Abstract
Cellular aging is accompanied by alterations in gene expression patterns. Here, using two models of replicative senescence, we describe the influence of the RNA-binding protein HuR in regulating the expression of several genes whose expression decreases during senescence. We demonstrate that HuR levels, HuR binding to target mRNAs encoding proliferative genes, and the half-lives of such mRNAs are lower in senescent cells. Importantly, overexpression of HuR in senescent cells restored a “younger” phenotype, while a reduction in HuR expression accentuated the senescent phenotype. Our studies highlight a critical role for HuR during the process of replicative senescence.
Human diploid fibroblasts exhibit a finite life span in culture. Following a limited number of cell divisions, they exit the cell cycle and may remain viable for long periods in a state known as replicative senescence (3). As this process is thought to reflect aspects of organismal aging, human primary cells have been used extensively to study cellular proliferation, immortality, and growth arrest. Over the years, and employing a variety of model systems, investigators have reported collections of genes whose expression levels are altered in senescent cells relative to young cells. Examples of genes whose expression is elevated in senescent cells are those for p16INK4, p21CIP1, cyclins D1 and E, APP, endothelin-1, fibronectin, interleukin-1, Cu, ZnSOD, GADD45, PAI-1, PAI-2, SPARC, IGFBP-3, collagenase, macrophage colony-stimulating factor, p53, bcl-2, and p33ING1. Examples of genes whose expression is reduced in senescent cells are those for cyclin A, cyclin B1, cyclin H, CAK, cdc2, MnSOD, c-fos, catalase, EPC-1, E2F-1, E2F-2, DP-1, elastin, thymidine kinase, IGF-II, egr-1, granulocyte-macrophage colony-stimulating factor, dihydrofolate reductase, PCNA, ribonucleotide reductase, and histones (2, 16, 21, 22, 26, 31, 33; for reviews, see references 4, 5, 14, 32, and 37).
Many investigators have postulated the existence of common regulatory mechanisms to account for such senescence-related alterations in gene expression. While a number of transcriptional regulators contributing to age-dependent gene expression have been identified, their influences on the senescent phenotype are not fully understood (8, 15, 20, 24). Based on the increasingly recognized participation of posttranscriptional regulatory mechanisms and the observation that many senescence-associated genes bear AU-rich elements, which are known targets of regulated mRNA turnover, in their 3′ untranslated regions (UTRs), we hypothesized that their orchestrated expression may be regulated, at least in part, through coordinate alterations in mRNA stability. Alterations in mRNA stability require the association of the mRNAs with RNA-binding proteins that either enhance or reduce their stabilities. Many RNA-binding proteins have been described, but only a few them, including the Elav (embryonic lethal abnormal visual)/Hu and AUF1 protein family, have been reported to affect mRNA half-life. The Elav/Hu family of RNA-binding proteins, including the ubiquitously expressed HuR and the neuronal-specific Hel-N1, HuC, and HuD, have been found to bind to critical mRNAs containing AU-rich elements (e.g., GLUT-1, c-myc, GAP-43, c-fos, PAI-2, VEGF, and p21 [10, 11, 25, 34, 35]) and either stabilize them, enhance their translation, or both (10, 11, 25). By contrast, AUF1 has generally been associated with enhanced mRNA turnover (6, 19).
We previously described the stress-triggered stabilization of the mRNA encoding the cyclin-dependent kinase (cdk) inhibitor p21 through complexing with the mRNA-binding protein HuR and subsequently reported that mRNAs encoding cyclins A and B1 are targeted and stabilized by HuR in a cell cycle-regulated fashion (34, 35). Here, we employ two established model systems of cellular senescence: in vitro passage of WI-38 human diploid fibroblasts (13) and the IDH4 human fibroblast model developed by Shay and colleagues, where the normal limitation of life span can be reversibly bypassed through inducible expression of the simian virus 40 (SV40) large T antigen (38). We report that senescent cells in both models had lower HuR levels; exhibited decreased binding to transcripts of c-fos, cyclin A, and cyclin B1; and displayed reduced stability and steady-state levels of the respective mRNAs, suggesting that reduced HuR binding to these mRNAs contributes to their lower expression with aging. Strikingly, transient overexpression of HuR in IDH4 cells restored a “younger” phenotype, while antisense-RNA-mediated reduction in HuR levels led to a more pronounced “old” phenotype. Our findings provide evidence that HuR serves to regulate the turnover of genes whose expression is coordinately downregulated during replicative senescence. Thus, a reduction in HuR during replicative senescence contributes directly to the senescent phenotype.
MATERIALS AND METHODS
Cell culture, cell transfections, and assessment of 3H-thymidine incorporation, fluorescence-activated cell sorter (FACS) distribution, and senescence-associated β-galactosidase (SA-β-gal) activity.
Human diploid IDH4 fibroblasts (generously provided by J. W. Shay) and early-passage (≈28 population doublings [pdl]) and late-passage (≈60 pdl) human diploid WI-38 fibroblasts were cultured in Dulbecco's modified essential medium (Gibco-BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum. Skin biopsy-derived human diploid fibroblasts were obtained from the CORIELL Cell Repositories (Camden, N.J.). The samples included fibroblasts from nine young individual donors (15 to 30 years old) and from nine elderly individual donors (80 to 94 years old). The cells were cultured in Dulbecco's modified essential medium plus 10% fetal bovine serum for approximately 3 to 4 pdl to obtain enough material for Western blot analysis. IDH4 cell culture medium was further supplemented with 1 μM dexamethasone (dex) for constitutive expression of SV40 large T antigen to suppress senescence and induce proliferation (38). To induce senescence of IDH4 cells, dex was removed from the culture media (and regular serum was replaced with charcoal-stripped serum), and the cells were assessed at different times (3 to 8 days) thereafter. Constructs pZeoSV2(−)HuR(S) and pZeoSV2(−)ASHuR (antisense) were previously described (17). All transfections were transient and were carried out using lipofectamine (Gibco-BRL), following the manufacturer's recommendations. This procedure resulted in a transfection efficiency of 75 to 90% based on parallel transfections using a green fluorescent protein-expressing plasmid.
For assessment of SA-β-gal activity, cells were seeded in 30-mm-diameter dishes, cultured in medium without dex for different lengths of time, and then fixed with a 3% formaldehyde solution. The cells were then washed and incubated with SA-β-gal staining solution (1 mg of X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside]/ml, 40 mM citric acid–sodium phosphate buffer [pH 6.0], 5 mM ferrocyanide, 5 mM ferricyanide, 150 mM NaCl, and 2 mM MgCl2) from 4 h to overnight to visualize SA-β-gal activity (7). 3H-thymidine incorporation assays and FACS analysis were performed as described previously (35).
Northern and Western blot analysis.
Northern blot analysis was carried out as previously described (12). Oligomers complementary to human cyclin D1, p21, and 18S rRNA (18) were 3′-end labeled using terminal transferase enzyme, while PCR-generated fragments of cyclin A, cyclin B1, DP-1, gadd153, c-fos, and β-actin cDNAs were random-primer labeled using Klenow enzyme; all labeling reactions were carried out in the presence of [α-32P]dATP. Signals on Northern blots were visualized and quantitated with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
For Western blot analysis, 40 μg of cell lysate was resolved on sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes. HuR was detected with the monoclonal anti-HuR antibody 19F12 (34); anti-AUF1 (39) and anti-BAF57c (36) antibodies were previously described. Monoclonal anti-β-actin antibody was from Santa Cruz Biotechnologies (Santa Cruz, Calif.). Signals were detected with the ECL reagent (Amersham) and quantitated using a Personal Densitometer (Molecular Dynamics).
Preparation of cell fractions and radiolabeled transcripts.
Lysis of cells and preparation of whole-cell lysates as well as cytoplasmic and nuclear fractions were carried out as described previously (34). For in vitro synthesis of all transcripts (either unlabeled or radiolabeled), RNA from IDH4 cells was reverse transcribed, and the cDNAs generated were used as templates in PCRs to amplify the 3′ UTRs of cyclin A, cyclin B1, cyclin E, and c-fos cDNAs, as described previously (34, 35). All 5′ primers contained the T7 RNA polymerase promoter sequence (T7): CCAAGCTTCTAATACGACTCACTATAGGGAGA. Oligonucleotides used to amplify the 3′ UTRs of cyclins A, B1, and E were described previously (35); oligonucleotides (T7)GCAATGAGCCTTCCTCTGAC and CATTCAACTTAAATGCTTTTATTG were used to prepare the c-fos 3′ UTR (region 1246 to 2101).
Binding assays.
Electrophoretic mobility shift assays to detect the formation of complexes between cellular proteins (10 μg of either cytoplasmic or nuclear fractions) and various radiolabeled transcripts (100,000 cpm of each RNA) were performed as described previously (34). For competition assays, 5×, 10×, or 20× excess unlabeled transcript was used. For supershift analysis, 10 μg of cytoplasmic protein was incubated with 2 μg each of anti-HuR antibodies or control antibodies recognizing p27, p38, and p53 (34).
cdk2 and cdc2 immunoprecipitation and kinase assays.
Immunoprecipitation-kinase assays were carried out as previously described (12). cdk2 and cdc2 were immunoprecipitated from 200-μg aliquots of lysate using either anti-cdk2 (Pharmingen) or anti-cyclin B1 (Santa Cruz) antibodies, respectively. Kinase activities were assayed using histone H1 (Ambion, Austin, Tex.) as a substrate and quantitated with a PhosphorImager.
RESULTS
mRNAs encoding senescence-associated genes exhibit different relative half-lives in young and senescent cells.
With rapidly accumulating experimental evidence that altered mRNA turnover regulates important cellular processes, we set out to investigate whether replicative senescence is also influenced by altered mRNA degradation by using two established models. The first consisted of standard in vitro passage of untransformed WI-38 human diploid fibroblasts (13). The second was the IDH4 human fibroblast model of reversible senescence developed by Wright and colleagues (38). In this model, constitutive dex-driven SV40 large-T-antigen expression allows IDH4 cells to suppress senescence and continue to proliferate as young cells. However, they can be induced to quickly undergo replicative senescence by removing dex from the culture medium, resulting in the loss of large-T-antigen expression (38). In each cell model, comparison of young cells (dex-treated IDH4 cells and WI-38 cells at ≈28 pdl) with senescent cells (IDH4 cells cultured without dex for 3 to 7 days and WI-38 cells at ≈60 pdl) revealed that senescent cells had considerably lower cdk activity, diminished rates of 3H-thymidine incorporation, and a reduced number of S- and G2-phase cells. Likewise, a neutral SA-β-gal activity that is largely absent from young cells (7) was greatly elevated in the senescent cultures (Fig. 1). SA-β-gal activity is used as a biomarker for replicative senescence, although the specific enzyme(s) involved remains poorly characterized. When the expression of senescence-regulated genes was studied (Fig. 2A), we observed that the cyclin D1 mRNA levels were moderately elevated in senescent cells, while mRNAs encoding the cdk inhibitors p16 (not shown) and p21 were much higher in senescent cells, in accordance with previous findings (9, 23, 27). Also in agreement with previous reports, levels of mRNAs encoding cyclin A, cyclin B1, and c-fos were greatly reduced in both senescent WI-38 (≈60 pdl) and IDH4 cells (7 days after dex was removed) (28, 30).
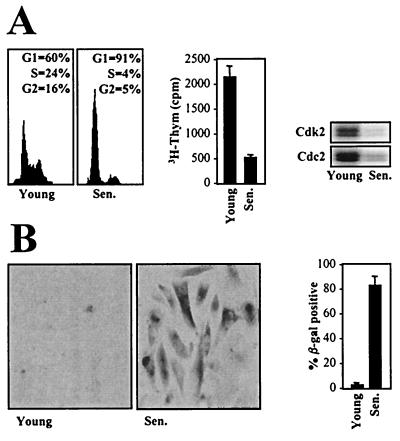
FIG. 1.
Phenotypic characterization of IDH4 cells cultured in the presence or absence of dex. IDH4 cells that were cultured in the presence (Young) or absence (Sen.) of dex for 7 days were subjected to FACS analysis, 3H-thymidine incorporation assays, and assessment of cdk2- and cdc2-associated kinase activity using histone H1 as a substrate (A) and to SA-β-gal staining (B) (left, representative fields; right, quantitation of SA-β-gal-positive IDH4 cells). The graphs represent the means + standard errors of the means of four independent experiments.
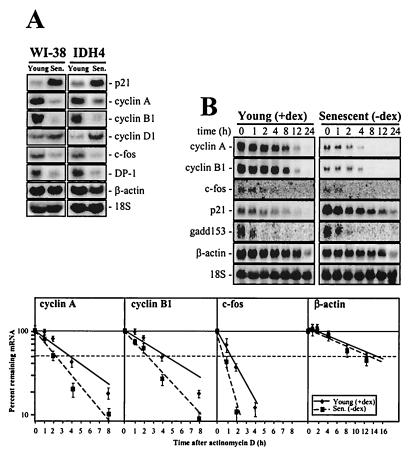
FIG. 2.
Senescence-associated gene expression in WI-38 and IDH4 fibroblasts. (A) Northern blot analysis of expression of the genes indicated using early-passage (Young; ≈28 pdl) and late-passage (senescent [Sen.]; ≈60 pdl) WI-38 cells, as well as young (dex-treated) and senescent (7 days after removing dex) IDH4 cells. (B) Stabilities of cyclin A, cyclin B1, c-fos, and β-actin mRNAs in IDH4 cells that were either dex-treated [Young (+dex)] or cultured without dex for 7 days [Senescent (−dex)] were assessed after the addition of 2 μg of actinomycin D/ml; preparation of RNA at the times indicated; measurement of cyclin A, cyclin B1, c-fos, and β-actin mRNA Northern blot signals; normalizing them to 18S rRNA; and plotting them on a logarithmic scale (bottom). Dashed horizontal lines, 50% of untreated. The data represent the means ± standard errors of the means of four independent experiments.
In order to determine if these age-related differences in gene expression were influenced by changes in the stabilities of the respective mRNAs, we examined mRNA half-life in young and senescent populations after addition of actinomycin D. As shown in Fig. 2B, proliferating (with dex) and senescent (without dex) IDH4 cells showed marked differences in the half-lives of mRNAs encoding cyclin A, cyclin B1, and c-fos. For these three mRNAs, treatment with actinomycin D resulted in faster transcript loss in the populations without dex, revealing that their stabilities were lower in senescent cells. These differences in mRNA stability were specific for a subset of genes, since the stabilities of other genes studied were unchanged. p21 mRNA stability did not seem to differ between proliferating and senescent populations, despite elevated p21 expression with senescence. Similarly, the gadd153 and β-actin mRNAs showed no differences in stability as a function of cell senescence. Basal gadd153 was unchanged between proliferating and senescent IDH4 cells, while basal β-actin mRNA levels were moderately reduced in senescent cultures (Fig. 2B). When the mRNA half-lives were calculated, they were found to be 4 h (in proliferating cells) versus 2 h (in senescent cells) for cyclin A, 4.3 versus 2 h for cyclin B1, and 1.2 versus 0.5 h for c-fos (Fig. 2B, graph). By contrast, the β-actin mRNA half-lives were essentially the same in proliferating and senescent IDH4 cells (about 13 to 14 h). A similar assessment of mRNA half-life in early-passage and senescent WI-38 cells revealed comparable differences, with young populations exhibiting longer half-lives of mRNAs encoding cyclin A, cyclin B1, and c-fos than did senescent cells (not shown).
HuR levels are lower in fibroblasts undergoing replicative senescence and in skin biopsy fibroblasts from elderly individuals.
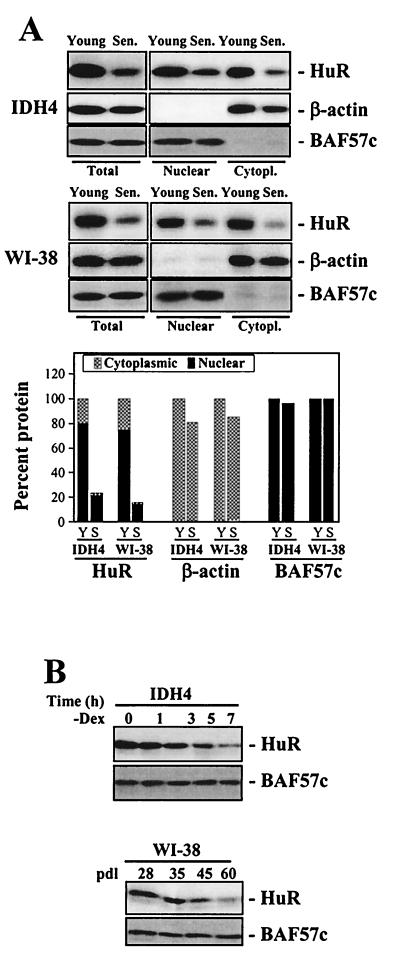
Since mRNAs encoding c-fos, cyclin A, and cyclin B1 have been reported to be targets of the RNA-binding protein HuR, and their half-lives are known to increase upon HuR binding, we investigated whether HuR levels change with senescence. As shown in Fig. 3A, HuR expression was found to be high in young cells but decreased significantly with replicative senescence in both WI-38 and IDH4 cells. This decrease affected total cellular HuR, with more pronounced reductions in cytoplasmic HuR than in nuclear HuR. It was important to assess the levels of cytoplasmic HuR because our earlier studies suggested that critical binding of HuR to target mRNAs leading to their altered half-lives occurred in the cytoplasm (34, 35). The quality of the subcellular fractionation, as well as differences in loading and transfer among samples, was monitored by hybridization with antibodies recognizing β-actin (a cytoplasmic protein) and BAF57c (a nuclear protein). As depicted in Fig. 3B, reductions in HuR levels occurred gradually as cells progressed towards senescence.
FIG. 3.
HuR expression in young and senescent human fibroblasts. (A) Western blot analysis of HuR expression in whole-cell (Total [20 μg]), nuclear (10 μg), or cytoplasmic (Cytopl. [40 μg]) lysates from either WI-38 or IDH4 populations (young or senescent [Sen.], as described in the legend to Fig. 1A). Western blot analysis of BAF57c and β-actin expression served to assess the quality of the cell fractionation procedure and to monitor differences in loading and transfer among samples. Western blot signals were quantitated and represented (graph) relative to the total protein present in whole-cell lysates from young cells. Y, young; S, senescent. (B) Western blot analyses depicting time-dependent changes in total HuR expression in dex-depleted (−Dex) IDH4 cells or in WI-38 cells of the indicated number of population doublings.
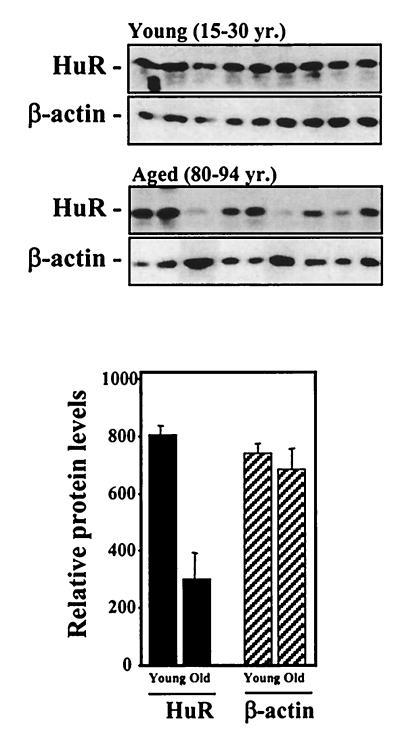
We extended our analysis of HuR expression to skin fibroblasts obtained from individual donors of different ages. As shown in Fig. 4, HuR expression in fibroblasts derived from young individuals (15 to 30 years of age) was, on average, about 2.5-fold higher than that seen in fibroblasts from elderly donors (80 to 94 years of age). Although the growth of these fibroblast cultures was not formally measured, there was a good correlation between the rate of cell growth and HuR expression levels: fast-growing cultures (those derived from young donors and several of those derived from elderly donors) exhibited high levels of HuR expression, with slow-growing cultures (derived from elderly donors) showing reduced HuR expression. While this system has important limitations (such as the small sample sizes available for experimentation), it provides evidence suggesting that HuR expression may also be altered during in vivo aging.
FIG. 4.
HuR expression in fibroblasts from skin biopsies from young and elderly individuals. Western blot analysis of expression of the proteins indicated was carried out using fibroblasts from skin biopsies obtained from individual donors who were either young (15 to 30 years old) or aged (80 to 94 years old). The fibroblasts were cultured in vitro for 3 to 4 pdl before Western blot analysis of whole-cell lysates. The error bars represent standard errors of the mean.
HuR binding to target mRNAs decreases with senescence.
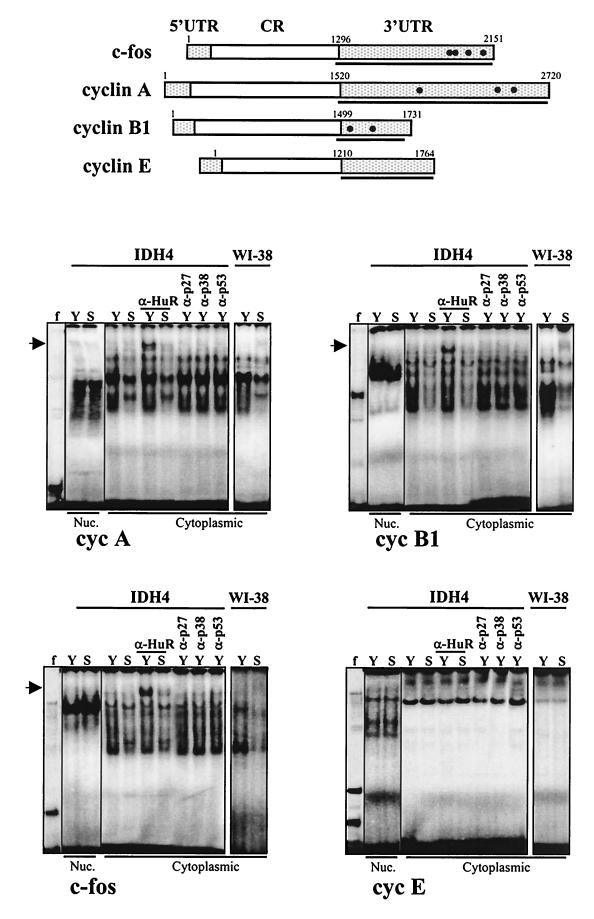
Lysates prepared from either young or senescent cells exhibited abundant binding to radiolabeled RNAs comprising the 3′ UTRs of the cyclin A, cyclin B1, and c-fos transcripts (Fig. 5). As shown, for each transcript tested, nuclear lysates prepared from young IDH4 cells exhibited essentially the same binding pattern and intensity as did lysates prepared from senescent cells (Fig. 5). Similar results were observed when WI-38 cells were used (not shown). Remarkably, however, proteins present in cytoplasmic lysates prepared from young cells (both proliferating IDH4 and early-passage WI-38 cells) revealed much more extensive binding to radiolabeled transcripts than did proteins present in cytoplasmic lysates from senescent cells. That HuR was part of these senescence-sensitive complexes was evidenced by the ability of an anti-HuR antibody to supershift some of the protein-RNA associations, while antibodies directed to unrelated proteins did not produce supershifts (Fig. 5). These age-dependent associations with HuR were specific for cyclin A, cyclin B1, and c-fos, since assessment of a control radiolabeled RNA (encoding the cyclin E 3′ UTR) in binding assays revealed no differences associated with cell senescence and similarly failed to produce supershifts (Fig. 5). Other control transcripts (those encompassing the coding region and 5′ UTRs of the three genes) likewise failed to show this age-dependent difference in complex formation, and no supershifts were observed (not shown).
FIG. 5.
HuR binding to target mRNAs in young and senescent human fibroblasts. Radiolabeled RNAs encoding the 3′ UTRs of c-fos, cyclin A, cyclin B1, and cyclin E are shown (top; underlined). Radiolabeled transcripts were incubated with proteins present in either nuclear (Nuc.) or cytoplasmic lysates of young (Y) or senescent (S) WI-38 or IDH4 cells (defined in the legend to Fig. 3), forming associations with slower electrophoretic mobilities (bottom). Complexes formed with lysates from IDH4 cells and radiolabeled transcripts were assayed for the ability to be supershifted by either anti-HuR antibodies (α-HuR), or control anti-p27, anti-p38, or anti-p53 antibodies (α-p27, α-p38, and α-p53, respectively), as shown. f, free probes; arrows, supershifted complexes.
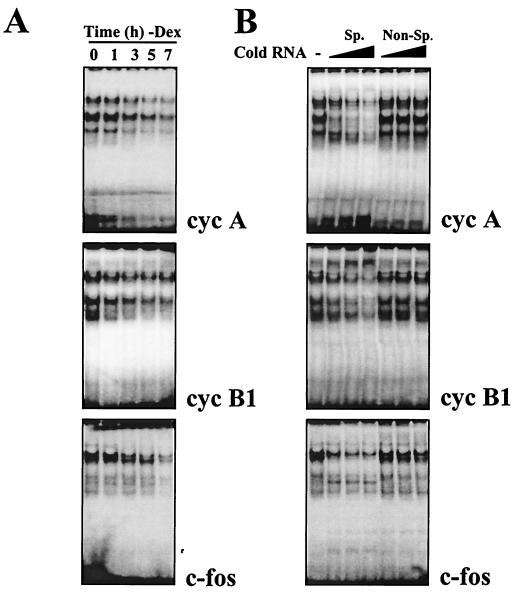
These age-dependent binding activities were further characterized, as shown in Fig. 6. Decreasing complex formation was seen in lysates from cells that were advancing towards senescence (Fig. 6A). Binding specificity was assessed using competing unlabeled transcripts. Complex formation was inhibited when lysates were preincubated with 5-, 10-, or 20-fold excess specific, or “self,” unlabeled transcript (that is, unlabeled cyclin A transcript in the case of shifts using radiolabeled cyclin A, unlabeled cyclin B1 transcript for cyclin B1 shifts, and similarly for c-fos). By contrast, similar fold excess of a nonspecific transcript (cyclin E 3′ UTR) failed to compete for binding (Fig. 6B).
FIG. 6.
Time course of complex formation in cells undergoing senescence, showing specificity of binding to target radiolabeled transcripts. (A) Cytoplasmic lysates from IDH4 cells cultured without dex (−Dex) for the indicated times were incubated with radiolabeled cyclin A, cyclin B1, or c-fos transcripts. (B) IDH4 cytoplasmic lysates were incubated with 5×, 10×, or 20× excess unlabeled competitor transcripts. Lanes Sp, competition using a specific transcript (unlabeled cyclin [cyc] A RNA competing for binding of radiolabeled cyclin A RNA, unlabeled cyclin B1 RNA competing for binding of radiolabeled cyclin B1 RNA, and likewise for c-fos RNA); lanes Non-Sp, competition using unlabeled cyclin E RNA as a nonspecific transcript in binding assays with radiolabeled cyclin A RNA, cyclin B1 RNA, and c-fos RNA.
Ectopic intervention to either elevate or reduce HuR expression can alter the half-lives of target senescence-associated mRNAs.
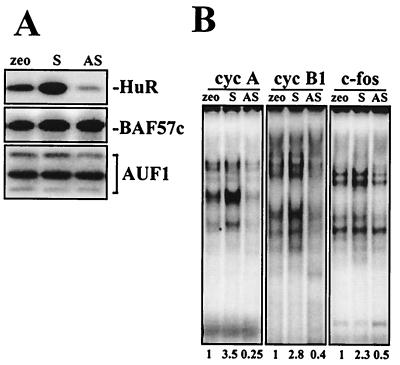
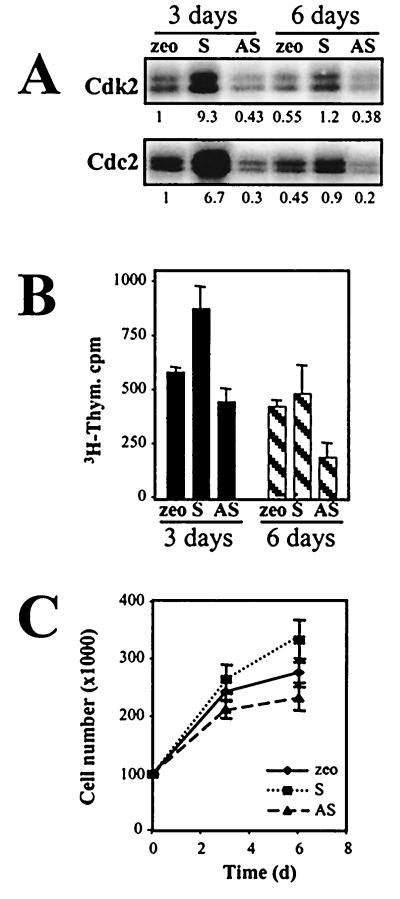
Next, we sought to determine if the observed differences in HuR levels in young and senescent cells directly influenced the levels of cyclin A, cyclin B1, and c-fos. To this end, we chose to utilize IDH4 cells grown without dex for 3 to 4 days, which rendered their phenotype intermediate between those of young (with dex) and senescent (without dex for 7 days) fibroblasts and allowed us to monitor HuR-dependent changes leading to the acquisition of younger or more “senescencelike” traits. Cells that had been transiently transfected with an empty vector (zeo) or with vectors expressing either HuR (S) or an antisense transcript complementary to HuR mRNA (AS) (see Materials and Methods) were grown without dex for 3 to 4 days. Parallel transfections using an enhanced green fluorescent protein expression vector revealed that 75 to 90% of the cells were transfected under our experimental conditions (not shown). WI-38 cells were not amenable to such transfection-driven changes in HuR expression levels due to their very low transfection rates. As shown in Fig. 7, by day 3 such transient overexpression of HuR(S) in senescent IDH4 cells led to an ≈4-fold increase in HuR levels, while HuR(AS) expression led to an ≈3.5-fold decrease in HuR levels relative to transfections using an empty vector. Levels of the constitutively expressed nuclear protein BAF57c served as a control for loading, while levels of the RNA-binding AUF1 protein family were not significantly altered. Accordingly, lysates prepared from each transfection group formed complexes with transcripts encoding the 3′ UTRs of cyclin A, cyclin B1, and c-fos that were in keeping with HuR levels: compared with control cells (zeo), binding was higher in HuR-overexpressing cells and lower in cells with reduced HuR expression (S and AS, respectively) (Fig. 7B).
FIG. 7.
Influence of HuR levels on the formation of protein complexes with the 3′ UTRs of senescence-associated genes. (A) Western blot analysis of expression of the indicated genes was carried out using IDH4 cells transiently transfected with either empty vector (zeo), HuR-expressing (S), or antisense-HuR-expressing (AS) plasmid pZeoSV2(−) and then cultured in the absence of dex for 3 days. (B) Binding of radiolabeled RNAs corresponding to the 3′ UTRs of cyclin (cyc) A, cyclin B1, and c-fos and proteins present in the cytoplasmic lysates (10 μg) of IDH4 cells cultured as described for panel A. Fold differences in intensity of shifted complexes, relative to those in zeo populations, are shown below the lanes.
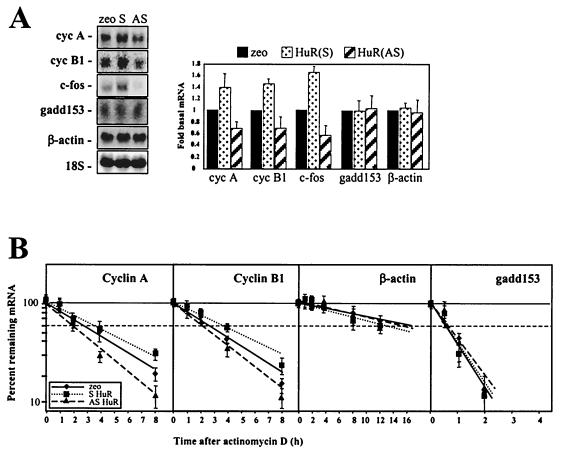
These interventions to either elevate or reduce HuR expression levels affected the steady-state levels and half-lives of target mRNAs. As shown in Fig. 8, the expression levels, as well as the half-lives, of mRNAs encoding cyclins A and B1 were longer in the HuR(S) transfection group and shorter in the HuR(AS) transfection group than they were in control, empty-vector-transfected group (as measured by day 3 without dex). For cyclin A mRNA, half-lives were 2.7 h for the zeo transfection group, 3.3 h for the HuR(S) group, and 2 h for HuR(AS); for cyclin B1 mRNA, half-lives were 2.8 h for zeo control, 3.8 h for HuR(S), and 2 h for HuR(AS). c-fos mRNA signals were too low to measure accurately in actinomycin D-based experiments. By contrast, HuR levels did not influence either the steady-state levels or the half-lives of two control mRNAs, those encoding gadd153 and β-actin (Fig. 8).
FIG. 8.
Influence of HuR levels on the steady-state levels and stabilities of senescence-associated genes. Three days after transfection and removal of dex (as described in the legend to Fig. 7), expression of cyclin A, cyclin B1, and c-fos was assessed in IDH4 cells. (A) Representative Northern blots depicting the steady-state levels of cyclin (cyc) A, cyclin B1, c-fos, gadd153, and β-actin mRNAs, as well as 18S rRNA, and quantitations from 10 independent experiments (bar graph, showing means + standard errors of the means [SEM]). (B) Cyclin A, cyclin B1, β-actin, and gadd153 mRNA stabilities, assessed as explained in the legend to Fig. 2B; the values represent the means ± SEM of seven independent experiments.
Taken together, these observations suggest that high HuR levels, such as are found in young cells, contribute to maintaining high expression of cyclin A, cyclin B1, and c-fos, all three important proliferation-associated genes, while decreased HuR expression in senescent cells contributes to lowering their expression.
Ectopic intervention to either elevate or reduce HuR expression in IDH4 cells can alter their senescence phenotype.
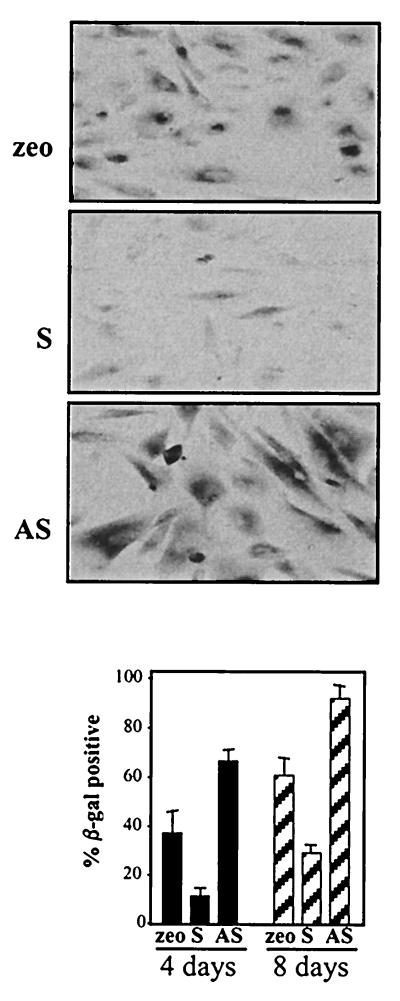
Given HuR's ability to regulate the mRNA levels of genes whose expression is reduced with senescence, we postulated that HuR expression levels could directly influence the implementation (onset and/or maintenance) of the senescence phenotype. Consistent with this view, overexpression of HuR in IDH4 cells grown without dex for 3 to 4 days revealed a younger phenotype than did vector-transfected (zeo) cells, as the cells exhibited higher cdk activity, proliferation, and 3H-thymidine incorporation (Fig. 9) while they displayed a lower proportion of SA-β-gal-positive cells (Fig. 10). By contrast, populations where HuR expression was suppressed displayed more pronounced characteristics of senescence, with lower basal cdk activity, fewer cells, less 3H-thymidine incorporation, and a dramatically higher number of enlarged, SA-β-gal-positive cells (Fig. 9 and 10).
FIG. 9.
Influence of HuR levels on the senescent phenotype. IDH4 cells transfected as described in the legend to Fig. 7 (zeo, S, and AS) were cultured without dex for either 3 or 6 days and then subjected to assessment and quantitation of cdk2- and cdc2-associated kinase activity using histone H1 as a substrate (A), 3H-thymidine (Thym.) incorporation (B), or total cell numbers (C). The graphs represent the means ± standard errors of the means of four independent experiments.
FIG. 10.
Influence of HuR levels on SA-β-gal activity. IDH4 cells transfected as described in the legend to Fig. 7 (zeo, S, and AS) were cultured without dex for 4 days and then stained to assess SA-β-gal activity. Top, representative fields; bottom, quantitation of SA-β-gal-positive IDH4 cells. The graph represents the means + standard errors of the means of four independent experiments.
DISCUSSION
The data presented here provide novel insight into the regulation of gene expression during replicative senescence. Using two established models of cellular senescence, we show that expression of the RNA-binding protein HuR, as well as binding of HuR to target mRNAs encoding cyclin A, cyclin B1, and c-fos, decreases with replicative senescence (Fig. 3 and 5). Both the half-lives and expression levels of these mRNAs are elevated in young cells, where HuR is abundant, but are low in senescent cells, where HuR expression is diminished. We further show that transfected IDH4 cells overexpressing HuR displayed elevated stability of target mRNAs, while those expressing lower HuR levels showed reduced target mRNA half-lives. Importantly, such manipulations of HuR expression affected the senescence phenotype of IDH4 cells. As shown in Fig. 9 and 10, cells overexpressing HuR displayed features of young cells (lower SA-β-gal activity and higher cdk activity and 3H-thymidine incorporation) compared with empty-vector-transfected cells, while cells expressing reduced HuR levels displayed an enhanced senescent phenotype (higher SA-β-gal activity and diminished cdk activity, proliferation, and 3H-thymidine incorporation).
To date, the expression of replicative-senescence-associated genes has been studied by comparing collections of genes expressed in various young and senescent cell systems, but the underlying mechanisms serving to coordinately regulate their expression (for example, transcriptional regulators) have remained elusive. Based on our results reported here, we propose that changes in mRNA stability may constitute an effective means of globally regulating age-related gene expression. Coordinate, HuR-dependent changes in the expression of cyclin A, cyclin B1, and c-fos (whose mRNAs are known targets of HuR [25, 35]) may have a direct impact on the senescence phenotype, since these genes are critical regulators of cellular proliferation and cell cycle progression. However, it is likely that additional HuR target genes collectively participate in the implementation of the senescence phenotype, its maintenance, or both. In this regard, HuR was found to bind transcripts corresponding to other senescence-related genes, such as those for MnSOD and DP-1 (not shown).
Based on earlier observations that (i) elevated p21 is a hallmark of entry into senescence, (ii) HuR stabilized the p21 mRNA after exposure to UV irradiation (34), and (iii) to our knowledge, no senescence-associated activators of p21 transcription have been reported, we initially set out to examine if HuR enhanced p21 mRNA in our study system. Surprisingly, HuR does not appear to be responsible for upregulating p21 expression during replicative senescence (as it does during the cellular stress response) based on our data presented here (Fig. 2). Systematic studies aimed at examining HuR-regulated changes in gene expression are under way in our laboratory using DNA arrays. We anticipate finding additional senescence-associated genes using this strategy.
According to the model emerging from the present study, high HuR expression in young cells has a direct impact on the cellular phenotype, characterized by a highly proliferative state (in which cyclin A, cyclin B1, and c-fos are known to be major participants). Likewise, low HuR expression in senescent cells contributes to maintaining reduced levels of such proliferative genes. A corollary of our hypothesis is that cells expressing high levels of HuR are likely to have high proliferation rates. In this regard, it is of interest to note that HuR was more highly expressed in skin fibroblasts from young individual donors (Fig. 4) and that high HuR expression levels were found in neoplastic tissues and were correlated with rapid growth, both in vivo and in vitro (1). Likewise, HuR was found to be highly expressed in virtually all tumors examined (H. Furneaux, personal communication). In conclusion, while HuR expression could be a consequence of replicative senescence, the ability of HuR manipulations to alter the senescent phenotype suggests a direct participation of HuR in the process of in vitro senescence.
Although the link between in vitro cellular senescence and human aging remains controversial, a diminution in proliferative capacity is also a hallmark of in vivo aging. Therefore, knowledge of the mechanisms serving to regulate gene expression during in vitro senescence is likely to aid our understanding of in vivo aging as well as contribute to our comprehension of age-related diseases, such as cancer and hyperplasia, where control of proliferation is lost. As in cancer, where clearly more genes are abnormally expressed than are mutated (29), it is plausible that conditions associated with aging also arise as a consequence of deregulated gene expression rather than mutations. Thus, while single-gene approaches to intervene in such conditions have been unfruitful, it is likely that strategies aimed at modulating global patterns of gene expression will prove more successful.
ACKNOWLEDGMENTS
We are grateful to Jerry W. Shay for providing the IDH4 cells, Weidong Wang for the anti-BAF57c antibody, Henry Furneaux for the anti-HuR antibody, and Gary Brewer for the anti-AUF1 antibody. We thank Theresa Marinucci for providing WI-38 cells and Mingyi Wang for photographic assistance.
REFERENCES
- 1.Blaxall B C, Dwyer-Nield L D, Bauer A K, Bohlmeyer T J, Malkinson A M, Port J D. Differential expression and localization of the mRNA binding proteins, AU-rich element mRNA binding protein (AUF1) and Hu antigen R (HuR), in neoplastic lung tissue. Mol Carcinog. 2000;28:76–83. [PubMed] [Google Scholar]
- 2.Buzby J S, Lee S M, Van Winkle P, DeMaria C T, Brewer G, Cairo M S. Increased GM-CSF mRNA stability in cord vs. adult mononuclear cells is translation dependent and associated with increased levels of A+U-rich element binding factor. Blood. 1996;88:2889–2897. [PubMed] [Google Scholar]
- 3.Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 4.Cristofalo V J, Pignolo R J, Cianciarulo F L, DiPaolo B R, Rotenberg M O. Changes in gene expression during senescence in culture. Exp Gerontol. 1992;27:429–432. doi: 10.1016/0531-5565(92)90077-d. [DOI] [PubMed] [Google Scholar]
- 5.Cristofalo V J, Volker C, Francis M K, Tresini M. Age-dependent modifications of gene expression in human fibroblasts. Crit Rev Eukaryot Gene Expr. 1998;8:43–80. doi: 10.1615/critreveukargeneexpr.v8.i1.30. [DOI] [PubMed] [Google Scholar]
- 6.DeMaria C T, Brewer G. AUF1 binding affinity to A+U-rich elements correlates with rapid mRNA degradation. J Biol Chem. 1996;271:12179–12184. doi: 10.1074/jbc.271.21.12179. [DOI] [PubMed] [Google Scholar]
- 7.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I O, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimri G P, Testori A, Acosta M, Campisi J. Replicative senescence, aging and growth-regulatory transcription factors. Biol Signals. 1996;5:154–162. doi: 10.1159/000109185. [DOI] [PubMed] [Google Scholar]
- 9.Dulic V, Drullinger L F, Lees E, Reed S I, Stein G H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-CDK2 and cyclin D1-CDK2 complexes. Proc Natl Acad Sci USA. 1993;90:11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan X C, Steitz J A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford L P, Watson J, Keene J D, Wilusz J. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev. 1999;13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorospe M, Liu Y, Xu Q, Chrest F J, Holbrook N J. Inhibition of G1 cyclin-dependent kinase activity during growth arrest of human breast carcinoma cells by prostaglandin A2. Mol Cell Biol. 1996;16:762–770. doi: 10.1128/mcb.16.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayflick L. The limited in vitro life time of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 14.Jansen-Durr P. The making and breaking of senescence: changes of gene expression during cellular aging and immortalization. Exp Gerontol. 1998;33:291–301. doi: 10.1016/s0531-5565(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 15.Lavrovsky Y, Chatterjee B, Clark R A, Roy A K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp Gerontol. 2000;35:521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee C-K, Kloop R G, Weindruch R, Prolla T A. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 17.Levy N S, Chung S, Furneaux H, Levy A P. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998;273:6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- 18.Lin S, Wang W, Wilson G M, Yang X, Brewer G, Holbrook N J, Gorospe M. Downregulation of cyclin D1 expression by prostaglandin A2 is mediated by enhanced cyclin D1 mRNA turnover. Mol Cell Biol. 2000;20:7903–7913. doi: 10.1128/mcb.20.21.7903-7913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loflin P, Chen C Y, Shyu A-B. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 1999;13:1884–1897. doi: 10.1101/gad.13.14.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyyappan M, Atadja P W, Riabowol K T. Regulation of gene expression and transcription factor binding activity during cellular aging. Biol Signals. 1996;5:130–138. doi: 10.1159/000109183. [DOI] [PubMed] [Google Scholar]
- 21.Meyyappan M, Wheaton K, Riabowol K T. Decreased expression and activity of the immediate-early growth response (Egr-1) gene product during cellular senescence. J Cell Physiol. 1999;179:29–39. doi: 10.1002/(SICI)1097-4652(199904)179:1<29::AID-JCP4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Millis A J T, Sottile J, Hoyle M, Mann D M, Diemer V. Collagenase production by early and late passage cultures of human fibroblasts. Exp Gerontol. 1989;24:559–575. doi: 10.1016/0531-5565(89)90060-0. [DOI] [PubMed] [Google Scholar]
- 23.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 24.Pang J H, Chen K Y. Global change of gene expression at late G1/S boundary may occur in Human IMR-90 diploid fibroblasts during senescence. J Cell Physiol. 1994;160:531–538. doi: 10.1002/jcp.1041600316. [DOI] [PubMed] [Google Scholar]
- 25.Peng S S, Chen C Y, Xu N, Shyu A B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignolo R J, Cristofalo V J, Rotenberg M O. Senescent WI-38 cells fail to express EPC-1, a gene induced in young cells upon entry into the G0 state. J Biol Chem. 1993;268:8949–8957. [PubMed] [Google Scholar]
- 27.Reznikoff C A, Yeager T R, Belair C D, Savelieva E, Puthenveettil J A, Stadler W M. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res. 1996;56:2886–2890. [PubMed] [Google Scholar]
- 28.Richter K H, Afshari C A, Annab B A, Burkhart L A, Owen R D, Boyd J, Barrett J C. Down-regulation of Cdc2 in senescent human and hamster cells. Cancer Res. 1991;51:6010–6013. [PubMed] [Google Scholar]
- 29.Sager R. Expression genetics in cancer: shifting the focus from DNA to RNA. Proc Natl Acad Sci USA. 1997;94:952–955. doi: 10.1073/pnas.94.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelton D N, Chang E, Whittier P S, Choi D, Funk W D. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 31.Suen Y, Lee S M, Schreurs J, Knoppel E, Cairo M S. Decreased macrophage colony-stimulating factor mRNA expression from activated cord versus adult mononuclear cells: altered posttranscriptional stability. Blood. 1994;84:4269–4277. [PubMed] [Google Scholar]
- 32.Thakur M K, Oka T, Natori Y. Gene expression and aging. Mech Ageing Dev. 1993;66:283–298. doi: 10.1016/0047-6374(93)90015-j. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Moerman E J, Jones R A, Thweatt R, Goldstein S. Characterization of IGFBP-3, PAI-1 and SPARC mRNA expression in senescent fibroblasts. Mech Ageing Dev. 1996;92:121–132. doi: 10.1016/s0047-6374(96)01814-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Furneaux H, Cheng H, Caldwell M C, Hutter D, Liu Y, Holbrook N J, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol. 2000;20:760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Lin S, Caldwell M C, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during the cell division cycle. EMBO J. 2000;19:2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Chi T, Xue Y, Zhou S, Kuo A, Crabtree G R. Architectural DNA binding by a high-mobility-group/kinesin-like subunit in mammalian SWI/SNF-related complexes. Proc Natl Acad Sci USA. 1998;95:492–498. doi: 10.1073/pnas.95.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong H, Riabowol K. Differential cdk-inhibitor gene expression in aging human diploid fibroblasts. Exp Gerontol. 1996;31:311–325. doi: 10.1016/0531-5565(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 38.Wright W E, Pereira-Smith O M, Shay J W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989;9:3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Wagner B J, Ehrenman K, Schaefer A W, DeMaria C T, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13:7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]