Abstract
Nitric oxide (NO) is a key factor in inflammation. Endothelial nitric oxide synthase (eNOS), whose activity increases after stimulation with proinflammatory cytokines, produces NO in endothelium. NO activates two pathways: 1) soluble guanylate cyclase-protein kinase G and 2) S-nitrosylation (NO-induced modification of free-thiol cysteines in proteins). S-nitrosylation affects phosphorylation, localization, and protein interactions. NO is classically described as a negative regulator of leukocyte adhesion to endothelial cells. However, agonists activating NO production induce a fast leukocyte adhesion, which suggests that NO might positively regulate leukocyte adhesion. We tested the hypothesis that eNOS-induced NO promotes leukocyte adhesion through the S-nitrosylation pathway. We stimulated leukocyte adhesion to endothelium in vitro and in vivo using tumor necrosis factor-α (TNF-α) as proinflammatory agonist. ICAM-1 changes were evaluated by immunofluorescence, subcellular fractionation, immunoprecipitation, and fluorescence recovery after photobleaching (FRAP). Protein kinase Cζ (PKCζ) activity and S-nitrosylation were evaluated by Western blot analysis and biotin switch method, respectively. TNF-α, at short times of stimulation, activated the eNOS S-nitrosylation pathway and caused leukocyte adhesion to endothelial cells in vivo and in vitro. TNF-α-induced NO led to changes in ICAM-1 at the cell surface, which are characteristic of clustering. TNF-α-induced NO also produced S-nitrosylation and phosphorylation of PKCζ, association of PKCζ with ICAM-1, and ICAM-1 phosphorylation. The inhibition of PKCζ blocked leukocyte adhesion induced by TNF-α. Mass spectrometry analysis of purified PKCζ identified cysteine 503 as the only S-nitrosylated residue in the kinase domain of the protein. Our results reveal a new eNOS S-nitrosylation-dependent mechanism that induces leukocyte adhesion and suggests that S-nitrosylation of PKCζ may be an important regulatory step in early leukocyte adhesion in inflammation.
NEW & NOTEWORTHY Contrary to the well-established inhibitory role of NO in leukocyte adhesion, we demonstrate a positive role of nitric oxide in this process. We demonstrate that NO induced by eNOS after TNF-α treatment induces early leukocyte adhesion activating the S-nitrosylation pathway. Our data suggest that PKCζ S-nitrosylation may be a key step in this process.
Keywords: leukocyte adhesion, nitric oxide, protein kinase, S-nitrosylation
INTRODUCTION
Nitric oxide (NO) is critical for endothelial function, and it is classically described as an inhibitor of leukocyte adhesion (1–13). NO activates mainly two pathways: 1) soluble guanylate cyclase-protein kinase G (GC-1-PKG) and 2) S-nitrosylation, which is the binding of NO to a thiol group in free cysteines and affects interactions between proteins, phosphorylation, and intracellular trafficking (14–17). NO is produced in endothelial cells by two isoforms: endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS). eNOS is constitutively expressed whereas iNOS expression is induced after sustained stimulation with proinflammatory agonists (18). Normally (in noninflammatory conditions), eNOS produces low NO levels that prevent or minimize leukocyte adhesion to endothelium (2, 4–6). This protective role of eNOS-derived NO is supported by the observation that inhibition of eNOS leads to leukocyte adhesion (7, 10–13). Under inflammatory conditions, high levels of iNOS-derived NO inhibit the expression of adhesion molecules through the S-nitrosylation of NF-κB, which blocks its binding to the target DNA (19).
Leukocyte adhesion is a highly regulated multistep process that involves the expression of adhesion proteins in the endothelium that bind to integrins in leukocytes to finally promote leukocyte extravasation in injured tissues (20–22). Firm leukocyte adhesion to endothelial cells is mediated by intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) that bind to leukocytes’ integrins VLA-4, LFA-1, and Mac-1 (23). Under acute inflammatory conditions, fast recruitment of leukocytes (minutes) involves P-selectin translocation (24), which mediates loose interaction between leukocyte and endothelium. Strong adhesion requires proteins synthesis induced mainly via NF-κB transcriptional regulation (25, 26). A different mechanism (independent of protein synthesis) mediates fast leukocyte adhesion through ICAM-1 clustering that involves lateral association of ICAM-1 molecules and cytoskeletal proteins, improving the affinity of ICAM-1 by its ligands in leukocytes (27–29). ICAM-1 phosphorylation induced by protein kinase Cζ (PKCζ) seems to be crucial for ICAM-1 clustering (27, 28).
Based on these reported observations and considering that some agents [TNF-α, platelet activating factor (PAF), and thrombin] (27, 30, 31) that increase leukocyte adhesion to endothelium at early or short exposure times stimulate the S-nitrosylation pathway to increase endothelial permeability (16, 17, 32), we investigated whether NO-induced S-nitrosylation is a mechanism involved in early leukocyte adhesion to endothelium. Our results support the concept that NO-induced S-nitrosylation is a fundamental process involved in early leukocyte adhesion to endothelium at early stages of inflammation. The mechanism involves PKCζ S-nitrosylation at Cys 503, association of PKCζ with ICAM-1, phosphorylation of ICAM-1, and ICAM clustering at the cell surface. This work demonstrates for the first time the stimulatory role of NO and S-nitrosylation in leukocyte adhesion at the onset of the inflammation.
MATERIALS AND METHODS
Reagents
TNF-α was purchased from Roche. 1H-[1,2, 4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and NG-methyl-l-arginine (l-NMA) were from Sigma Chemicals (St. Louis, MO). PKCζ inhibitor peptide was from Tocris. The working concentrations and application times were TNF-α (1 nM) for the indicated times in each figure, ODQ (10 µM) for 10 min before TNF-α, l-NMA (150 µM) for 45 min before TNF-α, and PKCζ inhibitor peptide (1 µM) for 20 min before TNF-α.
Antibodies
Mouse anti-ICAM-1 and rabbit anti-phospho-ICAM-Y512 were purchased from Santa Cruz. Rabbit anti-PKCζ, rabbit anti-pPKCζ (Thr410), rabbit anti-p-threonine, and rabbit anti-cortactin were from Cell Signaling. Mouse anti-peNOS-Ser1177 was from BD Transduction Laboratories and mouse anti-β-actin was from Sigma.
Intravital Microscopy and Image Analysis
Protocols for experiments on animals were approved by the Institutional Bioethics and Biosecurity Committee of the Universidad Austral de Chile and conducted according to National Institutes of Health (NIH) Guidelines for the use of animals in research. We used five animals in each experimental group. C57BL/6J (Jackson Laboratory, Bar Harbor, MA) male mice were anesthetized with ketamine (100 mg/kg ip)-xylazine (10 mg/kg ip). The cremaster was gently exteriorized through a midline scrotum incision and visualized with an inverted microscope (objective, ×32, 0.4 numerical aperture) equipped with a TV camera (Hamamatsu, Middlesex, NJ) connected to the microscope. Leukocytes adhering to the venular endothelium were recorded for 10 min, whereas a phosphate-buffered saline (PBS) solution was applied topically. One venule was recorded per animal. l-NMA was administered at 50 mg/kg for 30 min via the caudal tail vein in experiments designed to assess the role of NO.
Cell Culture
Immortalized human venous endothelial cells EAhy926 (ATCC CRL2922) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 2.5 µg/mL fungizone and 100 µM sodium hypoxanthine, 0.4 µM aminopterin, and 16 µM thymidine (HAT). EAhy926 cells have been authenticated as endothelial cells by ATCC using morphology, karyotyping, and PCR-based approaches to confirm their identity and to rule out intra- and interspecies contamination. The assays include the detection of species-specific variants of the cytochrome-c oxidase I gene (COI analysis) and short tandem repeat (STR) profiling. We used EAhy926 cells between passages 6–10 and additionally tested them for von Willebrand factor.
Polymorphonuclear Leukocyte Isolation
Whole blood was drawn from healthy human donors (the informed consent protocol was applied previously) on sodium citrate/dextran (6% p/vol; Sigma-Aldrich, St. Louis, MO). Isolation of polymorphonuclear leukocyte (PMN) was performed under sterile conditions using a dextran-PBS density gradient centrifugation technique. The leukocyte-enriched plasma was centrifuged, and the cellular pellet was layered on 50% Percoll solution gradient (Sigma-Aldrich) to separate the neutrophils (33).
Adhesion Assay
EAhy926 cells were grown to confluence on 24-well plates and then stimulated with 1 nM TNF-α in the presence or absence of l-NMA, ODQ, or PKCζ inhibitor peptide. Isolated PMNs were suspended in DMEM media at 1.5 × 106 cells/mL, activated with 1 nM TNF-α for 30 min at 37°C, and incubated with endothelial cells for 30 min at 37°C. Subsequently, unbound PMN were washed three times to remove them. Bound cells were lysed and assayed for myeloperoxidase (MPO) activity adding Hank’s balanced salt solution, 3,3′,5,5′-tetramethylbenzidine, and H2O2. The product was measured spectrophotometrically (Titertek, Multiscan) at 460 nm (33).
Western Blot Analysis
Confluent cells grown in 60-mm or 100-mm plates were serum starved overnight in DMEM 2% (vol/vol) FBS). TNF-α (1 nM) was applied to the cells for different times. Cells were washed two times with ice cold PBS and scraped in 300 µL lysis buffer 1, consisting of 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 1% Triton X-100, and protease and phosphatases inhibitor mixture, and incubated on ice with shaking for 30 min. Lysates were obtained by centrifugation at 10,000 g for 15 min at 4°C. Separated proteins were blotted to PVDF membranes and detected with specific antibodies. Proteins of interest were detected by ECL (Pierce). We analyzed Western blots densitometrically using the NIH ImageJ program.
Real-Time Detection of NO
We used a protocol previously described (34). Confluent EAHy926 cells were loaded with DAF-FM Diacetate (Invitrogen, Cat. No. D-23844) and examined for increases in NO following addition of TNF-α (1 nM). Spinning disk confocal imaging parameters: a 488-nm laser line (20w) atenued at 2% was used for excitation. To minimize exposure time, resolution was reduced at 2 × 2 binning on a CCD-based (EMCCD iXon Life 888, ANDOR, Oxford Instruments) wide field system. Exposure times were 50 ms. Multiple exposures over time were collected at one image per second for 360 s. The imaging sequence was initiated, and 10 s later the stimulus was added. For the analysis, 10 individual regions of interest (ROI) were used for each plate. Data are expressed as the mean fluorescence intensity (F) relative to the mean fluorescence intensity before stimulus application (F0). This value is expressed as F/F0.
ICAM-1 Cell Surface Immunofluorescence Microscopy
EAhy926 cells were cultured on glass coverslips, treated with TNF-α for the indicated times, and then washed with cold Dulbecco’s phosphate-buffered saline (DPBS) Ca2+, Mg2+ pH 7.2 on ice. Coverslips were blocked with 3% BSA in DPBS for 30 min on ice. Thereafter, cells were incubated with anti-ICAM antibody for 4 h at 4°C. After three washes in DPBS, cells were fixed in 100% methanol for 45 min at −20°C. Coverslips were air-dried and incubated with the secondary Alexa Fluor-conjugated antibody for 2 h at room temperature. Cells were extensively washed in DPBS, mounted on glass slides, and images were obtained using an epifluorescence microscope (Axioscop; Carl Zeiss) and Axio Vision Rel. software (Zeiss, Germany). Eight-bit images were prepared for publication in Adobe Photoshop.
Immunoprecipitation
EAhy926 cells stimulated with the different agents were lysed in immunoprecipitation buffer, consisting of 50 mM Tris·HCl (pH 7.4), 1% NP-40, 20 mM EDTA, and protease and phosphatase inhibitors mixture, and passed five times through a 28G1/2 needle. Samples were centrifuged at 14,000 rpm for 3 min at 4°C. Equal amounts of protein in the supernatant from control and TNF-α-treated cells were incubated with specific antibodies 3 h or overnight at 4°C. Protein A/G beads were added to samples for 2 h at 4°C; they were then pelleted by centrifugation and washed five times with immunoprecipitation buffer. Proteins of interest were detected using Western blot analysis and chemiluminescence. Quantification of changes from control was evaluated by densitometric analysis of Western blots using the NIH ImageJ Program.
Subcellular Fractionation
We used protocols previously described (27). Briefly, monolayers were washed in cold Tris-buffered saline (TBS) and lysed in 10 mM Tris·HCl (pH 7.4), 1 mM MgCl2, 5 mM EDTA, 10 mM EGTA, 1 mM NaVO4, and antiprotease mixture. The cell suspension was sonicated at 4°C for 10 s and then centrifuged at 100,000 g for 1 h at 4°C. The supernatant was saved as the cytosolic fraction. The pellet was suspended in lysis buffer with Triton X-100 1% and sonicated and incubated for 30 min at 4°C. Thereafter, suspended pellets were centrifuged at 13,000 rpm for 3 min at 4°C, and this second supernatant was considered as the membrane fraction. Membrane and cytosolic fractions were analyzed by Western blot analysis using anti-ICAM-1 antibody.
ICAM-GFP Dynamics Measurements on the Cell Surface by FRAP
EAhy926 cells were seeded on MatTek live cell imaging glass bottom dishes (MatTek Corporation, Ashland, MA) and transfected transiently with lipofectin (Invitrogen) to express transiently human ICAM-GFP (kindly donated by Dr. Francisco Sánchez-Madrid; Spanish National Center for Cardiovascular Research, Spain). After 48 h, the cells were washed twice with DMEM 10 mM HEPES, pH 7.4, 2% FBS and placed on temperature-controlled chamber UNO-Controller (Okolab SRL, Pozzuoli, Italy) mounted on a TCS SP8 Leica confocal microscope running Leica LAS X controller software (Leica Microsystems, Wetzlar, Germany). Different treatments protocols were applied: TNF-α for 5 min, l-NMA for 45 min, and l-NMA 45 min plus TNF-α 5 min. Fluorescence recovery after photobleaching (FRAP) for GFP was performed as follows: a multiline Argon laser was adjusted to 80% of potency (50 mW) for a single-shot irradiation at 488 nm by using FRAP-booster and zoom-in bleaching on a selected circular 20-pixel diameter region of interest (ROI) placed on ICAM-GFP cell surface located fluorescent signal. Pre- and postbleaching 144.72 × 144.72 µm (1,024 × 1,024 pixels) images were acquired every 3 s using 63× oil-immersion objective and a photomultiplier tube (PMT) gain adjusted to 650, 2 average, and 700 Hz frequency scanning at 1 mW 488 laser potency. Fluorescence recovery was recorded after photobleaching, and the resulting recovery curve was fitted to a single exponential curve using Leica LAS X software quantify tool. Recovery time and mobile fraction were obtained directly from curve fitting.
Biotin-Switch Assay
Total protein (100 µg) obtained from cellular lysates from control and TNF-α treated cells were denatured with sodium dodecyl sulfate (SDS) in the presence of methyl methanothiosulfonate (MMTS) (35). After acetone precipitation to remove excess MMTS, 1 mM ascorbate and 4 mM N-[6-(biotinamido)-hexyl]-3′-(2′-pyridyldithio) propionamide (biotin-HPDP) were added to reduce the S-NO bond and label the reduced thiol with biotin, respectively. Biotinylated proteins were precipitated with acetone, suspended, captured with streptavidin-agarose beads, and then separated by sodium dodecyl sulfate-PAGE. Proteins of interest were detected using Western blot analysis and chemiluminescence. We analyzed Western blots densitometrically using the NIH ImageJ program.
eNOS siRNA Transfection
We used specific siRNA (Ambion, Austin, TX) to deplete eNOS as we described previously (36). We transfected EAHy926 cells with small interfering (si)RNA using lipofectin and following the protocol recommended by the manufacturer (Invitrogen, Carlsbad, CA). We perform the experiment 96-h posttransfection. Western blot analysis was done to confirm eNOS depletion.
Identification of S-Nitrosylated Cysteines in PKCζ
The site of S-nitrosylation in PKCζ was identified by MS/MS mass spectrometry. Five micrograms of recombinant PKCζ (Cat. No. P2273, ThermoFisher) were treated with S-nitrosoglutathione (GSNO) at 37°C for 30 min. After the S-nitrosylation reaction, GSNO was removed, and all the free SH- were blocked by MMTS. Biotin-switch on S-nitrosylated PKCζ was performed with Biotin-HPDP. The result protein was split into two parts. One part was loaded on SDS-PAGE for trypsin digestion; the other part was run in Western blot analysis and detected using antibiotin antibody. Since both MMTS and Biotin-HPDP blocking could be reduced by dithiothreitol (DTT), trypsin digestion was performed without reduction. The peptides were C18 desalted and analyzed by LC-MS/MS on Orbitrap Fusion Lumos instrument. The MS/MS spectra were searched against SwissProt mouse database using Sequest search engines on Proteome Discoverer (V2.4) platform. The protein false discovery rate was less than 1%.
Statistical Analysis
Experiments were conducted in groups with a minimum of five independent experiments (n = 5). Data are expressed as means ± SE. Apparent differences were assessed for statistical significance using GraphPad Prism and Sigma Plot software. Details of the specific method are indicated in each figure. Significance was accepted at P < 0.05.
RESULTS
NO Signaling Regulates Positively Leukocyte Adhesion Induced by TNF-α In Vivo at Short Times of Stimulation
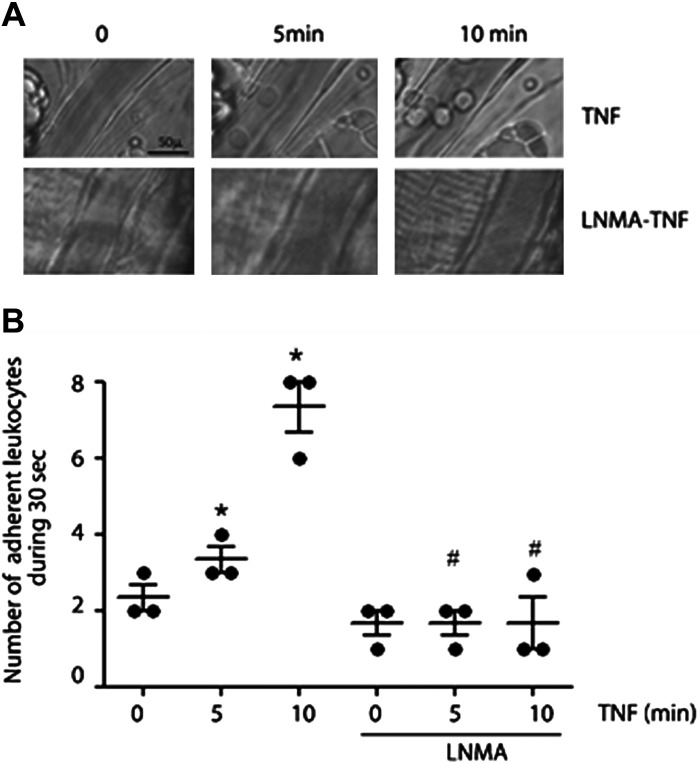
We first determined in vivo the effect of NO inhibition on leukocyte adhesion induced by TNF-α using intravital microscopy in the mouse cremaster muscle (30). We applied 1 nM TNF-α topically to the muscle and recorded leukocyte adherence. Leukocyte adhesion increased significantly at 5 and 10 min after TNF-α, and pretreatment of the mice with the nitric oxide synthases inhibitor l-NMA (50 mg/kg, injected via caudal vein 30 min before muscle exposure) inhibited TNF-α triggered leukocyte adhesion at 5 and 10 min (Fig. 1, A and B). These results demonstrated in vivo that leukocyte adhesion induced by TNF-α requires NO production. Based on the short time of TNF-α administration, we infer that eNOS is the isoform involved in the process.
Figure 1.
NO signaling regulates leukocyte adhesion in vivo. A: intravital microscopy of cremaster showing leukocyte adhesion at 5 and 10 min after treatment with 1 nM TNF-α. Pretreatment with l-NMA (50 mg/kg iv) inhibits TNF-α-induced leukocyte adhesion. B: statistical analysis of leukocyte adhesion. Two-way ANOVA and Tukey test, *P < 0.05 compared with time 0 of treatment with TNF-α; #P < 0.05 in comparison to the treatment with TNF-α. n = 3 different animals. l-NMA, NG-methyl-l-arginine; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
S-Nitrosylation Regulates TNF-α-Induced PMN Adhesion to Endothelial Cells at Short Times of Stimulation
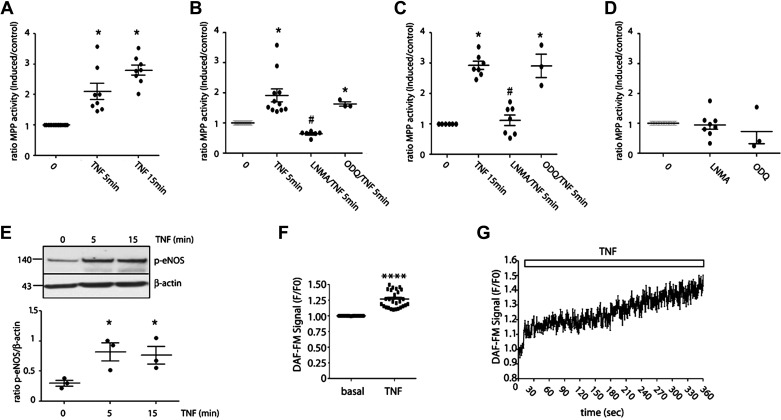
Next, we tested the effects of NO on leukocyte adhesion in vitro. TNF-α, applied for 5 and 15 min, induced significant PMN adhesion measured by the MPO activity (Fig. 2A). To test whether this effect depends on eNOS activation, we inhibited eNOS by incubating EAhy926 cells with l-NMA (150 µM for 45 min), a global nitric oxide synthase inhibitor. Since NO can independently activate sGC-PKG pathway and the S-nitrosylation pathway, we applied ODQ (10 µM for 10 min), a recognized sGC-PKG inhibitor, to differentiate between these two pathways. Inhibition of eNOS, but not inhibition of sGC-PKG, inhibited TNF-α-induced PMN adhesion at 5 min (Fig. 2B) and 15 min (Fig. 2C) indicating that this process depends on S-nitrosylation. The treatment with l-NMA and ODQ alone did not have direct effects on leukocyte adhesion (Fig. 2D). We measured eNOS phosphorylation at Ser1177 to verify activation of the enzyme and to confirm the involvement of eNOS-derived NO. TNF-α induced eNOS phosphorylation at times corresponding with its effects on PMN adhesion (Fig. 2E). To corroborate that eNOS phosphorylation is leading to NO production, we measured NO levels in response to TNF-α using DAF-FM. Figure 3F shows a significant increase in the accumulated fluorescence signal for NO after application of the agonist. Figure 3G illustrates a representative result of one experiment showing the increase in the fluorescence signal for NO in the time. These results demonstrate that the eNOS-NO-S-nitrosylation pathway regulates TNF-α-induced PMN adhesion at short times of stimulation.
Figure 2.
S-nitrosylation regulates early PMN adhesion induced by TNF-α in vitro. A: confluent monolayers of EAhy926 cells were incubated with 1 nM TNF-α for 5 and 15 min. Then, isolated human preactivated PMNs were added to the monolayers and allowed to adhere for 30 min at 37°C. Nonadherent PMN were washed away and MPO activity was assayed in the cell lysates. One-way ANOVA and Tukey’s multiple comparison test. *P < 0.05 compared with time 0. n = 8 independent experiments. Confluent monolayers of EAhy926 cells were preincubated with 300 µM l-NMA for 45 min or 10 µM ODQ for 10 min before TNF-α treatment for 5 (B) and 15 min (C). Increase in leukocyte adhesion induced by TNF-α was blocked by inhibition of eNOS with l-NMA but not by inhibition of GC-1-PKG with ODQ. One-way ANOVA and Bonferroni’s multiple comparison test. *P < 0.05 compared with time 0; #P < 0.05 compared with TNF-α treatment. n = 6 independent experiments. D: confluent monolayers of EAhy926 cells were preincubated with 300 µM l-NMA for 45 min or 10 µM ODQ for 10 min. Neither agent caused leukocyte adhesion. One-way ANOVA and Bonferroni’s multiple comparison test. n = 8 independent experiments. All data were normalized to adhesion seen at time 0. E: TNF-α significantly increases phosphorylation of eNOS at Ser1177 at 5 and 15 min. One-way ANOVA and Bonferroni’s multiple comparison test. *P < 0.05; n = 3 independent experiments. F: real-time detection of NO induced by TNF-α in live EAHy926 cells. Paired t test. ****P < 0.0001; n = 5 independent experiments. G: representative image of one experiment showing the increases in NO levels in the time after TNF-α application. eNOS, endothelial nitric oxide synthase; l-NMA, NG-methyl-l-arginine; NO, nitric oxide; ODQ, 1H-[1,2, 4]oxadiazolo[4,3-a]quinoxalin-1-one; PMN, polymorphonuclear leukocyte; TNF-α, tumor necrosis factor-α.
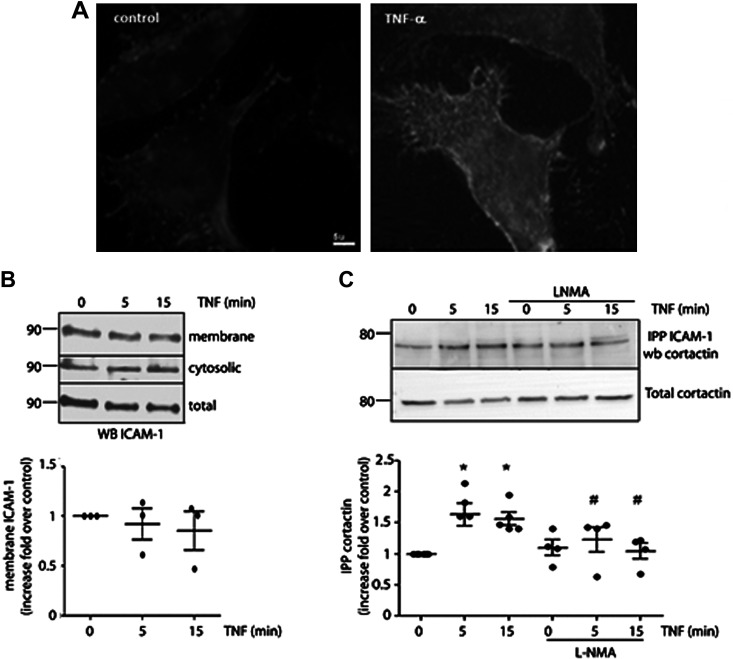
Figure 3.
TNF-α induces ICAM-1 clustering dependent on NO signaling. A: confluent monolayers of EAhy926 cells were incubated with 1 nM TNF-α for 15 min and processed by immunofluorescence to detect ICAM-1 in the cell surface. The images are representative of 3 independent experiments. B: confluent monolayers of EAhy926 cells were incubated with 1 nM TNF-α for different times and membrane and cytosolic fractions were isolated, separated by PAGE-SDS, and probed with anti-ICAM antibodies. One-way ANOVA and Dunnett’s multiple comparison test, n = 3 independent experiments. C: confluent monolayers of EAhy926 cells were incubated with 1 nM TNF-α for different times in the presence or absence of l-NMA; ICAM-1 was immunoprecipitated and the interaction with cortactin evaluated by Western blot analysis. Two-way ANOVA and Tukey’s test, *P < 0.05 in comparison to time 0, #P < 0.05 in comparison to the treatment with TNF-α; n = 4 independent experiments. Data were normalized to band intensity seen at time 0. ICAM-1, intercellular adhesion molecule 1; l-NMA, NG-methyl-l-arginine; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
NO Regulates Positively ICAM-1 Clustering Induced by TNF-α
It has been previously reported that the fast leukocyte adhesion induced by TNF-α is mediated by ICAM-1 clustering, which involves changes in constitutively expressed ICAM-1 improving the affinity of this protein by its ligands in leukocytes (27–29). By immunofluorescence experiments using an anti-ICAM-1 antibody that labels an extracellular epitope of ICAM-1, we observed an increase in ICAM-1 surface label after TNF-α treatment (Fig. 3A) with a punctate label in the cell surface characteristic of clustering. To discard any possible effects of vesicular traffic of ICAM-1 cytosolic pool to the plasma membrane, we performed subcellular fractionation experiments. EAhy926 cells were treated with 1 nM TNF-α for 5 and 15 min and then cytosolic and membrane fractions were analyzed by Western blot analysis. Figure 3B shows that TNF-α treatment did not cause any significant changes in the plasma membrane/cytosol distribution of ICAM-1. These results indicate that the enhanced surface label for ICAM-1 in immunofluorescence experiments are not due to ICAM-1 vesicular traffic to the plasma membrane, but rather to changes in ICAM-1 that improve its affinity for the antibody, which is strongly suggestive of clustering (27). During clustering, ICAM-1 also associates with cytoskeletal proteins such as cortactin (21); therefore, we evaluated the association between ICAM-1 and cortactin after stimulation of EAhy926 cells with TNF-α for 5 and 15 min. We observed that TNF-α increased the association between ICAM-1 and cortactin, which was inhibited by blocking NO production with l-NMA (Fig. 3C).
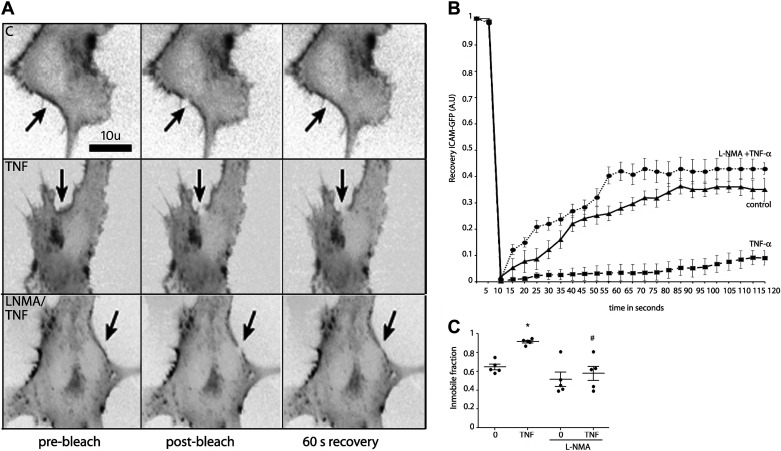
ICAM-1 clustering also increases the immobile fraction of the protein (37); therefore, to further verify ICAM-1 clustering, we performed FRAP experiments. We transfected EAhy926 cells transiently with human ICAM-1-GFP; then, we bleached a small area of the plasma membrane and monitored the fluorescence recovery of ICAM-1-GFP in real time. The results in Fig. 4A show a representative image of ICAM-1-GFP before bleaching, immediately after bleaching, and after recovery in nonstimulated conditions (C, top), under TNF-α treatment (TNF, middle) and with pretreatment with NOS inhibitor, l-NMA, before TNF-α treatment (LNMA/TNF, bottom). After TNF-α treatment (square), fluorescence recovery was significantly less than control (triangle) indicating that TNF-α increased the immobile fraction of ICAM-1 (Fig. 4B). The l-NMA pretreatment (circles) showed a similar recovery curve as control indicating that l-NMA inhibited the effect of TNF-α on the lateral mobility (Fig. 4B). Figure 4C shows the quantification of the immobile fraction indicating that TNF-α treatment increased the immobile fraction, and this effect is inhibited in the presence of l-NMA. These data indicate that ICAM-1 clustering induced by TNF-α depends on NO signaling.
Figure 4.
TNF-α-induced NO increases ICAM-1 immobile fraction. A: ICAM-1-GFP (grayscale, inverted contrast) was expressed in EAhy926 cells, and FRAP was recorded. Arrow depicts the FRAP region. Top shows FRAP without treatment applied. Middle shows ICAM-1-GFP FRAP after treatment with 1 nM TNF-α for 5 min. Bottom shows ICAM-1-GFP FRAP after pretreatment of the cells with l-NMA before TNF-α application for 5 min. B: recovery ICAM-GFP fluorescence curves after different treatments. C: quantification of the FRAP data show increase in the immobile fraction of ICAM-1-GFP following TNF-α treatment and inhibition of this effect by application of l-NMA. Two-way ANOVA and Bonferroni t test. *P < 0.05 in comparison to time 0, #P < 0.05 in comparison to the treatment with TNF-α; n = 5 independent experiments. FRAP, fluorescence recovery after photobleaching; ICAM-1, intercellular adhesion molecule 1; l-NMA, NG-methyl-l-arginine; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
TNF-α Leads to S-Nitrosylation of PKCζ and Regulation of Its Activity
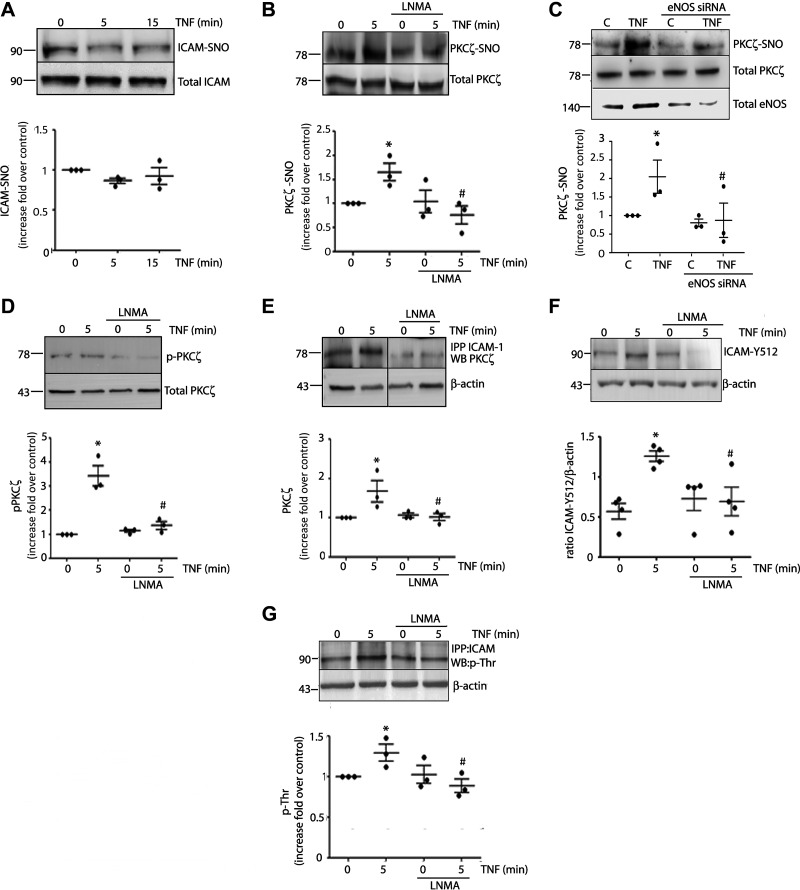
Next, we searched for possible S-nitrosylated proteins that might be responsible for ICAM-1 clustering. We first tested S-nitrosylation of ICAM-1. Figure 5A shows that application of TNF-α for 5 and 15 min failed to elicit S-nitrosylation of ICAM-1. We next tested PKCζ as a possible target because it phosphorylates ICAM-1 and promotes ICAM-1 clustering after stimulation with TNF-α (27, 28). Application of TNF-α for 5 min induced significant PKCζ S-nitrosylation in EAhy926 cells, which was inhibited by l-NMA (Fig. 5B). To confirm that PKCζ S-nitrosylation is being induced by eNOS, we deplete eNOS using siRNA. Figure 5C shows that in the presence of eNOS siRNA, there is an inhibition in PKCζ S-nitrosylation induced by TNF-α (top) coincident with lower expression of eNOS shown in the Western blot (bottom). Since NO-induced S-nitrosylation regulates protein phosphorylation (17, 38) and PKCζ is activated by phosphorylation, we investigated whether or not NO regulates PKCζ phosphorylation. Figure 5D shows that TNF-α significantly increases PKCζ phosphorylation in EAhy926 cells. The increase in PKCζ phosphorylation was blocked by l-NMA indicating that NO, and presumably S-nitrosylation, is required to activate PKCζ. Because S-nitrosylation regulates protein interactions (16, 17, 39) and PKCζ associates with ICAM-1 during ICAM-1 clustering (27, 40, 41), we investigated whether or not S-nitrosylation regulates PKCζ association with ICAM-1. Figure 5E shows that TNF-α (applied for 5 min) causes significant association between PKCζ and ICAM-1 in EAhy926 cells, as demonstrated by enhanced coimmunoprecipitation. Inhibition of NO blocks the association of PKCζ and ICAM-1 induced by TNF-α, indicating that NO production and signaling are required for PKCζ-ICAM-1 association. Finally, we tested ICAM-1 phosphorylation. Figure 5F shows that TNF-α significantly increases ICAM-1 phosphorylation at Tyr 512 and the inhibition of eNOS blocks TNF-α-induced ICAM-1 phosphorylation at this residue. In the same way, Fig. 5G shows that TNF-α treatment for 5 min induced ICAM-1 threonine phosphorylation, which was inhibited in the presence of l-NMA. Taken together, these data indicate that PKCζ is a target of S-nitrosylation by TNF-α and that NO signaling regulates PKCζ activation, the interaction of PKCζ with ICAM-1, and ICAM-1 phosphorylation at tyrosine and threonine.
Figure 5.
TNF-α leads to PKCζ S-nitrosylation, PKCζ activation, association with ICAM-1, and ICAM-1 phosphorylation. A: confluent monolayers of EAhy926 cells were incubated with 1 nM TNF-α for different times. Cell lysates were obtained and processed by biotin switch to detect S-nitrosylated ICAM-1. One-way ANOVA and Tukey’s test, n = 3 independent experiments. B: confluent monolayers of EAhy926 cells were incubated with 1 nM TNF-α for 5 min in the presence or absence of 300 µM l-NMA for 1 h. Cell lysates were obtained and processed to detect S-nitrosylated PKCζ by biotin switch. C: depletion of eNOS in EAHy926 cells abrogates PKCζ S-nitrosylation induced by TNF-α. EAHy926 cells transfected with scrambled siRNA served as a control. Western blot for eNOS confirms protein depletion. One-way ANOVA and Dunnett’s multiple comparison test, n = 3 independent experiments; *P < 0.05 in comparison to control (C), #P < 0.05 in comparison to TNF-α treatment in the absence of eNOS siRNA. Data were normalized at band intensity seen at control without eNOS siRNA treatment. Confluent monolayers of EAhy926 cells were incubated with 1 nM TNF-α for 5 min in the presence or absence of 300 µM l-NMA for 1 h. Cell lysates were obtained and processed to detect phosphorylated p-PKCζ by Western blot analysis (D) and interaction between ICAM-1 and PKCζ by immunoprecipitation (E). Two-way ANOVA and Holm–Sidak test, *P < 0.05 in comparison to time 0, #P < 0.05 in comparison to the treatment with TNF-α; n = 3 independent experiments. All data were normalized at band intensity seen at time 0. F: ICAM-Y512 by Western blot analysis. Two-way ANOVA and Holm–Sidak test, *P < 0.05 in comparison to time 0, #P < 0.05 in comparison to the treatment with TNF-α; n = 4 independent experiments. G: ICAM-1 Thr phosphorylation by immunoprecipitation with anti ICAM-1 antibody, followed by Western blot analysis against p-Thr. Two-way ANOVA and Holm–Sidak test, *P < 0.05 in comparison to time 0, #P < 0.05 in comparison to the treatment with TNF-α; n = 3 independent experiments. Data were normalized at band intensity seen at time 0. eNOS, endothelial nitric oxide synthase; ICAM-1, intercellular adhesion molecule 1; l-NMA, NG-methyl-l-arginine; PKCζ, protein kinase Cζ; TNF-α, tumor necrosis factor-α.
PKCζ Regulates Leukocyte Adhesion
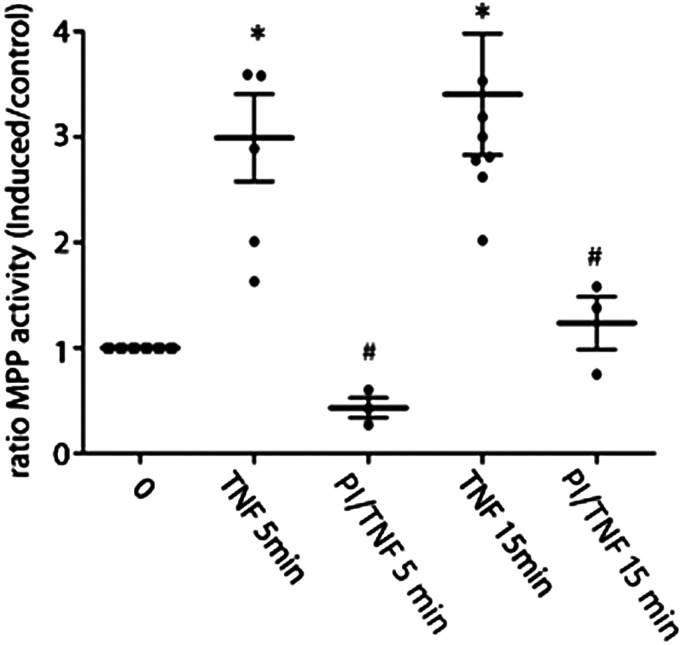
To demonstrate that PKCζ regulates leukocyte adhesion, we use a myristoylated membrane-permeable peptide that specifically inhibits PKCζ in adhesion assays (27). Figure 6 shows that the pretreatment of EAhy926 cells with the inhibitor peptide of PKCζ significantly reduces the early endothelial adhesion induced by TNF-α after 5 and 15 min of stimulation. These results demonstrate that PKCζ activation is required for leukocyte adhesion induced by TNF-α.
Figure 6.
PKCζ regulates leukocyte adhesion. Confluent monolayers of EAhy926 cells were preincubated with 1 µM inhibitor peptide for 20 min before TNF-α treatment for 5 and 15 min. Then, isolated human preactivated PMNs were added to the monolayers and allowed to adhere for 30 min at 37°C. Nonadherent PMNs were washed away and MPO activity was assayed in the cell lysates. Two-way ANOVA and Holm–Sidak test, *P < 0.05 in comparison to time 0, #P < 0.05 in comparison to the treatment with TNF-α; n = 3 independent experiments. Data were normalized at adhesion seen at time 0. MPO, myeloperoxidase; PKCζ, protein kinase Cζ; PMN, polymorphonuclear leukocyte; TNF-α, tumor necrosis factor-α.
NO Leads to S-Nitrosylation of PKCζ in Cys 503
To determine the cysteines nitrosylated by NO in PKCζ, we exposed 5 µg of purified PKCζ to S-nitrosoglutathione as a source of bioavailable NO. Analysis by mass spectroscopy identified Cys 503 as the only S-nitrosylated cysteine in PKCζ (Fig. 7). Cys 503 locates at the protein kinase domain of the protein (unit prot), and therefore, the modification of this cysteine may affect the kinase activity of the protein.
Figure 7.

Identification of S-nitrosylated Cys 503 in PKCζ. Protein sequence from human PKCζ. Purified PKCζ was S-nitrosylated with GSNO for 30 min, subjected to biotin switch assay followed by in-solution trypsin digestion. The kinase domain of PKCζ is shown in gray background. Cysteine 503 (red background) was the only S-nitrosylated cysteine according to mass spectrometry. GSNO, S-nitrosoglutathione; PKCζ, protein kinase Cζ.
DISCUSSION
We demonstrated a new NO-mediated regulatory mechanism of early leukocyte adhesion that requires eNOS-mediated S-nitrosylation at the beginning of the inflammatory response. In vivo, TNF-α-induced NO promoted leukocyte adhesion at the onset of the inflammatory response in the mouse cremaster. In vitro, TNF-α induced a rapid increase in leukocyte adhesion that was dependent on eNOS activation of the S-nitrosylation pathway. TNF-α-induced NO promoted changes characteristic of clustering in constitutively expressed ICAM1. TNF-α-induced NO also induced S-nitrosylation of PKCζ, increased PKCζ activity, improved association between PKCζ and ICAM-1, and ICAM-1 phosphorylation at Tyr and Thr. The inhibition of PKCζ blocked leukocyte adhesion induced by TNF-α. We identified Cys 503 as the residue S-nitrosylated in PKCζ.
Our results agree with other reports showing leukocyte adhesion at short times of stimulation using TNF-α as stimuli (27, 28, 37) and highlight eNOS-induced S-nitrosylation as an important regulator of this process. Previously, some reports demonstrated a positive role of NO in leukocyte adhesion; however, they focused on transcriptional regulation of adhesion proteins (42, 43). Our study is the first one demonstrating a positive role of NO in leukocyte adhesion at the onset of inflammation, independently of transcriptional regulation of adhesion molecules. Other studies have also demonstrated a role of nitric oxide in the transmigration process (44–47). However, leukocyte adhesion was not affected by NO inhibition in these studies.
Through cell surface immunofluorescence, we demonstrated that after TNF-α treatment, ICAM-1 shows a punctuate pattern in the cell surface. However, experiments of subcellular fractionation demonstrated that ICAM-1 did not increase its association with membrane fractions after TNF-α treatment. These results suggest that TNF-α treatment induces ICAM-1 clustering since clustering of adhesion proteins has been defined as the lateral association of proteins and does not involve traffic from intracellular pools to the plasma membrane (27, 29). ICAM-1 clustering strengthens the association between ICAM-1 and cytoskeletal actin-binding proteins and prevents shear stress detachment of adhered leukocytes under flow and favors firm attachment and transmigration (21, 48–51). In fact, a decrease in adhesion mechanisms associated with decreased neutrophil extravasation was reported in postcapillary venules of the cremaster of cortactin-deficient mice stimulated with TNF-α (52). We demonstrated that TNF-α induced a fast association between cortactin and ICAM-1, and that this interaction was inhibited by blocking NO production with l-NMA. In addition, we determined reduced lateral mobility of ICAM-1 after stimulation of endothelial cells through FRAP experiments. The increased immobile fraction probably accounts for the ICAM-1 fraction associated with cytoskeletal proteins during clustering. NOS inhibition blocked the increases of ICAM-1 immobile fraction strongly pointing that the clustering of the protein depends on NO signaling.
As our in vitro experiments showed dependency on the S-nitrosylation pathway for ICAM-1 clustering, we investigated S-nitrosylation of ICAM-1 in response to TNF-α treatment. ICAM-1 was not S-nitrosylated despite having several cysteines. A possible explanation is that S-nitrosylation requires closeness of NOS with its substrates (16, 32, 38) and cysteines in ICAM-1 have an extracellular location; therefore, NO produced intracellularly maybe not reach the adequate concentration in the extracellular space to S-nitrosylate cysteines in ICAM-1. Next, we focused our attention on PKCζ—a kinase that is regulated functionally via thiol groups (53) and phosphorylates ICAM-1 leading to clustering and leukocyte adhesion at short times of stimulation (27, 28). Our results demonstrated that PKCζ is S-nitrosylated at times that correlates with leukocyte adhesion and ICAM-1 clustering. This S-nitrosylation was dependent on NO induced by eNOS since depletion of this enzyme with siRNA treatment inhibited the increase in PKCζ S-nitrosylation induced by TNF-α treatment. Importantly, PKCζ S-nitrosylation correlated with activation of PKCζ measured as its phosphorylation at Thr 410 and with improved interaction of this protein with ICAM-1. Previously, it has been demonstrated that PKCζ mediates ICAM-1 phosphorylation in Tyr 512 through Src activation (28) and may also induce phosphorylation in three threonines located in ICAM-1 cytosolic tail (27). Our results showed that TNF-α-induced NO positively regulated ICAM-1 phosphorylation in Tyr 512 and threonine at times coincident with PKCζ S-nitrosylation and early leukocyte adhesion. We also demonstrated the key role of PKCζ in early leukocyte adhesion in in vitro adhesion assays where the administration of a specific PKCζ blocking peptide inhibited leukocyte adhesion induced by TNF-α at 5 and 15 min of treatment. Since it is well known that PKCζ induces ICAM-1 clustering (27, 28) to promote leukocyte adhesion, these data reinforced the concept that this early adhesion is produced by ICAM-1 clustering.
The above data suggest that PKCζ S-nitrosylation regulates its own activation, its interaction with ICAM-1, and its kinase activity on ICAM-1. PKCζ has 16 cysteines in the four different domains of the protein. Our in vitro mass spectrometry analysis identified Cys 503 as the only S-nitrosylated cysteine in PKCζ. Cys 503 is in the kinase domain of the protein (54) and therefore its S-nitrosylation might contribute to PKCζ kinase activity in vivo. It is also possible that S-nitrosylation in this site promotes a conformational change that contributes to an improved interaction with ICAM-1 to induce its phosphorylation. S-nitrosylation has been shown to either promote or inhibit protein phosphorylation depending on the specific protein (17, 55, 56). Our data contradict previous reports showing that NO induces inactivation of PKC and that S-nitrosylation of PKC inhibits its activity (57, 58). These results were obtained with PKCα; therefore, we can argue that differential regulation by S-nitrosylation may occur in different PKC isoforms.
Our investigation demonstrates a signaling pathway where NO is positioned upstream of ICAM-1 and regulates leukocyte adhesion by S-nitrosylation. Other studies showed a pathway in which ICAM-1 is upstream of eNOS to promote leukocyte transmigration (44, 45). In this pathway, ICAM-1 activation by ligation or crosslinking led to eNOS phosphorylation, NO production and VE-cadherin or PECAM phosphorylation causing leukocyte transmigration (45). In both studies, eNOS inhibition had no effect on leukocyte adhesion. The apparent discrepancies may be due to the different experimental setup used. We used endothelial cells stimulated with TNF-α to induce the activation of endogenous ICAM-1. The other studies used ICAM overexpressed in endothelial cells and crosslinking with antibodies to activate ICAM-1. Another important difference is the timing at which leukocyte adhesion was observed. In our experiments, we observed our effect from 5 to 15 min. In contrast, the other studies measure leukocyte adhesion at 30 min and 1 h. Our data suggest a pathway where TNF-α increases NO production, which induces ICAM-1 clustering through PKCζ S-nitrosylation. Based on the timing of stimulation (5–15 min), eNOS is the isoform responsible for NO production, which was demonstrated by eNOS phosphorylation at Ser1177 in response to TNF-α. Furthermore, the fact that PKCζ is S-nitrosylated is a strong indication that endothelial NO is produced. Even though recent evidence indicates that eNOS phosphorylation not always accounts for NO production (59), we have before demonstrated that TNF-α induces NO production in EAHy926 cells at short times of stimulation (16). It is possible that ICAM-1 in the clustering-adopted conformation may positively stimulate eNOS to further produce NO and more ICAM-1 clustering or VE-cadherin/PECAM phosphorylation. This way, the positive feedback on eNOS activity may regulate both leukocyte adhesion and transmigration.
Our study reporting NO as a signal that promotes leukocyte adhesion is in strong contrast with studies showing an inhibitory role of NO in leukocyte adhesion (1–13). In this regard, we must consider that the regulation of leukocyte adhesion to endothelial cells by NO is complex. We recently reviewed in detail this complexity (60), and we suggested that besides the differences in the cell and animal models used, this apparent discrepancy can be explained by the fact that the physiological effects of NO depend strongly on its concentration, which is in direct relationship to its localization (16, 32, 38, 69). In nonstimulated cells, basal NO production generated by eNOS located to the Golgi and caveolae maintains an antiadhesive phenotype by basal S-nitrosylation of proteins or mechanisms mediated by PKG, which require low levels of NO (61–64). In stimulated conditions, we have previously demonstrated that eNOS moves to the cytosol and releases higher concentrations of NO leading to S-nitrosylation of different proteins (16, 32, 65, 66). S-nitrosylation of PKCζ, as we demonstrated here, might promote leukocyte adhesion through ICAM-1 phosphorylation and clustering. Prolonged inflammatory stimulation induces iNOS expression (18). The high levels of NO produced by iNOS S-nitrosylate NFKB inhibiting its binding to DNA resulting in inhibition of leukocyte adhesion as a feedback negative mechanism (19). Thus, low concentrations of NO achieved by eNOS activation and short exposure times to agonists increase adhesion protein expression, whereas higher NO concentrations achieved by iNOS stimulation and longer times of stimulation will inhibit protein adhesion expression (67, 68). Thus, the reason why our results differ from others in the literature is because the short times of stimulation that we have used (5–15 min) focusing on the beginning of the inflammatory response.
Our study breaks the classic paradigm that NO inhibits leukocyte adhesion and reveals S-nitrosylation as an important mechanism that induces leukocyte adhesion at the onset of inflammation. Our results will help to better understand the early stages of the inflammatory process during host defense.
GRANTS
This work was financed by Vicerrectoría de Investigación, Desarrollo y Creación Artística (VIDCA)-Universidad Austral de Chile (UACh) Grant 2020 (to F.A.S.); National Fund for Scientific and Technological Development (FONDECYT) Grant 1201635 (to F.A.S., F.C., G.A., T.K., and P.E.); FONDECYT National Commission for Scientific and Technological Research (CONICYT) Grant PFB12/2007 (to G.A.); Programa de Apoyo a Centros con Financiamiento Basal Grant AFB 170005; and National Institutes of Health (NIH) Grants R56HL134842-01 (to W.N.D., P.E.M., and N.G.A.), R01HL146539 (to W.N.D., P.E.M., N.G.A.), HL087823, and NIH GM122940. The mass spectrometry data were obtained from an Orbitrap mass spectrometer funded in part by NIH Grants NS046593 and 1S10OD025047-01, for the support of proteomics research at Rutgers Biomedical and Health Sciences, Newark Campus. The imaging for DAF was obtained with a spinning disk microscope funded by (UMFLUCEL) Fondequip EQM 1501118 (to J.S.), Instituto de Fisiología, Facultad de Medicina, UACh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.A., P.E., F.A.S., and J.C. conceived and designed research; G.A., N.G.A., P.E.M., P.E., F.A.S., F.C., T.K., J.S., M.P.B., J.C., and A.S. performed experiments; G.A., P.E., F.A.S., F.C., T.K., J.S., and J.C. analyzed data; W.N.D., P.E., F.A.S., and J.S. interpreted results of experiments; F.A.S., J.S., and J.C. prepared figures; W.N.D. and F.A.S. drafted manuscript; G.A., W.N.D., P.E., F.A.S., M.P.B., K.B., and M.V.-G. edited and revised manuscript; G.A., N.G.A., P.E.M., W.N.D., P.E., F.A.S., F.C., T.K., J.S., M.P.B., K.B., J.C., M.V.-G., and A.S. approved final version of manuscript.
REFERENCES
- 1.Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res 72: 403–412, 1993. doi: 10.1161/01.RES.72.2.403. [DOI] [PubMed] [Google Scholar]
- 2.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. [PubMed] [Google Scholar]
- 3.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res 65: 1–21, 1989. doi: 10.1161/01.RES.65.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655, 1991. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moncada S. The 1991 Ulf von Euler lecture. The l-arginine: nitric oxide pathway. Acta Physiol Scand 145: 201–227, 1992. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsao PS, McEvoy LM, Drexler H, Butcher EC, Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by l-arginine. Circulation 89: 2176–2182, 1994. doi: 10.1161/01.CIR.89.5.2176. [DOI] [PubMed] [Google Scholar]
- 7.Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology 107: 1050–1058, 1994. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 8.Hickey MJ, Granger DN, Kubes P. Inducible nitric oxide synthase (iNOS) and regulation of leucocyte/endothelial cell interactions: studies in iNOS- deficient mice. Acta Physiol Scand 173: 119–126, 2001. doi: 10.1046/j.1365-201X.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- 9.Hickey MJ, Sharkey KA, Sihota EG, Reinhardt PH, Macmicking JD, Nathan C, Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte–endothelium interactions in endotoxemia. FASEB J 11: 955–964, 1997. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- 10.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, Huang PL, Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol 276: H1943–H1950, 1999. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 11.Hossain M, Qadri SM, Liu L. Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J Inflamm (Lond) 9: 28, 2012. doi: 10.1186/1476-9255-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu S, Zhou X, Yuan D, Xu Y, He P. Caveolin-1 scaffolding domain promotes leukocyte adhesion by reduced basal endothelial nitric oxide-mediated ICAM-1 phosphorylation in rat mesenteric venules. Am J Physiol Heart Circ Physiol 305: H1484–H1493, 2013. doi: 10.1152/ajpheart.00382.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao F, Lucke-Wold BP, Li X, Logsdon A, Xu LC, Xu S, LaPenna KB, Wang H, Talukder MAH, Siedlecki CA, Huber JD, Rosen CL, He P. Reduction of endothelial nitric oxide increases the adhesiveness of constitutive endothelial membrane ICAM-1 through Src-mediated phosphorylation. Front Physiol 8: 1124, 2017. doi: 10.3389/fphys.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron 46: 533–540, 2005. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA 89: 444–448, 1992. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marín N, Zamorano P, Carrasco R, Mujica P, González FG, Quezada C, Meininger CJ, Boric MP, Durán WN, Sánchez FA. S-Nitrosation of β-catenin and p120 catenin: a novel regulatory mechanism in endothelial hyperpermeability. Circ Res 111: 553–563, 2012. doi: 10.1161/CIRCRESAHA.112.274548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guequén A, Carrasco R, Zamorano P, Rebolledo L, Burboa P, Sarmiento J, Boric MP, Korayem A, Durán WN, Sánchez FA. S-nitrosylation regulates VE-cadherin phosphorylation and internalization in microvascular permeability. Am J Physiol Heart Circ Physiol 310: H1039–H1044, 2016. doi: 10.1152/ajpheart.00063.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall HE, Stamler JS. Inhibition of NF-κ B by S-nitrosylation. Biochemistry 40: 1688–1693, 2001. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 20.Fan Z, Ley K. Leukocyte arrest: biomechanics and molecular mechanisms of β2 integrin activation. Biorheology 52: 353–377, 2015. doi: 10.3233/BIR-15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer A, Te Riet J, Ritz K, Hoogenboezem M, Anthony EC, Mul FP, de Vries CJ, Daemen MJ, Figdor CG, van Buul JD, Hordijk PL. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J Cell Sci 127: 4470–4482, 2014. [Erratum in J Cell Sci. 2014 Nov 15;127: 4985, 2014] doi: 10.1242/jcs.154708. [DOI] [PubMed] [Google Scholar]
- 22.Kreuger J, Phillipson M. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov 15: 125–142, 2016. doi: 10.1038/nrd.2015.2. [DOI] [PubMed] [Google Scholar]
- 23.Leick M, Azcutia V, Newton G, Luscinskas FW. Leukocyte recruitment in inflammation: basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res 355: 647–656, 2014. doi: 10.1007/s00441-014-1809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689, 2007. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 25.Ala A, Dhillon AP, Hodgson HJ. Role of cell adhesion molecules in leukocyte recruitment in the liver and gut. Int J Exp Pathol 84: 1–16, 2003. doi: 10.1046/j.1365-2613.2003.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger DN, Senchenkova E. Chapter 7 leukocyte–endothelial cell adhesion. Inflammation and the Microcirculation. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. doi: 10.4199/C00013ED1V01Y201006ISP008. [DOI] [PubMed] [Google Scholar]
- 27.Javaid K, Rahman A, Anwar KN, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-α induces early-onset endothelial adhesivity by protein kinase Cζ dependent activation of intercellular adhesion molecule-1. Circ Res 92: 1089–1097, 2003. doi: 10.1161/01.RES.0000072971.88704.CB. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Vogel SM, Gao X, Javaid K, Hu G, Danilov SM, Malik AB, Minshall RD. Src phosphorylation of endothelial cell surface intercellular adhesion molecule-1 mediates neutrophil adhesion and contributes to the mechanism of lung inflammation. Arterioscler Thromb Vasc Biol 31: 1342–1350, 2011. doi: 10.1161/ATVBAHA.110.222208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermanowski-Vosatka A, Detmers PA, Götze O, Silverstein SC, Wright SD. Clustering of ligand on the surface of a particle enhances adhesion to receptor-bearing cells. J Biol Chem 263: 17822–17827, 1988. doi: 10.1016/S0021-9258(19)77909-5. [DOI] [PubMed] [Google Scholar]
- 30.Dillon PK, Fitzpatrick MF, Ritter AB, Duran WN. Effect of platelet-activating factor on leukocyte adhesion to microvascular endothelium. Time course and dose-response relationships. Inflammation 12: 563–573, 1988. doi: 10.1007/BF00914318. [DOI] [PubMed] [Google Scholar]
- 31.Sugama Y, Tiruppathi C, offakidevi K, Andersen TT, Fenton JW 2nd, Malik AB. Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. J Cell Biol 119: 935–944, 1992. doi: 10.1083/jcb.119.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamorano P, Marín N, Córdova F, Aguilar A, Meininger C, Boric MP, Golenhofen N, Contreras JE, Sarmiento J, Durán WN, Sánchez FA. S-nitrosylation of VASP at cysteine 64 mediates the inflammation-stimulated increase in microvascular permeability. Am J Physiol Heart Circ Physiol 313: H66–H71, 2017. doi: 10.1152/ajpheart.00135.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, Bhoola KD, Figueroa CD. Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol 80: 117–124, 2006. doi: 10.1189/jlb.1205744. [DOI] [PubMed] [Google Scholar]
- 34.Tjalkens RB, Carbone DL, Wu G. Detection of nitric oxide formation in primary neural cells and tissues. Methods Mol Biol 758: 267–277, 2011. doi: 10.1007/978-1-61779-170-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001: pl1, 2001. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez FA, Kim DD, Durán RG, Meininger CJ, Durán WN. Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules. Am J Physiol Heart Circ Physiol 295: H1642–H1648, 2008. doi: 10.1152/ajpheart.00629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Buul JD, van Rijssel J, van Alphen FP, Hoogenboezem M, Tol S, Hoeben KA, van Marle J, Mul EP, Hordijk PL. Inside-out regulation of ICAM-1 dynamics in TNF-α-activated endothelium. PLoS One 5: e11336, 2010. doi: 10.1371/journal.pone.0011336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Groszmann RJ, Shah VH, Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA 103: 19777–19782, 2006. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamorano P, Koning T, Oyanadel C, Mardones GA, Ehrenfeld P, Boric MP, González A, Soza A, Sánchez FA. Galectin-8 induces endothelial hyperpermeability through the eNOS pathway involving S-nitrosylation-mediated adherens junction disassembly. Carcinogenesis 40: 313–323, 2019. doi: 10.1093/carcin/bgz002. [DOI] [PubMed] [Google Scholar]
- 40.Wickley PJ, Ding X, Murray PA, Damron DS. Propofol-induced activation of protein kinase C isoforms in adult rat ventricular myocytes. Anesthesiology 104: 970–977, 2006. doi: 10.1097/00000542-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334: 661–665, 1988. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 42.Radisavljevic Z, Avraham H, Avraham S. Vascular endothelial growth factor up-regulates ICAM expression via the phosphatidylinositol 3OH-kinase/AKT/Nitric oxide pathway and modulates migration of brain microvascular endothelial cells. J Biol Chem 275: 20770–20774, 2000. doi: 10.1074/jbc.M002448200. [DOI] [PubMed] [Google Scholar]
- 43.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Weigand SJ, Rudge J, Yancpoulos GD, Adamis AP. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 160: 501–509., 2002. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinelli R, Gegg M, Longbottom R, Adamson P, Turowski P, Greenwood J. ICAM-1-mediated endothelial nitric oxide synthase activation via calcium and AMP-activated protein kinase is required for transendothelial lymphocyte migration. Mol Biol Cell 20: 995–1005, 2009. doi: 10.1091/mbc.e08-06-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu G, Place AT, Chen Z, Brovkovych VM, Vogel SM, Muller WA, Skidgel RA, Malik AB, Minshall RD. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 120: 1942–1952, 2012. doi: 10.1182/blood-2011-12-397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isenberg J, Tabatabai N, Spinelli HM. Nitric oxide modulation of low-density mononuclear cell transendothelial migration. Microsurgery 25: 452–456., 2005. doi: 10.1002/micr.20144. [DOI] [PubMed] [Google Scholar]
- 47.Franco-Penteado CF, Desouza I, Teixeira SA, Ribeiro-DaSilva G, De Nucci G, Antunes E. Role of nitric oxide on the increased vascular permeability and neutrophil accumulation induced by staphylococcal enterotoxin B into the mouse paw. Biochem Pharmacol 61: 1305–1311, 2001. doi: 10.1016/S0006-2952(01)00573-1. [DOI] [PubMed] [Google Scholar]
- 48.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J 16: 1257–1259, 2002. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 49.Scott HA, Quach B, Yang X, Ardekani S, Cabrera AP, Wilson R, Messaoudi-Powers I, Ghosh K. Matrix stiffness exerts biphasic control over monocyte-endothelial adhesion via Rho-mediated ICAM-1 clustering. Integr Biol (Camb) 8: 869–878, 2016. doi: 10.1039/c6ib00084c. [DOI] [PubMed] [Google Scholar]
- 50.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 157: 1233–1245, 2002. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Buul JD, Kanters E, Hordijk PL. Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler Thromb Vasc Biol 27: 1870–1876, 2007. doi: 10.1161/ATVBAHA.107.145821. [DOI] [PubMed] [Google Scholar]
- 52.Schnoor M, Lai FPL, Zarbock A, Kläver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med 208: 1721–1735, 2011. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kikkawa U, Ogita K, Ono Y, Asaoka Y, Shearman MS, Fujii T, Ase K, Sekiguchi K, Igarashi K, Nishizuka Y. The common structure and activities of four subspecies of rat brain protein kinase C family. FEBS Lett 223: 212–216, 1987. doi: 10.1016/0014-5793(87)80291-0. [DOI] [PubMed] [Google Scholar]
- 54.Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem 133: 1–7, 2003. [Erratum in J Biochem (Tokyo) 133: 395, 2003] doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 55.Erickson JR, Nichols C, Uchinoumi H, Stein ML, Bossuyt J, Bers DM. S-nitrosylation induces both autonomous activation and inhibition of calcium/calmodulin-dependent protein kinase II δ. J Biol Chem 290: 25646–25656, 2015. doi: 10.1074/jbc.M115.650234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Switzer CH, Glynn S A, Cheng RY, Ridnour LA, Green JE, Ambs S, Wink DA. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol Cancer Res 10: 1203–1215, 2012. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gopalakrishna R, Chen ZH, Gundimeda U. Nitric oxide and nitric oxide-generating agents induce a reversible inactivation of protein kinase C activity and phorbol ester binding. J Biol Chem 268: 27180–27185, 1993. doi: 10.1016/S0021-9258(19)74235-5. [DOI] [PubMed] [Google Scholar]
- 58.Choi H, Tostes RC, Webb RC. S-nitrosylation Inhibits protein kinase C-mediated contraction in mouse aorta. J Cardiovasc Pharmacol 57: 65–71, 2011. doi: 10.1097/FJC.0b013e3181fef9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eroglu E, Saravi SS, Sorrentino A, Steinhorn B, Michel T. Discordance between eNOS phosphorylation and activation revealed by multispectral imaging and chemogenetic methods. Proc Natl Acad Sci USA 40: 20210–20217, 2019. doi: 10.1073/PNAS.1910942116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aguilar G, Koning T, Ehrenfeld P, Sánchez FA. Role of NO and S-nitrosylation in the expression of endothelial adhesion proteins that regulate leukocyte and tumor cell adhesion. Front Physiol 11: 595526, 2020. doi: 10.3389/fphys.2020.595526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian Z, Gelzer-Bell R, Yang Sx SX, Cao W, Ohnishi T, Wasowska BA, Hruban RH, Rodriguez ER, Baldwin WM 3rd, Lowenstein CJ. Inducible nitric oxide synthase inhibition of weibel-palade body release in cardiac transplant rejection. Circulation 104: 2369–2375, 2001. doi: 10.1161/hc4401.098471. [DOI] [PubMed] [Google Scholar]
- 62.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115: 139–150, 2003. doi: 10.1016/S0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol 26: 1015–1021, 2006. doi: 10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]
- 64.Feron O, Balligand JL. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc Res 69: 788–797, 2006. doi: 10.1016/j.cardiores.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Sánchez FA, Savalia NB, Durán RG, Lal BK, Boric MP, Durán WN. Functional significance of differential eNOS translocation. Am J Physiol Heart Circ Physiol 291: H1058–H1064, 2006. doi: 10.1152/ajpheart.00370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sánchez FA, Rana R, González FG, Iwahashi T, Durán RG, Fulton DJ, Beuve AV, Kim DD, Durán WN. Functional significance of cytosolic endothelial nitric-oxide synthase (eNOS): regulation of hyperpermeability. J Biol Chem 286: 30409–30414, 2011. doi: 10.1074/jbc.M111.234294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Umansky V, Hehner SP, Dumont A, Hofmann TG, Schirrmacher V, Dröge W, Schmitz ML. Co-stimulatory effect of nitric oxide on endothelial NF-κB implies a physiological self-amplifying mechanism. Eur. J. Immunol 28: 2276–2282, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 68.Sektioglu IM, Carretero R, Bender N, Bogdan C, Garbi N, Umansky V, Umansky L, Urban K, von Knebel-Döberitz M, Somasundaram V, Wink D, Beckhove P, Hämmerling GJ. Macrophage-derived nitric oxide initiates T-cell diapedesis and tumor rejection. Oncoimmunology 5: e1204506, 2016. doi: 10.1080/2162402X.2016.1204506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, Gordon DM, Meininger CJ, Durán WN. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc Natl Acad Sci USA 106: 6849–6853, 2009. doi: 10.1073/pnas.0812694106. [DOI] [PMC free article] [PubMed] [Google Scholar]