Abstract
Context
Taohong Siwu decoction (THSWD) has been shown to promote heart repair in myocardial infarction.
Objective
To determine the effects of modified THSWD (THSWD plus four ingredients) on myocardial ischaemia and reperfusion (I/R) injury.
Materials and methods
Sixty Sprague-Dawley rats were randomly divided into the I/R group and three different modified THSWD dose groups (gavage administration, 1.215, 2.43, and 4.86 g, respectively). 2,3,5-Triphenyltetrazolium chloride and Evans blue staining were used to detect the infarct area at 24 h after treatment. The serum biochemical indexes and cell apoptosis were examined to determine myocardial injury. The number of endogenous stem cells, expression of stromal dell derived factor-1 (SDF-1) and stem cell factor (SCF), and cardiac function were measured at 4 weeks. The serum was collected for metabolomic analysis.
Results
The high-dose modified THSWD group presented a reduced infarction area (decreased by 21.3%), decreased levels of lactate dehydrogenase and creatinine kinase, attenuated cell apoptosis, and enhanced superoxide dismutase activity in early stage I/R compared with other groups. The serum SCF and SDF-1 levels were higher in the high-dose group than in the I/R group. At 4 weeks, the infarct size and collagen content were the lowest, and the ejection fraction and fractional shortening values were the highest in the high-dose group. Moreover, high-dose modified THSWD affected the metabolism of phosphonate and phosphonate, taurine, and hypotaurine.
Conclusions
Endogenous stem cell mobilization and metabolic regulation were related to the cardioprotection of modified THSWD. We provided a new strategy and direction for the treatment of cardiovascular diseases with traditional Chinese medicine.
Keywords: Apoptosis, traditional Chinese medicine, cardiovascular diseases, heart repair
Introduction
Ischaemic heart disease remains a major disease worldwide and is one of the main causes of human death (Severino et al. 2020). Recently, the mortality rate of acute myocardial infarction (MI) has rapidly increased, and infarct size is the main determinant of MI prognosis (Stone et al. 2016). The most common cause of acute MI is reduction or cessation of blood flow to the myocardium, leading to myocardial necrosis (Vafaie 2016). Reperfusion therapy is the most important treatment for acute ST-segment elevation MI. Recanalization of coronary artery occlusion within 12 h can decrease the infarct area and reduce mortality (Rentrop and Feit 2015; Esposito et al. 2018). However, reperfusion may further aggravate the death of myocardial cells, increasing infarct size, known as myocardial ischaemia and reperfusion (I/R) injury (Neri et al. 2017; Davidson et al. 2018). Therefore, finding ways to limit the death of cardiomyocytes during and after acute I/R has been the focus of extensive research over the past 30 years.
Traditional Chinese medicine (TCM) has many advantages for the prevention and treatment of cardiovascular diseases, such as low price, fewer side effects, and good acceptability by patients (Hao et al. 2017). The Taohong Siwu Decoction (THSWD) consists of Dihuang (DH; Rehmannia glutinosa Libosch [Scrophulariaceae]); Danggui (DG; Radix Angelica sinensis (Oliv.) Diels [Umbelliferae]); Chuanxiong (CX; Ligusticum chuanxiong Hort. [Umbelliferae]); Baishao (BS; Paeonia lactiflora Pall. [Ranunculaceae]); Honghua (HH; Carthamus tinctorius L. [Compositae]; Taoren (TR; Prunus persica (L.) Batsch [Rosaceae]). THSWD can activate blood circulation, remove blood stasis, and relieve collateral circulation (Tao et al. 2020; Wang et al. 2020). Its use originated from the Heart Tips of Yizong Jinjian Gynaecology (Volume 44), written by Wu Qian during the Qing Dynasty in 1742. Previous studies on cardiovascular diseases have demonstrated that THSWD improves blood lipid levels, reduces cardiomyocyte damage, promotes endothelial cell proliferation, and inhibits inflammation (Xu et al. 2006; Zhang et al. 2018; Fuping et al. 2019). Recently, the role of THSWD in MI has also been demonstrated. For example, we found that THSWD can protect cardiac function by improving the microenvironment and reducing mitochondrial fission after MI (Luo et al. 2019). THSWD has also been shown to reduce calcium overload and the inflammatory response of myoblast cells in I/R rats, thereby improving myocardial injury caused by I/R (Fuping et al. 2019).
In clinical practice, modifying THSWD by adding or removing some ingredients might have a better therapeutic effect than THSWD alone. Studies have shown that modified THSWD can improve the metabolic imbalance of thrombin and prostacyclin to treat intractable paediatric nephropathy (Yu et al. 2000). However, since myocardial I/R injury is dominated by qi deficiency and blood stasis, the function of modified THSWD warrants further research. Moreover, the current research on various modified THSWD in the treatment of MI is limited to the observation of their curative effects, and their mechanisms have not been clarified. Hence, in the present study, we constructed a rat myocardial I/R injury model to analyze the effects of modified THSWD on myocardial protection during early and long-term I/R injury. Additionally, we clarified the effects of modified THSWD on endogenous stem cell mobilization and related chemokine expression to explore the potential mechanisms. Furthermore, we used metabonomic analysis to further understand the effects of high-dose modified THSWD. Overall, we provide a practical basis and data reference for the mechanisms of action of modified THSWD and its clinical application in the treatment of cardiovascular diseases such as MI.
Materials and methods
I/R Rat model
Sixty Sprague-Dawley (SD) rats (180–200 g) were purchased from the Experimental Animal Centre of Shanghai University of TCM (Shanghai, China). The rats were anaesthetized with isoflurane (Hebei Yipin Pharmaceutical, Co., Ltd., Shijiazhuang, China) at a flow rate of 0.25 L/min, and fixed on the surgical table in a supine position. After successful endotracheal intubation, the endotracheal tube was connected to a rodent ventilator (Harvard Apparatus; Holliston, MA, USA). Next, the rats were maintained under anaesthesia, and the ventilation rate was set to 120/min. A longitudinal incision was made and the pectoralis major and pectoralis minor muscle layers were separated until the ribs were exposed. To expose the heart, the thoracic cavity of the rats was opened using a chest expander in the intercostal space of the third rib. The lower edge of the left anterior descending coronary artery (LAD) was passed through a suture needle, and a latex tube was placed at the ligation site to avoid damaging the artery. After successful ligation, the outer surface of the left ventricle anterior wall became pale. The chest expander was removed, and the surgical opening was closed for 30 min of ischaemia. After the ischaemia was completed, a chest expander was placed to expose the heart again to untie the knot and remove the latex tube. The incision was closed and disinfected using iodophor. This study was approved by the Animal Ethics Committee of Shanghai University of TCM (No. PZSHUTCM190628003). We attempted to minimize the number of rats used and animal suffering during the experimental procedure.
Intragastric administration of modified THSWD
Using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA), 60 SD rats were randomly divided into 4 groups: I/R, low-dose modified THSWD, medium-dose modified THSWD, and high-dose modified THSWD groups (n = 15). In the I/R group, rats were intragastrically administered physiological saline after I/R. In the modified THSWD group, rats received the appropriate dose (listed below) of modified THSWD for 5 days before the establishment of the I/R model. After successful establishment of the I/R model, rats continued to receive the corresponding dose of modified THSWD for 24 h to 4 weeks (1 mL/100 g, twice a day). The crude herbal extract for the preparation of modified THSWD was provided by the Shuguang Hospital affiliated with the Shanghai University of TCM (Shanghai, China), and its components were identified and verified by two experts in TCM and clinicians. Voucher specimens of the crude drugs were maintained appropriately. Low-dose modified THSWD contained TR (0.08 g), HH (0.08 g), DG (0.11 g), BS (0.09 g), CX (0.07 g), DH (0.135 g), Qianghuo (QH; Notopterygium incisum Ting ex H. T. Chang [Umbelliferae]) (0.135 g), Yinyanghuo (YYH; Epimedium brevicornu Maxim. [Berberidaceae]) (0.135 g), Danshen (DS; Salvia miltiorrhiza Bge. [Lamiaceae]) (0.27 g), and Astragalus membranaceus (Fisch.) Bge. [Leguminosae]) (0.2 g). Medium-dose modified THSWD contained TR (0.16 g), HH (0.16 g), DG (0.22 g), BS (0.18 g), CX (0.14 g), DH (0.27 g), QH (0.27 g), YYH (0.27 g), DS (0.54 g), and HQ (0.4 g). High-dose modified THSWD contained TR (0.32 g), HH (0.32 g), DG (0.44 g), BS (0.36 g), CX (0.28 g), DH (0.54 g), QH (0.54 g), YYH (0.54 g), DS (1.08 g), and HQ (0.8 g). The extraction procedure for the modified THSWD was as follows: first, crude herbal drugs were mixed with distilled water and soaked for 30 min; the mixture was extracted with boiling water for 30 min; the residue was also extracted using boiling water for 20 min, then filtered through 4 layers of gauze, and the filtrates were evaporated using rotary evaporator at 60 °C. After modelling, two rats died in the I/R group (n = 13), one died in the low-dose group (n = 14), two died in the medium-dose group (n = 13), and no rats died in the high-dose group (n = 15).
2,3,5-Triphenyltetrazolium chloride (TTC) and Evans blue staining
After I/R, the rats were treated with different doses of modified THSWD for 24 h, and the LAD was connected again for 2,3,5-triphenyltetrazolium chloride (TTC) and Evans Blue staining. Briefly, 2 mL of Evans blue stain (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) prepared with 2% phosphate buffer saline (PBS) was injected into the rats through the femoral vein. After injection, the heart was quickly harvested, rinsed in pre-cooled PBS, frozen at −20 °C for 20 min, and sliced on ice. Fresh tissue sections were placed in the TTC staining solution (Nanjing Jiancheng Bioengineering Institute) and incubated at 37 °C in the dark for 20 min. The tissues were then washed with PBS to remove the excess TTC staining solution and photographed immediately. The infarct size (%) was assessed by calculating the ratio of the infarct area to the area at risk (AAR) using the ImageJ software.
Detection of serum biochemical indexes
After successful I/R modelling and treatment with different doses of modified THSWD for 24 h, rat blood was collected to obtain serum. The levels of serum creatine kinase (CK) and lactate dehydrogenase (LDH) and the activity of superoxide dismutase (SOD) were determined using the CK assay kit, LDH assay kit, and SOD assay kit (Nanjing Jiancheng Bioengineering Institute), respectively, according to the manufacturer’s instructions.
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining
After treatment with modified THSWD for 24 h, the rats were euthanized, and their hearts were collected. The heart samples were fixed with 4% paraformaldehyde solution, dehydrated with sucrose solution, and embedded in OCT to prepare frozen sections. Sections were treated with Triton X-100 solution for 5 min and then incubated with terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) stain (Roche, Mannheim, Germany) for 60 min in the dark. After washing with PBS (0.01 M, pH 7.2–7.4), the sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime, Shanghai, China) for 5 min to label the nuclei. Next, the sections were photographed under a fluorescence microscope, and the number of TUNEL-positive cells was counted at least 5× high per field (HPF) to assess cell apoptosis.
Western blot
The total protein concentrations of tissue proteins were detected by the BCA protein assay (Beyotime, Shanghai, China). After electrophoresis and transmembrane electrophoresis, the membranes were blocked with 5% skim milk for 1 h and then incubated with Bcl-2 (Abcam, Cambridge, UK), Bax (Abcam, Cambridge, UK) and GAPDH (CST, USA) separately overnight at 4 °C. After that, the membranes were incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (CST, USA) and visualised using enhanced chemiluminescence (Merck Millipore, USA).
Echocardiography
After 4 weeks of treatment with different doses of modified THSWD, the rats were anaesthetized with 2% isoflurane and examined using a commercial echocardiography system (VisualSonics, Toronto, ON, Canada). Two-dimensional images were obtained along the long axis of the parasternum, and M-type echocardiography was performed in the flat papillary muscle position. The ejection fraction (EF, %) and fractional shortening (FS, %) of the three cardiac cycles were measured to calculate the average values.
Masson’s trichrome staining
After treatment with different doses of modified THSWD for 4 weeks, the heart was harvested after echocardiography, and frozen sections were prepared. Frozen sections were washed twice with PBS (0.01 M, pH 7.2–7.4) and then treated with Bouin’s solution followed by Masson’s trichrome staining according to the kit instructions (SenBeiJia Biological Technology Co., Ltd., Nanjing, China). The degree of myocardial fibrosis in each group was observed under a light microscope, and the infarct size (%) was calculated as follows: the sum of the epicardial perimeter and endocardial perimeter of the infarct area divided by the sum of the left ventricular epicardial perimeter and endocardial perimeter. The collagen content in the infarct area (%) was calculated as the ratio of the blue area to the total area using the ImageJ software.
Immunofluorescence staining
Frozen sections were washed with PBS (0.01 M, pH 7.2–7.4) and treated with Triton X-100 solution for 10 min. After incubation with normal goat serum at 37 °C for 30 min, the sections were incubated with primary antibodies against c-kit (1:400, Abcam, Cambridge, MA, USA) and Sca-1 (1:500, Sigma-Aldrich, St. Louis, MO, USA) overnight at 4 °C. Sections were then probed with a secondary antibody coupled with Alexa Fluor 488 (1:200; Invitrogen, Carlsbad, CA, USA) at 37 °C for 1 h and washed with PBS three times. The c-kit and Sca-1 positive cells (c-kit + and Sca-1+) in 200× HPF were observed under a fluorescence microscope and counted using the ImageJ software.
ELISA assay
The levels of stem cell factor (SCF) and stromal cell-derived factor-1 (SDF-1) in the serum of the I/R group and the high-dose modified THSWD group were measured according to the instructions of the rat SCF and SDF-1 ELISA kits (R&D Systems, Minneapolis, MN, USA).
Ultra-performance liquid chromatography (UHPLC)/mass spectrometry (MS)-based metabolomics
The serum of the rats in the I/R group and the high-dose modified THSWD group was prepared to obtain the extracts for ultra-high-performance liquid chromatography (UHPLC) (Nexera UHPLC LC-30A, Japan)-mass spectrometry (MS) (AB SCIEX TripleTOF® 5600+, USA). A mixture containing 20 μL of each sample extract was prepared for quality control to ensure that the results obtained from the global metabolic profile study were valid. The acquisition method was set up using alternative reverse-phase gradients. The optimal conditions for MS analysis were as follows: Ion Source Gas 1 (Gas 1): 50; Ion Source Gas 2 (Gas 2): 50; Curtain Gas (CUR): 25; Source Temperature: 500 °C (positive ion) and 450 °C (negative ion); Ion Sapary Voltage Floating (ISVF): 5500 V (positive ion) and 4400 V (negative ion); TOF MS scan range: 100–1200 Da; product ion scan range: 50–1000 Da; Time-of-flight (TOF) MS scan accumulation time: 0.2 sec; and product ion scan accumulation time: 0.01 sec. The secondary mass spectrum was obtained using information-dependent acquisition, and a high-sensitivity mode was adopted.
Statistical analyses
IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA) was used for statistical analysis and the data were expressed as mean ± standard deviation (SDs). Statistical differences between the groups were analyzed using unpaired Student’s t-tests (two groups) or one-way analysis of variance (ANOVA), followed by the LSD method (multiple groups). Statistical significance was defined as p < 0.05.
Results
Modified THSWD reduced infarction area in early-stage I/R rat models
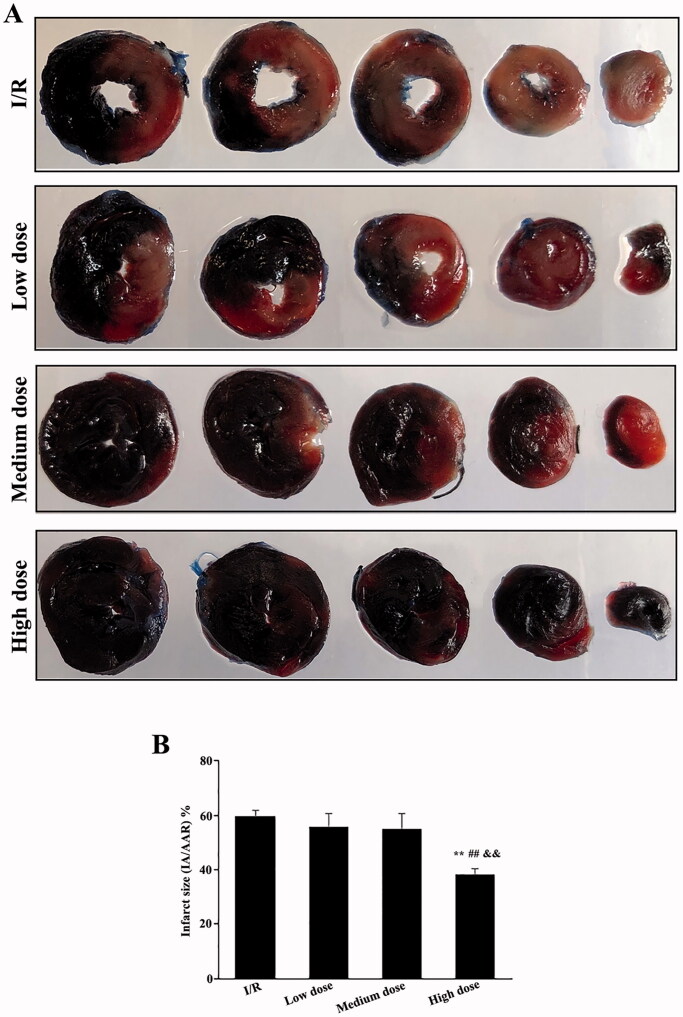
The protective effect of modified THSWD on the infarction area of early stage I/R rats was detected using TTC and Evans blue staining. A large, pale area was observed in the I/R group. No significant difference was detected between the low- and medium-dose modified THSWD groups and the I/R group, whereas the pale and light red areas of the high-dose modified THSWD group were significantly reduced (Figure 1(A)). The statistical analyses showed that the low- and medium-dose modified THSWD groups tended to present a reduced infarct area but without statistical difference compared with the I/R group. The infarct area of the high-dose modified THSWD group was significantly lower than that of the I/R and low- and medium-dose groups (Figure 1(B)).
Figure 1.
Effects of modified THSWD on the early myocardial infarction area in I/R rat models. (A) Representative photos of TTC and Evans blue staining. (B) Statistical analysis of the myocardial infarction area. **p < 0.01 compared with I/R group; ##p < 0.01 compared with low-dose modified THSWD group; && p < 0.01 compared with medium-dose modified THSWD group.
Modified THSWD protected early myocardial injury caused by I/R
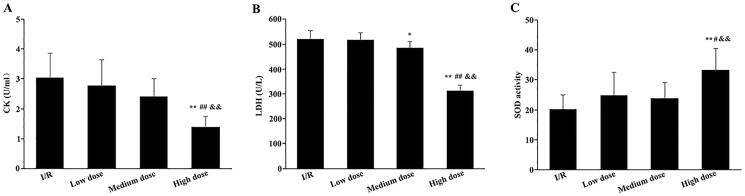
Furthermore, we evaluated the levels of CK and LDH, as well as the activity of SOD, to assess the protective effect of the modified THSWD on early myocardial injury in I/R rats. The levels of serum CK in the low- and medium-dose modified THSWD groups showed a decreasing trend but did not statistically differ from those in the I/R group. Meanwhile, the serum CK levels in the high-dose modified THSWD group were significantly lower than the other 3 groups (Figure 2(A)). No significant differences were detected between the low-dose modified THSWD and I/R groups regarding serum LDH content. The serum LDH levels in the medium and high-dose modified THSWD groups were significantly lower than those in the I/R group. Moreover, the serum LDH level in the high-dose modified THSWD group was markedly lower than that in the other groups (Figure 2(B)). Additionally, the serum SOD activity in the low and medium-dose modified THSWD groups showed an increasing trend but did not statistically differ from that in the I/R group. However, serum SOD activity in the high-dose modified THSWD group was significantly higher than that in the other groups (Figure 2(C)).
Figure 2.
Effects of modified THSWD on early myocardial injury caused by I/R. (A) Serum CK content. (B) Serum LDH content. (C) Serum SOD activity. *p < 0.05 and **p < 0.01 compared with I/R group; #p < 0.05 and ##p < 0.01 compared with low-dose modified THSWD group; && p < 0.01 compared with medium-dose modified THSWD group.
Modified THSWD inhibited early cardiomyocyte apoptosis
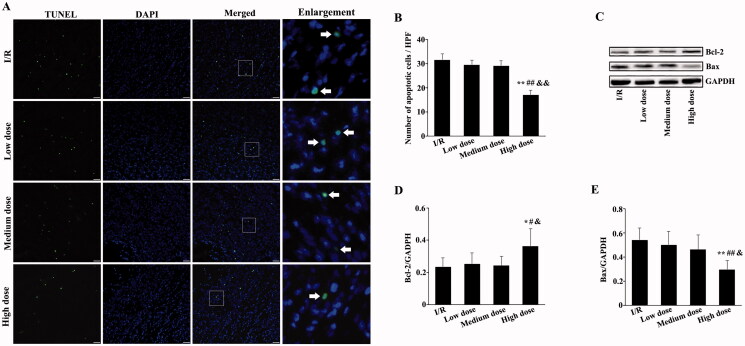
Moreover, to demonstrate the protective effect of modified THSWD on early cardiomyocyte injury in I/R rats, we used TUNEL staining to detect apoptosis of myocardial tissue in each group. TUNEL-positive cells were clear in the I/R and low- and medium-dose modified THSWD groups, whereas they were reduced in the high-dose modified THSWD group (Figure 3(A)). Although apoptosis in the low- and medium-dose modified THSWD groups showed a decreasing trend, it did not significantly differ from that in the I/R group. However, in the high-dose modified THSWD group, apoptosis was significantly reduced and was statistically different from that in the other three groups (Figure 3(B)). To further confirm the effect of modified THSWD on apoptosis, the expression of apoptosis-related proteins was detected by Western blot. The expression of Bcl-2 was significantly increased and the expression of Bax was obviously decreased in the high-dose modified THSWD group compared with the other three groups (Figure 3(C–E)).
Figure 3.
Effects of modified THSWD on early cell apoptosis in infarcted myocardium. (A) TUNEL staining of myocardial tissues (scale bar = 25 μm). (B) Statistical analysis of TUNEL-positive cells. (C–E) The levels of Bcl-2 and Bax were detected by Western blot. *p < 0.05 and **p < 0.01 compared with I/R group; #p < 0.05 and ##p < 0.01 compared with low-dose modified THSWD group; &p < 0.05 and &&p < 0.01 compared with medium-dose modified THSWD group.
High-dose modified THSWD promoted the recruitment of endogenous stem cells to the infarct sites
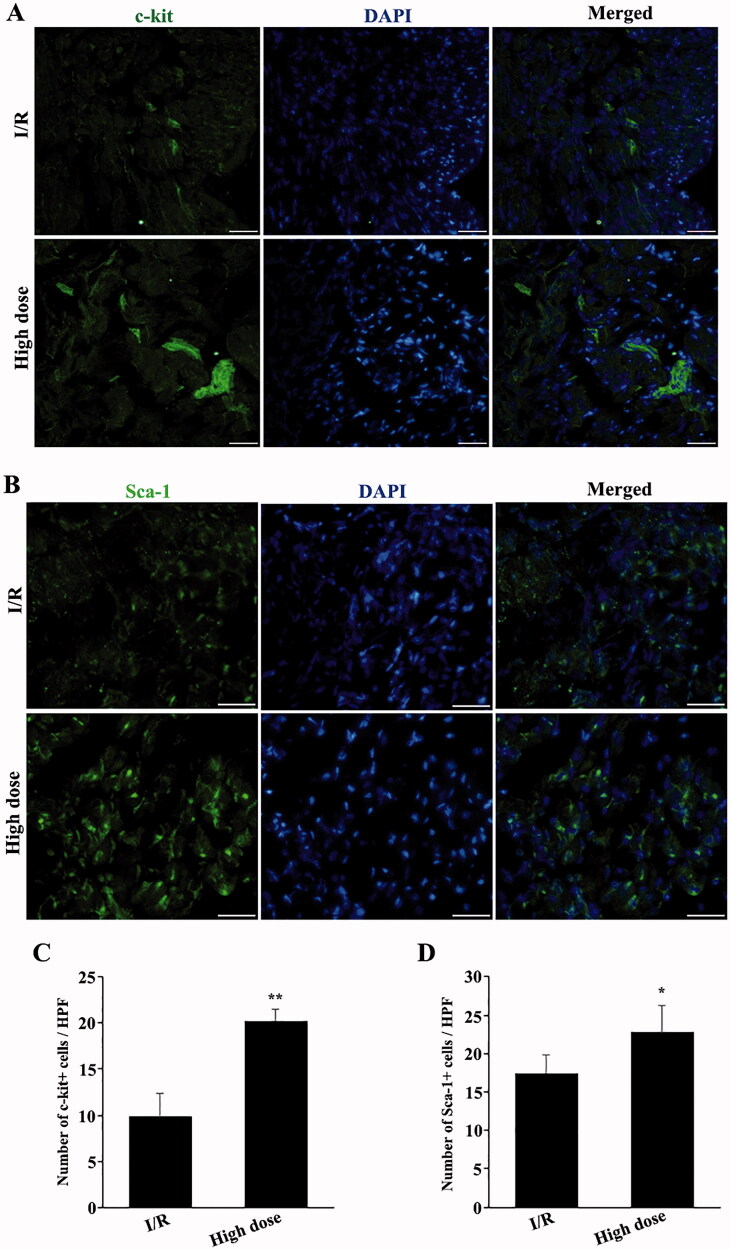
Endogenous stem cells can be recruited to infarct sites and play a role in protecting against myocardial injury by secreting different cytokines. Hence, to clarify whether high-dose modified THSWD could protect against I/R-induced myocardial injury by regulating the migration of endogenous stem cells, the number of c-kit + and Sca-1+ stem cells in and around the infarct area was determined by immunofluorescence staining after 24 h of reperfusion. Compared with the I/R group, high-dose modified THSWD treatment significantly increased the number of c-kit + and Sca-1+ cells (Figure 4(A–D)).
Figure 4.
Effects of high-dose modified THSWD on the recruitment of endogenous stem cells to the infarct sites. (A) c-kit immunofluorescence staining (scale bar = 25 μm). (B) Sca-1 immunofluorescence staining (scale bar = 25 μm). (C) Statistical analysis of the number of c-kit-positive cells. (D) Statistical analysis of Sca-1-positive cells. *p < 0.05 and **p < 0.01 compared with the I/R group.
High-dose modified THSWD increased the levels of serum chemokines
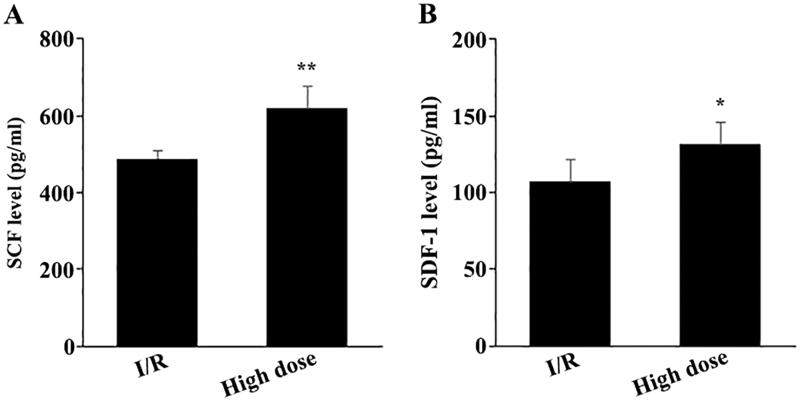
Furthermore, to explore the mechanisms by which high-dose modified THSWD promoted the recruitment of c-kit + and Sca-1+ stem cells to the infarct sites, we used ELISA to detect the levels of serum SCF and SDF-1 after 24 h of reperfusion. Compared with the I/R group, high-dose THSWD treatment significantly enhanced the levels of serum SCF and SDF-1 (Figure 5(A,B)).
Figure 5.
Effects of high-dose modified THSWD on the level of serum chemokines. Levels of serum SCF (A) and SDF-1 (B) were detected by ELISA. *p < 0.05 and **p < 0.01 compared with I/R group.
Modified THSWD improved cardiac function of late-stage I/R rat models
After 4 weeks of continuous administration of modified THSWD to I/R rats, we used echocardiography to evaluate the changes in cardiac function of rats in each group. Although the low- and medium-dose modified THSWD groups presented an increase in the EF value, they did not statistically differ from the I/R group. Meanwhile, the EF value of rats in the high-dose modified THSWD group was the highest and statistically different from that of the I/R and low- and medium-dose modified THSWD groups (Figure 6(A)). Regarding the FS, the differences between the groups showed a similar tendency (Figure 6(B)). These results suggest that modified THSWD could promote the recovery of heart function in I/R rats after 4 weeks of reperfusion and that high-dose modified THSWD had the best therapeutic effect. Besides, there was no significant difference in body weight among rats in each group after the treatment (Figure S1), which provided evidence for the safety of modified THSWD.
Figure 6.
Effects of modified THSWD on the cardiac function of I/R rat models. (A) EF and (B) FS were measured by echocardiography after 4 weeks of intragastric administration of different doses of modified THSWD. **p < 0.01 compared with I/R group; #p < 0.05 compared with low-dose modified THSWD group; &p < 0.05 compared with medium-dose modified THSWD group.
Modified THSWD inhibited infarct size and collagen content in late-stage I/R rat models
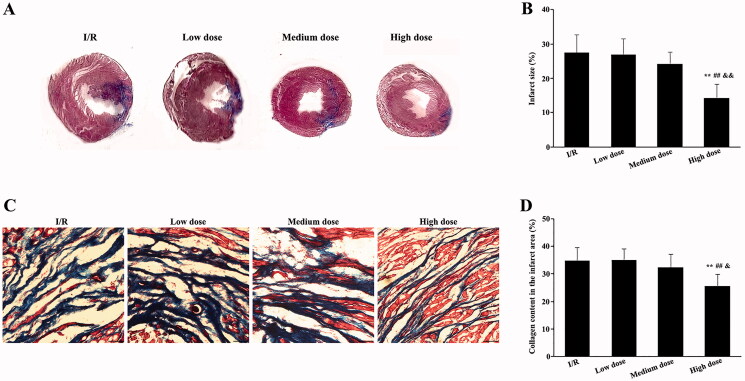
To explore the long-term therapeutic effect of modified THSWD, the infarct size and collagen content in the infarct area after 4 weeks of continuous administration were evaluated in I/R rats using Masson’s trichrome staining. The ventricular walls of rats in the I/R and low-dose modified THSWD groups became thinner, and the infarct area was clear. In contrast, infarct size in the medium-dose modified THSWD group decreased. The smallest infarct size was observed in the high-dose THSWD group (Figure 7(A)). No significant differences were detected in infarct size between the I/R and the low- and medium-dose modified THSWD groups. Meanwhile, the infarct size in the high-dose modified THSWD group was significantly reduced compared with that in the other groups (Figure 7(B)). Furthermore, the collagen content in the infarct area between the I/R and low- and medium-dose modified THSWD groups did not differ significantly. However, in the high-dose modified THSWD group, the collagen content in the infarct area was significantly decreased compared with that in the other groups (Figure 7(C,D)).
Figure 7.
Effects of modified THSWD on infarct size and collagen content in late-stage I/R. (A) Masson’s trichrome stainings showed the extent of myocardial infarction in each group. (B) Statistical analysis of infarct size. (C) Masson’s trichrome staining showed collagen content in the infarct area of each group (scale bar = 50 μm). (D) Statistical analysis of collagen content in the infarct area of each group. **p < 0.01 compared with I/R group; ##p < 0.01 compared with low-dose modified THSWD group; &&p < 0.01 compared with medium-dose modified THSWD group.
Effect of high-dose modified THSWD on the metabolomics of I/R rats
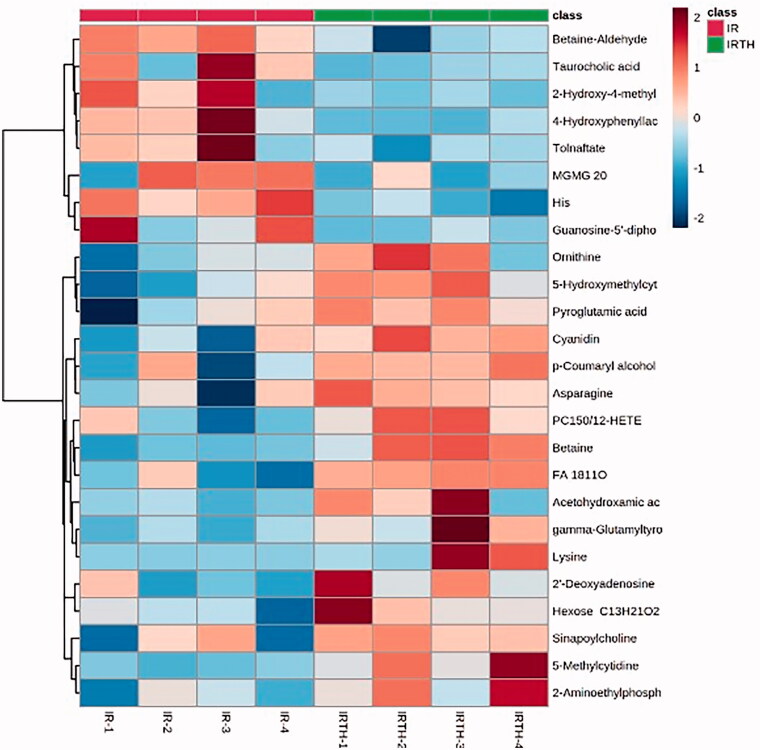
Furthermore, to investigate the long-term therapeutic effects of high-dose modified THSWD on I/R rats, we collected the serum of rats after 4 weeks of high-dose modified THSWD treatment. We performed metabolomic analyses using UPLC-MS. We found significant differences in the metabolite profiles between the two groups. The top 25 metabolites are displayed on a heatmap (Figure 8). We chose different metabolites between the high-dose modified THSWD and I/R groups based on the screening criteria [FC ≥1.5 (≤0.67) and p ≤ 0.05] (Table 1). High-dose modified THSWD increased the levels of glycine trimethyl internal salts and anthocyanins, which could reduce the risk of MI. Through the MetPA database analysis, we evaluated the related metabolic pathways. The results suggested that high-dose modified THSWD mainly affected the metabolism of phosphonate and phosphonate, as well as the metabolism of taurine and hypotaurine (Table 2).
Figure 8.
Metabolomics heat map analysis between high-dose modified THSWD and I/R groups.
Table 1.
Differences in metabolites between high-dose modified THSWD and I/R groups.
| Metabolite name | VIP (IR VS IRTH) | FC (IR VS IRTH) | P (IR VS IRTH) |
|---|---|---|---|
| Betaine-aldehyde | 0.18611 | 1.903717129 | 0.009180041 |
| Betaine | 0.48081 | 0.254398122 | 0.001683166 |
| 2-Aminoethylphosphonate | 1.2322 | 0.437002396 | 0.031954065 |
| Asparagine | 2.9817 | 0.603089351 | 0.041058393 |
| 5-Hydroxymethylcytosine | 0.031184 | 0.471764059 | 0.014465447 |
| 5-Methylcytidine | 0.023345 | 0.551517581 | 0.013641693 |
| 2′-Deoxyadenosine 5′-monophosphate | 0.019575 | 0.32659409 | 0.038055789 |
| Hexose + C13H21O2 | 0.0083564 | 0.481777011 | 0.049402505 |
| Guanosine-5′-diphosphate sodium salt | 0.035606 | 4.614189905 | 0.043187648 |
| Taurocholic acid | 0.019495 | 6.570718781 | 0.037130944 |
| 2-Hydroxy-4-methylpentanoic acid | 0.026869 | 2.922534627 | 0.044736211 |
| p-Coumaryl alcohol | 0.041263 | 0.564599973 | 0.030592737 |
| 4-Hydroxyphenyllactic acid | 0.027467 | 6.063247863 | 0.015778092 |
| Cyanidin | 0.019137 | 0.500136252 | 0.020556667 |
| FA 18:1 + 1O | 0.024723 | 0.430889102 | 0.004619752 |
| gamma-Glutamyltyrosine | 0.042221 | 0.347310325 | 0.032460331 |
| PC(15:0/12-HETE) | 0.043412 | 0.42674116 | 0.024070635 |
Table 2.
Differences in metabolite pathways between high-dose modified THSWD and I/R groups.
| Total | Expected | Raw p | Holm adjust | Impact | |
|---|---|---|---|---|---|
| Phosphonate and phosphinate metabolism | 6 | 0.031809 | 0.031442 | 1 | 0 |
| Taurine and hypotaurine metabolism | 8 | 0.042412 | 0.041729 | 1 | 0 |
| Alanine, aspartate and glutamate metabolism | 28 | 0.14844 | 0.13945 | 1 | 0 |
| Glycine, serine and threonine metabolism | 34 | 0.18025 | 0.16702 | 1 | 0.0465 |
| Primary bile acid2 biosynthesis | 46 | 0.24387 | 0.21984 | 1 | 0.02285 |
| Aminoacyl-tRNA biosynthesis | 48 | 0.25447 | 0.22835 | 1 | 0 |
| Purine metabolism | 66 | 0.3499 | 0.30137 | 1 | 0.0010 |
Note: Total, the Total number of metabolites in the target metabolic pathway; Raw p: p value of hypergeometric distribution test; Holm adjust, p value after correction of Holm false positive; Impact: the Impact value of metabolic pathway.
Discussion
The main purpose of THSWD is to nourish and promote blood circulation. Given the weak effect of THSWD on MI treatment, we prepared a modified THSWD to enhance its protective effect on myocardial I/R and explored its possible mechanisms. Based on the original formula of THSWD, we added 4 TCM to formulate the modified THSWD: Radix astragali, Salvia miltiorrhiza, Herba epimedii, and Rhizoma et radix notopterygii. Radix astragali is the root of the leguminous plant Astragalus mongolicus Bunge (Fabaceae) and its main chemical components are verbasil isoflavones; astragaloside IV; and astragalus saponins I, V, and III. For example, Radix astragali has a good therapeutic effect on ischaemic heart disease (Li et al. 1995). Previous studies have found that Radix astragali promotes angiogenesis in rats with ischaemic injury through the VEGF pathway (Zhang et al. 2011). Moreover, the antioxidant and nitric oxide-inducing properties of astragaloside IV can be used for protection against myocardial ischaemia (Zhang et al. 2006). For example, astragaloside IV can reduce myocardial I/R injury in rats by inhibiting the apoptotic signalling pathway mediated by calcium-sensitive receptors (Yin et al. 2019). Increasing evidence has shown that Salvia miltiorrhiza can prevent vascular diseases, especially atherosclerosis, and heart diseases, including MI, myocardial I/R injury, arrhythmia, myocardial hypertrophy, and myocardial fibrosis (Geng et al. 2015; Su et al. 2015; Wang et al. 2017). Herba epimedii has been found to increase blood flow to the heart and blood vessels and have favourable protective effects against myocardial ischaemia (Yang et al. 2006). The volatile oil from Rhizoma et radix notopterygii can prevent acute myocardial ischaemia caused by the pituitary gland and increase nutrient blood flow (Qin 1982). Therefore, modified THSWD can evacuate the internal and external evil winds, promote blood circulation, enhance collateral circulation, and relieve pain.
In the present study, we found that high-dose modified THSWD could protect against early myocardial injury caused by I/R and reduce the early infarct area. The high-dose modified THSWD group presented not only reduced levels of CK and LDH and cardiomyocyte apoptosis but also increased SOD activity. To evaluate the long-term therapeutic effect of modified THSWD, I/R rats were continuously administered modified THSWD for 4 weeks. Masson’s trichrome staining was used to detect infarct size and collagen content in the infarct area, and echocardiography was used to assess cardiac function changes. We showed that high-dose modified THSWD had a significant protective effect on the cardiac function of late-stage I/R rats, presenting reduced myocardial infarct size and collagen content in the infarct area and providing left ventricular contraction function. Overall, high-dose modified THSWD had a good therapeutic effect on myocardial I/R injury and deserves further clinical trials and research. Furthermore, low- and medium-dose modified THSWD had no discernible protective effects against I/R injury and were significantly weaker than high-dose modified THSWD. The medium-dose of modified THSWD was determined based on the body area according to the conversion from the human dose to rat dose. The human dosage of each component of modified THSWD was an effective dose which commonly used in clinical practice for cardiovascular disease. The low-dose of modified THSWD was determined as half of medium-dose. However, only the high-dose modified THSWD exerted a significant cardioprotective effect. Therefore, the dose of modified THSWD has a great impact on its protective effects and should receive more attention during clinical applications.
In the past 20 years, stem cell therapy has become a hot topic and focus of myocardial ischaemic injury research because of its unique advantages in the treatment of ischaemic heart disease (Yu et al. 2017; Khodayari et al. 2019). After myocardial ischaemia, the number and distribution of endogenous stem cells in the body may change (Fortini et al. 2011), which also implies that the activation of endogenous stem cells may be important for myocardial repair. For example, the treatment of myocardial ischaemic injury using autologous bone marrow stem cells can effectively improve the function of ischaemic tissue (Malecki et al. 2013). Herein, we showed that high-dose modified THSWD increased the migration of c-kit + and Sca-1+ stem cells to myocardial ischaemia sites, which might be one of the mechanisms by which modified THSWD improves cardiac function and reduces the infarct area. Although c-kit + stem cells cannot differentiate into cardiomyocytes, it has been reported that c-kit + stem cell transplantation can improve cardiac function after MI by regulating the immune response (Vagnozzi et al. 2020). Recently, Xing et al. (2021) reported that endogenous quiescent c-kit + cells improved heart function after MI via neovascularization of capillaries. Sca-1+ cells, rather than c-kit + cells, may, however, protect energy metabolism and heart function after MI (Marunouchi et al. 2019). Young Sca-1+ cells have previously been shown to improve cardiac regeneration by promoting the proliferation, migration, and reactivation of epithelial to mesenchymal transition via the TGF-β signalling pathway (Li et al. 2018).
Further, to explore the long-term therapeutic effect of high-dose modified THSWD on I/R rats, we collected serum after 4 weeks of treatment to perform metabolomic analysis. We found significant differences in the metabolites between the high-dose modified THSWD and I/R groups. Betaine aldehyde, betaine, 2-aminoethyl phosphonate, asparagine, 5-hydroxymethylcytosine, 5-methylcytidine, taurocholic acid, and γ-glutamyl tyrosine were among the different metabolites identified. The different metabolite pathways between the high-dose modified THSWD and I/R groups mainly included phosphonate and phosphonate metabolism; taurine and hypotaurine metabolism; alanine, aspartic acid, and glutamate metabolism; glycine, serine, and threonine metabolism; primary bile acid biosynthesis; aminoacyl-tRNA biosynthesis; and purine metabolism. Furthermore, the anthocyanin content in the high-dose modified THSWD group was much higher than that in the I/R group, which might also be one of the foundations of treatment efficacy. Previous studies have shown that the metabolome profile of the body changes after the occurrence of myocardial ischaemia (Sun et al. 2017; Wang et al. 2017). Additionally, an anthocyanin-rich extract of Brassica oleracea L. (Cruciferae) has cardioprotective effects following MI (Jana et al. 2017). A high intake of anthocyanins may also reduce the risk of MI in predominantly young women (Cassidy et al. 2013). Therefore, high-dose modified THSWD might play a protective role in the myocardium, partly by affecting metabolic pathways.
However, the current study has some limitations. First, we confirmed that the effects of modified THSWD on the improvement of cardiac function after I/R were better than those of THSWD alone after adding the four ingredients. However, whether these 4 components could play a therapeutic role requires further research. Second, although we found that treatment with modified THSWD increased the number of c-kit + and Sca-1+ stem cells in ischaemic sites, we did not detect engraftment and distribution of other stem cell types. Additionally, the number of stem cells in peripheral blood should be determined, and the source of stem cells needs to be clarified. Third, the modified THSWD could regulate metabolites and metabolic pathways. However, their specific roles remain unclear. Nevertheless, our study provides a basis and direction for modifying Chinese medicine by adding or subtracting some components to obtain better effects. In addition, we indicated that some mechanisms of TCM in the improvement of myocardial injury might be explained by the mobilisation of endogenous stem cells and the regulation of metabolites and metabolic pathways.
Conclusions
We showed that modified THSWD can improve cardiac function after I/R injury in rats and endogenous stem cell mobilization and metabolic regulation were related to the cardioprotection of modified THSWD. This study provided a promising direction and strategy for the treatment of cardiovascular diseases with traditional Chinese medicine.
Supplementary Material
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China [82174120], Natural Science Foundation of Shanghai [No. 21ZR1463100] and Shanghai Talent Development Funding Scheme [No. 2019090].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB.. 2013. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 127(2):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Arjun S, Basalay MV, Bell RM, Bromage DI, Botker HE, Carr RD, Cunningham J, Ghosh AK, Heusch G, et al. 2018. The 10th Biennial Hatter Cardiovascular Institute workshop: cellular protection-evaluating new directions in the setting of myocardial infarction, ischaemic stroke, and cardio-oncology. Basic Res Cardiol. 113(6):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito ML, Zhang Y, Qiao X, Reyelt L, Paruchuri V, Schnitzler GR, Morine KJ, Annamalai SK, Bogins C, Natov PS, et al. 2018. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J Am Coll Cardiol. 72(5):501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini C, Toffoletto B, Fucili A, Puppato E, Olivares A, Beltrami AP, Fiorelli V, Bergamin N, Cesselli D, Morelli C, et al. 2011. Circulating stem cell vary with NYHA stage in heart failure patients. J Cell Mol Med. 15(8):1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuping Z, Wuping L, Linhua W, Chengxi P, Fuqiang Z, Yi Z, Aijun W.. 2019. Tao-Hong-Si-Wu decoction reduces ischemia reperfusion rat myoblast cells calcium overloading and inflammation through the Wnt/IP3R/CAMKII pathway. J Cell Biochem. 120(8):13095–13106. [DOI] [PubMed] [Google Scholar]
- Geng ZH, Huang L, Song MB, Song YM.. 2015. Cardiovascular effects in vitro of a polysaccharide from Salvia miltiorrhiza. Carbohydr Polym. 121:241–247. [DOI] [PubMed] [Google Scholar]
- Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y.. 2017. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. 69(24):2952–2966. [DOI] [PubMed] [Google Scholar]
- Jana S, Patel D, Patel S, Upadhyay K, Thadani J, Mandal R, Das S, Devkar R.. 2017. Anthocyanin rich extract of Brassica oleracea L. alleviates experimentally induced myocardial infarction. PLOS One. 12(8):e0182137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodayari S, Khodayari H, Amiri AZ, Eslami M, Farhud D, Hescheler J, Nayernia K.. 2019. Inflammatory microenvironment of acute myocardial infarction prevents regeneration of heart with stem cells therapy. Cell Physiol Biochem. 53(5):887–909. [DOI] [PubMed] [Google Scholar]
- Li J, Li SH, Wu J, Weisel RD, Yao A, Stanford WL, Liu SM, Li RK.. 2018. Young bone marrow Sca-1 cells rejuvenate the aged heart by promoting epithelial-to-mesenchymal transition. Theranostics. 8(7):1766–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SQ, Yuan RX, Gao H.. 1995. Clinical observation on the treatment of ischemic heart disease with Astragalus membranaceus. Zhongguo Zhong xi yi jie he za zhi (In Chinese). 15:77–80. [PubMed] [Google Scholar]
- Luo ZR, Li H, Xiao ZX, Shao SJ, Zhao TT, Zhao Y, Mou FF, Yu B, Guo HD.. 2019. Taohong Siwu decoction exerts a beneficial effect on cardiac function by possibly improving the microenvironment and decreasing mitochondrial fission after myocardial infarction. Cardiol Res Pract. 2019:5198278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki M, Sabo C, Putzer E, Stampe C, Foorohar A, Quach C, Beauchaine M, Tombokan X, Anderson M.. 2013. Recruitment and retention of human autologous CD34+ CD117+ CD133+ bone marrow stem cells to infarcted myocardium followed by directed vasculogenesis: novel strategy for cardiac regeneration. Mol and Cell Ther. 1(1):4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunouchi T, Sasaki K, Yano E, Tanonaka K.. 2019. Transplantation of cardiac Sca-1-positive cells rather than c-Kit-positive cells preserves mitochondrial oxygen consumption of the viable myocardium following myocardial infarction in rats. J Pharmacol Sci. 140(3):236–241. [DOI] [PubMed] [Google Scholar]
- Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E.. 2017. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediators Inflamm. 2017:7018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin CL. 1982. Pharmacological study of the Chinese drug Qiang-Huo (Notopterygium incisium Ting). Zhong Yao Tong Bao. 7(1):31–32. [PubMed] [Google Scholar]
- Rentrop KP, Feit F.. 2015. Reperfusion therapy for acute myocardial infarction: concepts and controversies from inception to acceptance. Am Heart J. 170(5):971–980. [DOI] [PubMed] [Google Scholar]
- Severino P, D'Amato A, Pucci M, Infusino F, Adamo F, Birtolo LI, Netti L, Montefusco G, Chimenti C, Lavalle C, et al. 2020. Ischemic heart disease pathophysiology paradigms overview: from plaque activation to microvascular dysfunction. IJMS. 21(21):8118–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, et al. 2016. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 67(14):1674–1683. [DOI] [PubMed] [Google Scholar]
- Su CY, Ming QL, Rahman K, Han T, Qin LP.. 2015. Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chinese J Nat Med. 13:163–182. [DOI] [PubMed] [Google Scholar]
- Sun L, Liu J, Sun M, Lin L, Miao L, Ge Z, Yang B.. 2017. Comprehensive metabonomic analysis of heart tissue from isoproterenol-induced myocardial infarction rat based on reversed-phase and hydrophilic interaction chromatography coupled to mass spectrometry. J Sep Sci. 40(10):2198–2206. [DOI] [PubMed] [Google Scholar]
- Tao T, He T, Mao H, Wu X, Liu X.. 2020. Non-targeted metabolomic profiling of coronary heart disease patients with Taohong Siwu decoction treatment. Front Pharmacol. 11:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaie M. 2016. State-of-the-art diagnosis of myocardial infarction. Diagnosis. 3(4):137–142. [DOI] [PubMed] [Google Scholar]
- Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, et al. 2020. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 577(7790):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ma R, Liu C, Liu H, Zhu R, Guo S, Tang M, Li Y, Niu J, Fu M, et al. 2017. Salvia miltiorrhiza: a potential red light to the development of cardiovascular diseases. Curr Pharm Des. 23(7):1077–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu Z, Hu S, Duan X, Zhang Y, Peng C, Peng D, Han L.. 2020. Taohong Siwu decoction ameliorates ischemic stroke injury via suppressing pyroptosis. Front Pharmacol. 11:590453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang D, Wu J, Yu X, Lv J, Kong J, Zhu G, Su R.. 2017. Metabolic characterization of myocardial infarction using GC-MS-based tissue metabolomics. Int Heart J. 58(3):441–446. [DOI] [PubMed] [Google Scholar]
- Xing S, Tian JZ, Yang SH, Huang XT, Ding YF, Lu QY, Yang JS, Yang WJ.. 2021. Setd4 controlled quiescent c-Kit+ cells contribute to cardiac neovascularization of capillaries beyond activation. Sci Rep. 11(1):11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang S, Chen W, Chen G.. 2006. Effects of taohong siwu decoction II in the chick chorioallantoic membrane (CAM) assay and on B16 melanoma in mice and endothelial cells ECV304 proliferation. J Tradit Chin Med. 26(1):63–67. [PubMed] [Google Scholar]
- Yang Y, Liu N, Mo Z, Xie J, Liao J, Mo S.. 2006. Influence of a Chinese crude drug on Ca2+ influx and efflux in rat visceral organs: investigation and evaluation by 45Ca. Appl Radiat Isot. 64(2):241–246. [DOI] [PubMed] [Google Scholar]
- Yin B, Hou XW, Lu ML.. 2019. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol Sin. 40(5):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lu K, Zhu J, Wang J.. 2017. Stem cell therapy for ischemic heart diseases. Br Med Bull. 121(1):135–154. [DOI] [PubMed] [Google Scholar]
- Yu K, Huang X, Li W.. 2000. Clinical observation on treatment of pediatric intractable nephropathy with modified Taohong Siwu decoction. Zhongguo Zhong xi yi Jie he za Zhi. 20:831–833. [PubMed] [Google Scholar]
- Zhang L, Yang Y, Wang Y, Gao X.. 2011. Astragalus membranaceus extract promotes neovascularisation by VEGF pathway in rat model of ischemic injury. Pharmazie. 66(2):144–150. [PubMed] [Google Scholar]
- Zhang WD, Chen H, Zhang C, Liu RH, Li HL, Chen HZ.. 2006. Astragaloside IV from Astragalus membranaceus shows cardioprotection during myocardial ischemia in vivo and in vitro. Planta Med. 72(1):4–8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li P, Hua Y, Ji P, Yao W, Ma Q, Yuan Z, Wen Y, Yang C, Wei Y.. 2018. Urinary metabolomics study the mechanism of Taohong Siwu Decoction intervention in acute blood stasis model rats based on liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 1074–1075:51–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.