Abstract
Background
Dapsone (4,4'-diamino-diphenyl sulfone) is a synthetic derivative of sulfones, with the antimicrobial activity described since 1937. It is also a drug traditionally used in dermatological therapies due to its anti-inflammatory effect. In recent years its antioxidant, anti-excitotoxic, and antiapoptotic effects have been described in different ischemic damage models, traumatic damage, and models of neurodegenerative diseases, such as Parkinson's (PD) and Alzheimer's diseases (AD). Finally, dapsone has proven to be a safe and effective drug as a protector against heart, renal and pulmonary cells damage; that is why it is now employed in clinical trials with patients as a neuroprotective therapy by regulating the main mechanisms of damage that lead to cell death.
Objective
The objective of this study is to provide a descriptive review of the evidence demonstrating the safety and therapeutic benefit of dapsone treatment, evaluated in animal studies and various human clinical trials
Methods
We conducted a review of PubMed databases looking for scientific research in animals and humans, oriented to demonstrate the effect of dapsone on regulating and reducing the main mechanisms of damage that lead to cell death.
Conclusion
The evidence presented in this review shows that dapsone is a safe and effective neuro and cytoprotective treatment that should be considered for translational therapy.
Keywords: Dapsone, neuroprotection, cytoprotection, anti-inflammatory, antioxidant, antiexcitotoxic, antiapoptotic
1. INTRODUCTION
Neuronal damage is a pathological process observed in various neurological diseases such as neurodegenerative diseases, ischemic or hemorrhagic stroke, traumatic brain and spinal cord damage, and epilepsy. Ionic dysregulation is the main mechanism that leads to cell death in those diseases. The intracellular influx of calcium and the release of excitatory amino acids cause excitotoxicity, oxidative stress, inflammation, and apoptosis [1, 2]. In this scenario, it is essential to develop effective neuroprotective therapies that regulate or block the main mechanisms that lead to irreversible damage [3]. As research is looking for new neuroprotective therapies, dapsone appears as a compelling candidate due to its anti-inflammatory effect, particularly to preserve nervous tissue [4-6]. On the other hand, diseases of the central nervous system (CNS) share damage mechanisms similar to others that occur in heart, kidney and lung diseases. Interestingly, those pleiotropic therapeutic mechanisms of dapsone allowed some authors to consider this drug as a potential therapeutic resource in the severe form of the COVID-19 disease [7-10].
1.1. Dapsone
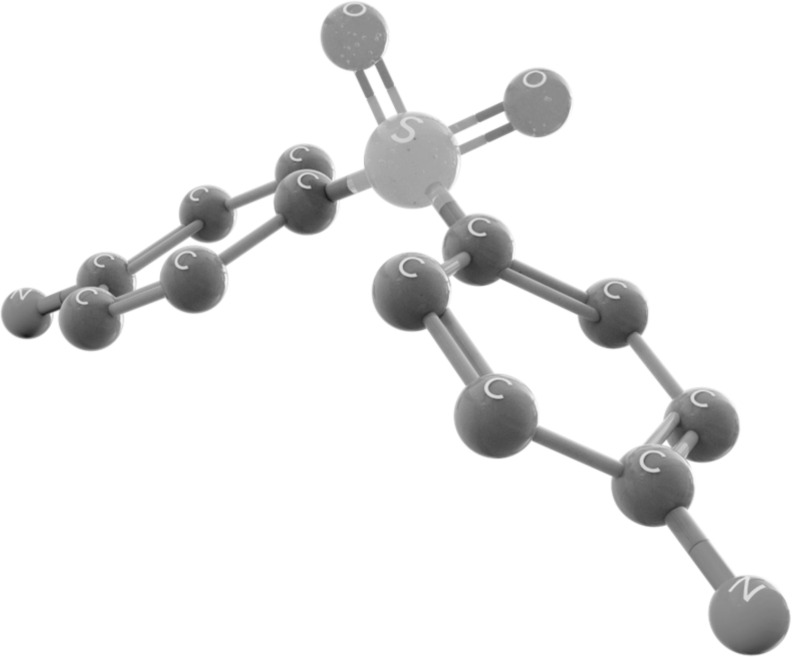
Dapsone (4,4'diaminodiphenylsulfone) was synthesized for the first time in 1908 by Emil Fromm, who is considered the father of sulfones [11]; its chemical structure consists of a central sulfur atom attached to two carbon atoms (Fig. 1). The use of sulfones as therapeutic agents appeared long after dapsone synthesis. Dapsone is classified as an antibiotic to treat infections due to bacteria, yeasts, and parasites [4, 12]. This sulphone has a general use for the treatment of leprosy (Mycobacterium leprae) [13-15] and by having anti-inflammatory effects and inhibiting the production of reactive oxygen species (ROS), reducing the effect of eosinophil peroxidase on mast cells and negatively regulating neutrophil chemotaxis, this allows its use in the treatment of a wide variety of inflammatory and skin conditions. such as dermatitis herpetiformis and acne vulgaris [16] (Fig. 2).
Fig. (1).
Dapsone chemical structure.
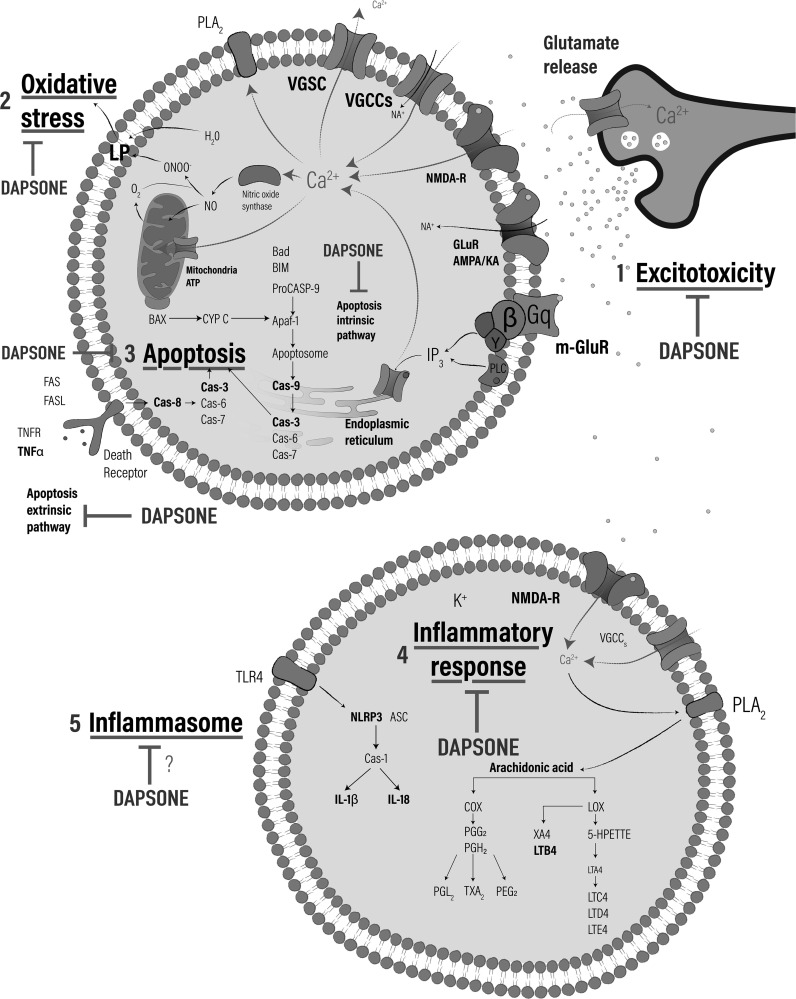
Fig. (2).
Neuroprotective and cytoprotective mechanisms of dapsone. 1) Antiexcitotoxic effect: Regulates glutamate release preventing the activation of metabotropic and ionotropic receptors (m-GluR y NMDA, respectively) as well as Glu receptors (AMPARs) and kainate receptors (KA), which leads to avoid membrane depolarization inhibiting the opening of voltage-gated calcium channels (VGCCs), voltage-dependent sodium channels (VGSC) and voltage gated-potassium channels (VGKC), regulating the signaling cascade and ions liberation. 2. Antioxidant effect: Decrease the formation of superoxide radical (O2-) and hydrogen peroxide (H2O2), produced in mitochondria during the excitotoxic and ischemic process, avoiding cell death and decrease the NO production and peroxynitrite (ONOO-). 3) Dapsone regulates formation of the multiprotein complex known as inflammasome active by Toll-like receptor 4 (TLR4) attenuating the assembly of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) interacting with the ASC and recruits the Caspase-1 (Cas-1) responsible of formation of proinflammatory interleukins (IL-1β y IL-18). 4) Antiinflamatory effect: regulates metabolism of arachidonic acid mediated by cyclooxygenase (COX) and lipoxygenase (LOX) through inhibition of phospholipase A2, reducing the formation of chemoattractants as prostaglandins (PGDs), thromboxanes (TXBs) and leukotrienes (LTs). 5) Antiapoptotic effect: Regulates both the intrinsic as extrinsic way of apoptosis mediated by the activation of several caspases.
Dapsone is rapidly and completely absorbed by the gastrointestinal tract with a bioavailability of nearly 90%. Peak serum concentrations are achieved between 2 to 8 hours after administration and its elimination takes about 20-30 hours [17, 18].
Once inside the body, dapsone passes into the enterohepatic circulation and is metabolized by the liver. Metabolic inactivation is carried out through cytochrome P450 (CYP) in hepatocytes. However, other cells such as polymorphonuclear cells (PMNs) and monocytes can contribute to its metabolism [19]. Dapsone metabolism can occur in two ways in hepatocytes: N-acetylation (by an N-acetyltransferase) or N-hydroxylation (by isoforms of CYP) such as cytochrome P450-2E1 (CYP2E1) and cytochrome P450-2C (CYP2C).
Acetylation generates Monoacetyldapsone (MADDS) [20, 21] and hydroxylation, dapsone hydroxylamine (DDS-NHOH) [22]. Dapsone is distributed into all organs and excreted through urine and a small amount can be excreted in feces [6].
The bacteriostatic effect is given by the regulation of folic acid synthesis (necessary for DNA synthesis) by inhibiting the enzyme dihydropteroate synthetase that incorporates Paraminobenzoic Acid (PABA) into dihydropteroate, which is the immediate precursor of folic acid [23].
The anti-inflammatory effect on PMNs is carried out by the inhibition of the formation of ROS [24], as well as by the inhibition of Myeloperoxidase (MPO), the enzyme responsible for producing Hypochlorous acid (HOCl), a highly reactive and cytotoxic compound [25, 26]. Dapsone also inhibits the cell migration process (adherence to endothelium and chemotaxis) of PMNs; by interfering with chemokine receptors (G protein-coupled receptors; (GPCRs), integrins (CD11b / CD18) [27-29], chemotaxis by Leukotriene B4 (LTB4) [30] and inhibition of the formation of the Interleukin-8 (IL-8) [31, 32]. Other cytokines also identified as a target for dapsone are Interleukin 1 beta (IL-1β) and Tumor Necrosis Factor-alpha (TNF-α) [33] (Fig. 2).
Dapsone has a well-known safety profile. Adverse effects occur between 0.5-3.6% of people taking dapsone pills for the control of infections [16]. Methemoglobinemia is the main adverse effect of dapsone, and Methemoglobin molecule possesses a high affinity for molecular iron that produces hypoxia by not allowing oxygen release in the tissues [23, 34, 35].
Clinically, hypoxemia manifestations as dyspnea, acrocyanosis, and headache, as well as the presence of hemolysis in laboratory tests, and dissociation between the concentration of oxyhemoglobin in blood and oxygen desaturation using the pulse oximeter characterizes this condition.
Methemoglobinemia and hemolysis, as the adverse effects of dapsone, may coincide with the genetic deficiency of 6-phosphate dehydrogenase, with chronic use of the drug and in some cases with doses higher than 200 mg/kg. A recent study demonstrated the utility of high doses of dapsone in three patients suffering from relapsing and remitting Lyme disease; they were treated with double doses of dapsone (200 mg/kg/day for a total of 7-8 weeks) showed remission of main Lyme symptoms for a period of 25 to 30 months with no side effects [36]. Furthermore, it is relevant to note that these adverse effects are reversible as medication stops [37-39], and some treatments, such as methylene blue and ascorbic acid, are available to resolve clinical methemoglobinemia [40].
2. METHODS
This review includes clinical trial reports and original studies using experimental models with rats, cats, ferrets and in vitro cells. The database consulted was from the National Library of Medicine, National Center for Biotechnology Information (NIH) PubMed, taking as inclusion criteria only works published in English, using the following keywords: Dapsone and Epilepsy, dapsone and excitotoxicity, dapsone and quinolinic acid, dapsone and seizures, dapsone and Parkinson models, dapsone and Alzheimer´s disease, multiple sclerosis and blood-brain barrier and anti-inflammatory treatments review, dapsone and cerebrovascular disease, dapsone and neuroprotection, dapsone and glioblastoma multiforme, cardioprotective effects of dapsone, dapsone and damage, dapsone and acne, dapsone and diabetes mellitus and dapsone and Lung. From 685 retrieved references, 161 articles were reviewed and discussed as they met the inclusion and exclusion criteria.
3. DISCUSSION
3.1. Epilepsy and Status Epilepticus
Epilepsy is one of the most common neurological problems, affecting 1% to 2% of the human world population. The International League Against Epilepsy (ILAE) defined epilepsy as a chronic neurological condition characterized by recurrent epileptic seizures. A seizure is a brain dysfunction with transitory paroxysms with a recurrent tendency and spontaneous termination [41]. Some works report that temporal lobe epilepsy in humans and animal models is associated with neuronal loss in the hippocampus during epileptogenesis [42, 43]. On the other hand, status epilepticus (SE) is a severe medical condition defined by the ILAE as a disorder that results from the failure of the mechanisms responsible for the termination or the initiation mechanisms of the crisis, which leads to abnormal and prolonged seizures: It is a condition frequently related with long-term consequences including neuronal death, neuronal damage, and disruption of neural networks, depending on the type and duration of seizures [44].
Regardless of the presence or not of any systemic, cardiovascular, or metabolic complication as well as pre-existing epilepsy, SE produces disseminated neuronal loss and reactive gliosis in the hippocampus, amygdala, the dorsomedial nucleus of the thalamus, in the cerebellar Purkinje cell layer, and the periamigdaloid (piriformis) region.
In patients with SE, the neuronal loss has a similar distribution to that seen in SE induced by domoic acid intoxication in humans and SE induced by Kainic acid and pilocarpine in rats [45].
There is a wide variety of medications used to treat epilepsy. These include sodium, potassium, and calcium transmembrane blockers, N-methyl-D-aspartate (NMDA) α-amino-3-hydroxy-5-methyl-4-isoazolepropionic acid (AMPA) channel blockers, gamma-amino butyric acid (GABA) agonists or enhancers, and synaptic vesicle blockers, all of which improved over time, resulting in a new generation of antiepileptic drugs. The therapeutic success of antiepileptic drugs depends on the tolerability of the drug, adherence to treatment and the reduction of adverse reactions (dizziness, headache, diplopia, and ataxia) [46] as well, in some cases, of the capacity to develop treatment resistance [47]. Most of these drugs have little or no neuroprotective effect; therefore, it seems reasonable to propose new therapeutic strategies with an alternative, complementary and neuroprotective approach with minimal adverse reactions even with prolonged use with not drug resistance (Fig. 2).
Recent work shows the efficacy of dapsone to treat epilepsy and SE in animals and humans (Table 1) [48-58]. In support of the above, some authors describe that the acute and chronic administration of dapsone (6.25, 9.375, 12.5 mg/kg) reduces the number of seizures generated by electrical stimulation of the amygdala and hippocampus in cats with no adverse reactions (maximum dose of 40 mg/kg) [48, 49]. On the other hand, the use of dapsone (9,375 and 125 mg/kg) administered 30 min before inducing seizures by kainic acid (AK) (10 mg/kg) decreases the number of seizures and mortality in a dose-dependent manner in rats [50]. Likewise, the use of dapsone and sodium phenobarbital (12.5/30 mg/kg, respectively) alone or in combination diminished KA-induced seizures, decreased lipoperoxidation, and significantly increases neuronal hippocampal viability in rats [51]. Similarly, the combination of dapsone and Diazepam (25/20 mg/kg, respectively) administered during seizures demonstrated therapeutic efficacy in a model of SE induced by KA by significantly reducing the electrical activity associated with this condition; this effect remained up to 24 hours after drugs administration.
Table 1.
Excitotoxicity and experimental epilepsy models and pharmacological effects of dapsone and analogs.
| Specie | Treatment | Doses | Model | Effect | References |
|---|---|---|---|---|---|
| Rat | Dapsone | 6.25-12,5 mg/Kg | Amygdaloid kindling model of epilepsy | ↓ Seizures | [48] |
| Dapsone | 6.25, 9.375 y 12.5 mg/Kg | Amygdala-kindled seizures and hippocampal-kindled seizures model | ↓ Seizures | [49] | |
| Dapsone | 9.375 y 125 mg/Kg | Temporal lobe epilepsy model (Kainic Acid) |
↓ Seizures ↓ Mortality |
[50] | |
| Dapsone | 12.5 mg/Kg and 25 mg/Kg | Excitotoxicity model by quinolinic acid (an NMDA agonist of glutamate receptors)- and kainic acid (a non-NMDA agonist of glutamate receptors |
↑Functional improvement Prevented the decrease of GABA Neuroprotection |
[56] | |
| Dapsone | 12.5 mg/Kg and 25 mg/Kg | Excitotoxicity model by quinolinic acid (an NMDA agonist of glutamate receptors) |
↓Lipid peroxidation Prevent the neuronal damage |
[57] | |
| Dapsone analogs (1) 4,4'-diaminodifenylsulfone N,N'-diformaldehyde sulfoxylate (2) 4,4'-diaminodiphenylsulfone N,N'-didextrose sulfonate (3) Sodium dibisulfite 4,4'-biscinamilidenamindiphenyl sulfone (4) N,N'-dimethyl-4,4'-dimethylphenylsulfone |
3.12 and 6.25 mg/Kg | Temporal lobe epilepsy model (Kainic Acid) |
↓ Seizures Only analogs 3 and 4 showed therapeutic effect |
[53] | |
| Dapsone analogs (1) N,N'-dimethyldapsone (2) N,N'-diethyldapsone (3) N,N'-dipropyldapsone (4) N,N'-dibutyldapsone and N,N'-ditosyldapsone |
12.5mg/Kg and equimolar doses respectively | Excitotoxicity model by quinolinic acid |
↓ Methemoglobin ↑Functional improvement Prevented the decrease of GABA |
[54] | |
| - | Dapsone/ Phenobarbital |
12.5/30 mg/Kg | Temporal lobe epilepsy model (Kainic Acid) |
↓ Lipid peroxidation ↓ Mortality ↑ Hippocampal neuronal cells viability |
[51] |
| - | Dapsone/ Diazepam |
25/20mg/Kg | Status Epilepticus model Kainic Acid |
↓ Seizures ↑ Hippocampal neuronal cells viability |
[52] |
| Kat | Dapsone | 13-23 mg/Kg | Amygdaloid kindling model of epilepsy | ↓ Seizures | [48] |
| Human | Dapsone | 100 mg/día | Open Clinical Trial | ↓ Seizures | [55] |
| - | Dapsone | - | Review | ↓ IL-8 ↓ Seizures |
[58] |
Abbreviations: NMDA: N-methyl-D-aspartate receptor, GABA: Gamma-aminobutyric acid.
A more significant number of viable hippocampal CA-3 pyramidal neurons were also found in animals treated with dapsone than in the control group (untreated). This study indicates that dapsone is not only an anticonvulsant drug but also a neuroprotective one [52]. Dapsone analogs have also been shown to have a neuroprotector effect by decreasing lipoperoxidation, increasing cell viability, preventing GABA reduction, and improving behavioral disturbances, after the application of quinolinic acid, a known agonist of the NMDA receptor. Some of those analogs, with N- substituents, also decreased methemoglobin levels compared to dapsone itself [53, 54] (Fig. 2).
A clinical study shows the therapeutic effect of dapsone in patients who develop resistance to conventional treatments. Administration of 100 mg/day decreased the frequency of seizures per month by more than 50%, after a follow-up for 3 months. In terms of therapeutic safety, there were no severe adverse effects related to dapsone daily administration. Only symptoms such as methemoglobinemia (at subclinical levels), headaches, drowsiness, and paleness were observed [55]. The studies carried out on the anticonvulsant effect of dapsone in vivo models, and one study in drug-resistant epileptic patients have shown encouraging results to consider dapsone and some of its analogs, a potential treatment to decrease the number and intensity of seizures and the consequent degeneration of nervous tissue, necessary for preserving CNS functions.
3.2. Parkinson's Disease
PD is a progressive neurodegenerative disorder characterized by the massive loss of dopaminergic neurons in the Substantia Nigra pars compacta (SNc) [59] and the generalized presence of intracytoplasmic and intraneuritic inclusions of α aggregates-synuclein (Lewy bodies (DLB)), and Lewy Neurites (LN) [60]. PD results from the interaction between genetic susceptibility and some identified environmental factors [61]. After AD, PD is the second most common chronic and systemic neurodegenerative disease in adults [62, 63].
In industrialized countries, the prevalence of this disease ranges between 0.3% and 1% in older than 60 years and reaches 3% in those older than 80 years or more, with incidence rates that vary between 0.08 and 0.18 per 1,000 people/year [64]. The main clinical characteristics of patients with PD are impairments in motor functions such as tremor at rest, rigidity, postural instability, and bradykinesia. It also causes non-motor manifestations, including cognitive, behavioral and pain [65]. Several studies have reported that motor and non-motor problems are related to aging, accelerating neuronal dysfunction, and extended loss of dopaminergic neurons in the SNc. Several studies have reported that motor and non-motor problems are related to aging, accelerating neuronal dysfunction, and extended loss of dopaminergic neurons in the SNc.
Disturbance of protein degradation by the ubiquitin-proteasome system might have a critical role in neurodegeneration. Parkin is a ubiquitin-protein ligase involved in protein degradation as collaborating with the ubiquitinconjugating enzyme UbcH7. PD patients show loss of this ubiquitin-protein ligase activity [66, 67].
Interestingly, long-term treatment with dapsone (2 mg/kg) restores parkin levels through its up-regulation. Furthermore, chronic treatment with dapsone prevents neuronal loss related to normal aging and reduces oxidative stress in mice with MPTP-induced PD [68].
On the other hand, a study on cellular aging, using an animal aging and lifespan model in worms (C. elegans) treated with paraquat (free radical-generating compound, used as a model of Parkinson´s disease), dapsone (5 mg/kg) reduces the production of free radicals and increased life expectancy and motor activity [69].
3.3. Dementia and Alzheimer's Disease
Dementia is a state of progressive cognitive impairment that leads to loss of independent functions and significant disruption of daily living activities [70]. More than 70 conditions cause the clinical syndrome of dementia [71]. Of these, AD represents 60-80% of all cases, followed by vascular dementia and other neurodegenerative dementias such as DLB dementia, dementia-Parkinson complex, and frontotemporal dementia [72]. Around 35.6 million people worldwide are affected by AD, mainly affecting people over 65 [73]. Therefore, AD should be suspected in a patient older than 60 years who suffers from cognitive impairment and progressive short-term memory impairment. Although AD definitive diagnosis requires a histopathological or post-mortem study [74], biological markers are now available to make an early diagnosis. Among these, quantification of total Tau protein (Tau), Phosphorylated Tau isoforms (P-Tau181 and P-Tau231), and β-amyloid peptide (Ab42), in cerebrospinal fluid, showed sensitivity and specificity of 80-90% [75].
Drugs used to treat AD, and other forms of degenerative dementia can resolve biochemical abnormalities due to neuronal loss but do not modify the underlying neuropathology or its progression. Cholinesterase inhibitors partially restore the acetylcholine deficiency that arises from the loss of neurons in the nucleus basalis of Meynert and the central septum area, projecting to the cortical regions. Memantine as an NMDA receptor inhibitor eventually attenuates the toxic effects of glutamate released by degenerating neurons, although its exact mechanism of action is uncertain. No drug has shown neuroprotective potential in humans [76].
Chronic inflammatory processes are related to the progression of different degenerative diseases, including AD. There is evidence that dapsone treatment by regulating brain cells' inflation decreases the progression of cognitive disorders in patients with AD [71]. A clinical study demonstrated that leprosy patients treated with dapsone had a lower incidence of dementia than tuberculosis treatment [77] and without treatment [78]. Dapsone does not seem to have a direct effect on the degenerative neuronal process once it shows high levels of β-amyloid in cell cultures and patients with AD [79, 80]; therefore, it is possible that dapsone does not modify the progression of the disease in advanced phases. However, a recent review [81] suggests that it is necessary to develop a combined drug therapy to attenuate chronic inflammation and confer neuroprotection in AD. In this report, it is proposed that the mechanisms to neuroprotect against AD must include: anti-inflammatory actions, show drug permeability through the Blood-Brain Barrier (BBB), evidence of an efficacy in blocking oxidative damage and the chemotactic response mediated by activated microglia; Evidence indicates that dapsone possesses these effects in different conditions both in clinical and experimental studies, and this strategy could be used to delay the progression of AD (Fig. 3).
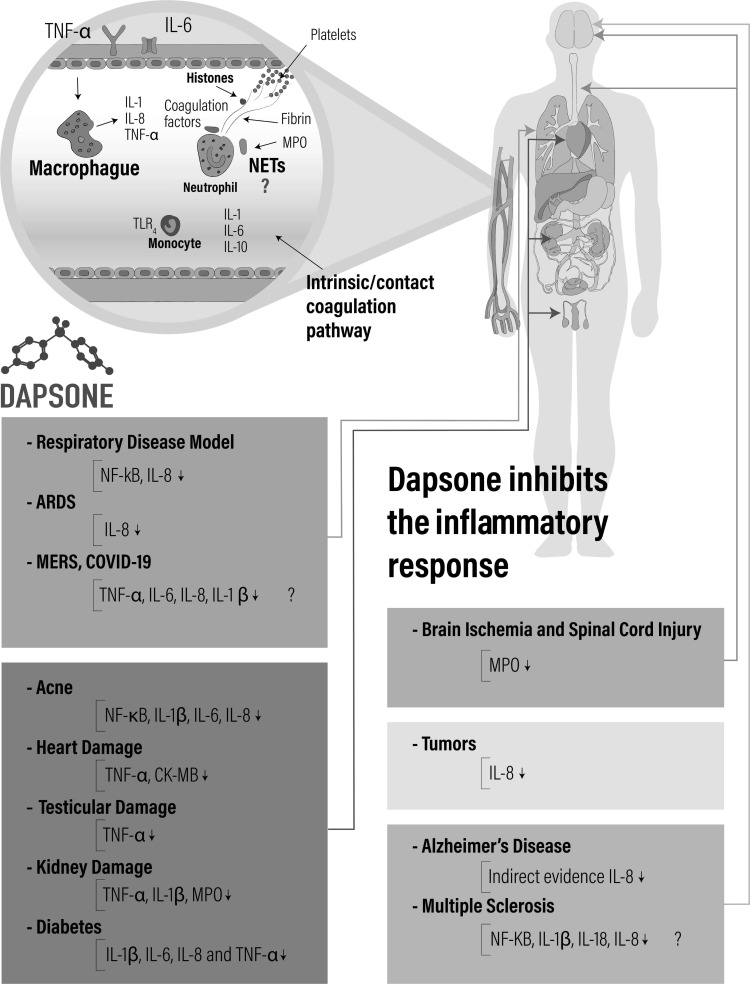
Fig. (3).
Anti-inflammatory effect of dapsone in several diseases. Alzheimer’s disease and dementia syndrome the evidence shows that dapsone decreased levels of IL-8. Multiple sclerosis, it is proposed that due to the histopathological characteristics of the disease, dapsone treatment may decrease the levels of various pro-inflammatory cytokines (IL-1β, IL-18 and IL-8) and the expression of the transcription factor NFkB. Respiratory disease model a decrease in IL8 and expression of the transcription NFκB factor were observed. Acute respiratory distress syndrome (ARDS), Middle East respiratory syndrome (MERS) and coronavirus disease 2019 (COVID-19), by pathophysiological characteristics the dapsone may be decrease the levels of various proinflammatory cytokines (IL-1β, IL-6, IL-8 and TNFα). Acnes showed diminished of IL-1β, IL-6, IL-8 and NFκB factor. Heart damage TNFα and Creatine kinase-myocardial (CK-MB) diminished levels. Testicular damage (TNFα diminished level). Kidney ischemia damage (TNFα, IL-1β and Myeloperoxidase (MPO) decreased levels). Diabetes (IL-1β, IL-8, IL-18 and TNFα diminished levels). Brain ischemia and Spinal cord injury MPO decreased level. Tumors IL-8 decreased level. Blood vessels the dapsone lowers the levels of IL-8, IL-6 and TNFα by blocking the inflammatory response mediated by macrophages and neutrophils present in the blood vessels, an effect that may prevent the formation of extracellular neutrophil networks (NETs) traps and the disseminated thrombosis characteristic of the COVID-19 disease.
Given these data, it is important to consider of interest to carry out new research to determine if the anti-inflammatory effect of dapsone is useful or not to modify the course of the disease.
3.4. Cognitive Dysfunction and Depression
Yang et al. (2017) [82] evaluated the effect of propofol on cognition in aged rats treated or not with dapsone, since this anesthetic commonly produces postoperative cognitive dysfunction in elderly patients. The results showed that propofol produces alterations in learning and memory in rats, associated with decreased autophagy in the hippocampus and that pretreatment with dapsone at a dose of 5 mg / kg or 10 mg / kg of body weight significantly improved the behavioral disorder and positively regulated the inhibited autophagic response.
Propofol is frequently related to postoperative cognitive dysfunction in elderly patients. Yang et al. (2017) [82] evaluated propofol's effect on cognition in aged rats treated or not with dapsone. They confirmed that propofol produces alterations in learning and memory in rats, associated with decreased autophagy in the hippocampus, and pretreatment with dapsone at a dose of 5 mg / kg or 10 mg / kg of body weight significantly improved the behavioral disorder and positively regulated the inhibited autophagic response.
Dapsone has also been proposed as an antidepressant treatment in experimental models of aged mice undergoing abdominal surgery since it has been described that depression induced by surgical stress and anxiety are common complications in patients after surgery, as well as recent studies have shown that oxidative stress is involved in the pathophysiology of depression and anxiety induced by surgical stress [83]. As dapsone is known to possess antioxidant properties, Zhang et al. (2015) [84] evaluated the effect of dapsone on depressive and anxious behavior and brain oxidative stress in a surgical stress model. The results demonstrated depressive and anxiety-like behaviors accompanied by elevated brain oxidative stress in aged mice undergoing abdominal surgery and that pretreatment with dapsone 5 mg / kg significantly improved the mice's behavior and decreased brain oxidative stress. They also observed that surgical stress increased the level of NADPH oxidase in the brain, while pretreatment with dapsone blocked the elevation of NADPH oxidase triggered by surgical stress. Therefore, the authors suggest that dapsone is effective in preventing oxidative damage to the brain induced by surgical stress by downregulating the level of NADPH oxidase in elderly mice.
Furthermore, because depression induced by surgical stress and anxiety are common complications in patients after surgery, dapsone has also been proposed as an antidepressant treatment in experimental models of aged mice undergoing abdominal surgery.
Recent studies have shown that oxidative stress is involved in the pathophysiology of depression and anxiety induced by surgical stress [83]. As dapsone is known to possess antioxidant properties, Zhang et al. (2015) [84] evaluated the effect of dapsone on depressive and anxious behavior and brain oxidative stress in a surgical stress model. The results demonstrated depressive and anxiety-like behaviors accompanied by elevated brain oxidative stress in aged mice undergoing abdominal surgery and that pretreatment with dapsone 5 mg/kg has a significant improvement on the mice behavior and decreased brain oxidative stress. They also observed that surgical stress increased NADPH oxidase's level in the brain, while pretreatment with dapsone blocked the elevation of NADPH oxidase triggered by surgical stress. Therefore, the authors suggest that dapsone effectively prevents oxidative damage to the brain induced by surgical stress by downregulating the level of NADPH oxidase in elderly mice.
3.5. Multiple Sclerosis and Other Autoimmune Diseases
Multiple Sclerosis (MS) is a chronic inflammatory disorder of the CNS characterized by demyelination and axonal and neuronal degeneration [85]. MS affects more than 2 million people worldwide [86], presenting itself as the most common cause of non-traumatic disability in young adults [87]. The most common symptoms include visual loss in one eye due to optic neuritis, limb weakness, sensory dysfunction due to transverse myelitis, double vision due to brain stem dysfunction, or ataxia due to cerebellar injury. These lesions occur throughout the white matter of CNS as focal areas of demyelination, inflammation, and glial reaction [86]. Activation of the inflammasome and the breakdown of the BBB seems to play fundamental roles in the autoimmune and pro-inflammatory responses in various neurological diseases, including MS and autoimmune encephalitis, since the excessive induction of Caspase-1 (Cas-1) leads to a programmed cell death process called pyroptosis characterized by the rapid induction of an inflammatory response leading to cell lysis [88]. As mentioned previously, MS is a demyelinating disease of the CNS and remains the most common autoimmune disorder affecting the CNS [86]. Although the cause of MS remains unclear, the pathological mechanisms present can be due to damage to myelin-producing cells (oligodendrocytes) or direct damage to the myelin layer by autoimmune T cells. Recent advances have indicated that inflammasomes contribute to the etiology of MS [89]. In this way, inflammasomes are multiprotein complexes of the innate immune response involved in the processing of Cas-1, the activation of the pro-inflammatory cytokines IL-1β and IL-18, as well as the mechanism of pyroptosis mediated by cell death and activation of the adaptive immune response [90]. Inhibition of the inflammasome is a therapeutic strategy used in patients with MS. Interferon- β (IFNβ) is one of the first-line treatments for MS and can inhibit the inflammasomes NLRP1 and NLRP3 [91]. Based on this information, dapsone may be useful in patients with MS since it can covalently compete with proteins that activate the NLRP3 inflammasome and to decrease the nuclear factor -kappa B (NF-kB) signaling that drives transcription of inflammatory cytokines and chemokines, including IL-1β, IL-18, and IL-8, and priming the expression of NLRP3 to its functional level [71]. On the other hand, the BBB rupture contributes to the development of the disease because the autoaggressive T cells cross this barrier, causing demyelination that eventually leads to progressive disability [92]. The BBB integrity maintenance represents a fundamental pillar for the correct functioning of the CNS; some misfunctioning of this barrier are associated with various neurodegenerative diseases [93, 94]. The BBB has high selective permeability to peripheral molecules and cells, which allows maintaining CNS homeostasis [95]. Endothelial Cells (EC) are the hallmark of this barrier. Specific proteins in the CE of the CNS allow tight junctions between the BBB cells, preventing a possible leakage of molecules or cells potentially damaging to the tissue. These proteins are claudins, occludins, and Zonula Occludens (ZO) cytoplasmic proteins [96, 97]. An experimental study using a model of disruption of BBB in rats by administering lipopolysaccharide demonstrated that the administration of 0.5 mg/kg, 2 mg/kg and 5 mg/kg of dapsone managed to normalize the BBB in a dose-dependent effect by regulating the levels of the Zonula occludens-1 (ZO-1), occludin and claudin-5, which are responsible for generating the tight junctions of the BBB [98]. In another similar study, dapsone induced recovery of the BBB integrity in a model of injury generated by a high-fat diet in mice. The continuous administration of dapsone reversed the lipid peroxidation induced by this diet and the loss of tight junction proteins after eight weeks [99].
Dapsone is a proven effective treatment for subacute cutaneous lupus erythematosus, particularly in refractory drug cases [100]. The potential mechanisms for this efficacy are attributed to the inhibition of the myeloperoxidase enzyme by dapsone, its inhibitory effect on the synthesis and release of prostaglandins, the inhibition of the production of leukotrienes C4 and the inhibition of the adherence of neutrophils to cells vascular endothelial cells and their subsequent extravasation through the endothelial vascular wall, thus preventing the migration of neutrophils to the injury sites [101].
Furthermore, dapsone has been tested to treat bullous systemic lupus erythematosus, a rare bullous condition associated with systemic lupus erythematosus [102]. The results showed that dapsone was effective in 90% of the cases to diminished dermal infiltration of polynuclear neutrophils, alignment of these cells at the basal membrane zone and leukocytoclastic. Therefore, the authors conclude that dapsone is the first-choice option for this disease.
Finally, the therapeutic efficacy of dapsone has been tested in autoimmune diseases such as pemphigus and pemphigoid, two variants of the autoimmune mucocutaneous bullous dermatoses [103]. The anti-inflammatory action of dapsone lies in its ability to suppress neutrophils migration and production of toxic secretory products that cause skin damage [6, 28]. Therefore, it is effective in various skin disorders associated with abnormal neutrophils accumulation such as dermatitis herpetiformis, linear IgA bullous dermatosis, pyoderma gangrenosum, PV, BP, sweet’s syndrome and vasculitis [5, 6]. All the patients showed a satisfactory response to dapsone, achieving disease remission in a short period with no serious side-effects necessitating treatment cessation.
3.6. Dapsone in Ischemic and Traumatic Diseases of the Central Nervous System
3.6.1. Cerebrovascular Diseases
Cerebrovascular disease (CVD) is a temporary or permanent alteration of the functioning of one or more parts of the brain caused by a circulatory disorder [104]. Clinically characterized as an acute-onset focal neurological deficit, which includes cerebral infarction, intracerebral hemorrhage and subarachnoid hemorrhage. The frequency of stroke subtypes is variable, while ischemic stroke occurs in approximately 70% of cases, and hemorrhagic stroke in 30% in different series [105]. In some reports, data from different hospitals showed that ischemic stroke occurs in 57% of cases, intracranial hemorrhage in 28%, and subarachnoid hemorrhage in 12.0%, and cerebral venous thrombosis in 3% [106].
The occlusive phenomenon originating ischemic stroke has different causes; large artery atherosclerosis, cardioembolism, small artery disease, and in some cases, the cause cannot be determined [107]. In this patient group, comorbidity is the rule; in people older than 60 years, arterial hypertension, diabetes mellitus, hyperlipidemia, heart disease and altered coagulation states (including transitory states such as pregnancy and the puerperium) are known risk factors and as well-defined causative agents [108].
Regardless of the etiology, interruption of blood flow to the brain triggers chemical events that alter tissue homeostasis, produce neurotoxicity and changes in gene expression, which lead to cell death, loss of nerve connections [109] and apoptosis [110]. That is why restoring cerebral blood flow in the shortest possible time is a priority, and thrombolytic therapy and thrombectomy have marked a before and after in patients' prognosis [111]. Unfortunately, full and timely recanalization is often not achieved, and in this clinical scenario is crucial to continue and propose new treatments with different approaches to efficiently decrease the degradation of nervous tissue [112]. In this sense, in an experimental study, the effect of dapsone at doses of 9.375 mg/kg and 12.5 mg/kg was evaluated in a model of cerebral ischemia in Wistar rats, the results allowed to demonstrate in the experimental group with dapsone, a smaller infarction area, better physical performance and they presented lower mortality than control rats [113]. In the effort of translational science, a pilot study was performed in patients who suffered from an ischemic stroke, using dapsone (200 mg) as treatment within the first 10 hours of evolution after stroke. The treated patients presented lower neurological deficits and better capacity to carry out daily living activities after 60 days of follow-up [114].
Dapsone has an antioxidant, anti-inflammatory and antiapoptotic effect proven in an ischemia/reperfusion model [115]. In other similar experimental models of ischemia, dapsone demonstrated a beneficial effect by achieving, in the experimental group, attenuation of different markers of nerve tissue damage such as edema, generation of ROS, area of infarction (injured tissue), neurological deficit and transcription factors associated with oxidative stress such as Nuclear factor-erythroid 2 related factor 2 (Nrf-2) [116]. Finally, in a recent work, using a model of occlusion of the middle cerebral artery in rats, the administration of dapsone, even six h after the injury, reduced the area of cerebral infarction and improved neurobehavioral performance (Fig. 3).
On the other hand, the results of an in vitro model of excitotoxicity with glutamate, demonstrated that dapsone could reduce neuronal damage by inhibiting the pro-apoptotic proteins c-Jun N-terminal kinase (JNK), Phosphatidyl 3,4,5-triphosphate 3 phosphatase (PTEN), calpain, caspase-3 (Cas-3), together with the activation of the survival protein Brain derived neurotrophic factor (BDNF), concluding that the treatment with dapsone can be an effective treatment with a wide therapeutic window [117].
3.6.2. Spinal Cord Injury
Spinal Cord Injury (SCI) is a serious condition that in most cases produces permanent disability and irreversible damage. The annual incidence of SCI depends on the geographical area and the socioeconomic environment, being approximately 10 to 80 patients per million inhabitants, with a prevalence of 235-1800 victims per million inhabitants [118, 119]. The clinical spectrum of SCI includes loss of voluntary mobility, decreased range of motion [120], autonomic dysfunction, peripheral vasodilation, alteration of thermoregulation, respiratory deficiencies [118] and alteration of sensitivity [120]. The SCI has complex pathophysiology, including neurotoxic processes, apoptosis, oxidative stress, inflammation and excitotoxicity [121, 122], making it difficult to develop new treatments focused on neuroprotection.
Regarding dapsone, the administration of (2.5 mg/kg to rats, up to 3 or 5 hours after the injury, managed to decrease the damage of the nervous tissue by diminishing the area of injured tissue, lipid peroxidation, the inflammatory response (decrease of neutrophil MPO activity) and contributing significantly to functional recovery. Furthermore, when testing a therapeutic window of 3 hours after the injury, this effect was more evident [123]. Subsequent studies demonstrated that treatment with dapsone reduces apoptosis in spinal cord tissue by reducing Caspase-9 (Cas-9), Caspase-8 (Cas-8) and Cas-3 [124]. In a recently published review work, the mechanisms and neuroprotective targets that lead to the therapeutic success of dapsone and other antibiotics after SCI are analyzed and synthesized, with evidence of the anti-inflammatory, antiapoptotic, antiexcitotoxic and antioxidant effects of the sulfone (Fig. 3). This review provides information on the role of dapsone in translational medicine to manage SCI and its complications [125].
3.7. Cancer
Cancer is an anormal and uncontrolled cellular growth in any organ or body structure [126]. Current cancer research focuses on early recognition of a malignant lesion delimiting the patient's healthy tissue and implementing radio and medical therapy. The knowledge of the tumor microenvironment allows for describing and predicting cancer cells' behavior and response to a specific treatment. This microenvironment is composed of fibroblasts, immune cells, cells that form new blood vessels, and the proteins produced by all cells present in the tumor that promote the growth of cancer cells [127].
In Glioblastoma (GB), the tumoral CE abnormally generates IL-8 and increases neutrophil recruitment. Neutrophils, in turn, produce Vascular Endothelial Growth Factor (VEGF), inducing angiogenesis and more CE that produce more IL-8 and greater recruitment of neutrophils as a vicious circle [128, 129]. This accumulation of neutrophils in the tumor tissue is a biological marker of poor prognosis [130, 131].
It has been suggested [132] that dapsone is potentially useful for the treatment of GB multiforme by inhibiting neutrophil accumulation, migration, and response to IL-8, as well as the production of IL-8, [133]. Furthermore, dapsone and its analogs have an antiproliferative effect in glioma cell cultures [134].
3.8. Dapsone as Cytoprotective Drug
3.8.1. Cardiac Damage Model
Doxorubicin, a drug frequently used in cancer treatment [135], has been associated with adverse effects such as ROS generation, inflammation, and cell death; damaging various organs such as the heart, brain, kidney and liver [136]. In an experimental study with rats, dapsone was applied at doses of 1, 3, and 10 mg/kg, 30 min after the administration of doxorubicin (2.5 mg/kg) for two weeks. Results showed that dapsone prevented damage to cardiac tissue by decrease lipid peroxidation, inflammatory response (decrease of TNF α expression) and diminished Creatine Kinase-myocardial (CK-MB), associated with destabilization of cardiac cell membranes, and a marker of cardiac cells´ damage [137].
3.8.2. Testicle Damage Model
Testicular ischemia occurs in patients with torsion of the spermatic cord. This pathology requires prompt attention to avoid irreversible ischemic damage that causes sterility and a possible orchiectomy [138, 139]. In an experimental study of testicular torsion in rats, dapsone, when administered (12.5 mg/kg) 30 min before testis torsion, showed a decrease of inflammatory cytokines (TNF α), lipid peroxidation and diminished lesion of the tissue [140].
3.8.3. Kidney Damage Model
Ischemic and reperfusion damage occurs in the kidney of patients with kidney transplantation, hydronephrosis, nephrectomy and acute kidney damage [141-143]. In an experimental study in rats, dapsone (20 mg/kg) administration significantly decreased kidney scars after pyelonephritis. This protective effect of the sulfone was due to its anti-inflammatory effect through MPO inhibition [144]. It is also possible to observe the protective effect of dapsone on the kidneys when this drug is administered 30 min before (1, 3 and 10 mg/kg) the production of ischemia. The levels of inflammatory cytokines (TNFα, IL-1β), cell death and tissue damage due to necrosis and hemorrhage, all decreased due to the use of dapsone [145].
3.8.4. Diabetes Model and Acne In Vitro Models
Another potential use of dapsone can be in wound healing of patients with diabetes, which undergo an intense inflammation process. Treatment with dapsone (30 mg/kg) improves wound healing in streptozotocin-induced diabetic rats, through the diminution of both IL-8 mediated neutrophil infiltration and oxidative stress [146]. In non-obese mice, treatment with dapsone allowed a lower possibility of developing insulin-dependent diabetes mellitus than in controls. The diminution of ROS by this drug can delay the onset of the disease and prevent lymphocytic infiltration of the islets of Langerhans in mice [147]. In this effect, the participation of dapsone-induced prevention of the over-activity of NADPH oxidase (Nox) generated by an increase in oxidative stress was reported [148]. Likewise, there are reports indicating that the use of dapsone (0.4 μg/mL, 4 μg/mL and 40 μg/mL) favors the recovery of the lesions and the inflammatory process induced by acne (Cutibacterium) by reducing the levels of IL-8 in human epidermal keratinocytes and IL-1β, Interleukin-6 (IL-6), IL-8, and TNFα in primary neonatal epidermal keratinocyte cells and human monocytes in response to Cutibacterium [149].
3.8.5. Dapsone in Models of Lung Damage
Experimental evidence in lung damage models demonstrates that IL-8 is an important activator and chemoattractant for neutrophils, produced by normal human bronchial epithelial cells through the p65 pathways of the Mtogen-Activated Protein kinase (MAPK) and NF-κB. Based on this information, Kanoh et al. (2011) [31] designed a study of lipopolysaccharide-induced lung damage in ferrets to evaluate the anti-inflammatory effect of dapsone. The study was conducted as follows: ferrets were exposed to intratracheal Liposaccharide (LPS) for five days to produce tracheal inflammation. Once inflammation was established, oral or nebulized dapsone was administered for five days. Histological tracheal tissue samples demonstrated that dapsone inhibited the secretion of IL-8 and decreased the level of IL-8 mRNA induced by LPS and decrease in phosphorylation of the transcription factor NF-κB p65; this is an essential mechanism since the activation of NF-κB p65 exacerbates the inflammatory response and consequent oxidative stress [150].
The protective effect of dapsone was tested in a lung damage model with paraquat in mice [151]. Results showed a reduction of lung damage due to the treatment with dapsone and low local expression of mRNA transcripts encoding molecules related to inflammation, including Endothelin-1 (ET-1), Macrophage Inflammatory Protein-1α (MIP-1α), and transforming growth factor β (TGF-β), decreased the expression of Nox mRNA and the activation of Protein kinase Cμ (PKCμ), induced by paraquat. Also, a decrease the oxidative stress was observed after dapsone, evaluated as a decreased generation of superoxide anions induced by paraquat. Based on this information, the authors propose that dapsone is an effective protective treatment against tissue damage induced by oxidative stress and inflammation (Fig. 3).
3.8.6. Acute Respiratory Distress Syndrome, Middle East Respiratory Syndrome CoV and COVID-19 Acute Respiratory Diseases
Acute Respiratory Distress Syndrome (ARDS) is associated with high fatality and high levels of IL-8. The IL-8 neutrophile attraction activity contributes to the alveolar damage of the ARDS, whereas neutrophil infiltration into alveoli is present in ARDS-related coronavirus infections (SARS-CoV) and the Middle East Respiratory Syndrome CoV (MERS-CoV) [152].
Regarding COVID-19 infection and SARS-CoV-2 syndrome, the viral infection process began when the viral spike protein bound to the receptor for Angiotensin Converting Enzyme 2 (ACE2), expressed in various types of cells; Type II pneumocytes, macrophages, and CE. Once the cell is infected, begins a highly inflammatory cell death process called pyroptosis, which results in the release of Damage-Associated Molecular Patterns (DAMP), leading to a hyper-inflammatory response [153], mediated by hyperactivated monocytes and activated monocyte-derived macrophages that release massive amounts of pro-inflammatory cytokines [154].
Patients with COVID-19 present thrombotic events that include activation of platelets leading to an overexpression of the coagulation cascade in a distinct manner from those seen with bacterial sepsis-induced coagulopathy and disseminated intravascular coagulation. COVID-19 infection blockage the conversion of angiotensin 2 to angiotensin 1-7, decreasing endothelial Nitric oxide (NO) production and its vasodilator and platelet aggregation blocking effect, allowing the accumulation of endothelial von Willebrand factor, that, in turn, increases thrombus production and platelet activation, by increasing D-dimer and fibrinogen levels with minimal abnormalities in prothrombin time and platelet count [155].
Furthermore, high levels of IL-1β and IL-6 in COVID-19 infection induce thrombocytosis and hyperfibrinogenemia. The hypercoagulable state due to sustained inflammation is named thromboinflammation. An interesting topic in the COVID-19 infection is the poor regulation of the so-called extracellular neutrophil networks (NETs) formed by chromatin, microbicidal proteins and oxidizing enzymes released by neutrophils to contain an infection. Inadequate regulation of NETs propagates inflammation and produces disseminated microvascular thrombosis [156]. The presence of NET remnants in the serum of patients hospitalized for COVID-19 is related to an increased risk of thrombotic events and increased mortality [157]. In in vitro studies, it is possible to demonstrate NET formation when neutrophils from healthy people are exposed to the serum of patients with COVID 19 [157]. COVID-19 patients also show high levels of some inflammatory cytokines such as IL-6, Interleukin-10 (IL-10), and TNFα and chemokines (XCL10/IP-10, CCL2/MCP-1 and CCL3/MIP-1α), however, the expression of this elevation depends on the disease's evolution and severity.
On the other hand, patients who develop severe illness from COVID-19 show an increase in the IL-8 concentration and, due to its chemotactic effect, a more than double increase of the number of neutrophils in the blood (10.6 x 109/L), compared to those patients who did not require intensive care (4.4 x 109/L) [158]. Changes in plasma levels of Cytokines, Chemokines, and Growth Factors (CCGF) and how these are related depend on the severity of COVID-19 disease. Xu et al. (2020) highlight the considerable elevation of IL-8 and IL-6 in COVID-19 patients with a fatal outcome, suggesting that the reduction in the levels of these cytokines is a possible therapeutic target [159].
Regarding those changes observed in patients with COVID-19, some authors have recently proposed the therapeutic benefit of dapsone [7,8] as a treatment in patients with lung damage generated by the SARS-CoV-2 virus, since it could modulate the production of pro-inflammatory cytokines [160], the secretion of IL-8 from bronchial epithelial cells, by regulating TNFα from activated mononuclear cells and the decrease in MPO activity [161]. Finally, Schön, et al., (2020) [10], in a recent review, discussed the therapeutic benefit of various immunological therapies that regulate the main inflammatory mechanisms in COVID-19, from a basic aspect and its clinical translational implications. In this review, they included dapsone because it can inhibit cytokine storm and neutrophil chemotaxis in the lungs (Fig. 3). Our group suggests dapsone as a potential, safe drug to be tested in patients developing severe COVID-19 infection by SARS-CoV2.
CONCLUSION
The neuro and cytoprotective effect of dapsone has been tested both in experimental models and in clinical studies in neurodegenerative, traumatic, and ischemic diseases of the CNS such as dementia, PD, AD, epilepsy, SE, cerebral infarction, spinal cord trauma, as well as in conditions such as diabetes mellitus, ischemic testicular damage, heart damage, kidney ischemia and lung damage, all associated to exacerbated inflammatory responses. After reviewing the literature, it is concluded that dapsone regulates or decreases the main mechanisms of damage such as oxidative stress, excitotoxicity, inflammation and various mechanisms of cell death. Likewise, its therapeutic safety has been demonstrated both in experimental and clinical studies as its anti-inflammatory efficacy has been widely demonstrated, proposing its use in acute diseases such as acute respiratory distress syndrome, MERS, and COVID-19. Therefore, we conclude that dapsone is a safe and effective neuro and cytoprotective treatment that must be considered translational therapy.
ACKNOWLEDGEMENTS
We thank Michel Nader Sayun for preparing and correcting the final version of the illustrations.
LIST OF ABBREVIATIONS
- ACE2
angiotensin converting enzyme 2
- AD
Alzheimer's disease
- AK
Kainic acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoazolepropionic acid
- ARDS
Acute respiratory distress syndrome
- BBB
Blood-brain barrier
- BDNF
Brain Derived Neurotrophic Factor
- Cas-1
Caspase-1
- Cas-3
Caspase-3
- Cas-8
Caspase-8
- Cas-9
Caspasa-9
- CE
Endothelial cells
- CCGF
growth factors
- CK-MB
Creatine kinase-myocardial
- CNS
Central nervous system
- CVD
Cerebrovascular disease
- CYP
Cytocrome P450
- CYP2C
Cytocrome P4502C
- CYP2E1
Cytocrome P4502E1
- DAMP
damage-associated molecular patterns
- DDS-NHOH
Dapsone hydroxylamine
- DLB
Lewy bodies
- ET-1
Endothelin-1
- GABA
Gamma-amino butyric acid
- GB
Glioblastoma
- GPCRs
G protein-coupled receptors
- HOCl
Hypochlorous acid
- IFNβ
Interferon- β
- ILAE
International League Against Epilepsy
- IL-1β
Interleukin 1β
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- IL-10
Interleukin-10
- JNK
c-Jun N-terminal kinase
- LTB4
Leukotriene B4
- LN
Lewy Neurites
- LPS
Liposaccharide
- MADDS
monoacetyldapsone
- MAPK
mitogen-activated protein kinase
- MERS-CoV
East respiratory syndrome CoV
- MIP-1α
protein-1α
- MPO
Myeloperoxidase
- MS
Multiple sclerosis
- NETs
Extracellular Neutrophil Networks
- NF-kB
Nuclear factor -kappa B
- NMDA
N-methyl-D-aspartate
- NO
Nitric oxide
- Nox
Nicotinamide adenine dinucleotide phosphate oxidase
- Nrf-2
Nuclear factor-erythroid 2 related factor 2
- PABA
Paraminobenzoic acid
- PD
Parkinson's disease
- PKCμ
activation of protein kinase Cμ
- PMNs
Polymorphonuclear cells
- PTEN
Phosphatidyl 3,4,5-triphosphate 3 phosphatase
- ROS
Reactive oxygen species
- SARS-CoV
coronavirus infections
- SE
Status epilepticus
- SNc
Substantia Nigra pars compacta
- SCI
Spinal Cord Injury
- Tau
Tau protein
- TGF-β
transforming growth factor β
- TNF-α
Tumor Necrosis Factor alpha
- VEGF
Vascular Endothelial Growth Factor
- ZO
Zonula occludens
- ZO-1
Zonula occludens-1
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This review was partially sponsored by Servicios al Enfermo Neurológico S.C., Mexico.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Leker R.R., Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: Neuroprotective opportunities. Brain Res. Brain Res. Rev. 2002;39(1):55–73. doi: 10.1016/S0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- 2.Kunz A., Dirnagl U., Mergenthaler P. Acute pathophysiological processes after ischaemic and traumatic brain injury. Best Pract. Res. Clin. Anaesthesiol. 2010;24(4):495–509. doi: 10.1016/j.bpa.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Mendel N.P., Boris D.M. Neuroprotection: The way of anti-inflammatory agents. Neuroprotection - new approaches and prospects. Intech Open; 2020. [Google Scholar]
- 4.Molinelli E., Paolinelli M., Campanati A., Brisigotti V., Offidani A. Metabolic, pharmacokinetic, and toxicological issues surrounding dapsone. Expert Opin. Drug Metab. Toxicol. 2019;15(5):367–379. doi: 10.1080/17425255.2019.1600670. [DOI] [PubMed] [Google Scholar]
- 5.Wozel G., Blasum C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014;306(2):103–124. doi: 10.1007/s00403-013-1409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y.I., Stiller M.J. Dapsone and sulfones in dermatology: Overview and update. J. Am. Acad. Dermatol. 2001;45(3):420–434. doi: 10.1067/mjd.2001.114733. [DOI] [PubMed] [Google Scholar]
- 7.Farouk A., Salman S. Dapsone and doxycycline could be potential treatment modalities for covid-19. Med. Hypotheses. 2020;140:109768. doi: 10.1016/j.mehy.2020.109768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altschuler E.L., Kast R.E. Covid-19 associated adult respiratory distress syndrome (ards). Med. Hypotheses. 2020;141:109774. doi: 10.1016/j.mehy.2020.109774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J.H., An H.K., Sohn M.G., Kivela P., Oh S. 4,4′-Diaminodiphenyl Sulfone (DDS) as an inflammasome competitor. Int. J. Mol. Sci. 2020;21(17):1–23. doi: 10.3390/ijms21175953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schön M.P., Berking C., Biedermann T., Buhl T., Erpenbeck L., Eyerich K., Eyerich S., Ghoreschi K., Goebeler M., Ludwig R.J., Schäkel K., Schilling B., Schlapbach C., Stary G., von Stebut E., Steinbrink K. Covid-19 and immunological regulations – from basic and translational aspects to clinical implications. German Soc. Dermatol. 2020;18(8):795–807. doi: 10.1111/ddg.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wozel G. The story of sulfones in tropical medicine and dermatology. Int. J. Dermatol. 1989;28(1):17–21. doi: 10.1111/j.1365-4362.1989.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolf R., Matz H., Orion E., Tuzun B., Tuzun Y. Dapsone. Dermatol. Online J. 2002;8(1):3–47. doi: 10.2165/00128415-201214270-00052. [DOI] [PubMed] [Google Scholar]
- 13.Faget Q., Rogge R., Johansen F., Dinan J. P. B. y E. C. The promin treatment of leprosy. Public Health Rep. 1943;34(3):298–310. [PubMed] [Google Scholar]
- 14.Shepard C.C. Leprosy today. N. Engl. J. Med. 1982;307(26):1640–1641. doi: 10.1056/NEJM198212233072608. [DOI] [PubMed] [Google Scholar]
- 15.Chaves L.L., Patriota Y., Soares-Sobrinho J.L., Vieira A.C.C., Costa Lima S.A., Reis S. Drug delivery systems on leprosy therapy: Moving towards eradication? Pharmaceutics. 2020;12(12):1202. doi: 10.3390/pharmaceutics12121202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghaoui N., Hanna E., Abbas O., Kibbi A.G., Kurban M. Update on the use of dapsone in dermatology. Int. J. Dermatol. 2020;59(7):7787–7795. doi: 10.1111/ijd.14761. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad R.A., Rogers H.J. Pharmacokinetics and protein binding interactions of dapsone and pyrimethamine. Br. J. Clin. Pharmacol. 1980;10(5):519–524. doi: 10.1111/j.1365-2125.1980.tb01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pieters F.A., Zuidema J. The pharmacokinetics of dapsone after oral administration to healthy volunteers. Br. J. Clin. Pharmacol. 1986;22(4):491–494. doi: 10.1111/j.1365-2125.1986.tb02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuidema J., Hilbers-Modderman E.S.M., Merkus F.W.H.M. Clinical pharmacokinetics of dapsone. Clin. Pharmacokinet. 1986;11(4):299–315. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]
- 20.Glazko A.J., Chang T., Baukema J., Chang S.F. S. A. y D. W. A. Central role of madds in the metabolism of DDS. Int. J. Lepr. 1969:462–463. [Google Scholar]
- 21.Karim A.K.M.B., Elfellah M.S., Evans D.A.P. Human acetylator polymorphism: Estimate of allele frequency in Libya and details of global distribution. J. Med. Genet. 1981;18(5):325–330. doi: 10.1136/jmg.18.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uehleke H. N-hydroxylation. Xenobiotica. 1971;1(4):327–338. doi: 10.3109/00498257109041497. [DOI] [PubMed] [Google Scholar]
- 23.Coleman M.D. Dapsone: Modes of action, toxicity and possible strategies for increasing patient tolerance. Br. J. Dermatol. 1993;129(5):507–513. doi: 10.1111/j.1365-2133.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 24.Vyas P.M., Roychowdhury S., Woster P.M., Svensson C.K. Reactive oxygen species generation and its role in the differential cytotoxicity of the arylhydroxylamine metabolites of sulfamethoxazole and dapsone in normal human epidermal keratinocytes. Biochem. Pharmacol. 2005;70(2):275–286. doi: 10.1016/j.bcp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Bozeman P.M., Learn D.B., Thomas E.L. Inhibition of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase by dapsone. Biochem. Pharmacol. 1992;44(3):553–563. doi: 10.1016/0006-2952(92)90449-S. [DOI] [PubMed] [Google Scholar]
- 26.Weiss S.J., Weiss S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 27.Booth S.A., Moody C.E., Dahl M.V., Herron M.J., Nelson R.D. Dapsone suppresses integrin-mediated neutrophil adherence function. J. Invest. Dermatol. 1992;98(2):135–140. doi: 10.1111/1523-1747.ep12555654. [DOI] [PubMed] [Google Scholar]
- 28.Debol S.M., Herron M.J., Nelson R.D. Anti-inflammatory action of dapsone: Inhibition of neutrophil adherence is associated with inhibition of chemoattractant-induced signal transduction. J. Leukoc. Biol. 1997;62(6):827–836. doi: 10.1002/jlb.62.6.827. [DOI] [PubMed] [Google Scholar]
- 29.Harvath L., Yancey K.B., Katz S.I. Selective inhibition of human neutrophil chemotaxis to n-formyl-methionyl-leucyl-phenyl-alanine by sulfones. J. Immunol. 1986;137(4):1305–1311. [PubMed] [Google Scholar]
- 30.Maloff B.L., Fox D., Bruin E., Di Meo T.M. Dapsone inhibits LTB4 binding and bioresponse at the cellular and physiologic levels. Eur. J. Pharmacol. 1988;158(1-2):85–89. doi: 10.1016/0014-2999(88)90256-7. [DOI] [PubMed] [Google Scholar]
- 31.Kanoh S., Tanabe T., Rubin B.K. Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with lipopolysaccharide and resolves airway inflammation in the ferret. Chest. 2011;140(4):980–990. doi: 10.1378/chest.10-2908. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt E., Reimer S., Kruse N., Bröcker E.B., Zillikens D. The IL-8 release from cultured human keratinocytes, mediated by antibodies to bullous pemphigoid autoantigen 180, is inhibited by dapsone. Clin. Exp. Immunol. 2001;124(1):157–162. doi: 10.1046/j.1365-2249.2001.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe M., Shimizu A., Yokoyama Y., Takeuchi Y., Ishikawa O. A possible inhibitory action of diaminodiphenyl sulfone on tumour necrosis factor-α production from activated mononuclear cells on cutaneous lupus erythematosus. Clin. Exp. Dermatol. 2008;33(6):759–763. doi: 10.1111/j.1365-2230.2008.02864.x. [DOI] [PubMed] [Google Scholar]
- 34.Grossman S.J., Jollow D.J. Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J. Pharmacol. Exp. Ther. 1988;244(1):118–125. [PubMed] [Google Scholar]
- 35.Mansouri A., Lurie A.A. Methemoglobinemia. Am. J. Hematol., 1993, 7-12. [DOI] [PubMed]
- 36.Horowitz R.I., Freeman P.R. Efficacy of double-dose dapsone combination therapy in the treatment of chronic lyme disease/post-treatment lyme disease syndrome (ptlds) and associated co-infections: A report of three cases and retrospective chart review. Antibiotics (Basel) 2020;9(11):1–24. doi: 10.3390/antibiotics9110725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barclay J.A., Ziemba S.E., Ibrahim R.B. Metahemoglobinemia inducida por dapsona: Un manual para facultativos. Ann. Pharmacother. 2011;45(9):1103–1115. doi: 10.1345/aph.1Q139. [DOI] [PubMed] [Google Scholar]
- 38.Paccor A., Matsuda M., Capurso C., Rizzo E., Larroca M.C. Methemoglobinemia due to dapsone: A pediatric case report. Arch. Argent. Pediatr. 2018;116(4):e612–e615. doi: 10.5546/aap.2018.e612. [DOI] [PubMed] [Google Scholar]
- 39.Skold A., Klein R. Symptomatic-low grade methemoglobinemia because of dapsone: A multiple hit hypothesis. Am. J. Ther. 2013;20(6):e729–e732. doi: 10.1097/MJT.0b013e318217a5af. [DOI] [PubMed] [Google Scholar]
- 40.Toker I., Yesilaras M., Tur F.C., Toktas R. Methemoglobinemia caused by dapsone overdose: Which treatment is best? Turk. J. Emerg. Med. 2016;15(4):182–184. doi: 10.1016/j.tjem.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardenas-Rodriguez N., Huerta-Gertrudis B., Rivera-Espinosa L., Montesinos-Correa H., Bandala C., Carmona-Aparicio L., Coballase-Urrutia E. Role of oxidative stress in refractory epilepsy: Evidence in patients and experimental models. Int. J. Mol. Sci. 2013;14(1):1455–76. doi: 10.3390/ijms14011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majores M., Schoch S., Lie A., Becker A.J. Molecular neuropathology of temporal lobe epilepsy: Complementary approaches in animal models and human disease tissue. Epilepsia. 2007;48(Suppl. 2):4–12. doi: 10.1111/j.1528-1167.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 43.Méndez-Armenta M., Nava-Ruíz C., Juárez-Rebollar D., Rodríguez-Martínez E., Yescas Gómez P. Oxidative stress associated with neuronal apoptosis in experimental models of epilepsy. Oxid. Med. Cell. Long. 2014;2014:293689. doi: 10.1155/2014/293689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blume W.T., Lüders H.O., Mizrahi E., Tassinari C., van Emde Boas W., Engel J., Jr Glossary of descriptive terminology for ictal semiology: Report of the ILAE task force on classification and terminology. Epilepsia. 2001;42(9):1212–1218. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- 45.Fujikawa D.G., Itabashi H.H., Wu A., Shinmei S.S. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41(8):981–991. doi: 10.1111/j.1528-1157.2000.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 46.Brodie M.J. Antiepileptic drug therapy the story so far. Seizure. 2010;19(10):650–655. doi: 10.1016/j.seizure.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 47.Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Allen Hauser W., Mathern G., Moshé S.L., Perucca E., Wiebe S., French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 48.Hamada K., Hiyoshi T., Kobayashi S., Ishida S., Yagi K., Seino M. Anticonvulsive effect of dapsone (4,4′-diaminodiphenyl sulfone) on amygdala-kindled seizures in rats and cats. Epilepsy Res. 1991;10(2–3):93–102. doi: 10.1016/0920-1211(91)90001-v. [DOI] [PubMed] [Google Scholar]
- 49.Ishida S., Hamada K., Yagi K., Seino M. Comparing the anticonvulsive effects of dapsone on amygdala-kindled seizures and hippocampal-kindled seizures in rats. Acta Neurol. Scand. 1992;85(2):132–135. doi: 10.1111/j.1600-0404.1992.tb04012.x. [DOI] [PubMed] [Google Scholar]
- 50.Altagracia M., Monroy-Noyola A., Osorio-Rico L., Kravzov J., Alvarado-Calvillo R., Manjarrez-Marmolejo J., Ríos C. Dapsone attenuates kainic acid-induced seizures in rats. Neurosci. Lett. 1994;176(1):52–54. doi: 10.1016/0304-3940(94)90869-9. [DOI] [PubMed] [Google Scholar]
- 51.Diaz-Ruiz A., Mendez-Armenta M., Galván-Arzate S., Manjarrez J., Nava-Ruiz C., Santander I., Balderas G., Ríos C. Antioxidant, anticonvulsive and neuroprotective effects of dapsone and phenobarbital against kainic acid-induced damage in rats. Neurochem. Res. 2013;38(9):1819–1827. doi: 10.1007/s11064-013-1087-z. [DOI] [PubMed] [Google Scholar]
- 52.Ríos C., Farfán-Briseño A.C., Manjarrez-Marmolejo J., Franco-Pérez J., Méndez-Armenta M., Nava-Ruiz C., Caballero-Chacón S., Ruiz-Diaz A., Baron-Flores V., Díaz-Ruiz A. Efficacy of dapsone administered alone or in combination with diazepam to inhibit status epilepticus in rats. Brain Res. 2019;1708:181–187. doi: 10.1016/j.brainres.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 53.López-Naranjo F., Castañeda-López C., Rojas-Oviedo I., Altagracia-Martínez M., Krazov-Jinich J., Manjarrez-Marmolejo J., Alvarado-Calvillo R. Anticonvulsant activity of dapsone analogs. Electrophysiologic evaluation. Arch. Med. Res. 2003;34(4):269–272. doi: 10.1016/S0188-4409(03)00045-6. [DOI] [PubMed] [Google Scholar]
- 54.Tristán-López L., Pérez-Álvarez V., Pérez-Severiano F., Montes S., Pérez-Neri I., Rivera-Espinosa L., Ríos C. Protective effect of N,N′-dialkylated analogs of 4,4′-diaminodiphenylsulfone in a model of intrastriatal quinolinic acid induced-excitotoxicity. Neurosci. Lett. 2012;528(1):1–5. doi: 10.1016/j.neulet.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 55.López-Gómez M., Corona T., Diaz-Ruiz A., Ríos C. Safety and tolerability of dapsone for the treatment of patients with drug-resistant, partial-onset seizures: An open-label trial. Neurol. Sci. 2011;32(6):1063–1067. doi: 10.1007/s10072-011-0612-6. [DOI] [PubMed] [Google Scholar]
- 56.Santamaría A., Ordaz-Moreno J., Rubio-Osornio M., Solís-Hernández F., Ríos C. Neuroprotective effect of dapsone against quinolinate- and kainate-induced striatal neurotoxicities in rats. Pharmacol. Toxicol. 1997;81(6):271–275. [PubMed] [Google Scholar]
- 57.Rodríguez E., Méndez-Armenta M., Villeda-Hernández J., Galván-Arzate S., Barroso-Moguel R., Rodríguez F., Ríos C., Santamaría A. Dapsone prevents morphological lesions and lipid peroxidation induced by quinolinic acid in rat corpus striatum. Toxicology. 1999;139(1-2):111–118. doi: 10.1016/S0300-483X(99)00116-X. [DOI] [PubMed] [Google Scholar]
- 58.Kast R.E., Lefranc F., Karpel-Massler G., Halatsch M.E. Why dapsone stops seizures and may stop neutrophils’ delivery of vegf to glioblastoma. Br. J. Neurosurg. 2012;26(6):813–817. doi: 10.3109/02688697.2012.674577. [DOI] [PubMed] [Google Scholar]
- 59.Luk K. C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J. Q., Lee V. M. Y. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science (80-.)., 2012, 338(6109), 949-953. [DOI] [PMC free article] [PubMed]
- 60.Deleidi M., Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell. Mol. Life Sci. 2013;70(22):4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30(5):244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 62.Beitz J.M. Parkinson’s disease: A review. Front. Biosci. 2014;6:65–74. doi: 10.2741/S415. [DOI] [PubMed] [Google Scholar]
- 63.Tysnes O.B., Storstein A. Epidemiology of Parkinson’s disease. J. Neural Trans. 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 64.Pringsheim T., Jette N., Frolkis A., Steeves T.D.L. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 65.Broen M.P.G., Braaksma M.M., Patijn J., Weber W.E.J. Prevalence of pain in Parkinson’s disease: A systematic review using the modified QUADAS tool. Mov. Disord. 2012;27(4):480–484. doi: 10.1002/mds.24054. [DOI] [PubMed] [Google Scholar]
- 66.Shimura H., Hattori N., Kubo Si., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000;25(3):302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 67.Hattori N., Mizuno P.Y. Pathogenetic mechanisms of parkin in Parkinson’s disease. Lancet. 2004;364(9435):722–724. doi: 10.1016/S0140-6736(04)16901-8. [DOI] [PubMed] [Google Scholar]
- 68.Lee Y.I., Kang H., Ha Y.W., Chang K.Y., Cho S.C., Song S.O., Kim H., Jo A., Khang R., Choi J.Y., Lee Y., Park S.C., Shin J.H. Diaminodiphenyl sulfone-induced parkin ameliorates age-dependent dopaminergic neuronal loss. Neurobiol. Aging. 2016;41:1–10. doi: 10.1016/j.neurobiolaging.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 69.Cho S.C., Park M.C., Keam B., Choi J.M., Cho Y., Hyun S., Park S.C., Lee J. DDS, 4,4′-diaminodiphenylsulfone, extends organismic lifespan. Proc. Natl. Acad. Sci. USA. 2010;107(45):19326–19331. doi: 10.1073/pnas.1005078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ljubenkov P.A., Geschwind M.D. Dementia. Semin. Neurol. 2016;36(4):397–404. doi: 10.1055/s-0036-1585096. [DOI] [PubMed] [Google Scholar]
- 71.Lee J.H., Choi S.H., Lee C.J., Oh S.S. Recovery of dementia syndrome following treatment of brain inflammation. Dement. Geriatr. Cogn. Disord. Extra. 2020;10(1):1–12. doi: 10.1159/000504880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garre-Olmo J. Epidemiology of Alzheimer’s disease and other dementias. Rev. Neurol. 2018;66(11):377–386. doi: 10.33588/rn.6611.2017519. [DOI] [PubMed] [Google Scholar]
- 73.Khan H., Ullah H., Aschner M., Cheang W.S., Akkol E.K. Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules. 2020;10(1):59. doi: 10.3390/biom10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H., Zheng Y. β amyloid hypothesis in Alzheimer’s disease: Pathogenesis, prevention,and management. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Med. Sinicae. 2019;41(5):702–708. doi: 10.3881/j.issn.1000-503X.10875. [DOI] [PubMed] [Google Scholar]
- 75.Hulstaert F., Blennow K., Ivanoiu A., Schoonderwaldt H.C., Riemenschneider M., De Deyn P.P., Bancher C., Cras P., Wiltfang J., Mehta P.D., Iqbal K., Pottel H., Vanmechelen E., Vanderstichele H. Improved discrimination of AD patients using β-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555–1562. doi: 10.1212/WNL.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 76.Bishara D., Sauer J., Taylor D. The pharmacological management of Alzheimer’s disease. Prog. Neurol. Psychiatry. 2015;19(4):9–16. doi: 10.1002/pnp.387. [DOI] [Google Scholar]
- 77.Goto M., Kimura T., Hagio S., Ueda K., Kitajima S., Tokunaga H., Sato E. Neuropathological analysis of dementia in a Japanese leprosarium. Dementia. 1995;6(3):157–161. doi: 10.1159/000106939. [DOI] [PubMed] [Google Scholar]
- 78.McGeer P.L., Harada N., Kimura H., McGeer E.G., Schulzer M. Prevalence of dementia amongst elderly japanese with leprosy: Apparent effect of chronic drug therapy. Dement. Geriatr. Cogn. Disord. 1992;3(3):146–149. doi: 10.1159/000107010. [DOI] [Google Scholar]
- 79.Endoh M., Kunishita T., Tabira T. No effect of anti-leprosy drugs in the prevention of Alzheimer’s disease and β-amyloid neurotoxicity. J. Neurol. Sci. 1999;165(1):28–30. doi: 10.1016/S0022-510X(99)00057-X. [DOI] [PubMed] [Google Scholar]
- 80.Eriksen J.L., Sagi S.A., Smith T.E., Weggen S., Das P., McLendon D.C., Ozols V.V., Jessing K.W., Zavitz K.H., Koo E.H., Golde T.E. NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Abeta 42 in vivo. J. Clin. Invest. 2003;112(3):440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLarnon J.G. Consideration of a pharmacological combinatorial approach to inhibit chronic inflammation in Alzheimer’s Disease. Curr. Alzheimer Res. 2019;16(11):1007–1017. doi: 10.2174/1567205016666191106095038. [DOI] [PubMed] [Google Scholar]
- 82.Yang N., Li L., Li Z., Ni C., Cao Y., Liu T., Tian M., Chui D., Guo X. Protective effect of dapsone on cognitive impairment induced by propofol involves hippocampal autophagy. Neurosci. Lett. 2017;649:85–92. doi: 10.1016/j.neulet.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 83.Patki G., Solanki N., Atrooz F., Allam F., Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang T., Tian X., Wang Q., Tong Y., Wang H., Li Z., Li L., Zhou T., Zhan R., Zhao L., Sun Y., Fan D., Lu L., Zhang J., Jin Y., Xiao W., Guo X., Chui D. Surgical stress induced depressive and anxiety like behavior are improved by dapsone via modulating NADPH oxidase level. Neurosci. Lett. 2015;585:103–108. doi: 10.1016/j.neulet.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 85.Faissner S., Plemel J.R., Gold R., Yong V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019;18(12):905–922. doi: 10.1038/s41573-019-0035-2. [DOI] [PubMed] [Google Scholar]
- 86.Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple sclerosis. New Engl. J. Med. 2018;378(2):169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamm C.P., Uitdehaag B.M., Polman C.H. Multiple sclerosis: Current knowledge and future outlook. Eur. Neurol. 2014;72(3-4):132–141. doi: 10.1159/000360528. [DOI] [PubMed] [Google Scholar]
- 88.Ming X., Li W., Maeda Y., Blumberg B., Raval S., Cook S.D., Dowling P.C. Caspase-1 expression in multiple sclerosis plaques and cultured glial cells. J. Neurol. Sci. 2002;197(1-2):9–18. doi: 10.1016/S0022-510X(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 89.Danikowski K.M., Jayaraman S., Prabhakar B.S. Regulatory t cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation. 2017;14(1):117. doi: 10.1186/s12974-017-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Govindarajan V., De Rivero V.J.P., Keane R.W. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J. Neuroinflammation. 2020;17(1):260. doi: 10.1186/s12974-020-01944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 92.Ortiz G.G., Pacheco-Moisés F.P., Macías-Islas M.Á., Flores-Alvarado L.J., Mireles-Ramírez M.A., González-Renovato E.D., Hernández-Navarro V.E., Sánchez-López A.L., Alatorre-Jiménez M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014;45(8):687–697. doi: 10.1016/j.arcmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Jiang X., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., Keep R.F., Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Obermeier B., Verma A., Ransohoff R.M. The blood-brain barrier.Handbook of clinical neurology; Elsevier, 2016, 133, pp. 39-59. [DOI] [PubMed] [Google Scholar]
- 96.Dejana E., Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr. Opin. Hematol. 2012;19(3):218–223. doi: 10.1097/MOH.0b013e3283523e1c. [DOI] [PubMed] [Google Scholar]
- 97.Huber J.D., Egleton R.D., Davis T.P. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24(12):719–725. doi: 10.1016/S0166-2236(00)02004-X. [DOI] [PubMed] [Google Scholar]
- 98.Zhou T., Zhao L., Zhan R., He Q., Tong Y., Tian X., Wang H., Zhang T., Fu Y., Sun Y., Xu F., Guo X., Fan D., Han H., Chui D. Blood-brain barrier dysfunction in mice induced by lipopolysaccharide is attenuated by dapsone. Biochem. Biophys. Res. Commun. 2014;453(3):419–424. doi: 10.1016/j.bbrc.2014.09.093. [DOI] [PubMed] [Google Scholar]
- 99.Zhan R., Zhao M., Zhou T., Chen Y., Yu W., Zhao L., Zhang T., Wang H., Yang H., Jin Y., He Q., Yang X., Guo X., Willard B., Pan B., Huang Y., Chen Y., Chui D., Zheng L. Dapsone protects brain microvascular integrity from high-fat diet induced LDL oxidation. Cell Death Dis. 2018;9(6):683. doi: 10.1038/s41419-018-0739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zampeli E., Moutsopoulos H.M. Dapsone: An old drug effective for subacute cutaneous lupus erythematosus. Rheumatology (Oxford) 2019;58(5):920–921. doi: 10.1093/rheumatology/key434. [DOI] [PubMed] [Google Scholar]
- 101.Moutsopoulos M., Zampeli E., Vlachoyiannopoulos G. Medications, therapeutic modalities, and regimens used in the management of rheumatic diseases. Rheumatol. Questions; 2018. pp. 153–157. [Google Scholar]
- 102.de Risi-Pugliese T., Cohen Aubart F., Haroche J., Moguelet P., Grootenboer-Mignot S., Mathian A., Ingen-Housz-Oro S., Hie M., Wendremaire N., Aucouturier F., Lepelletier F., Miyara M., Bader-Meunier B., Rémy P., Fabien N., Francès C., Barete S., Amoura Z. Clinical, histological, immunological presentations and outcomes of bullous systemic lupus erythematosus: 10 new cases and a literature review of 118 cases. Semin. Arthritis Rheum. 2018;48(1):83–89. doi: 10.1016/j.semarthrit.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 103.Alenazi S.A., Elmorsy E., Al-Ghafari A., El-Husseini A. Effect of amphotericin B-deoxycholate (Fungizone) on the mitochondria of Wistar rats’ renal proximal tubules cells. J. Appl. Toxicol. 2021;41(10):1620–1633. doi: 10.1002/jat.4151. [DOI] [PubMed] [Google Scholar]
- 104.Bamford J., Sandercock P., Dennis M., Burn J., Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi: 10.1016/0140-6736(91)93206-O. [DOI] [PubMed] [Google Scholar]
- 105.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B., Bin A.A., Birbeck G., Blyth F., Bolliger I., Boufous S., Bucello C., Burch M., Burney P., Carapetis J., Chen H., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahodwala N., De Leo D., Degenhardt L., Delossantos A., Denenberg J., Des Jarlais D.C., Dharmaratne S.D., Dorsey E.R., Driscoll T., Duber H., Ebel B., Erwin P.J., Espindola P., Ezzati M., Feigin V., Flaxman A.D., Forouzanfar M.H., Fowkes F.G., Franklin R., Fransen M., Freeman M.K., Gabriel S.E., Gakidou E., Gaspari F., Gillum R.F., Gonzalez-Medina D., Halasa Y.A., Haring D., Harrison J.E., Havmoeller R., Hay R.J., Hoen B., Hotez P.J., Hoy D., Jacobsen K.H., James S.L., Jasrasaria R., Jayaraman S., Johns N., Karthikeyan G., Kassebaum N., Keren A., Khoo J.P., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lipnick M., Lipshultz S.E., Ohno S.L., Mabweijano J., MacIntyre M.F., Mallinger L., March L., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGrath J., Mensah G.A., Merriman T.R., Michaud C., Miller M., Miller T.R., Mock C., Mocumbi A.O., Mokdad A.A., Moran A., Mulholland K., Nair M.N., Naldi L., Narayan K.M., Nasseri K., Norman P., O’Donnell M., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Pahari B., Pandian J.D., Rivero A.P., Padilla R.P., Perez-Ruiz F., Perico N., Phillips D., Pierce K., Pope C.A., III, Porrini E., Pourmalek F., Raju M., Ranganathan D., Rehm J.T., Rein D.B., Remuzzi G., Rivara F.P., Roberts T., De León F.R., Rosenfeld L.C., Rushton L., Sacco R.L., Salomon J.A., Sampson U., Sanman E., Schwebel D.C., Segui-Gomez M., Shepard D.S., Singh D., Singleton J., Sliwa K., Smith E., Steer A., Taylor J.A., Thomas B., Tleyjeh I.M., Towbin J.A., Truelsen T., Undurraga E.A., Venketasubramanian N., Vijayakumar L., Vos T., Wagner G.R., Wang M., Wang W., Watt K., Weinstock M.A., Weintraub R., Wilkinson J.D., Woolf A.D., Wulf S., Yeh P.H., Yip P., Zabetian A., Zheng Z.J., Lopez A.D., Murray C.J., AlMazroa M.A., Memish Z.A. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carlos C-B., José R-S.L., Erwin C., Antonio A., Carolina L-J., Luis M-B.M., Jorge V-C., Fernando B., José Antonio F., Bertha T., Idelfonso R-L., Ricardo R-G. Factores de riesgo, causas y pronóstico de los tipos de enferme-dad vascular cerebral en méxico: Estudio renamevasc, 2011, 12.
- 107.Boltze J., Aronowski J.A., Badaut J., Buckwalter M.S., Caleo M., Chopp M., Dave K.R., Didwischus N., Dijkhuizen R.M., Doeppner T.R., Dreier J.P., Fouad K., Gelderblom M., Gertz K., Golubczyk D., Gregson B.A., Hamel E., Hanley D.F., Härtig W., Hummel F.C., Ikhsan M., Janowski M., Jolkkonen J., Karuppagounder S.S., Keep R.F., Koerte I.K., Kokaia Z., Li P., Liu F., Lizasoain I., Ludewig P., Metz G.A.S., Montagne A., Obenaus A., Palumbo A., Pearl M., Perez-Pinzon M., Planas A.M., Plesnila N., Raval A.P., Rueger M.A., Sansing L.H., Sohrabji F., Stagg C.J., Stetler R.A., Stowe A.M., Sun D., Taguchi A., Tanter M., Vay S.U., Vemuganti R., Vivien D., Walczak P., Wang J., Xiong Y., Zille M. New mechanistic insights, novel treatment paradigms, and clinical progress in cerebrovascular diseases. Front. Aging Neurosci. 2021;13:623751. doi: 10.3389/fnagi.2021.623751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cipolla M.J., Liebeskind D.S., Chan S.L. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J. Cereb. Blood Flow Metab. 2018;38(12):2129–2149. doi: 10.1177/0271678X18800589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saver J.L. Time is brain--quantified. Stroke. 2006;37(1):263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 110.Cheon S.Y., Kim E.J., Kim J.M., Koo B.N. Cell type-specific mechanisms in the pathogenesis of ischemic stroke: The role of apoptosis signal-regulating kinase 1. Oxid. Med. Cell. Longev. 2018;2018:2596043. doi: 10.1155/2018/2596043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alonso de Leciñana M., Egido J.A., Casado I., Ribó M., Dávalos A., Masjuan J., Caniego J.L., Martínez V.E., Díez T.E. Alonso de Leciñana, M.; Egido, J. A.; Casado, I.; Ribó, M.; Dávalos, A.; Masjuan, J.; Caniego, J. L.; Martínez Vila, E.; Díez, T.E.; Fuentes, S.B.; Álvarez-Sabin, J.; Arenillas, J.; Calleja, S.; Castellanos, M.; Castillo, J.; Díaz-Otero, F.; López-Fernández, J. C.; Freijo, M.; Gállego, J.; García-Pastor, A.; Gil-Núñez, A.; Gilo, F.; Irimia, P.; Lago, A.; Maestre, J.; Martí-Fábregas, J.; Martínez-Sánchez, P.; Molina, C.; Morales, A.; Nombela, F.; Purroy, F.; Rodríguez-Yañez, M.; Roquer, J.; Rubio, F.; Segura, T.; Serena, J.; Simal, P.; Tejada, J.; Vivancos, J. Guía Para El Tratamiento Del Infarto Cerebral Agudo. Neurologia. Neurologia. 2014;(March):102–122. doi: 10.1016/j.nrl.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 112.Lansberg M.G., O’Donnell M.J., Khatri P., Lang E.S., Nguyen-Huynh M.N., Schwartz N.E., Sonnenberg F.A., Schulman S., Vandvik P.O., Spencer F.A., Alonso-Coello P., Guyatt G.H., Akl E.A. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis. Chest, 9th; American College of Chest Physicians, 2012, 141, pp. (2 SUPPL.)e601S-e636S. Evidence-Based Clinical Practice Guidelines. [DOI] [PMC free article] [PubMed]
- 113.Ríos C., Nader-Kawachi J., Rodriguez-Payán A.J., Nava-Ruiz C. Neuroprotective effect of dapsone in an occlusive model of focal ischemia in rats. Brain Res. 2004;999(2):212–215. doi: 10.1016/j.brainres.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 114.Nader-Kawachi J., Góngora-Rivera F., Santos-Zambrano J., Calzada P., Ríos C. Neuroprotective effect of dapsone in patients with acute ischemic stroke: A pilot study. Neurol. Res. 2007;29(3):331–334. doi: 10.1179/016164107X159234. [DOI] [PubMed] [Google Scholar]
- 115.Diaz-Ruiz A., Zavala C., Montes S., Ortiz-Plata A., Salgado-Ceballos H., Orozco-Suarez S., Nava-Ruiz C., Pérez-Neri I., Perez-Severiano F., Ríos C. Antioxidant, antiinflammatory and antiapoptotic effects of dapsone in a model of brain ischemia/reperfusion in rats. J. Neurosci. Res. 2008;86(15):3410–3419. doi: 10.1002/jnr.21775. [DOI] [PubMed] [Google Scholar]
- 116.Diaz-Ruiz A., Roldan-Valadez E., Ortiz-Plata A., Mondragón-Lozano R., Heras-Romero Y., Mendez-Armenta M., Osorio-Rico L., Nava-Ruiz C., Ríos C. Dapsone improves functional deficit and diminishes brain damage evaluated by 3-Tesla magnetic resonance image after transient cerebral ischemia and reperfusion in rats. Brain Res. 2016;1646:384–392. doi: 10.1016/j.brainres.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 117.Mahale A., Kumar R., Sarode L.P., Gakare S., Prakash A., Ugale R.R. Dapsone prolong delayed excitotoxic neuronal cell death by interacting with proapoptotic/survival signaling proteins. J. Stroke Cerebrovasc. Dis. 2020;29(8):104848. doi: 10.1016/j.jstrokecerebrovasdis.2020.104848. [DOI] [PubMed] [Google Scholar]
- 118.Hagen E.M. Acute complications of spinal cord injuries. World J. Orthop. 2015;6(1):17–23. doi: 10.5312/wjo.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wyndaele M., Wyndaele J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord. 2006;44(9):523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 120.Hastings J.D., Harvey L.A., Bruce J.A., Somers M.F. comp ensation allows reco very of functional in dependence in people with severe motor impairm ents folowing spinal cord injury. J. Rehabil. Med. 2012;44(5):477–478. doi: 10.2340/16501977-0965. [DOI] [PubMed] [Google Scholar]
- 121.Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Silva N.A., Sousa N., Reis R.L., Salgado A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 123.Diaz-Ruiz A., Salgado-Ceballos H., Montes S., Guizar-Sahagún G., Gelista-Herrera N., Mendez-Armenta M., Diaz-Cintra S., Ríos C. Delayed administration of dapsone protects from tissue damage and improves recovery after spinal cord injury. J. Neurosci. Res. 2011;89(3):373–380. doi: 10.1002/jnr.22555. [DOI] [PubMed] [Google Scholar]
- 124.Ríos C., Orozco-Suarez S., Salgado-Ceballos H., Mendez-Armenta M., Nava-Ruiz C., Santander I., Barón-Flores V., Caram-Salas N., Diaz-Ruiz A. Anti-apoptotic effects of dapsone after spinal cord injury in rats. Neurochem. Res. 2015;40(6):1243–1251. doi: 10.1007/s11064-015-1588-z. [DOI] [PubMed] [Google Scholar]
- 125.Afshari K., Momeni Roudsari N., Lashgari N.A., Haddadi N.S., Haj-Mirzaian A., Hassan Nejad M., Shafaroodi H., Ghasemi M., Dehpour A.R., Abdolghaffari A.H. Antibiotics with therapeutic effects on spinal cord injury: A review. Fundam. Clin. Pharmacol. 2021;35(2):277–304. doi: 10.1111/fcp.12605. [DOI] [PubMed] [Google Scholar]
- 126.Roy P.S., Saikia B.J. Cancer and cure: A critical analysis. Indian J. Cancer. 2016;53(3):441–442. doi: 10.4103/0019-509X.200658. [DOI] [PubMed] [Google Scholar]
- 127.Wang J.J., Lei K.F., Han F. Tumor microenvironment: Recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018;22(12):3855–3864. doi: 10.26355/EURREV_201806_15270. [DOI] [PubMed] [Google Scholar]
- 128.Schruefer R., Lutze N., Schymeinsky J., Walzog B. Human neutrophils promote angiogenesis by a paracrine feedforward mechanism involving endothelial interleukin-8. Am. J. Physiol. Heart Circ. Physiol. 2005;288(3):57–3. doi: 10.1152/ajpheart.00237.2004. [DOI] [PubMed] [Google Scholar]
- 129.Sun S., Wang Q., Giang A., Cheng C., Soo C., Wang C.Y., Liau L.M., Chiu R. Knockdown of CypA inhibits interleukin-8 (IL-8) and IL-8-mediated proliferation and tumor growth of glioblastoma cells through down-regulated NF-κB. J. Neurooncol. 2011;101(1):1–14. doi: 10.1007/s11060-010-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bambury R.M., Teo M.Y., Power D.G., Yusuf A., Murray S., Battley J.E., Drake C., O’Dea P., Bermingham N., Keohane C., Grossman S.A., Moylan E.J., O’Reilly S. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J. Neurooncol. 2013;114(1):149–154. doi: 10.1007/s11060-013-1164-9. [DOI] [PubMed] [Google Scholar]
- 131.McNamara M.G., Lwin Z., Jiang H., Templeton A.J., Zadeh G., Bernstein M., Chung C., Millar B.A., Laperriere N., Mason W.P. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J. Neurooncol. 2014;117(1):147–152. doi: 10.1007/s11060-014-1366-9. [DOI] [PubMed] [Google Scholar]
- 132.Kast R.E. Erlotinib augmentation with dapsone for rash mitigation and increased anti-cancer effectiveness. Springerplus. 2015;4(1):638. doi: 10.1186/s40064-015-1441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kast R.E., Hill Q.A., Wion D., Mellstedt H., Focosi D., Karpel-Massler G., Heiland T., Halatsch M.E. Glioblastoma-synthesized g-csf and gm-csf contribute to growth and immunosuppression: Potential therapeutic benefit from dapsone, fenofibrate, and ribavirin. Tumour Biol. 2017;39(5):1010428317699797. doi: 10.1177/1010428317699797. [DOI] [PubMed] [Google Scholar]
- 134.Karpel-Massler G., Kast R.E., Siegelin M.D., Dwucet A., Schneider E., Westhoff M.A., Wirtz C.R., Chen X.Y., Halatsch M.E., Bolm C. Anti-glioma activity of dapsone and its enhancement by synthetic chemical modification. Neurochem. Res. 2017;42(12):3382–3389. doi: 10.1007/s11064-017-2378-6. [DOI] [PubMed] [Google Scholar]
- 135.Meredith A.M., Dass C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016;68(6):729–741. doi: 10.1111/jphp.12539. [DOI] [PubMed] [Google Scholar]
- 136.Tacar O., Sriamornsak P., Dass C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 137.Sheibani M., Nezamoleslami S., Faghir-Ghanesefat H., Emami A.H., Dehpour A.R. Cardioprotective effects of dapsone against doxorubicin-induced cardiotoxicity in rats. Cancer Chemother. Pharmacol. 2020;85(3):563–571. doi: 10.1007/s00280-019-04019-6. [DOI] [PubMed] [Google Scholar]
- 138.Karaguzel E., Kadihasanoglu M., Kutlu O. Mechanisms of testicular torsion and potential protective agents. Nat. Rev. Urol. 2014;11(7):391–399. doi: 10.1038/nrurol.2014.135. [DOI] [PubMed] [Google Scholar]
- 139.Anthony Ta., Arcy F.T.D., Hoag N., Arcy J.D.P., Lawrentschuk N. Testicular torsion and the acute scrotum: Current emergency management. Eur. J. Emerg. Med. 2016;23(3):160–165. doi: 10.1097/MEJ.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 140.Dejban P., Rahimi N., Takzare N., Jahansouz M., Haddadi N.S., Dehpour A.R. Beneficial effects of dapsone on ischemia/reperfusion injury following torsion/detorsion in ipsilateral and contralateral testes in rat. Theriogenology. 2019;140:136–142. doi: 10.1016/j.theriogenology.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 141.Requião-Moura L.R. Durão Junior, Mde.S.; Matos, A.C.; Pacheco-Silva, A. Ischemia and reperfusion injury in renal transplantation: Hemodynamic and immunological paradigms. Einstein (Sao Paulo) 2015;13(1):129–135. doi: 10.1590/S1679-45082015RW3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salvadori M., Rosso G., Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015;5(2):52–67. doi: 10.5500/wjt.v5.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weight S.C., Bell P.R.F., Nicholson M.L. Renal ischaemia--reperfusion injury. Br. J. Surg. 1996;83(2):162–170. doi: 10.1046/j.1365-2168.1996.02182.x. [DOI] [PubMed] [Google Scholar]
- 144.Mochida O., Matsumoto T., Mizunoe Y., Sakumoto M., Abe J., Kumazawa J. Preventive effect of dapsone on renal scarring following mannose-sensitive piliated bacterial infection. Chemotherapy. 1998;44(1):36–41. doi: 10.1159/000007088. [DOI] [PubMed] [Google Scholar]
- 145.Nezamoleslami S., Sheibani M., Jahanshahi F., Mumtaz F., Abbasi A., Dehpour A.R. Protective effect of dapsone against renal ischemia-reperfusion injury in rat. Immunopharmacol. Immunotoxicol. 2020;42(3):272–279. doi: 10.1080/08923973.2020.1755308. [DOI] [PubMed] [Google Scholar]
- 146.Lan C.C.E., Wu C.S., Huang S.M., Wu I.H., Chen G.S. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes. 2013;62(7):2530–2538. doi: 10.2337/db12-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peterson K.P., Van Hirtum M., Peterson C.M. Dapsone decreases the cumulative incidence of diabetes in non-obese diabetic female mice. Proc. Soc. Exp. Biol. Med. 1997;215(3):264–268. doi: 10.3181/00379727-215-44137. [DOI] [PubMed] [Google Scholar]
- 148.Wang X., Elksnis A., Wikström P., Walum E., Welsh N., Carlsson P.O. The novel NADPH oxidase 4 selective inhibitor GLX7013114 counteracts human islet cell death in vitro. PLoS One. 2018;13(9):e0204271. doi: 10.1371/journal.pone.0204271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geyfman M., Debabov D., Poloso N., Alvandi N. Mechanistic insight into the activity of a sulfone compound dapsone on Propionibacterium (newly reclassified as cutibacterium) Acnes-mediated cytokine production. Exp. Dermatol. 2019;28(2):190–197. doi: 10.1111/exd.13869. [DOI] [PubMed] [Google Scholar]
- 150.Rahman I., Adcock I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28(1):219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 151.Cho S.C., Rhim J.H., Choi H.R., Son Y.H., Lee S.J., Song K.Y., Park S.C. Protective effect of 4,4′-diaminodiphenylsulfone against paraquat-induced mouse lung injury. Exp. Mol. Med. 2011;43(9):525–537. doi: 10.3858/emm.2011.43.9.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. Covid-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Merad M., Martin J.C. Pathological inflammation in patients with covid-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of covid-19 coagulopathy. Crit. Care. 2020;24(1):360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moschonas I.C., Tselepis A.D. SARS-CoV-2 infection and thrombotic complications: A narrative review. J. Thromb. Thrombolysis. 2021;52(1):111–123. doi: 10.1007/s11239-020-02374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Z.S., Shu T., Kang L., Wu D., Zhou X., Liao B.W., Sun X.L., Zhou X., Wang Y.Y. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal covid-19 patients. Signal Transduc. Target. Ther. Springer Nature. 2020;19(5):100. doi: 10.1038/s41392-020-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goldust M., Hartmann K., Abdelmaksoud A., Navarini A.A. Utility and risk of dermatologic medications during the COVID-19 pandemic. Dermatol. Ther. (Heidelb.) 2020;33(6):e13833. doi: 10.1111/dth.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.El Farnawany N. Dapsone old drug can be useful in management of covid-19.APIK J. Intern. Med., 2020, 8(3), 150-150. [DOI]