Abstract
Obesity, a chronic metabolic condition, is an increase in fat mass and blood lipid levels mainly causing atherosclerosis and hypertension, which further lead to cardiovascular complications. The objective of the study was to investigate the crude extract of Caralluma edulis (CE.Cr) for its potential against high-fat diet (HFD)-induced obesity and its related complications. Hyperlipidemia was induced in Wistar albino rats with HFD (1% cholesterol + 0.5% cholic acid) for 28 days. Treatment groups were administered with different doses of CE.Cr (100, 300 and 500 mg/Kg, p.o.) and the standard group received atorvastatin. At the end of study, sera were analyzed for biochemical markers and the aorta was dissected for microscopic examination. Antioxidant potential was evaluated and high-performance liquid chromatography (HPLC) analysis was performed. The hypotensive potential of CE.Cr was evaluated through an invasive technique. HPLC analysis of CE.Cr showed the presence of chlorogenic acid, caffeic acid, apigenin and naringenin. Histological examination of the aorta section showed anti-atherosclerotic effects which were also evident from decrease in serum total cholesterol, triglycerides and low-density lipoproteins levels. CE.Cr decreased mean arterial blood pressure and evoked significant hypotensive effects. The crude extract of C. edulis showed anti-obesity, antihypertensive, anti-atherosclerotic and antioxidant potential.
Keywords: Caralluma edulis, high-fat diet, obesity, hyperlipidemia, hypertension, atherosclerosis
Introduction
Obesity is a chronic metabolic condition caused by an imbalance in energy intake and expenditure and is defined by an increase in fat mass and blood lipid levels.1 Obesity is recognized as a risk factor for many disorders, including hyperlipidemia, hypertension and diabetes in both the developed and developing countries. The buildup of fat leads to the formation of atherosclerotic plaques, which can either expand towards the artery’s lumen or become unstable causing obstructed blood flow.2,3 Obesity is associated with the formation of free radicals, the activity of these free radicals increases in the absence of effective defense mechanism resulting in oxidative stress and other complications.4
The pathogenesis of atherosclerosis includes endothelial dysfunction, oxidized low-density lipoprotein (oxLDL) and proliferation of vascular smooth muscle cells that may also be significantly influenced by reactive oxygen species (ROS). ROS can be generated from a variety of sources such as xanthine oxidases, NO synthases, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase isoforms and metal-catalyzed reactions.5 LDL activates endothelial NADPH oxidase and promotes ROS formation. When ROS-induced OxLDL enters the intima through damaged endothelium, monocytes get changed into macrophages, which take up OxLDL and turn them into foam cells. The foam cells, containing lipids, can evolve into atheromas or atherosclerotic plaques in the arterial wall and if these plaques get ruptured, can result in ischemic heart disease, hypertension, stroke and even death.6 In recent decades, great interest has been observed in plants that can help to prevent atherosclerosis associated with obesity.
The world has witnessed growing scientific and commercial interest in medicinal plants, mainly due to the immense economic development and widespread cultural acceptability of plant-based products. Due to the greater risk of adverse effects as compared to the beneficial effects of currently available synthetic drugs, people are attracted towards natural therapies, among which herbal products are more common.7 Caralluma edulis (Edgew.), belonging to the family Asclepiadaceae, is an edible plant of extremely arid regions of the Thar and Cholistan deserts, and is commonly known as Seetu.8 C. edulis grows in Mauritania, Sudan, Pakistan, Eritrea, Somalia, Saudi Arabia, UAE, India, Iran and Afghanistan. The plant, as an ethnomedicine, is used as carminative, febrifuge and stomachic, and is also employed in the treatment of Alzheimer’s disease, fever, gastric disturbances, hypertension, rheumatism, leprosy and parasitic infections.9,10 In multiple ethnobotanical reports, it was found that natives consume it in various forms for the management of diabetes mellitus.11-13 The extracts of C. edulis are reported to have antioxidant, antidiabetic, antinociceptive, anti-inflammatory and hunger suppressant effects.14-17 The current study was designed to evaluate CE.Cr for its potential against obesity, hypertension, atherosclerosis and oxidative stress, and to provide scientific evidence for its folkloric uses.
Material and Methods
Animals
Wistar albino rats (180-250 g) of either sex (3 ♀ and 3 ♂), approximately 8–10 weeks old, were employed in experimental work and Swiss albino mice (20-30 g) were used for the acute toxicity study. Animals were housed in the animal house of the Pharmacology research laboratory, department of Pharmacology, faculty of Pharmacy, the Islamia University of Bahawalpur (IUB). Animals were kept in standard polycarbonate cages under controlled temperature (23 ± 2°C), along with 55 ± 5% humidity and a 12 h light/dark cycle. Food and water were provided ad libitum. Animals were acclimatized to experimental conditions for 1 week before start of the study. The study protocols were approved by pharmacy animal ethics committee (PAEC) under reference number PAEC/2020/25.
Plant Material
The stems of C. edulis were collected from the Cholistan desert of Bahawalpur, Pakistan. After collection, the plant material was identified by a botanist and its specimen was preserved in the herbarium of Pharmacology research laboratory, department of Pharmacology, faculty of Pharmacy, IUB, Pakistan. Voucher number; CE-SM-02-18-209, was issued for future reference. The plant material was washed, dried in the shade and carefully screened to remove any extraneous material.
Preparation of Crude Extract
1.5 kg dried stem of C. edulis was ground into coarse powder and soaked in 70:30 methanol and distilled water solution for 3 days thrice. The soaked plant was filtered through a muslin cloth and then with Whatman grade 1 filter paper, residues were discarded, and the filtrate was subjected to the rotary evaporator (Heidolph, Laborota 400-efficient, Germany) under reduced pressure to prepare a concentrated semi-solid paste.18,19 The thick semi-solid crude extract of C. edulis (CE. Cr) was weighed, labelled, and stored in a freezer for future use.
Phytochemical Analysis
CE.Cr was analyzed qualitatively for the presence of secondary metabolites like alkaloids, flavonoids, glycosides, tannins, anthraquinones, coumarins, saponins, polyphenols, carbohydrates, amino acids and proteins by standard procedures.20
High-Performance Liquid Chromatographic Analysis
HPLC analysis was carried out on Shimadzu LC10-AT VP Liquid Chromatograph equipped with SIL-20A autosampler (Shimadzu Scientific Instruments, Kyoto, Japan) and SPD-10AV UV VIS Detector. A shim-pack CLC-ODS (C-18, 25 cm × 4.6 mm, 5 µm), maintained at room temperature, was used for separation. The mobile phase consisted of binary solvent system; that is, solvent A (water: acetic acid-94:6, PH = 2.2) and the solvent B (acetonitrile) with the following gradient elution (linear gradient v/v): 0–15 minutes, 85% A: 15% B, 15–30 minutes, 55% A: 45% B and 30-35 minutes 0%A: 100% B (equilibration). The flow rate was 1.0 mL/min and the detection wavelength was 280 nm.21
In Vitro Antioxidant Activity by DPPH Assay
The antioxidant potential of CE.Cr was measured in terms of free radical scavenging or hydrogen donating ability of stable 1, 1- diphenyl 2-picrylhydrazyl (DPPH) free radical according to the protocol described by Jabeen et al with slight modifications.22 1 mL of 0.1 mM methanolic solution of DPPH was mixed with 1 mL of each CE.Cr methanolic solution of varying concentration (50-1000 μg/ml). L-Ascorbic acid solution was used as a reference standard, and a mixture of 1 mL DPPH and 1 mL methanol was used as control. The reaction was carried out in triplicate and all the solutions were incubated for 30 minutes and absorbance was measured at 517 nm using UV-Spectrophotometer. DPPH percent scavenging activity was calculated using the following formula
Ac is the Absorbance of control; whereas, As is the absorbance of sample
Normal Diet & High-Fat Diet
1 Kg normal diet was prepared by mixing 500 g of poultry feed, 350 g of choker and 150 g dry milk,9 obtained from the local market of Bahawalpur. The energy of a normal diet was calculated as 402 kcal/100 g, containing carbohydrate 54%, protein 22%, fat 10%, fibers 8%, minerals 2% and moisture 2%.
High-Fat Diet (HFD) consisted of fat 26%, carbohydrate 48%, and protein 20% with total energy value calculated as 510 kcal/100 g. 1% cholesterol and 0.5% cholic acid (Sigma Aldrich, USA) were mixed in the normal diet to induce hypercholesterolemia and butter was used as a source of fat. It is the most common method for developing hypercholesterolemia in the rat model; that is, 1% cholesterol to the normal diet is reported in 12.1% of the studies already published.23-27
Sample Size Calculation
The sample size or the number of animals in each group was measured by using power analysis method. A simple calculation can be carried out manually with the help of formula and complex calculations power analysis-based software is available28
where SD = standard deviation, d = difference between mean values and Z value was checked from Z-table.
Rat Model of Hypercholesterolemia
Albino rats were divided into five groups each comprising six animals. All the groups received tap water ad libitum and respective treatment for 28 days. Normal control group was given normal feed and the positive control group was fed on HFD. Both the groups were administered normal saline (1 mL/Kg/day, p.o.) by oral gavage feeding tubes. CE.Cr was given to the treatment groups, at doses of 100, 300 and 500 mg/Kg (p.o. per day), respectively, along with HFD. The standard control group was given atorvastatin (Pfizer, USA), at the dose of 5 mg/Kg/day; p.o. It is a synthetic HMG-CoA reductase inhibitor which lowers plasma cholesterol levels by inhibiting endogenous cholesterol synthesis. CE.Cr and atorvastatin were diluted in normal saline. After 28 days, all the animals were fasted overnight (for about 12-18 hours) before the induction of anesthesia. The aorta was dissected and blood samples were collected for the estimation of biochemical markers; that is, serum TC, LDL, HDL and TG levels.
Anti-obesity Potential of CE.Cr
The change in body weight of rats, over the course of 28 days, was used as a physical parameter of obesity since it is linked to body fat levels. The change in body weight was determined at 7, 14, 21 and 28th day of the study.
Estimation of Biochemical Markers
All the animals were anesthetized with ketamine (Abbott Laboratories, USA) and diazepam (Roche pharmaceuticals, Germany). Blood samples were collected through cardiac puncture at the end of the study, allowed to clot for 30 minutes, and centrifuged at 3000 rpm for 15 minutes to separate sera. Then the levels of TC, TGs, HDL and LDL were determined using assay kits of Human Diagnostics, Germany.
Anti-Atherosclerotic Potential (Histological Examination)
To evaluate the anti-atherosclerotic effects of CE.Cr, the aorta of experimental animals (one representative animal from each group) were detached, fixed in 10% formalin, embedded in paraffin, serially cut with a microtome (5 μm) and processed for hematoxylin and eosin staining. Histological studies of cross-sections of the aorta were made at 20× magnification using camera lucida drawings.29 The histological slides were graded using a semi-quantitative scoring system and were assigned severity grades according to the percentage of foam cells observed in each group; that is, 0 ≤ 1%, 1 < 25%, 2 = 25–50%, 3 = 50–75% and 4 > 75%.
Evaluation of Hypotensive Potential by Using Invasive Technique
CE.Cr was evaluated for its hypotensive effects by using invasive technique.30 Animals were anesthetized and fixed in a supine position on a dissecting table. Temperature was maintained with the help of an overhead lamp. The trachea, right jugular vein and left carotid artery were exposed by a small mid-tracheal incision. The trachea was cannulated with 18-gauge polyethylene tubing to facilitate spontaneous respiration. The right jugular vein was cannulated with polyethylene tubing PE-50 for intravenous injection of drugs and CE.Cr solutions. The left carotid artery was also cannulated with polyethylene tubing; PE-50, filled with heparinized saline (60 IU/ml) and was connected to a pressure transducer (MLT0699 disposable BP transducer) coupled with PowerLab 4/30 and LabChart Pro software (AD Instruments, Australia) for blood pressure (BP) and heart rate (HR) recordings. A system calibration was performed with the help of a mercury manometer connected to the pressure transducer before the start of the first experiment every day. The exposed surface of the cannulated area was covered with a piece of cotton swab moistened with warm saline. Heparinized saline (0.1 mL) was injected into cannulated rat to prevent blood clotting. Acetylcholine (1 μg/Kg) and Nor-adrenaline (1 μg/Kg) were used to check the hypotensive and hypertensive responsiveness of each animal before administration of CE.Cr. After 15–20 minutes of equilibration, 0.1 mL of CE.Cr was injected, at doses of 1, 3, 10 and 30 mg/Kg intravenously, followed by 0.1 mL of saline flush. BP was returned to the resting level before every next dosing. Mean arterial blood pressure (MABP) was calculated by adding the values of DBP and one-third of the pulse width. Change in blood pressure was measured as the difference between the steady-state values before administration of the dose and the lowest reading after administration of each dose of the test substance.
Diuretic Activity
Rats (other than those used in the HFD model) were randomly divided into different groups. The control group received normal saline (10 mL/kg, i.p.). Another group of animals was given frusemide (10 mg/kg, i.p.) as standard diuretic. The treatment groups of animals were injected with doses of 100, 300 and 500 mg/kg of CE.Cr, intraperitoneally. Immediately after dosing, animals were individually housed in the metabolic cages (Techniplast, Italy) and the urine was collected for 6 hours. Total urine volume was noted. Sodium and potassium urinary concentrations were measured by using a clinical flame photometer (Model 410C, Sherwood, UK).31
Acute Toxicity Study
Acute toxicity test was performed to evaluate the toxic potential of CE.Cr by following the OECD guidelines.32 Swiss albino mice weighing 20–30 g were divided into different groups, comprising of five mice in each group (2♀ and 3♂). Mice were fasted overnight and received only water ad libitum. Normal control group received normal saline (10 mL/kg, p.o.) and other groups received CE.Cr, at doses of 0.5, 1, 3 and 5 g/kg; p.o. All the animals were observed critically for 2 hours and then at the interval of 60 minutes for the next 6 hours, for any type of behavioral changes and then mortality was noted for the next 14 days.
Statistical Analysis
The results were expressed as mean ± SEM for six animals in each group. The results were statistically analyzed, using one-way ANOVA followed by Tukey’s test. The data were computed using GraphPad Prism version 8 and P < 0.05 was considered significant.18
Results
Phytochemical Analysis
CE.Cr was found to be rich in alkaloids, saponins, quinones, carbohydrates, flavonoids, tannins, phenols, terpenes, terpenoids and glycosides.
HPLC Analysis
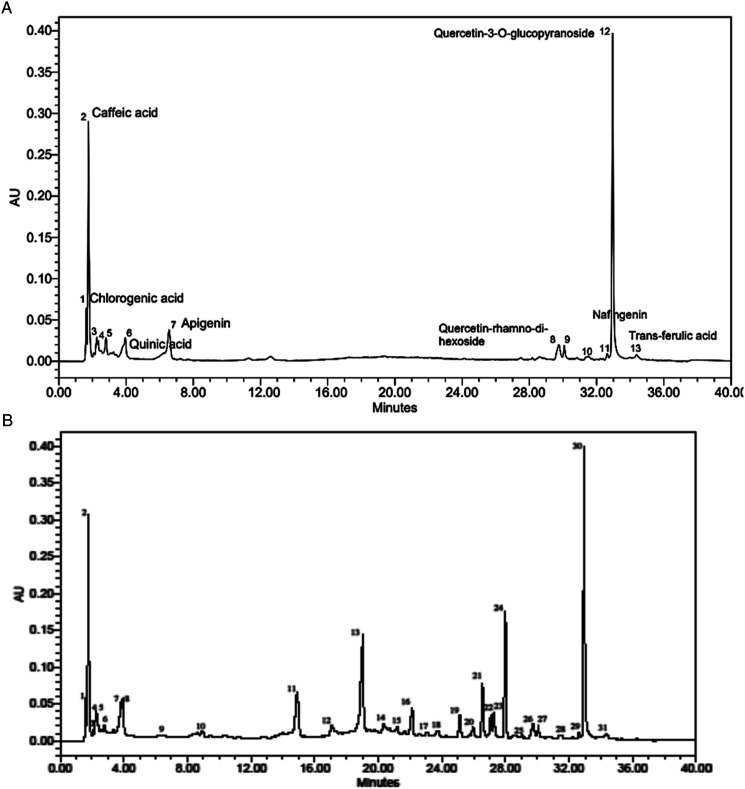
The constituents found in CE.Cr include chlorogenic acid, caffeic acid, vanillic acid, p-coumaric acid, hydroxycinnamic acid, quinic acid, apigenin, quercetin-rhamno-di-hexoside, syringic acid, hyperoside, naringenin, quercetin-3-O-glucopyanoside and trans-ferulic acid (Figure 1A) which have already been reported for their therapeutic importance. The chromatogram was compared with that of standard (Figure 1B and Table 1).
Figure 1.
HPLC chromatogram of (A) CE.Cr and (B) Standard phytochemical constituents.
Table 1.
HPLC Profile of Phytochemical Compounds Detected in CE.Cr.
| Retention Time | Phytochemical Compounds Identified | Percentage Similarity of Retention Time With Standard |
|---|---|---|
| 1.66 | Chlorogenic acid | 99 |
| 1.82 | Caffeic acid | 99 |
| 2.25 | Vanillic acid | 99 |
| 2.44 | p-Coumaric acid | 98 |
| 2.83 | Hydroxycinnamic acid | 98 |
| 3.98 | Quinic acid | 100 |
| 6.43 | Apigenin | 99 |
| 29.72 | Quercetin-rhamno-di-hexoside | 99 |
| 30.15 | Syringic acid | 99 |
| 31.44 | Hyperoside | 100 |
| 32.76 | Naringenin | 100 |
| 33.03 | Quercetin-3-O-glucopyranoside | 100 |
| 34.39 | Trans-ferulic acid | 100 |
Antioxidant Activity by DPPH
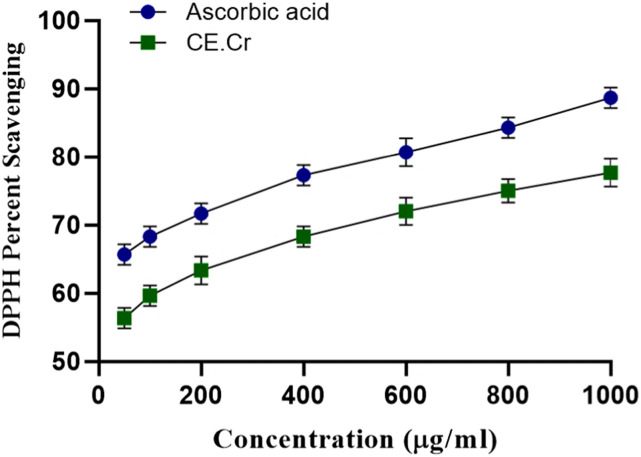
The present study suggested the remarkable antioxidant potential of CE.Cr. The results are shown in Figure 2. Antioxidant potential was increased in a graded manner. Maximum radical scavenging effects shown by CE.Cr was 78% which was comparable to that of ascorbic acid (89%), at the concentration of 1000 μg/mL.
Figure 2.
The effects of CE.Cr and ascorbic acid, the standard antioxidant, on DPPH free radical scavenging activity. The values are expressed as Mean ± SEM of three observations (n = 3).
Anti-Obesity Potential
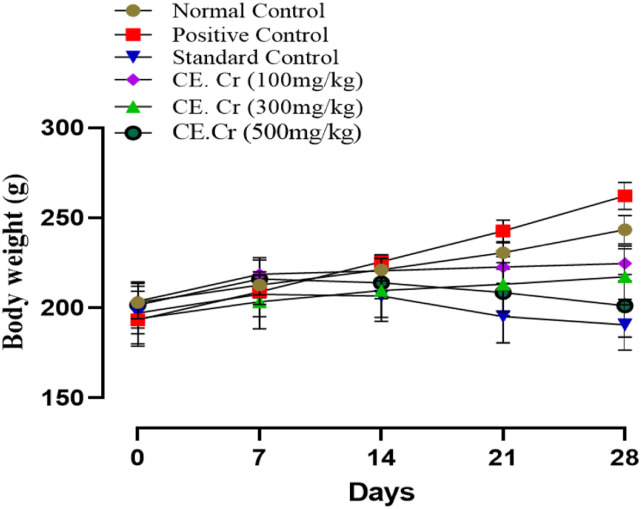
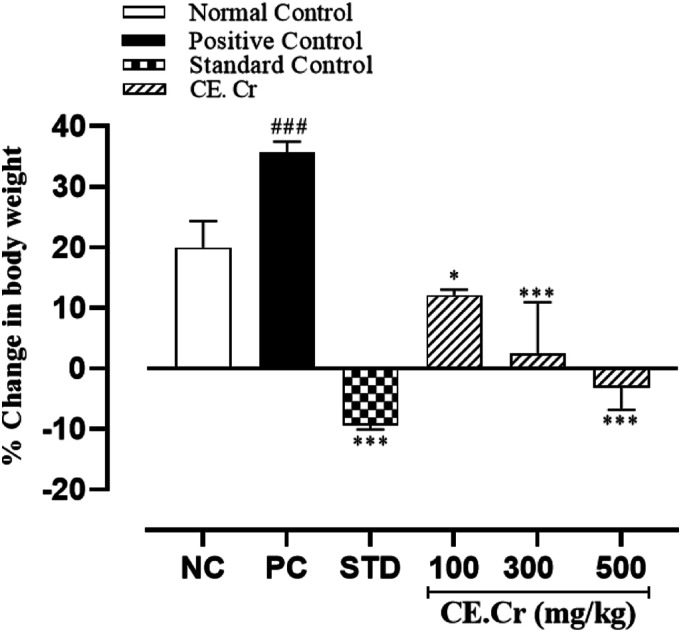
Throughout the experimental period, change in body weight of the normal and positive control groups was found to be increased; that is, 43.00 ± 2.51 and 68.50 ± 1.19 g, respectively, and percent increase in body weight was also observed (Figures 3 and 4). A decrease in body weight was observed in treatment groups, receiving different doses of CE.Cr and atorvastatin. CE.Cr, at doses of 300 and 500 mg/kg, prevented the increase in body weight and results were highly significant (P < 0.001) as compared to the positive control group (Figure 3). Whereas, there was no statistical difference found in the food consumption among different experimental groups (P > 0.05).
Figure 3.
The effects of CE.Cr and atorvastatin on change in body weight (g) of albino rats during 28 days of HFD-induced hypercholesterolemia model. Values are expressed as Mean ± SEM of six animals in each group (n = 6).
Figure 4.
The effects of CE.Cr and atorvastatin on percent change in body weight in albino rats during 28 days of HFD-induced hypercholesterolemia model. The values are expressed as Mean ± SEM of six animals in each group and the results are statistically analyzed using one way ANOVA, followed by Tukey–Kramer’s test. The results of treatment groups are compared with positive control group, and are considered non-significant (ns) if P > 0.05, significant (*) if P < 0.05, more significant (**) if P < 0.01 and highly significant (***) if P < 0.001. The results of positive control group are also compared with the normal control group and considered highly significant (###) if P < .001.
Biochemical Markers
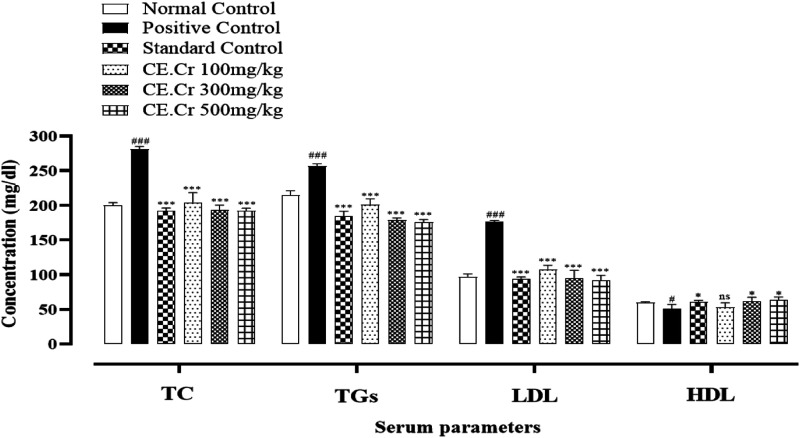
The serum levels of TC, TGs, LDL and HDL in experimental animals are shown in Figure 5. HFD increased serum TC levels in the positive control group and CE.Cr, at doses of 100, 300 and 500 mg/kg, reduced serum TC concentration. CE.Cr showed highly significant (P < .001) decrease in TC levels, at all doses, as compared to that of positive control group. HFD increased the levels of TGs in the positive control group which were reduced significantly in treatment groups, at all doses (100, 300 and 500 mg/kg). HFD decreased serum HDL levels in the positive control group and the levels were significantly (P < 0.01) increased, at doses of 300 and 500 mg/kg, as compared to the positive control group. CE.Cr exhibited a significant (P < 0.001) reduction in serum LDL levels, at doses of 100, 300, and 500 mg/kg.
Figure 5.
Effects of CE.Cr and atorvastatin on serum total Cholesterol (TC), triglycerides (TGs), low density lipoproteins (LDL) and high density lipoproteins (HDL) levels in albino rats during 28 days of HFD-induced hypercholesterolemia model. The values are expressed as Mean ± SEM of six animals in each group and the results are statistically analyzed using one way ANOVA followed by Tukey–Kramer’s test. The results of treatment groups are compared with positive control group and are considered non-significant (ns) if P > .05, significant (*) if P < .05, more significant (**) if P < .01 and highly significant (***) if P < .001. The results of positive control group are also compared with the normal control group and considered highly significant (###) if P < .001.
Hypotensive Effects
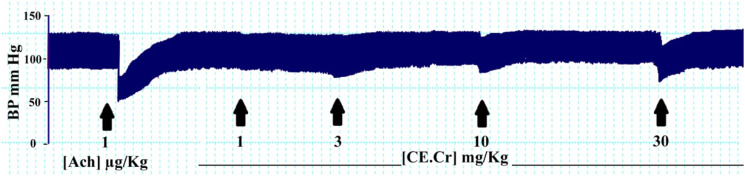
CE.Cr, at doses of 1, 3, and 10 mg/kg did not show significant decrease in mean arterial blood pressure (MABP); that is, 130 ± 2.42, 129 ± 2.87 and 121 ± 3.12 mmHg, respectively. CE.Cr produced significant hypotensive effects, at the dose of 30 mg/kg, as shown in Figure 6 and Table 2.
Figure 6.
The effects of CE.Cr on blood pressure of anesthetized rat using invasive technique and arrows indicate the time of administration of different doses.
Table 2.
Hypotensive Effects of CE.Cr.
| MABP (mmHg) | % Fall in MABP | |
|---|---|---|
| Normal control | 130 ± 1.33 | — |
| Acetylcholine (1 µg/Kg) | 67 ± 1.54*** | 50 |
| CE.Cr (1 mg/Kg) | 130 ± 2.42ns | 0 |
| CE.Cr (3 mg/Kg) | 129 ± 2.87ns | 4 |
| CE.Cr (10 mg/Kg) | 121 ± 3.12ns | 12.96 |
| CE.Cr (30 mg/Kg) | 95 ± 3.98** | 26.92 |
MABP = Mean arterial blood pressure.
The values are expressed as Mean ± SEM of three observations in each group and the results are statistically analyzed using one way ANOVA followed by Tukey–Kramer’s test. The results of treatment groups are compared with normal control group and are considered non-significant (ns) if P > 0.05, significant (*) if P < 0.05, more significant (**) if P < 0.01 and highly significant (***) if P < 0.001.
Diuretic Activity
Urinary volume (ml/100 g/6 h) was measured in different groups of animals, and mean value was calculated as 1.10 ± 0.13 mL for the normal control group, 4.31 ± 0.05 mL for the standard group and CE.Cr, at doses of 100, 300, and 500 mg/kg showed an increase in urinary volume; that is, 2.58 ± 0.7, 3.38 ± 0.28 and 3.84 ± 0.45 mL, respectively. In addition to an increase in urinary volume, CE.Cr also enhanced the Na+ and K+ excretion. CE.Cr, at doses of 300 and 500 mg/kg, showed highly significant increase in the sodium and potassium ions excretion and results were comparable with those of the standard drug, frusemide (Table 3).
Table 3.
The Effects of CE.Cr on Urine Volume (ml/100 g/6 h), Na+ and K+ Excretion (mmol) in Albino Rats.
| Urine Volume (ml/100 g/6h) | Diuretic Index | Na+ (mmol/L) | K+ (mmol/L) | |
|---|---|---|---|---|
| Control (NS, 10 mL/Kg; i.p.) | 1.10 ± .138 | — | 50.10 ± 3.47 | 12.0 ± .74 |
| Standard (Furosemide, 10 mg/Kg; i.p.) | 4.31 ± .056*** | 3.9 | 108.20 ± 4.41*** | 26.27 ± 2.61*** |
| CE.Cr (100 mg/Kg; i.p) | 2.58 ± .7*** | 2.53 | 69.43 ± 4.83** | 15.34 ± 1.25* |
| CE.Cr (300 mg/Kg; i.p) | 3.38 ± .28*** | 3.00 | 79.82 ± 2.53*** | 23.28 ± 1.56*** |
| CE.Cr (500 mg/Kg; i.p) | 3.84 ± .45*** | 3.4 | 82.34 ± 1.75*** | 26.56 ± 1.09*** |
The values are expressed as Mean ± SEM of six animals in each group and the results arestatistically analyzed using one way ANOVA followed by Tukey–Kramer’s test. The results of treatment groups are compared with control group and are considered non-significant (ns) if P > 0.05, significant (*) if P < 0.05, more significant (**) if P < 0.01 and highly significant (***) if P < 0.001.
Anti-Atherosclerotic Potential
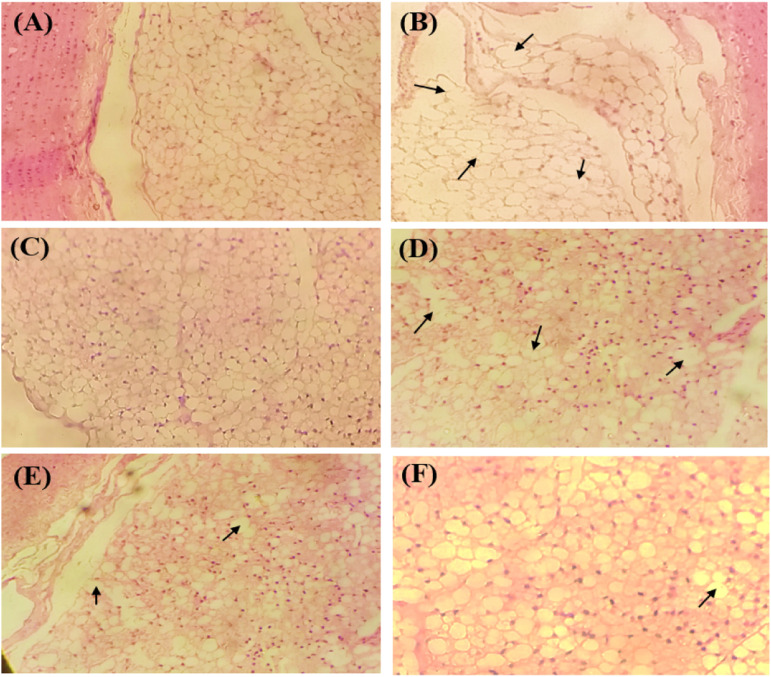
Histological studies of cross-sections of the aorta showed several foam cells in the positive control group with erosion of the endothelial membrane which was a sign of first-stage atherosclerosis. The positive control group was scored as 3 according to the severity grade (Table 4). The normal control group showed the uniform histological structure of the endothelial membrane. Experimental groups receiving different doses of CE.Cr showed restoration of normal endothelial cell morphology. Therefore, histological images showed the anti-atherosclerotic effects of CE.Cr, at doses of 300 and 500 mg/kg and were scored as 1, similar to that of the standard drug, atorvastatin (Figure 7).
Table 4.
The Effects of CE.Cr on Severity Score of Rat Aorta in HFD-Induced Hypercholesterolemia Model.
| Groups | Severity Score |
|---|---|
| Normal control | 0 |
| Positive control | 3 |
| Standard control | 1 |
| CE.Cr | |
| 100 mg/kg | 2 |
| 300 mg/kg | 1 |
| 500 mg/kg | 1 |
The histological slides were assigned severity grades according to the percentage of foam cells observed in each group; that is, 0 ≤ 1%, 1 < 25%, 2 = 25-50%, 3 = 50-75% and 4>75%.
Figure 7.
The effects CE.Cr and atorvastatin on the histological parameters of aorta cross section of albino rats, observed under polarized light microscope (100×) in HFD-induced hypercholesterolemia model; (A) Normal control, (B) Positive control, (C) Standard control, CE.Cr; (D) 100 mg/kg, (E) 300 mg/kg and (F) 500 mg/kg. The images are of one representative animal from each group and the arrows (→) show presence of foam cells.
Acute Toxicity Study
Acute toxicity assay showed no signs of toxicity and mortality and was found to be safe up to 5 g/kg and the results of highest dose (5 g/kg) were tabulated in Table 5.
Table 5.
The Behavioral Pattern of Mice Treated With CE.Cr (5 g/kg; p.o.) in Acute Toxicity Study.
| Parameters | 0 h | 2 h | 6 h | 24 h | 48 h | 7 days | 14 days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCG | CE.Cr | NCG | CE.Cr | NCG | CE.Cr | NCG | CE.Cr | NCG | CE.Cr | NCG | CE.Cr | NCG | CE.Cr | |
| Lacrimation | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Salivation | NF | NF | NF | P | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
| Hyperactivity | N | N | N | NF | N | N | N | N | N | N | N | N | N | N |
| Convulsions | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
| Ataxia | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
| Tremors | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
| Somatomotor activity | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Diarrhea | NF | NF | NF | P | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
| Lethargy | NF | NF | NF | P | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
| Sleep | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Coma | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
| Death | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF | NF |
NCG = Normal control group, CE.Cr = The group treated with the crude extract of Caralluma edulis.
N = Normal, P = Present, MD = Moderately decreased, NF = Not found.
Discussion
Obesity has been a major public health issue that may lead to a variety of consequences including hypertension and formation of atherosclerotic plaques. Medical trends are turning to the dietary modifications and natural medicines for the cure of obesity and its related complications. C. edulis, a traditional Cholistani plant, has been postulated to be a good source of human nutrition, because of its unique lipid-lowering impact and lack of toxicity and side effects, causing widespread concern among medical professionals and prompting substantial research.33 We explored the primary phytochemical ingredients of C. edulis and its anti-obesity, anti-atherosclerotic effects in a high-fat diet-induced rat model. The antioxidant, diuretic, and anti-hypertensive potential were also evaluated.
HFD-induced hyperlipidemia raised the levels of total cholesterol, triglyceride, and low-density lipoproteins as well as induced oxidative stress.34 Cholic acid plays a key role in liver inflammation, lowering bile acid synthesis and affecting TGs and HDL levels, all of which contribute to the development of atherosclerosis.35 Adding 0.25–0.5% cholic acid with cholesterol in a normal diet has been reported to increase cholesterol absorption thus causing atherosclerosis in experimental animals.36,37 In the current study, rats fed on HFD showed an increase in body weight and high levels of plasma TC, TGs, LDL, and oxidative damage to tissues of the aorta, as already reported in previous studies.38-40 Hypercholesterolemia causes an increase in free radical generation and change in lipid peroxide levels. It also promotes oxidative stress, which leads to higher amounts of oxidized LDL. This oxidized LDL enters the intima through the damaged endothelial cells, monocytes convert into macrophages, which take up oxidized LDL and turned into foam cells,41 as observed in the histological images of the aorta section in the positive control group. CE.Cr reduced serum lipid profile (plasma TC, TGs, LDL levels) and increased HDL levels, that may be due to the presence of polyphenols which have been reported to reduce cardiovascular disorders including atherosclerosis as they decrease oxidative stress biomarkers. Flavonoids and terpenoids have been reported to lower TC, TGs, LDL and VLDL levels by inhibiting pancreatic lipases, which convert TGs to fatty acids and glycerol.42 Polyphenol-rich plants reduce lipid peroxidation and boost glutathione peroxidase, which protects tissues by scavenging reactive oxygen species; such as superoxide anion and peroxynitrite.43 The presence of phytochemical compounds in CE.Cr including terpenoids, phenyl propanoids, and iridoids have been reported for their antioxidant activity which might be linked to the anti-hyperlipidemic effects.44
Reverse cholesterol transport, a mechanism in which excess tissue cholesterol is taken up and processed by HDL particles before being sent to the liver for metabolism, is largely responsible for maintaining cholesterol levels in the body. As a result, an increase in HDL levels lowers the risk of atherosclerosis.45 Chlorogenic acid and naringenin, found in CE.Cr, as evident from HPLC analysis, play a role in fat metabolism as they increase the activity of hepatic lipases and improve the lipid profile.46,47 Naringenin has been reported to modulate signaling pathways related to fatty acid metabolism, lowering their accumulation in the liver, thus preventing fatty liver.46 Quercetin rhamno-di-hexoside and quercetin-3-O-glucopyranoside have been documented as effective antioxidant agents.48 It has scientifically been proven that reduction in the raised levels of cholesterol, especially LDL levels, reduces the risk of cardiovascular complications and the risk of mortality, similarly reduction in TG levels reduces coronary heart diseases.49
The present study showed that CE.Cr produces hypotensive effects, at the dose of 30 mg/kg, that may be due to a combination of important phytoconstituents which show their effects through multiple mechanisms; such as, diuretic activity, ACE inhibitory potential, calcium channel blocking activity, potassium channel opening activity etc. Ferulic acid, found in CE.Cr has been reported to possess free radical scavenging properties and hypotensive effects in spontaneously hypertensive rats which was associated with nitric oxide (NO) mediated vasodilation.50 CE.Cr showed diuretic effects in albino rats which could be due to the presence of saponins and high flavonoid content. Diuretics are considered as one of the suitable choices for the management of uncomplicated hypertension and are often used in combination with antihypertensive drugs for moderate to severe hypertension.51 The presence of diuretic activity in CE.Cr is likely to complement its antihypertensive effects. These effects could possibly be due to the presence of polyphenols and glycosides; that is, chlorogenic acid, ferulic acid, hyperoside, naringenin, quercetin-rhamno-di-hexoside and quercetin-3-O-glucopyranoside.
Atherosclerosis is associated with increased levels of serum TC, LDL and TG, as well as low levels of HDL. HDL is inversely related to total body cholesterol, a decrease in plasma HDL concentration may hasten the onset of atherosclerosis, which leads to ischemic heart disease, by altering cholesterol clearance from the arterial wall.52 The histological studies showed that CE.Cr possesses anti-atherosclerotic effects which were evident from the restoration of normal endothelial cell morphology and a decrease in the number of foam cells as well as a decrease in the levels of LDL and TGs which are the main causes of atherosclerosis. Together, these findings proposed that anti-obesity, anti-atherosclerotic, anti-hypertensive and anti-oxidant effects of CE.Cr lie on its diversely active phytoconstituents and also target the interrelationship of these complications.
Conclusions
The results of the present study concluded that C. edulis possesses potential against obesity, hyperlipidemia and atherosclerosis as evident from the normalization of plasma TC, TGs, LDL and HDL levels, and restoration of endothelial cells integrity that may be due to the presence of polyphenols, glycosides and terpenoids which have been reported to possess cardioprotective properties. These protective effects could possibly be mediated through a combination of antioxidant, diuretic and antihypertensive activities. Therefore, this study provides scientific ground to the folkloric use of C. edulis in cardiovascular complications.
Limitations
HMG-CoA reductase pathway for hypercholesterolemia while the ACE system, calcium channel, and autonomic nervous system for hypertension are the major limitations of this study.
Acknowledgments
The authors acknowledge Bahauddin Zakariya University, Multan, Pakistan, for its support in anti-hypertensive experiments.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Ayesha Jamshed https://orcid.org/0000-0001-7321-8754
Hafiz Muhammad Farhan Rasheed https://orcid.org/0000-0003-2516-7387
Naveed Aslam https://orcid.org/0000-0002-6305-3447
References
- 1.Choi H, Eo H, Park K, et al. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem Biophys Res Commun. 2007;359(3):419-425. [DOI] [PubMed] [Google Scholar]
- 2.Kolodgie FD, Narula J, Guillo P, Virmani R. Apoptosis in human atherosclerotic plaques. Apoptosis. 1999;4(1):5-10. [DOI] [PubMed] [Google Scholar]
- 3.Xiong C, Peng Y, Liu B, Cui W, Liu X. Anti-obesity, anti-atherosclerotic and anti-oxidant effects of Pu-Erh tea on a high fat diet-induced obese rat model. J Biosci Med. 2019;7(02):120-130. [Google Scholar]
- 4.Rowicka G, Dyląg H, Ambroszkiewicz J, Riahi A, Weker H, Chełchowska M. Total oxidant and antioxidant status in prepubertal children with obesity. Oxid Med Cell Longev. 2017;2017:5621989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009;2(5):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y-C, Sheen J-M, Hu WL, Hung Y-C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid Med Cell Longev. 2017;2017:8526438. doi: 10.1155/2017/8526438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20(5):707-727. [DOI] [PubMed] [Google Scholar]
- 8.Patel AK, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS. In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f.: An endemic and endangered edible plant species of the Thar Desert. Sci Hortic. 2014;165:175-180. [Google Scholar]
- 9.Adnan M, Jan S, Mussarat S, et al. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R. Br. J Pharm Pharmacol. 2014;66(10):1351-1368. [DOI] [PubMed] [Google Scholar]
- 10.Ali H, Sannai J, Sher H, Rashid A. Ethnobotanical profile of some plant resources in Malam Jabba valley of Swat, Pakistan. J Med Plants Res. 2011;5(18):4676-4687. [Google Scholar]
- 11.Ahmad M, Qureshi R, Arshad M, Khan MA, Zafar M. Traditional herbal remedies used for the treatment of diabetes from district Attock (Pakistan). Pakistan J Bot. 2009;41(6):2777-2782. [Google Scholar]
- 12.Malik S, Ahmad S, Sadiq A, et al. A comparative ethno-botanical study of Cholistan (an arid area) and Pothwar (a semi-arid area) of Pakistan for traditional medicines. J Ethnobiol Ethnomed. 2015;11(1):1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaseen G, Ahmad M, Zafar M, et al. Traditional management of diabetes in Pakistan: Ethnobotanical investigation from traditional health practitioners. J Ethnopharmacol. 2015;174:91-117. [DOI] [PubMed] [Google Scholar]
- 14.Surveswaran S, Cai Y-Z, Xing J, Corke H, Sun M. Antioxidant properties and principal phenolic phytochemicals of Indian medicinal plants from Asclepiadoideae and Periplocoideae. Nat Prod Res. 2010;24(3):206-221. [DOI] [PubMed] [Google Scholar]
- 15.Shad AA, Bakht J, Shah HU, Hayat Y. Antioxidant activity and nutritional assessment of under-utilized medicinal plants. Pak J Pharm Sci. 2016;29(6):2039-2045. [PubMed] [Google Scholar]
- 16.Sayantan R, Abhishek S. Antidiabetic activity of Caralluma edulis bark and leaf extract against streptozotocin induced diabetic rats. NSHM J Pharm Healthc Manag. 2012;3:76-81. [Google Scholar]
- 17.Firdoos S, Khan AU, Ali F. Anti-nociceptive effect of Caralluma edulis on peripheral and central pain pathways. J Sains Malays. 2017;46(10):1859-1863. [Google Scholar]
- 18.Rasheed HMF, Rasheed F, Qureshi AW, Jabeen Q. Immunostimulant activities of the aqueous methanolic extract of Leptadenia pyrotechnica, a plant from Cholistan desert. J Ethnopharmacol. 2016;186:244-250. [DOI] [PubMed] [Google Scholar]
- 19.Gul H, Jamshed A, Jabeen Q. Pharmacological Investigation of Asphodelus tenuifolius Cav. for its potential against thrombosis in experimental models. Dose Response. 2022;20(3):15593258221127566. doi: 10.1177/15593258221127566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslam N, Janbaz KH. Studies on antidiarrheal and laxative activities of aqueous-ethanol extract of Asphodelus tenuifolius and underlying mechanisms. BMC Compl Alternative Med. 2019;19(1):307. doi: 10.1186/s12906-019-2740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haider SI, Asif A, Rasheed HMF, Akram A, Jabeen Q. Caralluma tuberculata exhibits analgesic and anti-arthritic potential by downregulating pro-inflammatory cytokines and attenuating oxidative stress. Inflammopharmacology. 2022;30(2):621-638. doi: 10.1007/s10787-022-00949-5. [DOI] [PubMed] [Google Scholar]
- 22.Jabeen Q, Khan MS, Qureshi AW, Rasheed HMF. Effect of Abutilon indicum in thyroxine-induced hyperthyroidism in rat. Bangladesh J Pharmacol. 2021;16(3):103-113. [Google Scholar]
- 23.Lee E, Lee MS, Chang E, et al. High hydrostatic pressure extract of mulberry leaves ameliorates hypercholesterolemia via modulating hepatic microRNA-33 expression and AMPK activity in high cholesterol diet fed rats. Food Nutr Res 2021;65:64-74. doi: 10.29219/fnr.v65.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leontowicz M, Leontowicz H, Jesion I, et al. Actinidia arguta supplementation protects aorta and liver in rats with induced hypercholesterolemia. Nutr Res. 2016;36(11):1231-1242. DOI: 10.1016/j.nutres.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Khan TJ, Ahmed YM, Zamzami MA, et al. Effect of atorvastatin on the gut microbiota of high fat diet-induced hypercholesterolemic rats. Sci Rep. 2018;8(1):662. doi: 10.1038/s41598-017-19013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokozawa T, Ishida A, Cho E, Nakagawa T. The effects of Coptidis Rhizoma extract on a hypercholesterolemic animal model. Phytomedicine. 2003;10(1):17-22. [DOI] [PubMed] [Google Scholar]
- 27.Wickramasinghe ASD, Attanayake AP, Kalansuriya P. Biochemical characterization of high fat diet fed and low dose streptozotocin-induced diabetic Wistar rat model. J Pharmacol Toxicol Methods. 2022;113:107144. DOI: 10.1016/j.vascn.2021.107144. [DOI] [PubMed] [Google Scholar]
- 28.Charan J, Kantharia N. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ntchapda F, Maguirgue K, Adjia H, Etet PFS, Dimo T. Hypolipidemic, antioxidant and anti-atherosclerogenic effects of aqueous extract of Zanthoxylum heitzii stem bark in diet-induced hypercholesterolemic rats. Asian Pac J Trop Med. 2015;8(5):359-365. doi: 10.1016/S1995-7645(14)60344-8. [DOI] [PubMed] [Google Scholar]
- 30.Jabeen Q, Aslam N. Hypotensive, angiotensin converting enzyme (ACE) inhibitory and diuretic activities of the aqueous-methanol extract of Ipomoea reniformis. Iran J Pharm Res (IJPR). 2013;12(4):769-776. [PMC free article] [PubMed] [Google Scholar]
- 31.Jamshed A, Jabeen Q. Pharmacological evaluation of Mentha piperita against urolithiasis: An In Vitro and In Vivo Study. Dose-Response. 2022;20(1):15593258211073087. doi: 10.1177/15593258211073087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamshed A, Jabeen Q. Prophylactic and curative potential of peppermint oil against calcium oxalate kidney stones. Pak J Pharm Sci. 2021;34:34-1872. [PubMed] [Google Scholar]
- 33.Ansari B, Behl T, Pirzada AS, Khan H. Caralluma edulis (Apocynaceae): A comprehensive review on its traditional uses, phytochemical profile and pharmacological effects. Curr Top Med Chem. 2022;22(18):1501-1514.doi: 10.2174/1568026622666220527092825. [DOI] [PubMed] [Google Scholar]
- 34.Lei S, He S, Li X, Zheng B, Zhang Y, Zeng H. Effect of lotus seed resistant starch on small intestinal flora and bile acids in hyperlipidemic rats. Food Chem. 2022;404:134599. doi: 10.1016/j.foodchem.2022.134599. [DOI] [PubMed] [Google Scholar]
- 35.Buettner R, Parhofer K, Woenckhaus M, et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36(3):485-501. [DOI] [PubMed] [Google Scholar]
- 36.Nishina PM, Lowe S, Verstuyft J, Naggert JK, Kuypers FA, Paigen B. Effects of dietary fats from animal and plant sources on diet-induced fatty streak lesions in C57BL/6J mice. JLR (J Lipid Res). 1993;34(8):1413-1422. [PubMed] [Google Scholar]
- 37.Ashfaq A, Khan A-u, Minhas AM, Aqeel T, Assiri AM, Bukhari IA. Anti-hyperlipidemic effects of Caralluma edulis (Asclepiadaceae) and Verbena officinalis (Verbenaceae) whole plants against high-fat diet-induced hyperlipidemia in mice. Trop J Pharmaceut Res. 2017;16(10):2417-2423. [Google Scholar]
- 38.Shrivastava AK, Thapa S, Shrestha L, Mehta RK, Gupta A, Koirala N. Phytochemical screening and the effect of Trichosanthes dioica in high-fat diet induced atherosclerosis in Wistar rats. Food Frontiers. 2021;2(4):527-536. doi: 10.1002/fft2.91. [DOI] [Google Scholar]
- 39.Sinaga E, Suprihatin Y, Iswahyudi M, Setyowati S, Prasasty VD. Effect of supplementation of Rhodomyrtus tomentosa fruit juice in preventing hypercholesterolemia and atherosclerosis development in rats fed with high fat high cholesterol diet. Biomed Pharmacother. 2021;142:111996. doi: 10.1016/j.biopha.2021.111996. [DOI] [PubMed] [Google Scholar]
- 40.Cai X, Liu Z, Dong X, et al. Hypoglycemic and lipid lowering effects of theaflavins in high-fat diet-induced obese mice. 10.1039/D1FO01966J. Food Funct. 2021;12(20):9922-9931. doi: 10.1039/D1FO01966J. [DOI] [PubMed] [Google Scholar]
- 41.Vellasamy DM, Lee S-J, Goh KW, et al. Targeting immune senescence in atherosclerosis. Int J Mol Sci. 2022;23(21):13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morikawa T, Xie Y, Asao Y, et al. Oleanane-type triterpene oligoglycosides with pancreatic lipase inhibitory activity from the pericarps of Sapindus rarak. Phytochemistry. 2009;70(9):1166-1172. [DOI] [PubMed] [Google Scholar]
- 43.Chedea VS, Macovei ȘO, Bocsan IC, et al. Grape pomace polyphenols as a source of compounds for management of oxidative stress and inflammation—A possible alternative for non-steroidal anti-inflammatory drugs? Molecules. 2022;27(20):6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y, Xie H, Chen H, et al. Terpenoids and phenylpropanoids isolated from the twings and leaves of Abelia macrotera and their anti inflammatory activities. Chem Biodivers. 2022;19:e202200870. [DOI] [PubMed] [Google Scholar]
- 45.Barter P. The role of HDL-cholesterol in preventing atherosclerotic disease. Eur Heart J Suppl. 2005;7(suppl_F):F4-F8. [Google Scholar]
- 46.Zobeiri M, Belwal T, Parvizi F, et al. Naringenin and its nano-formulations for fatty liver: Cellular modes of action and clinical perspective. Curr Pharmaceut Biotechnol. 2018;19(3):196-205. [DOI] [PubMed] [Google Scholar]
- 47.Karthikesan K, Pari L, Menon VP. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem Biol Interact. 2010;188(3):643-650. doi: 10.1016/j.cbi.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 48.Akram A, Jabeen Q. Pharmacological evaluation of Typha domingensis for its potentials against diet-induced hyperlipidemia and associated complications. Trop J Pharmaceut Res. 2021;21(3):563-569. [Google Scholar]
- 49.Bubb KJ, Nelson AJ, Nicholls SJ. Targeting triglycerides to lower residual cardiovascular risk. Expet Rev Cardiovasc Ther. 2022;20(3):185-191. doi: 10.1080/14779072.2022.2058489. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe Y, Nessa N, Toba H, Kobara M, Nakata T. Angelica acutiloba exerts antihypertensive effect and improves insulin resistance in spontaneously hypertensive rats fed with a high-fat diet. Pharmacology. 2022;107(3-4):188-196. doi: 10.1159/000520982. [DOI] [PubMed] [Google Scholar]
- 51.Krakoff LR. Diuretics for hypertension. Circulation. 2005;112(10):e127-e129. doi: 10.1161/CIRCULATIONAHA.105.570192. [DOI] [PubMed] [Google Scholar]
- 52.Duan Y, Gong K, Xu S, Zhang F, Meng X, Han J. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct Targeted Ther. 2022;7(1):265. doi: 10.1038/s41392-022-01125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]