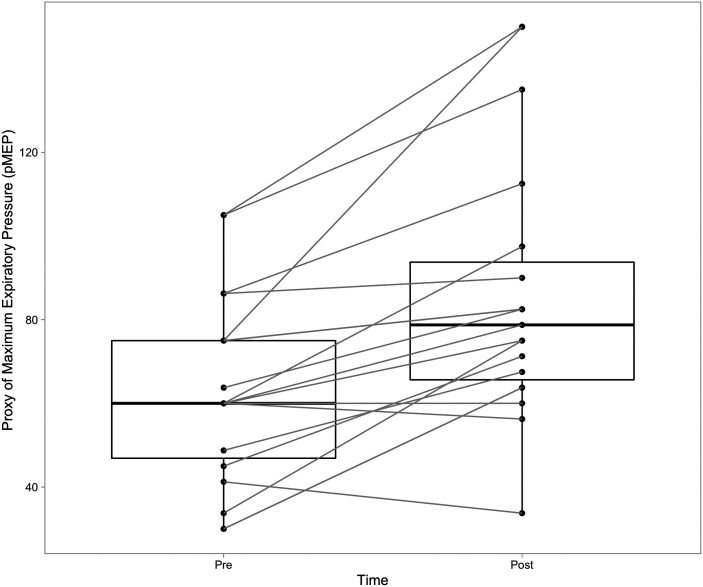
Mean pMEP before treatment was 65.6 cm H2O (SD = 24.5) and significantly increased to 83.6 cm H2O (SD = 27.2) after 2 weeks of training, t(19) = 6.02, p < .001, d = 1.35. The correlation between pMEP at pretreatment and after 2 weeks of training was r = .871 (p < .001). pMEP further increased to 87.4 cm H2O (SD = 31.4) after an additional 2 weeks of treatment (i.e., a total of 4 weeks); however, the additional increase in pMEP between 2 and 4 weeks of training was not significant, t(19) = −1.33, p = .198, d = 0.298. The correlation between pMEP after 2 weeks of training and after 4 weeks of training was r = .918 (p < .001). The overall improvement from pre to post 4 weeks of treatment was significant, t(19) = 4.93, p < .001, d = 1.13 (see Figure 2). The correlation between pMEP from pre to post 4 weeks of treatment was r = .78 (p < .001).
Figure 2.
Change in proxy of maximum expiratory pressure (pMEP) pre–post treatment. pMEP increased after 4 weeks of expiratory muscle strength training. Pre = pretreatment; Post = post 4 weeks of treatment.
CST
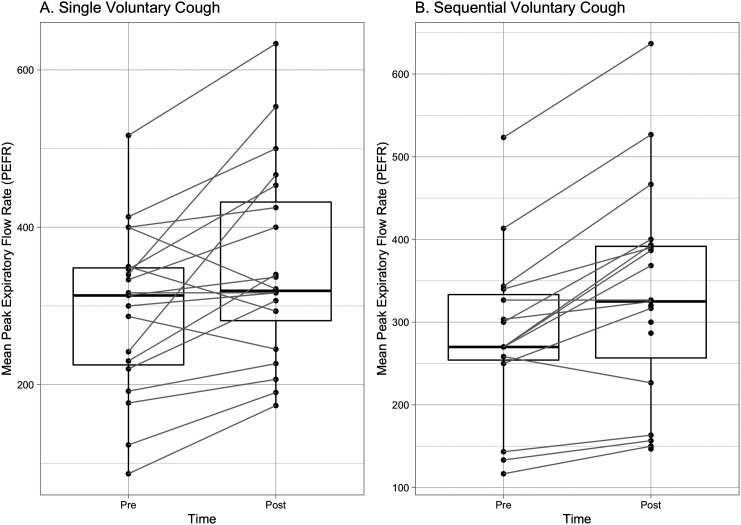
Mean PEFR for single voluntary coughs pretreatment was 293 L/min (SD = 108) and significantly increased to 350 L/min (SD = 127) posttreatment, t(97.13) = 5.68, p < .001, d = 1.15. The correlation between single voluntary cough PEFR at pre- and posttreatment was r = −.26 (p = .052). Mean PEFR for sequential voluntary coughs pretreatment was 284 L/min (SD = 106) and significantly increased to 331 L/min (SD = 132) posttreatment, t(83.62) = 7.14, p < .001, d = 1.56 (see Figure 3). The correlation between sequential voluntary cough PEFR at pre- and posttreatment was r = −.43 (p = .003).
Figure 3.
Change in peak expiratory flow rate (PEFR) pre–post treatment. PEFR increased after 2 weeks of cough skill training. This figure uses the mean PEFR for each participant to allow for a clearer visual representation of within-participant data. Pre = pretreatment; Post = post 2 weeks of treatment.
Session Factors Influencing Feasibility
Qualitative analysis revealed several patient factors impacting EMST/CST trial success, such as difficulty with lip seal, positioning, achieving an adequate breath, and sequencing the steps required to complete the exercise (see Table 4). The primary technology-related issue identified was momentary audio/video freezing, which impaired clinician ability to determine if a trial was successful. Additionally, one participant had difficulty reading the numbers on the peak flow meter, and the clinician had difficulty visualizing the numbers when the patient held the device up to the screen. Clinician-directed facilitatory supports included providing additional modeling and instructions and incorporating care-partner support. Care-partner–directed facilitatory supports most commonly included rotating the EMST-150 device to set the training target, holding the participant's cheeks to prevent air escape around the EMST-150 device, and reading the numbers on the peak flow meter. When care partners were not present, clinician-directed facilitatory supports were frequently repeated (e.g., provided another visual model) or adapted (e.g., rephrased the instructions, provided more specific cueing, used an analogy such as “bear down like you're lifting weights”) to facilitate target success. The number of participants for whom each “session factor” occurred can be found in Table 4. Interrater reliability (percent absolute agreement) of session factors identified from the three key sessions for each participant was ≥ 80% (patient factors impacting trial success = 82.4%, clinician-directed facilitatory supports = 80%, care-partner–directed facilitatory supports = 92.3%, technology-related issues = 100%). None of the above session factors prevented successful treatment delivery. The one participant for whom feasibility was questionable is described below in the Discussion section.
Table 4.
Session factors influencing feasibility.
| Patient factors impacting trial success | Clinician-directed facilitatory supports | Care-partner–directed facilitatory supports | Technology-related issues |
|---|---|---|---|
| Inadequate lip seal/air escaping (n = 15) | Additional visual model (n = 5) | Rotating EMST devices (n = 6) | Participant difficulty reading the numbers on the side of the peak flow meter and appearing blurred when showing the clinician (n = 1) |
| Inadequate preparatory breath/insufficient air (n = 8) | Repeat instructions/additional explanation or cues (n = 8) | Repeat instructions/provide verbal cue or encouragement (n = 4) | |
| Poor coordination of treatment steps (n = 3) | Incorporating caregiver physical support (n = 3) | Holding cheeks to obtain lip seal (n = 3) | Momentary audio and/or video freezing that impaired clinicians' to hear or see a cough or EMST burst (n = 2) |
| Poor posture (n = 3) | Adjusting posture (n = 2) | ||
| Throat-clearing or blowing instead of coughing (n = 9) | Tracking data (n = 2) | ||
| Incorrect use or positioning of peak flow meter (n = 4) | Reading numbers on the peak flow meter (n = 4) |
Note. EMST = expiratory muscle strength training.
Discussion
Telehealth is a rapidly expanding service delivery model for health care delivery and has the potential to increase access to specialty speech-language pathology services such as the management of airway protective dysfunction—an urgent public health need and priority (U.S. Department of Health & Human Services, 2019; Weidner & Lowman, 2020). To date, no studies have assessed the feasibility of airway protective treatments, specifically EMST and CST, via telehealth in patients with neurodegenerative movement disorders. This study demonstrated that completing EMST and CST exercises and obtaining treatment-related assessments to obtain pMEP and PEFR are feasible via telehealth. These findings are particularly important because of the progressive decline of cough and swallowing function in neurodegenerative diseases (Aiello et al., 2008; Ebihara et al., 2003; Hegland et al., 2014; Pitts et al., 2010; Tabor-Gray, Gallestagui, et al., 2019), the impact of this decline on health and quality of life (Fall et al., 2003; Sohn et al., 2018; Takizawa et al., 2016), and the known benefits of these interventions for improving airway protective function when performed in person. Specifically, EMST has repeatedly demonstrated improvements to cough and swallowing (Claus et al., 2021; Plowman et al., 2019; Troche et al., 2010), and CST has been shown to improve various components of cough function (Borders, Curtis, et al., 2021; Curtis et al., 2020). Thus, delivery of EMST and CST via telehealth is a crucial step in expanding the provision of these important airway protective treatments.
Practical Feasibility
Completing EMST and obtaining pMEP, as well as completing CST and obtaining PEFR, were each feasible within a 30-min treatment session. The total time taken to obtain outcome measures and complete each treatment was less than 30 min. At baseline, the mean time taken to obtain pMEP and complete EMST was 17.48 min and that to obtain PEFR and complete CST was 17.69 min. Time then decreased by the end of treatment—taking 12.27 min to obtain pMEP and complete EMST and 12.43 min to obtain PEFR and complete CST. These data suggest that even with significant variability in patient diagnoses, disease duration, and cognitive status, it is feasible to complete EMST or CST training within 30 min. We also found that the time taken to obtain pMEP and PEFR and complete these exercises decreased over time, supporting the use of these treatments via telehealth within a clinical setting.
In all EMST studies to date, MEP has been obtained by a clinician or researcher using a respiratory manometer. However, it is not financially feasible to provide this device to patients for use at home via telehealth. Even when treatment can be initiated in person, EMST may require weekly re-assessment of MEP to reset the device training level; although manufacturer guidelines recommend increasing the device by a “quarter-turn” each week of training, this approach has not been empirically tested. Future research will be necessary to determine the impact of these different practices on treatment outcomes. The finding in this study that pMEP can be obtained from the EMST-150 device provides preliminary support for expanding EMST delivery, as it relates to setting EMST training level and tracking change over time, via telehealth. Future study is needed to fully assess the validity of telehealth delivery, with important implications for expanding access to this robust treatment approach for patients who cannot access in-person appointments. The challenges of the proxy measure of MEP (i.e., pMEP) cannot be ignored and are described more fully in the Limitations section. Although more straightforward, obtaining PEFR via telehealth has also not been previously studied. This study suggests that obtaining PEFR via telehealth is possible. The findings of this study are the first to suggest that individuals with movement disorders can obtain pMEP and PEFR at home. This is especially important given that pMEP and PEFR need to be obtained weekly when completing EMST and CST in order to re-adjust training targets accordingly.
Preliminary Treatment Effect
EMST
The significant increase in pMEP observed from pre- to posttreatment preliminarily suggests the presence of a treatment effect when EMST was completed via telehealth. Although we cannot make direct comparisons between pMEP and actual MEP, when examining the magnitude of change, the large increase in pMEP in this study is consistent with improvements to MEP typically seen following in-person EMST treatment. Previous studies in healthy young and older adults as well as disease groups including those with PD, MS, and ALS have found that following 4 or 5 weeks of EMST, MEP measured by a respiratory manometer increased anywhere from 15% to 50% (Chiara et al., 2006; Kim et al., 2009; Reyes et al., 2018, 2020; Troche et al., 2015). In this study, the average improvement in pMEP based on the EMST-150 conversion estimate was 36%. This provides initial support for the utility of EMST for improving expiratory muscle strength when administered via telehealth.
The increase in pMEP in this study occurred most significantly in the first 2 weeks of training (30%), and although pMEP continued to increase in Weeks 3 and 4, the additional 6% increase (i.e., from Week 2 to Week 4) was nonsignificant. This finding is in line with studies that suggest neural adaptations to exercise occur in the initial stages of training (e.g., the first 4 weeks) resulting in rapid strength gain, with sustained yet less steep improvements later on (Baker et al., 2005; Kim et al., 2009; Sale, 1988; Saleem et al., 2005). To date, MEP has usually been reported before the start of treatment and again after 4 or 5 weeks of training (Chiara et al., 2006; Kuo et al., 2017; Plowman et al., 2019), with no midtreatment probes. However, a few cohort studies have explored changes in MEP over the course of training or following shorter training periods. Similar to the findings of this study, a recent study identified an increase in MEP following 2 weeks of EMST training in persons with PD that is similar to the gains observed following longer training periods in other studies (Srp et al., 2021). Baker et al. (2005) also found the most significant increase in MEP was in the first 2 weeks of training, with a nonsignificant but steady increase in the third and fourth weeks (Baker et al., 2005), and Kim et al. (2009) found the most significant improvement in the first week of training with a continued steady improvement in the remaining weeks (Kim et al., 2009). Our finding is in contrast to Anand et al. (2012) who found a relatively equal gradual increase in MEP across all four training weeks (Anand et al., 2012), although week-to-week change was not statistically analyzed in that study and the participants were healthy adults. Therefore, change in pMEP in this study seems to be in accordance with patterns of change previously identified in the literature; however, further studies should explore patterns and rate of change of MEP in response to EMST, as it relates to optimal treatment duration.
CST
The improvement of single and sequential voluntary cough PEFR in this study is consistent with findings from previous studies on patients with movement disorders, demonstrating improvements in measures of cough effectiveness following cough training (Borders, Curtis, et al., 2021; Curtis et al., 2020). Inadequate cough strength contributes to the progressive decline in cough effectiveness and impaired airway protection (Pitts et al., 2008; Plowman, Watts, Robison, et al., 2016; Silverman et al., 2016), and reduced voluntary cough strength has specifically been associated with impaired ability to clear aspirate material from the airway (Borders & Troche, 2021). More specifically, Borders and Troche (2021) identified that small differences in PEFR (e.g., a change of 0.50 L/s) correspond to functional differences in the amount of aspirate material expelled from the airway. This has significant implications for pulmonary sequelae including aspiration pneumonia (Bianchi et al., 2012; Ebihara et al., 2012; Sohn et al., 2018). Therefore, the improvement in PEFR identified following CST in this study, a change of 57 L/min (i.e., 0.95 L/s) for single voluntary coughs and a change of 47 L/min (i.e., 0.78 L/s) for sequential voluntary coughs, has important implications for the utility of cough training as a treatment approach to improve airway protection that can be delivered via telehealth.
Session Factors Influencing Feasibility
Qualitative data from a video review of telehealth sessions may help to refine the use of EMST and CST via telehealth. The fact that both treatments utilized devices seems to have facilitated treatment feasibility—providing the clinician and participant with real-time feedback regarding whether the target was achieved. However, the use of these devices also presented challenges. There are technical parameters surrounding device use for both EMST and CST such as holding the device correctly, achieving sufficient lip seal, sequencing the steps to perform the exercise, and understanding if the target is achieved. During typical in-person treatment sessions, clinicians may provide tactile support, mouthpiece alternatives, and positioning adjustments to facilitate accurate performance. When additional physical support was needed during telehealth sessions in this study, clinicians utilized care partners for assistance by having them assist with various components of EMST, such as rotating the EMST device to set the training target, holding the device, or holding the participant's cheeks to achieve a lip seal. These results demonstrate that physical assistance may be necessary for a subgroup of patients. For greater detail regarding outcomes for those with versus without care partners, see Supplemental Materials S1 and S2. All participants who did not have care partners (n = 12) were able to complete the treatments independently. Future studies should systematically consider caregiver participation.
One participant (Participant 18) could not be included in quantitative feasibility analyses of EMST and CST exercise duration, given that he did not complete 25 repetitions (as per the protocol) in any treatment session. There are many possibilities as to why this occurred, and the feasibility of the exercises themselves versus of the telehealth modality cannot easily be disentangled for this participant. The clinician cited time constraints and clinician-perceived patient fatigue and used clinical judgment to complete fewer exercise repetitions, taking a conservative approach to ensure patient safety. Of note, MEP and PEFR durations were appropriate, the patient was able to participate in EMST and CST exercises, and sessions were frequently shorter than 30 min. However, MEP and PEFR did not improve over the course of treatment for this participant. This may be due to insufficient exercise intensity and/or patient factors such as disease severity and cognitive impairment that impacted feasibility for this participant. A care partner was present at all sessions but participated minimally (see Table 3). Perhaps, in this case, greater care-partner involvement would enhance feasibility. The impact of diagnosis, disease severity, cognition, and care-partner involvement on feasibility are important variables to consider in subsequent studies, as it remains possible that feasibility may be limited for certain subgroups of patients with a more severe disease profile—all factors that may impact feasibility in-person and via telehealth.
Limitations and Future Directions
Given that this was an initial feasibility study, there was a small sample size, no control group, and no in-person comparison group. Some participants began EMST and CST concurrently, whereas others received 2 weeks of EMST before initiating CST. It is possible that there were cross-treatment effects and/or interferences and that these may have occurred differentially for those who received the treatments concurrently versus consecutively. However, the primary goal of this study was to assess practical feasibility, which was confirmed despite the combined treatment approach and treatment schedules. It is also possible that completing treatment consecutively versus concurrently may have influenced adherence, and this should be explored in future studies. Given that adherence was not a primary outcome of this study, exercise logs were not systematically requested upon study completion and were only obtained for a portion of participants in our sample. Future studies should consider adherence as a component of treatment feasibility.
The approach used to obtain pMEP in this study generally follows the guidelines outlined by the Aspire Products company (Aspire Products, LLC) on how to obtain MEP using the EMST-150 device (EMST150, 2021). This procedure is not outlined in the ATS/ERS guidelines on respiratory muscle testing, and although this approach is utilized clinically, it is unknown how closely pMEP values map onto MEP measured via a respiratory manometer. Therefore, pMEP values obtained in this study should not be directly compared to normative data in the literature and should not be used to identify patients whose MEP may be below normal limits. Future research is needed to understand how pMEP obtained from the EMST-150 device correlates with MEP standardly obtained via a respiratory manometer.
There have been conflicting reports in the literature as to how well peak flow rates measured by handheld digital and analog devices correlate with peak flow rates measured via the gold standard pneumotachograph. Some studies have identified excellent concordant validity (Sancho et al., 2004; Silverman et al., 2014; Tabor-Gray, Vasilopoulos, & Plowman, 2019), whereas others have not (Kulnik et al., 2015; Sancho et al., 2004), specifically suggesting that handheld devices may have a tendency to overinflate values below 270 L/min (Sancho et al., 2004). Therefore, direct comparisons to normative data obtained via a pneumotachograph may not be appropriate. Nonetheless, cough peak flow meters have been used in a number of clinically relevant studies, identifying their utility as a screening and treatment tool (Ebihara et al., 2003; Silverman et al., 2014; Tabor-Gray, Vasilopoulos, & Plowman, 2019). Since the same devices were used for obtaining outcome measures and completing treatment for both EMST and CST, practice effects may have inflated the improvement observed in pMEP and PEFR outcomes. Therefore, the treatment effect of EMST and CST should be considered preliminary and needs to be confirmed by future research examining changes to other physiologic and functional outcomes.
All participants were community-dwelling individuals, and no participants had severely impaired cognition—the two participants with diagnoses of dementia had care partners who assisted. Future studies in larger and more diverse cohorts are necessary to confirm the feasibility of these exercises via telehealth across varying diagnoses and cognitive abilities as well as in acute and subacute populations and those with more severe airway protective deficits. Lastly, only a subset of participants in this study had care partners who assisted with the evaluation and treatment sessions, and the way in which care partners assisted was not standardized. This allowed for ecologically valid conclusions regarding feasibility of these treatments in the natural environments of persons with neurodegenerative diagnoses. However, future study is required to examine the influence of caregiver involvement on feasibility and whether caregivers are required for certain patient groups.
Conclusions
This study provides initial support for the feasibility of two device-driven treatment approaches—EMST and CST—to improve airway protection via telehealth for individuals with movement disorders. The findings of this study demonstrate the practical feasibility of completing EMST and CST, as well as obtaining the associated outcome measures, with a resulting improvement in both pMEP and PEFR. This has important implications for initiating treatment via telehealth in cases where patients are unable to access in-person clinics due to geographical distance and mobility limitations as well as the possible persistence of the coronavirus pandemic, particularly given that these treatments are aerosol generating. Increased access to airway protective treatment may also facilitate earlier intervention before the onset of severe airway protective dysfunction. Given the current lack of speech-language pathology services via telehealth for patients with movement disorders (Feeney et al., 2021), this study is an important initial step toward expanding the provision of these airway protective interventions to the telehealth modality as well as providing clinicians with guidance regarding key challenges and facilitatory strategies. Future studies should investigate EMST and CST treatment paradigms via telehealth as compared to in-person services and their impact on functional and patient-centered outcomes.
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Compliance With Ethical Standards
Ethical Approval: All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was obtained from the institutional review board.
Informed Consent: Informed consent was obtained from all participants before enrollment in this research study.
Supplementary Material
Acknowledgments
Alcalay's research is funded by the National Institutes of Health (NIH), Department of Defense, Parkinson's Foundation, and Michael J. Fox Foundation. He has consulted for Caraway, Avrobio, Merck, GSK, Sanofi, and Ono Therapeutics. Kuo's research is funded by the NIH and the National Ataxia Foundation. He has consulted for Praxis, Neurocrine, uniQure, and Sage Therapeutics. Troche's research is funded by the Michael J. Fox Foundation and the Cure PSP Foundation. Briana Kiefer's research is funded by the NIH (Grant F31DC019281-01).
Funding Statement
Alcalay's research is funded by the National Institutes of Health (NIH), Department of Defense, Parkinson's Foundation, and Michael J. Fox Foundation. He has consulted for Caraway, Avrobio, Merck, GSK, Sanofi, and Ono Therapeutics. Kuo's research is funded by the NIH and the National Ataxia Foundation. He has consulted for Praxis, Neurocrine, uniQure, and Sage Therapeutics. Troche's research is funded by the Michael J. Fox Foundation and the Cure PSP Foundation. Briana Kiefer's research is funded by the NIH (Grant F31DC019281-01).
References
- Aiello, M. , Rampello, A. , Granella, F. , Maestrelli, M. , Tzani, P. , Immovilli, P. , Franceschini, M. , Olivieri, D. , & Chetta, A. (2008). Cough efficacy is related to the disability status in patients with multiple sclerosis. Respiration, 76(3), 311–316. https://doi.org/10.1159/000119641 [DOI] [PubMed] [Google Scholar]
- Akbar, U. , Dham, B. , He, Y. , Hack, N. , Wu, S. , Troche, M. , Tighe, P. , Nelson, E. , Friedman, J. H. , & Okun, M. S. (2015). Incidence and mortality trends of aspiration pneumonia in Parkinson's disease in the United States, 1979–2010. Parkinsonism & Related Disorders, 21(9), 1082–1086. https://doi.org/10.1016/j.parkreldis.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Anand, S. , El-Bashiti, N. , & Sapienza, C. (2012). Effect of training frequency on maximum expiratory pressure. American Journal of Speech-Language Pathology, 21(4), 380–386. https://doi.org/10.1044/1058-0360(2012/11-0048) [DOI] [PubMed] [Google Scholar]
- Argolo, N. , Sampaio, M. , Pinho, P. , Melo, A. , & Nóbrega, A. C. (2013). Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson's disease? NeuroRehabilitation, 32(4), 949–955. https://doi.org/10.3233/NRE-130918 [DOI] [PubMed] [Google Scholar]
- Athukorala, R. P. , Jones, R. D. , Sella, O. , & Huckabee, M.-L. (2014). Skill training for swallowing rehabilitation in patients with Parkinson's disease. Archives of Physical Medicine and Rehabilitation, 95(7), 1374–1382. https://doi.org/10.1016/j.apmr.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Baker, S. , Davenport, P. , & Sapienza, C. (2005). Examination of strength training and detraining effects in expiratory muscles. Journal of Speech, Language, and Hearing Research, 48(6), 1325–1333. https://doi.org/10.1044/1092-4388(2005/092) [DOI] [PubMed] [Google Scholar]
- Bakheit, A. M. O. (2001). Management of neurogenic dysphagia. Postgraduate Medical Journal, 77(913), 694–699. https://doi.org/10.1136/pmj.77.913.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascuñana-Ambrós, H. , Renom-Guiteras, M. , Nadal-Castells, M. J. , Beranuy-Rodríguez, M. , Perrot-González, J. C. , Ramirez-Mirabal, E. , Trejo-Omeñaca, A. , & Monguet-Fierro, J. M. (2021). Swallowing muscle training for oropharyngeal dysphagia: A non-inferiority study of online versus face-to-face therapy. Journal of Telemedicine and Telecare. Advance online publication. https://doi.org/10.1177/1357633X211035033 [DOI] [PubMed] [Google Scholar]
- Beck, C. A. , Beran, D. B. , Biglan, K. M. , Boyd, C. M. , Dorsey, E. R. , Schmidt, P. N. , Simone, R. , Willis, A. W. , Galifianakis, N. B. , Katz, M. , Tanner, C. M. , Dodenhoff, K. , Aldred, J. , Carter, J. , Fraser, A. , Jimenez-Shahed, J. , Hunter, C. , Spindler, M. , Reichwein, S. , … Zhu, W. (2017). National randomized controlled trial of virtual house calls for Parkinson disease. Neurology, 89(11), 1152–1161. https://doi.org/10.1212/WNL.0000000000004357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, C. , Baiardi, P. , Khirani, S. , & Cantarella, G. (2012). Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. American Journal of Physical Medicine & Rehabilitation, 91(9), 783–788. https://doi.org/10.1097/PHM.0b013e3182556701 [DOI] [PubMed] [Google Scholar]
- Borders, J. C. , Curtis, J. A. , Sevitz, J. S. , Vanegas-Arroyave, N. , & Troche, M. S. (2021). Immediate effects of sensorimotor training in airway protection (smTAP) on cough outcomes in progressive supranuclear palsy: A feasibility study. Dysphagia, 37, 74–83. https://doi.org/10.1007/s00455-021-10251-1 [DOI] [PubMed] [Google Scholar]
- Borders, J. C. , Sevitz, J. S. , Malandraki, J. B. , Malandraki, G. A. , & Troche, M. S. (2021). Objective and subjective clinical swallowing outcomes via telehealth: Reliability in outpatient clinical practice. American Journal of Speech-Language Pathology, 30(2), 598–608. https://doi.org/10.1044/2020_AJSLP-20-00234 [DOI] [PubMed] [Google Scholar]
- Borders, J. C. , & Troche, M. S. (2021). Voluntary cough effectiveness and airway clearance in neurodegenerative disease. Journal of Speech, Language, and Hearing Research, 65(2), 431–449. https://doi.org/10.1044/2021_JSLHR-21-00308 [DOI] [PubMed] [Google Scholar]
- Bowen, D. J. , Kreuter, M. , Spring, B. , Cofta-Woerpel, L. , Linnan, L. , Weiner, D. , Weiner, D. , Bakken, S. , Kaplan, C. P. , Squiers, L. , Fabrizio, C. , & Fernandez, M. (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36(5), 452–457. https://doi.org/10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandimore, A. E. , Hegland, K. W. , Okun, M. S. , Davenport, P. W. , & Troche, M. S. (2017). Voluntary upregulation of reflex cough is possible in healthy older adults and Parkinson's disease. Journal of Applied Physiology, 123(1), 19–26. https://doi.org/10.1152/japplphysiol.00612.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, C. L. , Ward, E. C. , Gray, A. , Baker, L. , Cowie, B. , Winter, N. , Rusch, R. , Saxon, R. , Barnes, S. , & Turvey, J. (2019). Implementation of speech pathology telepractice services for clinical swallowing assessment: An evaluation of service outcomes, costs and consumer satisfaction. Journal of Telemedicine and Telecare, 25(9), 545–551. https://doi.org/10.1177/1357633X19873248 [DOI] [PubMed] [Google Scholar]
- Burns, C. L. , Ward, E. C. , Hill, A. J. , Kularatna, S. , Byrnes, J. , & Kenny, L. M. (2017). Randomized controlled trial of a multisite speech pathology telepractice service providing swallowing and communication intervention to patients with head and neck cancer: Evaluation of service outcomes. Head & Neck, 39(5), 932–939. https://doi.org/10.1002/hed.24706 [DOI] [PubMed] [Google Scholar]
- Cassel, S. G. (2016). Case reports: Trial dysphagia interventions conducted via telehealth. International Journal of Telerehabilitation, 8(2), 71–76. https://doi.org/10.5195/IJT.2016.6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara, T. , Martin, A. D. , Davenport, P. W. , & Bolser, D. C. (2006). Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: Effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Archives of Physical Medicine and Rehabilitation, 87(4), 468–473. https://doi.org/10.1016/j.apmr.2005.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, H. M. , Stierwalt, J. A. G. , Tosakulwong, N. , Botha, H. , Ali, F. , Whitwell, J. L. , & Josephs, K. A. (2020). Dysphagia in progressive supranuclear palsy. Dysphagia, 35(4), 667–676. https://doi.org/10.1007/s00455-019-10073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus, I. , Muhle, P. , Czechowski, J. , Ahring, S. , Labeit, B. , Suntrup-Krueger, S. , Wiendl, H. , Dziewas, R. , & Warnecke, T. (2021). Expiratory muscle strength training for therapy of pharyngeal dysphagia in Parkinson's disease. Movement Disorders, 36(8), 1815–1824. https://doi.org/10.1002/mds.28552 [DOI] [PubMed] [Google Scholar]
- Clavé, P. , Kraa, M. D. , Arreola, V. , Girvent, M. , Farré, R. , Palomera, E. , & Serra-Prat, M. (2006). The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Alimentary Pharmacology & Therapeutics, 24(9), 1385–1394. https://doi.org/10.1111/j.1365-2036.2006.03118.x [DOI] [PubMed] [Google Scholar]
- Collins, A. , Burns, C. L. , Ward, E. C. , Comans, T. , Blake, C. , Kenny, L. , Greenup, P. , & Best, D. (2017). Home-based telehealth service for swallowing and nutrition management following head and neck cancer treatment. Journal of Telemedicine and Telecare, 23(10), 866–872. https://doi.org/10.1177/1357633X17733020 [DOI] [PubMed] [Google Scholar]
- Constantinescu, G. , Rieger, J. , Seikaly, H. , & Eurich, D. (2021). Adherence to home-based swallowing therapy using a mobile system in head and neck cancer survivors. American Journal of Speech-Language Pathology, 30(6), 2465–2475. https://doi.org/10.1044/2021_AJSLP-21-00026 [DOI] [PubMed] [Google Scholar]
- Crizzle, A. M. , Classen, S. , & Uc, E. Y. (2012). Parkinson disease and driving: An evidence-based review. Neurology, 79(20), 2067–2074. https://doi.org/10.1212/WNL.0b013e3182749e95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, J. A. , Dakin, A. E. , & Troche, M. S. (2020). Respiratory–swallow coordination training and voluntary cough skill training: A single-subject treatment study in a person with Parkinson's disease. Journal of Speech, Language, and Hearing Research, 63(2), 472–486. https://doi.org/10.1044/2019_JSLHR-19-00207 [DOI] [PubMed] [Google Scholar]
- D'Amelio, M. , Ragonese, P. , Morgante, L. , Reggio, A. , Callari, G. , Salemi, G. , & Savettieri, G. (2006). Long-term survival of Parkinson's disease. Journal of Neurology, 253(1), 33–37. https://doi.org/10.1007/s00415-005-0916-7 [DOI] [PubMed] [Google Scholar]
- Dorsey, E. R. , Bloem, B. R. , & Okun, M. S. (2020). A new day: The role of telemedicine in reshaping care for persons with movement disorders. Movement Disorders, 35(11), 1897–1902. https://doi.org/10.1002/mds.28296 [DOI] [PubMed] [Google Scholar]
- Dorsey, E. R. , Deuel, L. M. , Voss, T. S. , Finnigan, K. , George, B. P. , Eason, S. , Miller, D. , Reminick, J. I. , Appler, A. , Polanowicz, J. , Viti, L. , Smith, S. , Joseph, A. , & Biglan, K. M. (2010). Increasing access to specialty care: A pilot, randomized controlled trial of telemedicine for Parkinson's disease. Movement Disorders, 25(11), 1652–1659. https://doi.org/10.1002/mds.23145 [DOI] [PubMed] [Google Scholar]
- Ebihara, S. , Ebihara, T. , & Kohzuki, M. (2012). Effect of aging on cough and swallowing reflexes: Implications for preventing aspiration pneumonia. Lung, 190(1), 29–33. https://doi.org/10.1007/s00408-011-9334-z [DOI] [PubMed] [Google Scholar]
- Ebihara, S. , Saito, H. , Kanda, A. , Nakajoh, M. , Takahashi, H. , Arai, H. , & Sasaki, H. (2003). Impaired efficacy of cough in patients with Parkinson disease. Chest, 124(3), 1009–1015. https://doi.org/10.1378/chest.124.3.1009 [DOI] [PubMed] [Google Scholar]
- Ebihara, S. , Sekiya, H. , Miyagi, M. , Ebihara, T. , & Okazaki, T. (2016). Dysphagia, dystussia, and aspiration pneumonia in elderly people. Journal of Thoracic Disease, 8(3), 632–639. https://doi.org/10.21037/jtd.2016.02.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson, M. J. , Stevenson, E. A. , Feldman, B. A. , Lim, J. , Beck, C. A. , Beran, D. B. , Schmidt, P. N. , Biglan, K. M. , Simone, R. , Willis, A. W. , Dorsey, E. R. , & Boyd, C. M. (2018). Telemedicine for Parkinson's disease: Limited engagement between local clinicians and remote specialists. Telemedicine and E-Health, 24(9), 722–724. https://doi.org/10.1089/tmj.2017.0210 [DOI] [PubMed] [Google Scholar]
- EMST150. (2021). About Aspire products [Video] . https://emst150.com/about#public-training-videos
- Fall, P.-A. , Saleh, A. , Fredrickson, M. , Olsson, J.-E. , & Granérus, A.-K. (2003). Survival time, mortality, and cause of death in elderly patients with Parkinson's disease. A 9-year follow-up. Movement Disorders, 18(11), 1312–1316. https://doi.org/10.1002/mds.10537 [DOI] [PubMed] [Google Scholar]
- Feeney, M. P. , Xu, Y. , Surface, M. , Shah, H. , Vanegas-Arroyave, N. , Chan, A. K. , Delaney, E. , Przedborski, S. , Beck, J. C. , & Alcalay, R. N. (2021). The impact of COVID-19 and social distancing on people with Parkinson's disease: A survey study. NPJ Parkinson's Disease, 7(1), 10. https://doi.org/10.1038/s41531-020-00153-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana, G. A. , & Lavorini, F. (2006). Cough motor mechanisms. Respiratory Physiology & Neurobiology, 152(3), 266–281. https://doi.org/10.1016/j.resp.2006.02.016 [DOI] [PubMed] [Google Scholar]
- Fullard, M. E. , Thibault, D. P. , Hill, A. , Fox, J. , Bhatti, D. E. , Burack, M. A. , Dahodwala, N. , Haberfeld, E. , Kern, D. S. , Klepitskava, O. S. , Urrea-Mendoza, E. , Myers, P. , Nutt, J. , Rafferty, M. R. , Schwalb, J. M. , Shulman, L. M. , & Willis, A. W. (2017). Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology, 89(11), 1162–1169. https://doi.org/10.1212/WNL.0000000000004355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén-Solà, A. , Messagi Sartor, M. , Bofill Soler, N. , Duarte, E. , Barrera, M. C. , & Marco, E. (2017). Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: A randomized controlled trial. Clinical Rehabilitation, 31(6), 761–771. https://doi.org/10.1177/0269215516652446 [DOI] [PubMed] [Google Scholar]
- Haas, B. M. , Trew, M. , & Castle, P. C. (2004). Effects of respiratory muscle weakness on daily living function, quality of life, activity levels, and exercise capacity in mild to moderate Parkinson's disease. American Journal of Physical Medicine & Rehabilitation, 83(8), 601–607. https://doi.org/10.1097/01.PHM.0000133436.61009.02 [DOI] [PubMed] [Google Scholar]
- Haulman, A. , Geronimo, A. , Chahwala, A. , & Simmons, Z. (2020). The use of telehealth to enhance care in ALS and other neuromuscular disorders. Muscle & Nerve, 61(6), 682–691. https://doi.org/10.1002/mus.26838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk, A.-W. , & Roos, R. A. C. (2012). Aspiration pneumonia and death in Huntington's disease. PLOS Currents, 4, RRN1293. https://doi.org/10.1371/currents.RRN1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegland, K. W. , Okun, M. S. , & Troche, M. S. (2014). Sequential voluntary cough and aspiration or aspiration risk in Parkinson's disease. Lung, 192(4), 601–608. https://doi.org/10.1007/s00408-014-9584-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegland, K. W. , Troche, M. S. , & Davenport, P. W. (2013). Cough expired volume and airflow rates during sequential induced cough. Frontiers in Physiology, 4, 167. https://doi.org/10.3389/fphys.2013.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, H.-F. , & Shannon, S. E. (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. https://doi.org/10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Kaneoka, A. , Pisegna, J. M. , Inokuchi, H. , Ueha, R. , Goto, T. , Nito, T. , Stepp, C. E. , LaValley, M. P. , Haga, N. , & Langmore, S. E. (2018). Relationship between laryngeal sensory deficits, aspiration, and pneumonia in patients with dysphagia. Dysphagia, 33(2), 192–199. https://doi.org/10.1007/s00455-017-9845-8 [DOI] [PubMed] [Google Scholar]
- Kantarcigil, C. , & Malandraki, G. A. (2017). First step in telehealth assessment: A randomized controlled trial to investigate the effectiveness of an electronic case history form for dysphagia. Dysphagia, 32(4), 548–558. https://doi.org/10.1007/s00455-017-9798-y [DOI] [PubMed] [Google Scholar]
- Kantarcigil, C. , Sheppard, J. J. , Gordon, A. M. , Friel, K. M. , & Malandraki, G. A. (2016). A telehealth approach to conducting clinical swallowing evaluations in children with cerebral palsy. Research in Developmental Disabilities, 55, 207–217. https://doi.org/10.1016/j.ridd.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Kim, J. , Davenport, P. , & Sapienza, C. (2009). Effect of expiratory muscle strength training on elderly cough function. Archives of Gerontology and Geriatrics, 48(3), 361–366. https://doi.org/10.1016/j.archger.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Kulnik, S. T. , MacBean, V. , Birring, S. S. , Moxham, J. , Rafferty, G. F. , & Kalra, L. (2015). Accuracy of portable devices in measuring peak cough flow. Physiological Measurement, 36(2), 243–257. https://doi.org/10.1088/0967-3334/36/2/243 [DOI] [PubMed] [Google Scholar]
- Kuo, Y.-C. , Chan, J. , Wu, Y.-P. , Bernard, J. R. , & Liao, Y.-H. (2017). Effect of expiratory muscle strength training intervention on the maximum expiratory pressure and quality of life of patients with Parkinson disease. NeuroRehabilitation, 41(1), 219–226. https://doi.org/10.3233/NRE-171474 [DOI] [PubMed] [Google Scholar]
- Levin, M. F. , & Demers, M. (2020). Motor learning in neurological rehabilitation. Disability and Rehabilitation, 43(24), 3445–3453. https://doi.org/10.1080/09638288.2020.1752317 [DOI] [PubMed] [Google Scholar]
- Malandraki, G. A. , Arkenberg, R. H. , Mitchell, S. S. , & Malandraki, J. B. (2021). Telehealth for dysphagia across the life span: Using contemporary evidence and expertise to guide clinical practice during and after COVID-19. American Journal of Speech-Language Pathology, 30(2), 532–550. https://doi.org/10.1044/2020_AJSLP-20-00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki, G. A. , & Kantarcigil, C. (2017). Telehealth for dysphagia rehabilitation: The present and the future. Perspectives of the ASHA Special Interest Groups, 2(18), 42–48. https://doi.org/10.1044/persp2.SIG18.42 [Google Scholar]
- Malandraki, G. A. , Kaufman, A. , Hind, J. , Ennis, S. , Gangnon, R. , Waclawik, A. , & Robbins, J. (2012). The effects of lingual intervention in a patient with inclusion body myositis and Sjögren's syndrome: A longitudinal case study. Archives of Physical Medicine and Rehabilitation, 93(8), 1469–1475. https://doi.org/10.1016/j.apmr.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Malandraki, G. A. , Markaki, V. , Georgopoulos, V. C. , Bauer, J. L. , Kalogeropoulos, I. , & Nanas, S. (2013). An international pilot study of asynchronous teleconsultation for oropharyngeal dysphagia. Journal of Telemedicine and Telecare, 19(2), 75–79. https://doi.org/10.1177/1357633x12474963 [DOI] [PubMed] [Google Scholar]
- Malandraki, G. A. , Rajappa, A. , Kantarcigil, C. , Wagner, E. , Ivey, C. , & Youse, K. (2016). The intensive dysphagia rehabilitation approach applied to patients with neurogenic dysphagia: A case series design study. Archives of Physical Medicine and Rehabilitation, 97(4), 567–574. https://doi.org/10.1016/j.apmr.2015.11.019 [DOI] [PubMed] [Google Scholar]
- Marik, P. E. , & Kaplan, D. (2003). Aspiration pneumonia and dysphagia in the elderly. Chest, 124(1), 328–336. https://doi.org/10.1378/chest.124.1.328 [DOI] [PubMed] [Google Scholar]
- McEachern, W. , Kirk, A. , Morgan, D. G. , Crossley, M. , & Henry, C. (2008). Reliability of the MMSE administered in-person and by telehealth. Canadian Journal of Neurological Sciences, 35(5), 643–646. https://doi.org/10.1017/S0317167100009458 [DOI] [PubMed] [Google Scholar]
- Miles, A. , Jardine, M. , Johnston, F. , de Lisle, M. , Friary, P. , & Allen, J. (2017). Effect of lee Silverman voice treatment (LSVT LOUD®) on swallowing and cough in Parkinson's disease: A pilot study. Journal of the Neurological Sciences, 383, 180–187. https://doi.org/10.1016/j.jns.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Moon, J. H. , Jung, J.-H. , Won, Y. S. , Cho, H.-Y. , & Cho, K. (2017). Effects of expiratory muscle strength training on swallowing function in acute stroke patients with dysphagia. Journal of Physical Therapy Science, 29(4), 609–612. https://doi.org/10.1589/jpts.29.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell, K. , Hyers, M. , Stuchiner, T. , Lucas, L. , Schwartz, K. , Mako, J. , Spinelli, K. J. , & Yanase, L. (2017). Telehealth stroke dysphagia evaluation is safe and effective. Cerebrovascular Diseases, 44(3–4), 225–231. https://doi.org/10.1159/000478107 [DOI] [PubMed] [Google Scholar]
- Morse, J. M. (2015). Critical analysis of strategies for determining rigor in qualitative inquiry. Qualitative Health Research, 25(9), 1212–1222. https://doi.org/10.1177/1049732315588501 [DOI] [PubMed] [Google Scholar]
- Mosley, P. E. , Moodie, R. , & Dissanayaka, N. (2017). Caregiver burden in Parkinson disease: A critical review of recent literature. Journal of Geriatric Psychiatry and Neurology, 30(5), 235–252. https://doi.org/10.1177/0891988717720302 [DOI] [PubMed] [Google Scholar]
- Muratori, L. M. , Lamberg, E. M. , Quinn, L. , & Duff, S. V. (2013). Applying principles of motor learning and control to upper extremity rehabilitation. Journal of Hand Therapy, 26(2), 94–103. https://doi.org/10.1016/j.jht.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, T. , Maeda, K. , Tahira, K. , Taniguchi, K. , Mori, K. , Kiyomiya, H. , & Akagi, J. (2018). Silent aspiration predicts mortality in older adults with aspiration pneumonia admitted to acute hospitals. Geriatrics & Gerontology International, 18(6), 828–832. https://doi.org/10.1111/ggi.13250 [DOI] [PubMed] [Google Scholar]
- Nordio, S. , Innocenti, T. , Agostini, M. , Meneghello, F. , & Battel, I. (2018). The efficacy of telerehabilitation in dysphagic patients: A systematic review. Acta Otorhinolaryngologica Italica, 38(2), 79–85. https://doi.org/10.14639/0392-100X-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara, R. , & Jackson, S. (2017). Integrating telehealth services into a remote allied health service: A pilot study. The Australian Journal of Rural Health, 25(1), 53–57. https://doi.org/10.1111/ajr.12189 [DOI] [PubMed] [Google Scholar]
- Ortega, O. , Martín, A. , & Clavé, P. (2017). Diagnosis and management of oropharyngeal dysphagia among older persons, state of the art. Journal of the American Medical Directors Association, 18(7), 576–582. https://doi.org/10.1016/j.jamda.2017.02.015 [DOI] [PubMed] [Google Scholar]
- Pitts, T. , Bolser, D. , Rosenbek, J. , Troche, M. , Okun, M. S. , & Sapienza, C. (2009). Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest, 135(5), 1301–1308. https://doi.org/10.1378/chest.08-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts, T. , Bolser, D. , Rosenbek, J. , Troche, M. , & Sapienza, C. (2008). Voluntary cough production and swallow dysfunction in Parkinson's disease. Dysphagia, 23(3), 297–301. https://doi.org/10.1007/s00455-007-9144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts, T. , Troche, M. , Mann, G. , Rosenbek, J. , Okun, M. S. , & Sapienza, C. (2010). Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest, 138(6), 1426–1431. https://doi.org/10.1378/chest.10-0342 [DOI] [PubMed] [Google Scholar]
- Plowman, E. K. , Tabor-Gray, L. , Rosado, K. M. , Vasilopoulos, T. , Robison, R. , Chapin, J. L. , Gaziano, J. , Vu, T. , & Gooch, C. (2019). Impact of expiratory strength training in amyotrophic lateral sclerosis: Results of a randomized, sham-controlled trial. Muscle & Nerve, 59(1), 40–46. https://doi.org/10.1002/mus.26292 [DOI] [PubMed] [Google Scholar]
- Plowman, E. K. , Watts, S. A. , Robison, R. , Tabor, L. , Dion, C. , Gaziano, J. , Vu, T. , & Gooch, C. (2016). Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia, 31(3), 383–390. https://doi.org/10.1007/s00455-015-9687-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman, E. K. , Watts, S. A. , Tabor, L. , Robison, R. , Gaziano, J. , Domer, A. S. , Richter, J. , Vu, T. , & Gooch, C. (2016). Impact of expiratory strength training in amyotrophic lateral sclerosis. Muscle & Nerve, 54(1), 48–53. https://doi.org/10.1002/mus.24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Reyes, A. , Castillo, A. , & Castillo, J. (2020). Effects of expiratory muscle training and air stacking on peak cough flow in individuals with Parkinson's disease. Lung, 198(1), 207–211. https://doi.org/10.1007/s00408-019-00291-8 [DOI] [PubMed] [Google Scholar]
- Reyes, A. , Castillo, A. , Castillo, J. , & Cornejo, I. (2018). The effects of respiratory muscle training on peak cough flow in patients with Parkinson's disease: A randomized controlled study. Clinical Rehabilitation, 32(10), 1317–1327. https://doi.org/10.1177/0269215518774832 [DOI] [PubMed] [Google Scholar]
- Reyes, A. , Cruickshank, T. , Nosaka, K. , & Ziman, M. (2015). Respiratory muscle training on pulmonary and swallowing function in patients with Huntington's disease: A pilot randomised controlled trial. Clinical Rehabilitation, 29(10), 961–973. https://doi.org/10.1177/0269215514564087 [DOI] [PubMed] [Google Scholar]
- Rogus-Pulia, N. M. , & Plowman, E. K. (2020). Shifting tides toward a proactive patient-centered approach in dysphagia management of neurodegenerative disease. American Journal of Speech-Language Pathology, 29(2S), 1094–1109. https://doi.org/10.1044/2020_AJSLP-19-00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnefarth, M. , Hanisch, N. , Brandt, A. U. , Mähler, A. , Endres, M. , Paul, F. , & Doss, S. (2020). Dysphagia affecting quality of life in cerebellar ataxia—A large survey. The Cerebellum, 19(3), 437–445. https://doi.org/10.1007/s12311-020-01122-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale, D. G. (1988). Neural adaptation to resistance training. Medicine and Science in Sports and Exercise, 20(Suppl. 1), S135–S145. https://doi.org/10.1249/00005768-198810001-00009 [DOI] [PubMed] [Google Scholar]
- Saleem, A. F. , Sapienza, C. M. , & Okun, M. S. (2005). Respiratory muscle strength training: Treatment and response duration in a patient with early idiopathic Parkinson's disease. NeuroRehabilitation, 20(4), 323–333. https://doi.org/10.3233/nre-2005-20407 [PubMed] [Google Scholar]
- Sancho, J. , Servera, E. , Diaz, J. , & Marin, J. (2004). Comparison of peak cough flows measured by pneumotachograph and a portable peak flow meter. American Journal of Physical Medicine & Rehabilitation, 83(8), 608–612. https://doi.org/10.1097/01.PHM.0000133431.70907.A2 [DOI] [PubMed] [Google Scholar]
- Schneider, R. B. , & Biglan, K. M. (2017). The promise of telemedicine for chronic neurological disorders: The example of Parkinson's disease. The Lancet Neurology, 16(7), 541–551. https://doi.org/10.1016/S1474-4422(17)30167-9 [DOI] [PubMed] [Google Scholar]
- Sharma, S. , Ward, E. C. , Burns, C. , Theodoros, D. , & Russell, T. (2013). Assessing dysphagia via telerehabilitation: Patient perceptions and satisfaction. International Journal of Speech-Language Pathology, 15(2), 176–183. https://doi.org/10.3109/17549507.2012.689333 [DOI] [PubMed] [Google Scholar]
- Shinn, E. H. , Jensen, K. , McLaughlin, J. , Garden, A. S. , Fellman, B. M. , Liang, L. , & Peterson, S. K. (2019). Interactive website for head and neck cancer patients: Adherence and coping program to prevent dysphagia after radiation. Internet Interventions, 18, 100289. https://doi.org/10.1016/j.invent.2019.100289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, E. P. , Carnaby, G. , Singletary, F. , Hoffman-Ruddy, B. , Yeager, J. , & Sapienza, C. (2016). Measurement of voluntary cough production and airway protection in Parkinson disease. Archives of Physical Medicine and Rehabilitation, 97(3), 413–420. https://doi.org/10.1016/j.apmr.2015.10.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, E. P. , Carnaby-Mann, G. , Pitts, T. , Davenport, P. , Okun, M. S. , & Sapienza, C. (2014). Concordance and discriminatory power of cough measurement devices for individuals with Parkinson disease. Chest, 145(5), 1089–1096. https://doi.org/10.1378/chest.13-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, D. , Park, G.-Y. , Koo, H. , Jang, Y. , Han, Y. , & Im, S. (2018). Determining peak cough flow cutoff values to predict aspiration pneumonia among patients with dysphagia using the citric acid reflexive cough test. Archives of Physical Medicine and Rehabilitation, 99(12), 2532–2539.e1. https://doi.org/10.1016/j.apmr.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Srp, M. , Korteová, R. , Kliment, R. , Jech, R. , Růžička, E. , & Hoskovcová, M. (2021). Expiratory muscle strength training in patients with Parkinson's disease: A pilot study of Mobile monitoring application. Movement Disorders Clinical Practice, 8(7), 1148–1149. https://doi.org/10.1002/mdc3.13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer, H. M. , Abrams, R. , Webster, K. , Kizner, J. , Beadle, B. , Holsinger, F. C. , Quon, H. , & Richmon, J. (2018). Feasibility of a mobile application to enhance swallowing therapy for patients undergoing radiation-based treatment for head and neck cancer. Dysphagia, 33(2), 227–233. https://doi.org/10.1007/s00455-017-9850-y [DOI] [PubMed] [Google Scholar]
- Sura, L. , Madhavan, A. , Carnaby, G. , & Crary, M. A. (2012). Dysphagia in the elderly: Management and nutritional considerations. Clinical Interventions in Aging, 7, 287–298. https://doi.org/10.2147/CIA.S23404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor-Gray, L. C. , Gallestagui, A. , Vasilopoulos, T. , & Plowman, E. K. (2019). Characteristics of impaired voluntary cough function in individuals with amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 20(1–2), 37–42. https://doi.org/10.1080/21678421.2018.1510011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor-Gray, L. , Vasilopoulos, T. , & Plowman, E. K. (2019). Concordant validity of a digital peak cough flow meter to assess voluntary cough strength in individuals with ALS. Dysphagia, 35(4), 568–573. https://doi.org/10.1007/s00455-019-10060-7 [DOI] [PubMed] [Google Scholar]
- Tabor-Gray, L. , Vasilopoulos, T. , & Plowman, E. K. (2020). Differences in voluntary and reflexive cough strength in individuals with amyotrophic lateral sclerosis and healthy adults. Muscle & Nerve, 62(5), 597–600. https://doi.org/10.1002/mus.27040 [DOI] [PubMed] [Google Scholar]
- Takizawa, C. , Gemmell, E. , Kenworthy, J. , & Speyer, R. (2016). A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson's disease, Alzheimer's disease, head injury, and pneumonia. Dysphagia, 31(3), 434–441. https://doi.org/10.1007/s00455-016-9695-9 [DOI] [PubMed] [Google Scholar]
- Troche, M. S. , Brandimore, A. E. , Godoy, J. , & Hegland, K. W. (2014). A framework for understanding shared substrates of airway protection. Journal of Applied Oral Science, 22(4), 251–260. https://doi.org/10.1590/1678-775720140132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche, M. S. , Brandimore, A. E. , Okun, M. S. , Davenport, P. W. , & Hegland, K. W. (2014). Decreased cough sensitivity and aspiration in Parkinson disease. Chest, 146(5), 1294–1299. https://doi.org/10.1378/chest.14-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche, M. S. , Okun, M. S. , Rosenbek, J. C. , Musson, N. , Fernandez, H. H. , Rodriguez, R. , Romrell, J. , Pitts, T. , Wheeler-Hegland, K. M. , & Sapienza, C. M. (2010). Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurology, 75(21), 1912–1919. https://doi.org/10.1212/WNL.0b013e3181fef115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche, M. S. , Rosenbek, J. C. , Okun, M. S. , & Sapienza, C. M. (2015). Detraining outcomes with expiratory muscle strength training in Parkinson disease. Journal of Rehabilitation Research and Development, 51(2), 305–310. https://doi.org/10.1682/JRRD.2013.05.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troche, M. S. , Schumann, B. , Brandimore, A. E. , Okun, M. S. , & Hegland, K. W. (2016). Reflex cough and disease duration as predictors of swallowing dysfunction in Parkinson's disease. Dysphagia, 31(6), 757–764. https://doi.org/10.1007/s00455-016-9734-6 [DOI] [PubMed] [Google Scholar]
- Turner, A. P. , Wallin, M. T. , Sloan, A. , Maloni, H. , Kane, R. , Martz, L. , & Haselkorn, J. K. (2013). Clinical management of multiple sclerosis through home telehealth monitoring. International Journal of MS Care, 15(1), 8–14. https://doi.org/10.7224/1537-2073.2012-012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye, C. B. , Gardner, P. A. , Dion, G. R. , Simpson, C. B. , & Dominguez, L. M. (2021). Impact of fiberoptic endoscopic evaluation of swallowing outcomes and dysphagia management in neurodegenerative diseases. The Laryngoscope, 131(4), 726–730. https://doi.org/10.1002/lary.28791 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services. (2019). Increase the use of telehealth to improve access to health services—AHS‑R02. Healthy People 2030. https://health.gov/healthypeople/objectives-and-data/browse-objectives/health-it/increase-use-telehealth-improve-access-health-services-ahs-r02
- van de Wetering-van Dongen, V. A. , Kalf, J. G. , van der Wees, P. J. , Bloem, B. R. , & Nijkrake, M. J. (2020). The effects of respiratory training in Parkinson's disease: A systematic review. Journal of Parkinson's Disease, 10(4), 1315–1333. https://doi.org/10.3233/JPD-202223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, A. P. , Fendel, L. , Paige Brubacher, K. , Chan, V. , & Maule, R. (2015). Dysphagia in spinocerebellar ataxia and multiple system atrophy-cerebellar. Speech, Language and Hearing, 18(1), 39–43. https://doi.org/10.1179/2050572814Y.0000000047 [Google Scholar]
- Wall, L. R. , Ward, E. C. , Cartmill, B. , Hill, A. J. , Isenring, E. , Byrnes, J. , & Porceddu, S. V. (2020). Prophylactic swallowing therapy for patients with head and neck cancer: A three-arm randomized parallel-group trial. Head & Neck, 42(5), 873–885. https://doi.org/10.1002/hed.26060 [DOI] [PubMed] [Google Scholar]
- Walshe, M. (2014). Oropharyngeal dysphagia in neurodegenerative disease. Journal of Gastroenterology and Hepatology, 29(9), 1265–1271.24955455 [Google Scholar]
- Ward, E. C. , & Burns, C. L. (2014). Dysphagia management via telerehabilitation: A review of the current evidence. Journal of Gastroenterology and Hepatology Research, 3(5), 1088–1094. https://doi.org/10.6051/j.issn.2224-3992.2014.03.408-17 [Google Scholar]
- Ward, E. C. , Sharma, S. , Burns, C. , Theodoros, D. , & Russell, T. (2012a). Managing patient factors in the assessment of swallowing via Telerehabilitation. International Journal of Telemedicine and Applications, 2012, 1–6. https://doi.org/10.1155/2012/132719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E. C. , Sharma, S. , Burns, C. , Theodoros, D. , & Russell, T. (2012b). Validity of conducting clinical dysphagia assessments for patients with normal to mild cognitive impairment via telerehabilitation. Dysphagia, 27(4), 460–472. https://doi.org/10.1007/s00455-011-9390-9 [DOI] [PubMed] [Google Scholar]
- Weidner, K. , & Lowman, J. (2020). Telepractice for adult speech-language pathology services: A systematic review. Perspectives of the ASHA Special Interest Groups, 5(1), 326–338. https://doi.org/10.1044/2019_PERSP-19-00146 [Google Scholar]
- Westfall, J. , Kenny, D. A. , & Judd, C. M. (2014). Statistical power and optimal design in experiments in which samples of participants respond to samples of stimuli. Journal of Experimental Psychology: General, 143(5), 2020–2045. https://doi.org/10.1037/xge0000014 [DOI] [PubMed] [Google Scholar]
- Zoom Video Communications Inc. (2016). Security guide. https://d24cgw3uvb9a9h.cloudfront.net/static/81625/doc/Zoom-Security-White-Paper.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author upon reasonable request.