Abstract
Arrhythmogenic right ventricular cardiomyopathy/dysplasia is inherited cardiomyopathy that has a propensity for ventricular arrhythmia, ventricular dysfunction, and sudden cardiac death. High-intensity exercise is associated with early disease manifestation and increased risk of malignant arrhythmia and sudden death. Exercise restriction should be advised as an integral part of disease management. This overview summarizes the medical literature on the impact of exercise in triggering ventricular arrhythmias and disease progression.
Keywords: Arrhythmogenic cardiomyopathy, desmosomal mutation, dysplasia, exercise, metabolic equivalent, plakophilin-2
INTRODUCTION
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) was initially described in 1977, then its clinical features were reported in 1982.[1,2] ARVC/D is an inherited cardiomyopathy[1,3,4,5] with a prevalence of 1 in 5000 individuals.[6] It is characterized by ventricular arrhythmia, right ventricular dysfunction, and sudden cardiac death,[1,3,4,5] especially in young and competitive athletes.[4,6]
The diagnosis of ARVC/D accounts for up to 20% of those who experience sudden cardiac death and is more frequent among athletes.[1] Although the etiology is not fully known, there are underlying genetic abnormalities.[6] ARVC/D is mainly caused by inherited mutations in the desmosomal complex proteins.[5,7] Mutations are usually present in the desmosomal proteins plakophilin-2 (PKP2), desmoplakin, desmoglein-2, and, rarely, plakoglobin and desmocollin-2.[2] ARVC/D is a genetically complex condition with marked intrafamilial phenotype diversity.[8]
However, the genetic cause is unknown in 40%–50% of patients.[2] The main pathophysiological characteristic of the disease is the progressive loss of myocytes and replacement by fibrous or fibrofatty tissue of the right ventricular myocardium,[1,2,6,7,9] that create substrates for ventricular arrhythmia.[9]
The clinical manifestations of the disease range from asymptomatic patients with sudden cardiac death as the first symptom to patients with chronic symptoms and recurrent palpitations with or without right or biventricular failure.[6]
ARVC/D is diagnosed by major and minor Task Force Criteria.[7,10,11] Diagnostic modalities include electrocardiography (e.g., Epsilon wave in approximately one-third of patients), echocardiography, computerized tomography, magnetic resonance imaging, and right ventricular cineangiography to detect right ventricular morphologic abnormalities.[2,6]
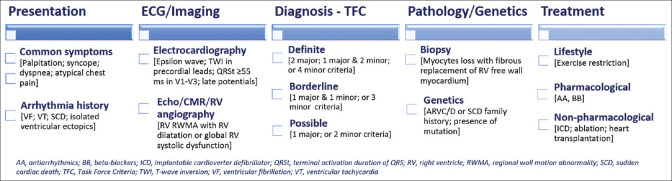
The crucial goal of therapy is preventing ventricular arrhythmias, sudden cardiac death, and disease progression, with medications, implantable cardioverter-defibrillator (ICD), and radiofrequency ablation as indicated.[3,6] Figure 1 summarizes the clinical approach to ARVC/D and highlights the classic features and assessment.[1,2,11,12,13] Physical activity has been recognized as a risk factor for disease manifestation and progression.[7] The present overview[14] aims to summarize medical literature on the role of sport participation in triggering ventricular arrhythmias and disease progression in ARVC/D patients by surveying the literature and describing its characteristics.
Figure 1.
Clinical approach to arrhythmogenic right ventricular cardiomyopathy/dysplasia
METHODS
An electronic PubMed search was conducted to identify studies that examined sport participation by patients with ARVC/D. The Medical Subject Headings (MeSH) terms used and combined were “Arrhythmogenic Right Ventricular Dysplasia” [Mesh] AND “Exercise” [Mesh], which resulted in 72 records. Seven animal or preclinical[15,16,17,18,19,20,21] and 18 clinical studies[22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] were selected and discussed. [Table 1] presents the definitions of terms used in the identified studies.[22,23,25,26,27,28,29,30,32,33,36]
Table 1.
Relevant terms and definitions used in the literature
| Term | Subterm | Definition |
|---|---|---|
| Exercise | - | Physical activity performed on a regular basis during a specified period[26] |
| Exercise levels | - | Inactive, recreational, and competitive/professional[23] |
| Exercise intensity | Vigorous | Activity ≥6 MET[22,32] |
| High | Activity ≥9 MET-h/day[28] | |
| Activity >6 MET, for example, running, aerobics, fast swimming, or competitive sports[26] | ||
| High/competitive | Activity >3 h/week[25] | |
| Moderate | Activity 1-3 h/week[25] | |
| Moderate-low | Activity <9 MET-h/day[28] | |
| Low | Activity 3-6 MET, for example, walking, dancing, or weightlifting[26] | |
| Minimal/inactive | <1 h/week[25] | |
| Aerobic | Participation in vigorous-intensity endurance (aerobic) athletics[33] | |
| Others | Light, moderate, or vigorous using language and definitions from the multiethnic study of atherosclerosis typical week physical activity survey[29,32,33] | |
| Exercise duration | - | Actual time in motion expressed as average hours per week[26] |
| - | Calculated for every activity separately and summed to achieve the total hours spent exercising before clinical presentation then the value is annualized[27,29] | |
| Sport | High dynamic | Such as basketball, soccer, hockey, skiing, running, biking, and tennis[23,25] |
| Low-to-moderate dynamic | Such as bowling, golf, weightlifting, wrestling, baseball, or softball[23] | |
| Athletes | - | Subjects with a history of physical activity with intensity ≥6 MET for ≥4 h/week (≥1440 MET × min/week) during minimum 6 years[22] |
| - | Subjects performing at least 3 h/week of sports with a moderate to intense dynamic component (at least 6 MET in intensity) in the 3 years preceding presentation, translating to 936 MET-h per year[29] | |
| Endurance | Participants in sports with high dynamic demand (>70% max O2), as defined by the 36th Bethesda conference classification of sports (task force 8), done for at least 50 h/year at vigorous intensity[27,33] | |
| Exercise intensity or dose calculation | - | According to a 2011 compendium of physical activities expressed as MET,[26,27,32,36] for example, 1 MET is energy spent sitting at rest, 3 MET walking at a slow pace on even ground, 7 MET jogging, and 12 MET running vigorously[27] |
| Some studies reported physical activity in MET-h, while others reported activity in hours per given period and intensity, for example, MET-h/year[30] | ||
| One MET | Represents an individual’s energy expenditure while sitting quietly and is approximated to 3.5 mL O2/kg min or 1 kcal/kg h[22] | |
| MET-h | A tool for measuring energy expenditure associated with physical activity[27,32] | |
| Calculated by using the metabolic equivalent of task hours[29,32,36] | ||
| MET-h/week | Calculated by multiplying exercise intensity (in MET) by weekly exercise duration (h)[26,36] | |
| MET-h/year | MET value for each exercise activity multiplied by its duration and annualized to obtain MET-h per year[27,29,32] | |
| Individual’s level of physical fitness | - | Measured with VO2 maximum, i.e., the amount of oxygen consumed at peak aerobic effort[30] |
| Minimum required physical activity* | - | Calculated by applying established metabolic conversion equations to minimum VO2 maximum (in MET) required to pass service-specific physical fitness test[30] |
*For military retention. MET: Metabolic equivalent, MET-h: MET of task-hours
RESULTS AND DISCUSSION
Preclinical studies
The heterozygous plakoglobin deficiency or decreased desmosomal protein expression in mice led to ARVC/D. The affected mice had significant alterations in right ventricular volume (i.e., increased) and function (i.e., decreased), in addition to spontaneous ventricular ectopy without histological abnormalities in the right ventricle.
Endurance training enhanced the manifestations of right ventricular dysfunction and arrhythmias.[15] Subsequent studies confirmed that PKP2 loss in mice led to an exercise-dependent ARVC/D phenotype[16] or acceleration of disease progression.[17] van Opbergen explored the cellular changes that trigger the arrhythmogenic state and unveiled that exercise can dysregulate the intracellular calcium homeostasis in the PKP2-deficient murine model through the activation of intracellular beta-adrenergic receptors and hyperphosphorylation of phospholamban. It was suggested that membrane-permeable beta-blockers may be more effective in ARVC/D patients.[18] Interestingly, reducing the ventricular load (i.e., with furosemide and nitrates) prevented ARVC/D induced by training in plakoglobin-deficient mice.[19] While the heterozygous plakoglobin deficiency did not affect left ventricular size or function,[15] homozygous desmoplakin deficiency was manifested as a biventricular form of ARVC/D. The affected mice exhibited biventricular function impairment and premature death, in which catecholamine stimulation and exercise exacerbated ventricular arrhythmia.[20] A study on a murine model confirmed the effect of chronic endurance exercise on desmoplakin mutation-induced ARVC/D pathogenesis.[21]
CLINICAL STUDIES
Impact of exercise and its intensity
Saberniak et al. examined exercise impact on myocardium in individuals with ARVC/D (n = 110). Athletes (n = 37) as compared to nonathletes (n = 73) had impaired left and right ventricular function. Furthermore, exercise intensity was correlated with reduced biventricular functions.[22] In their study, Ruwald et al. questioned the enrolled probands (n = 108), within three sports categories, about their exercise levels before and after their ARVC/D diagnosis. They found that there was a twofold increase in the risk of ventricular arrhythmia and death within the competitive sports category as compared with recreational (hazard ratio 1.99, 95% confidence interval (CI) 1.21–3.28, P = 0.007) and inactive (hazard ratio 2.05, 95% CI 1.07–3.91, P = 0.03) sports categories. There was no difference in risk between the latter two categories (hazard ratio 1.03, 95% CI 0.54–1.97, P = 0.930). Individuals participating in competitive sports developed symptoms at an earlier age compared to those in the other categories (at 30 ± 12 vs. 38 ± 17 vs. 41 ± 11 years, significant for both comparisons) and without significant difference in age between the latter two categories.[23]
An observational study indicated that recreational sport did not affect the long-term outcomes of radiofrequency catheter ablation therapy of ventricular tachycardia in ARVC/D patients when compared with the inactive lifestyle. Advanced age was the only variable that was significantly associated with the recurrence of ventricular tachycardia (hazard ratio 1.15, 95% CI 1.05–1.26, P = 0.004).[24]
Through a clinical interview, Ruiz et al. collected data from individuals about their intensity of exercise in the 10 years before the diagnosis and defined it as high, moderate, and minimal (i.e., >3 h, 1–2 h, and <1 h per week, respectively). The occurrence of the first major arrhythmia and severe right ventricular impairment was earlier in the high-intensity group, then in the moderate intensity, and at a later age in the minimal-intensity groups.[25] Similarly, in a study that enrolled 173 ARVC/D patients, Lie et al. found that life-threatening ventricular arrhythmia was significantly more frequent with high-intensity (74% vs. 20%, P < 0.001) and long-duration (65% vs. 31%, P < 0.001) exercises. However, high-intensity, not long-duration, exercise was a predictor of arrhythmias (odds ratio 3.8; 95% CI 1.3–11, P < 0.001).[26]
When individuals without desmosomal mutations (i.e., gene elusive) were compared with those with desmosomal mutations, gene-elusive individuals were significantly more likely to perform endurance sports and more intense exercise before presentation, especially among those presenting at an age younger than 25 years. Their family history of the disease was less prevalent than that for individuals with desmosomal mutations (9% vs. 40%, P < 0.001), proposing an environmental influence. Gene-elusive patients who performed the most intense exercise before their disease presentation were younger at presentation (P = 0.025) and had shorter arrhythmia-free survival during the follow-up (P = 0.002).[27] Finally, the first study to investigate the association of high-level exercise with ARVC/D secondary to TMEM43 p.S358L mutation (n = 80) suggested that exercise ≥9 metabolic equivalents of task-hour (MET-h)/day increased the risk of malignant ventricular arrhythmia.[28]
Exercise restriction
In a study that recruited 129 ARVC/D patients with an ICD, Wang et al. reported that restricting the dose and duration of exercise was associated with decreased ventricular arrhythmia rates (hazard ratio 0.14, 95% CI 0.04–0.44 and hazard ratio 0.23, 95% CI 0.07–0.81, respectively). The benefit of restricting exercise was seen more in those with an ICD and those without desmosomal mutations but not in athletes.[29] The study by Segre et al. aimed to define a therapeutic window for exercise and find a balance between maintaining a safe level of exercise and continuing military service in ARVC/D gene-positive participants. As reported in the literature, 700–1100 MET-h/year was not associated with inferior clinical outcomes, the study found that a member of the military service needs 600–700 MET-h/year for passing the physical fitness test.[30]
In a longitudinal review of 109 charts, physical activity restriction was significantly more common (80%) in phenotype-positive children with inherited cardiomyopathies or arrhythmias, including ARVC/D, compared with their phenotype-negative counterparts (37%). Nonetheless, 38% of children continued their participation in competitive sports.[31] Genotype-positive ARVC/D family members with desmosomal variants should limit their athletic activities as reported by Wang et al. The investigators demonstrated that high exercise dose and duration led to ARVC/D manifestations. However, the risk of developing ARVC/D or ventricular arrhythmia was much lower when exercising within the guideline's recommended limits.[32]
In a study on patients with desmosomal mutations, 87 carriers were interviewed about their regular exercise since the age of 10. Endurance and frequent exercise increased the risk of ventricular tachycardia/fibrillation, heart failure, and ARVC/D. Moreover, the study findings supported exercise restriction for mutation carriers as well.[33] A study on 28 family members with PKP2 mutation carriers reported that those restricting exercise below 650 MET-h/year (i.e., the upper bound of American guidelines’ goal) did not experience ventricular tachycardia/fibrillation events and had lower odds to be diagnosed with ARVC/D (adjusted odds ratio 0.07; 95% CI 0.01–0.38, P = 0.002).[34]
Risk prediction
Predictors for the first life-threatening ventricular arrhythmia in ARVC/D probands and mutation-positive family members (n = 117) included a history of high-intensity exercise (adjusted hazard ratio 4.7, 95% CI 1.2–17.5, P = 0.02), T-wave inversions ≥V3 (adjusted hazard ratio 4.7, 95% CI 1.6–13.9, P = 0.005), and greater left ventricular mechanical dispersion (adjusted hazard ratio 1.4, 95% CI 1.2–1.6, P < 0.001).
In individuals without risk factors, the median survival without arrhythmia was 12 years as compared to 1.2 years in those with all risk factors.[35] Bosman et al. interviewed 176 ARVC/D patients without sustained ventricular arrhythmia and quantified the effect of exercise, at diagnosis, on the incidence of ventricular arrhythmia. They found that exercise was associated with a sustained risk of arrhythmia when its intensity is above 15 to 30 MET-h/week.[36] Gasperetti et al. examined the consequences of detraining 25 athletes on ARVC/D progression after the initial diagnosis and evaluated the reliability of the Cadrin-Tourigny arrhythmic risk prediction algorithm. Clinical detraining significantly reduced premature ventricular complex burden over 5 years. The authors concluded that the algorithm does not require correction to be applied to ARVC/D athletes.[37] Lie reported that higher exercise exposure resulted in worse left ventricular function at the first encounter with ARVC/D patients (n = 168) but did not affect the progression rate during the follow-up. However, left ventricular function deterioration was associated with the incidence of subsequent ventricular arrhythmia and may be accounted for in risk stratification.[38]
Finally, a study concluded that sex hormone serum levels may be used in risk stratification for ARVC/D patients. It found that increased testosterone and reduced estradiol levels can affect disease pathology and are associated with major arrhythmic cardiovascular events.[39]
GUIDELINES RECOMMENDATIONS
The international guidelines including the European Society of Cardiology and the Sports Cardiology Section of the European Association of Preventive Cardiology discourage patients or athletes with arrhythmogenic cardiomyopathy to participate in high-intensity or competitive sports. This recommendation also extends to genetic carriers of the pathogenic variants of the disease regardless of the presence of overt disease phenotype.[13,40,41] These athletes should limit the exercise to leisure-time activities and should continue regular clinical follow-ups.[41] The American Heart Association/American College of Sports Medicine recommended activities of moderate intensity of at least 150 or vigorous-intensity activity of at least 75 min per week for cardiovascular health in healthy adults.[42] Wang et al. discussed that such recommendation is equivalent to activity ranges from 390 to 650 MET-h/year and considered 650 MET-h/year (i.e., the upper bound) as a cutoff in their study analyses.[32]
SUMMARY
ARVC/D has an inherent propensity for ventricular arrhythmia, ventricular dysfunction, and sudden cardiac death. Clinical and preclinical studies showed that high-intensity exercise is associated with early disease manifestation and increased risk of malignant arrhythmias and sudden cardiac death. Recreational sport or low-to-moderate intensity exercise may not be detrimental and ARVC/D individuals should not be completely deprived of the benefits of exercise. Exercise restriction advice is an integral part of management for both individuals with established disease and those who carry gene mutations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, et al. Arrhythmogenic right ventricular dysplasia: A United States experience. Circulation. 2005;112:3823–32. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 2.Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:784–804. doi: 10.1016/j.jacc.2018.05.065. [DOI] [PubMed] [Google Scholar]
- 3.Orgeron GM, Calkins H. Advances in the diagnosis and management of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Curr Cardiol Rep. 2016;18:53. doi: 10.1007/s11886-016-0732-y. [DOI] [PubMed] [Google Scholar]
- 4.Coelho SA, Silva F, Silva J, António N. Athletic training and arrhythmogenic right ventricular cardiomyopathy. Int J Sports Med. 2019;40:295–304. doi: 10.1055/a-0750-5848. [DOI] [PubMed] [Google Scholar]
- 5.Prior D, La Gerche A. Exercise and arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ. 2020;29:547–55. doi: 10.1016/j.hlc.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Francés RJ. Arrhythmogenic right ventricular dysplasia/cardiomyopathy. A review and update. Int J Cardiol. 2006;110:279–87. doi: 10.1016/j.ijcard.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Murray B. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): A review of molecular and clinical literature. J Genet Couns. 2012;21:494–504. doi: 10.1007/s10897-012-9497-7. [DOI] [PubMed] [Google Scholar]
- 8.Quarta G, Muir A, Pantazis A, Syrris P, Gehmlich K, Garcia-Pavia P, et al. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: Impact of genetics and revised task force criteria. Circulation. 2011;123:2701–9. doi: 10.1161/CIRCULATIONAHA.110.976936. [DOI] [PubMed] [Google Scholar]
- 9.Zorzi A, Cipriani A, Bariani R, Pilichou K, Corrado D, Bauce B. Role of exercise as a modulating factor in arrhythmogenic cardiomyopathy. Curr Cardiol Rep. 2021;23:57. doi: 10.1007/s11886-021-01489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–8. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–14. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulot JS, Jouven X, Empana JP, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–84. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 13.Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: An international task force consensus statement. Eur Heart J. 2015;36:3227–37. doi: 10.1093/eurheartj/ehv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant MJ, Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof P, Fabritz L, Zwiener M, Witt H, Schäfers M, Zellerhoff S, et al. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation. 2006;114:1799–806. doi: 10.1161/CIRCULATIONAHA.106.624502. [DOI] [PubMed] [Google Scholar]
- 16.Cruz FM, Sanz-Rosa D, Roche-Molina M, García-Prieto J, García-Ruiz JM, Pizarro G, et al. Exercise triggers ARVC phenotype in mice expressing a disease-causing mutated version of human plakophilin-2. J Am Coll Cardiol. 2015;65:1438–50. doi: 10.1016/j.jacc.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Cerrone M, Marrón-Liñares GM, van Opbergen CJ, Costa S, Bourfiss M, Pérez-Hernández M, et al. Role of plakophilin-2 expression on exercise-related progression of arrhythmogenic right ventricular cardiomyopathy: A translational study. Eur Heart J. 2022;43:1251–64. doi: 10.1093/eurheartj/ehab772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Opbergen CJ, Bagwan N, Maurya SR, Kim JC, Smith AN, Blackwell DJ, et al. Exercise causes arrhythmogenic remodeling of intracellular calcium dynamics in plakophilin-2-deficient hearts. Circulation. 2022;145:1480–96. doi: 10.1161/CIRCULATIONAHA.121.057757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabritz L, Hoogendijk MG, Scicluna BP, van Amersfoorth SC, Fortmueller L, Wolf S, et al. Load-reducing therapy prevents development of arrhythmogenic right ventricular cardiomyopathy in plakoglobin-deficient mice. J Am Coll Cardiol. 2011;57:740–50. doi: 10.1016/j.jacc.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Lyon RC, Mezzano V, Wright AT, Pfeiffer E, Chuang J, Banares K, et al. Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum Mol Genet. 2014;23:1134–50. doi: 10.1093/hmg/ddt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martherus R, Jain R, Takagi K, Mendsaikhan U, Turdi S, Osinska H, et al. Accelerated cardiac remodeling in desmoplakin transgenic mice in response to endurance exercise is associated with perturbed Wnt/β-catenin signaling. Am J Physiol Heart Circ Physiol. 2016;310:H174–87. doi: 10.1152/ajpheart.00295.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail. 2014;16:1337–44. doi: 10.1002/ejhf.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruwald AC, Marcus F, Estes NA, 3rd, Link M, McNitt S, Polonsky B, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: Results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36:1735–43. doi: 10.1093/eurheartj/ehv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müssigbrodt A, Czimbalmos C, Stauber A, Bertagnolli L, Bode K, Dagres N, et al. Effect of exercise on outcome after ventricular tachycardia ablation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Int J Sports Med. 2019;40:657–62. doi: 10.1055/a-0962-1325. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz Salas A, Barrera Cordero A, Navarro-Arce I, Jiménez Navarro M, García Pinilla JM, Cabrera Bueno F, et al. Impact of dynamic physical exercise on high-risk definite arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Electrophysiol. 2018;29:1523–9. doi: 10.1111/jce.13704. [DOI] [PubMed] [Google Scholar]
- 26.Lie ØH, Dejgaard LA, Saberniak J, Rootwelt C, Stokke MK, Edvardsen T, et al. Harmful effects of exercise intensity and exercise duration in patients with arrhythmogenic cardiomyopathy. JACC Clin Electrophysiol. 2018;4:744–53. doi: 10.1016/j.jacep.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Sawant AC, Bhonsale A, te Riele AS, Tichnell C, Murray B, Russell SD, et al. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc. 2014;3:e001471. doi: 10.1161/JAHA.114.001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulin FL, Hodgkinson KA, MacLaughlan S, Stuckless SN, Templeton C, Shah S, et al. Exercise and arrhythmic risk in TMEM43 p.S358L arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020;17:1159–66. doi: 10.1016/j.hrthm.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Orgeron G, Tichnell C, Murray B, Crosson J, Monfredi O, et al. Impact of exercise restriction on arrhythmic risk among patients with arrhythmogenic right ventricular cardiomyopathy. J Am Heart Assoc. 2018;7:e008843. doi: 10.1161/JAHA.118.008843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segre EM, Hellwig LD, Turner C, Dobson CP, Haigney MC. Exercise dose associated with military service: Implications for the clinical management of inherited risk for arrhythmogenic right ventricular cardiomyopathy. Mil Med. 2020;185:e1447–52. doi: 10.1093/milmed/usaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christian S, Somerville M, Giuffre M, Atallah J. Physical activity restriction for children and adolescents diagnosed with an inherited arrhythmia or cardiomyopathy and its impact on body mass index. J Cardiovasc Electrophysiol. 2018;29:1648–53. doi: 10.1111/jce.13713. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Tichnell C, Murray BA, Agafonova J, Cadrin-Tourigny J, Chelko S, et al. Exercise restriction is protective for genotype-positive family members of arrhythmogenic right ventricular cardiomyopathy patients. Europace. 2020;22:1270–8. doi: 10.1093/europace/euaa105. [DOI] [PubMed] [Google Scholar]
- 33.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–7. doi: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawant AC, Te Riele AS, Tichnell C, Murray B, Bhonsale A, Tandri H, et al. Safety of American Heart Association-recommended minimum exercise for desmosomal mutation carriers. Heart Rhythm. 2016;13:199–207. doi: 10.1016/j.hrthm.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 35.Lie ØH, Rootwelt-Norberg C, Dejgaard LA, Leren IS, Stokke MK, Edvardsen T, et al. Prediction of life-threatening ventricular arrhythmia in patients with arrhythmogenic cardiomyopathy: A primary prevention cohort study. JACC Cardiovasc Imaging. 2018;11:1377–86. doi: 10.1016/j.jcmg.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Bosman LP, Wang W, Lie ØH, van Lint FH, Rootwelt-Norberg C, Murray B, et al. Integrating exercise into personalized ventricular arrhythmia risk prediction in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2022;15:e010221. doi: 10.1161/CIRCEP.121.010221. [DOI] [PubMed] [Google Scholar]
- 37.Gasperetti A, Dello Russo A, Busana M, Dessanai M, Pizzamiglio F, Saguner AM, et al. Novel risk calculator performance in athletes with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020;17:1251–9. doi: 10.1016/j.hrthm.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Lie ØH, Chivulescu M, Rootwelt-Norberg C, Ribe M, Bogsrud MP, Lyseggen E, et al. Left ventricular dysfunction in arrhythmogenic cardiomyopathy: Association with exercise exposure, genetic basis, and prognosis. J Am Heart Assoc. 2021;10:e018680. doi: 10.1161/JAHA.120.018680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akdis D, Saguner AM, Shah K, Wei C, Medeiros-Domingo A, von Eckardstein A, et al. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: From a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur Heart J. 2017;38:1498–508. doi: 10.1093/eurheartj/ehx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 41.Pelliccia A, Solberg EE, Papadakis M, Adami PE, Biffi A, Caselli S, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC) Eur Heart J. 2019;40:19–33. doi: 10.1093/eurheartj/ehy730. [DOI] [PubMed] [Google Scholar]
- 42.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]