Abstract
Introduction
The BBV152 coronavirus disease 2019 (COVID-19) vaccine (COVAXIN) has recently been approved for adolescents.
Objective
We provide the first real-world safety data of COVAXIN use in adolescents and compare with adults.
Methods
A prospective observational study was initiated in January 2022. Enrolled adolescents and adults were contacted by telephone after 14 days of receiving the BBV152 vaccine. The primary outcome was vaccine safety assessed as rate of adverse events following immunization (AEFIs). Severity grading of AEFIs was done using the Food and Drug Administration (FDA) scale. Interim results are presented.
Results
A total of 698 adolescents and 326 adults were enrolled. AEFIs after the first dose developed in 243 out of 670 adolescents (36.3%), with 21% reporting only local AEFIs and 15.2% reporting systemic AEFIs. Among 340 adolescents who had received the second dose of vaccine, 129 (37.9%) developed AEFIs, with only local involvement in 20.3% and systemic involvement in 17.6%. Injection site pain and fever were the common AEFIs. The majority of AEFIs were mild-moderate. Nearly 0.9% of adolescents receiving the first dose reported severe AEFIs. Atypical AEFIs were observed in 0.6–0.9% of adolescents. The majority of the AEFIs resolved in 1–2 days. AEFIs were persistent in > 2% of adolescents at day 14 after the second dose, and also in 3.7% of adults overall at follow-up. No difference was observed in AEFI incidence and patterns between adolescents and adults. Regression analysis showed females and those with a history of allergy to be, respectively, at 1.6 times and 3 times increased risk of AEFIs among adolescents.
Conclusions
COVAXIN carries an overall favorable short-term safety profile in adolescents. The observed AEFI rates in adolescents are much lower than that reported with mRNA vaccines, but head–head comparisons in the same population are required to generate relative vaccine safety data. Female adolescents and those with a history of allergy need watchfulness for severe and persistent AEFIs. With some AEFIs persisting at 14 days, a longer follow-up is recommended to strengthen the safety data of COVAXIN.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-022-01226-8.
Key Points
| The short-term safety of the BBV152 coronavirus disease 2019 (COVID-19) vaccine in the 15- to 18-year-old age group seems favorable. |
| Watchfulness for severe and persistent adverse events may be needed for females and those with a history of allergy. |
| Vigilance is required for atypical events such as increased bleeding. |
| Long-term safety data need to be generated for BBV152 vaccine despite the favorable short-term results. |
Introduction
Vaccines based on novel and pre-existing platforms have been licensed for use to curtail the coronavirus disease 2019 (COVID-19) pandemic. These include messenger RNA (mRNA)-based vaccines, viral vectored vaccines, and inactivated severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccines. Initially high-risk groups were prioritized for vaccination, and later on, the program was extended to the general population. The World Health Organization (WHO) recommends extension of vaccination to children 5–17 years of age once the high-risk population has been adequately vaccinated [1]. Pfizer’s mRNA based BNT162b2 vaccine was the first to get Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval for vaccinating children against COVID-19 [2]. The inactivated CoronaVac in China has also been granted emergency use approval for children and adolescents [3]. In India, COVISHIELD, based on the ChAdOx1-nCoV-19 chimpanzee adenoviral platform, and COVAXIN (BBV152), an inactivated SARS-CoV-2 vaccine, were mostly used for mass vaccination of the adult population. In late December 2021, COVAXIN was granted emergency use authorization for use in adolescents 15–18 years of age, and mass vaccination for adolescents was initiated in the first week of January 2022. There is a paucity of data on the safety profile of COVAXIN specific to the adolescent subset. The only evidence available to date is a pre-print of an open-label phase 2/3 trial of the BBV152 vaccine by the manufacturer, Bharat Biotech, which enrolled only 176 adolescents [4]. Since the performance and safety of vaccines might differ in larger population samples in the real world and also on long-term follow-up, we decided to conduct a prospective observational study of the BBV152 vaccine (COVAXIN) in adolescents aged 15–18 years. Here, we provide the interim results of the study and the first real-world safety data of COVAXIN in adolescents. A comparative analysis of vaccine safety in adolescents and adults is also presented. A pre-print version of the article is already available [5].
Methods
Study Design and Setting
This is a prospective observational study conducted in a tertiary hospital of north India. The study started in January 2022, and with a target of 1 year follow-up of all participants, it is expected to be continued until May 2023. Adolescents visiting the COVID-19 vaccination center of the hospital at the time of either their first or second dose were recruited in the study. As mentioned earlier, COVID-19 vaccination for adolescents began in the country during the first week of January 2022. Since the present study could be started in the second week of January 2022, after obtaining ethical permission, a subset of participants had already received their first dose of vaccine. In the absence of adolescent-specific safety data on COVAXIN, and in order to bring the safety data into the public domain in a timely manner, it was decided that this subset should not be missed out on and would be enrolled while receiving the second dose. In this group, the adverse events following immunization (AEFIs) following the first dose of vaccine were evaluated retrospectively. This was also done to ensure that any serious, severe, or atypical AEFIs, which are less likely to be affected by recall bias, could be identified, and reported in the public domain. The AEFI specific information was obtained from these participants and their accompanying guardians at the time of the second dose and was recorded in the pre-designed case report form. Adult participants in the study were recruited while visiting the vaccination center at the time of either their first, second, or booster dose. As per the Ministry of Health and Family Welfare (MoHFW) guidelines, adults at high risk of COVID-19 were eligible for a booster if they had received the second dose at least 9 months (39 weeks) back [6]. Because of the inconsistent availability of COVAXIN and personal health concerns, some of the adults receiving the second dose had received the first dose more than 3 months back, although guidelines mandate a 4- to 6-week gap between the first and second doses for both adults and adolescents. Because of the significant time lag and the possibility of recall bias, retrospective evaluation of AEFIs was not performed in adults who were enrolled at the time of either second or booster dose. All adult participants were monitored prospectively to assess the AEFIs.
Here, we report the first short-term safety results of all participants—adolescents and adults who successfully completed at least 14 days of follow-up after any dose of the BBV152 vaccine (COVAXIN). The authors UK, SSC, and VJ had access to the complete data.
Study Participants
All individuals who received COVAXIN in the study center during the period of enrolment were included in the study. Participants were adolescents 15–18 years of age and adults ≥ 19 years of age. Informed consent was taken from the adults. In the case of adolescents, informed consent was taken from the accompanying guardian, along with written assent of the adolescent. Adolescents who visited the center without guardians were excluded, as were the vaccinees who refused to provide consent/assent.
Safety Analysis
AEFIs were recorded at 14 days after vaccination through telephonic interview, and the following detailed data for safety analysis were extracted:
Incidence of AEFIs
Type and pattern of AEFIs (Medical Dictionary for Regulatory Activities [MedDRA®] low level terms [LLT] and system organ class [SOC] terminology used)
Distribution of AEFIs with respect to age and sex
Outcomes of AEFIs
Interventions done to manage AEFIs
Seriousness of AEFIs as per adapted WHO and FDA definitions (Supplementary Table 1, see the electronic supplementary material)
Severity of AEFIs for local adverse events (AEs) and systemic AEs [these were recorded as per the FDA severity grading scale (Supplementary Table 1)]
AEFIs requiring hospitalization
Any vaccine–disease interaction resulting in AEFIs
Any vaccine–drug interaction resulting in AEFIs
Vaccination Procedure and Enrolment in Study
Participants receiving any dose of COVAXIN were recruited for the present study. COVAXIN is administered intramuscularly in the deltoid at the dose of 0.5 mL as a two-dose schedule, with an interval of 4–6 weeks between the first and second dose. Since mid-January 2022, the booster dose of vaccine was recommended for adults with a gap of 9 months from the second dose [6]. Post vaccination, all participants were routinely monitored at the study site for 30 min. They were informed of the AEs expected after vaccination and of the detailed study procedure, including advice to record AEFIs. They were contacted by phone after 14 days after vaccination. Specifically, they were questioned about local site symptoms such as pain, erythema, swelling, tenderness, and any limitation of physical activity. Also, enquiry was made about the occurrence of systemic events such as fever, fatigability, myalgia, arthralgia, headache, nausea, vomiting, diarrhea, rash, chest tightness, dyspnea, or any other complaint. With the third wave of the COVID-19 pandemic ongoing, individuals were given instructions regarding reverse transcriptase polymerase chain reaction (RT-PCR) based nasal or oropharyngeal swab tests for SARS-CoV-2 in any event of them developing COVID-19 like symptoms.
Data Sources/Measurement
Data related to demography and medical history were recorded in a pre-designed case report form. The medical history included history of SARS-CoV-2 positivity at any time in the past, existing co-morbidities, concurrent drug history, and history of allergy to any known stimuli including, but not limited to, drugs, vaccines, food products, and dust. For adolescents visiting the center for the second dose, information regarding AEFIs during the first dose was recorded in the case report form. For all participants, the data recorded included severity of AEFIs, interventions required for the management of AEFIs, outcomes of AEFIs, and time to complete recovery.
Sample Size
The available safety data from controlled settings shows the AEFI rate to vary between 12 and 21% in the general population [7, 8]. The primary objective of the study was to evaluate the safety profile of COVAXIN in adolescents. Considering a 15% rate of occurrence of any AEFI, a margin of error of 4%, and a 10% rate of drop out, the expected sample size for the present study was calculated to be 355. The study also aimed to compare the vaccine safety in adolescents with the adult population. Considering the feasibility concerns, it was decided to enroll at least 1000 participants, with an adolescent:adult ratio of around 2:1. The enrolment was stopped after 1024 participants were recruited.
Statistical Analysis
For data such as incidence, severity, and outcomes of AEFIs, values were recorded as frequencies as well as percentages. Chi-square test was applied for dichotomous variables such as sex, presence of co-morbidities, and prior COVID-19 to find associations between these potential risk factors and the development of AEFIs. Comorbidities in adolescents being quite low in number were analyzed as a single composite variable (comorbidities) rather than separate entities like diabetes, hypertension, hypothyroidism, etc. Variables with a statistically significant association (P < 0.05) on bivariate analysis or those deemed to be clinically relevant were incorporated into the final regression model. In this model, an AEFI occurring after any dose was the composite dependent variable. In addition, a comparative AEFI analysis was done only between adolescents and adults enrolled while receiving the first dose and monitored prospectively. This was done to ensure that the analysis was unadulterated by the dose of vaccine or recall bias. Results were analyzed using SPSS version 16.
Results
Figure 1 (as per the STrengthening the Reporting of OBservational studies in Epidemiology [STROBE] guidelines) shows the recruitment of vaccinees for the present study. Of the 698 adolescents enrolled, 339 and 359 were recruited at the time of the first and second doses of COVAXIN, respectively. Of the 339 adolescents visiting the center for the first dose, 28 were lost to follow-up, and among adolescents recruited at the time of the second dose, 19 were lost to follow-up. Loss to follow-up was defined as no response to three telephonic calls made at different times by the study team. Thus, AEFIs after 14 days post-first dose were assessed in a total of 670 adolescents, and AEFIs after 14 days post-second dose were assessed in 340 adolescents. A total of 326 adults were recruited in the study, of whom 201, 113, and 12 were visiting the center for first, second, and booster doses of COVAXIN, respectively. Of the 326 adults enrolled, 31 were lost to follow-up, and AEFI data after 14 days of vaccination was available for the remaining 295. The baseline characteristics of the study participants are mentioned in Table 1.
Figure 1.
STROBE flowchart showing enrolment of adolescents and adults in the study. AEFI adverse event following immunization, STROBE STrengthening the Reporting of OBservational studies in Epidemiology
Table 1.
Baseline characteristics of enrolled adolescents and adults
| Adolescents (N = 698) | Adults (N = 326) | ||
|---|---|---|---|
| Female/male | 368/330 | 165/161 | |
| Age (range in years) | 15–18 | 19–82 (median 30) | |
| Body mass index (mean ± SD) | 19.7 ± 3.3 | 22.2 ± 3.7 | |
| Comorbidities | 33 (4.7) | 66 (20.2) | |
| Asthma | 8 | DM | 20 |
| Epilepsy | 4 | Hypertension | 20 |
| Skin disease | 7 | Hypothyroidism | 7 |
| Hypothyroidism | 4 | Migraine | 3 |
| PCOD | 2 | Asthma | 2 |
| Others | 8 | Kidney disease | 2 |
| History of allergy, N (%)* | 40 (5.7) | 27 (8.3) | |
| Prior SARS-CoV-2 infection, N (%) | 42 (6) | 26 (7.9) |
All percentages expressed with respect to total enrolled adolescents and adults, respectively
DM diabetes mellitus, PCOD polycystic ovarian disease, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2
*Among adolescents with a history of allergy, common allergens were dust, smoke, food products such as egg, and curd (yoghurt), perfumes, sunlight, cold items, plastic, and soap
AEFIs After First Dose in Adolescents
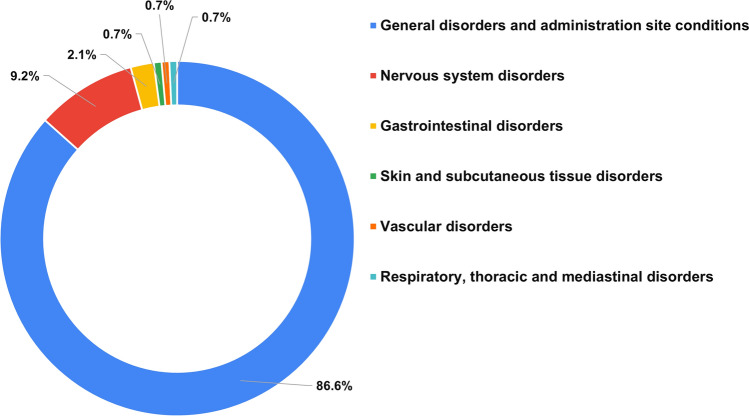
Among the 670 adolescents for whom data were available, 243 reported systemic or local AEFIs (36.3%). The majority of AEFIs involved the injection site and were reported by 183 adolescents (27.3%). Systemic involvement with or without local site involvement was reported by 102 (15.2%). Individually, injection site pain was the commonest AEFI reported (179, 26.7%) followed by fever (12.2%) and weakness (1.9%). The majority of AEFIs occurred within 24 h of receiving the vaccine (n = 233, 95.9% of all AEFIs). Most local site AEFIs were mild (152/183, 83.1%), and most systemic AEFIs were of ‘moderate’ grade (51/102, 50%). Altogether, six adolescents (0.9% of all adolescents: five females, one male) complained of systemic AEFIs of ‘severe’ grade, and no ‘serious’ AEFIs were noticed. Complete recovery from AEFIs was observed in all adolescents except three (two females, one male), and time to recovery (TTR) varied from 0.5 to 7 days. Four atypical AEFIs were reported in four adolescents (0.6%). These included a case of severe menstrual bleeding with no endocrinal disturbance in the past, a case of disturbing burning sensation in lower limbs in a male with insignificant past history, a case of recurrence of seizure within 5 days of the first dose in a female with pre-vaccination uncontrolled seizure disorder, and a case of hypothyroidism within 3 weeks of the first dose in a female with pre-vaccination neck swelling [Table 2 and Supplementary Table 2 (see the electronic supplementary material)]. The latter two were reported to the study team at the time of the second dose. The MedDRA SOCs of reported AEFIs are shown in Fig. 2. Details of severe, atypical, and persisting AEFIs are provided in Supplementary Table 2.
Table 2.
AEFIs in adolescents and adults after first, second and booster doses of BBV152 vaccine (COVAXIN)
| Adolescents after first dose (n = 670) | Adolescents after second dose (n = 340) | Adults after first dose (n = 181) | Adults after second dose (n = 102) | Adults after booster dose (n = 12) | |
|---|---|---|---|---|---|
| Vaccinees developing AEFIs, n (%) | 243 (36.3) | 129 (37.9) | 93 (51.4) | 38 (37.3) | 5 (41.7) |
| Vaccinees with local/systemic AEFIs! | |||||
| Only local | 141 (21) | 69 (20.3) | 53 (29.3) | 15 (14.7) | 0 (0) |
| Both systemic and local | 42 (6.3) | 27 (7.9) | 20 (11.0) | 4 (3.9) | 1 (8.3) |
| Only systemic | 60 (8.9) | 33 (9.7) | 20 (11.0) | 19 (18.6) | 4 (33.3) |
| Severity of local AEFIs!! | |||||
| Mild | 152 (83.1) | 90 (93.7) | 71 (97.3) | 15 (78.9) | 1 (100.0) |
| Moderate | 31 (16.9) | 6 (6.3) | 2 (2.7) | 4 (21.1) | 0 (0) |
| Severity of systemic AEFIs!!! | |||||
| Mild | 45 (44.1) | 27 (45.0) | 19 (47.5) | 13 (56.5) | 4 (80.0) |
| Moderate | 51 (50.0) | 33 (55.0) | 20 (50.0) | 10 (43.5) | 1 (20.0) |
| Severe | 6 (5.9) | 0 (0) | 1 (2.5) | 0 (0) | 0 (0) |
| Individual AEFIs (LLT)$ | |||||
| Injection site pain | 179 (26.7) | 96 (28.2) | 71 (39.2) | 18 (17.6) | 1 (8.3) |
| Fever | 82 (12.2) | 48 (14.1) | 29 (16.0) | 13 (12.7) | 1 (8.3) |
| Weakness | 13 (1.9) | 3 (0.9) | 7 (3.9) | 3 (2.9) | 1 (8.3) |
| Headache | 10 (1.5) | 11 (3.2) | 7 (3.9) | 4 (3.9) | 0 (0) |
| General body pain | 7 | 8 | 3 | 5 | 0 |
| Injection site swelling | 5 | 0 | 1 | 0 | 0 |
| Abdominal distress | 4 | 0 | 1 | 1 | 1 |
| Diarrhea | 2 | 0 | 0 | 0 | 0 |
| Dizziness | 2 | 1 | 3 | 0 | 0 |
| Itching | 2 | 0 | 1 | 1 | 0 |
| Vomiting | 0 | 2 | 0 | 0 | 0 |
| Allergy (skin allergy increased) | 0 | 1 | 1 | 0 | 0 |
| Anorexia | 0 | 2 | 1 | 1 | 0 |
| Burning sensation in lower limbs | 1 | 0 | 0 | 0 | 0 |
| Cold (like features) | 1 | 0 | 1 | 0 | 0 |
| Convulsions* | 1 | 1 | 0 | 0 | 0 |
| Cough | 0 | 1 | 1 | 0 | 1 |
| Drowsiness | 0 | 1 | 0 | 0 | 0 |
| Dyspepsia (increased) | 0 | 0 | 1 | 0 | 0 |
| Dyspepsia (flatulence) | 0 | 0 | 1 | 0 | 0 |
| Epistaxis | 0 | 1 | 1 | 0 | 0 |
| Eye discomfort | 0 | 0 | 1 | 0 | 0 |
| Fall in blood pressure | 0 | 0 | 1 | 0 | 0 |
| Fatigue | 1 | 1 | 0 | 2 | 0 |
| Heavy menstrual bleeding | 1 | 0 | 1 | 0 | 0 |
| Hypothyroidism** | 1 | 0 | 0 | 0 | 0 |
| Joint pain (upper limb) | 1 | 0 | 0 | 0 | 0 |
| Nausea | 1 | 1 | 1 | 0 | 0 |
| Pain of lower extremities | 1 | 0 | 0 | 0 | 0 |
| Throat sore | 0 | 0 | 0 | 0 | 1 |
| Unilateral leg swelling | 0 | 0 | 0 | 1 | 0 |
| TTR (range in days) | 0.5–7 | 0.5–14 | 1–12 | 1–7 | 2–7 |
| AEFIs persisting at 14-days follow-up | 3& | 8 | 5 | 5 | 1 |
AEFI adverse event following immunization, LLT low level term, TTR time to recovery
*In a female with pre-vaccination uncontrolled seizure disorder
**In a female with pre-vaccination neck swelling
!All percentages are expressed with respect to total vaccinees in group
!!All percentages are expressed with respect to total number of local AEFIs
!!!All percentages are expressed with respect to total number of systemic AEFIs
$All percentages are expressed with respect to total vaccinees in group; percentages mentioned only for prominent AEFIs; some vaccinees developed more than one AEFI
&In one vaccinee, AEFI after first dose persisted until the time of second dose
Figure 2.
MedDRA SOCs of AEFIs following first dose of BBV152 in adolescents (percentages are out of total AEFI SOCs; in the case of development of more than one AEFI from the same SOC in an individual vaccinee, the SOC has been counted once). AEFI adverse event following immunization, MedDRA Medical Dictionary for Regulatory Activities, SOC system organ class
AEFIs After Second Dose in Adolescents
Of 340 adolescents interviewed after the second dose, AEFIs were reported by 129 (37.9%). Among these, local site and systemic AEFIs were noticed in 96 (28.2%) and 60 adolescents (17.6%), respectively. Injection site pain was the commonest (28.2%), followed by fever (14.1%) and headache (3.2%). The majority of the local AEFIs were of ‘mild’ grade, and the majority of the systemic AEFIs were ‘moderate.’ No ‘severe’ or ‘serious’ AEFIs were observed (Table 2). The majority of AEFIs occurred within 24 h of the vaccine (122, 94.6%), and recovery was seen in all except eight (2.3%) until the date of interview. TTR varied from 0.5 to 14 days. Eight adolescents (six females, two males) had persisting AEFIs at 14 days; none were of ‘severe’ or ‘serious’ grade. Three atypical AEFIs were reported in three adolescents (0.9%). These included one case each of epistaxis, recurrence of seizures in a female with uncontrolled seizure disorder pre-vaccination (same female, as after the first dose), and aggravation of pre-vaccination skin allergy. The MedDRA SOCs of reported AEFIs are shown in Fig. 3. The details of atypical and persisting AEFIs are described in Supplementary Table 2 (see the electronic supplementary material).
Figure 3.
MedDRA SOCs of AEFIs following second dose of BBV152 in adolescents (percentages are out of total AEFI SOCs; in the case of development of more than one AEFI from the same SOC in an individual vaccinee, the SOC has been counted once). AEFI adverse event following immunization, MedDRA Medical Dictionary for Regulatory Activities, SOC system organ class
AEFIs in Adults After First, Second, and Booster Dose of COVAXIN
After excluding those lost to follow-up, AEFIs were assessable in 181 adults after the first dose and in 102 adults after the second dose. AEFIs occurred in 93 (51.4%) after the first dose and 38 (37.3%) after the second dose of the vaccine (Table 2). Injection site pain and fever were common AEs after both doses, and the majority of the AEFIs were mild-moderate. Only one severe and no serious AEFI was reported in adults. Among atypical AEFIs reported in two adults (0.7%), epistaxis and heavy menstrual bleed were reported in one case each after the first dose. AEFIs after a booster dose were reported in 41.7% and are mentioned in Table 2. AEFIs until the day of telephonic follow-up were persisting in 11 (eight females, three males) adults (3.7%). The majority of these AEFIs belonged to the SOC of ‘general disorders and administration site conditions’ and ranged in severity from mild to moderate. Details of severe, atypical, and persisting AEFIs in adults are given in Supplementary Table 2 (see the electronic supplementary material).
Comparative Analysis Between Adolescents Receiving First Dose and Adults Receiving First Dose
Direct comparison of AEFI occurrence was performed between adults and adolescents recruited at the time of receiving the first dose (n = 540). After excluding 48 participants lost to follow-up, this analysis was conducted in the set of 492 participants (311 adolescents and 181 adults). No statistically significant difference was observed in the local (41.8% vs 40.3%, P = 0.75), systemic (19% vs 22.1%, P = 0.40), or overall occurrence (50.8% vs 51.4%, P = 0.90) of AEFIs between adolescents and adults.
Risk Factors of AEFIs in Adolescents
Bivariate analysis (Table 3a) was performed to determine association between AEFIs occurring after any dose and potential risk factors. This was performed only in adolescents for whom there was complete information of AEFIs available following both doses (n = 344). Variables selected were sex, history of previous COVID-19, presence of co-morbidities, and history of allergy. In unadjusted analysis, a statistically significant positive association for occurrence of AEFIs was observed with female sex, as well as history of allergy. Association of both these factors remained statistically significant even on logistic regression. Females were at 1.6 times and those with history of allergy were at 3 times higher risk of development of AEFIs with respect to respective comparators (Table 3b).
Table 3.
Risk factors of occurrence of AEFIs in adolescents: (a) bivariate analysis of association between potential risk factors and AEFIs; (b) logistic regression analysis to determine risk factors of AEFIs
| Adolescents, n = 344* | (a) | Adolescents, n = 344* | (b) | ||
|---|---|---|---|---|---|
| Vaccinees developing AEFIs (% of total vaccinees in group) |
P value | aOR (CI) | P value | ||
| Sex (n) | Sex | ||||
| Females (189) | 105 (55.5) | 0.03 | Females | 1.57 (1.02–2.42) | 0.04 |
| Males (155) | 68 (43.9) | Males (reference) | |||
| Allergy history (n) | Allergy history | ||||
| Yes (20) | 15 (75) | 0.02 | Yes | 3 (1.07–8.6) | 0.036 |
| No (324) | 158 (48.8) | No (reference) | |||
| Prior COVID-19 (n) | |||||
| Yes (27) | 16 (59.3) | 0.33 | |||
| No (317) | 157 (49.5) | ||||
| Comorbidities (n) | |||||
| Yes (21) | 14 (66.7) | 0.12 | |||
| No (323) | 159 (49.2) | ||||
AEFI adverse events following immunization, aOR adjusted odds ratio, CI confidence interval, COVID-19 coronavirus disease 2019
Significant P values and odds ratios marked in bold
*After excluding vaccinees who did not develop AEFIs after first dose and were lost to follow-up after second dose (n = 15)
Description of AEFIs in Female Adolescents and Adolescents with History of Allergy
With female sex and history of allergy emerging as determinants of AEFIs in adolescents, a separate descriptive analysis was performed to underscore the type of AEFIs in female adolescents (n = 189) and adolescents with a history of allergy (n = 20) for whom information was available for both doses. Injection site pain and fever were the common AEFIs following the first dose of the vaccine, reported by 39 (20.6%) and 24 (12.7%) in female participants, respectively. The two were also the leading AEFIs following the second dose of the vaccine, developing, respectively, in 66 (34.9%) and 23 (12.2%) in female participants. The majority of the AEFIs belonged to the MedDRA SOC of ‘general disorders and administration site conditions,’ followed by ‘nervous system disorders.’ T cases of atypical AEFIs were reported. One was a case of recurrence of seizure in a female with a history of uncontrolled seizure disorder, and the other was hypothyroidism in a female with pre-vaccination history of neck swelling.
Injection site pain and fever were also common AEFIs in adolescents with a history of allergy. Injection site pain occurred in 30% after the first dose and 40% after the second dose, and fever developed in 20% after the first dose and 25% after the second dose in adolescents. T atypical AEFIs were reported in this risk group, while no anaphylactic events were reported. One was a case of recurrence of convulsions in a female with a history of uncontrolled seizure disorder and allergy to sunlight (same patient as described above), and the other was a case of exaggerated skin allergy and generalized burning sensation in a male with a history of allergy to sunlight. Though the majority of AEFIs were mild-moderate and recovered fully, female adolescents were more likely to develop persistent (2.2% vs 0.9%) and severe (1.3% vs 0.3%) AEFIs compared to males.
Discussion
After vaccination for COVID-19, serious AEs such as the syndrome of thrombosis and thrombocytopenia, acute cardiac events, and new onset as well as flares of autoimmune diseases have been reported in adults [9–11]. Myocarditis, though rare, has been observed in adults with mRNA-based COVID-19 vaccines. With recommendations to vaccinate adolescents, myocarditis, as assessed by passive surveillance methods, is being reported in this age group also at an increased frequency [12]. Rates of AEs may be high in adolescents with underlying inflammatory diseases. In adolescents with juvenile rheumatic diseases, an overall favorable short-term safety profile was demonstrated. However, certain complications such as renal failure, pulmonary hemorrhage, and flares of lupus were reported in the vaccine group [13]. Since causality association is not always established in such cases, a longer and disease-specific follow-up is warranted for risk stratification of individuals. The commonly used passive surveillance methods often suffer from underreporting of AEFIs. The actual risk of rare but severe AEs needs evaluation using a prospective cohort-based design. To fill this gap, the present study was planned to generate evidence on the short- and long-term safety profile of COVAXIN in adolescents monitored prospectively at decided intervals. The study is also the first real-world study generating safety data for COVAXIN in adolescents.
Nearly one third of selected adolescents developed AEFIs. Both local and systemic AEFIs were slightly more common after the second dose in adolescents. A higher reactogenicity after the second dose has previously been demonstrated for mRNA-based COVID-19 vaccines [14]. The viral vectored vaccines and inactivated vaccines, on the other hand, have displayed lesser reactogenicity with the second dose [7, 8, 15, 16]. The observation of increased reactogenicity with the second dose in the present study, however, needs a cautious interpretation. Though AEFIs assessed retrospectively were enquired actively from both adolescents and accompanying guardians, the component of recall bias cannot be excluded.
The observed incidence of local and systemic AEFIs in adolescents is higher than reported in the pre-print version of the phase 2/3 trial of COVAXIN by Bharat Biotech (n = 176) [4]. Close to 0.9% of adolescents developed systemic AEFIs of ‘severe’ grade after the first dose. Both local and systemic reactogenicity rates observed in adolescents were higher than those reported previously in adults receiving inactivated COVID-19 vaccines [8, 17]. Apart from adolescents, a higher rate of occurrence of AEFIs (37–51%) was observed in adults too, contrary to the published studies showing close to 12–21% rates [7, 8]. Notwithstanding the higher rate of AEFIs, the majority of these were mild-moderate and recovered over a median time of 1–2 days.
Regression analysis showed female adolescents and those with a history of allergy to be at 1.6 times and 3 times higher risk of developing AEFIs, commonly from the SOC of general disorders. These two co-variates have recently been projected as risk factors of AEFI after COVAXIN in adults by a group from eastern India [16]. Female sex, a history of allergy, and presence of hypothyroidism have been proposed as determinants of AEFI after COVISHIELD in adults [15]. The association of AEFIs with hypothyroidism could not be examined in the present study because of a small number of individuals with thyroid disorders.
Other vaccines approved for adolescents include CoronaVac of Sinovac (China) and the BNT162b2 mRNA vaccine of Pfizer. Compared to what has been previously reported with CoronaVac, both local and systemic AEFIs were higher with COVAXIN in adolescents, particularly after the second dose [18]. However, AEFI rates with BBV152 were lower compared to mRNA-based vaccines, for which high local (85%) and systemic (55–66%) reactogenicity rates have been observed in adolescents [14]. Whether these differences can also be attributed to variable study designs or ethnic diversity or vaccine types can be verified from head–head comparisons of different vaccines in the same population setting. Among atypical AEs, lymphadenopathy has been reported in 0.8% of adolescent BNT162b2 recipients [14]. In the current study, of the total interviewed adolescents, six atypical AEFIs (0.9%) were reported. Among these, were two cases of increased bleeding, which recovered fully. Abnormal bleeding, though recovered, was also reported by two adult participants. Monitoring of such atypical events along with performance of causality association should be incorporated in future vaccination drives. Among other atypical AEFIs, two were persisting until the time of interview and included a case of disturbing burning sensation in lower limbs and a case of aggravation of pre-vaccination skin allergy. The remaining two cases included recurrence of seizure after both doses in a female with pre-vaccination uncontrolled seizure disorder and hypothyroidism diagnosed within 3 weeks of the first dose in a female with pre-vaccination neck swelling. Both of these participants have been started on the necessary medications by their treating physicians and shall be monitored in the scheduled follow-ups. In all, AEFIs were persistent in > 2% of adolescents at day 14 after the second dose, and also in 3.7% of adults overall, and require further follow-up to predict the course.
Limitations
Before the study was planned, the authors in their clinical practice had observed atypical AEFIs, particularly after BBV152, to be occurring in adults within 7–10 days of vaccination. Based on this and considering the manpower and feasibility issues, one-time telephonic follow-up after 14 days was planned for the present study. Because of administrative issues related to obtaining ethical approval, there was a delay of 7–10 days between the initiation of adolescent vaccination and the start of the study. As a result, nearly half of the adolescents were recruited at the time of their second dose, and AEFIs after the first dose in this subset were assessed retrospectively. Though this was done to reduce time delays in highlighting adolescent-specific safety data in the public domain, an element of recall bias cannot be ruled out in such retrospective evaluation. This bias, to some extent, can also explain a relatively higher rate of AEFIs in adolescents after the second dose of vaccine compared to the first dose. However, the main motive of this retrospective assessment was the timely detection and reporting of adolescent-specific serious, severe, and atypical AEFIs, which are less likely to be affected by recall bias. Though the vaccinees recruited prospectively were informed of the AEFIs expected after vaccine, the need to record them in diaries, and about the telephonic call from the study team, no pre-designed format pertaining to AEFIs was provided to them, mainly because of the varying educational standards of the participants. A pre-designed format could have further reduced errors due to recall bias and improved the overall AEFI reporting. The study was primarily based on telephonic interviews. The telephonic interview is a time-saving tool for obtaining required and sensitive information in detail particularly in resource-limited settings. It is also a practical approach in the time of a pandemic, when travel-related restrictions because of lockdown policies hamper the hospital visit. However, it is inferior to direct observations made by physicians and data retrieval methods based on clinical records. The latter could be arranged in the current study only for a few atypical AEFIs. The study did not involve any physical examinations of the enrolled participants. Further, due to funding issues, no routine blood or radiological investigations could be performed. Therefore, certain AEFIs such as vital sign variations and serious AEFIs such as thrombosis/thrombocytopenia and myocarditis might have been missed, especially when the manifestations were not florid, leading to underestimation of the AEFI incidence. These investigations may be incorporated into future follow-ups with availability of funds.
Some of the AEFIs such as fever, cough, and sore throat overlap with clinical features of COVID-19, the third wave of which was ongoing in the community during the time of adolescent vaccination. Despite the RT-PCR advice given to the vaccinees, the test was not performed by the majority because of the continuous media campaign of common AEFIs expected after vaccination. Further, compared to adolescents, adults were recruited in small numbers. However, nearly a similar number of adolescents and adults recruited prospectively after the first dose were selected to provide unadulterated comparative analysis.
The study design being focused only on a small population of vaccinated individuals is useful for hypothesis generation and detection of early safety signals. However, no conclusions can be drawn on the risk of atypical AEFIs with the vaccine. Future studies with larger sample sizes, involving a comparator arm of unvaccinated adolescents or involving comparisons with the background rate of such events, could provide insights on the possible risk of atypical AEFIs with the BBV152 vaccine.
Conclusion
COVAXIN carries an overall favorable short-term safety profile in adolescents. The AEFI rates with COVAXIN are much lower compared to those reported with mRNA vaccines in adolescents, though future studies with direct comparisons of different vaccine types in the same population setting are needed to generate evidence on relative vaccine safety. Vigilance for severe and persistent AEFIs is needed while vaccinating females and those with a history of allergy, and watchfulness is advised for bleeding events. A longer follow-up, as planned in the study, may unravel more information about the long-term safety profile of COVAXIN in adolescents.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
UK would like to thank the Institutions of Eminence Scheme of the Banaras Hindu University for research support; SSC would like to thank the Institutions of Eminence Scheme of the Banaras Hindu University and the Indian Council of Medical Research for research support.
Declarations
Funding
No funding support.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
No human experimentation was performed. All procedures were performed as per the Declaration of Helsinki and its further modifications. Study was conducted after permission from Institute Ethics Committee of the Institute of Medical Sciences, Banaras Hindu University (Ethical Approval number: Dean/2022/EC/3210 and Dean/2021/EC/2526).
Consent to participate
Written informed consent/assent obtained from all participants.
Consent for publication
Written informed consent/assent obtained from all participants.
Availability of data and material
Since this is a preliminary analysis, associated data may be made available by the corresponding author on reasonable request, following applicable regulations.
Code availability
Not applicable.
Author contributions
UK: Planned the study, designed the study methodology, supervised data collection and data verification, conducted the review of the literature, performed the statistical analysis, and drafted the first version of the article. AKL, MC, AJ, AD: Assisted in the review of the literature and data collection and recording. SK: Planned the study and supervised data collection. VJ, KP: Supervised data collection and data verification and assisted in the review of the literature. SSC: Supervised the study, assisted in data verification and visualization, and edited the final version of manuscript. All authors read and approved the final version.
Contributor Information
Vaibhav Jaisawal, Email: drvaibhav29@gmail.com.
Kishor Patwardhan, Email: kpatwardhan@bhu.ac.in.
Sankha Shubhra Chakrabarti, Email: sankha.chakrabarti1@bhu.ac.in.
References
- 1.World Health Organization. Interim statement on COVID-19 vaccination for children and adolescents. https://www.who.int/news/item/24-11-2021-interim-statement-on-covid-19-vaccination-for-children-and-adolescents.
- 2.US FDA. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic [Internet]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use [cited 2022 Apr 7].
- 3.China.org.cn. Sinovac COVID-19 vaccine safe for children, adolescents [Internet]. http://www.china.org.cn/china/2021-07/02/content_77602836.htm [cited 2022 Apr 7].
- 4.Vadrevu KM, Reddy S, Jogdand H, Ganneru B, Mirza N, Tripathy VN, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine (BBV152) in children from 2 to 18 years of age: an open-label, age-de-escalation phase 2/3 study. medRxiv [Internet]. 2021;2021.12.28.21268468. http://medrxiv.org/content/early/2021/12/29/2021.12.28.21268468.abstract.
- 5.Kaur U, Anju KL, Chauhan M, Joshi A, Kansal S, Jaisawal V, et al. A prospective observational study on BBV152 coronavirus vaccine use in adolescents and comparison with adults- first real-world safety analysis. medRxiv [Internet]. 2022;2022.04.08.22273634. http://medrxiv.org/content/early/2022/04/10/2022.04.08.22273634.abstract. [DOI] [PMC free article] [PubMed]
- 6.MoHFW. Guidelines for COVID-19 vaccination of children between 15-18 years and precaution dose to HCWs, FLWs & 60+ population with comorbidities [Internet]. https://www.mohfw.gov.in/pdf/GuidelinesforCOVID19VaccinationofChildrenbetween15to18yearsandPrecautionDosetoHCWsFLWs&60populationwithcomorbidities.pdf [cited 2022 Apr 7].
- 7.Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R, Chakrabarti SS, Gambhir IS, Verma A, Kumar I, Ghosh S, et al. Acute cardiac events after ChAdOx1 nCoV-19 corona virus vaccine: report of three cases. Am J Ther. 2022 doi: 10.1097/MJT.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Kaur U, Singh A, Chakrabarti SS. Refractory hyper-eosinophilia associated with newly diagnosed rheumatoid arthritis following inactivated BBV152 COVID-19 vaccine. J Med Virol. 2022;94:3482–3487. doi: 10.1002/jmv.27742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June Choe Y, Yi S, Hwang I, Kim J, Park Y-J, Cho E, et al. Safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents. Vaccine. 2022;40:691–694. doi: 10.1016/j.vaccine.2021.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heshin-Bekenstein M, Ziv A, Toplak N, Hagin D, Kadishevich D, Butbul YA, et al. Safety and immunogenicity of BNT162b2 mRNA COVID-19 vaccine in adolescents with rheumatic diseases treated with immunomodulatory medications. Rheumatology (Oxford) 2022 doi: 10.1093/rheumatology/keac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenck RW, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur U, Ojha B, Pathak BK, Singh A, Giri KR, Singh A, et al. A prospective observational safety study on ChAdOx1 nCoV-19 corona virus vaccine (recombinant) use in healthcare workers—first results from India. EClinicalMedicine. 2021;38:101038. doi: 10.1016/j.eclinm.2021.101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parida SP, Sahu DP, Singh AK, Alekhya G, Subba SH, Mishra A, et al. Adverse events following immunization of COVID-19 (Covaxin) vaccine at a tertiary care center of India. J Med Virol. 2022;94:2453–2459. doi: 10.1002/jmv.27655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–1653. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.