Significance
Harnessing the heterogeneity of the cancer antigenic landscape to induce antitumor T cells is at the basis of personalized vaccines and adoptive T cell–transfer therapies. However, these highly personalized approaches remain associated with limited efficacy against most solid tumors and manufacturing complexities, whose high costs of development and implementation have limited their impact as public health interventions. Using murine cancer models, we report a tumor antigen–agnostic approach based on intratumoral injection of virus-derived peptide epitopes to mobilize vigorous antiviral T cell responses. This strategy does not require the characterization of tumor-associated antigens, and it can mobilize vigorous, cytotoxic T cell responses in the tumor microenvironment and promote epitope spreading against tumor-associated antigens.
Keywords: antiviral immunity, intratumoral immunotherapy, cytomegalovirus, antigen spreading, tumor microenvironment
Abstract
Tumor infiltration by T cells profoundly affects cancer progression and responses to immunotherapy. However, the tumor immunosuppressive microenvironment can impair the induction, trafficking, and local activity of antitumor T cells. Here, we investigated whether intratumoral injection of virus-derived peptide epitopes could activate preexisting antiviral T cell responses locally and promote antitumor responses or antigen spreading. We focused on a mouse model of cytomegalovirus (CMV), a highly prevalent human infection that induces vigorous and durable T cell responses. Mice persistently infected with murine CMV (MCMV) were challenged with lung (TC-1), colon (MC-38), or melanoma (B16-F10) tumor cells. Intratumoral injection of MCMV-derived T cell epitopes triggered in situ and systemic expansion of their cognate, MCMV-specific CD4+ or CD8+ T cells. The MCMV CD8+ T cell epitopes injected alone provoked arrest of tumor growth and some durable remissions. Intratumoral injection of MCMV CD4+ T cell epitopes with polyinosinic acid:polycytidylic acid (pI:C) preferentially elicited tumor antigen–specific CD8+ T cells, promoted tumor clearance, and conferred long-term protection against tumor rechallenge. Notably, secondary proliferation of MCMV-specific CD8+ T cells correlated with better tumor control. Importantly, intratumoral injection of MCMV-derived CD8+ T cell–peptide epitopes alone or CD4+ T cell–peptide epitopes with pI:C induced potent adaptive and innate immune activation of the tumor microenvironment. Thus, CMV-derived peptide epitopes, delivered intratumorally, act as cytotoxic and immunotherapeutic agents to promote immediate tumor control and long-term antitumor immunity that could be used as a stand-alone therapy. The tumor antigen–agnostic nature of this approach makes it applicable across a broad range of solid tumors regardless of their origin.
The advent of high-throughput sequencing has revolutionized our understanding of the genetic and molecular heterogeneity of cancer and provides an opportunity to develop highly personalized cancer diagnostics and therapies (1). Notably, the heterogeneity of the tumor antigenic landscape is reflected by the tumor-specific nature of tumor-associated antigens and mutated neoantigens. Deciphering the tumor antigenic landscape is at the basis of most cancer immunotherapies, such as personalized vaccines or adoptive cell transfer (2–5). Tumor antigen–targeted approaches can result in the diversification of immune responses or antigen spreading against tumor antigens that are not included in the initial treatment (e.g., cancer vaccines) (6). However, personalized tumor antigen–targeted approaches remain associated with limited efficacy against most solid tumors, manufacturing complexities, and high costs of development and implementation, thus limiting their impact as public health interventions.
The tumor immunosuppressive microenvironment in solid tumors negatively affects the induction, trafficking, and local activity of antitumor T cells. Intratumoral (i.t.) delivery of immunomodulating agents has the potential to safely overcome local immunosuppression (7). For example, i.t. delivery of cytokines and chemokines can specifically recruit and activate immune cells in the tumor bed (8). Seminal clinical studies have shown that in situ targeting of nucleic acid sensing pathways can induce durable tumor remission (9, 10). Oncolytic viruses, which have been shown to promote antitumor immunity in preclinical studies (11, 12), constitute another class of local immunotherapy against solid tumors (13, 14). Talimogene laherparepvec (T-Vec), an oncolytic type I herpes simplex virus, has recently been approved as a first-in-class therapy for advanced melanoma; although it is injected directly into some malignant lesions (15), it also promotes clinical responses in distant uninjected tumors (16).
In this study, we investigated a tumor antigen–agnostic approach based on the induction of in situ anamnestic responses of preexisting antiviral T cells by the i.t. injection of virus-derived minimal peptide epitopes, with or without the innate immune modifier polyinosinic acid:polycytidylic acid (pI:C). Cytomegalovirus (CMV), a beta herpes virus, is a promising candidate for implementing this approach. First, human CMV (HCMV) causes predominantly asymptomatic infections and, with 83% estimated global seroprevalence, it is one of the most prevalent human viral infections (17). Second, HCMV infections induce massive, long-term CD8+ and CD4+ T cell responses that increase with age, a phenomenon referred as memory inflation (18), which can result in more than 10% of all circulating CD8+ and CD4+ cells being specific for a limited number of HCMV epitopes in older individuals (19). Third, HCMV-specific T cell responses have been shown to remain functional, even in patients with advanced cancer (20). In this study, we used a murine model of CMV (MCMV) that faithfully recapitulates the hallmarks of latent HCMV infection and associated long-term, functional T cell responses. Specifically, we evaluated whether anti-CMV T cell responses could be harnessed and redirected into tumors to kill cancer cells, cause tumor regression, activate the tumor immune microenvironment, and elicit adaptive antitumor immunity to epitopes not included in the treatment (i.e., antigen spreading) (21).
Results
Inflationary and Noninflationary MCMV–Specific CD8+ T Cells Infiltrate Solid Tumors.
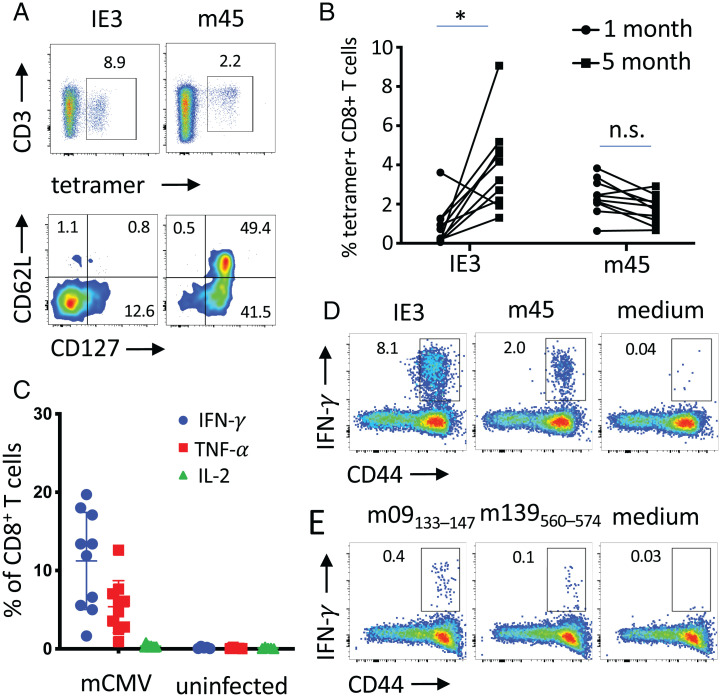
HCMV infection induces polyfunctional CD8+ T cell responses that increase with age and remain active in cancer patients (20). MCMV infection faithfully mimics CMV infection in humans characterized by broad T cell reactivity and memory inflation (22). First, we used MCMV-infected C57BL/6 mice to confirm the inflationary and noninflationary nature of CD8+ T cell responses against major histocompatibility complex-I (MHC-I) epitopes derived from the IE3 and m45 MCMV proteins (22). MHC-I tetramer staining analysis on gated CD8+ T cells confirmed strong responses against both inflationary IE3 and noninflationary m45 epitopes (Fig. 1 A and B). Indeed, IE3-specific CD8+ T cells increased between 1 and 5 mo after infection, whereas the m45-specific CD8+ T cells remained stable in blood (Fig. 1B). Inflationary IE3-specific CD8+ T cells displayed a terminally differentiated phenotype characterized by low expression levels of CD127 and CD62L molecules (23). In contrast, noninflationary m45-specific CD8+ T cells displayed a mixed effector-memory/central memory phenotype characterized by high expression of CD127 and a bimodal expression pattern of CD62L (23) (Fig. 1A). Restimulation of blood samples with a mixture of seven MHC-I MCMV epitopes (IE3, m38, m45, m57, m139, m141, and m164) resulted in a notable production of IFN-γ (mean positive, 11%), TNF-α to a lesser extent (mean positive, 5.5%), and no IL-2 (Fig. 1C) by CD44+CD8+ T cells, confirming the high magnitude of the responses against MCMV. Despite the striking differences in the memory phenotype of IE3- and m45-specific CD8+ T cells, both inflationary and noninflationary CD8+ T cells produced significant amounts of IFN-γ (Fig. 1D and SI Appendix, Fig. S1). Cytokine production was also assessed by MCMV-specific CD44+CD4+ T cells restimulated in vitro with m09 and m139 MHC-II MCMV-derived peptides and found to be one order of magnitude lower compared with the CD8+ T cell responses (Fig. 1E).
Fig. 1.
Memory phenotype and cytokine production by MCMV-specific T cells in MCMV infected C57BL/6 mice. (A) representative fluorescence-activated cell sorting plot of IE3 and m45 tetramer staining of CD8+ T cells and expression of CD127 and CD62L by tetramer-positive CD8+ T cells in blood. (B) IE3 and m45 tetramer+ CD8+ T cells for each individual mice are shown at 1 mo and 5 mo after infection. (C) Production of IFN-γ, TNF-α, and IL2 by CD8+ T cells after in vitro stimulation of splenocytes with a pool of seven MCMV peptides (IE3, m38, m45, m57, m139, m141, and m164). (D and E) Production of IFN-γ by spleen (D) CD8+ T cells and (E) CD4+ T cells after in vitro stimulation with indicated MCMV peptides. Statistical significance was assessed by paired Student t test. *P < 0.05, representative of two experiments (n = 10). n.s., not significant.
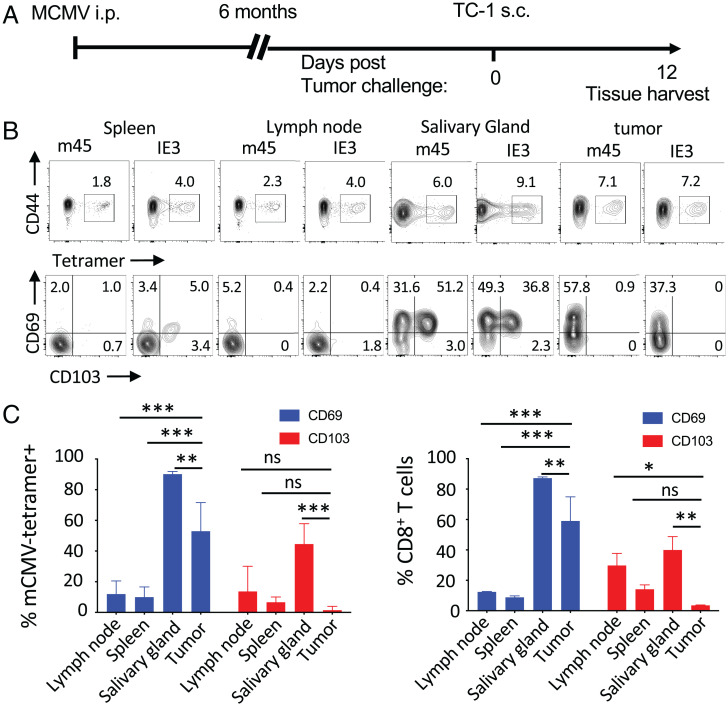
Next, we assessed the tissue distribution of inflationary and noninflationary MCMV-specific T cells in latently infected mice challenged with subcutaneous tumors. C57BL/6 mice were inoculated subcutaneously 6 mo after MCMV infection, with TC-1 tumor cells that express human papillomavirus type 16 (HPV16) E6 and E7 oncogenes and mutated H-Ras (Fig. 2A). By 12 d, established tumor masses were heavily infiltrated with both inflationary (IE3) and noninflationary (m45) MCMV-specific CD8+ T cells without i.t. injection of MCMV peptide epitopes. Surprisingly, the infiltration of tumors by MCMV-specific CD8+ T cells was comparable to infiltration in the salivary glands, a major tissue reservoir for CMV (24) (Fig. 2B). Indeed, tumors were more infiltrated by MCMV-specific CD8+ T cells than were secondary lymphoid organs, suggesting that MCMV-specific CD8+ T cells were poised to traffic into a fast-growing tumor. We asked whether these tumor-infiltrating, MCMV-specific CD8+ T cells were bona fide resident cells by assessing in tetramer+ CD8+ T cells the expression of CD69 and CD103, markers of tissue residence and intraepithelial lymphocytes, respectively (Fig. 2B). Unlike spleen and lymph node, CD69 was expressed by the majority (55%) of the tumor-infiltrating, MCMV-specific CD8+ T cells, but CD103 was not expressed (Fig. 2 B and C). These data suggest that a substantial fraction of the tumor-infiltrating, MCMV-specific CD8+ T cells had differentiated into resident cells. In contrast to tumors, salivary gland–infiltrating, MCMV-specific CD8+ T cells did express CD103. This difference might be due to the absence of detectable viral replication in the tumors, as no viral DNA or mRNA was found in tumor samples, but both were found in salivary gland tissues (SI Appendix, Fig. S2). Alternatively, TC-1 tumors might already be too dedifferentiated to provide tissue residency signals typical of a normal epithelium, as bulk CD8+ T cells did not express CD103 (24).
Fig. 2.
Inflationary and noninflationary MCMV-specific CD8+ T cells infiltrate solid tumors. (A) C57BL/6 mice were infected with MCMV and implanted 6 mo later with TC-1 tumors. When tumor reached 100 mm3, infiltration by MCMV T cells was assessed by tetramer staining. (B) Representative fluorescence-activated cell sorting plot of IE3 and m45 tetramer staining of CD8+ T cells in lymph nodes, spleen, salivary gland, and tumor tissues, and expression of CD69 and CD103 by tetramer-positive CD8+ T cells. (C) Data are shown as mean percentage ± SD of expression of CD69 (blue bars) and CD103 (red bars) by MCMV tetramer-positive and bulk CD8+ T cells in tumor-draining lymph nodes, spleen, salivary gland, and tumors. Statistical significance was assessed by Dunn test. ***P < 0.001, **P < 0.01, *P < 0.05, representative of two experiments (n = 4). i.p., intraperitoneal; ns, not significant; s.c., subcutaneous.
Intratumoral Administration of MCMV Epitopes Induces Broad Cellular Infiltration and Local Immune Activation of the Tumor Microenvironment.
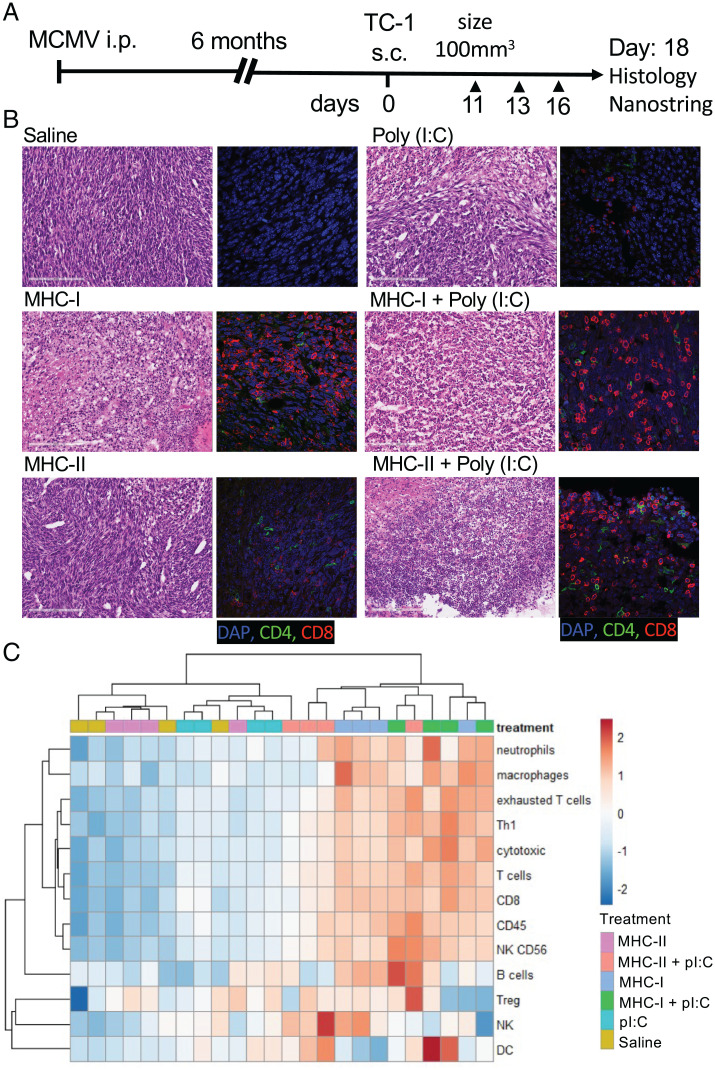
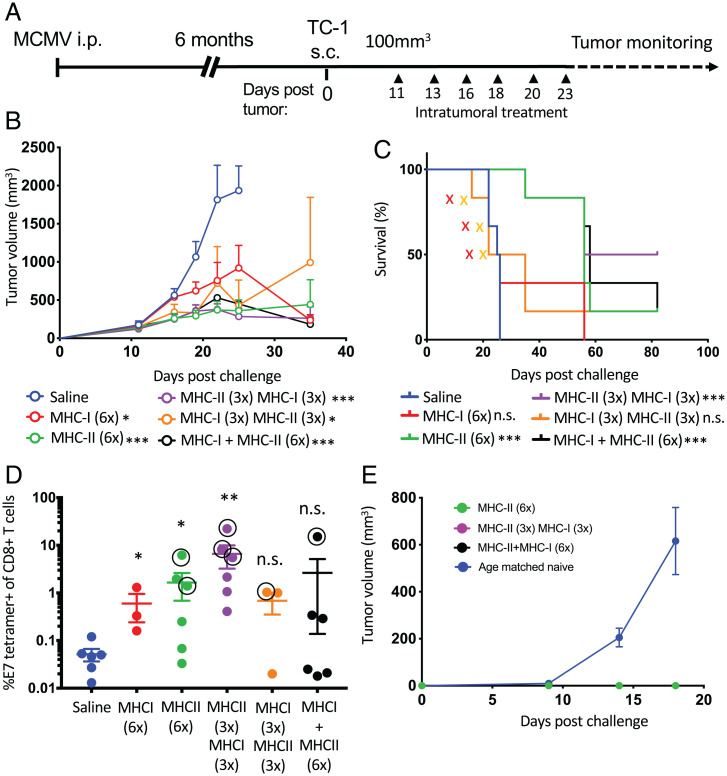
We next sought to determine whether tumor-infiltrating, MCMV-specific T cells could be activated in situ with their cognate peptide epitopes with or without pI:C and whether in situ activation of MCMV-specific T cells could alter the tumor immune microenvironment. We chose pI:C as a molecular adjuvant because it can induce strong secretion of inflammatory cytokines, in particular, type 1 IFN, by TC-1 cells in vitro after transfection (SI Appendix, Fig. S3). Latently MCMV-infected C57BL/6 mice were inoculated with TC-1 tumor cells subcutaneously. Once TC-1 tumor volume reached 100 mm3, they were injected i.t. with IE3, m38 and m45 MCMV MHC-I peptides (1 μg each) or m139 MCMV MHC-II peptide (3 μg) with or without pI:C (50 μg). Tumors were injected three times (Fig. 3A) and 2 d after the third injection, we analyzed by microscopy the consequences of i.t. injection of MCMV-derived peptide epitopes on the tumor tissues. First, MCMV-derived MHC-I–restricted epitopes with or without pI:C induced wide areas of necrotic tissue (Fig. 3B), supporting the notion that MCMV-specific CD8+ T cells exerted cytotoxic functions in the tumor. Lymphocytic infiltration was also noted and confirmed by immunofluorescence analysis of CD8+ and CD4+ T cell infiltrates. In contrast, tissue sections from tumors treated with MCMV-derived MHC-II peptide plus pI:C displayed both CD4+ and CD8+ lymphocytic infiltration but no necrotic tissue (Fig. 3B).
Fig. 3.
Intratumoral administration of MCMV epitopes induces profound changes in the nature of the tumor cellular immune infiltrate. (A) Experimental design. MCMV-infected C57BL/6 mice were transplanted with TC-1 tumors. When tumors reached 100 mm3, they were injected i.t. three times with saline, pI:C– (50 μg), MHC-I– (1 μg each: IE3, m38, and m45), and MHC-II– (3 μg, m139) restricted peptides with or without pI:C. (B) Hematoxylin and eosin staining and immunofluorescence microscopy analysis of CD8+ and CD4+ T cells in the tumor bed. (C) Heat map of z-scores of cellular immune infiltrate scores deconvoluted from the Nanostring PanCancer Immune profiling data set; single experiment (n = 4). DC, dendritic cell; Treg, T regulatory cell; i.p., intraperitoneal; s.c., subcutaneous.
Next, using the Nanostring mouse nCounter PanCancer Immune Profiling assay (Nanostring), we characterized the tumor cellular infiltration after the injection of MCMV peptide epitopes, summarized as a heat map of z-scores for each indicated cell type (Fig. 3C). Intratumoral injection of MHC-I peptide with or without pI:C and MHC-II peptide plus pI:C induced an increase in CD8, cytotoxic, and Th1 cells that was also associated with cell types that were not initially targeted by the MHC-I– and MHC-II–restricted peptides (Fig. 3C). Bystander natural killer (NK) cells, B cells, neutrophils, and macrophages were also increased upon i.t. injection of MHC-I–restricted peptide with or without pI:C and MHC-II–restricted peptide plus pI:C (Fig. 3C and Dataset S1). Notably, the dendritic cell score was only increased in the groups treated with pI:C alone or in combination with MCMV-derived epitopes (Fig. 3C). Together, these data indicate that i.t. injection of MCMV-derived epitopes causes profound changes in the immune activation status and cellular composition of the tumor microenvironment.
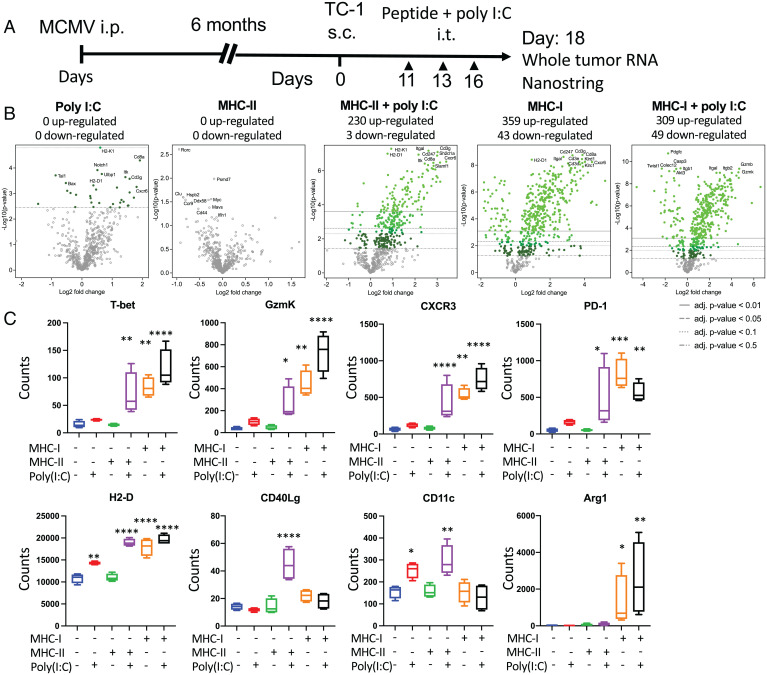
Using the same Nanostring dataset as previously described, we further analyzed the modification of the tumor immune microenvironment after i.t. injections (Fig. 4A). After normalization, differential expression of individual genes in each group was analyzed relative to saline-treated samples as a reference (Fig. 4B and Dataset S2). Intratumoral injection with pI:C induced minimal changes in the number of genes up- or down-regulated (Fig. 4B), and injection of MHC-II epitope alone had no effect. However, the combination of MHC-II epitope with pI:C led to the massive modification of the tumor gene expression profile with the up- and down-regulation of 230 and 3 genes, respectively (Fig. 4B). Interestingly, the i.t. injection of MHC-I–restricted MCMV epitopes without pI:C induced the up- and down-regulation of 359 and 43 genes, respectively (Fig. 4B). When combined with pI:C, the MHC-I epitopes induced changes in the expression of genes whose numbers were similar to MHC-I peptide alone, with the up- and down-regulation of 309 and 49 genes, respectively (Fig. 4B).
Fig. 4.
Intratumoral administration of MCMV epitopes induces broad cellular infiltration and local immune activation of the tumor microenvironment. (A) MCMV-infected C57BL/6 mice were transplanted with TC-1 tumors. When tumors reached 100 mm3, they were injected i.t. three times with saline, pI:C– (50 μg), MHC-I– (1 μg each: IE3, m38, and m45), and MHC-II– (3 μg, m139) restricted peptides with or without pI:C. Tumor RNA was extracted 48 h after the third injection and analyzed with the Nanostring PanCancer Immune Profiling panel (n = 4). (B) Volcano plot representation of the differential expression of each group compared with saline. Adjusted (adj.) P values were generated using the Benjamini–Yekutieli procedure. (C) Representative differentially regulated genes after i.t. injection for T cell function, antigen presentation, tissue damage, and inflammation. Statistical significance was assessed by Dunn test. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. Single experiment (n = 4). i.p., intraperitoneal; s.c., subcutaneous.
Heat-map visualization and hierarchical clustering of gene expression of all samples indicated that samples from tumors injected with MHC-I peptide with or without pI:C and MHC-II peptide plus pI:C clustered together (SI Appendix, Fig. S4A). However, the magnitude of gene regulation was less pronounced in groups treated with MHC-II peptide plus pI:C than in groups treated with MHC-I peptide with or without pI:C. Specifically, single-gene analysis showed pronounced up-regulation of genes associated with T cell function and activation, such as T-bet, granzyme k, CXCR3, and PD1, in tumor samples injected with MHC-I restricted with or without pI:C or MHC-II plus pI:C (Fig. 4C). H2-D gene expression was increased in all tumor samples injected with MHC-I–restricted epitopes with or without pI:C, MHC-II–restricted epitopes plus pI:C, and, to a lesser extent, with pI:C alone (Fig. 4C). Expression of the CD40Lg gene was increased only in the samples from tumors injected with MHC-II–restricted epitopes plus pI:C, and CD11c was increased in groups injected with MHC-II–restricted epitopes plus pI:C and, to a lesser extent, with pI:C alone (Fig. 4C). The i.t. treatment with MHC-I–restricted epitopes induced the up-regulation of Arg1, S100A8, and CD36 genes, which are involved in response to tissue damage and/or sterile inflammation (Fig. 4C and SI Appendix, Fig. S4B). Together, these results suggest that local immune activation with tissue-damage signatures were associated with MHC-I peptide i.t. injection, whereas a local immune activation signature alone was associated MHC-II peptide plus pI:C injection.
Intratumoral Injection of MHC-I–Restricted MCMV Epitopes Delays Tumor Growth.
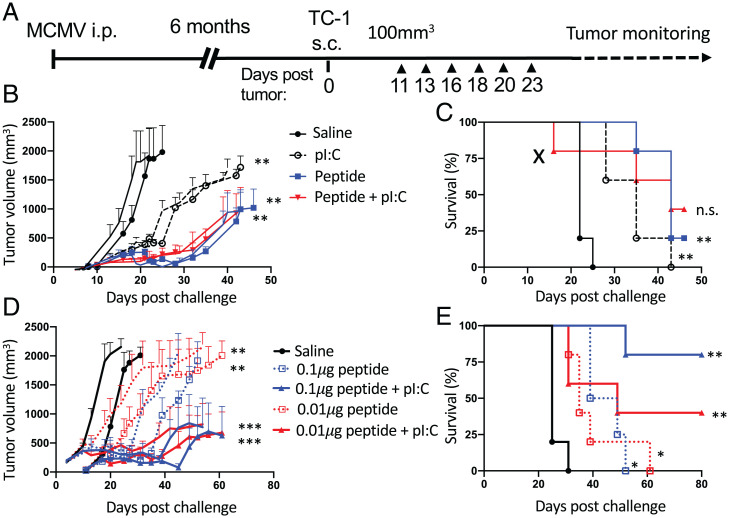
Next, we investigated whether the local immune activation and tissue-damage response caused by MCMV peptide i.t. injection could affect tumor growth and survival in the TC-1 subcutaneous model. In latently MCMV-infected mice, TC-1 subcutaneous tumors were injected six times with a mixture of inflationary (IE3), noninflationary (m45), and intermediate (m38) MHC-I minimal epitopes with or without pI:C (Fig. 5A). Compared with i.t. saline injection, i.t. injection of 1 μg of each of MHC-I–restricted peptide mixture with or without pI:C caused significant delay in tumor growth and improved survival (Fig. 5 B and C). In addition, pI:C alone caused a significant, but less pronounced, delay in tumor growth.
Fig. 5.
Intratumoral injection of MHC-I restricted MCMV epitopes delays tumor growth. (A) Experimental design. C57BL/6 mice were transplanted with TC-1 tumors 6 mo after MCMV infection. Tumor growth was monitored every second day. Tumors were injected six times with saline, pI:C (50 μg), and IE3, m38, and m45 MHC-I epitopes with or without pI:C (B and C) with 1 μg of each peptide or (D and E) with 0.01 μg or 0.1 μg of each peptide. Tumor volume was monitored two to three times a week. (B and D) Tumor growth is shown as mean tumor volume for each group and SE. (C and E) Survival curve for each group is shown. (X indicates treatment immune-related toxicity). Statistical significance was assessed by Mantel–Cox test for survival analysis and Dunn's test for tumor volume analysis. ***P < 0.001, **P < 0.01, *P < 0.05, representative of two experiments (n = 5). i.p., intraperitoneal; n.s., not significant; s.c., subcutaneous.
However, we observed occasional acute toxicity toward the end of the series of i.t. injections of MHC-I–restricted MCMV epitope in combination with pI:C, including death before the control animals succumbed to their tumors (Fig. 5C). To determine whether decreasing the dose of injected peptide would remain effective while reducing the acute toxicity, we assessed i.t. injection with lower doses of MHC-I–restricted peptide with or without pI:C. The peptides alone at reduced doses of 0.1 μg or 0.01 μg were equally able to significantly delay tumor growth without toxicity (Fig. 5D). When combined with pI:C, reduced doses of MHC-I–restricted peptides were devoid of immune-related toxicity and still able to induce long-term survival after the end of the treatment (Fig. 5 D and E). The observation that a low dose of 0.01 μg of peptide retained antitumor effect without toxicity suggests potential for scaling up in a context of clinical evaluation.
Sequential I.T. Administration of MHC-II– and MHC-I–Restricted MCMV Epitopes Promotes Long-Term Tumor Control and Antitumor Immunity.
The Nanostring analysis of the tumor microenvironment indicated that the consequences of the injection of MHC-I– and MHC-II–restricted peptide were substantially overlapping in terms of gene expression. However, while injection of MHC-I–restricted peptides induced a T cell/IFN-γ signature together with a strong tissue-damage response and sterile inflammation, MHC-II–restricted peptide injection was not associated with a tissue-damage response and induced a T cell/IFN-γ signature together with increased antigen-presentation functions. We reasoned that sequential injection of the MHC-I– and MHC-II–restricted peptides might help to harness both local immune activation and tumor killing that would favor a strong antitumor T cell response and long-term control. To evaluate this hypothesis, TC-1 subcutaneous tumors in MCMV-infected mice were injected six times with a mixture of MHC-I (IE3, m38, m45) or MHC-II (m139) MCMV epitopes plus pI:C or, sequentially, MHC-I plus pI:C followed by MHC-II plus pI:C (n = 3 times for each peptide) or conversely with MHC-II plus pI:C followed with MHC-I plus pI:C (Fig. 6A). Tumor growth was significantly delayed in all groups injected with MCMV peptides (Fig. 6B). Interestingly, complete regression and long-term cure were only observed in the groups that received MHC-II peptide alone or in combination with MHC-I peptides (Fig. 6C). Of note, i.t. injection of MHC-I peptides led to the expansion of m45-specific (noninflationary), but not IE3-specific (inflationary), CD8+ T cells (SI Appendix, Fig. S5 B and C).
Fig. 6.
Sequential i.t. administration of MHC-II– and MHC-I–restricted MCMV epitopes promotes long-term tumor control and antitumor immunity. (A) Experimental design. MCMV-infected C57BL/6 mice were transplanted with TC-1 tumors. Tumors were injected six times with MCMV-derived peptides plus pI:C. Saline (n = 6 times), MHC-I (IE3, m38, and m45, 1 μg each; n = 6 times), MHC-II (m139, 3 μg; n = 6 times), sequentially MHC-II (m139, 3 μg; n = 3 times), then MHC-I (IE3, m38, and m45, 1 μg each; n = 3 times), sequentially MHC-I (IE3, m38, and m45, 1 μg each; n = 3 times) then MHC-II (m139, 3 μg; n = 3 times), or MHC-I (IE3, m38, and m45, 1 μg each) admixed with MHC-II (m139, 3 μg; n = 6 times). (B) Tumor volume measurement and (C) survival (X indicates treatment immune-related toxicity). (D) E7-specific tetramer+CD8+ T cells in blood 48 h after treatment (complete cure, circled). (E) TC-1 tumor rechallenge of long-term survivors 4 mo after clearance of primary tumor. Tumor volume was monitored two to three times a week. (B and E) Tumor growth is shown as mean tumor volume for each group and SE. (C) Survival curve for each group is shown (n = 6). Statistical significance was assessed by Mantel–Cox test for survival analysis and Dunn’s test for tumor-volume analysis. ***P < 0.001, **P < 0.01, *P < 0.05, representative of two experiments (n = 6). i.p., intraperitoneal; n.s., not significant; s.c., subcutaneous.
To assess the induction of tumor antigen–specific immunity, we measured the presence of circulating CD8+ T cells in blood against the HPV16 E7 oncoprotein by MHC tetramer staining (Fig. 6D). Interestingly, high levels of E7-specific CD8+ T cells were detected in all groups that received i.t. injection of the MHC-II peptide (Fig. 6D). More remarkably, 50% of mice in the group that received MHC-II peptide followed by MHC-I peptide developed a high level of E7-specific CD8+ T cells (1% to 10% of total CD8+ T cells). The induction of high-level tetramer+CD8+ T cells correlated with complete cure and long-term survival, compared with partial responders (Fig. 6D, circled data point, and SI Appendix, Fig. S5C).
Next, we sought to determine whether the induction of CD8+ T cell responses against E7 oncogenes or unidentified tumor antigens was associated to long-term immunity. Four months after treatment, tumor-free mice from the first challenge were rechallenged subcutaneously with 50,000 TC-1 cells (Fig. 6E). Age-matched naïve mice were used as a tumor-challenge positive control. While all naïve control mice developed palpable subcutaneous tumors within 12 wk, we did not detect any palpable tumor in the long-term survivors, suggesting that long-term antitumor immunity had been established upon treatment with MHC-II peptide plus pI:C during the primary tumor challenge (Fig. 6E).
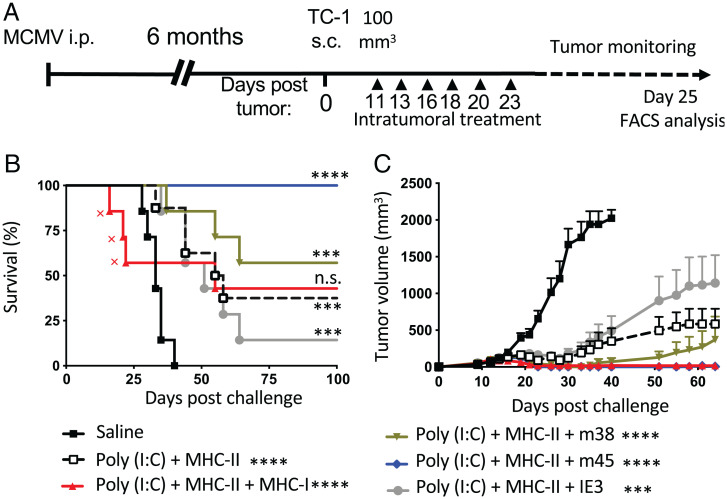
Tumor Regression Upon I.T. Injection with MCMV MHC-I–Restricted Epitopes Is Preferentially Conferred by Noninflationary Epitopes.
We next asked whether inflationary (IE3 or m38) or noninflationary (m45) MCMV-specific CD8+ T cells preferentially contributed to tumor regression upon intratumoral recall. TC-1 tumor–bearing mice were injected i.t. with IE3, m38, or m45 alone, or as pool together with pI:C and the MHC-II (m139) peptide (Fig. 7A). Groups injected with the combination containing only the noninflationary epitope (m45) showed tumor control comparable with the combination of the mixture of MCMV peptides (IE3, m38, and m45) but with no treatment-related toxicity. The tumor control was even superior to that of the groups injected with MHC-II peptide (m139) alone or combined with the inflationary peptides, IE3 or m38 (Fig. 7 B and C). Interestingly, production of IFN-γ and TNF-α was readily observed after ex vivo peptide stimulation by noninflationary (m45) or inflationary (IE3 or m38) MCMV-specific CD8+ T cells, but m45-specific CD8+ T cells displayed a slightly higher functional avidity (SI Appendix, Fig. S6).
Fig. 7.
Tumor regression upon i.t. injection with MCMV MHC-I–restricted epitopes conferred by noninflationary epitopes. (A) Experimental design. Mice were treated six times with MCMV-derived peptides plus pI:C (50 μg). Group treatments were as follows: saline (n = 6 times), MHC-II (1 μg; n = 6 times), MHC-II admixed with MHC-I MCMV pool (IE3, m38, and m45, 1 μg each; n = 6 times) or individually (1 μg; n = 6 times). (B) Survival curves (X indicates treatment immune-related toxicity). (C) Tumor volume was monitored three times a week and is shown as mean tumor volume for each group and SE. (B and C) Statistical significance was assessed by Mantel–Cox test for survival analysis w and Dunn's test for tumor-volume analysis. P values are shown directly in the survival graph and next to the legend for the tumor growth analysis (****P < 0.0001, ***P < 0.001, n.s.: not significant), representative of a single experiment (n = 7). FACS, fluorescence-activated cell sorting; i.p., intraperitoneal; s.c., subcutaneous.
To track MCMV-specific CD8+ and CD4+ T cells in tumors and tumor-draining lymph nodes, we performed tetramer staining after three i.t. injections of MHC-I (IE3, m38, and m45) and MHC-II (m25) peptides for which IAb tetramers were available. Noninflationary m45-specific CD8+ T cells were specifically expanded after i.t. injection with the corresponding peptide alone, whereas their inflationary IE3-specific counterparts remained unchanged in the tumors and, to a lesser extent, in the draining lymph nodes (SI Appendix, Fig. S7 A and B). Together, these data are consistent with the notion that inflationary T cells display a more terminally differentiated phenotype, whereas the noninflationary cells display a memory phenotype. We assessed the proliferative response of IE3- and m45-specific CD8+ T cells by measuring the expression of the Ki67 nuclear antigen, a proliferation marker. Surprisingly, Ki67 was equally induced in inflationary and noninflationary CD8+ T cells in tumor-draining lymph nodes (SI Appendix, Fig. S7C) and in tumors (SI Appendix, Fig. S7D) after i.t. peptide injection. Therefore, the lack of IE3-specific CD8+ T cell expansion in blood (SI Appendix, Fig. S5B) or in the tumor (SI Appendix, Fig. S7B) upon i.t. injection might result from decreased survival capacity rather than impaired proliferative potential (25).
To track the recall of CD4+ T cell responses in vivo upon i.t. injection with a CMV m25 peptide (SI Appendix, Fig. S7 A and B), we used H2-Ab tetramer harboring the MHC-II m25 MCMV epitope. We observed the expansion of m25-specific CD4+ T cells in both draining lymph nodes and tumor tissue, and up-regulation of Ki67 (SI Appendix, Fig. S7 C and D). These findings suggest that the proliferation and expansion of the CD4+ T cells might contribute to the antitumor effect, as for m45-specific CD8+ T cells.
Intratumoral Injection of MCMV-Derived Epitopes in Poorly Immunogenic B16-F10 Melanoma Delays Tumor Growth and Induces T Cell Infiltration and Immune Activation.
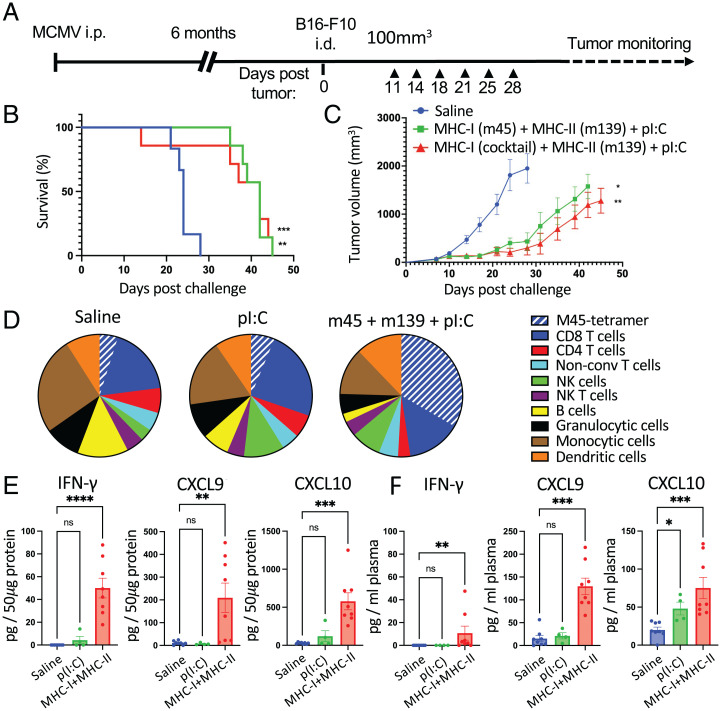
We next assessed the potential of i.t. injection of MCMV-derived peptide epitopes in an immunologically “cold” tumor model. We used the well-established melanoma model B16-F10, which does not respond to immune checkpoint blockade and is poorly immunogenic despite a high mutation burden. MCMV-infected, B16-F10–bearing mice were treated by repeated i.t. injection of combinations of MCMV-derived MHC-I– and MHC-II–restricted epitopes with pI:C (Fig. 8A). Treatment with MHC-I (m45) and MHC-II (m139) peptides significantly delayed tumor growth and improved survival compared with saline-treated groups (Fig. 8 B and C), but treatment with pI:C alone did not delay B16-F10 tumor growth (SI Appendix, Fig. S8). Flow cytometry analysis of the tumor immune infiltrate of B16-F10 tumors after i.t. treatment with MCMV-derived peptides showed a pronounced increase in CD8+ T cells (CD3+CD8+) and m45-specific CD8+ T cells, and a modest increase in NK cells (CD3−NK1.1+). The treatment led to a decrease in infiltrating B cells (CD19+), granulocytic cells (CD11b+LyG+), and CD4+ T cells (CD3+CD4+). Infiltrating monocytic cells (CD11b+Ly6C+Ly6G−) and dendritic cells (MHC-II+CD11c+) remained unchanged (Fig. 8D and SI Appendix, Fig. S9). To further characterize the immune modulation in the B16-F10 tumor tissue, we used the multiplex cytokine/chemokine measurement kit (Biolegend, CRS Legendplex). We found that i.t. treatment with MCMV-derived peptide led to the increase in tumor-tissue concentration of IFN-γ, CXCL9, and CXCL10 (Fig. 8E), which have been mostly associated with antitumor response, and increase of CCL2 and CCL4 (SI Appendix, Fig. S10A) that can exert pro- or antitumor effects depending on the immune status of the tumor (26). Interestingly, the i.t. injection in B16-F10 tumors led to an increase of plasma cytokines and chemokine (Fig. 8F and SI Appendix, Fig. S10B). Surprisingly, although TNF-α was not significantly induced in the treated tumor, it was induced in the plasma of treated mice (SI Appendix, Fig. S10). Finally, injection of pI:C alone did not significantly alter the tumor or plasma concentrations of any of the analyzed cytokines and chemokines (SI Appendix, Fig. S10). Together, these results show that MCMV-specific T cells remain functional in an immunologically cold tumor environment and that the MCMV-derived peptides administered i.t. can cause profound activation of the tumor immune microenvironment and potent antitumor activity.
Fig. 8.
Intratumoral injection of MCMV-derived epitopes in poorly immunogenic B16-F10 melanoma delays tumor growth and induces in situ infiltration and broad immune activation. (A) Experimental design. MCMV-infected C57BL/6 mice were transplanted with B16-F10 tumors. Mice were treated six times with saline pI:C (50 μg) alone or admixed with MHC-I– (m45 alone; 1 μg; or a mixture of m38, m45, and IE3, 0.1 μg each) and MHC-II– (m139; 1 μg) restricted peptides. (B) Survival was monitored. (C) Tumor growth is shown as the mean of tumor volume for each group with SE. (D–F) For the tumor microenvironment analysis, mice were treated twice, and samples were collected between 24 h and 36 h after the second i.t. injection. (D) Tumor-cell suspension was analyzed by fluorescence-activated cell sorting (FACS) to assess the presence of the indicated cells: m45-tetramer+CD3+CD8+ (m45-tetramer), CD3+CD8+ (CD8 T cells), CD3+CD4+ (CD4 T cells), CD3+CD4−CD8− (nonconventional T cells), CD3−NK1.1+ (NK), CD3+NK1.1+(NKT), CD19+ (B cells), CD11b+LyG+ (granulocytic cells), and CD11b+Ly6C+Ly6G− (monocytic cells), and MHC-II+CD11c+ (dendritic cells). The pie charts represent the average proportion of each cell type in each treatment group. (E and F) Cytokine/chemokine production was measured using Legendplex cytokine release syndrome panel. (E) Tumor lysate (pg/50 μg total protein) and (F) plasma production (pg/mL plasma) of IFN-γ, CXCL-9, and CXCL-10. Data are shown as individual values and mean ± SEM (n = 4–8, two independent experiments). Statistical significance was assessed by one-way ANOVA followed by Tukey’s test for multiple comparison analysis. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. i.d., intradermal; i.p., intraperitoneal; ns, not significant.
Finally, we investigated whether i.t. injection of MCMV epitopes could lead to a more pronounced antitumor effect, using a spontaneous colon adenocarcinoma model, MC-38, which harbors a high number of mutations and responds to immune checkpoint blockade (27). As in the previously assessed tumor models, i.t. injection of a combination of MCMV-derived peptides with pI:C provoked, in some mice, a sustained reduction in subcutaneous MC-38 tumor size, leading to its complete clearance that appeared more pronounced than in the B16-F10 model (SI Appendix, Fig. S11). However, we did not observe increased CD8+ T cell reactivity to the previously reported MC-38 neoepitopes derived from Adpgk- and Resp1-mutated antigens (SI Appendix, Fig. S11), which represent a fraction of the neoepitope repertoire that remains to be identified in MC-38 (28).
Discussion
In this study, we show that preexisting antiviral CD4+ and CD8+ T cell immunity can be leveraged for the i.t. treatment of solid tumors. Compared with other human viruses, CMV may be a superior candidate for intratumoral recall for multiple reasons. First, unlike some chronic viral infections, such as hepatitis C, HCMV infection elicits T cell responses that remain functional and do not show exhaustion (29). Second, HCMV prevalence is high in most human populations and increases with age (30). Third, in contrast to acute viral infections, CMV infection elicits long-lived T cell responses. Importantly, HCMV-specific T cell responses remain functional in cancers such as chronic lymphocytic leukemia, where the majority of the patient’s CD8+ T cells are anergic (20). Our results support the notion the CMV-specific T cells remain highly functional after tumor challenge in immunologically “hot” and “cold” transplantable murine tumor models and, upon recruitment, can effectively overcome the immunosuppressive tumor environment in the absence of immune checkpoint blockade.
Recent studies have shown that tumors are populated by antimicrobial T cells (31), which sometimes display hallmarks of tissue residency (32). Solid-tumor infiltration by bystander antiviral T cells is an intriguing phenomenon and opens the possibility that antiviral T cell immunity might contribute to cancer progression or to therapeutic response (31, 33). Our results show that MCMV-specific T cells populated subcutaneous tumors and expressed CD69, a marker of resident memory T cells, in contrast to their circulating T cell counterparts. This infiltration occurred in absence of intratumoral MCMV replication, as we did not detect any MCMV genomic DNA or mRNA in the tumor samples during the latent phase or after i.t. treatment (SI Appendix, Fig. S2). Intratumoral infection of naïve animals with MCMV virus has been shown to alter the tumor microenvironment through its interaction with macrophages (34). In contrast, our data indicate that intratumoral reactivation of cognate MCMV T cells by minimal peptide epitopes in latently infected mice is key to the antitumor effect and likely does not depend on intratumoral or systemic reactivation of MCMV. Notably, our results are consistent with a recent study showing that adoptively transferred T-cell receptor transgenic OT-1 CD8+ T cells differentiated into tumor-resident memory T cells could be mobilized by i.t. injection of ovalbumin-derived peptide and caused some tumor regression in syngeneic murine tumor models (32). However, sustained remission in the majority of animals required systemic administration of anti-PD1 antibodies. Virus-derived peptides were also shown to activate intratumoral resident CD8+ T cells in human tumor explants. However, to our knowledge, the recruitment of virus-specific CD4+ T cells with or without a Toll-like receptor (TLR) agonist has not been previously evaluated.
The syngeneic TC-1 tumor model used in our studies has proved a valuable model for preclinical evaluation of cancer vaccines and immunotherapies against HPV-associated cancers (35). TC-1 cells express the oncoproteins HPV16 E6 and E7 and a mutated H-RAS. The oncoprotein E7 harbors an immunodominant MHC-I epitope, and induction of E7-specific CD8+ T cells confers protection from subsequent tumor challenge. However, effective therapeutic vaccination against TC-1 generally requires combining vaccination with other agents such as chemotherapy (36) or immune checkpoint inhibitors to treat established tumors (37). In contrast, our approach did not require vaccination with a tumor-associated antigen or combining it with immune checkpoint blockade to consistently eradicate large tumor masses.
We were surprised that co-injection of the MHC-II MCMV epitopes and pI:C together, but not separately, had strong antitumor effects and profoundly modulated the tumor immune microenvironment. Noteworthy, these responses were at least as effective as injection of the MHC-I MCMV epitopes plus pI:C and did not result in any obvious toxicities. The synergistic activity of the vaccine adjuvant pI:C and MHC-II MCMV epitopes was associated with activation and amplification of MCMV-specific CD4+ T cells. The mobilization of MCMV-specific CD4+ T cells could facilitate epitope spreading to released tumor neoantigens, as we observed against an E7 epitope, a model tumor-restricted antigen. Interestingly, MCMV CD4+ T cells expanded and up-regulated the proliferation marker Ki67 in the tumor-draining lymph nodes as well as in the tumors. Future studies will assess the role of MCMV-specific CD4+ T cells in epitope spreading to tumor antigens, in particular, their potential role in providing T cell help in the tumor-draining lymph nodes, to modulate the tumor immune microenvironment and/or to mediate direct cytotoxicity. In addition, the associated increase in intratumoral NK cell signature suggests that recruited NK cells may also play a role in tumor killing and/or dendritic cell recruitment and activation (38).
Initial experiments indicated that pI:C can induce strong secretion of inflammatory cytokines produced by tumor cells in vitro after transfection (SI Appendix, Fig. S3). In contrast, in vivo i.t. injection of pI:C alone had a modest effect on the tumor microenvironment, which was associated with a dendritic cell signature as previously reported (39). Various formulations of pI:C are being investigated in preclinical models for i.t. delivery (40, 41) or evaluated clinically as adjuvants for cancer vaccines (42), which opens a path for the clinical evaluation of pI:C in combination with CMV-derived epitopes [ClinicalTrials.gov identifiers NCT01984892 (43) and NCT02423863 (44)].
Cancer disproportionally affects older individuals, and assessing immunotherapy in older mice should reflect more faithfully the context in humans. Additionally, aging has been linked to increased toxicity to tumor immune therapies in preclinical models (45). Therefore, to approximate the delay between primary CMV infection and the onset of cancer development in humans, mice were challenged with tumors in the latent phase of MCMV infection after memory inflation had been established. We observed occasional acute toxicity after repeated i.t. injection of MHC-I–restricted peptide epitopes, which was eliminated by lower dose of peptides or injecting only noninflationary CD8+ epitopes while retaining antitumor efficacy. Killing of peptide-bound cells outside the tumor by increasing the number of the peptide-specific CD8+ T cells generated by each round of peptide injection into shrinking tumor beds is likely responsible for this toxicity. Therefore, slow-release formulations for low-molecular-weight therapeutics such as antibodies or peptides may be helpful to maximize local efficacy and minimize systemic toxicity of i.t. approaches.
The expansion of intratumoral and systemic CD4+ T cells and noninflationary CD8+ T cells paired with the maintenance of inflationary CD8+ T cells after cognate peptide injection, despite their strong tumor cytotoxic activity, is notable for two reasons. First, it suggests that repeated treatment of large tumors might not deplete the effector memory T cell pool. Second, it suggests that activation of a pathologic CMV infection would be an unlikely outcome of the treatment, as we did not observe reactivation of MCMV upon i.t. treatment.
It is surprising that the mobilization of the noninflationary compartment conferred the best antitumor response in our murine models. While CMV-specific T cell memory inflation is well established in humans (18), the notion that the dichotomy between inflationary (terminally differentiated) and noninflationary (effector/central memory) CD8+ T cells is encoded at the level of antigen specificity comes from the murine model of CMV infection (22). However, exhaustive analysis of CMV-specific T cell responses in various human tissues shows a broad range of CD8+ T cell memory phenotypes, including the effector/central memory type (19). These findings indicate that the selection of epitope targeting central-memory CD8+ T cells is feasible and could be critical to maximize antitumor effect and de novo proliferation in patients with cancer.
Harnessing bystander antiviral T cells with minimal peptide epitopes presents practical advantages over classically used intratumoral therapies such as live virus or those that are cytokine or chemokine based (8). First, mobilizing antiviral CD4+ and CD8+ T cells can simultaneously induce production of several cytokines and chemokines, leading to the activation of multiple immune pathways and broad cellular infiltration. Second, mobilizing cytotoxic CD8+ T cells with MHC-I–restricted peptides acts as a debulking agent with immediate reduction of the tumor mass and release of tumor antigens. Third, short peptides can be easily synthesized in vitro compared with more complex biologicals. Fourth, the production of a CMV peptide mixture is scalable, as even minute amounts of peptide are effective in vivo due to the high affinity of the antiviral T-cell receptors. Fifth, compared with oncolytic viruses or viral vaccines, minimal peptide epitopes are less susceptible to neutralization by preexisting or drug-induced antibodies. Finally, minimal viral peptide epitopes can be presented by virtually any MHC-I– or MHC-II–expressing cell, in contrast to synthetic long peptides, which require intracellular antigen processing and/or cross-presentation. Also, direct binding to MHC molecules at the cell surface bypasses immune evasion mechanisms affecting the MHC presentation pathways. This is particularly relevant as antiviral memory cytotoxic CD8+ T cells normally exert their function in a context of high MHC-peptide presentation by infected cells and professional antigen-presenting cells.
The selection of immunogenic and protective neoepitopes is a hurdle in the development of antigen-specific cancer vaccines. This is a particular challenge with mutations in nondriver cancer genes, which can be easily down-regulated by cancer cells to escape immune recognition. Immune evasion through selective regulation of human leukocyte antigen (HLA) was shown to be associated with a higher mutation burden and immune reactivity, thereby reducing the pool of potential antigenic targets (46). Therefore, the dynamic nature of the cancer immunopeptidome landscape is a major hurdle to the development of targeted vaccines against cancer. In contrast, a tumor antigen–agnostic intratumoral approach could overcome the challenges of personalized identification of cancer immunopeptidome and potentially induce T cell responses against multiple tumor antigens that would be more resistant to immune escape. Diversification of T cell responses toward tumor antigens that are not included in a tumor antigen–based vaccine has been proposed to contribute to clinical response (47). This diversification, referred to as “epitope spreading” or “antigen cascade,” appears to result from increased cross-presentation of tumor antigens released after tumor lysis mediated by the original T cells (47). The diversification of antitumor T cell responses has been reported for immune checkpoint blockade as well. Notably, Yost et al. (48) have shown that the clonal composition of tumor T cell infiltrates before and after PD-L1 blockade was markedly expanded, suggesting de novo generation or amplification of a new repertoire of antitumor T cells. We believe that harnessing preexisting antiviral T cell responses presents the advantage of mobilizing consistently stronger T cell responses to released tumor antigens and disrupting the suppressive microenvironment, and thereby has the potential to maximize epitope spreading.
The three transplantable solid tumor models used in this study have been invaluable for early-stage investigation of drugs targeting the tumor microenvironment, although they develop rapidly and do not fully recapitulate the slow progression of solid tumors in humans. Establishment of the human tumor microenvironment usually takes years and is characterized by the slow accumulation of mutations, vascularization, and development of immune suppression and immunoediting (49). Indeed, differences between transplantable murine models and naturally occurring tumors in humans might explain why it has been difficult to develop drugs targeting innate immune-recognition pathways (49). However, given the fundamental nature of the mechanisms of peptide presentation and T cell activation within mammals, it is likely that a substantial fraction of human solid tumors would respond to CMV minimal peptide i.t. injection.
Whether CMV-specific T cells are unique compared with other virus or vaccine-induced T cells remain to be addressed. In this regard, it is interesting to note that Epstein-Barr virus–specific CD8+ T cells in patients with chronic lymphocytic leukemia do not retain the same degree of functionality as CMV-specific ones (50). In addition, functional CMV T cells were found to populate colon carcinoma and lung cancer (31, 51), and circulating CMV T cells were shown to respond to PD1 treatment in patients with melanoma (52, 53).
Translation of the CMV approach to human trials could be relatively straightforward, since immunodominant minimal HCMV epitopes have been identified and could provide a limited pool of peptides for broad HLA coverage (54). Indeed, a commercial test composed of 22 CMV-derived peptides is widely used to screen for cellular immunity against CMV in patients at risk for viral reactivation due to immunosuppressive treatment (e.g., organ transplant) (55, 56). This type of test could be used to identify patients most likely to benefit from i.t. treatment with the corresponding CMV peptides. We believe that it would be feasible to translate this approach in most human populations, based on a defined mixture of CD4+ and CD8+ peptides effective across most HLA types.
Intratumoral delivery of drugs has recently gained traction in the clinical and commercial sectors, following the recent approval of the oncolytic virus T-Vec for intralesional injection of melanoma (15). This major breakthrough has opened a path for the development of new intratumoral therapies and offers a new framework for regulatory agencies to evaluate such therapies (57). Clinical response in the noninjected lesions was an important criterion required for approval of T-Vec. Our approach indicates that i.t. injection of MCMV peptide epitopes induces long-term protection against secondary challenge (i.e., the TC-1 model). In addition to superficial tumors such as melanoma, the recent advances in image-guided biopsy permit the application of intratumoral therapies to tumors located deeper in tissues (e.g., breast, pancreas, prostate) (58, 59).
In conclusion, we show that CMV-specific T cell responses can be harnessed and redirected into tumors to kill cancer cells, cause tumor regression, activate the tumor immune microenvironment, and elicit antitumor immunity. CMV-derived peptides could, therefore, be considered as combined cytotoxic and immunotherapeutic drugs. We believe that i.t. injection of minimal viral peptide epitopes represents a strategy broadly applicable to many patients and across cancer types. This simple tumor antigen–agnostic approach could be implemented as a potentially low-cost, off-the-shelf, stand-alone therapy or in combination with current cancer therapies such as immune checkpoint inhibitor antibodies.
Methods
Cell Lines and Viruses.
TC-1 cells were obtained from Dr. Tzyy-Choou Wu (The Johns Hopkins University) (35) and maintained in Roswell Park Memorial Institute (RPMI; GIBCO) containing 10% (volume per volume) fetal bovine serum (FBS; SIGMA), l-glutamine (GIBCO), and 100 μg/mL G418 (InvivoGen). B16-F10 cells were acquired from ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. MC-38 cells (60) were obtained from Dr. James Hodge (National Cancer Institute, NIH) and maintained in DMEM (GIBCO) containing 10% FBS, l-glutamine, nonessential amino acids (GIBCO), sodium pyruvate (GIBCO) and HEPES (GIBCO). Mouse M2-10B4 fibroblasts were purchased from ATCC and maintained in RPMI-Glutamax medium (Life Technologies) containing 10% FBS. Cell lines were negative for mycoplasma and maintained in culture at 37 °C, 5% CO2. Highly virulent salivary gland–derived MCMV stocks were obtained from salivary gland homogenates of 6-wk-old infected BALB/c mice after serial passages (SI Appendix, Materials and Methods).
Mice and Experimental Procedures.
BALB/c and C57BL/6 female mice were purchased from the Jackson Laboratory and housed under specific pathogen-free conditions at the animal care facilities of the National Cancer Institute. All animal protocols used in this study were approved by the National Cancer Institute Animal Care and Use Committee. Mice aged 8 to 10 wk were infected intraperitoneally with MCMV (5 × 103 pfu) diluted in 200 μL of PBS.
Tumor cells were injected subcutaneously (2.5 × 105 TC-1 and 5 × 105 MC-38) or intradermally (5 × 105 B16-F10) into the right flank of MCMV-infected mice at least 4 mo after MCMV infection. At this time, MCMV is latent with no detectable replication (61). Tumors were measured twice per week with digital calipers, and tumor size was calculated according to the formula W2 × L/2 (where L = length and W = width). For survival experiments, humane end point was based on tumor volume (>2,000 mm3) or average diameter (>20 mm) and overall condition of the animal.
When tumors reached a volume between 50 and 150 mm3, mice were randomized into different experimental treatment groups and injected i.t. (day 0) with the following formulations: MCMV minimal peptide (Genscript; SI Appendix, Table S1) epitopes to recall MCMV-specific CD8+ and CD4+ T cells referred to as MHC-I–restricted (MHC-I peptides) or MHC-II–restricted (MHC-II peptides), as described in SI Appendix, Table S1. MHC-I or MHC-II peptides admixed with 50 μg pI:C) (low molecular weight; InvivoGen), a TLR 3 and RIG-I–like receptor agonist that potentiates tumor antigen cross-presentation (62). Mice injected with saline (InvivoGen) or pI:C alone were used as controls. For each injection, the formulation was diluted with saline to 50 μL. Tumors were injected three times per week for 1 wk (n = 3 doses) or 2 wk (n = 6 doses).
Preparation of Cell Suspensions for Flow Cytometry and Elispot.
Tumors, lymph nodes, and spleens were finely minced and incubated at 37 °C at 250 rpm in RPMI supplemented with 2% FBS, 0.5 mg/mL Collagenase A (Roche), and 0.1 mg/mL DNase1 (Roche) for 30 min (tumor) or 15 min (lymph nodes and spleen). After incubation, single suspensions were filtered through a 70-μm mesh. Remaining erythrocytes were lysed in ammonium–chloride–potassium buffer (Life Technologies). Circulating leukocytes were obtained from ammonium–chloride–potassium–treated whole-blood samples. Single-cell suspensions were stained with fluorochrome-conjugated antibodies and MHC tetramers for flow cytometry analysis of T cell responses and for the characterization of the tumor leukocyte infiltrate (SI Appendix, Materials and Methods). Single-cell suspensions were incubated with the indicated peptide epitopes (SI Appendix, Table S1) for in vitro, antigen-specific T cell stimulation (SI Appendix, Materials and Methods) for intracellular cytokine staining or ELISPOT. For details, see SI Appendix, Materials and Methods.
Tissue Processing for RNA and Protein-Content Quantification and Microscopy Analyses.
For gene expression analysis, tumor tissues were collected 48 h after the last of three i.t. injections and flash-frozen in liquid nitrogen. RNA was isolated using TRIzol and stored at −80 °C until analysis with the Pancancer immune profiling panel (nCounter, Nanostring). For protein content analysis, ethylenediaminetetraacetic acid–potassium–treated plasma and tumor-tissue lysates were obtained 24 h to 36 h after the last i.t. treatment and stored at −80 °C until analysis with the cytokine-release syndrome panel (Legendplex, Biolegend). For microscopy analysis, fresh tumor tissues were fixed in paraformaldehyde, incubated in sucrose, and then embedded in optimal cutting temperature compound (Tissue-Tek). Frozen tissue blocks were stored frozen at −80 °C until histology and immunofluorescence analyses. For details, see SI Appendix, Materials and Methods.
Statistical Analyses.
Statistical analyses were performed using Prism software (GraphPad Software, Inc.). Paired or unpaired Mann–Whitney tests were used to analyze statistical differences between two groups. For multiple comparisons, Dunn’s test was used. The Mantel–Cox test was used for survival analysis. Treatments were compared using the extra sum-of-squares F test. Values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank the NIH tetramer facility for providing critical reagents; the Center for Cancer Research (CCR) Confocal Core facility; the CCR genomic core facility; the CCR flow cytometry facility; and Dr. Mark Simpson and Dr. Christophe Cataisson for helpful discussions.
Footnotes
Reviewers: O.J.F., University of Pittsburgh School of Medicine; and P.R., Department of Oncology, University of Lausanne.
Competing interest statement: The authors declare a competing interest. N.C. and J.T.S. have filed an employee invention report through the National Cancer Institute, NIH, related to Novel Cancer Treatment Utilizing Preexisting Microbial Immunity.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2116738119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix. Nanostring datasets are deposited in the Gene Expression Omnibus and publicly available at, reference Series GSE205574 (63).
References
- 1.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Alexandrov L. B., Nik-Zainal S., Siu H. C., Leung S. Y., Stratton M. R., A mutational signature in gastric cancer suggests therapeutic strategies. Nat. Commun. 6, 8683 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott P. A., et al. , An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin U., et al. , Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017). [DOI] [PubMed] [Google Scholar]
- 5.van Montfoort N., et al. , NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell 175, 1744–1755.e15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brossart P., The role of antigen spreading in the efficacy of immunotherapies. Clin. Cancer Res. 26, 4442–4447 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Marabelle A., Kohrt H., Levy R., Intratumoral anti-CTLA-4 therapy: Enhancing efficacy while avoiding toxicity. Clin. Cancer Res. 19, 5261–5263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marabelle A., Tselikas L., de Baere T., Houot R., Intratumoral immunotherapy: Using the tumor as the remedy. Ann. Oncol. 28 (suppl. 12), xii33–xii43 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Salazar A. M., Erlich R. B., Mark A., Bhardwaj N., Herberman R. B., Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: Case report, hypothesis, and clinical trial. Cancer Immunol. Res. 2, 720–724 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Kyi C., et al. , Therapeutic immune modulation against solid cancers with intratumoral poly-ICLC: A pilot trial. Clin. Cancer Res. 24, 4937–4948 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrales L., et al. , Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagiv-Barfi I., et al. , Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl. Med. 10, eaan4488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricca J. M., et al. , Pre-existing immunity to oncolytic virus potentiates its immunotherapeutic efficacy. Mol. Ther. 26, 1008–1019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell S. J., For the success of oncolytic viruses: Single cycle cures or repeat treatments? (One cycle should be enough). Mol. Ther. 26, 1876–1880 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribas A., et al. , Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bommareddy P. K., Shettigar M., Kaufman H. L., Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 18, 498–513 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Zuhair M., et al. , Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 29, e2034 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Klenerman P., Oxenius A., T cell responses to cytomegalovirus. Nat. Rev. Immunol. 16, 367–377 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Gordon C. L., et al. , Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J. Exp. Med. 214, 651–667 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.te Raa G. D., et al. , CMV-specific CD8+ T-cell function is not impaired in chronic lymphocytic leukemia. Blood 123, 717–724 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Gulley J. L., et al. , Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J. Natl. Cancer Inst. 109, djw261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munks M. W., et al. , Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177, 450–458 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Huster K. M., et al. , Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. U.S.A. 101, 5610–5615 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith C. J., Caldeira-Dantas S., Turula H., Snyder C. M., Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep. 13, 1137–1148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton B. L., Williams J. B., Cabanov A., Spranger S., Gajewski T. F., Intratumoral CD8+ T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol. Res. 6, 14–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagarsheth N., Wicha M. S., Zou W., Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17, 559–572 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juneja V. R., et al. , PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 214, 895–904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav M., et al. , Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Cicin-Sain L., Arens R., Exhaustion and inflation at antipodes of T cell responses to chronic virus infection. Trends Microbiol. 26, 498–509 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Cannon M. J., Schmid D. S., Hyde T. B., Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 20, 202–213 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Simoni Y., et al. , Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Rosato P. C., et al. , Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 10, 567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strickley J. D., et al. , Immunity to commensal papillomaviruses protects against skin cancer. Nature 575, 519–522 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erkes D. A., Wilski N. A., Snyder C. M., Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor-associated macrophages to promote cancer immunity. Hum. Vaccin. Immunother. 13, 1778–1785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin K. Y., et al. , Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 56, 21–26 (1996). [PubMed] [Google Scholar]
- 36.Tseng C. W., et al. , Pretreatment with cisplatin enhances E7-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. Clin. Cancer Res. 14, 3185–3192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moynihan K. D., et al. , Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Böttcher J. P., et al. , NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammerich L., et al. , Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 25, 814–824 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Amos S. M., et al. , Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol. Immunother. 60, 671–683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmon H., et al. , Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins K. A., Bavari S., Salazar A. M., Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev. Vaccines 14, 447–459 (2015). [DOI] [PubMed] [Google Scholar]
- 43.ClinicalTrials.gov, Treatment of solid tumors with intratumoral Hiltonol® (poly-ICLC) (Hiltonol). NCT01984892. https://clinicaltrials.gov/ct2/show/NCT01984892. Accessed 23 May 2022.
- 44.ClinicalTrials.gov, In situ, autologous therapeutic vaccination against solid cancers with intratumoral Hiltonol® (poly-ICLC). NCT02423863. https://clinicaltrials.gov/ct2/show/NCT02423863?term=NCT02423863&draw=2&rank=1. Accessed 23 May 2022.
- 45.Bouchlaka M. N., et al. , Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J. Exp. Med. 210, 2223–2237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGranahan N., et al. ; TRACERx Consortium, Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 171, 1259–1271.e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butterfield L. H., et al. , Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin. Cancer Res. 9, 998–1008 (2003). [PubMed] [Google Scholar]
- 48.Yost K. E., et al. , Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerin M. V., Finisguerra V., Van den Eynde B. J., Bercovici N., Trautmann A., Preclinical murine tumor models: A structural and functional perspective. eLife 9, e50740 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofland T., et al. , Functional differences between EBV- and CMV-specific CD8+ T cells demonstrate heterogeneity of T cell dysfunction in CLL. HemaSphere 4, e337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leem G., et al. , Tumour-infiltrating bystander CD8+ T cells activated by IL-15 contribute to tumour control in non-small cell lung cancer. Thorax 10.1136/thoraxjnl-2021-217001(2021). [DOI] [PubMed] [Google Scholar]
- 52.Wieland A., et al. , T cell receptor sequencing of activated CD8 T cells in the blood identifies tumor-infiltrating clones that expand after PD-1 therapy and radiation in a melanoma patient. Cancer Immunol. Immunother. 67, 1767–1776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fairfax B. P., et al. , Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat. Med. 26, 193–199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhanwani R., et al. , Profiling human cytomegalovirus-specific T cell responses reveals novel immunogenic open reading frames. J. Virol. 95, e0094021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giulieri S., Manuel O., QuantiFERON®-CMV assay for the assessment of cytomegalovirus cell-mediated immunity. Expert Rev. Mol. Diagn. 11, 17–25 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Clari M. A., et al. , Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8(+) T-cell response in allogeneic stem cell transplant recipients. Clin. Vaccine Immunol. 19, 791–796 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu K., Clinical considerations for oncolytic viral therapies: A regulatory perspective. Clin. Pharmacol. Ther. 101, 580–582 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Tacher V., et al. , Factors associated with success of image-guided tumour biopsies: Results from a prospective molecular triage study (MOSCATO-01). Eur. J. Cancer 59, 79–89 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Lester S. C., How image-guided core needle biopsies altered the treatment of breast disease: Challenges accepted and opportunities taken. Breast J. 26, 1156–1159 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg S. A., Spiess P., Lafreniere R., A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233, 1318–1321 (1986). [DOI] [PubMed] [Google Scholar]
- 61.Reddehase M. J., Podlech J., Grzimek N. K., Mouse models of cytomegalovirus latency: Overview. J. Clin. Virol. 25 (suppl. 2), S23–S36 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Aznar M. A., et al. , Immunotherapeutic effects of intratumoral nanoplexed poly I:C. J. Immunother. Cancer 7, 116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.N. Çuburu et al., GSE205574. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE205574. Accessed 17 June 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix. Nanostring datasets are deposited in the Gene Expression Omnibus and publicly available at, reference Series GSE205574 (63).