Abstract
Background
Obesity has reached epidemic proportions around the world. Effectiveness of hormonal contraceptives may be related to metabolic changes in obesity or to greater body mass or body fat. Hormonal contraceptives include oral contraceptives (OCs), injectables, implants, hormonal intrauterine contraception (IUC), the transdermal patch, and the vaginal ring. Given the prevalence of overweight and obesity, the public health impact of any effect on contraceptive efficacy could be substantial.
Objectives
To examine the effectiveness of hormonal contraceptives in preventing pregnancy among women who are overweight or obese versus women with a lower body mass index (BMI) or weight.
Search methods
Until 4 August 2016, we searched for studies in PubMed (MEDLINE), CENTRAL, POPLINE, Web of Science, ClinicalTrials.gov, and ICTRP. We examined reference lists of pertinent articles to identify other studies. For the initial review, we wrote to investigators to find additional published or unpublished studies.
Selection criteria
All study designs were eligible. The study could have examined any type of hormonal contraceptive. Reports had to contain information on the specific contraceptive methods used. The primary outcome was pregnancy. Overweight or obese women must have been identified by an analysis cutoff for weight or BMI (kg/m2).
Data collection and analysis
Two authors independently extracted the data. One entered the data into RevMan and a second verified accuracy. The main comparisons were between overweight or obese women and women of lower weight or BMI. We examined the quality of evidence using the Newcastle‐Ottawa Quality Assessment Scale. Where available, we included life‐table rates. We also used unadjusted pregnancy rates, relative risk (RR), or rate ratio when those were the only results provided. For dichotomous variables, we computed an odds ratio with 95% confidence interval (CI).
Main results
With 8 studies added in this update, 17 met our inclusion criteria and had a total of 63,813 women. We focus here on 12 studies that provided high, moderate, or low quality evidence. Most did not show a higher pregnancy risk among overweight or obese women. Of five COC studies, two found BMI to be associated with pregnancy but in different directions. With an OC containing norethindrone acetate and ethinyl estradiol (EE), pregnancy risk was higher for overweight women, i.e. with BMI ≥ 25 versus those with BMI < 25 (reported relative risk 2.49, 95% CI 1.01 to 6.13). In contrast, a trial using an OC with levonorgestrel and EE reported a Pearl Index of 0 for obese women (BMI ≥ 30) versus 5.59 for nonobese women (BMI < 30). The same trial tested a transdermal patch containing levonorgestrel and EE. Within the patch group, obese women in the "treatment‐compliant" subgroup had a higher reported Pearl Index than nonobese women (4.63 versus 2.15). Of five implant studies, two that examined the six‐capsule levonorgestrel implant showed differences in pregnancy by weight. One study showed higher weight was associated with higher pregnancy rate in years 6 and 7 combined (reported P < 0.05). In the other, pregnancy rates differed in year 5 among the lower weight groups only (reported P < 0.01) and did not involve women weighing 70 kg or more.
Analysis of data from other contraceptive methods indicated no association of pregnancy with overweight or obesity. These included depot medroxyprogesterone acetate (subcutaneous), levonorgestrel IUC, the two‐rod levonorgestrel implant, and the etonogestrel implant.
Authors' conclusions
The evidence generally did not indicate an association between higher BMI or weight and effectiveness of hormonal contraceptives. However, we found few studies for most contraceptive methods. Studies using BMI, rather than weight alone, can provide information about whether body composition is related to contraceptive effectiveness. The contraceptive methods examined here are among the most effective when used according to the recommended regimen.
We considered the overall quality of evidence to be low for the objectives of this review. More recent reports provided evidence of varying quality, while the quality was generally low for older studies. For many trials the quality would be higher for their original purpose rather than the non‐randomized comparisons here. Investigators should consider adjusting for potential confounding related to BMI or contraceptive effectiveness. Newer studies included a greater proportion of overweight or obese women, which helps in examining effectiveness and side effects of hormonal contraceptives within those groups.
Keywords: Female; Humans; Pregnancy; Body Mass Index; Obesity; Pregnancy Rate; Body Weight; Contraception; Contraception/methods; Contraceptive Agents, Female; Contraceptive Agents, Female/administration & dosage; Overweight; Pregnancy, Unplanned; Prospective Studies; Randomized Controlled Trials as Topic
Plain language summary
Hormones for birth control in overweight or obese women
Excess body weight has become a health problem around the world. Being overweight or obese may affect how well some birth control methods work to prevent pregnancy. Hormonal birth control includes pills, the skin patch, the vaginal ring, implants, injectables, and hormonal intrauterine contraception (IUC).
Until 4 August 2016, we did computer searches for studies of hormonal birth control among women who were overweight or obese. We looked for studies that compared overweight or obese women with women of normal weight or body mass index (BMI). The formula for BMI is weight (kg) / height (m)2. We included all study designs. For the original review, we wrote to investigators to find other studies we might have missed.
With 8 studies added in this update, we had 17 with a total of 63,813 women. We focus here on 12 studies with high, moderate, or low quality results. Most did not show more pregnancies for overweight or obese women. Two of five studies using birth control pills found differences between BMI groups. In one, overweight women had a higher pregnancy risk. The other found a lower pregnancy rate for obese women versus nonobese women. The second study also tested a new skin patch. Obese women in the patch group had a higher pregnancy rate. Of five implant studies, two showed differences among weight groups. They studied the older six‐capsule implant. One study showed a higher pregnancy rate in years 6 and 7 combined for women weighing 70 kg or more. The other reported pregnancy differences in year 5 among the lower weight groups only. Results for other methods of birth control did not show overweight or obesity related to pregnancy rate. Those methods included an injectable, hormonal IUC, and the two‐rod and single‐rod implants.
These studies generally did not show an association of BMI or weight with the effect of hormonal methods. We found few studies for most methods. Studies using BMI rather than weight can show whether body fat is related to how well birth control prevents pregnancy. The methods studied here work very well when used according to directions. The overall study quality was low for this review, especially in the older reports. However, many studies would have higher quality for their original purpose than for the comparisons here.
Summary of findings
for the main comparison.
| Combination oral contraceptives or transdermal patch for contraception in overweight or obese women | ||||
|
Patient or population: women with need for contraception Settings: clinical trials sites Intervention: overweight or obese women Comparison: women not overweight | ||||
| Outcome | Reported relative effect | Participants (study) | Evidence quality (GRADE) | Comparison groups; intervention |
| Pregnancy | RR 2.49 (95% CI 1.01 to 6.13) | 1139 (Burkman 2009) | Moderate | BMI ≥ 25 vs < 25; COC NETA 1 mg + EE 20 µg |
| Pregnancy | Pearl Index 0 vs 5.59 | 375 (Kaunitz 2014) |
Low | BMI ≥ 30 vs < 30; COC LNG 100 µg + EE 10 µg |
| Pregnancy | Pearl Index 4.63 vs 2.15 ("treatment‐compliant") | BMI ≥ 30 vs < 30; experimental patch LNG 120 µg + EE 30 µg daily | ||
| BMI: body mass index; CI: confidence interval; EE: ethinyl estradiol; LNG: levonorgestrel; NETA: norethindrone acetate; RR: relative risk | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
2.
| Levonorgestrel implant, 6 capsules, for contraception in overweight or obese women | ||||
|
Patient or population: women with need for contraception Settings: clinical trials sites Intervention: overweight or obese women Comparison: women not overweight | ||||
| Outcomes | Reported relative effect | Participants (study) | Quality of evidence (GRADE) | Comparison groups |
| Pregnancy | Life‐table rates, year 5a: 40 to 49 kg group < 50 to 59 kg group < 60 to 60 kg group (P < 0.01) | 16,282 (Grubb 1995) |
Low | Weight (kg): 40 to 49; 50 to 59; 60 to 69; ≥ 70 |
| Pregnancy | Pearl rates, years 6 + 7 combined: 0.21; 0.29; 0.82; 0.86 respectively (P < 0.05) | 10,781 (Gu 1995) |
Low | Weight (kg): < 50; 50 to 59; 60 to 69; ≥ 70 |
| CI: confidence interval; LNG: levonorgestrel | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aReport did not provide specific numbers
Background
Description of the condition
Obesity has reached epidemic proportions around the world, and the prevalence of overweight is not limited to any particular income level. Worldwide, the prevalence of overweight and obesity among adults increased 25% from 1980 to 2013 (Ng 2014). In the US, more than a third of adults were considered obese in 2011 to 2012, and more than two‐thirds were either overweight or obese (Ogden 2014). In many European countries, the prevalence of obesity has tripled since the 1980s (WHO 2012). Obesity rates are 20% to 30% among women in Europe, Latin America, and Central Asia, while the prevalence is 34% in North Africa and Middle East and 37% in southern sub‐Saharan Africa (Ng 2014).
Overweight and obesity are generally determined with the body mass index (BMI), which is based on weight and height (BMI = weight [kg] / height [m]2) (CDC 2016). BMI does not distinguish between lean and fat body mass, but for most people (other than highly trained athletes) a higher BMI reflects more body fat (CDC 2016). Commonly used BMI categories are 25 to 29.9 (kg/m2) for overweight and 30 or higher for obesity. Older cutoffs were derived from the National Health and Nutrition Examination Survey II (NHANES II). With those criteria, women with a BMI greater than 27.3 are overweight and those with a BMI greater than 32 are considered obese.
Overweight and obese women may have a higher risk for failure of hormonal contraceptives (Grimes 2005; Callegari 2014). Higher BMI or body weight may be associated with unintended pregnancy while using oral contraceptives (Holt 2005; Dinger 2011). Some research has indicated little association after adjusting for demographics or socioeconomic factors (Brunner 2005; Brunner Huber 2007). From a study of more than 34,000 women who gave birth in a large maternity hospital, nearly a third stated their pregnancy was unplanned and half of those women were obese (McKeating 2015). Contraceptive failure with a hormonal method was more likely for overweight and obese women. Overweight women were also more likely than normal weight women to have conceived with a non‐hormonal method. The risk of oral contraceptive failure among overweight or obese women may depend on whether the assessment is based on 'perfect use' or 'typical use' (Trussell 2009).
Description of the intervention
Hormonal contraceptives include oral contraceptives (OCs), injectables, implants, hormonal intrauterine contraception, the transdermal patch, and the vaginal ring. Worldwide, intrauterine contraception (IUC) is the most commonly used reversible method among women married or in union (UN 2015). While much IUC use is non‐hormonal globally, most IUC use in the US is now hormonal (Kavanaugh 2015). Worldwide, after IUC, the method used most frequently is oral contraceptives, particularly in Europe and the Americas. In the "least developed countries" injectables are most commonly used, followed by oral contraceptives (UN 2015).
How the intervention might work
Effectiveness of hormonal contraceptives may be related to metabolic changes in obesity or greater body mass or body fat (Grimes 2005; Trussell 2009). Since contraceptive studies historically excluded overweight women (Lopez 2014; Yamazaki 2015), little was known about how overweight women metabolized hormonal contraceptives when we conducted the initial review. Pharmacokinetic studies have shown that obesity affects drug levels across a range of contraceptive methods, but findings vary regarding the impact on the pharmacodynamics (end‐organ activity). Studies have shown differences between obese versus normal‐weight women using a COC (Edelman 2009) or a vaginal ring (Westhoff 2012b), but ovarian suppression appeared to be similar for obese and nonobese women (Westhoff 2009; Westhoff 2012b). A current area of investigation involves methods of compensating for pharmacokinetic changes with OCs related to obesity (Edelman 2014). Small studies have shown pharmacokinetic differences, which may not be clinically significant, between obese and normal‐weight users of the injectable depot medroxyprogesterone acetate (DMPA) (Segall‐Gutierrez 2010) or the etonogestrel implant (Mornar 2012). A small cross‐sectional study of the etonogestrel implant found that serum etonogestrel levels were comparable across BMI groups (not overweight, overweight but not obese, obese) (Morrell 2016). A trial of a gestodene transdermal patch included women with BMI ≤ 30, > 30 and ≤ 35, and > 35 in similar proportions (Westhoff 2014). The patch provided effective inhibition of ovulation, which was not related to BMI. Another trial compared new IUC (LNG20) with the currently marketed levonorgestrel IUC. A conference report of a subsample analysis showed plasma levonorgestrel levels were lower in obese women versus nonobese women within each contraceptive group (Creinin 2015).
Why it is important to do this review
When we conducted the initial review, we wanted to identify what was known about the relationship between excess body mass or weight and the effectiveness of hormonal contraceptives. Given the prevalence of overweight and obesity, the public health impact of any effect on contraceptive efficacy could be substantial. The results may inform investigators in the field and help healthcare providers assist women in making contraceptive choices.
Objectives
To examine the effectiveness of hormonal contraceptives in preventing pregnancy among women who are overweight or obese versus women with a lower BMI or weight.
Methods
Criteria for considering studies for this review
Types of studies
We included studies of hormonal contraceptive effectiveness among overweight or obese women. Reports had to identify the specific contraceptive methods used. Because we did not anticipate finding randomized controlled trials stratified by body weight, we included all study designs. The main comparisons were overweight or obese women versus women of lower BMI or weight. Therefore, the comparisons of interest were possible in single‐arm studies, i.e. those with only one intervention. All languages of publication were eligible for inclusion.
We eliminated studies focused on women with specific health problems, such as HIV or diabetes. We also excluded studies of contraceptives as treatment for specific disorders, e.g. acne, hirsutism, or polycystic ovary syndrome.
Types of participants
Participants were the women in the studies who used the hormonal contraceptive for contraception. Overweight or obese women must have been identified by an analysis cutoff for BMI or weight. The comparison group could have been women in a lower BMI or weight group. As noted earlier, several criteria for defining overweight or obese are still used, including a BMI (kg/m2) ≥ 25 for overweight and ≥ 30 for obesity (CDC 2016) as well as the NHANES II cutoffs (> 27.3 for overweight and > 32 for obesity) (Burkman 2009). Some investigators analyzed the outcome by body weight quartiles, deciles or groups, e.g. < 70 kg or ≥ 70 kg. We included studies with differing criteria, as practices differ across time periods and by country and we initially anticipated finding few studies. The report had to provide the BMI or weight cutoff points.
Types of interventions
The study must have examined the use of one or more hormonal contraceptives. The focus could have been any hormonal contraceptive, i.e. an oral contraceptive, a transdermal skin patch, a vaginal ring, an injectable contraceptive, a subdermal implant, or hormonal intrauterine contraception. Treatment duration must have been at least three cycles or three months.
Types of outcome measures
Primary outcomes
The main outcome was pregnancy. We examined BMI or weight as a potential predictor of contraceptive effectiveness. For consideration, studies must have assessed pregnancies.
We did not include ovulation, since it is not a useful surrogate endpoint for pregnancy. A valid surrogate marker captures the effect of the treatment on the true outcome (Grimes 2010).
Secondary outcomes
Other outcomes include side effects, e.g. unscheduled bleeding and rare events such as venous thromboembolism. We also looked for adherence data, by BMI or weight groups, to see if they differed in contraceptive use patterns.
Weight gain was not an outcome of interest for this review. Other reviews have focused on the effect of combination contraceptives (Gallo 2014) or progestin‐only methods (Lopez 2013) on weight gain.
Search methods for identification of studies
Electronic searches
Until 4 August, we searched PubMed (MEDLINE), the Cochrane Central Register of Controlled Trials (CENTRAL), POPLINE, and Web of Science. We also searched for current trials through ClinicalTrials.gov and ICTRP. Appendix 1 shows the recent search strategies and Appendix 2 contains the earlier strategies.
Searching other resources
We examined reference lists of relevant articles to identify additional studies. For the initial review, we contacted investigators in the field to seek additional unpublished trials or published studies.
Data collection and analysis
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature searches with no language limitations. One author reviewed the search results and identified reports for inclusion or exclusion. A second author examined the reports for appropriate categorization.
Studies could have been randomized controlled trials (RCTs) or non‐randomized studies (NRS), e.g. prospective single‐arm or multi‐arm studies, case‐control studies, or observational studies of contraceptive users. We considered post hoc analysis from these types of studies as long as the analysis met the eligibility criteria.
Data extraction and management
One author extracted the data and entered the information into RevMan. Another author conducted a second data extraction and verified correct data entry. We resolved any discrepancies by discussion or with a third author if necessary.
Assessment of risk of bias in included studies
We examined RCTs for methodological quality, according to recommended principles (Higgins 2011). The randomization was unrelated to the BMI or weight comparisons, but provides an indicator of study quality. We considered randomization method, allocation concealment, blinding, and losses to follow‐up and early discontinuation.
For NRS, we used the Newcastle‐Ottawa Quality Assessment Scale (NOS) (Higgins 2011; Wells 2014). Of the two NOS versions for case‐control and cohort studies, the latter was more pertinent here (Appendix 3). The NOS investigators are examining the criterion validity and construct validity of this scale as well as the inter‐rater and intra‐rater reliability. The scale does not yet have an overall scoring or threshold for 'good' or 'poor' quality. The NOS has eight items within three domains: selection (representativeness), comparability (due to design or analysis), and outcomes (assessment and follow‐up). A study can receive one star (✸) for meeting each criterion. The exception is comparability, which can have two stars (for design and analysis). We adapted NOS items for this project as suggested by the developers (Wells 2014).
We recorded whether pregnancies and body weight were measured or self‐reported. Pregnancies may be under‐reported when relying on self reports from interviews or questionnaires rather than testing. Such under‐reporting is unlikely to differ by BMI or weight group. However, body weight is frequently underestimated by a few pounds (Holt 2005). The result would be categorizing more women in a lower weight group, which would bias the effect estimate toward no difference.
Measures of treatment effect
The main comparisons for this review were between overweight or obese women and women of lower BMI or weight. The comparisons were possible in studies having only one intervention. For example, for the primary outcome of pregnancy, we compared pregnancies among 'overweight' women with those of 'normal' or 'healthy' weight women who used the specific contraceptive. Definitions of overweight and normal, or the cutoffs for BMI or weight, depended on the analytic methods used for the study reports. For two‐arm studies, we compared the BMI or weight groups within each contraceptive method group.
Oral contraceptive studies tend to have relatively high discontinuation rates. Time‐to‐event measures such as life‐table or incidence rates are commonly used, as they are based on actual exposure to the contraceptive and prevent an imbalance in discontinuations from distorting the comparisons. We extracted life‐table rates (actuarial or continuous) where available. We also used unadjusted pregnancy rates, relative risk (RR), or rate ratio when those were the only results reported by the investigator.
With non‐randomized studies, investigators need to control for confounding factors. When available, we used adjusted measures that the investigators reported. The effect measure may have been hazard ratio, rate ratio, or relative risk. Where only the crude number of events was available for dichotomous outcomes, we computed the Mantel‐Haenszel odds ratio (OR) with 95% confidence interval (CI). An example is the proportion of women that reported bleeding or spotting problems.
Dealing with missing data
We wrote to the study investigators for missing data and clarification of issues related to participants and methods. Responses and any data provided are in Characteristics of included studies. We limited our requests to studies less than 10 years old. Investigators are unlikely to have access to information from older studies.
Assessment of heterogeneity
We expected study populations, designs, and interventions to be heterogeneous. We described the clinical and methodological diversity (or heterogeneity) of the studies. We did not pool data from studies that had different contraceptive methods (e.g. COC and transdermal patch), different doses of the same method, or different criteria for analyzing BMI or body weight. Therefore, we did not conduct meta‐analysis. Statistical heterogeneity is not an issue when a comparison has a single study.
Data synthesis
To assess the quality of evidence and address confidence in the effect estimates, we applied principles from GRADE (Grades of Recommendation, Assessment, Development and Evaluation) (Higgins 2011; GRADE 2013). If meta‐analysis is not viable because of varied interventions or outcome measures, a typical 'Summary of findings' table is not feasible. Also, the criteria for quality assessment differ for non‐randomized versus randomized comparisons. We do provide 'Summary of findings' tables for the main results, although we did not conduct a formal GRADE assessment for all outcomes (GRADE 2013).
We based our assessment of the body of evidence on the quality of evidence from the studies. In 2016, we revised the Risk of bias tables to accommodate the criteria for both RCTs and NRS. We used the Newcastle‐Ottawa Quality Assessment Scale for all studies (Appendix 3). The comparisons were non‐randomized, since participants could not be randomized by BMI or weight, though the sample could have been stratified. Evidence quality included the design, implementation, and reporting of the study. We examined the evidence for the primary outcome of pregnancy. We list the criteria for downgrading below.
BMI not analyzed
NRS: high risk of bias in selection (NOS)
NRS: not controlling for relevant confounding in design or analysis, e.g. age, education, prior contraceptive use
Follow‐up less than 12 months for pregnancy outcome
Loss to follow‐up > 20%, combined loss to follow‐up and discontinuation > 50%, or retrospective chart review of selected cases
Sensitivity analysis
In the initial review in 2010, we synthesized results from the studies that had confirmed pregnancies and measured body weight (not self‐reported). In 2016, we conducted the quality assessment using the NOS in lieu of the earlier sensitivity analysis.
Results
Description of studies
Results of the search
The 2012 searches produced 421 references: 395 from the database searches, 8 from other sources such as reference lists, and 18 trials from searches of clinical trials sites. We included two new studies (with four reports) (Westhoff 2012a; Xu 2012). We excluded six (with seven reports) after examining the full text (Excluded studies). A conference presentation was awaiting classification due to limited data; we included the full report in 2016 (Kaunitz 2014). After reviewing the abstracts, we retained 22 references for background information and discarded 366 for not meeting the eligibility criteria.
In 2016, the database searches yielded 681 references. After removing 280 duplicates, we had 401 unduplicated references (Figure 1). Through other searching, we identified four articles with relevant outcome and design information from three older studies. With those 4 articles plus 4 reports from other sources, the new total was 409 unduplicated references. After discarding 393 based on title or abstract, we examined the full text of 16 articles and then excluded two studies. The remaining 14 articles represented 8 new studies. Searches of ClinicalTrials.gov and ICTRP produced 26 unduplicated listings of clinical trials. No ongoing trial appeared to meet our eligibility criteria.
1.

Study flow diagram, 2016
Included studies
With the 8 studies added in this update, 17 studies met our inclusion criteria and had a total of 63,813 women. The median sample size was 1736. The hormonal contraceptives that they studied varied.
Six studies examined COCs.
-
norgestimate 180/215/250 µg + ethinyl estradiol (EE) 25 µg
norethindrone acetate (NETA) 1 mg + EE 20 µg
-
nomegestrol acetate 2.5 mg + 17β‐estradiol (E2) 1.5 mg
drospirenone 3 mg + EE 30 μg
Westhoff 2012a: levonorgestrel 100 µg + EE 20 µg (84 days)/ EE 10 µg (7 days)
Kaunitz 2014: levonorgestrel 100 µg + EE 20 µg
Nakajima 2016: norethindrone acetate 1 mg + EE 10 µg
-
Yamazaki 2015 (data from seven trials)
3 norgestimate (NGM) formulations combined in analysis (NGM 180/215/250 µg + EE 25 µg; NGM 180/250 µg + EE 25 µg; NGM 60/180 µg + EE 20 µg)
norethindrone acetate 1 mg + EE 10 µg/ EE 10 µg
norethindrone 800 µg + EE 25 µg
levonorgestrel 90 µg + EE 20 µg
levonorgestrel 100 µg + EE 20/10 µg
levonorgestrel 150 µg + EE 20/25/30/ 10 µg
desogestrel 150 µg + EE 20/10 µg
Two studies examined different transdermal patches. One analyzed data from three trials (Zieman 2002); the other had a COC comparison group listed above (Kaunitz 2014).
Zieman 2002: norelgestromin 150 µg + EE 20 µg daily release (data from three studies)
Kaunitz 2014; levonorgestrel 120 µg + EE 30 µg daily release (experimental; not yet marketed)
Of seven implant studies, six examined levonorgestrel implants; one of which included two different types (Sivin 1998b). The seventh study examined the etonogestrel implant.
levonorgestrel 6 capsules, 216 mg (Grubb 1995; Gu 1995; Sivin 1998b; Sivin 1998c)
levonorgestrel 2 rods, 150 mg (Sivin 1997; Sivin 1998a; Sivin 1998b)
etonogestrel 1 rod, 68 mg (Xu 2012)
Three studies examined other methods.
Jain 2004: depot medroxyprogesterone acetate, subcutaneous formulation (DMPA‐SC) containing 104/0.65 mL
Gemzell‐Danielson 2015: levonorgestrel intrauterine contraception (LNG‐IUC), with one releasing 8 µg/day (LNG‐IUS 8) and one releasing 13 µg/day (LNG‐IUS 13)
WHO 1990: experimental vaginal ring (not marketed); levonorgestrel (LNG) 20 µg daily release, intended for 90‐day use
The treatment duration varied. Four studies lasted one year or 13 cycles (WHO 1990; Jain 2004; Westhoff 2012a; Nakajima 2016) and four had durations of 6 and 13 cycles within the same study or analysis (Zieman 2002; Burkman 2009; Kaunitz 2014; Yamazaki 2015). The IUC study lasted three years (Gemzell‐Danielson 2015). Implant studies had durations of three years (Xu 2012), five years (Grubb 1995; Sivin 1998a; Sivin 1998b; Sivin 1998c), and seven years (Gu 1995; Sivin 1997).
Seven reports used data from RCTs (Sivin 1997; Sivin 1998b; Zieman 2002; Burkman 2009; Kaunitz 2014; Gemzell‐Danielson 2015; Yamazaki 2015). The main comparisons in those studies were between contraceptive methods, and the randomization was not stratified by weight. Zieman 2002 pooled data from two RCTs and one uncontrolled study. Yamazaki 2015 was a meta‐analysis of individual participant data from seven trials. The remaining reports were from prospective non‐comparative trials.
Nine studies used BMI cutoffs for overweight or obesity (Jain 2004; Burkman 2009;Westhoff 2012a; Mansour 2012; Xu 2012; Kaunitz 2014; Gemzell‐Danielson 2015; Nakajima 2016; Yamazaki 2015). Burkman 2009 and Westhoff 2012a also used a body weight dichotomy at 70 kg and deciles for BMI and weight. The results for cycle control under Burkman 2009 came from an earlier report in which the investigators used quartiles of body weight (lb). Zieman 2002 presented results by body weight deciles and a dichotomy at 90 kg. Four older studies used body weight groups of 10 kg each (WHO 1990; Grubb 1995; Gu 1995; Sivin 1997). Three older studies reported body weights of women who became pregnant (Sivin 1998a; Sivin 1998b; Sivin 1998c).
Excluded studies
We excluded 14 studies. Common reasons were no pregnancy outcome data or insufficient analysis by BMI or weight group. Studies of emergency contraception did not have sufficient treatment duration. Details are in Characteristics of excluded studies.
Risk of bias in included studies
The comparisons of interest involved weight or BMI groups. We used the Newcastle‐Ottawa Scale to assess the quality of evidence, as noted earlier; Table 3 summarizes the results. Figure 2 illustrates the risk of bias for the review overall, and Figure 3 shows the risk for each study.
2. Evidence quality.
| Study | NOS selection criterion | NOS comparability | BMI analyzed | Follow‐up < 12 months | Loss or chart review | Evidence qualitya |
| Burkman 2009 | _ | ‐1 | _ | _ | _ | Moderate |
| Gemzell‐Danielson 2015 | _ | ‐1 | _ | _ | _ | Moderate |
| Grubb 1995 | _ | ‐1 | ‐1 | _ | _ | Low |
| Gu 1995 | _ | ‐1 | ‐1 | _ | _ | Low |
| Jain 2004 | _ | _ | _ | _ | _ | Highb |
| Kaunitz 2014 | _ | ‐1 | _ | ‐1 | _ | Low |
| Mansour 2012 | _ | ‐1 | _ | _ | _ | Moderate |
| Nakajima 2016 | ‐1 | ‐1 | _ | _ | _ | Low |
| Sivin 1997 | _ | _ | ‐1 | _ | _ | Moderateb |
| Sivin 1998a | _ | ‐1 | ‐1 | _ | _ | Low |
| Sivin 1998b | _ | ‐1 | ‐1 | _ | ‐1 | Very low |
| Sivin 1998c | _ | ‐1 | ‐1 | _ | ‐1 | Very low |
| Westhoff 2012a | _ | ‐1 | _ | _ | _ | Moderate |
| WHO 1990 | ‐1 | ‐1 | ‐1 | _ | _ | Very low |
| Xu 2012 | _ | ‐1 | _ | _ | ‐1 | Low |
| Yamazaki 2015 | ‐1 | _ | _ | ‐1 | ‐1 | Very low |
| Zieman 2002 | ‐1 | ‐1 | ‐1 | _ | _ | Very low |
aDowngraded for the following: (1) BMI not analyzed; (2) high risk of bias in selection (NOS); (3) not controlling for relevant confounding; (4) follow‐up < 12 months for pregnancy; (5) loss to follow‐up > 20%, combined loss to follow‐up and discontinuation > 50%, or retrospective chart review of selected cases. bNo pregnancy; not downgraded for lack of adjustment for potential confounding BMI = body mass index NOS = Newcastle‐Ottawa Quality Assessment Scale
2.
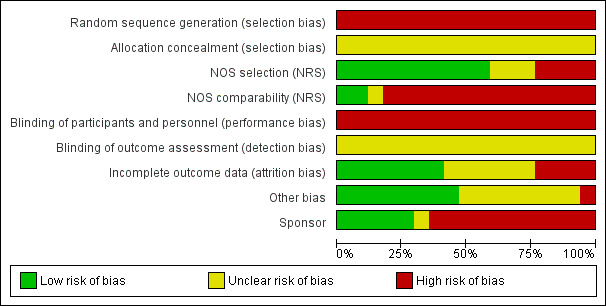
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For the RCTs, we extracted information on how the randomization sequence was generated and the allocation concealment. The randomization method indicates overall study quality, but is unrelated to our comparisons of interest.
NOS selection
In some studies, participants had volunteered for trials of specific contraceptive methods, and therefore may not represent typical contraceptive users. For all studies, the overweight and obese groups came from the same source as the obese women. Both groups were exposed to the intervention. We edited the NOS terminology for the comparison groups. Instead of 'exposed' and 'non‐exposed, we used 'overweight or obese' and 'not overweight or obese'. The specific definitions vary by study (Characteristics of included studies).
For studies of OCs and the transdermal patch, exposure to the contraceptive came from self reports. A few studies also used participant diaries or collected unused pill packs. Studies of long‐acting methods, such as implants or IUC, had objective measures of exposure. The DMPA trials had study records of injections.
NOS comparability
Most studies did not adjust for potential confounding factors. Two did not identify any pregnancies and therefore adjustment for potential confounding was less relevant for evidence quality (Sivin 1997; Jain 2004). We did not downgrade those studies for NOS comparability. In the post hoc analysis of three trials, Zieman 2002 used proportional hazards regression that included age, race, and baseline body weight and BMI. In analyzing data from seven trials, Yamazaki 2015 used age and race as dichotomous variables: age < versus ≥ median; race Black or African American versus non‐Black.
Blinding
Blinding was not applicable for single‐arm studies (WHO 1990; Grubb 1995; Gu 1995; Sivin 1998a; Sivin 1998c; Jain 2004; Westhoff 2012a; Nakajima 2016). Nor was it feasible in some studies due to visible differences in the contraceptive methods (Sivin 1998b; Xu 2012; Kaunitz 2014). In Gemzell‐Danielson 2015, blinding investigators was not feasible due to differences in the devices but participants were blinded to device type.
Of the remaining studies, Sivin 1997 was blinded. Burkman 2009 had blinding of the NGM arm; the trial originally had three NGM arms. The trials included in Zieman 2002 were open label, as was Mansour 2012. Yamazaki 2015 did not mention blinding for the included trials.
Incomplete outcome data
Three reports had some evidence of incomplete outcome data. The investigators in WHO 1990 dropped women from the study if they had three expulsions in one week or more than five within four weeks. Burkman 2009 excluded cycles with incorrect pill intake as well as cycles lacking data on dosing and bleeding. Mansour 2012 excluded those who did not take any study drug.
Three studies had loss to follow‐up greater than 20% or combined loss to follow‐up and discontinuation greater than 50%. High losses to follow‐up threaten validity (Strauss 2005).
Sivin 1998b: discontinuation by 5 years, 55% 2‐rod and 60% Norplant; loss to follow‐up, 14% 2‐rod and 18% Norplant
Sivin 1998c: loss to follow‐up 42% by 5 years
Xu 2012: loss to follow‐up 23% by 3 years
Yamazaki 2015 did not have information on losses in the seven trials included in those analyses.
Other potential sources of bias
Outcome analysis
Six reports specified that pregnancy was tested and weight was measured (Jain 2004; Burkman 2009; Westhoff 2012a; Kaunitz 2014; Gemzell‐Danielson 2015; Nakajima 2016). Two studies conducted gynecologic exams at each follow‐up and measured or recorded weight during the clinic visits (WHO 1990; Sivin 1998c). Six reports mentioned pregnancy assessment but not weight measurement. Zieman 2002 and Yamazaki 2015 used serum or urine tests for pregnancy, Grubb 1995 mentioned objective assessment of pregnancy, Sivin 1998a and Sivin 1998b provided pelvic or gynecologic exams at each follow‐up, and Xu 2012 used pregnancy tests to confirm "suspect" pregnancies. Three reports did not have any information on the assessment methods for pregnancy or weight (Gu 1995; Sivin 1997; Mansour 2012).
Corporate sponsorship
Eleven studies had high risk of bias due to funding from pharmaceutical corporations; some had investigators who were employees of those companies. One was unclear; the lead investigator was an employee of a pharmaceutical research institute. Five did not appear to have corporate sponsorship or investigators and therefore had low risk of bias.
Effects of interventions
For most included studies, the nonobese group were those with a BMI less than 30 and the obese group included those with a BMI of 30 or greater. If a study compared other BMI or weight groups, we provide the specific cutoffs below.
Combined oral contraceptives
Five trials examined one or two COC formulations each (Burkman 2009; Mansour 2012; Westhoff 2012a; Kaunitz 2014; Nakajima 2016). We grouped the results by type of progestin. A sixth report was a meta‐analysis of individual participant data from seven trials (Yamazaki 2015). We present the results separately. Although some of the COC formulations overlap with some in the review, others are not used in practice. Also, the analysis combined three norgestimate formulations from one trial.
Burkman 2009 studied norgestimate (NGM) 180/215/250 µg + EE 25 µg (N = 1671) as well as a COC with norethindrone acetate (below). For NGM, pregnancy risk did not differ by body weight or BMI groups (Analysis 1.1). The report stated that women with a BMI greater than 32.4 were excluded from participation. However, that may have been an error, as the report also stated the highest BMI was 47.6. An earlier publication reported on cycle control (Hampton 2008). Of eight comparisons for breakthrough bleeding or spotting, three showed a significant difference. Women above the 75th percentile for weight (more than 155 lb) were less likely to report breakthrough bleeding or spotting at cycle 13 than those below the 25th percentile (123 lb or less) (Analysis 1.4) or those in the 25th to 75th percentiles (124 to 155 lb) (Analysis 1.5). Burkman 2009 had adherence data from daily diaries, but the investigators reported the results by COC not BMI group.
1.1. Analysis.
Comparison 1 COC: norgestimate (NGM) 180/215/250 µg + EE 25, Outcome 1 Relative risk of pregnancy by body weight or BMI.
| Relative risk of pregnancy by body weight or BMI | ||
|---|---|---|
| Study | Comparison group | Reported relative risk (95% CI) |
| Burkman 2009 | Weight (kg) ≥ 70 (N = 711) versus < 70 (N = 2099) | 1.41 (0.56 to 3.54) |
| Burkman 2009 | BMI (kg/m2) ≥ 25 (N = 876) versus < 25 (N = 1934) | 1.39 (0.57 to 3.40) |
| Burkman 2009 | BMI (kg/m2) > 27.3 versus ≤ 27.3 | 1.41 (0.51 to 3.89) |
1.4. Analysis.
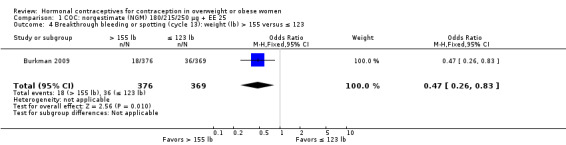
Comparison 1 COC: norgestimate (NGM) 180/215/250 µg + EE 25, Outcome 4 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus ≤ 123.
1.5. Analysis.
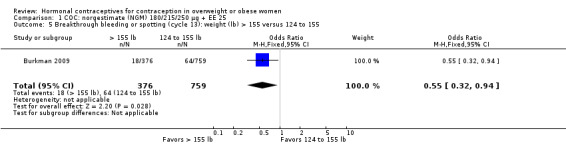
Comparison 1 COC: norgestimate (NGM) 180/215/250 µg + EE 25, Outcome 5 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus 124 to 155.
Estrogen dose differed for two trials of a COC containing norethindrone acetate (NETA). With NETA 1 mg + EE 20 µg (Burkman 2009) (N = 1139), women with a BMI of 25 or more had a higher pregnancy risk compared with those who had a BMI less than 25 (reported relative risk [RR] 2.49, 95% CI 1.01 to 6.13) (Analysis 2.1). Pregnancy risk did not differ significantly between groups when using a BMI cutoff of 27.3. Women above the 75th percentile (more than 155 lb) were less likely to report breakthrough bleeding or spotting at cycle 6 than the women below the 25th percentile (123 lb or less) (Analysis 2.2). The other trial used NETA 1 mg + EE 10 µg (Nakajima 2016) (N = 1581). According to the Pearl Index, obese women (BMI > 30) did not have decreased efficacy compared with women in the lower BMI groups (Analysis 3.1). For those aged 18 to 35 years old, the Pearl Index was 2.50 versus 3.04 for the overweight group (BMI 25 to 30) and 3.06 for those with a lower BMI (< 25). The pattern was similar in the analysis of those aged 18 to 45 years. The adherence data show that obese women took an average of 25.8 pills per cycle compared with 26.2 pills each of the groups with a lower BMI (Analysis 3.2). Bleeding data came from participant diaries. For unscheduled bleeding or spotting in cycle 13, the mean number of days was 2.2 for obese women, 1.9 for the overweight group, and 1.6 for those with a lower BMI (Analysis 3.3). Some of the more common treatment‐related adverse events were more common among obese women, i.e. headache, upper respiratory tract infection, nasopharyngitis, and nausea (Analysis 3.4).
2.1. Analysis.
Comparison 2 COC: norethindrone acetate 1 mg + EE 20 µg, Outcome 1 Relative risk of pregnancy by body weight or BMI.
| Relative risk of pregnancy by body weight or BMI | ||
|---|---|---|
| Study | Comparison group | Reported relative risk (95% CI) |
| Burkman 2009 | Weight (kg) ≥ 70 versus < 70 | 1.12 (0.40 to 3.12) |
| Burkman 2009 | BMI (kg/m2) ≥ 25 versus < 25 | 2.49 (1.01 to 6.13) |
| Burkman 2009 | BMI (kg/m2) > 27.3 versus ≤ 27.3 | 0.97 (0.28 to 3.33) |
2.2. Analysis.
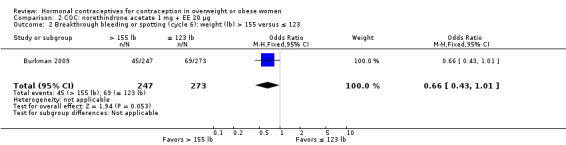
Comparison 2 COC: norethindrone acetate 1 mg + EE 20 µg, Outcome 2 Breakthrough bleeding or spotting (cycle 6): weight (lb) > 155 versus ≤ 123.
3.1. Analysis.
Comparison 3 COC: norethindrone acetate 1 mg + EE 10 µg, Outcome 1 Pearl Index by BMI group.
| Pearl Index by BMI group | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | 28‐day cycles N | Pearl Index (95% CI) |
| Nakajima 2016 | age 18 to 35 years | _ | _ | _ | _ |
| Nakajima 2016 | < 25 | 707 | 16 | 6806 | 3.06 (1.75 to 4.96) |
| Nakajima 2016 | 25 to 30 | 346 | 8 | 3419 | 3.04 (1.31 to 5.99) |
| Nakajima 2016 | > 30 | 219 | 4 | 2081 | 2.50 (0.68 to 6.39) |
| Nakajima 2016 | age 18 to 45 years | _ | _ | _ | _ |
| Nakajima 2016 | < 25 | 840 | 16 | 8353 | 2.49 (1.42 to 4.04) |
| Nakajima 2016 | 25 to 30 | 435 | 8 | 4484 | 2.32 (1.00 to 4.57) |
| Nakajima 2016 | > 30 | 279 | 4 | 2753 | 1.89 (0.51 to 4.83) |
3.2. Analysis.
Comparison 3 COC: norethindrone acetate 1 mg + EE 10 µg, Outcome 2 Adherence by BMI (mean pills per 28‐day cycle).
| Adherence by BMI (mean pills per 28‐day cycle) | |||
|---|---|---|---|
| Study | BMI < 25 | BMI 25 to 30 | BMI > 30 |
| Nakajima 2016 | 26.2 | 26.2 | 25.8 |
3.3. Analysis.
Comparison 3 COC: norethindrone acetate 1 mg + EE 10 µg, Outcome 3 Unscheduled bleeding or spotting by BMI (cycle 13).
| Unscheduled bleeding or spotting by BMI (cycle 13) | ||||
|---|---|---|---|---|
| Study | Measure | BMI < 25 | BMI 25 to 30 | BMI > 30 |
| Nakajima 2016 | Mean days | 1.6 | 1.9 | 2.2 |
| Nakajima 2016 | Mean intensity of episodes (1 = light; 2 = normal; 3 = heavy) |
1.8 | 1.7 | 1.8 |
3.4. Analysis.
Comparison 3 COC: norethindrone acetate 1 mg + EE 10 µg, Outcome 4 Treatment‐emergent adverse events by BMI.
| Treatment‐emergent adverse events by BMI | ||||
|---|---|---|---|---|
| Study | Adverse event | BMI < 25 % | BMI 25 to 30 % | BMI > 30 % |
| Nakajima 2016 | Headache | 7.0 | 5.6 | 9.6 |
| Nakajima 2016 | Upper respiratory tract infection | 6.4 | 5.2 | 7.3 |
| Nakajima 2016 | Nasopharyngitis | 4.8 | 5.0 | 6.3 |
| Nakajima 2016 | Sinusitis | 4.5 | 6.0 | 6.0 |
| Nakajima 2016 | Nausea | 4.6 | 4.5 | 6.0 |
| Nakajima 2016 | Bacterial vaginitis | 3.5 | 5.4 | 2.7 |
| Nakajima 2016 | Dysmenorrhea | 4.0 | 5.0 | 4.6 |
Two trials examined LNG 100 µg + EE 20 µg in different regimens. With an extended regimen (84 days) (Westhoff 2012a ) (N = 1736), pregnancy rates did not differ significantly by BMI groups of less than 25 versus 25 or greater (Analysis 4.1; Analysis 4.2). Similarly, with 70 kg as the cutoff for weight, pregnancy rates did not differ significantly (Analysis 4.3; Analysis 4.4). The investigators also examined deciles for weight and BMI and found no trends in the crude pregnancy rates. Kaunitz 2014 used a standard regimen of LNG 100 µg + EE 20 µg (N = 1129) as the comparison in a trial of a transdermal patch releasing levonorgestrel. Results for the patch are in the relevant section below. With the LNG COC, the reported Pearl Index for nonobese women was 5.59 (95% CI 0.70 to 10.47) compared with zero for obese women (Analysis 5.1). For the subgroup of women who were "treatment‐compliant," the investigators found a similar pattern. The nonobese women had a Pearl Index of 5.26 (95% CI 0.12 to 10.41) versus zero for the obese women (Analysis 5.2). The BMI groups did not differ significantly in adherence to the treatment regimens, according to self‐reported data and plasma levels of LNG and EE (Analysis 5.3).
4.1. Analysis.
Comparison 4 COC: levonorgestrel 100 µg + EE 20/10 µg (extended regimen), Outcome 1 Crude pregnancy rate by BMI at 1 year.
| Crude pregnancy rate by BMI at 1 year | |||
|---|---|---|---|
| Study | BMI (kg/m2) | Crude pregnancy rate | 95% CI |
| Westhoff 2012a | < 25 | 2.22% | 1.34 to 3.44 |
| Westhoff 2012a | ≥ 25 | 1.94% | 1.13 to 3.08 |
4.2. Analysis.
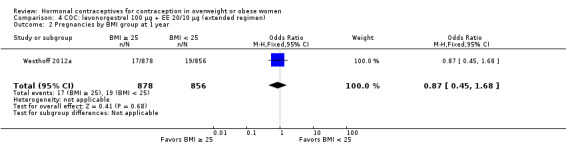
Comparison 4 COC: levonorgestrel 100 µg + EE 20/10 µg (extended regimen), Outcome 2 Pregnancies by BMI group at 1 year.
4.3. Analysis.
Comparison 4 COC: levonorgestrel 100 µg + EE 20/10 µg (extended regimen), Outcome 3 Crude pregnancy rate by weight group at 1 year.
| Crude pregnancy rate by weight group at 1 year | |||
|---|---|---|---|
| Study | Weight group | Crude pregnancy rate | 95% CI |
| Westhoff 2012a | < 70 kg | 1.97% | 1.19 to 3.05 |
| Westhoff 2012a | >= 70 kg | 2.21% | 1.29 to 3.51 |
4.4. Analysis.
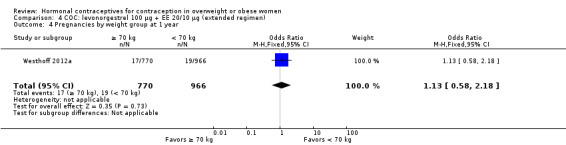
Comparison 4 COC: levonorgestrel 100 µg + EE 20/10 µg (extended regimen), Outcome 4 Pregnancies by weight group at 1 year.
5.1. Analysis.
Comparison 5 COC: levonorgestrel 100 µg + EE 20 µg, Outcome 1 Pearl Index by BMI group for cycles 1 to 6.
| Pearl Index by BMI group for cycles 1 to 6 | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | Cycles N | Pearl Index (95% CI) |
| Kaunitz 2014 | < 30 | 221 | 5 | 1164 | 5.59 (0.70 to 10.47) |
| Kaunitz 2014 | ≥ 30 | 89 | 0 | 454 | 0 |
5.2. Analysis.
Comparison 5 COC: levonorgestrel 100 µg + EE 20 µg, Outcome 2 Pearl Index by BMI group for cycles 1 to 6 (treatment‐compliant).
| Pearl Index by BMI group for cycles 1 to 6 (treatment‐compliant) | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | Cycles N | Pearl Index (95% CI) |
| Kaunitz 2014 | < 30 | 190 | 4 | 988 | 5.26 (0.12 to 10.41) |
| Kaunitz 2014 | ≥ 30 | 74 | 0 | 381 | 0 |
5.3. Analysis.
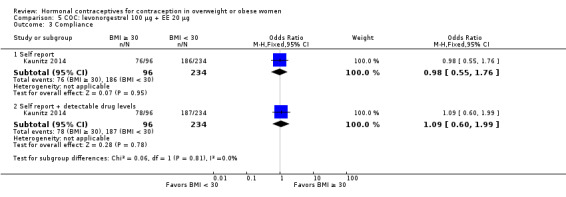
Comparison 5 COC: levonorgestrel 100 µg + EE 20 µg, Outcome 3 Compliance.
Mansour 2012 examined pregnancies by BMI group with nomegestrol acetate 2.5 mg + 17β‐estradiol (E2) 1.5 mg (N = 1613) and with drospirenone 3 mg + EE 30 µg (N = 539). The investigators used four BMI groups: < 18.5; 18.5 to < 25; 25 to < 30; ≥ 30. Four pregnancies occurred with nomegestrol acetate + E2 (Analysis 6.1) and three with drospirenone + EE (Analysis 7.1). All pregnancies were in women with BMI 18.5 to < 25, a group considered not overweight. That normal weight group included 73% (1556/2121) of the women. The conference abstract did not include the distribution by BMI within treatment group. The investigators reported that BMI was not associated with pregnancy but did not provide any statistical test.
6.1. Analysis.
Comparison 6 COC: nomegestrol acetate 2.5 mg + E2 1.5 mg, Outcome 1 Pregnancy by BMI group.
| Pregnancy by BMI group | |||
|---|---|---|---|
| Study | BMI (kg/m2) | Pregnancy n | Pearl Index (95% CI) |
| Mansour 2012 | (N = 1587) | _ | 0.31 (0.08 to 0.79) |
| Mansour 2012 | < 18.5 | 0 | _ |
| Mansour 2012 | 18.5 to < 25 | 4 | _ |
| Mansour 2012 | 25 to < 30 | 0 | _ |
| Mansour 2012 | ≥ 30 | 0 | _ |
7.1. Analysis.
Comparison 7 COC: drospirenone 3 mg + EE 30 μg, Outcome 1 Pregnancy by BMI group.
| Pregnancy by BMI group | |||
|---|---|---|---|
| Study | BMI (kg/m2) | Pregnancy n | Pearl Index (95% CI) |
| Mansour 2012 | (N = 534) | _ | 0.66 (0.14 to 1.94) |
| Mansour 2012 | < 18.5 | 0 | _ |
| Mansour 2012 | 18.5 to < 25 | 3 | _ |
| Mansour 2012 | 25 to < 30 | 0 | _ |
| Mansour 2012 | ≥ 30 | 0 | _ |
Analysis of data from multiple COC trials
Yamazaki 2015 analyzed data from seven trials that examined various COC formulations (N = 14,024). We present the results in one section here, due to the type of analysis and having limited information on the individual trials. The investigators reported the incident rate ratio (IRR) for each analysis. They also provided the hazard ratio adjusted for age and race: age < median versus ≥ median; race Black or African American versus non‐Black. The IRR and the adjusted hazard ratio (AHR) showed no significant difference between the BMI groups in the trials of desogestrel 150 µg + EE (20, 10 µg) (Analysis 8.1), levonorgestrel 100 µg + EE (20 µg) (10 µg) (Analysis 9.1), or levonorgestrel 90 µg + EE 20 µg (Analysis 11.1). For levonorgestrel 150 µg + EE (20, 25, 30 µg)/(10 µg), the IRR indicated a higher pregnancy rate for obese women compared with nonobese women, but the AHR did not show a significant difference between the BMI groups (Analysis 10.1). The groups also did not differ significantly in the trials of norethindrone 800 µg + EE 25 µg (Analysis 12.1), norethindrone acetate 1 mg + EE 10 µg (Analysis 13.1), or three norgestimate preparations combined (NGM 180, 215, 250 µg + EE 25 µg; NGM 180, 250 µg + EE 25 µg; NGM 60, 180 µg + EE 20 µg) (Analysis 14.1). The investigators conducted meta‐analysis with individual participant data from the seven trials, using a fixed‐effect model. The AHR for the meta‐analysis indicated obese women had a higher pregnancy risk compared with nonobese women (reported AHR 1.44, 95% CI 1.06 to 1.95).
8.1. Analysis.
Comparison 8 COC: desogestrel 150 μg + EE 20/10 μg, Outcome 1 Pregnancy rate by BMI group.
| Pregnancy rate by BMI group | |||||||
|---|---|---|---|---|---|---|---|
| Study | BMI (kg/m2) | n | Pregnancy N | 28‐day cycles N | Pearl Index (95% CI) | Incident rate ratio (95% CI) | Reported adjusted hazard ratio (95% CI) |
| Yamazaki 2015 | < 30 | 727 | 6 | 3754 | 2.08 (0.97 to 4.52) | _ | _ |
| Yamazaki 2015 | ≥ 30 | 310 | 6 | 1534 | 5.08 (2.39 to 11.1) | 2.44 (0.79 to 7.56) | 2.67 (0.84 to 8.51) |
9.1. Analysis.
Comparison 9 COC: levonorgestrel 100 µg + EE 20/10 µg, Outcome 1 Pregnancy rate by BMI group.
| Pregnancy rate by BMI group | |||||||
|---|---|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | 28‐day cycles N | Pearl Index (95% CI) | Incident rate ratio (95% CI) | Reported adjusted hazard ratio (95% CI) |
| Yamazaki 2015 | < 30 | 1287 | 24 | 13,291 | 2.35 (1.58 to 3.49) | _ | _ |
| Yamazaki 2015 | ≥ 30 | 446 | 11 | 4741 | 3.02 (1.70 to 5.40) | 1.29 (0.63 to 3.10) | 1.32 (0.63 to 2.73) |
11.1. Analysis.
Comparison 11 COC: levonorgestrel 90 µg + EE 20 µg, Outcome 1 Pregnancy rate by BMI group.
| Pregnancy rate by BMI group | |||||||
|---|---|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | 28‐day cycles N | Pearl Index (95% CI) | Incident rate ratio (95% CI) | Reported adjusted hazard ratio (95% CI) |
| Yamazaki 2015 | < 30 | 1377 | 17 | 10,681 | 2.07 (1.30 to 3.31) | _ | _ |
| Yamazaki 2015 | ≥ 30 | 381 | 9 | 2857 | 4.10 (2.18 to 7.77) | 1.98 (0.88 to 4.44) | 1.81 (0.79 to 4.12) |
10.1. Analysis.
Comparison 10 COC: levonorgestrel 150 µg + EE (20/25/30)/ 10 µg, Outcome 1 Pregnancy rate by BMI group.
| Pregnancy rate by BMI group | |||||||
|---|---|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | 28‐day cycles N | Pearl Index (95% CI) | Incident rate ratio (95% CI) | Reported adjusted hazard ratio (95% CI) |
| Yamazaki 2015 | < 30 | 2144 | 43 | 21,090 | 2.65 (1.97 to 3.57) | _ | _ |
| Yamazaki 2015 | ≥ 30 | 847 | 27 | 7924 | 4.43 (3.05 to 6.44) | 1.67 (1.03 to 2.70) | 1.54 (0.94 to 2.51) |
12.1. Analysis.
Comparison 12 COC: norethindrone 800 μg + EE 25 μg, Outcome 1 Pregnancy rate by BMI group.
| Pregnancy rate by BMI group | |||||||
|---|---|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | 28‐day cycles N | Pearl Index (95% CI) | Incident rate ratio (95% CI) | Reported adjusted hazard ratio (95% CI) |
| Yamazaki 2015 | < 30 | 1076 | 15 | 10,612 | 1.84 (1.12 to 3.03) | _ | _ |
| Yamazaki 2015 | ≥ 30 | 175 | 4 | 1642 | 3.17 (1.29 to 8.05) | 1.72 (0.57 to 5.18) | 1.87 (0.61 to 5.72) |
13.1. Analysis.
Comparison 13 COC: norethindrone acetate 1 mg + EE 10 µg/ EE 10 µg, Outcome 1 Pregnancy rate by BMI group.
| Pregnancy rate by BMI group | |||||||
|---|---|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | 28‐day cycles N | Pearl Index (95% CI) | Incident rate ratio (95% CI) | Reported adjusted hazard ratio (95% CI) |
| Yamazaki 2015 | < 30 | 1083 | 25 | 10,094 | 3.22 (2.19 to 4.75) | _ | _ |
| Yamazaki 2015 | ≥ 30 | 211 | 3 | 1898 | 2.05 (0.75 to 6.00) | 0.64 (0.19 to 2.12) | 0.80 (0.24 to 2.67) |
14.1. Analysis.
Comparison 14 COC: norgestimate 180/215/250 µg + EE 25 µg or NGM 250 µg + EE 25 µg or NGM 60/180 µg + EE 020 µg, Outcome 1 Pregnancy rate by BMI group.
| Pregnancy rate by BMI group | |||||||
|---|---|---|---|---|---|---|---|
| Study | BMI (kg/m2) | N | Pregnancy n | 28‐day cycles N | Pearl Index (95% CI) | Incident rate ratio (95% CI) | Reported adjusted hazard ratio (95% CI) |
| Yamazaki 2015 | < 30 | 3623 | 60 | 20,505 | 3.80 (2.96 to 4.90) | _ | _ |
| Yamazaki 2015 | ≥ 30 | 337 | 5 | 1918 | 3.39 (1.49 to 7.91) | 0.89 (0.36 to 2.22) | 0.80 (0.32 to 2.01) |
Transdermal contraceptive patch
Zieman 2002 studied the patch containing norelgestromin 150 µg + EE 20 µg (N = 3318). The investigators stated that baseline body weight was associated with pregnancy risk in a proportional hazards model, which included age, race, BMI, and body surface area (reported P < 0.001). Pooled data from three trials showed 15 pregnancies over one year in 3319 women (Analysis 15.1). The top three deciles of women weighed 69 kg or more and were about 30% of the sample. Of the 15 pregnancies, 7 were in the top decile (80 kg or more). Five of the seven were among those weighing 90 kg (198 lb) or more, who constituted 3% of the study population. Reportedly, pregnancies were not clustered in any BMI subgroup (no data provided).
15.1. Analysis.
Comparison 15 Skin patch: norelgestromin 150 µg + EE 20 µg, Outcome 1 Pregnancies by body weight decile.
| Pregnancies by body weight decile | ||
|---|---|---|
| Study | Body weight decile (kg) | Pregnancy n |
| Zieman 2002 | < 52 | 1 |
| Zieman 2002 | 52 to < 55 | 2 |
| Zieman 2002 | 55 to < 58 | 0 |
| Zieman 2002 | 58 to < 60 | 0 |
| Zieman 2002 | 60 to < 63 | 2 |
| Zieman 2002 | 63 to < 66 | 0 |
| Zieman 2002 | 66 to < 69 | 1 |
| Zieman 2002 | 69 to < 74 | 0 |
| Zieman 2002 | 74 to < 80 | 2 |
| Zieman 2002 | ≥ 80 (80 to < 90) (≥ 90) | 7 (2) (5) |
Kaunitz 2014 examined an experimental patch releasing levonorgestrel 120 µg + EE 30 µg (N = 1129). The comparison was an LNG COC as noted above. For obese women, the Pearl Index was 4.58 (95% CI 0.57 to 8.59) compared with 4.40 (95% CI 1.92 to 6.89) for nonobese women (Analysis 16.3). Of those who were "treatment‐compliant," the Pearl Index for obese women was higher than that for nonobese women, i.e. 4.63 (95% CI 0.10 to 9.17) versus 2.15 (95% CI 0.27 to 4.04), respectively (Analysis 16.4). The BMI groups did not differ significantly for adherence to the treatment regimens, according to self‐reported data and plasma levels of LNG and EE (Analysis 16.5).
16.3. Analysis.
Comparison 16 Skin patch: levonorgestrel 120 µg + EE 30 μg, Outcome 3 Pearl Index by BMI group for cycles 1 to 6.
| Pearl Index by BMI group for cycles 1 to 6 | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | n | Pregnancies N | 28‐day cycles N | Pearl Index (95% CI) |
| Kaunitz 2014 | < 30 | 680 | 12 | 3543 | 4.40 (1.92 to 6.89) |
| Kaunitz 2014 | ≥ 30 | 273 | 5 | 1418 | 4.58 (0.57 to 8.59) |
16.4. Analysis.
Comparison 16 Skin patch: levonorgestrel 120 µg + EE 30 μg, Outcome 4 Pearl Index by BMI group for cycles 1 to 6 (treatment‐compliant).
| Pearl Index by BMI group for cycles 1 to 6 (treatment‐compliant) | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | n | Pregnancies N | 28‐day cycles N | Pearl Index (95% CI) |
| Kaunitz 2014 | < 30 | 583 | 5 | 3019 | 2.15 (0.27 to 4.04) |
| Kaunitz 2014 | ≥ 30 | 222 | 4 | 1122 | 4.63 (0.10 to 9.17) |
16.5. Analysis.
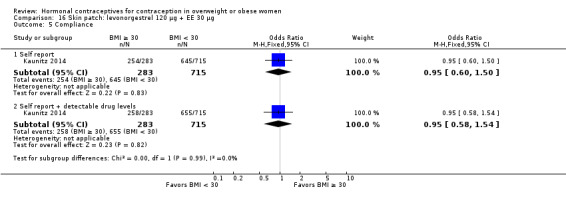
Comparison 16 Skin patch: levonorgestrel 120 µg + EE 30 μg, Outcome 5 Compliance.
Depot medroxyprogesterone acetate, subcutaneous (DMPA‐SC)
With data from two trials, Jain 2004 examined a subcutaneous form of depot medroxyprogesterone acetate (DMPA‐SC) containing 104 mg/0.65 mL. No pregnancies occurred in one year (Analysis 17.1). Overweight or obese women were 27% of the sample in the European and Asian trial, but only 6% had a BMI greater than 30. In contrast, 44% of the women in the 'Americas' trial were overweight or obese with nearly 18% having a BMI greater than 30.
17.1. Analysis.
Comparison 17 Injectable: DMPA‐SC 104 mg/0.65 mL, Outcome 1 Pregnancies by BMI group at 1 year.
| Pregnancies by BMI group at 1 year | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | Americas trial N | Americas trial Pregnancy n | Europe and Asia trial N | Europe and Asia trial Pregnancy n |
| Jain 2004 | ≤ 25 | 403 | 0 | 779 | 0 |
| Jain 2004 | > 25 to 30 | 189 | 0 | 219 | 0 |
Levonorgestrel‐releasing intrauterine contraception (LNG‐IUC)
Gemzell‐Danielson 2015 was a subanalysis from a trial of low‐dose LNG‐IUC. One method released 8 µg/day (LNG‐IUS 8) (N = 1432) and the other 13 µg/day (LNG‐IUS 13) (N = 1452). Within both LNG‐IUC groups, the Pearl Index was similar for nonobese (BMI < 30) and obese (BMI ≥ 30) women at year 1 and year 3 (Analysis 18.1; Analysis 18.2). Based on the 95% CI for each BMI group, the investigators concluded that BMI was not significantly associated with the cumulative failure rates in either LNG‐IUC group at one year or three years (Analysis 18.3; Analysis 18.4). The report did not include a statistical test for the comparisons.
18.1. Analysis.
Comparison 18 LNG‐IUS 8 µg/day versus LNG‐IUS 13 µg/day, Outcome 1 Pearl Index by BMI (year 1).
| Pearl Index by BMI (year 1) | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | Pregnancy n/ N | Reported Pearl Index (95% CI) LNG‐IUS 8 | Pregnancy n/ N | Reported Pearl Index (95% CI) LNG‐IUS 13 |
| Gemzell‐Danielson 2015 | < 30 | 4/1187 | 0.40 (0.11 to 1.01) | 1/1198 | 0.10 (0.00 to 0.54) |
| Gemzell‐Danielson 2015 | ≥ 30 | 1/244 | 0.48 (0.01 to 2.69) | 1/250 | 0.47 (0.01 to 2.64) |
18.2. Analysis.
Comparison 18 LNG‐IUS 8 µg/day versus LNG‐IUS 13 µg/day, Outcome 2 Pearl Index by BMI (year 3).
| Pearl Index by BMI (year 3) | |||||
|---|---|---|---|---|---|
| Study | BMI (kg/m2) | Pregnancy n/ N | Reported unadjusted Pearl Index (95% CI) LNG‐IUS 8 | Pregnancy n/ N | Reported unadjusted Pearl Index (95% CI) LNG‐IUS 13 |
| Gemzell‐Danielson 2015 | < 30 | 9/1187 | 0.35 (0.16 to 0.67) | 6/1198 | 0.23 (0.08 to 0.49) |
| Gemzell‐Danielson 2015 | ≥ 30 | 1/244 | 0.20 (0.00 to 1.09) | 4/250 | 0.74 (0.20 to 1.90) |
18.3. Analysis.
Comparison 18 LNG‐IUS 8 µg/day versus LNG‐IUS 13 µg/day, Outcome 3 Kaplan‐Meier cumulative failure rate by BMI (1 year).
| Kaplan‐Meier cumulative failure rate by BMI (1 year) | |||
|---|---|---|---|
| Study | BMI (kg/m2) | Reported cumulative failure rate (95% CI) LNG‐IUS 8 | Reported cumulative failure rate (95% CI) LNG‐IUS 13 |
| Gemzell‐Danielson 2015 | < 30 | 0.004 (0.001 to 0.010) | 0.001 (0.000 to 0.008) |
| Gemzell‐Danielson 2015 | ≥ 30 | 0.005 (0.001 to 0.034) | 0.005 (0.001 to 0.037) |
18.4. Analysis.
Comparison 18 LNG‐IUS 8 µg/day versus LNG‐IUS 13 µg/day, Outcome 4 Kaplan‐Meier cumulative failure rate by BMI (3 years).
| Kaplan‐Meier cumulative failure rate by BMI (3 years) | |||
|---|---|---|---|
| Study | BMI (kg/m2) | Reported cumulative failure rate (95% CI) LNG‐IUS 8 | Reported cumulative failure rate (95% CI) LNG‐IUS 13 |
| Gemzell‐Danielson 2015 | < 30 | 0.010 (0.005 to 0.019) | 0.007 (0.003 to 0.016) |
| Gemzell‐Danielson 2015 | ≥ 30 | 0.005 (0.001 to 0.033) | 0.022 (0.008 to 0.057) |
Implants
Levonorgestrel, six capsules
Four studies examined the older six‐capsule implant containing levonorgestrel 216 mg (Norplant).
The analysis of Grubb 1995 included data from multiple trials (N = 16,282). The report showed outcome data by weight group in a figure without specific rates or counts, so we present the results descriptively here. Weight groups reportedly differed in their fifth‐year pregnancy rates (life‐table method). Women weighing 40 to 49 kg had a lower rate than those weighing 50 to 59 kg, who had a lower rate than the group weighing 60 to 69 kg (reported P < 0.01). Seven pregnancies occurred in the group that weighed 70 kg or more, but the rate did not differ significantly from the group weighing 60 to 69 kg. The report did not provide numbers for women in each weight group.
In Gu 1995 (N = 10,718), higher weight was associated with pregnancy rate. The investigators combined the Pearl rates for years 6 and 7 for statistical precision. The Pearl rates were similar to the life‐table rates in those years (Analysis 19.1). For years 6 and 7, the Pearl rate for those weighing 70 kg or more was 0.86 versus 0.21, 0.29, and 0.82 for the other three weight groups (reported P < 0.05). The women in this highest weight group were 3% of the study sample. The report also provided cumulative pregnancy rates by body weight groups (Analysis 19.2).
Two studies of similar design reported pregnancies by body weight. Sivin 1998b did not detect any pregnancies in years 1 to 4 (N = 598). Two pregnancies occurred in year 5, both in women who weighed 70 kg or more, resulting in a higher pregnancy rate (reported P < 0.001). This weight group was 11% of the sample at admission (Analysis 19.3). Results from this study for the two‐rod implant are below. In Sivin 1998c (N = 511), one pregnancy occurred by three years and the woman weighed 70 kg or more (Analysis 19.3). That higher weight group was 27% of the sample at admission. The investigators identified two more pregnancies in year 5, also in the highest weight group.
19.1. Analysis.
Comparison 19 Implant: levonorgestrel, 6 capsules (216 mg), Outcome 1 Annual pregnancy rates per 100 women by body weight.
| Annual pregnancy rates per 100 women by body weight | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Body weight (kg) | Year 5 | Year 5 | Year 6 | Year 6 | Year 7 | Year 7 | Years 6 + 7 |
| Gu 1995 | _ | Pearl | LIfe table | Pearl | LIfe table | Pearl | LIfe table | Pearl |
| Gu 1995 | < 50 | 0.26 | 0.25 | 0.26 | 0.25 | 0.14 | 0.16 | 0.21 ± 0.10 |
| Gu 1995 | 50 to 59 | 0.59 | 0.59 | 0.30 | 0.30 | 0.27 | 0.26 | 0.29 ± 0.09 |
| Gu 1995 | 60 to 69 | 0.77 | 0.74 | 0.82 | 0.85 | 0.82 | 0.79 | 0.82 ± 0.23 |
| Gu 1995 | ≥ 70 | 3.01 | 3.00 | 0.00 | 0.00 | 2.35 | 2.10 | 0.86 ± 0.61 |
19.2. Analysis.
Comparison 19 Implant: levonorgestrel, 6 capsules (216 mg), Outcome 2 Cumulative pregnancy rates per 100 women by body weight.
| Cumulative pregnancy rates per 100 women by body weight | |||||
|---|---|---|---|---|---|
| Study | Body weight (kg) | Enrollment N | Year 5 | Year 6 | Year 7 |
| Gu 1995 | < 50 | 2692 | 0.77 | 1.02 | 1.18 |
| Gu 1995 | 50 to 59 | 5343 | 1.46 | 1.76 | 2.02 |
| Gu 1995 | 60 to 69 | 2318 | 2.08 | 2.91 | 3.68 |
| Gu 1995 | ≥ 70 | 365 | 4.58 | 4.58 | 6.62 |
19.3. Analysis.
Comparison 19 Implant: levonorgestrel, 6 capsules (216 mg), Outcome 3 Pregnancy by body weight per year.
| Pregnancy by body weight per year | |||||
|---|---|---|---|---|---|
| Study | Body weight (kg) | Enrollment % | Years 1 to 3 n | Year 4 n | Year 5 n |
| Sivin 1998b | _ | (N = 598) | _ | _ | _ |
| Sivin 1998b | < 50 | 18% | 0 | 0 | 0 |
| Sivin 1998b | 50 to 59 | 43% | 0 | 0 | 0 |
| Sivin 1998b | 60 to 69 | 28% | 0 | 0 | 0 |
| Sivin 1998b | ≥ 70 | 11% | 0 | 0 | 2 |
| Sivin 1998c | _ | (N = 501) | _ | _ | _ |
| Sivin 1998c | < 50 | 5% | 0 | 0 | 0 |
| Sivin 1998c | 50 to 59 | 35% | 0 | 0 | 0 |
| Sivin 1998c | 60 to 69 | 33% | 0 | 0 | 0 |
| Sivin 1998c | ≥ 70 | 27% | 1 | 0 | 2 |
Levonorgestrel, two rods
Three studies of similar design examined the two‐rod implant containing levonorgestrel 150 mg. Pregnancies were few in these studies, which provided counts by weight or weight group of the women. Sivin 1997 included an implant in use at the time (i.e. "original" or Norplant‐2) (N = 199) and a newer implant with a different elastomer (later known as Jadelle®) (N = 199). The study found no pregnancy in the first three years with the original or newer implant (Analysis 20.1). Follow‐up of the newer two‐rod implant showed five pregnancies, with two in year 5 and three in year 7. Only one occurred in the group that weighed 70 kg or more (Analysis 20.2), which represented 16% of the study population at admission. In Sivin 1998a (N = 594), three pregnancies occurred over three years among women who weighed 63, 65, and 80 kg (Analysis 20.2). No pregnancy occurred in years 4 or 5. Sivin 1998b found no pregnancies through year 4 (Analysis 20.2). Of three pregnancies in year 5, none occurred in the group weighing 70 kg or more.
20.1. Analysis.
Comparison 20 Implant: levonorgestrel, 2 rods (150 mg), Outcome 1 Pregnancy by body weight, 3 years.
| Pregnancy by body weight, 3 years | ||||
|---|---|---|---|---|
| Study | Weight (kg) | Original implant Enrollment % | Implant with new elastomer Enrollment % | Cumulative pregnancy n |
| Sivin 1997 | _ | (N = 199) | (N = 199) | _ |
| Sivin 1997 | < 50 | 19% | 16% | 0 |
| Sivin 1997 | 50 to 59 | 36% | 38% | 0 |
| Sivin 1997 | 60 to 69 | 29% | 30% | 0 |
| Sivin 1997 | ≥ 70 | 16% | 16% | 0 |
20.2. Analysis.
Comparison 20 Implant: levonorgestrel, 2 rods (150 mg), Outcome 2 Pregnancy by body weight, 1 to 7 years (implant with new elastomer).
| Pregnancy by body weight, 1 to 7 years (implant with new elastomer) | ||||||
|---|---|---|---|---|---|---|
| Study | Weight (kg) | Enrollment % | Years 1 to 3 n | Year 4 n | Year 5 n | Year 7 n |
| Sivin 1997 | _ | (N = 199) | _ | _ | _ | _ |
| Sivin 1997 | < 50 | 16% | 0 | 0 | 0 | 0 |
| Sivin 1997 | 50 to 59 | 38% | 0 | 0 | 1 | 1 |
| Sivin 1997 | 60 to 69 | 30% | 0 | 0 | 0 | 2 |
| Sivin 1997 | ≥ 70 | 16% | 0 | 0 | 1 | 0 |
| Sivin 1998a | _ | (N = 594) | _ | _ | _ | _ |
| Sivin 1998a | < 50 | 12% | 0 | 0 | 0 | _ |
| Sivin 1998a | 50 to 59 | 36% | 0 | 0 | 0 | _ |
| Sivin 1998a | 60 to 69 | 26% | 2 | 0 | 0 | _ |
| Sivin 1998a | ≥ 70 | 26% | 1 | 0 | 0 | _ |
| Sivin 1998b | _ | (N = 600) | _ | _ | _ | _ |
| Sivin 1998b | < 50 | 18% | 0 | 0 | 1 | _ |
| Sivin 1998b | 50 to 59 | 41% | 0 | 0 | 0 | _ |
| Sivin 1998b | 60 to 69 | 30% | 0 | 0 | 2 | _ |
| Sivin 1998b | ≥ 70 | 11% | 0 | 0 | 0 | _ |
Etonogestrel, one rod
Xu 2012 analyzed data from women using the implant containing etonogestrel 68 mg (N = 1168). One pregnancy occurred over three years, i.e. in 1377 woman‐years of use (Analysis 21.1). The woman who became pregnant had a baseline BMI 30.7 kg/m2. Of the implant users, 28% were overweight (BMI 25 to 29.9) and 35% were classed as obese (BMI ≥ 30).
21.1. Analysis.
Comparison 21 Implant: etonogestrel, 1 rod (68 mg), Outcome 1 Pregnancy rate per 100 woman‐years by BMI group (3 years).
| Pregnancy rate per 100 woman‐years by BMI group (3 years) | ||||
|---|---|---|---|---|
| Study | BMI (kg/m2) | Enrollment % | N | Pregnancy rate |
| Xu 2012 | 18.5 to 24.9 | 37% | 439 | 0 |
| Xu 2012 | 25 to 29.9 | 28% | 324 | 0 |
| Xu 2012 | ≥ 30 | 35% | 405 | 0.23 |
Progestin‐only vaginal ring
An experimental ring with levonorgestrel 5 mg was never marketed (WHO 1990) (N = 1005). The group weighing 70 kg or more had a cumulative discontinuation rate for pregnancy of 8.2 (Analysis 22.1), and represented about 10% of the sample. Rates for the other weight groups were 1.8 (≤ 49 kg), 2.6 (50 to 59 kg), and 5.3 (60 to 69 kg). Weight was associated with pregnancy (reported P = 0.0013). Cox proportional hazards indicated pregnancy risk increased by 61% with a 10 kg increase in body weight. The risk of pregnancy more than doubled with a 20 kg increase (e.g. from 60 to 80 kg). The estimated hazards ratio was 2.60 for this increase in body weight, but the report did not provide information on statistical significance. The cumulative life‐table rate for a woman weighing 80 kg was 9.8, i.e. more than twice the 4.4 rate for a woman weighing 60 kg (Analysis 22.2).
22.1. Analysis.
Comparison 22 Vaginal ring: levonorgestrel 5 mg, Outcome 1 Discontinuation rate due to pregnancy at 12 months by body weight.
| Discontinuation rate due to pregnancy at 12 months by body weight | ||||
|---|---|---|---|---|
| Study | Body weight (kg) | N | Pregnancy n | Cumulative discontinuation rate due to pregnancy |
| WHO 1990 | < 49 | 227 | 3 | 1.8 |
| WHO 1990 | 50 to 59 | 425 | 7 | 2.6 |
| WHO 1990 | 60 to 69 | 253 | 10 | 5.3 |
| WHO 1990 | ≥ 70 | 100 | 6 | 8.2 |
22.2. Analysis.
Comparison 22 Vaginal ring: levonorgestrel 5 mg, Outcome 2 Pregnancy life‐table rates by body weight.
| Pregnancy life‐table rates by body weight | ||
|---|---|---|
| Study | Weight (kg) | Pregnancy rate |
| WHO 1990 | 40 | 1.7 |
| WHO 1990 | 50 | 2.7 |
| WHO 1990 | 60 | 4.4 |
| WHO 1990 | 70 | 7.1 |
| WHO 1990 | 80 | 9.8 |
Discussion
Summary of main results
We summarized results for each study in Table 4, which also has the quality of evidence assessment from Table 3. In this section we focus on the 12 studies with high, moderate, or low quality evidence. Most did not indicate a higher risk of pregnancy for overweight or obese women. Four reported a difference in pregnancy risk by BMI or body weight group.
3. Pregnancy by contraceptive method.
| Study | N | Contraceptive method | Comparison groups | Reported results for pregnancy | Quality of evidencea |
| Combination oral contraceptive | |||||
| Burkman 2009 | 1671 | Norgestimate 180/215/250 µg + EE 25 µg | BMI ≥ 25 vs < 25; BMI > 27.3 vs ≤ 27.3; weight ≥ 70 kg vs < 70 kg |
NS | Moderate |
| 1139 | NETA 1 mg + EE 20 µg | RR 2.49, 95% CI 1.01 to 6.13 (BMI ≥ 25 vs < 25) | |||
| Nakajima 2016 | 1581 | NETA 1 mg + EE 10 µg | BMI < 25, 25 to 30, > 30 | NS | Low |
| Westhoff 2012a | 1736 | LNG 100 µg + EE 20 µg, extended regimen | BMI < 25 vs ≥ 25; weight < 70 kg vs ≥ 70 kg | NS | Moderate |
| Kaunitz 2014 | 375 | LNG 100 µg + EE 10 µg | BMI ≥ 30 vs < 30 | Pearl Index 0 vs 5.59 | Low |
| Mansour 2012 | 1613 | Nomegestrol acetate 2.5 mg + 17β‐estradiol (E2) 1.5 mg | BMI < 18.5, 18.5 to < 25, 25 to < 30, ≥ 30 | NS | Moderate |
| 539 | Drospirenone 3 mg + EE 30 µg | NS | |||
| Yamazaki 2015 | 14,024 |
|
BMI ≥ 30 vs < 30 |
1 trial of LNG 150 µg + EE 20/25/30 µg/ 10 µg: IRR 1.67, 95% CI 1.03 to 2.70 Meta‐analysis from 7 trials: IRR 1.43, 95% CI 1.07 to 1.92 |
Very low |
| Transdermal patch | |||||
| Zieman 2002 | 3471 | Norelgestromin 150 µg + EE 20 µg daily | Body weight | HR, P < 0.001 | Very low |
| Kaunitz 2014 | 1129 | LNG 120 µg + EE 30 µg daily (experimental) | BMI ≥ 30 vs < 30 | NS overall; "treatment‐compliant" Pearl Index 4.63 vs 2.15 | Low |
| DMPA‐SC | |||||
| Jain 2004 | 1787 | DMPA 104/ 0.65 mL, subcutaneous | BMI ≤ 25; 25 to 30; > 30 kg/m2 | 1 year, n = 0 | High |
| LNG‐IUS | |||||
| Gemzell‐Danielson 2015 | 2884 | LNG‐IUS 8 µg/day or 13 µg/day | BMI < 30 vs ≥ 30 | NS | Moderate |
| Implants | |||||
| Grubb 1995 | 16,282 | LNG 6 capsules | Weight (kg) < 40, 40 to 49, 50 to 59, 60 to 69, ≥ 70 | LIfe table rate (year 5), P < 0.01; weight (kg) 60 to 69 vs 50 to 59 vs 40 to 49 | Low |
| Gu 1995 | 10,718 | LNG 6 capsules | Weight (kg) < 50, 50 to 59, 60 to 69, ≥ 70 | Pregnancy rate (years 6 + 7), Chi statistic, P < 0.05 | Low |
| Sivin 1998c | 501 | LNG 6 capsules | Weight (kg) | 3 years, n = 1 | Very low |
| Sivin 1998b | 598 | LNG 6 capsules | Weight (kg) < 50, 50 to 59, 60 to 69, 70 to 79, ≥ 80 | Cumulative 5‐year rate, P < 0.001; weight ≥ 70 kg vs other | Very low |
| 600 | LNG 2 rods | Weight (kg) | NS | ||
| Sivin 1997 | 398 | LNG 2 rods (original and newer) | Weight (kg) < 50, 50 to 59, 60 to 69, ≥ 70 | NS | Moderate |
| Sivin 1998a | 594 | LNG 2 rods | Weight (kg) | NS | Low |
| Xu 2012 | 1168 | Etonogestrel 1 rod | BMI 18.5 to 24.9, 25 to 29.9, ≥ 30 | NS | Low |
| Vaginal ring | |||||
| WHO 1990 | 1005 | Vaginal ring LNG 20 µg daily, 90‐day use (experimental) | Weight (kg) ≤ 49, 50 to 59, 60 to 69, ≥ 70 | Cumulative discontinuation rate for pregnancy, Chi statistic, P < 0.0013 | Very low |
aSee Table 3
DMPA: depot medroxyprogesterone acetate HR: hazard ratio IRR: incident rate ratio LNG: levonorgestrel LNG‐IUC: levonorgestrel‐releasing intrauterine contraception MPA: medroxyprogesterone acetate NETA: norethisterone acetate NET‐EN: norethisterone enanthate NS: no significant difference between groups PI: Pearl Index RR: relative risk
Two of five COC studies found BMI was associated with pregnancy but in different directions (Table 1). A study of a COC containing norethindrone acetate and EE showed a higher risk for pregnancy with BMI of 25 or higher versus BMI less than 25. A trial using a COC with levonorgestrel and EE reported a lower Pearl Index for obese women (BMI 30 or higher) versus nonobese women. For the experimental patch in the same trial, which released levonorgestrel plus EE, obese women had a higher Pearl Index than nonobese women (Table 1). The difference was evident within the subgroup that was "treatment‐compliant," determined from diaries and plasma levels of levonorgestrel and EE. Trials of subcutaneous DMPA and levonorgestrel IUC indicated no association of pregnancy with overweight or obesity.
Of five implant studies, two that examined the six‐capsule levonorgestrel implant reported differences in pregnancy among weight groups (Table 2). One study showed higher weight was associated with a higher pregnancy rate in years 6 and 7 combined. In the other study, differences in pregnancy did not involve the highest weight group. Pregnancy rates differed in year 5 among the lower weight groups only.
Reports from 2004 or later used BMI, while those through 2002 analyzed body weight alone. As noted earlier, a higher BMI generally reflects more body fat. Weight alone indicates total body mass but not whether the person is overweight for a particular height. For a woman weighing 70 kg (154 lb) and 1.65 meters (65 inches) tall, BMI would be 25.7, indicating she was slightly overweight (CDC 2016). At 1.5 meters, her BMI would be 31.1 and in the obese range. Studies using body weight groups implicitly addressed a different issue than those using BMI. They may have examined whether a different dose was needed for larger women. Studying BMI might inform whether body composition, especially the amount of fat, plays a role in effectiveness.
Overall completeness and applicability of evidence
The range of contraceptive methods and of formulations within methods limits the conclusions about specific types. The five COC trials examined seven different formulations, only one of which showed a higher risk for overweight or obese women by BMI. The meta‐analysis report included data from seven trials with COCs of various formulations. The actual meta‐analysis showed increased risk for obese women, but only one of the individual seven trials did.
We also included two studies of transdermal patches with different progestins and single studies of an injectable, levonorgestrel intrauterine contraception, and an older experimental vaginal ring. Of the implant studies, a newer one examined the etonogestrel implant. Three older studies reported on the two‐rod implant and four examined the six‐capsule implant. Higher risk for heavier women by body weight alone came from reports that examined the transdermal patch, the older six‐capsule implant, and the early experimental ring. We found no studies of the currently available vaginal ring. A study of an experimental patch showed a difference by BMI for the "treatment‐compliant" subgroup.
Contraceptive studies historically restricted participation to women whose weight was within 115% or 120% of ideal (Lopez 2014; Yamazaki 2015). The studies in this review varied in their eligibility criteria, with later studies have a higher BMI limit. The trials in Zieman 2002 limited weight to 135% of ideal, though many obese women were likely excluded with that cutoff. For Jain 2004, 44% were reportedly overweight or obese in one trial and 27% in the other. In Yamazaki 2015, obese women in the seven trials ranged from 9% to 30% of participants. Three of the seven trials had a BMI limit of 35 and four had no limitations. Of the newer studies in this review, two had a BMI limit of 35 (Mansour 2012; Nakajima 2016) and four had no restriction on BMI or body weight (Westhoff 2012a; Xu 2012; Kaunitz 2014; Gemzell‐Danielson 2015). In Westhoff 2012a, half the women had a BMI greater than 25 and more than 20% had a BMI over 31. Xu 2012 recruited women from the community as well as clinics, so 28% were overweight and 35% were obese. Obese women were 17% of the sample in Gemzell‐Danielson 2015 and 29% in Kaunitz 2014.
Studies of emergency contraception (EC) did not meet our eligibility criterion for length of use. Other sources provide information on the safety and effectiveness of EC for obese women. A recent review identified four analyses of levonorgestrel EC and ulipristal acetate use among obese women (Jatlaoui 2016). Three of the analyses had overlapping data. Professional organizations in the US and Europe have also reviewed the evidence and made recommendations regarding EC for overweight and obese women (ASEC 2015; ECEC 2015).
Pharmacokinetic studies did not have our primary outcome of pregnancy. Earlier, we discussed how such research may elucidate whether and how obesity affects contraceptive efficacy (How the intervention might work).
Quality of the evidence
We considered the overall quality of evidence to be low for the objectives of this review (Table 3). Six studies provided high or moderate quality evidence, while six had low quality and five had very low quality evidence. For many trials, the quality would have been higher for their original purpose rather than for the non‐randomized comparisons here. At least seven RCTs would have been moderate or high quality rather than low or moderate quality. The main reason for downgrading was lack of adjustment for potential confounding such as age, education, or prior contraceptive use. In addition, the older studies analyzed pregnancy by weight groups rather than BMI categories.
The number of studies and evidence quality varied by contraceptive method. The evidence was moderate to low quality for the COC studies overall. For the patch and implant studies, the quality of evidence was low or very low with one exception. Most of the implant trials were conducted 15 to 30 years ago, as was the experimental ring study. The DMPA‐SC study provided high quality information and the LNG‐IUS trial had moderate quality evidence for this review.
We looked for associations between corporate sponsorship and study quality or study results for our review. Eleven of the 17 studies had high risk of bias due to sponsorship and other involvement of pharmaceutical companies. The remaining six had low or unclear risk for sponsorship; all provided evidence of low or very low quality in our review. For the four studies in the 'Summary of findings' tables, risk due to sponsorship was high for two, unclear for one and low for one. Consequently, we did not identify any pattern between sponsorship and study quality or results here.
RCTs are generally considered the gold standard for experimental research. Study design is usually stronger for RCTs than for other types of studies. However, the main comparisons in this review were not the contraceptive methods but the weight or BMI groups, which were not used for stratification. Allocation concealment and blinding are less relevant when the comparisons differ from those of the original study, and were not applicable to the single‐arm studies.
Agreements and disagreements with other studies or reviews
A European panel examined the evidence on safety and effectiveness of contraceptives for obese women (Merki‐Feld 2015). Of combined hormonal methods, the report noted that COC efficacy did not appear to differ for obese women, though data were limited for women with a BMI over 35. For the transdermal patch, one analysis indicated it was less effective with weight more than 90 kg. They did not find any evidence on a monthly vaginal ring. For progestin‐only methods, the report recommended caution regarding the etonogestrel implant due to evidence of reduced etonogestrel plasma levels among obese women. The panel considered DMPA effective for overweight and obese women. They also considered LNG‐IUC to be effective for obese women and stated that lower plasma levonorgestrel with obesity should not be related to efficacy due to local effects of IUC.
Risk of unintended pregnancy depends on regimen adherence, often presented as method failure versus user failure (Trussell 2009). Adherence is less of a concern for long‐acting methods such as implants and intrauterine contraception than for COCs, the ring, or the patch. Two included studies provided adherence data by BMI. For a new LNG patch and a COC comparison, the BMI groups did not differ significantly in "compliance" as determined from self reports in diaries and detectable plasma drug levels (Kaunitz 2014). In a COC study, the BMI groups did not differ significantly in pill intake according to the returned blister packs (Nakajima 2016). In a COC trial not included here, nonadherence was associated with poverty level, which is related to female obesity and low education in the USA (Westhoff 2012c). Outside of clinical trials, nonadherence may help explain disparate findings regarding obesity and efficacy, especially with short‐acting methods.
Contraceptive use patterns may help explain the higher rates of unintended pregnancy among obese women (Callegari 2014). In 2013, nearly half of the 146,336 clients from family planning sites in the US were overweight or obese (Kohn 2015). Obese women, who were 22% of the sample, were more likely than nonobese women to use long‐acting reversible contraception (LARC) method and to use condoms. They were less likely to use 'any hormonal method,' especially OCs or the transdermal patch. A US national survey showed that 22% of obese women at risk for unintended pregnancy did not use any contraception (Callegari 2014). Less than half used a prescription method, i.e. a more effective method such as pills, an injectable, or intrauterine contraception. Women who used no method or a nonprescription method were less likely to have discussed contraception with a health care provider in the past year.
Several relevant studies did not meet the inclusion criteria (Table 27). Most analyzed data on any oral contraceptive rather than one specific formulation. An analysis of data from vaginal ring trials did not provide the duration of use. Many of these reports have been cited in reviews of body weight and COC effectiveness (Trussell 2009; Merki‐Feld 2015). Most used BMI rather than body weight for constructing the comparison groups. Once the investigators in these additional OC studies adjusted the models for potential confounding, overweight or obese women had pregnancy risk similar to that for women of normal BMI (Brunner 2005; Brunner Huber 2006; Brunner Huber 2007; Dinger 2009) or weight (Vessey 2001). One adjusted analysis showed some difference for very obese women versus all other women (Dinger 2011). Two studies with unadjusted rates showed no relationship of weight to pregnancy risk (Westhoff 2005; Westhoff 2008). Two often‐cited OC studies using adjusted models were the exceptions: (1) women with greater body weight had a higher risk (Holt 2002); (2) women with a higher BMI, but not necessarily greater body weight, had higher risk (Holt 2005).
1. Additional studies of interest.
| Study | Type of study | Data collected | N | Hormonal contraceptive | Analytic method | Comparison groups | Reported pregnancy (95% CI) |
| Brunner 2005 | Retrospective cohort analysis | 1993 to 1995 | 1916 | Any OC | Hazard ratio (HR) adjusted for age, demographics, parity, dual method use | BMI < 20, referent 20 to 24.9, 25 to 29.9, ≥ 30 | NS |
| Weight by 20 lb increments, 80 to > 190; referent 111 to 130 | NS | ||||||
| Brunner Huber 2006 | Case‐cohort | 1999 and 2000 | 358 | Any OC | OR (logistic model adjusted for education, ethnicity, income) | BMI < 20, referent 20 to 24.9, 25 to 29.9, ≥ 30 | NS |
| OR (logistic model as above + age) | BMI > 25 vs < 25 | NS | |||||
| Brunner Huber 2007 | Retrospective cohort analysis | 2002 to 2003 | 1301 | Any OC | Hazard ratio (adjusted for age, ethnicity, parity) | BMI < 20, referent 20 to 24.9, 25 to 29.9, ≥ 30 | NS |
| BMI < 18.5, referent 18.5 to 24.9, 25 to 29.9, ≥ 30 | NS | ||||||
| Dinger 2009 | Prospective; active surveillance | 2000 to 2005 | 58,674 | Any OC | Life‐table rates | BMI < 20, 20 to 24.9, 25 to 29.9, ≥ 30 Examined by type of progestin | NS; CMA higher failure rate for BMI ≥ 30 |
| Weight (kg): < 55, 55 to 59.9, 60 to 64.9, 65 to 69.9, 70 to 74.9, ≥ 75 | Results similar to BMI analysis | ||||||
| Dinger 2011 | Prospective; active surveillance (USA) | 2005 to 2008 | 52,218 | Any OC | Hazard ratio (adjusted for age, parity, education) | BMI ≥ 35 vs < 35 | HR 1.5 (1.3 to 1.8) |
| Holt 2002 | Retrospective cohort analysis | 1990 to 1994 | 618 | Any OC | Relative risk (RR) adjusted for parity | Weight (kg) quartiles: < 56.5, 56.5 to < 62.5, 62.5 to < 70.5, ≥ 70.5 | ≥ 70.5 vs < 70.5: RR 1.6 (1.1 to 2.4) |
| Holt 2005 | Case control | 1998 to 2001 | 816 | Any OC | OR (logistic model adjusted for age, reference year, and parity) | BMI quartiles: ≤ 21.3, > 21.3 to 23.6, > 23.6 to 27.3, > 27.3 (split at 32.2) | > 27.3 vs ≤ 21.3: OR 1.62 (1.02 to 2.57) |
| > 27.3 vs ≤ 27.3: OR 1.58 (1.11 to 2.24) | |||||||
| > 32.2 vs ≤ 27.3: OR 1.72 (1.04 to 2.82); | |||||||
| Weight quartiles (kg): ≤ 56.7, > 56.7 to 63.5, > 63.5 to 74.8, > 74.8 (split at 86.2) | All OC users: NS | ||||||
| Consistent OC users, > 74.8 vs ≤ 74.8: OR 1.71 (1.08 to 2.71) | |||||||
| Consistent OC users, > 86.2 vs > 74.8 to 86.2: OR 1.95 (1.06 to 3.67) | |||||||
| Vessey 2001 | Prospective | Recruited 1968 to 1974; follow‐up to 1994 | 17,032 | Any OC | Life‐table rates adjusted for age and parity | Weight (kg): < 51, 51 to 57, 58 to 64, 64 to 70, 70 to 76, ≥ 77 | NS |
| Weight (kg) < 82 vs ≥ 82 | NS | ||||||
| Westhoff 2005a | Trial database | Not specified | 3259 | Vaginal ring; duration not specified | Rate | Body weight deciles (weight per decile not reported) | Pregnancy, 106 to 188 lb, n = 27; ≥ 189 lb, n = 0 |
| Westhoff 2008a | 5 multicenter trials | 1999 to 2007 | 6465 | 4 COCs | Crude rate | Weight (kg) < 90 vs ≥ 90 | ≥ 90 kg 0.70% vs < 90 kg 0.99% |
aAbstract; Westhoff 2008 includes data in Westhoff 2012a COC: combination oral contraceptive NS: no significant difference between comparison groups OC: oral contraceptive
Authors' conclusions
Implications for practice.
The evidence generally did not indicate an association between higher BMI or weight and effectiveness of hormonal contraceptives. However, we found few studies for methods other than oral contraceptives. Studies using BMI, rather than weight alone, can provide information about whether body composition is related to contraceptive effectiveness. The contraceptive methods examined here are among the most effective when used according to the recommended regimen.
Implications for research.
We considered the overall quality of evidence to be low for the intent of this review. The quality of many trials would have been higher for their original purpose rather than the non‐randomized comparisons here. The more recent reports provided evidence of varying quality. For the older studies, the evidence was generally low or very low quality. Most studies did not adjust for potential confounding, and the older studies used weight rather than BMI groups. Investigators should include greater numbers of overweight or obese women, as the newer studies did. This will help in examining the effectiveness and side effects of hormonal contraceptives within those groups. Investigators should also consider adjusting for potential confounding related to BMI.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2016 | New search has been performed | Search updated |
| 28 June 2016 | New citation required but conclusions have not changed | From 8 studies added, few associations of obesity or overweight with pregnancy: levonorgestrel (LNG) patch, using BMI; LNG COC, using BMI; LNG 6‐capsule implant, using weight |
| 12 May 2016 | Amended | Included 5 new studies (Kaunitz 2014; Mansour 2012; Gemzell‐Danielson 2015; Yamazaki 2015; Nakajima 2016) plus 3 older studies with limited outcome data (Sivin 1998a; Sivin 1998b; Sivin 1998c) Added 'Summary of findings' tables |
History
Protocol first published: Issue 4, 2010 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 4 February 2013 | New citation required but conclusions have not changed | Two new studies were included (Westhoff 2012a; Xu 2012). Added one 'additional study' Table 27 (Dinger 2011) and one report to Studies awaiting classification (now Kaunitz 2014). We summarized the quality assessment of the evidence. |
| 1 February 2012 | New search has been performed | Searches were updated. An earlier ongoing trial was excluded (Mornar 2012). |
Acknowledgements
Carol Manion of FHI 360 developed the search strategies for several databases.
Appendices
Appendix 1. 2016 search
MEDLINE via PubMed (4 August 2016)
(Contraceptive Agents, Female[MESH] OR Contraceptive Devices, Female[MESH] OR contraception[MeSH] OR contracept*[tiab]) AND (obesity[tiab] OR obese[tiab] OR weight[tiab] OR body mass index[MeSH] OR body weight[Mesh]) NOT (cancer*[ti] OR polycystic[ti] OR exercise[ti] OR physical activity[ti] OR postmenopaus*[ti] OR body weight changes) AND ( ( "2011/10/01"[PDat] : "3000/12/31"[PDat] ) AND Humans[Mesh])
CENTRAL (5 April 2016 (Cochrane Library Issue 3, 2016))
overweight OR obese OR obesity OR weight OR body mass index OR BMI in Abstract AND contracept* in Abstract NOT premenstrual OR dysmenor* OR endometr* OR *androgen* OR HIV OR polycystic OR PCOS OR cancer OR exercise OR anorexia OR bulimic in Record Title NOT postmenopausal OR post‐menopausal OR hormone therapy OR male hormonal in Record Title Publication Year from 2012 to 2016 in Trials
POPLINE
(5 April 2016)
Keyword: contraception AND All fields: efficacy OR effectiveness OR "contraceptive failure") AND (obesity OR obese OR overweight OR "body weight" OR "body mass index" OR BMI NOT All fields: spermicid* OR "weight changes" OR "barrier methods" OR cancer OR polycystic OR exercise OR postmenopausal OR "hormone therapy" OR "hormone replacement" Years: 2015 to 2016
(18 September 2015)
All Fields: contracept* AND (efficacy OR effectiveness OR "contraceptive failure") AND (obesity OR obese OR overweight OR "body weight" OR "body mass index" OR BMI) NOT (spermicid* OR "weight changes" OR "barrier methods" OR cancer OR polycystic OR exercise OR postmenopausal OR "hormone therapy" OR "hormone replacement") Years: From 2012 to 2015 Filter by Keywords: Research Report
Web of Science (5 April 2016)
TOPIC: (contracept*) AND TOPIC: (overweight OR obese OR obesity OR body mass index OR BMI) NOT TITLE: (cancer OR polycystic OR PCOS OR exercise OR physical activity OR postmenopaus* OR body weight change*) Refined by: WEB OF SCIENCE CATEGORIES: ( OBSTETRICS GYNECOLOGY OR ENDOCRINOLOGY METABOLISM OR REPRODUCTIVE BIOLOGY ) AND DOCUMENT TYPES: ( ARTICLE OR MEETING ABSTRACT ) Timespan: 2013‐2015. Indexes: SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, CCR‐EXPANDED, IC.
ClinicalTrials.gov (5 April 2016)
Search terms: (overweight OR obese OR obesity OR weight OR body mass index OR BMI) NOT (child OR children OR infant) Recruitment: All Studies Condition: NOT (diabetes OR tuberculosis OR acne OR HIV OR postmenopausal OR polycystic OR PCOS OR cancer OR anorexia) Intervention: contraception OR contraceptive Gender: Studies with female participants First received: 01 June 2012 to 5 April 2016
ICTRP (5 April 2016)
Title: overweight OR obese OR obesity OR weight OR body mass index OR BMI Condition: contraceptive OR contraception Recruitment status: All Date of Registration: 01 June 2012 to 5 April 2016
Appendix 2. Previous searches
2012 to 2013 search
MEDLINE via PubMed (01 January 2009 to 01 February 2013)
(Contraceptive Agents, Female[MESH] OR Contraceptive Devices, Female[MESH] OR contraception[MeSH] OR contracept*[tiab]) AND (obesity[tiab] OR obese[tiab] OR weight[tiab] OR body mass index[MeSH] OR body weight[Mesh]) NOT (cancer*[ti] OR polycystic[ti] OR exercise[ti] OR physical activity[ti] OR postmenopaus*[ti] OR body weight changes)
CENTRAL (2009 to 20 November 2012)
overweight OR obese OR obesity OR weight OR body mass index OR BMI in Abstract AND contracept* in Abstract NOT premenstrual OR dysmenor* OR endometr* OR *androgen* OR HIV OR polycystic OR PCOS OR cancer OR exercise OR anorexia OR bulimic in Record Title NOT postmenopausal OR post‐menopausal OR hormone therapy OR male hormonal in Record Title
POPLINE (2009 to 20 November 2012)
global: contracept* AND (efficacy OR effectiveness OR "contraceptive failure") AND (obesity OR obese OR overweight OR "body weight" OR "body mass index" OR BMI) NOT (spermicid* OR "weight changes" OR "barrier methods" OR cancer OR polycystic OR exercise OR postmenopausal OR "hormone therapy" OR "hormone replacement")
Limited to research reports
ClinicalTrials.gov (01 June 2009 to 20 November 2012)
Search terms: (overweight OR obese OR obesity OR weight OR body mass index OR BMI) NOT (child OR children OR infant) Condition: NOT (diabetes OR tuberculosis OR acne OR HIV OR postmenopausal OR polycystic OR PCOS OR cancer OR anorexia) Intervention: contraceptive OR contraception
ICTRP (01 June 2009 to 26 November 2012)
Title: overweight OR obese OR obesity OR weight OR body mass index OR BMI Condition: contraceptive OR contraception
2009 search
MEDLINE via PubMed (23 August 2009)
1. Search for clinical trials: (Contraceptive Agents, Female[MESH] OR Contraceptive Devices, Female[MESH] OR contraception[MeSH] OR contracept*[tiab]) AND (obesity[tiab] OR obese[tiab] OR weight[tiab] OR body mass index[MeSH] OR body weight[Mesh]) NOT (cancer*[ti] OR polycystic[ti] OR exercise[ti] OR physical activity[ti] OR postmenopaus*[ti] OR body weight changes) AND (Clinical Trial[ptyp] OR Randomized Controlled Trial[ptyp])
2. Search for other types of studies: (Contraceptive Agents, Female[MESH] OR Contraceptive Devices, Female[MESH] OR contraception[MeSH] OR contracept*[tiab]) AND (obesity[ti] OR obese[ti] OR overweight[ti] OR weight[ti] OR body mass index[ti] OR BMI[ti]) NOT (cancer*[ti] OR polycystic[ti] OR exercise[ti] OR physical activity[ti] OR postmenopaus*[ti] OR body weight changes) NOT (Editorial[ptyp] OR Letter[ptyp] OR Practice Guideline[ptyp] OR Review[ptyp])
POPLINE (29 August 2009)
(contraceptive agents, female/contracept*/oral contraceptives/contraceptive methods/vaginal rings/injectables/contraceptive implants/contraceptive patch*/skin patch*/vaginal contraceptive ring*/IUD, hormone releasing/(IUD & hormon*) & (efficacy/effective*/contraception failure) & (obesity/obese/overweight/body weight/weight/body mass index/ BMI) !(spermicid*/vaginal spermicides/barrier methods/weight changes/cancer/polycystic/exercise/postmenopaus*/hormone therapy/hormone replacement therapy/HRT)
CENTRAL (22 October 2009)
overweight OR obese OR obesity OR weight OR body mass index OR BMI in Abstract AND contraceptive OR contraception in Abstract NOT premenstrual OR dysmenor* OR endometr* OR *androgen* OR HIV OR polycystic OR PCOS OR cancer OR exercise OR anorexia OR bulimic in Record Title NOT postmenopausal OR post‐menopausal OR hormone therapy OR male hormonal in Record Title
EMBASE (20 October 2009)
1) s contraceptive agents or contraceptive device or contraception or female contraceptive device or contracept? 2) s obesity or obese or weight 3) s weight, mass and size e weight, mass and size 4) s e3 5) s body()mass()index or BMI 6) s s2 or s4 or s5 7) s s1 and s6 8) s cancer or polycystic or exercise or physical()activity or postmenopaus? or body()weight()change? 9) s s7 not s8 10) s efficacy or effective? 11) s s9 and s10 12) s clinical trial or clinical study or multicenter study or phase 1 clinical trial or phase 2 clinical trial or phase 3 clinical trial or phase 4 clinical trial or randomized controlled trial or controlled clinical trial 13) s s11 and s12
ClinicalTrials.gov (10 September 2009)
Search terms: overweight OR obese OR obesity OR weight OR body mass index OR BMI Condition: NOT (HIV OR polycystic OR PCOS OR cancer OR anorexia) Intervention: contraceptive OR contraception
ICTRP (15 September 2009)
Title: overweight OR obese OR obesity OR weight OR body mass index OR BMI Condition: contraceptive OR contraception
Appendix 3. Newcastle‐Ottawa Quality Assessment Scale
Cohort studies
Note: A study can be awarded a maximum of one star (✸) for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability.
Selection
1) Representativeness of the exposed cohort
a) truly representative of the average _______________ (describe) in the community ✸
b) somewhat representative of the average ______________ in the community ✸
c) selected group of users eg nurses, volunteers
d) no description of the derivation of the cohort
2) Selection of the non exposed cohort
a) drawn from the same community as the exposed cohort ✸
b) drawn from a different source
c) no description of the derivation of the non exposed cohort
3) Ascertainment of exposure
a) secure record (eg surgical records) ✸
b) structured interview ✸
c) written self report
d) no description
4) Demonstration that outcome of interest was not present at start of study
a) yes ✸
b) no
Comparability
1) Comparability of cohorts on the basis of the design or analysis
a) study controls for _____________ (select the most important factor) ✸
b) study controls for any additional factor ✸ (This criteria could be modified to indicate specific control for a second important factor.)
Outcome
1) Assessment of outcome
a) independent blind assessment ✸
b) record linkage ✸
c) self report
d) no description
2) Was follow‐up long enough for outcomes to occur
a) yes (select an adequate follow up period for outcome of interest) ✸
b) no
3) Adequacy of follow up of cohorts
a) complete follow up ‐ all subjects accounted for ✸
b) subjects lost to follow up unlikely to introduce bias ‐ small number lost ‐ > ____ % (select an adequate %) follow up, or description provided of those lost) ✸
c) follow up rate < ____% (select an adequate %) and no description of those lost
d) no statement
Data and analyses
Comparison 1. COC: norgestimate (NGM) 180/215/250 µg + EE 25.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relative risk of pregnancy by body weight or BMI | Other data | No numeric data | ||
| 2 Breakthrough bleeding or spotting (cycle 6): weight (lb) >155 versus ≤ 123 | 1 | 745 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.43] |
| 3 Breakthrough bleeding or spotting (cycle 6): weight (lb) > 155 versus 124 to 155 | 1 | 1135 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.52] |
| 4 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus ≤ 123 | 1 | 745 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.26, 0.83] |
| 5 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus 124 to 155 | 1 | 1135 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.94] |
1.2. Analysis.
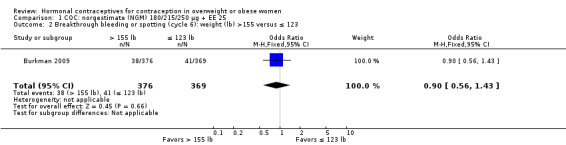
Comparison 1 COC: norgestimate (NGM) 180/215/250 µg + EE 25, Outcome 2 Breakthrough bleeding or spotting (cycle 6): weight (lb) >155 versus ≤ 123.
1.3. Analysis.

Comparison 1 COC: norgestimate (NGM) 180/215/250 µg + EE 25, Outcome 3 Breakthrough bleeding or spotting (cycle 6): weight (lb) > 155 versus 124 to 155.
Comparison 2. COC: norethindrone acetate 1 mg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relative risk of pregnancy by body weight or BMI | Other data | No numeric data | ||
| 2 Breakthrough bleeding or spotting (cycle 6): weight (lb) > 155 versus ≤ 123 | 1 | 520 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.01] |
| 3 Breakthrough bleeding or spotting (cycle 6): weight (lb) > 155 versus 124 to 155 | 1 | 783 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.13] |
| 4 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus ≤ 123 | 1 | 520 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.43] |
| 5 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus 124 to 155 | 1 | 783 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.78, 1.91] |
2.3. Analysis.
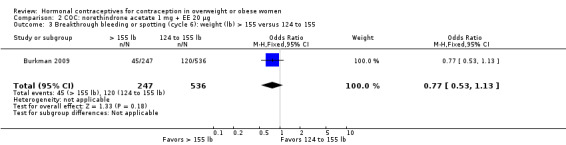
Comparison 2 COC: norethindrone acetate 1 mg + EE 20 µg, Outcome 3 Breakthrough bleeding or spotting (cycle 6): weight (lb) > 155 versus 124 to 155.
2.4. Analysis.

Comparison 2 COC: norethindrone acetate 1 mg + EE 20 µg, Outcome 4 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus ≤ 123.
2.5. Analysis.
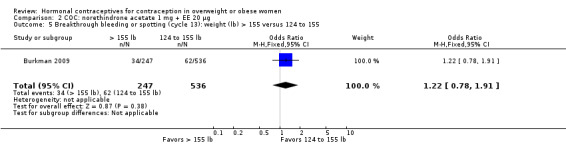
Comparison 2 COC: norethindrone acetate 1 mg + EE 20 µg, Outcome 5 Breakthrough bleeding or spotting (cycle 13): weight (lb) > 155 versus 124 to 155.
Comparison 3. COC: norethindrone acetate 1 mg + EE 10 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pearl Index by BMI group | Other data | No numeric data | ||
| 2 Adherence by BMI (mean pills per 28‐day cycle) | Other data | No numeric data | ||
| 3 Unscheduled bleeding or spotting by BMI (cycle 13) | Other data | No numeric data | ||
| 4 Treatment‐emergent adverse events by BMI | Other data | No numeric data |
Comparison 4. COC: levonorgestrel 100 µg + EE 20/10 µg (extended regimen).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Crude pregnancy rate by BMI at 1 year | Other data | No numeric data | ||
| 2 Pregnancies by BMI group at 1 year | 1 | 1734 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.45, 1.68] |
| 3 Crude pregnancy rate by weight group at 1 year | Other data | No numeric data | ||
| 4 Pregnancies by weight group at 1 year | 1 | 1736 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.58, 2.18] |
Comparison 5. COC: levonorgestrel 100 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pearl Index by BMI group for cycles 1 to 6 | Other data | No numeric data | ||
| 2 Pearl Index by BMI group for cycles 1 to 6 (treatment‐compliant) | Other data | No numeric data | ||
| 3 Compliance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Self report | 1 | 330 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.55, 1.76] |
| 3.2 Self report + detectable drug levels | 1 | 330 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.60, 1.99] |
Comparison 6. COC: nomegestrol acetate 2.5 mg + E2 1.5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy by BMI group | Other data | No numeric data |
Comparison 7. COC: drospirenone 3 mg + EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy by BMI group | Other data | No numeric data |
Comparison 8. COC: desogestrel 150 μg + EE 20/10 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate by BMI group | Other data | No numeric data |
Comparison 9. COC: levonorgestrel 100 µg + EE 20/10 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate by BMI group | Other data | No numeric data |
Comparison 10. COC: levonorgestrel 150 µg + EE (20/25/30)/ 10 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate by BMI group | Other data | No numeric data |
Comparison 11. COC: levonorgestrel 90 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate by BMI group | Other data | No numeric data |
Comparison 12. COC: norethindrone 800 μg + EE 25 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate by BMI group | Other data | No numeric data |
Comparison 13. COC: norethindrone acetate 1 mg + EE 10 µg/ EE 10 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate by BMI group | Other data | No numeric data |
Comparison 14. COC: norgestimate 180/215/250 µg + EE 25 µg or NGM 250 µg + EE 25 µg or NGM 60/180 µg + EE 020 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate by BMI group | Other data | No numeric data |
Comparison 15. Skin patch: norelgestromin 150 µg + EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancies by body weight decile | Other data | No numeric data |
Comparison 16. Skin patch: levonorgestrel 120 µg + EE 30 μg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Crude pregnancy rate by BMI group | Other data | No numeric data | ||
| 2 Crude pregnancy rate by BMI group (treatment‐compliant) | Other data | No numeric data | ||
| 3 Pearl Index by BMI group for cycles 1 to 6 | Other data | No numeric data | ||
| 4 Pearl Index by BMI group for cycles 1 to 6 (treatment‐compliant) | Other data | No numeric data | ||
| 5 Compliance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Self report | 1 | 998 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.60, 1.50] |
| 5.2 Self report + detectable drug levels | 1 | 998 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.54] |
16.1. Analysis.
Comparison 16 Skin patch: levonorgestrel 120 µg + EE 30 μg, Outcome 1 Crude pregnancy rate by BMI group.
| Crude pregnancy rate by BMI group | ||
|---|---|---|
| Study | BMI (kg/m2) | Crude pregnancy rate (95 % CI) |
| Kaunitz 2014 | < 30 | 1.76% (0.96 to 3.15) |
| Kaunitz 2014 | ≥ 30 | 1.83% (0.68 to 4.45) |
16.2. Analysis.
Comparison 16 Skin patch: levonorgestrel 120 µg + EE 30 μg, Outcome 2 Crude pregnancy rate by BMI group (treatment‐compliant).
| Crude pregnancy rate by BMI group (treatment‐compliant) | ||
|---|---|---|
| Study | BMI (kg/m2) | Crude pregnancy rate |
| Kaunitz 2014 | < 30 | 0.86% (0.32 to 2.11) |
| Kaunitz 2014 | ≥ 30 | 1.80% (0.58 to 4.85) |
Comparison 17. Injectable: DMPA‐SC 104 mg/0.65 mL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancies by BMI group at 1 year | Other data | No numeric data |
Comparison 18. LNG‐IUS 8 µg/day versus LNG‐IUS 13 µg/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pearl Index by BMI (year 1) | Other data | No numeric data | ||
| 2 Pearl Index by BMI (year 3) | Other data | No numeric data | ||
| 3 Kaplan‐Meier cumulative failure rate by BMI (1 year) | Other data | No numeric data | ||
| 4 Kaplan‐Meier cumulative failure rate by BMI (3 years) | Other data | No numeric data |
Comparison 19. Implant: levonorgestrel, 6 capsules (216 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Annual pregnancy rates per 100 women by body weight | Other data | No numeric data | ||
| 2 Cumulative pregnancy rates per 100 women by body weight | Other data | No numeric data | ||
| 3 Pregnancy by body weight per year | Other data | No numeric data |
Comparison 20. Implant: levonorgestrel, 2 rods (150 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy by body weight, 3 years | Other data | No numeric data | ||
| 2 Pregnancy by body weight, 1 to 7 years (implant with new elastomer) | Other data | No numeric data |
Comparison 21. Implant: etonogestrel, 1 rod (68 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate per 100 woman‐years by BMI group (3 years) | Other data | No numeric data |
Comparison 22. Vaginal ring: levonorgestrel 5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation rate due to pregnancy at 12 months by body weight | Other data | No numeric data | ||
| 2 Pregnancy life‐table rates by body weight | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burkman 2009.
| Methods | Design: post hoc analysis from RCT Location: 100 sites in USA; 10 in Canada Time frame: first publication Hampton 2001 Sample size calculation and outcome of focus: based on US regulatory requirements of at least 10,000 cycles for evaluation of safety and efficacy of OCs with ≥ 200 participants evaluated for 13 cycles |
|
| Participants | 2810 women Inclusion criteria: sexually active, healthy women; aged 18 to 45 years; at risk for pregnancy with regular menses Report states 32.4 was upper limit for BMI, but also notes highest BMI was 47.6. Exclusion criteria: pregnancy or lactation in past 42 days; contraindication to OCs; certain diseases; smoker aged 35 or more years; receipt of certain drugs or devices; DMPA use in past 6 months; alcohol or substance abuse in past 12 months |
|
| Interventions | Combination oral contraceptives
Duration: first 1/3 of participants assigned to 13 treatment cycles; remaining 2/3 assigned to 6 treatment cycles |
|
| Outcomes | Pregnancy (serum β‐hCG); cycle control and side effects; body weight Daily diary cards for pill intake, cycle control and side effects; reviewed at scheduled visits Side effects recorded if reported in response to general question or observed during physical examination. Follow‐up: 6 or 13 cycles Comparison groups for pregnancy:
Comparison groups for cycle control (Hampton 2008): weight ≤ 25th percentile (≤ 123 lb); 25th to 75th percentile (124 to 155 lb); > 75th percentile (> 155 lb) |
|
| Notes | Adherence data from daily diaries reported per COC (not BMI) group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons RCT information: communication with investigators indicated computer‐generated random allocation sequence; ratio 3:3:3:2; blocks of size 11:9; balanced using permuted blocks and stratified by study center |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis Investigators communicated allocation concealment was by centralized voice‐activated randomization system. |
| NOS selection (NRS) | Unclear risk | Overweight or obese: participants in RCT of 2 COCs (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: daily diaries reviewed at visits (unclear risk) |
| NOS comparability (NRS) | High risk | Design:_ Analysis: no adjustment for potential confounding; post hoc analysis of RCT data by BMI or weight groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups NGM + EE regimen blinded (no detail); study originally had 3 NGM arms; NETA + EE open |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 6.5% triphasic; 5.8% monophasic; no numerator provided Excluded from analysis cycles in which data lacking on dosing and bleeding and cycles with incorrect pill intake. Analysis population differs across reports and 75th percentile of weight differs. |
| Other bias | Low risk | Outcome assessment: pregnancy (serum β‐hCG); body weight and height measured at baseline and study visits; analyzed weight and BMI groups |
| Sponsor | High risk | Study supported by Ortho‐McNeil Janssen Scientific Affairs, LL, division of Ortho‐McNeil Pharmaceutical, Inc (Raritan, NJ) Investigators: 2 from Ortho‐McNeil Janssen; 1 from Johnson & Johnson Regenerative Therapeutics |
Gemzell‐Danielson 2015.
| Methods | Design: post hoc subgroup analysis of data from multicenter RCT (Nelson 2013), which provided design information Location: 138 centers across 11 countries (6 Europe, 2 South America, 2 North America, 1 Mexico) Time frame: August 2007 through July 2011 Sample size calculation and outcome of focus: not done for post hoc subgroup analysis; for original study, sufficient women in each treatment for Pearl Index with 95% CI, difference between upper limit of CI and point estimate < 1 for each treatment year |
|
| Participants | 2884 women at baseline, healthy, nulliparous or parous Inclusion criteria: aged 18 to 35 years, regular menstrual cycles, requested contraception Exclusion criteria: contraindication for use of LNG‐IUS in place at time of protocol development but without specific uterine dimension criteria; vaginal or cesarean delivery or abortion in past 6 weeks; known or suspected pregnancy; lactation; infected abortion or postpartum endometritis in past 3 months; distortion of uterine cavity likely to cause problems with placement, retention, or removal of device; unexplained abnormal uterine bleeding; history of ectopic pregnancy; genital malignancy or untreated cervical dysplasia; previous or current pelvic inflammatory disease; genital infection not yet successfully treated |
|
| Interventions | Levonorgestrel‐releasing intrauterine system (LNG‐IUS)
Insertion first 7 days of menstrual cycle; up to 2 placement attempts per woman Ultrasound used to confirm correct placement |
|
| Outcomes | For BMI group comparisons: Pearl Index (pregnancies per 100 woman‐years); cumulative failure rate For comparison by age and parity: discontinuation; expulsion; perforation; pelvic inflammatory disease Follow‐up: every 3 months for 1 year, then every 6 months through 3 years Comparison groups for analysis: BMI < 30 versus ≥ 30 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons RCT information: computer‐generated random number sequence in blocks of 4; 1:1 allocation; balanced for sites |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis Numbered randomization cards, used in ascending order, to inform investigator which device to place No mention of envelopes |
| NOS selection (NRS) | Low risk | Overweight or obese: participants in RCT of 2 IUC (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: gynecologic exam each visit with vaginal ultrasound for IUC position (low risk) |
| NOS comparability (NRS) | High risk | Design:_ Analysis: no adjustment for confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Participants blinded to device type; not feasible to blind investigators due to visible difference in device (length of hormone reservoir) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Full analysis set: all women with IUC placement attempt; BMI data missing 1 LNG‐IUS 8 and 4 LNG‐IUS 13) Loss to follow‐up: not specified (grouped with non‐AE reasons for discontinuation) Study completion: year 1, age 18 to 25, 77% LNG‐IUS 8 and 81% LNG‐IUS 13; age 26 to 35, 84% both groups; year 3, age 18 to 25, 51% LNG‐IUS 8 and 58% LNG‐IUS 13; age 26 to 35, 61% |
| Other bias | Low risk | Outcome assessment: gynecologic exam each visit; pregnancy test at end of 3‐year study and when clinically indicated; weight measured at baseline and annually |
| Sponsor | High risk | Study funded by Bayer HealthCare Pharmaceuticals; medical writing support in development of manuscript provided by Chameleon Communications, with financial support of Bayer HealthCare Investigators: 3 employees of Bayer (roles: involved in design of study, verified data in manuscript, and study statistician; all contributed to writing of manuscript) |
Grubb 1995.
| Methods | Design: non‐comparative trials conducted by Family Health International and Population Council Location: 17 countries (Brazil, Chile, Ecuador, El Salvador, Bangladesh, Nepal, Pakistan, Philippines, Singapore, Sri Lanka, Ghana, Haiti, Kenya, Nigeria, Senegal, Zaire, Zambia) Time frame: 1984 to 1991 Sample size calculation: no information |
|
| Participants | 16,282 women Inclusion criteria: 18 to 40 years old; sexually active; previously pregnant; no injectable use in past 6 months; within first 7 days of menstrual cycle; and able to return for follow‐up Exclusion: history of liver disease, jaundice, sickle‐cell anemia, or herpes; evidence on exam of thromboembolic disease, hypertension, pelvic inflammatory disease, undiagnosed vaginal bleeding, cancer or pregnancy In 1988, dropped original exclusion criterion regarding postpartum women (6 months postpartum or breastfeeding); all postpartum women included. |
|
| Interventions | Six‐capsule levonorgestrel implant (Norplant®) Follow‐up: 1, 3, and 6 months; then every 6 or 12 months until removal or 5 years |
|
| Outcomes | Life table rates for pregnancy overall; rates by weight group in figure without sufficient data for analysis Rates per country in table but not by weight Comparison groups: 5 body weight categories (< 40, 40 to 49, 50 to 59, 60 to 69, and ≥ 70 kg) |
|
| Notes | Sample sizes not reported for weight groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | Low risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of implants (low risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding; pregnancy by weight group not primary outcome |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm studies |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: not specified; discontinuation rates by country (N = 17) not overall; 6 countries had 5‐year discontinuation ≥ 50%; 5 countries had 2‐year to 4‐year discontinuation 41% to 49% |
| Other bias | Unclear risk | Outcome assessment: pregnancy rate was "objective‐determined"; no method specified for weight assessment at admission; analyzed weight not BMI groups |
| Sponsor | Unclear risk | Investigators: 1 (lead) from RW Johnson Pharmaceutical Research Institute Partial support for study from Family Health International (now FHI 360) with funds from US Agency for International Development |
Gu 1995.
| Methods | Design: non‐comparative trial Location: China Time frame: enrollment from 1984 to 1987 Sample size calculation: no information |
|
| Participants | 10,718 women at admission; 7554 at end of year 5 Inclusion criteria: 18 to 40 years old; had at least one living child; good health; no contraindication to hormonal contraceptives Exclusion criteria: pregnant or breastfeeding |
|
| Interventions | Six‐capsule levonorgestrel implant (Norplant®) Option of continuing into year 6, then again for year 7 Follow‐up: 1, 3, and 6 months; semi‐annually through 24 months; annually through 5 years |
|
| Outcomes | Pregnancy: life table estimates and Pearl rates Comparison groups: 4 weight categories (< 50, 50 to 59, 60 to 69, and ≥ 70 kg) |
|
| Notes | Pregnancy rates for years 1 to 4 in earlier report (Gu 1994) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | Low risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of implants (low risk) |
| NOS comparability (NRS) | High risk | Design: Analysis: no adjustment for potential confounding; pregnancy by weight group not primary outcome |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 1% |
| Other bias | Unclear risk | Outcome assessment: pregnancy status determined at each visit (method not specified); weight assessed at admission (method not specified); analyzed weight not BMI groups |
| Sponsor | Low risk | Initial funding and supplies from Rockefeller Foundation and UNFPA (now United Nations Population Fund) |
Jain 2004.
| Methods | Design: 2 non‐comparative trials Location: 1 trial in North and South America (36 sites); 1 trial in Europe and Asia (64 sites) Time frame: no information Sample size calculation: no information |
|
| Participants | 1787 women; 18 to 49 years old; sexually active and wanting long‐term contraception Inclusion criteria: no OC use for past 2 months; regular menstruation in past 3 months; willing to rely on DMPA‐SC for 1 year Exclusion criteria: used OCs, implants, or hormonal IUD in past 2 months or DMPA in past 10 months; pregnant or infertile; abnormal Pap; undiagnosed genital bleeding; other contraindication to hormonal contraceptives |
|
| Interventions | Subcutaneous form of depot medroxyprogesterone acetate (DMPA‐SC) 104/0.65 mL injected every 3 months Follow‐up: 1 year |
|
| Outcomes | Treatment failure: cumulative pregnancy rate at 1 year, i.e. positive pregnancy test prior to next scheduled injection Secondary outcomes: amenorrhea; irregular bleeding; adverse events Comparison groups (pregnancy): BMI ≤ 25; 25 to 30; > 30 kg/m2 |
|
| Notes | Overweight or obese: nearly 44% in 'Americas' trial; about 27% in European and Asian trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | Low risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of injections (low risk) |
| NOS comparability (NRS) | Low risk | Design: _ Analysis: no adjustment for potential confounding, but no pregnancy detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm studies |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 6.8% in Americas trial; 3% in European and Asian trial |
| Other bias | Low risk | Outcome assessment: pregnancy test prior to next scheduled injection; weight assessed as safety endpoint; analyzed BMI groups |
| Sponsor | High risk | Investigators: 1 from Pfizer Corporation and 1 from Pfizer Inc Statistical expertise from Pfizer Global Research and Development |
Kaunitz 2014.
| Methods | Design: multicenter randomized parallel‐group (cycles 1 to 6) with one‐arm crossover (cycles 7 to 13) study Location: Massachusetts, Ohio, Louisiana, Washington (USA) Time frame: August 2010 to November 2011 Sample size calculation: based on US FDA requirements of 10,000 28‐day treatment cycles of exposure to patch with at least 200 women completing 1 year of therapy. One‐arm crossover design adopted for this purpose. Assuming 25% dropout by end of cycle 6 and 40% dropout by end of 1 year of therapy |
|
| Participants | 1504 sexually active women randomized; 1387 in safety population (exposed to study drugs) in first 6 cycles Inclusion criteria: any body mass index; requesting contraception; 17 to 40 years of age; smokers < 35 years of age; well‐controlled hypertension and diabetes mellitus without vascular disease; regular menses; good general health confirmed by medical history, physical and gynecological examinations, clinical chemistry, and liver function values; appropriate candidate for combination estrogen‐progestin contraception (AG200‐15 N = 1129; COC N = 375) |
|
| Interventions |
AG200‐15 group treated for 1 year (13 cycles); COC group treated for 6 cycles with COC, then 7 cycles of AG200‐15 |
|
| Outcomes | Pregnancy rates (Pearl Index), treatment compliance from Kaunitz 2015 report (cycles with perfect use from diaries and plasma levels of LNG and EE) Follow‐up: 6 cycles before crossover of COC group to patch Comparison groups: BMI < 30 and ≥ 30 for first 6 cycles |
|
| Notes | Investigator provided accurate CIs for Pearl Index with supporting table; report error (did not include point estimate) Adverse event data not presented by BMI group in this report; Kaunitz 2015 article had safety data by BMI for 2 trials combined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons Randomized with interactive voice response system |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis Interactive voice response system |
| NOS selection (NRS) | Low risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: diaries for pill or patch + plasma levels of LNG and EE (low risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Open‐label; blinding of methods not feasible due to differences in contraceptive methods |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: for comparison part of study (cycles 1 to 6) 11.0% COC and 12.1% patch; patch users cycles 7 to 13, 8.1%; patch users cycles 1 to 13, 15.6% Discontinuation (with loss to follow‐up): 36.9% patch group and 27.6% COC group in first 6 cycles |
| Other bias | Unclear risk | Outcome assessment: home pregnancy test; if positive, confirmed via hierarchy (ultrasonography, exam or pregnancy outcome, date of first positive pregnancy test, daily diary information, serum b‐hCG level, qualitative urine b‐hCG) Weight assessed by investigator at study each study visit (Kaunitz 2015 safety and tolerability report) |
| Sponsor | High risk | Study supported by Agile Therapeutics, Inc, Princeton, NJ Investigators: 1 employee of Agile Therapeutics, Inc Editorial assistance provided by Phase Five Communications Inc, with financial support provided by Agile Therapeutics, Inc |
Mansour 2012.
| Methods | Design: multicenter RCT Location: 95 practices in Europe, Asia, Australia Time frame: May 2006 to April 2008 Sample size calculation: based on precision of Pearl Index in women ≤ 35 years old, 80% power; used guidance of Committee for Medicinal Products for Human Use |
|
| Participants | 2152 healthy, sexually active women Inclusion criteria: aged 18 to 50 years; BMI 17 to 35 kg/m2; needed contraception and did not plan to use condoms Exclusion criteria: contraindication for contraceptive steroids; abnormal cervical smear at screening; clinically relevant abnormal laboratory result at screening; use of injectable hormonal contraception within 6 months if 3‐month duration, within 4 months if 2‐month duration, within 2 months if 1‐month duration; present use or use in past 2 months of phenytoin, barbiturates, primidone, carbamazepine, oxcarbazepine, topiramate, felbamate, rifampicin, nelfinavir, ritonavir, griseofulvin, ketoconazole, sex steroids (except allowed contraceptive methods used before and after treatment period), or herbal remedies containing Hypericum perforatum (St John’s Wort) |
|
| Interventions | Group 1: Nomegestrol acetate (NOMAC) 2.5 mg + 17β‐estradiol (E2) 1.5 mg (24/4) (N = 1613)
Group 2: Drospirenone (DRSP) 3 mg + EE 30 μg (21/7) (N = 539) Duration: 13 treatment cycles |
|
| Outcomes | Pearl Index; percentage pregnant Other outcomes, including adherence, not examined by BMI Comparison groups: BMI < 18.5; 18.5 to < 25; 25 to < 30; ≥ 30 |
|
| Notes | Study funded by Merck Sharp & Dohme (MSD); 3 authors employed by MSD (Oss, the Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons RCT information: computer‐generated randomization schedule with blocks of four; allocated 3:1 (NOMAC + E2: DRSP + EE); stratified by age (18 to 35 years; 36 to 50 years) |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis Interactive voice‐response system; central allocation in order of randomization call |
| NOS selection (NRS) | Unclear risk | Overweight or obese: participants in RCT of OCs (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: counted dispensed and unused tablets and examined participants' records in electronic diaries (unclear risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Open label for methods |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: NOMAC 2.5%; DRSP 3.2% Loss overall (discontinuations, exclusions): NOMAC 471/1613 (29%); DRSP 129/539 (24%) Excluded those who did not take any study drug: NOMAC n = 22 (1.4%); DRSP n = 4 (0.7%) |
| Other bias | Unclear risk | Outcome assessment: no information regarding pregnancy or weight assessment |
| Sponsor | High risk | Merck provided funding for study Investigators: 3 from Merck; Oss, Netherlands |
Nakajima 2016.
| Methods | Design: post‐hoc analysis of Phase 3 open‐label uncontrolled multicenter efficacy and safety study Location: multicenter USA (68 centers in 21 states) Time frame: 2006 to 2008 Sample size calculation: no mention |
|
| Participants | 1581 women; 18 to 45 years (original study had 1657 participants; recruited locally from practices and by advertising) Inclusion criteria for original study: heterosexually active women; BMI ≤ 35; negative serum pregnancy test; willing to use study drug as only method of contraception From original report: regular menstrual cycles Exclusion criteria for original study: BMI > 35 (5 women with BMI > 35 inadvertently enrolled in original study; included in analysis) From original report: use of progestational implants, progestin, estrogen or estrogen and progestational injectable drug therapy within 9 months; IUC within 3 months; abnormal Pap test suggestive of low‐grade squamous intraepithelial lesion or more serious problem; untreated Chlamydia infection; breastfeeding; abnormal finding or condition on medical history, screening, physical and gynecologic examination, known or suspected malignant disease, cardiovascular disease, uncontrolled hypertension, thrombophlebitis or thromboembolic disorder, or undergoing treatment with anticoagulants; history of drug addiction or alcohol abuse (within 2 years); smoked ≥ 15 cigarettes per day if aged ≥ 35 years |
|
| Interventions | COC: norethindrone acetate 1 mg + EE 10 µg (ultra‐low dose) | |
| Outcomes | Pearl Index, adherence from returned blister packs, unscheduled bleeding and spotting from participant diaries, amenorrhea, other adverse events Follow‐up: 13 cycles Comparison groups: normal weight (BMI < 25; 54%); overweight (BMI 25 to 30; 28%); obese (BMI > 30; 18%) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparison |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | High risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: OC intake based on returned blister packs (high risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: original study (Archer 2013) 14% (227/1660); dropout rate 40% to 43% reportedly similar across BMI groups; 20% to 22% did not complete 2 cycles |
| Other bias | Unclear risk | Outcome assessment: returned blister packs for adherence; bleeding data by patient diary; serum and urine pregnancy tests at visits Weight and gynecological assessments at baseline and final visit; adverse events recorded at each visit |
| Sponsor | High risk | Study sponsored by Actavis, Inc. Sponsor involved in study design, analysis and interpretation of data; in writing of report and in decision to submit article for publication |
Sivin 1997.
| Methods | Design: randomized controlled trial Location: 8 centers; 4 USA (Los Angeles, New York City (2), San Francisco) and other 4 most likely Chile, Dominican Republic, Singapore, Thailand Time frame: enrollment 1990 to 1992 Sample size calculation: none apparent; 400 subjects divided equally among 8 clinics |
|
| Participants | 398 healthy women; 18 to 40 years old; sought implant contraception Inclusion criteria: no contraindication to implant use; willing to undergo study procedures; lived in area accessible to clinic Exclusion criteria: history of cancer, severe cardiovascular problem, hyperlipidemia, or diabetes mellitus; no OC use in past month or injectable steroids in past year |
|
| Interventions | Levonorgestrel implants, 2 rods, 150 mg total
Follow‐up: 1, 3, 6, 9, and 12 months; then semi‐annually through 3 years LNG rod group: at 3 years, invited to continue 2 more years (Sivin 1997); at 5 years, invited to continue 2 more years (Sivin 2001) Follow‐up: annual data reported after 3 years |
|
| Outcomes | Pregnancy (assessment method not specified); serum levonorgestrel concentrations Comparison groups for analysis: 4 weight groups (< 50, 50 to 59, 60 to 69, ≥ 70 kg) Weight at admission reported; measurement method not specified |
|
| Notes | Highest weight group (≥ 70 kg) about 16% of sample | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons RCT information: "linear congruential method"; block of 50 per clinic with 25 assigned to each group |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis Implants in sealed opaque envelopes numbered according to randomization lists (sequential) |
| NOS selection (NRS) | Low risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of implants (low risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding but no pregnancy occurred |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Implants were similar in appearance; investigators and participants could not distinguish |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: not specified 1 set of each implant contaminated and not used. Not all subjects had 3 years of use by December 1994. |
| Other bias | High risk | Outcome assessment: did not specify method for pregnancy assessment or weight at admission; analyzed weight not BMI groups |
| Sponsor | High risk | Study financed by cooperative agreements from US Agency for International Development and grants from United Nations Population Fund, George J Hecht Family Trust, Rockefeller Foundation, Andrew W Mellon Foundation, and Wyeth‐Ayerst Research; study was part of Contraceptive Development program at Population Council |
Sivin 1998a.
| Methods | Design: multicenter study; non‐comparative Location: 4 sites; 3 USA (Los Angeles, New York City, San Francisco) plus Dominican Republic Time frame: initiated 1990; ended December 1997 Sample size calculation: |
|
| Participants | 594 women Inclusion criteria: sexually active; in good health; aged 18 to 40 years; no known contraindication to levonorgestrel implants Exclusion criteria: history or current evidence of cancer, undiagnosed, abnormal genital bleeding, hyperprolactinemia or bloody breast discharge, hyperlipidemia, severe cardiovascular problems, mental illness, diabetes mellitus, epilepsy, severe or frequent headaches, pelvic inflammatory disease (PID) since the last pregnancy, or ectopic pregnancy |
|
| Interventions | Levonorgestrel implant, 2 rods (Jadelle®) containing LNG 150 mg (total) Duration: 3 years; extended to 5 years for active participants with new consent |
|
| Outcomes | Pregnancy (gross cumulative rate/100 continuing users); discontinuation due to menstrual or medical problems (gross cumulative rate/100 continuing users) Follow‐up: annual for 5 years Provided weight at admission for women who became pregnant; measurement method not specified |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | Low risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of implants (low risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: pregnancy counts by body weight; no analysis by body weight |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 16.5% (year 1, 2.2%; year 2, 3%; year 3, 3.4%; year 4, 3.7%; year 5, 4.2%) |
| Other bias | Unclear risk | Outcome assessment: for pregnancy, date of last menses by self report and gynecological exam at each follow‐up; assessment of weight not specified |
| Sponsor | High risk | Support from Wyeth‐Ayerst Research, United Nations Population Fund, and US Agency for International Development; study was part of Contraceptive Development Program at Population Council |
Sivin 1998b.
| Methods | Design: randomized controlled trial Location: 7 centers (Chile (2), Egypt, Finland, Singapore, Thailand, USA) Time frame: enrollment 1990 to 1994 Sample size estimation and outcome of focus: assumed 50/100 acceptors would continue; at 3 years, pregnancy rate 2.0/100 ± SE 0.66/100; distinguish difference in pregnancy of 2/100 between 2 implant types; 5 clinics to enroll 200 each and 2 clinics to enroll 100 each (USA and Finland) |
|
| Participants | 1198 healthy women; 18 to 40 years old; sought implant contraception Inclusion criteria: no contraindication to implant use; willing to undergo study procedures Exclusion criteria: cancer; severe cardiovascular problem; hyperlipidemia; diabetes mellitus; mental illness; epilepsy; severe or frequent headaches; undiagnosed genital bleeding; hyperprolactinemia or bloody breast discharge; pelvic inflammatory disease since last pregnancy or ectopic pregnancy |
|
| Interventions | Levonorgestrel implants 1) Norplant (N = 598); 6 capsules containing LNG 216 mg (total) 2) LNG rod (Jadelle®) (N = 600); 2 rods containing LNG 150 mg (total); elastomer in core differed from earlier implant | |
| Outcomes | Pregnancy (cumulative rate), discontinuation due to menstrual or medical problems Follow‐up: 1, 3, 6 months; then semi‐annually to 5 years Provided weight at admission for women who became pregnant |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons RCT information: "linear congruential method"; blocks of 50 per clinic |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis Implants in sealed opaque envelopes numbered sequentially according to randomization lists |
| NOS selection (NRS) | Low risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of implants (low risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: pregnancy counts by body weight; no adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups, nor methods due to apparent differences |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: LNG 2‐rod 13.9% (5 years); Norplant 17.6% (5 years) Discontinuation: year 3, 2‐rod 32% (190/600) and Norplant 31% (187/598); year 5, 2‐rod 55% (328/600) and Norplant 60% (359/598) 2 sets of Norplant contaminated and not used (1198/1200 analyzed) |
| Other bias | Unclear risk | Outcome assessment: for pregnancy, pelvic exam at each scheduled visit; assessment of weight not specified |
| Sponsor | Low risk | Study support from US Agency for International Development, United Nations Population Fund, Andrew W Mellon Foundation, Rockefeller Foundation, and George J Hecht Family Trust; study was part of Contraceptive Development program at Population Council |
Sivin 1998c.
| Methods | Design: multicenter study Location: 4 sites in USA (Los Angeles, New Jersey, New York City, San Francisco) Time frame: initiated mid‐1990; ended March 1997 Sample size calculation: expected 50% to 60% enrolled available for cumulative 5‐year pregnancy rate. With 500 women admitted, 5‐year cumulative pregnancy rate 2/100 with standard error 0.8 to 0.9 per 100, which would distinguish 5‐year cumulative pregnancy rate 2/100 or fewer from fixed value of 4/100 or more, close to that stated in product label |
|
| Participants | 511 women (501 with weight data) Inclusion criteria: age 18 to 40 years; regularly exposed to pregnancy risk; in good health Exclusion criteria: evidence of current hypertension, hepatitis or liver damage, thromboembolic disease, bloody breast discharge, malignancy of any kind, mental illness, severe hirsutism, pelvic inflammatory disease, or salpingitis; history of severe cardiovascular disease, persistent abnormal undiagnosed genital bleeding, diabetes mellitus, malignancy, severe or frequent headaches, mental illness, or epilepsy; not pregnant since episode of pelvic infection or history of ectopic pregnancy |
|
| Interventions | Norplant; 6 capsules containing LNG 216 mg (total) | |
| Outcomes | Pregnancy (gross rate/100 continuing users); discontinuation due to menstrual or medical problem (average annual first occurrence/100 years during 5‐year course) Follow‐up: annually for 5 years Reported weight at admission for women who became pregnant |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | Low risk | Overweight or obese: study participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of implants (low risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: pregnancy counts by body weight; no analysis by weight or adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 42% (year 1, 11%; year 2, 12%; year 3, 6%; year 4, 5%; year 5, 8%) |
| Other bias | Low risk | Outcome assessment: gynecologic exams at each follow‐up and where indicated at unscheduled visits; weight measured at admission and each follow‐up |
| Sponsor | High risk | Funded in part by Wyeth Ayerst Research and US Agency for International Development |
Westhoff 2012a.
| Methods | Design: secondary analysis from multicenter single‐treatment trial Location: USA; 56 clinical sites Time frame: enrolled June 2005 to June 2007 Sample size calculation: none apparent; sample size based on extent of exposure required to adequately characterize efficacy and safety; efficacy based on minimum exposure of equivalent of 10,000 28‐day cycles, with minimum of 200 completing 1 full year of treatment |
|
| Participants | 1736 women, aged 18 to 35 years, in "typical use" cohort of main study (i.e. all women 18 to 35 years old who were dispensed product) Inclusion criteria for larger study: 18 to 40 years of age; sexually active and at risk of getting pregnant; regular menstrual cycles; agreed to use the study medication as their only form of contraception Exclusion criteria for larger study: any contraindication for use of OCs; history of drug or alcohol abuse, HIV or hepatitis C positivity; history of noncompliance with chronic medications; recent surgical or medical abortion, miscarriage, or vaginal or cesarean delivery unless 3 or more consecutive spontaneous menstrual cycles or withdrawal bleeding episodes prior to enrollment; breastfeeding; history of using long‐acting forms of hormonal contraception within pre‐specified time periods prior to screening; use of non‐contraceptive hormonal therapy within 3 months of screening |
|
| Interventions | COC: 91‐day extended‐regimen of levonorgestrel 100 µg + 20 EE 20 µg (84 days)/EE 10 µg (7 days) Duration: 1 year |
|
| Outcomes | Pregnancy by positive urine pregnancy test Follow‐up: every 3 months for 1 year Weight and BMI from baseline measures: weight by deciles and dichotomous (< 70 kg or ≥ 70 kg); BMI by deciles and dichotomous (< 25 and ≥ 25) Daily paper diaries of pill‐taking and bleeding patterns for larger study; not analyzed by weight or BMI |
|
| Notes | Analysis based on ages 18 to 35 years to avoid underestimating method failure rate due to lower probability of pregnancy after age 35; study included women 18 to 40 years old. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | Unclear risk | Overweight or obese: participants in trial of OC (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: OC intake from paper diaries and return of used pill packs (unclear risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up in larger study: 14% (304/2185) Early discontinuations in larger study: 29% (632/2185); included participant request (10.3%), adverse event (11.6%), noncompliance with protocol (3.7%), pregnancy (1.6%), other (1.7%) |
| Other bias | Low risk | Outcome measure: pregnancy test conducted at each study visit; analyzed by weight and BMI groups (body weight measured) |
| Sponsor | High risk | Study supported by Duramed Research (acquired by Teva Pharmaceuticals/Women's Health and supported medical writing assistance for manuscript) Investigators: 1 employee of Teva Pharmaceuticals and 1 former employee of Duramed Research |
WHO 1990.
| Methods | Design: non‐comparative trial Location: 19 centers in Africa, China, Europe, Asian, and Latin America Time frame: 1980 to 1986 Sample size calculation: no mention |
|
| Participants | 1005 women attending family planning services Inclusion criteria: 18 to < 35 years old; regular menses for at least 2 months; sexually active (2 times/week); at least one living child or abortion by this marriage; no injectable or implant contraceptive for past 3 months and no OC for past month; able to use menstrual diary card Exclusion criteria: malignancy, genital prolapse, severe incontinence of urine, severe or chronic constipation, recurrent urinary tract infections; pelvic inflammatory disease, purulent cervical discharge, or vaginal infection; congenital disorder of renal or hepatic excretion; dyspareunia or coital difficulty; recurrent jaundice; pruritus of late pregnancy; liver disease in past 6 months |
|
| Interventions | Vaginal ring releasing levonorgestrel 20 µg daily; ring designed for 90‐day protection (continuous use), after which new ring provided Duration: 1 year Follow‐up: monthly until June 1982, when schedule was changed to every 3 months |
|
| Outcomes | Pregnancy Comparison groups for analysis: 4 weight groups (≤ 49, 50 to 59, 60 to 69, ≥ 70 kg) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS |
| Allocation concealment (selection bias) | Unclear risk | NA |
| NOS selection (NRS) | High risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: self report of ring insertion and of removal and reinsertion, if applicable (high risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Single‐arm study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: reportedly 12.7% at 1 year (99 women; denominator not specified); includes late for follow‐up as per protocol Dropped from study if 3 expulsions in 1 week or > 5 in 4 weeks |
| Other bias | Low risk | Outcome assessment: gynecological exam at each follow‐up visit; weight recorded at each visit (assessment method not specified) (low risk); analyzed weight rather than BMI (high risk) |
| Sponsor | Low risk | Study from World Health Organization (WHO); Special Programme of Research, Development, and Research Training in Human Reproduction |
Xu 2012.
| Methods | Design: analysis of data from CHOICE project; prospective cohort study of 9256 women, designed to promote long‐acting reversible methods Location: St. Louis, MO area (USA) Time frame: enrollment August 2007 to October 2010 Sample size calculation: none; post hoc estimation |
|
| Participants | 1168 of first 8445 participants enrolled in CHOICE chose implant; full cohort not analyzed since follow‐up data not available Inclusion criteria: aged 14 to 45 years; participants must initiate new contraceptive method |
|
| Interventions | Subdermal implant containing etonogestrel 68 mg (N = 1168) Participants selected baseline contraceptive method; could change methods at any time during study. |
|
| Outcomes | Pregnancy (per woman‐years of use); method failure defined as conception during method use Follow‐up: 3 and 6 months, then every 6 months until 36 months; if enrolled January 2010 or later, followed 24 months Comparison groups: BMI (18.5 to 24.9, 25 to 29.9, ≥ 30) |
|
| Notes | Reported pregnancies for LNG‐IUC and Cu T380A users combined; not included here due to grouping of hormonal and non‐hormonal IUC Investigators estimated survival curves for implants versus IUC within 3 BMI groups; reported no significant difference |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS Participant selected contraceptive method |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis |
| NOS selection (NRS) | Low risk | Overweight or obese: from community (low risk) Not overweight or obese: same source (low risk) Exposure to intervention: records of implants (low risk) |
| NOS comparability (NRS) | High risk | Design: _ Analysis: no adjustment for potential confounding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Not feasible to blind methods due to differences |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 12 months 7%; 24 months 13%; 36 months 23%; reportedly did not vary by contraceptive method |
| Other bias | Unclear risk | Outcome assessment: suspect pregnancies confirmed with urine pregnancy test; BMI calculated with baseline measurements |
| Sponsor | Low risk | Funded by anonymous foundation, grant from Doris Duke Charitable Foundation to Washington University in St Louis School of Medicine to fund Clinical Research Fellow (Xu), and award from US National Institute of Child Health and Human Development. |
Yamazaki 2015.
| Methods | Design: meta‐analysis using individual participant data (IPD); most were multicenter, open‐label, non‐comparative trials; if comparator arm, only study drug arm included Location: North America Time frame: new drug applications in FDA database, reviewed 2000 to 2012 Sample size calculation: no mention |
|
| Participants | 14,024 women in 7 COC trials Inclusion criteria for trial: comparable to Phase 3 clinical trials with hormonal contraceptives for prevention of pregnancy, typically with participants aged 18 to 49 years, at risk of pregnancy, 1‐year treatment (13 cycles); conducted in North America; acceptable quality per FDA review regarding pregnancy recording, assessment of conception during treatment period, and provision of back‐up contraceptive use; IPD retrievable in analyzable format; sample size ≥ 200; obese sample size ≥ 10% of analysis sample or ≥ 100 subjects; observation period > 6 cycles Women in pregnancy intent‐to‐treat cohort, aged 18 to 35, included in meta‐analysis if BMI, age, and race known |
|
| Interventions | COCs from 7 trials (as listed in report)
|
|
| Outcomes | Pregnancy during hormonal contraceptive use or within 7 days after last hormonal contraceptive administration (Pearl Index and incidence rate ratio) Follow‐up: > 6 cycles Comparison groups: obese (BMI ≥ 30) and nonobese (BMI < 30) |
|
| Notes | Unable to obtain clarification from investigator on COC formulations Obese women: reportedly 9% to 30% of women in these trials (typically excluded from trials during this time) Transdermal patch trial (N = 1523) not used here; investigators combined outcome data with COC data in 1 analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparison |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis |
| NOS selection (NRS) | High risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: self report of contraceptive use by trial diary (high risk) |
| NOS comparability (NRS) | Low risk | Design: _ Analysis: Cox proportional hazards regression included age and race (age < median vs ≥ median; race Black or African American vs non‐Black); no adjustment for potential confounding in rate ratio Other potential confounding factors not commonly available in data sets submitted to FDA and definitions inconsistent across trials |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Most trials were non‐comparative |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: no information; unclear whether only complete records selected (or in data sets from trials) |
| Other bias | Low risk | Outcome assessment: pregnancy confirmed by urine or serum pregnancy test; method for weight assessment not specified |
| Sponsor | Low risk | Funding through Intramural Research Science Program of Office of Women’s Health (OWH) at U.S. Food and Drug Administration (FDA) |
Zieman 2002.
| Methods | Design: post hoc analysis with data from 2 RCTs and 1 non‐comparative trial Location: (1) Audet 2001, 39 centers USA and 6 centers Canada; (2) Urdl 2005, 54 centers Europe and 11 in South Africa; (3) Smallwood 2001, 73 centers (31 USA, 36 Europe, 4 Israel, and 2 Australia) Time frame: October 1997 to June 1999 (Audet 2001; Urdl 2005) Sample size calculations: Audet 2001: 400 person‐years of observation for patch, based on regulatory guidance for Pearl Index; endpoint breakthrough bleeding or spotting, difference between groups 6% detected at alpha 0.05 with power 0.82, assuming data for 80% enrolled Urdl 2005: 400 person‐years of observation for patch, based on regulatory guidance for Pearl Index; endpoint breakthrough bleeding or spotting, difference 7.5% between groups detected at alpha 0.05 with power 0.85, assuming data for 80% enrolled Smallwood 2001: 850 person‐years of observation for patch, based on regulatory guidance for Pearl Index |
|
| Participants | 3471 women from 3 studies Inclusion criteria: sexually active; healthy; aged 18 to 45 years; within 35% of ideal body weight; blood pressure < 140/90 mm Hg; not pregnant in 42 days prior to study admission; not lactating; normal menses including 1 normal period since last pregnancy Exclusion criteria: contraindication to hormonal contraceptives; dermal hypersensitivity; smoking if > 35 years old; alcohol or substance abuse in past 12 months; injectable progestin in past 6 months; experimental drug or device use in past 30 days |
|
| Interventions | Transdermal patch (norelgestromin 150 µg + EE 20 µg daily) Duration: 13 cycles for first 1/3 enrolled and 6 cycles for other 2/3 Follow‐up: cycles 1, 3, 6; and cycles 9 and 13 for those with 13 treatment cycles Comparison groups: baseline weight deciles; < 90 versus ≥ 90 kg; BMI also examined but report had no detail | |
| Outcomes | Pregnancy (serum β‐hCG) when pregnancy suspected; urine pregnancy test 10 days after final cycle; if pregnant, ultrasound to establish date Method and user failure estimated overall but not by body weight groups | |
| Notes | 2 studies had comparison COC groups that investigator did not include in this analysis Adherence reported overall, not for post‐hoc analysis by weight and BMI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: non‐randomized comparisons RCT information: studies 1 & 2, interactive voice‐activated randomization system with permuted blocks stratified by study center; study 2 used 4:3 ratio Study 3 (Smallwood 2001), non‐comparative |
| Allocation concealment (selection bias) | Unclear risk | NA for this analysis Studies 1 & 2: interactive voice‐activated randomization system |
| NOS selection (NRS) | High risk | Overweight or obese: trial participants (high risk) Not overweight or obese: same source (low risk) Exposure to intervention: self report of patch application and of removal, if applicable (high risk) |
| NOS comparability (NRS) | Unclear risk | Design: _ Analysis: post hoc analysis with proportional hazards regression models including age, race, and baseline body weight and BMI; pregnancies reported by body weight decile |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind BMI or weight groups Open label for methods |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: study 1, 4%; study 2, 2%; study 3, not reported Efficacy analysis: returned at least 1 diary card (96% of patch) Non‐completers: 19% to 31% |
| Other bias | Low risk | Outcome assessment: serum β‐hCG when pregnancy suspected; urine pregnancy test 10 days after final cycle; if pregnant, ultrasound to establish date; body weight assessment method not specified; analyzed by weight group |
| Sponsor | High risk | Supported by RW Johnson Pharmaceutical Research Institute, Raritan NJ, later known as Johnson & Johnson Pharmaceutical Research & Development, LLC Investigators: 3 employees of Johnson & Johnson Pharmaceutical Research & Development, LLC |
BMI: body mass index COC: combination oral contraceptive EE: ethinyl estradiol NA: not applicable NRS: non‐randomized study OC: oral contraceptive
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Banerjee 1984 | Association of body weight and method failures focused on 'thin' women (≤ 40 kg versus > 40 kg). |
| Berenson 2011 | No pregnancy outcome data |
| Casey 2011 | No pregnancy outcome data; reported BMI was not statistically significant risk for bleeding or implant removal |
| Cirkel 1990 | Side effects reported by weight group; pregnancy reported overall. |
| Du 2000 | Mentions higher pregnancy rates associated with heavier body weight, but provides no data. |
| Gemzell‐Danielsson 2015b | Analyses of data from 3 emergency contraception trials; did not meet eligibility criteria of 3 treatment months or cycles. |
| Graesslin 2008 | Insufficient data on weight; for efficacy analysis, 14.5% > 70 kg Six pregnancies confirmed occurred within 14 days of removal; US FDA required those counted as in‐treatment; pregnancies not categorized by weight Report provides postmarketing surveillance data. Method failures in figure by body weight (10 kg groups) without actual counts or percentages |
| Kapp 2015 | Analyses of data from 2 emergency contraception trials; did not meet eligibility criteria of 3 treatment months or cycles. |
| McNicholas 2013 | Analysis combined OC, patch, and ring users |
| Moreau 2012 | Analyses of data from 2 emergency contraception trials (1 also in Kapp 2015); did not meet eligibility criteria of 3 treatment months or cycles. |
| Mornar 2012 | Previously an ongoing trial; published article indicates study did not measure our outcomes. |
| Sivin 1988 | Data appeared to overlap those in Gu 1995. |
| Sivin 2000 | Data appeared to overlap those in Sivin 1997. |
| Weisberg 1999 | Insufficient data on weight; no cutoffs or percent of sample provided |
Contributions of authors
2010: For the initial review, LM Lopez reviewed the search results, conducted the primary data extraction and drafted the review. DA Grimes (DAG) (formerly of FHI 360) was an author of the initial review. He conducted the second data extraction and verified correct data entry. M Chen reviewed the statistical reporting and provided guidance on statistical presentation and interpretation. All authors reviewed and commented on the manuscript.
2013: LM Lopez and C Otterness (formerly of FHI 360) reviewed search results, conducted the data extraction, and incorporated the new studies. All authors reviewed and commented on the manuscript.
2016: LM Lopez and A Bernholc reviewed search results and conducted the primary data extraction and the secondary extraction for some reports. LM Lopez drafted the review. T Grey did part of the secondary data extraction and checked the results and discussion text for consistency. M Chen reviewed the Risk of bias tables and the statistical presentation and interpretation. All authors reviewed and commented on the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
For conducting the review and updates at FHI 360
Declarations of interest
Some of the trials in Grubb 1995 were conducted at FHI 360 (formerly Family Health International), where review authors are employed (LL, AB, MC, TG) or were employed (DAG, CO). None of the review authors were involved in Grubb 1995.
L Lopez. A Bernholc, T Grey, M Chen, C Otterness, FM Helmerhorst have no interests to declare.
A Edelman: Agile Pharmaceutical, consultant and served on an advisory board; Merck, trainer and served as an expert advisor; Up To Date, Inc, continuing author; FHI 360 (where review was conducted), serves on Data and Safety Monitoring Board for novel contraceptive injectable (review authors from FHI 360 are not involved in that project).
C Westhoff conducted Westhoff 2012a and studies in Table 27. She is advisor to Agile Therapeutics, which funded Kaunitz 2014, and consults with Bayer and Merck.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Burkman 2009 {published and unpublished data}
- Burkman RT, Fisher AC, Wan GJ, Barnowski CE, LaGuardia KD. Association between efficacy and body weight or body mass index for two low‐dose oral contraceptives. Contraception 2009;79(6):424‐7. [DOI] [PubMed] [Google Scholar]
- Hampton RM, Short M, Bieber E, Bouchard C, Ayotte N, et al. Comparison of a novel norgestimate/ethinyl estradiol oral contraceptive (Ortho Tri‐Cyclen Lo) with the oral contraceptive Loestrin Fe 1/20. Contraception 2001;63(6):289‐95. [DOI] [PubMed] [Google Scholar]
- Hampton RM, Zhang HF, Barnowski C, Wan GJ. Bleeding patterns with monophasic and triphasic low‐dose ethinyl estradiol combined oral contraceptives. Contraception 2008;77(6):415‐9. [DOI] [PubMed] [Google Scholar]
- Zhang H, LaGuardia K, Creanga D. Higher body weight and body mass index are not associated with reduced efficacy in Ortho Tri‐Cyclen Lo users (abstract). Obstetrics and Gynecology 2006;107(4 Suppl):50S. [Google Scholar]
Gemzell‐Danielson 2015 {published data only (unpublished sought but not used)}
- Gemzell‐Danielsson K, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. The effect of age, parity and body mass index on the efficacy, safety, placement and user satisfaction associated with two low‐dose levonorgestrel intrauterine contraceptive Systems: subgroup analyses of data from a Phase III trial. PloS Pne 2015;10(9):e0135309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunitz AM, Dermout S, Tuppurainen M, Jensen J, Rosen K, Gemzell‐Danielsson K. Efficacy and safety of two low‐dose levonorgestrel intrauterine systems according to women's age: a global, multicentre, open‐label, randomized 3‐year Phase III Pearl Index study (conference poster abstract). European Journal of Contraception and Reproductive Health Care 2013;18(S1):S191‐2. [Google Scholar]
- Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, et al. Two low‐dose levonorgestrel intrauterine contraceptive systems. Obstetrics and Gynecology 2013;122(6):1205‐13. [DOI] [PubMed] [Google Scholar]
Grubb 1995 {published data only}
- Grubb GS, Moore D, Anderson NG. Pre‐introductory clinical trials of Norplant implants: a comparison of seventeen countries' experience. Contraception 1995;52(5):287‐96. [DOI] [PubMed] [Google Scholar]
Gu 1995 {published data only}
- Gu S, Sivin I, Du M, Zhang L, Ying L, Meng F, et al. Effectiveness of Norplant implants through seven years: a large‐scale study in China. Contraception 1995;52(2):99‐103. [DOI] [PubMed] [Google Scholar]
- Gu SJ, Du MK, Zhang LD, Liu YL, Wang SH, Sivin I. A 5‐year evaluation of NORPLANT contraceptive implants in China. Obstetrics and Gynecology 1994;83(5 Pt 1):673‐8. [PubMed] [Google Scholar]
Jain 2004 {published data only}
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA‐SC. Contraception 2004;70(4):269‐75. [DOI] [PubMed] [Google Scholar]
Kaunitz 2014 {published and unpublished data}
- Kaunitz AM, Archer DF, Mishell DR Jr, Foegh M. Safety and tolerability of a new low‐dose contraceptive patch in obese and nonobese women. American Journal of Obstetrics and Gynecology 2015;212(3):318.e1‐8. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM, Portman D, Westhoff CL, Archer DF, Mishell DR Jr, Foegh M. Self‐reported and verified compliance in a phase 3 clinical trial of a novel low‐dose contraceptive patch and pill. Contraception 2015;91(3):204‐10. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM, Portman D, Westhoff CL, Archer DF, Mishell DR Jr, Rubin A, et al. Low‐dose levonorgestrel and ethinyl estradiol patch and pill: a randomized controlled trial. Obstetrics and Gynecology 2014;123(2 Pt 1):295‐303. [DOI] [PubMed] [Google Scholar]
Mansour 2012 {published data only}
- Mansour D, Sundstrom Poromaa I, Sommer W, Korver T. Effect of body mass index and age on the contraceptive effectiveness of nomegestrol acetate/17beta‐oestradiol and drospirenone/ethinylestradiol. European Journal of Contraception and Reproductive Health Care 2012; Vol. 17, issue Suppl 1:S95.
- Mansour D, Verhoeven C, Sommer W, Weisberg E, Taneepanichskul S, Melis GB, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β‐oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. European Journal of Contraception and Reproductive Health Care. 2011/10/15 2011; Vol. 16, issue 6:430‐43. [DOI] [PMC free article] [PubMed]
Nakajima 2016 {published data only}
- Archer DF, Nakajima ST, Sawyer AT, Wentworth J, Trupin S, Koltun WD, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low‐dose oral contraceptive. Obstetrics and Gynecology 2013;122(3):601‐7. [DOI] [PubMed] [Google Scholar]
- Nakajima ST, Pappadakis J, Archer DF. Body mass index does not affect the efficacy or bleeding profile during use of an ultra‐low‐dose combined oral contraceptive. Contraception 2016;93(1):52‐7. [DOI] [PubMed] [Google Scholar]
Sivin 1997 {published data only}
- Sivin I, Lahteenmaki P, Ranta S, Darney P, Klaisle C, Wan L, et al. Levonorgestrel concentrations during use of levonorgestrel rod (LNG ROD) implants. Contraception 1997;55(2):81‐5. [DOI] [PubMed] [Google Scholar]
- Sivin I, Wan L, Ranta S, Alvarez F, Brache V, Mishell DR, et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001;64(1):43‐9. [DOI] [PubMed] [Google Scholar]
Sivin 1998a {published data only}
- Sivin I, Alvarez F, Mishell D R Jr, Darney P, Wan L, Brache V, et al. Contraception with two levonorgestrel rod implants. A 5‐year study in the United States and Dominican Republic. Contraception 1998;58(5):275‐82. [DOI] [PubMed] [Google Scholar]
Sivin 1998b {published data only}
- Sivin I, Campodonico I, Kiriwat O, Holma P, Diaz S, Wan L, et al. The performance of levonorgestrel rod and Norplant contraceptive implants: a 5 year randomized study. Human Reproduction 1998;13(12):3371‐8. [DOI] [PubMed] [Google Scholar]
- Sivin I, Viegas O, Campodonico I, Diaz S, Pavez M, Wan L, et al. Clinical performance of a new two‐rod levonorgestrel contraceptive implant: a three‐year randomized study with Norplant implants as controls. Contraception 1997;55(2):73‐80. [DOI] [PubMed] [Google Scholar]
Sivin 1998c {published data only}
- Sivin I, Mishell DR Jr, Darney P, Wan L, Christ M. Levonorgestrel capsule implants in the United States: a 5‐year study. Obstetrics and Gynecology 1998;92(3):337‐44. [DOI] [PubMed] [Google Scholar]
Westhoff 2012a {published data only}
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low‐dose, 91‐day, extended‐regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010;81(1):41‐8. [DOI] [PubMed] [Google Scholar]
- Westhoff CL, Hait HI, Reape KZ. Body weight does not impact pregnancy rates during use of a low‐dose extended‐regimen 91‐day oral contraceptive. Contraception. 2011/11/10 2012; Vol. 85, issue 3:235‐9. [DOI] [PubMed]
WHO 1990 {published data only}
- World Health Organization, Task Force on Long‐Acting Systemic Agents for Fertility Regulation. Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial. I. Contraceptive efficacy and side effects. Contraception 1990;41(2):105‐24. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Task Force on Long‐Acting Systemic Agents for Fertility Regulation. Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial. II. Expulsions and removals. Contraception 1990;41(2):125‐41. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Task Force on Long‐Acting Systemic Agents for Fertility Regulation. Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial. III. The relationship between pregnancy rate and body weight. World Health Organization. Contraception 1990;41(2):143‐50. [DOI] [PubMed] [Google Scholar]
Xu 2012 {published data only}
- Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long‐acting reversible contraception. American Journal of Obstetrics and Gynecology. 2010/06/15 2010; Vol. 203, issue 2:115 e1‐7. [DOI] [PMC free article] [PubMed]
- Xu H, Wade JA, Peipert JF, Zhao Q, Madden T, Secura GM. Contraceptive failure rates of etonogestrel subdermal implants in overweight and obese women. Obstetrics and Gynecology. 2012/06/09 2012; Vol. 120, issue 1:21‐6. [DOI] [PMC free article] [PubMed]
Yamazaki 2015 {published data only (unpublished sought but not used)}
Zieman 2002 {published data only}
- Audet MC, Moreau M, Koltun WD, Waldbaum AS, Shangold G, Fisher AC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. Journal of the American Medical Association 2001;285(18):2347‐54. [DOI] [PubMed] [Google Scholar]
- Smallwood GH, Meador ML, Lenihan JP, Shangold GA, Fisher AC, Creasy GW. Efficacy and safety of a transdermal contraceptive system. Obstetrics and Gynecology 2001;98(5 Pt 1):799‐805. [DOI] [PubMed] [Google Scholar]
- Urdl W, Apter D, Alperstein A, Koll P, Schonian S, Bringer J, et al. Contraceptive efficacy, compliance and beyond: factors related to satisfaction with once‐weekly transdermal compared with oral contraception. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;121(2):202‐10. [DOI] [PubMed] [Google Scholar]
- Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertility and Sterility 2002;77(2 Suppl 2):S13‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Banerjee 1984 {published data only}
- Banerjee SK, Baweja R, Bhatt RV, Chatterjee A, Choudhury SD, Coyaji B, et al. Comparative evaluation of contraceptive efficacy of norethisterone oenanthate (200 mg) injectable contraceptive given every two or three monthly. Indian Council of Medical Research Task Force on Hormonal Contraception. Contraception 1984, issue 6:561‐74. [DOI] [PubMed]
Berenson 2011 {published data only}
- Berenson AB, Berg P, Williams KJ, Rahman M. Effect of injectable and oral contraceptives on glucose and insulin levels. Obstet Gynecol. 2010/12/22 2011; Vol. 117, issue 1:41‐7. [DOI] [PMC free article] [PubMed]
Casey 2011 {published data only}
Cirkel 1990 {published data only}
- Cirkel U, Schneider HPG. [Incidence of side effects caused by a three‐stage preparation administered to 10,034 women] [[Nebenwirkungsinzidenz eines Dreistufenpräparates unter der Anwendung bei 10.034 Frauen]]. Geburtshilfe und Frauenheilkunde 1991;50(12):969‐73. [DOI] [PubMed] [Google Scholar]
Du 2000 {published data only}
- Du MK, Chow LP, Zheng HM, Chen CH. A 10‐year follow‐up study of contraceptive Norplant implants. International Journal of Gynaecology & Obstetrics. 2000/03/04 2000; Vol. 68, issue 3:249‐56. [DOI] [PubMed]
- Du MK, Zheng HM, Chen HC, Chow LP. Study of Norplant implants in Shanghai: three‐year experience. International Journal of Gynaecology & Obstetrics. 1990/12/01 1990; Vol. 33, issue 4:345‐57. [DOI] [PubMed]
Gemzell‐Danielsson 2015b {published data only}
- Gemzell‐Danielsson K, Kardos L, Hertzen H. Impact of bodyweight/body mass index on the effectiveness of emergency contraception with levonorgestrel: a pooled‐analysis of three randomized controlled trials. Current Medical Research and Opinion 2015;31(12):2241‐8. [DOI] [PubMed] [Google Scholar]
Graesslin 2008 {published data only}
- Graesslin O, Korver T. The contraceptive efficacy of Implanon®: a review of clinical trials and marketing experience. European Journal of Contraception and Reproductive Health Care 2008;13(Suppl 1):4‐12. [DOI] [PubMed] [Google Scholar]
Kapp 2015 {published data only}
- Glasier A, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011/09/17 2011; Vol. 84, issue 4:363‐7. [DOI] [PubMed]
- Kapp N, Abitbol JL, Mathe H, Scherrer B, Guillard H, Gainer E, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception 2015;91(2):97‐104. [DOI] [PubMed] [Google Scholar]
McNicholas 2013 {published data only (unpublished sought but not used)}
- McNicholas C, Zhao Q, Secura G, Allsworth JE, Madden T, Peipert JF. Contraceptive failures in overweight and obese combined hormonal contraceptive users. Obstetrics and Gynecology 2013;121(3):585‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreau 2012 {published data only}
- Moreau C, Trussell J. Results from pooled Phase III studies of ulipristal acetate for emergency contraception. Contraception. 2012/07/10 2012. [DOI] [PMC free article] [PubMed]
Mornar 2012 {published data only}
- Mornar S, Chan L‐N, Mistretta S, Neustadt A, Martins S, Gilliam M. Pharmacokinetics of the etonogestrel contraceptive implant in obese women. American Journal of Obstetrics and Gynecology August 2012; Vol. 207, issue 110:e1‐6. [NCT00724438] [DOI] [PubMed]
- Neustadt A, Gilliam M. Pharmacokinetics of Implanon in obese women. http://clinicaltrials.gov/ct2/show/NCT00724438 (accessed 15 Sep 2009). [NCT00724438]
Sivin 1988 {published data only}
- Sivin I. International experience with NORPLANT® and NORPLANT®‐2 contraceptives. Studies in Family Planning 1988;19(2):81‐94. [PubMed] [Google Scholar]
Sivin 2000 {published data only}
Weisberg 1999 {published data only}
- Weisberg E, Fraser IS, Lacarra M, Mishell DR, Jr, Alvarez F, et al. Efficacy, bleeding patterns, and side effects of a 1‐year contraceptive vaginal ring. Contraception 1999;59(5):311‐8. [DOI] [PubMed] [Google Scholar]
Additional references
ASEC 2015
- American Society for Emergency Contraception. Efficacy of emergency contraception and body weight: Current understanding and recommendations. http://americansocietyforec.org/uploads/3/2/7/0/3270267/asec_ec_efficacy_and_weight_statement.pdf. [Princeton, New Jersey], American Society for Emergency Contraception [ASEC], 2015 Jan., January 2015 (accessed 12 April 2016).
Brunner Huber 2006
- Brunner Huber LR, Hogue CJ, Stein AD, Drews C, Zieman M. Body mass index and risk for oral contraceptive failure: a case‐cohort study in South Carolina. Annals of Epidemiology 2006;16(8):637‐43. [DOI] [PubMed] [Google Scholar]
Brunner 2005
- Brunner LR, Hogue CJ. The role of body weight in oral contraceptive failure: results from the 1995 National Survey of Family Growth. Annals of Epidemiology 2005;15(7):492‐9. [DOI] [PubMed] [Google Scholar]
Brunner Huber 2007
- Brunner Huber LR, Toth JL. Obesity and oral contraceptive failure: findings from the 2002 National Survey of Family Growth. American Journal of Epidemiology 2007;166(11):1306‐11. [DOI] [PubMed] [Google Scholar]
Callegari 2014
- Callegari LS, Nelson KM, Arterburn DE, Prager SW, Schiff MA, Schwarz EB. Factors associated with lack of effective contraception among obese women in the United States. Contraception 2014;90(3):265‐71. [DOI] [PubMed] [Google Scholar]
CDC 2016
- Centers for Disease Control and Prevention. Defining Adult Overweight and Obesity. www.cdc.gov/obesity/adult/defining.html (accessed 12 May 2016).
Creinin 2015
- Creinin MD, Baker JB, Eisenberg L, Ginde S, Turok DK, Westhoff CL. Levonorgestrel levels in nonobese and obese women using LNG20, a new intrauterine contraceptive. Obstetrics and Gynecology 2015;125 Suppl 1:84S‐5S. [Google Scholar]
Dinger 2009
- Dinger JC, Cronin M, Mohner S, Schellschmidt I, Minh TD, Westhoff C. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. American Journal of Obstetrics and Gynecology 2009;201(3):263.e1‐9. [DOI] [PubMed] [Google Scholar]
Dinger 2011
ECEC 2015
- European Consortium for Emergency Contraception. Efficacy of emergency contraception and body weight. Current understanding and recommendations. www.ec‐ec.org/custom‐content/uploads/2015/12/ECEC‐EC‐Body‐Weight‐November‐2015.pdf November 2015 (accessed 12 Aprl 2016).
Edelman 2009
- Edelman AB, Carlson NE, Cherala G, Munar MY, Stouffer RL, Cameron JL, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic‐pituitary‐ovarian activity. Contraception 2009;80(2):119‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edelman 2014
- Edelman AB, Cherala G, Munar MY, McInnis M, Stanczyk FZ, Jensen JT. Correcting oral contraceptive pharmacokinetic alterations due to obesity: a randomized controlled trial. Contraception 2014;90(5):550‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallo 2014
- Gallo MF, Lopez LM, Grimes DA, Carayon F, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD003987.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook [updated October 2013]. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (accessed 29 December 2015).
Grimes 2005
- Grimes DA, Shields WC. Family planning for obese women: challenges and opportunities. Contraception 2005;72(1):1‐4. [DOI] [PubMed] [Google Scholar]
Grimes 2010
- Grimes DA, Schulz KF, Raymond EG. Surrogate end points in women's health research: science, protoscience, and pseudoscience. Fertility and Sterility 2010;93(6):1731‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 26 Mar 2012).
Holt 2002
- Holt VL, Cushing‐Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstetrics and Gynecology 2002;99(5 Pt 1):820‐7. [DOI] [PubMed] [Google Scholar]
Holt 2005
- Holt VL, Scholes D, Wicklund KG, Cushing‐Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstetrics and Gynecology 2005;105(1):46‐52. [DOI] [PubMed] [Google Scholar]
Jatlaoui 2016
- Jatlaoui TC, Curtis KM. Safety and effectiveness data for emergency contraceptive pills among women with obesity: a systematic review. Contraception 2016 May 24 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Kavanaugh 2015
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long‐acting reversible contraceptive methods among U.S. women, 2009‐2012. Obstetrics and Gynecology 2015;126(5):917‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohn 2015
- Kohn JE, Lopez PM, Simons HR. Weight and body mass index among female contraceptive clients. Contraception 2015;91(6):470‐3. [DOI] [PubMed] [Google Scholar]
Lopez 2013
- Lopez LM, Edelman A, Chen M, Otterness C, Trussell J, HelmerhorstM. Progestin‐only contraceptives: effects on weight. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008815.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2014
- Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD006133.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKeating 2015
- McKeating A, O'Higgins A, Turner C, McMahon L, Sheehan SR, Turner MJ. The relationship between unplanned pregnancy and maternal body mass index 2009‐2012. European Journal of Contraception and Reproductive Health Care 2015;20(6):409‐18. [DOI] [PubMed] [Google Scholar]
Merki‐Feld 2015
- Merki‐Feld GS, Skouby S, Serfaty D, Lech M, Bitzer J, Crosignani PG, et al. European society of contraception statement on contraception in obese women. European Journal of Contraception and Reproductive Health Care 2015;20(1):19‐28. [DOI] [PubMed] [Google Scholar]
Morrell 2016
- Morrell KM, Cremers S, Westhoff CL, Davis AR. Relationship between etonogestrel level and BMI in women using the contraceptive implant for more than 1 year. Contraception 2016;93(3):263‐5. [PUBMED: 26577754] [DOI] [PubMed] [Google Scholar]
Ng 2014
- Ng M, Robinson M, Thomson B, Graetz N, Margano C, Mullany EC. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9945):766‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogden 2014
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011‐2012. JAMA 2014;311(8):806‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segall‐Gutierrez 2010
- Segall‐Gutierrez P, Taylor D, Liu X, Stanczyk F, Azen S, Mishell DR. Follicular development and ovulation in extremely obese women receiving depo‐medroxyprogesterone acetate subcutaneously. Contraception 2010;81(6):487‐95. [DOI] [PubMed] [Google Scholar]
Sivin 2001
- Sivin I, Wan L, Ranta S, Alvarez F, Brache V, Mishell DR, et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001;64(1):43‐9. [DOI] [PubMed] [Google Scholar]
Strauss 2005
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005. [Google Scholar]
Trussell 2009
- Trussell J, Schwarz EB, Guthrie K. Obesity and oral contraceptive pill failure. Contraception 2009;79(5):334‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2015
- United Nations, Department of Economic and Social Affairs. World Contraceptive Patterns 2015. www.un.org/en/development/desa/population/publications/ (accessed 23 February 2015).
Vessey 2001
- Vessey M. Oral contraceptive failures and body weight: findings in a large cohort study. Journal of Family Planning and Reproductive Health Care 2001;27(2):90‐1. [DOI] [PubMed] [Google Scholar]
Wells 2014
- GA Wells, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 6 October 2015).
Westhoff 2005
- Westhoff C. Higher body weight does not affect NuvaRing's efficacy (abstract). Obstetrics and Gynecology 2005;105(4 Suppl):56S. [Google Scholar]
Westhoff 2008
- Westhoff C. Subject weight and oral contraceptive efficacy in recent clinical trials (abstract). Contraception 2008;78(2):167. [Google Scholar]
Westhoff 2009
- Westhoff C, Torgal A, Mayeda ER, Lerner J, Paik M. Ovarian suppression during oral contraceptive use in normal‐weight and obese women. Contraception 2009;80(2):210. [DOI] [PubMed] [Google Scholar]
Westhoff 2012b
- Westhoff CL, Torgal AH, Mayeda ER, Petrie K, Thomas T, Dragoman M, et al. Pharmacokinetics and ovarian suppression during use of a contraceptive vaginal ring in normal‐weight and obese women. American Journal of Obstetrics and Gynecology 2012;207(1):39.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westhoff 2012c
- Westhoff CL, Torgal AT, Mayeda ER, Shimoni N, Stanczyk FZ, Pike MC. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception 2012;85(5):465‐9. [DOI] [PubMed] [Google Scholar]
Westhoff 2014
- Westhoff CL, Reinecke I, Bangerter K, Merz M. Impact of body mass index on suppression of follicular development and ovulation using a transdermal patch containing 0.55‐mg ethinyl estradiol/2.1‐mg gestodene: a multicenter, open‐label, uncontrolled study over three treatment cycles. Contraception 2014;90(3):272‐9. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Obesity. http://www.euro.who.int/en/what‐we‐do/health‐topics/noncommunicable‐diseases/obesity (accessed 27 Dec 2012).


