Abstract
This year marks the tenth anniversary of cell therapy with chimeric antigen receptor (CAR)-modified T cells for refractory leukemia. The widespread commercial approval of genetically engineered T cells for a variety of blood cancers offers hope for patients with other types of cancer, and the convergence of human genome engineering and cell therapy technology holds great potential for generation of a new class of cellular therapeutics. In this Review, we discuss the goals of cellular immunotherapy in cancer, key challenges facing the field and exciting strategies that are emerging to overcome these obstacles. Finally, we outline how developments in the cancer field are paving the way for cellular immunotherapeutics in other diseases.
Cell therapies entered routine medical practice as blood transfusion in the 1930s, based on the discovery of the blood-group system by Karl Landsteiner in 1900. T cells were first used for cancer therapy in the setting of allogeneic (donor) stem cell transplantation and autologous (patient’s own) cell therapy with tumor-infiltrating lymphocytes (TILs) for metastatic melanoma. In addition to CAR-modified T cells, which target cell surface antigens, the US Food and Drug Administration (FDA) currently lists chondrocytes, cord blood, dendritic cells (DCs), fibroblasts, keratinocytes and thymus as approved cellular and tissue-based therapeutics1. The approval of CAR T cells for children and young adults with refractory leukemia was first reported a decade ago2, and the emergence of cellular therapeutics and gene therapies as a new pillar in medicine is leading the pharmaceutical industry to diversify from its previous focus on small-molecule drug discovery and recombinant-protein therapeutics3.
The trajectories of cellular immunotherapies in cancer, regenerative medicine and beyond are likely to involve ever-increasing levels of genetic engineering. For example, red blood cell transfusion has been used recursively for decades, as a lifesaving procedure for millions of patients with transfusion-dependent sickle-cell anemia and other hemoglobinopathies. Recently, in a hallmark pilot trial for sickle-cell disease and thalassemia, two patients were given a single infusion of autologous hematopoietic stem cells (HSCs) engineered with the CRISPR–Cas9 system and both became transfusion-independent as a result4. The prospect of a cure for hemoglobinopathies by means of genetically engineered HSCs is on the horizon, offers hope to patients afflicted with the most common genetic diseases, and has major implications for the pharmaceutical industry, FDA regulation policies, and the cellular immunotherapy space at large. Most notably, the manufacturing of genetically modified HSCs will require higher standards and more stringent regulation than that of red blood cells derived from healthy donors.
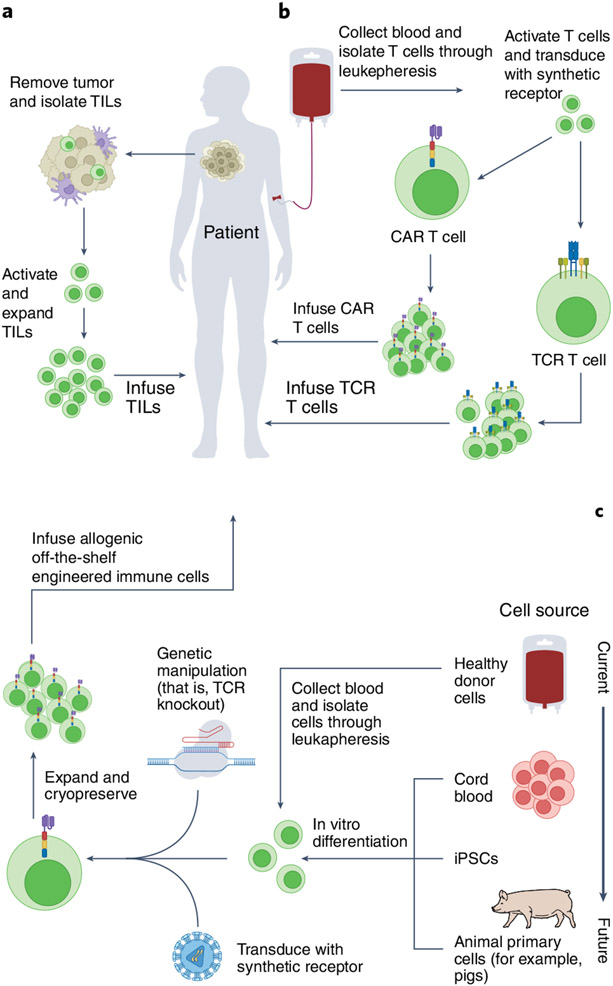
In the field of oncology, there are hundreds of ongoing clinical trials with autologous engineered T cells5. In addition, of increasing importance are the many ongoing trials using engineered allogeneic cells comprising T cells, natural killer (NK) cells, stem cells and other cell types. For considerations on the emerging field of xenogeneic (of a different species) cell therapies, see ref.6. These distinctions of cell type and source (Fig. 1) are particularly imperative as we seek to understand mechanisms underlying cell rejection, engraftment success, efficacy, immunotoxicities and FDA regulation.
Fig. 1 ∣. Autologous and allogeneic engineered cell manufacturing.
a, Tumor mass is excised and TILs are isolated. Once collected, autologous TILs are activated, engineered and expanded ex vivo under optimized culture conditions, prior to being reinfused into the patient. b, Peripheral blood is collected and T cells are isolated through leukapheresis. Upon isolation, T cells are activated ex vivo and virally transduced to express a synthetic CAR receptor or an engineered TCR. Following expansion, genetically modified autologous T cells are infused into the patient. c, Cells from various sources are either directly collected from peripheral blood mononuclear cells or are differentiated in vivo. To prevent alloreactivity, human genome engineering, such as removal of the endogenous TCRs in allogeneic T cells, is conducted, and synthetic receptors are inserted. Cells are cryopreserved and banked following manufacturing. Figure created using BioRender.
A premise of this Review is that oncology is paving the way for the use of engineered cells to deliver powerful effects outside of oncology, leading to the potential cure of autoimmunity, inflammation and genetic disorders, and to advances in the field of regenerative medicine. The fields of transplant, tolerance and rejection have set the stage for cellular therapy as we know it today. Here, we outline the path from early transplant biology to more recent discoveries. As we highlight mechanisms of cellular tolerance, rejection and immunotoxicities, we discuss the importance of leveraging this knowledge with advances in genomic engineering and combination therapy regimens, to accelerate progress in the emerging field of engineered cellular immunotherapeutics.
Impact of tolerance and rejection mechanisms on cellular therapies
The holy grail of cell therapy is achieving both long-term engraftment and durable remission. However, the initial success of allogeneic cell therapies has been tempered by the short persistence of engineered T cells7. Development of efficacious allogeneic engineered cell therapies will require elucidation and manipulation of cellular rejection circuits to permit long-term engraftment. Here, we consider lessons from the field of immunology that are related to tolerance in the healthy state and mechanisms of engineered cell rejection. See Box 1 for considerations related to HSC transplantation (HSCT), the first successful allogeneic cell therapy to enter the clinic.
Box 1 ∣. Lessons learned from graft-versus-host disease.
Long before the current age of engineered cell therapies, myeloablative therapies with HSCT were implemented to combat hematological malignancies. Despite successful transplantation, the initial autologous HSCT recipients quickly relapsed149, and allogeneic HSCT was found to be more effective in this setting. An unexpected finding was that the nature of the infused bone marrow conferred a major and durable antileukemic effect: HLA-identical sibling (allogeneic) marrow was superior to HLA-identical monozygotic twin marrow150. The antileukemic effects of allogeneic HSCT were correlated with onset of GVHD and a graft-versus-leukemia (GVL) effect151. These landmark studies were the first to demonstrate that the human immune system could mediate potent antitumor effects.
Donor T cells present in the graft were necessary to elicit the GVL effect; these cells engage the immune system to mediate killing of host leukemic cells and HSCs. While we now understand that the origins of the GVL effect and GVHD are rooted in minor and MHC mismatches152, the mechanisms are still being explored153. The field was thus left with a similar paradox to one faced in the field of cellular immunotherapy: how can tumor immune tolerance be broken without disrupting peripheral tolerance? The field of GVHD has and continues to shed light on basic and translational aspects of tolerance and serves as an example for leveraging this understanding in the broader context of cancer.
With the initial investigation of GVHD came an improved understanding of immune tolerance. For decades, the field exemplified the translation of bench to bedside and back to bench research, presenting new opportunities to further our understanding of tolerance in human immunology. Cellular mechanisms that maintain central and peripheral tolerance are under attack during GVHD, providing a pathogenic link between alloimmunity and the development of engrafted donor autoreactive T cells that play a pivotal role in the pathophysiology of chronic GVHD154. Novel strategies designed to promote both tissue tolerance and immune tolerance may be essential to decrease GVHD severity without suppressing T-cell responses, thus preserving the beneficial GVL effect153,155.
Perspectives on immune tolerance.
The ultimate goal of cell, tissue and organ transplantation is the development of strategies that enable the acceptance of cells in the recipient without the need for immunosuppression. The possibility of this aim was supported by the discovery of freemartin cattle, dizygotic twins who shared the same placental blood supply in utero. In the last century, Medawar has shown that freemartin cattle have microchimerism as adults, and that these animals accept skin grafts from their genetically disparate twin, yet reject skin grafts from unrelated cattle8. However, intensive research in humans has not been able to replicate this experiment of nature to induce tolerance at will, that is, permitting infusion of transplanted allogeneic cells.
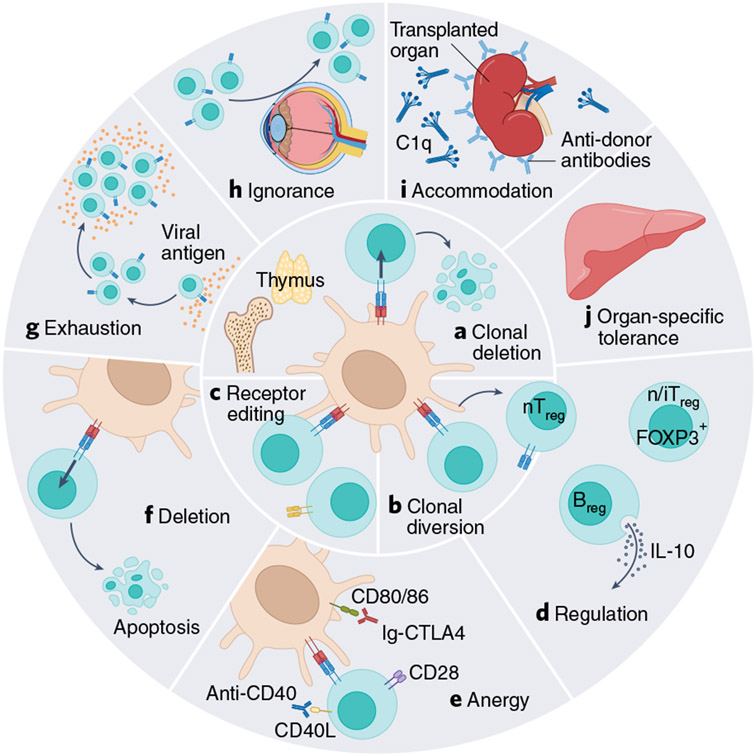
In the healthy state, immune tolerance is maintained through (1) central and (2) peripheral tolerance, and each arm works in tandem to prevent the onset of autoimmunity while enabling rejection of foreign grafts (Fig. 2). Immunologists have recognized at least ten mechanisms of tolerance at the level of development in the thymus and in the peripheral compartments. Engineered therapeutic cells will need to obey or circumvent these basic immunologic mechanisms to avoid rejection, and will need to do so at varying degrees, depending on the desired site of engraftment. Organ transplant studies have revealed that certain organs (such as the liver) are innately more tolerogenic than others9. Similar to organ- and tissue-specific tolerance, tumors generate their own homeostatic niche by adapting mechanisms of native immunological tolerance to evade attack10. In their evasion of the antitumor attack, tumors seek to phenocopy nearly every mechanism of immune tolerance11. Thus, the engraftment and persistence of engineered cells in the TME may differ from that in healthy organs.
Fig. 2 ∣. Mechanisms of tolerance.
Central (a–c) and peripheral (d–j) tolerance mechanisms a, Clonal deletion. A developing T cell (in the thymus) or B cell (in the bone marrow) recognizes a self-antigen and is deleted by apoptosis. b, Clonal diversion. A developing T cell receives a medium-strength signal through its receptor in the thymus, which induces FOXP3 expression and differentiation into a natural Treg cell (nTreg). c, Receptor editing. A developing B cell recognizes a self-antigen in the bone marrow and undergoes further genetic recombination events to produce a new antigen receptor on its surface that no longer responds to self-antigen. d, Regulation. nTreg cells (from the thymus), inducible Treg (iTreg) cells (generated in the periphery), regulatory B cells (Breg) and CD8+ T suppressor cells work through various contact-dependent and contact-independent modes to suppress immune responses in the periphery. e, Anergy. A state of unresponsiveness induced when a T cell receives a signal through its cognate antigen receptor (TCR-Ag-MHC) in the absence of costimulation (CD28-B7 or CD40-CD40L). f, Deletion. Strong signals through the cognate antigen receptors on lymphocytes can bring about activation-induced cell death. g, Exhaustion. The persistence of antigen during an ongoing immune response can lead to a state of hyporesponsiveness. h, Immunologic ignorance. Some organs (such as the anterior chamber of the eyes) are immune-privileged, and lymphocytes have diminished access to these tissues. i, Accommodation. B cells produce antibodies that fix complement and damage a transplanted organ; but in the presence of a persistent antigen, the B-cell and antibody repertoires change, and produce antibodies that protect from complement fixation, thereby protecting the transplanted organ from damage. j, Organ-specific tolerance. Some organs are more tolerogenic than others, such as the liver (adapted from Ezekian at al. with permission127).
Mechanisms of engineered cell rejection.
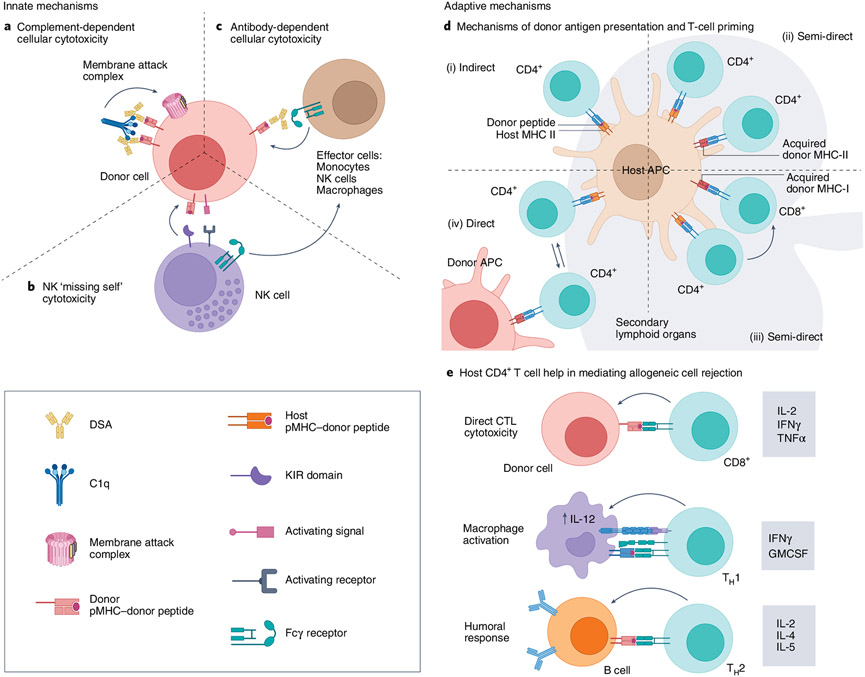
From early studies of transplantation and rejection, it became clear that rejection was mediated through an immunological event. As scientists pieced together mechanisms underlying graft recognition, tolerance, rejection and graft-versus-host disease (GVHD; Box 1), they concurrently translated these findings to improve patient outcomes. The concept of ‘hybrid resistance’ was first described by Cudkowicz and Stimpfling12 in their study of murine bone marrow transplant (BMT) rejection. This resistance referred to the rejection of parental BMT by F1 progeny, even in irradiated mice. Despite the prominent role of T cells in mediating skin-graft rejection, BMT rejection was later demonstrated to be mostly driven by host NK cells owing to missing major histocompatibility complex-I (MHC-I) molecules, similar to the ‘missing self’ concept identified in allogeneic rejection13,14. Contrary to the role of host NK cells in mediating rejection, donor NK cell alloreactivity plays a role in both preventing GVHD and abrogating leukemia relapse and graft rejection in humans15. The conserved function of NK cells in mice and humans, despite divergences in expression of proteins (for example, ly49 in mice and killer-cell immunoglobulin-like receptors (KIRs) and CD56 in humans) and respective complement pathways, has enabled an enhanced understanding of ‘self’ versus ‘missing self’16. Once more, concepts of hybrid resistance were likely the first hints at the immune cell inhibitory axis, which now serves as the basis for checkpoint immunotherapies. Therefore, early work investigating these concepts of allorecognition paved the way for BMT, checkpoint immunotherapies and, ultimately, cellular immunotherapies17.
Over half a century later, mechanisms underlying cell rejection are still being investigated (Fig. 3). While early concepts of host NK ‘missing self’ recognition explain rejection in naive mice, alloantigen-presensitized mice depend more heavily on macrophage-mediated mechanisms instead18. Importantly, macrophage-mediated mechanisms in alloantigen-presensitized models were inducible by either CD4+ T-cell help (which provides CD40/40L engagement) or CD40 stimulation alone, and have also been shown to ameliorate GVHD in mice18. The adaptive inflammatory immune response is initiated following host T-cell recognition of non-self/donor antigen in secondary lymphoid organs (Fig. 3d,e)19. Novel visualization techniques have recently enabled the investigation of allogeneic T-cell trafficking through secondary lymphoid organs20. Allogeneic T cells were rapidly rejected, even in mice devoid of host T cells, and were found to rapidly die by apoptosis. Donor T-cell fragments were then endocytosed and presented on resident DCs, which induced host T-cell activation. Others have since identified this allogeneic T-cell mechanism of DC activation as restricted to resident DCs expressing the chemokine receptor XCR1, highlighting its potential to be leveraged in novel contexts as a vaccine vector21.
Fig. 3 ∣. Innate and adaptive mechanisms of cell recognition and rejection.
The contribution of each pathway in allogeneic cell rejection can vary depending on donor cell source, transplant location, and host levels of immunogenicity. Innate mechanisms of rejection are shown in parts a–c. a, Complement-dependent cellular cytotoxicity is initiated after pre-existing donor-specific anti-HLA antibodies (DSAs) recognize non-self MHC-I. Complement component C1q then recognizes these DSA–pMHC I complexes and initiates the complement cascade, leading to the formation of membrane attack complexes, which induce donor cell apoptosis (as seen in HSCT128 and solid organ transplant129). b, NK cell ‘missing self’ cytotoxicity: NK cells have activating and inhibiting receptors (that is, KIR in humans and Ly49 in mice). In the presence of an activating signal, KIR detection of self MHC-I will prevent killing by NK cells. However, if self MHC-I is not detected on the target cell, NK cytotoxicity will not be inhibited. c, Antibody-dependent cellular cytotoxicity can be mediated by DSAs and effector cells bearing Fcγ receptors (that is, monocytes, NK cells and macrophages). d, Adaptive mechanisms of rejection. Donor antigen presentation and priming can be mediated by direct and indirect mechanisms. CD4+ T cells can be primed indirectly by donor peptide loaded on host MHC-II (i) or semi-directly on recycled donor MHC-II (ii). Another form of semi-direct antigen recognition is when MHC-I molecules are recycled from donor cells, which results in CD8+ T cell recognition of donor antigen in the presence of indirectly activated CD4+ T cells (iii). Finally, host CD4+ T cells can also become activated directly on donor antigen-presenting cells19 (iv). e, Host CD4+ T cells promote activation of CD8+ T cells, NK cells, macrophages and B cells. Activated CD4+ T cells (called type 1 helper T cells, TH1 cells) can provide proinflammatory cytokines to improve direct cytotoxicity of T cells and other effector-mediated cellular cytotoxicity mechanisms. TH1-type cytokines can also activate macrophages, which enhances IL-12 secretion and helps maintain the TH1 subset. In presensitized models, CD4+ T cells can also bolster a macrophage-driven phenotype through CD40–CD40L interactions130. TH2-skewed CD4+ T cells also enhance antibody-dependent cellular cytotoxicity through antigen-specific activation of B cells and secretion of cytokines that are important for class switching (IL-4/IL-5) and proliferation (IL-2). pMHC, peptide-bound MHC; APC, antigen-presenting cell; IFNγ, interferon γ; GMCSF, granulocyte–macrophage colony-stimulating factor; CTL, cytotoxic T lymphocytes. Figure created using BioRender.
To date, mechanisms of allogeneic rejection in the context of transplantation are still being investigated, and often reveal the dynamic balance of allogeneic cell rejection and GVHD at the molecular level. These mechanisms and the dynamic balance of efficacy and immunotoxicity can also serve as a baseline as we seek to understand mechanisms of engineered T-cell engraftment and rejection, particularly in novel allogeneic strategies.
Understanding the dichotomy of solid tumor and blood cancer
CAR T-cell therapies have achieved durable responses in hematological malignancies, as the overall response rate across many trials in patients with refractory leukemia, lymphoma and myeloma is 50–90% (ref. 22). However, in the context of solid tumors, CAR T-cell therapies are still in the early stages of development, and their efficacy is limited. This dichotomy is mediated in part by characteristics of the bone marrow niche of hematological malignancies and the additional barriers posed by the solid tumor microenvironment (TME), such as diminished infiltration and function of CAR T cells, and more complex mechanisms of tumor escape23.
The success of CAR T-cells in patients with leukemia, lymphoma and multiple myeloma would have been predicted in part by previous favorable results with allogeneic HSCT; in contrast, outcomes with allogeneic HSCT in patients with solid tumors have generally been disappointing24. In blood cancers, the availability of lineage-restricted antigens (for example, CD19 and B-cell maturation antigen (BCMA)) provides targets with acceptable toxicity, given expected on-target but off-tumor toxicity on healthy cells. The exception to this is acute myeloid leukemia (AML), in which acceptable surface targets that spare healthy HSCs are lacking; yet by analogy to allogeneic HSCT, a potent antileukemic effect would be predicted, given the strong graft-versus-leukemia effect (Box 1) mediated by T cells and NK cells in this context. The use of T-cell receptor (TCR)-engineered T cells, which target intracellular tumor antigens presented on MHC-I molecules, appears highly promising in AML25.
Several scientific questions remain open and will need to be addressed so that there are uniform responses to engineered cell therapies for all forms of hematopoietic cancer. The bone marrow niche was initially thought to maintain HSCs and has since been demonstrated to be the principal site of residence for human plasma cells and memory T cells26. Recent studies indicate that the bone marrow environment is composed of multiple micro-niches with distinct metabolic features and stromal cell populations27. Leukemia and myeloma cells survive in distinct niches, but engineered T cells may have differential trafficking and persistence in these niches—perhaps contributing to the superior durability of responses to CD19-directed versus BCMA-directed CAR T-cell therapy. Cancer-specific issues remain a challenge, such as the observation that AML is more immunosuppressive than acute lymphocytic leukemia (ALL)28. This is likely related to the close relationship between AML stem cells and HSCs29, and the fact that several mechanisms may have evolved to protect HSCs from immune attack.
One observation now apparent from CD19 CAR T cells is that they induce durable responses in patients with many forms of B-cell malignancies22. The persistence of CD19 CAR T cells is dependent on the CAR endodomains and can be reliably measured by assessing the duration of B-cell aplasia after infusion30. In our initial studies in patients treated with the CD19-directed CAR T-cell therapy tisagenlecleucel, we demonstrated ongoing B-cell aplasia and persistence of functional CAR T cells for a decade31. Emerging data suggest that long-term persistence of CD19 CAR T cells is unusual when compared with other cell therapies with CAR T and TCR T cells that target other cell lineages. This is likely owing to the ongoing production by HSCs of pre-B cells in the marrow that express CD19, thus presenting a target for CAR T cells, even after sterile elimination of tumor target cells has been achieved. A lesson from this observation is that various boosting strategies may be able to enhance persistence of engineered cells in the treatment of non-B-cell blood cancers and solid tumors32,33.
Solid tumors, including some forms of lymphoma, create a robustly immunosuppressive microenvironment composed of numerous cell types and extracellular matrix (ECM)34,35. Infusions of TILs, while commonly effective in metastatic melanoma36, are rarely effective in other solid cancers such as adenocarcinoma. The increased interstitial pressure, creating compressive forces and high tensile strength created by the ECM combine to thwart T-cell infiltration. Thus, new approaches to control solid tumor biomechanical forces (such as heparanse37) and improve T-cell trafficking are required.
One implication of the biomechanical properties of the solid TME is that therapeutic cell doses used in many studies may be inadequate. Recent modeling of human and rodent circulatory systems has revealed that the delivery rate of CAR T cells to solid tumors is 10,000-fold greater in mice than in humans38, providing a potential explanation for the disappointing results of cell therapy trials in patients with solid tumors. This work challenges allometric-based approaches in preclinical studies that are traditionally used in dosing considerations for solid tumors39. While engineered T-cell therapy dosing has always required additional individualized features in the transition from mice to humans (for example, consideration of tumor mass and receptor expression), these findings suggest that improved dosing for solid tumors follows a less linear path than current algorithms suggest.
Solid tumors also harbor a strong tolerogenic environment, leading to T-cell exhaustion and dysfunction40-42. In the presence of continuous stimulation by tumor antigens, T cells develop a progressive state of hypofunctionality characterized by distinct epigenetic, metabolic and phenotypic signatures43. To assess this dysfunction in CAR T cells directed at pancreatic cancer, our group has recently developed an in vitro model of continuous antigen stimulation, enabling the investigation of dynamic temporal changes in the induction of T cell dysfunction44. CD8+ CAR T cells displayed altered chromatin dynamics and upregulated many genes typically associated with NK cells. The NK-like T-cell transition was associated with the upregulation of ID3 and SOX4, and knockout of the transcription factors encoded by these genes prevented or delayed the state of T-cell dysfunction. New strategies to overcome the toxic effects of the solid TME are imperative as the field seeks to improve the therapeutic efficacy engineered cells for solid tumors.
Toxicities from engineered immune cell therapies
The toxicities of engineered cellular immunotherapies are distinct from those of other classes of therapies, such as cytotoxic chemotherapies, and, surprisingly, they are also distinct from those of immune checkpoint inhibitors45. This realization has required the development of classification systems that are dedicated to the grading of adverse events resulting from engineered cell therapies46,47.
As with all therapeutics, toxicities with engineered cells can be classified as on-target or off-target. The longest running clinical experience in the CAR T-cell field has been with CD19-directed CAR T cells, for which on-target toxicities, including B-cell aplasia, cytokine-release syndrome (CRS) and immune-effector-cell-associated neurotoxicity syndrome (ICANS), have been described46. ICANS was initially thought to result from a cascade of off-target effects due to systemic inflammation and cytokine release48; however, some aspects of the syndrome may be related to the expression of CD19 in pericytes in the central nervous system49, an unexpected observation because CD19 was initially described as a B-lineage-restricted protein. An important and somewhat surprising recent finding suggests that the host microbiome has substantial effects on the efficacy and toxicity of CAR T cells (Box 2).
Box 2 ∣. The emerging role of the microbiome in cell transfer.
Emerging innovations in sequencing and computational modeling have enabled the assessment of both diversity and abundance of particular taxa in the host microbiome. Although it is still not fully understood how the localized microbial environment instructs systemic immunity, emerging evidence has highlighted its role as a predictive biomarker of response to chemotherapy and immune checkpoint blockade (ICB)156,157.
For instance, the chemotherapeutic agent cyclophosphamide exerts its effects through a gut-induced TH17 response, which increases tumor susceptibility to the chemotherapeutic itself158; this response is diminished by antibiotics that alter the microbial diversity159. The impact of specific bacterial species on ICB efficacy varies between treatment regimens, but species associated with increased efficacy typically enhance pathways of host immunogenicity (such as DC and T-cell activation and proinflammatory cytokine responses) and reduce host tolerance mechanisms of regulatory immunosuppression (Treg cells, TGF-β and IL-10)160. While overabundance of Bacteroidetes species is associated with a poor antitumor response in PD-1-targeting ICB therapy, overabundance of Firmicutes species is associated with increased host immunogenicity and improved efficacy161. A diverse microbiota is also associated with improved outcome after HSCT162. Antibiotic use may deplete Bacteroidetes species, and it may affect other aspects of community structure and metabolite pools; therefore, more mechanistic work is needed to determine the distinct drivers of community structure and metabolite composition.
Uribe-Herranz and coworkers showed that the efficacy of TCR-engineered T cells was improved in mice with fecal microbiota transplant or vancomycin, both increasing CD8a+ DCs and IL-12 (ref. 163). This was surprising in that most of the activity of adoptively transferred T cells was thought to arise from cell-intrinsic effects imparted during cell culture. In the context of human CAR T-cell therapy, distinct intestinal species abundances have correlated with increased therapeutic efficacy164, but these studies were limited to a small cohort. More recently, however, Smith et al.165 conducted a multi-institutional study exploring the associations between prior antibiotic exposure and subsequent CD19 CAR T-cell efficacy and toxicity. These retrospective studies revealed that specific antibiotic use (that is, piperacillin/tazobactam, meropenem, and imipenem/cilastatin) increased the likelihood of ICANS in patients with ALL or non-Hodgkin’s lymphoma. The conservation across domains suggests an association of microbiota and ICANS through a gut–brain axis165. This study also assessed the fecal microbiota and found that the predicted probability of toxicity was correlated with high Bacteroides species abundance. Conversely, the predicted probability of efficacy was correlated with high Ruminococcus species abundance, corroborating their prior findings from their 2019 cohort in which complete responders had higher abundances of Ruminococcaceae and Lachnospiraceae family members164. Intriguingly, similar relative abundances have correlated with an increased ratio of Treg/TH17 cells, suggesting their role in balancing anti-inflammatory and proinflammatory responses during GVHD in allogeneic HSCT166 and potentially in adoptive cell therapies as well. Other considerations include the role of current myeloablation techniques in barrier destruction and enhanced engagement of innate immunity. Further research is warranted as we stretch the bounds of engineered therapies and move toward allogeneic cell sources, as intestinal microbial diversity loss is acutely correlated with lower survival in allogeneic HSCT162,166.
CRS and ICANS toxicities are now regarded as a class effect of CAR T cells, as these syndromes have been observed in patients treated with CAR T cells targeting CD19, BCMA, and many other cell surface structures50. Increasing clinical work has led to the emergence of specific toxicity profiles for the various targets. Although both CD19- and BCMA-targeted CAR T cells trigger ICANS, the clinical aspects differ; for example, patients with multiple myeloma treated with BCMA-specific CAR T cells may develop a Parkinsonian syndrome51, potentially related to expression of BCMA in the central nervous system50.
Unlike some other therapies (such as chemotherapy), the toxicities of which are predominantly off-target, the toxicity of engineered cells is predominantly on-target. Therefore, CRS and ICANS induced by engineered cells may be more a feature of increasingly potent therapies than a class effect. Further, these toxicities are generally not observed in preclinical studies owing to a lack of sufficiently representative animal models. Emerging single-cell technologies can precisely map target expression levels and thereby predict on-target, off-tumor toxicities with CAR T cells50. However, this remains a challenging technical issue with TCR T cells, which can cause severe and unexpected toxicity52. An emerging lesson for the field is that the expression of targets in healthy patients may not be the same as in patients with cancer. The inflammation triggered by initially on-target recognition by engineered cells can trigger the subsequent induction of off-tumor target expression that was not present at the time of cell infusion50. Real-world data indicate that, with increasing clinical experience, the severity of CRS and ICANS has decreased, likely reflecting earlier intervention and possibly the treatment of patients with lower disease burden47,53.
Genotoxicity is an important challenge with engineered cell therapies. As engineered receptors are primarily introduced by viral-vector integration, safety concerns arise from the potential for insertional mutagenesis and cellular transformation. Clinical experience indicates that these risks are cell-type-specific. For example, the risk of transformation is higher with HSC than with T cells54. Our group has found that CAR T cells can safely persist for a decade in patients31,55, and despite the many thousands of patients who have been treated with engineered T cells globally, there are, to our knowledge, only two reported instances of T-cell transformation56. Both these cases of CAR T lymphomas occurred in the setting of allogeneic HSCT, with a CAR T product manufactured using the piggyBac non-viral gene transfer technology57. In a pilot trial of multiplex engineering of T cells using CRISPR–Cas9 technology, we found that chromosomal translocations, rather than off-target edits, were the primary safety concern58.
The emerging clinical efficacy of engineered HSCs and engineered induced pluripotent stem cells (iPSCs; generated by reprogramming of adult cells) highlights an urgent need to further characterize and enhance the safety of engineered stem cells. In preclinical models, tumor formation from engineered iPSCs is increased when cells are derived from patients with some baseline genetic abnormalities59. The convergence of multiplex human genome engineering with cell therapies illustrates the increasing need to refine genome-editing technologies and to develop assays for monitoring the safety of infused cellular products.
In addition to cell-type specificity, another issue facing the field is the broad safety of allogeneic cell therapies60, which have substantially higher safety hurdles than do autologous cell therapies. Autologous T cells have specialized cell-intrinsic and cell-extrinsic safety features, such as control of TCR clonal abundance and resistance to oncogene transformation, which may provide an additional measure of safety lacking in the T cells, HSCs and iPSCs used for allogeneic therapies61,62. It is reassuring that, at present, the collective safety of engineered cell therapies compares favorably to cytotoxic agents that have been the standard of care for the previous 50 years53,63.
Combination cancer therapy
The feasibility of engineered cells of diverse types and diverse sources (autologous versus allogeneic) creates the permutational possibility of many combination strategies. As well as combining different cellular therapies, it is also possible to combine cellular therapies with other drugs or recombinant proteins. There is often confusion between the terms ‘combination therapy’ and ‘combination product’. Combination therapy refers to the common practice in medicine of the administration of, for example, several chemotherapy reagents to a patient in combination for treatment of a particular cancer. In contrast, a combination product is defined by health authorities as a product that involves a drug and/or a biologic and/or a medical device. Thus, combining an infusion composed of dendritic cells with one composed of T cells would be a combination therapy but not a combination product, because both components are considered biologics by the FDA. In contrast, engineered T cells injected into the liver with a specific catheter for treatment of cancer would be a combination product. For information on the FDA guidance and policy, see ref. 5. Below, we discuss potential combination strategies, some of which would be considered combination products from a regulatory perspective.
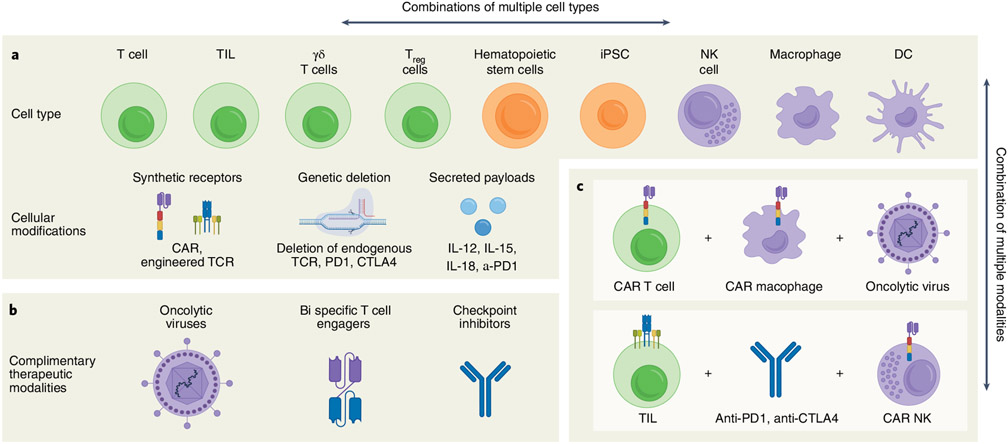
Combination therapies can alleviate the unique hurdles of engineered cellular therapies to enhance antitumor effects (Fig. 4). Indeed, preclinical studies of combination therapy incorporating oncolytic virotherapy64-66, immune checkpoint blockade67, bispecific antibodies68 or small molecules with cellular therapy have demonstrated enhanced antitumor activity. Oncolytic adenoviruses (OAds) have engineered modifications that allow them to selectively enter tumor cells and replicate, resulting in tumor cell death through oncolysis (thereby reducing the modalities of tumor escape); they can also be modified to express therapeutic transgenes in the TME69,70 and have demonstrated safety in clinical trials71,72. Oncolytic virotherapy and CAR and TCR T-cell therapy are synergistic modalities for solid tumor treatment. Combinatorial studies have exploited the transgene delivery potential of OAds to drive tumor-antigen-specific expression (that is, CD19)73. Preclinical studies published by our group have demonstrated that the new combination of OAds expressing tumor necrosis factor (TNF)-α and interleukin (IL)-2 with mesothelin-targeting CAR T cells was able to modulate the immunosuppressive TME and induce CAR-dependent and CAR-independent host immunity in pancreatic cancer74.
Fig. 4 ∣. Permutations of cell therapies for cancer treatment.
a, The availability of numerous types of cell therapies creates the possibility for a wide range of combinatorial therapeutic approaches. Within each cell type, there is a wide range of cellular modifications that can be made, ranging from addition of synthetic receptors93,131-138 and secreted payloads75,139-141 to genetic deletions58,142,143. This diverse toolbox of cell therapies can be used in numerous combinations, which may be more effective than each one as monotherapy. Note that only a subset of examples is listed for each category of cellular modification. b, There are additional therapeutic modalities that have the potential to synergize with cell therapies144-148, thus further increasing the number of potential therapeutic combinations. c, Two examples of potential therapeutic combinations are shown that could improve the ability to directly target tumor cells and increase the endogenous immune response. Figure created using BioRender.
Immune checkpoint blockade is another approach to overcome tumor-associated immune suppression75 and revitalize T cells76. In clinical trials, the combination of pembrolizumab with mesothelin-targeting CAR T cells further enhances the persistence and function of the latter in patients with malignant pleural diseases67. Effective checkpoint blockade and expression of checkpoint molecules are key to the success of such combinatorial therapies; for instance, one study demonstrated that two CARs, one targeting EGFRvIII and the other targeting IL-13Ra2, preferred different checkpoint blockades within the same tumor model77.
An alternative approach to checkpoint blockade is to redirect non-specific bystander T cells against tumors by engineering CAR T cells to produce bispecific antibodies, as shown in a preclinical study for the treatment of glioblastoma68. Research by our team has also explored the combination of folate receptor alpha-targeting CAR T cells with OAds expressing a localized bispecific antibody targeting EGFR, which led to enhanced T cell activation and antitumor effects78.
Overall, combination strategies involving engineered cell therapy for solid tumors show promising results; the interaction between the OAd and tumor immune cells and the selection of appropriate CAR signaling domains and of effective checkpoint blockade have all been highlighted as key determinants of antitumor efficacy.
New directions in cancer and beyond
Convergence of genome engineering and synthetic hematopoietic systems.
The ability to rewrite the human genome at will was a long-held goal of gene therapy79. Over the past two decades, advances in recombinant nuclease technology have made gene correction a possibility for genetic disorders of the bone marrow. In addition, this rapidly advancing field now permits the installation of new therapeutic functions in cells, which is a central goal of the field of synthetic biology. In principle, ‘living drugs’ created from engineered hematopoietic stem cells can be created to cure essentially all the disorders of the hematopoietic and immune systems, such as cancer and hemoglobinopathies (including sickle-cell disease and severe thalassemia, which affect over 300,000 births annually80). To this end, advances in human genome engineering, when combined with advances in synthetic biology, have vastly improved the potential of cellular therapies. Here, we address some of the opportunities and challenges on the near-term horizon.
Synthetic hematopoiesis.
In our view, advances in the field of HSC transplantation and genome engineering have reduced correction of hemoglobinopathies to an engineering problem. The proof of principle is being established in early-stage trials and the scaling up of the technologies will become a principal challenge. This will likely open up the field of synthetic hematopoiesis, whereby new functions can be added to HSC and their progeny. In the field of infectious diseases, on the basis of genetic evidence from the CCR5Δ32 polymorphism and other antiviral approaches, it is likely that HIV-proof immune systems could be used to induce functional eradication of the virus in patients with HIV/AIDS81. Engineering HSCs has broad applications in cancer and regenerative medicine, and these opportunities will depend on the identification of safe-harbor spots in the genome to install genes and gene circuits82.
Epitope deletion and replacement strategies.
One of the most promising cellular therapy combination approaches in cancer treatment involves engineered HSCs with CAR T cells, given their long lifespans and feasibility of administration as single agents. The absence of cancer-restricted surface markers is a major impediment to antigen-specific immunotherapy for solid tumors and several hematologic malignancies. For example, CD33-targeting CAR T cells can effectively kill CD33+ AML cells; but, as CD33 is also expressed in HSCs, the treatment causes severe myelotoxicity. To address this issue, a study conducted in mice and nonhuman primates showed that deletion of CD33 from normal HSCs did not restrict their long-term engraftment and generated progeny that were resistant to CD33-specific CAR T cells83. Therefore, infusion of CD33-deleted hematopoietic stem cells could allow efficient elimination of CD33+ leukemia cells by CAR T cell therapy, without myelotoxicity. This principle of creating a cancer-specific neoantigen with a combination of CAR T and gene-edited HSCs will be tested in a planned clinical trial.
It is likely that this strategy of using genome-edited HSCs can be extended to target other ‘Achilles heel’ molecules on HSCs, whereby off-tumor expression leads to unacceptable toxicity following CAR T cell therapy—for example, CD34, CD45 and CD123 (all promising targets for blood cancer). Given the ability to map the extracellular binding sites of CAR targets and recently developed gene-editing strategies (that is, base editing and prime editing), it may be possible to remove extracellular epitopes on HSCs to avoid targeting by CAR T cells—which would continue to target wild-type epitopes on malignant cells. The feasibility of this epitope-replacement strategy depends on structural knowledge of the extracellular domains of the targeted molecules and characterization of the binding site of various antibody fragments used in CAR T cells.
Avoiding tumor escape and striving for synthetic lethality.
Tumor escape is an inevitable occurrence with potent cancer therapies23. Synthetic lethality was first described in the early 1920s, and later coined in the 1940s84. It describes individual genetic mutations that were only lethal upon combination in Drosophila. Since then, this concept has been successfully applied in the discovery of druggable cancer-specific targets85 (for example, BRCA−/− breast cancer cell sensitivities to PARP inhibitors86).
Now in the age of immunotherapies, we have sought out similar multifaceted models to induce immune synthetic lethality and improve antitumor immunity. For instance, tumor-cell-intrinsic JAK1 loss-of-function mutations have been shown to drive resistance to immune checkpoint blockade87. In the context of TCR-engineered T-cell therapies exploiting driver oncogene neoantigens88, tumors often develop MHC-I escape to evade attack. Therefore, to achieve immune synthetic lethality in TCR therapies, mechanisms of MHC loss must be addressed—for example, through small molecules. Similar two-pronged approaches have been applied in CD19-targeting CAR T cells; in this context, we found that pretreatment of patients with leukemia with the Bruton tyrosine kinase/IL-2-inducible kinase (BTK/ITK) inhibitor (ibrutinib), which decreases the expression of PD-1 on B cells, improved expansion of CD19 CAR T cells89. This is likely mediated through subsequent effects on B-cell receptor signaling that enhanced their attack by adoptively transferred CAR T cells90.
Another approach is to combine two effector strategies in the same cells, such as the development of engineered cells that simultaneously express a CAR and a TCR91, or CAR T cells secreting bispecific engagers targeting alternative structures in the tumor or its microenvironment68, which would ensure that tumor cells require several alterations to escape92. Yet another form of synthetic lethality could be the combination of different cell types, for example CAR T cells that kill by lytic mechanisms and CAR macrophages that kill by phagocytosis93, thereby overcoming some potential forms of tumor escape. A bioinformatics challenge facing the field is the identification of various forms of immune synthetic lethality so that strategies to target these vulnerabilities can be developed.
Applications across the disease spectrum.
Beyond their application in cancer, adoptive cell therapy advances have also revolutionized the field of immunotherapy in other disease contexts94,95. In addition to the initial success of HSC engineering in hemoglobinopathies and HIV, progress has been made in the treatment of autoimmune disorders96. Following the implementation of regulatory T cell (Treg) therapies to expedite tolerance induction of donor organ transplants (NCT02371434), ex-vivo-expanded Treg therapies have been explored in multiple preclinical models of autoimmunity, including type I diabetes (T1D), multiple sclerosis (MS), systemic lupus erythematosus (SLE) and pemphigus vulgaris (PV)96. Engineered T cells have been leveraged to mitigate pathogenic antibodies such as those found in SLE97 and PV98. Chimeric autoantibody receptors (CAAR) directed against the PV autoantigen desmoglein have demonstrated antigen-specific cytotoxicity in vivo and in vitro98, and are currently being investigated in a multi-institutional clinical trial (NCT04422912). In refractory SLE, conventional CD19 CAR T cells have been leveraged to deplete B cells, which led to a complete response in a pilot clinical trial involving a single patient99. Finally, engineered iPSC-based therapies have also been explored with promising results in the treatment of T1D100.
Engineered cell therapy strategies have also been applied in the treatment of cardiac fibrosis, for which few anti-fibrotic therapies exist101. Recent studies have demonstrated that re-engineering T cells with a CAR against fibroblast activation protein (FAP) mediates reduction in cardiac fibrosis and restores function in vivo by inhibiting stromagenesis and angiogenesis102. Anti-fibrotic CAR T cells have also been generated in vivo using modified messenger RNA in T-cell-targeted lipid nanoparticles103. As in T1D treatment, iPSCs have also been extended to treatment of cardiac fibrosis. Human iPSC-derived macrophages have been shown to improve liver fibrosis and stimulate regeneration in vivo104. There is increasing awareness of the immune system’s role in cardiac function and injury response105.
CAR T cells for myeloablation.
There is a long-standing unmet medical need for strategies to safely ablate hematopoiesis and/or lymphopoiesis to facilitate engraftment of therapeutic cells106,107. This issue has vexed researchers in the transplant field for many years, where high-dose chemotherapy has been the standard of care to facilitate engraftment of transplanted stem cells. Related to this is the requirement for transient immunosuppression to facilitate engraftment of adoptively transferred cells107. One strategy involves targeting of HSCs and hematopoietic progenitor cells with depleting antibodies, instead of chemotherapy. A promising target is the panhematopoietic CD45 tyrosine phosphatase, and studies with CD45 antibodies conjugated to radioisotopes or other toxins have been reported or are ongoing108. A phase 3 trial with an 131I-labeled anti-CD45 antibody as an adjunct for conditioning has been recently completed (NCT02665065). Targeting the tyrosine kinase c-Kit (also known as CD117) on host HSCs is another strategy to facilitate allo-HSC engraftment109. However, a limitation of the use of antibodies labeled with radioconjugates is that they have the potential to mediate genotoxicity in the hematopoietic niche, whereas antibody–drug conjugates against HSCs have the potential to circumvent this limitation.
We suggest that CAR T cells may be able to eradicate host hematopoiesis even more efficiently than antibody-facilitated host conditioning. For example, most antibody-directed therapies rely on auxiliary mechanisms to eradicate targets, such as antibody-dependent cellular cytotoxicity, a process that may be impaired in heavily pretreated patients. A single infusion of CAR T cells targeting CD19 has been shown to be able to eradicate all cells (both normal and malignant) of the B-cell lineage31. On the basis of this remarkable finding, we posit that CAR T cells targeting lineage-restricted molecules on HSCs may provide a ‘deep cleaning’ (>6 log depletion) of host hematopoiesis and facilitate a variety of stem-cell-directed therapies. Such myeloablative strategies with engineered CAR T cells could be transient or permanent, depending on CAR T designs110. Another myeloablative approach using engineered HSC and T cells is described above in the section on epitope deletion and replacement strategies.
Clinical considerations: bottlenecks and challenges
Autologous CAR T cells are personalized, living drugs that require a rethinking of the traditional manufacturing paradigm used for other biologics, such as recombinant proteins and vaccines111. The current approach to manufacturing autologous CAR T-cell therapies relies on a centralized model, in which patient materials are cryopreserved, shipped to the manufacturing facility, genetically modified and expanded, cryopreserved a second time, and then shipped back to the clinical site112,113. In addition to complex logistics, manufacturing of currently approved CAR T-cell products requires manual processing steps that must be conducted by highly trained personnel in good manufacturing practice (GMP)-certified facilities114. This cumbersome, centralized model presents a bottleneck for translation of preclinical candidates into phase 1 trials and will present challenges for commercialization of CAR T-cell and HSC therapies directed for indications with larger patient populations. It also presents challenges for translation in emerging markets that lack the required infrastructure for receipt and storage of cryopreserved materials.
Several approaches are being pursued to address the manufacturing challenges for autologous CAR T-cell therapy. Off-the-shelf CAR T-cell products—whether derived from allogeneic T cells or iPSCs—have received considerable attention owing to the elimination of the complications associated with a personalized therapy, and are currently being evaluated in numerous clinical trials7,115. Although the use of non-patient starting material considerably reduces the cost per dose and simplifies logistics116, manufacturing is still highly complex owing to the need for genetic editing117, and durability is not yet on par with autologous therapies7,118.
Another method to mitigating the manufacturing bottleneck is the use of in vivo cell engineering, in which a patient’s T cells are transduced or transfected directly within the patient. In vivo cell transduction eliminates the ex vivo manufacturing step, and if successful, would enormously simplify the delivery of CAR T cell therapy and allow for translation to a broader patient population. To date, there have been multiple demonstrations of in vivo CAR T-cell generation in mice using CD4− and CD8-targeted lentivirus119,120, as well as an engineered adeno-associated virus121. Given the risks of off-target transduction, a potentially safer approach might be to transiently transfect T cells using T-cell-targeted lipid nanoparticles loaded with mRNA encoding the CAR. This approach was used to successfully treat cardiac fibrosis in mice using CD5-targeted lipid nanoparticles containing a mRNA encoding a FAP-directed CAR103.
As with food products, cell therapies may be broadly classified as natural or genetically modified. The policy of the FDA is to regulate cell and tissue products that are not homologous and are more than minimally manipulated122. Broadly speaking, the homologous use of cells refers to regenerative medicine applications that repair or replace damaged cells and tissues. Cell products that are more than minimally manipulated have altered characteristics (such as being genetically engineered), another feature that triggers full regulation by health authorities. These distinctions have major implications for clinical medicine and the development of cellular therapeutics. The prospect of the cure for hemoglobinopathies with genetically engineered HSCs is on the horizon, offers hope to afflicted patients, and has major implications for the pharmaceutical industry and healthcare policies. The manufacturing of genetically modified autologous HSCs will require higher standards and more stringent regulation than for red blood cells derived from healthy donors.
Summary and perspectives
Transplant studies have laid the groundwork for our current understanding of immune tolerance and highlight the underlying complexities of cellular therapeutics today. The expansion of the cellular immunotherapeutic field to numerous diseases, cell sources, and cell types indubitably reflects the careful integration of engineering innovations with a deepened understanding of underlying mechanisms of action.
Lessons learned from cancer immunotherapies have set the stage for application of engineered cellular therapy to autoimmune diseases, genetic disorders, infectious diseases and regenerative medicine. However, we need to identify more selective targets, leveraging our understanding of peripheral tolerance. TCRs targeting shared mutations in driver oncogene pathways hold promise for treatment of solid tumors88, as do CARs that bind to tumor-specific glycans, splice variants, peptide central targets presented by human leukocyte antigens123,124. Advances in synthetic protein engineering and computational modeling also offer novel opportunities to increase CAR T-cell specificity in the TME125,126.
Going forward, as the field implements allogeneic engineered cell therapies, we are likely to face obstacles in engraftment and persistence. However, as we break down their mechanisms, these hurdles are likely to be overcome by novel applications of synthetic biology and increased precision in genome editing. Science, medicine and the public need to embrace these developments to realize the full potential of engineered cell therapies.
Acknowledgements
The authors would like to thank R. Young for insightful discussions, and the authors apologize to colleagues for work that we were unable to cite owing to space constraints. This was supported by 1P01CA214278, R01CA226983 and the Parker Institute for Cancer Immunotherapy (C.H.J.); the National Science Foundation Graduate Fellowship DGE-1321851 (A.V.F.); the National Institute of Health T32 CA009140 (T.B.); and the Go for IT Fondazione CRUI/MIUR (Italy) Fellowship 2020 (G.G.).
Footnotes
Competing interests
C.H.J. has received grant support from Novartis, and has patents related to CAR therapy with royalties paid from Novartis to the University of Pennsylvania. C.H.J. is also a scientific co-founder and holds equity in Capstan Therapeutics and Tmunity Therapeutics. C.H.J. serves on the board of AC Immune and is a scientific advisor to Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Cellcarta, Celldex, Danaher, Decheng, ImmuneSensor, Poseida, Verismo, Viracta, and WIRB-Copernicus group.
References
- 1.Combination Products (FDA, accessed 1 March 2022); https://www.fda.gov/combination-products [Google Scholar]
- 2.Grupp SA et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med 368, 1509–1518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischbach MA, Bluestone JA & Lim WA Cell-based therapeutics: the next pillar of medicine. Sci. Transl. Med 5, 179ps177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frangoul H et al. CRISPR–Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med 384, 252–260 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Approved Cellular and Gene Therapy (FDA, 2022); https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products [Google Scholar]
- 6.Elisseeff J, Badylak SF & Boeke JD Immune and genome engineering as the future of transplantable tissue. N. Engl. J. Med 385, 2451–2462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin R et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Anderson D, Billingham RE, Lampkin GH & Medawar PB The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity 5, 379–397 (1951). [Google Scholar]
- 9.Martínez-Llordella M et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am. J. Transplant 7, 309–319 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Chen DS & Mellman I Oncology meets immunology: the cancer–immunity cycle. Immunity 39, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Beatty GL & Gladney WL Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res 21, 687–692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cudkowicz G & Stimpfling J Deficient growth of C57bl marrow cells transplanted in F1 hybrid mice: association with the histocompatibility-2 locus. Immunology 7, 291 (1964). [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy WJ, Kumar V & Bennett M Acute rejection of murine bone marrow allografts by natural killer cells and T cells. Differences in kinetics and target antigens recognized. J. Exp. Med 166, 1499–1509 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiessling R et al. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur. J. Immunol 7, 655–663 (1977). [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri L et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Huntington ND, Vosshenrich CA & Di Santo JP Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol 7, 703–714 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Raulet DH Bone marrow cell rejection, MHC, NK cells, and missing self recognition: ain’t that peculiar (with apologies to Marvin Gaye). J. Immunol 195, 2923–2925 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Xiao X, Demirci G, Madsen J & Li XC Innate NK cells and macrophages recognize and reject allogeneic nonself in vivo via different mechanisms. J. Immunol 188, 2703–2711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marino J, Paster J & Benichou G Allorecognition by T lymphocytes and allograft rejection. Front. Immunol 7, 582 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda Y et al. Visualizing the rapid and dynamic elimination of allogeneic T cells in secondary lymphoid organs. J. Immunol 201, 1062–1072 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Kitazawa Y et al. Novel targeting to XCR1+ dendritic cells using allogeneic T cells for polytopical antibody responses in the lymph nodes. Front. Immunol 10, 1195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.June CH & Sadelain M Chimeric antigen receptor therapy. N. Engl. J. Med 379, 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn GP, Bruce AT, Ikeda H, Old LJ & Schreiber RD Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol 3, 991–998 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Ringdén O, Karlsson H, Olsson R, Omazic B & Uhlin M The allogeneic graft-versus-cancer effect. Br. J. Haematol 147, 614–633 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Chapuis AG et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med 25, 1064–1072 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Q & Frenette PS Niches for hematopoietic stem cells and their progeny. Immunity 48, 632–648 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu VW & Scadden DT Heterogeneity of the bone marrow niche. Curr. Opin. Hematol 23, 331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khaldoyanidi S, Nagorsen D, Stein A, Ossenkoppele G & Subklewe M Immune biology of acute myeloid leukemia: implications for immunotherapy. J. Clin. Oncol 39, 419–432 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Passegué E, Jamieson CH, Ailles LE & Weissman IL Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl Acad. Sci. USA 100, 11842–11849 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paszkiewicz PJ et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Invest 126, 4262–4272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melenhorst JJ et al. Decade-long remissions of leukemia sustained by the persistence of activated CD4+ CAR T-cells. Nature 602, 503–509 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L et al. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinhard K et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D & Coussens LM Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Jain RK, Martin JD & Stylianopoulos T The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng 16, 321–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seitter SJ et al. Impact of prior treatment on the efficacy of adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma. Clin. Cancer Res 27, 5289–5298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caruana I et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med 21, 524–529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown LV, Gaffney EA, Ager A, Wagg J & Coles MC Quantifying the limits of CAR T-cell delivery in mice and men. J. R. Soc. Interface 18, 20201013 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majzner RG & Mackall CL Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med 25, 1341–1355 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Thommen DS & Schumacher TN T cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauken KE & Wherry EJ Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36, 265–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong M, Clubb JD & Chen YY Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 38, 473–488 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Blank CU et al. Defining ‘T cell exhaustion’ Nat. Rev. Immunol 19, 665–674 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Good CR et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell 184, 6081–6100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.June CH, Warshauer JT & Bluestone JA Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med 23, 540–547 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Lee DW et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl 25, 625–638 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Sheth VS & Gauthier J Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 56, 552–566 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taraseviciute A et al. Chimeric antigen receptor t cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 8, 750–763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker KR et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lareau CA, Parker KR & Satpathy AT Charting the tumor antigen maps drawn by single-cell genomics. Cancer Cell 39, 1553–1557 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Oekelen O et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med 27, 2099–2103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cameron BJ et al. Identification of a titin-derived HLA-A1–presented peptide as a cross-reactive target for engineered MAGE A3–directed T cells. Sci. Transl. Med 5, 197ra103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang K & Nastoupil LJ Real-world experiences of CAR T-cell therapy for large B-cell lymphoma: how similar are they to the prospective studies? J. Immunother. Precis. Oncol 4, 150–159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrari G, Thrasher AJ & Aiuti A Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet 22, 216–234 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Scholler J et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med 4, 132Ra153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Micklethwaite KP et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood 138, 1391–1405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schambach A, Morgan M & Fehse B Two cases of T cell lymphoma following Piggybac-mediated CAR T cell therapy. Mol. Ther 292, 631–633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stadtmauer EA et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nori S et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 4, 360–373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben-David U & Benvenisty N The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 11, 268–277 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Hataye J, Moon JJ, Khoruts A, Reilly C & Jenkins MK Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312, 114–116 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Newrzela S et al. Resistance of mature T cells to oncogene transformation. Blood 112, 2278–2286 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Kaldor JM et al. Leukemia following chemotherapy for ovarian cancer. N. Engl. J. Med 322, 1–6 (1990). [DOI] [PubMed] [Google Scholar]
- 64.Ajina A & Maher J Prospects for combined use of oncolytic viruses and CAR T-cells. J. Immunother. Cancer 5, 90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guedan S & Alemany R CAR-T cells and oncolytic viruses: joining forces to overcome the solid tumor challenge. Front Immunol. 9, 2460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biegert GWG, Shaw AR & Suzuki M Current development in adenoviral vectors for cancer immunotherapy. Mol. Ther. Oncolytics 23, 571–581 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adusumilli PS et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 11, 2748–2763 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi BD et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol 37, 1049–1058 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Siurala M et al. Adenoviral delivery of tumor necrosis factor-alpha and interleukin-2 enables successful adoptive cell therapy of immunosuppressive melanoma. Mol. Ther 24, 1435–1443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosewell Shaw A et al. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T Cells against metastatic head and neck cancer. Mol. Ther 25, 2440–2451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim KH et al. A phase I clinical trial of Ad5/3-Δ24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol 130, 518–524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranki T et al. Phase I study with ONCOS-102 for the treatment of solid tumors — an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 4, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park AK, et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med 12, eaaz1863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe K et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 3, e99573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rafiq S et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol 36, 847–856 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia Y, Medeiros LJ & Young KH Immune checkpoint blockade: releasing the brake towards hematological malignancies. Blood Rev. 30, 189–200 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Yin Y et al. Checkpoint blockade reverses anergy in IL-13Rα2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol. Ther. Oncolytics 11, 20–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wing A et al. Improving CAR T-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol. Res 6, 605–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedmann T & Roblin R Gene therapy for human genetic disease? Science 175, 949–955 (1972). [DOI] [PubMed] [Google Scholar]
- 80.Williams TN & Weatherall DJ World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb. Perspect. Med 2, a011692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ndung’u T, McCune JM & Deeks SG Why and where an HIV cure is needed and how it might be achieved. Nature 576, 397–405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pellenz S et al. New human chromosomal sites with “safe harbor” potential for targeted transgene insertion. Hum. Gene Ther 30, 814–828 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim MY et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell 173, 1439–1453.e1419 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dobzhansky T Genetics of natural populations. XIII. Recombination and variability in populations of Drosophila pseudoobscura. Genetics 31, 269 (1946). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Setton J et al. Synthetic lethality in cancer therapeutics: the next generation. Cancer Discov. 11, 1626–1635 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farmer H et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Zaretsky JM et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med 375, 819–829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klebanoff CA & Wolchok JD Shared cancer neoantigens: making private matters public. J. Exp. Med 215, 5–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fraietta JA et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127, 1117–1127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okazaki T, Maeda A, Nishimura H, Kurosaki T & Honjo T PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl Acad. Sci. USA 98, 13866–13871 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davenport AJ et al. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol. Res 3, 483–494 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Singh N et al. Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T-cell dysfunction. Cancer Discov. 10, 552–567 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klichinsky M et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol 38, 947–953 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellis GI, Sheppard NC & Riley JL Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet 22, 427–447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alfageme-Abello O, Porret R, Perreau M, Perez L & Muller YD Chimeric antigen receptor T-cell therapy for HIV cure. Curr. Opin. HIV AIDS 16, 88–97 (2021). [DOI] [PubMed] [Google Scholar]
- 96.Esensten JH, Muller YD, Bluestone JA & Tang Q Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J. Allergy Clin. Immunol 142, 1710–1718 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Haddadi M-H et al. Autoimmunity as a target for chimeric immune receptor therapy: a new vision to therapeutic potential. Blood Rev. 41, 100645 (2020). [DOI] [PubMed] [Google Scholar]
- 98.Ellebrecht CT et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mougiakakos D et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med 385, 567–569 (2021). [DOI] [PubMed] [Google Scholar]
- 100.Maxwell KG & Millman JR Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep. Med 2, 100238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fang L, Murphy AJ & Dart AM A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Front. Pharm 8, 186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aghajanian H et al. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rurik JG et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pouyanfard S et al. Human induced pluripotent stem cell-derived macrophages ameliorate liver fibrosis. Stem Cells 39, 1701–1717 (2021). [DOI] [PubMed] [Google Scholar]
- 105.Rurik JG, Aghajanian H & Epstein JA Immune cells and immunotherapy for cardiac injury and repair. Circ. Res 128, 1766–1779 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Czechowicz A, Kraft D, Weissman IL & Bhattacharya D Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 318, 1296–1299 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Louis CU et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood, J. Am. Soc. Hematol 113, 2442–2450 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matthews DC et al. Phase I study of 131I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood 94, 1237–1247 (1999). [PubMed] [Google Scholar]
- 109.Czechowicz A et al. Selective hematopoietic stem cell ablation using CD117-antibody–drug-conjugates enables safe and effective transplantation with immunity preservation. Nat. Commun 10, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Williams JasperZ.. et al. Precise T cell recognition programs designed by transcriptionally linking multiple receptors. Science 370, 1099–1104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roddie C, O’Reilly M, Dias Alves Pinto J, Vispute K & Lowdell M Manufacturing chimeric antigen receptor T cells: issues and challenges. Cytotherapy 21, 327–340 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Levine BL & June CH Perspective: assembly line immunotherapy. Nature 498, S17 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Aijaz A et al. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng 2, 362–376 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levine BL, Miskin J, Wonnacott K & Keir C Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev 4, 92–101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheridan C Off-the-shelf, gene-edited CAR-T cells forge ahead, despite safety scare. Nat. Biotechnol 40, 5–8 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Harrison RP, Zylberberg E, Ellison S & Levine BL Chimeric antigen receptor-T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy 21, 224–233 (2019). [DOI] [PubMed] [Google Scholar]
- 117.Depil S, Duchateau P, Grupp SA, Mufti G & Poirot L ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov 19, 185–199 (2020). [DOI] [PubMed] [Google Scholar]
- 118.DiNofia AM & Grupp SA Will allogeneic CAR T cells for CD19+ malignancies take autologous CAR T cells ‘off the shelf’? Nat. Rev. Clin. Oncol 18, 195–196 (2021). [DOI] [PubMed] [Google Scholar]
- 119.Pfeiffer A et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med 10, e9158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Agarwal S et al. In vivo generation of CAR T cells selectively in human CD4+ lymphocytes. Mol. Ther 28, 1783–1794 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nawaz W et al. AAV-mediated in vivo CAR gene therapy for targeting human T-cell leukemia. Blood Cancer J. 11, 119 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use (FDA, 2020); https://www.fda.gov/media/109176/download [Google Scholar]
- 123.Posey AD Jr. et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yarmarkovich M et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 599, 477–484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Lajoie MJ et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dannenfelser R et al. Discriminatory power of combinatorial antigen recognition in cancer T cell therapies. Cell Syst. 11, 215–228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ezekian B et al. Contemporary strategies and barriers to transplantation tolerance. Transplantation 102, 1213–1222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ciurea SO et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant 21, 1392–1398 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sacks SH & Zhou W The role of complement in the early immune response to transplantation. Nat. Rev. Immunol 12, 431–442 (2012). [DOI] [PubMed] [Google Scholar]
- 130.Liu C et al. Agonistic antibody to CD40 boosts the antitumor activity of adoptively transferred T cells in vivo. J. Immunother 35, 276–282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baeuerle PA et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun 10, 2087 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson LA et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol 177, 6548–6559 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oei VYS et al. Intrinsic functional potential of NK-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol. Res 6, 467–480 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Li Y, Hermanson DL, Moriarity BS & Kaufman DS Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sadeghzadeh M et al. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 254, 117580 (2020). [DOI] [PubMed] [Google Scholar]
- 136.Iriguchi S et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun 12, 430 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maldini CR, Ellis GI & Riley JL CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol 18, 605–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rozenbaum M et al. Gamma-delta CAR-T cells show CAR-directed and independent activity against leukemia. Front Immunol. 11, 1347 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chmielewski M & Abken H CAR T cells releasing IL-18 convert to T-bet. Cell Rep. 21, 3205–3219 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Koneru M, Purdon TJ, Spriggs D, Koneru S & Brentjens RJ IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors. Oncoimmunology 4, e994446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krenciute G et al. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res 5, 571–581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ren J et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res 23, 2255–2266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rupp LJ et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep 7, 737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rezaei R et al. Combination therapy with CAR T cells and oncolytic viruses: a new era in cancer immunotherapy. Cancer Gene Ther. 10.1038/s41417-021-00359-9 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Fukuhara H, Ino Y & Todo T Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 107, 1373–1379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aalipour A et al. Viral delivery of CAR targets to solid tumors enables effective cell therapy. Mol. Ther. Oncolytics 17, 232–240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hipp S et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 31, 1743–1751 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Bagchi S, Yuan R & Engleman EG Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev. Pathol 16, 223–249 (2021). [DOI] [PubMed] [Google Scholar]
- 149.Thomas ED, Lochte HL, Cannon JH, Sahler OD & Ferrebee JW Supralethal whole body irradiation and isologous marrow transplantation in man. J. Clin. Investig 38, 1709–1716 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weiden PL et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med 300, 1068–1073 (1979). [DOI] [PubMed] [Google Scholar]
- 151.Blazar BR, Murphy WJ & Abedi M Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol 12, 443–458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Griffioen M, van Bergen CA & Falkenburg J Autosomal minor histocompatibility antigens: how genetic variants create diversity in immune targets. Front. Immunol 7, 100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hill GR, Betts BC, Tkachev V, Kean LS & Blazar BR Current concepts and advances in graft-versus-host disease immunology. Annu. Rev. Immunol 39, 19–49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tivol E, Komorowski R & Drobyski WR Emergent autoimmunity in graft-versus-host disease. Blood 105, 4885–4891 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu SR & Reddy P Tissue tolerance: a distinct concept to control acute GVHD severity. Blood 129, 1747–1752 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zitvogel L, Ayyoub M, Routy B & Kroemer G Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016). [DOI] [PubMed] [Google Scholar]
- 157.Sepich-Poore GD et al. The microbiome and human cancer. Science 371, eabc4552 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Viaud S et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kuczma MP et al. The impact of antibiotic usage on the efficacy of chemoimmunotherapy is contingent on the source of tumor-reactive T cells. Oncotarget 8, 111931 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Honda K & Littman DR The microbiome in infectious disease and inflammation. Annu. Rev. Immunol 30, 759–795 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tanoue T et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Peled JU et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N. Engl. J. Med 382, 822–834 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uribe-Herranz M et al. Gut microbiota modulates adoptive cell therapy via CD8a dendritic cells and IL-12. JCI Insight 3, e94952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smith M et al. Intestinal microbiome analyses identify biomarkers for patient response to CAR T cell therapy. Biol. Blood Marrow Transplant 25, S177 (2019). [Google Scholar]
- 165.Smith M, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nguyen CL, Docampo MD, van den Brink MR & Markey KA The role of the intestinal microbiota in allogeneic HCT: clinical associations and preclinical mechanisms. Curr. Opin. Genet. Dev 66, 25–35 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]