ABSTRACT
Flaviviruses have a cytoplasmic replicative cycle, and crucial events, such as genome translation and replication, occur in the endoplasmic reticulum. However, some viral proteins, such as C, NS1, and NS5 from Zika virus (ZIKV) containing nuclear localization signals (NLSs) and nuclear export signals (NESs), are also located in the nucleus of Vero cells. The NS2A, NS3, and NS4A proteins from dengue virus (DENV) have also been reported to be in the nucleus of A549 cells, and our group recently reported that the NS3 protein is also located in the nucleus of Huh7 and C636 cells during DENV infection. However, the NS3 protease-helicase from ZIKV locates in the perinuclear region of infected cells and alters the morphology of the nuclear lamina, a component of the nuclear envelope. Furthermore, ZIKV NS3 has been reported to accumulate on the concave face of altered kidney-shaped nuclei and may be responsible for modifying other elements of the nuclear envelope. However, nuclear localization of NS3 from ZIKV has not been substantially investigated in human host cells. Our group has recently reported that DENV and ZIKV NS3 alter the nuclear pore complex (NPC) by cleaving some nucleoporins. Here, we demonstrate the presence of ZIKV NS3 in the nucleus of Huh7 cells early in infection and in the cytoplasm at later times postinfection. In addition, we found that ZIKV NS3 contains an NLS and a putative NES and uses the classic import (importin-α/β) and export pathway via CRM-1 to be transported between the cytoplasm and the nucleus.
IMPORTANCE Flaviviruses have a cytoplasmic replication cycle, but recent evidence indicates that nuclear elements play a role in their viral replication. Viral proteins, such as NS5 and C, are imported into the nucleus, and blocking their import prevents replication. Because of the importance of the nucleus in viral replication and the role of NS3 in the modification of nuclear components, we investigated whether NS3 can be localized in the nucleus during ZIKV infection. We found that NS3 is imported into the nucleus via the importin pathway and exported to the cytoplasm via CRM-1. The significance of viral protein nuclear import and export and its relationship with infection establishment is highlighted, emphasizing the development of new host-directed antiviral therapeutic strategies.
KEYWORDS: viral proteases, flavivirus, ZIKV, nuclear localization signal, nuclear export signal, NLS, NES, nuclear export, nuclear localization
INTRODUCTION
Zika virus (ZIKV) is an emerging arbovirus in multiple countries around the world and is associated with microcephaly and Guillain-Barré syndrome cases (1, 2). ZIKV belongs to the Flaviviridae family and contains a single-stranded, positive-sense RNA genome that encodes a polyprotein that gives rise to three structural proteins (C, E, and prM) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) involved in viral morphogenesis and replication (3–6). NS3 and its cofactor NS2B are indispensable for processing the viral polyprotein and replication because NS3 has both protease and helicase functions. Due to its important role in viral replication, NS3 has been explored as a pharmacological target against ZIKV (7–9).
Although ZIKV has a cytoplasmic replication cycle, some viral proteins, such as C, NS1, and NS5, which contain nuclear location signals (NLSs), have also been observed in the nuclei of Vero cells (10–12). C, NS4B, and NS5 proteins of other Flaviviruses, such as Japanese encephalitis virus (JEV), West Nile virus (WNV), and Dengue virus (DENV), have been observed in the nuclei of infected cells (13). Although little is known about the role that these proteins play in the nucleus, mutations in the NLSs of C and NS5 proteins, which impede nuclear localization, reduce the production of viral progeny (13–18).
Nuclear import and export of proteins with molecular weights greater than 40 to 50 kDa are regulated by the activity of nuclear transport receptors (NTRs), such as importins, exportins, transporters, and small GTPases of the Ran family that transport cargo molecules through nuclear pore complexes (19, 20).
Both ZIKV proteins C and NS5 are imported into the nucleus through the classical importin-α/β pathway and are exported through the CRM-1 exportin pathway. Importin-α recognizes specific NLSs containing repeated amino acids of arginine (Arg or R) and lysine (Lys or K) in both C and NS5 (21, 22). However, nuclear export sequences (NESs), recognized by the exportin CRM-1, are composed of leucine-rich sequences of hydrophobic amino acids, such as valine (Val), isoleucine (Ile), phenylalanine (Phe), or methionine (Met), which are found in conserved motifs in cargo and viral proteins (23–25).
During infection of Vero cells with ZIKV, NS3 protease localizes in the perinucleus and affects the morphology of the nuclear lamina, a component of the nuclear envelope, forming extrusion sites in the nucleus that may affect the function of the centrosome (11). Moreover, NS3 is accumulated in the concave face of the kidney-shaped altered nuclei and can alter other components of the nuclear envelope (26). Recently, our research group described that NS3 participates in the processing of some nucleoporins, components of the nuclear pore complex (27).
Our research group recently described the presence of DENV NS3 in the nuclei of Huh-7 cells at early times of viral infection (8 and 12 h) (28) and have also reported the presence of DENV NS3 in the nuclei of C6/36 mosquito cells (29). Therefore, this work aims to determine whether ZIKV NS3 can localize in the nuclei of Huh-7 cells and which pathways are involved in this process.
RESULTS
ZIKV NS3 protein localizes in the nuclei of Huh-7 cells early in infection and in the cytoplasm at later times postinfection.
Flaviviruses have a cytoplasmic replication cycle and translate and replicate their genomes in the endoplasmic reticulum (ER) (30). However, some viral proteins, such as C, NS1, and NS5, contain nuclear localization signals (NLSs) and are also present in the nuclei of the infected cells (10–12). Our group has described that DENV NS3 protein localizes in the nuclei of C636 mosquito cells, and, more recently, we reported that DENV NS3 localizes early in infection in the nuclei of Huh-7 hepatocarcinoma cells (28, 29). However, no information regarding the subcellular localization of ZIKV NS3 protein has been described.
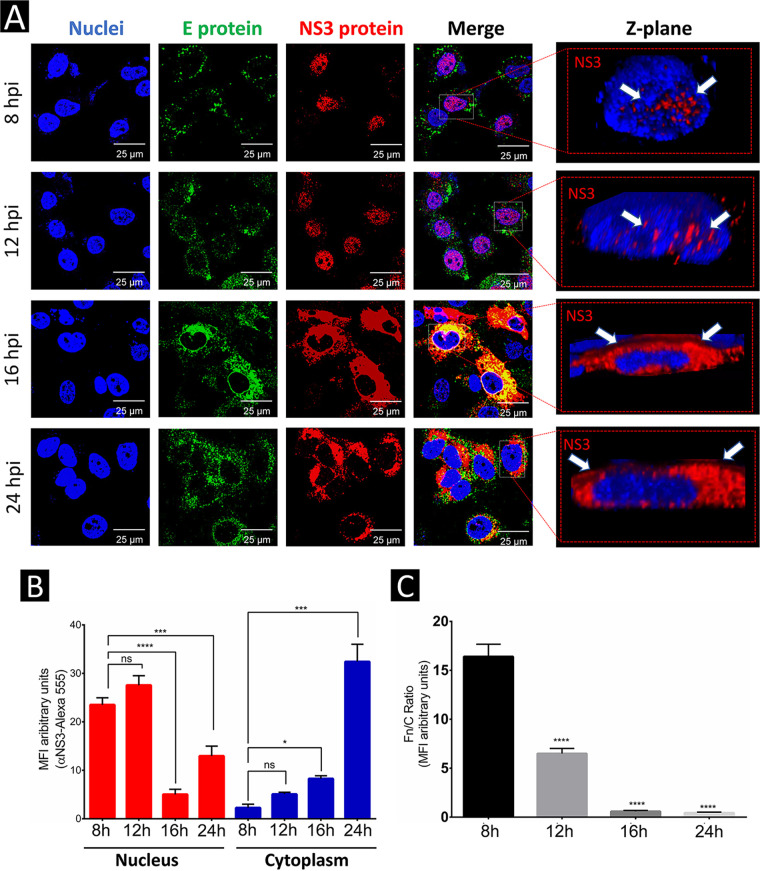
To study the subcellular localization of ZIKV NS3 protein, Huh-7 cells were infected with ZIKV at a multiplicity of infection (MOI) of 3 and were analyzed by indirect immunofluorescence (IIF) and confocal microscopy at 8, 12, 16, and 24 h postinfection (hpi). As in DENV infection, NS3 protein from ZIKV was predominantly observed in the nuclei and to a lesser extent in the cytoplasm of Huh-7 cells at 8 and 12 hpi (Fig. 1A). No significant differences in the amount of nuclear NS3 protein at these two times postinfection were observed (Fig. 1B).
FIG 1.
ZIKV NS3 protein localizes in the nucleus early in infection and in the cytoplasm at later times postinfection in Huh-7 cells. (A) The localization of NS3 and E proteins in mock-infected or ZIKV-infected Huh7 cells at 8, 12, 16, and 24 h postinfection (hpi) was analyzed by confocal microscopy using anti-NS3 (red) and anti-E antibodies (green) and Hoechst staining of nuclei (blue). To determine the nuclear localization of ZIKV NS3, clipped three-dimensional images (yz clip 3D images) were analyzed, as mentioned in the Materials and Methods. (B and C) The MFI (B) was determined for selected regions of interest in the nucleus and cytoplasm (30 cells per condition), and the Fn/Fc ratio (C) was determined for the selected regions in each immunofluorescence confocal microscopy image; *, P = 0.05; **, P = 0.001; ***, P = 0.0001; ****, P < 0.0001.
In contrast to what was observed earlier, at 16 and 24 hpi, the localization of ZIKV NS3 protein was predominantly in the cytoplasm and to a much lesser extent in the nucleus (Fig. 1A and B). These results were confirmed by a decrease in the fluorescence ratio between nuclear and cytoplasmic levels of NS3 (Fn/Fc) (Fig. 1C).
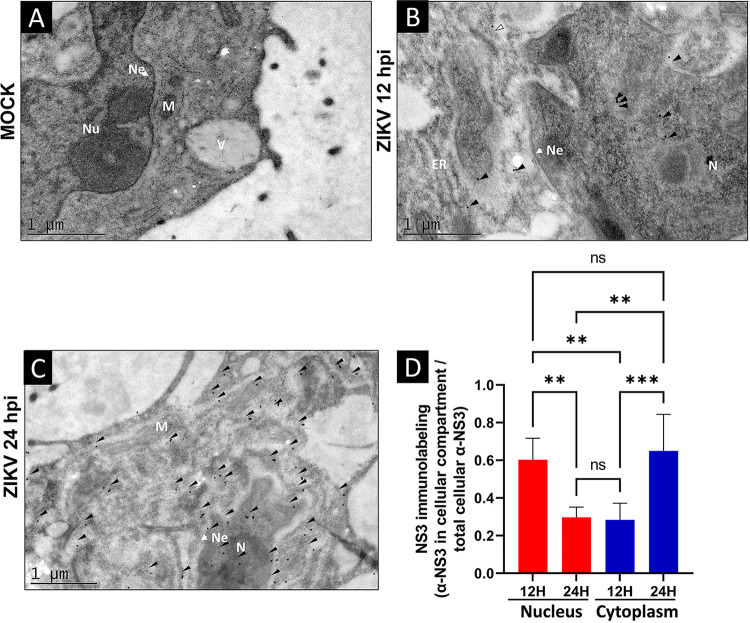
To further confirm the subcellular localization of ZIKV NS3 protein at 12 and 24 hpi, transmission electron microscopy (TEM) assays were performed using specific anti-NS3 antibodies. In agreement with the results shown in Fig. 1, NS3 protein was observed predominantly in the nuclei (N) and to a lesser extent in the cytoplasm (C) at 12 hpi (Fig. 2B and D). However, at 24 hpi, NS3 was observed mainly in the cytoplasm and to a lesser extent in the nuclei (Fig. 2C and D). As expected, some alterations in the ER, such as distended cisterns, were also observed in cells infected with ZIKV (Fig. 2B and C), in contrast to uninfected cells (Fig. 2A).
FIG 2.
The ZIKV NS3 protein is present in the nucleus of Huh7 cells during ZIKV infection. (A to C) Mock-infected (A) or ZIKV-infected Huh7 cells were fixed and processed for TIM using an anti-NS3 antibody at 12 hpi (B) and at 24 hpi (C). (D) NS3 immunolabeling in arbitrary units was determined by selected regions of interest in the nucleus and cytoplasm (6 cells per condition). Anti-NS3 in the cellular compartment was divided by the total cellular anti-NS3 of each condition. The NS3-specific tagging signal is shown as dark dots and is indicated by an arrowhead; Ne, nuclear envelope; N, nucleus; ER, endoplasmic reticulum; M, mitochondria; N, XYZ; ns, not significant; *, P = 0.05; **, P = 0.001; ***, P = 0.0001; ****, P < 0.0001.
Ivermectin (IVM) and leptomycin B (LMB) inhibit nuclear import and export of ZIKV NS3 in Huh-7 cells.
The C and NS5 proteins from ZIKV use the classical nuclear import (importin-α/β) and export (CRM-1) pathways to be imported and exported from the nucleus. IVM is a specific drug used to inhibit the classical import pathway (importin-α/β); thus, it has been widely used to determine which proteins use this pathway for internalization into the nucleus. However, LMB is a specific inhibitor of the classical nuclear export pathway (CRM-1) and was previously used to study the nuclear export of C, NS5, and other proteins (31–33).
Thus, to analyze the participation of both pathways in the import and export of NS3 protein in infected cells, IVM and LMB were used. To determine the cell viability of Huh-7 cells in the presence of both drugs, we tested the concentrations used previously to analyze the import and export of ZIKV NS5 protein (31); therefore, concentrations of 12, 25, 50, 75, and 100 μM IVM and 5, 10, and 15 ng/mL LMB were tested. No significant differences in cell viability were observed at the different concentrations tested or in the cells treated with respective vehicle controls (dimethyl sulfoxide [DMSO] and ethanol) (Fig. S1A and B in the supplemental material).
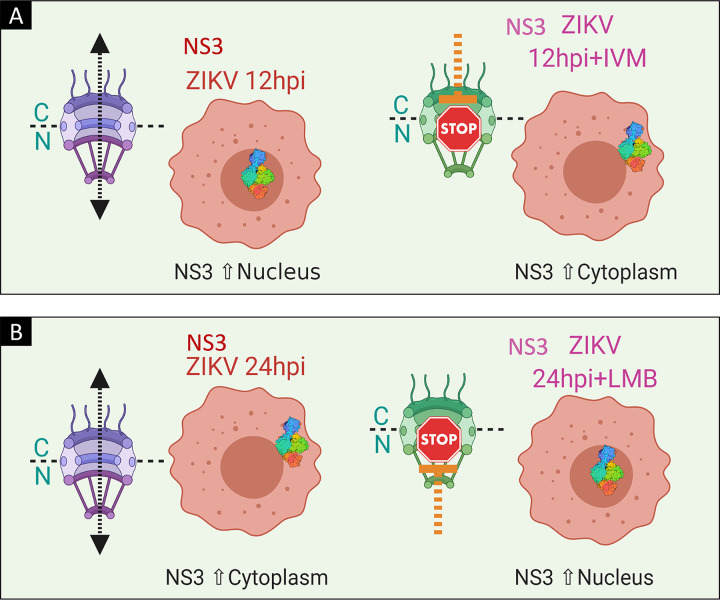
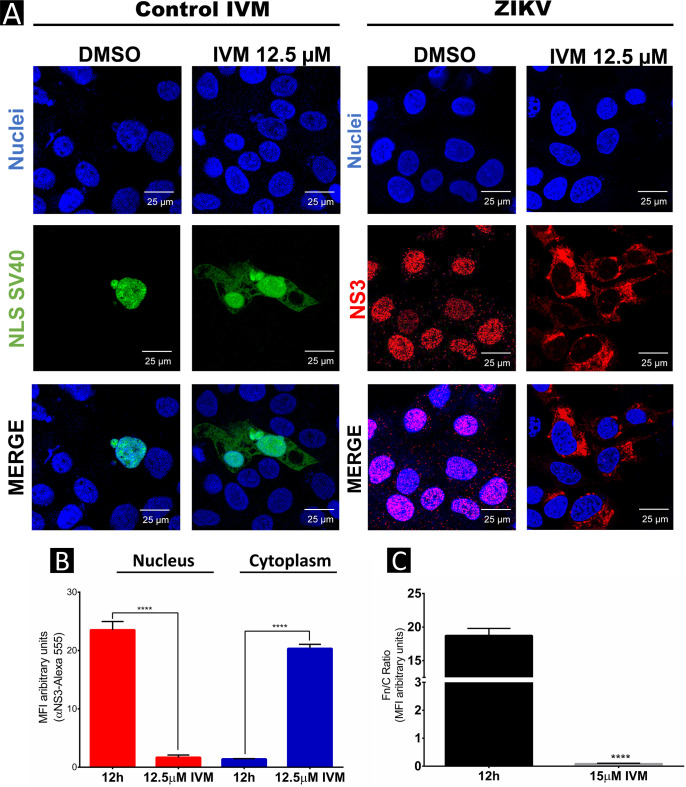
Once we analyzed cell viability in the presence of both drugs, the subcellular localization of ZIKV NS3 was analyzed. Because our previous experiments indicated that at 12 hpi, the ZIKV NS3 protein is in the nucleus, if NS3 uses the importin-α/β pathway, IVM would inhibit NS3 import (Fig. 3A). Therefore, Huh-7 cells were infected with ZIKV at an MOI of 3 for 2 h, virus was removed, and culture medium with IVM at a concentration of 12.5 μM was added for 12 hpi. As a positive control, Huh-7 cells were transfected with the NLS-SV40-tetraGFP plasmid, which contains the NLS of the SV40 T antigen coupled to tetra-green fluorescent protein (tetra-GFP), and were treated with IVM for 24 h. Cellular localization of ZIKV NS3 was analyzed by confocal microscopy using an anti-NS3 antibody. As a control for effects of IVM on the import pathway, an anti-importin-α (anti-Imp-α) antibody was used.
FIG 3.
Schematic of the localization of the ZIKV NS3 protein during inhibition of the importin-α and exportin CRM-1 pathways using IVM and LMB, respectively. (A) Localization of NS3 at 12 hpi and during importin-α inhibition with IVM. (B) NS3 localization at 24 hpi and during CRM-1 exportin inhibition with LMB.
As expected, localization of NLS-SV40-tetraGFP was observed mainly in the nuclei of transfected cells; however, after treatment with IVM, NLS-SV40-tetraGFP was present in the nuclei and the cytoplasm, indicating that IVM was able to inhibit this pathway. In the presence of IVM, the subcellular localization of ZIKV NS3 protein at 12 hpi was also affected because its location was observed mainly in the cytoplasm, in contrast to the infected, non-treated cells, where NS3 was observed in the nuclei (Fig. 4A and B), indicating that NS3 is imported to the nucleus by the importin-α/β pathway. These results were confirmed by a decrease in the nuclear fluorescence/cytoplasm fluorescence (Fn/Fc) ratio of NS3 in IVM-treated and untreated cells (Fig. 4C).
FIG 4.
Ivermectin inhibits nuclear import of ZIKV NS3 protein in Huh-7 cells. (A) Localization of NS3 and NLS-SV40-tetraGFP proteins in untreated or IVM-treated Huh7 cells was analyzed by confocal microscopy using anti-NS3 (red) and Hoechst staining of nuclei (blue). To determine the nuclear localization of ZIKV NS3 at 12 hpi, three-dimensional clipped images (yz clip 3D images) were analyzed, as mentioned in the Materials and Methods. (B) MFI was determined for selected regions of interest in the nucleus and cytoplasm (30 cells per condition). (C) The Fn/Fc ratio was determined for the latter selected regions of interest in each immunofluorescence confocal microscopy image in the different conditions; *, P = 0.05; **, P = 0.001; ***, P = 0.0001; ****, P < 0.0001.
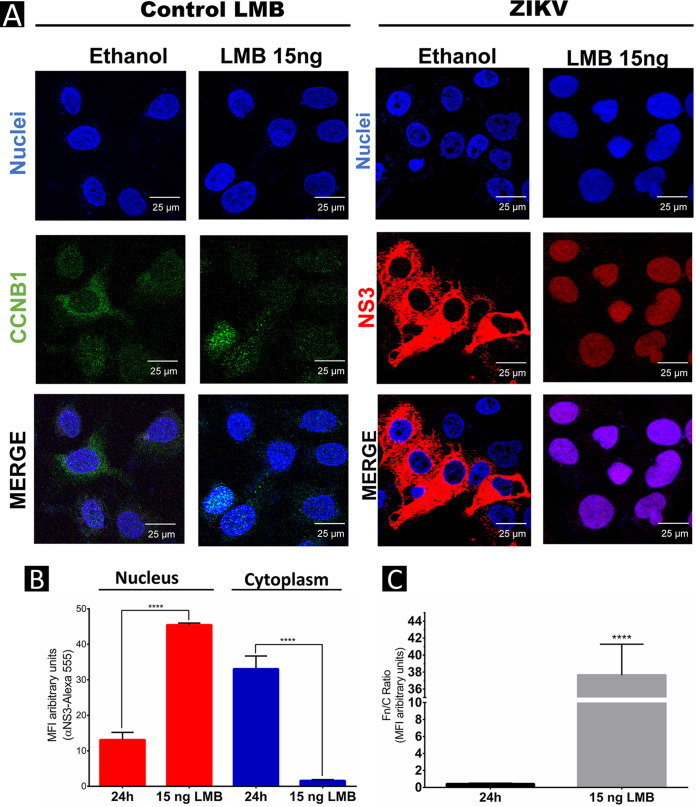
Because our previous experiments showed that at 24 hpi, the ZIKV NS3 protein is located mainly in the cytoplasm of the infected cells, to analyze if this protein is exported by the CRM-1 pathway, infected cells were treated with LMB (Fig. 3B). Huh-7 cells were infected with ZIKV at an MOI of 3 for 2 h, virus was removed, and fresh medium with LMB at a concentration of 15 ng/mL was added, as previously reported (32). The subcellular localization of ZIKV NS3 was analyzed at 24 hpi by IIF using an anti-NS3 antibody. As a control for LMB activity on the export pathway, subcellular localization of the CCNB1 protein, which uses the CRM-1 pathway for its export, was also assessed.
As expected, in the mock LMB-untreated cells, CCNB1 was observed in the cytoplasm and perinuclear area, while in the mock LMB-treated cells, CCNB1 was observed mainly in the nuclei (Fig. 5A), confirming that LMB was inhibiting the CRM-1 export pathway. As observed before, the subcellular localization of the ZIKV NS3 protein at 24 hpi was in the cytoplasm; however, increasing concentrations of LMB caused its retention in the nucleus, indicating that this protein uses the CRM-1 nuclear export pathway to relocate from the nucleus to the cytoplasm at late times postinfection (Fig. 5A and B).
FIG 5.
Leptomycin B inhibits nuclear export of ZIKV NS3 in Huh-7 cells. (A) Localization of NS3 and CCNB1 proteins in mock-infected, LMB-treated, or ZIKV-infected Huh7 cells at 24 h postinfection (hpi) was analyzed by confocal microscopy using anti-NS3 (red) and anti-CCNB1 antibodies (green) and Hoechst staining of nuclei (blue). To confirm the nuclear localization of ZIKV NS3 protein, three-dimensional clipped images (yz clip 3D images) were analyzed, as mentioned in the Materials and Methods. (B) MFI was determined for selected regions of interest in the nucleus and cytoplasm (30 cells per condition). (C) The Fn/Fc ratio was determined for these latter selected regions in each immunofluorescence confocal microscopy image in the different conditions; *, P = 0.05; **, P = 0.001; ***, P = 0.0001; ****, P < 0.0001.
This increase in nuclear localization of NS3 protein in the presence of LMB was quantified, and significant differences in NS3 nuclear and cytoplasmic localization between untreated and LMB-treated cells were observed (Fig. 5B). These results were confirmed by the increase in Fn/Fc ratio of NS3 protein in LMB-treated and untreated cells (Fig. 5C).
In silico prediction of nuclear localization signals (NLSs) and nuclear export signals (NESs) in ZIKV NS3 protein.
Importin-α/β-directed transport requires specific sequences that are recognized in the cargo proteins that cross the nuclear membrane from the cytoplasm. These NLSs contain repeated amino acids of arginine (Arg or R) and lysine (Lys or K) as the monopartite classic NLS reported in the SV40 large T antigen, which consists of the five amino acids KKKRK. Furthermore, some proteins possess a bipartite NLS composed of two groups of basic amino acids and separated by approximately 10 amino acids (21, 22).
To determine if the ZIKV NS3 protein contains an NLS, an in silico analysis using NLS predictors, such as cNLS Mapper, Wregex, and Prosite, was performed (34–36). One monopartite (210 REAIKKRLRTV) and three bipartite (169 RREEETPVECFEPSMLKKQL, 202 RRVLPEIVREAIKKRLR, and 583 RHGEKRVLKPRWMDARVCSDHAALKSFKE) NLSs were predicted in the ZIKV NS3 protein. Interestingly, the monopartite NLS 210 is contained in the bipartite NLS 202. Therefore, this sequence could function as a monopartite or bipartite NLS (Fig. 6A to D).
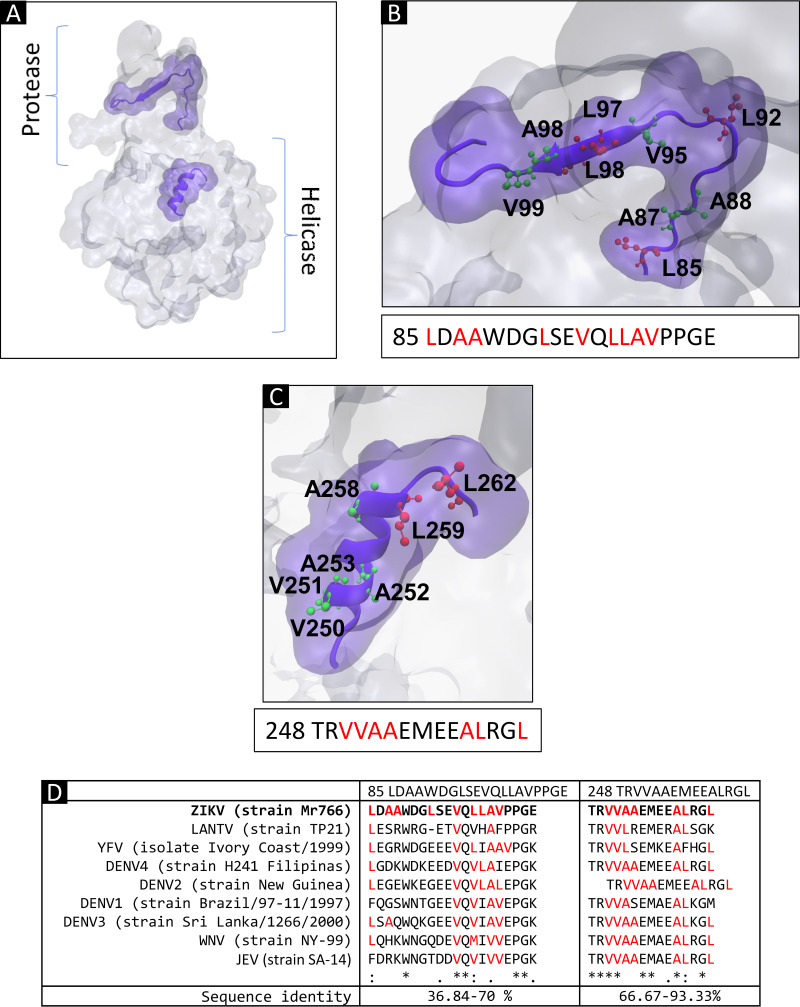
FIG 6.
In silico prediction of nuclear localization signals (NLSs) in ZIKV NS3 protein. (A) Three-dimensional structure of NS3 showing the location of the putative NLS identified with the cNLS Mapper software and WREGEX. (B) Structure and sequence of putative NLS 210. (C) Putative NLS 169. (D) Putative NLS 583. (E) ClustalW alignment of putative NLSs from other flaviviruses.
Nuclear localization of the NS3 protein has been described in other members of the Flaviviridae family, such as hepatitis C virus (HCV) (37, 38), JEV, WNV (39), and DENV (28, 29). Thus, the degree of conservation of these sequences in different members of the Flavivirus genus was analyzed. Sequence alignment performed with ClustalW2 (40) revealed a high degree of conservation of the monopartite/bipartite NLS (202/210), with an identity of 41.18 to 76.47%, and the bipartite NLS 583, with an identity of 27.59 to 89.66%, in the different members of the Flavivirus genus analyzed (Fig. 6E).
However, NESs are composed of sequences rich in leucine or hydrophobic amino acids, such as valine (Val or V), isoleucine (Ile or I), phenylalanine (Phe or F), or methionine (Met or M) (23–25). To identify these sequences in the ZIKV NS3 protein in silico, the NES predictors LocNES and NetNES were used (41, 42). Two probable NESs (85 LDAAWDGLSEVQLLAVPPGE and 248 TRVVAAEMEEALRGL) were identified (Fig. 7A to C). Analysis of the degree of conservation of these sequences in the different members of the Flavivirus genus found that NES 248 was the sequence with the highest sequence identity (66.67 to 93.33%) (Fig. 7D).
FIG 7.
In silico prediction of nuclear export signals (NESs) in ZIKV NS3 protein. (A) Three-dimensional structure of NS3 showing the location of the putative NES identified with LocNES and WREGEX software. (B) Structure and sequence of putative NES 85. (C) Putative NES 248. (D) ClustalW alignment of putative NESs of different flaviviruses.
Molecular docking analysis of the interaction between importin-α and the NLS of the ZIKV NS3 protein.
Once a putative monopartite (210 REAIKKRLRTV) and three putative bipartite (169 RREEETPVECFEPSMLKKQL, 202 RRVLPEIVREAIKKRLR, and 583 RHGEKRVLKPRWMDARVCSDHAALKSFKE) NLSs were identified in silico, we wanted to determine if they were responsible for the nuclear import of ZIKV NS3 via the importin-α/β pathway. Thus, a molecular docking assay was performed using importin-α and ZIKV NS3.
Structural modeling of the ZIKV NS3 protein was performed because the protease/helicase region is not reported in databases such as the Protein Data Bank (PDB). The structural homology modeling servers I-TASSER, RaptorX, and Swiss-Model were used to model the ZIKV NS3 protein, taking DENV-4 NS3 protein (PDB ID: 2WHX) as a template (43). By the Ramachandran graph, different modeled structures of NS3 were evaluated using the SAVES v6.0 server (44). According to the percentage of residues in the allowed areas of the graph, we chose the model with the highest percentage (92%) of residues in the permitted areas of the Ramachandran plot, obtained with Swiss-Model (data not shown). This model was used in the molecular docking test.
For the molecular docking test, we used the HDOCK server (45). ZIKV NS5 NLS (RKRPRV), SV40 large T antigen NLS (PKKKRKV), and HAT-KAT8 NLS (RNQKRKHDEI) sequences were used as positive controls that bind to importin-α, and ATP synthase-alpha (located in mitochondria) and Fas-L member 6 (located in the cell membrane) were used as negative controls. We found that the docking scores (DSs) obtained from the positive controls ranged between −199.21 and −215.02 kcal/mol between the different NLSs and importin-α, and the DSs obtained for the negative controls ranged between −72 and −86 kcal/mol (Fig. S2).
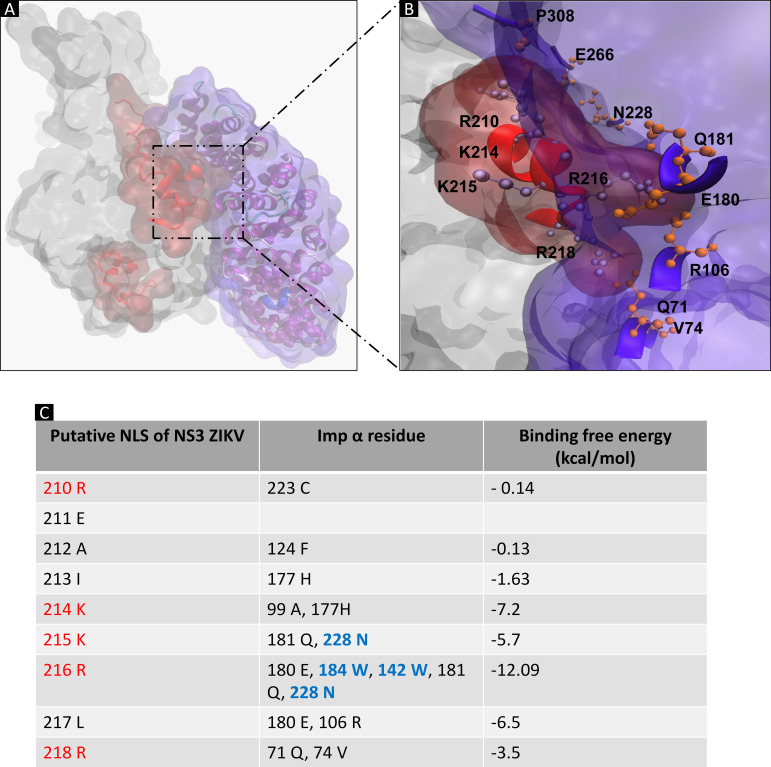
Once the ability of the server to predict the specific binding between the different NLSs and importin-α was corroborated, the putative NLS responsible for the nuclear import of ZIKV NS3 protein by the importin-α/β pathway was determined by molecular docking assay by using importin-α (PDB ID: 5W41) and the ZIKV NS3 protein, as previously modeled. Importin-α is composed of two domains, an autoinhibitory N-terminal domain called importin-β binding (IBB), and a C-terminal arm domain (armadillo), which recognizes and binds to the NLSs of the cargo proteins (46). The ZIKV NS3 protein binds to the armadillo region of importin-α with a DS of −213.21 kcal/mol binding energy as in the previous controls (Fig. 8A and B).
FIG 8.
Molecular docking of NS3 ZIKV-importin-α. (A) Molecular docking of NS3 ZIKV-importin-α. (B) Binding of the armadillo domains of importin-α and the putative NLS 210 of the ZIKV NS3 protein. Molecular docking analysis was performed using HDOCK software. The 3D protein structure of importin-α used was PDB ID 4WV6. (C) MM/GBSA analysis of the binding of the armadillo domains of importin-α and the putative NLS 210 of the ZIKV NS3 protein, predicting the free energy of binding of the protein-protein complex for each residue using HawkDock software.
Next, an molecular mechanics with generalised Born and surface area solvation (MM/GBSA) analysis with the HawkDock server (47), which predicts the free energy of a protein-protein complex for each residue, was performed. We found a high binding energy at residues 210 to 218 (REAIKKRLR) of ZIKV NS3 protein (Fig. 8C), a region previously predicted as a putative NLS (NLS 210). Interestingly, residues K214, K215, and R216 provided the highest energy between NS3 and importin-α.
A mutation on the putative NLS 210 from ZIKV NS3 protein prevents its nuclear localization.
To determine if an alteration of the NLS could affect the import of ZIKV NS3 protein to the nucleus, we first analyzed its subcellular location during transfection at 8, 12, 16, 24, and 48 h posttransfection (hpt) by confocal microscopy.
No differences in NS2B3-wild type (WT) subcellular localization at 8, 12, 16, and 24 hpt were found, because the expressed protein was located mainly in the cytoplasm and, to a lesser extent, in the nuclei of transfected cells. However, a significant amount of NS2B3-WT in the nucleus was observed at 48 hpt; thus, the nuclear import and export assays were performed at this time posttransfection (Fig. S3).
To determine the role of amino acids KKR in the import of the ZIKV NS3 protein, site-directed mutagenesis of the plasmid NS2B3-210NLS (kindly donated by Jonathan Ball, University of Nottingham, UK) containing the NS2B3 sequence from ZIKV was performed using the Q5 site-directed mutagenesis kit (NEB). Residues K214, K215, and R216, which provided the highest binding energy between NS3 and importin-α proteins, were changed to A214, A215, and A216 by direct mutagenesis, and the plasmid NS2B3-210NLS-Mut was obtained. The oligonucleotides containing the REAIAAALR mutation were designed following the manufacturer’s instructions (Fig. S4).
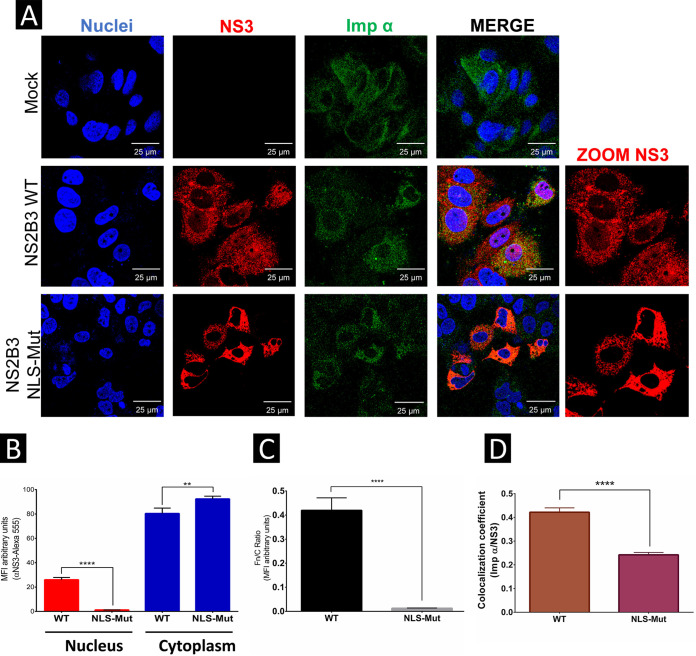
Huh-7 cells were independently transfected with the NS2B3-WT and NS2B3-210NLS-Mut plasmids, and the subcellular localization of ZIKV NS3 protein was analyzed at 48 h posttransfection by confocal microscopy. NS2B3-WT was located mainly in the cytoplasm and to a lesser extent in the nuclei of transfected cells, while NS2B3-210NLS-Mut was observed only in the cytoplasm (Fig. 9A and B). The Fn/Fc ratio indicated a more significant amount of nuclear NS3 protein in cells transfected with NS2B3-WT than in cells transfected with NS2B3-210NLS-Mut (Fig. 9C). Pearson’s correlation coefficient analysis indicated a positive correlation between importin-α and NS2B3-WT but not NS2B3-210NLS-Mut (Fig. 9D), indicating that the NLS 210 region is involved in nuclear localization of the NS3 protein.
FIG 9.
Mutation in the putative NLS 210 of the ZIKV NS3 protein prevents its nuclear localization. (A) Localization of mutated NS2B3-WT or NLS 210 and importin-α proteins in Huh7 cells was analyzed 48 h posttransfection by confocal microscopy using anti-NS3 (red) and anti-importin-α (green) antibodies and Hoechst staining of nuclei (blue). To determine the nuclear localization of ZIKV NS3, three-dimensional clipped images (yz clip 3D images) were analyzed, as mentioned in the Materials and Methods. (B) MFI was determined for selected regions of interest in the nucleus and cytoplasm (30 cells per condition). (C) We determined the Fn/Fc ratio for these regions of interest in each immunofluorescence confocal microscopy image. (D) Image analysis results presented as the mean and SEM of the Person’s correlation coefficients (30 cells at each time point); *, P = 0.05; **, P = 0.001; ***, P = 0.0001; ****, P < 0.0001.
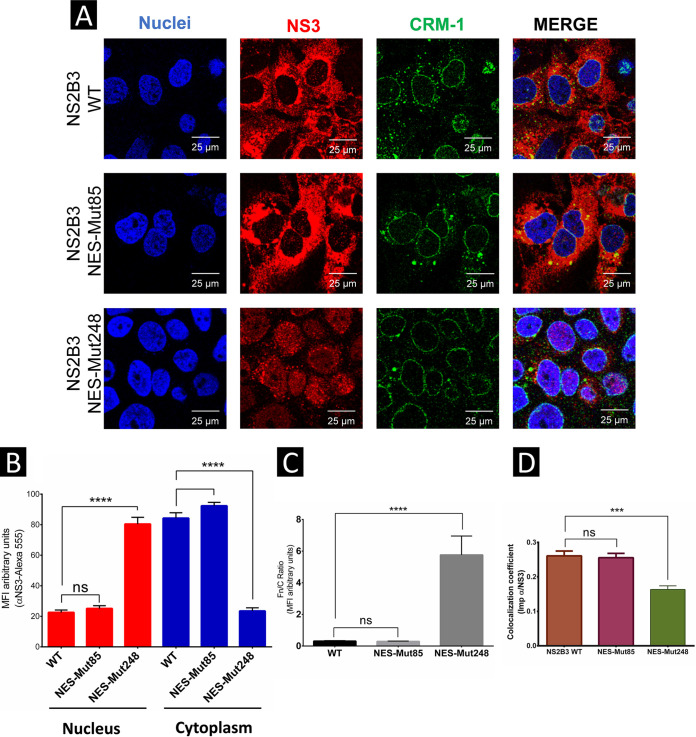
Putative NES 248 deletion of the ZIKV NS3 protein retains NS3 in the nucleus.
Although we could not predict the binding of CRM-1 exportin to ZIKV NS3 by molecular docking analysis due to its structural complexity in its repeated domains, the deletion of both putative NESs (85 or 248) in the NS2B3 sequence was performed by direct mutagenesis to determine which sequence participates in the nuclear export of ZIKV NS3. Oligonucleotides containing the deletion of putative NESs 85 or 248 were designed following the manufacturer’s instructions (Fig. S5).
Huh-7 cells were independently transfected with NS2B3-WT, NS2B3-NES-Mut85, or NES-Mut248 plasmids, and the subcellular localization of ZIKV NS3 protein was analyzed 48 hpt by confocal microscopy. Both NS2B3-WT and NES-Mut85 were localized mainly in the cytoplasm and to a lesser extent in the nuclei of transfected cells, while NS2B3 NES-Mut248 was found mainly in the cell nuclei and to a lesser extent in the cytoplasm compared to NS2B3-WT (Fig. 10A and B).
FIG 10.
Putative NES 248 deletion of the ZIKV NS3 protein retains NS3 in the nucleus. (A) Localization of NS2B3-WT, NES-Mut85, or NES-248 proteins and CRM-1 exportin in Huh7 cells was analyzed 48 h posttransfection by confocal microscopy using anti-NS3 (red) and anti-CRM-1 (green) and Hoechst staining of nuclei (blue). To determine the nuclear localization of ZIKV NS3, three-dimensional clipped images (yz clip 3D images) were analyzed, as mentioned in the Materials and Methods. (B) MFI was determined for selected regions of interest in the nucleus and cytoplasm (30 cells per condition). (C) We determined the Fn/Fc ratio for these regions of interest in each immunofluorescence confocal microscopy image. (D) Image analysis results presented as the mean and SEM of the Person’s correlation coefficients (30 cells at each time point); *, P = 0.05; **, P = 0.001; ***, P = 0.0001; ****, P < 0.0001.
The Fn/Fc ratio indicated a significantly greater amount of nuclear NS3 protein in cells transfected with NS2B3-NES-Mut248 than in cells transfected with NS2B3-WT and NES-Mut85 (Fig. 10C), indicating that region NES 248 participates in the nuclear export of NS3. However, the Pearson correlation coefficient for colocalization between CMR-1 and NES-Mut248 (r = 0.163 ± 0.01) was significantly lower than those coefficients calculated for colocalization between CRM-1 and NS2B3-WT and between CRM-1 and NES-Mut85 (r = 0.261 ± 0.01 and r = 0.255 ± 0.012, respectively) (Fig. 10D), corroborating that NES 248 participates in the binding of NS3 with the exportin CRM-1 and its nuclear export.
DISCUSSION
Although flavivirus replication takes place in the endoplasmic reticulum, the migration of viral proteins to the nucleus is common and is required in several viral replicative cycles (13, 48). The C protein, which participates in viral assembly and encapsidation, and the NS5 protein, which has methyltransferase and RNA-dependent RNA polymerase activities, are two of the proteins known to migrate to the nucleus during flavivirus infection (15, 29).
Although the function of the C protein in the nucleus is not yet fully understood, mutations in its NLS, which prevents its translocation to the nucleus, reduces viral progeny production, indicating the relevance of its nuclear presence. Import of the C protein to the nucleus occurs via the classical pathway through importin-α/β (16, 49, 50).
However, the NS5 polymerase, the largest and most conserved protein among flaviviruses, contains a conserved NLS whose integrity is involved in the formation of virions. Due to its large size (105 kDa), NS5 requires the active importin-α/β pathway to locate to the nucleus. Inhibition of this pathway with ivermectin reduces the formation of virions, highlighting the importance of the nucleus in flavivirus replication. In addition, inhibition of the CRM-1 export pathway with leptomycin B causes the accumulation of NS5 in the nucleus, increasing viral yield (15, 32, 51).
Shuttling of viral and nuclear proteins between the nucleus and the cytoplasm requires interaction with the nuclear envelope, which is composed of the nuclear lamina, a lipid bilayer, and the nuclear pore complexes (13, 27, 52). NS3 and its cofactor NS2B of ZIKV have a trypsin-like protease activity (53), which is responsible for the processing of the polyprotein that gives rise to the mature viral proteins. Moreover, like other viral proteases, NS3 participates in establishment of infection, altering components of the nuclear envelope, such as the nuclear lamina, which might be required for efficient viral replication and timely evasion of the immune response (11).
We have recently described that ZIKV and DENV NS3 proteins alter the integrity of nuclear pore complexes by processing some nucleoporins (27). Considering its role in the degradation of components of the nucleus, we analyzed whether ZIKV NS3 protein can be imported into the nucleus and found that at early times postinfection (8 and 12 hpi), it is present in the nuclei of infected cells. However, at 16 and 24 hpi, we found that ZIKV NS3 is in the cytoplasm of infected cells (Fig. 1A to C and 2B and C). These results are in agreement with our previous findings demonstrating the presence of DENV NS3 in the nuclei of C6/36 mosquito and human Huh-7 cells (28, 29, 54, 55).
Considering the presence of NS3 protein in the nucleus at early times postinfection with ZIKV, we analyzed the mechanism by which this protein is imported and exported from the nucleus. Other studies have described the interaction between the NS3 protein of other flaviviruses (e.g., ZIKV, DENV, WNV, JEV, and tick-borne encephalitis virus [TBEV]) and exportin CRM-1 and importin-β, required for the transport trimer (cargo-importin-α/β). However, these interactions were determined from high-throughput proteomics analyses aimed at identifying interactions between human host proteins and flavivirus NS3 and NS5 proteins (56). Given this, the participation of both importin-α/β and CRM-1 pathways in NS3 protein import and export from nuclei was analyzed using specific inhibitors such as IVM and LMB, respectively (Fig. 3A and B). Inhibition of the importin-α/β pathway with IVM prevented the nuclear import of NS3 protein at 12 hpi (Fig. 4A to C), and inhibition of the exportin CRM-1 pathway with LMB impeded the nuclear export of NS3 protein at 24 hpi (Fig. 5A to C), indicating that ZIKV NS3 protein uses these two classical pathways, as has been reported for other ZIKV proteins such as NS5 and C (15, 16, 29, 49, 50), highlighting the importance of nuclear-cytoplasmic trafficking during the establishment of infection.
It is well known that nuclear import of proteins through the importin-α/β pathway requires the presence of NLSs, while export by CRM-1 requires the presence of NESs. For this reason, the presence of putative NLSs and NESs in the amino acid sequence of ZIKV NS3 was analyzed (11). Our in silico results indicated the presence of three putative NLSs (Fig. 6A to D) and two putative NESs (Fig. 7A to C). Furthermore, sequence alignment of different NS3 proteins demonstrated that these NLSs and NESs are conserved in other flavivirus members (Fig. 6E and 7D). Nuclear localization of the NS3 protein of other flaviviruses, such as DENV, Japanese encephalitis virus, Langat virus (LANTV), and HCV, (38, 54) support these results.
The relevance of these sequences in the interaction between ZIKV NS3 protein and the armadillo domains of importin-α (KPNA2), which are responsible for recognizing the cargo protein and subsequent import into the nucleus, were predicted by molecular docking (22). Interestingly, one of the portions of NS3 that binds importin-α is the putative NLS 210 REAIKKRLRTV (Fig. 8A to C), previously described by others (11).
The functional role of NLS 210 in the nuclear import of NS3 protein was demonstrated by mutagenesis assays. Substitution of the KKR amino acid sequence by AAA (alanine triplet) prevented nuclear import of NS3, indicating that NLS 210 is the sequence involved in import of NS3 to the nucleus (Fig. 9A to D).
Although we could not predict CRM-1 binding to ZIKV NS3 by molecular docking analysis due to the complexity of the CRM-1 exportin, the role of NES 248 in nuclear export of NS3 was demonstrated by direct mutagenesis assays. The 15-amino-acid deletion (TRVVAAEMEEALRGL) containing NES 248 prevented nuclear export of the ZIKV NS3 protein, indicating its role in NS3 export to the cytoplasm (Fig. 10A to D).
Taken together, our results demonstrate that ZIKV NS3 protein contains both an NLS and an NES involved in the nuclear import and export of the protein during infection. However, we do not know what other elements are involved in the subcellular localization of NS3 protein during the viral replicative cycle. It is possible that conformational changes of the NS3 protein, phosphorylation levels, or its association with other proteins could determine its location in specific moments of the replicative cycle. Conformational changes in NS3 domains in the presence of genomic RNA (57–59) and during the formation of the replicase complex (NS2B-NS3-NS5-NS4B) (30, 60) have been reported. Conformational changes in NS3 protein also occur after NS2B-NS3 transfection, where the NS3 protease domain is activated by binding the NS2B cofactor (61, 62). Moreover, NS2B is also involved in the nuclear presence of NS3 protein in transfected cells (11). Therefore, the conformational changes in NS3 protein caused by NS2B could be required for its localization in the nucleus. The roles of NS3 conformational changes, phosphorylation levels, and its interaction with other proteins in its subcellular localization require further analysis.
Our findings using inhibitors of nuclear import and export indicate that the nuclear presence of ZIKV NS3, like other viral proteins such as NS5, NS1, and C of several flaviviruses (31, 33, 63, 64), is crucial for viral replication. During DENV infection, LMB treatment inhibits nuclear export via CRM-1 of NS5, resulting in its accumulation in the nucleus and an increase of virion production up to 30 h postinfection. However, how this treatment favors viral production has not been investigated. On the other hand, during treatment for DENV infection with LMB, the suppression of interleukin-8 (IL-8) induction is dependent on CRM-1 (32). Because IL-8 is related to antiviral activity (65), treatment with LMB may inhibit the export of mRNAs that encode proteins with antiviral activity, favoring viral replication, and may be a mechanism of immune evasion.
Therefore, new therapeutic strategies to inhibit viral replication and virus production should be focused on the inhibition of viral proteins imported to the nucleus.
MATERIALS AND METHODS
Cell culture and virus.
The Huh-7 human hepatoma cell line (kindly donated by Rivas from Universidad Autonoma de Nuevo Leon) was cultured in advanced Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM glutamine, penicillin (5 × 104 U/mL), streptomycin (50 μg/mL), 1 mL/L of amphotericin B (Fungizone), and 8% fetal bovine serum (FBS) at 37°C and in a 5% CO2 humidified atmosphere.
The propagation of ZIKV strain MEX_CIENI551 was performed in CD-1 suckling mouse brains (provided by Unidad de Producción y Experimentación de Animales de Laboratorio [UPEAL]). Virus titer was determined by focus-forming assay in Huh-7 cells. Brain extracts from mock-infected CD-1 mice were used as controls.
Subsequently, cells were infected with ZIKV at an MOI of 3, and infection was allowed for 8, 12, 16, 24, and 48 h, as appropriate.
Generation of the NLS or NES mutation of NS3 protein and expression in Huh7 cells.
The plasmid containing the NS2B3 sequence (kindly donated by Jonathan Ball of the University of Nottingham, UK) was propagated in competent Escherichia coli DH5α cells and purified using the Zyppy plasmid miniprep kit (Zymo Research) following the instructions provided by the supplier.
Mutation of the putative NLS or NES in the NS3 protein sequence was performed in the plasmid NS2B3 using the Q5 hot start high-fidelity DNA polymerase PCR kit (New England Biolabs). The primer pair forward (agcaCTCCGTACTGTGATCTTAG) and reverse (gctgcTATGGCTTCACGGACTATTTC) was used to introduce mutations in the putative NLS 210, following the manufacturer’s recommendations (98°C for 30 s, 25 cycles of 98°C for 10 s, 60°C for 20 s, and 72°C for 1 min). Deletions of the putative NESs were performed with the primers GTGCCCCCCGGAGAGAGA (forward) and CTTCCATGGACCACAGTATGACAC (reverse) for the NES 85 deletion and CTTCCATGGACCACAGTATGACAC (forward) and CTTCCATGGACCACAGTATGACAC (reverse) for the NES 248 deletion. Both deletions were performed with the kit and conditions described above.
Huh7 cells at a confluence of 70 to 80% were transfected with the different plasmids by electroporation following the protocol of Hashemi et al., with some modifications (66). Briefly, 1 × 107 cells were washed with phosphate-buffered saline (PBS) and resuspended in 200 μL of Opti-MEM with 5 μg of DNA. The cells were transferred to a Gene Pulser cuvette with a 4-mm electrode gap.
Electroporation of the plasmid with the NS3 or NLS SV40-tetraGFP protein sequences was performed on a Gene Pulser Xcell (Bio-Rad, Germany), with electric field strength and pulse length of 170 V and 40 ms, respectively, in exponential decay. Cells were cultured in advanced DMEM with 15% FBS, and transfection was evaluated at 48 h.
Confocal microscopy assays.
Subconfluent monolayers of Huh7 cells were cultured on coverslips for infection with ZIKV or transfection with NS2B3 plasmids. Cells were fixed with 4% paraformaldehyde (PFA), permeabilized and blocked with 0.2% saponin and 1% FBS-PBS, and incubated with the primary antibodies to NS3 protein (rabbit polyclonal, 1:200, GTX-124252), E protein (mouse monoclonal, 1:200, 4G2), importin-α (mouse monoclonal, 1:100, sc-101292), and CRM-1 (mouse monoclonal, 1:100, sc-74454) and with secondary antibodies conjugated to Alexa 488 anti-mouse and to Alexa 594 anti-rabbit (Life Technologies) diluted in saponin and FBS-PBS.
Cell nuclei were stained with Hoechst (Santa-Cruz), and samples were visualized on a Leica TCS SP8 confocal microscope. The images obtained were processed with Leica Application Suite X Core Offline software.
Transmission immunoelectron microscopy (TIM) assays.
Huh7 cells grown in T-75 flasks (Corning) were mock infected or infected with ZIKV at an MOI of 3. After 8 and 24 h, the cells were fixed with 4% paraformaldehyde/0.5% glutaraldehyde for 1 h at room temperature (RT), dehydrated with increasing concentrations of ethanol, embedded in acrylic resin (LR White), and polymerized under UV irradiation at 4°C overnight. Resin-embedded cell sections of 70 nm were mounted on Formvar-covered nickel grids and incubated in PBS with 10% FBS for 1 h to block nonspecific binding and were reacted with an anti-NS3 antibody diluted 1:20 in PBS with 5% FBS. The samples were washed three times with PBS and 10% FBS and were incubated with an anti-rabbit IgG secondary antibody conjugated to 20-nm colloidal gold particles (Ted Pella Inc., Redding, CA, USA) at RT for 1 h. Finally, sections were contrasted with uranyl acetate and lead citrate before being examined under a Jeol JEM-1011 transmission electron microscope (Jeol Ltd., Tokyo, Japan).
In silico prediction of NLSs or NESs of flavivirus NS3 protein.
The amino acid sequence of the NS3 protein from ZIKV strain Mr-766 (access number Q32ZE1) was collected from the UniProt KnowledgeBase (UniProtKB). NLSs of ZIKV NS3 were identified using the cNLS Mapper software (server for prediction of importin-α-dependent nuclear localization signals) (34) with a predetermined cutoff score (5.0), while for NES prediction in CRM1 cargo proteins, LocNES (a computational tool for locating classical NESs) was used (41). TRG_NLS_MonoExtN_4 and TRG_NES_CRM1_1 motifs were determined with WREGEX (weighted regular expression) v2.1, a server for amino acid motif searching (35). To compare whether the putative ZIKV NS3 nuclear localization sequences were conserved in flaviviruses, a multiple sequence alignment was performed using ClustalW. The NS3 protein sequences were obtained from UniProtKB IDs and correspond to DENV (P14340), LANTV (P29837), yellow fever virus (YFV) (Q6J3P1), DENV4 (Q58HT7), DENV1 (P27909), DENV3 (Q6YMS4), WNV (Q9Q6P4), and JEV (P27395).
Three-dimensional (3D) modeling of NS3 protein from ZIKV and molecular docking of NS3-importin-α.
The amino acid sequence of the NS3 protein from ZIKV strain Mr-766 (access number Q32ZE1) was collected from the UniProtKB and used for 3D structure prediction performed using RaptorX (67) and analyzed with the VMD v1.9.3 software. Validation was performed using the Ramachandran graph and the SAVES v6.0 server; 91% of the amino acids were in the allowed area of psi and phi angles (0.2; values not shown).
Molecular docking of NS3-importin-α was analyzed with the HDOCK server using the previously modeled ZIKV NS3 and the structure of importin-α (PDB ID: 4WV6). As positive controls, the NLS of the ZIKV NS5 protein (PDB ID: 5W41) and the SV40 NLS (1EJL) were used because both use the classical pathway of nuclear import. The cytoplasmic protein ATP synthase α (PDB ID: 1QO1) and the cellular membrane protein Fas-L member 6 (PDB ID: 5L19), which lack NLSs, were used as negative controls.
Cell viability to IVM or LMB.
Cell viability was evaluated with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazole bromide (MTT) method. Briefly, Huh-7 cells were cultured in 96-well plates (70 to 80% confluence) and treated for 48 h at 37°C with the vehicles (DMSO and ethanol) and drugs at different concentrations (IVM [12, 25, 50, 75, and 100 μM] or LMB [1, 3, and 5 ng]). MTT (10 μL) was added in 100 μL of culture medium per well and incubated for 3 h at 37°C, according to the manufacturer’s instructions. The MTT was removed, and 100 μL of DMSO was added per well. To remove the background, the plate was read at a wavelength of 630 nm, and the absorbances obtained were subtracted from the absorbances obtained at a wavelength of 562 nm. Absorbance was determined in the spectrophotometer at a wavelength of 562 nm.
Classical nuclear import (importin-α/β) inhibition with IVM and nuclear export (CRM-1) inhibition with LMB.
Huh7 cells cultured with DMEM/7.5% (vol/vol) FBS were mock infected or infected with ZIKV at an MOI of 3 for 2 h at 37°C with 5% CO2 in advanced DMEM without serum, followed by treatment with 12.5 μM IVM (Sigma, 18898-250MG) diluted in DMSO, which inhibits protein import into the nucleus via importin-α/β1 (33). The infection was allowed for 12 h. Huh-7 cells transfected with the plasmid containing NLS SV40-tetraGFP were treated with IVM as a control.
To inhibit the nuclear export pathway by CRM-1, cells were mock infected or infected for 2 h, followed by treatment with 15 nM LMB diluted in ethanol added to the fresh culture medium (Santa Cruz, 87081-35-4) (32). Infection was allowed for 24 h.
The localization of NS3 protein in cells infected with ZIKV and treated with IVM or LMB was analyzed using indirect immunofluorescence. As a control of the inhibition of nuclear import and export by IVM and LMB, the localization of NLS SV40-tetraGFP in transfected cells treated with IVM was analyzed. On the other hand, the localization of CCNB1 in untreated and LMB-treated cells was analyzed.
Imaging and statistical analysis.
Images obtained by confocal microscopy were analyzed using the Icy image analysis software. Three different images for each condition (8, 12, 16, 24, and 48 h or NS2B3 WT/Mut-NLS as appropriate) were imported, and the mean fluorescence intensity (MFI) for pixel values were obtained for every selected region of interest (ROI) to quantify the nuclear fluorescence (Fn) in relation with the cytoplasm fluorescence (Fc). The Fn/Fc ratio was determined according to Fn/Fc = (Fn − Fb)/Fc − Fb, where Fb is the background fluorescence (68). Pearson’s correlation coefficients were determined for selected ROIs. MFI arbitrary units and correlation coefficients were expressed as means, and standard error of the mean (SEM) was determined. The ordinary one-way analysis of variance (ANOVA) with Dunnett multiple comparisons post hoc testing was used to determine significant differences among means of each condition (12, 16, 24, and 48 h or NS2B3-Mut-NLS and NES-Mut as indicated) against the control (8 h) or NS2B3-WT using RStudio version 1.4 and R version 4.1 software. The results were considered statistically significant when two-sided P values were less than 0.05.
Ethics statement.
This study was conducted by the Official Mexican Standard Guidelines for Production, Care, and Use of Laboratory Animals (NOM-062-ZOO-1999), and the protocol (number 048-02) was approved by the Animal Care and Use Committee (CICUAL) at CINVESTAV-IPN, Mexico.
ACKNOWLEDGMENTS
We thank Bibiana Chávez-Munguía and Lizbeth Iliana Salazar Villatoro for their valuable help, assistance, and preparation of the electron microscopy imaging samples. We also thank Fernando Medina and Clotilde Cancio Lonches for methodological support and Jaime Zarco for technical assistance. The biostatistical data analysis and graph generation were performed with the assistance of the MDatos research consultancy. The vatillos team.
L.A.D.J.-G., S.N.P.-R., J.M.R.-R., and C.D.C.-R. generated experimental data. L.A.D.J.-G., J.M.R.-R., J.F.O.-R., C.N.F.-M., B.C., A.L.G.-E., and R.M.d.Á. participated equally in the manuscript writing. A.L.G.-E. and R.M.d.Á. coordinated and edited the manuscript. All authors have read and agreed with the published version of the manuscript.
This research was supported by CONACYT (Consejo Nacional de Ciencia y Tecnología, Mexico), A1-S-9005 from Rosa María del Ángel, and CB-250696 from Ana Lorena Gutiérrez Escolano. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Rosa María del Ángel, Email: rmangel@cinvestav.mx.
Susana López, Instituto de Biotecnologia/UNAM.
REFERENCES
- 1.Hennessey M. 2016. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep 65:55–58. 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 2.WHO. 2019. Zika: the continuing threat. Bull World Health Organ 97:6–7. 10.2471/BLT.19.020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzon L, Trevisan M, Sinigaglia A, Lavezzo E, Palù G. 2016. Zika virus: from pathogenesis to disease control. FEMS Microbiol Lett 363:fnw202. 10.1093/femsle/fnw202. [DOI] [PubMed] [Google Scholar]
- 4.Gu SH, Song DH, Lee D, Jang J, Kim MY, Jung J, Woo KI, Kim M, Seog W, Oh HS, Choi BS, Ahn J-S, Park Q, Jeong ST. 2017. Whole-genome sequence analysis of Zika virus, amplified from urine of traveler from the Philippines. Virus Genes 53:918–921. 10.1007/s11262-017-1500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villordo SM, Carballeda JM, Filomatori CV, Gamarnik AV. 2016. RNA structure duplications and flavivirus host adaptation. Trends Microbiol 24:270–283. 10.1016/j.tim.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Chan JF-W, Tee K-M, Choi GK-Y, Lau SK-P, Woo PC-Y, Tse H, Yuen K-Y. 2016. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerging Microbes Infect 5:e22. 10.1038/emi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruba N, Martinez JIR, Grzywa R, Wysocka M, Skoreński M, Burmistrz M, Łęcka M, Lesner A, Sieńczyk M, Pyrć K. 2016. Substrate profiling of Zika virus NS2B-NS3 protease. FEBS Lett 590:3459–3468. 10.1002/1873-3468.12443. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Phoo WW, Loh YR, Zhang Z, Ng EY, Wang W, Keller TH, Luo D, Kang C. 2017. Structural characterization of the linked NS2B-NS3 protease of Zika virus. FEBS Lett 591:2338–2347. 10.1002/1873-3468.12741. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zhang Z, Phoo WW, Loh YR, Wang W, Liu S, Chen MW, Hung AW, Keller TH, Luo D, Kang C. 2017. Structural dynamics of Zika virus NS2B-NS3 protease binding to dipeptide inhibitors. Structure 25:1242–1250. 10.1016/j.str.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Hertzog J, Dias Junior AG, Rigby RE, Donald CL, Mayer A, Sezgin E, Song C, Jin B, Hublitz P, Eggeling C, Kohl A, Rehwinkel J. 2018. Infection with a Brazilian isolate of Zika virus generates RIG-I stimulatory RNA and the viral NS5 protein blocks type I IFN induction and signaling. Eur J Immunol 48:1120–1136. 10.1002/eji.201847483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou W, Cruz-Cosme R, Armstrong N, Obwolo LA, Wen F, Hu W, Luo M-H, Tang Q. 2017. Molecular cloning and characterization of the genes encoding the proteins of Zika virus. Gene 628:117–128. 10.1016/j.gene.2017.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Yang SNY, Smith K, Forwood JK, Jans DA. 2017. Nuclear import inhibitor N-(4-hydroxyphenyl) retinamide targets Zika virus (ZIKV) nonstructural protein 5 to inhibit ZIKV infection. Biochem Biophys Res Commun 493:1555–1559. 10.1016/j.bbrc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Denman AJ, Mackenzie JM. 2017. The IMPORTance of the nucleus during flavivirus replication. Viruses 9:14. 10.3390/v9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks AJ, Johansson M, John AV, Xu Y, Jans DA, Vasudevan SG. 2002. The interdomain region of dengue NS5 protein that binds to the viral helicase NS3 contains independently functional importin β1 and importin α/β-recognized nuclear localization signals. J Biol Chem 277:36399–36407. 10.1074/jbc.M204977200. [DOI] [PubMed] [Google Scholar]
- 15.Johansson M, Brooks AJ, Jans DA, Vasudevan SG. 2001. A small region of the dengue virus-encoded RNA-dependent RNA polymerase, NS5, confers interaction with both the nuclear transport receptor importin-β and the viral helicase, NS3. J Gen Virol 82:735–745. 10.1099/0022-1317-82-4-735. [DOI] [PubMed] [Google Scholar]
- 16.Mori Y, Okabayashi T, Yamashita T, Zhao Z, Wakita T, Yasui K, Hasebe F, Tadano M, Konishi E, Moriishi K, Matsuura Y. 2005. Nuclear localization of Japanese encephalitis virus core protein enhances viral replication. J Virol 79:3448–3458. 10.1128/JVI.79.6.3448-3458.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangiambut S, Keelapang P, Aaskov J, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. 2008. Multiple regions in dengue virus capsid protein contribute to nuclear localization during virus infection. J Gen Virol 89:1254–1264. 10.1099/vir.0.83264-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang S-H, Syu W-J, Huang K-J, Lei H-Y, Yao C-W, King C-C, Hu S-T. 2002. Intracellular localization and determination of a nuclear localization signal of the core protein of dengue virus. J Gen Virol 83:3093–3102. 10.1099/0022-1317-83-12-3093. [DOI] [PubMed] [Google Scholar]
- 19.Wente SR, Rout MP. 2010. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol 2:a000562. 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabachinski G, Schwartz TU. 2015. The nuclear pore complex—structure and function at a glance. J Cell Sci 128:423–429. 10.1242/jcs.083246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christie M, Chang C-W, Róna G, Smith KM, Stewart AG, Takeda AAS, Fontes MRM, Stewart M, Vértessy BG, Forwood JK, Kobe B. 2016. Structural biology and regulation of protein import into the nucleus. J Mol Biol 428:2060–2090. 10.1016/j.jmb.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Kosugi S, Hasebe M, Matsumura N, Takashima H, Miyamoto-Sato E, Tomita M, Yanagawa H. 2009. Six classes of nuclear localization signals specific to different binding grooves of importin α. J Biol Chem 284:478–485. 10.1074/jbc.M807017200. [DOI] [PubMed] [Google Scholar]
- 23.Hutten S, Kehlenbach RH. 2007. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol 17:193–201. 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 24.la Cour T, Gupta R, Rapacki K, Skriver K, Poulsen FM, Brunak S. 2003. NESbase version 1.0: a database of nuclear export signals. Nucleic Acids Res 31:393–396. 10.1093/nar/gkg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D, Farmer A, Chook YM. 2010. Recognition of nuclear targeting signals by karyopherin-β proteins. Curr Opin Struct Biol 20:782–790. 10.1016/j.sbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortese M, Goellner S, Acosta EG, Neufeldt CJ, Oleksiuk O, Lampe M, Haselmann U, Funaya C, Schieber N, Ronchi P, Schorb M, Pruunsild P, Schwab Y, Chatel-Chaix L, Ruggieri A, Bartenschlager R. 2017. Ultrastructural characterization of Zika virus replication factories. Cell Rep 18:2113–2123. 10.1016/j.celrep.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Jesús-González LA, Cervantes-Salazar M, Reyes-Ruiz JM, Osuna-Ramos JF, Farfán-Morales CN, Palacios-Rápalo SN, Pérez-Olais JH, Cordero-Rivera CD, Hurtado-Monzón AM, Ruíz-Jiménez F, Gutiérrez-Escolano AL, del Ángel RM. 2020. The nuclear pore complex: a target for NS3 protease of dengue and Zika viruses. Viruses 12:583. 10.3390/v12060583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacios-Rápalo SN, De Jesús-González LA, Reyes-Ruiz JM, Osuna-Ramos JF, Farfan-Morales CN, Gutiérrez-Escolano AL, del Ángel RM. 2021. Nuclear localization of non-structural protein 3 (NS3) during dengue virus infection. Arch Virol 166:1439–1446. 10.1007/s00705-021-05026-w. [DOI] [PubMed] [Google Scholar]
- 29.Reyes-Ruiz JM, Osuna-Ramos JF, Cervantes-Salazar M, Lagunes Guillen AE, Chávez-Munguía B, Salas-Benito JS, Del Ángel RM. 2018. Strand-like structures and the nonstructural proteins 5, 3 and 1 are present in the nucleus of mosquito cells infected with dengue virus. Virology 515:74–80. 10.1016/j.virol.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Brand C, Bisaillon M, Geiss BJ. 2017. Organization of the flavivirus RNA replicase complex. Wiley Interdiscip Rev RNA 8:10.1002/wrna.1437. 10.1002/wrna.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser JE, Rawlinson SM, Wang C, Jans DA, Wagstaff KM. 2014. Investigating dengue virus nonstructural protein 5 (NS5) nuclear import. Methods Mol Biol 1138:301–328. 10.1007/978-1-4939-0348-1_19. [DOI] [PubMed] [Google Scholar]
- 32.Rawlinson SM, Pryor MJ, Wright PJ, Jans DA. 2009. CRM1-mediated nuclear export of dengue virus RNA polymerase NS5 modulates interleukin-8 induction and virus production. J Biol Chem 284:15589–15597. 10.1074/jbc.M808271200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. 2012. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 443:851–856. 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosugi S, Hasebe M, Tomita M, Yanagawa H. 2009. Systematic identification of yeast cell cycle-dependent nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc Natl Acad Sci USA 106:10171–10176. 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieto G, Fullaondo A, Rodriguez JA. 2014. Prediction of nuclear export signals using weighted regular expressions (Wregex). Bioinformatics 30:1220–1227. 10.1093/bioinformatics/btu016. [DOI] [PubMed] [Google Scholar]
- 36.Sigrist CJA, de Castro E, Cerutti L, Cuche BA, Hulo N, Bridge A, Bougueleret L, Xenarios I. 2013. New and continuing developments at PROSITE. Nucleic Acids Res 41:D344–D347. 10.1093/nar/gks1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Zhao Y, Gao Y, Hu W, Qu Y, Lou N, Zhu Y, Zhang X, Yang H. 2017. Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation. J Exp Clin Cancer Res 36:42. 10.1186/s13046-017-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wölk B, Sansonno D, Kräusslich HG, Dammacco F, Rice CM, Blum HE, Moradpour D. 2000. Subcellular localization, stability, and trans-cleavage competence of the hepatitis C virus NS3-NS4A complex expressed in tetracycline-regulated cell lines. J Virol 74:2293–2304. 10.1128/jvi.74.5.2293-2304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchil PD, Kumar AVA, Satchidanandam V. 2006. Nuclear localization of flavivirus RNA synthesis in infected cells. J Virol 80:5451–5464. 10.1128/JVI.01982-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R. 2019. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu D, Marquis K, Pei J, Fu S-C, Cağatay T, Grishin NV, Chook YM. 2015. LocNES: a computational tool for locating classical NESs in CRM1 cargo proteins. Bioinformatics 31:1357–1365. 10.1093/bioinformatics/btu826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.la Cour T, Kiemer L, Mølgaard A, Gupta R, Skriver K, Brunak S. 2004. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng Des Sel 17:527–536. 10.1093/protein/gzh062. [DOI] [PubMed] [Google Scholar]
- 43.Luo D, Wei N, Doan DN, Paradkar PN, Chong Y, Davidson AD, Kotaka M, Lescar J, Vasudevan SG. 2010. Flexibility between the protease and helicase domains of the dengue virus NS3 protein conferred by the linker region and its functional implications. J Biol Chem 285:18817–18827. 10.1074/jbc.M109.090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agnihotry S, Pathak RK, Singh DB, Tiwari A, Hussain I. 2022. Chapter 11—Protein structure prediction, p 177–188. In Singh DB, Pathak RK (ed), Bioinformatics. Academic Press, Cambridge, MA. [Google Scholar]
- 45.Yan Y, Tao H, He J, Huang S-Y. 2020. The HDOCK server for integrated protein–protein docking. Nat Protoc 15:1829–1852. 10.1038/s41596-020-0312-x. [DOI] [PubMed] [Google Scholar]
- 46.Pumroy RA, Cingolani G. 2015. Diversification of importin-α isoforms in cellular trafficking and disease states. Biochem J 466:13–28. 10.1042/BJ20141186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng G, Wang E, Wang Z, Liu H, Zhu F, Li D, Hou T. 2019. HawkDock: a web server to predict and analyze the protein–protein complex based on computational docking and MM/GBSA. Nucleic Acids Res 47:W322–W330. 10.1093/nar/gkz397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neufeldt CJ, Cortese M, Acosta EG, Bartenschlager R. 2018. Rewiring cellular networks by members of the Flaviviridae family. Nat Rev Microbiol 16:125–142. 10.1038/nrmicro.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhuvanakantham R, Chong M-K, Ng M-L. 2009. Specific interaction of capsid protein and importin-α/β influences West Nile virus production. Biochem Biophys Res Commun 389:63–69. 10.1016/j.bbrc.2009.08.108. [DOI] [PubMed] [Google Scholar]
- 50.Netsawang J, Noisakran S, Puttikhunt C, Kasinrerk W, Wongwiwat W, Malasit P, Yenchitsomanus P, Limjindaporn T. 2010. Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res 147:275–283. 10.1016/j.virusres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Pryor MJ, Rawlinson SM, Butcher RE, Barton CL, Waterhouse TA, Vasudevan SG, Bardin PG, Wright PJ, Jans DA, Davidson AD. 2007. Nuclear localization of dengue virus nonstructural protein 5 through its importin α/β-recognized nuclear localization sequences is integral to viral infection. Traffic 8:795–807. 10.1111/j.1600-0854.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 52.Robijns J, Houthaeve G, Braeckmans K, De Vos WH. 2018. Chapter 5—Loss of nuclear envelope integrity in aging and disease, p 205–222. In Galluzzi L (ed), International Review of Cell and Molecular Biology. Academic Press, Cambridge, MA. [DOI] [PubMed] [Google Scholar]
- 53.Mishra PM, Uversky VN, Giri R. 2018. Molecular recognition features in Zika virus proteome. J Mol Biol 430:2372–2388. 10.1016/j.jmb.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Ma Y, Lu H, Wang W, Zhu J, Zhao W, Cui F. 2021. Membrane association of importin α facilitates viral entry into salivary gland cells of vector insects. Proc Natl Acad Sci USA 118:e2103393118. 10.1073/pnas.2103393118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao W, Zhu J, Lu H, Zhu J, Jiang F, Wang W, Luo L, Kang L, Cui F. 2022. The nucleocapsid protein of rice stripe virus in cell nuclei of vector insect regulates viral replication. Protein Cell 13:360–378. 10.1007/s13238-021-00822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Breton M, Meyniel-Schicklin L, Deloire A, Coutard B, Canard B, de Lamballerie X, Andre P, Rabourdin-Combe C, Lotteau V, Davoust N. 2011. Flavivirus NS3 and NS5 proteins interaction network: a high-throughput yeast two-hybrid screen. BMC Microbiol 11:234. 10.1186/1471-2180-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson RB, Hendrix J, Geiss BJ, McCullagh M. 2020. RNA-dependent structures of the RNA-binding loop in the flavivirus NS3 helicase. J Phys Chem B 124:2371–2381. 10.1021/acs.jpcb.0c00457. [DOI] [PubMed] [Google Scholar]
- 58.Jain R, Coloma J, García-Sastre A, Aggarwal AK. 2016. Structure of the NS3 helicase from Zika virus. Nat Struct Mol Biol 23:752–754. 10.1038/nsmb.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao X, Li Y, Jin X, Li Y, Guo F, Jin T. 2016. Molecular mechanism of divalent-metal-induced activation of NS3 helicase and insights into Zika virus inhibitor design. Nucleic Acids Res 44:10505–10514. 10.1093/nar/gkw941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li K, Phoo WW, Luo D. 2014. Functional interplay among the flavivirus NS3 protease, helicase, and cofactors. Virol Sin 29:74–85. 10.1007/s12250-014-3438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo D, Xu T, Hunke C, Grüber G, Vasudevan SG, Lescar J. 2008. Crystal structure of the NS3 protease-helicase from dengue virus. J Virol 82:173–183. 10.1128/JVI.01788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zephyr J, Kurt Yilmaz N, Schiffer CA. 2021. Viral proteases: structure, mechanism and inhibition. Enzymes 50:301–333. 10.1016/bs.enz.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji W, Luo G. 2020. Zika virus NS5 nuclear accumulation is protective of protein degradation and is required for viral RNA replication. Virology 541:124–135. 10.1016/j.virol.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Tay MYF, Fraser JE, Chan WKK, Moreland NJ, Rathore AP, Wang C, Vasudevan SG, Jans DA. 2013. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor ivermectin. Antiviral Res 99:301–306. 10.1016/j.antiviral.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Medin CL, Fitzgerald KA, Rothman AL. 2005. Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J Virol 79:11053–11061. 10.1128/JVI.79.17.11053-11061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashemi A, Roohvand F, Ghahremani MH, Aghasadeghi MR, Vahabpour R, Motevali F, Memarnejadian A. 2012. Optimization of transfection methods for Huh-7 and Vero cells: a comparative study. Cytol Genet 46:347–353. 10.3103/S0095452712060035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morten K, Haipeng W, Sheng W, Jian P, Zhiyong W, Hui L, Jinbo X. 2012. Template-based protein structure modeling using the RaptorX web server. Nat Protoc 7:1511–1522. 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez-Denman AJ, Russo A, Wagstaff KM, White PA, Jans DA, Mackenzie JM. 2018. Nucleocytoplasmic shuttling of the West Nile virus RNA-dependent RNA polymerase NS5 is critical to infection. Cell Microbiol 20:e12848. 10.1111/cmi.12848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5. Download jvi.01773-22-s0001.pdf, PDF file, 2.6 MB (2.6MB, pdf)