Abstract
Unlike ubiquitin, the ubiquitin-like protein modifier SUMO-1 and its budding yeast homologue Smt3p have been shown to be more important for posttranslational protein modification than for protein degradation. Here we describe the identification of the SUMO-1 homologue of fission yeast, which we show to be required for a number of nuclear events including the control of telomere length and chromosome segregation. A disruption of the pmt3+ gene, the Schizosaccharomyces pombe homologue of SMT3, was not lethal, but mutant cells carrying the disrupted gene grew more slowly. The pmt3Δ cells showed various phenotypes such as aberrant mitosis, sensitivity to various reagents, and high-frequency loss of minichromosomes. Interestingly, we found that pmt3+ is required for telomere length maintenance. Loss of Pmt3p function caused a striking increase in telomere length. When Pmt3p synthesis was restored, the telomeres became gradually shorter. This is the first demonstration of involvement of one of the Smt3p/SUMO-1 family proteins in telomere length maintenance. Fusion of Pmt3p to green fluorescent protein (GFP) showed that Pmt3p was predominantly localized as intense spots in the nucleus. One of the spots was shown to correspond to the spindle pole body (SPB). During prometaphase- and metaphase, the bright GFP signals at the SPB disappeared. These observations suggest that Pmt3p is required for kinetochore and/or SPB functions involved in chromosome segregation. The multiple functions of Pmt3p described here suggest that several nuclear proteins are regulated by Pmt3p conjugation.
Ubiquitin is a small (76-residue), abundant protein conserved in all eukaryotic cells. It exists in several cellular compartments, such as the cytosol, nucleus, and cell surface. It is well known that ubiquitin regulates the function and stability of target proteins through its posttranslational conjugation to target proteins. Before conjugation to target proteins, ubiquitin must be processed by a C-terminal hydrolase. The first step of the ubiquitin conjugation pathway is the ATP-dependent formation of a thioester bond between the conserved C-terminal glycine of processed ubiquitin and the active-site cysteine residue of an E1 ubiquitin-activating enzyme. The second step is the transfer of activated ubiquitin to the active-site cysteine of an E2 ubiquitin-conjugating enzyme. In the final step, the E2 enzyme may cooperate with an E3 ubiquitin protein ligase to form an isopeptide bond between the C-terminal glycine of ubiquitin and the ɛ-amino groups of lysine residues of target proteins. Ubiquitin covalently conjugated to target proteins can be removed by a ubiquitin isopeptidase (89).
Recently, a number of novel ubiquitin-like proteins were independently discovered in a number of species, suggesting that ubiquitin is part of a family of related proteins involved in the covalent modification of proteins. The first example of such a protein was the 15-kDa interferon-inducible, ubiquitin cross-reacting protein UCRP (25). UCRP contains two ubiquitin-related domains that are 43 and 62%, respectively, homologous to ubiquitin. It is conjugated to a number of unknown proteins and may serve as a trans-acting binding factor to direct the association of modified target proteins to intermediate filaments in the cytoplasm (25, 45). Other novel modification proteins such as Saccharomyces cerevisiae Rub1p and mammalian Nedd8 have also been reported (40, 44, 74). Rub1p and Nedd8 are also members of the ubiquitin-like protein family and display high homologies with ubiquitin, a probable functional homologue. Cdc53p/cullin, a subunit of the multifunctional SCF ubiquitin ligase, is a major substrate for Rub1p/Nedd8 conjugation (40, 74).
SUMO-1 was isolated as a protein covalently linked to RanGAP1, the Ran GTPase-activating protein of human cells (47). Modification by SUMO-1 targets the cytosolic RanGAP1 to the nuclear pore complex by promoting binding of the SUMO-1-modified RanGAP1 to RanBP2 (47, 48, 54, 75). In addition, it was suggested that the reversible modification of RanGAP1 by SUMO-1 had a regulatory role in the association of RanGAP1 with nuclear envelope (48). SUMO-1 was independently identified in two-hybrid screenings under names such as PIC1, which interacts with the PML component of nuclear multiprotein complex that is disrupted in acute promyelocytic leukemia (6), GMP1, which interacts with RanGAP1 (53), sentrin, which interacts with Fas/APO-1 or the tumor necrosis factor receptor 1 death domain (73), and UBL1, which interacts with the human Rad51/Rad52 proteins involved in DNA recombination and DNA double-strand break repair (81). There are at least two other proteins that are closely related to SUMO-1 (designated as SMT3C) (41), SMT3A (41) and SMT3B (10, 41, 49), in human cells, but there is only a single SUMO-1 homologue, Smt3p, in S. cerevisiae (33, 59). SMT3, an essential S. cerevisiae gene encoding a 11.5-kDa protein, was originally isolated as a multicopy suppressor of mutations in the MIF2 gene, which encodes a CENP-C-like centromere-binding protein (59). Smt3p is 48% identical to SUMO-1/PIC1/GMP1/sentrin/UBL1 and 17% identical to ubiquitin. Smt3p also becomes conjugated to several proteins posttranslationally (33). However, the precise function and target proteins of Smt3p remain to be determined.
The three-dimensional structure of SUMO-1 was determined (4). Although SUMO-1 has only 18% amino acid sequence identity with ubiquitin, its overall structure closely resembles that of ubiquitin. Lys48 of ubiquitin, required for the generation of ubiquitin polymers, is replaced by Gln at the corresponding position in SUMO-1 (Gln69). This explains why SUMO-1 has not been observed to form polymers (48). On the other hand, the positions of two C-terminal Gly residues required for isopeptide bond formation are conserved between ubiquitin and SUMO-1. The most prominent feature of SUMO-1 is its long and highly flexible N terminus, which protrudes from the core of the protein and which is absent in ubiquitin (4).
It has been proposed that several features of the ubiquitin pathway are conserved in the early steps of the Smt3p/SUMO-1 conjugation pathway (33). Like ubiquitin, Smt3p is proteolytically processed to expose its mature C terminus. Then, Smt3p is activated in an ATP-dependent manner by an activating enzyme made up of a heterodimer of Uba2p and Aos1p (33). Uba2p is a 71-kDa protein with high homology to the C-terminal regions of E1 ubiquitin-activating enzymes and displays conservation of the active-site cysteine residue participating in thioester formation. Aos1p is a 40-kDa protein with extensive similarity to the N-terminal regions of E1 ubiquitin-activating enzymes (33). A significant body of evidence indicates that the E2 enzyme for Smt3p/SUMO-1 conjugation is Ubc9p (78). A human Ubc9 homologue interacts with SUMO-1 in two-hybrid screening (80), and antibodies against the Xenopus laevis Ubc9 homologue can coimmunoprecipitate complexes containing SUMO-1-modified RanGAP1 (75). In addition, it was demonstrated that Ubc9p is the only E2-conjugating enzyme for Smt3p (32). UBC9 is an essential gene, and conditional ubc9ts mutants arrest at the G2/M of the cell cycle with concomitant accumulation of both B-type (78) and G1 (5) cyclins. Recently, it was shown that modification of RanGAP1 by SUMO-1 requires the Ubc9 homologue in Xenopus eggs (76) and human cells (42). Although the Ubc9 E2-conjugating enzymes of Smt3p/SUMO-1 are related to those of ubiquitin, they do not share the same target proteins (32, 42). These results suggest that the two pathways are distinct. Recently, a protease specific for ubiquitin-like proteins, Ulp1p, which cleaves proteins modified by Smt3p and SUMO-1 but not by ubiquitin, was reported (43). Ulp1p-related proteins are conserved in many organisms. Ulp1p plays an essential role in the G2/M phase of the cell cycle, indicating that Ulp1p-mediated Smt3p-protein deconjugation is required for cell cycle progression.
In this work, we describe the characterization of a Schizosaccharomyces pombe homologue of Smt3p/SUMO-1. We designated the gene encoding this S. pombe homologue pmt3+. Our results clearly showed that Pmt3p is required for a number of nuclear events including the control of telomere length and chromosome segregation.
MATERIALS AND METHODS
Fission yeast strains, media, and methods.
Fission yeast strains were grown and used as described by Moreno et al. (62). All fission yeast strains used in this study are listed in Table 1. Standard genetic methods and staining with 4′,6-diamidino-2-phenylindole (DAPI) were as described elsewhere (62). Cell number was determined with a Sysmex CDA-500 cell counter. Transformation of S. pombe was achieved by the lithium acetate (72) or the electroporation (35) method. Vegetative growth under nonselective conditions was in YPD (2% peptone, 1% yeast extract, 2% glucose) or YE (0.5% yeast extract, 3% glucose) medium, supplemented as required. The synthetic minimal medium used was EMM2 (60), supplemented as required. The thiamine-regulatable promoter nmt1 was repressed by adding 5 μg of thiamine per ml to EMM2 medium (55).
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h− leu1-32 | 36 |
| TP4-1D | h+ leu1-32 ura4-D18 his2 ade6-M216 | 36 |
| TP4-5A | h− leu1-32 ura4-D18 ade6-M210 | 36 |
| JN629 | h− leu1-32 pmt3Δ::ura4 ura4-D18 ade6-M216 | This work |
| JN630 | h− leu1-32 pmtΔ3::ura4 ura4-D18 | This work |
| JN632 | h− leu1-32 cdc2-3w | 66 |
| JN633 | h− leu1-32 cds1Δ::ura4 ura4-D18 | 66 |
| YM75 | h+ leu1-32 ura4-D18 ade6-704 Ch10 | 70 |
| YM76 | h+ leu1-32 pmt3Δ::ura4 ura4-D18 ade6-704 Ch10 | This work |
| YM77 | h+ leu1-32 ura4-D18 ade6-704 | This work |
| YM78 | h+ leu1-32 ura4-D18 ade6-704 pmt3+:pYC11-GFPpmt3 | This work |
Cloning of a genomic fragment and the full-length cDNA of the pmt3+ gene.
A genomic fragment of the pmt3+ gene was cloned by screening of an ordered S. pombe cosmid library (61) with the pmt3+ cDNA obtained by two-hybrid screening. The full-length cDNA of the pmt3+ gene was isolated by the screening of a λZapII S. pombe cDNA library (from about 5 × 104 plaques) (34), using plaque hybridization with the pmt3+ cDNA obtained by two-hybrid screening as a probe. Sequencing was carried out on both strands by using an ABI PRISM dye terminator cycle sequencing ready reaction kit (Perkin-Elmer). Multiple alignments were carried out by using the CLUSTAL W program (86).
Construction of the pmt3 deletion strain.
A 4.2-kb EcoRI genomic fragment containing the pmt3+ gene was subcloned from a pmt3+-containing cosmid after restriction mapping. The 1.8-kb ura4+ gene was inserted at the unique ApaLI site of a 4.2-kb pmt3+ genomic fragment which had been previously blunt ended and ligated to an XbaI linker. A 6.0-kb fragment carrying the pmt3::ura4 construct was transformed into diploid strain TP4-1D/TP4-5A (h+/h− his2/+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M216/ade6-M210) (36). Stable transformants were isolated, and gene disruption was confirmed by Southern blot analysis. For analysis of the phenotype of haploids with a disruption of the pmt3+ gene, tetrad analyses were performed by standard methods (62).
Analysis of HU and UV responses.
To test the response to hydroxyurea (HU), wild-type (HM123 [h− leu1-32]), pmt3Δ (JN630 [h− leu1-32 ura4-D18 pmt3Δ::ura4]), cdc2-3w (JN632 [h− leu1-32 cdc2-3w]), and cds1Δ (JN633 [h− leu1-32 ura4-D18 cds1Δ::ura4]) cells were grown to a mid-log phase (0.5 × 107 to 1 × 107 cells/ml) in YE medium at 30°C. HU was added to the culture to a final concentration of 12 mM at time zero. Portions were taken and plated on YE plates, and cell viability was determined (66).
To test UV sensitivity, wild-type (HM123) cells, pmt3Δ cells (JN630), and pmt3Δ cells containing plasmid pREP1x-pmt3, which expresses pmt3+, were grown to mid-log phase (0.5 × 107 to 1 × 107 cells/ml) in EMM2 supplemented with leucine or EMM2 containing 5 μg of thiamine per ml (for pmt3Δ cells containing pmt3+ expression plasmid) at 30°C. Then, cells were plated in duplicate onto EMM2 plates supplemented with leucine or EMM2 plates containing thiamine (5 μg/ml) at 800 cells per plate and irradiated in a Stratalinker (Stratagene) at doses ranging from 0 to 150 J/m2 (66). Cell viability was determined by counting colonies following incubation at 30°C for 4 to 6 days.
Determination of minichromosome loss rates.
Ch10 is a linear minichromosome that carries the sup3-5 tRNA gene, which suppresses the ade6-704 mutation (70). A colony color assay was performed to measure the stability of Ch10. S. pombe ade6-704 cells form red colonies. If the minichromosome Ch10 is stably maintained in the ade6-704 cells during colony formation, sup3-5 on Ch10 will suppress the ade6-704 mutation and the cells will form white Ade+ colonies. The rate of loss of Ch10 from wild-type (YM75 [h+ leu1-32 ura4-D18 ade6-704 Ch10]) and pmt3Δ (YM76 [h+ leu1-32 ura4-D18 pmt3Δ::ura4 ade6-704 Ch10]) cells under normal conditions was calculated by two different methods (67). Each experiment was carried out at least four times.
In the first method, YM75 and YM76 strains were grown in the absence of adenine to select for Ch10-harboring cells. Several colonies were dispersed in 1 ml of YE medium. Cell number was measured in a cell counter. The cells were then diluted into YE medium, sonicated briefly, and plated onto YE plates at a concentration of ∼103 cells/plate. After 3 to 5 days growth at 30°C, the proportion of Ade− (completely pink) colonies was calculated. The overall loss rates of Ch10 from wild-type and pmt3Δ cells were taken to be an average of the loss rates observed in four experiments.
In the second method, cultures of cells were grown under selective conditions to a density of 1 × 106 to 3 × 106 cells per plate to ensure maintenance of Ch10. The cells were then harvested, resuspended in an equal volume of YE medium, and incubated for three generations. Samples were removed at the beginning and the end of the incubation period, and cell number and the proportion of Ade− segregants were determined as follows. The wild-type and pmt3Δ cells were plated on EMM2 plates supplemented with adenine (7 μg/ml), which renders Ade− colonies pink. The rate of loss of Ch10 was calculated by using the following formula, previously described by Murakami et al. (67): rate of loss = 1−e(1/n)lnRn/R0 where R0 and Rn are the proportions of Ade+ cells at 0 and n generations after removal of selection, respectively.
Measurements of telomere length.
S. pombe chromosome DNA was prepared by a glass bead-phenol extraction protocol (29). Chromosome DNA was digested with ApaI, resolved on a 1% agarose gel, and transferred onto a nylon membrane (GeneScreen Plus; NEN). The 0.3-kb ApaI-EcoRI fragment of pAMP2 (52), which contains the S. pombe telomeric repeat sequences, was used as a probe for telomeric repeat sequences in the Amersham ECL system.
Expression of Pmt3p and HA3 tagging Pmt3p in S. pombe.
The pmt3+ cDNA from the two-hybrid screen was digested with BamHI and XhoI and cloned into the BamHI-XhoI sites of pREP1 after inserting a XhoI linker into the SmaI site of the multicloning site downstream of the thiamine-regulatable nmt1 promoter (55). The resulting construct was termed pREP1x-pmt3. Tagging of Pmt3p with three copies of the hemagglutinin (HA) epitope was done as follows. The pmt3+ cDNA was amplified by PCR using oligonucleotide primers located at either end of the open reading frame, and the amplified DNA was digested with NotI and BamHI. Primers were 5′-TAGCGGCCGCATGTCTGAATCACCATCA-3′ (a sense primer to create a NotI site at the start codon of pmt3+ cDNA; the NotI site is underlined) and 5′-CCGGATCCAAGGCATAGATGGGTGCA-3′ (an antisense primer to create a BamHI site downstream of pmt3+ cDNA; the BamHI site is underlined). The NotI-BamHI fragment of pmt3+ cDNA was cloned into the NotI-BglII sites of the strongest nmt1 promoter (nmt) HA3-tagging vector pSLF173 (23) after converting an ura4 marker to a LEU2 marker and into the weakest nmt1 promoter (nmt**) HA3-tagging vector pSLF373 (23) after converting an ura4 marker to a LEU2 marker. The resulting constructs were termed pSLF173L-pmt3 and pSLF373L-pmt3, respectively.
Preparation of S. pombe cell lysates and Western blot analysis.
Whole-cell extracts from S. pombe cells for Western analysis were prepared by the boiling sodium dodecyl sulfate (SDS)-glass bead method as described below (50). Ten milliliters of logarithmic-phase S. pombe cell cultures (about 5 × 106/ml) was harvested. Pellets were washed twice with water, resuspended in 100 μl of water, and heated at 90°C for 5 min. Then 120 μl of 2× Laemmli buffer (4% SDS, 20% glycerol, 0.6 M β-mercaptoethanol, 0.12 M Tris-HCl [pH 6.8]) containing 8 M urea was added to the samples, which were vigorously vortexed with an equal volume of acid-washed glass beads for 3 min and then heated again 90°C for 5 min. The whole-cell extracts were analyzed by SDS-polyacrylamide gel electrophoresis using 10% polyacrylamide gels and then transferred to Immobilon transfer membranes (Millipore) by using a wet-type transfer system. Anti-HA monoclonal antibody 12CA5 was purchased from BAbCo. Horseradish peroxidase-conjugated goat anti-mouse secondary antibody was purchased from Bio-Rad. Western blot detection was done with the ECL Plus system as described by the manufacturer (Amersham).
GFP tagging of Pmt3p.
The plasmid for green fluorescent protein (GFP) tagging of Pmt3p was constructed as follows. For the GFP fusion at the N terminus of Pmt3p, GFP(S65A) (63) was amplified by PCR with a 5′ primer and a 3′ primer. Primers were 5′-CCGGATCCGGCGGCCGCATGAGTAAAGGAGAAGAA-3′ [a sense primer to create a BamHI site before the start codon of GFP(S65A); the BamHI site is underlined] and 5′-GCGAATTCCTTTTGTATAGTTCATCCATGC-3′ [an antisense primer to remove the stop codon of GFP(S65A) and to create an EcoRI site; the EcoRI site is underlined]. The PCR product of the GFP(S65A) fragment whose stop codon was removed was digested with BamHI and EcoRI (fragment 1). For preparation of the pmt3+ promoter region, the pmt3+ genomic DNA was amplified by PCR with a 5′ primer and a 3′ primer. Primers were 5′-GATATCTTGAATAACTTC-3′ (a sense primer containing an EcoRV site, which is complementary to the 5′-terminal sequence shown in Fig. 1A; the EcoRV site is underlined) and 5′-GCGGATCCAGATACTATATAAAATC-3′ (an antisense primer to create a BamHI site, which is complementary to the region [positions −25 to −9 in Fig. 1A]) upstream of the initiator ATG of pmt3+; the BamHI site is underlined). The PCR product of the pmt3+ promoter region was digested with EcoRV and BamHI (fragment 2). For preparation of the pmt3+ coding and 3′ noncoding regions, the pmt3+ genomic DNA was amplified by PCR with a 5′ primer and a 3′ primer. Primers were 5′-GCGAATTCTGAATCACCATCAGC-3′ (a sense primer to create an EcoRI site and to remove the initiating ATG codon of pmt3+, which is complementary to the region [positions +4 to +20 in Fig. 1A] just downstream of the initiator ATG of pmt3+; the EcoRI site is underlined) and 5′-AAGCTTCAAGAAAATTTAGC-3′ (an antisense primer containing a HindIII site, which is complementary to the 3′-terminal sequence shown in Fig. 1A; the HindIII site is underlined). The PCR product of the pmt3+ coding and 3′ noncoding regions was digested with EcoRI and HindIII (fragment 3). Fragments 1, 2, and 3 were cloned together into the EcoRV-HindIII site of pBluescript II KS+ (77) to construct a plasmid for GFP tagging of Pmt3p (pBS-GFPpmt3). The resulting plasmid, pBS-GFPpmt3, contained the native promoter of pmt3+ and the full pmt3+ coding region, whose N terminus was ligated in frame to the GFP gene. To integrate the GFP-tagged pmt3+ gene onto the genome, the EcoRV-HindIII fragment containing the pmt3+ promoter, the GFP-tagged pmt3+ coding region, and the 3′ noncoding region of pBS-GFPpmt3 was isolated and cloned into the SmaI-HindIII site of an integration vector, pYC11 (13). The resulting plasmid, pYC11-GFPpmt3, was used for transformation of the wild-type strain (YM77 [h+ leu1-32 ura4-D18 ade6-704]), and stable Leu+ transformants (YM78 [h+ leu1-32 ura4-D18 ade6-704 pmt3+:pYC11- GFPpmt3]) were selected.
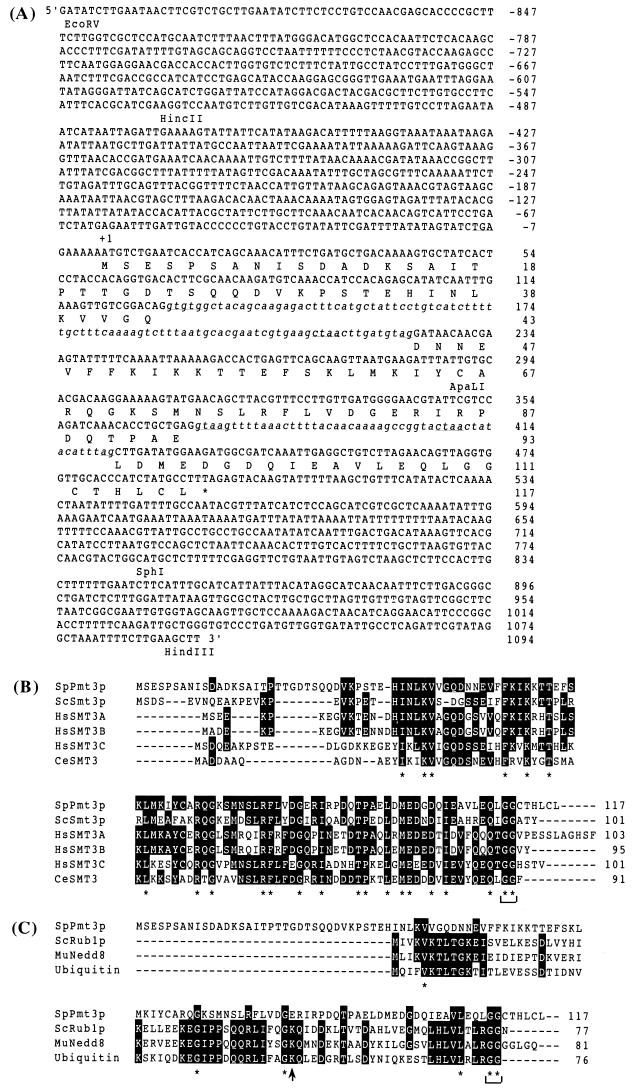
FIG. 1.
(A) Nucleotide sequence of S. pombe pmt3+ gene and predicted amino acid sequence of Pmt3p. The predicted amino acid sequence is shown in single-letter code below the nucleotide sequence. Intron sequences are shown in lowercase letters. Intron consensus sequences (92) are underlined. An in-frame stop codon upstream of the putative initiator ATG is also underlined. (B) Sequence alignment of a novel ubiquitin-like SUMO-1 family. Multiple alignment between S. pombe Pmt3p, S. cerevisiae Smt3p, human SMT3A (41), SMT3B (49), SMT3C (SUMO-1) (47), and Caenorhabditis elegans SMT3 (14) was done with the CLUSTAL W algorithm (86). Asterisks under amino acids indicate exact matches between the six aligned sequences. Dashes within the coding sequence indicate spaces inserted to achieve an optimal match. A bracket indicates the conserved diglycine, the expected site of precursor cleavage. (C) Sequence alignment of ubiquitin and ubiquitin-like proteins. Multiple alignment between S. pombe Pmt3p, S. cerevisiae Rub1p (44), mouse Nedd8 (39), and S. cerevisiae ubiquitin was done with the CLUSTAL W algorithm (86). Asterisks under amino acids indicate exact matches between the four sequences. Dashes within the coding sequence indicate spaces inserted to achieve an optimal match. A bracket indicates the conserved diglycine. An arrow indicates the site of the Lys48 of ubiquitin required to generate ubiquitin polymer.
Fluorescence microscopy.
Immunofluorescent staining and in situ hybridization of cells were done according to a protocol provided by Y. Chikashige (11), which is a modification of the protocols of Chikashige et al. (12) and Dernburg et al. (17). For GFP-Pmt3-expressing cells, cells were fixed with 3% formaldehyde at room temperature for 60 min. TAT1 mouse monoclonal anti-α-tubulin antibody (90) and anti-Sad1 rabbit polyclonal antibody (27) were used to stain microtubule and spindle pole bodies (SPB), respectively. Secondary antibodies used were goat anti-mouse immunoglobulin G (IgG) Cy3-conjugated antibody, goat anti-rabbit Cy3-conjugated antibody (Jackson Laboratory), goat anti-rabbit Oregon green-conjugated antibody (Molecular Probes), and donkey anti-mouse Cy5-conjugated antibody (Amersham). DNA was stained with DAPI (1 μg/ml). Microscopic images were obtained by using a Delta Vision system (Applied Precision) with an Olympus oil immersion objective lens (Dplan Apo 100/NA1.3). Several z-axis sections of 0.1-μm intervals were combined by the quick projection program to avoid overlooking any signals within a cell.
Nucleotide sequence accession number.
The nucleotide sequence of pmt3+ has been deposited in the GenBank database under accession no. AB017187.
RESULTS
Identification of S. pombe Smt3p/SUMO-1 homologue.
Two-hybrid screening of an S. pombe cDNA library was done with an accessory factor of DNA polymerase δ, PCNA, as bait. Two novel cDNAs were isolated, one of which was found to encode a homologue of the S. cerevisiae Smt3p after DNA sequencing. The SMT3 gene was originally isolated as a multicopy suppressor of the S. cerevisiae mif2ts mutation (59) and was suggested to be a novel type ubiquitin-related factor. Therefore, we named this gene pmt3+ (S. pombe homologue of SMT3). Although we confirmed the in vivo interaction between PCNA and Pmt3p by immunoprecipitation (data not shown), we have found no genetic relationship between PCNA and Pmt3p.
We searched and obtained the full-length pmt3+ cDNA and the corresponding 4.2-kb genomic DNA fragment as described in Materials and Methods. Genomic sequence analysis indicated that the pmt3+ gene contains three exons (total of 351 bp) and two introns with appropriate consensus splicing signal sequence (Fig. 1A). Since the sequences upstream of the putative initiator ATG include an in-frame stop codon, we concluded that this cDNA contains the full-length pmt3+ gene and that the original cDNA from two-hybrid screening also contained the full-length gene (Fig. 1A). The presence and positions of introns were confirmed by sequencing of the genomic pmt3+ gene and the pmt3+ cDNA. The pmt3+ open reading frame encodes a predicted protein of 117 amino acids with a molecular mass of 13 kDa. Amino acid sequence comparisons revealed that S. pombe Pmt3p is 39% identical to S. cerevisiae Smt3p and human SMT3C (SUMO-1) (47), 13% identical to S. cerevisiae Rub1p (44) and mouse Nedd8 (39), and 11% identical to ubiquitin (Fig. 1B and 1C). The conserved diglycine (GG) sequence corresponding to the C-terminal Gly75 and Gly76 of ubiquitin, which was shown to be crucial for conjugation between ubiquitin and ubiquitin pathway enzymes, was completely conserved among ubiquitin and ubiquitin-related proteins, including Pmt3p (Fig. 1B and C). However, ubiquitin Lys48, required for the generation of ubiquitin polymers, was replaced by Gln at the same position in Pmt3p (Gln83), which suggests that Pmt3p may not form polymers as proposed for SUMO-1 (Fig. 1C) (4).
Gene disruption of the pmt3+ gene and phenotypes of pmt3Δ cells.
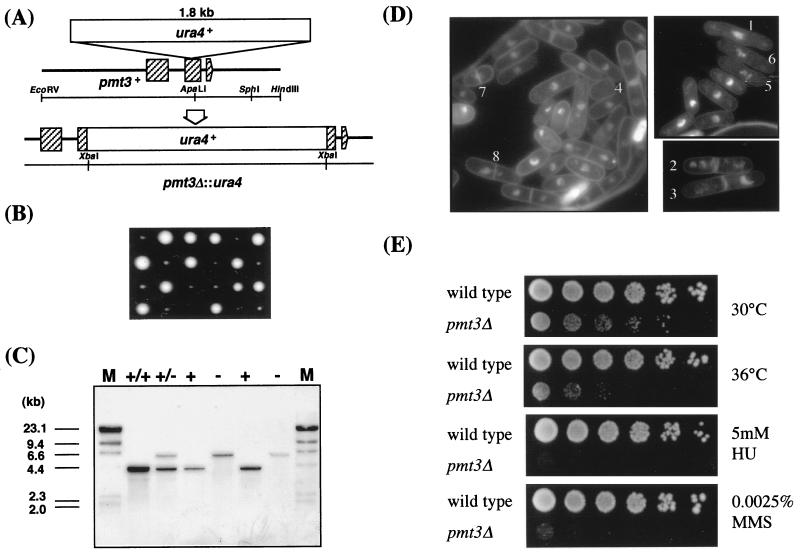
Gene disruption of pmt3+ was carried out as described in Materials and Methods. Precise replacement of one of the two chromosomal copies of pmt3+ in an S. pombe diploid strain by the pmt3::ura4 fragment was confirmed by Southern blot analysis of genomic DNA (Fig. 2A and C). All dissected tetrad spores of the Ura+ diploid gave two Ura− colonies and two tiny Ura+ colonies (Fig. 2B). Thus, the pmt3Δ cells were viable but grew more slowly than wild-type cells (the doubling time of pmt3Δ cells was about 5 h at 30°C in YE medium) (Fig. 2E). DAPI staining and microscopic analysis indicated that about 23% of pmt3Δ cells display aberrant cellular and nuclear morphologies (Fig. 2D); 6% were generally elongated (Fig. 2D-1), 4% showed a displaced nucleus (Fig. 2D-2 and 2D-3), and 2% showed enucleated daughter cells (Fig. 2D-4), indicating that cells had undergone cytokinesis without prior completion of mitosis. In addition, the chromatin of 4% of the cells was highly condensed (Fig. 2D-5 and 2D-6), indicating strong mitotic delay. Furthermore, 7% of the cells displayed the typical cut phenotype where cells remain connected by threads of chromatin (Fig. 2D-7 and 2D-8). These phenotypes are reminiscent of the constant-cut phenotypes of rad31+ and hus5+ mutants or disruptants (3, 21, 79) (see Discussion). Missegregation of the chromosomes was also confirmed by flow cytometric (fluorescence-activated cell sorting [FACS]) analysis. Wild-type cells showed the normal 2C DNA content peak. The profile of pmt3Δ cells showed a decrease of the number of cells with 2C DNA content and an increase in the number of cells with a DNA content greater or less than 2C DNA content (at times −2 and 0 h in Fig. 3C). This profile is consistent with the faulty chromosome segregation observed by DAPI staining.
FIG. 2.
Gene disruption of the pmt3+ gene. (A) Diagrammatic representation of disruption of the pmt3+ gene by one-step gene replacement. The ura4+ gene was used to disrupt the coding region of pmt3+ as indicated, and the resulting plasmid with the disrupted pmt3+ gene was used to transform the wild-type diploid strain. Hatched boxes indicate the three exons of the pmt3+ gene. (B) Tetrad analysis of a heterozygous diploid. A diploid which consisted of one copy of wild-type pmt3+ and its disrupted allele was manipulated and incubated on a YE plate supplemented with adenine at 30°C for 4 days. (C) Demonstration of pmt3 deletion by genomic Southern blot analysis. DNA was prepared from wild-type (+/+) and the heterozygous (+/−) diploid strains, digested with EcoRI, separated by agarose gel electrophoresis, blotted onto a nylon membrane, and probed with the 4.2-kb EcoRI fragment containing the pmt3+ gene. The 4.2-kb band represents the pmt3+ locus; the 6.0-kb bands represent the pmt3::ura4 allele, in which the ura4+ gene was inserted into the ApaLI site of pmt3+. M, size markers. (D) DAPI staining of pmt3Δ cells at the permissive temperature (30°C). Several phenotypes in addition to size heterogeneity can be seen: 1, elongated cell; 2 and 3, displaced nuclei; 4, nucleate daughter cell; 5 and 6, highly condensed chromosomes; 7 and 8, typical cut abortive mitosis. (E) Various phenotypes of pmt3Δ cells. pmt3Δ cells are hypersensitive to high temperature, HU, and MMS. Wild-type (HM123) and pmt3Δ (JN630) cells were spotted onto YE and YE plates containing 5 mM HU or 0.0025% MMS and incubated for 4 days at 30 or 36°C.
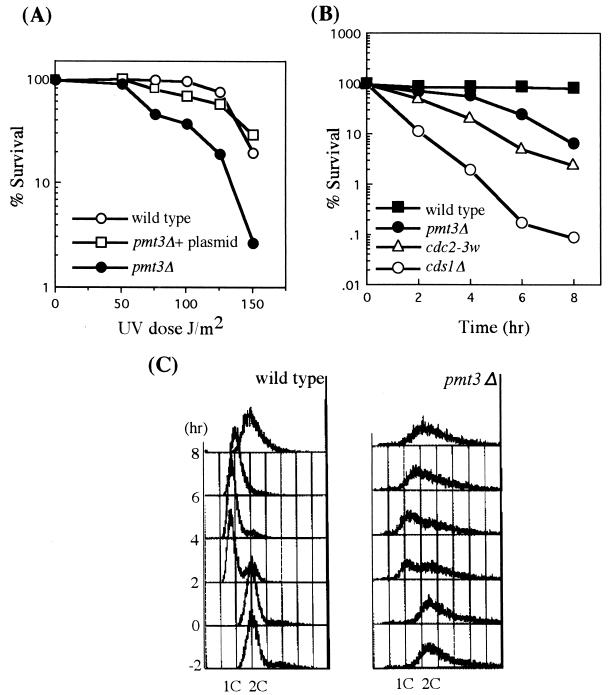
FIG. 3.
UV and HU sensitivity of pmt3Δ cells. (A) UV sensitivity of pmt3Δ cells. Viabilities of wild-type (HM123) and pmt3Δ (JN630) cells after UV irradiation were measured. Cells in mid-log phase were plated and exposed to UV at a dose of 0 to 150 J/m2. Sample plates were incubated, and cell viabilities were determined. UV sensitivity of pmt3Δ cells could be completely restored by introducing the plasmid expressing pmt3+. (B) HU sensitivity of pmt3Δ cells. Viabilities of wild-type (HM123), pmt3Δ (JN630), cdc2-3w (JN632), and cds1Δ (JN633) cells after incubation for 0 to 8 h at 30°C in YE medium in the presence of 12 mM HU were determined. Cells were grown to mid-log phase in YE medium at 30°C. HU was added to the culture at a final concentration of 12 mM at time zero. Samples were withdrawn at the indicated times and plated on YE plate, and cell viability was determined. (C) DNA contents of wild-type (HM123) and pmt3Δ (JN630) cells after HU treatment described for panel B were analyzed by flow cytometry. At various time points, cells were fixed and stained by propidium iodide, and their DNA contents were determined by FACS analysis.
In addition to the constant-cut phenotype described above, we found that pmt3Δ cells showed sensitivities to a number of stresses including high temperature, DNA damage induced by UV light or the DNA-damaging agent methyl methanesulfonate (MMS), inhibition of DNA synthesis by HU. The pmt3Δ cells incubated for 12 h at 36°C were 35% as viable as those incubated at 30°C (Fig. 2E). The frequency of elongated and cut phenotype cells after incubation for 12 h at 36°C was higher than at 30°C (data not shown). A plate concentration of 0.0025% MMS severely inhibited growth of the pmt3Δ cells but had little effect on the growth of wild-type cells (Fig. 2E). pmt3Δ cells were moderately sensitive to low doses of UV radiation, as their viability decreased more than 10-fold after a 150-J/m2 dose of UV radiation (Fig. 3A). In addition to the sensitivities to high temperature, UV, and MMS, a plate concentration of 5 mM HU severely inhibited growth of the pmt3Δ cells (Fig. 2E). The sensitivity of pmt3Δ cells to HU was examined quantitatively and compared to those of wild-type, cdc2-3w, and cds1Δ cells. As shown in Fig. 3B, pmt3Δ cells showed moderate sensitivity to HU treatment like that of cdc2-3w cells. During an 8-h incubation in the presence of 12 mM HU, the viability of pmt3Δ cells dropped more than 10-fold, while the viability of wild-type cells remained largely unchanged. The viabilities of cdc2-3w and cds1Δ cells, which are replication checkpoint-defective strains, dropped more than 50- and 1,000-fold, respectively (Fig. 3B). Furthermore, FACS analysis showed that the majority of pmt3Δ cells arrest with a 1C DNA content like that of wild-type cells after exposure to HU (Fig. 3C). These results suggested that pmt3Δ cells retain S/M phase checkpoint control (see Discussion).
Requirement of Pmt3p for chromosome segregation.
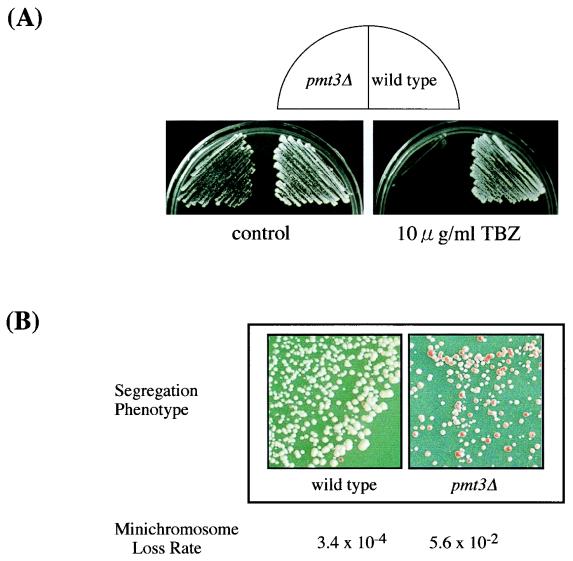
Since the DAPI staining and FACS profile described above suggested faulty segregation of chromosomes in pmt3Δ cells, we tested the sensitivity of pmt3Δ cells to the microtubule-destabilizing agent thiabendazole (TBZ). TBZ is known to bind tubulin molecules and to inhibit their polymerization. Increased sensitivity to microtubule-destabilizing benzimidazole compounds is a characteristic of defective microtubule components or microtubule-interacting proteins. A plate concentration of 10 μg of TBZ per ml severely inhibited the growth of the pmt3Δ cells but had little effect on the growth of wild-type cells (Fig. 4A). This result also suggested that pmt3+ may be involved in assembly of the microtubule system.
FIG. 4.
(A) TBZ sensitivity of pmt3Δ cells. Wild-type (HM123) and pmt3Δ (JN630) cells were streaked on YE and YE plates containing TBZ (10 μg/ml) and incubated for 4 days at 30°C. (B) Frequency of minichromosome loss in wild-type and pmt3Δ cells. The genomic pmt3+ gene was disrupted in a strain carrying the minichromosome. Single colonies of isogenic wild-type (YM75) and pmt3Δ (YM76) cells were grown at 30°C. See Materials and Methods for an explanation of minichromosome loss rate.
To analyze the chromosome segregation defect in pmt3Δ cells in more detail, we measured the rate of loss of a nonessential minichromosome from wild-type and pmt3Δ cells. Wild-type and pmt3Δ cells containing the ade6-704 allele at the genomic ade6 locus and a single copy of the nonessential centromeric minichromosome Ch10 (70) were constructed (YM75 and YM76, respectively [Table 1 and Materials and Methods]). The stability of minichromosome in wild-type and pmt3Δ cells was determined by two different methods described in Materials and Methods. In the first method, the ratio of cells that had lost the minichromosome in pmt3Δ cells (30%) was 50-fold higher than that in wild-type cells (0.6%). In the second method, the rate of minichromosome loss for wild-type cells was 3.4 × 10−4 per generation under normal growth conditions (Fig. 4B). However, pmt3Δ cells showed more than a 100-fold increase in minichromosome loss rate, with a loss rate of 5.6 × 10−2 per generation. These results suggest that pmt3Δ cells lack the functions required for accurate chromosome segregation, which may account for the longer doubling time, the aberrant cell morphology, and the low viability of pmt3Δ cells.
Striking increase in telomere length in pmt3Δ cells.
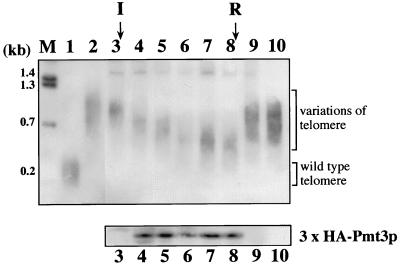
We found another interesting phenotype in pmt3Δ cells, that loss of Pmt3p function caused a striking increase in telomere length. S. pombe chromosomes I and II contain ApaI restriction sites which are located on the centromere-proximal side of the telomeric repeats (84). When the chromosome DNA of wild-type cells was digested with ApaI and analyzed for telomere length by Southern blot analysis with a telomeric repeat DNA fragment as the probe, a band smear of about 0.3 kb, which corresponds to the length of the telomeric repeat in wild-type cells, was detected (Fig. 5, lane 1). As shown in lane 2, disruption of the pmt3+ gene caused elongation of telomeres up to a length intermediate (0.8 to 1.2 kb) between that of the wild-type and taz1Δ cells (15), although the signal intensities were not quantitatively estimated in this experiment.
FIG. 5.
Southern blot analysis of telomere length in wild-type and pmt3Δ cells. Genomic DNA was extracted, digested with ApaI, and separated on 1% agarose gels. DNAs were blotted onto a nylon membrane and probed with the 0.3-kb EcoRI-ApaI fragment of pAMP2. Positions of DNA size markers and their sizes are given on the left. Brackets indicate the positions of wild-type telomeres and the heterogeneous telomeres of pmt3Δ cells. I, the point where expression of the pmt3+ gene was induced; R, the point where expression of the pmt3+ gene was rerepressed. Lane M, BstEII-digested bacteriophage lambda DNA; lane 1, wild-type (HM123); lane 2, pmt3Δ (JN630); lanes 3 to 10, pmt3Δ cells transformed with pSFL373L-pmt3 carrying pmt3+ cDNA under control of the nmt** promoter. Time courses of the restoration of wild-type telomere length after induction of pmt3+ gene expression and telomere elongation after repression of pmt3+ gene expression are shown in lanes 3 to 10. DNA was isolated before induction (0 generation; lane 3) and 18 (lane 4), 36 generations (lane 5), 54 (lane 6), 72 (lane 7), and 90 generations (lane 8) after induction. pmt3+ gene expression was repressed again at point R. Lanes 9 and 10, 18 and 36 generations after repression. The lower panel indicates expression levels of Pmt3p detected by Western blot analysis using monoclonal antibody 12CA5.
We investigated whether the increase in telomere length could be restored by reintroduction of the gene. pmt3Δ cells were transformed with pSFL373L-pmt3, which carries the HA3-tagged full-length pmt3+ cDNA under the control of the inducible nmt1** promoter (the weakest nmt1 promoter). pmt3Δ transformants containing pSFL373L-pmt3 displayed near-normal phenotypes of pmt3+ cells when grown in the absence of thiamine (induced conditions) but not in the presence of thiamine (repressed conditions). The elongated telomere did not recover its normal length under repressed conditions (Fig. 5, lane 3). First, we cultured the cells under repressed conditions and then removed thiamine to induce the expression of pmt3+. Chromosomal DNA was prepared every 18 generations after the removal of thiamine. As seen in Fig. 5, lane 3 to 10, the telomeres became gradually shorter and achieved a length of about 0.5 kb after about 90 generations of growth under induced conditions. This result suggested that the elongated telomeres were shortened by a mechanism that maintains telomere length in the pmt3+ background. The rate of telomere shortening in pmt3Δ cells expressing pmt3+ was approximately 4 to 8 bp per generation, which roughly correlates with the length of the consensus repeat unit of the S. pombe telomere. When pmt3+ gene expression was again repressed by the addition of thiamine at 90 generations after induction (lanes 9 and 10), the lengths of the telomeres immediately increased and attained the level seen in pmt3Δ cells within 18 generations (lanes 3, 8, 9, and 10). The amounts of HA3-tagged Pmt3 protein expressed by the plasmid were confirmed by Western blotting (lanes 3 to 10). These results strongly indicated that pmt3+ was directly or indirectly involved in the maintenance of correct telomere length. This is the first demonstration that one of the Smt3p/SUMO-1 family proteins is involved in telomere length maintenance.
Pmt3p-protein conjugation in S. pombe.
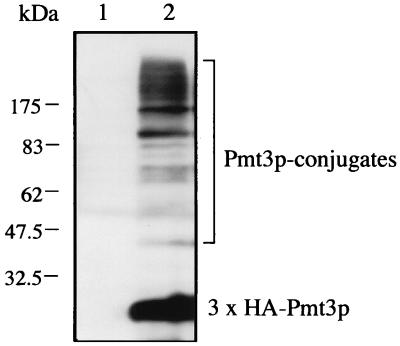
The results mentioned above suggest that Pmt3p is required in multiple cellular events. We speculated that Pmt3p exerts its function through its covalent attachment to target proteins like its homologues SUMO-1 and Smt3p. To confirm that S. pombe Pmt3p is conjugated to cellular proteins in vivo, Pmt3p was amino-terminally tagged with three copies of the HA epitope. The gene encoding this fusion protein was placed under the control of the inducible nmt1 promoter (pSLF173L-pmt3). Western blot analysis using an anti-HA antibody revealed the expression in pmt3Δ cells of HA3-Pmt3p in the presence of thiamine. Migration of the HA3-tagged Pmt3p was far slower than that predicted from the sequence. This is consistent with the previous reports that both mammalian SUMO-1 and S. cerevisiae Smt3p show anomalously slow migration on SDS-gels (6, 33, 48). In addition, several other anti-HA-reactive proteins with sizes ranging from about 40 to more than 175 kDa were observed as distinct bands (Fig. 6). Considering the pleiotropic phenotypes of pmt3Δ cells, these bands most likely represent HA3-Pmt3p conjugated to proteins of different sizes whose identities remain to be determined, although some of the bands may be degradation products of the higher-molecular-mass species. We also detected a similar but less clear conjugation pattern in the strain with the integrated GFP-Pmt3 fusion protein described below (data not shown).
FIG. 6.
Pmt3p-protein conjugation in vivo. pmt3Δ cells were transformed with a plasmid carrying the gene for HA3-tagged Pmt3p (3 × HA-Pmt3p) under control of the inducible nmt1 promoter (pSLF173L-pmt3) and a control vector (pSLF173L). The strains were grown in EMM2 medium supplemented with thiamine to repress the overexpression of HA3-Pmt3p. Protein extracts were prepared as described in Materials and Methods and subjected to Western blot analysis with anti-HA antibody 12CA5. High-molecular-mass Pmt3p-protein conjugates are indicated by a bracket.
Localization of Pmt3p.
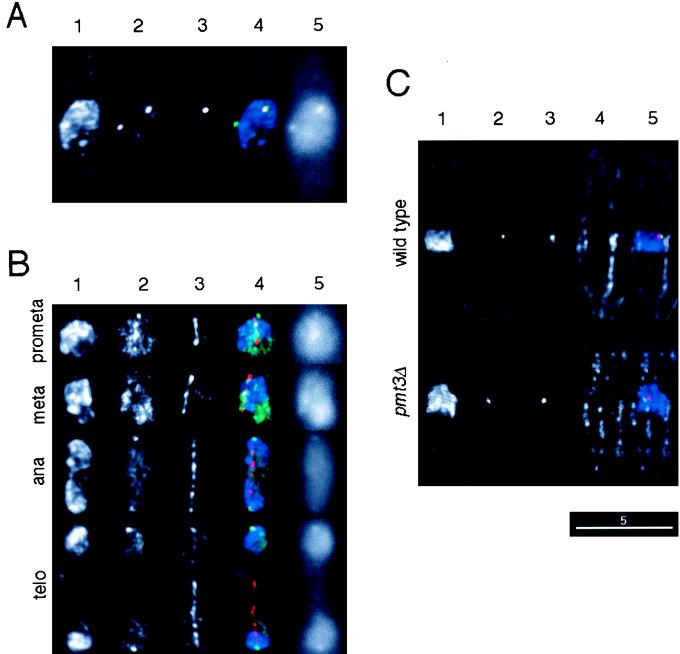
Various phenotypes of pmt3Δ cells described above predict that several nuclear proteins are regulated by conjugation of Pmt3p. To determine the intracellular localization of Pmt3p, GFP (63, 69) was fused to the N terminus of Pmt3p. The fusion gene under the control of the pmt3+ promoter was integrated into wild-type cells (see Materials and Methods). We confirmed that the pmt3Δ phenotypes were rescued by expression of the GFP-Pmt3 fusion protein, indicating that this fusion protein was functional (data not shown). The green fluorescence of the fusion protein was observed predominantly in the nucleus. Interestingly, intense signals appeared as one or more spots in the nuclei. To identify the nature of the spots, the cells expressing the fusion protein were fixed and stained with anti-Sad1 antibody, which recognizes SPB. The pattern of GFP fluorescence in the fixed cells was not significantly different from that of the live cells (data not shown). In interphase cells, the SPB signals always colocalized with the fluorescent spots. In Fig. 7A, one of the two spots of the green fluorescence signal can be seen to be colocalized with the anti-Sad1 signal. To examine the localization of GFP-Pmt3p during mitosis, cells expressing the fusion protein were fixed and stained with the anti-α-tubulin antibody. The GFP-Pmt3p spots were not detected at the spindle poles during prometaphase and metaphase. Instead, a relatively bright region or intense spots were observed between the spindle poles (Fig. 7B). After anaphase, GFP-Pmt3p spots reappeared at the spindle poles. The behavior of the bright signals at the SPB is reminiscent of centromeres which cluster and associate with SPB throughout the cell cycle except around metaphase in S. pombe (24). We compared centromere positioning in wild-type and pmt3Δ cells by using a combination of immunofluorescence and in situ hybridization with a probe specific for centromeric repeated sequences. The positioning of centromeres in pmt3Δ cells was indistinguishable from that in wild-type cells (Fig. 7C), suggesting that Pmt3p is not essential for centromere clustering at the SPB. Another feature of GFP-Pmt3p localization is that the GFP signal, which was dispersed throughout the nucleus in interphase, seemed to be excluded from chromosome DNA by condensed chromosomes during mitosis (Fig. 7B). These observations indicate that Pmt3p may be required for kinetochore and/or SPB functions during chromosome segregation.
FIG. 7.
Cellular localization of the Pmt3p and centromere positioning. (A and B) Cells expressing the GFP-Pmt3 fusion protein (YM78 [h+ leu1-32 ura4-D18 ade6-704 pmt3+:pYC11-GFPpmt3]) were grown exponentially in EMM2 supplemented with adenine and uracil at 26°C. The cells were fixed with formaldehyde and stained with anti-Sad1 rabbit polyclonal antibody followed by Cy3-conjugated anti-rabbit IgG secondary antibody (A) or anti-α-tubulin mouse monoclonal antibody followed by Cy3-conjugated anti-mouse IgG secondary antibody (B). Fluorescence images for a given cell: 1, DAPI; 2, GFP; 3, Cy3; 4, merged images of blue for DAPI, green for GFP, and red for Cy3; 5, GFP image before deconvolution. (C) Wild-type cells (HM123) or pmt3Δ (JN630) cells were grown exponentially in EMM2 supplemented with leucine at 26°C. The cells were fixed by the formaldehyde-glutaraldehyde method and stained with anti-Sad1 rabbit polyclonal antibody followed by the Oregon green-conjugated anti-rabbit IgG secondary antibody and with anti-α-tubulin mouse monoclonal antibody followed by the Cy5-conjugated anti-mouse IgG secondary antibody. The immunostained cells were fixed with formaldehyde again, and in situ hybridization probed with Cy3-labeled pRS140 which contains a centromeric repeated sequence of S. pombe (13), was carried out. Fluorescence images for a given cell: 1, DAPI; 2, Oregon green; 3, Cy3; 4, Cy5; 5, merged images of blue for DAPI, green for Oregon green, red for Cy3, and white for Cy5. The images were not deconvolved except for images 5 of panels A and B. All images are shown at the same magnification. Scale bar, 5 μm.
DISCUSSION
Members of the Smt3p/SUMO-1 family are homologous to ubiquitin and play major regulatory roles in such processes as the control of cellular protein localization and/or protein-protein interaction through their posttranslational conjugation to several proteins. However, the precise nature of the processes controlled by Smt3p/SUMO-1 remains obscure. In this work, we isolated an S. pombe homologue of the Smt3p/SUMO-1 family. We showed that Pmt3p is conjugated to target proteins like Smt3p/SUMO-1 and that Pmt3p modification is involved in multiple processes such as chromosome segregation and telomere length maintenance.
Our genetical analyses showed that the pmt3+ gene is not essential for viability, although pmt3-disrupted cells grew more slowly and showed several phenotypes. Unlike humans, which have at least three SUMO-1 homologues (41), there is only a single homologue (Smt3p) in S. cerevisiae. S. cerevisiae SMT3 is an essential gene, and its temperature-sensitive mutant displays G2/M defects (58). We speculate that this difference in cell viability between S. cerevisiae SMT3 and S. pombe pmt3+ may be dependent on the nature of the modified proteins; i.e., one or more of the Smt3p-modified proteins may be essential for cell viability in S. cerevisiae but not in S. pombe. Alternatively, there might be multiple SUMO-1 homologues having functions overlapping that of Pmt3p in S. pombe.
In S. cerevisiae, a heterodimer of Uba2p and Aos1p is an ATP-dependent Smt3p-activating enzyme (33) and Ubc9p is the only E2-conjugating enzyme for Smt3p (32). UBA2, AOS1, and UBC9 are all essential genes, consistent with the absolute requirement for the SMT3 function described above (5, 32, 33). On the other hand, in S. pombe, the rad31+ and hus5+ genes were isolated as a UBA-related AOS1 homologue (79) and as a UBC9 homologue (3), respectively. Recently, we characterized another UBA-related gene in S. pombe, the S. cerevisiae UBA2 homologue, and named it fub2+ (fission yeast UBA2) gene (84a). These fub2+, rad31+, and hus5+ genes are all dispensable for cell growth, although each of the disruptants grew slowly, showed aberrant mitosis, and displayed increased sensitivities to factors such as high temperature, HU, TBZ, UV, and MMS, characteristics that are entirely consistent with the phenotypes of pmt3Δ cells (3, 79, 84a). These facts strongly suggest that in S. pombe, Pmt3p may be activated by a heterodimer of Fub2p and Rad31p, the E1-activating enzyme for Pmt3p, and that the activated Pmt3p is transferred to the active-site cysteine of Hus5p, the E2-conjugating enzyme for Pmt3p.
The fact that pmt3Δ cells were strikingly sensitive to HU, UV, and MMS suggested that pmt3+ may be essential for the checkpoint coupling mitosis to the completion of DNA replication and the DNA damage response. Unlike the checkpoint rad mutants, however, pmt3Δ cells showed only a moderate loss of viability after treatment with HU (Fig. 3B). Microscopic analysis of the cell morphology indicated that treated pmt3Δ cells were more elongated than untreated pmt3Δ cells and that there was no increase in the frequency of cut cells during the first few hours after treatment with HU (data not shown). Furthermore, FACS analysis showed that pmt3Δ cells can arrest the cell cycle after exposure to HU (Fig. 3C). These results suggest that pmt3+ is involved in the DNA damage tolerance process rather than the checkpoint itself. The cut phenotype of pmt3Δ cells may be attributed to a defect in chromosome segregation because the cut phenotype is also observed in mutants with defects in chromosome segregation (28) and pmt3Δ cells are defective in chromosome segregation as discussed below. Consistent with this notion, similar results were reported for rad31+ and hus5+, and both genes have been shown to be required for the DNA damage tolerance process (3, 79). Therefore, it is tempting to speculate that Pmt3p may directly or indirectly target and modify a protein involved in the recovery process after checkpoint arrest.
Telomeres are specialized nucleoprotein structures at the ends of eukaryotic chromosomes which have been shown to be essential for maintaining chromatin integrity. However, considerably less research has been dedicated to understanding telomere structure and the mechanism of telomere regulation in S. pombe. S. pombe telomeres consist of 200 to 300 bp of DNA with the repeat unit consensus sequence 5′-(TTACAG)1–8-3′ on the 3′-end strand (18, 84). Unexpectedly, we found that the large and rapid elongation of telomere length in the pmt3+ disruptant cells suggests that Pmt3p functions in the regulation of telomere length (Fig. 5). This is the first demonstration of involvement of one of the Smt3p/SUMO-1 family proteins in telomere length maintenance. Our preliminary results showing that the S. cerevisiae ubc9ts mutant displays no telomere elongation at the restrictive temperature suggest that the Smt3p ubiquitin-like pathway does not play an important role in the control of telomere length at least in S. cerevisiae (84a). Three possibilities may be considered. (i) Pmt3p might modify and inhibit a negative regulator of telomere length. A candidate for a negative regulator of telomere length is the S. pombe telomere-binding protein, Taz1p. Disruption of the taz1+ gene causes a massive increase in telomere length and a defect of telomere loci silencing (15). However, we could not detect any Pmt3p-dependent modification of Taz1p in vivo by Western blot analysis or any silencing defect at telomeric loci in pmt3Δ cells (data not shown). Thus, these results predict the existence of a negative regulator other than Taz1p which would be inhibited by Pmt3p modification. (ii) Telomerase-independent, recombination-dependent mechanism of telomere repeat synthesis has been observed in S. cerevisiae undergoing senescence (46, 56). The observation that telomere length was immediately reelongated to the level of pmt3Δ cells after the repression of pmt3+ expression (Fig. 5) suggests the involvement of this mode of recombination. Therefore, it is also possible that certain factors involved in recombination are modified, resulting in the inhibition of the telomere elongation in this recombinational mode. The S. pombe homologues of human Rad51 and Rad52, which were shown to interact with both SUMO-1 and human Ubc9 by the two-hybrid assay, respectively (37, 80, 81), are attractive candidates for modification and inhibition of recombinational activity by Pmt3p. (iii) It is known that specific DNA replication mutations affect telomere length in S. cerevisiae (1, 7, 9, 82). We have found that telomeres are strikingly elongated in S. pombe polαts and polδts mutants (84a). Thus, it is tempting to speculate that Pmt3p may control telomere length by interacting with PCNA, an accessory factor of DNA polymerase δ, because Pmt3p was originally isolated as a PCNA-interacting factor in our two-hybrid screening assay.
The defect of chromosome segregation in pmt3Δ cells seems to be plausible because the S. cerevisiae SMT3 gene was originally isolated as a high-copy-number suppressor of mutations in MIF2, which encodes a CENP-C like centromere-binding protein (59). Furthermore, a previous report on the human homologue to S. cerevisiae Ubc9p suggested that it interacts with three subunits of S. cerevisiae centromere DNA-binding core complex, CBF3 (31). Thus, it appears very likely that the function of Smt3p/Ubc9p in the G2/M phase of S. cerevisiae may be related to its ability to regulate centromere proteins involved in chromosome segregation during mitosis. Our observation that the GFP-Pmt3 fusion protein is predominantly colocalized with the SPB with which clusters of centromeres associate and that this colocalization disappears during prometaphase and metaphase is reminiscent of the requirement for Pmt3p in kinetochore functions (Fig. 7A and B). It is also possible that Pmt3p modifies SPB proteins that release the conjugated Pmt3p moiety at mitosis or proteins that disappear from the SPB as a result of dissociation or degradation. There are many proteins associated with the SPB in S. pombe (2, 8, 20, 26, 27, 30, 38, 51, 64, 68, 71, 83, 85, 88, 91). Their activities change dynamically at mitosis and are involved in chromosome separation and/or cytokinesis. Our GFP-Pmt3p fusion system may provide a useful means to identify target proteins involved in chromosome segregation.
The nature of the additional GFP-Pmt3p spots that did not overlap with the anti-Sad1 signal is unknown. The number and position of the dots varied depending on the cell under observation. The GFP-Pmt3p spots may be related to mammalian PML bodies, which are the major targets of SUMO-1 conjugation (19, 65). The diffuse nature of GFP-Pmt3p fluorescence in interphase nuclei (Fig. 7A) may indicate Pmt3p involvement in DNA replication, repair, and/or recombination, a notion that is also supported by the phenotypes of pmt3Δ cells. The diffuse green fluorescence moved to the DAPI-negative part of the nucleus at mitosis (Fig. 7B). This behavior suggests that Pmt3p may be involved in the regulation of condensation and decondensation of chromosomes or the nuclear matrix association of interphase chromatin.
A distinct nuclear rim localization, consistent with the localization of SUMO-1-modified RanGAP1 (47, 53), can be observed in Cos-7 cells expressing low levels of HA-tagged SUMO-1 (48), and the human homologue of Ubc9 colocalizes with RanGAP1 at the nuclear envelope (42). These observations indicate that RanGAP1 is the major substrate of SUMO-1 modification in mammalian cells. Furthermore, it was shown that mutation of the Drosophila semushi gene, which encodes a Ubc9 homologue, blocks nuclear import of Bicoid during embryogenesis (22). These results suggest the possibility that the pleiotropic phenotype observed in the pmt3Δ cells is caused by the abnormal nuclear transport. However, Rna1p, the S. cerevisiae RanGAP1 homologue, is not detected in modified form by Western blotting using antibodies against Rna1p (16), indicating that Rna1p is unlikely to be a substrate of the Smt3p modification pathway. Using the GFP-Pmt3 fusion protein, we could not detect any specific GFP staining of the nuclear envelope in S. pombe (Fig. 7A), suggesting that the putative S. pombe RanGAP1 homologue may not be a substrate of the Pmt3p modification pathway. It should be noted that neither S. cerevisiae (87) nor S. pombe (57) RanGAP1 homologue has the conserved C-terminal domain that includes the site of SUMO-1 ligation (54).
We have identified the pmt3+ gene by two-hybrid screening using S. pombe PCNA as bait. We have confirmed that Pmt3p can interact with PCNA in vivo by immunoprecipitation using anti-PCNA antibodies (data not shown). Therefore, we believe that Pmt3p and PCNA may directly or indirectly interact each other. However, we cannot rule out the possibility that Pmt3p can transiently modify PCNA or that PCNA associates or cooperates with a protein modified by Pmt3p, as was shown to be the case for the association between RanBP2 and SUMO-1-modified RanGAP1 (47, 48, 75). PCNA is required for DNA replication, for DNA repair, and maybe for the recovery pathway after DNA replication arrest induced by DNA synthesis inhibitors or DNA damage. Pmt3p may directly or indirectly modify and regulate PCNA in the recovery pathway.
ACKNOWLEDGMENTS
We thank Y. Nakaseko for technical advice and helpful comments. We are grateful to M. Yanagida for help in the screening of the ordered S. pombe cosmid library, H. Murakami and H. Okayama for the gift of cdc2-3w and cds1::ura4 strains, K. Moriyoshi for the gift of the GFP(S65A) plasmid, A. Matsuura for the gift of plasmid pAMP2 (used as a telomere probe), F. Matsunaga for assistance with FACS analysis, S. L. Forsburg for the gift of the HA3-tagged expression vector, Y. Chikashige for advice on fluorescence microscopy, and I. Hagan for the gift of the anti-Sad1 antibody. This work was technically assisted by Y. Fujimoto and T. Nishi.
This work was supported by a grant-in-aid from the Ministry of Education, Science, and Culture of Japan and the Agricultural Chemical Research Foundation.
REFERENCES
- 1.Adams A K, Holm C. Specific DNA replication mutations affect telomere length in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4614–4620. doi: 10.1128/mcb.16.9.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa C E, Ducommun B, Beach D, Hyams J S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature (London) 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khodairy F, Enoch T, Hagan I M, Carr A M. The Schizosaccharomyces pombe hus5 gene encodes a ubiquitin conjugating enzyme required for normal mitosis. J Cell Sci. 1995;108:475–486. doi: 10.1242/jcs.108.2.475. [DOI] [PubMed] [Google Scholar]
- 4.Bayer P, Arndt A, Metzger S, Mahajan R, Melchior F, Jaenicke R, Becker J. Structure determination of the small ubiquitin-related modifier SUMO-1. J Mol Biol. 1998;280:275–286. doi: 10.1006/jmbi.1998.1839. [DOI] [PubMed] [Google Scholar]
- 5.Blondel M, Mann C. G2 cyclins are required for the degradation of G1 cyclins in yeast. Nature (London) 1996;384:279–282. doi: 10.1038/384279a0. [DOI] [PubMed] [Google Scholar]
- 6.Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. PIC1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene. 1996;13:971–982. [PubMed] [Google Scholar]
- 7.Bolwig C, Stillman B. Abstracts of the 1997 Meeting on Eukaryotic DNA Replication. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. A mutation in PRI2 affects telomere length in Saccharomyces cerevisiae; p. 37. [Google Scholar]
- 8.Bridge A J, Morphew M, Bartlett R, Hagan I M. The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev. 1998;12:927–942. doi: 10.1101/gad.12.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson M J, Hartwell L. CDC17: an essential gene that prevents telomere elongation in yeast. Cell. 1985;42:249–257. doi: 10.1016/s0092-8674(85)80120-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen A, Mannen H, Li S S. Characterization of mouse ubiquitin-like SMT3A and SMT3B cDNAs and gene/pseudogenes. Biochem Mol Biol Int. 1998;46:1161–1174. doi: 10.1080/15216549800204722. [DOI] [PubMed] [Google Scholar]
- 11.Chikashige, Y. Personal communication.
- 12.Chikashige Y, Ding D Q, Imai Y, Yamamoto M, Haraguchi T, Hiraoka Y. Meiotic nuclear reorganization: switching the position of centromeres and telomeres in the fission yeast Schizosaccharomyces pombe. EMBO J. 1997;16:193–202. doi: 10.1093/emboj/16.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chikashige Y, Kinoshita N, Nakaseko Y, Matsumoto T, Murakami S, Niwa O, Yanagida M. Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites. Cell. 1989;57:739–751. doi: 10.1016/0092-8674(89)90789-7. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury B K, Li S S. Identification and characterization of the SMT3 cDNA and gene from nematode Caenorhabditis elegans. Biochem Biophys Res Commun. 1997;234:788–791. doi: 10.1006/bbrc.1997.6709. [DOI] [PubMed] [Google Scholar]
- 15.Cooper J P, Nimmo E R, Allshire R C, Cech T R. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature (London) 1997;385:744–747. doi: 10.1038/385744a0. [DOI] [PubMed] [Google Scholar]
- 16.Corbett A H, Koepp D M, Schlenstedt G, Lee M S, Hopper A K, Silver P A. Rna1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J Cell Biol. 1995;130:1017–1026. doi: 10.1083/jcb.130.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dernburg A F, Sedat J W. Mapping three-dimensional chromosome architecture in situ. Methods Cell Biol. 1998;53:187–233. doi: 10.1016/s0091-679x(08)60880-8. [DOI] [PubMed] [Google Scholar]
- 18.Duffy M, Chambers A. DNA-protein interactions at the telomeric repeats of Schizosaccharomyces pombe. Nucleic Acids Res. 1996;24:1412–1419. doi: 10.1093/nar/24.8.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duprez E, Saurin A J, Desterro J M, Lallemand-Breitenbach V, Howe K, Boddy M N, Solomon E, de The H, Hay R T, Freemont P S. SUMO-1 modification of the acute promyelocytic leukaemia protein PML: implications for nuclear localisation. J Cell Sci. 1999;112:381–393. doi: 10.1242/jcs.112.3.381. [DOI] [PubMed] [Google Scholar]
- 20.Eng K, Naqvi N I, Wong K C, Balasubramanian M K. Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr Biol. 1998;8:611–621. doi: 10.1016/s0960-9822(98)70248-9. [DOI] [PubMed] [Google Scholar]
- 21.Enoch T, Carr A M, Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 1992;6:2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- 22.Epps J L, Tanda S. The Drosophila semushi mutation blocks nuclear import of Bicoid during embryogenesis. Curr Biol. 1998;8:1277–1280. doi: 10.1016/s0960-9822(07)00538-6. [DOI] [PubMed] [Google Scholar]
- 23.Forsburg S L, Sherman D A. General purpose tagging vectors for fission yeast. Gene. 1997;191:191–195. doi: 10.1016/s0378-1119(97)00058-9. [DOI] [PubMed] [Google Scholar]
- 24.Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas A L, Ahrens P, Bright P M, Ankel H. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J Biol Chem. 1987;262:11315–11323. [PubMed] [Google Scholar]
- 26.Hagan I, Yanagida M. Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature (London) 1992;356:74–76. doi: 10.1038/356074a0. [DOI] [PubMed] [Google Scholar]
- 27.Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano T, Funahashi S, Uemura T, Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cut mutants that block nuclear division but not cytokinesis. EMBO J. 1986;5:2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 30.Horio T, Uzawa S, Jung M K, Oakley B R, Tanaka K, Yanagida M. The fission yeast gamma-tubulin is essential for mitosis and is localized atmicrotubule organizing centers. J Cell Sci. 1991;99:693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Koltin Y. Two-hybrid interaction of a human UBC9 homolog with centromere proteins of Saccharomyces cerevisiae. Mol Gen Genet. 1996;251:153–160. doi: 10.1007/BF02172913. [DOI] [PubMed] [Google Scholar]
- 32.Johnson E S, Blobel G. Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J Biol Chem. 1997;272:26799–26802. doi: 10.1074/jbc.272.43.26799. [DOI] [PubMed] [Google Scholar]
- 33.Johnson E S, Schwienhorst I, Dohmen R J, Blobel G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 1997;16:5509–5519. doi: 10.1093/emboj/16.18.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawamukai M, Gerst J, Field J, Riggs M, Rodgers L, Wigler M, Young D. Genetic and biochemical analysis of the adenylyl cyclase-associated protein, cap, in Schizosaccharomyces pombe. Mol Biol Cell. 1992;3:167–180. doi: 10.1091/mbc.3.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly T J, Martin G S, Forsburg S L, Stephen R J, Russo A, Nurse P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell. 1993;74:371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita K, Nemoto T, Nabeshima K, Kondoh H, Niwa H, Yanagida M. The regulatory subunits of fission yeast protein phosphatase 2A (PP2A) affect cell morphogenesis, cell wall synthesis and cytokinesis. Genes Cells. 1996;1:29–45. doi: 10.1046/j.1365-2443.1996.02002.x. [DOI] [PubMed] [Google Scholar]
- 37.Kovalenko O V, Plug A W, Haaf T, Gonda D K, Ashley T, Ward D C, Radding C M, Golub E I. Mammalian ubiquitin-conjugating enzyme Ubc9 interacts with Rad51 recombination protein and localizes in synaptonemal complexes. Proc Natl Acad Sci USA. 1996;93:2958–2963. doi: 10.1073/pnas.93.7.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumada K, Nakamura T, Nagao K, Funabiki H, Nakagawa T, Yanagida M. Cut1 is loaded onto the spindle by binding to Cut2 and promotes anaphase spindle movement upon Cut2 proteolysis. Curr Biol. 1998;8:633–641. doi: 10.1016/s0960-9822(98)70250-7. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Yoshida Y, Noda M. Cloning of a cDNA which encodes a novel ubiquitin-like protein. Biochem Biophys Res Commun. 1993;195:393–399. doi: 10.1006/bbrc.1993.2056. [DOI] [PubMed] [Google Scholar]
- 40.Lammer D, Mathias N, Laplaza J M, Jiang W, Liu Y, Callis J, Goebl M, Estelle M. Modification of yeast Cdc53p by the ubiquitin-related protein Rub1p affects function of the SCFcdc4 complex. Genes Dev. 1998;12:914–926. doi: 10.1101/gad.12.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapenta V, Chiurazzi P, van der Spek P, Pizzuti A, Hanaoka F, Brahe C. SMT3A, a human homologue of the S. cerevisiae SMT3 gene, maps to chromosome 21qter and defines a novel gene family. Genomics. 1997;40:362–366. doi: 10.1006/geno.1996.4556. [DOI] [PubMed] [Google Scholar]
- 42.Lee G W, Melchior F, Matunis M J, Mahajan R, Tian Q, Anderson P. Modification of Ran GTPase-activating protein by the small ubiquitin-related modifier SUMO-1 requires Ubc9, an E2-type ubiquitin-conjugating enzyme homologue. J Biol Chem. 1998;13:6503–6507. doi: 10.1074/jbc.273.11.6503. [DOI] [PubMed] [Google Scholar]
- 43.Li S J, Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature (London) 1999;398:246–251. doi: 10.1038/18457. [DOI] [PubMed] [Google Scholar]
- 44.Liakopoulos D, Doenges G, Matuschewski K, Jentsch S. A novel protein modification pathway related to the ubiquitin system. EMBO J. 1998;17:2208–2214. doi: 10.1093/emboj/17.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loeb K R, Haas A L. Conjugates of ubiquitin cross-reactive protein distribute in a cytoskeletal pattern. Mol Cell Biol. 1994;14:8408–8419. doi: 10.1128/mcb.14.12.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundblad V, Blackburn E H. An alternative pathway for yeast telomere maintenance rescues est1− senescence. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- 47.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 48.Mahajan R, Gerace L, Melchior F. Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J Cell Biol. 1998;140:259–270. doi: 10.1083/jcb.140.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mannen H, Tseng H M, Cho C L, Li S S. Cloning and expression of human homolog HSMT3 to yeast SMT3 suppressor of MIF2 mutations in a centromere protein gene. Biochem Biophys Res Commun. 1996;222:178–180. doi: 10.1006/bbrc.1996.0717. [DOI] [PubMed] [Google Scholar]
- 50.Masai H, Miyake T, Arai K. hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J. 1995;14:3094–3104. doi: 10.1002/j.1460-2075.1995.tb07312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masuda H, Sevik M, Cande W Z. In vitro microtubule-nucleating activity of spindle pole bodies in fission yeast Schizosaccharomyces pombe: cell cycle-dependent activation in Xenopus cell-free extracts. J Cell Biol. 1992;117:1055–1066. doi: 10.1083/jcb.117.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuura A, Naito T, Ishikawa F. Genetic control of telomere integrity in Schizosaccharomyces pombe: rad3+ and tel1+ are parts of two regulatory networks independent of the downstream protein kinases chk1+ and cds1+ Genetics. 1999;152:1501–1512. doi: 10.1093/genetics/152.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matunis M J, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matunis M J, Wu J, Blobel G. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J Cell Biol. 1998;140:499–509. doi: 10.1083/jcb.140.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 56.McEachern M J, Blackburn E H. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 1996;10:1822–1834. doi: 10.1101/gad.10.14.1822. [DOI] [PubMed] [Google Scholar]
- 57.Melchior F, Weber K, Gerke V. A functional homologue of the RNA1 gene product in Schizosaccharomyces pombe: purification, biochemical characterization, and identification of a leucine-rich repeat motif. Mol Biol Cell. 1993;4:569–581. doi: 10.1091/mbc.4.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meluh P B, Koshland D. Abstracts of the 1998 Yeast Genetics and Molecular Biology Meeting. College Park: University of Maryland; 1998. Ubiquitin-related Smt3p, required for cell cycle progression in budding yeast, is attached to a subset of nuclear proteins and to a component of the mother-bud junction; p. 300. [Google Scholar]
- 59.Meluh P B, Koshland D. Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol Biol Cell. 1995;6:793–807. doi: 10.1091/mbc.6.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchison J M. Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:131–165. [Google Scholar]
- 61.Mizukami T, Chang W I, Garkavtsev I, Kaplan N, Lombardi D, Matsumoto T, Niwa O, Kounosu A, Yanagida M, Marr T G. A 13 kb resolution cosmid map of the 14 Mb fission yeast genome by nonrandom sequence-tagged site mapping. Cell. 1993;73:121–132. doi: 10.1016/0092-8674(93)90165-m. [DOI] [PubMed] [Google Scholar]
- 62.Moreno S, Klar A, Nurse P. Molecular genetics analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 63.Moriyoshi K, Richards L J, Akazawa C, O’Leary D D, Nakanishi S. Labeling neural cells using adenoviral gene transfer of membrane-targeted GFP. Neuron. 1996;16:255–260. doi: 10.1016/s0896-6273(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 64.Moser M J, Flory M R, Davis T N. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J Cell Sci. 1997;110:1805–1812. doi: 10.1242/jcs.110.15.1805. [DOI] [PubMed] [Google Scholar]
- 65.Muller S, Matunis M J, Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17:61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature (London) 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- 67.Murakami S, Yanagida M, Niwa O. A large circular minichromosome of Schizosaccharomyces pombe requires a high dose of type II DNA topoisomerase for its stabilization. Mol Gen Genet. 1995;246:671–679. doi: 10.1007/BF00290712. [DOI] [PubMed] [Google Scholar]
- 68.Nabeshima K, Kurooka H, Takeuchi M, Kinoshita K, Nakaseko Y, Yanagida M. p93dis1, which is required for sister chromatid separation, is a novel microtubule and spindle pole body-associating protein phosphorylated at the Cdc2 target sites. Genes Dev. 1995;9:1572–1585. doi: 10.1101/gad.9.13.1572. [DOI] [PubMed] [Google Scholar]
- 69.Nabeshima K, Saitoh S, Yanagida M. Use of green fluorescent protein for intracellular protein localization in living fission yeast. Methods Enzymol. 1997;283:459–471. doi: 10.1016/s0076-6879(97)83037-6. [DOI] [PubMed] [Google Scholar]
- 70.Niwa O, Matsumoto T, Chikashige Y, Yanagida M. Characterization of Schizosaccharomyces pombe minichromosome deletion derivatives and a functional allocation of their centromere. EMBO J. 1989;8:3045–3052. doi: 10.1002/j.1460-2075.1989.tb08455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohkura H, Hagan I M, Glover D M. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 72.Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okura T, Gong L, Kamitani T, Wada T, Okura I, Wei C F, Chang H M, Yeh E T. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J Immunol. 1996;157:4277–4281. [PubMed] [Google Scholar]
- 74.Osaka F, Kawasaki H, Aida N, Saeki M, Chiba T, Kawashima S, Tanaka K, Kato S. A new NEDD8-ligating system for cullin-4A. Genes Dev. 1998;12:2263–2268. doi: 10.1101/gad.12.15.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saitoh H, Pu R, Cavenagh M, Dasso M. RanBP2 associates with Ubc9p and a modified form of RanGAP1. Proc Natl Acad Sci USA. 1997;94:3736–3741. doi: 10.1073/pnas.94.8.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saitoh H, Sparrow D B, Shiomi T, Pu R T, Nishimoto T, Mohun T J, Dasso M. Ubc9p and the conjugation of SUMO-1 to RanGAP1 and RanBP2. Curr Biol. 1998;15:121–124. doi: 10.1016/s0960-9822(98)70044-2. [DOI] [PubMed] [Google Scholar]
- 77.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 78.Seufert W, Futcher B, Jentsch S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature (London) 1995;373:78–81. doi: 10.1038/373078a0. [DOI] [PubMed] [Google Scholar]
- 79.Shayeghi M, Doe C, Tavassoli M, Watts F. Characterization of Schizosaccharomyces pombe rad31, a UBA-related gene required for DNA damage tolerance. Nucleic Acids Res. 1997;25:1162–1169. doi: 10.1093/nar/25.6.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen Z, Pardington-Purtymun P E, Comeaux J C, Moyzis R K, Chen D J. Associations of UBE2I with RAD52, UBL1, p53, and RAD51 proteins in a yeast two-hybrid system. Genomics. 1996;37:183–186. doi: 10.1006/geno.1996.0540. [DOI] [PubMed] [Google Scholar]
- 81.Shen Z, Pardington-Purtymun P E, Comeaux J C, Moyzis R K, Chen D J. UBL1, a human ubiquitin-like protein associating with human RAD51/RAD52 proteins. Genomics. 1996;36:271–279. doi: 10.1006/geno.1996.0462. [DOI] [PubMed] [Google Scholar]
- 82.Smith J S, Caputo E, Boeke J D. A genetic screen for ribosomal DNA silencing defects identifies multiple DNA replication and chromatin-modulating factors. Mol Cell Biol. 1999;19:3184–3197. doi: 10.1128/mcb.19.4.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sohrmann M, Schmidt S, Hagan I, Simanis V. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sugawara N. Ph.D. thesis. Cambridge, Mass: Harvard University; 1989. [Google Scholar]
- 84a.Tanaka, K. Unpublished data.
- 85.Tange Y, Horio T, Shimanuki M, Ding D Q, Hiraoka Y, Niwa O. A novel fission yeast gene, tht1+, is required for the fusion of nuclearenvelopes during karyogamy. J Cell Biol. 1998;140:247–258. doi: 10.1083/jcb.140.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Traglia H M, Atkinson N S, Hopper A K. Structural and functional analyses of Saccharomyces cerevisiae wild-type and mutant RNA1 genes. Mol Cell Biol. 1989;9:2989–2999. doi: 10.1128/mcb.9.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.West R R, Vaisberg E V, Ding R, Nurse P, McIntosh J R. cut11+: a gene required for cell cycle-dependent spindle pole body anchoring in the nuclear envelope and bipolar spindle formation in Schizosaccharomyces pombe. Mol Biol Cell. 1998;9:2839–2855. doi: 10.1091/mbc.9.10.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilkinson K D. Roles of ubiquitinylation in proteolysis and cellular regulation. Annu Rev Nutr. 1995;15:161–189. doi: 10.1146/annurev.nu.15.070195.001113. [DOI] [PubMed] [Google Scholar]
- 90.Woods A, Sherwin T, Sasse R, MacRae T H, Baines A J, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto A, West R R, McIntosh J R, Hiraoka Y. A cytoplasmic dynein heavy chain is required for oscillatory nuclear movement of meiotic prophase and efficient meiotic recombination in fission yeast. J Cell Biol. 1999;145:1233–1250. doi: 10.1083/jcb.145.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang M Q, Marr T G. Fission yeast gene structure and recognition. Nucleic Acids Res. 1994;22:1750–1759. doi: 10.1093/nar/22.9.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]