Abstract
The Cu,Zn superoxide dismutase (SOD) of Aspergillus fumigatus has previously been purified and shown to be immunoreactive to the sera of patients with aspergillosis; however, the purification of large quantities of the enzyme for expanded immunological analysis is both difficult and time-consuming. Accordingly, a λEMBL3 A. fumigatus genomic library was screened with degenerate oligonucleotides based on N-terminal amino acid sequence data; from this initial screen a 1,400-bp fragment was identified, labelled, and used to screen an A. fumigatus λgt11 cDNA library. A full-length cDNA encoding Cu,Zn SOD was subsequently identified and cloned. The cDNA encodes a protein of 154 amino acids, which does not have a signal peptide. The A. fumigatus Cu,Zn SOD possesses the typical metal binding ligands of fungal Cu,Zn SODs (six histidines and one aspartic acid) and has significant overall homology with Cu,Zn SODs in general. A recombinant A. fumigatus Cu,Zn SOD has been expressed in Pichia pastoris, is enzymatically active, and has biochemical and biophysical properties that are similar to those of the native enzyme. A sheep polyclonal antibody raised against purified native A. fumigatus Cu,Zn SOD was reactive to the recombinant enzyme by immunoenzyme development of Western blots. Sixty percent of serum samples from patients with A. fumigatus infections were reactive against the recombinant Cu,Zn SOD via immunoenzyme development of Western blots, indicating that the recombinant protein may be useful in the serodiagnostic identification of A. fumigatus infections.
Aspergillus fumigatus is an important opportunistic fungal pathogen that causes a wide spectrum of diseases in humans, the most important of which are allergic bronchopulmonary aspergillosis, aspergilloma (via the colonization of existing cavities in the lung), and disseminated aspergillosis (which occurs in immunocompromised individuals) (20). The latter is of particular concern; and bone marrow transplant patients, those undergoing renal transplantation, and patients with acute leukemia (4, 9, 27) are at particular risk. Infection is often not recognized at an early stage, and as a consequence, disseminated aspergillosis is frequently fatal in such circumstances. The prognosis can be improved if infection is detected promptly and if appropriate therapy is instigated. Isolation and culture of the organism, together with its histopathological identification, remain the definitive means of diagnosing invasive aspergillosis (19, 20). There are, however, problems associated with these approaches, and these relate both to the differentiation of A. fumigatus colonization and active infection and to the invasive nature of the methods, such as bronchoalveolar lavage (29), which may have to be used to obtain samples.
Both antigen detection (28) and PCR-based (29) methodologies have recently been applied to the diagnosis of Aspergillus infections; however, the detection of patients' antibody responses to Aspergillus infections may still be useful in the recognition of disease. A number of serodiagnostically useful antigens have now been characterized; and these include an 18-kDa RNase (22), an 88-kDa dipeptidyl peptidase (2), a 33-kDa alkaline protease (21), a 90-kDa catalase (23), and a 19-kDa Cu,Zn superoxide dismutase (SOD) (13, 15). Cu,Zn SOD has been shown to be recognized by 84.6% of patients' serum samples via immunoenzyme development of Western blots. Unfortunately, purification of the A. fumigatus Cu,Zn SOD is a time-consuming process, requiring liquid isoelectric focusing and gel filtration fast protein liquid chromatography (15, 16), and in order to further study the suitability of this enzyme as an immunodiagnostic marker, large quantities must be made available. Several of the A. fumigatus antigens detailed above have now been produced in recombinant form (2, 6, 20) in order to further elucidate their usefulness as diagnostic markers. In this report we describe the identification of the cDNA encoding the A. fumigatus Cu,Zn SOD, the expression of the recombinant enzyme in Pichia pastoris, and its biochemical and immunological comparison with the native enzyme.
MATERIALS AND METHODS
Identification of cDNA encoding Cu,Zn SOD and expression and purification of recombinant enzyme.
Escherichia coli LE392 was used as a host strain for propagation of bacteriophages, and E. coli DH5α was used for plasmid transformation (24, 25). A preexisting λEMBL3 genomic library from A. fumigatus CHUV 192-88 (6, 18) was screened by using 32P-labelled degenerate oligonucleotide probes (pooled primers A, B, and C; Table 1) designed from the N-terminal amino acid sequence of the A. fumigatus Cu,Zn SOD (16, 24). DNA from positive clones was subcloned into pMTL21 (7) for sequencing by Microsynth (Basel, Switzerland). A partial Cu,Zn SOD sequence was obtained, 32P-labelled, and used to screen a preexisting A. fumigatus λgt11 cDNA library from the CHUV 192-88 strain (6). Positive clones were subcloned for sequencing as described above. PCR of the A. fumigatus cDNA was performed with two homologous primers (PCR primers SOD 1 and 2; Table 1) encoding the N-terminal sequence and the C-terminal extremity of the Cu,Zn SOD to which a sequence for a His tag had been added. The SOD PCR product digested by XhoI and NotI was cloned into the expression vector pPiCZα and was transformed into the P. pastoris yeast expression system, and transformants were selected as described previously (6). Recombinant Cu,Zn SOD was produced from selected transformants in methanol yeast-based medium by previously described methods (6). Culture filtrate was harvested by centrifugation (at 5,000 × g for 5 min), and the recombinant enzyme was purified by a combination of liquid isoelectric focusing with the Rotofor system (Bio-Rad, Hemel, United Kingdom) (12, 15) and nickel chelating resin (ProBond; Invitrogen Corp., CH Groningen, The Netherlands). Rotofor fractions containing activity were diluted 1:2 in distilled water, and 5-ml volumes were mixed with 0.5 ml of resin (prepared according to the manufacturer's instructions and prerinsed in binding buffer [20 mM sodium phosphate, 0.5 M sodium chloride {pH 7.8}]). The mixture was then gently rocked for 10 min to facilitate binding and was then poured into a column. The latter was then washed twice with binding buffer, followed by two washes with wash buffer (20 mM sodium phosphate, 0.5 M sodium chloride [pH 6.0]). The protein was eluted by applying 1-ml volumes of 300 mM imidazole elution buffer (300 mM imidazole in wash buffer); the eluted fractions were collected and monitored by the standard SOD assay. As a negative control P. pastoris expressing the Candida albicans secreted aspartyl proteinase (SAP1) (5, 17) gene was used (gift from S. Beggah, Lausanne). SOD purification was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 15% polyacrylamide gels as described previously (12). Protein bands were visualized with either Coomassie brilliant blue R-250 or silver stain (Bio-Rad). N-terminal amino acid sequencing of the recombinant Cu,Zn SOD was performed as described previously (16).
TABLE 1.
Oligonucleotides used as probes in screening of the A. fumigatus genomic library and as primers in PCR of A. fumigatus cDNA
| Primer or probe | Nucleotide sequence |
|---|---|
| A | GT(T/C/G) AAG GC(C/T) GT(T/C/G) GC(T/C) GT |
| B | GG(T/C) GAC TC(T/C) AAG AT(T/C) AC(T/C) GG(T/C) AC(T/C) GT(G/C/G)AC(T/C) TTC GA |
| C | GG(T/C) GAC AGC AAG AT(T/C) AC(T/C) GG(T/C) AC(T/C) GT(T/C/G) AC(T/C) TTC GA |
| PCR primer SOD 1 | CAA CTC GAG AAA AGA GTC AAG GCT GTT GCT GTC CTC |
| PCR primer SOD 2 | TAA GCG GCC GCA TTA AGC GGC GAT ACC AAT GAC |
The pI of the recombinant enzyme was deduced from the Rotofor profile. Finally, native A. fumigatus Cu,Zn SOD was purified from mycelial homogenate by using isolate NCPF 2010 (National Collection of Pathogenic Fungi, Mycological Reference Laboratory, Bristol, United Kingdom) as described previously (15, 16).
Characterization of A. fumigatus recombinant Cu,Zn SOD.
All studies were undertaken with purified recombinant and native SOD (2 to 5 μg per assay) and in quadruplicate. SOD activity was assayed as described previously (15).
Effect of pH on SOD activity.
The following buffer systems were used: pH 8.5 to 11.0 carbonate buffer (sodium carbonate-sodium bicarbonate, 50 mM) and pH 7.5 to 9.5 Tris HCl (50 mM) together with the assay procedure described previously (15).
Effects of SOD inhibitors.
The effects of inhibitors (potassium cyanide [KCN], dithiocarbamate [DDC], and EDTA) on the activity of the native and recombinant Aspergillus Cu,Zn SODs were determined as described previously (15). KCN was also used at a final working concentration of 30 mM as a specific inhibitor of Cu,Zn SOD activity to measure the potential contribution to total SOD activity in the P. pastoris culture supernatant made by other classes of SODs (15, 16).
Relative activity of recombinant Cu,Zn SOD at 20 and 37°C.
The relative activities of the native and recombinant enzymes at 20 and 37°C were compared as described previously (16).
Western blotting and immunoenzyme development with sheep polyclonal antisera and human sera.
Purified recombinant Cu,Zn SOD and native Cu,Zn SOD were subject to SDS-PAGE on 15% gels (see above) and Western blotted as described previously (12). The blots were blocked overnight with 2% casein in phosphate-buffered saline (PBS; 0.01 M; pH 7.4)–Tween 20 (0.05%) and dried. Intact blots were either developed with a sheep anti-SOD polyclonal antisera (14) (used at dilutions of 1:50 and 1:500) or cut into strips to be developed with human sera (used at 1:50 dilutions) in PBS-Tween with 0.5% casein (12, 13). After washing in PBS-Tween, the blots were incubated with either peroxidase-linked donkey anti-sheep immunoglobulin G (IgG; Jackson Immunochemicals, West Grove, Pa.) (used at a dilution of 1:250) or peroxidase-linked goat anti-human IgG (Jackson Immunochemicals) (used at a dilution of 1:250). After further washing with PBS-Tween and PBS, the blots were developed with 3,3′-diaminobenzidine and 4-chloronaphthol (12). A total of 20 serum samples from patients with confirmed A. fumigatus infections were used for the immunoenzyme development of blots, together with pooled normal human sera from 10 individuals and pooled sera from patients with C. albicans and Cryptococcus neoformans infections (10 serum samples from each group). Sera were kindly donated by the Public Health Laboratories, Colindale, London, United Kingdom, and the Corporacion para Investigaciones Biologicas, Medellin, Colombia. All serum samples were confirmed to be culture positive or were confirmed to be positive postmortem. In the case of the Aspergillus-infected sera, all infections were attributed to A. fumigatus.
Nucleotide sequence accession number.
The A. fumigatus Cu,Zn SOD has been registered in the GenBank database and its sequence has been given the accession no. AF128886.
RESULTS
Screening of genomic A. fumigatus library and cloning of A. fumigatus Cu,Zn SOD cDNA.
Six positive clones were identified from the screening of 40,000 recombinant plaques from the A. fumigatus genomic library. Further examination revealed from one of these clones a 3-kb EcoRI fragment of interest, which was then subcloned. Sequencing of the plasmid insert identified a portion that spanned 1,400 bp and that encoded a sequence with homology to Cu,Zn SODs (data not shown). This fragment was subsequently used to screen a cDNA library, and of 7,000 recombinant phage plaques, 14 clones were identified and purified. Restriction analysis of these clones with the EcoRI enzyme demonstrated that six of these clones contained a 0.8-kb insert. Subcloning and nucleotide sequence analysis of the 0.8-kb insert confirmed that it encoded a Cu,Zn SOD (Fig. 1). The cDNA encoding the Cu,Zn SOD had both a methionine residue in the N-terminal position and an Ochre termination codon, indicating that the full-length sequence was present. The Cu,Zn SOD sequence lacked a signal peptide but had six His and one Asp residues which act as the metal binding ligands in other fungal Cu,Zn SODs (8) (Fig. 1). The predicted molecular mass of the A. fumigatus Cu,Zn SOD is 18,573 Da, and its predicted pI is 6.04; both values are comparable to those estimated for the native enzyme (15). Comparison of the A. fumigatus Cu,Zn SOD sequence with other sequences in the GenBank database revealed significant identity with both fungal Cu,Zn SODs (e.g., 76% identity with the C. albicans Cu,Zn SOD and 74% identity with the Neurospora crassa Cu,Zn SOD) and Cu,Zn SODs from a range of diverse sources (e.g., 61% identity with the Mus musculus Cu,Zn SOD and 58% identity with the Homo sapiens Cu,Zn SOD).
FIG. 1.
Nucleotide sequence and amino acid residues encoded by the A. fumigatus Cu,Zn SOD cDNA. Residues coordinating copper and zinc are indicated with asterisks (six His residues [underlined] and one Asp residue).
Purification of recombinant A. fumigatus Cu,Zn SOD.
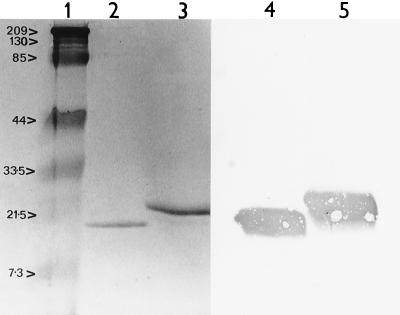
Transformation of P. pastoris with the A. fumigatus Cu,Zn SOD cDNA PCR product resulted in the secretion of Cu,Zn SOD into the culture medium. Secretion of the Cu,Zn SOD was accomplished by the fusion of the recombinant protein to the N-terminal peptide encoding the Saccharomyces cerevisiae alpha-factor secretion signal. The negative control P. pastoris culture expressing the SAP1 gene of C. albicans demonstrated no SOD activity in the culture filtrate. The total yield of recombinant protein prior to purification was 20 to 50 mg/liter. Purification of the recombinant enzyme was successfully performed by using a combination of liquid isoelectric focusing and nickel chelating resin to yield a main band at 21.5 kDa identifiable on a silver-stained gel (Fig. 2). In addition, there was a much fainter band at approximately 19.5 kDa. Immunoenzyme development of both native and recombinant proteins with the anti-Cu,Zn SOD antibody revealed intense reactivity, with an apparent overlap in band width. Two bands were recognized by the sheep polyclonal antibody previously raised against purified native protein (13) by immunoenzyme development of Western blots (Fig. 2); one was at approximately the same relative molecular mass as the native species and one was slightly larger.
FIG. 2.
Analysis of recombinant A. fumigatus Cu,Zn SOD by SDS-PAGE and Western blotting. Lane 1, molecular size markers (numbers on the left are in kilodaltons); lane 2, silver-stained purified native Cu,Zn SOD run by SDS-PAGE on a 15% polyacrylamide gel (reduced with 2-mercaptoethanol); lane 3, silver-stained recombinant Cu,Zn SOD (reduced with 2-mercaptoethanol); lanes 4 and 5, native and recombinant Cu,Zn SOD (reduced with 2-mercaptoethanol) transferred to Immobilon-P and subjected to immunoenzyme development with sheep anti-Cu,Zn SOD polyclonal antibody.
Characterization of recombinant Cu,Zn SOD.
A comparison of the biochemical and biophysical properties of the recombinant Cu,Zn SOD in relation to those described for the native form of the enzyme is provided in Table 2. The relative molecular mass of the recombinant form of the enzyme was determined to be 21.5 kDa, whereas that of the native form of the protein is 19.5 kDa. The isoelectric points of the recombinant and native forms of the enzyme were very similar, and the N-terminal amino acid sequence of the recombinant form of the protein matched that determined for the native form of the enzyme (Table 2). The activities of both the recombinant and native forms of the enzyme were pH independent, and the recombinant enzyme demonstrated an enzyme inhibition profile similar to that of the native form, with KCN and DDC completely inhibiting the activity, while EDTA had no effect. Finally, the recombinant and native forms of the enzyme demonstrated identical activities at 20 and 37°C (Table 2).
TABLE 2.
Biochemical and biophysical characteristics of recombinant Cu,Zn SOD
| Characteristic | Native Cu,Zn SOD | Recombinant Cu,Zn SOD |
|---|---|---|
| Reduced molecular mass (kDa) | 19.5 | 21.5 |
| pI | 5.9 | 6.0a |
| pH | Independent (over pH 7–11) | independent (over pH 7–11) |
| % Inhibition resulting from inhibition with: | ||
| KCN | 100 | 100 |
| DDC | 100 | 100 |
| EDTA | 0 | 0 |
| Relative activity at 37°C vs 20°C (%) | 100 | 100 |
| N-terminal amino acid sequence | VKAVAVLRGD | VKAVAVLRGD |
Determined by liquid isoelectric focusing (Rotofor).
Immune recognition.
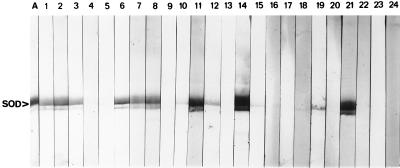
In total, 12 of the 20 (60%) serum samples from patients with confirmed A. fumigatus infections recognized the recombinant Cu,Zn SOD via immunoenzyme development of Western blots. Pooled normal human sera and pooled sera from patients with C. albicans and C. neoformans infections were all unreactive to the recombinant Cu,Zn SOD (Fig. 3). Seven serum samples were from patients with aspergilloma, all of which reacted positively with the antigen. Of the sera from the four patients with disseminated aspergillosis, only one reacted positively with the Cu,Zn SOD. Two of the six patients with bronchopulmonary aspergillosis were antibody positive, and two of the three patients with allergic bronchopulmonary aspergillosis were also positive (Table 3).
FIG. 3.
Immunoenzyme development of Western blots of recombinant Cu,Zn SOD with immune human sera. Lane A, recombinant Cu,Zn SOD; lanes 1 to 20 (corresponding to patients 1 to 20 in Table 3, respectively), sera from patients with confirmed Aspergillus infection; lane 21, sheep anti-Cu,Zn SOD polyclonal antibody; lane 22, pooled normal human sera; lane 23, pooled sera from patients with candidiasis; lane 24, pooled sera from patients with cryptococcosis. For the pooled sera in lanes 22 to 24, 10 individual serum samples from patients (patients with cryptococcosis or candidiasis or healthy humans) were combined to produce the appropriate pooled sera.
TABLE 3.
Source and reactivity of human sera to recombinant A. fumigatus Cu,Zn SOD
| Patient no. | Disease type | Serum reaction |
|---|---|---|
| 1 | Disseminated aspergillosis | + |
| 2 | Aspergilloma | + |
| 3 | Aspergilloma | + |
| 4 | Disseminated aspergillosis | − |
| 5 | Bronchopulmonary aspergillosis | − |
| 6 | Aspergilloma | + |
| 7 | Bronchopulmonary aspergillosis | + |
| 8 | Aspergilloma | + |
| 9 | Cranial aspergillosis | − |
| 10 | Aspergilloma | + |
| 11 | Bronchopulmonary aspergillosis | + |
| 12 | Aspergilloma | + |
| 13 | Bronchopulmonary aspergillosis | − |
| 14 | Allergic bronchopulmonary aspergillosis | + |
| 15 | Aspergilloma | + |
| 16 | Bronchopulmonary aspergillosis | − |
| 17 | Brochopulmonary aspergillosis | − |
| 18 | Disseminated aspergollosis | − |
| 19 | Allergic brochopulmonary aspergillosis | + |
| 20 | Allergic brochopulmonary aspergillosis | − |
DISCUSSION
The A. fumigatus cDNA of the Cu,Zn SOD revealed that the protein had significant homology not just with equivalent enzymes from other fungi but also with Cu,Zn SODs from the mouse and the human. This sequence conservation is not surprising given that the overall degree of sequence homology between the Cu,Zn SODs from higher and lower eukaryotes is between 50 and 56% (11). However, these figures cover a wide degree of variation over the molecule as a whole. Thus, three areas of Cu,Zn SODs are known to be almost completely conserved: the subunit interface, the metal binding ligands, and the residues responsible for the electric field gradient which channels superoxide to the Cu(II) active site (11). In contrast, pronounced variation in sequence may occur at the surfaces of Cu,Zn SODs (10, 26), and this heterogeneity in amino acid content and hence structure at the surface of the molecule may have a profound effect on antigenicity. Given that sera from patients with candidiasis and cryptococcosis do not recognize A. fumigatus Cu,Zn SOD, it would appear that there are sufficient differences in antigenicity between the Cu,Zn SODs from fungal pathogens to confer a degree of specificity.
The A. fumigatus Cu,Zn SOD does not possess a signal peptide, which is perhaps surprising, given that previous reports have demonstrated that this enzyme is detectable in filtrates of mid-logarithmic-phase cultures (15, 16). However, there is evidence for mechanisms of protein export which do not involve the mediation of signal peptides (3), although there is no way of knowing at this stage whether the A. fumigatus Cu,Zn SOD leaves the cell via such processes. Indeed, immunocytochemistry has also demonstrated the presence of the enzyme in the cell wall of hyphae (14), and it is possible that the presence of Cu,Zn SOD in the culture filtrate arises from the release of the enzyme from the cell wall rather than from active export, although release as a result of cell death cannot be ruled out in such circumstances.
The recombinant Cu,Zn SOD appears to be approximately 2 kDa larger than the native enzyme. This apparent difference is most likely due to the presence of the His tag. There also appears to be a second very faint band of the same size as the native protein which is also recognized by the sheep anti-Cu,Zn SOD polyclonal antibody. This faint band represents the recombinant protein without the His tag. Notwithstanding this small apparent size discrepancy, the recombinant enzyme appears to be practically identical to the native enzyme when a range of biophysical and biochemical properties are analyzed. Of particular note is the pH independence of the recombinant enzyme (which is common to many Cu,Zn SODs [11]), the inhibition of the enzyme by KCN and DCC (known inhibitors of this class of enzyme [1]), and the ability of the enzyme to maintain full activity at 37°C. The last characteristic is not shared by all the equivalent enzymes from other members of the genus Aspergillus (16), and it has previously been speculated that the thermotolerance of the A. fumigatus Cu,Zn SODs has arisen as an adaptation to the microenvironment (self-heating compost heaps) in which A. fumigatus is found (16); this explanation still appears to have some merit.
The immunoreactivity of a sheep polyclonal antibody raised against purified native Cu,Zn SOD to the recombinant form of the enzyme confirms the antigenic similarity of the two forms of the enzyme. This antigenic relatedness is confirmed by the ability of human immune sera to recognize the recombinant enzyme, and it would appear that the latter could be used to substitute for the native form of the enzyme in serodiagnostic assays. The recombinant form seems to be particularly useful for the detection of antibody responses in patients with aspergilloma. The overall percentage of patients' sera that recognize the recombinant form of the enzyme is lower than that originally reported for the native form (13). While this may reflect the fact that different sera were used in this study compared to the original report, it might also have arisen as a result of differences between the recombinant and native forms of the enzyme. However, the biophysical and biochemical analyses of the recombinant protein, coupled with the reactivity of the sheep polyclonal antibody, suggest strongly that the recombinant form of the protein is effectively identical to the native form.
The Western blot assay in which the immunoreactivity of the recombinant form of the enzyme has been demonstrated is not, however, a user-friendly format for performing routine diagnostic assays. Accordingly the recombinant Cu,Zn SOD is being assessed both by itself and in combination with other recombinant A. fumigatus antigens as a cocktail for use in an enzyme-linked immunosorbent assay for the detection of antibody responses (J. P. Latgé, personal communication). The use of a cocktail of antigens in such a test would appear to be imperative, as individual antigens are seldom, if ever, recognized by all patients in any given group, and this is clearly true of the A. fumigatus Cu,Zn SOD.
ACKNOWLEDGMENTS
We thank the Special Trustees of Guys Hospital, the British Council/Swiss National Foundation, and the Biochemical Society for financial support.
We thank Siham Beggah for providing recombinant control samples and Christophe Zaugg for computer analysis.
REFERENCES
- 1.Asada K, Yoshikawa K, Takahashi M, Maeda Y, Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975;250:2801–2807. [PubMed] [Google Scholar]
- 2.Beauvais A, Monod M, Debeaupuis J P, Diaquin M, Kobayashi H, Latgé J P. Biochemical and antigenic characterization of a new dipeptidyl-peptidase isolated from Aspergillus fumigatus. J Biol Chem. 1997;272:6238–6244. doi: 10.1074/jbc.272.10.6238. [DOI] [PubMed] [Google Scholar]
- 3.Binet R, Letoffe S, Ghigo J M, Delepelaire P, Wandersman C. Protein secretion by gram negative bacterial ABC exporters. Folia Microbiol. 1997;42:179–183. doi: 10.1007/BF02818975. [DOI] [PubMed] [Google Scholar]
- 4.Bodey G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, Mall G, Martino P, Meurnier F, Milliken S, Naoe S, Okudaira M, Scevola D, Van't Wout J. Fungal infections in cancer patients: an international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992;11:99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 5.Borg-von Zepelin M, Beggah S, Boggian K, Sanglard D, Monod M. The expression of the secreted aspartyl proteineases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol Microbiol. 1998;28:543–554. doi: 10.1046/j.1365-2958.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 6.Calera J A, Paris S, Monod M, Hamilton A J, Debeaupuis J P, Diaquin M, Lopez-Medrano R, Leal F, Latgé J P. Cloning and disruption of the antigenic catalase gene of Aspergillus fumigatus. Infect Immun. 1997;65:4718–4724. doi: 10.1128/iai.65.11.4718-4724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers S P, Prior S E, Barstow D A, Minton N P. The pMTL nic-cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988;68:139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 8.Chary P R, Hallewell R A, Natvig D O. Structure, exon pattern and chromosome mapping of the gene for cytosolic copper-zinc superoxide dismutase (sod-1) from Neurospora crassa. J Biol Chem. 1990;265:18961–18967. [PubMed] [Google Scholar]
- 9.DeGregorio M W, Lee W M F, Linker C A, Jacobs R A, Ries C A. Fungal infections in patients with acute leukemia. Am J Med. 1982;73:543–548. doi: 10.1016/0002-9343(82)90334-5. [DOI] [PubMed] [Google Scholar]
- 10.Fridovich I. Superoxide dismutases. Adv Enzymol Rel Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- 11.Gralla E B, Kosman D J. Molecular genetics of superoxide dismutases in yeasts and related fungi. Adv Genet. 1992;30:251–319. doi: 10.1016/s0065-2660(08)60322-3. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton A J, Goodley J. Purification of the 115-kilodalton exoantigen of Cryptococcus neoformans and its recognition by immune sera. J Clin Microbiol. 1993;31:335–339. doi: 10.1128/jcm.31.2.335-339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton A J, Holdom M D, Hay R J. Specific recognition of purified Cu,Zn superoxide dismutase from Aspergillus fumigatus by immune human sera. J Clin Microbiol. 1995;33:495–496. doi: 10.1128/jcm.33.2.495-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton A J, Holdom M D, Jeavons L. Expression of the Cu,Zn superoxide dismutase of Aspergillus fumigatus as determined by immunochemistry and immunoelectron microscopy. FEMS Immunol Med Microbiol. 1996;14:95–102. doi: 10.1111/j.1574-695X.1996.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 15.Holdom M D, Hay R J, Hamilton A J. Purification, N-terminal amino acid sequence and partial characterization of a Cu,Zn superoxide dismutase from the pathogenic fungus Aspergillus fumigatus. Free Rad Res. 1995;22:519–531. doi: 10.3109/10715769509150324. [DOI] [PubMed] [Google Scholar]
- 16.Holdom M D, Hay R J, Hamilton A J. The Cu,Zn superoxide dismutases of Aspergillus flavus, Aspergillus niger, Aspergillus nidulans, and Aspergillus terreus: purification and biochemical comparison with the Aspergillus fumigatus Cu,Zn superoxide dismutase. Infect Immun. 1996;64:3326–3332. doi: 10.1128/iai.64.8.3326-3332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hube B, Turver C J, Odds F C, Eiffert H, Boulnois G J, Kochel H, Rüchel R. Sequence of the Candida albicans gene encoding the secretory aspartate proteinase. J Med Vet Mycol. 1991;29:129–132. [PubMed] [Google Scholar]
- 18.Jaton-Ogay K, Suter M, Crameri R, Falchetto R, Fatih A, Monod M. Nucleotide sequence of a genomic and a cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiol Lett. 1992;92:163–168. doi: 10.1016/0378-1097(92)90506-j. [DOI] [PubMed] [Google Scholar]
- 19.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea and Febiger; 1992. pp. 201–247. [Google Scholar]
- 20.Latgé J P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latgé J P, Debeaupuis J P, Sarfati J, Diaquin M, Paris S. Cell wall antigens in Aspergillus fumigatus. Arch Med Res. 1993;24:269–274. [PubMed] [Google Scholar]
- 22.Latgé J P, Moutaouakil M, Debeaupuis J P, Bouchara J P, Haynes K, Prevost M C. The 18-kilodalton antigen secreted by Aspergillus fumigatus. Infect Immun. 1991;59:2586–2594. doi: 10.1128/iai.59.8.2586-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Medrano R, Ovejero M C, Calera J A, Puente P, Leal F. An immunodominant 90-kilodalton Aspergillus fumigatus antigen is the subunit of a catalase. Infect Immun. 1995;63:4774–4780. doi: 10.1128/iai.63.12.4774-4780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monod M. Construction of a genomic libraray for A. fumigatus. In: Maresca B, Kobayashi G S, editors. Molecular biology if pathogenic fungi. A laboratory manual. New York, N.Y: Telos Press; 1994. pp. 29–32. [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schininà M E, Barra D, Simmaco M, Bossa F, Rotilio G. Primary structure of porcine Cu,Zn superoxide dismutase. FEBS Lett. 1985;186:267–270. doi: 10.1016/0014-5793(85)80722-5. [DOI] [PubMed] [Google Scholar]
- 27.Sherertz R J, Belani A, Kramer B S, Elfenbein G J, Weiner R S, Sullivan M L, Thomas R G, Samsa G P. Impact of air filtration on nosocomial Aspergillus infections. Unique risk of bone marrow transplant recipients. Am J Med. 1987;83:709–718. doi: 10.1016/0002-9343(87)90902-8. [DOI] [PubMed] [Google Scholar]
- 28.Sulahian A, Tabouret M, Ribaud P, Sarfati J, Gluckman E, Latgé J P, Derouin F. Comparison of an enzyme immunoassay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur J Clin Microbiol Infect Dis. 1996;15:139–145. doi: 10.1007/BF01591487. [DOI] [PubMed] [Google Scholar]
- 29.Verweij P E, Latgé J P, Rijs A J, Melchers W J, DePauw B E, Hoogkamp-Korstanje J A, Meis J F. Comparison of antigen detection and PCR assay using bronchoalveolar lavage fluid for diagnosing invasive pulmonary aspergillosis in patients receiving treatment for hematological malignancies. J Clin Microbiol. 1995;33:3150–3153. doi: 10.1128/jcm.33.12.3150-3153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]