Abstract
In Covid-19, systemic disturbances may progress due to development of cytokine storm and dysregulation of and plasma osmolarility due to high release of pro-inflammatory cytokines and neuro-hormonal disorders. Arginine vasopressin (AVP) which is involve in the regulation of body osmotic system, body water content, blood pressure and plasma volume, that are highly disturbed in Covid-19 and linked with poor clinical outcomes. Therefore, this present study aimed to find the potential association between AVP serum level and inflammatory disorders in Covid-19. It has been observed by different recent studies that physiological response due to fever, pain, hypovolemia, dehydration, and psychological stress is characterized by activation release of AVP to counter-balance high blood viscosity in Covid-19 patients. In addition, activated immune cells mainly T and B lymphocytes and released pro-inflammatory cytokines stimulate discharge of stored AVP from immune cells, which in a vicious cycle trigger release of pro-inflammatory cytokines. Vasopressin receptor antagonists have antiviral and anti-inflammatory effects that may inhibit AVP-induced hyponatremia and release of pro-inflammatory cytokines in Covid-19. In conclusion, release of AVP from hypothalamus is augmented in Covid-19 due to stress, high pro-inflammatory cytokines, high circulating AngII and inhibition of GABAergic neurons. In turn, high AVP level leads to induction of hyponatremia, inflammatory disorders, and development of complications in Covid-19 by activation of NF-κB and NLRP3 inflammasome with release of pro-inflammatory cytokines. Therefore, AVP antagonists might be novel potential therapeutic modality in treating Covid-19 through mitigation of AVP-mediated inflammatory disorders and hyponatremia.
Keywords: Covid-19, Arginine vasopressin, Hyponatremia
Graphical Abstract
1. Background
From the beginning of coronavirus disease 2019 (Covid-19) pandemic there is a substantial worldwide impact on the health system due to high rate of hospitalization and high fatality rate especially in patients admitted in the intensive care unit [1]. Up to date, the total infected patients globally reach to more than 170 million with more than 3 million confirmed death cases in late May of 2021. Nonetheless, the ongoing and oncoming rise of infected cases is still alarming primarily with emerging of variant strains of this pandemic [2]. Covid-19 is caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an enveloped positive sense single strand RNA virus from Betacoronaviridae family, it has 79% similarity with SARS-CoV pandemic 2003 and about 40% with Middle East Respiratory Syndrome coronavirus (MERS-CoV) 2012. Most of SARS-CoV-2 variant strains have a high similarity with novel SARS-CoV-2 ranged from 81% to 96% [3].
The pathophysiological role of SARS-CoV-2 infection is mediated by direct viral toxicity, abnormal immune response, endothelial dysfunction, dysregulated renin-angiotensin system (RAS), and thrombo-inflammation that lead to pulmonary and extra-pulmonary manifestations [4]. The angiotensin converting enzyme 2 (ACE2) is regarded as the main receptor for entry of SARS-CoV-2 that is expressed in various tissue principally in lung epithelial alveolar type II cells, endothelial cells, enterocytes and cardiomyocytes [5], [6]. The pulmonary manifestations of SARS-CoV-2 infections are asymptomatic in 80% of infected cases however 15% of infected patients may develop pneumonitis and acute lung injury (ALI), while 5% of infected patients experience a critical Covid-19 due to progression of acute respiratory distress syndrome (ARDS) [7]. Moreover, systemic disturbances may progress due to development of cytokine storm and dysregulation of and plasma osmolarility due to high release of pro-inflammatory cytokines and neuro-hormonal disorders respectively [8].
Both serum sodium and arginine vasopressin (AVP) that involved in the regulation of body osmotic system, body water content, blood pressure and plasma volume, are highly disturb in Covid-19 and linked with poor clinical outcomes [9]. Therefore, this present study aimed to find the potential association between AVP serum level and inflammatory disorders in Covid-19.
2. Physiological profile of arginine vasopressin
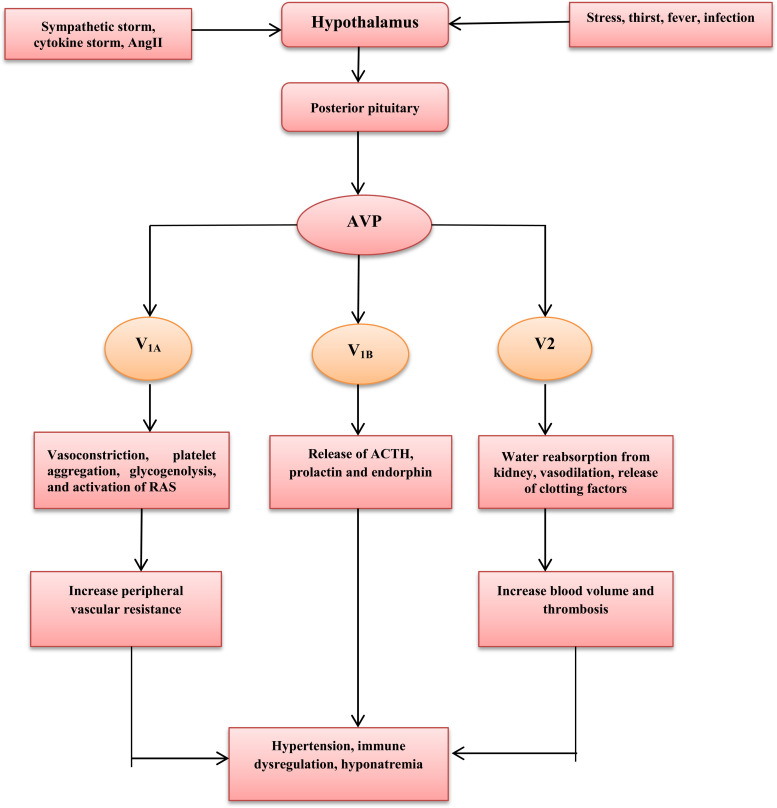
AVP is a nonapeptide primarily synthesized by paraventricular (PVN) and supraoptic neurons of the hypothalamus that stored in the posterior hypothalamus. Expression of AVP gene induces release of prepro-AVP, which proteolysed to release AVP, neurophysin II and copeptin [10]. High plasma osmolarility during thirst and dehydration is regarded as the main determinant of AVP release; however stress nausea, vomiting, acidosis, fever, pro-inflammatory cytokines, sympathetic stimulation, hypovolemia and catecholamines also stimulate AVP release [11]. AVP is also considered as a neurotransmitter regulates activity of AVPergic neurons in response to the stimulating factors [12]. Neuronal aquaporin 4 receptor and transient receptor potential cation channel sense blood and cerebrospinal fluid osmolarility that control release of AVP [13]. Moreover, expression of hypothalamic AVP gene is regulated by gut microbiota since antibiotic therapy in mice decrease gut microbiota and expression of hypothalamic AVP gene [14]. Similarly, AVP affects growth of gut microbiota through modulation of gut inflammatory and stress response suggesting that gut microbiota may metabolize AVP [15]. It has been reported that AVP affects glucose metabolism, it stimulates glycogenolysis and glucagon release leading to hyperglycemia and metabolic disturbances [16].
AVP acts through G-protein coupled receptors V1 (V1A), V2, and V3 (V1B). V1A receptors are expressed in vascular smooth muscle, myocardium, hepatocytes, and platelets that involved in vasoconstrictions, platelet aggregation, myocardial hypertrophy, and glycogenolysis. V3 (V1B) receptors are expressed in the anterior pituitary responsible for release of prolactin, adrenocorticotrophic hormone (ACTH) and endorphin. V2 receptors are expressed in renal collecting duct, vascular smooth muscle, and vascular endothelium responsible for water reabsorption from kidney, vasodilation, and release of clotting factor VIII and von Willebrand factor [17], [18]. Therefore, AVP leads to increase blood pressure directly through V1A-induced vasoconstriction or indirectly through activation of RAS, sympathetic flow and baroreflex sensitivity [19]. Despite of clinical significance of AVP, its measurement is limited due to short half-life, so instead copeptin, which is synthesized at the same time of AVP and correlated with plasma osmolarity, can be used as a prognostic factor due to long half-life. Therefore, copeptin is considered as a surrogate biomarker that reflects synthesis, level, and biological activity of AVP [20], [21].
Thus, AVP controls blood pressure, plasma volume, kidney water reabsorption, and synthesis of clotting factors [ Fig. 1].
Fig. 1.
The potential mechanisms of arginine vasopressin (AVP) release. Arginine vasopressin and infections.
It has been observed that AVP and copeptin are strongly increased in patients with septic shock that associated with systemic inflammatory disorders compared with infected patients without systemic inflammatory reactions [22]. Chassin et al. illustrated that administration of AVP V2 receptor agonist deamino-8-D-AVP reduces inflammatory changes in patients with pyelonephritis due to inhibition release of chemokines and NF-κB pathway, suggesting immune-regulatory effect of AVP [23]. However, AVP antagonist in patients with pulmonary tuberculosis restores the activity of protective immunity to counterbalance high AVP level in late active phase of disease [24]. In addition, beyond procalcitonin and C-reactive protein (CRP), AVP might be a promising biomarker in the diagnosis and poor prognosis of pneumonia [25]. Therefore, copeptin is regarded as a predictor biomarker for assessment severity and prognosis of acquired pneumonia [26]. Lopez et al. showed that high AVP in bacterial sepsis and pneumonia is associated with increased pulmonary microvascular permeability, thus AVP antagonist tolvaptan may reduce the severity in septic patients [27]. Similarly, copeptin serum level is correlated with severity of ventilator associated pneumonia [28]. These findings suggest that AVP plays a role in the pathophysiology of pneumonia and development of sepsis. However, elevation of AVP serum levels might be a compensatory mechanism to counteract pro-inflammatory cytokines-induced vasodilatory shock during sepsis.
On the other, dilution hyponatremia due to increasing AVP may impact the course of infection during sepsis. It has been illustrated that hyponatremia and elevated AVP are linked with poor clinical outcome and high mortality in patients with community acquired pneumonia [29].
Of note, hyponatremia is also associated with respiratory viral infections such as influenza virus (H1N1), parainfluenza virus, avian influenza (H7N9) and adenovirus [30]. In addition, hyponatremia is commonly connected to the human immune deficiency virus (HIV) infection mainly when associated with central nervous system (CNS) and pulmonary co-infections due to development of syndrome of inappropriate antidiuretic hormone (SIADH) [31]. Hyponatremia is regarded as a predictor of immune dysfunction in HIV infection as it inversely correlated with CD4 T lymphocyte number and function [32].
Moreover, development of hyponatremia is also reported in other viral infections including Hantavirus, Ebola virus, and herpes simplex virus [33]. Indeed, CNS viral infections are associated with development of SIADH and hyponatremia in about 60% due to vomiting, dehydration, fever, acidosis and stress that activate release of AVP from hypothalamus [34]. Besides, drugs that are used in the management of secondary bacterial infections in patients with viral pneumonia like trimethprim-sulfamethoxazole antibiotic may lead to hyponatremia in 60% of treated patients due to induction of natriuresis [35]. The underlying mechanism of SIADH and hyponatremia in respiratory viral infections might be neutrophilia and higher concentrations of pro-inflammatory cytokines that activate hypothalamus for release of AVP with subsequent hyponatremia [36]. Likewise, antiviral protease inhibitors ribavirin and paritaprevir therapy may lead to SIADH and hyponatremia [37].
3. Arginine vasopressin and Covid-19
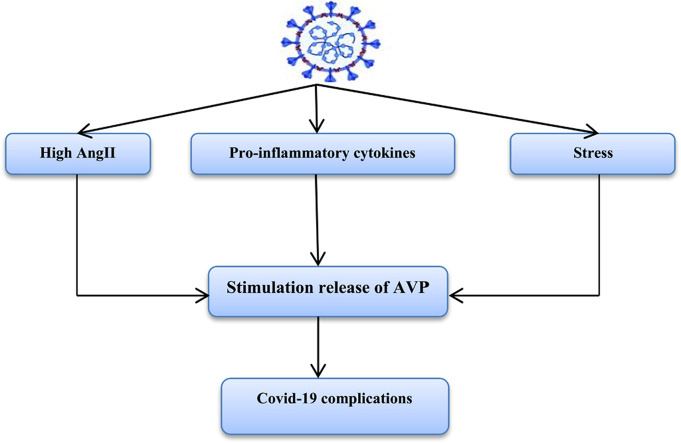
It has been observed by different recent studies that physiological response due to fever, pain, hypovolemia, dehydration and psychological stress is characterized by activation release of AVP to counter-balance high blood viscosity in Covid-19 patients [38]. Abnormal activation of vasopressin system during SARS-CoV-2 infection is linked with poor clinical outcomes and prognosis in hospitalized Covid-19 patients with complications [9]. Tzoulis et al., longitudinal, retrospective cohort study confirmed that high AVP-induced hyponatremia is associated with poor prognosis due to high risk of respiratory failure in hospitalized Covid-19 patients [39]. In addition, hyponatremia without elevation of AVP is also linked with complications and mortality in Covid-19 patients [40], thus increasing AVP and/or hyponatremia are more correlated with Covid-19 severity. Besides, various case-report studies illustrated that SARS-CoV-2 infection is associated with development of SIADH due to progression of acute kidney injury-induced hyponatremia [41] or ALI-induced pulmonary vasoconstriction [42] that together activate release of AVP by osmotic-independent pathway. The potential mechanisms for activation release of AVP in Covid-19 are stressful factors, hypoxic pulmonary vasoconstriction, and reduction of left atrium filling due to dehydration and hypovolemia, and high pro-inflammatory cytokines [42].
Acute inflammatory response with a higher concentration of pro-inflammatory cytokines mainly IL-6 leads to significant stimulation of hypothalamic AVP with progression of hyponatremia. Besides, IL-6 inhibitors attenuate systemic inflammation and development of SIADH suggesting that IL-6 is a prototype pro-inflammatory cytokine, which activate release of hypothalamic AVP [43]. In Covid-19, IL-6 is highly activated and linked with development of cytokine storm and ALI/ARDS, and IL-6 blockade may mitigate systemic inflammatory reactions and ALI [44]. Therefore, IL-6 has dual effect in the stimulation of AVP release either directly as it cross blood brain barrier (BBB) and initiates activation of hypothalamus or indirectly through induction of ALI-induced pulmonary vasoconstriction with reduction of cardiac atrial stretching [45]. As well, AVP stimulates release of IL-6 through activation of NF-κB pathway by V1A receptor leading to myocardial inflammation in the experimental heart failure [46]. Taken together, there is significant interaction between AVP and IL-6 during acute inflammatory reactions suggesting immunoregulatory effect of AVP on the innate immune response. Furthermore, low dose of AVP leads to immunomodulatory effect mainly in the lung through V2 receptor [47]. Both corticotrophin releasing hormone (CRH) and AVP are present in the immune cells and can be release in response to inflammation and stress. Also, AVP receptors are expressed on the immune cells that involved in antibody production and release of pro-inflammatory cytokines [48]. AVP also activates Th1 immune response leading to augmentation of mixed cellular immune response with further abnormal immune response [49].
Interestingly, abnormal immune response in Covid-19 that characterized by lymphopenia and abnormal T cell functions leading to cytokine storm and abnormal immune response-induced tissue injury [50]. Activated immune cells mainly T and B lymphocytes and released pro-inflammatory cytokines stimulate discharge of stored AVP from immune cells, which in a vicious cycle trigger release of pro-inflammatory cytokines [51]. Therefore, aberrant immune response in SARS-CoV-2 infection might be the potential cause for elevation of AVP [52].
Therefore, source of high AVP in Covid-19 is from hypothalamus and immune cells that together contribute into progression of inflammatory and immunological disorders in severe cases. In the recent researches, SARS-CoV-2 directly triggers macrophage and other immune cells through activation of NF-κB pathway for release of pro-inflammatory cytokines and progression of cytokine storm [53]. Besides, released AVP in Covid-19 may provoke inflammatory reactions through release of pro-inflammatory cytokines due to activation of NF-κB pathway [54]. Into the bargain, AVP-induced hyponatremia disturbs cellular osmolality and activate NLRP3 inflammasome for secretion of IL-1β, which cross BBB and stimulate hypothalamus for secretion of AVP. In turn, AVP increases progression of hyponatremia with more activation of NLRP3 inflammasome/IL-1β axis and development of SIADH [55]. In addition, AVP and associated hyponatremia trigger NLRP3 inflammasome activation through IL-6 dependent pathway causing more propagation of inflammatory reactions in Covid-19 patients [56], [57].
It has been observed that platelet activations, endothelial dysfunction and thrombosis in SARS-CoV-2 are due to activation of mitogen-activated protein kinase (MAPK) leading to pulmonary micro-vascular thrombosis and progression of ALI/ARDS [58]. Notably, AVP triggers MAPK-induced endothelial dysfunction with direct effect for release of clotting factors [59], [60]. Remarkably, administration of AVP reduces development of ALI [61] however, Barcroft et al., illustrated in a case-report study that intramyometrial infiltration of AVP during laparoscopic myomyectomy leads to acute pulmonary edema and coronary vasospasm [62]. Thus, dose-dependent effect of AVP may affect the cardiopulomary outcomes.
It has been shown that high mobility group box 1(HMGB1) may lead to ALI in SARS-CoV-2 infection through activation of autophagy, which involved in the pathogenesis of SARS-CoV-2-induced ALI [63]. Yang et al. [64] illustrated that autophagy and endocytic pathway are involved in SARS-CoV-2 infections and linked with Covid-19 severity. As well, AVP induces autophagy pathway leading to progressive cell death and inflammation with activation of oxidative stress-induced release of HMGB1 [65], [66]. Therefore, both SARS-CoV-2 infection and high AVP may augment autophagy-induced ALI.
On the other hand, SARS-CoV-2-induced downregulation of ACE2 with subsequent increase of AngII serum level since ACE2 metabolize AngII to vasodilator and protective Ang 1–7 [67]. High circulating AngII level provokes endothelial dysfunction, activation release of pro-inflammatory cytokines, thrombosis, and progression of ALI/ARDS [68]. Elevated AngII stimulates hypothalamus to release of AVP, which causes hyponatremia and release of more inflammatory cytokines [69]. However, central administration of Ang1–7 inhibits release of AVP from hypothalamus via anti-inflammatory effect [70]. Furthermore, high AVP in SARS-CoV-2 infection might be due to AngII-induced hypothalamic activation or due to AngII-induced immune cells activation that also release AVP.
Of interest, the inhibitory GABAerigic neurons inhibit pre-sympathetic hypothalamic PVN neurons. These GABAerigic neurons have a higher expression of ACE2 receptors, thus down-regulation of these receptors during SARS-CoV-2 infection may suppress these inhibitory interneurons with activation of hypothalamic sympathetic neurons leading to sympathetic storm [71]. Excitingly, central sympathetic stimulation due to SARS-CoV-2 infection increases circulating catecholamine, which activates macrophages and neutrophils for release of pro-inflammatory cytokines. Correspondingly, activated macrophages and neutrophils also release catecholamine, which act in a paracrine manner for augmentation release of pro-inflammatory cytokines [72]. Kim et al. illustrated that GABAerigic neurons dysfunction activate release of AVP [73]. In addition, sympathetic storm trigger release of AVP release and vice versa via neuro-hormonal loop [74]. Thus, SARS-CoV-2 infection is associated with high AVP and sympathetic flow due to inhibition of central GABAerigic neurons.
These findings give a clue that AVP action is augmented during SARS-CoV-2 infection and may participate for development of Covid-19 complications [ Fig. 2].
Fig. 2.
The interaction between SARS-CoV-2 and Arginine vasopressin(AVP): SARS-CoV-2 through downregulation of ACE2, induction of acute kidney injury (AKI), acute lung injury(ALI), release of pro-inflammatory cytokines (PIC) and inhibition of GABAergic neurons stimulate hypothalamus for secretion of AVP. Released AVP activates NF-κB, NLRP3 inflammasome,release of PIC and induction of Autophagy leading to acute lung injury(ALI) acute respiratory distress syndrome (ARDS).
4. Arginine vasopressin antagonists and Covid-19
Vasopressin receptor antagonists (VRAs) drugs that block AVP receptors used in the treatment of hyponatremia as in SIADH, heart failure and liver cirrhosis [75]. VRAs might be non-selective like conivaptan, which block V1 and V2 receptors, V1A receptor antagonist like relcovaptan, V1B receptor antagonist like nelivaptan, and selective V2 receptor antagonist like tolvaptan [76]. VRAs may have potential role in the management of hyponatermia in different viral infections including Covid-19 through block AVP effects, and silico study found that conivaptan inhibits SARS-CoV-2 3C-like protease and viral RNA-dependent polymerase [77]. Xiao et al., illustrated that VRAs may have anti-SARS-CoV-2 effects [78]. Interestingly, tolvaptan has in vitro and vivo antiviral effects against Zika virus [79].
On the other hand, tolvaptan has anti-inflammatory and anti-fibrotic effects through inhibition of monocyte chemotractic protein-1(MCP-1) and transforming growth factor β 1(TGF-β1) [80]. Both of MCP-1 and TGF-β1 are involved in the inflammatory process during SARS-CoV-2 infection.
Therefore, the antiviral and anti-inflammatory effects of VRAs may inhibit AVP-induced hyponatremia and release of pro-inflammatory cytokines in Covid-19.
5. Conclusion
Release of AVP from hypothalamus is augmented in Covid-19 due to stress, high pro-inflammatory cytokines, high circulating AngII and inhibition of GABAergic neurons. In turn, high AVP level leads to induction of hyponatremia, inflammatory disorders, and development of complications in Covid-19 by activation of NF-κB and NLRP3 inflammasome with release of pro-inflammatory cytokines. Therefore, AVP antagonists might be novel potential therapeutic modality in treating Covid-19 through mitigation of AVP-mediated inflammatory disorders and hyponatremia. Appreciation of these findings should trigger further research actions to confirm this association in a new perspective.
Ethics approval and consent to participate
Not applicable.
CRediT authorship contribution statement
Hayder M. Al-kuraishy: Conceptualization, Methodology, Validation, Resources, Writing – original draft, Supervision, Project administration. Ali I. Al-Gareeb: Conceptualization, Software. Safaa Qusti: Methodology, Software, Writing – original draft, Writing – review & editing. Eida M. Alshammari: Methodology, Writing – original draft, Writing – review & editing. Francis O. Atanu: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing. Gaber El-Saber Batiha: Conceptualization, Validation, Resources, Writing – original draft, Supervision, Project administration.
All authors have read and agreed to the published version of the manuscript.
Consent for publication
Not applicable.
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Al-kuraishy H.M., Al-Naimi M., Lungnier C., Al-Gareeb A.I. Macrolides and COVID-19: an optimum premise. Biomed. Biotechnol. Res. J. 2020;4:189–192. [Google Scholar]
- 2.Al-Kuraishy H., Hussien N., Al-Naimi M., Al-Buhadily A., Al-Gareeb A., Lungnier C. Is ivermectin–azithromycin combination the next step for COVID-19? Biomed. Biotechnol. Res. J. 2020;4:101–103. doi: 10.4103/bbrj.bbrj_109_20. [DOI] [Google Scholar]
- 3.Laamarti M., Alouane T., Kartti S., Chemao-Elfihri M.W., Hakmi M., Essabbar A., Laamarti M., Hlali H., Bendani H., Boumajdi N., Benhrif O., Allam L., El Hafidi N., El Jaoudi R., Allali I., Marchoudi N., Fekkak J., Benrahma H., Nejjari C., Amzazi S., Belyamani L., Ibrahimi A. Large scale genomic analysis of 3067 SARS-CoV-2 genomes reveals a clonal geo-distribution and a rich genetic variations of hotspots mutations. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-kuraishy H.M., Al-Gareeb A.I., Qusty N., Cruz-Martins N., El-Saber Batiha G. Sequential doxycycline and colchicine combination therapy in Covid-19: the salutary effects. Pulm. Pharmacol. Ther. 2021;67 doi: 10.1016/j.pupt.2021.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugnier C., Al-Kuraishy H.M., Rousseau E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem. Pharmacol. 2021;185 doi: 10.1016/j.bcp.2021.114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Kuraishy H., Hussien N., Al-Naimi M., Al-Buhadily A., Al-Gareeb A., Lungnier C. Renin–angiotensin system and fibrinolytic pathway in COVID-19: one-way skepticism. Biomed. Biotechnol. Res. J. 2020;4:33–40. doi: 10.4103/bbrj.bbrj_105_20. [DOI] [Google Scholar]
- 7.Al-Kuraishy H.M., Al-Gareeb A.I., Alblihed M., Cruz-Martins N., Batiha G.E.-S. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type II diabetes mellitus: the anti-inflammatory role of metformin. Front. Med. 2021;8 doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Kuraishy H.M., Al-Gareeb A.I., Almulaiky Y.Q., Cruz-Martins N., El-Saber Batiha G. Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of Covid-19: the enigmatic entity. Eur. J. Pharmacol. 2021;904 doi: 10.1016/j.ejphar.2021.174196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregoriano C., Molitor A., Haag E., Kutz A., Koch D., Haubitz S., Conen A., Bernasconi L., Hammerer-Lercher A., Fux C.A., Mueller B., Schuetz P. Activation of vasopressin system during COVID-19 is associated with adverse clinical outcomes: an observational study. J. Endocr. Soc. 2021;5(6):45. doi: 10.1210/jendso/bvab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Kuraishy H.M. Desmopressin acetate effects on human vigilance task and psychomotor performances in normal healthy volunteers: randomized single blind clinical trail. Iraqi J. Community Med. 2012:25. [Google Scholar]
- 11.Kanbay M., Yilmaz S., Dincer N., Ortiz A., Sag A.A., Covic A., Sánchez-Lozada L.G., Lanaspa M.A., Cherney D.Z.I., Johnson R.J., Afsar B. Antidiuretic hormone and serum osmolarity physiology and related outcomes: what is old, what is new, and what is unknown? J. Clin. Endocrinol. Metab. 2019;104(11):5406–5420. doi: 10.1210/jc.2019-01049. [DOI] [PubMed] [Google Scholar]
- 12.Bankir L.A.-O., Bichet D.G., Morgenthaler N.G. Vasopressin: physiology, assessment and osmosensation. J. Intern. Med. 2017;282(4):284–297. doi: 10.1111/joim.12645. [DOI] [PubMed] [Google Scholar]
- 13.Danziger J., Zeidel M.L. Osmotic homeostasis. Clin. J. Am. Soc. Nephrol. CJASN. 2015;10:852–862. doi: 10.2215/CJN.10741013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desbonnet L., Clarke G., Traplin A., O’Sullivan O., Crispie F., Moloney R.D., Cotter P.D., Dinan T.G., Cryan J.F. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Fields C.T., Chassaing B., Paul M.J., Gewirtz A.T., de Vries G.J. Vasopressin deletion is associated with sex-specific shifts in the gut microbiome. Gut Microbes. 2018;9(1):13–25. doi: 10.1080/19490976.2017.1356557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taveau C., Chollet C., Bichet D.G., Velho G., Guillon G., Corbani M., Roussel R., Bankir L.A.-O., Melander O., Bouby N. Acute and chronic hyperglycemic effects of vasopressin in normal rats: involvement of V(1A) receptors. Am. J. Physiol. -Endocrinol. Metab. 2017;312(3):E127–E135. doi: 10.1152/ajpendo.00269.2016. [DOI] [PubMed] [Google Scholar]
- 17.Kumari P., Srivastava A., Ghosh E., Ranjan R., Dogra S., Yadav P.N., Shukla A.K. Core engagement with β-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. Mol. Biol. Cell. 2017;28(8):1003–1010. doi: 10.1091/mbc.E16-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F., Ye C., Ma X., Yin W.A.-O., Croll T.I., Zhou Q., He X., Zhang X.A.-O., Yang D.A.-O., Wang P., et al. Molecular basis of ligand recognition and activation of human V2 vasopressin receptor. Cell Res. 2021;19:1–3. doi: 10.1038/s41422-021-00480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komnenov D., Quaal H., Rossi N.A.-O.X. V(1a) and V(1b) vasopressin receptors within the paraventricular nucleus contribute to hypertension in male rats exposed to chronic mild unpredictable stress. Integr. Comp. Physiol. 2021;320(3):R213–R225. doi: 10.1152/ajpregu.00245.2020. [DOI] [PubMed] [Google Scholar]
- 20.Morgenthaler N.G., Struck J., Fau - Alonso C., Alonso C.Fau, Bergmann A., Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 2006;52(1):112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 21.Gansevoort R.T., van Gastel M.D.A., Chapman A.B., Blais J.D., Czerwiec F.S., Higashihara E., Lee J., Ouyang J., Perrone R.D., Stade K., Torres V.E., Devuyst O., TEMPO I. Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease. Kidney Int. 2019;96(1):159–169. doi: 10.1016/j.kint.2018.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jochberger S., Dörler J.Fau, Luckner G., Luckner G.Fau, Mayr V.D., Wenzel V., Ulmer H., Morgenthaler N.G., Hasibeder W.R., Dünser M.W., Ulmer H. The vasopressin and copeptin response to infection, severe sepsis, and septic shock. Crit. Care Med. 2009;37(2):476–482. doi: 10.1097/CCM.0b013e3181957532. [DOI] [PubMed] [Google Scholar]
- 23.Chassin C., Hornef M.W., Bens M., Lotz M., Goujon J.-M., Vimont S., Arlet G., Hertig A., Rondeau E., Vandewalle A. Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin. J. Exp. Med. 2007;204:2837–2852. doi: 10.1084/jem.20071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zetter M., Barrios-Payán J., Mata-Espinosa D., Marquina-Castillo B., Quintanar-Stephano A., Hernández-Pando R. Involvement of vasopressin in the pathogenesis of pulmonary tuberculosis: a new therapeutic target? Front. Endocrinol. 2019;10:351. doi: 10.3389/fendo.2019.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karakioulaki M., Stolz D. Biomarkers in pneumonia-beyond procalcitonin. Int. J. Mol. Sci. 2019;20(8):2004. doi: 10.3390/ijms20082004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed G.B., Saed M.A., Abdelhakeem A.A., Salah K., Saed A.M. Predictive value of copeptin as a severity marker of community-acquired pneumonia. Electron. Physician. 2017;9:4880–4885. doi: 10.19082/4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez E., Fukuda S., Modis K., Fujiwara O., Enkhtaivan B., Trujillo-Abarca R., Ihara K., Lima-Lopez F., Perez-Bello D., Szabo C., Prough D.S., Enkhbaatar P. Arginine vasopressin receptor 2 activation promotes microvascular permeability in sepsis. Pharmacol. Res. 2021;163 doi: 10.1016/j.phrs.2020.105272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seligman R., Papassotiriou J Fau - Morgenthaler N.G., Morgenthaler Ng Fau - Meisner M., Meisner M Fau - Teixeira P.J.Z., Teixeira P.J. Copeptin, a novel prognostic biomarker in ventilator-associated pneumonia. Crit. Care. 2008;12(1):1–9. doi: 10.1186/cc6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krüger S., Ewig S., Giersdorf S., Hartmann O., Frechen D., Rohde G., Suttorp N., Welte T. Dysnatremia, vasopressin, atrial natriuretic peptide and mortality in patients with community-acquired pneumonia: results from the german competence network CAPNETZ. Respir. Med. 2014;108(11):1696–1705. doi: 10.1016/j.rmed.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira D.B., Durigon El Fau - Carvalho A.C.L., Carvalho Ac Fau - Leal A.L., Leal Al Fau - Souza T.S., Souza Ts Fau - Thomazelli L.M., Thomazelli Lm Fau - Moraes C.T.P., Moraes Ct Fau - Vieira S.E., Vieira Se Fau - Gilio A.E., Gilio Ae Fau - Stewien K.E., Stewien K.E. Epidemiology and genetic variability of human metapneumovirus during a 4-year-long study in Southeastern Brazil. J. Med. Virol. 2009;81(5):915–921. doi: 10.1002/jmv.21436. [DOI] [PubMed] [Google Scholar]
- 31.Shu Z., Tian Z., Chen J., Ma J., Abudureyimu A., Qian Q., Zhuo L. HIV/AIDS-related hyponatremia: an old but still serious problem. Ren. Fail. 2018;40:68–74. doi: 10.1080/0886022X.2017.1419975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Królicka A.L., Kruczkowska A., Krajewska M., Kusztal M.A. Hyponatremia in infectious diseases-a literature review. Int. J. Environ. Res. Public. Health. 2020;17:5320. doi: 10.3390/ijerph17155320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West T.E., von Saint André-von Arnim A. Clinical presentation and management of severe ebola virus disease. Ann. Am. Thorac. Soc. 2014;11(9):1341–1350. doi: 10.1513/AnnalsATS.201410-481PS. [DOI] [PubMed] [Google Scholar]
- 34.Lim A.K.H., Paramaswaran S., Jellie L.J., Junckerstorff R.K. A cross-sectional study of hyponatremia associated with acute central nervous system infections. J. Clin. Med. 2019;8:1801. doi: 10.3390/jcm8111801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppens R., Yang J., Ghosh S., Gill J., Chambers C., Easaw J.C. Evaluation of laboratory disturbance risk when adding low-dose cotrimoxazole for PJP prophylaxis to regimens of high-grade glioma patients taking RAAS inhibitors. J. Oncol. Pharm. Pract. 2019;25(6):1366–1373. doi: 10.1177/1078155218792985. [DOI] [PubMed] [Google Scholar]
- 36.Gaglani B., Gupta S., Chavez O., Libardo R. Influenza as a cause of SIADH related hyponatremia: a case report. J. Clin. Diagn. Res.: JCDR. 2017;11(5):10. doi: 10.7860/JCDR/2017/25785.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar R., Hsiang J.C., Tan J., Thurairajah P.H. Ombitasvir/paritaprevir/ritonavir+dasabuvir and ribavirin associated drug-induced liver injury and syndrome of inappropriate secretion of anti-diuretic hormone: a case report. Clin. Mol. Hepatol. 2019;25(3):326–330. doi: 10.3350/cmh.2018.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joob B., Wiwanitkit V. Blood viscosity of COVID-19 patient: a preliminary report. Am. J. Blood Res. 2021;11(1):93–95. [PMC free article] [PubMed] [Google Scholar]
- 39.Tzoulis P.A.-O.X., Waung J.A., Bagkeris E., Hussein Z., Biddanda A., Cousins J., Dewsnip A., Falayi K., McCaughran W., Mullins C., et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J. Clin. Endocrinol. Metab. 2021;106(6):1637–1648. doi: 10.1210/clinem/dgab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W., Lv X., Li C., Xu Y., Qi Y., Zhang Z., Li M., Cai F., Liu D., Yue J., Ye M., Chen Q., Shi K. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern. Emerg. Med. 2021;16:853–862. doi: 10.1007/s11739-020-02515-9. 1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemcioglu E., Karabuga B., Ercan A., Erden A. A case of inappropriate antidiuretic hormone secretion syndrome associated with COVID-19 pneumonia. Acta Endocrinol. 2020;16:110–111. doi: 10.4183/aeb.2020.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yousaf Z., Al-Shokri S.D., Al-Soub H., Mohamed M.F.H. COVID-19-associated SIADH: a clue in the times of pandemic! Am. J. Physiol. Endocrinol. Metab. 2020;318:E882–E885. doi: 10.1152/ajpendo.00178.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodax J.K., Bialo S.R., Yalcindag A. SIADH in systemic JIA resolving after treatment with an IL-6 inhibitor. Pediatrics. 2018;141:141. doi: 10.1542/peds.2016-4174. [DOI] [PubMed] [Google Scholar]
- 44.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quintanar-Stephano A., Viñuela-Berni V., Macías-Segura N., Valdez-Urias F., Kovacs K.T. Effects of arginine vasopressin (AVP) deficiency, conivaptan and desmopressin on clinical symptoms and blood cytokine levels in rats with experimental autoimmune encephalomyelitis. FASEB J. 2018;32 doi: 10.1096/fasebj.2018.32.1_supplement.741.7. (doi:https://doi.org/) [DOI] [Google Scholar]
- 46.Sun S.-Z., Cao H., Yao N., Zhao L.-L., Zhu X.-F., Ni E.-A., Zhu Q., Zhu W.-Z. β-arrestin 2 mediates arginine vasopressin-induced IL-6 induction via the ERK(1/2)-NF-ΚB signal pathway in murine hearts. Acta Pharmacol. Sin. 2020;41:198–207. doi: 10.1038/s41401-019-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyd J.H., Holmes C.L., Wang Y., Roberts H., Walley K.R. Vasopressin decreases sepsis-induced pulmonary inflammation through the V2R. Resuscitation. 2008;79:325–331. doi: 10.1016/j.resuscitation.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Baker C., Richards L.J., Dayan C.M., Jessop D.S. Corticotropin-releasing hormone immunoreactivity in human T and B cells and macrophages: colocalization with arginine vasopressin. J. Neuroendocrinol. 2003;15:1070–1074. doi: 10.1046/j.1365-2826.2003.01099.x. [DOI] [PubMed] [Google Scholar]
- 49.Park S.J., Shin J.I. Inflammation and hyponatremia: an underrecognized condition? Korean J. Pediatr. 2013;56:519–522. doi: 10.3345/kjp.2013.56.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y.A.-O. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020;5(1):1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabrina M.Scroggins, Lydia E.Sharp, Jenna M.Lund, Eric J.Devor, Akshaya Warrier, Wendy S.Hamilton, Donna A.Santillan, Mark K.Santillan. Correction. Circulation. 2015;131:535. doi: 10.1161/hyp.74.suppl_1.P132. (doi:doi:) [DOI] [Google Scholar]
- 52.Habib M.B., Sardar S., Sajid J. Acute symptomatic hyponatremia in setting of SIADH as an isolated presentation of COVID-19. IDCases. 2020;21:00859. doi: 10.1016/j.idcr.2020.e00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carcaterra M., Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: a physio-pathological theory. Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu F., Sun S., Wang X., Ni E., Zhao L., Zhu W. GRK2 mediates arginine vasopressin-induced interleukin-6 production via nuclear factor-ΚB signaling neonatal rat cardiac fibroblast. Mol. Pharmacol. 2017;92(3):278–284. doi: 10.1124/mol.116.107698. [DOI] [PubMed] [Google Scholar]
- 55.Kim J.H., Park J.H., Eisenhut M., Yu J.W., Shin J.I. Inflammasome activation by cell volume regulation and inflammation-associated hyponatremia: a vicious cycle. Med. Hypotheses. 2016;93:117–121. doi: 10.1016/j.mehy.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Paniri A., Akhavan-Niaki H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: role of LncRNAs in cytokine storm modulation. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Carvalho H., Letellier T., Karakachoff M., Desvaux G., Caillon H., Papuchon E., Bentoumi-Loaec M., Benaouicha N., Canet E., Chapelet G., et al. Hyponatremia is associated with poor outcome in COVID-19. J. Nephrol. 2021:1–8. doi: 10.1007/s40620-021-01036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asiedu S.A.-O., Kwofie S.A.-O., Broni E.A.-O., Wilson M.A.-O. Computational identification of potential anti-inflammatory natural compounds targeting the P38 mitogen-activated protein kinase (MAPK): implications for COVID-19-induced cytokine storm. Biomolecules. 2021;11(5):653. doi: 10.3390/biom11050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Datta A.A.-O., Yang C.R., Salhadar K., Park E., Chou C.L., Raghuram V., Knepper M.A.-O. Phosphoproteomic identification of vasopressin-regulated protein kinases in collecting duct cells. Br. J. Pharmacol. 2021;178(6):1426–1444. doi: 10.1111/bph.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saad A.F., Maybauer M.O. The role of vasopressin and the vasopressin type V1a receptor agonist selepressin in septic shock. J. Crit. Care. 2017;40:41–45. doi: 10.1016/j.jcrc.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Rostron A.J., Avlonitis Vs Fau - Cork D.M.W., Cork Dm Fau - Grenade D.S., Grenade Ds Fau - Kirby J.A., Kirby Ja Fau - Dark J.H., Dark J.H. Hemodynamic resuscitation with arginine vasopressin reduces lung injury after brain death in the transplant donor. Transplantation. 2008;85(4):597–606. doi: 10.1097/TP.0b013e31816398dd. [DOI] [PubMed] [Google Scholar]
- 62.Barcroft J.F., Al-Kufaishi A., Lowe J., Quinn S. Risk of vasopressin use: a case of acute pulmonary oedema, post intramyometrial infiltration of vasopressin in laparoscopic myomectomy. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-231331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun J. The hypothesis that SARS-CoV-2 affects male reproductive ability by regulating autophagy. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang N., Shen H.M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020;16(10):1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagiwara D., Arima H., Morishita Y., Wenjun L., Azuma Y., Ito Y., Suga H., Goto M., Banno R., Sugimura Y., Shiota A., Asai N., Takahashi M., Oiso Y. Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus. Cell Death Dis. 2014;5(3):1148. doi: 10.1038/cddis.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamagata K., Sone N., Suguyama S., Nabika T. Different effects of arginine vasopressin on high-mobility group box 1 expression in astrocytes isolated from stroke-prone spontaneously hypertensive rats and congenic SHRpch1_18 rats. Int. J. Exp. Pathol. 2016;97(2):97–106. doi: 10.1111/iep.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Chen B., Li Y., Zhang L., Wang Y., Yang S., Xiao X., Qin Q.A.-O. The use of renin-angiotensin-aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: a systematic review and meta-Analysis. J. Med. Virol. 2021;93(3):1370–1377. doi: 10.1002/jmv.26625. [DOI] [PubMed] [Google Scholar]
- 68.Darif D., Hammi I., Kihel A., El Idrissi Saik I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb. Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akihiko Kato, Janet D.Klein, Chi Zhang, Jeff M.Sands. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am. J. Physiol. -Ren. Physiol. 2000;279:F835–F840. doi: 10.1152/ajprenal.2000.279.5.F835. [DOI] [PubMed] [Google Scholar]
- 70.Passaglia P., de Lima Faim F., Batalhão M.E., Stabile A.M., Bendhack L.M., Antunes-Rodrigues J., Lacchini R., Capellari Carnio E. Central administration of angiotensin-(1-7) improves vasopressin impairment and hypotensive response in experimental endotoxemia. Cells. 2021;10(1):105. doi: 10.3390/cells10010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukerjee S., Gao H., Xu J., Sato R., Zsombok A., Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74(5):1181–1191. doi: 10.1161/HYPERTENSIONAHA.119.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gubbi S., Nazari M.A., Taieb D., Klubo-Gwiezdzinska J., Pacak K. Catecholamine physiology and its implications in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:978–986. doi: 10.1016/S2213-8587(20)30342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim Y.B., Kim Ys Fau - Kim W.B., Kim Wb Fau - Shen F.-Y., Shen Fy Fau - Lee S.W., Lee Sw Fau - Chung H.J., Chung Hj Fau - Kim J.S., Kim Js Fau - Han H.C., Han Hc Fau - Colwell C.S., Colwell Cs Fau - Kim Y.I., Kim Y.I. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ. Res. 2013;113(12):1296–1307. doi: 10.1161/CIRCRESAHA.113.301814. [DOI] [PubMed] [Google Scholar]
- 74.Kim J.S., Kim W.B., Kim Y.B., Lee Y., Kim Y.S., Shen F.Y., Lee S.W., Park D., Choi H.J., Hur J., Park J.J., Han H.C., Colwell C.S., Cho Y.W., Kim Y.I. Chronic hyperosmotic stress converts GABaergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J. Neurosci. 2011;31(37):13312–13322. doi: 10.1523/JNEUROSCI.1440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rondon-Berrios H., Berl T. Vasopressin receptor antagonists in hyponatremia: uses and misuses. Front. Med. 2017;4:141. doi: 10.3389/fmed.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedman B., Cirulli J. Hyponatremia in critical care patients: frequency, outcome, characteristics, and treatment with the vasopressin V2-receptor antagonist tolvaptan. J. Crit. Care. 2013;28(2):219. doi: 10.1016/j.jcrc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Gul S., Ozcan O., Asar S., Okyar A., Barıs I., Kavakli I.H. In silico identification of widely used and well-tolerated drugs as potential SARS-CoV-2 3C-like protease and viral RNA-dependent RNA polymerase inhibitors for direct use in clinical trials. J. Biomol. Struct. Dyn. 2020:1–20. doi: 10.1080/07391102.2020.1802346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao X., Wang C., Chang D., Wang Y., Dong X., Jiao T., Zhao Z., Ren L., Dela Cruz C.S., Sharma L., et al. Identification of potent and safe antiviral therapeutic candidates against SARS-CoV-2. Front. Immunol. 2020:11. doi: 10.3389/fimmu.2020.586572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X., Luo R.-H., Yao Z., Zheng C., Tang Q., Pang W.-Y., Wang F., Yang L., Xiong S., Yongtang Z. Tolvaptan, an FDA-approved drug, inhibits zika virus infection both in vitro and in vivo. J. Chin. Pharm. Sci. 2021;30:218–229. [Google Scholar]
- 80.Takanori Yamazaki, Yasukatsu Izumi, Yasuhiro Nakamura, Takehiro Yamaguchi, Akihisa Hanatani, Kenei Shimada, Hiroshi Iwao, Minoru Yoshiyama. Correction. Circulation. 2015;131:535. doi: 10.1161/circ.126.suppl_21.A9412. (doi:doi:) [DOI] [Google Scholar]