Abstract
Transient receptor potential (TRP) channels are one primary type of calcium (Ca2+) permeable channels, and those relevant transmembrane and intracellular TRP channels were previously thought to be mainly associated with the regulation of cardiovascular and neuronal systems. Nowadays, however, accumulating evidence shows that those TRP channels are also responsible for tumorigenesis and progression, inducing tumor invasion and metastasis. However, the overall underlying mechanisms and possible signaling transduction pathways that TRP channels in malignant tumors might still remain elusive. Therefore, in this review, we focus on the linkage between TRP channels and the significant characteristics of tumors such as multi-drug resistance (MDR), metastasis, apoptosis, proliferation, immune surveillance evasion, and the alterations of relevant tumor micro-environment. Moreover, we also have discussed the expression of relevant TRP channels in various forms of cancer and the relevant inhibitors’ efficacy. The chemo-sensitivity of the anti-cancer drugs of various acting mechanisms and the potential clinical applications are also presented. Furthermore, it would be enlightening to provide possible novel therapeutic approaches to counteract malignant tumors regarding the intervention of calcium channels of this type.
KEY WORDS: TRP channels, Cancer progression, Tumor microenvironment, Tumor-associated immunocytes, Intracellular mechanism, Programmed cancer cell death, Targeted tumor therapy
Abbreviations: 4α-PDD, 4α-phorbol-12,13-didecanoate; ABCB, ATP-binding cassette B1; AKT, protein kinase B; ALA, alpha lipoic acid; AMPK, AMP-activated protein kinase; APB, aminoethoxydiphenyl borate; ATP, adenosine triphosphate; CaR, calcium-sensing receptor; CaSR, calcium sensing receptor; CBD, cannabidiol; CRAC, Ca2+ release-activated Ca2+ channel; DAG, diacylglycerol; DBTRG, Denver Brain Tumor Research Group; ECFC, endothelial colony-forming cells; ECM, enhanced extracellular matrix; EGF, epidermal growth factor; EMT, epithelial–mesenchymal transition; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; ETS, erythroblastosis virus E26 oncogene homolog; FAK, focal adhesion kinase; GADD, growth arrest and DNA damage-inducible gene; GC, gastric cancer; GPCR, G-protein coupled receptor; GSC, glioma stem-like cells; GSK, glycogen synthase kinase; HCC, hepatocellular carcinoma; HIF, hypoxia-induced factor; HSC, hematopoietic stem cells; IP3R, inositol triphosphate receptor; KO, knockout; LOX, lipoxygenase; LPS, lipopolysaccharide; LRP, lipoprotein receptor-related protein; MAPK, mitogen-activated protein kinase; MLKL, mixed lineage kinase domain-like protein; MMP, matrix metalloproteinases; mTOR, mammalian target of rapamycin; NFAT, nuclear factor of activated T-cells; NLRP3, NLR family pyrin domain containing 3; NO, nitro oxide; Nrf2, nuclear factor erythroid 2-related factor 2; NSCLC, non-small cell lung cancer; PCa, prostate cancer; PDAC, pancreatic ductal adenocarcinoma; pFRG/RTN, parafacial respiratory group/retrotrapezoid nucleus; NEDD4, neural precursor cell expressed, developmentally down-regulated 4; P-gp, P-glycoprotein; PHD, prolyl hydroxylases; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; PKC, protein kinase C; PKD, polycystic kidney disease; RNS/ROS, reactive nitrogen species/reactive oxygen species; RTX, resiniferatoxin; SMAD, Caenorhabditis elegans protein (Sma) and mothers against decapentaplegic (Mad); SOCE, store operated calcium entry; SOR, soricimed; STIM1, stromal interaction molecules 1; TEC, tumor endothelial cells; TGF, transforming growth factor-β; TNF-α, tumor necrosis factor-α; TRPA/C/M/ML/N/P/V, transient receptor potential ankyrin/canonical/melastatin/mucolipon/NOMPC/polycystin/vanilloid; UPR, unfolded protein response; VEGF, vascular endothelial growth factor; VIP, vasoactive intestinal peptide; VPAC, vasoactive intestinal peptide receptor subtype
Graphical abstract
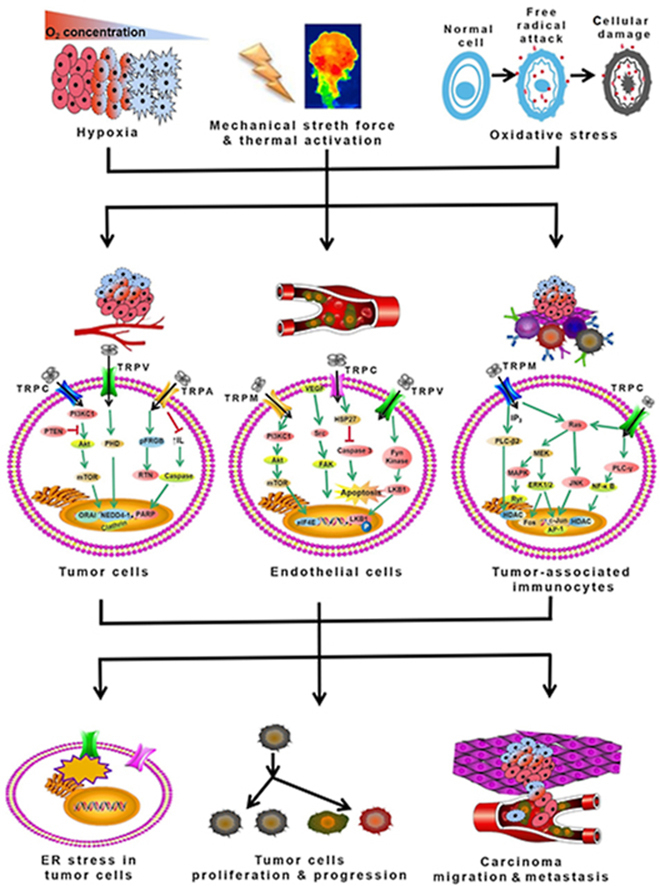
Transient receptor potential (TRP) channels are sensitive to various stimuli such as hypoxia, stretch and reactive oxygen species. We elucidate the mechanisms between TRP channels and cancer progression and propose potential approaches of tumor therapy.
1. Introduction
As the second leading cause of the mortality rate globally, cancer is characterized by several shared features, such as unrestrained cellular proliferation, cell death resistance, persistent angiogenesis, and metastasis to other adjacent or remote tissues and organs. Calcium is regarded as one of those secondary messengers involved in lots of cellular processes. For conventional cardiovascular and central & peripheral nervous systems, it is mainly responsible for cellular growth, muscle contraction, and neuronal plasticity and regeneration. Moreover, besides those diversified physiological roles, calcium signaling transduction pathways also take part in several pathological conditions, including changes in apoptosis, differentiation, metabolism, and gene transcription.
Located in the plasma membrane, calcium channels enable the access of Ca2+ due to the existence of the concentration gradient of Ca2+ across the plasma membrane (the difference between the intracellular and extracellular sides). The calcium channels could be divided into two major categories: voltage-gated channels and non-voltage-gated channels. Voltage-gated calcium channels (CaV family, VGCC), including five kinds of L-, P-, N-, R- and T-type calcium channels (L/P/N/R/TTCCs). Besides CaV expression, non-voltage-gated channels are mainly existent in Ca2+ accession into non-excitable cells with four major types: store-operated calcium channels (SOCs), ligand-gated calcium channels (LGCC, like P2X purinergic ionotropic receptor families), Ca2+ release-activated Ca2+ channel (CRAC), Na+/Ca2+ exchanger (NCX) and receptor-operated channels1 or secondary messenger-operated channels associated with GPCR activation [like transient receptor potential (TRP) superfamily of channels), stretch operated channels (members of TRP superfamily of channels], etc.2.
In recent decades, the TRP family is a brand new interest of study area which is worth notifying, this family was first discovered in Drosophila melanogaster in 1969, but their structures remain unclear until the 1990s. The TRP superfamily could be further subdivided into seven subfamilies with two groups based on their sequential homology. One group has the subfamilies of TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), and TRPA (ankyrin), while the other group has three subfamilies defined as TRPP (polycystin), TRPML (mucolipin), and TRPN (Drosophila NOMPC). However, it is almost impossible to foresee the mechanism(s) of the stimulation of a given channel based on a certain subfamily. What's more, capsaicin would activate the heat-sensitive TRPV1 whilst menthol triggers cold-sensitive TRPM8 channels. Unlike the shared structural similarities, the ionic signature of each TRP member is particular to the activation and stimuli response.
The molecular topological TRP channel contains six trans-membrane (TM1-6) α-helix segments with a re-entrant pore loop between TM5 and TM6 as well as cytosolic –NH2 and –COOH terminal tails. Thus, a tetrameric complex assembly formed by TRP channels makes a central cation conducting pathway via TM5 and TM6 subunits as well as the pore loop inter-connected. In spite of the absence of voltage-sensor in TM4, the structure is considerably illuminated in the voltage-gated potassium channels3.
This review mainly focuses on the role of TRP calcium-permeable channels signaling in cancer from molecular mechanisms to therapeutics. Even though cancer therapies and drugs have been improved significantly in the last years, novel and more efficient treatments are still needed to fight against such indiscriminate diseases. Calcium channels and transporters are widely expressed in the plasma membrane of carcinoma cells, where they may represent an excellent therapeutic target. From this viewpoint, the study on the role of ion channels in both initial and advanced stages of the disease should be facilitative to explore the possible clinical value of these membrane proteins as novel targets for therapy. We will consider the role of TRP channel-signaling pathways in cancer with particular attention to the mechanisms regarding various inner and outer stimulations in this review. The relevant chemical and biological channel modulators and their therapeutic use in treating cancer diseases will also be discussed in this review.
2. Introduction of major TRP channel types
As is mentioned in the above section, TRP channels are a group of relatively non-specific cationic channels located mainly on the plasma membrane site of animal tissues. These channels respond to various heterogeneous stimuli, including endogenous and exogenous chemical mediators, physical stimuli, such as mechanical force (stretch-sensitive) and temperature (thermo-sensitive), free cytosolic Ca2+ ions, depletion of Ca2+ stores in the endoplasmic reticulum (ER), and many others. In addition, many TRP channels are voltage-sensitive and ligand-gated with some unique features. These channels were initially discovered in the trp mutant strain of the fruit fly as D. melanogaster in 1969, and subsequently cloned in 1989. Later on, TRP channels have been found to be expressed mainly in various tissues in many mammalian species.
These TRP channels mediate various physiological and pathological functions, such as the sensation of pain, coldness, warmth or hotness, vision, taste, inflammation, and pressure. Therefore, the members of the TRP ion channel family are considered polymodal with diversified features. Many a TRP ion channel mediates calcium influx into the cells. Under normal homeostasis, the tight control of cytoplasmic calcium levels is critical for regulating many cellular processes, such as motility, secretion, action potential generation and propagation, the release of neurotransmitters, and gene expression. However, excessive cytoplasmic calcium levels are cytotoxic. Therefore, TRP channelopathies with either gain or loss of function can lead to pathophysiology. We would mainly discuss the functions of some major TRP channels in the following contents.
2.1. TRPV channels
Transient receptor potential vanilloid (TRPV) channels are a family of ligand-gated ion channels that mainly own sensory receptors that respond to thermal, mechanical, or chemical stimulations. This subfamily is constituted by six members, and the most investigated channel is TRPV1 as an ion channel stimulated by vanilloid capsaicin, shedding light on the nomination of this subfamily4. Those TRPV subfamilies own comparable structures with an ankyrin-repeat domain in the wake of six-transmembrane helix domains within the amino-terminal region. The carboxyl-terminal in the cytoplasmatic side comprises the TRP domain and other binding sites for those lipid regulators with identified architectures5. In several cell types, TRPV channels are ubiquitously expressed throughout the body and associated with neuronic disease such as stress, anxiety, depression, schizophrenia, etc.6. Apart from the roles of temperature and pain-sensing, TRPV channels could modify the advent of Rho GTPase7,8.
2.2. TRPC channels
The mammalian TRPC members resemble the Drosophila TRP and TRPL since they could be stimulated from receptors that signal through phospholipase C (PLC). The involvement of TRPC channels in cancer cell proliferation, invasion, and chemoresistance has also been discovered in recent years. This channel is nominated as “canonical” since the shared similarities of the TRP channel from Drosophila9. This channel has intracellular N- and C-terminal domains and six trans-membrane-spanning domains. Hetero-multimerization of those channels with the TRPC or other sub-families is reported, resulting in the functional non-selective ion channels with the relative concentration of calcium and sodium. What's more, PIP(4,5)2 and PIP2-derivated materials like diacylglycerol (DAG), inositol triphosphate (IP3), PI(4)P, and PI regulate the activity of TRPC channels. TRPC exists in various tissues like the kidney, hippocampus, salivary glands, pancreatic β cells, vascular smooth muscle, and heart with a wide range of physiological functions10.
2.3. TRPM channels
Eight members consist of the TRPM subfamily defined as TRPM1–8 due to the first member. Higher-grade human prostate cancer (PCa) samples were accompanied by TRPM2 overexpression, among which distinct autophagic–apoptotic gene expression levels were observed. In addition, both monovalent and divalent cationic channels were identified with the former as TRPM4 and TRPM5 and the latter of TRPM7 and TRPM8. Other TRPM channels are non-selective cationic current channels11,12.
Within the amino-terminal region, TPRM channels have a common melastatin homology region (MHR) domain which is likewise in other TRP members. However, diversified carboxyl-terminal are found between the TRPM membranes13. For TRPM4 and TRPM8 channels, they own coiled-coil domains and binding motifs for varied regulatory molecules. Therefore, the TRPM subfamily is quite a diversified subgroup within the TRP channel family for the particular structures and physical functions14.
2.4. TRPA channels
TRPA is a subtype of TRP ion channels, which is widely studied in the field of pain and neurogenic inflammation, and A stands for ankyrin. This subfamily of channels comprises around 1000 amino acids with the ratio of PCa/PNa of 0.8–1.4. It also contains a TRP-box-like motif, including an inositol phosphate-binding region with a coiled-coil region15. This channel type could be activated by diversified metabolites of oxidative stress, i.e., reactive oxygen species (ROS), methylglyoxal, and acrolein. In addition, these Ca2+-permeable cation channels are activated by a broad range spectrum of hazardous external/internal stimuli like sharp coldness, stimulating compounds, reactive chemical species, and endogenous signals associated with cell damage16.
As far as our knowledge could reach, TRPA1 is the only member within the TRPA family in mammals. However, in Caenorhabditis elegans and Drosophila, there are respectively two and four members17. The pain-detecting sensory nerves within the plasma membrane contain TRPA1, and it would activate the pain-sensing pathways to stimulate avoidance behaviors and promotion of long-lasting responses like inflammation. As a result, blockade TRPA1 is promising to reduce pain18.
2.5. TRPP channels
Three human genes nominated as polycystic kidney disease 2 (PKD2, TRPP2), PKD2-like 1 (PKD2L1, TRPP3), and PKD2-like2 (PKD2L2, TRPP5) encode the TRPP channel proteins. This protein channel is a 110 kDa protein, participating in autosomal dominant polycystic kidney disease (ADPKD) as a calcium-permeable non-selective channel. The transmembrane segments of S1–S6 share the identical sequential similarity of homology, whereas the amino and carboxyl terminals have few similarities. There are conserved orthologues within the mammalian TRPP channels with ∼90% similarity for TRPP2/3 and ∼80% for TRPP5. The polycystin-1 family proteins modify the TRPP ion channels into the receptor–channel complexes, establishing the core of the signaling pathway in which calcium is the second messenger19.
2.6. TRPN channels
This type of TRP channel doesn't encode any mammal proteins, only to be present in worms, flies, and zebrafish. This channel is appropriate for mechano-transduction, and it affects the behaviors of insects and nematodes as well as the transduction of vibratory stimuli in zebrafish hair cells20.
2.7. TRPML channels
Three subfamily members as TRPML1, TRPML2, and TRPML3, which consist TRPML group, share ∼75% amino acid similarity. For TRPM1, it is a 580 amino acid molecule with the weight of 65 kDa. Its mutation could be responsible for lysosomal storage disorder mucolipidosis IV, accounting for the iron ions going through the endosome/lysosome membrane into the cell21. On the other hand, 533 amino acids compose TRPML3 with a molecular weight of about 64 kDa. This channel is mainly expressed in the inner ear, being able to inwardly rectify the monovalent cation channel permeable to calcium proton secretion would alleviate its activity22.
2.8. Summary
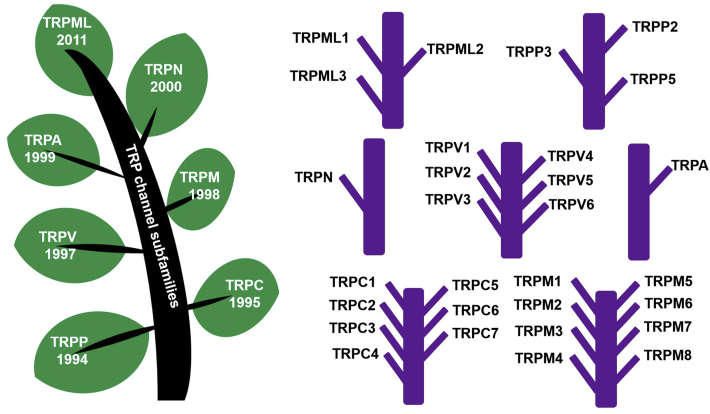
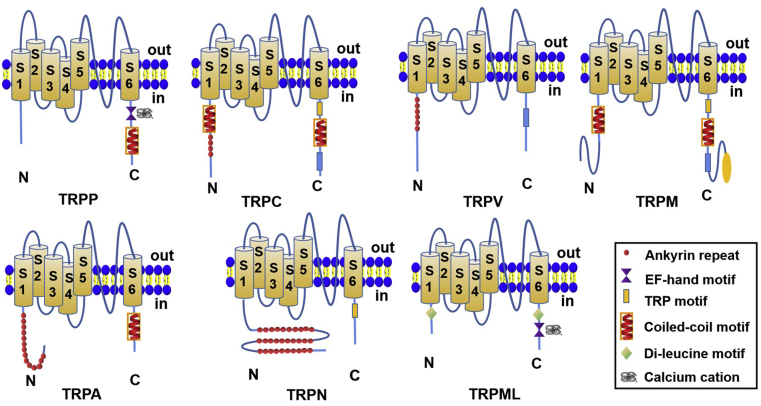
To conclude, those major TRP channels in tumors would be the targets worth further investigation to shed light on possible therapeutic approaches to alleviate or even cure the diseases. However, due to the limited space in this review, we will not further discuss other types of TRP channels, and their functions and expressions in tumors will be discussed in the following sections. In Fig. 1, the general classifications and relevant subfamilies of TRP channels are chronologically presented for their discovery time with the phylogenetic tree. In Fig. 2, the detailed structural variances of each TRP channel subfamily type are stated respectively14,17.
Figure 1.
The polymorphic diagram of the transient receptor potential (TRP) channel superfamilies, indicating TRPC (canonical), TRPM (melastatin) and TRPV (vanilloid), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucolipin) families. They are arranged with a chronological manner, and the year in which they were initially discovered is listed just below the channel names within the tree leaves. The specific TRP channel names in each subfamily are presented in the right part of this figure.
Figure 2.
The schematic depiction of the TRP channel subfamilies, each respectively showing the representative of a subfamily.
3. Dysregulated expression of TRP channels in malignant tumor cells, vascular endothelial cells, and tumor-associated immunocytes within the microenvironment
Aberrant transmembrane or intracellular ion channel expression is observed in either tumor cells or non-tumor cells. Several keynote factors such as hypoxia, elevated oxidative stress, abnormal redox state, and altered mechanical stretch force contribute to the dysregulated expression of TRP channels. The TRP channel expression in various cancer types and the modulators that have already or will enter the clinic is discussed in Table 123, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63.
Table 1.
TRP channel expression in various cancer types with clinical phase situations and challenges.
| Channel Name | Subtype | Cancer type | Clinical-pathological correlation mechanism | Modulator and EC50 value | Clinical phase situation | Challenges |
|---|---|---|---|---|---|---|
| TRPA | 1 | Breast cancer, NSCLC, malignant peripheral nerve sheath tumor | Protects cell from apoptotic death23,24; promotes cell survival and proliferation25 | AM-0902: 0.131 μmol/L | Pre-clinical evaluation (in vitro and in vivo)23 | Exhibits a short plasma half-life |
| TRPC | 1 | Breast cancer, pancreatic cancer, glioblastoma, lung cancer, colon cancer | Induces EMT; promotes invasion and proliferation26 | SKF96365, MRS1845 were non-selective TRPC inhibitors; No clear IC50 data was available | Pre-clinical evaluation (in vitro)27 | Lack of selective TRPC1 inhibitors |
| 3 | Ovarian cancer, breast cancer, glioblastoma | Promotes cell proliferation28; protects cell from apoptotic death29 | Pyr3: 700 nmol/L | Pre-clinical evaluation (in vitro)29 | Lack of sufficient experimental evidence | |
| 4 | Medulloblastoma | Enhances migration30 | N/A | N/A | N/A | |
| 5 | Colon cancer, chemo-resistant breast cancer | Promotes cell migration, invasion, and proliferation31; mediates the EMT of cancer cell32; | N/A | N/A | N/A | |
| 6 | Prostate cancer, glioblastoma, esophageal squamous cell carcinoma, breast cancer, hepatocellular cancer, gastric cancer33 | Mediates cell proliferation, invasiveness and angiogenesis34,35 | 2-APB, SKF96365 were non-selective TRPC inhibitors; no clear IC50 data was available | Pre-clinical evaluation (in vitro)34 | Lack of selective TRPC6 inhibitors | |
| TRPM | 2 | Breast cancer, gastric cancer, lung cancer, prostate cancer, pancreatic cancer, melanoma, leukemia, neuroblastoma36 | Protects cell from apoptotic death37; promotes cell survival and proliferation | N-(p-Amylcinnamoyl) anthranilic acid: 1.7 μmol/L; 2-APB: 1 μmol/L | Pre-clinical evaluation (in vitro)38,39 | Lack of selective TRPM2 inhibitors38 |
| 4 | Prostate cancer, cervical cancer, breast cancer, colorectal cancer | Increases of migration, invasion and proliferation of cancers33 | TRPM4-IN-1 (CBA): 1.5 μmol/L |
Pre-clinical evaluation (in vitro)40 | Lack of sufficient experimental evidence | |
| 7 | Gastric cancer, breast cancer, invasive ductal adenocarcinoma/lymph nodes, pancreatic cancer | Maintains cell proliferation and survival41 | Ginsenoside Rd: 131.2 μmol/L; waixenicin A: 7 μmol/L | Pre-clinical evaluation (in vitro)42,43 | Lack of selective inhibitors and TRPM7 exists a hetero-tetramer with TRPM6 increasing the difficulty of anti-TRPM7 drug | |
| 8 | Breast ductal adenocarcinoma, prostate cancer, bladder carcinoma, osteosarcoma | Mediates the EMT44; represses apoptotic cell death; promotes migration and invasion45 | Capsazepine: 18 μmol/L; RQ-00203078: 8.3 nmol/L; WS-12: 30 nmol/L; D-3263-hydrochloride, no clear EC50 data was available. | Pre-clinical evaluation (in vitro)46, 47, 48; D-3263-hydrochloride in phase I (NCT00839631) | TRPM8 modulators can affect core body temperature, designing a prodrug which can only activated by cancer cells may reduce toxicities | |
| TRPML | 1 | Triple-negative breast cancer, melanoma | Increases of proliferation, invasion and proliferation of cancers49 | MK6-83: 110 nmol/L | Pre-clinical evaluation (in vitro)50 | Lack of selective inhibitors |
| 2 | Breast cancer, prostate cancer, pancreatic cancer, melanoma, leukemia, neuroblastoma | Maintains cell proliferation and survival49 | N/A | N/A | N/A | |
| TRPP | 1 | N/A | Promotes EMT and migration51 | N/A | N/A | N/A |
| 2 | ||||||
| TRPV | 1 | Prostate cancer, papillary thyroid carcinoma, oral squamous cell carcinoma, breast cancer | Promotes cell apoptosis52 and decreases cell proliferation53 | Capsaicin: 20 μmol/L; arvanil: 50 nmol/L | Pre-clinical evaluation (in vitro54 and in vivo55) | Limited antitumor efficacy, with side effects |
| 2 | Pancreas cancers, triple-negative breast cancer, esophageal squamous cell carcinoma, urothelial cancer | Decreases cell proliferation and induces apoptotic cell death; induces cell migration and invasion56 | Cannabidiol: 22.2 μmol/L; Delta 9-THC, no clear EC50 data was available | Phase I57, phase II (NCT01812603) | Not a TRPV2-selective agonist, the antitumor effects was associated with other targets | |
| 4 | Gastric cancer, breast cancer, glioma cancer | Promotes migration and invasion58 | RN1734: 2.3 μmol/L | Pre-clinical evaluation (in vitro59 and in vivo60) | Further research is needed as the precise role of TRPV4 likely depends on multiple factors including channel expression and originating tissue type61 | |
| 6 | Breast cancer, colon cancer, ovary cancer, prostate cancer, thyroid carcinomas, pancreas cancers | Increases cell proliferation, metastasis and inhibition of apoptosis62 | Ruthenium Red: 9 μmol/L; TH-1177: 675 μmol/L; SOR-C13: 14 nmol/L; SOR-C27: 65 nmol/L63 | Phase I24 | Little is known about its exact role in initiation and/or progression for most of cancers |
I, inhibitor, A, activator, N/A, not available.
3.1. Dysregulated TRP channels expression in malignant tumor cells
In malignant tumors, dysregulated TRP channel expression is ubiquitous among the cells. In the following content, we will discuss several distinctive characteristics of tumor cells within which TRP channels play essential roles in such conditions.
3.1.1. Hypoxia
Hypoxia means the deficiency of oxygen, and it is commonplaces that this situation occurs in malignant tumor tissues. The methylation of tumor suppression genes is mainly induced by the environment of hypoxia, losing their due physiological functions by lowering or abolishing its expression. Hypoxia is the consisting barrier to successful therapy of cancer, and those ion channels are deemed as potential targets to conduct signaling transduction which is related to hypoxia-induced factor 1α (HIF-1α) proteins.
In the astrocytes which would further progress into glioblastoma, the TRPA1 cation channels would mediate the selective sensing under moderate oxygen concentration between 6% and 9%, which has a linkage with anterior inferior cerebellar artery (AICA) in the parafacial respiratory group/retrotrapezoid nucleus (pFRG/RTN) region within the ventral medullary surface. The ceased prolyl hydroxylases/neural precursor cell expressed developmentally down-regulated 4 (PHD/NEDD4-1)- mediated internalization underlay TRPA1 mediates the plasma membrane. The stimulation of TRPA1 induced by hypoxia in the plasma membrane contributes to the calcium influx, greatly accelerating adenosine triphosphate (ATP) release from the pFRG/RTN astrocytes so as to modify the neuron circuit within the respiratory center, causing the amplified inspiratory discharge rhythm64. In human glioblastoma, Denver Brain Tumor Research Group (DBTRG) cells triggered TRPA1 under hypoxia elevates apoptotic, inflammatory, and oxidant effects while those effects were attenuated by alpha lipoic acid (ALA) treatment, which might be useful in combatting combat hypoxia-induced syndromes in the neurons. By triggering calcium entry, generation of ROS and cytokines through the TRPA1 channel, hypoxia causes DBTRG cell death, suggesting a positive feedback of TRPA1 channel and apoptotic effects65.
For TRPV3, it is another TRPV channel type. we could infer that hypoxia might influence tumor progression by impacting specific TRP channels via activation of such signaling pathways. Under the condition of hypoxia, a sharp accumulation of TRPA1 in the plasma membrane of pFRG/RTN astrocytes. A reversible coupling of TRPA1 with superoxide-dependent internalization occurs through PHD and NEDD4-1, indicating a TRPA1-modulating respiratory center-induced astrocytic ATP release is stimulated by hypoxia66. In neuroblastoma cells, mitochondrial ROS were considerably elevated within cells while TRPM2 function was suppressed by TRPM2-S, which the antioxidant Mito-TEMPO pretreatment could reverse during doxorubicin treatment. The TRMP2 pore mutant E96D suppressed calcium accession, causing the accumulation of mitochondrial ROS in the wake of doxorubicin treatment. There also seems to be a positive correlation between calcium entry and HIF-1/2α expression. Thus, this channel would be the target of cellular bioenergetics and tumor growth67.
TRPC6 contributes to the basal Ca2+ entry induced by hypoxia. Orai1 or TRPC1 silencing, as well as SKF-96365, diminished the hypoxia-induced calcium entry elevation. Meanwhile, overexpression of Orail and TRPC1 in hypoxia is observed when compared with normoxic conditions. In these cells, TRPC1 serves as a constitutively active channel, and so as Orail, both of which diminished calcium entry respectively. Overexpression of TRPC6 elevated hypoxia, inducing a continuous basal calcium concentration. Proper knockdown of TRPC6 by shRNA or negative dominant expression as well as pharmacological inhibition by SKF-96365 attenuated both hypoxia-induced [Ca2+]i elevation and the 1-oleoyl-2-acetyl-sn-glycerol (OAG)-stimulated Ca2+ entry, but failed to affect the hypoxia-induced Ca2+ oscillation patterns68. When it comes to the homologues of the Drosophila protein, mothers against decapentaplegic (Mad) and the C. elegans protein Sma (SMAD) and its downstream component transforming growth factor-β (TGF-β), stimulation of SMAD2/3 dependent-TGF-β signaling pathway activated TRPC6 helps facilitate up-regulation of expression of α-smooth muscle actin (αSMA) and collagen under hypoxia conditions, shifting from the quiescent to the activation state of hepatic stellate cells under hypoxia conditions. Also, hypoxic condition enables the overexpression of TRPC6 in U-373MG glioma cell line monitoring cellular proliferation, angiogenesis, and invasion with a nuclear factor of activated T-cells (NFAT)-dependent manner35. Among other glioma cell lines such as U-87MG and U-251MG under hypoxia, insulin-like growth factor I receptor (IGF1R)-PLCγ-IP3R (inositol triphosphate receptor) pathway-induced, resulting in calcium's entry regulating HIF-1α accumulation seemingly to be independent of transcription or translocation. HIF-1α under hypoxic conditions also regulates the glucose transporter 1 (GLUT1) expression and glucose uptake via a TRPC6-dependent manner, and such regulation is operated through HIF-1α under hypoxic conditions with no effect on lactate level68.
Thus, hypoxia is an essential factor to regulate TRP channel expression, and a combination with this factor with others is necessary for the behaviors of tumor cells.
3.1.2. Oxidative stress and red-ox state stimuli
For redox states, the pH value of normal tissues is about 7.4, while its tumoral sites have a lower pH value at about 5.6. The lower pH value in the tumor environment is due to glycolysis, producing various anaerobic metabolites such as lactate, pyruvate, and phosphoglycerate compounds. Redox state is tightly associated with pH values since the transfer of electrons directly determines the numbers of the protons and the acidity. Also, inappropriate treatment with the advent of oxidative stress would result in tumor progression and metastasis. Thus, both ROS and RNS play essential roles in a number of physiological processes, and the interaction between NO and calcium could modify a variety of physiological and pathological functions with tightly coordinated mechanisms69.
An up-regulation of calcium-dependent anti-apoptotic signaling pathways is verified by TRPA1 so as to enhance ROS tolerance; during this process, the oxidant-defense transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) straightly regulates TRPA1 expression. Suppression of TRPA1 inhibits the growth of xenograft tumors, enhancing chemosensitivity. Therefore, an oxidative-stress defense program with TRPA1 could be utilized for targeting cancer treatments23. The reactive species within the biological system, like the reactive disulfides, could own varied potentials to perform such reactions. Through reactive disulfides with different redox potentials to tell the capability of TRPA1 sensing oxygen, stimulated under both hypoxia and hyperoxia conditions, contributing to the oxygen-sensing mechanisms. When the oxygen environment is routine around 21%, TRPA1 activity is suppressed by PHDs activations with an oxygen-dependent manner. When hyperoxia condition is met, direct O2 action overwhelmingly holds back the TRPA1 sensitivity to Cys-mediated oxidation. Such hyperoxia and hypoxia-generated cationic currents have been diminished in the vagal and sensory neurons in the Trpa1 knockout (KO) mice, suggesting a complete novel oxygen-sensing mechanism mediated by TRPA169. Due to the insufficient blood vessel supply and the relatively colossal respiration rate in the malignant cells. A serial adaptation of processes could further evolute the aggressive tumor type. Thus, TRPA1 overexpression mediates a non-canonical ROS defense program in human cancer70.
For TRPV, TRPV1 overexpression might have an association with PTEN, and a poor prognosis has been postulated in cervical cancer. The underlying mechanisms involve possible participation of ROS induced by TRPV1 activation. This would be helpful to predict the clinical prognosis71.
Thus, the interplay between calcium and ROS signaling in cancer would further enlighten the potential therapeutic approaches of malignant tumors.
3.1.3. Thermal activation and mechanical stretch force stimulation
When it comes to the mechanical stretch forces, the TRP channels have a distinctive characteristic: it is sensitive to mechanical force influences, which is a key component to distinguish this channel from others. Certain mechanical forces would open some TRP channels defined as stretch-activated channels. Several TRP channels are gated from opening by applying certain mechanical forces and thus function as stretch-activated channels gated either by direct physical forces such as membrane stretch or by the downstream signaling cascades. As a result, the forcing opened TRP channels of mechanical gating is a unifying activation mechanism. Cancer and stroma cells are also influenced by the mechanosensitive TRP channels. Those prominent exogenous challenges in the tumor cells are confronted during the various processes of tumor growth, causing the mechanosensitive TRP channels as potential therapeutic targets.
Mechanical stretch tension causes a considerable increment of cyclic adenosine monophosphate (cAMP) level and thus promotes the activation of several TRP channels linked to cellular migration and the ensuing metastasis72. TRPM7 partially participates in the mechanosensory complex to promote breast cancer during the process of metastasis, formation, and invasiveness of breast cancers. This process consists of the mitogen-activated protein kinase (MAPK) phosphorylation at the kinase domain of such a channel73. Metastasis is crucial for cancer progression, and TRP plays a role in this process. Myosin II-based cellular tension and modifying focal adhesion are regulated by TRPM7 via F-actin and paxillin74. The mutual regulatory relationship between TRP channels and the cytoskeleton could occur in various manners. TRPM7 regulates the dynamics of actomyosin via kinase-dependent phosphorylation in the cytoskeleton parts75. A complete actomyosin cytoskeleton is indispensable via the interaction between TRPC1 and the calcium sensor of stromal interaction molecules 1 (STIM1). In prostate adenocarcinoma, the down-regulation of TRPV4 in those tumor endothelial cells (A-TECs) demonstrated better motility because of the lower mechano-sensitivity since the enhanced extracellular matrix (ECM) stiffness76. The resultant outcome of excessive tumor growth is prominent vascularization77.
The cytoskeletons of breast cancer cells are adjusted by TRPV4, making them migrate. Calcium-dependent activation of protein kinase B (AKT) and the down-regulation of E-cadherin cell cortex proteins ensure the occurrence of this process78. The mechano-transduction is modified via TRPM4 in the cell movement to promote the focal adhesion disassembly to stimulate the focal adhesion kinase (FAK), setting off the Rac1 and actin to have the cytoskeleton reorganized78. For thermo-sensitive influence, functional thermal-sensitive TRPV was seen in non-tumor esophageal squamous cells with an up-regulation in carcinoma stages. TRPV3 overexpression in non-small cell lung cancer (NSCLC) shows a positive relationship between TNM (T: tumor size; N: regional lymph node; M: metastasis) stages and differentiation with a shorter survival rate in NSCLC patients. In the proliferation, migration, and invasion of gastric cancer cells, the involvement of calcium sensing receptor (CaSR) and TRPV4 was through a Ca2+-conducted Ca2+/AKT/β-catenin relay. Thus, CaSR/TRPV4/Ca2+ might be a potential target for cancer prevention and therapy2. TRPM4 overexpression was also declared in cervical cancer, and blockade of TRPM4 altered the cellular size respondent to hypertonicity due to the difference of mechanical force79.
Those findings would provide new insights into the role of TRP channels in tumor progression and cytoskeletal structure, which would be a useful tool for further investigation of tumor therapy. Thus, the gating of these channels would change the step and pace of tumorigenesis and metastatic cascade. Nonetheless, the changes in the physical properties of the tumor microenvironment suggest that the gating of these channels may be altered in tumorigenesis and along the metastatic cascade.
3.2. Dysregulated TRP channels expression in vascular endothelial cells
Angiogenesis marks the generation progression and development of tumors, and recent investigations found that TRP channels have a tight linkage with angiogenesis among various types of tumors. The detailed content is illustrated in the following paragraphs.
In breast cancer tumor endothelial cells (BTECs), both TRPM8 and TRPV4 are down-regulated80. However, a considerable up-regulation of TRPA1, TRPV2, and TRPC3 is observed in prostate-derived TECs (PTECs). The deregulation of those TRP channels associated with the prostate has prominent effects on endothelial cell biology, i.e., TRPV2 responsible for the proliferation, TRPC3 for TEC-mediated interplay with tumor cells, and TRPA1 for angiogenesis. Thus, they would be therapeutic targets to combat breast cancer81.
TRPC1 is also upregulated in the renal cancer-derived endothelial colony-forming cells (ECFCs), and an ensuing store operated calcium entry (SOCE) amplitude and stimulating ECFC proliferation are discovered82. When compared with normal human microvascular endothelial cells, the deregulation of TRP is quite weird and results in the sequencing intracellular calcium deregulation83. TRPC1 has pro-metastatic characters, and it could be applied in many cancers. The ciRS-7/miR-135a-5p/TRPC1 axis activated by TRPC1 aggravated epithelial–mesenchymal transition (EMT) in gastric cancer (GC), and this TRPC1 would be a potential target for end-stage GC patients. The authors also found that in GC cells, miR-135a-5p regulated TRPC1 by targeting its 3′-UTR. Thus, ciRS-7 acted as a sponge of miR-135a-5p in GC84. In Trpv4-KO mice, the tumor vasculature showed a more significant percentage of the hyper-permeability without the generation of pericytes or the dilation of microvessels, which is an effective approach to alleviate the therapeutic outcome of anti-cancer treatments85. In tumor-derived ECFCs, TRPC1 is strongly remodeled for vascular endothelial growth factor (VEGF) is not competent enough to cause the oscillations of pro-angiogenetic of calcium oscillations. This characteristic is essential to consolidate the viewpoint that anti-VEGF drugs incur primary and secondary resistance due to TRPC1 (and SOCE) inhibition could offer a bright future to interfere with tumor vascularization86.
Tumor proliferation, migration and survival demand angiogenesis under both physiological conditions such as the growth and renewal of blood vessels and pathological conditions like cardiovascular diseases and the initiation of tumor progression. There would be a close association between the TRP channels and the angiogenesis effect. The ensuing focus would be whether those TRP-generated calcium signals induce various cellular functions and other aspects. The Trpv4-knockout mice show the novel tumor blood vessels with enlarged diameters and densities. Meanwhile, the shrinkage of tumor capillary endothelial cells within the surrounding areas took place. Thus, the abnormalized formation of blood vessels could enhance the progression of lung cancer. In some sense, the activation of TRPV4 with the agonist of GSK1016790A normalized the vascular endothelium and the permeability of the chemotherapeutic drugs, ultimately reducing tumor cell secretion and suppressing the tumor growth77. Up-regulation of TRPV4 channels in the endothelium may essentially impact the early phases of angiogenesis in breast cancer. A significant enhancement of TRPV4 expression is seen in BTECs for malignant angiogenesis. Meanwhile, arachidonic acid (AA) or 4α-phorbol-12,13-didecanoate (4α-PDD)-induced migration accompanied with TRPV4 stimulation in BTECs instead of normal human cardiac microvascular endothelial cells80. During the initial stage of the tubulogenic process rather than the capillary-like network, AA promotes the access of extracellular Ca2+ entry into BTECs87. Substantial reduction of TRPV4 resulted in the VE-cadherin expression for the intercellular contacts, further contribute to the increased vascular leakage88. TRPC1 in tumor-derived ECFC might be considerably undergone vascular remodeling process. A significant upregulation of TRPC and SOCE was seen in renal carcinoma cells (RCC)82.
Thus, the TRP channel plays an indispensable role in tumor vasculature and the spread of angiogenesis. Therefore, proper treatment of the TRP channel would be a useful tool to alleviate tumor growth.
3.3. Dysregulated TRP channels expression in tumor-associated immunocytes within the microenvironment
The solid tumor has a complicated mixture of cells, both cancerous and non-cancerous. In general, these non-cancerous cells, plus factors including the ECM, cytokines, growth factors, and hormones, consisting of the microenvironment of tumors. Cytokines are the general name of bioactive small molecular peptides and glycoproteins. The function of cytokines is to regulate the immunological functions, mediate inflammatory response, stimulate the proliferation and differentiation of hematopoietic stem cells (HSCs), and participate in tissue repair and wound healing. Macrophages are essential in initiating immunological reactions such as immune surveillance stimulation, bactericide, remodeling, and repairment of tissues with both beneficial and hazardous effects in various diseases relying on the cell activation state and the microenvironment where they are present89.
The expression of those TRP members within the monocytes and macrophages90. Those channels enable the identification of exogenous signals such as exogenous damage-associated molecular pattern molecules as well as the endogenous danger signaling transductions during the injury period91. Microarray-based studies demonstrated that an up-regulation of TRPM4 transcripts was observed in human large B cell lymphoma. Also, within lymphoid tissues, human normal B cells could express a relatively lower level of TRPM4, while in diffuse large B cell lymphoma, higher TRPM4 protein levels were observed. An overexpression of TRPM4 level was kept in a quarter of all the cases in large B cell lymphoma when compared with human normal B cells within lymphoid-rich tissues (reactive tonsil, lymph node, and appendix)92.
TRP channels might regulate the varied macrophages differentiation. In Helicobacter pylori-infected Trpm2 knockout mice, alleviated bacterial colonization and aggravated gastric inflammation were diagnosed when compared with their counterparts. The absence of TRPM2 in the H. pylori-infected macrophages stimulates elevated inflammatory cytokines as well as M1 polarization. Triggering TRPM2-deficient macrophages with H. pylori resulted in calcium overload, extracellular signal-regulated kinase 1/2 (ERK1/2), and nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase activation93. Within lymphoid tissues (reactive tonsil, lymph node, and appendix), an insufficient level of TRPM4 was observed. However, a higher TRPM4 protein level was confirmed in diffuse large B cell lymphoma accounting for 26% of all cases, akin to prostate cancer with aggressive clinical parameters, resulting in a worse prognosis92. Differentially regulated TRPM7 expression and activity in bone-marrow-derived murine M1 and M2 macrophages94. The IL-4 triggered M2 macrophages have a more prominent TRPM7 current (∼4.7 fold), but the TRPM7 mRNA levels keep static upon cell polarization94.
The TRPM7 inhibitors of NS8593 and FTY720 would suppress the IL-4 and M-CSF-induced macrophage proliferation to suppress M2 polarization. Furthermore, suppression of TRPM7 diminished the levels of phosphorylation. Phosphoinositide 3-kinase (PI3K) and the downstream ERK1/2 phosphorylation levels were suppressed when TRPM7 was inhibited, causing apoptosis in the rat hepatic stellate cells95,96. An adoptive transfer of macrophages within the TRPM8-deficient mice exacerbates colitis. Meanwhile, the overexpression of IL-10 would rescue the sub-population of M2 macrophage. The M1 macrophage phenotype and pro-inflammatory activity were facilitated via tumor necrosis factor-α (TNF-α) generation in TRPM8-positive macrophages. As a result, triggering the TRPM8 channel in the murine peritoneal macrophages stimulates the calcium transient currents in wild-type rather than TRPM8-deficient mice, demonstrating defective phagocytosis and enhanced motility97. The TRPC6 channel translocated into the phagosomal membrane to elevate its function since in cystic fibrosis patients could restore the alveolar macrophage function via the enhancement of TRPC698.
For the TRPC1 channels, TLR4-dependent TRPC1 activation stimulates the ER storage. In the wake of activation of PLCγ, the protein kinase C-alpha (PKCα) is mediated by the calcium entry of TRPC1, leading to NF-κB/Jun kinase nuclear translocation as well as cytokine secretion-induced tissue destruction99.
Therefore, it is an efficient approach to investigate the roles of TRP channels in the immunocytes and tumor microenvironments, and it is believed that certain modifications within such microenvironments could facilitate potent therapeutic outcomes.
3.4. Summary of the characteristics and functions of TRP channels in tumors
TRP channels are often the effectors of dysregulated physiological functions for the altered expression, making a severe modification in the cells’ major signaling pathways. Likewise, altered expression of TRP channels in certain pathological functions such as tumor and inflammation could be fully utilized to regulate the entry of suitable reagents to unveil the non-permeable potential drugs and improve the therapeutic index of existing drugs via favorably aiding the uptake in affected cells and tissues. We just reviewed the apparent features of TRP channels in tumors, and thereafter, further investigations of the underlying intracellular and intranuclear mechanisms are prone to be elucidated.
4. The underlying intracellular mechanisms of TRP channels in tumor progression
In the above sections, we have mentioned the functions of TRP channels via an apparent aspect, indicating the TRP channels modulate the microenvironment of tumors and exert certain roles. After that, two possible mechanisms of TRP channels driving the enhancement of intracellular calcium concentration are investigated: the excretion of calcium from the internal stores like the ER and mitochondria or either via the stimulation of calcium entry pathways in the plasma membrane100.
Thus, the modified calcium signaling might alter the behaviors of cells such as gene transcription, cell proliferation, differentiation, migration and metastasis, invasion with calcium-dependent signaling cascades. In such processes, MAPK/ERK and PI3K/AKT pathways are probably involved. Both calmodulin and ERK are triggered upon calcium entry into tumor cells. The cytoplasmic downstream targets of ERK activation encompass the MAPK-activated protein kinases of ribosomal S6 kinase, mitogen, and stress-activated protein kinase MAPK interacting protein kinase, as well as the protease calpain. As a result, cell cycle progression, cellular survival, and nuclear signaling are tightly regulated by those proteins101.
4.1. The effects of TRP channels in ER stress in tumor cells
Interference of calcium homeostasis in the ER is an essential stimulation of ER stress, and the majority of the TRP family has been involved and mediate the intra- and extra-cellular homeostasis to initiate ER stress via diversified mechanisms.
The TRP channels and ER stress have a mutual function, reciprocally facilitating each other to amplify those actions. TPPC channels have been initially identified to play a role in ER stress-induced apoptosis; thus, TRP channels play crucial roles in maintaining ER calcium homeostasis and disrupting channel function, leading to ER stress102. TRPC1 is essential for maintaining ER calcium homeostasis and the reduction leading to prolonged unfolded protein response (UPR) pathway activation with a deterioration of AKT, resulting in neuro-degradation103. Be that as it may, mammalian target of rapamycin (mTOR) is also involved in such processes, and functional TRPC1 overexpression prevented neurotoxin-induced ER stress as well as UPR via AKT/mTOR signaling restoration and increasing dopaminergic (DA) neuron survival. Thus, prolonged ER stress might induce cellular death, launching a protective response mediated partially by autophagy. Meanwhile, TRPC3 also impacts ER stress in the epithelial cells extracted from mouse pancreatic and parotid glands; the absence of TRPC3 function ameliorates ER stressed induced-UPR via pERK signaling104. In tumor cells, ER-induced activation of calcium/calmodulin dependent protein kinase II (CAMKII) in human coronary artery endothelial cells (HCAECs) demonstrated that calcium influx via TRPC3 is indispensable UPR and the ultimate ER-stress-driven apoptosis105,106. Excessive calcium entry into cells under the condition of albumin overload results in the calcium entry and the ensuing ER stress protein glucose-regulated protein 78 (GFP78) accumulation as well as the ultimate apoptosis107.
Human lung cells have witnessed that TRPV1 agonists inside the ER deteriorated calcium homeostasis so as to trigger eukaryotic translation initiation factor 2 alpha kinase 3 (EIF2αK3)-dependent ER stress responses108. Overexpression of stress response-induced genes such as growth arrest and DNA damage-inducible gene 153 (GADD153), GADD45α, ATF3, CCNG2, and BiP/GRP78 mRNA and the simultaneous decrease of cyclin D (CCND) indicates the stress induced-agents like dithiothreitol (DTT) and thapsigargin109. Furthermore, phenomena such as ER stress, GADD153 expression, and lung cell injury are generated by inflammation-induced TRPV1 agonists110. However, ER-bound TRPV1 channels have a relatively lower sensitivity for its agonist capsaicin, indicating the critical safety mechanism as protecting neurons from calcium exhaustion from ER, which results in ER stress, abnormal folding of protein response, and cell death111.
ER stress and the crosstalk with autophagy are vital for the maintenance of cellular energetics and cell survival. The spatially restricted subcellular domain of calcium entry into the cells is achieved by many proteins and ion channels. Further investigation is needed to detect these intricate relationships, and a complicated signaling pathway for autophagic activation would be further analyzed.
4.2. The effects of TRP channels in tumor cell proliferation and progression
Tumor cells are characterized as unrestrained proliferation and duplication. There are intertwined underlying mechanisms worth investigating. It is believed that calcium could be one promoter to regulate the extent and rate of tumor cell proliferation. Thus, we will carefully review the roles of TRP channels in cellular proliferation.
The resultant suppression of nonobese diabetic-severe combined immunodeficiency (NOD-SCID) mouse xenograft models was subsequently observed112. Hepatocellular carcinoma (HCC) cells, Huh-7, and hepatocytes from HCC patients are harvested, and a research on TRPC6-mediated Ca2+ entry in HCC cell proliferation was carried out. Cell proliferation and store-operated calcium entry were detected in the wake of TRPC6 overexpression, which could be attenuated by its knockdown. Similar effects happened by the knockdown of STIM and Orai. This effect is more prominent in HCC when compared with normal liver tissue, suggesting the TRPC6-mediated calcium accession into HCC cells is essential for the HCC cells proliferation and oncogenesis113. The elevated expression of TRPV4 in healthy or inflamed skin when compared with lower or absent expression in skin cancer like precancerous lesions and non-melanoma. The discharge of IL-8 was activated via the triggering of TRPV4, which in turn downregulates the TRPV4 expression. The hypothesis made that lower expression in skin cancer would affect the ATP secretion along with autocrine communication between those keratinocytes via the regulation of calcium homeostasis. Thereafter, the diminished extracellular calcium concentration was also suppressed, resulting in the cells’ intact and eventual tumor formation114,115.
The basic biological events of cells contain proliferation and differentiation, and cancer cells have the capability of unlimited replicative potential, unlike most of the normal cell lineages with a limited number of successive growth-and-division cycles. The imitation point of tumorigenesis is the moment when those cancerous cells become immortal. TRPC6 was crucial in cell growth with a positive relationship of its expression when compared with the tumor grade. Inhibition of this TRP channel reduced cell growth, leading to glioblastoma arrest at the G2 phase, with promoted anti-tumor levels. As a result, the antiproliferative therapeutic efficacy of ionizing radiation has been further advanced to clonogenic ability with the state transition of the G2/M phase2. For TRPC6, its association with the pathophysiology in breast cancer when investigating the role of calcium-sensing receptor (CaR) within the cell line of MCF7 in the luminal A ER + breast cancer. The dual distinct cationic current induced by CaR activation through a highly Ca2+-sensitive and another secondary Na+ influx with the latter quite be dependent on the former, which is uniquely active upon the stimulation of calcium current. Thus, those two CaR-activated cationic influx TRPC1 and/or TRPC6-dependent mediated by hetero-multimers, making contributions to the biophysical characteristic of the channels and the intracellular calcium depletion116,117.
TRPM4 channel expression is observed in several cancer types such as prostate, urinary bladder, liver, cervical, colorectal, as well as large B cell lymphoma. For prostate cancer, benign and malignant prostate tissues with TRPM4 protein are observed. The ensuing studies confirmed TRPM4 upregulation in prostate cancerous tissues. Essentially, a higher level of TRPM4 protein has a positive correlation with a higher risk of recurrence following radical prostatectomy118. For the hepatic stellate cells (HSC), its proliferation and upregulation would be regulated by TRPV4. The mRNA and protein expression levels of TRPV4 in rat HSC-T6 cell line far outweighed the control group in the wake of the addition of transforming growth factor β1. Nevertheless, Ruthenium Red (non-specific antagonist of TRPV4) or synthetic siRNA targeting TRPV4 suppressed TGF-β1-induced HSC-T6 cell proliferation119. Upon the activation of TRPV4, HSCs could be transformed into the myofibroblasts and subsequently secrete a considerable amount of collagen, resulting in fibrosis and causes damages to liver tissue, cirrhosis, and liver cancer. Inhibition of TRPV4 with its antagonist of HC-067047 in hepatocellular carcinoma diminished the cellular proliferation, resulting in apoptosis with decreased migration capability via the alleviation of the EMT process both in vitro and in vivo120. Intraperitoneal injection of HC-067047 in oligodendrocytes, the proliferation is also promoted upon TRPV4 activation with increased mRNA levels in both primary cultures and in vivo models. The triggering of TRPV4 by GSK1016790A elevated OPC proliferation which could also be abolished by the co-treatment with HC-067047121. When it comes to the TRPM7 channel, this tension-triggered enhances the calcium entry via IP3R2 stimulation with the resultant local calcium concentration fluctuating for cellular migration122. Its overexpression is associated with the loss of cellular adhesion via calpain II123. The bladder cancer cells have a significantly elevated level of TRPM8, and the knockdown/inhibition of those cells could result in attenuated cell proliferation and migration. What's more, synthetic siRNA targeting TRPM8 treatment enhanced the ROS levels together with enhanced levels of catalase, heme oxygenase-1 (HO-1), and superoxide dismutase 2 (SOD2). Moreover, transplantation of the mouse model with stable TRPM8-deficient T24 cell line suggests such knockdown of TRPM8 has holdback the in vivo tumor growth124.
Strangely, TRPA1 is linked to tumor suppression with the inhibition of cancer progression as the down-regulation takes place in tumor cells125. Subsequently, TRPA1 showed tumor-promoting effects in small cell lung cancer (SCLC) cell lines, with well-stated roles in neuroendocrine functions126.
Thus, proper regulation of TRP channels would be an effective approach to combat cancer to restrain tumor growth via suppressing cellular proliferation.
4.3. The effects of TRP channels in tumor cell migration and metastasis
EMT refers to the tumor cells’ properties tightly linked with the invasiveness as well as the metastatic dissemination. It also associates with the degeneration of components of the extracellular matrix. During this process, the cells become spindle-shaped fibroblast-like ones with enhanced motility127.
The occurrence of EMT is mediated via TRPV4, and E-cadherin is one of the vital inhibitors to suppress the neonatal epithelial tumor cells by their movement and invasion. Diminished expression of E-cadherin is regarded as the prominent marker of EMT128.
For migration and metastasis, TRPV4 enhances the abilities of the relevant proteins associated with the tumor metastasis. Constant stimulation of TRPV4 enables the gene expression for the ability of metastasis while reducing the metastasis inhibition genes. The administration of TRPV4 agonist 4α-PDD causes the adhesion-associated tumor suppressor genes (i.e., Fn1, Clu, Tubb2c, and Spp1) to diminish in the mouse breast cancer cell line 4T0778. Meanwhile, the metastasis-promoting gene of talin secreted via exosomes demonstrates an increment129. In mice cerebral edema, the expression of hippocampus matrix metalloproteinases (MMP) such as MMP-2/MMP-9 (associated with the metastasis of many types of cancers) levels were considerably suppressed by the TRPV4 antagonist of HC-067046129. For vasoactive intestinal peptide receptor subtype 1 (VPAC1) as a G-protein coupled receptor (GPCR), it is predominantly activated by vasoactive intestinal peptide (VIP). Its activation facilitates the migration and invasion of the gastric cancerous cells via TRPV4-dependent calcium entry, amplifying the VIP expression. Thus, the VPAC1/TRPV4/calcium axis signaling axis caused substantial lung metastasis of gastric cancer cells in vivo via VIP elevation60.
In the bladder cancer cells, the inhibition of the overexpressed TRPV4 could significantly downregulate the expression of E-cadherin. TRPV4 suppression-induced AKT and FAK activation would further alter the E-cadherin expression level130. The cytoskeletal remodeling is triggered by TRPV4 activation in breast cancer cells. TRPV4 not only regulates the microtubule–microfilament polymerization but also causes the dynamic alterations of the microvilli and pseudopods, affecting the cellular motility. Contributing to the occurrence of tumor EMT modified via intracellular calcium signaling131. It is prominent that the straight binding of TRPA1 N-terminal ankyrin repeats into the C-terminal proline-rich motif of fibroblast growth factor receptor 2 (FGFR2), which subsequently activates the receptor, leading to the lung adenocarcinoma progression and metastasis. Conversely, the depletion of TRPA1 happens by the transfer of TRPA1-targeting exosomal microRNA (miRNA1723 142-3p) from brain astrocytes to cancer cells132.
Ryanodine receptor (RyR) stimulation results in the TRPM7 to migration of human nasopharyngeal cancer cells, and a subsequent increment of intracellular calcium levels and migration occur. This proves the hypothesis that TRPM7 has a direct impact on various cancer cells through the mechano-transduction mediated by intracellular calcium. Thus, TRPM7 overexpression is linked to the poor prognosis and metastasis as well as the enhancement of both phenomena in nonmetastatic cancer cells133. Overexpression of VPAC1 is considerably reported, and the VPAC/TRPV4/Ca2+ signaling axis has been confirmed involved in gastric cancer. The secretion of intrinsic VIP would enforce a positive feedback regulatory system via a Ca2+-dependent manner. VPAC1 triggered the TRPV4 channel through the PLC/DAG/PKC signaling pathway. Proper interference of VPAC1/TRPV4/Ca2+ signaling or VIP suppression would be helpful to be a potential prognostic marker to cure gastric cancer to suppress the metastasis degree60.
The progressive and invasive manner of pancreatic ductal adenocarcinoma (PDAC) is also partially attributed to TRPM7. TRPM7 silencing in the PDAC cells alleviates the cancer cell invasion. The MMP-2 released via Hsp90a/uPA/MMP-2 proteolytic axis triggers TRPM7, breaking the ECM to promote the invasive capacity of the tumor cells134. TRPM2 modifies the migration and invasion of the gastric cancerous cells in vitro, as well as that representative EMT markers such as N-cadherin, snail, slug, integrins, and MMPs to modify their expressions in vivo135.
Ovarian cancer patients with shorter survival periods have higher EMT processes, and TRPM7 channel overexpression is observed in such a process. Intracellular Ca2+ reduction via TRPM7 silencing or inhibition attenuated the EMT process, migration, invasion, and wound healing of ovarian cancer cells by inhibiting the PI3K/AKT activation, suggesting the preventive approach of metastasis in ovarian cancer136.
For TRPC1, it mediates the calcium entry and the MAPK and PI3K/AKT signaling pathways; it also modifies HIF1α, calpains as well as those MMPs. The directionality of the migrating cells such as the renal transformed epithelial cells137. The epidermal growth factor (EGF)-evoked migration is also aroused by TRPC1 in aggressive glioma cells, and the suppression of the channel ameliorates the state of cells138. Besides, in the leading edge of migration cells, TRPC1 facilitates the localization of the lipid rafts. Therefore, TRPC1 has been regarded as an essential player in both normal and cancerous cellular function139. TRPC2-regulated migration is dependent on Rac, calpain, and MMP-2 during embryogenesis in the thyroid cells140. Furthermore, activation of Ca2+ activated K+ channel 3.1 (KCa3.1) elicited TRPC3-evoked calcium entry with elevated calpain activity and concomitant cell migration141. For melanoma cell migration, it is enhanced by TRPC3 stimulation both in vitro and in vivo142. Both TRPC3 and TRPC6 account for the invasive manner of cancerous bladder cells, and within those cells, the regulation of histone variant macroH2A expressional levels arouse143. In follicular thyroid cancer ML-1 cells, TRPC1 is a strong regulator in both migration and invasion. The underlying mechanisms seem to be dependent on the MAPK/ERK1/2, MMP-2 and-9, and HIF1α144. As for the TRPC2 channel, it mainly regulates normal cells. But TRPC3 channel is a very interesting subject worth further investigation in various tumor cells, such as human ovarian cancer cells, in which an enhancement of TRPC3 expression is observed. Furthermore, the micro-injection of TRPC3-knockdown human ovarian adenocarcinoma SKOV3 cells diminished the tumor formations in nude mice28. Both cell and animal studies showed TRPC3 promoted tumor formation and cell migration in melanoma, causing deleterious results to the patients142. In stellar pancreatic cells, TRPC3-triggered calcium entry and calpain activity along with concomitant cell migration141. For bladder cancer cells, the migration and invasion behavior is also found dependent on both TRPC3 and TRPC6, and to our interest, the expressional levels in those cells are tightly regulated by the histone variant macroH2A143.
Thus, the intertwined relationship between metastasis and TRP channels are crucial targets for further investigations to seek effective and efficient approaches to cure or at least alleviate cancer.
4.4. TRP channels on novel patterns of programmed cellular death in cancer cells
Three novel modules of cell death patterns as pyroptosis, necroptosis and ferroptosis were found in recent decades. Discovered and defined by Yuan's laboratory in 2005, necroptosis is a type of death modality linked with inflammation and immunal responses, demonstrating many morphological similairities to necrosis, i.e., organelle swelling, cytoplasmic membrane breakage, and cellular collapse145. Pyroptosis, associated with inflammation, has the features of plasma membrane pores, is featured by the disruption of plasma membrane wholeness, and in the wake of those above characterizations, there is an ouflow of cytologic inflammatory contents146. In such processes, caspase plays an indispensable role, with the classic caspase-1/NLR family pyrin domain containing 3 (NLRP3)/gasdermin D(GSDMD) pathway srecting IL-1β and IL-18147 and atypical caspase-4/5/11 pathways148. As for ferroptosis, it is a Fe-dependent affregation of lipid peroxides, leading to the degradation of intracellular antioxidant pool with an elevation of intracellular iron149. When ferroptosis takes place, the integrity of cellular membrane is jeopardized with the mitochondrial membrane shrinkage, rupturing the out membrane of mitochondria as well the chromatin condensation of nulei150.
There are limited amount of literature reports indicating the association of those three novel pattern of cellular death with TRP channels. For pyroptosis, the TRPM2/NLRP3 inflammasone pathway induced by mitochondrial ROS generation is reported to own a tight linkage with gout151,152. Also TRPM2 demonstrated inhibitory effects in lipopolysaccharide (LPS)–ATP-induced pyroptosis via downregulation of ROS generation in macrophages stemmed from bone marrow, Trpm2−/− mice group showed higher caspase-1-P10 with elevated ASC oligomerization. Thus, ablation of TRPM2 woule be beneficial to trigger caspase-1 and pyroptosis through regulation of ROS generation as an approach of immunal adjustment153.
RIP-induced mixed lineage kinase domain-like protein (MLKL, a trimer) phosphorylation constitutes a homotrimer sited at the plasma membrane in the TNF-induced necroptosis. Thereafter, TRPM7 worked as its downstream substrate and mediates the calcium influx, resulting in the disruption of plasma membrane and shedding light on the role that TRPM7 plays in the association of MLKL with the receptor-interacting protein kinase 3 (RIP3) necrosome154. Moreover, TRPM4 is involved in LPS-induced vascular endothelial necrosis by mediating Na+ influx, cell depolarization, and increased cell volume155.
As to ferroptosis, it would be linked with oxidative glutamate toxicity or oxytosis, TRPC channels might be involved in such process via the 12/15-lipoxygenase (12/15-LOX) and the 12/15 LOX as the substrates156.
Despite substantial efforts have been invested to TRP channels in novel mechanisms of cell death, the above-mentioned patterns of cancer cell death is poorly elucidated. To achieve deeper apprehension within this field, TRP channels on the novel patterns of cellular death in tumors would be potential areas of further investigation.
4.5. Summary of the intracellular mechanisms of TRP channels in tumors
The essential interactions between tumor cells and tumor microenvironments are tightly regulated and modified by those transports and channels. We have briefly summarized the potential underlying mechanisms to tell which signaling pathways might be involved within such processes. By far, many factors are to be uncovered, and thorough investigation towards the underlying mechanisms would facilitate the therapeutic approaches of cancer.
5. Current and potential clinical approaches targeting TRP channels in tumor therapy
The widespread TRP channels throughout the body provide us with lots of potential candidate targets for cancer chemotherapy. Comprehension of how the intracellular Ca2+ signaling networks, especially the structural assembly of those channels, are important for the progression of drug design and development with a special focus on cellular membrane potentials and specific affinity of their inhibitors or regulators. Theoretically, calcium-permeable TRP channels could be attractant therapeutic targets for triggering those channels results in elevated intracytoplasmic calcium levels, leading to the apoptotic pathways. Nevertheless, problematic outcomes take place when a higher concentration of blockers is adopted in most tumors with intolerable side effects. For those marketized chemicals or antibodies targeting those above-mentioned cancer-relevant Ca2+ channels, regardless of in the preclinical research or in clinical trial periods, some of those items have shown promising outcomes to counteract cancer. This may be a novel approach to evade the side effects that could be induced by routinely adopted clinical methods such as radiation, chemotherapy, and surgery.
TRP channels are newly-discovered types of calcium channels with downstream components like Ca2+-activated K+ channels and so on. The following contents are mainly presenting the chemical compounds that are associated with TRP channel-related therapy. It is also promising that the macro- and micro-molecular chemical compounds will be developed targeting various TRP channels in the near future. We believe it would be a novel approach to combat tumors of varied styled since this interference of calcium is a useful tool to suppress of tumor growth. The chemo-sensitivity relationship between TRP channels and therapeutic reagents are listed in Table 223,157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176.
Table 2.
Drug-resistance regarding TRP channels and relevant involving mechanisms.
| Category | Drug name | TRP channel involved | Mechanism involved |
|---|---|---|---|
| Immune modulator | Vacquinol-1 | TRPM7 | Vacquinol-1 induced cell death in the glioblastoma cells is through the TRPM7 ATP-inducible inhibitory effect. The glioblastoma cultures with different grades of malignancy demonstrates different grades of sensitivity to methuosis157. |
| Cytotoxic/cytostatic agents | Gemcitabine | TRPM7 | RNAi-induced TRPM7 silencing fail to cause apoptosis, while TRPM7−/− cells results in replicative senescence. Combination of anti-TRPM7 siRNA with gemcitabine enhanced cytotoxicity via the pathway of p16 (CDKN2A) and WRN158. |
| TRPM8 | Proliferation and invasion were suppressed after RNA interference-mediated silencing TRPM8, and the multidrug resistance-associated proteins, P-gp, multi-drug resistance protein 2 (MRP-2), lipoprotein receptor-related protein (LRP), was significantly reduced in response to TRPM8 silence159. | ||
| Carboplatin | TRPA1 | TRPA1 enhanced resistance to the ROS-generation drug, and its inhibition suppresses the xenograft tumor growth and elevate the drug sensitivity. NRF2 as the oxidant-defense transcription factor, directly controls TRPA1 expression, to protect tumor cells from oxidative stress by ROS- neutralizing23. | |
| Cisplatin | TRPV1 | ROS level, lipid peroxidation, PARP1, caspase 3 and 9 expression levels are increased through activating TRPV1 in the cells by the Cisp and ALA treatments with reduced glutathione peroxidase (GPx)160. | |
| 5-Fluorouracil (5-Fu) | TRPC5 | TrpC5 in ATP-binding cassette B1 (ABCB1) induction and drug resistance in human colorectal cancer cells via promoting nuclear β-catenin accumulatio with ABCB and cyclin D1 over-expression161,162. | |
| Temozolomide | TRPV2 | TRPV2-dependent autophagy provokes the Aml-1a-dependent differentiation, eliminating the 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) chemoresistance163. | |
| Docetaxel (DTX) | TRPM2 | ROS activates TRPM2 and apoptosis, ROS generation and mitochondrial membrane depolarization levels were increased in glioblastoma cells were respectively treated with Se and DTX. Combined usage of Se and DTX would be linked with TRPM2-mediated elevation in oxidative stress and inward calcium channel164. | |
| Carmustine | TRPV2 | TRPV2 pore deletion abolishes cannabidiol(CBD)-induced Ca2+ permeation, totally turnovers the CBD-induced potentiation of drug cytotoxicity and increases the chemoresistance of glioma cells165. | |
| Doxorubicin (Adriamycin) | TRPC5 | NFATc3 is associated with TRPC5 activity to P-gp production, thus the TRPC5–NFATc3–P-gp signaling cascade in P-gp induction in drug-resistant cancer cells166. | |
| Pharmacological inhibition and gene-silencing show the prosurvival autophagy via calmodulin-dependent protein kinase kinase β/AMP-activated protein kinase α/mTOR (CaMKKβ/AMPKα/mTOR) pathway. Moreover, adriamycin-resistant MCF-7/ADM cells with high basal autophagic level could be silenced via inhibition of autophagy to counterbalance the resistance to adriamycin167. | |||
| TRPV2 | TRPV2 silencing in glioma cells is associated with FAS-induced apoptosis dependent on ERK activation. Furthermore, inhibition of ERK activation by the specific MAPK inhibitor of PD98-59 decrease the BCL-XL protein levels, and facilitate the FAS expression, restoring the AKT/PKB leading to the U87MG cell survival and proliferation, and elevated the sensitivity to Fas-induced apoptosis168. | ||
| Doxorubicin-mediated cell death is considerably prominent (smaller IC50 value) with the usage of CBD. Thus, CBD might promote doxorubicin-mediated cell death via the elevated entry of doxorubicin through TRPV2 channels to inhibit the clearance by suppression of P-glycoprotein ATPase transporter169. | |||
| Epirubicin | TRPM8 | TRPM8 is over-expression in human osteosarcoma with impaired regulation of intracellular calcium concentration. Thereafter, the AKT-glycogen synthase kinase-3β (GSK-3β) pathway and the suppression of phosphorylation of P44/P42, ERK1/2 and FAK. Knockdown of TRPM8 not only negatively influences the cell proliferation and metastasis but also enhances epirubicin-induced cell apoptosis170. | |
| Bortezomib | TRPV2 | The enhancement of TRPV2-induced glioma stem-like cells (GSCs) differentiation coincide with their proliferation. Inhibition of the balance between proliferation and differentiation of GSCs would lead to more specific and efficacious pharmacological approaches171. | |
| Dacarbazine | TRPA1 | TRPA1 is directly activated by dacarbazine and sensitize TRPA1 via elevated oxidative stress. Meanwhile, the nociception was attenuated by the TRPA1 antagonist or its ablation172. | |
| Gene therapy | miR-320a | TRPC5 | The transcription factor of erythroblastosis virus E26 oncogene homolog 1 (ETS-1) inhibited miR-320a expression with activated hypo-methylation of the ETS-1 promoter. Also, the down-regulation of miR-320a and high expression of TRPC5, NFATc3, and ETS-1 were verified in clinically chemo-resistant samples173. |
| Hormone | Tamoxifen | TRPV6 | TRPV6 can be modified by hormones such as estrogen, progesterone with a profound influence on the breast cancer proliferation. TRPV6 would be a novel target for calcium channel inhibitors to treat breast adenocarcinoma174. |
| Androgen | TRPV2 | PCa is dependent on TRPV2 for migration and invasion with enhanced MMP9 and cathepsin B. TRPV2 is the potential prognostic marker for PCa175. | |
| Estrogen | TRPV6 | In estrogen receptor (ER)-negative breast cancer cells, increased TRPV6 expression is a distinctive characteristic of human epidermal growth factor receptor 2 positive (HER-2+) cells. Patients with high TRPV6 expression has lower survival rate when compared with their counterparts with lower TRPV6 expression176. |
5.1. TRPC channels
The luminal A ER+ breast cancer cell lines such as MCF7 and triple-negative breast cancer (TNBC) MDA-MB-231 have overexpression of TRPC6 when compared with the epithelial breast cell line of MCF10A. For cellular proliferation and invasion that TRPC6 takes part in, the physiological and pathological functions were uniquely and considerably diminished in the tumoral cell lines. The proper intervention of TRPC6 would adjust the calcium cation hemostasis state in breast cancer177. Non-selective TRPC channel blockers SKF96365, MRS1845 and 2-aminoethoxydiphenyl borate (2-APB) suppressed cell proliferation and cytokinesis in D54MG glioblastoma cells27. TRPC1 channels promote the proliferation of human malignant gliomas intrinsically. Suppression of TRPC1 via shRNA or SKF96365 in D54MG glioma cells inhibits cell proliferation and incomplete cell division. In vivo results also indicated that shRNA suppression of TRPC1 expression reduced flank tumor size. In the D54MG glioma cells, shRNA knockdown comprised the EGF gradient-dependent chemo-tactical migration. Caveolar lipid rafts mark the TRPC1 channels, associating with cholesterol exhaustion, mediating glioma chemotactic metastasis, which could be the target by pharmacological inhibitors for TRPC channels178. SKF-96365 is an imidazole compound belonging to antimycotics that could inhibit both CRACs and some TRP channels. Initially, in human blood cells such as platelets, neutrophils, and endothelial cells, this chemical substance suppresses receptor-mediated Ca2+ entry, while before long, TRPCs blockade makes this compound suppress ovarian cancer cell growth and tumorigenesis to a larger extent27.
In CNE2 nasopharyngeal tumor cells, siRNA-mediated silencing downregulates the TRPC1 and significantly attenuated the adhesive and invasive abilities, indicating the efficacy of suppressing TRPC1 would be a potential approach of tumor therapy179. Overexpression of TRPC3 increment was observed in breast cancer cells. A specific TRPC3 blocker Pyr3 and dominant negative TRPC3 reduced proliferation, induced apoptosis and sensitized cell death to chemotherapeutic agents29.
5.2. TRPA channels
For the oxidative stress-dependent TRPA1, anti-cancer treatments with chemicals like bortezomib, oxaliplatin, cisplatin, and paclitaxel induces severe cold and mechanical allodynia. Further, blockade of TRPA1 alleviated cold and mechanical allodynia induced by anti-cancer chemicals like paclitaxel or oxaliplatin180. For the pro-inflammatory/proalgesic effects, TRPA1 also serves as the keynote mediator when the three aromatase inhibitors as exemestane, letrozole, and anastrozole, are used in breast cancer therapy181. In addition, TRPA1 has been reported to promote resistance to ROS-producing chemotherapies, TRPA1 inhibitor AM-0902 showed a significant inhibition of xenograft tumor growth and enhanced chemosensitivity23.
5.3. TRPV channels
TH-1177 was then modified to own higher specificity for TRPV6 as the primary category in cancer compared with TRPV5, and this novel chemical compound has been further demonstrated to be more efficient in combating prostate and breast cancer182. TRPV4 channel regulates the vessel maturation and angiogenesis in tumors, and GSK1016790A is its agonist of best activity with over hundreds folds potency than the routine clinical application of 4α-PDD. Combined usage of GSK1016790A with other anti-tumor reagents such as cisplatin infiltrated the tumor tissues with more depth. Melanoma A2058 and A375 cells showed decreased viability by TRPV2 and TRPV4 agonists, which would lead to cellular apoptosis or necrosis183. Soricimed-C13 (SOR-C13) and SOR-C27 are two TRPV6 inhibitors that resembling the C-terminus of soricidin analog, and they demonstrate competitive affinity in ovarian cancer cells. TRPV6 channel overexpression has been applied in phase I clinical trial for advanced cancer184. Resiniferatoxin (RTX), as a potent TRPV1 activator, is applied to own a permanent analgesic effect via the impairment of the signaling potential of susceptible sensory neurons185. RTX administration witnessed the fantastic decline in the pain behaviors in the wake of RTX intrathecal administration within a variety of species, including canine with osteosarcoma-induced pain186. The TRPM8 antagonists would induce probable hypothermia in the patients. The identification and optimization of those antagonists could make the PF-05105679 undergo clinical trials with ensured efficacy in one test akin to the opioid medication derivate oxycodone for severe pain alleviation187,188.
When it comes to malignant tumors, TRPV channels differentially contribute to diversified breast cancer, the specific activators as well as inhibitors of TRPV4 and TRPV6 channels would contribute to exert therapeutic effects to combat breast cancer. TRPV channels have mutating genetic modifications such as TRPV4 of S823D, S824D, and T813D, and TRPV6 of S691D, S692D, and T702. The mutant has more potent invasiveness in colon adenocarcinoma SW480 cells. S692D and T702D mutants show elevated mutant channel activity with TRPV6 rather than their TRPV4 counterparts. Thus, varied mechanisms would converge into the identical goal to the global effect in the deleterious phenotype. This highlights the potential importance of searching for TRP mutations involved in cancer189. On the contrary, shRNA targeting TRPV4 caused the knockdown of TRPV4 and inhibited AA-generated BTECs migration; within this process, AA-induced intracellular calcium entry has a larger extent of migration instead of non-migrating BTECs since the TRPV4 expression in the cellular surface overexpression by a mere exposure of AA itself80. Overexpression of TRPV1 ion channel can promote cisplatin to take part in the esophageal squamous cell carcinoma (ESCC) chemotherapy via inhibition tumor cells proliferation190.
5.4. TRPM channels
D-3263 is a TRPM8 activator and induces cancer cell apoptosis, which is a proper candidate for patients with solid tumors. For gastric cells, TRPM2 knockdown suppressed cellular proliferation, resulting in apoptosis and mitochondrial dysfunction. Within this process, ATP generation along with autophagy was attenuated, which would be an ensuing target for tumor therapy. As for human prostate cancer cells of DU145 and PC3, resveratrol diminished TRPM7 expression and function as well as alleviated TGF β-induced cell migration2. Another feature that might underpin the primary and secondary resistance to anti-VEGF drugs, TRPC1 (and SOCE) inhibition, could represent a promising strategy to interfere with tumor vascularization191.
Apart from the conventional drugs, proper interference of the TRP channel would be an appropriate approach to perform as the means to combat cancer. Prostate patients presented higher gene expression of TRPM4. The knockdown of the TRPM4 channel would deactivate the migration rather than proliferation in the prostate cells such as DU145 and PC3 cells192. The silencing of TRPM7 via proper knockdown of MDA-MB-231 or MDA-MB-435 in breast cancer cells would enhance the contractility and the amount of focal adhesions, which greatly diminishes the migratory and invasive potentials74.
To summarize, both small molecular drugs and gene delivery-associated therapy are also efficient and novel approaches to target the therapy of tumors.
6. Overall conclusions and future directions
Nowadays, a transition of tumor therapy from conventional surgery, chemotherapy, and radiation to novel immune therapy targeted therapy and ion channel therapy. This makes us contemplate that TRP would be a potent therapeutic target of tumor therapy. TRP channels resemble promising targets for manipulations via pharmacological or genetic perturbation. One vital challenge is to test the appropriate kinetics of TRP channels as long-term silencing or suppression of calcium channels might be deleterious to the cells. Tough as those challenges, the insight that TRP channels make contributions to have the interior remodeling in the immune effector cells, which might own the potential to discover the targets in cancer immunotherapy.
What comes manifest is that the disruption of calcium homoeostasis is essential for the generation and progression of malignant tumors, playing a role as the driving force of the acquirement of aggressive phenotype. The alteration of expression levels of transporters and ion channels regulate the intracellular concentrations of calcium both in pro- and post-survival circumstance. Evidence also shows the chemotherapeutic and genetic sensitivity and potency have been considerably modified as the potential limitation in the adoption and usage of therapeutic regimens nowadays, creating a tremendous challenge of tumor therapy such as drug tolerance. Thus, therapeutic regimens and several efforts are spent to overcome it. As a result, aiming at the TRP expression and activity, should be taken into account as the promising and fascinating strategy.
Moreover, the ability that animals have to substitute a silenced TRP channel so as to supply the required cation influx to meet the physiological functions would be passed down by those tumor cells, making an arduous task ahead of the scientific community. Thus, the advent of certain specific inhibitors, as well as the natural compounds with anti-tumor efficacy without doing harm to the non-tumoral cells, would be academia's other focus. What's beneficial for us is that the characterization of TRP structures sheds light on a great terrain for the discovery of novel antagonists to amiloride the therapeutic approaches nowadays whilst alleviate the hazardous effects of those treatments. Latest scientific news reported that American scientists David Julius and Ardem Patapoutian won the 2021 Nobel Prize for Physiology or Medicine, and their work was mainly on the discovery of the cellular receptors such as PIEZO and TRP channels in the skin, sensing temperature and touch which could pave the way for new pain-killers, indicating TRP channels would be one hotspot in the future research directions, especially for cancer treatment and the alleviation of the tumor-induced intolerable pain.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81930102 to Bo Yang) and the National Natural Science Foundation of China (No. 81773754 to Ling Ding).
Author contributions
Ling Ding and Bo Yang conceived the review topic. Tiecheng Zhong drafted and edited the manuscript. Wenxin Zhang, Hongjie Guo, Xiaohui Pan, Xi Chen and Qiaojun He helped revised the manuscript.
Conflicts of interest
The authors declare that this review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
Contributor Information
Bo Yang, Email: yang924@zju.edu.cn.
Ling Ding, Email: ld362@zju.edu.cn.
References
- 1.Villalta P.C., Rocic P., Townsley M.I. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L652–L659. doi: 10.1152/ajplung.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong T., Pan X., Wang J., Yang B., Ding L. The regulatory roles of calcium channels in tumors. Biochem Pharmacol. 2019;169:113603. doi: 10.1016/j.bcp.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Gees M., Colsoul B., Nilius B. The role of transient receptor potential cationchannels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Yuan P. Structural biology of thermoTRPV channels. Cell Calcium. 2019;84:102106. doi: 10.1016/j.ceca.2019.102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R., Bansal Y., Parhar I., Kuhad A., Soga T. Neuropsychiatric implications of transient receptor potential vanilloid (TRPV) channels in the reward system. Neurochem Int. 2019;131:104545. doi: 10.1016/j.neuint.2019.104545. [DOI] [PubMed] [Google Scholar]
- 7.Bevan S., Quallo T., Andersson D.A. TRPV1. Handb Exp Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Elias A., Mrkonjic S., Jung C., Pardo-Pastor C., Vicente R., Valverde M.A. The TRPV4 channel. Handb Exp Pharmacol. 2014;222:293–319. doi: 10.1007/978-3-642-54215-2_12. [DOI] [PubMed] [Google Scholar]
- 9.Clapham D.E., Runnels L.W., Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 10.Chen X.J., Sooch G., Demaree I.S., White F.A., Obukhov A.G. Transient receptor potential canonical (TRPC) channels: then and now. Cells. 2020;9:1983. doi: 10.3390/cells9091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launay P., Fleig A., Perraud A.L., Scharenberg A.M., Penner R., Kinet J.P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 12.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 13.Nadler M.J., Hermosura M.C., Inabe K., Perraud A.L., Zhu Q., Stokes A.J., et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 14.Fleig A., Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Lavanderos B., Silva I., Cruz P., Orellana-Serradell O., Saldias M.P., Cerda O. TRP channels regulation of Rho GTPases in brain context and diseases. Front Cell Dev Biol. 2020;8:582975. doi: 10.3389/fcell.2020.582975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cvetkov T.L., Huynh K.W., Cohen M.R., Moiseenkova-Bell V.Y. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J Biol Chem. 2011;286:38168–38176. doi: 10.1074/jbc.M111.288993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatachalam K., Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clapham D.E. Structural biology: pain-sensing TRPA1 channel resolved. Nature. 2015;520:439–441. doi: 10.1038/nature14383. [DOI] [PubMed] [Google Scholar]
- 19.Hofherr A., Kottgen M. TRPP channels and polycystins. Adv Exp Med Biol. 2011;704:287–313. doi: 10.1007/978-94-007-0265-3_16. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Moon S., Cha Y., Chung Y.D. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn-Schmiedeberg’s Arch Pharmacol. 2005;371:307–314. doi: 10.1007/s00210-005-1034-x. [DOI] [PubMed] [Google Scholar]
- 22.Puertollano R., Kiselyov K. TRPMLs: in sickness and in health. Am J Physiol Ren Physiol. 2009;296:F1245–F1254. doi: 10.1152/ajprenal.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi N., Chen H.Y., Harris I.S., Stover D.G., Selfors L.M., Bronson R.T., et al. Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell. 2018;33:985–1003.e7. doi: 10.1016/j.ccell.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu S., Hirte H., Welch S., Ilenchuk T.T., Lutes T., Rice C., et al. First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Invest N Drugs. 2017;35:324–333. doi: 10.1007/s10637-017-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer E.A., Stohr S., Meister M., Aigner A., Gudermann T., Buech T.R. Stimulation of the chemosensory TRPA1 cation channel by volatile toxic substances promotes cell survival of small cell lung cancer cells. Biochem Pharmacol. 2013;85:426–438. doi: 10.1016/j.bcp.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Elzamzamy O.M., Penner R., Hazlehurst L.A. The role of TRPC1 in modulating cancer progression. Cells. 2020;9:388. doi: 10.3390/cells9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bomben V.C., Sontheimer H. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia. 2010;58:1145–1156. doi: 10.1002/glia.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang S.L., Cao Q., Zhou K.C., Feng Y.J., Wang Y.Z. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28:1320–1328. doi: 10.1038/onc.2008.475. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Qi Y.X., Qi Z., Tsang S.Y. TRPC3 regulates the proliferation and apoptosis resistance of triple negative breast cancer cells through the TRPC3/RASA4/MAPK pathway. Cancers (Basel) 2019;11:558. doi: 10.3390/cancers11040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei W.C., Huang W.C., Lin Y.P., Becker E.B.E., Ansorge O., Flockerzi V., et al. Functional expression of calcium-permeable canonical transient receptor potential 4-containing channels promotes migration of medulloblastoma cells. J Physiol. 2017;595:5525–5544. doi: 10.1113/JP274659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z., Zhu Y., Dong Y., Zhang P., Han X., Jin J., et al. Overexpression of TrpC5 promotes tumor metastasis via the HIF-1alpha-Twist signaling pathway in colon cancer. Clin Sci (Lond) 2017;131:2439–2450. doi: 10.1042/CS20171069. [DOI] [PubMed] [Google Scholar]
- 32.He D.X., Ma X. Transient receptor potential channel C5 in cancer chemoresistance. Acta Pharmacol Sin. 2016;37:19–24. doi: 10.1038/aps.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldias M.P., Maureira D., Orellana-Serradell O., Silva I., Lavanderos B., Cruz P., et al. TRP channels interactome as a novel therapeutic target in breast cancer. Front Oncol. 2021;11:621614. doi: 10.3389/fonc.2021.621614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding X., He Z., Zhou K., Cheng J., Yao H., Lu D., et al. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst. 2010;102:1052–1068. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- 35.Chigurupati S., Venkataraman R., Barrera D., Naganathan A., Madan M., Paul L., et al. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res. 2010;70:418–427. doi: 10.1158/0008-5472.CAN-09-2654. [DOI] [PubMed] [Google Scholar]
- 36.Miller B.A. TRPM2 in cancer. Cell Calcium. 2019;80:8–17. doi: 10.1016/j.ceca.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S.J., Hoffman N.E., Shanmughapriya S., Bao L., Keefer K., Conrad K., et al. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2alpha. J Biol Chem. 2014;289:36284–36302. doi: 10.1074/jbc.M114.620922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klumpp D., Misovic M., Szteyn K., Shumilina E., Rudner J., Huber S.M. Targeting TRPM2 channels impairs radiation-induced cell cycle arrest and fosters cell death of T cell leukemia cells in a Bcl-2-dependent manner. Oxid Med Cell Longev. 2016;2016:8026702. doi: 10.1155/2016/8026702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Togashi K., Inada H., Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br J Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borgstrom A., Hauert B., Kappel S., Zoni E., Kiener M., Stoklosa P., et al. Small molecular inhibitors block TRPM4 currents in prostate cancer cells, with limited impact on cancer hallmark functions. J Mol Biol. 2021;433:166665. doi: 10.1016/j.jmb.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 41.Sterea A.M., Egom E.E., El Hiani Y. TRP channels in gastric cancer: new hopes and clinical perspectives. Cell Calcium. 2019;82:102053. doi: 10.1016/j.ceca.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Kim B.J. Involvement of melastatin type transient receptor potential 7 channels in ginsenoside Rd-induced apoptosis in gastric and breast cancer cells. J Ginseng Res. 2013;37:201–209. doi: 10.5142/jgr.2013.37.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zierler S., Yao G., Zhang Z., Kuo W.C., Porzgen P., Penner R., et al. Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem. 2011;286:39328–39335. doi: 10.1074/jbc.M111.264341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chodon D., Guilbert A., Dhennin-Duthille I., Gautier M., Telliez M.S., Sevestre H., et al. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer. 2010;10:212. doi: 10.1186/1471-2407-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Mikrani R., He Y., Faran Ashraf Baig M.M., Abbas M., Naveed M., et al. TRPM8 channels: a review of distribution and clinical role. Eur J Pharmacol. 2020;882:173312. doi: 10.1016/j.ejphar.2020.173312. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L., Barritt G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto Y., Ohkubo T., Ikebe T., Yamazaki J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int J Oncol. 2012;40:1431–1440. doi: 10.3892/ijo.2012.1340. [DOI] [PubMed] [Google Scholar]
- 48.Beck B., Bidaux G., Bavencoffe A., Lemonnier L., Thebault S., Shuba Y., et al. Prospects for prostate cancer imaging and therapy using high-affinity TRPM8 activators. Cell Calcium. 2007;41:285–294. doi: 10.1016/j.ceca.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Jung J., Cho K.J., Naji A.K., Clemons K.N., Wong C.O., Villanueva M., et al. HRAS-driven cancer cells are vulnerable to TRPML1 inhibition. EMBO Rep. 2019;20 doi: 10.15252/embr.201846685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morelli M.B., Amantini C., Tomassoni D., Nabissi M., Arcella A., Santoni G. Transient receptor potential mucolipin-1 channels in glioblastoma: role in patient's survival. Cancers (Basel) 2019;11:525. doi: 10.3390/cancers11040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van den Eynde C., De Clercq K., Vriens J. Transient receptor potential channels in the epithelial-to-mesenchymal transition. Int J Mol Sci. 2021;22:8188. doi: 10.3390/ijms22158188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu T.T., Peters A.A., Tan P.T., Roberts-Thomson S.J., Monteith G.R. Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium. 2014;56:59–67. doi: 10.1016/j.ceca.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Bode A.M., Cho Y.Y., Zheng D., Zhu F., Ericson M.E., Ma W.Y., et al. Transient receptor potential type vanilloid 1 suppresses skin carcinogenesis. Cancer Res. 2009;69:905–913. doi: 10.1158/0008-5472.CAN-08-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amantini C., Mosca M., Nabissi M., Lucciarini R., Caprodossi S., Arcella A., et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 55.Stock K., Kumar J., Synowitz M., Petrosino S., Imperatore R., Smith E.S., et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat Med. 2012;18:1232–1238. doi: 10.1038/nm.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santoni G., Amantini C., Maggi F., Marinelli O., Santoni M., Nabissi M., et al. The TRPV2 cation channels: from urothelial cancer invasiveness to glioblastoma multiforme interactome signature. Lab Invest. 2020;100:186–198. doi: 10.1038/s41374-019-0333-7. [DOI] [PubMed] [Google Scholar]
- 57.Guzman M., Duarte M.J., Blazquez C., Ravina J., Rosa M.C., Galve-Roperh I., et al. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95:197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chinigo G., Fiorio Pla A., Gkika D. TRP channels and small GTPases interplay in the main hallmarks of metastatic cancer. Front Pharmacol. 2020;11:581455. doi: 10.3389/fphar.2020.581455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie R., Xu J., Xiao Y., Wu J., Wan H., Tang B., et al. Calcium promotes human gastric cancer via a novel coupling of calcium-sensing receptor and TRPV4 channel. Cancer Res. 2017;77:6499–6512. doi: 10.1158/0008-5472.CAN-17-0360. [DOI] [PubMed] [Google Scholar]
- 60.Tang B., Wu J., Zhu M.X., Sun X., Liu J., Xie R., et al. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene. 2019;38:3946–3961. doi: 10.1038/s41388-019-0709-6. [DOI] [PubMed] [Google Scholar]
- 61.Lawhorn B.G., Brnardic E.J., Behm D.J. TRPV4 antagonists: a patent review (2015–2020) Expert Opin Ther Pat. 2021;31:773–784. doi: 10.1080/13543776.2021.1903432. [DOI] [PubMed] [Google Scholar]
- 62.Stewart J.M. TRPV6 as A target for cancer therapy. J Cancer. 2020;11:374–387. doi: 10.7150/jca.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowen C.V., DeBay D., Ewart H.S., Gallant P., Gormley S., Ilenchuk T.T., et al. In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.SheikhBahaei S. Physiology: new insights into central oxygen sensing. Curr Biol. 2020;30:R1004–R1006. doi: 10.1016/j.cub.2020.06.101. [DOI] [PubMed] [Google Scholar]
- 65.Deveci H.A., Akyuva Y., Nur G., Naziroglu M. Alpha lipoic acid attenuates hypoxia-induced apoptosis, inflammation and mitochondrial oxidative stress via inhibition of TRPA1 channel in human glioblastoma cell line. Biomed Pharmacother. 2019;111:292–304. doi: 10.1016/j.biopha.2018.12.077. [DOI] [PubMed] [Google Scholar]
- 66.Uchiyama M., Nakao A., Kurita Y., Fukushi I., Takeda K., Numata T., et al. O2-dependent protein internalization underlies astrocytic sensing of acute hypoxia by restricting multimodal TRPA1 channel responses. Curr Biol. 2020;30:3378–3396.e7. doi: 10.1016/j.cub.2020.06.047. [DOI] [PubMed] [Google Scholar]
- 67.Bao L., Chen S.J., Conrad K., Keefer K., Abraham T., Lee J.P., et al. Depletion of the human ion channel TRPM2 in neuroblastoma demonstrates its key role in cell survival through modulation of mitochondrial reactive oxygen species and bioenergetics. J Biol Chem. 2016;291:24449–24464. doi: 10.1074/jbc.M116.747147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S., Wang J., Wei Y., Liu Y., Ding X., Dong B., et al. Crucial role of TRPC6 in maintaining the stability of HIF-1alpha in glioma cells under hypoxia. J Cell Sci. 2015;128:3317–3329. doi: 10.1242/jcs.173161. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi N., Kuwaki T., Kiyonaka S., Numata T., Kozai D., Mizuno Y., et al. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- 70.Riffle S., Hegde R.S. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J Exp Clin Cancer Res. 2017;36:102. doi: 10.1186/s13046-017-0570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chay D.B., Han G.H., Cho H., Kim S., Kim J.H. High expression of TRPV-1 is associated with poor prognosis in cervical cancer. Gynecol Oncol. 2019;154:270. [Google Scholar]
- 72.Howe A.K. Cross-talk between calcium and protein kinase A in the regulation of cell migration. Curr Opin Cell Biol. 2011;23:554–561. doi: 10.1016/j.ceb.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen W.L., Barszczyk A., Turlova E., Deurloo M., Liu B., Yang B.B., et al. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget. 2015;6:16321–16340. doi: 10.18632/oncotarget.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Middelbeek J., Kuipers A.J., Henneman L., Visser D., Eidhof I., van Horssen R., et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–4261. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- 75.Clark K., Langeslag M., van Leeuwen B., Ran L., Ryazanov A.G., Figdor C.G., et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez J.J., Salido G.M., Pariente J.A., Rosado J.A. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 77.Adapala R.K., Thoppil R.J., Ghosh K., Cappelli H.C., Dudley A.C., Paruchuri S., et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35:314–322. doi: 10.1038/onc.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee W.H., Choong L.Y., Mon N.N., Lu S., Lin Q., Pang B., et al. TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci Rep. 2016;6:27903. doi: 10.1038/srep27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomi S., Nakada T., Kashihara T., Ikeda U., Yamaha M. TRPM4 channels mediate hypertonicity-induced, Ca2+-impermeable, non-selective cation currents in a cervical cancer cell cine, HeLa cells. Shinshu Med J. 2014;62:33–34. [Google Scholar]
- 80.Fiorio Pla A., Ong H.L., Cheng K.T., Brossa A., Bussolati B., Lockwich T., et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31:200–212. doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernardini M., Brossa A., Chinigo G., Grolez G.P., Trimaglio G., Allart L., et al. Transient receptor potential channel expression signatures in tumor-derived endothelial cells: functional roles in prostate cancer angiogenesis. Cancers (Basel) 2019;11:956. doi: 10.3390/cancers11070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lodola F., Laforenza U., Bonetti E., Lim D., Dragoni S., Bottino C., et al. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Genova T., Grolez G.P., Camillo C., Bernardini M., Bokhobza A., Richard E., et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J Cell Biol. 2017;216:2107–2130. doi: 10.1083/jcb.201506024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Z., Ren L., Zhao Q., Lu G., Ren M., Lu X., et al. TRPC1 exacerbate metastasis in gastric cancer via ciRS-7/miR-135a-5p/TRPC1 axis. Biochem Biophys Res Commun. 2020;529:85–90. doi: 10.1016/j.bbrc.2020.05.181. [DOI] [PubMed] [Google Scholar]
- 85.Moccia F. Endothelial Ca2+ signaling and the resistance to anticancer treatments: partners in crime. Int J Mol Sci. 2018;19:217. doi: 10.3390/ijms19010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Persoons E., Hennes A., De Clercq K., Van Bree R., Vriens G.,O.D.F., et al. Functional expression of TRP ion channels in endometrial stromal cells of endometriosis patients. Int J Mol Sci. 2018;19:2467. doi: 10.3390/ijms19092467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fiorio Pla A., Grange C., Antoniotti S., Tomatis C., Merlino A., Bussolati B., et al. Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol Cancer Res. 2008;6:535–545. doi: 10.1158/1541-7786.MCR-07-0271. [DOI] [PubMed] [Google Scholar]
- 88.Cappelli H.C., Kanugula A.K., Adapala R.K., Amin V., Sharma P., Midha P., et al. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer Lett. 2019;442:15–20. doi: 10.1016/j.canlet.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feske S., Wulff H., Skolnik E.Y. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parenti A., De Logu F., Geppetti P., Benemei S. What is the evidence for the role of TRP channels in inflammatory and immune cells?. Br J Pharmacol. 2016;173:953–969. doi: 10.1111/bph.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santoni G., Cardinali C., Morelli M.B., Santoni M., Nabissi M., Amantini C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation. 2015;12:21. doi: 10.1186/s12974-015-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loo S.K., Ch'ng E.S., Md Salleh M.S., Banham A.H., Pedersen L.M., Moller M.B., et al. TRPM4 expression is associated with activated B cell subtype and poor survival in diffuse large B cell lymphoma. Histopathology. 2017;71:98–111. doi: 10.1111/his.13204. [DOI] [PubMed] [Google Scholar]
- 93.Beceiro S., Radin J.N., Chatuvedi R., Piazuelo M.B., Horvarth D.J., Cortado H., et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017;10:493–507. doi: 10.1038/mi.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schilling T., Miralles F., Eder C. TRPM7 regulates proliferation and polarisation of macrophages. J Cell Sci. 2014;127:4561–4566. doi: 10.1242/jcs.151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fang L., Zhan S., Huang C., Cheng X., Lv X., Si H., et al. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol Appl Pharmacol. 2013;272:713–725. doi: 10.1016/j.taap.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 96.Zhu Y., Men R., Wen M., Hu X., Liu X., Yang L. Blockage of TRPM7 channel induces hepatic stellate cell death through endoplasmic reticulum stress-mediated apoptosis. Life Sci. 2014;94:37–44. doi: 10.1016/j.lfs.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 97.Khalil M., Babes A., Lakra R., Forsch S., Reeh P.W., Wirtz S., et al. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol. 2016;9:1500–1513. doi: 10.1038/mi.2016.16. [DOI] [PubMed] [Google Scholar]
- 98.Riazanski V., Gabdoulkhakova A.G., Boynton L.S., Eguchi R.R., Deriy L.V., Hogarth D.K., et al. TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc Natl Acad Sci U S A. 2015;112:E6486–E6495. doi: 10.1073/pnas.1518966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou X., Ye Y., Sun Y., Li X., Wang W., Privratsky B., et al. Transient receptor potential channel 1 deficiency impairs host defense and proinflammatory responses to bacterial infection by regulating protein kinase Cα signaling. Mol Cell Biol. 2015;35:2729–2739. doi: 10.1128/MCB.00256-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kadio B., Yaya S., Basak A., Dje K., Gomes J., Mesenge C. Calcium role in human carcinogenesis: a comprehensive analysis and critical review of literature. Cancer Metastasis Rev. 2016;35:391–411. doi: 10.1007/s10555-016-9634-0. [DOI] [PubMed] [Google Scholar]
- 101.Kemeny A., Kodji X., Horvath S., Komlodi R., Szoke E., Sandor Z., et al. TRPA1 acts in a protective manner in imiquimod-induced psoriasiform dermatitis in mice. J Invest Dermatol. 2018;138:1774–1784. doi: 10.1016/j.jid.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 102.Ogata M., Hino S., Saito A., Morikawa K., Kondo S., Kanemoto S., et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harding H.P., Calfon M., Urano F., Novoa I., Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 104.Kim M.S., Lee K.P., Yang D., Shin D.M., Abramowitz J., Kiyonaka S., et al. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115.e4. doi: 10.1053/j.gastro.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Timmins J.M., Ozcan L., Seimon T.A., Li G., Malagelada C., Backs J., et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smedlund K., Tano J.Y., Vazquez G. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ Res. 2010;106:1479–1488. doi: 10.1161/CIRCRESAHA.109.213314. [DOI] [PubMed] [Google Scholar]
- 107.Chen S., He F.F., Wang H., Fang Z., Shao N., Tian X.J., et al. Calcium entry via TRPC6 mediates albumin overload-induced endoplasmic reticulum stress and apoptosis in podocytes. Cell Calcium. 2011;50:523–529. doi: 10.1016/j.ceca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 108.Thomas K.C., Sabnis A.S., Johansen M.E., Lanza D.L., Moos P.J., Yost G.S., et al. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J Pharmacol Exp Ther. 2007;321:830–838. doi: 10.1124/jpet.107.119412. [DOI] [PubMed] [Google Scholar]
- 109.Schroder M., Kaufman R.J. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 110.Thomas K.C., Roberts J.K., Deering-Rice C.E., Romero E.G., Dull R.O., Lee J., et al. Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L111–L119. doi: 10.1152/ajplung.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gallego-Sandin S., Rodriguez-Garcia A., Alonso M.T., Garcia-Sancho J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J Biol Chem. 2009;284:32591–32601. doi: 10.1074/jbc.M109.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fang Y., Liu G., Xie C., Qian K., Lei X., Liu Q., et al. Pharmacological inhibition of TRPV4 channel suppresses malignant biological behavior of hepatocellular carcinoma via modulation of ERK signaling pathway. Biomed Pharmacother. 2018;101:910–919. doi: 10.1016/j.biopha.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 113.El Boustany C., Bidaux G., Enfissi A., Delcourt P., Prevarskaya N., Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47:2068–2077. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- 114.Olivan-Viguera A., Garcia-Otin A.L., Lozano-Gerona J., Abarca-Lachen E., Garcia-Malinis A.J., Hamilton K.L., et al. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moriuchi M., Nakano Y., Tsurekawa Y., Piruzyan M., Matsuyama S., Nohara H., et al. Taurine inhibits TRPV-dependent activity to overcome oxidative stress in Caenorhabditis elegans. Biol Pharm Bull. 2018;41:1672–1677. doi: 10.1248/bpb.b18-00370. [DOI] [PubMed] [Google Scholar]
- 116.El Hiani Y., Ahidouch A., Roudbaraki M., Guenin S., Brule G., Ouadid-Ahidouch H. Calcium-sensing receptor stimulation induces nonselective cation channel activation in breast cancer cells. J Membr Biol. 2006;211:127–137. doi: 10.1007/s00232-006-0017-2. [DOI] [PubMed] [Google Scholar]
- 117.Rodland K.D. The role of the calcium-sensing receptor in cancer. Cell Calcium. 2004;35:291–295. doi: 10.1016/j.ceca.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 118.Gao Y., Liao P. TRPM4 channel and cancer. Cancer Lett. 2019;454:66–69. doi: 10.1016/j.canlet.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 119.Song Y., Zhan L., Yu M., Huang C., Meng X., Ma T., et al. TRPV4 channel inhibits TGF-beta1-induced proliferation of hepatic stellate cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dou C., Liu Z., Tu K., Zhang H., Chen C., Yaqoob U., et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology. 2018;154:2209–2221.e14. doi: 10.1053/j.gastro.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ohashi K., Deyashiki A., Miyake T., Nagayasu K., Shibasaki K., Shirakawa H., et al. TRPV4 is functionally expressed in oligodendrocyte precursor cells and increases their proliferation. Pflügers Archiv. 2018;470:705–716. doi: 10.1007/s00424-018-2130-3. [DOI] [PubMed] [Google Scholar]
- 122.Wei C., Wang X., Chen M., Ouyang K., Song L.S., Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Su L.T., Agapito M.A., Li M., Simonson W.T., Huttenlocher A., Habas R., et al. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang G., Cao R., Qian K., Peng T., Yuan L., Chen L., et al. TRPM8 inhibition regulates the proliferation, migration and ROS metabolism of bladder cancer cells. OncoTargets Ther. 2020;13:8825–8835. doi: 10.2147/OTT.S257056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yokota T., Ehlin-Henriksson B., Hansson G.K. Scavenger receptors mediate adhesion of activated B lymphocytes. Exp Cell Res. 1998;239:16–22. doi: 10.1006/excr.1997.3876. [DOI] [PubMed] [Google Scholar]
- 126.Du G.J., Li J.H., Liu W.J., Liu Y.H., Zhao B., Li H.R., et al. The combination of TRPM8 and TRPA1 expression causes an invasive phenotype in lung cancer. Tumour Biol. 2014;35:1251–1261. doi: 10.1007/s13277-013-1167-3. [DOI] [PubMed] [Google Scholar]
- 127.Brabletz T., Kalluri R., Nieto M.A., Weinberg R.A. EMT in cancer. Nat Rev Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 128.Lu Z., Ghosh S., Wang Z., Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 129.Lee W.H., Choong L.Y., Jin T.H., Mon N.N., Chong S., Liew C.S., et al. TRPV4 plays a role in breast cancer cell migration via Ca2+-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis. 2017;6:e338. doi: 10.1038/oncsis.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Janssen D.A., Hoenderop J.G., Jansen K.C., Kemp A.W., Heesakkers J.P., Schalken J.A. The mechanoreceptor TRPV4 is localized in adherence junctions of the human bladder urothelium: a morphological study. J Urol. 2011;186:1121–1127. doi: 10.1016/j.juro.2011.04.107. [DOI] [PubMed] [Google Scholar]
- 131.Nieto M.A., Huang R.Y., Jackson R.A. Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 132.Berrout J., Kyriakopoulou E., Moparthi L., Hogea A.S., Berrout L., Ivan C., et al. TRPA1–FGFR2 binding event is a regulatory oncogenic driver modulated by miRNA-142-3p. Nat Commun. 2017;8:947. doi: 10.1038/s41467-017-00983-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen J.P., Luan Y., You C.X., Chen X.H., Luo R.C., Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca2+ influx. Cell Calcium. 2010;47:425–432. doi: 10.1016/j.ceca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 134.Rybarczyk P., Vanlaeys A., Brassart B., Dhennin-Duthille I., Chatelain D., Sevestre H., et al. The transient receptor potential melastatin 7 channel regulates pancreatic cancer cell invasion through the Hsp90alpha/uPA/MMP2 pathway. Neoplasia. 2017;19:288–300. doi: 10.1016/j.neo.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Almasi S., Sterea A.M., Fernando W., Clements D.R., Marcato P., Hoskin D.W., et al. TRPM2 ion channel promotes gastric cancer migration, invasion and tumor growth through the AKT signaling pathway. Sci Rep. 2019;9:4182. doi: 10.1038/s41598-019-40330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu L., Wu N., Wang Y., Zhang X., Xia B., Tang J., et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J Exp Clin Cancer Res. 2019;38:106. doi: 10.1186/s13046-019-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fabian A., Fortmann T., Dieterich P., Riethmuller C., Schon P., Mally S., et al. TRPC1 channels regulate directionality of migrating cells. Pflügers Archiv. 2008;457:475–484. doi: 10.1007/s00424-008-0515-4. [DOI] [PubMed] [Google Scholar]
- 138.Bomben V.C., Turner K.L., Barclay T.T., Sontheimer H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J Cell Physiol. 2011;226:1879–1888. doi: 10.1002/jcp.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nesin V., Tsiokas L. Trpc1. Handb Exp Pharmacol. 2014;222:15–51. doi: 10.1007/978-3-642-54215-2_2. [DOI] [PubMed] [Google Scholar]
- 140.Wildi-Runge S., Stoppa-Vaucher S., Lambert R., Turpin S., Van Vliet G., Deladoey J. A high prevalence of dual thyroid ectopy in congenital hypothyroidism: evidence for insufficient signaling gradients during embryonic thyroid migration or for the polyclonal nature of the thyroid gland? J Clin Endocrinol Metab. 2012;97:E978–E981. doi: 10.1210/jc.2011-3156. [DOI] [PubMed] [Google Scholar]
- 141.Storck H., Hild B., Schimmelpfennig S., Sargin S., Nielsen N., Zaccagnino A., et al. Ion channels in control of pancreatic stellate cell migration. Oncotarget. 2017;8:769–784. doi: 10.18632/oncotarget.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oda K., Umemura M., Nakakaji R., Tanaka R., Sato I., Nagasako A., et al. Transient receptor potential cation 3 channel regulates melanoma proliferation and migration. J Physiol Sci. 2017;67:497–505. doi: 10.1007/s12576-016-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim J.M., Heo K., Choi J., Kim K., An W. The histone variant MacroH2A regulates Ca2+ influx through TRPC3 and TRPC6 channels. Oncogenesis. 2013;2:e77. doi: 10.1038/oncsis.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Asghar M.Y., Magnusson M., Kemppainen K., Sukumaran P., Lof C., Pulli I., et al. Transient receptor potential canonical 1 (TRPC1) channels as regulators of sphingolipid and VEGF receptor expression: implications for thyroid cancer cell migration and proliferation. J Biol Chem. 2015;290:16116–16131. doi: 10.1074/jbc.M115.643668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 146.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 147.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 148.Baker P.J., Boucher D., Bierschenk D., Tebartz C., Whitney P.G., D'Silva D.B., et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015;45:2918–2926. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- 149.Kagan V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Haneklaus M., O'Neill L.A. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 152.Zhong Y., Zhai Y., Liang S., Mori Y., Han R., Sutterwala F., et al. TRPM2 links oxidative stress to the NLRP3 inflammasome activation (P1268) J Immunol. 2013;190:12. [PMC free article] [PubMed] [Google Scholar]
- 153.Wang H., Zhou X., Li H., Qian X., Wang Y., Ma L. Transient receptor potential melastatin 2 negatively regulates LPS-ATP-induced caspase-1-dependent pyroptosis of bone marrow-derived macrophage by modulating ROS production. BioMed Res Int. 2017;2017:2975648. doi: 10.1155/2017/2975648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cai Z., Jitkaew S., Zhao J., Chiang H.C., Choksi S., Liu J., et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Becerra A., Echeverria C., Varela D., Sarmiento D., Armisen R., Nunez-Villena F., et al. Transient receptor potential melastatin 4 inhibition prevents lipopolysaccharide-induced endothelial cell death. Cardiovasc Res. 2011;91:677–684. doi: 10.1093/cvr/cvr135. [DOI] [PubMed] [Google Scholar]
- 156.Maher P., van Leyen K., Dey P.N., Honrath B., Dolga A., Methner A. The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium. 2018;70:47–55. doi: 10.1016/j.ceca.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sander P., Mostafa H., Soboh A., Schneider J.M., Pala A., Baron A.K., et al. Vacquinol-1 inducible cell death in glioblastoma multiforme is counter regulated by TRPM7 activity induced by exogenous ATP. Oncotarget. 2017;8:35124–35137. doi: 10.18632/oncotarget.16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yee N.S., Zhou W., Lee M., Yee R.K. Targeted silencing of TRPM7 ion channel induces replicative senescence and produces enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinoma. Cancer Lett. 2012;318:99–105. doi: 10.1016/j.canlet.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu J., Hu G., Gong Y., Yu Q., He B., Li W., et al. Silencing of TRPM8 inhibits aggressive tumor phenotypes and enhances gemcitabine sensitivity in pancreatic cancer. Pancreatology. 2018;18:935–944. doi: 10.1016/j.pan.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 160.Nur G., Naziroglu M., Deveci H.A. Synergic prooxidant, apoptotic and TRPV1 channel activator effects of alpha-lipoic acid and cisplatin in MCF-7 breast cancer cells. J Recept Signal Transduct Res. 2017;37:569–577. doi: 10.1080/10799893.2017.1369121. [DOI] [PubMed] [Google Scholar]
- 161.Wang T., Chen Z., Zhu Y., Pan Q., Liu Y., Qi X., et al. Inhibition of transient receptor potential channel 5 reverses 5-fluorouracil resistance in human colorectal cancer cells. J Biol Chem. 2015;290:448–456. doi: 10.1074/jbc.M114.590364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Campbell J., Vaghela K., Rogers S., Pyer M., Simon A., Waller J. Promoting prompt help-seeking for symptoms—assessing the impact of a gynaecological cancer leaflet on presentations to primary care: a record-based randomised control trial. BMC Publ Health. 2018;18:997. doi: 10.1186/s12889-018-5920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nabissi M., Morelli M.B., Amantini C., Liberati S., Santoni M., Ricci-Vitiani L., et al. Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner. Int J Cancer. 2015;137:1855–1869. doi: 10.1002/ijc.29573. [DOI] [PubMed] [Google Scholar]
- 164.Ertilav K., Naziroglu M., Ataizi Z.S., Braidy N. Selenium enhances the apoptotic efficacy of docetaxel through activation of TRPM2 channel in DBTRG glioblastoma cells. Neurotox Res. 2019;35:797–808. doi: 10.1007/s12640-019-0009-5. [DOI] [PubMed] [Google Scholar]
- 165.Nabissi M., Morelli M.B., Santoni M., Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34:48–57. doi: 10.1093/carcin/bgs328. [DOI] [PubMed] [Google Scholar]
- 166.Ma X., Cai Y., He D., Zou C., Zhang P., Lo C.Y., et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc Natl Acad Sci U S A. 2012;109:16282–16287. doi: 10.1073/pnas.1202989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang P., Liu X., Li H., Chen Z., Yao X., Jin J., et al. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Sci Rep. 2017;7:3158. doi: 10.1038/s41598-017-03230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nabissi M., Morelli M.B., Amantini C., Farfariello V., Ricci-Vitiani L., Caprodossi S., et al. TRPV2 channel negatively controls glioma cell proliferation and resistance to Fas-induced apoptosis in ERK-dependent manner. Carcinogenesis. 2010;31:794–803. doi: 10.1093/carcin/bgq019. [DOI] [PubMed] [Google Scholar]
- 169.Neumann-Raizel H., Shilo A., Lev S., Mogilevsky M., Katz B., Shneor D., et al. 2-APB and CBD-mediated targeting of charged cytotoxic compounds into tumor cells suggests the involvement of TRPV2 channels. Front Pharmacol. 2019;10:1198. doi: 10.3389/fphar.2019.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang Y., Yang Z., Meng Z., Cao H., Zhu G., Liu T., et al. Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int J Biol Sci. 2013;10:90–102. doi: 10.7150/ijbs.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morelli M.B., Nabissi M., Amantini C., Farfariello V., Ricci-Vitiani L., di Martino S., et al. The transient receptor potential vanilloid-2 cation channel impairs glioblastoma stem-like cell proliferation and promotes differentiation. Int J Cancer. 2012;131:E1067–E1077. doi: 10.1002/ijc.27588. [DOI] [PubMed] [Google Scholar]
- 172.Brusco I., Li Puma S., Chiepe K.B., da Silva Brum E., de David Antoniazzi C.T., de Almeida A.S., et al. Dacarbazine alone or associated with melanoma-bearing cancer pain model induces painful hypersensitivity by TRPA1 activation in mice. Int J Cancer. 2020;146:2797–2809. doi: 10.1002/ijc.32648. [DOI] [PubMed] [Google Scholar]
- 173.He D.X., Gu X.T., Jiang L., Jin J., Ma X. A methylation-based regulatory network for microRNA 320a in chemoresistant breast cancer. Mol Pharmacol. 2014;86:536–547. doi: 10.1124/mol.114.092759. [DOI] [PubMed] [Google Scholar]
- 174.Bolanz K.A., Hediger M.A., Landowski C.P. The role of TRPV6 in breast carcinogenesis. Mol Cancer Therapeut. 2008;7:271–279. doi: 10.1158/1535-7163.MCT-07-0478. [DOI] [PubMed] [Google Scholar]
- 175.Monet M., Lehen'kyi V., Gackiere F., Firlej V., Vandenberghe M., Roudbaraki M., et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–1235. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- 176.Peters A.A., Simpson P.T., Bassett J.J., Lee J.M., Da Silva L., Reid L.E., et al. Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor-negative breast cancer. Mol Cancer Therapeut. 2012;11:2158–2168. doi: 10.1158/1535-7163.MCT-11-0965. [DOI] [PubMed] [Google Scholar]
- 177.Jardin I., Diez-Bello R., Lopez J.J., Redondo P.C., Salido G.M., Smani T., et al. TRPC6 channels are required for proliferation, migration and invasion of breast cancer cell lines by modulation of Orai1 and Orai3 surface exposure. Cancers (Basel) 2018;10:331. doi: 10.3390/cancers10090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maklad A., Sharma A., Azimi I. Calcium signaling in brain cancers: roles and therapeutic targeting. Cancers (Basel) 2019;11:145. doi: 10.3390/cancers11020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.He B., Liu F., Ruan J., Li A., Chen J., Li R., et al. Silencing TRPC1 expression inhibits invasion of CNE2 nasopharyngeal tumor cells. Oncol Rep. 2012;27:1548–1554. doi: 10.3892/or.2012.1695. [DOI] [PubMed] [Google Scholar]
- 180.Nassini R., Gees M., Harrison S., De Siena G., Materazzi S., Moretto N., et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 181.Fusi C., Materazzi S., Benemei S., Coppi E., Trevisan G., Marone I.M., et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun. 2014;5:5736. doi: 10.1038/ncomms6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Landowski C.P., Bolanz K.A., Suzuki Y., Hediger M.A. Chemical inhibitors of the calcium entry channel TRPV6. Pharm Res (N Y) 2011;28:322–330. doi: 10.1007/s11095-010-0249-9. [DOI] [PubMed] [Google Scholar]
- 183.Li L., Chen C., Chiang C., Xiao T., Chen Y., Zhao Y., et al. The impact of TRPV1 on cancer pathogenesis and therapy: a systematic review. Int J Biol Sci. 2021;17:2034–2049. doi: 10.7150/ijbs.59918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xue H., Wang Y., MacCormack T.J., Lutes T., Rice C., Davey M., et al. Inhibition of transient receptor potential vanilloid 6 channel, elevated in human ovarian cancers, reduces tumour growth in a xenograft model. J Cancer. 2018;9:3196–3207. doi: 10.7150/jca.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iadarola M.J., Gonnella G.L. Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain J. 2013;6:95–107. doi: 10.2174/1876386301306010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brown D.C., Agnello K., Iadarola M.J. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain. 2015;156:1018–1024. doi: 10.1097/j.pain.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Andrews M.D., Af Forselles K., Beaumont K., Galan S.R., Glossop P.A., Grenie M., et al. Discovery of a selective TRPM8 antagonist with clinical efficacy in cold-related pain. ACS Med Chem Lett. 2015;6:419–424. doi: 10.1021/ml500479v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Winchester W.J., Gore K., Glatt S., Petit W., Gardiner J.C., Conlon K., et al. Inhibition of TRPM8 channels reduces pain in the cold pressor test in humans. J Pharmacol Exp Ther. 2014;351:259–269. doi: 10.1124/jpet.114.216010. [DOI] [PubMed] [Google Scholar]
- 189.Arbabian A., Iftinca M., Altier C., Singh P.P., Isambert H., Coscoy S. Mutations in calmodulin-binding domains of TRPV4/6 channels confer invasive properties to colon adenocarcinoma cells. Channels (Austin) 2020;14:101–109. doi: 10.1080/19336950.2020.1740506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.OuYang Y., Zhang X., Liu X., Liu X., Cao Y., Qiu J., et al. TRPV 1 regulates the proliferation and cisplatin sensitivity of esophageal cancer. Int J Radiat Oncol Biol Phys. 2020;108 [Google Scholar]
- 191.Dragoni S., Turin I., Laforenza U., Potenza D.M., Bottino C., Glasnov T.N., et al. Store-operated Ca2+ entry does not control proliferation in primary cultures of human metastatic renal cellular carcinoma. BioMed Res Int. 2014;2014:739494. doi: 10.1155/2014/739494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Holzmann C., Kappel S., Kilch T., Jochum M.M., Urban S.K., Jung V., et al. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget. 2015;6:41783–41793. doi: 10.18632/oncotarget.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]