Abstract
Microscopic detection of parasites has been the reference standard for malaria diagnosis for decades. However, difficulty in maintaining required technical skills and infrastructure has spurred the development of several nonmicroscopic malaria rapid diagnostic devices based on the detection of malaria parasite antigen in whole blood. The ParaSight F test is one such device. It detects the presence of Plasmodium falciparum-specific histidine-rich protein 2 by using an antigen-capture immunochromatographic strip format. The present study was conducted at outpatient malaria clinics in Iquitos, Peru, and Maesod, Thailand. Duplicate, blinded, expert microscopy was employed as the reference standard for evaluating device performance. Of 2,988 eligible patients, microscopy showed that 547 (18%) had P. falciparum, 658 (22%) had P. vivax, 2 (0.07%) had P. malariae, and 1,750 (59%) were negative for Plasmodium. Mixed infections (P. falciparum and P. vivax) were identified in 31 patients (1%). The overall sensitivity of ParaSight F for P. falciparum was 95%. When stratified by magnitude of parasitemia (no. of asexual parasites per microliter of whole blood), sensitivities were 83% (>0 to 500 parasites/μl), 87% (501 to 1,000/μl), 98% (1,001 to 5,000/μl), and 98% (>5,000/μl). Device specificity was 86%.
Microscopic analysis of appropriately stained thick and thin blood smears has been the standard diagnostic technique for identifying malaria infections for more than a century. The technique is capable of accurate and reliable diagnosis when performed by skilled microscopists using defined protocols (2, 31). These qualifiers, skill of the microscopist and use of proven, defined procedures, frequently present the greatest obstacles to fully achieving the potential accuracy of microscopic diagnosis. Although there is a logistical burden associated with performing a time-, labor-, and equipment-intensive procedure such as diagnostic microscopy, it is the training required to establish and sustain competent performance of microscopy that poses the greatest difficulty in employing this diagnostic technology.
The problems associated with implementing and sustaining a level of skilled microscopy appropriate for clinical diagnosis, particularly in the field setting, have prompted the development of a variety of malaria rapid diagnostic devices (MRDDs). The World Health Organization has recognized the need to overcome deficiencies associated with diagnostic microscopy and supports the development of nonmicroscopic alternatives (33, 35). The current generation of MRDDs is based on antigen capture immunoassay methodologies utilizing immunochromatographic strip (ICS) technology. One such device, the ParaSight F test (Becton Dickinson Diagnostic Systems, Cockeysville, Md.), employs monoclonal antibodies, directed against Plasmodium falciparum histidine-rich protein 2 (HRP-2), immobilized on a nitrocellulose strip (27). The ParaSight F dipstick format has been available as a commercial product outside North America for several years; it has not been approved by the Food and Drug Administration for use as an in vitro diagnostic aid in the United States.
A number of reports evaluating ParaSight F performance in various laboratory and field settings have been published. These reports have typically cited good sensitivity and specificity (3, 5, 6, 8, 9, 14, 22, 27–29). Unfortunately, many of these studies base their conclusions on small study cohorts and do not provide a statistically powerful discrimination of assay performance. Further, given the relatively small patient enrollments in most earlier studies, evaluation of assay performance at stratified levels of parasitemia has been limited. The present study was designed to precisely define ParaSight F test sensitivity and specificity in symptomatic patients, within narrow confidence intervals, and to assess possible changes in assay sensitivity as the level of parasitemia in peripheral blood changed.
MATERIALS AND METHODS
Participants.
Study participants were symptomatic patients who presented on their own initiative to existing outpatient malaria clinics. These local malaria clinics, in Iquitos, Peru, and Maesod, Thailand, are operated under the direction of local public health authorities, fully independently of the present study. Patients were enrolled in 1998, from 28 May through 28 August in Thailand and from 23 June through 17 August in Peru. Patients who met the specific inclusion criteria of this study, i.e., symptoms of fever (oral temperature, ≧38°C), or headache, or history of fever within the past 72 h, and an age of ≧15 years (Thailand) or ≧1 year (Peru), were enrolled in the study (differences in host country policies concerning the enrollment of children in medical studies dictated the difference in age cutoff between the two sites). Patients who were currently taking antimalarial therapy or who had been treated with antimalarial drugs within the past 2 weeks were excluded from the evaluation. At enrollment, each patient donated approximately 2 ml of blood collected by venipuncture into tubes (Vacutainer blood collection system) containing tripotassium salt of EDTA. Immediately following collection, precise volumes of well-mixed whole blood were micropipetted onto precleaned glass microscope slides for the preparation of thick (6-μl sample volume) and thin (4-μl sample volume) peripheral blood smears. A total of three slides were prepared for each patient, each slide containing both a thick smear and a thin smear. One slide was promptly provided to the local clinical staff for them to stain and examine. Using this slide, the local clinical staff, not the study team, made all diagnostic and treatment decisions for patients enrolled in the study. The remaining two slides were saved by the study team for later staining and examination; these slides were used to determine diagnostic endpoints for device performance measures. Blood specimens, still in the primary EDTA-containing collection tubes, were packed into insulated containers cooled with refreezable ice packs and transported from the enrollment site to a testing station. At the testing station, specimens were analyzed within 6 h of collection for a complete blood count and assayed with the ParaSight F test.
ParaSight F test.
Samples were applied to the ParaSight F test strip according to the instructions included in the assay kit. This dipstick method is based on antigen capture ICS technology. In brief, mouse monoclonal immunoglobulin G1 antibodies directed against epitopes of P. falciparum HRP-2 were immobilized as a test line on a nitrocellulose strip during the manufacturing process. Individual test strips also contained an internal control line consisting of immobilized recombinant HRP-2 that ultimately appeared as a dashed pink line when the assay procedure was properly performed. Three drops of lysing buffer, dispensed from a manufacturer-provided plastic reagent vial, was mixed with 50 μl of whole blood, measured in a heparinized capillary tube included in the test kit. One drop of the resulting hemolysate was placed into a test sample well, into which the end of the test strip was then inserted. The lysate was wicked up the test strip until the sample lysate was completely adsorbed onto the nitrocellulose strip matrix. Next, one drop of a detector reagent containing a secondary ligand, liposomal-encapsulated dye-conjugated rabbit anti-HRP-2 antibody, was added to the test well and allowed to wick up the ParaSight F test strip. As a final step, after the detector reagent had been entirely absorbed onto the test strip, two drops of a wash solution was placed in the test well and also wicked up completely. The development of a solid pink line, corresponding to the immobilized line of mouse anti-P. falciparum HRP-2 on the test strip, in conjunction with the development of the dashed pink control line, was interpreted as a positive test result. Test results were interpreted as negative (only control line visible), positive for P. falciparum (both control and test lines visible), or uninterpretable (darkly stained background obscuring test or control lines, or absence of the control line). In addition to reading test lines as positive or negative, the intensity of the P. falciparum reaction line was scored as 0 (negative), 0.25, 0.5, 1, 2, 3, or 4 by comparing the test line intensity against photographic standards provided by the manufacturer. In this numerical scheme, a line with a score of 0.25 was the least intense and a line with a score of 4 was the most intense. Any visible evidence of a test line, no matter how faint, associated with a visible control dashed line, was interpreted as a positive test result for the assay device.
Reference microscopy.
Two slides, designated slide 1 and slide 2 for study purposes, were prepared for each patient enrolled into the study, each having both a thick and a thin blood smear. Smears were sheltered from dust and insects, air dried, and stained with 3% Giemsa according to the study protocol. Two skilled microscopists, designated microscopists A and B, independently examined slide 1 for each patient. Microscopists A and B were blinded to each other's interpretations as well as to the results of the ParaSight F test. Two hundred thick film oil immersion high-power fields were examined before a slide was interpreted as negative for malaria. The interpretation was determined to be positive if asexual P. falciparum stages were observed. The presence of gametocytes in the absence of asexual parasite forms was interpreted as a negative result for the purpose of assay evaluation. In cases where P. falciparum was observed during microscopic examination, the number of asexual forms was counted with a concomitant enumeration of white blood cells (WBCs). If, after 200 WBCs were counted, 10 or more asexual parasite stages were counted, then the total number of asexual parasites was recorded. If malaria parasites were present but numbered fewer than 10 parasites per 200 WBCs, then the microscopists continued to examine the smear, counting asexual stage parasites and WBCs, until at least 500 WBCs had been counted. For all positive smears, the number of asexual parasites counted was multiplied by the patient's WBC count, and the resulting value divided by the total number of WBCs counted during the micro examination. The final data endpoint was a calculated parasitemia expressed as the number of asexual stage parasites per microliter of whole blood.
Validation and control. (i) Blinding of results.
Technicians performing the ParaSight F tests were blinded to each of the other measurements collected during the study. Care was taken to ensure that technicians using the rapid diagnostic device were blinded to patient histories and examinations, WBC determinations, and patient demographics. In all cases, the results of the ParaSight F test were determined prior to diagnostic microscopy, with strict blinding between the rapid test results and technicians performing the microscopy.
(ii) Microscopy.
The independent readings for slide 1 determined by microscopists A and B were compared for concordance in three areas: (i) agreement about the presence of asexual forms of Plasmodium, (ii) agreement about the species of Plasmodium when present, and (iii) agreement on the calculated level of parasitemia within a factor of 2. When all three conditions for concordance were met, the mean parasitemia value from the two independent readings was recorded as the “true” diagnostic outcome. When the results of microscopists A and B were discordant for any of the three criteria cited, a third, senior microscopist, designated microscopist C, examined both study slides 1 and 2. The procedures used by microscopist C to review the slides were the same as those used by microscopists A and B. The cumulative findings of microscopist C for slides 1 and 2 were then considered the “true” diagnostic outcome for the specimen, with the interpretations of microscopists A and B no longer being considered. All technicians serving as study microscopists were prospectively certified as skilled and competent through documentation of training, experience, and past performance. Microscope optics were standardized in both field sites using identical model microscopes (model CH-2; Olympus America, Inc., Melville, N.Y.), objectives (100× DPlan 100 1.25 oil 160/0.17), and equivalent eyepieces (10×, WHK 10×/20L or WHK 10×/20L-H).
(iii) WBC determinations.
Conventional control measures were applied to semiautomated hematology analyzers used in the study. The Thai study team analyzed specimens by particle counting and sizing (Coulter Ac-T 10; Beckman-Coulter, Inc., Fullerton, Calif.), and a three-level commercial hematology control material (4C Plus; Beckman-Coulter, Inc.) was used to monitor analyzer performance. WBC counting was validated daily and additional replicate testing was performed to ascertain compatibility between the values reported from two identical analyzers used at the testing station. Manual WBC counts were performed weekly throughout the study using prepared dilutions (Unopette; Becton Dickinson Clinical Laboratory Solutions, Franklin Lakes, N.J.) and a hemacytometer, the purpose being to maintain a ready backup method for WBC counts as well as to apply an external monitor of analyzer function. At the Iquitos test station, a centrifugal hematology system (QBC II; Becton Dickinson Diagnostic Systems) was used to provide WBC counts. The Peruvian study team conformed to the manufacturer's recommendation to validate instrument reader function with a calibrated standard each day prior to specimen analysis. The same system of manual WBC counts and daily verification of results between multiple analyzers was applied in the Peruvian setting.
(iv) ParaSight F assays.
Assay control materials were supplied by the manufacturer and used to validate assay performance daily prior to analyzing patient specimens. Control materials consisted of a known positive sample containing recombinant P. falciparum HRP-2 shown to reproducibly generate a positive test result with the production lot of ParaSight F used in this study and a similarly prepared negative sample lacking the HRP-2 analyte. All assays were reported as positive, negative, or uninterpretable per specific instructions provided in the test kit. Test whose results were reported as uninterpretable after the initial procedure were repeated in an attempt to resolve the discrepant event, i.e., failure to observe a control line or a darkly stained background that potentially obscured a positive reaction line. Repeatedly uninterpretable results were reported as such and were incorporated into calculations of sensitivity or specificity as “false” results. Each investigator and technician performing the assay was prospectively trained and certified by the study principal investigator. Additionally, for quality control purposes, at least once each day, the technician's interpretation of a ParaSight F result was promptly reviewed by an on-site investigator. At least once each week, the technician's performance of one ParaSight F assay, from start to finish, was directly observed by the on-site investigator. The ParaSight F assays evaluated in this study were from a single production run (single lot), manufactured in a process consistent with current good manufacturing practice standards established by the U.S. Food and Drug Administration to govern the production of in vitro diagnostic devices.
(v) Protocol review and approval.
The study protocol was reviewed by the Institutional Review Board, Walter Reed Army Institute of Research, and the Human Subjects Safety Review Board, U.S. Army Medical Research and Materiel Command, and approved as Walter Reed Army Institute of Research protocol 687. The study protocol was further approved by the Ministry of Health in Iquitos, Peru, and performed under the direction of the Direccion de Salud de Loreto. Similarly, the study protocol was also approved by the Thai Ministry of Public Health and implemented under the guidance of the Vector-Borne Disease Control Office #1, Phrabuddhabat, Thailand.
Data analysis.
Study size was determined by two factors: first, the need to characterize ParaSight F performance at prospectively defined levels of parasitemia, and second, the need to generate sensitivity and specificity results within narrow 95% confidence intervals (CI95). These primary considerations were analyzed within the historical epidemiology and seasonal transmission patterns for malaria in the selected study sites to calculate cohort size requirements. Device performance endpoints for sensitivity and specificity were based on the diagnostic results of blood smear interpretation. Standard definitions of sensitivity [true positives/(true positives + false negatives)] and specificity [true negatives/(true negatives + false positives)] were applied to the study dataset. Device sensitivity was calculated for each of the predetermined parasitemia ranges: >0 to 500, 501 to 1,000, 1,001 to 5,000, and >5,000 parasites/μl. Confidence intervals were computed for device sensitivity and specificity under the assumptions that the diagnostic accuracy of the test followed a binomial distribution. Results from patient specimens not supported by complete and accurate documentation were rejected prior to analysis of device performance. The association between magnitude of parasitemia and ParaSight F line intensity was displayed in box plots of intensity group median parasitemia with an accompanying display of value dispersion. The effect of low line intensity on device performance was evaluated by redefining the ParaSight F line intensity numerical score threshold for a negative test result and then analyzing the effects on assay sensitivity and specificity.
RESULTS
A total of 3,006 self-presenting, symptomatic patients were enrolled during the study period, 844 (28%) and 2,162 (72%) in Peru and Thailand, respectively. Of this number, 13 patients (0.43%) were excluded from further consideration as a result of incomplete documentation of patient history (8 patients [0.26%]), failure to document informed consent (3 [0.11%]), or subsequent withdrawal of consent to participate, i.e., unwillingness to donate blood (2 [0.06%]). Among 2,993 blood specimens from evaluable patients, five gave assay results (0.16%) which were determined to be invalid because of incomplete documentation of the assay result (3 [0.10%]), failure to record line intensity (1 [0.03%]), or ambiguity associated with test outcome (1 [0.03%]). Data generated from the remaining 2,988 specimens were included in the study data set for evaluating device performance.
Microscopic examination of 2,988 peripheral blood smears showed that 1,238 (41.4%) were positive for malaria and the remaining 1,750 (58.6%) were negative for asexual parasite stages (Table 1). Species representation was as follows: 547 (18.3%) had P. falciparum, 658 (22.0%) had P. vivax, 2 (0.07%) had P. malariae, and 31 (1.0%) had mixed infections presenting with both P. falciparum and P. vivax. A summary of discordance between primary study slide readers for the 2,988 study smears is presented in Table 2. Disagreement on calculated parasitemia greater than twofold was the greatest single source of discordance between study microscopists, accounting for 104 (56.8%) of 183 microscopic results that required resolution by microscopist C. Within this specific subgroup of discordant results, 31 (29.8%) involved parasitemia levels of ≤100 parasites/μl. Of 40 cases where disagreement on the presence of asexual stages of P. falciparum was noted, 31 (77.5%) involved parasite densities of ≤100 parasites/μl and 21 (52.5%) involved parasitemias of ≤50 parasites/μl.
TABLE 1.
Summary of microscopic findings
| Resulta | No. (%) of samples
|
||
|---|---|---|---|
| At study site
|
Total | ||
| Peru | Thailand | ||
| Positive for: | |||
| P. falciparum | 115 (13.7) | 432 (20.0) | 547 (18.3) |
| P. vivax | 208 (24.8) | 450 (20.9) | 658 (22.0) |
| Mixed P. falciparum and P. vivax | 12 (1.4) | 19 (0.9) | 31 (1.0) |
| P. malariae | 0 (0) | 2 (0.09) | 2 (0.07) |
| Negativeb | 503 (60.0) | 1,247 (58.1) | 1,750 (58.6) |
| Study total | 838 | 2,150 | 2,988 |
Positive results required the presence of asexual Plasmodia stages.
Blood smears containing Plasmodium sp. gametocytes in the absence of same species, asexual stages were interpreted as negative n = 3 for P. falciparum and, n = 2 for P. vivax.
TABLE 2.
Summary of discordant microscopic results
| Area of microscopists' disagreement | No. (%) of samples
|
||
|---|---|---|---|
| At study site
|
Total (n = 2,988) | ||
| Peru (n = 838) | Thailand (n = 2,150) | ||
| Presence of asexual parasitesa | 21 (2.5) | 19 (0.8) | 40 (1.3) |
| Species identification | 1 (0.1) | 25 (1.1) | 26 (0.9) |
| Calculated parasitemia (>2-fold difference) | 22 (2.6) | 82 (3.8) | 104 (3.4) |
| Disagreement in multiple areasb | 6 (0.7) | 7 (0.3) | 13 (0.4) |
| Study total | 50 (5.9) | 133 (6.2) | 183 (6.1) |
Agreement not required for presence or absence of gametocytes.
Results differed in species identification plus quantification of parasitemia, or in identification of a second species, i.e., mixed infection reported by only one of the primary microscopists.
Cross-tabulation of ParaSight F test results against reference microscopy results revealed that the greatest proportion of false-negative results occurred in the lowest range of P. falciparum parasitemia, with 14 of 81 (17.2%) specimens with >0 to 500 parasites/μl falsely interpreted as negative by ParaSight F (Table 3). Increased parasite density was associated with a decreased incidence of false-negative results by ParaSight F, with 12.9, 1.9, and 2.1% of specimens interpreted as assay negative at levels of 501 to 1,000, 1,001 to 5,000, and >5,000 parasites/μl, respectively. With regard to false-positive ParaSight F results, the proportion of false-positive results was equivalent in specimens microscopically negative for any Plasmodium sp. (249 of 1,750, 14.2%) and in those microscopically positive for non-P. falciparum Plasmodium (96 of 658, 14.6%). Approximately one-third of mixed infections (10 of 31, 32.2%) containing asexual stages of P. falciparum were interpreted as negative by the ParaSight F test, although in mixed-species infections it was not possible to precisely quantify the portion of the total parasitemia attributable to P. falciparum relative to the second species, P. vivax. The lack of an accurate method of determining P. falciparum parasitemia in a mixed infection and the low number of mixed infections in the study cohort (1.03%) prompted the exclusion of mixed infections from calculations of overall device performance.
TABLE 3.
Cross-tabulation of ParaSight F assay results against microscopy results
| Microscopy result | No. of samples with ParaSight F result
|
Total | ||
|---|---|---|---|---|
| Negative | Positive | Uninterpretablea | ||
| Positive for asexual stages of P. falciparum (no. of parasites/μl) | ||||
| >0–500 | 14 | 67 | 0 | 81 |
| 501–1,000 | 4 | 27 | 0 | 31 |
| 1,001–5,000 | 2 | 102 | 0 | 104 |
| >5,000 | 7 | 324 | 0 | 331 |
| Total | 27 | 520 | 0 | 547 |
| Negative for asexual stages of P. falciparum | ||||
| Negative for Plasmodium | 1,495 | 249 | 6 | 1,750 |
| Positive for P. vivax | 561 | 96 | 1 | 658 |
| Positive for P. malariae | 1 | 1 | 0 | 2 |
| Total | 2,057 | 346 | 7 | 2,410 |
| Positive for mixed infection (P. falciparum and P. vivax) | 10 | 21 | 0 | 31 |
| Study total | 2,094 | 887 | 7 | 2,988 |
Results were considered uninterpretable if they failed to demonstrate a clear positive or negative endpoint after repeat testing; these results were considered “false” for the purpose of determining device performance.
The performance characteristics of the ParaSight F test are presented in Table 4. Overall device sensitivity for detecting the presence of P. falciparum relative to reference microscopy was 95%, with a CI95 of 93 to 99% for the total study cohort. The Peruvian arm of the study had a slightly lower overall sensitivity, 89%, than the Thai arm, 97%. The difference in overall device sensitivity between the two study sites was statistically significant, as was the difference in device sensitivity in the parasitemia range of >5,000 parasites/μl. Overall device sensitivity was ≧90% when infections exceeded 1,000 parasites/μl, and performance was observed to progressively decline in the lower parasitemia ranges, reflecting a similar trend in both study sites. Device specificity for excluding the presence of P. falciparum was 86% (CI95, 84 to 92%) with site-specific values of 95% (Peru) and 82% (Thailand). The difference in assay specificity between the study sites was statistically significant. ParaSight F sensitivity and specificity did not differ to a statistically significant degree for blood samples obtained from men versus those from women or those from adults (age, ≧18 years) versus those from children (data not shown).
TABLE 4.
Performance characteristics of ParaSight F by study site and combined total
| Parameter | % (CI95)
|
||
|---|---|---|---|
| Peru | Thailand | Study total | |
| Sensitivitya | |||
| Any parasitemia | 89 (81–94) | 97 (95–98) | 95 (93–99) |
| >0–500 | 75 (43–95) | 84 (73–92) | 84 (73–91) |
| 501–1,000 | 70 (35–93) | 95 (76–100) | 87 (72–96) |
| 1,001–5,000 | 100 (86–100) | 98 (91–100) | 98 (93–100) |
| >5,000 | 90 (80–96) | 100 (99–100) | 98 (96–100) |
| Specificity | 95 (93–97) | 82 (84–87) | 86 (84–92) |
Values are numbers of parasites per microliter of blood. Mixed infections (P. falciparum and P. vivax) were excluded from calculations of device performance (n = 31).
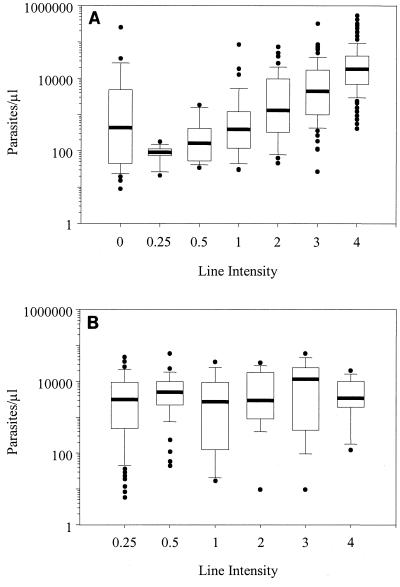
Figure 1 illustrates the association between HRP-2-positive line intensity and parasite density for P. falciparum-positive (Fig. 1A) and non-P. falciparum Plasmodium-positive (Fig. 1B) specimens. Line intensity of true-positive assay results, i.e., a test line intensity of ≧0.25 in Fig. 1A, was observed to increase in association with increased P. falciparum parasitemia, but considerable dispersion of values outlying the 10th and 90th percentiles was evident in the higher line intensity groups. There was no apparent relationship between the median parasitemias of line intensity subgroups for ParaSight F assays that generated false-positive results in specimens containing non-P. falciparum Plasmodium spp. (Fig. 1B).
FIG. 1.
Box plots of asexual parasite density and test line intensity (excluding mixed infections). (A) Positive assay results from P. falciparum-positive specimens showed an increase in test line intensity with increased parasitemia (n = 547). (B) Test line intensity of positive assays from non-P. falciparum Plasmodium-positive specimens was not associated with predictable changes in parasitemia (n = 97). The bold horizontal lines indicate the median parasitemia for each intensity group, and the white boxes show the 25th and 75th percentiles. The whisker caps extending from each box indicate the 10th and 90th percentiles, with significant outliers shown as individual points.
Cross-tabulation of test line intensity against parasite density showed that 507 of 547 (92.6%) P. falciparum-positive specimens generated line intensities of ≧1 by ParaSight F (Table 5). Of 2,403 specimens that were P. falciparum negative by microscopy, 2,330 (96.9%) had assay line intensities of ≤0.05. Thirty-two (1.8%) specimens microscopically negative for asexual Plasmodium generated test line intensities of ≧1. Although it was not originally intended to further stratify parasitemia into subgroups within the >0- to 500-parasite/μl range, we were interested in characterizing the lower limits of device performance at reduced parasitemia levels. The ParaSight F device demonstrated a lower sensitivity for detection of P. falciparum in the range of >0 to 100 parasites/μl (Table 5). While the numbers of samples with confirmed asexual parasites in these groups were too small to substantiate statistical significance within narrow confidence intervals, the rapid assay identified 44 of 49 (90%) of P. falciparum-positive samples in the range of 101 to 500 parasites/μl.
TABLE 5.
Cross-tabulation of ParaSight F line intensity against calculated parasitemia
| Microscopy result | n | No. of samples with ParaSight F line intensity
|
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 1 | 2 | 3 | 4 | ||
| Positive for P. falciparum (no. of parasites/μl) | ||||||||
| >0–500 | 81 | 14 | 6 | 6 | 19 | 20 | 14 | 2 |
| >0–100 | 32 | 9 | 3 | 3 | 8 | 8 | 1 | 0 |
| 101–200 | 19 | 2 | 3 | 1 | 6 | 3 | 4 | 0 |
| 201–300 | 8 | 0 | 0 | 0 | 3 | 3 | 2 | 0 |
| 301–400 | 10 | 2 | 0 | 1 | 1 | 3 | 3 | 0 |
| 401–500 | 12 | 1 | 0 | 1 | 1 | 3 | 4 | 2 |
| 501–1,000 | 31 | 4 | 0 | 0 | 5 | 4 | 13 | 5 |
| 1,001–5,000 | 104 | 2 | 0 | 1 | 8 | 15 | 27 | 51 |
| >5,000 | 331 | 7 | 0 | 0 | 4 | 20 | 53 | 247 |
| Total | 547 | 27 | 6 | 7 | 36 | 59 | 107 | 305 |
| Negative for P. falciparum | ||||||||
| No Plasmodium | 1,744 | 1,495 | 180 | 37 | 14 | 11 | 5 | 2 |
| Positive Pv | 657 | 561 | 42 | 14 | 15 | 9 | 12 | 4 |
| Positive Pm | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Total | 2,403 | 2,057 | 222 | 51 | 29 | 20 | 18 | 6 |
| Positive for mixed infection (P. falciparum and P. vivax) | 31 | 10 | 0 | 1 | 2 | 2 | 9 | 7 |
| Study totala | 2,981 | 2,094 | 228 | 59 | 67 | 81 | 134 | 318 |
Excludes ParaSight F results that were uninterpretable following repeated assay because line intensity could not be reliably determined (n = 7).
The impact of low line intensity on ParaSight F device performance is presented in Table 6. Modifying the definition of a negative test result to include faint lines, i.e., line intensities of 0.25 and 0.5, as a negative assay outcome had no significant effect on device sensitivity. This manipulation did improve overall device specificity, particularly when the faintest visible reaction intensity, 0.25, was redefined as a negative result.
TABLE 6.
Effect of redefined threshold for negative interpretations on ParaSight F performance
| Parameter and line intensity defined as negative result | % (CI95)
|
||
|---|---|---|---|
| Peru | Thailand | Study total | |
| Sensitivity | |||
| 0 (base case) | 89 (81–94) | 97 (95–98) | 95 (93–97) |
| 0 and 0.25 | 88 (80–93) | 96 (93–97) | 94 (92–96) |
| 0, 0.25, and 0.5 | 87 (79–93) | 94 (92–96) | 93 (90–95) |
| 0, 0.25, 0.5, and 1 | 83 (74–89) | 87 (83–90) | 86 (83–89) |
| Specificity | |||
| 0 (base case) | 95 (93–97) | 82 (80–83) | 86 (84–87) |
| 0 and 0.25 | 97 (95–98) | 94 (93–95) | 95 (94–96) |
| 0, 0.25, and 0.5 | 98 (96–99) | 97 (96–98) | 97 (96–98) |
| 0, 0.25, 0.5, and 1 | 99 (98–100) | 98 (97–99) | 98 (97–99) |
DISCUSSION
This study was designed to evaluate the diagnostic accuracy of the ParaSight F assay in symptomatic patients living in locales where malaria is endemic and presenting for medical care on their own initiative. The dipstick provided accurate detection of parasitemia in a patient population enrolled in two study sites characterized by distinct geographic, population, and epidemiologic features. When a rigorously applied microscopic reference method confirmed the presence of any asexual forms of P. falciparum in peripheral blood samples, overall device sensitivity was very good, with the rapid assay identifying 95% of all P. falciparum-positive specimens. This level of performance approaches the diagnostic accuracy of skilled microscopy for P. falciparum and clearly exceeds the quality of microscopy reported in a variety of health care settings (1, 4, 7, 11, 13, 18, 20, 23, 29). Device sensitivity was achieved without an appreciable loss of specificity in the study population. In rural malaria clinics, or in instances where skilled microscopy cannot be readily employed or sustained, the ParaSight F test provides an acceptable aid to the diagnosis of malaria in symptomatic patients at risk for P. falciparum infection. A quick and accurate diagnostic test for P. falciparum would facilitate early intervention and appropriate patient management (4, 17, 18, 32, 34, 35).
The overall high level of sensitivity reported here has precedents in previous reports of HRP-2-based malaria diagnostic assays (5, 9, 14, 27). The present study, though, subjected the device to stringent evaluation at multiple levels of parasite density to more accurately determine the diagnostic utility of the MRDD across a broader spectrum of symptomatic parasitemias. Consistent with previous studies, our findings showed reduced assay sensitivity when parasitemia fell below 500 parasites/μl (5, 14). We observed that the lower limit of parasitemia at which acceptable device performance is seen approximates 100 parasites/μl (Table 5). These data indicate that the ParaSight F test strips evaluated in this study were optimized for performance at parasite densities of ≧100 parasites/μl. Improved sensitivity at even lower levels of parasitemia would likely result in a loss of device specificity, as observed when low-intensity test lines were included as negative assay results (Table 6). However, because device sensitivity improved in association with higher levels of parasitemia, testing of serially obtained blood specimens with this device offers the potential to salvage a diagnosis of malaria when an initial test result is negative in a patient who presents with a low peripheral parasitemia. Considering the increase in parasite burden that occurs during the asexual amplification cycle, repeat blood sampling at 12- or 24-h intervals, and retesting, should yield specimens with higher parasitemias, associated with higher levels of device sensitivity. Using a protocol for serial sampling and testing with ParaSight F would be comparable to the practice of repeated microscopic examination of serially drawn blood in initially blood smear-negative, symptomatic patients with a significant history of exposure to malaria.
The redefinition of negative results at various thresholds of test line intensity illustrates one effect of faint line intensity on device performance. While redefining test line values of 0.25 and 0.5 as negative results did not significantly diminish overall sensitivity, the effect on device specificity was beneficial (Table 6). Improved specificity resulted from reclassifying 72% (180 of 249) and 43% (42 of 96) of false-positive results at the 0.25 level of intensity for specimens diagnosed as negative for Plasmodium or positive for P. vivax, respectively (Table 5). The observation that these very faint lines (intensity of 0.25) are consistently false positive suggests that this aspect of test functioning should be improved. Most users will find the assay easier to interpret accurately if they can rely on obviously positive or negative test lines instead of trying to gauge test line intensity. A diligent effort to standardize reference microscopy was emphasized in the execution of this study. The low overall discordance rate of 6.1% noted between the primary readers is strong evidence of consistent and skilled microscopy. Not surprisingly, the greatest single basis of disagreement was parasite density, particularly at levels of <100 parasites/μl (10, 26). Six additional instances of discordant microscopy were observed at parasitemias of ≧50,000 parasites/μl.
The observation of seven specimens from the Peru study site with P. falciparum parasitemias of >5,000 parasites/μl (median = 16,203; range = 5,386 to 252,367) that tested negative by the ParaSight F method is concerning. Two specimens from Thailand with parasitemias of 1,228 and 3,280 were also assay negative. The reason for these false-negative assay results in patients with higher parasitemias is not clear, but similar findings have been reported in other studies (5, 14, 17, 19, 25, 32). Reports of HRP-2-negative variants of P. falciparum have been presented (I. Traore, O. Koita, and O. Doumbo, Am. J. Trop. Med. Hyg., abstr. 502, p. 272, 1997), but the observation of false-negative rapid malaria diagnostic assays is not limited to HRP-2-based methods. Repeatedly negative tests have been reported with ICS-based rapid diagnostic tests that target other malarial antigens (16, 17, 24). Other factors, perhaps related to geographic and antigenic variation or patient immune status, merit consideration in future studies of nonmicroscopic malaria assays.
It is not entirely clear why performance differences between the two study sites were observed in some instances. Considerable rigor was exerted in standardizing the protocol execution between the sites and in validating performance parameters. Confounding variables, such as tester-dependent bias or subtle differences in sample handling, may have played a role in the variation observed between the Thai and Peruvian study results.
Immunocapture ICS techniques detect parasite antigenemia, and assay results should not be regarded as a direct equivalent of viable parasitemia. In particular, test methods based on HRP-2 antigen capture have been reported to detect persistent peripheral antigenemia for upwards of 28 days after cure of P. falciparum (12, 14) and in aparasitemic women with placental malaria (21). The consequence of persistent assay positivity for clinical utility and patient management remains to be determined, but it is not likely to be a major factor in the diagnosis of malaria in nonimmune travelers or other populations with limited exposure, such as deployed military forces.
The present study highlights the chronology of rapid malaria diagnostic device development. The ParaSight F test was a pioneer industry effort in large-scale, process-controlled manufacturing of an MRDD product. This study clearly defines and validates device performance in a large, multisite trial. These findings provide an unequivocal demonstration of the potential clinical utility of ICS technology for the diagnosis of malaria in symptomatic patients. The HRP-2 antigen capture system employed by the ParaSight F test format is sufficiently evolved to withstand the rigors of field use and to provide a substantive diagnostic adjunct in a clinical setting.
Despite the high level of diagnostic accuracy that ParaSight F has demonstrated, the assay is inherently limited by its capability to identify only one species causing human malaria. Continued development of nonmicroscopic malaria diagnostic products beyond single species assays is essential if such devices are to reach a high level of clinical utility. Recognition of this need, and progress towards satisfying it, are suggested by recent reports of multispecies assays with performance approaching that documented here for a P. falciparum-specific device (15, 22, 24, 30).
ACKNOWLEDGMENTS
We thank Joe Perrone and David Baggett of Becton Dickinson Diagnostic Systems for providing access to the ParaSight F test kits used in this study. We also acknowledge Janelle Rhorer of Statistics Collaborative, Washington, D.C., for statistical analysis of our data. Finally, we express our appreciation for the skill and dedication of the field study teams deployed by Navy Medical Research Center, Lima, Peru, and the Armed Forces Research Institute for Medical Sciences, Bangkok, Thailand.
REFERENCES
- 1.Aron J L. Malaria epidemiology and detectability. Trans R Soc Med Hyg. 1982;76:595–601. doi: 10.1016/0035-9203(82)90219-x. [DOI] [PubMed] [Google Scholar]
- 2.Bain B J, Chiodini P L, England J M, Bailey J W. The laboratory diagnosis of malaria. The Malaria Working Party of The General Haematology Task Force of the British Committee for Standards in Haematology. Clin Lab Haematol. 1997;19:165–170. [PubMed] [Google Scholar]
- 3.Banchongaksorn T, Prajakwong S, Rooney W, Vickers P. Operational trial of ParaSight-F (dipstick) in the diagnosis of falciparum malaria at the primary health care level. Southeast Asian J Trop Med Public Health. 1997;28:243–246. [PubMed] [Google Scholar]
- 4.Barat L, Chipipa J, Kolczak M, Sukwa T. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am J Trop Med Hyg. 1999;60:1024–1030. doi: 10.4269/ajtmh.1999.60.1024. [DOI] [PubMed] [Google Scholar]
- 5.Beadle C, Long G W, Weiss W R, McElroy P D, Maret S M, Oloo A J, Hoffman S L. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. doi: 10.1016/s0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 6.Caraballo A, Ache A. The evaluation of a dipstick test for Plasmodium falciparum in mining areas of Venezuela. Am J Trop Med Hyg. 1996;55:482–484. doi: 10.4269/ajtmh.1996.55.482. [DOI] [PubMed] [Google Scholar]
- 7.Collier J A, Longmore J M. The reliability of the microscopic diagnosis of malaria in the field and in the laboratory. Ann Trop Med Parasitol. 1983;77:113–117. doi: 10.1080/00034983.1983.11811683. [DOI] [PubMed] [Google Scholar]
- 8.Craig M H, Sharp B L. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Trans R Soc Trop Med Hyg. 1997;91:279–282. doi: 10.1016/s0035-9203(97)90074-2. [DOI] [PubMed] [Google Scholar]
- 9.Dietze R, Perkins M, Boulos M, Luz F, Reller B, Corey G R. The diagnosis of Plasmodium falciparum infection using a new antigen detection system. Am J Trop Med Hyg. 1995;52:45–49. doi: 10.4269/ajtmh.1995.52.45. [DOI] [PubMed] [Google Scholar]
- 10.Dowling M A, Shute G T. A comparative study of thick and thin blood films in the diagnosis of scanty malaria parasitaemia. Bull WHO. 1966;34:249–267. [PMC free article] [PubMed] [Google Scholar]
- 11.Durrheim D N, Becker P J, Billinghurst K. Diagnostic disagreement—the lessons learnt from malaria diagnosis in Mpumalanga. S Afr Med J. 1997;87:1016. [PubMed] [Google Scholar]
- 12.Fiaz M A, Rashid R, Palit R, Rahman M R, Bin Yunus E, Hussain A, Rahman E, Talukdar K R, Bangali A M, Montanari R M. ParaSight-F test results in cerebral malaria patients before and after treatment in Chittagong Medical College, Bangladesh. Trans R Soc Med Hyg. 2000;94:56–57. doi: 10.1016/s0035-9203(00)90439-5. [DOI] [PubMed] [Google Scholar]
- 13.Gautam A S, Sharma R C, Bhatt R M, Gupta D K. Microscopic diagnosis of malaria in Kheda district of Gujarat. Indian J Malariol. 1992;29:83–87. [PubMed] [Google Scholar]
- 14.Humar A, Ohrt C, Harrington M A, Pillai D, Kain K C. Parasight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am J Trop Med Hyg. 1997;56:44–48. doi: 10.4269/ajtmh.1997.56.44. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal J, Sher A, Hira P R, Al-Owaish R. Comparison of the OptiMAL test with PCR for diagnosis of malaria in immigrants. J Clin Microbiol. 1999;37:3644–3646. doi: 10.1128/jcm.37.11.3644-3646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jelinek T, Grobusch M P, Nothdurft H D. Use of dipstick tests for the rapid diagnosis of malaria in nonimmune travelers. J Travel Med. 2000;7:175–179. doi: 10.2310/7060.2000.00055. [DOI] [PubMed] [Google Scholar]
- 17.Jelinek T, Grobusch M P, Schwenke S, Steidl S, von Sonnenburg F, Nothdurft H D, Klein E, Loscher T. Sensitivity and specificity of dipstick tests for rapid diagnosis of malaria inimmune travelers. J Clin Microbiol. 1999;37:721–723. doi: 10.1128/jcm.37.3.721-723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kain K C, Harrington M A, Tennyson S, Keystone J S. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis. 1998;27:142–149. doi: 10.1086/514616. [DOI] [PubMed] [Google Scholar]
- 19.Kilian A H, Kabagambe G, Byamukama W, Langi P, Weis P, von Sonnenburg F. Application of the ParaSight-F dipstick test for malaria diagnosis in a district control program. Acta Trop. 1999;72:281–293. doi: 10.1016/s0001-706x(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 20.Kilian A H, Metzger W G, Mutschelknauss E J, Kabagambe G, Langi P, Korte R, von Sonnenburg F. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop Med Int Health. 2000;5:3–8. doi: 10.1046/j.1365-3156.2000.00509.x. [DOI] [PubMed] [Google Scholar]
- 21.Leke R F, Djokam R R, Mbu R, Leke R J, Fogako J, Megnekou R, Metenou S, Sama G, Zhou Y, Cadigan T, Parra M, Taylor D W. Detection of the Plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J Clin Microbiol. 1999;37:2992–2996. doi: 10.1128/jcm.37.9.2992-2996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lema O E, Carter J Y, Nagelkerke N, Wangai M W, Kitenge P, Gikunda S M, Arube P A, Munafu C G, Materu S F, Adhiambo C A, Mukunza H K. Comparison of five methods of malaria detection in the outpatient setting. Am J Trop Med Hyg. 1999;60:177–182. doi: 10.4269/ajtmh.1999.60.177. [DOI] [PubMed] [Google Scholar]
- 23.Milne L M, Kyi M S, Chiodini P L, Warhurst D C. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J Clin Pathol. 1994;47:740–742. doi: 10.1136/jcp.47.8.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer C J, Lindo J F, Klaskala W I, Quesada J A, Kaminsky R, Baum M K, Ager A L. Evaluation of the OptiMAL test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J Clin Microbiol. 1998;36:203–206. doi: 10.1128/jcm.36.1.203-206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pieroni P, Mills C D, Ohrt C, Harrington M A, Kain K C. Comparison of the ParaSight-F test and the ICT Malaria Pf test with the polymerase chain reaction for the diagnosis of Plasmodium falciparum malaria in travellers. Trans R Soc Trop Med Hyg. 1998;92:166–169. doi: 10.1016/s0035-9203(98)90730-1. [DOI] [PubMed] [Google Scholar]
- 26.Raghavan K. Statistical considerations in the microscopical diagnosis of malaria, with special reference to the role of cross-checking. Bull W H O. 1966;34:788–791. [PMC free article] [PubMed] [Google Scholar]
- 27.Shiff C J, Premji Z, Minjas J N. The rapid manual ParaSight-F test. A new diagnostic tool for Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1993;87:646–648. doi: 10.1016/0035-9203(93)90273-s. [DOI] [PubMed] [Google Scholar]
- 28.Singh N, Valecha N, Sharma V P. Malaria diagnosis by field workers using an immunochromatographic test. Trans R Soc Trop Med Hyg. 1997;91:396–397. doi: 10.1016/s0035-9203(97)90254-6. [DOI] [PubMed] [Google Scholar]
- 29.Stow N W, Torrens J K, Walker J. An assessment of the accuracy of clinical diagnosis, local microscopy and a rapid immunochromatographic card test in comparison with expert microscopy in the diagnosis of malaria in rural Kenya. Trans R Soc Trop Med Hyg. 1999;93:519–520. doi: 10.1016/s0035-9203(99)90359-0. [DOI] [PubMed] [Google Scholar]
- 30.Tjitra E, Suprianto S, Dyer M, Currie B J, Anstey N M. Field evaluation of the ICT Malaria P.f/P.v immunochromatographic test for detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in eastern Indonesia. J Clin Microbiol. 1999;37:2412–2417. doi: 10.1128/jcm.37.8.2412-2417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warhurst D C, Williams J E. ACP broadsheet no. 148. July 1996. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533–538. doi: 10.1136/jcp.49.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wongsrichanalai C, Chuanak N, Tulyayon S, Thanoosingha N, Laoboonchai A, Thimasarn K, Brewer T G, Heppner D G. Comparison of a rapid field immunochromatographic test to expert microscopy for the detection of Plasmodium falciparum asexual parasitemia in Thailand. Acta Trop. 1999;73:263–273. doi: 10.1016/s0001-706x(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Malaria diagnosis: memorandum from a WHO meeting. Bull W H O. 1988;66:575–594. [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. New perspectives: malaria diagnosis. Joint WHO/USAID Informal Consultation. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 35.World Health Organization. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. WHO Informal Consultation on Recent Advances in Diagnostic Techniques and Vaccines for Malaria. Bull W H O. 1996;74:47–54. [PMC free article] [PubMed] [Google Scholar]