Abstract
Background: The association between fibroblast growth factor 21 (FGF21) and cardiovascular disease (CVD) risk remains unclear. We conducted this systematic review and meta-analysis to evaluate the association between FGF21 and CVDs, and relevant vascular parameters.
Methods: PubMed and Web of Science databases were systematically searched to identify relevant studies published before March 2021. The FGF21 concentration was compared between individuals with and without CVDs. The effect of FGF21 on CVD risk was assessed by using hazard ratio (HR) and odds ratio (OR). The association between FGF21 and vascular parameters was assessed by Pearson's r. Study quality was assessed using Newcastle–Ottawa Scale and Joanna Briggs Institution Checklist.
Results: A total of 29,156 individuals from 30 studies were included. Overall, the serum FGF21 concentration was significantly higher in CVD patients (p < 0.001), especially for coronary artery disease (CAD) (p < 0.001) and hypertension (p < 0.001). The pooled OR (p = 0.009) and HR (p < 0.001) showed that the risk of CVDs increased with FGF21. The linear association between FGF21 and vascular parameters, including pulse wave velocity (r = 0.32), carotid intima-media thickness (r = 0.21), ankle-brachial index (r = 0.33), systolic blood pressure (r = 0.13), and diastolic blood pressure (r = 0.05), was insignificant. The incidence of overall CVDs (p = 0.03) was significantly higher in individuals with higher FGF21 levels.
Conclusion: High-level serum FGF21 concentration is closely associated with an increased risk of CVDs, which may be independent of vascular parameters. A standard FGF21 classification threshold needs to be established before clinical use for CVD risk assessment.
Systematic Review Registration:https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=241968, identifier: CRD42021241968.
Keywords: fibroblast growth factor 21, coronary heart disease, hypertension, cardiovascular disease, risk factor, meta-analysis
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death globally and are composed of heart and blood vessel diseases. According to data from the World Health Organization, CVDs caused 17.9 million deaths in 2016, representing 31% of all global deaths (1). Early identification of risk factors to prevent or treat CVDs is very important for reducing morbidity. Various biomarkers have been investigated for their roles in the diagnosis and prognosis of CVDs, including blood lipids, blood glucose, weight, and age (2).
Fibroblast growth factors (FGFs) are a family of signaling proteins, in which FGF21 is a metabolic regulating hormone in energy homeostasis. Due to the lack of a heparin binding domain, FGF21 can be released in the circulation and function in an endocrine manner (3, 4). Because of its ability to regulate carbohydrate and lipid metabolism, FGF21 is considered to have multiple beneficial effects on major cardiovascular risk factors, such as hyperlipidemia, obesity, and diabetes (2). In addition, an increasing number of studies evaluated the potential role of FGF21 as a CVD biomarker. Among them, some studies reported a significant association between FGF21 and CVDs, while others found the association insignificant (5). Therefore, we conducted this systematic review and meta-analysis to evaluate the association between FGF21 and CVDs, especially for those closely related to metabolic abnormalities including coronary heart disease (CHD) or coronary artery disease (CAD), atrial fibrillation, cerebral infarction, and hypertension (2). We also evaluated the relationship of FGF21 with vascular parameters, including carotid intima-media thickness (cIMT), pulse wave velocity (PWV), ankle-brachial index (ABI), and blood pressure (BP).
Materials and Methods
This work was executed in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-analysis (PRISMA) guidelines (6). It was also registered in the International Prospective Register of Systematic Reviews (PROSPERO) before screening studies for inclusion (ID: CRD42021241968).
Literature Search
We conducted a systematic literature search by searching PubMed and Web of Science in March 2021. Studies that assessed the association between serum FGF21 concentration and CVDs and relative vascular parameters were identified through full-text review. The following terms and their combinations were employed: “fibroblast growth factor 21,” ”FGF21,” “coronary artery disease,” “coronary heart disease,” “artery stiffness,” “aortic aneurysm,” “hypertension,” “blood pressure,” ”pulse wave velocity,” “atherosclerosis,” “ischemic heart disease,” “cerebral infarction,” “myocardial infarction,” “angina,” “atrial fibrillation,” “cardiovascular,” ”cardiac,” ”cardio-ankle vascular index,” “carotid intima-media thickness,” “ankle-brachial index,” and “flow-mediated dilatation.”
Selection Criteria
The inclusion criteria were as follows: (1) studies that assessed the association between serum FGF21 concentration and CVDs, including CHD or CAD, atrial fibrillation, cerebral infarction and hypertension, or relative vascular parameters, including cIMT, PWV, ABI, BP, cardio-ankle vascular index, and flow-mediated dilatation; (2) the results contained at least one set of the following statistics: (a) mean with standard deviation or median with quartile of serum FGF21 concentration in patients with or without CVDs, (b) odds ratios (ORs) or hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) of serum FGF21 concentration and incidence of CVDs, (c) Pearson correlation coefficient (Pearson's r) of serum FGF21 concentration and vascular parameters, and (d) incidence of CVDs in individuals with different serum FGF21 levels; and (3) adult clinical studies that were published in English.
The exclusion criteria were as follows: (1) studies that were reviews, letters, meeting abstracts, case reports, commentary, or editorials; (2) studies that only reported on rheumatic heart disease, cardiomyopathy, or microvascular disease, and not CHD, atrial fibrillation, cerebral infarction, or hypertension; (3) duplicate studies with overlapping data; (4) studies that reported invalid data that could not be pooled; and (5) studies on pregnant women.
According to the selection criteria, the initial screening of studies was based on titles and abstracts. Then, the full texts of the potential studies were assessed. An additional manual search of references from identified studies was also performed. All studies were independently screened by two reviewers (YZ and JY). A third researcher (NY) was consulted to resolve disagreements.
Data Extraction and Quality Assessment
Two reviewers independently extracted data from the included studies. Basic information and patient baseline characteristics of all studies were extracted. To assess the association between serum FGF21, CVDs, and vascular parameters, the following data were extracted: (1) mean with standard deviation or median with quartile of serum FGF21 concentration; (2) ORs or HRs with corresponding 95% CIs; (3) Pearson's r; and (4) incidence of CVDs.
Quality assessment was independently performed by two reviewers (ZQ and HN). Discrepancies were resolved by discussion with a third reviewer (ZY). The quality of cohort studies and case–control studies was assessed by using the Newcastle–Ottawa Quality Assessment Scale (NOS) (7). Studies scoring > 5 were considered to be high-quality. The quality of cross-sectional studies was assessed by using the Joanna Briggs Institution (JBI) Checklist for Analytical Cross-Sectional Study (joannabriggs.org/research/critical?appraisal-tools.html). Publication bias was assessed by funnel plots if the number of included cohorts was ≥10. Publication bias was considered to be significant if the funnel plot was asymmetric.
Data Analysis
The mate analysis was performed by using RevMan 5.3 (the Nordic Cochrane Center, Copenhagen, Denmark). To achieve conservative results, a random-effects model was employed for pooled analysis. Heterogeneity was tested by using the Chi-squared test and I2 statistic. p < 0.05 or I2 > 50% indicated that the heterogeneity was significant. The overall effects were determined by the Z-test, and p < 0.05 was considered statistically significant. Subgroup analysis was conducted according to specific CVDs or disease outcomes.
Due to the differences in the number of FGF21 levels among studies, we chose to extract the CVD incidence from the individuals with the highest or lowest FGF21 level in each study.
Medians with quartiles were transformed into means with standard deviations for pooled estimates by using the webpage tool in the BOX-COX manner developed by McGrath et al. (8).
Pearson's r was transformed into Fisher's Z-value for pooled estimates. The resulting value was then weighted with the inverse of the variance of the correlation coefficients. The 95% CI of the pooled weighted Fisher's Z coefficients was also calculated, after which all of the values were back-transformed into r using the following formula (9). The linear association was considered to be very high, high, moderate, low, and irrelevant when summary |r| was larger than 0.8, between 0.6 and 0.8, between 0.4 and 0.6, between 0.2 and 0.4, and smaller than 0.2, respectively.
Results
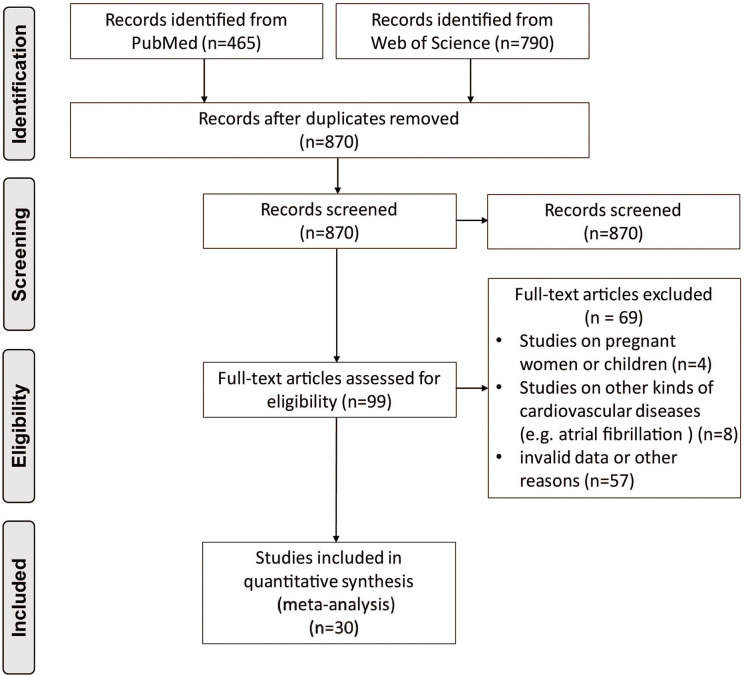
After removing duplicate articles, 870 articles were identified in the initial database search. After screening titles and abstracts, 99 articles remained for further full-text evaluation. Finally, 30 articles with 29,156 individuals were included in the meta-analysis (10–39). The flow diagram of study screening is shown in Figure 1. Tables 1–4 summarize the basic information and patient baseline characteristics of these studies. Supplementary Table 1 summarizes the patient selection criteria of these studies. Among these studies, 14 reported serum FGF21 concentration in patients with or without CVDs (10–23), 14 reported ORs or HRs of serum FGF21 concentration and incidence of CVDs (11, 14, 17, 18, 20, 24–32), 8 reported Pearson's r of serum FGF21 concentration and vascular parameters (12, 15, 16, 25, 30, 33–35), and 6 reported incidence of CVDs in individuals with different serum FGF21 levels (27, 30, 36–39).
Figure 1.
Preferred reporting items for systemic reviews and meta-analysis flow diagram of literature screening.
Table 1.
Baseline characteristics of studies reported serum FGF21 concentration in patients with or without CVDs.
| References | Region | Study design | Disease | CVDs group (n) | Control group (n) | Male (%) | Age (years) # | FGF21 concentration (pg/ml)* | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CVDs | Control | CVDs | Control | CVDs | Control | ||||||
| Wu et al. (10) | China | Cross-section | Stable CAD | 116 | 45 | 72.4 | 71.1 | 63.4 ± 9.0 | 61.2 ± 8.7 | ||
| Wu et al. (11)## | China | Cross-section | Developed IHD | 36 | 608 | 55.6 | 38.8 | 71.6 ± 14.1 | 57.6 ± 13.5 | 479.5 (302.4–627.0) | 325.2 (189.0–498.9) |
| Atherosclerosis | 186 | 185 | 61.8 | 51.4 | 58.6 ± 8.4 | 54.4 ± 9.0 | 266.7 (135.5–415.2) | 198.4 (99.9–373.6) | |||
| Basurto et al. (13)## | Mexico | Cross-section | Subclinical atherosclerosis | 75 | 65 | 0 | 0 | 53.0 (49.0–61.0)* | 50.0 (46.0–53.0)* | 127.0 (46.9–200.0) | 185.2 (95.0–260.4) |
| Chen et al. (14)## | China | Prospective cohort | AMI | 183 | 55 | 84.7 | 60.0 | 63.6 ± 10.9 | 66.9 ± 9.5 | 143.8 (75.2–254.3) | 121.0 (57.1–179.6) |
| Cheng et al. (15)** | China | Cross-section | stable angina pectoris | 66 | 55 | 54.6 | 38.2 | 60.7 ± 9.5 | 59.4 ±9.2 | ||
| Trakarnvanich et al. (16)** | Thailand | Cross-section | Cardiovascular events | 12 | 78 | 64.4 | 47.2 ±11.8 | ||||
| Lee et al. (17) | China | Prospective cohort | Incident CHD | 147 | 3,381 | 66.7 | 51.1 | 65.6 ± 12.1 | 60.6 ± 12.8 | 222.7 (92.8–438.4) | 151.1 (75.6–274.6) |
| Zhang et al. (18)## | China | Cross-section | AMI | 55 | 45 | 81.0 | 56.3 | 64 ± 11 | 63 ± 10 | 25 (16–34) | 14 (11–20) |
| Kim et al. (19)## | Korea | Cross-section | CAD without diabetes | 30 | 30 | 70.0 | 76.7 | 60.5 ± 11.0 | 58.5 ± 10.4 | 277.1 (155.6–476.6) | 141.7 (73.9–180.9) |
| CAD with diabetes | 30 | 30 | 20.0 | 30.0 | 61.7 ± 11.0 | 63.1 ± 10.4 | 278.5 (190.5–875.1) | 224.5 (146.8–337.4) | |||
| Semba et al. (22)## | USA | Case-control | Hypertension | 235 | 509 | 54.5 | 47.7 | – | – | 269 (161–457) | 208 (117–335) |
| Shen et al. (20) ## | China | Cross-section | CAD without NAFLD | 136 | 47 | 72.1 | 48.9 | 68.2 ± 9.9 | 64.6 ± 9.9 | ||
| CAD with NAFLD | 43 | 27 | 62.8 | 58.3 | 65.5 ± 10.9 | 61.3 ± 7.7 | |||||
| Chow et al. (12)## | China | Cross-section | Hypertension | 363 | 307 | 45.2 | 39.4 | 58.2 ± 12.9 | 283.0 (175.8–455.3) | 197.3 (126.2–330.3) | |
| Lee et al. (21) | Korea | Retrospective cohort | CAD | 60 | 129 | 57.1 | 50.3 ± 7.6 | 62.1 ± 9.8 | |||
| Lin et al. (23) | USA | Cross-section | CHD | 135 | 61 | 49.6 | 50.8 | 69 ± 5.8 | 68.6 ± 10.8 | ||
FGF21, fibroblast growth factor 21; CVD, cardiovascular disease; CAD, coronary artery disease; IHD, ischemic heart disease; AMI, acute myocardial infarction; CHD, coronary heart disease; NAFLD, non-alcoholic fatty liver disease.
Mean ± standard deviation.
Median (interquartile range).
Original data was median with quartile.
Data was transformed by the logarithm of 10.
Table 4.
Baseline characteristics of studies reported incidence of CVDs in individuals with different FGF21 levels.
| References | Region | Study design | Disease | Male (%) | Age (year)# | FGF21 level (pg/mL) classification and case (n) |
|---|---|---|---|---|---|---|
| Gan et al. (36) | China | Prospective cohort | Hypertension, CHD (ACS and stent implantation) | 62.9 | 54.2 ± 13.7 | ≤ 103.8 (283), 108.6–184.9 (283), 199.3–271.2 (283), ≥276.1 (283) |
| Wu et al. (27) | Australia | Prospective cohort | MI | 66.9 | 68.6 ± 12.9 | <113.7 (177), 113.7–227.3 (177), ≥227.3 (177) |
| Kohara et al. (37) | Japan | Cross-section | Total CVD | 58.9 | 66.1 ± 12.9 | 1,029 (704–1,518) (44) 2,989 (2,184–5,973) (46) |
| Rusu et al. (38) | Romania | Prospective cohort | Total CVD | 57.1 | 59.9 ± 12.5 | <19.75 (17), ≥34.55 (18) |
| Ong et al. (39) | Australia | Prospective cohort | Total CVD | 62.7 | 62.3 ± 6.9 | <239.3 (3,232), 239.3–412.8 (3,233), ≥412.8 (3,232) |
FGF21, fibroblast growth factor 21; CHD, coronary heart disease; ACS, acute coronary syndrome; MI, myocardial infraction; CVD, cardiovascular disease.
Mean ± standard deviation.
Table 2.
Baseline characteristics of studies reported serum FGF21 concentration on the risk of CVDs.
| References | Region | Study design | Disease | Outcome | Cases (n) | Male (%) | Age (years)# | Median follow-up (range) |
|---|---|---|---|---|---|---|---|---|
| Wu et al. (11) | China | Prospective cohort | Total ASCVD | HR | 705 | 38.8 | 57.6 ± 13.5 | 74 months |
| Subclinical atherosclerosis | OR | 371 | 56.6 | 56.5 ± 8.9 | – | |||
| Ong et al. (24) | Australia | Prospective cohort | Total CVD, hard CVD | HR | 5,767 | 47.9 | 62.6 ± 10.2 | 14 years |
| Ong et al. (26) | Australia | Prospective cohort | MCVE | HR | 1,992 | 80.1 | 61.3 ± 8.9 | 4.9 years |
| Yafei et al. (25) | Egypt | Cross-section | Subclinical atherosclerosis | OR | 120 | 36.7 | 51.1 ± 7.7 | – |
| Chen et al. (14) | China | Prospective cohort | MACE | HR | 238 | 79.0 | 64.4 ± 10.7 | 24 months |
| Wu et al. (27) | China | Prospective cohort | MACE | HR | 88 | 66.9 | 68.6 ±12.9 | 2.3 ± 1.3 years (mean ± SD) |
| Shen et al. (28) | China | Prospective cohort | MACE | HR | 169 | 65.5 | – | 57 months |
| Shen et al. (29) | China | Prospective cohort | Cardiac death | HR | 218 | 65.6 | 66.3 ± 10.1 | 5.0 years |
| Lee et al. (17) | China | Prospective cohort | Incident CHD | HR | 3,528 | 51.8 | 60.8 ± 12.8 | 3.8 (2.8–5.0) years |
| Li et al. (30) | China | Prospective cohort | Cardiac death | HR | 1,668 | 65.5 | 4.9 years | |
| Zhang et al. (18) | China | Cross-section | AMI | OR | 100 | 78.0 | – | – |
| Xiao et al. (31) | China | Cross-section | Male subclinical atherosclerosis | OR | 107 | 100 | 52.6 ± 9.7 | – |
| Female subclinical atherosclerosis | OR | 105 | 0 | 55.6 ± 6.9 | ||||
| Shen et al. (20) | China | Cross-section | CAD | OR | 253 | 64.8 | 66.3 ± 10.1 | – |
| Lenart-Lipinska et al. (32) | Poland | Prospective cohort | CVD | HR | 87 | 51.7 | 61 (57–66)* | 24 months |
| CVD mobidity | HR |
FGF21, fibroblast growth factor 21; ASCVD, atherosclerotic cardiovascular disease; CVD, cardiovascular disease; MCVE, major cardiovascular event; MACE, major adverse cardiovascular event; CHD, coronary heart disease; AMI, acute myocardial infraction; CAD, coronary artery disease.
Mean ± standard deviation.
Median (interquartile range).
Table 3.
Baseline characteristics of studies reported linear association of serum FGF21 concentration and vascular parameters.
| References | Region | Study design | Vascular parameters | Male (%) | Age (years)# | Cases (n) |
|---|---|---|---|---|---|---|
| Lee et al. (33) | Singapore | Cross-section | PWV | 53.8 | 42.2 ± 15.8 | 78 |
| Yamamoto et al. (35) | Japan | Cross-section | SBP, DBP | 21 | 76.2 ± 8.0 | 73 |
| Sunaga et al. (34) | Japan | Cross-section | SBP, DBP | 79.6 | 70.5 ± 9.7 | 93 |
| Yafei et al. (25) | Egypt | Cross-section | PWV, cIMT, ABI, SBP, DBP | 36.7 | 51.1 ± 7.7 | 120 |
| Cheng et al. (15) | China | Cross-section | SBP, DBP | 55.3 | 60.1 ± 8.9 | 197 |
| Trakarnvanich et al. (16) | Thailand | Cross-section | cIMT | 64.4 | 47.2 ± 11.8 | 90 |
| Li et al. (30) | China | Prospective cohort | SBP, DBP | 65.5 | 63.5 ± 1.2 | 1,668 |
| Chow et al. (12) | China | Cross-section | cIMT | 42.5 | 58.2 ± 12.9 | 670 |
FGF21, fibroblast growth factor 21; PWV, pulse wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; cIMT, carotid intima-media thickness; ABI, ankle-brachial index.
Mean ± standard deviation.
According to NOS, all cohort studies and case–control studies were considered to be high quality (Supplementary Tables 2, 3). According to the JBI checklist, all cross-sectional studies were also considered to be high quality (Supplementary Table 4).
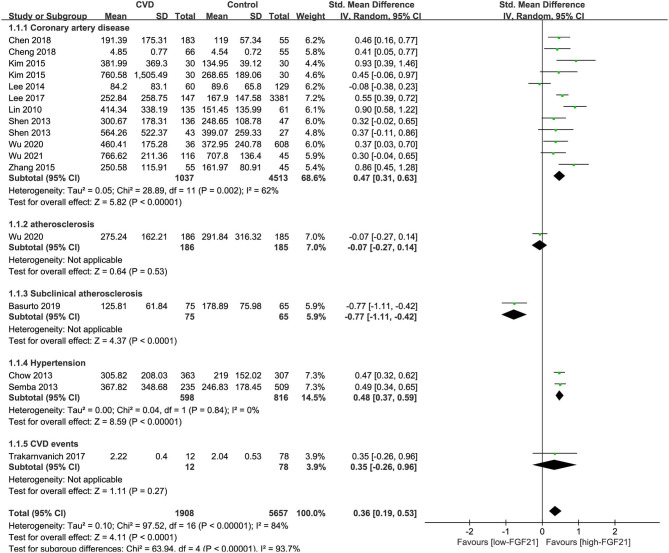
Differences in Serum FGF21 Concentration in Individuals With or Without CVDs
The median serum FGF21 concentration in patients with or without CVDs is shown in Table 1. Three studies also provided serum FGF21 concentration of each patient in scatter plots (10, 14, 23) (Supplementary Figure 1). Among 14 studies, 9 studies reported that serum FGF21 concentration was significantly higher in patients with CVDs, including CAD and hypertension (11, 12, 14, 15, 17–19, 22, 23). However, one study reported that serum FGF21 concentration was significantly lower in patients with subclinical atherosclerosis (13). Overall, the serum FGF21 concentration was significantly higher in CVD patients than in those without CVDs [standard mean difference (SMD) = 0.58, 95% CI: 0.33–0.84, p < 0.001], especially for CAD (SMD = 0.75, 95% CI: 0.42–1.09, p < 0.001) and hypertension (SMD = 0.48, 95% CI: 0.37–0.59, p < 0.001) (Figure 2). The heterogeneity was significant among studies. Publication bias was assessed by a funnel plot, which indicated moderate publication bias (Supplementary Figure 2).
Figure 2.
Forest plot of serum FGF21 concentration in individuals with or without CVDs. FGF21, fibroblast growth factor 21; CVD, cardiovascular disease. Mean value refers to the mean serum FGF21 concentration in each group.
Association Between Serum FGF21 Concentration and Prevalence of CVDs
Five studies reported the ORs of FGF21 concentration and CVDs by logistic regression (Figure 3A). The pooled results showed that the risk of CAD (OR = 5.99, 95% CI: 1.13–31.77, p = 0.04) and overall CVDs (OR = 1.68, 95% CI: 1.14–2.48, p = 0.009) increased with FGF21. The heterogeneity was significant among studies.
Figure 3.
Forest plot of serum FGF21 concentration on the risk of CVDs. (A) Odds ratio; (B) Hazard ratio. FGF21, fibroblast growth factor 21; CVD, cardiovascular disease.
Eleven studies reported the HRs of FGF21 concentration and CVDs (Figure 3B). The pooled results showed that the risk of hard CVD (HR = 1.43, 95% CI: 1.13–1.82, p = 0.003), total CVD (HR = 1.37, 95% CI: 1.20–1.56, p < 0.001), and cardiac death (HR = 2.65, 95% CI: 1.17–6.01, p = 0.02) increased with FGF21. The heterogeneity was significant among studies. Publication bias was assessed by a funnel plot, which indicated high publication bias (Supplementary Figure 3).
Linear Association Between FGF21 and Vascular Parameters
No association was observed between FGF21 and systolic BP (summary r = 0.13, Figure 4A) or diastolic BP (summary r = 0.05 Figure 4B). For the linear association between FGF21 and PWV, cIMT, and ABI, Fisher's Z was 0.32, 0.21, and −0.33, respectively (Figures 4C–E). The corresponding summary r values were 0.31, 0.24, and −0.31, which indicates a low association.
Figure 4.
Forest plot of linear association between FGF21 and vascular parameters. (A) Systolic blood pressure; (B) diastolic blood pressure; (C) pulse wave velocity; (D) carotid intima-media thickness; (E) ankle-brachial index. FGF21, fibroblast growth factor 21. Linear association between FGF21 and vascular parameters was assessed by Pearson's r. Pearson's r was transformed into Fisher's Z-value for pooled estimates.
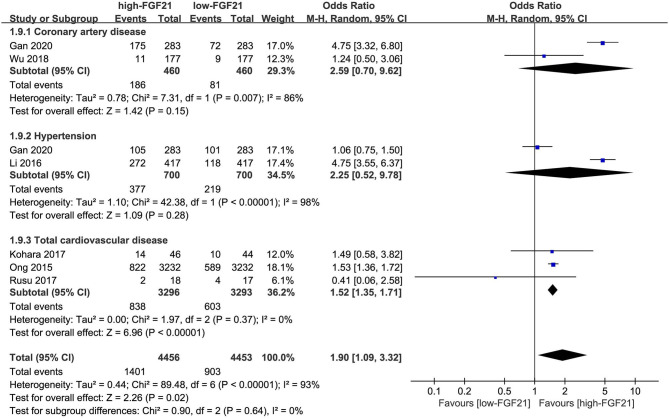
Incidence of CVDs in Individuals With Different FGF21 Levels
The incidences of overall CVDs (OR = 2.10, 95% CI: 1.09–4.06, p = 0.03) and hypertension (OR = 4.75, 95% CI: 3.55–6.37, p < 0.001) were significantly higher in individuals with higher FGF21 levels (Figure 5). However, the difference in CAD incidence between individuals with different FGF21 levels was insignificant (p = 0.16).
Figure 5.
Forest plot of incidence of cardiovascular disease in individuals with different FGF21 levels. FGF21, fibroblast growth factor 21. Events refer to the numbers of corresponding disease that were diagnosed in each group.
Discussion
FGF21 is involved in glucose and lipid metabolism. Studies have reported that FGF21 is increased in patients with non-alcoholic fatty liver disease, obesity, and diabetes (40). Therefore, FGF21 has been considered as a new biomarker for metabolic syndrome. For CVDs, metabolic syndrome is a well-known precursor (2), which suggests a possible link of FGF21 to CVDs. Our meta-analysis also demonstrated that FGF21 was increased in patients with CVDs, which may be caused by a compensatory response to underlying metabolic stress (41). Some studies reported the cutoff value (ranged from 123.0 to 321.5 pg/ml) of serum FGF21 as a predictor for CVDs according to Youden index (11, 14, 17, 25, 28, 29) (Supplementary Table 5).
Experimental data from animal studies revealed the association between FGF21 and CVDs. A study demonstrated that the administration of exogenous FGF21 significantly improved lipid metabolic disorders and reduced atherosclerotic plaque areas in animal models (42). In detail, FGF21 can reduce cholesterol synthesis by suppressing hepatic sterol regulatory element-binding protein 2 (43), increase lipoprotein catabolism in adipose tissue, and reduce hepatic very low-density lipoprotein export (44). These critical events are major contributors to FGF21's ability to enhance lipid profiles, which are partly mediated by CD36 and lipoprotein lipase. The mechanisms of atherosclerosis prevention induced by FGF21 may be associated with suppression of endoplasmic reticulum stress-mediated apoptosis (45). FGF21 can stimulate adiponectin secretion, which has a large effect on the inhibition of neointima formation through inhibition of canonical events, including macrophage infiltration, and smooth muscle proliferation (43, 45, 46). These events are generally believed to cause endoplasmic reticulum stress within the vasculature. The antioxidative function of FGF21 is also involved in the therapeutic effect in atherosclerotic Wistar rats, including increased levels of superoxide dismutase, reduced form of glutathione, and reduced form of malondialdehyde (47). Our previous study also demonstrated that FGF21 can protect vascular endothelial cells from H2O2-induced premature cell senescence and intracellular accumulation of reactive oxygen species through SIRT1 (48). In addition, Wang et al. also demonstrated the cardioprotective effects of FGF21 against doxorubicin-induced toxicity through the SIRT1/LKB1/AMPK pathway (49). These results indicate that FGF21 is not only a biomarker of CVDs but may also have a protective effect on the cardiovascular system. Lin et al. reported that serum FGF21 increased in apoE−/− mice, and FGF21 deficiency enhanced atherosclerotic deterioration and mortality (43). Considering the mechanisms of atherosclerosis prevention induced by FGF21, the increased FGF21 may not be the basis for atherosclerotic pathogenesis while it may compensate for the increase during atherosclerosis and bring beneficial effects instead (5).
Based on strong preclinical evidence of the therapeutic effect of FGF21 in metabolic syndrome, clinical studies were conducted to evaluate the effect of FGF21 variants or analogs. PF-05231023, an FGF21 analog, was reported to have beneficial effects on body weight, lipoprotein profile, and adiponectin concentrations in overweight/obese subjects with type 2 diabetes (50). During the treatment of diabetic patients using at least 25 mg of PF-05231023 once a week, the minimum observed concentration was 0.768 μg/ml. It can also significantly lower triglycerides in the absence of weight loss in monkeys (51). LY2405319, another FGF21 analog, was found to improve lipid levels, lipoprotein profile, body weight, fasting insulin, and adiponectin levels in obese patients with type 2 diabetes in a randomized, placebo-controlled, double-blind clinical trial (52). During the treatment, average steady-state circulating plasma concentrations of LY2405319 on day 28 were 17.5 ± 4 ng/ml in subjects who received the 3-mg daily dose, and levels were 67.3 ± 25 ng/ml and 150 ± 49 ng/ml in subjects treated with 10 and 20 mg, respectively. Therefore, administration of exogenous supra-physiological doses of FGF21 variants or analogs may also provide a therapeutic benefit on CVDs. Unfortunately, no clinical study has directly evaluated the therapeutic effects of FGF21 analogs on CVDs at present. In our study, the risk of CVDs increased with FGF21, which also indicates the need for supra-physiological doses of FGF21 to achieve therapeutic efficacy. Clinical evidence is needed to investigate the role and the effective dose of FGF21 analogs in the prevention or treatment of CVDs as a novel therapeutic agent.
Arterial stiffness is one of the earliest indicators of changes in vascular wall structure and function, which can be assessed by using various indicators, including PWV, ABI, cardio-ankle vascular index, and cIMT (53). Increased arterial stiffness has a major effect on pulse pressure, which directly leads to abnormal blood pressure (54). A large number of clinical studies have demonstrated an association between arterial stiffness and atherosclerotic burden as well as between arterial stiffness and incident cardiovascular events. The association between arterial stiffness and atherosclerosis might be incidental, as the two processes occur at similar sites of the arterial tree and both progress with age, or might be explained by the impact of common risk factors, such as metabolic syndrome (55). However, the differences between atherosclerosis and arteriosclerosis should still be noted. Arteriosclerosis mainly refers to stiffening of the normally flexible walls due to loss of elasticity of the arterial musculature. The loss or disorder of elastin is the main reason for the thickening of the arterial walls. In atherosclerosis, there is a gradual increase in the deposition of plaque (consisting of calcium, white blood cells and clumps of platelets, cholesterol, and lipids) within the lumen leading to narrowing or complete blockage of the artery. The development of atherosclerosis is closely related to hyperglycemia and hyperlipidemia (56). Therefore, FGF21 has a stronger association with atherosclerosis than arteriosclerosis. In our meta-analysis, the association between FGF21 and vascular parameters was weak. Therefore, FGF21 may act as a CVD biomarker independent of vascular parameters, such as PWV, ABI, cIMT, and BP.
Several limitations of our meta-analysis must be taken into consideration. First, due to different patient selection criteria and baseline characteristics, such as age and gender, significant heterogeneity was observed in our meta-analysis. Second, the definition of CVDs and CHD varied among the included studies. Third, the follow-up time varied among each included study, which may bring bias in the estimation of pooled HR of FGF21 concentration and CVDs. Fourth, our meta-analysis only included CHD, hypertension, and overall CVDs. Studies on other CVDs such as peripheral arterial disease, rheumatic heart disease, atrial fibrillation, cerebral infarction, cardiomyopathy, or microvascular disease were not included. Fifth, original data reported in articles were transformed for meta-analysis, especially for data of median value with quartile. Those original data with skewed distribution may not be suitable for meta-analysis. Sixth, the threshold for FGF21 level classification varied among studies. A standard FGF21 classification threshold needs to be established before clinical use for CVD risk assessment. Several modalities were applied to reduce these limitations. First, we conducted a systematic, comprehensive search in two databases. Second, we strictly stipulated the inclusion criteria, eliminating the bias caused by some potential confounding factors, and the data were independently extracted by two reviewers. Third, we conducted a subgroup analysis of specific CVDs.
Conclusion
High-level serum FGF21 concentration is closely associated with an increased risk of CVDs, which may be independent of vascular parameters, including cIMT, PWV, ABI, and BP. A standard FGF21 classification threshold needs to be established before clinical use for CVD risk assessment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CZ and LZ: conceptualization. YZ: methodology. JY and NY: investigation. ZQ and HN: data curation. ZY, DY, and XW: formal analysis. YZ, LR, and YH: writing, review, and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the patients included in the studies we reviewed.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (Grant Numbers: 81873533 and 81570411; principal investigator: LZ) and the National Key Research and Development Program of China (Grant Number: 2020YFC2008000; principal investigator: CZ).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.705273/full#supplementary-material
References
- 1.Cardiovascular Diseases (CVDs) . Available online at: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed June 11, 2021).
- 2.Domouzoglou EM, Naka KK, Vlahos AP, Papafaklis MI, Michalis LK, Tsatsoulis A, et al. Fibroblast growth factors in cardiovascular disease: the emerging role of FGF21. Am J Physiol Heart Circ Physiol. (2015) 309:H1029–38. 10.1152/ajpheart.00527.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. (2011) 149:121–30. 10.1093/jb/mvq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. (2004) 20:563–9. 10.1016/j.tig.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 5.Cheng P, Zhang F, Yu L, Lin X, He L, Li X, et al. Physiological and pharmacological roles of FGF21 in cardiovascular diseases. J Diabetes Res. (2016) 2016:1540267. 10.1155/2016/1540267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. (2003) 7:iii-x:1–173. 10.3310/hta7270 [DOI] [PubMed] [Google Scholar]
- 8.McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020). 10.1177/0962280219889080. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; (2009). p. 41–3. [Google Scholar]
- 10.Wu Y, Chen Z, Duan J, Huang K, Zhu B, Yang L, et al. Serum levels of FGF21, beta-Klotho, and BDNF in Stable coronary artery disease patients with depressive symptoms: a cross-sectional single-center study. Front Psychiatry. (2021) 11:587492. 10.3389/fpsyt.2020.587492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Qian L, Zhang L, Zhang J, Zhou J, Li Y, et al. Fibroblast growth factor 21 is related to atherosclerosis independent of nonalcoholic fatty liver disease and predicts atherosclerotic cardiovascular events. J Am Heart Assoc. (2020) 9:e015226. 10.1161/JAHA.119.015226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, et al. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. (2013) 33:2454–9. 10.1161/ATVBAHA.113.301599 [DOI] [PubMed] [Google Scholar]
- 13.Basurto L, Gregory MA, Hernandez SB, Sanchez-Huerta L, Martinez AD, Manuel-Apolinar L, et al. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp Gerontol. (2019) 124:110624. 10.1016/j.exger.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Lu N, Zheng M. A high circulating FGF21 level as a prognostic marker in patients with acute myocardial infarction. Am J Transl Res. (2018) 10:2958–66. [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J, Su X, Qiao L, Zhai C, Chen W. Circulating level of fibroblast growth factor 21 is independently associated with the risks of unstable angina pectoris. Biosci Rep. (2018) 38:BSR20181099. 10.1042/BSR20181099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trakarnvanich T, Prommool S, Kurathong S, Teepprasan T, Wang Y. Associations among cardio-ankle vascular index, carotid intima-media thickness, and fibroblast growth factor-21 levels in kidney transplant patients. Transplant Proc. (2017) 49:1791–6. 10.1016/j.transproceed.2017.06.038 [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Woo YC, Chow WS, Cheung C, Fong C, Yuen M, et al. Role of circulating fibroblast growth factor 21 measurement in primary prevention of coronary heart disease among chinese patients with type 2 diabetes mellitus. J Am Heart Assoc. (2017) 6:e005344. 10.1161/JAHA.116.005344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Chu S, Ding W, Wang F. Serum level of fibroblast growth factor 21 is independently associated with acute myocardial infarction. PLoS ONE. (2015) 10:e0129791. 10.1371/journal.pone.0129791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WJ, Kim SS, Lee HC, Song SH, Bae MJ, Yi YS, et al. Association between serum fibroblast growth factor 21 and coronary artery disease in patients with type 2 diabetes. J Korean Med Sci. (2015) 30:586–90. 10.3346/jkms.2015.30.5.586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, et al. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol. (2013) 12:124. 10.1186/1475-2840-12-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, Lim S, Hong ES, Kim JH, Moon MK, Chun EJ, et al. Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clin Endocrinol. (2014) 80:57–64. 10.1111/cen.12134 [DOI] [PubMed] [Google Scholar]
- 22.Semba RD, Crasto C, Strait J, Sun K, Schaumberg DA, Ferrucci L. Elevated serum fibroblast growth factor 21 is associated with hypertension in community-dwelling adults. J Hum Hypertens. (2013) 27:397–9. 10.1038/jhh.2012.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS ONE. (2010) 5:e15534. 10.1371/journal.pone.0015534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong KL, Campbell S, Wu BJ, McClelland RL, Kokkinos J, Szklo M, et al. Relationship of fibroblast growth factor 21 with subclinical atherosclerosis and cardiovascular events: multi-ethnic study of atherosclerosis. Atherosclerosis. (2019a) 287:46–53. 10.1016/j.atherosclerosis.2019.06.898 [DOI] [PubMed] [Google Scholar]
- 25.Yafei S, Elsewy F, Youssef E, Ayman M, El-Shafei M. Fibroblast growth factor 21 association with subclinical atherosclerosis and arterial stiffness in type 2 diabetes. Diabetes Metab Syndr. (2019) 13:882–8. 10.1016/j.dsx.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 26.Ong KL, Hui N, Januszewski AS, Kaakoush NO, Xu A, Fayyad R, et al. High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the Treating to New Targets TNT Study). Metabolism. (2019b) 93:93–9. 10.1016/j.metabol.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Wu CH, Chou RH, Kuo CS, Huang PH, Chang CC, Leu HB, et al. Circulating fibroblast growth factor 21 is associated with subsequent renal injury events in patients undergoing coronary angiography. Sci Rep. (2018) 8:12425. 10.1038/s41598-018-35360-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Y, Zhang X, Xu Y, Xiong Q, Lu Z, Ma X, et al. Serum FGF21 is associated with future cardiovascular events in patients with coronary artery disease. Cardiology. (2018) 139:212–218. 10.1159/000486127 [DOI] [PubMed] [Google Scholar]
- 29.Shen Y, Zhang X, Pan X, Xu Y, Xiong Q, Lu Z, et al. Contribution of serum FGF21 level to the identification of left ventricular systolic dysfunction and cardiac death. Cardiovasc Diabetol. (2017) 16:106. 10.1186/s12933-017-0588-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Zhang Y, Ding D, Yang Y, Chen Q, Su D, et al. Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J Clin Endocrinol Metab. (2016) 101:4886–94. 10.1210/jc.2016-2308 [DOI] [PubMed] [Google Scholar]
- 31.Xiao Y, Liu L, Xu A, Zhou P, Long Z, Tu Y, et al. Serum fibroblast growth factor 21 levels are related to subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. (2015) 14:72. 10.1186/s12933-015-0229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenart-Lipinska M, Matyjaszek-Matuszek B, Gernand W, Nowakowski A, Solski J. Serum fibroblast growth factor 21 is predictive of combined cardiovascular morbidity and mortality in patients with type 2 diabetes at a relatively short-term follow-up. Diabetes Res Clin Pract. (2013) 101:194–200. 10.1016/j.diabres.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 33.Lee SY, Burns SF, Ng K, Stensel DJ, Zhong L, Tan F, et al. Pulse wave velocity is associated with increased plasma oxLDL in ageing but not with FGF21 and habitual exercise. Antioxidants. (2020) 9:221. 10.3390/antiox9030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunaga H, Koitabashi N, Iso T, Matsui H, Obokata M, Kawakami R, et al. Activation of cardiac AMPK-FGF21 feed-forward loop in acute myocardial infarction: role of adrenergic overdrive and lipolysis byproducts. Sci Rep. (2019) 9:11841. 10.1038/s41598-019-48356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto S, Koyama D, Igarashi R, Maki T, Mizuno H, Furukawa Y, et al. Serum endocrine fibroblast growth factors as potential biomarkers for chronic kidney disease and various metabolic dysfunctions in aged patients. Intern Med. (2020) 59:345–55. 10.2169/internalmedicine.3597-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan F, Huang J, Dai T, Li M, Liu J. Serum level of fibroblast growth factor 21 predicts long-term prognosis in patients with both diabetes mellitus and coronary artery calcification. Ann Palliat Med. (2020) 9:368–74. 10.21037/apm.2020.03.28 [DOI] [PubMed] [Google Scholar]
- 37.Kohara M, Masuda T, Shiizaki K, Akimoto T, Watanabe Y, Honma S, et al. Association between circulating fibroblast growth factor 21 and mortality in end-stage renal disease. PLoS ONE. (2017) 12:e0178971. 10.1371/journal.pone.0178971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rusu CC, Racasan S, Kacso IM, Moldovan D, Potra A, Tirinescu D, et al. The metabolic hormone FGF21 is associated with endothelial dysfunction in hemodialysis patients. Int Urol Nephrol. (2017) 49:517–23. 10.1007/s11255-016-1474-x [DOI] [PubMed] [Google Scholar]
- 39.Ong KL, Januszewski AS, O'Connell R, Jenkins AJ, Xu A, Sullivan DR, et al. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the fenofibrate intervention and event lowering in diabetes study. Diabetologia. (2015) 58:464–73. 10.1007/s00125-014-3458-7 [DOI] [PubMed] [Google Scholar]
- 40.Lakhani I, Gong M, Wong WT, Bazoukis G, Lampropoulos K, Wong SH, et al. Fibroblast growth factor 21 in cardio-metabolic disorders: a systematic review and meta-analysis. Metabolism. (2018) 83:11–7. 10.1016/j.metabol.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 41.Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol. (2013) 78:489–96. 10.1111/cen.12095 [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Lu Y, Fu K, Wang S, Zhao D, Peng H, et al. Impact of exogenous fibroblast growth factor 21 on atherosclerosis in apolipoprotein E deficient mice. Zhonghua Xin Xue Guan Bing Za Zhi. (2014) 42:126–31. 10.3760/cma.j.issn.0253-3758.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 43.Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. (2015) 131:1861–71. 10.1161/CIRCULATIONAHA.115.015308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metabolism. (2016) 23:441–53. 10.1016/j.cmet.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Qi YF, Chang JR, Lu WW, Zhang JS, Wang SP, et al. Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE(-/-) mice. Heart Vessels. (2015) 30:657–68. 10.1007/s00380-014-0557-9 [DOI] [PubMed] [Google Scholar]
- 46.Hui X, Feng T, Liu Q, Gao Y, Xu A. The FGF21-adiponectin axis in controlling energy and vascular homeostasis. J Mol Cell Biol. (2016) 8:110–9. 10.1093/jmcb/mjw013 [DOI] [PubMed] [Google Scholar]
- 47.Zhu W, Wang C, Liu L, Li Y, Li X, Cai J, et al. Effects of fibroblast growth factor 21 on cell damage in vitro and atherosclerosis in vivo. Can J Physiol Pharmacol. (2014) 92:927–35. 10.1139/cjpp-2014-0227 [DOI] [PubMed] [Google Scholar]
- 48.Yan J, Wang J, Huang H, Huang Y, Mi T, Zhang C, et al. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H2O2-induced premature senescence through SIRT1. Am J Transl Res. (2017) 9:4492–501. [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Wang Y, Zhang Z, Liu Q, Gu J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. (2017) 8:e3018. 10.1038/cddis.2017.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. (2016) 23:427–40. 10.1016/j.cmet.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 51.Kim AM, Somayaji VR, Dong JQ, Rolph TP, Weng Y, Chabot JR, et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metab. (2017) 19:1762–72. 10.1111/dom.13023 [DOI] [PubMed] [Google Scholar]
- 52.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. (2013) 18:333–40. 10.1016/j.cmet.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 53.Li X, Lyu P, Ren Y, An J, Dong Y. Arterial stiffness and cognitive impairment. J Neurol Sci. (2017) 380:1–10. 10.1016/j.jns.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 54.Safar ME. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. (2018) 15:97–105. 10.1038/nrcardio.2017.155 [DOI] [PubMed] [Google Scholar]
- 55.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. (2016) 77:1–7. 10.1016/j.vph.2015.11.083 [DOI] [PubMed] [Google Scholar]
- 56.Tolle M, Reshetnik A, Schuchardt M, Hohne M, van der Giet M. Arteriosclerosis and vascular calcification: causes, clinical assessment and therapy. Eur J Clin Invest. (2015) 45:976–85. 10.1111/eci.12493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.