Abstract
The use of multiparametric prostate magnetic resonance imaging (mpMRI) is recommended, in the European Association of Urology (EAU) guidelines, for local staging of patients with prostate cancer (PCa). Systemic staging is recommended only for patients with unfavourable intermediate and high-risk disease, with bone and lymph node assessments usually being performed using bone scan (BS) and computed tomography (CT), respectively. Magnetic resonance imaging (MRI) is the imaging technique with the highest sensitivity for the detection of bone metastases and has shown promising results also for lymph node assessments. In this report we illustrate how MRI provided a comprehensive assessment of local disease as well as bone and lymph node metastases in a patient with PCa. (www.actabiomedica.it)
Keywords: Prostate cancer, lymph node metastases, bone metastases, magnetic resonance imaging, diffusion-weighted imaging, multiparametric prostate magnetic resonance imaging, Prostate Imaging Reporting And Data System v2.1, next-generation imaging, 68Gallium prostate-specific membrane antigen positron emission tomography
Background
The most common sites of metastases in patients with advanced prostate cancer (PCa) are bone and lymph nodes, where metastases are present in over 80% of cases (1–4). Consequently, the detection of bone and lymph node metastases is fundamental for staging, prognosis and treatment planning in patients with high-risk PCa.
For the diagnosis of bone metastases, current European Association of Urology (EAU) guidelines (5) recommend at least the use of bone scan (BS), though it is recognized to have a low sensitivity and specificity in identifying lytic metastases (6).
Magnetic resonance imaging (MRI) is well established in the clinical management of patients with PCa. In addition to its fundamental role in primary disease detection, MRI has also shown high sensitivity for the diagnosis of bone metastases and performs similarly to computed tomography (CT) in the assessment of lymph nodes (7,8). Thus, MRI can provide a complete picture of disease in PCa.
In this report we illustrate how MRI provided a comprehensive assessment of local disease and lymph node and bone metastases in a patient with PCa.
Case description
A 68-year-old male with a previous history of surgery, radiotherapy and chemotherapy for squamous carcinoma of the pyriform sinus (staging: pT2 pN3b grading: G2 – TNM 8 edition; 3 years prior), as well as surgery for pulmonary adenocarcinoma (staging: T4 N0 M0 grading: G2 – TNM 8 edition; 1 year prior), underwent a neck-chest-abdomen-pelvis CT examination as part of routine follow-up.
The CT images showed the presence of pathologic lymph nodes in the right common and right external iliac region that did not appear to be related to tumours in patient’s known history, but suggested the presence of an undiagnosed PCa (Figure. 1). Further clinical investigations were therefore considered necessary.
Figure 1.
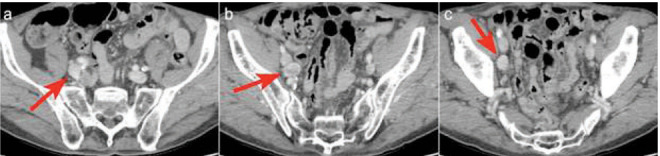
CT scan showed the presence of pathologic lymph nodes in the right common and right external iliac region that did not appear to be related to tumours in patient’s known history, but suggested the presence of an undiagnosed PCa.a
The patient subsequently underwent urological evaluation, and was found to have a PSA dosage of 4.4 ng/ml.
At one month, a multiparametric prostate magnetic resonance imaging (mpMRI) was performed using a 3 Tesla MR scanner. In accordance with the protocol of Prostate Imaging Reporting and Data System Version 2.1 (PI-RADS v2.1) guidelines, this included: T2-weighted images, Diffusion-Weighted Imaging (DWI) and Dynamic Contrast Enhanced Magnetic Resonance Imaging (DCE-MRI) (9).
Assessment of the mpMRI images was performed following the reporting criteria of PI-RADS v2.1 by a radiologist with more than 12 years of experience in PCa. A 15 mm lesion was found in the right peripheral zone of the prostate (Figure. 2). The lesion was given a PI-RADS score of 5, indicating a lesion with a high probability of clinically significant disease. Given the size and the bulging of the glandular profile, the lesion was considered to have at high risk of extraprostatic extension.
Figure 2.
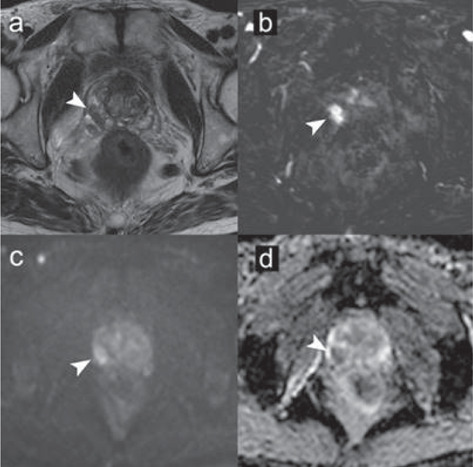
mpMRI performed with 3T MR scanner according to PI-RADS v2.1 protocol guidelines: (a) T2-weighted image, (b) dynamic contrast-enhanced image, (c) diffusion-weighted image for b=1000 s/mm2, (d) ADC map. According to the PI-RADS v2.1 criteria, a 15 mm lesion was found in the right peripheral zone of the prostate. The lesion was assigned a PI-RADS score of 5, indicating a lesion with a high probability of clinically significant disease. Given the size and the bulging of the glandular profile, the lesion was also considered to have at high risk of extraprostatic extension.
MRI of the pelvis confirmed the presence of pathological lymph nodes in the right common and right external iliac region, and further revealed two secondary bone lesions: one in the left sacrum and one in the right ischial tuberosity (Figure. 3). The bone lesions were not visible at the CT evaluation.
Figure 3.
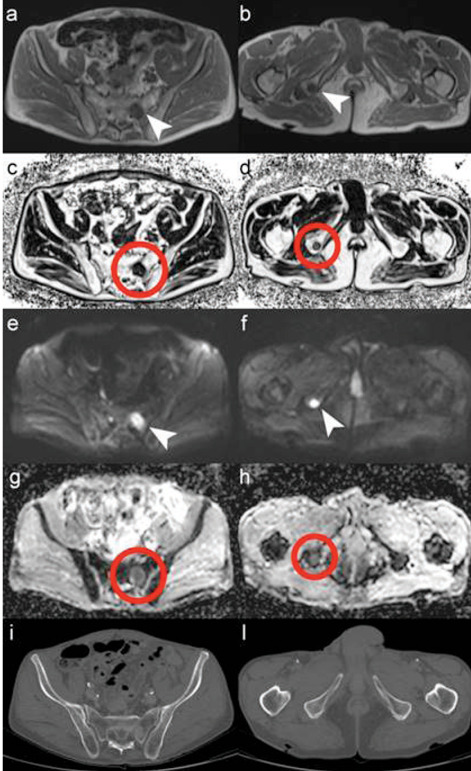
MRI of pelvis acquired with 3T MR scanner: (a, b) T1-weighted, (c, d) fat fraction (rF%), (e, f) diffusion weighted image (DWI) and (g, h) apparent diffusion coefficient map (ADC map) compared with CT images (i, l). MRI showed the presence of two bone metastases: one in the left sacrum (white arrows and red circles in a, c, e, g) and one in the right ischial tuberosity (arrows and circles in b, d, f, h), neither was visible in the CT evaluation (i, l).
Subsequently, the patient underwent a MRI-targeted prostate biopsy using MR/ultrasound (US) fusion, which confirmed the presence of PCa (Gleason Score 4+4=8).
An 18F-fluorocholine positron emission tomography (PET)/CT examination was then performed, revealing the presence of lesions with high phospholipid metabolism in the right prostate, the ipsilateral seminal vesicles, pelvic lymph nodes and in the skeleton corresponding to the sites of disease seen in the mpMRI exam (Figure. 4).
Figure 4.
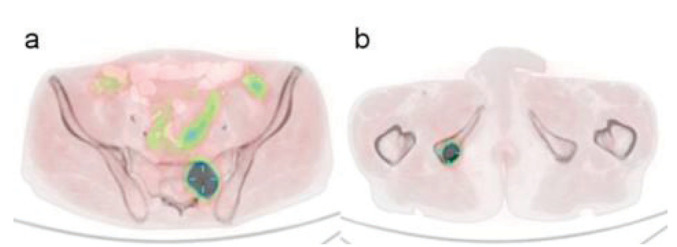
18F-fluorocholine PET/CT examination revealed lesions with high phospholipid metabolism in the skeleton corresponding to the sites of disease seen in the MRI exam: left sacrum in (a) and right ischial tuberosity in (b).
After four months, the patient underwent a CT-guided biopsy of the left sacral wing lesion (Figure. 5): histological examination confirmed the presence of a bone metastasis from PCa.
Figure 5.
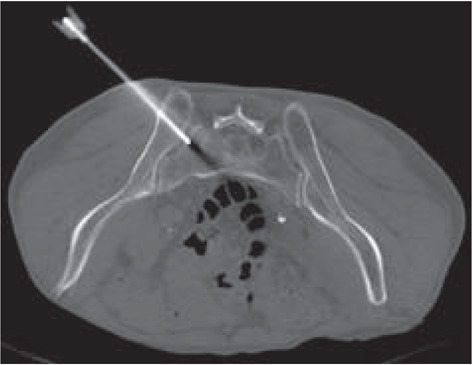
The patient underwent a CT-guided biopsy of the left sacral wing lesion: histological examination confirmed the presence of a bone metastasis from PCa.
The final diagnosis was de novo oligometastatic hormone sensitive PCa (cT3b cN1 cM1b).
Discussion
In this patient, with a history of two previous tumours (squamous carcinoma of the pyriform sinus and lung cancer), a routine follow-up CT examination showed the presence of pathological pelvic lymph nodes that did not appear to be related to the known history, but rather suggested the presence of PCa, finally confirmed by mpMRI of the prostate and then by biopsy. This case highlights the importance of being open to the possibility of finding cancers of new onset during the follow-up examinations of oncologic patients.
Our observations are limited by the fact that the patient did not undergo a BS for excluding bone metastases as suggested by the currently guidelines (5), but underwent a second level diagnostic investigation, such as MRI of the pelvis and retroperitoneum, with standard and DWI sequences, currently recognized as a superior modality for the evaluation of lymph node and bone metastases in advanced PCa (10–13). This investigation confirmed the presence of locoregional lymph node disease and demonstrated, in addition, the presence of two lytic lesions of the pelvic bones.
PCa is typically associated with osteoblastic metastasis, but it is now well-established that an osteolytic component may coexist within osteoblastic metastases, and even completely lytic metastases are not uncommon (14,15). Reflecting known limitations of CT in the detection of lytic bone lesions (6), the routine follow-up CT failed to detect the two lytic pelvic bone metastases subsequently identified at MRI examination.
To appreciate the different sensitivities of BS, CT and MRI for lytic bone lesions, it is useful to consider the progression of events in the evolution of bone metastases. Bone remodelling requires an initial phase of bone destruction mediated by osteoclasts, which may or may not be followed by a phase of new bone formation mediated by osteoblasts: bone production does not occur without prior bone absorption (16). Mundy and Guise introduced a “vicious cycle” model (17) that describes how PCa cells promote the activation of osteoblasts and of osteoclasts to elicit an osteoblastic, osteolytic or mixed bone response (18). In particular, tumour cells release growth factors, such as interleukin 6 (IL-6) and parathyroid hormone-related peptide (PTHrP), that determine the expression of Receptor Activator of Nuclear Factor KB Ligand (RANKL) in osteoblasts, whose activity in turn triggers the differentiation of progenitor cells into osteoclasts, and thus activates bone resorption. The pathological bone resorption determines the release of further growth factors, such as transforming growth factor-beta (TGF-b) and insulin-like growth factor (IGF-1), that, in turn, stimulate the growth of the tumour tissue, forming a positive feedback loop (Figure. 6).
Figure 6.
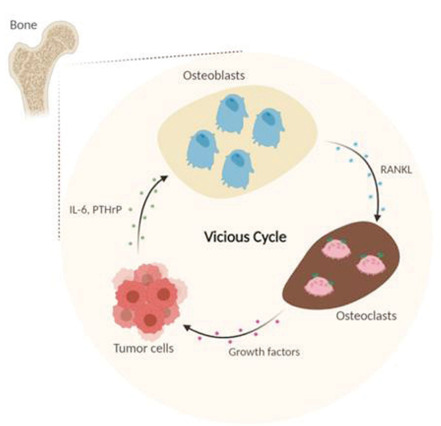
The “vicious cycle” model describes how PCa cells promote the activation of osteoblasts and of osteoclasts to elicit an osteoblastic, osteolytic or mixed bone response. In particular, tumour cells release growth factors, such as interleukins 6 (IL-6) and parathyroid hormone-related peptide (PTHrP), that determine the expression of Receptor Activator of Nuclear Factor KB Ligand (RANKL) in osteoblasts, whose activity in turn triggers the differentiation of progenitor cells into osteoclasts, and thus activates bone resorption. The pathological bone resorption determines the release of further growth factors, such as transforming growth factor-beta (TGF-b) and insulin-like growth factor (IGF-1), that, in turn, stimulate the growth of the tumour tissue, forming a positive feedback loop. Adapted from: (19).
BS has a rather poor sensitivity of 59% for detecting bone metastases (4), because it only relies on the osteoblastic reaction of normal bone to tumour cells. Therefore, our patient’s lytic metastases probably would not have been recognized on BS as well as on CT. Moreover, BS reliability is affected by the presence of a high number of false positives (6).
CT has the ability to depict both osteoblastic and osteolytic lesions, but its sensitivity for detecting bone metastases is only 73% (20), because it cannot discriminate early lytic metastases that do not involve the cortical bone, from normal bone marrow.
68Gallium prostate-specific membrane antigen positron emission tomography (68Ga-PSMA-PET) is a next-generation imaging (NGI) technique for assessment of PCa due to its high lesion detection rate, even at very low serum PSA levels (21,22). Its clinical use might improve detection and localization of PCa and could have a strong impact on the management of PCa, with the perspective of performing targeted treatments in recurrences and in oligometastatic disease, deferring the use of androgen deprivation therapy in cases of biochemical recurrence with negative findings on imaging studies (23).
Due to its high contrast resolution, MRI has the inherent ability to directly visualize metastases in the bone marrow, independent from their osteoblastic or osteolytic activity, that, as explained above, may occur in the evolution of bone disease (4). The addition of DWI to morphological MR images further increases the diagnostic performances of MRI for bone metastases detection, with a sensitivity of 96% reported in a meta-analysis including 1031 patients with PCa (8,24,25).
The presence of bone metastases has a strong impact on the quality of life of patients with PCa. It leads to skeletal morbidity, manifested as so-called skeletal-related events (SREs), that include pain symptoms requiring radiotherapy treatment (33%), pathological fractures (25%), spinal cord compression (8%) and hypercalcaemia (6). Early detection of bone metastases is, therefore, crucial for the clinical management of PCa patients and is important for tumour staging, evaluating the patient’s prognosis, and determining further therapeutic decisions (2,26,27). Indeed, patients with bone metastases may be eligible for endocrine therapy or other systemic therapies aimed at blocking the pathogenic cascade of bone resorption. Drugs which block the activity of osteoclasts, such as bisphosphonates or denosumab, are widely used in patients with PCa, in order to prevent the pathological reabsorption of bone and indirectly inhibit tumour growth. The addition of local surgical or radiotherapy is then sought only in selected cases. A recent meta-analysis shows that in patients with oligometastatic low volume PCa at onset, prostate irradiation prolongs survival without compromising quality of life (28), and recently published results suggest that patients with a limited number of metastases can achieve long-term disease control, or even cure, if all sites of disease undergo ablative treatment (29).
In light of the consequences, it is important not to disregard any type of bone metastases. Although CT and BS are the most common imaging methods currently used for the staging of patients with high-risk PCa, they have known limitations for the early detection of bone metastases. MRI is the most sensitive technique for the detection of bone metastases, superior to BS and choline-PET (5), and therefore should be used for bone assessments in high-risk PCa, when available (30).
Conclusion
Many patients with advanced PCa have bone metastases at diagnosis. Bone metastases have a strong negative impact on the quality of life and survival expectancy of these patients.
It is well known that CT and BS are not sufficient for a confident early detection of osteolytic bone metastases in high-risk PCa patients. NGI techniques, such as MRI with DWI, have higher sensitivity and specificity than conventional imaging techniques for bone metastasis detection and their use is encouraged for the staging of unfavourable intermediate- and high-risk PCa by the ESMO and ASCO guidelines (31,32), but at present their use is still limited. Large-scale adoption of these techniques could lead to improved patient management. However, future comparative studies are needed to evaluate the impact of staging advanced PCa patients by NGI techniques on survival.
Consent:
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Funding:
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds and by Fondazione IEO-CCM.
Conflict of Interest:
Dr Summers is co-owner of QMRI Tech, a company that provides support for medical physics activities and MRI research. The other authors declare that they have no conflicts of interest.
References
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- Beheshti M, Langsteger W, Fogelman I. Prostate cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:396–407. doi: 10.1053/j.semnuclmed.2009.05.003. doi: 10.1053/j.semnuclmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Giovanella L, Castellani M, Suriano S, Ruberto T, Ceriani L, Tagliabue L, et al. Multi-field-of-view SPECT is superior to whole-body scanning for assessing metastatic bone disease in patients with prostate cancer. Tumori. 2011;97:629–33. doi: 10.1177/030089161109700515. doi: 10.1700/989.10723. [DOI] [PubMed] [Google Scholar]
- Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–13. doi: 10.1007/s00256-014-1903-9. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- Mottet N, Cornford P, van der Bergh RCN, Briers E, De Santis M, Fanti S, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer [Internet] 2019 [2021 Apr 9] [Google Scholar]
- Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, et al. Bone metastases. Nat Rev Dis Prim. 2020;6:83. doi: 10.1038/s41572-020-00216-3. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- Padhani AR, Lecouvet FE, Tunariu N, Koh D, De Keyzer F, Collins DJ, et al. METastasis Reporting and Data System for Prostate Cancer: Practical Guidelines for Acquisition, Interpretation, and Reporting of Whole-body Magnetic Resonance Imaging-based Evaluations of Multiorgan Involvement in Advanced Prostate Cancer. Eur Urol. 2017;71:81–92. doi: 10.1016/j.eururo.2016.05.033. doi: 10.1016/j.eururo.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Diagnostic Performance of Magnetic Resonance Imaging for the Detection of Bone Metastasis in Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2018;73:81–91. doi: 10.1016/j.eururo.2017.03.042. doi: 10.1016/j.eururo.2017.03.042. [DOI] [PubMed] [Google Scholar]
- Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019;76:340–51. doi: 10.1016/j.eururo.2019.02.033. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- Lecouvet FE, El Mouedden J, Collette L, Coche E, Danse E, Jamar F, et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol. 2012;62:68–75. doi: 10.1016/j.eururo.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Akduman EI, Momtahen AJ, Balci NC, Mahajann N, Havlioglu N, Wolverson MK. Comparison Between Malignant and Benign Abdominal Lymph Nodes on Diffusion-weighted Imaging. Acad Radiol. 2008;15:641–6. doi: 10.1016/j.acra.2007.12.023. doi: 10.1016/j.acra.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Eiber M, Beer AJ, Holzapfel K, Tauber R, Ganter C, Weirich G, et al. Preliminary Results for Characterization of Pelvic Lymph Nodes in Patients With Prostate Cancer by Diffusion-Weighted MR-Imaging. Invest Radiol. 2010;45:15–23. doi: 10.1097/RLI.0b013e3181bbdc2f. doi: 10.1097/RLI.0b013e3181bbdc2f. [DOI] [PubMed] [Google Scholar]
- Kim JK, Kim KA, Park B-W, Kim N, Cho K-S. Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: Early experience. J Magn Reson Imaging. 2008;28:714–9. doi: 10.1002/jmri.21480. doi: 10.1002/jmri.21480. [DOI] [PubMed] [Google Scholar]
- Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91:718–29. doi: 10.1002/jcb.10662. doi: 10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- Roland SI. Calcium studies in ten cases of osteoblastic prostatic metastasis. J Urol. 1958;79:339–42. doi: 10.1016/S0022-5347(17)66278-5. doi: 10.1016/s0022-5347(17)66278-5. [DOI] [PubMed] [Google Scholar]
- Sottnik JL, Keller ET. Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med. 2013;13:626–39. doi: 10.2174/1566524011313040012. doi: 10.2174/1566524011313040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR, Guise TA. Hypercalcemia of malignancy. Am J Med. 1997;103:134–45. doi: 10.1016/s0002-9343(97)80047-2. doi: 10.1016/s0002-9343(97)80047-2. [DOI] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhou H, Dunstan CR, Sutherland RL, Seibel MJ. The role of the bone microenvironment in skeletal metastasis. J Bone Oncol. 2013;2:47–57. doi: 10.1016/j.jbo.2012.11.002. doi: 10.1016/j.jbo.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H-L, Liu T, Wang X-M, Xu Y, Deng S-M. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–17. doi: 10.1007/s00330-011-2221-4. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, Specificity, and Predictors of Positive 68 Ga–Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;70:926–37. doi: 10.1016/j.eururo.2016.06.021. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68 Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2018;4:686–93. doi: 10.1016/j.euf.2016.11.002. doi: 10.1016/j.euf.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Jambor I, Kuisma A, Ramadan S, Huovinen R, Sandell M, Kajander S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016;55:59–67. doi: 10.3109/0284186X.2015.1027411. doi: 10.3109/0284186X.2015.1027411. [DOI] [PubMed] [Google Scholar]
- Johnston EW, Latifoltojar A, Sidhu HS, Ramachandran N, Sokolska M, Bainbridge A, et al. Multiparametric whole-body 3.0-T MRI in newly diagnosed intermediate-and high-risk prostate cancer: diagnostic accuracy and interobserver agreement for nodal and metastatic staging. Eur Radiol. 2019;29:3159–69. doi: 10.1007/s00330-018-5813-4. doi: 10.1007/s00330-018-5813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman I, Cook G, Israel O, Van der Wall H. Positron emission tomography and bone metastases. Semin Nucl Med. 2005;35:135–42. doi: 10.1053/j.semnuclmed.2004.11.005. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol. 2004;171:2122–7. doi: 10.1097/01.ju.0000123981.03084.06. doi: 10.1097/01.ju.0000123981.03084.06. [DOI] [PubMed] [Google Scholar]
- Burdett S, Boevé LM, Ingleby FC, Fisher DJ, Rydzewska LH, Vale CL, et al. Prostate Radiotherapy for Metastatic Hormone-sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-analysis. Eur Urol. 2019;76:115–24. doi: 10.1016/j.eururo.2019.02.003. doi: 10.1016/j.eururo.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma DA, Olson R, Harrow S, Gaede S, Louie A V, Haasbeek C, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38:2830–8. doi: 10.1200/JCO.20.00818. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasoglou V, Larbi A, Collette L, Annet L, Jamar F, Machiels J, et al. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified “all-in-one” imaging approach? Prostate. 2014;74:469–77. doi: 10.1002/pros.22764. doi: 10.1002/pros.22764. [DOI] [PubMed] [Google Scholar]
- Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119–34. doi: 10.1016/j.annonc.2020.06.011. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- Trabulsi EJ, Rumble RB, Jadvar H, Hope T, Pomper M, Turkbey B, et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J Clin Oncol. 2020;38:1963–96. doi: 10.1200/JCO.19.02757. doi: 10.1200/JCO.19.02757. [DOI] [PubMed] [Google Scholar]


