Abstract
Background
The Central Brain Tumor Registry of the United States (CBTRUS) contains information on all primary brain and other central nervous system (CNS) tumors diagnosed in the United States (US). Here we summarize the 2021 CBTRUS annual statistical report for clinicians.
Methods
Incidence survival data are obtained from the Centers for Disease Control’s National Program of Cancer Registries (NPCR) and National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Survival data are obtained from NPCR. Mortality data are obtained from the National Vital Statistics System. Incidence and mortality rates are age-adjusted using the 2000 US population and presented per 100,000 population.
Results
An annual average of 86,355 cases of primary malignant and nonmalignant CNS tumors were diagnosed over the period 2014–2018, corresponding to an average annual age-adjusted incidence rate of 24.25. The most commonly occurring malignant tumor was glioblastoma (14.3%), and the most common predominately nonmalignant tumor was meningioma (39%). Over the 2014–2018 period, there were 16,606 annual average deaths due to malignant primary CNS tumors, corresponding to an average annual age-adjusted mortality rate of 4.43. In this report we detail key incidence, survival, and mortality statistics for major primary CNS tumor histologies, highlighting relevant differences by age, sex, and race.
Conclusions
This summary describes the most up to date population-based incidence of primary malignant and nonmalignant brain and other CNS tumors in the US, and mortality and survival for primary malignant tumors and aims to serve as a useful resource for clinicians.
Keywords: brain tumors, CBTRUS, CNS tumors, epidemiology, incidence, mortality, neuro-oncology, survival
The Central Brain Tumor Registry of the United States (CBTRUS), in collaboration with the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR), and the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER) publishes a comprehensive annual statistical report on primary brain and other central nervous system (CNS) tumors reported in the United States (US).1,2 CBTRUS is a unique professional research organization that focuses exclusively on providing high-quality statistical data on the population-based incidence of primary brain and other CNS tumors in the US (for more information on CBTRUS see: http://www.cbtrus.org/about/).2 CBTRUS was incorporated as a nonprofit organization in 1992 following a study conducted by the American Brain Tumor Association (ABTA) to determine the feasibility of a population-based central registry focused on all primary brain and other CNS tumors in the US. CBTRUS is currently the only population-based site-specific registry in the US that works in partnership with a public cancer surveillance organization, the CDC’s NPCR, and from which data are directly received through the NPCR Cancer Surveillance System (NPCR-CSS) Submission Specifications mechanism3 under a special agreement.
By US law, all primary cancer including all primary malignant brain and other CNS tumors must be reported to central (state or territorial) cancer registries (CCR), as mandated by Public Law 102–515, the Cancer Registries Amendment Act of 1992.4 This mandate was expanded to include nonmalignant CNS tumors with the 2002 passage of Public Law 107–260, starting January 1, 2004.5 Previous to 2004, CBTRUS reported data on both malignant and nonmalignant brain and other CNS tumors from CCR through individual agreements with a subset of US state cancer registries that voluntarily provided these data. The CBTRUS analytic dataset used in this summary includes incidence data from two sources: (1) CDC’s NPCR and (2) the NCI’s SEER. De-identified data on cancer diagnoses and mortality are reported from hospitals and other medical facilities to state-level CCRs, which then curate and transmit these data to either NPCR (48 CCRs) or SEER (4 CCRs). CBTRUS combines these data to capture incidence data from 52 CCRs (50 US states plus Puerto Rico and the District of Columbia). Death certificate data from all 50 US states and the District of Columbia allows CBTRUS to estimate mortality due to malignant primary brain and other CNS tumors. A special dataset from NPCR containing data from 42 CCR enables CBTRUS to provide survival estimates (Supplementary Table 1). Data on CNS metastases are not recorded by CBTRUS Reports as these tumors are not included in US cancer collection practices.
In this paper, we summarize the key findings of the CBTRUS 2021 Report (ref), organized by major histologic categories. We focus on the age-adjusted incidence rates for diagnosis years 2014–2018, mortality due to malignant brain and other CNS tumors for years 2014–2018, and survival rates of patients with CNS tumors for years 2001–2017. This represents the first summary of the CBTRUS annual statistical report for practicing clinicians, with additional clinically-relevant analyses focused on WHO grade in glioma and meningioma and for site in ependymoma.
Materials and Methods
CBTRUS Data and Classification
An overview of CBTRUS data processing procedures is provided in Ostrom, et al.1,6 Briefly, cancer registrars will preferentially identify cases from pathological records, but if these are not available radiographic diagnosis records will be used.7 Survival data were obtained for 42 of 52 US CCR from NPCR for the years 2001–2017.8 Mortality data were obtained from the National Center for Health Statistics’ (NCHS) National Vital Statistics System (NVSS) from 50 states and the District of Columbia for 2014–2018.9
The specific sites used by CBTRUS are based on the WHO International Classification of Diseases for Oncology, Third Edition (ICD-O-3) topography codes found in World Health Organization (WHO) CNS classifications.10 See Supplementary Table 2 for the CBTRUS primary site groupings. Histology groupings used by CBTRUS are based on morphology codes found in ICD-O-3 and the 2016 World Health Organization (WHO) Classification of Tumours of the Central Nervous System.1,11 Gliomas are one of the most common types of primary brain and other CNS tumors. This broad category includes astrocytoma (including glioblastoma), oligodendroglioma, ependymoma, oligoastrocytoma (mixed glioma), and a few rare histologies. Because there is no standard definition for glioma, CBTRUS defines glioma as ICD-O-3 histology codes 9380-9384, and 9391-9460. Statistics produced by CBTRUS for lymphomas refer to those that occur only in the CNS (ie primary CNS lymphoma). See Supplementary Table 3 for the CBTRUS Histology Grouping scheme. The definition of malignant versus nonmalignant corresponds to behavior codes of/3 for malignant and behavior codes/0 benign and/1 uncertain for nonmalignant tumors, with the exception of pilocytic astrocytoma which is assigned an ICD-O-3 uncertain behavior code of/1 and is considered WHO grade 1, but is historically reported as malignant by tumor registries; accordingly, CBTRUS reports pilocytic astrocytoma as malignant.12
Statistics are presented by age group at diagnosis as based on standard surveillance age groups, defined as pediatric (age 0–14 years), adolescent and young adult (AYA [age 15–39 years]), and older adults (age 40+ years). CBTRUS classifies race categories using standard racial groups reported by US cancer registration (White, Black, American Indian/Alaskan Native [AIAN], and Asian/Pacific Islander [API]). Individuals categorized as other race, unspecified, and unknown race are included in statistics that are not race-specific. Hispanic ethnicity is defined using the North American Association of Central Cancer Registries (NAACCR) Hispanic Identification Algorithm, version 2, data element, which utilizes a combination of cancer registry data fields (Spanish/Hispanic Origin data element, birthplace, race, and surnames) to directly and indirectly classify cases as Hispanic or non-Hispanic.13
Incidence and Mortality Rates
Incidence rates measure the number of patients who are newly diagnosed with disease in each given time period. Average annual age-adjusted incidence rates (AAAIR) and 95% confidence intervals (95% CI) were estimated per 100,000 population based on five-year age groups and were standardized to the 2000 US standard population.14 Population data for each geographic region were obtained from the SEER program website15 for the purpose of rate calculation.
Mortality rates are a measure of the number of patients who die each year due to a specific disease. The mortality data used in this report are from the National Center for Health Statistics’ (NCHS) National Vital Statistics System (NVSS) and include deaths where primary brain or other CNS tumor was listed as primary/underlying cause of death on the death certificate for individuals from all 50 states and the District of Columbia. These data were obtained from NVSS9 (includes death certification data for 100% of the US population) for malignant brain and other CNS tumors and comparison via SEER*Stat (for malignant brain tumors only). NVSS data are not collected through the cancer registration system. Average annual age-adjusted mortality rates (AAAMR) and 95% confidence intervals (95% CI) were estimated per 100,000 population based on five-year age groups and were standardized to the 2000 US standard population.14 Counts, rates, ratios, proportions, and other relevant statistics were calculated using R 4.0.5 statistical software16 and/or SEER*Stat 8.3.9.17 Figures were created in R 4.0.5. According to the CBTRUS agreement with NPCR, rates are suppressed when counts are fewer than 16 within a group but included in totals, except when data are suppressed from only one group, to prevent identification of the number in the suppressed group.
Calculation of Relative and Overall Survival
Survival calculations are measured using two key metrics: relative survival rates and median survival time. The relative survival rate refers to the proportion of cancer patients who are alive at a specified timepoint after diagnosis who would not otherwise have died from other causes. Relative survival is calculated from the observed survival, which represents the fraction of cancer patients alive at a specified time after diagnosis; and the estimated survival, which is the fraction of the general population (of the same age) that is expected to survive to the specified time. SEER*Stat 8.3.9 statistical software17 was used to estimate 1-, 5-, and 10-year relative survival rates for primary malignant brain and other CNS tumor cases diagnosed between 2004 and 2017 in 42 NPCR CCRs. This software utilizes lifetable (actuarial) methods to compute survival estimates and accounts for current follow-up. The median survival time is defined as the time after which 50% of patients had either died or been “censored” (survival data no longer known). Median survival times are calculated using the Kaplan–Meier method for all malignant CNS tumors diagnosed between 2004 and 2017 in R 4.0.5 statistical software.16 Second or later primary tumors, cases diagnosed at autopsy, cases in which race or sex is coded as other or unknown, and cases known to be alive but for whom follow-up time could not be calculated, were excluded from all survival data analyses.
Results
Overview of CNS Tumor Incidence and Mortality
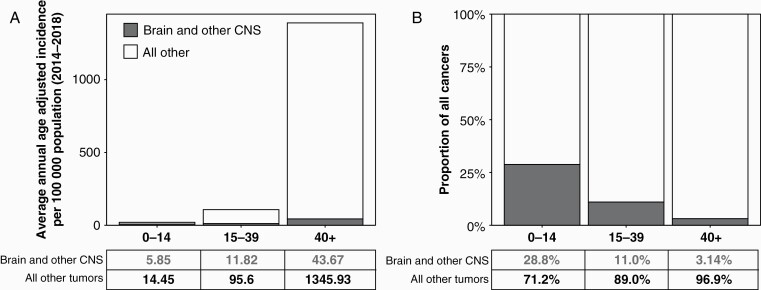
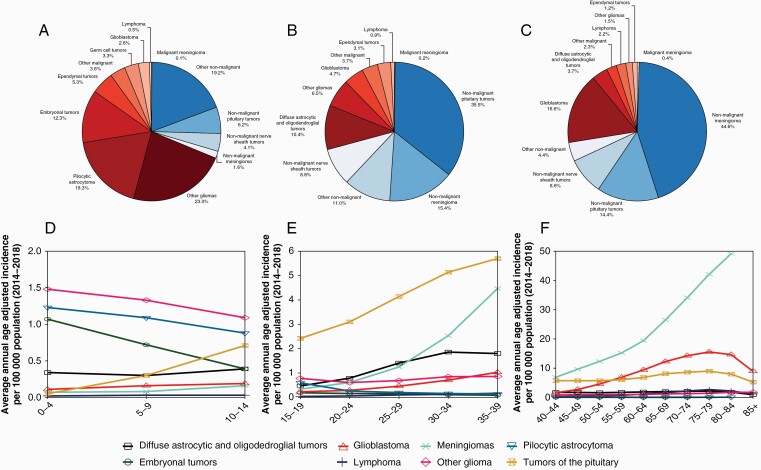
A total of 431,773 cases of brain and other CNS tumors were diagnosed in the US from 2014 to 2018, for an annual average of 86,355 CNS tumors. This corresponds to an AAAIR of 24.25 (95% CI: 24.17–24.32) (Table 1). Of these tumors, 25,105 (AAAIR: 7.06, 95% CI: 7.02–7.10) were classified as malignant, while 61,250 (AAAIR: 17.19, 95% CI: 17.12–17.25) were nonmalignant. The incidence of brain tumors increased with age. In children age 0–14 years, there were 3,562 brain and other CNS tumors diagnosed annually (AAAIR: 5.85, 95% CI: 5.76–5.93); in the AYA age group (age 15–39 years), 12,512 (AAAIR: 11.82, 95% CI: 11.73–11.92); and in older adults aged 40+ years, 70,281 (AAAIR: 43.67, 95% CI: 43.53–43.82). In children, the majority of tumors were malignant (66.5%), as compared to AYA and older adults where nonmalignant tumors represented the majority of new diagnoses (72.2% and 72.6%, respectively). While their incidence increased with age, relative to all cancer types CNS tumors are less common with age (Figure 1). Brain and other CNS tumors are the most common cancers in children, the second most common in AYA, and the eighth most common in older adults. Overall, meningiomas were the most common CNS tumor histology, followed by pituitary tumors and glioblastoma. Glioblastoma was the most common malignant CNS tumor (49.1%), while meningioma was the most common nonmalignant tumor (54.5%) (Figure 2A–C). Incidence of specific brain tumor histologies varied substantially by age (Figure 2D–F). In children, pilocytic astrocytomas (AAAIR: 1.07, 95% CI: 1.03–1.10) and other gliomas (AAAIR: 0.87, 95% CI: 0.84–0.91; this designation likely includes other circumscribed gliomas other than pilocytic astrocytoma and ependymoma, see Table 7 in Ostrom, et al1) were the most common histologies, while glioblastomas (AAAIR: 0.15, 95% CI: 0.14–0.17) and meningiomas (AAAIR: 0.10, 95% CI: 0.09–0.11) were extremely uncommon (Figure 2D). Pituitary region tumors became increasingly common with age and formed the dominant histology in the AYA age group (AAAIR: 4.15, 95% CI: 4.10–4.21), after which their annual incidence was eclipsed by other histologies (AAAIR in older adults: 6.54, 95% CI: 6.48–6.60) (Figure 2E and F). The incidence of meningiomas increased markedly with age, becoming the second most common histology in AYA (AAAIR: 1.93, 95% CI: 1.89–1.97) and most common in older adults (AAAIR: 19.56, 95% CI: 19.46–19.66). Glioblastomas occured primarily in older adults (AAAIR: 6.97, 95% CI: 6.91–7.03), particularly in patients older than 65 years.
Table 1.
Annual Average Total,a Average Annual Age-Adjusted Incidence Rates,b Average Annual Age-Adjusted Mortality Rates,c and 5-year Relative Survival with 95% Confidence Intervals for Brain and Other CNS Tumors by Behavior, Sex, Age Group at Diagnosis, Race, and Hispanic Ethnicity, (CBTRUS: Data provided by CDC’s NPCR and NCI’s SEER Program, 2014–2018; NCHS’s NVSS Program, 2014–2018; CDC’s NPCR Program, 2001–2017)
| Group | Incidence Rate | Mortality Rate | 5-year Relative Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Malignantc | Nonmalignantd | Malignant Only | Malignant | Nonmalignant | |||||||
| Annual Average Cases | Rate (95% CI) | Annual Average Cases | Rate (95% CI) | Annual Average Cases | Rate (95% CI) | Annual Average Deaths | Rate (95% CI) | N | RS (95% CI) | N | RS (95% CI) | |
| Total | 86,355 | 24.25 (24.17–24.32) | 25,105 | 7.06 (7.02–7.10) | 61,250 | 17.19 (17.12–17.25) | 16,606 | 4.43 (4.40–4.46) | 296,432 | 35.6% (35.4%–35.8%) | 635,732 | 91.8% (91.7%–91.9%) |
| Sex | ||||||||||||
| Male | 35,900 | 21.35 (21.25–21.46) | 13,973 | 8.28 (8.22–8.34) | 21,927 | 13.07 (12.99–13.15) | 9,354 | 5.40 (5.35–5.45) | 163,873 | 34.6% (34.3%–34.9%) | 225,537 | 91.0% (90.8%–91.2%) |
| Female | 50,454 | 26.95 (26.85–27.06) | 11,132 | 5.98 (5.93–6.03) | 39,323 | 20.97 (20.88–21.07) | 7,252 | 3.60 (3.56–3.63) | 132,559 | 36.9% (36.6%–37.2%) | 410,195 | 92.2% (92.1%–92.4%) |
| Age | ||||||||||||
| 0–14 years | 3,562 | 5.85 (5.76–5.93) | 2,367 | 3.88 (3.82–3.96) | 1,195 | 1.96 (1.91–2.01) | 425 | 0.70 (0.67–0.73) | 32,998 | 74.8% (74.3%–75.3%) | 13,974 | 97.5% (97.2%–97.8%) |
| 15–39 years | 12,512 | 11.82 (11.73–11.92) | 3,473 | 3.25 (3.20–3.30) | 9,039 | 8.57 (8.49–8.65) | 1,008 | 0.97 (0.94–0.99) | 48,434 | 71.5% (71.0%–71.9%) | 99,184 | 98.2% (98.1%–98.3%) |
| 40+ years | 70,281 | 43.67 (43.53–43.82) | 19,273 | 11.79 (11.72–11.87) | 51,009 | 31.88 (31.76–32.01) | 15,173 | 9.14 (9.08–9.21) | 215,000 | 21.0% (20.8%–21.2%) | 522,574 | 90.3% (90.2%–90.5%) |
| Race | ||||||||||||
| White | 70,297 | 24.24 (24.16–24.32) | 21,841 | 7.55 (7.51–7.60) | 48,456 | 16.69 (16.62–16.76) | 14,981 | 4.83 (4.80–4.87) | 259,999 | 34.3% (34.1%–34.5%) | 513,053 | 91.9% (91.8%–92.0%) |
| Black | 10,273 | 24.58 (24.36–24.80) | 1,924 | 4.44 (4.35–4.53) | 8,349 | 20.14 (19.94–20.34) | 1,115 | 2.66 (2.59–2.73) | 23,017 | 41.5% (40.8%–42.2%) | 83,012 | 89.8% (89.5%–90.2%) |
| AIAN | 570 | 14.62 (14.06–15.20) | 144 | 3.54 (3.27–3.82) | 426 | 11.08 (10.59–11.59) | 68 | 1.82 (1.62–2.03) | 1,619 | 46.8% (44.1%–49.4%) | 4,120 | 94.6% (93.4%–95.5%) |
| API | 3,879 | 19.52 (19.24–19.80) | 863 | 4.40 (4.27–4.53) | 3,016 | 15.12 (14.88–15.37) | 442 | 2.27 (2.18–2.37) | 8,798 | 45.9% (44.8%–47.1%) | 25,230 | 92.9% (92.4%–93.3%) |
| Ethnicity | ||||||||||||
| Non-Hispanic | 76,494 | 24.68 (24.60–24.77) | 22,435 | 7.31 (7.26–7.35) | 54,059 | 17.38 (17.31–17.45) | 15,346 | 4.61 (4.58–4.65) | 264,940 | 34.1% (33.9%–34.3%) | 562,889 | 91.6% (91.5%–91.7%) |
| Hispanic | 9,861 | 22.12 (21.91–22.32) | 2,670 | 5.77 (5.66–5.87) | 7,191 | 16.35 (16.17–16.53) | 1,232 | 3.03 (2.95–3.11) | 31,492 | 48.6% (48.0%–49.2%) | 72,843 | 93.4% (93.1%–93.7%) |
Abbreviations: AIAN, American Indian/Alaska Native; API, Asian or Pacific Islander; CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NPCR, National Program of Cancer Registries; RS, Relative Survival; SEER, Surveillance, Epidemiology, and End Results Program.
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cAssigned behavior code of /3 (see Supplementary Table 3).
dAssigned behavior code of /0 or /1 (see Supplementary Table 3).
Figure 1.
A) Average annual age-adjusted incidence of brain and other CNS tumors and all other cancers by age group at diagnosis, and B) proportion of total cancers occurring in brain and other CNS by age group at diagnosis (CBTRUS: US Cancer Statistics - NPCR and SEER, 2014–2018).
Figure 2.
Distribution of all primary brain and other CNS tumors by behavior for A) children ages 0–14 years old, B) adolescents and young adults 15–39 years and C) adults 40+ years and age-adjusted incidence rates of brain and other CNS tumors by selected histologies for D) children ages 0–14 years old, E) adolescents and young adults 15–39 years and F) adults 40+ years (CBTRUS: US Cancer Statistics - NPCR and SEER, 2014–2018).
Over the 2014–2018 period, there were 83,029 deaths due to malignant brain and CNS tumors, or 16,606 deaths per year, corresponding to an AAAMR of 4.43 (95% CI: 4.40–4.46) (Table 1). AAAMR increased with age, with the AAAMR being 0.70 (95% CI: 0.67–0.73) in children, 0.97 (95% CI: 0.94–0.99) in AYA, and 9.14 in older adults (95% CI: 9.08–9.21).1 Similar to incidence, mortality relative to other cancers decreased with age, with malignant brain and other CNS tumors being the most common cause of cancer death in children, the second most common cause of cancer death in AYA, and the 12th most common cause of cancer death in older adults.
For each of the following major CNS tumor histological types, the annual incidence, averaged over the five-year period 2014–2018, AAAIR, AAAMR, and survival data (if available, for malignant tumors only) are presented. As these rates usually vary with patient age, we divided the data into three broad categories by age as described above. Significant sex and racial differences in incidence rates are also discussed.
Gliomas
Gliomas are named due to their histological resemblance to glial cells from which they were historically thought to arise: astrocytes, oligodendrocytes, and ependymal cells. They may be divided into two broad categories: circumscribed gliomas that have well-demarcated borders and can be completely resected surgically; and diffusely infiltrating gliomas whose borders are poorly delineated and cannot be completely removed surgically. Circumscribed gliomas include pilocytic astrocytomas and ependymomas and are generally low-grade tumors that follow a more indolent course. Diffusely infiltrating gliomas include diffuse astrocytic and oligodendroglial tumors. These may be low or high grade, are generally more clinically aggressive, and harbor an inevitable tendency to recur. Based on the 2016 WHO classification and ICD-O-3 codes, the CBTRUS divides gliomas into the following categories: diffuse astrocytic and oligodendroglial tumors; other astrocytic tumors (including pilocytic astrocytomas and unique astrocytoma variants); ependymal tumors; and other gliomas.
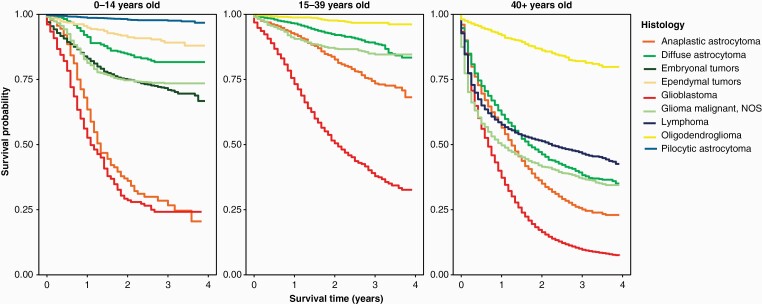
Overall, gliomas comprised 51.3% of CNS tumors in children, of which pilocytic astrocytoma accounted for the largest proportion, totaling 18.3% of CNS tumors, and ependymal tumors comprised 5.3%.1 The diffuse astrocytic tumors, including diffuse and anaplastic astrocytoma and glioblastoma, together comprised 7.9% of CNS tumors in this age group (Figure 2A). Of note, 14.8% of tumors in children were classified as “other gliomas”. In contrast, in older adults (65+), gliomas comprised 22.4% of all CNS tumors and 78.2% of all malignant CNS tumors of which diffuse astrocytic and oligodendroglial tumors comprised the largest proportion of this group (19.3% of all CNS tumors). Incidence of these tumors varied significantly across age groups (Figure 2D–F). Diffuse astrocytic, oligodendroglial, and ependymal tumors were more common in males as compared to females, while pilocytic astrocytoma did not demonstrate a sex preference. Diffuse astrocytic, oligodendroglial, ependymal tumors, and pilocytic astrocytomas were all more commonly diagnosed in patients who are White as compared to patients who are Black (Table 1). The main glioma subtypes displayed marked differences in survival (Table 2, Figure 3).
Table 2.
Annual Average Total Cases,a, and Average Annual Age-Adjusted Incidence Ratesb with 95% Confidence Intervals for Selected Histologies by Grade, Sex, Age Group at Diagnosis, Race, and Hispanic Ethnicity, (CBTRUS: Data provided by CDC’s NPCR and NCI’s SEER Program,2014–2018)
| Histology | Total | Sex | Age at Diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | 0–14 Years | 15–39 Years | 40+ Years | ||||||||
| Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | |
| All gliomasc | 21,146 | 5.95 (5.91–5.99) | 11,959 | 7.05 (6.99–7.11) | 9,187 | 4.98 (4.94–5.03) | 1,825 | 2.99 (2.93–3.06) | 3,152 | 2.95 (2.91–3.00) | 16,169 | 9.90 (9.83–9.97) |
| Glioblastoma | 12,340 | 3.23 (3.20–3.26) | 7,175 | 4.04 (4.00–4.09) | 5,165 | 2.53 (2.50–2.56) | 92 | 0.15 (0.14–0.17) | 584 | 0.56 (0.54–0.58) | 11,664 | 6.97 (6.91–7.03) |
| Pilocytic astrocytoma | 1,061 | 0.36 (0.35–0.37) | 546 | 0.36 (0.35–0.38) | 515 | 0.36 (0.34–0.37) | 650 | 1.07 (1.03–1.10) | 293 | 0.27 (0.26–0.28) | 118 | 0.08 (0.08–0.09) |
| Diffuse and anaplastic astrocytoma | 2,981 | 0.88 (0.87–0.90) | 1,655 | 1.01 (0.99–1.03) | 1,326 | 0.76 (0.74–0.78) | 187 | 0.31 (0.29–0.33) | 905 | 0.84 (0.81–0.86) | 1,888 | 1.21 (1.18–1.23) |
| WHO Grade 2d | 883 | 0.27 (0.26–0.28) | 501 | 0.31 (0.30–0.33) | 383 | 0.23 (0.22–0.24) | 56 | 0.09 (0.08–0.10) | 374 | 0.35 (0.33–0.36) | 453 | 0.30 (0.29–0.31) |
| WHO Grade 3 | 1,417 | 0.41 (0.40–0.42) | 786 | 0.48 (0.46–0.49) | 630 | 0.35 (0.34–0.37) | 43 | 0.07 (0.06–0.08) | 393 | 0.37 (0.35–0.38) | 980 | 0.62 (0.61–0.64) |
| Oligodendroglial tumorse | 1,109 | 0.34 (0.33–0.35) | 614 | 0.39 (0.37–0.40) | 495 | 0.30 (0.29–0.32) | 18 | 0.03 (0.02–0.04) | 391 | 0.37 (0.35–0.39) | 701 | 0.48 (0.46–0.49) |
| WHO Grade 2 | 607 | 0.19 (0.19–0.2) | 334 | 0.21 (0.20–0.23) | 273 | 0.17 (0.16–0.18) | – | – | 250 | 0.24 (0.22–0.25) | 346 | 0.24 (0.23–0.26) |
| WHO Grade 3 | 355 | 0.11 (0.10–0.11) | 196 | 0.12 (0.11–0.13) | 159 | 0.09 (0.09–0.10) | – | – | 98 | 0.09 (0.08–0.10) | 256 | 0.17 (0.16–0.18) |
| Ependymal tumors | 1,385 | 0.42 (0.41–0.43) | 798 | 0.49 (0.48–0.51) | 587 | 0.36 (0.34–0.37) | 188 | 0.31 (0.29–0.33) | 384 | 0.36 (0.34–0.38) | 813 | 0.53 (0.51–0.55) |
| Spinal ependymomaf | 666 | 0.20 (0.20–0.21) | 362 | 0.23 (0.21–0.24) | 304 | 0.18 (0.17–0.19) | 29 | 0.05 (0.04–0.06) | 226 | 0.21 (0.20–0.23) | 410 | 0.27 (0.26–0.28) |
| Supratentorial ependymomag | 116 | 0.04 (0.03–0.04) | 59 | 0.04 (0.03–0.04) | 56 | 0.04 (0.03–0.04) | 38 | 0.06 (0.05–0.07) | 32 | 0.03 (0.03–0.03) | 45 | 0.03 (0.03–0.03) |
| Infratentorial ependymomah | 460 | 0.14 (0.13–0.14) | 292 | 0.17 (0.17–0.18) | 169 | 0.10 (0.09–0.11) | 78 | 0.13 (0.11–0.14) | 94 | 0.09 (0.08–0.10) | 288 | 0.18 (0.17–0.19) |
| Meningiomas | 33,686 | 9.12 (9.08–9.17) | 9,099 | 5.41 (5.36–5.46) | 24,588 | 12.41 (12.34–12.49) | 62 | 0.10 (0.09–0.11) | 1,954 | 1.93 (1.89–1.97) | 31,670 | 19.56 (19.46–19.66) |
| WHO Grade 1 | 9,696 | 2.66 (2.64–2.69) | 2,632 | 1.53 (1.50–1.56) | 7,064 | 3.72 (3.68–3.76) | – | – | – | – | 8,849 | 5.5 (5.44–5.55) |
| WHO Grade 2 | 1,895 | 0.53 (0.51–0.54) | 802 | 0.47 (0.45–0.48) | 1,092 | 0.58 (0.57–0.60) | – | – | – | – | 1,667 | 1.04 (1.02–1.06) |
| WHO Grade 3 | 165 | 0.04 (0.04–0.05) | 84 | 0.05 (0.04–0.05) | 81 | 0.04 (0.04–0.05) | – | – | – | – | 150 | 0.09 (0.08–0.10) |
| Lymphoma | 1,696 | 0.45 (0.44–0.46) | 863 | 0.50 (0.48–0.52) | 832 | 0.41 (0.40–0.43) | 18 | 0.03 (0.02–0.04) | 108 | 0.10 (0.09–0.11) | 1,570 | 0.96 (0.93–0.98) |
| Embryonal tumors | 650 | 0.22 (0.21–0.23) | 389 | 0.26 (0.25–0.27) | 261 | 0.18 (0.17–0.19) | 439 | 0.72 (0.69–0.75) | 152 | 0.14 (0.13–0.15) | 59 | 0.04 (0.04–0.05) |
| Medulloblastomai | 456 | 0.16 (0.15–0.16) | 290 | 0.19 (0.18–0.21) | 166 | 0.12 (0.11–0.12) | 297 | 0.49 (0.46-0.51) | 127 | 0.11 (0.11–0.12) | 31 | 0.02 (0.02–0.03) |
| ATRTj | 83 | 0.03 (0.03–0.03) | 42 | 0.03 (0.02–0.03) | 42 | 0.03 (0.03–0.03) | 75 | 0.12 (0.11–0.14) | – | – | – | – |
| Germ cell tumors | 250 | 0.08 (0.08–0.09) | 187 | 0.12 (0.11–0.13) | 63 | 0.04 (0.04–0.05) | 117 | 0.19 (0.18–0.21) | 120 | 0.11 (0.10–0.12) | – | – |
| Nerve sheath tumors | 7,329 | 2.05 (2.03–2.07) | 3,526 | 2.06 (2.03–2.09) | 3,804 | 2.05 (2.02–2.08) | 150 | 0.25 (0.23–0.26) | 1,104 | 1.06 (1.03–1.09) | 6,075 | 3.77 (3.73–3.81) |
| Tumors of the pituitary | 14,789 | 4.36 (4.33–4.40) | 6,598 | 3.92 (3.87–3.96) | 8,191 | 4.90 (4.85–4.95) | 219 | 0.36 (0.34–0.38) | 4,440 | 4.15 (4.10–4.21) | 10,130 | 6.54 (6.48–6.6) |
| Craniopharyngioma | 628 | 0.19 (0.18–0.20) | 322 | 0.20 (0.19–0.21) | 306 | 0.18 (0.17–0.19) | 135 | 0.22 (0.21–0.24) | 142 | 0.13 (0.12–0.14) | 351 | 0.22 (0.21–0.23) |
| Histology | Race | Ethnicity | ||||||||||
| White | Black | AIAN | API | Non-Hispanic | Hispanic | |||||||
| Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | Annual Average | Rate (95% CI) | |
| All gliomas | 18,592 | 6.45 (6.41–6.50) | 1,533 | 3.50 (3.42–3.58) | 116 | 2.79 (2.56–3.04) | 637 | 3.20 (3.09–3.32) | 19,013 | 6.21 (6.17–6.26) | 2,133 | 4.55 (4.46–4.64) |
| Glioblastoma | 11,114 | 3.52 (3.49–3.55) | 761 | 1.78 (1.72–1.84) | 53 | 1.43 (1.25–1.62) | 290 | 1.46 (1.38–1.53) | 11,329 | 3.33 (3.3–3.36) | 1,010 | 2.51 (2.44–2.58) |
| Pilocytic astrocytoma | 855 | 0.38 (0.37–0.39) | 127 | 0.27 (0.25–0.29) | – | – | 47 | 0.25 (0.22–0.28) | 891 | 0.39 (0.38–0.40) | 170 | 0.25 (0.23–0.27) |
| Diffuse and anaplastic astrocytoma | 2,599 | 0.97 (0.95–0.99) | 218 | 0.50 (0.47–0.53) | 18 | 0.40 (0.32–0.49) | 107 | 0.52 (0.48–0.57) | 2,671 | 0.94 (0.92–0.96) | 310 | 0.62 (0.58–0.65) |
| WHO Grade 2 | 763 | 0.30 (0.29–0.31) | 66 | 0.15 (0.13–0.17) | – | – | 35 | 0.17 (0.15–0.20) | 785 | 0.29 (0.28–0.30) | 98 | 0.18 (0.17–0.20) |
| WHO Grade 3 | 1,254 | 0.46 (0.45–0.47) | 94 | 0.22 (0.20–0.24) | – | – | 44 | 0.21 (0.19–0.25) | 1,282 | 0.44 (0.43–0.45) | 135 | 0.27 (0.25–0.29) |
| Oligodendroglial tumors | 970 | 0.38 (0.37–0.39) | 66 | 0.15 (0.14–0.17) | – | – | 43 | 0.20 (0.18–0.23) | 971 | 0.36 (0.35–0.37) | 138 | 0.26 (0.24–0.28) |
| WHO Grade 2 | 536 | 0.22 (0.21–0.23) | 34 | 0.08 (0.07–0.10) | – | – | 19 | 0.09 (0.07–0.11) | 534 | 0.21 (0.2–0.21) | 73 | 0.14 (0.12–0.15) |
| WHO Grade 3 | 311 | 0.12 (0.11–0.12) | 20 | 0.05 (0.04–0.06) | – | – | 17 | 0.08 (0.07–0.10) | 310 | 0.11 (0.11–0.12) | 45 | 0.09 (0.08–0.10) |
| Ependymal tumors | 1,178 | 0.46 (0.44–0.47) | 119 | 0.26 (0.24–0.29) | – | – | 53 | 0.26 (0.23–0.29) | 1,184 | 0.43 (0.42–0.45) | 201 | 0.37 (0.35–0.39) |
| Spinal ependymoma | 580 | 0.23 (0.22–0.23) | – | – | – | – | 26 | 0.13 (0.10–0.15) | 576 | 0.21 (0.20–0.22) | 90 | 0.17 (0.15–0.18) |
| Supratentorial ependymoma | 91 | 0.04 (0.03–0.04) | – | – | – | – | – | – | 97 | 0.04 (0.03–0.04) | 19 | 0.03 (0.03–0.04) |
| Infratentorial ependymoma | 389 | 0.15 (0.14–0.15) | 46 | 0.10 (0.09–0.12) | – | – | – | – | 392 | 0.14 (0.13–0.15) | 68 | 0.13 (0.11–0.14) |
| Meningiomas | 27,236 | 8.90 (8.85–8.95) | 4,308 | 10.70 (10.56–10.85) | 205 | 5.81 (5.44–6.2) | 1,514 | 7.87 (7.69–8.05) | 30,379 | 9.25 (9.20–9.29) | 3,308 | 8.53 (8.39–8.66) |
| WHO Grade 1 | 7,774 | 2.61 (2.59–2.64) | 1,251 | 2.97 (2.90–3.05) | 63 | 1.67 (1.48–1.87) | 481 | 2.36 (2.27–2.46) | 8,673 | 2.72 (2.69–2.74) | 1,023 | 2.38 (2.31–2.45) |
| WHO Grade 2 | 1,444 | 0.49 (0.48–0.5) | 298 | 0.71 (0.67–0.74) | – | – | 112 | 0.55 (0.50–0.60) | 1,715 | 0.54 (0.53–0.56) | 180 | 0.42 (0.39–0.45) |
| WHO Grade 3 | 132 | 0.04 (0.04–0.05) | – | – | – | – | – | – | 148 | 0.04 (0.04–0.05) | – | – |
| Lymphoma | 1,422 | 0.46 (0.44–0.47) | 138 | 0.33 (0.30–0.35) | – | – | 100 | 0.51 (0.46–0.56) | 1,490 | 0.45 (0.44–0.46) | 206 | 0.52 (0.49–0.55) |
| Embryonal tumors | 514 | 0.23 (0.22–0.24) | 75 | 0.16 (0.14–0.17) | – | – | 39 | 0.21 (0.18–0.24) | 509 | 0.23 (0.22–0.24) | 141 | 0.21 (0.19–0.22) |
| Medulloblastoma | 361 | 0.16 (0.16–0.17) | 50 | 0.10 (0.09–0.12) | – | – | 28 | 0.15 (0.13–0.18) | 355 | 0.16 (0.15–0.17) | 101 | 0.15 (0.14–0.16) |
| ATRT | 62 | 0.03 (0.03–0.03) | – | – | – | – | – | – | 65 | 0.03 (0.03–0.03) | 18 | 0.03 (0.02–0.03) |
| Germ cell tumors | 192 | 0.08 (0.08–0.09) | 27 | 0.06 (0.05–0.07) | – | – | 25 | 0.13 (0.11–0.15) | 192 | 0.08 (0.08–0.09) | 58 | 0.09 (0.08–0.10) |
| Nerve sheath tumors | 6,222 | 2.16 (2.13–2.18) | 455 | 1.05 (1.00–1.09) | 47 | 1.13 (0.98–1.29) | 436 | 2.09 (2.00–2.18) | 6,661 | 2.16 (2.13–2.18) | 669 | 1.42 (1.37–1.47) |
| Tumors of the pituitary | 10,583 | 3.94 (3.90–3.97) | 2,940 | 6.91 (6.80–7.03) | 129 | 3.09 (2.84–3.35) | 820 | 3.93 (3.81–4.06) | 12,352 | 4.28 (4.24–4.31) | 2,437 | 4.93 (4.83–5.02) |
| Craniopharyngioma | 454 | 0.18 (0.17–0.18) | 125 | 0.28 (0.26–0.30) | – | – | 33 | 0.16 (0.14–0.19) | 529 | 0.20 (0.19–0.20) | 99 | 0.18 (0.16–0.20) |
Abbreviations: AIAN, American Indian/Alaska Native; API, Asian or Pacific Islander; ATRT, Atypical teratoid/rhabdoid tumors; CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results Program.
aAnnual average cases are calculated by dividing the five-year total by five.
bRates are per 100,000 and are age-adjusted to the 2000 US standard population.
cICD-O-3 histology codes 9380-9384, 9391-9460.
dMay not sum to total of all cases in histology due to missing grade information.
eIncludes oligodendroglioma and anaplastic oligodendroglioma.
fIncludes ependymal tumors occurring in sites spine and cauda equina.
gIncludes ependymal tumors occurring in sites cerebellum, frontal lobe, occipital lobe, temporal love, and parietal lobe.
hIncludes ependymal tumors occurring in sites cerebrum, ventricle, and brain stem.
iICD-O-3 code 9470-9472, 9474-9477.
jICD-O-3 code 9508/3.
– Counts and rates are not presented when fewer than 16 cases were reported for the specific category. The suppressed cases are included in the counts and rates for totals.
Figure 3.
Kaplan–Meier survival curves for the five most common histologies within age group at diagnosis (ages 0–14 years old, 15–39 years old and 40+ years old). (Data provided by CDC’s National Program of Cancer Registries, 2001–2017).
Diffuse Astrocytic and Oligodendroglial Tumors
The 2021 WHO classification uses IDH mutation and 1p/19q codeletion information to define the diffuse astrocytic and olidendroglial glioma subtypes.18 IDH mutation without 1p/19q codeletion defines the grades 2, 3, and 4 astrocytomas, approximately corresponding to the traditionally used diagnoses of diffuse astrocytoma, anaplastic astrocytoma, and secondary glioblastoma, respectively. IDH mutation with 1p/19q define the grades 2 and 3 oligodendroglial tumors. Finally, IDH wildtype without 1p/19q codeletion largely define glioblastoma (WHO grade 4). Because molecular data have only been collected beginning in diagnosis year 2018, in this paper we use the histologic diagnoses recorded in cancer registries that relied on the diagnostic criteria in effect at the time of their diagnosis. Data on IDH mutation status for diagnosis year 2018 are available for 75% of diffuse astrocytoma, 85% of anaplastic astrocytoma, and 78% of glioblastoma cases. Approximately 58% of tumors classified as diffuse astrocytoma (ICD-O-3 code 9400/3 only), 50% of anaplastic astrocytoma (ICD-O-3 code 9401/3 only), and 3% of glioblastoma (ICD-O-3 codes 9440/3 and 9445/3 only) were IDH-mutant (see Table 4 in Ostrom, et al1). Therefore, a significant portion of diffuse and anaplastic astrocytoma cases would likely be considered glioblastomas in the 2021 WHO classification due to their IDH-wildtype status.
The overall incidence of diffuse astrocytic and oligodendroglial tumors in the US was 16,625 cases per year (AAAIR 4.52, 95% CI: 4.49–4.55).1 They were more common with increasing age (AAAIR 0.31 (95% CI: 0.29–0.33) in children, 0.84 (95% CI: 0.81–0.86) in AYA, and 1.21 (95% CI: 1.18–1.23) in older adults). Oligodendroglial tumors occur almost exclusively in the AYA (AAAIR 0.37, 95% CI: 0.35–0.39) and older adult (AAAIR: 0.48, 95% CI: 0.46–0.49) age groups. Glioblastomas are overwhelmingly more common in older adults (AAAIR: 0.15 (95% CI: 0.14–0.17) in children, 0.56 (95% CI: 0.54–0.58) in AYA, and 6.97 (95% CI: 6.91–7.03) in older adults) (Table 2).
Diffusely infiltrating gliomas had significantly worse prognosis than circumscribed gliomas and displayed marked variability in survival depending upon histologic subtype (Table 3). Oligodendroglial tumors had the highest survival, with oligodendrogliomas showing a median survival of 199 months (16.5 years) and anaplastic oligodendrogliomas (WHO grade 3) showing a median survival of 97 months (8 years). In contrast, infiltrating astrocytomas had shorter survival times: 59 months for diffuse astrocytoma (WHO grade 2), 20 months for anaplastic astrocytoma (WHO grade 3), and 8 months for glioblastoma (WHO grade 4). The overall 1-, 5-, and 10-year relative survival rates for glioblastoma were 40.9%, 6.6%, and 4.3%, respectively. Survival in glioblastoma was worst in the elderly, with those over age 65 having a 1-, 5-, and 10-year relative survival rates of 24.0%, 2.7%, and 2.1%, respectively; patients in the 40–64-year-old age group showed 1-, 5-, and 10-year relative survival rates of 52.8%, 7.6%, and 4.5%, respectively. The best survival rates were observed in children (1-, 5-, and 10-year survival rates 56.3%, 20.6%, and 17.6%) and AYA (1-, 5-, and 10-year survival rates 75.3%, 26.0%, 18.4%), It is important to note that these glioblastoma data included both IDH wildtype and IDH-mutant tumors, since the data were collected prior to the 2021 WHO revision that excluded IDH-mutant tumors from the glioblastoma diagnosis. This is particularly pronounced in the AYA age group, where 2018 collection data indicated that 18% of glioblastoma diagnoses were IDH mutated
Table 3.
One-, Five-, and Ten-Year Relative Survival Ratesa,b (RS) with 95% Confidence Intervals for Malignant Brain and Other Central Nervous System Tumors by Selected Histologies and Behavior, Overall and by Age Group at Diagnosis, (CBTRUS: Data provided by CDC’s NPCR Program, 2001–2017)
| Histology | Age Group | Median Survival (95% CI) | N | 1-Year RS (95% CI) | 5-Year RS (95% CI) | 10-Year RS (95% CI) |
|---|---|---|---|---|---|---|
| All gliomasc | 0–14d | ** (**–**) | 23,155 | 89.0% (88.6%–89.5%) | 78.1% (77.6%–78.7%) | 75.5% (74.9%–76.1%) |
| 15–39e | ** (192–**) | 39,620 | 91.9% (91.6%–92.2%) | 71.5% (71.0%–72.0%) | 60.0% (59.4%–60.6%) | |
| 40–64 | 17 (17–17) | 100,922 | 63.4% (63.1%–63.7%) | 25.7% (25.4%–26.0%) | 20.3% (20.0%–20.6%) | |
| 65+ | 5 (4–5) | 79,026 | 27.6% (27.2%–27.9%) | 6.6% (6.3%–6.8%) | 5.0% (4.7%–5.2%) | |
| All ages | 16 (16–16) | 242,723 | 59.1% (58.9%–59.3%) | 32.5% (32.3%–32.7%) | 27.6% (27.4%–27.8%) | |
| Glioblastoma | 0–14 | 13 (13–14) | 1,216 | 56.3% (53.4%–59.1%) | 20.6% (18.2%–23.1%) | 17.6% (15.3%–20.1%) |
| 15–39 | 23 (23–24) | 7,058 | 75.3% (74.2%–76.3%) | 26.0% (24.9%–27.1%) | 18.4% (17.3%–19.5%) | |
| 40–64 | 12 (12–12) | 63,788 | 52.8% (52.4%–53.2%) | 7.6% (7.4%–7.9%) | 4.5% (4.3%–4.7%) | |
| 65+ | 4 (4–4) | 61,911 | 24.0% (23.7%–24.4%) | 2.7% (2.6%–2.9%) | 2.1% (1.9%–2.2%) | |
| All ages | 8 (8–8) | 133,973 | 40.9% (40.7%–41.2%) | 6.6% (6.4%–6.7%) | 4.3% (4.1%–4.4%) | |
| Pilocytic astrocytoma | 0–14 | ** (**–**) | 8,691 | 98.8% (98.5%–99.0%) | 96.8% (96.4%–97.2%) | 95.3% (94.7%–95.8%) |
| 15–39 | ** (**–**) | 4,312 | 98.4% (97.9%–98.7%) | 94.8% (94.0%–95.5%) | 92.9% (91.9%–93.8%) | |
| 40–64 | ** (**–**) | 1,177 | 93.9% (92.3%–95.2%) | 81.6% (78.9%–84.0%) | 79.0% (75.7%–81.9%) | |
| 65+ | 83 (53–125) | 262 | 81.6% (75.8%–86.2%) | 65.1% (56.8%–72.2%) | 64.6% (55.6%–72.2%) | |
| All ages | ** (**–**) | 14,442 | 98.0% (97.7%–98.2%) | 94.4% (94.0%–94.8%) | 92.8% (92.2%–93.3%) | |
| Diffuse and anaplastic astrocytoma | 0–14 | ** (**–**) | 3,198 | 86.0% (84.7%–87.1%) | 68.7% (67.0%–70.4%) | 66.2% (64.4%–67.9%) |
| 15–39 | 137 (129–148) | 11,632 | 93.5% (93.0%–93.9%) | 70.8% (69.8%–71.7%) | 53.9% (52.8%–55.1%) | |
| 40–64 | 26 (25–27) | 16,033 | 71.8% (71.1%–72.5%) | 35.3% (34.5%–36.1%) | 26.2% (25.4%–27.0%) | |
| 65+ | 5 (5–6) | 8,946 | 33.3% (32.3%–34.3%) | 9.1% (8.4%–9.9%) | 5.7% (5.0%–6.5%) | |
| All ages | 34 (33–35) | 39,809 | 70.8% (70.3%–71.3%) | 42.9% (42.3%–43.4%) | 33.4% (32.9%–34.0%) | |
| WHO Grade 2 Diffuse and anaplastic astrocytoma | 0–14 | ** (**–**) | 403 | 94.2% (91.3%–96.2%) | 82.7% (78.1%–86.5%) | – |
| 15–39 | ** (**–**) | 1,986 | 98.1% (97.3%–98.6%) | 83.4% (80.8%–85.6%) | – | |
| 40–64 | 66 (58–73) | 1,645 | 88.2% (86.4%–89.7%) | 53.7% (50.2%–57.1%) | – | |
| 65+ | 15 (13–18) | 618 | 58.5% (54.2%–62.5%) | 23.8% (18.8%–29.1%) | – | |
| All ages | ** (**–**) | 4,652 | 89.0% (88.0%–89.9%) | 65.3% (63.4%–67.2%) | – | |
| WHO Grade 3 Diffuse and anaplastic astrocytoma | 0–14 | 16 (14–20) | 276 | 66.4% (60.4%–71.8%) | 24% (18.5%–30.0%) | – |
| 15–39 | ** (**–**) | 2,120 | 92.9% (91.7%–94%) | 64% (61%–66.9%) | – | |
| 40–64 | 23 (22–25) | 3,337 | 72.6% (71.0%–74.2%) | 28.2% (26.1%–30.2%) | – | |
| 65+ | 6 (5–7) | 1,812 | 34.6% (32.3%–36.9%) | 5.4% (3.9%–7.1%) | – | |
| All ages | 24 (22–25) | 7,545 | 69% (67.9%–70.0%) | 32.6% (31.1%–34%) | – | |
| Oligodendroglial tumors | 0–14 | ** (**–**) | 405 | 95.5% (93.0%–97.2%) | 89.7% (86.1%–92.4%) | 86.7% (82.5%–89.9%) |
| 15–39 | ** (**–**) | 5,991 | 97.9% (97.4%–98.2%) | 88.4% (87.5%–89.3%) | 73.6% (72.2%–75.0%) | |
| 40–64 | 146 (140–152) | 8,239 | 92.8% (92.2%–93.4%) | 74.2% (73.1%–75.3%) | 60.2% (58.8%–61.5%) | |
| 65+ | 24 (22–28) | 1,601 | 66.8% (64.3%–69.2%) | 37.6% (34.6%–40.5%) | 24.9% (21.5%–28.4%) | |
| All ages | 165 (160–174) | 16,236 | 92.2% (91.8%–92.7%) | 76.5% (75.8%–77.3%) | 62.8% (61.9%–63.8%) | |
| WHO Grade 2 Oligodendroglial tumors | 0–14 | ** (**–**) | 71 | ** | ** | – |
| 15–39 | ** (**–**) | 1,433 | 99.1% (98.3%–99.5%) | 94.8% (92.8%–96.2%) | – | |
| 40–64 | ** (**–**) | 1,631 | 97.9% (96.9%–98.5%) | 88.4% (85.7%–90.6%) | – | |
| 65+ | ** (53–**) | 223 | 88.5% (82.6%–92.5%) | 63.7% (52.0%–73.3%) | – | |
| All ages | ** (**–**) | 3,358 | 97.8% (97.2%–98.3%) | 90% (88.4%–91.5%) | – | |
| WHO Grade 3 Oligodendroglial tumors | 0–14 | – | – | – | – | – |
| 15–39 | ** (**–**) | 572 | 97.1% (95.2%–98.2%) | 81.7% (76.8%–85.7%) | – | |
| 40–64 | ** (**–**) | 1,088 | 93.2% (91.4%–94.6%) | – | – | |
| 65+ | 29 (25–54) | 244 | 72.1% (65.4%–77.7%) | 44.8% (35.3%–53.8%) | – | |
| All ages | ** (**–**) | 1,915 | 91.8% (90.3%–93.0%) | 69% (66.0%–71.8%) | – | |
| Ependymal tumors | 0–14 | ** (**–**) | 2,420 | 94.5% (93.5%–95.4%) | 76.6% (74.7%–78.4%) | 67.0% (64.6%–69.2%) |
| 15–39 | ** (**–**) | 3,047 | 97.1% (96.4%–97.7%) | 91.4% (90.2%–92.4%) | 87.2% (85.7%–88.6%) | |
| 40–64 | ** (**–**) | 4,233 | 95.2% (94.5%–95.9%) | 89.4% (88.3%–90.5%) | 86.6% (85.0%–88.0%) | |
| 65+ | 106 (97–119) | 1,250 | 85.7% (83.4%–87.8%) | 77.4% (73.7%–80.6%) | 69.5% (63.5%–74.6%) | |
| All ages | ** (**–**) | 10,950 | 94.5% (94.1%–95.0%) | 85.8% (85.0%–86.6%) | 80.7% (79.7%–81.7%) | |
| Spinal ependymomaf | 0–14 | ** (**–**) | 164 | 97.5% (93.5%–99.1%) | 93.4% (88.0%–96.4%) | 86.2% (77.7%–91.7%) |
| 15–39 | ** (**–**) | 1,498 | 98.9% (98.2%–99.3%) | 95.4% (94.1%–96.5%) | 93.3% (91.4%–94.7%) | |
| 40–64 | ** (**–**) | 2,552 | 99.1% (98.4%–99.4%) | 97.2% (96.1%–98.1%) | 96.2% (94.5%–97.4%) | |
| 65+ | 139 (127–155) | 658 | 95.2% (92.6%–96.9%) | 92.9% (87.4%–96.1%) | 86.9% (77.9%–92.4%) | |
| All ages | ** (**–**) | 4,872 | 98.5% (98.0%–98.8%) | 96.0% (95.1%–96.8%) | 94.0% (92.6%–95.2%) | |
| Supratentorial ependymomag | 0–14 | ** (**–**) | 508 | 93.9% (91.4%–95.7%) | 81.8% (77.8%–85.2%) | 73.5% (68.4%–78.0%) |
| 15–39 | ** (**–**) | 389 | 95.3% (92.7%–97.1%) | 82.9% (78.3%–86.6%) | 75.7% (70.2%–80.3%) | |
| 40–64 | 83 (60–165) | 291 | 86.0% (81.3%–89.6%) | 58.1% (51.5%–64.1%) | 49.4% (42.1%–56.2%) | |
| 65+ | 18 (13–26) | 93 | 64.0% (52.6%–73.4%) | 21.5% (12.0%–32.9%) | – | |
| All ages | ** (**–**) | 1,281 | 90.5% (88.6%–92.0%) | 72.8% (70.0%–75.4%) | 64.5% (61.2%–67.6%) | |
| Infratentorial ependymomah | 0–14 | ** (**–**) | 1,097 | 95.1% (93.6%–96.2%) | 74.4% (71.4%–77.1%) | 63.6% (60.0%–67.0%) |
| 15–39 | ** (**–**) | 832 | 95.3% (93.5%–96.5%) | 89.5% (87.0%–91.5%) | 84.0% (80.7%–86.8%) | |
| 40–64 | ** (200–**) | 973 | 89.5% (87.3%–91.3%) | 82.1% (79.2%–84.7%) | 78.0% (74.1%–81.3%) | |
| 65+ | 86 (67–104) | 355 | 78.7% (73.6%–82.9%) | 69.1% (62.0%–75.2%) | 55.5% (44.6%–65.2%) | |
| All ages | ** (**–**) | 3,257 | 91.7% (90.7%–92.6%) | 80.0% (78.4%–81.5%) | 72.7% (70.6%–74.6%) | |
| Meningiomas | 0–14 | ** (**–**) | 58 | 89.6% (78.3%–95.2%) | 77.8% (64.0%–86.8%) | 72.0% (56.6%–82.7%) |
| 15–39 | ** (**–**) | 396 | 94.2% (91.3%–96.1%) | 83.4% (79.0%–86.9%) | 78.0% (72.8%–82.4%) | |
| 40–64 | ** (188–**) | 2,070 | 90.6% (89.2%–91.8%) | 76.3% (74.1%–78.3%) | 69.2% (66.6%–71.7%) | |
| 65+ | 43 (40–49) | 2,318 | 76.8% (74.8%–78.6%) | 55.7% (52.9%–58.5%) | 48.0% (44.4%–51.5%) | |
| All ages | 103 (94–110) | 4,842 | 84.4% (83.2%–85.5%) | 67.5% (65.8%–69.2%) | 60.8% (58.7%–62.9%) | |
| Lymphoma | 0–14 | ** (**–**) | 179 | 91.4% (86.1%–94.7%) | 85.3% (78.7%–89.9%) | 80.4% (72.0%–86.5%) |
| 15–39 | 104 (70–137) | 1,809 | 62.2% (59.8%–64.4%) | 53.4% (51.0%–55.8%) | 49.3% (46.6%–51.8%) | |
| 40–64 | 36 (32–39) | 7,579 | 63.2% (62.0%–64.3%) | 44.2% (43.0%–45.5%) | 33.7% (32.3%–35.1%) | |
| 65+ | 7 (6–7) | 8,786 | 44.1% (43.0%–45.2%) | 24.7% (23.6%–25.9%) | 17.5% (16.1%–18.9%) | |
| All ages | 15 (14–16) | 18,353 | 54.4% (53.6%–55.1%) | 36.7% (35.9%–37.5%) | 28.6% (27.7%–29.5%) | |
| Embryonal tumors | 0–14 | ** (**–**) | 6,705 | 81.2% (80.2%–82.1%) | 62.5% (61.3%–63.7%) | 57.8% (56.4%–59.1%) |
| 15–39 | ** (**–**) | 2,466 | 90.2% (89.0%–91.3%) | 70.2% (68.2%–72.2%) | 60.1% (57.8%–62.4%) | |
| 40–64 | 64 (49–78) | 725 | 75.1% (71.7%–78.1%) | 51.8% (47.8%–55.7%) | 41.9% (37.5%–46.2%) | |
| 65+ | 6 (5–10) | 140 | 41.3% (32.7%–49.6%) | – | – | |
| All ages | ** (**–**) | 10,036 | 82.4% (81.7%–83.2%) | 63.0% (62.0%–64.0%) | 56.5% (55.4%–57.6%) | |
| Medulloblastomai | 0–14 | ** (**–**) | 4,218 | 89.8% (88.8%–90.7%) | 72.6% (71.1%–74.0%) | 67.1% (65.4%–68.7%) |
| 15–39 | ** (**–**) | 1,894 | 93.1% (91.8%–94.2%) | 79.3% (77.2%–81.2%) | 68.6% (66.0%–71.1%) | |
| 40–64 | 130 (105–186) | 406 | 84.4% (80.4%–87.7%) | 69.2% (64.0%–73.9%) | 55.6% (49.0%–61.7%) | |
| 65+ | – | – | – | – | – | |
| All ages | ** (**–**) | 6,548 | 90.3% (89.5%–91.0%) | 74.1% (72.9%–75.2%) | 66.7% (65.3%–68.0%) | |
| ATRTj | 0–14 | 13 (11–15) | 984 | 52.9% (49.7%–56.0%) | 33.0% (29.9%–36.2%) | 30.7% (27.5%–34.0%) |
| 15–39 | – | – | – | – | – | |
| 40–64 | – | – | – | – | – | |
| 65+ | – | – | – | – | – | |
| All ages | 13 (12–16) | 1,044 | 53.6% (50.5%–56.6%) | 33.6% (30.6%–36.8%) | 31.0% (27.9%–34.3%) | |
| Germ cell tumors | 0–14 | ** (**–**) | 1,344 | 92.8% (91.2%–94.1%) | 87.2% (85.1%–89.0%) | 83.4% (80.8%–85.6%) |
| 15–39 | ** (**–**) | 1,507 | 94.1% (92.8%–95.2%) | 87.9% (86.0%–89.6%) | 85.4% (83.2%–87.4%) | |
| 40–64 | 135 (127–**) | 74 | 84.8% (73.9%–91.4%) | 71.5% (58.4%–81.1%) | 66.1% (50.4%–77.8%) | |
| 65+ | – | – | – | – | – | |
| All ages | ** (**–**) | 2,938 | 93.2% (92.2%–94.0%) | 86.9% (85.6%–88.2%) | 83.9% (82.2%–85.4%) | |
| Nerve sheath tumors | 0–14 | – | – | – | – | – |
| 15–39 | ** (148–**) | 166 | 81.7% (74.8%–86.9%) | 69.3% (61.4%–76.0%) | 64.9% (56.5%–72.1%) | |
| 40–64 | ** (**–**) | 301 | 86.9% (82.4%–90.3%) | 78.3% (72.8%–82.9%) | 76.8% (70.2%–82.1%) | |
| 65+ | 97 (73–145) | 160 | 84.5% (76.8%–89.8%) | 73.1% (61.8%–81.5%) | 67.4% (49.6%–80.0%) | |
| All ages | ** (**–**) | 669 | 85.1% (82.0%–87.7%) | 74.7% (70.8%–78.2%) | 71.7% (66.7%–76.0%) | |
| Tumors of the pituitary | 0–14 | – | – | – | – | – |
| 15–39 | ** (**–**) | 95 | 98.0% (91.5%–99.5%) | 89.0% (79.8%–94.2%) | 89.0% (79.8%–94.2%) | |
| 40–64 | ** (**–**) | 231 | 89.1% (84.1%–92.6%) | 80.6% (73.9%–85.7%) | 77.1% (68.6%–83.6%) | |
| 65+ | 76 (59–117) | 127 | 85.9% (77.2%–91.5%) | 75.9% (62.8%–84.9%) | 63.5% (44.2%–77.8%) | |
| All ages | ** (165–**) | 456 | 90.2% (86.8%–92.8%) | 81.2% (76.3%–85.2%) | 76.9% (70.3%–82.3%) |
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; NOS, not otherwise specified; NPCR, National Program of Cancer Registries; RS, Relative Survival.
aThe cohort analysis of survival rates was utilized for calculating the survival estimates presented in this table. Long-term cohort-based survival estimates reflect the survival experience of individuals diagnosed over the time period, and they may not necessarily reflect the long-term survival outlook of newly diagnosed cases.
bRates are an estimate of the percentage of patients alive at one, two, five, and ten years, respectively. Rates were not presented for categories with 50 or fewer cases and were suppressed for rates where fewer than 16 cases were surviving within a category.
cICD-O-3 histology codes 9380-9384, 9391-9460.
dChildren as defined by the National Cancer Institute, see: http://www.cancer.gov/researchandfunding/snapshots/pediatric.
eAdolescents and Young Adults (AYA), as defined by the National Cancer Institute, see: http://www.cancer.gov/cancertopics/aya.
fDefined as ependymal tumors occurring in sites spine and cauda equina.
gDefined as ependymal tumors occurring in sites cerebellum, frontal lobe, occipital lobe, temporal love, and parietal lobe.
hDefined as ependymal tumors occurring in sites cerebrum, ventricle, and brain stem.
iICD-O-3 code 9470-9472, 9474-9477.
jICD-O-3 code 9508/3.
– Rates were not presented for categories with 50 or fewer cases and were suppressed for rates where fewer than 16 cases were surviving within a category.
** Could not be calculated.
While overall incidence of glioma in the US has generally been found to be stable over time, incidence of specific glioma subtypes has changed (See Figure 23 in Ostrom, et al.1). Some of this change is due to changes in diagnostic classification, particularly the elimination of oligoastrocytoma as a distinct entity. Several analyses conducted since the introduction of the Stupp protocol19 in 2005 have suggested slight increases in glioblastoma survival over time.20–22
Circumscribed Gliomas
Pilocytic astrocytomas were more common in the youngest age group, with an AAAIR of 1.07 in children but only 0.08 in older adults (Table 2). Pilocytic astrocytomas had a high survival rate, with 10-year survival of >90% for children and young adults, and >70% for older adults. The median survival for pilocytic astrocytoma could not be calculated due to its overall high survival rate.
Ependymal tumors are comprised of 9 distinct subtypes that are classified according to anatomical site and molecular features and which display distinct age preferences and clinical behaviors. Anatomically, ependymal tumors were broadly divided into 3 groups: supratentorial, posterior fossa, and spinal ependymomas. Spinal ependymomas were most common in young adults while supratentorial and posterior fossa ependymomas occurred primarily in children. CBTRUS groups ependymal tumors broadly into malignant and nonmalignant subtypes. The malignant subtypes consist of WHO grade 3 anaplastic ependymomas and the nonmalignant subtype comprise WHO grades 1 and 2 ependymal tumors, including subependymomas and myxopapillary ependymomas. Nonmalignant ependymal tumors had 10-year survival rates of >90% for all age groups. Malignant ependymal tumors demonstrated 1-year survival rates of >90%, but survival decreased over time, particularly in young patients: 10-year survival rates were 67% for children, 78% for AYA, and 83% for older adults. Ependymal tumors in the spine had the best prognosis, with a 10-year relative survival rate of 94%. Supratentorial ependymomas had 1-, 5-, and 10-year relative survival rates of 90.5%, 72.8%, and 64.5% respectively. Infratentorial (posterior fossa) ependymomas had 1-, 5-, and 10-year relative survival rates of 91.7%, 80.0%, and 72.7% respectively. With the 2021 WHO classification revision,23 these tumors will now be subdivided into 9 subgroups based on anatomical, histopathological, and molecular markers
Meningiomas
Meningiomas are slow-growing extra-axial tumors that arise from the meninges. Due to their long latency period of approximately 20–30 years and the fact that many diagnoses are made incidentally when neuroimaging is performed for other indications, meningiomas are likely under-diagnosed.24 Overall, 62.5% of meningioma cases are diagnosed solely by imaging, though this percentage drops to 18.2% of malignant meningioma cases (see Table 13 in Ostrom, et al1). While radiographic diagnoses are collected by all cancer registries, the completeness of radiographic diagnosis data varies from state to state and these tumors are likely underreported. Despite this, meningiomas are still the most common primary intracranial tumor, with an average of 33,686 cases per year in the US based on 2014–2018 data, which is approximately double that found for diffuse astrocytic and oligodendroglial tumors. However, meningiomas were far more clinically indolent than infiltrating glioma, with an overall 10-year survival of 83.7% for nonmalignant meningiomas (WHO grades 1 and 2; see Table 24 in Ostrom, et al1) and 60.8% for malignant meningiomas (WHO grade 3) (Table 3). Most meningiomas (80%) were WHO grade 1 tumors, 18.3% were WHO grade 2, and only 1.6% were malignant WHO grade 3 tumors. Meningiomas were approximately twice as common in females (AAAIR: 12.41, 95% CI: 12.34–12.49) as compared to males (AAAIR: 5.41, 95% CI: 5.36–5.46) (See Figure 12 in Ostrom, et al1). This male-female discrepancy became less pronounced at the extremes of the age spectrum. No statistically significant sex discrepancy was seen in malignant meningiomas. Patients who are Black (AAAIR: 10.70, 95% CI: 10.56–10.85) had a higher incidence than patients who are Whites (AAAIR: 8.90, 95% CI: 8.85–8.95) (See Figure 14 in Ostrom, et al1).
Lymphomas
Primary CNS lymphoma (PCNSL) is a rare form of extranodal lymphoma that arises in the CNS without evidence of systemic involvement at presentation. These are rare tumors, with an annual incidence of 1,696 cases in the US (AAAIR: 0.45, 95%CI: 0.44–0.46), and occur predominantly in older adults (Table 2). There was a slight male predominance (AAAIR for males: 0.50, 95% CI: 0.48–0.52; for females: 0.41, 95% CI: 0.40–0.43). Lymphomas were more commonly diagnosed in patients who are White (AAAIR: 0.46, 95%CI: 0.44–0.47) as compared to patients who are Black (AAAIR: 0.33, 95%CI: 0.30–0.35) (Table 2). They are aggressive tumors with a median survival of 15 months (Table 3). Of the cases reported to NPCR and SEER databases from 2004 to 2017, the overall 1-year relative survival was 55.3%, 5-year relative survival 38.3%, and 10-year relative survival 30.5% (Table 3). However, survival rates for PCNSL have increased markedly since the introduction of high-dose intravenous methotrexate-based chemotherapy in the 1990s, with the median survival rates for patients diagnosed in the 2010s more than double (26 months) of those diagnosed in the 1990s (13 months).25 Improved survival is strongly associated with younger age.
Nerve Sheath Tumors
Nerve sheath tumors include schwannomas and neurofibromas. Schwannomas are benign, slow-growing WHO grade 1 tumors composed exclusively of well-differentiated Schwann cells and are associated with loss of the NF-2 gene product merlin (neurofibromin). Neurofibromas are composed of neoplastic Schwann cells and nonneoplastic intermixed elements. They are also slow-growing WHO grade 1 tumors, although there is a small risk of transformation to malignant peripheral nerve sheath tumors (MPNST), particularly for large plexiform neurofibromas. They are associated with NF1. There were 7,329 newly diagnosed cases per year of nerve sheath tumors in the US, with an AAAIR of 2.05 (95% CI: 2.03–2.07) (Table 2). There was no sex predilection. These tumors were more commonly diagnosed in older adults (AAAIR 3.77, 95% CI: 3.73–3.81) and are rare in children (AAAIR: 0.25, 95% CI: 0.23–0.26). They were approximately twice as common in patients who are Whites (AAAIR: 2.16, 95% CI: 2.13–2.18) and patients who are Asian/Pacific Islanders (AAAIR: 2.09, 95% CI: 2.00–2.18) as compared to patients who are Blacks (AAAIR: 1.05, 95% CI: 1.00–1.09) and patients who are American Indians (AAAIR: 1.13, 95% CI: 0.98–1.29). Survival is excellent for these tumors, with 10-year survival rates of >99%. For rare malignant variants (MPNST), the 1-year relative survival was 85.1%, 5-year relative survival 74.7%, and 10-year relative survival 71.7% (Table 3).
Sellar Region Tumors
Tumors of the sellar region include pituitary tumors (primarily pituitary adenoma) and craniopharyngioma. Sellar region tumors had an estimated annual incidence of 15,417 (AAAIR: 4.55, 95% CI: 4.52–4.59), were slightly more common in females (AAAIR: 5.08, 95% CI: 5.03–5.13) as compared to males (AAAIR: 4.11, 95% CI: 4.07–4.16), and were significantly more common in patients who are Black (AAAIR: 7.19, 95% CI: 7.08–7.31) as compared to patients who are White (AAAIR: 4.11, 95% CI: 4.08–4.15) (see Tables 3 and 14 in Ostrom, et al1). Pituitary tumors were more common with increasing age (AAAIR: 6.54 in older adults, 95% CI: 6.48–6.60; 4.15 in AYA, 95% CI: 4.10–4.21; and 0.36 in children, 95% CI: 0.34–0.38) (Table 2). Craniopharyngiomas were considerably less common than pituitary tumors and have higher incidence in children and older adults (AAAIR: 0.22 in older adults, 95% CI: 0.21–0.23; 0.13 in AYA, 95% CI: 0.12–0.14; and 0.22 in children, 95% CI: 0.21–0.24) (Table 2). Pituitary tumors are overwhelmingly nonmalignant, with only 0.2% of such tumors reported as malignant (primarily pituitary carcinoma). Craniopharyngiomas are classified as nonmalignant, though despite their low mortality (See Table 24 in Ostrom, et al1) can cause significant morbidity. Sellar region tumors have favorable survival rates, with 10-year relative survival of >90% for pituitary tumors and approximately 80% for craniopharyngiomas.
Embryonal Tumors
Embryonal tumors are aggressive WHO grade 4 tumors that primarily affect young children, with a median age at diagnosis of 8 years. They include medulloblastoma (AAAIR in children: 0.49, 95% CI: 0.46–0.51), atypical teratoid/rhabdoid tumor (AT/RT, AAAIR in children: 0.12, 95% CI: 0.11–0.14), and the rare embryonal tumors with multilayered rosettes (ETMR). Approximately 650 embryonal tumors are diagnosed annually in the US (Table 2). Medulloblastoma was more common in males; ATRT did not demonstrate a sex predilection. Medulloblastomas comprise four distinct subgroups defined by molecular criteria that display distinct clinical behavior, response to therapy, and prognosis. Children with medulloblastoma had 1-, 5-, and 10-year relative survival rates of 89.8%, 72.6%, and 67.1%, respectively. Children with AT/RT had 1-, 5-, and 10-year relative survival rates of 52.9%, 33.0%, and 30.7%, respectively.
Germ Cell Tumors
CNS germ cell tumors are the CNS homologs of their gonadal counterparts. They are divided into germinomas and nongerminomatous variants. The nongerminomatous variants include embryonal carcinoma, yolk sac tumor, choriocarcinoma, teratoma, and mixed germ cell tumors. CNS germ cell tumors exhibit a bimodal distribution with a peak incidence in the early teens and a smaller peak in young children <3 years old. There were 250 new cases diagnosed annually on average in the US between 2014 and 2018. Males were more commonly affected than females (AAAIR 0.12 for males, 95% CI: 0.11–0.13; 0.04 for females, 95% CI: 0.04–0.05, Table 2). CNS germ cell tumors are more common in East Asia as compared to the US and Europe.26 In the US, patients who are Asian/Pacific Islander were significantly more likely to be diagnosed with CNS germ cell tumors (AAAIR 0.13, 95% CI: 0.11–0.15) as compared to patients who are White (AAAIR 0.08, 95% CI: 0.08–0.09) and patients who are Black (AAAIR 0.06, 95% CI: 0.05–0.07) (Table 2). Children with CNS germ cell tumors had 1-, 5-, and 10-year relative survival rates of 92.8%, 87.2%, and 83.4% (80.8%–85.6%), respectively (Table 3).
Conclusions and Perspective
This report summarizes the incidence, mortality, and survival for the major categories of primary brain and other CNS tumors, with an emphasis on statistics most relevant to the practicing clinician. The CBTRUS dataset used in this summary is the most complete record of primary CNS tumors diagnosed in the years 2014–2018. Despite the wealth of information contained in this dataset, several inherent limitations should be considered when using and interpreting these data:
Radiographic Diagnoses
US cancer registration permits the reporting of cases diagnosed by both histologic confirmation (where a tissue specimen was examined by a pathologist to confirm the diagnosis) and radiographic confirmation (where diagnosis was made based solely on imaging). Histologic diagnosis affords greater diagnostic certainty, enables the assignment of a WHO grade, and is generally the preferred diagnostic method. However, some tumors, such as meningiomas and pilocytic astrocytomas of the optic pathway, display characteristic imaging findings that enable their diagnoses based solely on imaging criteria. The diagnostic certainty for these radiographically-diagnosed tumors is inherently somewhat lower.
Changing Diagnostic Criteria and the Collection of Molecular Marker Data
Cases transmitted to CCR undergo no central pathology review, and histology code assignment at case registration is based on histology information contained in the patient’s medical record. The WHO Classification of Tumours of the Central Nervous System was revised in 1993,27 2000,28 2007,29 2016,11 and 2021.18 As of 2018, the US cancer registration uses the 2016 classification for data abstraction, but tumors included in this report may have been diagnosed using the prevailing criteria at the time of diagnosis and subsequent case registration. This means that despite changes to histologic classification that have occurred over time, it is not possible to reclassify tumors based on new criteria but only to assign them to revised histology groupings (Supplementary Table 3) as CBTRUS has done with the data in its report covering 2014–2018. Only the data collected in 2018 include molecular biomarker information for certain histologies, including gliomas and medulloblastomas. In addition to changes in histologic criteria, there has historically been significant inter-rater variability in histopathological diagnosis of glioma.30,31 However, the accuracy of histopathological diagnosis will improve as molecular marker testing is increasingly implemented in clinical practice.
The 2021 WHO classification further expands the role of molecular pathology in defining CNS tumors, particularly for the diffuse astrocytic and oligodendroglial tumors where IDH mutation and 1p/19q codeletion status are the defining molecular markers.18 It is important that key stakeholders, including clinicians, researchers, cancer registrars, and standard setters, work together to ensure that WHO classifications are implemented in a meaningful and timely way. Cancer registries have begun to record key molecular marker information including IDH1/2 mutation, 1p/19q codeletion, and MGMT promoter methylation status for gliomas; and medulloblastoma molecular subtypes (WNT- and SHH-activated, including TP53 mutational status.1 While such molecular information could not be included in the current summary, we anticipate that future versions will be able to include stratification by such information as the data accrue.
Mortality and Survival Data
There are several limitations specific to mortality and survival data. Mortality statistics depend on the clinician that completes an individual’s death certificate. In cases where cancer contributed to a death but is not listed as the underlying cause of death, the death may be incorrectly attributed to another cause. Survival data used for this report are collected by NPCR for 42 of the 52 CCR in the US, primarily through linkage with death certificate and other administrative records, including health care provider or social security records. These methods have been shown to produce reliable and robust estimates of cancer survival,32,33 although survival may be overestimated in cases where death information is missing, such as when a patient leaves the state or country. Survival data used in this report cover the period 2001–2017 and do not include cases with molecular marker data, which only began to be collected for specific histologies in diagnosis years 2018. For histologies where molecular markers are not critical to diagnosis, resulting survival data continue to represent the “gold standard” of population-based survival statistics for primary brain and CNS tumors. For histologies which now incorporate molecular data, particularly the diffuse astrocytic and oligodendroglial tumors, survival statistics based on histologic groupings continue to represent accurate population-based survival for individuals classified under the prevailing criteria at their time of diagnosis.
In conclusion, we summarize incidence, mortality, and survival statistics for major histologic groupings of primary CNS tumors in the US. We hope that this will serve as a resource for clinicians in the field and anticipate future reports to reflect increasingly molecular-based WHO diagnostic criteria.
Supplementary Material
Acknowledgments
The CBTRUS data were provided through an agreement with the Centers for Disease Control’s (CDC) National Program of Cancer Registries. In addition, CBTRUS used data from the research data files of the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program, and the National Center for Health Statistics National Vital Statistics System. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056 Amendment/Modification No: 0002, the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or of the National Cancer Institute.
Conflict of interest statement: The authors have no conflicts of interest to disclose.
References
- 1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021; 23(Supplement_3):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kruchko C, Ostrom QT, Gittleman H, Barnholtz-Sloan JS. The CBTRUS story: providing accurate population-based statistics on brain and other central nervous system tumors for everyone. Neuro Oncol. 2018; 20(3):295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC). National Program of Cancer Registries Cancer Surveillance System Rationale and Approach. 1999; http://www.cdc.gov/cancer/npcr/pdf/npcr_css.pdf. Accessed July 21, 2020.
- 4. Cancer Registries Amendment Act, 102nd Cong. § 515. 1992; https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg3372.pdf [Google Scholar]
- 5. Benign Brain Tumor Cancer Registries Amendment Act, 107th Cong. § 260. 2002; http://www.gpo.gov/fdsys/pkg/PLAW-107publ260/pdf/PLAW-107publ260.pdf. Accessed July 21, 2020.
- 6. Central Brain Tumor Registry of the United States SEER*Stat Database. CDC National Program of Cancer Registries and NCI Surveillance, Epidemiology and End Results Incidence Data, 2020 submission (2000–2018). 2021. [Google Scholar]
- 7. Johnson C, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards B.. The 2007 Multiple Primary and Histology Coding Rules. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 8. U.S. Cancer Statistics Working Group. NPCR Survival Analytical Database, November 2020 Submission, Diagnosis Year 2001-2017.2021.
- 9. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Mortality - All COD, Aggregated with State, Total U.S. (1969-2018) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2020 Underlying mortality data provided by NCHS.2020; (www.cdc.gov/nchs).
- 10. Surveillance Research Program - National Cancer Institute. ICD-0-3 SEER Site/Histology Validation List.2019; https://seer.cancer.gov/icd-o-3/sitetype.icdo3.20190618.pdf. Accessed July 14, 2020.
- 11. Louis DN OH, Wiestler OD, Cavanee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 12. Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Pilocytic astrocytomas: where do they belong in cancer reporting? Neuro Oncol. 2020; 22(2):298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. September 2012; https://www.naaccr.org/wp-content/uploads/2016/11/NHIA-v2.2.1.pdf. Accessed July 21, 2020.
- 14. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006; 15(6):547–569. http://www.ncbi.nlm.nih.gov/pubmed/17260923. [DOI] [PubMed] [Google Scholar]
- 15. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Populations - Total U.S. (1990-2019) - Linked To County Attributes - Total U.S., 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Released December 2020. 2020; http://seer.cancer.gov/popdata/.
- 16. R Core Team. R: A Language and Environment for Statistical Computing. 2021; http://www.R-project.org/. Accessed April 20, 2021. [Google Scholar]
- 17. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.3.9. 2020; www.seer.cancer.gov/seerstat. Accessed April 7, 2021.
- 18. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 20. Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012; 107(1):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012; 107(2):359–64. [DOI] [PubMed] [Google Scholar]
- 22. Sheikh S, Radivoyevitch T, Barnholtz-Sloan JS, Vogelbaum M. Long-term trends in glioblastoma survival: implications for historical control groups in clinical trials. Neurooncol Pract. 2020; 7(2):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO Classification of Tumours of the Central Nervous System, 5th ed. In: WHO Classification of Tumours Editorial Board, ed. Lyon, France: International Agency for Research on Cancer; 2021. [Google Scholar]
- 24. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010; 99(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018; 20(5):687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCarthy BJ, Shibui S, Kayama T, et al. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol. 2012; 14(9):1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993; 3(3):255–268. [DOI] [PubMed] [Google Scholar]
- 28. Kleihues P, Cavenee W, eds. Tumours of the Nervous System: World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2000. [Google Scholar]
- 29. Louis D, Wiestler O, Cavanee W, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 30. van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. 2010; 120(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aldape K, Simmons ML, Davis RL, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer 2000; 88(10):2342–2349. [PubMed] [Google Scholar]
- 32. Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries’ (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014; 2014(49):198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilson RJ, O’Neil ME, Ntekop E, Zhang K, Ren Y. Coding completeness and quality of relative survival-related variables in the National Program of Cancer Registries Cancer Surveillance System, 1995-2008. J Registry Manag. 2014; 41(2):65–71; quiz 96. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.