Abstract
Although brain tumors occur less frequently than other forms of cancer, they have one of the bleakest prognoses with low survival rates. The conventional treatment for brain tumors includes surgery, radiotherapy, and chemotherapy. However, resistance to treatment remains a problem with recurrence shortly following. The resistance to treatment may be caused by cancer stem cells (CSCs), a subset of brain tumor cells with the affinity for self-renewal and differentiation into multiple cell lineages. An emerging approach to targeting CSCs in brain tumors is through repurposing the lipid-lowering medication, lovastatin. Lovastatin is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor that impacts the mevalonate pathway. The inhibition of intermediates in the mevalonate pathway affects signaling cascades and oncogenes associated with brain tumor stem cells (BTSC). In this review, we show the possible mechanisms where lovastatin can target BTSC for different varieties of malignant brain tumors.
Keywords: brain cancer, signaling pathways, statins, stem cells
Brain Cancer Epidemiology
Out of all the solid cancers, brain tumors have the highest morbidity and mortality rates 1 . Approximately only one-third of patients with a form of brain or other central nervous system (CNS) tumor will survive at least 5 years after diagnosis 2 . Brain tumors account for less than 2% of malignancies, yet they are the leading cause of cancer-related deaths in children and fourth leading cause in adults 1 . In 2021, 83,570 people living in the United States are estimated to be diagnosed with some form of brain or CNS tumor and 18,600 individuals will succumb to the disease 2 . The incidence of brain tumors is increasing in certain cohorts possibly due to advances in primary brain tumor detection or improvement in the treatment of systematic cancers 3 . There are over 120 different types of brain tumors. These lesions can be grouped into either primary brain tumors which are defined based on the cell of origin or secondary brain tumors which originate from metastatic cells of peripheral sites 4 . The most common malignant primary brain tumor is glioma, of which over 50% comprise grade IV glioblastoma (GBM) 4 . Malignant brain tumors are more common and are approximated to outnumber primary brain tumors by a factor of four 4 . Metastasis to the brain occurs in approximately 10% to 30% of cancer patients with 70% to 80% of those individuals developing multiple lesions, most commonly in the cerebrum5,6.
Management of Malignant Brain Tumors
The treatment of malignant primary brain tumors includes a combination of surgical resection, radiation, chemotherapy, and symptomatic control. Surgical resection is the initial approach for treating most malignant primary brain tumors 7 . Studies suggest that extent of resection is correlated with both progression-free and overall survival 8 . Radiation is often used in conjunction with surgery, but as the study by Grunert et al. 9 notes, there is potential for inducing cognitive decline, forming new tumors and developing more proliferative and treatment-resistant cancer strains. In the case of chemotherapy, a higher dosage is necessary in treating brain cancer compared with other cancers due to the blood–brain barrier 10 . This problem has led to the continued development of alternative strategies like the use of nanotechnology to deliver the medications 11 . However, as of yet, there is no widely accepted standard for the treatment of metastatic brain tumors, and the response will depend on the tumor type and overall prognosis 3 . Despite advances in treatment protocols, malignant brain tumors remain a clinical challenge with recurrence a few months after treatment and median survival of 8 months for brain metastasis and 14.2 months for malignant primary brain tumors 5 .
Brain Tumor Stem Cells
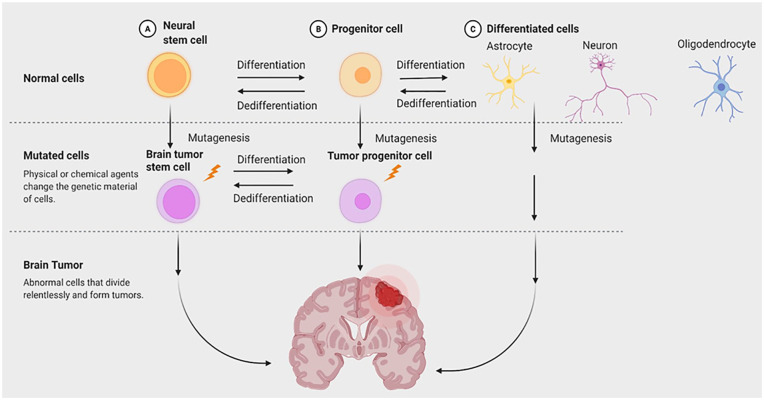
The cancer stem cell (CSC) hypothesis can apply to multiple solid tumors, particularly in the brain. Singh et al. 12 in 2004 was the first study that identified a subpopulation of CD133+ human brain tumor cells in vitro with stem cell activity. Future studies expand on this work and show that brain tumor stem cells (BTSCs) have properties similar to neural stem cells (NSCs) such as the ability to grow as neurospheres, perpetual self-renewal, and extensive brain parenchymal migration13,14 (see Fig. 1). Another similarity between BTSCs and NSCs is the molecular pathways involving self-renewal. In particular, the Shh and Notch pathways are implicated in the proliferative capacity of human glioma cells and initiation of medulloblastomas 1 . BTSCs possess certain traits that impact treatment outcomes and recurrence rates of malignant brain tumors. Postoperative wounds can change the tissue microenvironment initiating differentiation or de-differentiation of persistent BTSCs 15 . BTSCs also overexpress multidrug resistance proteins that protect them from cytotoxic drugs that kill differentiated brain tumor cells 12 . Human glioma cells can activate DNA repair mechanisms more efficiently than regular brain tumor cells, making the subpopulation of tumor cells more resistant to radiotherapy 16 . With the discovery of BTSCs and their role in conventional treatment failures of malignant brain tumors, additional strategies suggest treating brain cancer based on a stem cell model.
Figure 1.
Diagram of neural versus cancer stem cell differentiation. The model is a simplified version of the differentiation between neural stem cells (yellow) and brain tumor stem cells (purple) in the formation of brain tumors. Neural stem cells and brain tumor stem cells share similar characteristics such as self-renewal and differentiation. Neural stem cells differentiate into unrestricted progenitor cells which give rise to either glial progenitors or neural progenitors. The glial precursors differentiate into astrocytes and oligodendrocytes while the neural precursor will form the neuron. Similarly, brain tumor stem cells have the ability to generate a wide range of differentiated progeny. Brain tumor stem cells can develop from mutations accumulated in neural stem cells and in both their restricted and unrestricted progeny that have the ability to self-renew.
Cancer Stem Cells
Recent studies show that only a small subset of tumor cells display key characteristics similar to somatic stem cells such as long-term replicative potential, extensive self-renewal, and multilineage differentiation 14 . Inappropriate activation of certain pathways, such as Wnt/β-catenin, Sonic hedgehog (Shh), Notch, and bone morphogenetic protein, involved in the differentiation and self-renewal of stem cells are implicated in a variety of cancers 15 . This concept, which was built from prior work on acute myeloid leukemia, established evidence for the existence of CSCs 17 .
Statins for Treating Brain Cancer
Statins are a class of drugs commonly used in the treatment of cardiovascular disease as a result of their inhibitory effects on 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-controlling enzyme of the mevalonate pathway and cholesterol biosynthesis 18 . Clinical data have frequently demonstrated that statins are a safe and effective therapy in treating cardiovascular disease across a wide range of demographics 19 , significantly reducing low-density lipoprotein and triglyceride levels while improving high-density lipoprotein levels in patients 18 . Moreover, statin therapies are reliably found to reduce mortality and morbidity due to complications such as strokes or cardiovascular events18,19. Despite their overall benefit, statins are still noted for carrying a wide range of adverse effects such as muscle breakdown and dysfunction, hepatic dysfunction, increased risk of diabetes mellitus, and renal insufficiency 18 . In addition, other products potentially involved in cell survival, such as coenzyme Q10, are also targeted by statin-mediated inhibition of HMG-CoA, yielding mitochondrial defects and pro-apoptotic signaling 19 . The toxicity of atorvastatin has been examined along with temozolomide and radiotherapy for GBM treatment 20 . Patients were started on 40 mg of atorvastatin per day for the first 21 days of the study after which the dosage was increased to 80 mg per day and continued until early termination by the participants, disease progression, or serious adverse effects 20 . In total, 11% of the patients stopped the treatment during the adjuvant and concomitant therapy periods 20 , suggesting that the adverse effects of statins do not follow a strict dose-dependent pattern. Future studies investigating dosage and adverse effects may help optimize statin therapy to minimize side effects 20 .
Growing interest has shifted toward investigating the non-cardiovascular benefits of statin therapy as a result of the application of their pro-apoptotic effects in cancer therapy. Early comparative analysis in Danish populations showed that statin-users had a slightly reduced overall incidence of cancer compared with non-users and users of other lipid-lowering drugs, although there was no demonstrable effect on any organ-specific cancers 21 . More recently, similar nationwide study populations have shown that statin use reduced cancer-related mortality, although they again did not significantly influence cancer incidence 22 . This distinction has been reiterated in studies investigating prostate, colorectal, and breast cancer, wherein it was reported that stains could improve outcomes and protect against advanced progression of cancer without affecting cancer incidence23–25.
Outside of clinical data, there is evidence of statin demonstrating anticancer effects through modulation of stem cells in laboratory settings. Statin treatment demonstrated reduced cell proliferation in human embryonic stem cell lines, as well as ovarian and colorectal cancer cell lines, as a result of inhibition of the mevalonate pathway 26 . Furthermore, in work done in mesenchymal stem cells (MSCs), statins were also shown to reduce differentiation potential in addition to promoting senescence and apoptosis 27 . However, certain evidence suggests that statins are not always pro-apoptotic modulators of stem cells. In the context of stem cell-based transplantation for heart disease, certain statins have been shown to promote stem cell survival, differentiation, and promotion 28 , with lovastatin being among them.
Lovastatin, or Monacolin K, was first isolated and described in 1979 by Japanese biochemist Akira Endo, being noted primarily for its use as a potential therapeutic for hypercholesterolemia 29 . Further work has since demonstrated that lovastatin also possesses both anticancer effects, particularly in preventing metastasis in breast cancers30,31 and neuroprotective effects among differentiated nerve cells by inhibiting apoptosis 32 . Lovastatin has also shown to promote differentiation and proliferation among various stem cell types, including human gingiva-derived stem cells, MSCs, and NSCs33–35. Differentiation into osteoblastic lineages is predominantly reported in literature following lovastatin treatment of stem cells34,36; however, MSC differentiation into neuroglial cells has also been observed 35 . Among MSCs and NSCs in particular, lovastatin is also shown to protect against apoptosis induced as a result of both hypoxia 37 , as in the case of MSCs, and oxidative stress, as in the case of NSCs 33 . Taken all together, these characteristics would seem to suggest that lovastatin could potentially play a role in both treating brain cancer and promoting subsequent regeneration of lost brain matter.
Lovastatin for Targeting Brain Cancer Stem Cells
Lovastatin Targeting GBM Stem Cells
Glioblastomas (GBM) contain CSCs located in the tumor bulk 38 . CSCs in GBM contribute to tumor initiation, recurrence, and resistance to chemotherapy and radiotherapy. Statins show promise as a repurposed drug in the treatment of GBM 39 . As discussed above, statins inhibit the enzyme HMG-CoA reductase which prevents the synthesis of mevalonate and downstream intermediates involved in CSC self-renewal, differentiation, and proliferation. Although the characterization of the antiproliferative effects lovastatin has with GBM stem cells is not completely understood, certain studies highlight the possible mechanisms where lovastatin can target GBM CSCs.
One potential mechanism where lovastatin can inhibit glioma stem cell proliferation is through S-phase kinase-associated protein 2 (Skp2) degradation. Skp2 is a member of the F-box protein family that targets the cell cycle through degradation of CDK inhibitors p27Kip1 and p21Cip1/Waf140. Lovastatin downregulates Skp2 possibly from the depletion of geranylgeranyl pyrophosphate, a downstream intermediate of the mevalonate pathway 41 . In 2020, Yi et al. 30 demonstrated that lovastatin induced degradation of Skp2 in glioma cells which lead to decreased glioma neurosphere formation, proliferation, and stem cell markers Sox2 and Nestin.
Another plausible mechanism where lovastatin can potentially target glioma stem cells is through modulation of doublecortin (DCX) expression. DCX is a brain-specific gene involved in neuroblast and differentiating neuron migration in the developing brain 42 . Previous studies show DCX synthesis induces terminal differentiation of BTSCs 43 . In 2011, Santra et al. 44 demonstrated that statin treatment had favorable effects on mice survival by inducing apoptosis of BTSCs by strengthening the activity of DCX and neurabin II. They found that statin treatment led to activation of c-jun NH2-terminal kinase 1 which in turn increased activation of the caspase-3 pathway via effects on DCX and neurabin II.
In addition to its role as a potential targeted therapy, lovastatin is generating interest in its use as an adjuvant therapy for glioma tumors. One example of lovastatin as an adjuvant in the treatment of GBM would be its use in combination with Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL). Evidence shows that lovastatin may inhibit the nuclear factor-κB pathway which sensitizes TRAIL mediated apoptosis through upregulation of death receptor 5 on the cell surface of GBM cell lines 45 . There is also evidence of the interaction between lovastatin and radiation in the treatment of GBM. In 2008, Gabryś et al. 46 studied the effects of lovastatin with and without radiation in the treatment of U87MG glioma cells. The investigators reported that lovastatin by itself decreased cell proliferation but did not enhance the effect of radiation therapy.
Lovastatin Targeting Medulloblastoma Stem Cells
Medulloblastomas contain a subpopulation of CSCs responsible for tumor proliferation, metastasis, recurrence, and resistance to conventional treatment 47 . Preclinical studies show lovastatin prevents proliferation and induces apoptosis in medulloblastoma cell lines48,49; however, the exact relation between lovastatin and medulloblastoma CSC remains unclear.
One studied target for lovastatin inhibition of medulloblastoma CSCs is Myc expression. Myc amplification is associated with group 3 medulloblastomas which has the worst prognosis of the four groups of medulloblastomas 50 . Previous studies show that c-myc and n-myc are critical for neurosphere formation and expansion of CD133+ malignant medulloblastoma tumor cells51,52. Lovastatin is shown to indirectly target c-myc through the modulation of miRNA 53 . In 2012, Takwi found that lovastatin induced miR-33b expression which led to the decreased expression of c-myc and function of the medulloblastoma cells 53 .
Another potential pathway where lovastatin can target medulloblastoma CSCs is through modulation of Shh signaling. Shh is associated with group 2 medulloblastomas which comprises 25% of all cases 50 . One of the major drivers of the Shh pathway is protein patched homolog 1 (Ptch) 54 . Tumors in Ptch+/- medulloblastoma mice were found to contain self-renewing, long-term stem cells 54 . In 2018, Gordon et al. 55 studied the interaction between statin therapy, Shh signaling, and medulloblastoma progression. The investigators found that cholesterol dysregulation mediates Shh medulloblastomas. They believed that the loss of cholesterol homeostasis may have been caused by a mutation in Ptch1. Inhibition of cholesterol biosynthesis by statin treatment repressed medulloblastoma proliferation.
Conclusion
Malignant brain tumors house a subpopulation of CSCs which are responsible for recurrence, metastasis, and maintenance of the tumor. Conventional therapy such as surgical resection, radiotherapy, and chemotherapy only target the bulk tumor leading to proliferation of the CSCs. Lovastatin shows potential as a novel therapy for malignant brain tumors because of its ability to target BTSC. Prior studies demonstrate efficacious treatment of glioma and medulloblastoma stem cells with lovastatin. Lovastatin can potentially target glioma stem cells through the modulation of Skp2 and DCX. Potential mechanisms for lovastatin treatment for medulloblastoma stem cells include a change in Myc expression and Shh signaling. Although the initial preclinical results for lovastatin have been generally positive, further research should be conducted both in vitro and in vivo to elucidate the exact mechanisms of how lovastatin interacts with BTSCs.
Acknowledgments
Figure 1 was created with BioRender.com.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Cesario V. Borlongan  https://orcid.org/0000-0002-2966-9782
https://orcid.org/0000-0002-2966-9782
References
- 1. Rahman R, Heath R, Grundy R. Cellular immortality in brain tumours: an integration of the cancer stem cell paradigm. Biochim Biophys Acta. 2009;1792(4):280–88. [DOI] [PubMed] [Google Scholar]
- 2. Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, Fuchs HE, Waite KA, Jemal A, Siegel RL, Barnholtz-Sloan JS. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021;71(5):381–406. [DOI] [PubMed] [Google Scholar]
- 3. McFaline-Figueroa JR, Lee EQ. Brain tumors. Am J Med. 2018;131(8):874–82. [DOI] [PubMed] [Google Scholar]
- 4. Shah K. Stem cell-based therapies for tumors in the brain: are we there yet? Neuro Oncol. 2016;18(8):1066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aparicio-Blanco J, Torres-Suárez AI. Towards tailored management of malignant brain tumors with nanotheranostics. Acta Biomater. 2018;73:52–63. [DOI] [PubMed] [Google Scholar]
- 6. Vargo MM. Brain tumors and metastases. Phys Med Rehabil Clin N Am. 2017;28(1):115–41. [DOI] [PubMed] [Google Scholar]
- 7. Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70(4):299–312. [DOI] [PubMed] [Google Scholar]
- 8. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269–82. [DOI] [PubMed] [Google Scholar]
- 9. Grunert M, Kassubek R, Danz B, Klemenz B, Hasslacher S, Stroh S, Schneele L, Langhans J, Ströbele S, Barry SE, Zhou S, et al. Radiation and brain tumors: an overview. Crit Rev Oncog. 2018;23(1–2):119–38. [DOI] [PubMed] [Google Scholar]
- 10. Shah V, Kochar P. Brain cancer: implication to disease, therapeutic strategies and tumor targeted drug delivery approaches. Recent Pat Anticancer Drug Discov. 2018;13(1):70–85. [DOI] [PubMed] [Google Scholar]
- 11. Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48(11):2967–3014. [DOI] [PubMed] [Google Scholar]
- 12. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. [DOI] [PubMed] [Google Scholar]
- 13. Liu JM, Mao BY, Hong S, Liu YH, Wang XJ. The postoperative brain tumour stem cell (BTSC) niche and cancer recurrence. Adv Ther. 2008;25(5):389–98. [DOI] [PubMed] [Google Scholar]
- 14. Dirks PB. Brain tumor stem cells: the cancer stem cell hypothesis writ large. Mol Oncol. 2010;4(5):420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piccirillo SG, Binda E, Fiocco R, Vescovi AL, Shah K. Brain cancer stem cells. J Mol Med (Berl). 2009;87(11):1087–95. [DOI] [PubMed] [Google Scholar]
- 16. Palm T, Schwamborn JC. Brain tumor stem cells. Biol Chem. 2010;391(6):607–17. [DOI] [PubMed] [Google Scholar]
- 17. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–48. [DOI] [PubMed] [Google Scholar]
- 18. Ramkumar S, Raghunath A, Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol Sin. 2016;32(6):631–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8(6):373–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altwairgi AK, Alghareeb WA, AlNajjar FH, Alhussain H, Alsaeed E, Balbaid AAO, Aldanan S, Orz Y, Alsharm AA. Atorvastatin in combination with radiotherapy and temozolomide for glioblastoma: a prospective phase II study. Invest New Drugs. 2021;39(1):226–31. [DOI] [PubMed] [Google Scholar]
- 21. Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sørensen HT, Olsen JH. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114(4):643–47. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2013;368(6): 576–77. [DOI] [PubMed] [Google Scholar]
- 23. Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut. 2010;59(11):1572–85. [DOI] [PubMed] [Google Scholar]
- 24. Beckwitt CH, Brufsky A, Oltvai ZN, Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018;20(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–25. [DOI] [PubMed] [Google Scholar]
- 26. Gauthaman K, Fong CY, Bongso A. Statins, stem cells, and cancer. J Cell Biochem. 2009;106(6):975–83. [DOI] [PubMed] [Google Scholar]
- 27. Izadpanah R, Schächtele DJ, Pfnür AB, Lin D, Slakey DP, Kadowitz PJ, Alt EU. The impact of statins on biological characteristics of stem cells provides a novel explanation for their pleiotropic beneficial and adverse clinical effects. Am J Physiol Cell Physiol. 2015;309(8):C522–31. [DOI] [PubMed] [Google Scholar]
- 28. Xu H, Yang YJ, Yang T, Qian HY. Statins and stem cell modulation. Ageing Res Rev. 2013;12(1):1–7. [DOI] [PubMed] [Google Scholar]
- 29. Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J Antibiot (Tokyo). 1979;32(8):852–54. [DOI] [PubMed] [Google Scholar]
- 30. Yi H, Wu M, Zhang Q, Lu L, Yao H, Chen S, Li Y, Zheng C, He G, Deng X. Reversal of HER2 negativity: an unexpected role for lovastatin in triple-negative breast cancer stem cells. J Cancer. 2020;11(13):3713–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng C, Yan S, Lu L, Yao H, He G, Chen S, Li Y, Peng X, Cheng Z, Wu M, Zhang Q, et al. Lovastatin inhibits EMT and metastasis of triple-negative breast cancer stem cells through dysregulation of cytoskeleton-associated proteins. Front Oncol. 2021;11:656687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiong Z, Cao X, Wen Q, Chen Z, Cheng Z, Huang X, Zhang Y, Long C, Zhang Y, Huang Z. An overview of the bioactivity of monacolin K / lovastatin. Food Chem Toxicol. 2019;131: 110585. [DOI] [PubMed] [Google Scholar]
- 33. Abdanipour A, Tiraihi T, Noori-Zadeh A, Majdi A, Gosaili R. Evaluation of lovastatin effects on expression of anti-apoptotic Nrf2 and PGC-1α genes in neural stem cells treated with hydrogen peroxide. Mol Neurobiol. 2014;49(3):1364–72. [DOI] [PubMed] [Google Scholar]
- 34. Kim BB, Tae JY, Ko Y, Park JB. Lovastatin increases the proliferation and osteoblastic differentiation of human gingiva-derived stem cells in three-dimensional cultures. Exp Ther Med. 2019;18(5):3425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee OK, Ko YC, Kuo TK, Chou SH, Li HJ, Chen WM, Chen TH, Su Y. Fluvastatin and lovastatin but not pravastatin induce neuroglial differentiation in human mesenchymal stem cells. J Cell Biochem. 2004;93(5):917–28. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Cui Q, Kao C, Wang GJ, Balian G. Lovastatin inhibits adipogenic and stimulates osteogenic differentiation by suppressing PPARgamma2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone. 2003;33(4):652–59. [DOI] [PubMed] [Google Scholar]
- 37. Xu R, Chen J, Cong X, Hu S, Chen X. Lovastatin protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3K/Akt and ERK1/2. J Cell Biochem. 2008;103(1):256–69. [DOI] [PubMed] [Google Scholar]
- 38. Bahmad HF, Daher D, Aljamal AA, Elajami MK, Oh KS, Alvarez Moreno JC, Delgado R, Suarez R, Zaldivar A, Azimi R, Castellano A, et al. Repurposing of anticancer stem cell drugs in brain tumors. J Histochem Cytochem. 2021;69(12):749–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yanae M, Tsubaki M, Satou T, Itoh T, Imano M, Yamazoe Y, Nishida S. Statin-induced apoptosis via the suppression of ERK1/2 and Akt activation by inhibition of the geranylgeranyl-pyrophosphate biosynthesis in glioblastoma. J Exp Clin Cancer Res. 2011;30(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu J, Su HK, Yu ZH, Xi SY, Guo CC, Hu ZY, Qu Y, Cai HP, Zhao YY, Zhao HF, Chen FR, et al. Skp2 modulates proliferation, senescence and tumorigenesis of glioma. Cancer Cell Int. 2020;20:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vosper J, Masuccio A, Kullmann M, Ploner C, Geley S, Hengst L. Statin-induced depletion of geranylgeranyl pyrophosphate inhibits cell proliferation by a novel pathway of Skp2 degradation. Oncotarget. 2015;6(5):2889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ortensi B, Setti M, Osti D, Pelicci G. Cancer stem cell contribution to glioblastoma invasiveness. Stem Cell Res Ther. 2013;4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Santra M, Zheng X, Roberts C, Santra S, Lu M, Panda S, Jiang F, Chopp M. Single doublecortin gene therapy significantly reduces glioma tumor volume. J Neurosci Res. 2010; 88(2):304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Santra M, Santra S, Buller B, Santra K, Nallani A, Chopp M. Effect of doublecortin on self-renewal and differentiation in brain tumor stem cells. Cancer Sci. 2011;102(7):1350–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu PC, Lu G, Deng Y, Wang CD, Su XW, Zhou JY, Chan TM, Hu X, Poon WS. Inhibition of NF-κB pathway and modulation of MAPK signaling pathways in glioblastoma and implications for lovastatin and tumor necrosis factor-related apoptosis inducing ligand (TRAIL) combination therapy. PLoS ONE. 2017;12(1):e0171157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gabryś D, Dörfler A, Yaromina A, Hessel F, Krause M, Oertel R, Baumann M. Effects of lovastatin alone or combined with irradiation on tumor cells in vitro and in vivo. Strahlenther Onkol. 2008;184(1):48–53. [DOI] [PubMed] [Google Scholar]
- 47. Bahmad HF, Elajami MK, El Zarif T, Bou-Gharios J, Abou-Antoun T, Abou-Kheir W. Drug repurposing towards targeting cancer stem cells in pediatric brain tumors. Cancer Metastasis Rev. 2020;39(1):127–48. [DOI] [PubMed] [Google Scholar]
- 48. Wang W, Macaulay RJ. Cell-cycle gene expression in lovastatin-induced medulloblastoma apoptosis. Can J Neurol Sci. 2003;30(4):349–57. [DOI] [PubMed] [Google Scholar]
- 49. Macaulay RJ, Wang W, Dimitroulakos J, Becker LE, Yeger H. Lovastatin-induced apoptosis of human medulloblastoma cell lines in vitro. J Neurooncol. 1999;42(1):1–11. [DOI] [PubMed] [Google Scholar]
- 50. Archer TC, Mahoney EL, Pomeroy SL. Medulloblastoma: molecular classification-based personal therapeutics. Neurotherapeutics. 2017;14(2):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garg N, Bakhshinyan D, Venugopal C, Mahendram S, Rosa DA, Vijayakumar T, Manoranjan B, Hallett R, McFarlane N, Delaney KH, Kwiecien JM, et al. CD133+ brain tumor-initiating cells are dependent on STAT3 signaling to drive medulloblastoma recurrence. Oncogene. 2017;36(5):606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ahmad Z, Jasnos L, Gil V, Howell L, Hallsworth A, Petrie K, Sawado T, Chesler L. Molecular and in vivo characterization of cancer-propagating cells derived from MYCN-dependent medulloblastoma. PLoS ONE. 2015;10(3):e0119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takwi AA, Li Y, Becker Buscaglia LE, Zhang J, Choudhury S, Park AK, Liu M, Young KH, Park WY, Martin RC, Li Y. A statin-regulated microRNA represses human c-Myc expression and function. EMBO Mol Med. 2012;4(9):896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bahmad HF, Poppiti RJ. Medulloblastoma cancer stem cells: molecular signatures and therapeutic targets. J Clin Pathol. 2020;73(5):243–49. [DOI] [PubMed] [Google Scholar]
- 55. Gordon RE, Zhang L, Peri S, Kuo YM, Du F, Egleston BL, Ng JMY, Andrews AJ, Astsaturov I, Curran T, Yang ZJ. Statins synergize with hedgehog pathway inhibitors for treatment of medulloblastoma. Clin Cancer Res. 2018;24(6):1375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]