Abstract
The lung epithelium has long been overlooked as a key player in tuberculosis disease. In addition to acting as a direct barrier to Mycobacterium tuberculosis (Mtb), epithelial cells (EC) of the airways and alveoli act as first responders during Mtb infections; they directly sense and respond to Mtb by producing mediators such as cytokines, chemokines and antimicrobials. Interactions of EC with innate and adaptive immune cells further shape the immune response against Mtb. These three essential components, epithelium, immune cells and Mtb, are rarely studied in conjunction, owing in part to difficulties in coculturing them. Recent advances in cell culture technologies offer the opportunity to model the lung microenvironment more closely. Herein, we discuss the interplay between lung EC, immune cells and Mtb and argue that modelling these interactions is of key importance to unravel early events during Mtb infection.
Keywords: tuberculosis, airway epithelium, innate immunity, lymphocyte biology, respiratory infection, bacterial infection
Introduction
While breathing, the entire surface of the lungs is exposed to numerous foreign particles. These include micro-organisms such as bacteria, viruses and fungi. Despite the abundance of microbes that enter the lung, infections are relatively rare. This is the result of a tightly regulated host defence system, comprised of specialised epithelial responses, innate and adaptive immunity and the microbiota1 that synergistically provide protection against infectious agents invading the lung. However, failure or microbial evasion of these defence systems may result in pulmonary infections.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), has been regarded a pandemic by the WHO for decades. Mtb has infected approximately a quarter of the world population and kills over 1.5 million people every year, making it the leading cause of death from a single infectious agent over the last two centuries.2 3 A proportion of individuals exposed to Mtb remains uninfected,4 or possibly clears the infection at an early stage. In those who do acquire the infection, Mtb can persist for decades without causing disease, a condition known as latent TB infection.
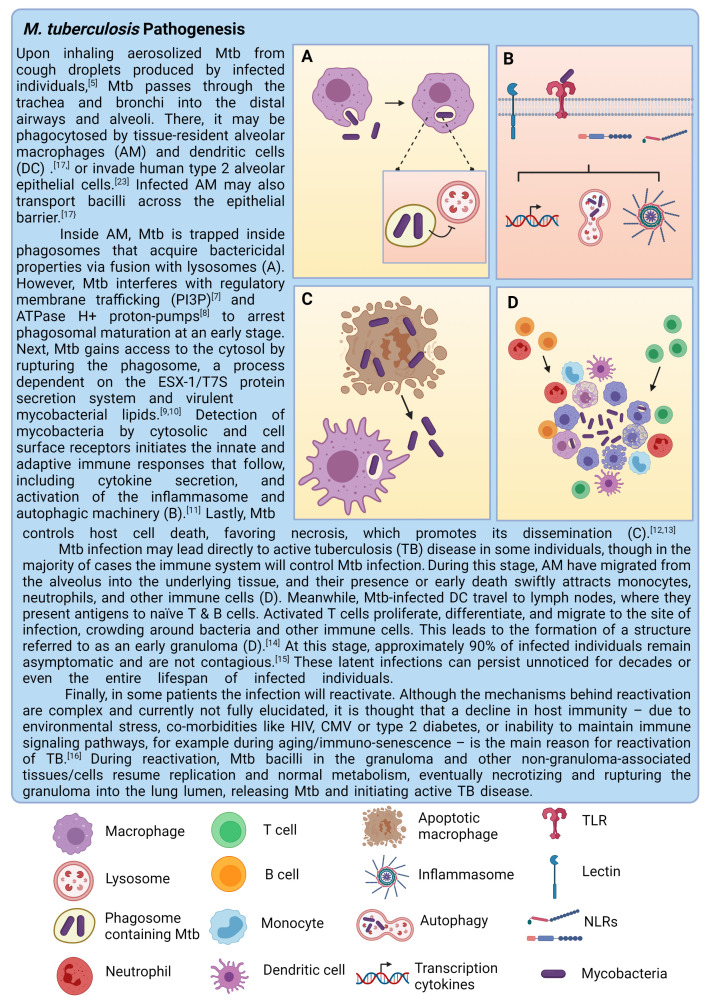
Mtb bacilli hide deep in the lungs, complicating detection.5 Therefore, relatively little is known about the early stages of infection and the key players involved. According to the field’s current understanding, Mtb preferentially targets macrophages, in particular those that reside in the alveoli,6 and employs various strategies to survive intracellularly.7–13 The primary pathology resulting from these infected macrophages is the formation of granulomas, organised structures consisting of various immune cells that contain the infection.14–16 The initial infection and disease progression of TB are summarised in detail in figure 1. This focus on the granuloma disease stage, however, does not yet take into account the role of the lung epithelium, which represents a major part of the pathogens’ microenvironment. Moreover, lung epithelial cells (EC) directly interact with alveolar macrophages (AM) and orchestrate the macrophage-driven immune response.17 Other immune players involved in TB control may also behave differently in the presence of EC. Therefore, local interactions between Mtb, the lung epithelium and the immune system are of particular interest to understand the initiation and subsequent pathophysiology of the disease. Finally, in vitro models used in many studies do not recapitulate the complex microenvironment surrounding Mtb infections. Interactions between epithelium, immune cells and Mtb and the importance of modelling the Mtb infection niche in vitro will be discussed in greater detail in this review.
Figure 1.
Tuberculosis pathology including granuloma formation. This figure was created with BioRender.com.
Key players in respiratory mycobacterial immunity
Epithelial–Mtb interactions
Current findings suggest that the epithelium is less susceptible to infection with Mtb than AM, which may have contributed to the relatively unexplored role of lung epithelium in antimycobacterial immunity. The cellular composition of epithelium varies depending on the region of the lung, and it may therefore respond differently to mycobacterial challenge. Here, we distinguish between airway and alveolar epithelium when discussing lung epithelial contributions to mycobacterial immunity.
The alveolar epithelium consists of large type 1 alveolar cells (AEC1) responsible for gas exchange and cuboidal AEC2 responsible for secretory functions (figure 2), including surfactant production.18 AEC2 also have a progenitor function, as they can self-renew and give rise to AEC1 and other subsets.19 The airway epithelium, on the other hand, is pseudostratified and mostly consists of multiciliated cells and secretory goblet cells and club cells, interspersed with neuroendocrine cells and basal cells (figure 3). More recently, other small cell populations have been discovered: tuft cells and ionocytes. Basal cells act as progenitor cells and are mainly responsible for self-renewal of the airways.20
Figure 2.
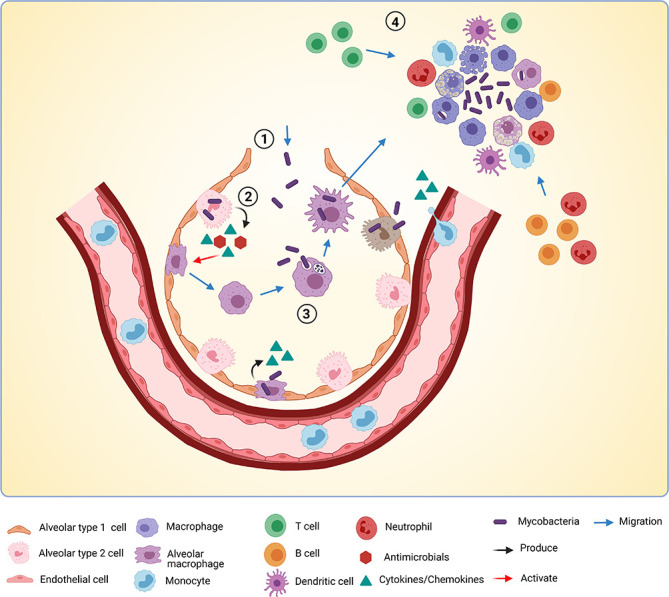
Early Mtb infection events in the alveoli. A model of early Mtb (Mycobacterium tuberculosis) infection events in the alveoli based on the field’s current understanding discussed in this review. Mtb enters the alveolus (1) and is subsequently sensed by alveolar type 2 cells, which produce cytokines, chemokines and antimicrobials (2). Mtb also directly infects alveolar type 2 cells and alveolar macrophages (AM). Cytokines produced by alveolar type 2 cells help activate AM, which migrate towards Mtb bacilli. (3) Mtb is phagocytosed by AM and transported over the alveolar barrier to underlying tissue. (4) Infected AM die and attract more macrophages, monocytes from the bloodstream, neutrophils and adaptive immune players such as T cells, B cells and dendritic cells (DCs). This marks the start of granuloma formation. This figure was created with BioRender.com.
Figure 3.
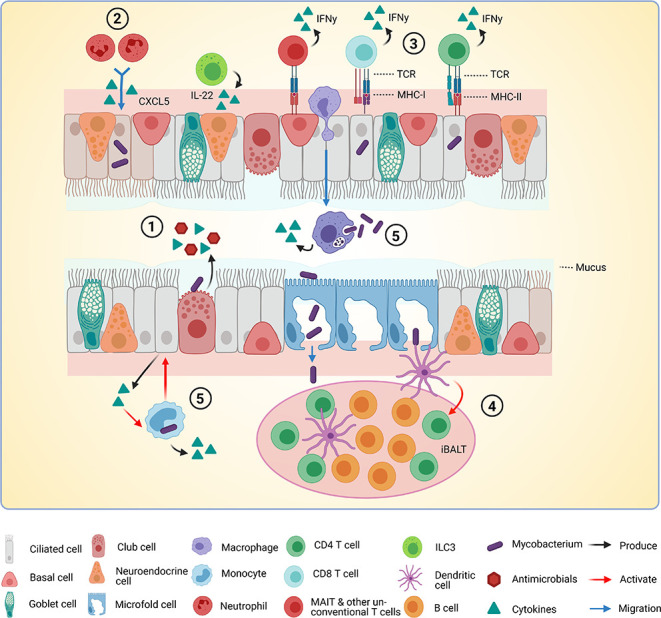
Interactions between Mtb, epithelium and immune cells in the airways. A model of interactions between Mtb (Mycobacterium tuberculosis), epithelial cells (EC) and immune cells in the airways based on findings discussed in this review. (1) Mtb is sensed by EC via pathogen recognition receptors. Cytokines, chemokines and antimicrobials are released into the lumen of the airway. (2) Cytokines released by infected EC attract immune cells. In this case, CXCL5 attracts neutrophils to the site of infection. (3) Infected EC present antigens to T cells via major histocompatibility complex class I (MHC-I) and MHC-II. T cells become activated and produce interferon gamma (IFN-γ). (4) Microfold cells transport Mtb through the epithelial barrier to the underlying inducible bronchus-associated lymphoid tissue (iBALT). Presence of M cells is not yet verified completely in humans. Dendritic cells (DCs) sense Mtb and activate T cells in the BALT. (5) Infected macrophages and monocytes produce cytokines and may activate EC to produce more cytokines in a positive feedback loop. This figure was created with BioRender.com.
Epithelial sensing of Mtb
EC express a large repertoire of pattern recognition receptors (PRRs) to sense micro-organisms. These include the membrane-bound Toll-like receptors (TLRs) and C-type lectins, cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors and nucleic acid sensors (table 1). On contact with pathogens, PRRs are activated and EC secrete an array of pro-inflammatory and anti-inflammatory cytokines as well as chemokines which attract different immune cell subsets (table 1). Interestingly, CD4+ T cell-associated type 1 helper (Th1) and Th2 cytokines were also shown to regulate expression of different TLRs in primary airway EC (PBEC), illustrating the complex cytokine milieu required to coordinate appropriate responses to pathogens.21 In addition, antimicrobial peptides (AMPs) with direct effects on pathogens are secreted by airway and alveolar epithelium (table 1). For example, β-defensins are AMPs with broad-spectrum activity against bacteria, fungi and certain viruses and are produced by airway and alveolar EC in response to mycobacterial infections. Human β-defensin-2 (hBD2) controls Mtb growth22 and has chemotactic activity for memory lymphocytes and immature dendritic cells (DCs).23 LL-37, a cathelicidin AMP, is also produced by PBEC, AM and the AEC2-like cell line A549 (an overview of epithelial and immune cell lines that are used in culture models is provided in online supplemental table S1) in response to mycobacterial infection.24 25 LL-37 and β-defensin-2 (hBD2) expression can be upregulated by vitamin D, and physiological vitamin D levels contributed to restriction of Mtb or Mtb/HIV replication in macrophages.26 Interestingly, vitamin D-induced interleukin-1 (IL)-1β secretion by macrophages stimulated antimycobacterial capacity of primary small airway EC in a coculture model and reduced bacterial burden.27 These effects were dependent on epithelial production of hBD2 and IL-1 receptor type 1 signalling. On the other hand, IL-1-mediated crosstalk between EC and Mtb-infected AM was shown to promote dissemination of infected cells.28 Other products secreted by EC might also have indirect effects on mycobacteria. For example, various hydrolases secreted in the alveolar surfactant were shown to modify the Mtb cell wall.29 This resulted in a significant decrease in Mtb association with and infection of macrophages.
Table 1.
Overview of pattern recognition receptors (PRRs), antimicrobial peptides (AMPs) and proteins and cytokines and chemokines produced by lung epithelial cells and their modulation by mycobacterial exposure
| Airway epithelium | Alveolar epithelium | ||||
| Primary | Cell line | Primary | Cell line | ||
| PRRs | TLRs | TLR121 48 90 | TLR148 90 | TLR291 | TLR291 |
| TLR221 48 90 | TLR248 90 | TLR392 | TLR392 | ||
| TLR321 48 90 | TLR348 90 | TLR491 | TLR491 | ||
| TLR421 48 90 | TLR448 90 93 | ||||
| TLR521 48 90 | TLR548 90 93 | ||||
| TLR621 48 90 | TLR648 90 | ||||
| TLR790 | TLR748 | ||||
| TLR890 | TLR990 | ||||
| TLR948 90 | TLR1090 | ||||
| TLR1090 | |||||
| TLR coreceptors | CD1490 | CD1491 | |||
| MD291 | |||||
| NLRs | NOD148 | NOD193 | NOD194 | NOD194 | |
| NLRP195 | NOD293 | ||||
| NLRP396 | |||||
| Lectins | Dectin-197 | Dectin-197 | |||
| MINCLE98 | L-SIGN99 | ||||
| MINCLE98 | |||||
| Nucleic acid sensors | RIG1100 | RIG1101 | RIG192 | RIG192 | |
| MDA5101 | MDA5102 | ||||
| LGP2101 | |||||
| Other | SDC440 | ||||
| AMPs and proteins | Defensins | hBD180 | hBD2103 | ||
| hBD280 | |||||
| Cathelicidins | LL-3780 | LL-3725 | |||
| Other | Lactoferrin80 | Hepcidin104 | |||
| Hepcidin80 | |||||
| Cytokines | Pro-inflammatory | IL-621 44 48 | IL-848 90 93 105 | IL-18106 | IL-6107 |
| IL-821 48 80 | IL-32108 | IL-891 105 107 | |||
| IL-32108 | IL-17107 | ||||
| IL-32108 | |||||
| IFN-γ107 | |||||
| TNF-α107 | |||||
| Anti-inflammatory | IL-1044 | IL-10105 | IL-1RA107 | ||
| IL-2244 | IL-4107 | ||||
| IL-9107 | |||||
| IL-10105 | |||||
| IL-13107 | |||||
| Chemotactic | CCL2021 | CCL291 107 | |||
| CXCL1021 | CCL3107 | ||||
| GM-CSF21 80 | CCL4107 | ||||
| CCL5107 | |||||
| CXCL1092 107 | |||||
| GM-CSF107 | |||||
| G-CSF107 | |||||
PRRs expressed by human lung epithelial cells (blue). AMPs and cytokines expressed or produced by human lung epithelium on mycobacterial stimulation (green).
GM-CSF, Granulocyte-Macrophage Colony Stimulating Factor; IFN-γ, interferon gamma; IL, interleukin; NK, Natural Killer; NLRs, NOD-like receptors; SDC4, syndecan 4; TLR, Toll-like receptor; TNF-α, Tumor Necrosis Factor - Alpha.
thoraxjnl-2021-217997supp001.pdf (86.5KB, pdf)
Different epithelial cell types employ various defence strategies that contribute to clearing infections. Secretory goblet cells produce mucus which is mechanically transported up the airways by ciliary motion of multiciliated cells,30 resulting in physical removal of the pathogen. AEC2 secrete surfactant containing the aforementioned antimicrobial mediators and reactive oxygen species, to keep pathogens, including Mtb,31 at bay. EC can therefore be considered sentinels, initiators and modulators of immunity, thus contributing directly to infection-driven pathogenesis, including the regulation of innate and adaptive immune responses.
Epithelial infection by Mtb
Besides sensing pathogens and acting as an early warning and defence system, EC of the lung can also be directly infected by mycobacteria. PBEC seemed relatively insensitive to infection with Mtb32 in vitro, though Mycobacterium avium and Mycobacterium abscessus achieved higher infection rates when used in supraphysiological quantities.33
In contrast to the bronchial EC described above, alveolar EC might be more susceptible to Mtb infection. Early reports described successful infection of the cell line A549 by Mtb and the attenuated vaccine strain Mycobacterium bovis Bacille Calmette-Guerin (BCG).34 35 Moreover, both species were able to migrate across a bilayer model of A549 and endothelial cells, in the absence of macrophages.36–38 During migration of Mtb, bacteria were observed intracellularly.37 Cell death and a decreased capacity to maintain cellular tight junctions was indicated by a drop in transmembrane electrical resistance (TEER) and increased passage of dextran through the cell layer.36 37 Therefore, it appears that Mtb kills infected cells to gain passage through the alveolar barrier. Another study using primary rat alveolar cell layers found a marked decrease in TEER on Mtb infection, which appeared to result from excessive tumor necrosis factor - alpha (TNF-α) production by the epithelium.39 Furthermore, dissemination-attenuated strains with decreased migratory capacities demonstrated a role for Mtb virulence factor ESAT-6 in the direct crossing of the alveolar barrier.38 These studies only included A549 cells and validation in primary alveolar EC will be important.
EC are non-professional phagocytes, although mechanisms of mycobacterial adherence and internalisation by EC have been described. The proteoglycan syndecan 4 (SDC4) was identified as an attachment receptor for BCG on alveolar A549 cells, and this interaction involved host SDC and mycobacterial heparin-binding haemagglutinin adhesin.40 Furthermore, SDC4-knockout mice were more resistant to Mtb H37Rv colonisation.40 Other adhesins expressed by Mtb that directly bind EC are malate synthase and ESAT-6, which may also facilitate dissemination of mycobacteria by epithelial barrier disruption via cell lysis.38
Inside EC, Mtb is initially processed via the phagocytic pathway, where it is localised in late endosomal compartments in alveolar and airway epithelium.41 42 However, fusion of Mtb-containing compartments with lysosomes was blocked in EC, as is the case in macrophages. In A549 cells, Mtb was found inside vesicles labelled with the autophagy marker LC3, indicating that Mtb is processed via the autophagy pathway.41
Epithelial–immune cell interaction during Mtb infection
Innate immune cell activation by Mtb-exposed epithelium
Sometimes the direct response mounted by the lung epithelium is not sufficient to eradicate invading pathogens. EC also secrete inflammatory mediators that may affect the type and magnitude of the immune response that follows. Culture of the monocyte cell line U937 in presence of Mtb-infected A549 cells resulted in reduced Mtb-triggered TLR signalling in the monocytes, compared with infected monocytes alone.43 This suggests that EC influence the inflammatory response of immune cells. Additionally, submerged PBEC cultures produced anti-inflammatory cytokines IL-10 and IL-22 in response to BCG infection.44 This suggests that airway and alveolar EC act as moderators of inflammation during mycobacterial infection. In line with these observations, some studies found increased levels of IL-10 in sputum of patients with TB. This correlated with higher Mtb antigen burden,45 suggesting that IL-10 could undermine effective clearance of Mtb. This effect is likely caused by the negative influence of IL-10 on Th1 T cells and macrophages,46 which play a pivotal role in protective immunity against Mtb.
Neutrophils are attracted more strongly to PBEC infected with Pseudomonas aeruginosa (common in cystic fibrosis patients) than with a non-pathogenic Escherichia coli strain.47 In this manner, EC filter pathogenic signals, levelling subsequent immune activation by moderating PRR expression.48 A tightly regulated balance is important as EC recruiting too many leukocytes risk excessive inflammation and lung injury as a result. Neutrophils, for example, are considered protective during early Mtb infections, but pathogenic at later stages where they are associated with poor prognosis. CXCL5 is a chemokine produced by EC and important in the initial recruitment of neutrophils. Cxcl5 −/− mice exhibited enhanced survival after Mtb infection compared with wild-type mice. This resistance to Mtb infection was due to impaired neutrophil recruitment, which reduced pulmonary inflammation. This suggests that excessive epithelial-derived CXCL5 is critical for leukocyte-driven destructive inflammation in TB.49
In turn, innate immune cells influence the rate of infection in EC. Mtb and BCG first cultured intracellularly in murine macrophages had a higher association with A549 cells and infected a higher percentage of them,35 suggesting enhancement of mycobacterial virulence after exposure to the intracellular environment of macrophages. By contrast, Mtb and M. avium growth in the macrophage cell line Mono-Mac-6 was reduced by A549 cell-derived soluble factors including TNF-α and granulocyte-macrophage colony stimulating factor (GM-CSF).50 Lastly, in a contact-independent model, PBEC were activated by Mtb-infected monocytes to produce antimicrobials and cytokines that were not inducible by direct infection of the bronchial cells themselves,32 highlighting the crosstalk between epithelial and immune cells.
Antigen presentation and T cell activation by the epithelium
Antigen presentation to T cells is required for activation of adaptive immune responses against Mtb. While this role is often fulfilled by DC, there is evidence for antigen presentation to T cells by EC. Several studies have identified expression of major histocompatibility complex class II (MHC-II) molecules on human bronchial (figure 3) and alveolar EC, which would imply direct activation of CD4+ T cells by the epithelium.51–53 Human nasal and bronchial EC were able to stimulate allogenic T cells, an effect abrogated by addition of anti-MHC-II antibodies.53 Functional MHC-II has also been identified on pulmonary EC of other species. Rats constitutively expressed MHC-II on AEC2, and to a lower degree on bronchial epithelium, suggesting cell type-specific regulation of expression.54 Murine AEC2 also expressed MHC-II and were able to present antigens to primed CD4+ T cells when infected with Mtb.55 Furthermore, expression of costimulatory molecules has been demonstrated in human epithelium and appears to be cell type specific. CD86 was found on both PBEC and A549 cells.52 56 CD80, on the other hand, was only found on alveolar EC.56
CD8+ T cells play an additional role in protection against intracellular pathogens such as Mtb. In a study by Harriff et al, interferon gamma (IFN-γ) production by CD8+ T cells was stimulated by bronchial epithelium following Mtb infection.42 These authors also showed that the bronchial epithelial basal cell line BEAS-2B activated several Mtb-specific, classically (HLA-B45) and non-classically (HLA-E, MR-1) restricted T cell clones to a higher degree than DCs after infection with Mtb, as measured by IFN-γ producing T cells. This is perhaps a surprising observation, since bronchial epithelium was infected with lower efficiency compared with DCs.
Further studies highlighting the importance of EC in shaping T cell responses studied BCG vaccination in mouse and rhesus macaque models. In these studies, mucosal vaccination-induced superior protection against Mtb compared with intradermal vaccination.57 Perdomo et al described the recruitment of significantly higher frequencies and absolute numbers of effector memory T cells (TEM) and tissue-resident memory T cells (TRM) after mucosal vaccination compared with subcutaneous vaccination.58 Particularly the TRM subset has been suggested to confer protection against infections, including Mtb.59 TRM cells are hardly detectable in the circulation, but abundantly present in tissues. Comparison of different administration routes showed substantially more antigen-responsive TRM cells in all lung parenchymal tissues after intravenous BCG immunisation.60 Furthermore, approximately 75% of all CD4+ and CD8+ T cells were TRM following direct endobronchial instillation of BCG.
Other types of adaptive immune cells may participate in anti-Mtb immunity too. Mucosal-associated invariant T (MAIT) cells were found enriched in the airways,61 and Mtb-infected airway EC were capable of inducing IFN-γ production from MAIT cells.42 Furthermore, BCG vaccination induced transient expansion of the peripheral MAIT cell population in humans and specifically activated MAIT cells in non-human primates.62 63 Few studies to date looked at interactions between lung epithelium, regulatory T cells and innate lymphoid cells (ILCs). In Mtb-infected mice, IL-22-producing ILC3s prolonged survival and prevented epithelial cell damage.64 Interestingly, EC may also regulate IL-17-producing T helper cells (Th17) via a complex feedback loop. The enzyme indoleamine-2,3-dioxygenase (IDO) is expressed by lung EC. Following IFN-γ release by T cells, downstream IDO products secreted by EC were found to selectively inhibit IL-17 production by Th17 cells.65
Microfold cells (M cells) transport Mtb to bronchus-associated lymphoid tissue (BALT)
M cells are specialised EC present in areas of the airways where inducible BALT (iBALT) exists or can be induced.66 These specialised M cells transport macromolecules and microbes from the airway into the subepithelial region, a process called antigen transcytosis. Subsequently, these antigens induce maturation of immature DCs, which in turn activate naive T cells residing in the iBALT. In mice, M cells enabled transport of particles and microbes, including Mtb, across the epithelial barrier.67 Scavenger receptor B1 on M cells was reported to bind the major Mtb virulence factor EsxA, facilitating Mtb adherence to and translocation through the epithelial layer in vitro.68 Furthermore, the TB granuloma is often surrounded by clusters of cells that are remarkably similar in structure to iBALT and perform tertiary lymphoid functions.66 iBALT is not present in healthy adults and is formed following a period of inflammation in the airways. However, iBALT induced by a previous unrelated infection or allergic reaction, smoking or chronic inflammation (asthma) may persist for weeks or longer.66 69 Even so, it is unlikely that M cells and iBALT are present in healthy individuals when Mtb first enters their lungs, so the importance of M cells in initial Mtb infection biology is debatable. Although M cells have been induced in human bronchial epithelial cell lines68 and their presence was demonstrated in multiple animal models, it has not been verified whether M cells or comparable cells do exist in human lungs. Taking these considerations into account, M cells could potentially function as gateways for Mtb (figure 3), though their relevance to human Mtb infection biology requires further investigation.
The findings discussed so far support the view of a tightly regulated interplay between the epithelium, immune cells and Mtb during different stages of infection. The next section of this review shall focus on possibilities to integrate these three key players in models for in vitro studies.
Importance of complex microenvironment representation for mechanistic studies
Studying early phases in Mtb infection in humans in vivo is complicated; the lung is not readily accessible and patients are often diagnosed at later stages of infection. Though animal models have contributed valuable scientific insights in the past, most are not very representative of human lung physiology. For example, epithelial cell composition varies between similar regions of human and mouse lungs as well as the distribution of cartilage.70 A caveat for the zebrafish model is the fact that it does not possess lungs. In vitro models provide an alternative to study Mtb infections more closely. However, these models often lack sufficient complexity to represent the microenvironment of the lung. For example, immortalised or tumour cell lines of lung epithelia generally consist of one cell type and are derived from one donor; depending on culture conditions they are poorly or not at all differentiated. In addition, most culture systems lack crosstalk with other players. Recent developments in cell culture technology have resulted in a larger array of in vitro models with higher levels of complexity to mimic the desired microenvironment. Though studies using such models in combination with Mtb are currently scarce, we argue that they are of great importance in further unravelling early TB infection phases.
Air–liquid interface (ALI) cultures of (primary) lung EC
ALI cultures consist of an epithelial cell layer grown on a permeable membrane. Cells are exposed to air on their apical side, while nutrients are supplied from the basal side, promoting polarisation in all epithelial cultures and differentiation in airway EC cultures. This model allows EC to be cultured in a way that resembles their physiological environment more closely than submerged cultures.71
Infectious agents and immune cell subsets can be added to the air-exposed (apical) side or to the medium (basal side) of the ALI model. Several studies have used Mtb and BCG to infect bronchial ALI cultures, but encountered some issues. The epithelium was resistant to infection in models using primary material32 and cell lines.72 In one study, macrophages were integrated into the epithelial layer before infection, and the success of infecting the tissue seemed macrophage dependent. However, integration of macrophages also resulted in uncontrolled mycobacterial growth.61 Seeding Mtb-infected macrophages together with uninfected monocytes before addition of the bronchial epithelial cell line 16HBE on top resulted in clustering of monocytes around infected macrophages.73 This might indicate early-stage granuloma formation. Alternatively, Mtb-infected macrophages may be introduced to the basal compartment of Transwell systems.43 While such methods do illustrate the multifunctionality of the ALI model, they bypass the epithelium as a first point of host–pathogen contact. Airway ALI models have been successfully infected with various bacteria, fungi and viruses, suggesting that bronchial EC are less susceptible to Mtb infection in comparison to other pathogens.
Alveolar ALI models may be more hospitable hosts to mycobacteria. Most studies use A549 cells to study Mtb infection rates and migration through the alveolar barrier. It should be noted that A549 cells can be cultured at ALI for a short period only, due to leakage through the cell layer during longer air exposure due to their limited ability to form a barrier. A549 cells are sometimes cultured as a bilayer with endothelial cells.38 Within 3 days, approximately 1% of Mtb inoculum migrated across such a bilayer model, while dissemination-attenuated strains such as BCG achieved less than 0.5% migration.38 This supports the view that macrophages or monocytes are not required to transport the bacteria across the alveolar barrier.
Organoids
Organoid cultures are three-dimensional (3D) models that recapitulate certain organ environments. They are self-organising and self-renewing structures generated from adult stem/progenitor cells isolated directly from a target organ or from embryonic or human induced pluripotent stem cells (hiPSC).74 Airway EC containing basal cells or AEC2 are isolated from the airways or alveoli, respectively, to generate organoids.75 76 They are grown in extracellular matrix gels to provide support for 3D growth.
Organoid models of the lungs are disadvantaged in the sense that no ALI can be established as the apical side of the cells is generally facing toward the lumen of the organoid which is filled with liquid and mucus. Apical pathogen exposure can therefore be challenging. To overcome this issue, pathogens can be injected into the lumen of organoids with a microinjector.77 Although this process is labor intensive, it creates a representative infection model by exposing cells’ apical side to pathogens. Immune cells can be added to microinjected organoid cultures, creating a coculture where immune cells and bacteria are separated by the epithelial barrier. However, since organoids are cultured in matrix gels, immune cells may have difficulty accessing the infected organoids. Finally, organoids can be sheared into pieces before pathogen addition, exposing both apical and basal sides of the cells,78 or the polarity of organoids can be reversed to have the apical side facing outwards.79
While many studies successfully used lung organoids to model SARS-CoV-2,76 79 and other viral infections, organoids have not been used extensively for host–pathogen studies with mycobacteria. Primary bronchial epithelial organoids microinjected with Mtb and cocultured with macrophages showed that Mtb remained viable inside the airway organoid lumen up to 21 days after injection, with approximately 2% of bacteria associating with EC.80 Additionally, within 3 days, monocyte-derived macrophages migrated towards the infected organoids, though they did not traverse into the lumen of the organoids. Other pathogens and leucocyte subsets have been used in different combinations cocultured with organoids of different organs including the intestines, gallbladder and brain.81 Collectively, these studies show that the organoid is an emerging in vitro model for host–pathogen interactions and merits further investigation regarding Mtb infections.
Organs-on-chips
Organs-on-chips aim to recreate in vivo-like tissue microenvironments having multiple compartments, including dynamically controlled flow of nutrients in the vascular compartment or stretching to mimic breathing-like motions of the lung.82 In addition, organs-on-chips require a smaller culture volume and fewer cells, while allowing precise control of microenvironmental parameters. A variety of lung-on-chip models have been developed over the past years in which EC are used in combination with vascular cells, immune cells derived from primary sources and/or hiPSC cell lines. Despite the rather novel and developing technology, cocultures with Mtb and other pathogens have already been reported. A recent study using a lung-on-chip model of murine alveoli demonstrated that alveolar surfactant played an essential role in controlling mycobacterial growth.31 A different study used a novel variant of the chip technology to model the immune compartment of granulomas, in combination with endothelial cells to model granuloma-associated angiogenesis.83 EC were not incorporated in this model, though further development could include the addition of EC and fibroblasts. Another in vitro granuloma model which was used to study anti-TB drug efficacy was comprised of primary human monocytes and T cells. These cells formed granulomas in a 3D matrix,84 85 but no EC were included in the model.
Tissue explants
Human lung tissue explants can be used to study Mtb infection, as they retain a high degree of tissue relevance. Tissue explants contain all relevant cell types, including myeloid cells and lymphocytes, as well as fibroblasts, endothelial cells and others. Precision cut lung slices (PCLS) are prepared by cutting thin sections of agarose-perfused resected tissue. They maintain the 3D architecture of the lung, and when embedded in specialised hydrogels can be kept in culture for at least 21 days.86 In PCLS cultures, Mtb and BCG localised near alveolar septa and were in proximity to AEC2.87 No evidence was found of infection of AEC2, though intracellular mycobacteria were observed in AM in this model.
Larger tissue explants have also been used for mycobacterial infection studies. In human tissue explants, alveolar EC are readily infected by Mtb. Cytokine expression was analysed in the alveolar epithelium and an array of leucocyte subsets present in the tissue, including AM, natural killer (NK) cells, γ/δ T cells, MAIT cells and ILCs.88 Another study confirmed the localisation of Mtb in alveolar EC and found approximately 3.5% of the infected cells were AEC2, while over 74% of infected cells were macrophages.89
While tissue explants are arguably the most representative model of the lung and the insights gleaned from tissue explants are valuable, the model does not lend itself to long-term culturing and longer infection times that might be necessary to study certain aspects of mycobacterial infections.
Future perspectives
Historically, Mtb is humankind’s deadliest pathogen and is likely to remain a serious threat until a vaccine with high efficacy is developed; currently, the BCG vaccine provides around 50% protection on average. Difficulties in TB vaccine development might stem from a lack of knowledge about early interactions of the pathogen with the mucosal epithelia and immune players in the lung. Herein, we have attempted to provide a comprehensive overview of current research into the roles of airway and alveolar epithelium during TB infections. Far from a simple physical barrier, the epithelium in the lung appears central to directing host responses: from pathogen detection and direct killing via AMPs to the recruitment of various immune cells and antigen presentation to lymphocytes. This close interaction with the immune system might shape disease outcome in TB infections and warrants further investigation.
The covert nature of (latent) Mtb infections presents a challenge to researchers modelling early disease stages in vitro. Increasingly advanced cell culture systems provide promising platforms for host–Mtb research and also offer the possibility of adding other cell types or pathogens of interest in numerous combinations. The models discussed here are useful for studying early infection events and interactions between Mtb and the human host. In the future, they could be expanded to model later stages of TB disease as well. It will be of particular interest to see if granulomas can be modelled accurately, with the inclusion of EC.
In conclusion, more in-depth research regarding the role of lung epithelia during initial stages of Mtb infections is required to elucidate the complex mechanisms behind TB disease outcome. We anticipate that future studies integrating the interplay between the epithelium, immune cells and Mtb will become a new research priority in TB research.
Footnotes
SAJ and AMvdD contributed equally.
Contributors: SAJ and AMvdD contributed equally to this work.
Funding: This work was supported by a grant from the Leiden-Edinburgh joint PhD programme for Integrated One Health Solutions, awarded by the universities of Leiden and Edinburgh in December 2019, in a joint project with Professor David Dockrell and Professor Anura Rambukkana whose helpful discussions are gratefully acknowledged.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1. Khan R, Petersen FC, Shekhar S. Commensal bacteria: an emerging player in defense against respiratory pathogens. Front Immunol 2019;10:1–9. 10.3389/fimmu.2019.01203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paulson T. Epidemiology: a mortal foe. Nature 2013;502:S2–3. 10.1038/502S2a [DOI] [PubMed] [Google Scholar]
- 3. Licence: CC BY-NC-SA 3.0 IGO . Global tuberculosis report 2020. Geneva: World Health Organization, 2020. [Google Scholar]
- 4. Sloot R, Schim van der Loeff MF, Kouw PM, et al. Risk of tuberculosis after recent exposure. A 10-year follow-up study of contacts in Amsterdam. Am J Respir Crit Care Med 2014;190:1044–52. 10.1164/rccm.201406-1159OC [DOI] [PubMed] [Google Scholar]
- 5. Nardell EA. Transmission and institutional infection control of tuberculosis. Cold Spring Harb Perspect Med 2016;6:a018192. 10.1101/cshperspect.a018192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol 2013;35:563–83. 10.1007/s00281-013-0388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vergne I, Chua J, Lee H-H, et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2005;102:4033–8. 10.1073/pnas.0409716102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong D, Bach H, Sun J, et al. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc Natl Acad Sci U S A 2011;108:19371–6. 10.1073/pnas.1109201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Augenstreich J, Arbues A, Simeone R, et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol 2017;19:e12726. 10.1111/cmi.12726 [DOI] [PubMed] [Google Scholar]
- 10. Gröschel MI, Sayes F, Simeone R, et al. Esx secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol 2016;14:677–91. 10.1038/nrmicro.2016.131 [DOI] [PubMed] [Google Scholar]
- 11. Watson RO, Bell SL, MacDuff DA, et al. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 2015;17:811–9. 10.1016/j.chom.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ottenhoff THM. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol 2012;20:419–28. 10.1016/j.tim.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 13. Srinivasan L, Ahlbrand S, Briken V. Interaction of Mycobacterium tuberculosis with host cell death pathways. Cold Spring Harb Perspect Med 2014;4:a022459. 10.1101/cshperspect.a022459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pagán AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med 2015;5:a018499. 10.1101/cshperspect.a018499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gideon HP, Flynn JL. Latent tuberculosis: what the host “sees”? Immunol Res 2011;50:202–12. 10.1007/s12026-011-8229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veatch AV, Kaushal D. Opening Pandora’s Box: Mechanisms of Mycobacterium tuberculosis Resuscitation. Trends Microbiol 2018;26:145–57. 10.1016/j.tim.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhattacharya J, Westphalen K. Macrophage-epithelial interactions in pulmonary alveoli. Semin Immunopathol 2016;38:461–9. 10.1007/s00281-016-0569-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pai S, Muruganandah V, Kupz A. What lies beneath the airway mucosal barrier? throwing the spotlight on antigen-presenting cell function in the lower respiratory tract. Clin Transl Immunology 2020;9:1–13. 10.1002/cti2.1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi J, Park J-E, Tsagkogeorga G, et al. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 2020;27:366–82. 10.1016/j.stem.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol 2021;14:978–90. 10.1038/s41385-020-00370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ritter M, Mennerich D, Weith A, et al. Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response. J Inflamm 2005;2:1–15. 10.1186/1476-9255-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kisich KO, Heifets L, Higgins M, et al. Antimycobacterial agent based on mRNA encoding human beta-defensin 2 enables primary macrophages to restrict growth of Mycobacterium tuberculosis. Infect Immun 2001;69:2692–9. 10.1128/IAI.69.4.2692-2699.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang D, Chertov O, Bykovskaia SN, et al. Beta-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999;286:525–8. 10.1126/science.286.5439.525 [DOI] [PubMed] [Google Scholar]
- 24. Méndez-Samperio P, Miranda E, Trejo A. Expression and Secretion of Cathelicidin LL-37 in Human Epithelial Cells after Infection by Mycobacterium bovis Bacillus Calmette-Guérin. Clin Vaccine Immunol 2008;15:1450–5. 10.1128/CVI.00178-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rivas-Santiago B, Hernandez-Pando R, Carranza C, et al. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun 2008;76:935–41. 10.1128/IAI.01218-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung C, Silwal P, Kim I, et al. Vitamin D-cathelicidin axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw 2020;20:1–26. 10.4110/in.2020.20.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verway M, Bouttier M, Wang T-T, et al. Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog 2013;9:e1003407. 10.1371/journal.ppat.1003407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen SB, Gern BH, Delahaye JL, et al. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 2018;24:439–46. 10.1016/j.chom.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arcos J, Sasindran SJ, Fujiwara N, et al. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol 2011;187:372–81. 10.4049/jimmunol.1100823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 2017;9:a028241–18. 10.1101/cshperspect.a028241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thacker VV, Dhar N, Sharma K, et al. A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. Elife 2020;9:1–22. 10.7554/eLife.59961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reuschl A-K, Edwards MR, Parker R, et al. Innate activation of human primary epithelial cells broadens the host response to Mycobacterium tuberculosis in the airways. PLoS Pathog 2017;13:e1006577–26. 10.1371/journal.ppat.1006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuyama M, Martins AJ, Shallom S, et al. Transcriptional response of respiratory epithelium to nontuberculous mycobacteria. Am J Respir Cell Mol Biol 2018;58:241–52. 10.1165/rcmb.2017-0218OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun 1996;64:1400–6. 10.1128/iai.64.4.1400-1406.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDonough KA, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun 1995;63:4802–11. 10.1128/iai.63.12.4802-4811.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bermudez LE, Sangari FJ, Kolonoski P, et al. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect Immun 2002;70:140–6. 10.1128/IAI.70.1.140-146.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Birkness KA, Deslauriers M, Bartlett JH, et al. An In Vitro Tissue Culture Bilayer Model To Examine Early Events in Mycobacterium tuberculosis Infection. Infect Immun 1999;67:653–8. 10.1128/IAI.67.2.653-658.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryndak MB, Chandra D, Laal S. Understanding dissemination of Mycobacterium tuberculosis from the lungs during primary infection. J Med Microbiol 2016;65:362–9. 10.1099/jmm.0.000238 [DOI] [PubMed] [Google Scholar]
- 39. Zhang M, Kim KJ, Iyer D, et al. Effects of Mycobacterium tuberculosis on the bioelectric properties of the alveolar epithelium. Infect Immun 1997;65:692–8. 10.1128/iai.65.2.692-698.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zimmermann N, Saiga H, Houthuys E, et al. Syndecans promote mycobacterial internalization by lung epithelial cells. Cell Microbiol 2016;18:1846–56. 10.1111/cmi.12627 [DOI] [PubMed] [Google Scholar]
- 41. Fine KL, Metcalfe MG, White E, et al. Involvement of the autophagy pathway in trafficking of Mycobacterium tuberculosis bacilli through cultured human type II epithelial cells. Cell Microbiol 2012;14:1402–14. 10.1111/j.1462-5822.2012.01804.x [DOI] [PubMed] [Google Scholar]
- 42. Harriff MJ, Cansler ME, Toren KG, et al. Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8+ T cells. PLoS One 2014;9:e97515–12. 10.1371/journal.pone.0097515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Sun Y, Xu J, et al. Epithelial Cells Attenuate Toll-Like Receptor-Mediated Inflammatory Responses in Monocyte-Derived Macrophage-Like Cells to Mycobacterium tuberculosis by Modulating the PI3K/Akt/mTOR Signaling Pathway. Mediators Inflamm 2018;2018:1–19. 10.1155/2018/3685948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lutay N, Håkansson G, Alaridah N, et al. Mycobacteria bypass mucosal NF-kB signalling to induce an epithelial anti-inflammatory IL-22 and IL-10 response. PLoS One 2014;9:e86466–11. 10.1371/journal.pone.0086466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huard RC, Chitale S, Leung M, et al. The Mycobacterium tuberculosis complex-restricted gene CFP32 encodes an expressed protein that is detectable in tuberculosis patients and is positively correlated with pulmonary interleukin-10. Infect Immun 2003;71:6871–83. 10.1128/IAI.71.12.6871-6883.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murray PJ, Wang L, Onufryk C, et al. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol 1997;158:315–21. [PubMed] [Google Scholar]
- 47. Yonker LM, Mou H, Chu KK, et al. Development of a primary human co-culture model of inflamed airway mucosa. Sci Rep 2017;7:1–12. 10.1038/s41598-017-08567-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayer AK, Muehmer M, Mages J, et al. Differential recognition of TLR-Dependent microbial ligands in human bronchial epithelial cells. J Immunol 2007;178:3134–42. 10.4049/jimmunol.178.5.3134 [DOI] [PubMed] [Google Scholar]
- 49. Nouailles G, Dorhoi A, Koch M, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest 2014;124:1268–82. 10.1172/JCI72030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sato K, Tomioka H, Shimizu T, et al. Type II alveolar cells play roles in macrophage-mediated host innate resistance to pulmonary mycobacterial infections by producing proinflammatory cytokines. J Infect Dis 2002;185:1139–47. 10.1086/340040 [DOI] [PubMed] [Google Scholar]
- 51. Scordo JM, Knoell DL, Torrelles JB. Alveolar epithelial cells in Mycobacterium tuberculosis infection: active players or innocent Bystanders? J Innate Immun 2016;8:3–14. 10.1159/000439275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wosen JE, Mukhopadhyay D, Macaubas C, et al. Epithelial MHC class II expression and its role in antigen presentation in the gastrointestinal and respiratory tracts. Front Immunol 2018;9:1–14. 10.3389/fimmu.2018.02144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kalb TH, Chuang MT, Marom Z, et al. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol 1991;4:320–9. 10.1165/ajrcmb/4.4.320 [DOI] [PubMed] [Google Scholar]
- 54. Steiniger B, Sickel E, Molecules CIIMHC. Class II MHC molecules and monocytes/macrophages in the respiratory system of conventional, germ-free and interferon-gamma-treated rats. Immunobiology 1992;184:295–310. 10.1016/S0171-2985(11)80588-7 [DOI] [PubMed] [Google Scholar]
- 55. Debbabi H, Ghosh S, Kamath AB, et al. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4 + T cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L274–9. 10.1152/ajplung.00004.2005 [DOI] [PubMed] [Google Scholar]
- 56. Papi A, Stanciu LA, Papadopoulos NG, et al. Rhinovirus infection induces major histocompatibility complex class I and costimulatory molecule upregulation on respiratory epithelial cells. J Infect Dis 2000;181:1780–4. 10.1086/315463 [DOI] [PubMed] [Google Scholar]
- 57. Dijkman K, Sombroek CC, Vervenne RAW, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med 2019;25:255–62. 10.1038/s41591-018-0319-9 [DOI] [PubMed] [Google Scholar]
- 58. Perdomo C, Zedler U, Kühl AA, et al. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. mBio 2016;7:1–11. 10.1128/mBio.01686-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ogongo P, Porterfield JZ, Leslie A. Lung tissue resident memory T-cells in the immune response to Mycobacterium tuberculosis. Front Immunol 2019;10:1–11. 10.3389/fimmu.2019.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Darrah PA, Zeppa JJ, Maiello P, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020;577:95–102. 10.1038/s41586-019-1817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 2010;8:e1000407–14. 10.1371/journal.pbio.1000407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Greene JM, Dash P, Roy S, et al. MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol 2017;10:802–13. 10.1038/mi.2016.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Suliman S, Murphy M, Musvosvi M, et al. MR1-Independent activation of human mucosal-associated invariant T cells by mycobacteria. J Immunol 2019;203:2917–27. 10.4049/jimmunol.1900674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tripathi D, Radhakrishnan RK, Thandi RS, et al. Correction: IL-22 produced by type 3 innate lymphoid cells (ILC3s) reduces the mortality of type 2 diabetes mellitus (T2DM) mice infected with Mycobacterium tuberculosis. PLoS Pathog 2021;17:e1009578–21. 10.1371/journal.ppat.1009578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Desvignes L, Ernst JD. Interferon-γ-Responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 2009;31:974–85. 10.1016/j.immuni.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hwang JY, Randall TD, Silva-Sanchez A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front Immunol 2016;7:1–17. 10.3389/fimmu.2016.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nair VR, Franco LH, Zacharia VM, et al. Microfold cells actively translocate Mycobacterium tuberculosis to initiate infection. Cell Rep 2016;16:1253–8. 10.1016/j.celrep.2016.06.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan HS, Nair VR, Ruhl CR, et al. Identification of scavenger receptor B1 as the airway microfold cell receptor for Mycobacterium tuberculosis. eLife 2020;9:1–20. 10.7554/eLife.52551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 2000;68:1–8. 10.1159/000028109 [DOI] [PubMed] [Google Scholar]
- 70. Wansleeben C, Barkauskas CE, Rock JR, et al. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol 2013;2:131–48. 10.1002/wdev.58 [DOI] [PubMed] [Google Scholar]
- 71. Upadhyay S, Palmberg L. Air-Liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol Sci 2018;164:21–30. 10.1093/toxsci/kfy053 [DOI] [PubMed] [Google Scholar]
- 72. Parasa VR, Rahman MJ, Ngyuen Hoang AT, et al. Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue. Dis Model Mech 2014;7:281–8. 10.1242/dmm.013854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Braian C, Svensson M, Brighenti S, et al. A 3D human lung tissue model for functional studies on Mycobacterium tuberculosis infection. J Vis Exp 2015;104:1–9. 10.3791/53084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Clevers H. Modeling development and disease with organoids. Cell 2016;165:1586–97. 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- 75. Sachs N, Papaspyropoulos A, Zomer‐van Ommen DD, et al. Long‐term expanding human airway organoids for disease modeling. Embo J 2019;38:1–20. 10.15252/embj.2018100300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Katsura H, Sontake V, Tata A, et al. Human lung stem cell-based Alveolospheres provide insights into SARS-CoV-2-Mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 2020;27:890–904. 10.1016/j.stem.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bartfeld S, Clevers H. Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. J Vis Exp 2015;105:1–9. 10.3791/53359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Tang P, Cai S, et al. Organoid based personalized medicine: from bench to bedside. Cell Regen 2020;9:1–33. 10.1186/s13619-020-00059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Salahudeen AA, Choi SS, Rustagi A, et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 2020;588:670–5. 10.1038/s41586-020-3014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Iakobachvili N, Knoops K. Mycobacteria-host interactions in human bronchiolar airway organoids. bioRxiv 2020:2020.11.12.379586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dutta D, Clevers H. Organoid culture systems to study host-pathogen interactions. Curr Opin Immunol 2017;48:15–22. 10.1016/j.coi.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gkatzis K, Taghizadeh S, Huh D, et al. Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur Respir J 2018;52:1800876. 10.1183/13993003.00876-2018 [DOI] [PubMed] [Google Scholar]
- 83. Berry SB, Gower MS, Su X, et al. A modular microscale granuloma model for Immune-Microenvironment signaling studies in vitro. Front Bioeng Biotechnol 2020;8:1–13. 10.3389/fbioe.2020.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bielecka MK, Tezera LB, Zmijan R, et al. A bioengineered three-dimensional cell culture platform integrated with Microfluidics to address antimicrobial resistance in tuberculosis. MBio 2017;8. 10.1128/mBio.02073-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tezera LB, Bielecka MK, Chancellor A, et al. Dissection of the host-pathogen interaction in human tuberculosis using a bioengineered 3-dimensional model. eLife 2017;6. 10.7554/eLife.21283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bailey KE, Pino C, Lennon ML, et al. Embedding of Precision-Cut Lung Slices in Engineered Hydrogel Biomaterials Supports Extended Ex Vivo Culture. Am J Respir Cell Mol Biol 2020;62:14–22. 10.1165/rcmb.2019-0232MA 10.1165/rcmb.2019-0232MA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carranza-Rosales P, Carranza-Torres IE, Guzmán-Delgado NE, et al. Modeling tuberculosis pathogenesis through ex vivo lung tissue infection. Tuberculosis 2017;107:126–32. 10.1016/j.tube.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maertzdorf J, Tönnies M, Lozza L, et al. Mycobacterium tuberculosis Invasion of the Human Lung: First Contact. Front Immunol 2018;9:1346. 10.3389/fimmu.2018.01346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ganbat D, Seehase S, Richter E, et al. Mycobacteria infect different cell types in the human lung and cause species dependent cellular changes in infected cells. BMC Pulm Med 2016;16:1–16. 10.1186/s12890-016-0185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sha Q, Truong-Tran AQ, Plitt JR, et al. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol 2004;31:358–64. 10.1165/rcmb.2003-0388OC [DOI] [PubMed] [Google Scholar]
- 91. Thorley AJ, Grandolfo D, Lim E, et al. Innate immune responses to bacterial ligands in the peripheral human lung--role of alveolar epithelial TLR expression and signalling. PLoS One 2011;6:e21827. 10.1371/journal.pone.0021827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu W, Zhang W, Duggan ES, et al. Rig-I and TLR3 are both required for maximum interferon induction by influenza virus in human lung alveolar epithelial cells. Virology 2015;482:181–8. 10.1016/j.virol.2015.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roussel L, Rousseau S. Il-17 primes airway epithelial cells lacking functional cystic fibrosis transmembrane conductance regulator (CFTR) to increase NOD1 responses. Biochem Biophys Res Commun 2010;391:505–9. 10.1016/j.bbrc.2009.11.088 [DOI] [PubMed] [Google Scholar]
- 94. Barton JL, Berg T, Didon L, et al. The pattern recognition receptor Nod1 activates CCAAT/enhancer binding protein β signalling in lung epithelial cells. Eur Respir J 2007;30:214–22. 10.1183/09031936.00143906 [DOI] [PubMed] [Google Scholar]
- 95. Kummer JA, Broekhuizen R, Everett H, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 2007;55:443–52. 10.1369/jhc.6A7101.2006 [DOI] [PubMed] [Google Scholar]
- 96. Hirota JA, Hirota SA, Warner SM, et al. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. J Allergy Clin Immunol 2012;129:1116–25. 10.1016/j.jaci.2011.11.033 [DOI] [PubMed] [Google Scholar]
- 97. Heyl KA, Klassert TE, Heinrich A, et al. Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. MBio 2014;5:1–9. 10.1128/mBio.01492-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Human Protein Atlas . tissue expression of MINCLE (CLEC4E) [Internet], 2021. Available: https://www.proteinatlas.org/ENSG00000166523-CLEC4E/tissue/lung [Accessed 17 Jun 2021].
- 99. Jeffers SA, Tusell SM, Gillim-Ross L, et al. Cd209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A 2004;101:15748–53. 10.1073/pnas.0403812101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wu W, Zhang W, Booth JL, et al. Human primary airway epithelial cells isolated from active smokers have epigenetically impaired antiviral responses. Respir Res 2016;17:1–11. 10.1186/s12931-016-0428-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yin X, Riva L, Pu Y, et al. Mda5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep 2021;34:108628. 10.1016/j.celrep.2020.108628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Y, Renner DM, Comar CE, et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc Natl Acad Sci U S A 2021;118:e2022643118. 10.1073/pnas.2022643118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rivas-Santiago B, Schwander SK, Sarabia C, et al. Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun 2005;73:4505–11. 10.1128/IAI.73.8.4505-4511.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sow FB, Nandakumar S, Velu V, et al. Mycobacterium tuberculosis components stimulate production of the antimicrobial peptide hepcidin. Tuberculosis 2011;91:314–21. 10.1016/j.tube.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 105. Méndez-Samperio P, Miranda E, Vázquez A. Expression and Secretion of CXCL-8 and CXCL-10 From Mycobacterium Bovis BCG-Infected Human Epithelial Cells: Role of IL-4. Mediators Inflamm 2006;2006:1–6. 10.1155/MI/2006/67451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pechkovsky DV, Goldmann T, Vollmer E, et al. Interleukin-18 expression by alveolar epithelial cells type II in tuberculosis and sarcoidosis. FEMS Immunol Med Microbiol 2006;46:30–8. 10.1111/j.1574-695X.2005.00013.x [DOI] [PubMed] [Google Scholar]
- 107. Mvubu NE, Pillay B, McKinnon LR, et al. Mycobacterium tuberculosis strains induce strain-specific cytokine and chemokine response in pulmonary epithelial cells. Cytokine 2018;104:53–64. 10.1016/j.cyto.2017.09.027 [DOI] [PubMed] [Google Scholar]
- 108. Bai X, Ovrutsky AR, Kartalija M, et al. Il-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. Int Immunol 2011;23:679–91. 10.1093/intimm/dxr075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-217997supp001.pdf (86.5KB, pdf)