Abstract
Accurate diagnosis of cribriform Gleason pattern 4 (CrP4) prostate adenocarcinoma (PCa) is important due to its independent association with adverse clinical outcomes and as a growing body of evidence suggests that it impacts clinical decision making in PCa management. To identify reproducible features for diagnosis of CrP4, we assessed interobserver agreement among 27 experienced urologic pathologists of 60 digital images from 44 radical prostatectomies (RP) that represented a broad spectrum of potential CrP4. The following morphologic features were correlated with the consensus diagnosis (defined as 75% agreement) for each image: partial vs. transluminal glandular bridging, intraglandular stroma, <12 vs. ≥12 lumina, well vs. poorly formed lumina, mucin (mucinous fibroplasia, extravasation, or extracellular pool), size (compared to benign glands and number of lumina), number of attachments with gland border by tumor cells forming a “glomeruloid-like” pattern, a clear luminal space along the periphery of gland occupying <50% of glandular circumference, central nerve, dense (cell mass occupying >50% of luminal space) vs. loose, and regular vs. irregular contour. Interobserver reproducibility for the overall diagnostic agreement was fair (k=0.40). Large CrP4 had better agreement (k=0.49) compared to small CrP4 (k=0.40). Transluminal bridging, dense cellular proliferation, a clear luminal space along the periphery of gland occupying <50% of gland circumference, lack of intraglandular mucin, and lack of contact between the majority of intraglandular cells with stroma were significantly associated with consensus for CrP4. In contrast, partial bridging, majority of intraglandular cells in contact with stroma, mucinous fibroplasia, only one attachment to the gland border by tumor cells forming a “glomeruloid-like” pattern, and a clear luminal space along the periphery of gland accounting for >50% of the glandular circumference were associated with consensus against CrP4. In summary, we identified reproducible morphological features for and against CrP4 diagnosis, which could be used to refine and standardize the diagnostic criteria for CrP4.
Keywords: Prostate adenocarcinoma, cribriform, gleason grade, grade group, reproducibility, consensus
Introduction
The term “cribriform” is derived from the Latin word cribrum (i.e. sieve). It describes glands composed of sheets of tumor cells that form cohesive rounded or irregularly shaped trabeculae with perforations or multiple “punched out” lumina [1,2]. There is growing evidence that cribriform pattern 4 (CrP4) prostate adenocarcinoma (PCa) in both biopsies and radical prostatectomies (RP) is associated with adverse clinical outcomes, including worse biochemical recurrence-free, metastasis-free, and cancer-specific survival than those without [3,4]. Specifically, CrP4 carries a much higher risk of disease progression compared to the other Gleason grade 4 patterns [5-7]. Among men with grade group (GG) 2 at biopsy, some studies have demonstrated a higher risk of failure in the presence of cribriform morphology, while patients without cribriform architecture carried the same risk as GG 1 cancer, implying that CrP4 diagnosis might affect clinical decision-making [4,8-10]. Whereas the value of cribriform architecture has mostly been studied for Gleason score (GS) 7 patients, some studies have demonstrated its independent prognostic value in men with GS 8 and GS 9-10 PCa [6,11,12]. However, the majority of studies addressing the significance of cribriform carcinoma have not distinguished between intraductal carcinoma of the prostate (IDC-P) and CrP4 to determine outcomes, and have referred to these lesions collectively as “cribriform growth” [2-4,10]. Cribriform PCa also has unique adverse molecular features with significantly more frequent PTEN and p27 loss at RNA and protein levels, defects in DNA repair genes, and higher frequency of mutational events similar to metastatic prostate cancer as opposed to non-cribriform Gleason pattern 4 PCa [3,13-17]. Due to multiple lines of evidence supporting the association with adverse outcomes, both the Genitourinary Pathology Society (GUPS) and the International Society of Urologic Pathology (ISUP) recommended reporting of CrP4 in needle biopsy and RP specimens [1,18].
Although the cribriform pattern has the best interobserver reproducibility among genitourinary pathologists (ranging from 54%-79%) compared with other Gleason 4 patterns, there is still a significant variability and ambiguity in diagnosing this pattern [19]. Specifically, differentiation of CrP4 from complex fused glands, complex glands with papillary proliferation, glands with partial or roman bridging, complex “glomeruloid-like” growth pattern, or PCa exhibiting complex cribriform-like morphology but with intra- or extraglandular mucin or involving a nerve creates significant challenges in classification, diagnostic reproducibility, and reporting of CrP4. In this study, we focused specifically on the classification and practice-related issues that may impact the diagnostic reproducibility of CrP4. Our objectives were to assess the diagnostic reproducibility amongst urological pathologists with expertise in prostate cancer using a wide range of potential CrP4 lesions, identify morphological features that are associated with consensus for and against CrP4, and provide recommendations for diagnostic criteria for CrP4.
Materials and methods
Case selection and construction of survey
Sixty digital images of hematoxylin and eosin-stained slides from 44 prospectively collected RP cases with potentially CrP4 lesions were obtained from the pathology archives of the lead author (R.B.S.). The study was approved by the University of Texas Southwestern Medical Center institutional review board. In all cases, the lesions selected as potentially CrP4 were confirmed as invasive based on morphological features and/or by the lack of basal cells staining. Sixty images with ×100 magnification were used, and in 4 cases, additional images with ×200 magnification were provided. Wherever possible, adjacent benign glands were included for size comparison with the cancer glands in question. The images were distributed electronically to 27 urologic pathologists using a Google Forms survey. A multiple-choice questionnaire was included for each digital image, and participants were asked if a particular image would be classified as large CrP4, small CrP4, or non-cribriform Gleason pattern 4, based on the criteria they apply in their respective routine clinical practice. In some instances, additional question choices included if pattern 3 or 5 or “other” options are applicable, with fill-in responses. Finally, five additional practice-specific questions were included: the years of urologic pathology practice (including fellowship training), whether they routinely report the presence or absence of CrP4 in their practice, whether they differentiate small versus large CrP4 if they report CrP4, which criteria or definition they use to differentiate small from large CrP4, and whether they report “cribriform cancer glands” when pattern 5, especially solid tumor/necrosis, is present elsewhere in the specimen.
Pathological analysis
The selection of cases for the study was primarily based on morphologic criteria for the diagnosis of CrP4 as outlined in the 2014 modified ISUP Gleason grading system [20]. In addition, some cases represented a spectrum of possible CrP4 not included in the 2014 system that may prompt consideration for CrP4. Such lesions included variably sized PCa glands exhibiting “glomeruloid-like” morphology, with intraluminal cellular proliferation with a variable number of attachments to the peripheral layer of the gland, prominent mucinous features (specifically mucinous fibroplasia, mucin extravasation, or extracellular mucin pool), cribriform-like morphology with a central nerve, poorly formed “rosette-like” formations without clear lumina, papillae with fibrovascular cores, and complex fused glands. These cases were selected to represent a wide range of lesions from unequivocal CrP4 to those with overlapping morphology between CrP4 and non-cribriform pattern 4, pattern 3, or 5. A representative example of classic and other potential CrP4 spectrums is illustrated in Figures 1, 2, 3 and 4.
Figure 1.
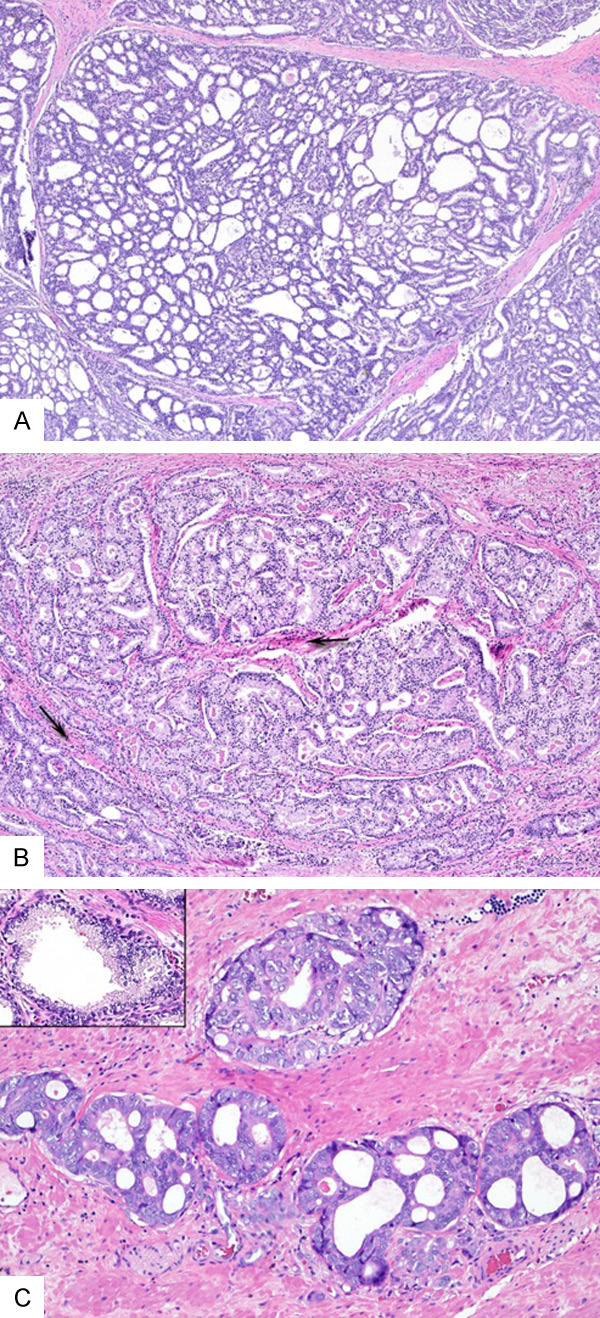
(A-C) Examples of cribriform lesions that achieved consensus for CrP4. (A) A large CrP4 showing dense cellular proliferation with numerous well-formed lumina (>12) and transluminal bridging, forming a “sieve-like” growth. There is no intraglandular stroma or blood vessels. (B) A large CrP4 with branching contour. Despite stoma between branching cribriform glands (arrows), there is no intraglandular stroma or vessels. (C) Small CrP4 that are the size of adjacent benign glands (insert) and have dense transluminal cellular proliferation with <12 well-formed lumina (A-C, Hematoxylin and eosin, original magnifications ×4 (A and B), ×10 (C)).
Figure 2.
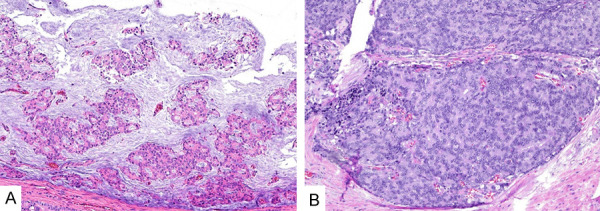
(A, B) Examples of unusual morphologic patterns that achieved consensus for CrP4. (A) An example of mucinous prostate adenocarcinoma (PCa) showing multiple complex confluent nests with well-formed lumina floating within pools of mucin. Seventy-four % of participants classified it as large CrP4, 4% as small CrP4, and the remaining 22% as non-cribriform pattern 4. (B) An example of dense transluminal proliferation with poorly formed “rosette-like” multiple lumina. Seventy-eight % of participants classified this example as CrP4 and the remaining 22% as pattern 5 (A, B, Hematoxylin and eosin, original magnification ×10 (A and B)).
Figure 3.
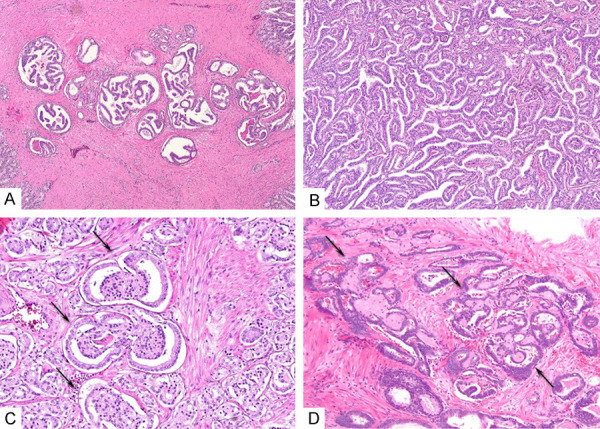
(A-D) Examples of lesions that achieved consensus against CrP4. (A) Cancer glands showing loose cellular proliferation forming multiple incomplete (partial) bridging. Ninety % of participants classified this lesion as a non-cribriform pattern 4. (B) PCa exhibiting complex proliferation of interconnecting tumor cells with well-formed slit-like lumina. However, the majority of tumor cells are in contact with stroma of blood vessels, suggestive of a papillary process. Ninety-six % of participants classified this as a non-cribriform pattern 4. (C) Small to medium cancer glands showing glomeruloid morphology (arrows). Intraluminal proliferation is attached to the border of the gland with one attachment and a clear luminal space along gland periphery occupies >50% of gland circumference. Ninety-three % of participants classified this as an example of glomerulation pattern 4. (D) PCa with intraluminal mucinous fibroplasia (arrows), creating a complex architecture mimicking CrP4. 96% classified it as either pattern 3 (63%) or as non-cribriform pattern 4 (33%) (A-D, Hematoxylin, and eosin, original magnification ×4 (A) and ×10 (B-D)).
Figure 4.
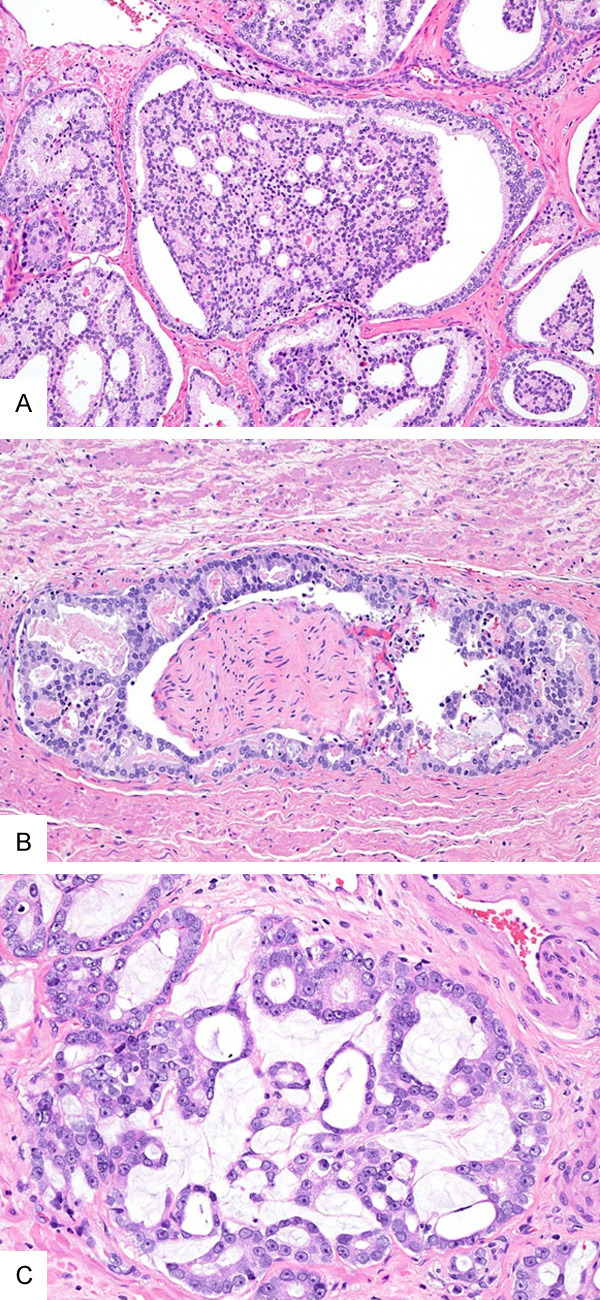
(A-C) Examples that did not achieve consensus for or against Crp4. (A) A PCa showing large gland “glomeruloid-like” architecture. There are multiple attachments to the gland border and a clear luminal space along the periphery occupy >50% of gland circumference. Fifty-two % of participants classified it as large CrP4, 11% as small CrP4, and 37% as glomerulation pattern 4. (B) A PCa forms complex dense cellular proliferation with multiple well-formed lumina around the nerve. Thirty-three % participants classified it as small CrP4, 26% large CrP4, 26% non-cribriform pattern 4 and 15% pattern 3. (C) A PCa with mucin extravasation. Sixty-three % of participants classified it as non-cribriform pattern 4, 11% as large CrP4, 22% as small CrP4, and 4% as pattern 3 (A-C, Hematoxylin, and eosin, original magnification ×10 (A) and ×20 (B, C)).
Each image was independently assessed for the following 11 objectively defined morphologic features by 2 authors (R.B.S. and M.Z.): partial glandular bridging defined as cellular column/trabeculae projecting into, but not completely spanning the glandular lumen vs. transluminal bridging where cellular column/trabeculae span glandular lumen completely; a majority of intraglandular cells are in contact with stroma vs. a majority of intraglandular cells are not in contact with stroma; the number of lumina <12 vs. ≥12; well-formed lumina defined as “punched out”, well-defined luminal spaces vs. poorly formed lumina with rudimentary luminal formation lacking a “punched out”, well-defined luminal spaces; presence of intra- or extraglandular mucin characterized by either mucinous fibroplasia forming intraluminal eosinophilic nodules or aggregates, extravasated pattern characterized by extraglandular mucin rupture or extracellular pools of mucin vs. lack of intra- or extraglandular mucin; size in comparison to adjacent benign glands and based on number of lumens; one attachment to the gland border by tumor cells forming a “glomeruloid-like” pattern vs. >1 attachments to the gland border by tumor cells forming a “glomeruloid-like” pattern; a clear luminal space along the periphery of gland occupying <50% of glandular circumference vs. a clear luminal space along the periphery of gland occupying >50% of glandular circumference; presence of nerve in the middle or surrounded by cribriform-like glands; cellular proliferation defined as dense when cellular mass is ≥50% and luminal space <50% of gland and loose when cellular mass is <50% and luminal space is ≥50% of gland; and contour defined as regular when smooth and round without branching vs. irregular when not round and branching. As previously reported, cancer glands with a diameter of at least twice the size of the adjacent benign gland and/or ≥12 lumina were classified as large glands [6,21]. A representative example of morphological description utilized to classify these 60 images is illustrated in Figures 1, 2, 3 and 4.
For all RP cases, the assessment of GG, extraprostatic extension (EPE), seminal vesicle invasion (SVI), lymph node metastasis, adverse pathological features (specifically IDC-P), and pathological stage (pT) were documented. For the purpose of the study, adverse pathology was defined as the presence of any of these features in RP: GG ≥3, IDC-P, extraprostatic extension (pT3a), seminal vesicle invasion (pT3b), or lymph node metastasis.
Statistical analysis
Interobserver reproducibility for overall and size-based subpattern (small vs. large CrP4) was assessed using Fleiss’s kappa. The statistical significance of Kappa value is defined as <0.2 (poor), 0.21-0.4 (fair), 0.41-0.60 (moderate), 0.61-0.80 (good) and 0.81-1.0 (very good). A consensus was defined as at least 20/27 (75%) of participants agreed on whether CrP4 was present or not. Fisher exact test was performed to evaluate the association between morphologic features and the consensus results, as well as the correlation of consensus results with adverse pathologic features at RP. The value of p≤0.05 was considered statistically significant.
Results
Practice pattern-based survey questions
The mean years of experience of urologic pathology practice including fellowship training for 27 urologic pathologist participants were 21 (range, 2-40 years). The majority (78%) of participants indicated that they routinely report the presence or absence of CrP4 in biopsy and RP specimens. However, only 26% indicated that they distinguish between small vs. large CrP4. Two participants (8%) reported only the presence of large CrP4 and do not mention it when absent. The responses were variable regarding the definition/criteria used to classify small vs. large CrP4. Twenty-six % of participants used the size of the cribriform gland twice that of the adjacent benign gland, 19% used ≥12 lumens, 2 (8%) used a combination of both, 1 (4%) used the size of cribriform gland greater than the largest benign gland, 1 (4%) used the size of cribriform >0.5 mm, and 1 (4%) used the longest cross-section distance >0.25 mm as criteria for large CrP4. Twenty-six % did not use any specific criteria to distinguish small vs. large CrP4. Four (16%) participants indicated that there are no compelling data to suggest the importance of separating small CrP4 from large CrP4. Finally, 48% of participants reported CrP4 even when pattern 5 is present and 48% did not report the CrP4 when pattern 5 is present.
Interobserver agreement (κ) for diagnosis of CrP4
The overall agreement for all 60 images was fair (k=0.40). Large CrP4 had better reproducibility (moderate, k=0.49) than small CrP4 (fair, k=0.40).
Consensus diagnosis for and against CrP4
Table 1 summarizes the consensus diagnosis of 60 images. A consensus diagnosis was reached when 75% of participants agreed on a diagnosis. Twenty-four (40%) images reached a consensus for CrP4 diagnosis, of which 5 had 100% agreement. Twelve (20%) images had a consensus against CrP4 diagnosis, of which, 2 had 100% agreement. Twenty-four (40%) images did not reach a consensus for or against the CrP4 classification (no consensus).
Table 1.
Consensus classification of 60 images among 27 participants
| Consensus diagnosis | Consensus/total number (%) |
|---|---|
| For Cribriform | 24/60 (40%) |
| 100% agreement | 5 |
| ≥75% agreement | 24 |
| Against cribriform | 12/60 (20%) |
| 100% agreement | 2 |
| >75% agreement | 12 |
| No consensus | 24/60 (40%) |
Correlation of histologic features with consensus diagnoses
Table 2 shows the correlation of 11 morphologic features with consensus diagnosis for CrP4, against CrP4 and no consensus. The presence of the following 5 morphologic features was significantly associated with a consensus diagnosis for CrP4: transluminal bridging, dense cellular proliferation, a clear luminal space along the periphery of gland occupying <50% of the glandular circumference, lack of intraglandular mucin, and the majority of intraglandular cells lacking contact with the stroma (P<0.05). In contrast, the following morphologic features were associated with the consensus against CrP4: partial bridging, majority of intraglandular cells in contact with stroma, mucinous fibroplasia, only one attachment to the gland border by tumor cells forming a “glomeruloid-like” pattern, and a clear luminal space along the periphery of gland accounting for >50% of the glandular circumference (P<0.05). Histological features that are “diagnostic of” and “against” CrP4 classification are summarized in Table 3. Representative examples of the morphologic features that achieved consensus for CrP4 are illustrated in Figure 1A-C and some unusual morphologic patterns that achieved consensus for CrP4 are illustrated in Figure 2A, 2B. Representative examples of the morphologic features that achieved consensus against CrP4 are illustrated in Figure 3A-D and examples of the features that did not achieve consensus for or against CrP4 are illustrated in Figure 4A-C.
Table 2.
Correlation of morphologic features with consensus diagnosis
| Morphological features | Consensus for cribriform (n=24) # (%), p | Consensus against cribriform (n=12) # (%), p | No consensus (n=24) # (%) |
|---|---|---|---|
| Glandular bridging | |||
| Transluminal | 24 (100), 0.0001 | 2 (17) | 18 (75) |
| Partial | 0 | 10 (83), 0.00001 | 6 (25) |
| Majority of intraglandular cells in contact with stroma | |||
| No | 24 (100), 0.0001 | 4 (33) | 16 (67) |
| Yes | 0 | 8 (67), 0.0014 | 8 (33) |
| Number of lumina | |||
| <12 | 10 (42) | 9 (75) | 13 (55) |
| ≥12 | 14 (58), 0.1886 | 3 (25), 0.1155 | 11 (45) |
| Lumina | |||
| Well-formed | 22 (92), 0.0563 | 8 (67) | 17 (71) |
| Poorly formed | 2 (8) | 4 (33), 0.2711 | 7 (29) |
| Mucin | |||
| No | 23 (96), 0.0401 | 9 (75), 0.4027 | 18 (75) |
| Fibroplasia | 0 (0) | 3 (25), 0.00064 | 0 (0) |
| Extravasation | 0 (0) | 0 (0) | 6 (25) |
| Pools | 1 (4) | 0 (0) | 0 (0) |
| Size | |||
| Twice benign glands | |||
| Small | 7 (29), 0.5822 | 6 (50) | 8 (33) |
| Large | 17 (71) | 6 (50), 0.3118 | 16 (67) |
| Number of lumina | |||
| Small | 10 (42), 0.1886 | 9 (75) | 13 (54) |
| Large | 14 (58) | 3 (25), 0.1155 | 11 (46) |
| Contact of intraluminal cells with gland periphery | |||
| 1 attachment | 0 (0), 0.5119 | 2 (17) | 0 |
| >1 attachment | 24 (100) | 10 (83), 0.0373 | 24 (100) |
| Luminal space at gland periphery occupies | |||
| <50% circumference | 24 (100), 0.000001 | 1 (8) | 12 (50) |
| >50% circumference | 0 | 11 (92), 0.000 | 12 (50) |
| Central nerve | |||
| Not present | 24 (100), 0.0768 | 11 (92) | 20 (83) |
| Present | 0 | 1 (8), 1 | 4 (17) |
| Cellular proliferation | |||
| Dense | 21 (87), 0.0023 | 2 (17) | 15 (63) |
| Loose | 3 (13) | 10 (83), 0.0003 | 9 (37) |
| Contour | |||
| Regular | 15 (63), 1 | 4 (33) | 18 (75) |
| Irregular | 9 (37) | 8 (67), 0.0436 | 6 (25) |
Table 3.
Histologic features that are “diagnostic of” and “against” cribriform prostate adenocarcinoma
| For Cribriform | Against Cribriform |
|---|---|
| -Transluminal bridging | -Partial bridging |
| -Majority of intraglandular cells are not in contact with stroma | -Majority of intraglandular cells are in contact with stroma |
| -Lack of intraglandular mucin | -Presence of mucinous fibroplasia |
| -A clear luminal space along the periphery of the gland accounting for <50% of the gland circumference | -Single attachment to gland border by tumor cells forming “glomeruloid-like” pattern |
| -Dense cellular proliferation | -A clear luminal space along the periphery of the gland accounting for >50% of the gland circumference |
Correlation of adverse pathologic features at radical prostatectomy with consensus diagnosis
Table 4 shows the correlation of the adverse pathological features at RP with the consensus diagnosis for CrP4 compared to the consensus diagnosis against CrP4. CrP4 with consensus cases had significantly higher GG ≥3 (79% vs. 33%), presence of intraductal carcinoma (83% vs. 25%), and pT3a/T3b stage (71% vs. 17%) (P<0.05). The presence of lymph node metastasis (25% vs. 0%) reached marginal significance.
Table 4.
Association of cribriform lesions with consensus with adverse pathology features at radical prostatectomy
| Pathologic outcome | Consensus for Cribriform # (%) | Consensus Against Cribriform # (%) |
|---|---|---|
| Grade group ≥3 | 19/24 (79%)* | 4/12 (33%)* |
| Presence of IDC-P | 20/24 (83%)* | 3/12 (25%)* |
| pT3a/pT3b | 17/24 (71%)* | 2/12 (17%)* |
| Lymph node metastasis | 6/24 (25%) | 0/12 (0%) |
IDC-P: intraductal carcinoma of the prostate;
P≤0.05.
Discussion
A growing body of evidence suggests that cribriform morphology may potentially affect clinical decision-making in PCa management. van Leenders et al. have recently proposed significant modification to the Gleason system by incorporating cribriform morphology to improve its prognostic utility [22]. In the current study, nearly 85% of participants indicated that they routinely report the presence or absence of CrP4 in prostate biopsies and RP specimens, indicating an acceptance of CrP4 as an adverse pathological feature that should be reported. Using any morphological marker for important clinical decision-making requires that its diagnosis should be reproducible among pathologists. The overall interobserver agreement for a diagnosis of CrP4 by a group of urological pathologists was only fair (k=0.40), which is lower than the reproducibility (53-79%) reported by earlier studies [19], due to the inclusion in this study of more difficult cases. Nevertheless, it suggests that there is significant interpretational disagreement in diagnosing cribriform PCa. To address these issues, we correlated various morphologic features of CrP4 with consensus diagnosis, attempting to extract the key morphological features that urologic pathologists rely on to diagnose or rule out CrP4.
Cribriform cancer pattern is defined as sheets of tumor cells that form cohesively rounded or irregularly shaped trabeculae with perforations or multiple “punched out” lumina. Cribriform glands may be spherical or oblong and may sometimes have irregular borders [1]. However, this definition does not provide clear guidance regarding how to differentiate CrP4 from complex fused glands, complex glands with papillary proliferation or partial bridging, complex glomeruloid-like growth pattern, or PCa exhibiting complex cribriform-like morphology but with intra- or extraglandular mucin. In order to provide guidance regarding these issues, there have been recent attempts to improve the histological definition of the cribriform gland, specifically pertaining to CrP4. Van Leenders et al. proposed a definition of cribriform architecture as an epithelial sheet in which the majority of tumor cells do not contact the surrounding stroma, with a gland-like space surrounding less than half of the sheet circumference, and with regular intercellular lumens clearly visible on hematoxylin and eosin (H&E)-stained sections [2]. Recently, van der Kwast et al. proposed a consensus definition of CrP4 as “a confluent sheet of contiguous malignant epithelial cells with multiple glandular lumina that are easily visible at low power. There should be no intervening stroma or mucin separating individual or fused glandular structures” [23]. To derive this consensus definition, the Delphi method was applied among the 12 panelists on a set of 32 images during 2 initial rounds of the study. Using the same set of 32 images, in a subsequent study they analyzed morphological features to identify those that define the essence of the CrP4 [24]. They found the presence of intervening stroma, mucin, predominant papillary pattern, or an irregular outer boundary detracted participants from calling CrP4 while all consensus cases had ≥9 lumens.
Compared with the two aforementioned publications, this study has several important differences and strengths. First, more (27) pathologists participated in this study, therefore, this study represents a broader opinion of expert urologic pathologists’ diagnostic criteria of cribriform lesions. Second, more images (60) with a much broader morphological spectrum were used to represent a wide range of lesions that may be considered CrP4, from unequivocal CrP4 to those with morphology overlapping between CrP4 and non-cribriform pattern 4, pattern 3, or 5. These lesions included variably sized PCa glands exhibiting “glomeruloid-like” morphology, those with intraluminal cellular proliferation with a variable number of attachments to the peripheral layer of the gland, prominent mucinous features (specifically mucinous fibroplasia, mucin extravasation, or extracellular mucin pool), cribriform-like morphology with a central nerve, poorly formed “rosette-like” formations without clear lumina, papillae with fibrovascular cores, and complex fused glands (Figures 1, 2, 3 and 4). Third, more morphological features were investigated to identify that were associated with consensus for and against CrP4 diagnosis, including those that were not addressed by the ISUP study, such as glandular bridging (transluminal vs. partial), number of contacts of intraluminal cells with gland periphery, whether peripheral luminal space occupies more or less than 50% of glandular circumference and whether a nerve was present within the cribriform lesions. In addition to following two morphological features: a lack of contact of the intraglandular cells with stroma and lack of intraglandular mucin, we found that transluminal bridging and a clear luminal space along the periphery of the gland accounting for <50% of the glandular circumference were two additional important criteria for CrP4 diagnosis (Figures 1 and 2). Specifically, all cases that reached consensus for CrP4 demonstrated transluminal bridging, a lack of contact of the intraglandular cells with stroma and a clear luminal space along the periphery of the gland accounting for <50% of the glandular circumference. These features are not new diagnostic criteria for CrP4; rather they identify a group of cribriform lesions that have achieved consensus among GU pathologists. Importantly, we also identified diagnostic features that were associated with the consensus against CrP4: partial bridging, a majority of intraglandular cells in contact with the stroma, mucinous fibroplasia, only one attachment of tumor cells to gland border for “glomeruloid-like” proliferation, and a clear luminal space along the periphery of gland accounting for >50% of the glandular circumference (Figure 3). Two cases showing partial bridging were diagnosed as “against CrP4”, and all 3 cases exhibiting complex mucinous fibroplasia also reached a consensus against CrP4 (Figure 3). Therefore, our study not only attempts to address issues pertaining to the definition but also for interpretation aspects of CrP4. We show that the diagnosis of CrP4 is not only based on the presence of the classic definitional features of the CrP4 diagnosis but importantly depends on ruling out features against the CrP4 diagnosis. One may still classify a lesion as CrP4 if all five morphological features associated with CrP4 consensus are not present as long as it lacks features against CrP4 consensus diagnosis.
In some studies, CrP4 has been divided into small and/or large CrP4, based on its size in relation to adjacent benign glands, the number of lumina, and the greatest cross-sectional diameter [6,21,25]. Specifically, a cribriform gland twice the size of the adjacent benign glands and exhibiting >12 lumina has been proposed as large CrP4 [6,21]. Hollemans et al. used a criterion of twice the size of the diameter of adjacent benign glands and demonstrated that, in a multivariable analysis, only large CrP4 was an independent predictive factor for biochemical recurrence-free survival in GG 2 patients [21]. However, most studies, when assessing for outcomes associations, have either not differentiated CrP4 based on size, or have utilized varying definitions for the size cut-off. In this study, we showed that large CrP4 had a better agreement (k=0.49) compared with small CrP4 (k=0.40). However, we found that the size of CrP4, when compared with the adjacent benign glands, or defined based on the number of lumina, was not associated with a consensus diagnosis for CrP4 or non-CrP4. This is further evident from the variable responses on this issue as only 26% of participants indicated that they routinely distinguish between small vs. large CrP4. For these reasons, in our opinion, routine reporting of CrP4 as two separate categories (large or small) requires additional studies demonstrating its value of such division.
We have also demonstrated that for certain PCa morphologic patterns, there remains considerable diagnostic variability among experts. Examples in this category included variably sized “glomeruloid-like” PCa glands with more than one point of attachment to gland periphery, complex cribriform-like proliferation around nerve and PCa with extravasated mucin imparting complex patterns (Figure 4). The glomeruloid pattern has been defined as dilated cancer glands of variable size, with a cellular mass protruding into the lumen yet not attaching to the opposite side of the gland wall (one attachment to gland periphery), superficially resembling a glomerulus [20]. Notably, because of its resemblance to the cribriform pattern, particularly when the gland is large, some have suggested that a glomeruloid pattern may represent an early stage of cribriform cancer [26]. However, subsequent studies have suggested that a glomeruloid pattern, regardless of size, has outcome associations intermediate between pattern 3 and CrP4 [4,5,7]. For this “glomeruloid-like” category, two examples with a single attachment of tumor cells to the gland periphery were uniformly associated with consensus for a glomeruloid pattern 4 but for 9 lesions (two classified as small gland and 7 as large glands) showing multiple contacts to the gland border had no consensus for CrP4 vs. non-cribriform pattern 4 diagnosis (Figure 4A). In a significant number of these lesions (89%), a clear luminal space along the periphery of the gland that occupied >50% of the glandular circumference was present, a feature that in this study was correlated “against CrP4” consensus diagnosis (Figure 4A). Another significant area of diagnostic variability was the presence of a nerve in the center of the PCa glands showing complex architecture that resembles CrP4 (Figure 4B). Of 5 such examples, one case reached a consensus for Gleason pattern 3, while the remaining 4 cases had no consensus. Interestingly, two participants indicated that they do not grade components of PCa in regions of perineural invasion. Traditionally, some experts have cautioned against over-grading when the tumor is present around the nerve and recommend a conservative approach, particularly in needle biopsy. One case had all and remaining 3 had variable of five diagnostic features of CrP4. Importantly, all lacked features that were associated against CrP4 diagnosis, arguing that CrP4 can be encountered around nerve. Finally, all 6 cases of PCa exhibiting mucin extravasation had no consensus classification (Figure 4C). It is well known that PCas with extracellular mucin, extravasated mucin, and/or fibroplasia may represent patterns 3 or 4, dependent upon the complexity of the glandular structures, and the overall grade of these lesions should be based on the underlying architecture [20]. Interestingly, the one included example of PCa with cribriform architecture embedded in extracellular mucin pools did reach consensus for CrP4 (Figure 2A). This finding is similar to the architectural pattern described by McKenney et al., which they termed “mucinous adenocarcinoma with marked epithelial complexity, either solid or complex confluent cribriform” [7]. In their study, this specific mucin pattern was associated with aggressive outcomes, compared to other mucin patterns which tend to have better outcomes [7,20].
The current study has a few limitations. First, the study was conducted using prospectively collected cases and was enriched for difficult-to-classify cases, which may not represent the routine morphologic spectrum of CrP4. Likewise, the survey did not address the issue of extent of CrP4, which may potentially influence outcomes. Despite these limitations, this study showed that certain morphologic features are quite reproducible for the diagnosis of CrP4 and may be used to establish consensus diagnostic criteria for CrP4. The recommendations from this study are provided in Table 3. We also found that there is considerable overlap between the spectrum of CrP4 within certain morphologic patterns with the potential for over-grading or diagnosing CrP4. Finally, further validation of our proposed diagnostic criteria for CrP4 with cases that are annotated with clinical outcome data would be desirable.
In summary, we have demonstrated only fair diagnostic agreement amongst urologic pathologists for CrP4 diagnosis, suggesting a need for standardization of diagnostic criteria. We identified the following morphologic features associated with consensus CrP4 diagnosis: “a dense sheet of tumor cells forming multiple lumens with transluminal bridging, imparting a “sieve-like” architecture, in which a majority of intraglandular cells are not in direct contact with stroma or mucin, and a clear luminal space along the periphery of gland accounts for <50% of the glandular circumference”. We hope that these suggestions will represent a step toward standardization of prostate cancer grading based on reproducible morphologic features.
Acknowledgements
Daniel M Berney is supported by Orchid.
Disclosure of conflict of interest
None.
References
- 1.Epstein JI, Amin MB, Fine SW, Algaba F, Aron M, Baydar DE, Beltran AL, Brimo F, Cheville JC, Colecchia M, Comperat E, da Cunha IW, Delprado W, DeMarzo AM, Giannico GA, Gordetsky JB, Guo CC, Hansel DE, Hirsch MS, Huang J, Humphrey PA, Jimenez RE, Khani F, Kong Q, Kryvenko ON, Kunju LP, Lal P, Latour M, Lotan T, Maclean F, Magi-Galluzzi C, Mehra R, Menon S, Miyamoto H, Montironi R, Netto GJ, Nguyen JK, Osunkoya AO, Parwani A, Robinson BD, Rubin MA, Shah RB, So JS, Takahashi H, Tavora F, Tretiakova MS, True L, Wobker SE, Yang XJ, Zhou M, Zynger DL, Trpkov K. The 2019 Genitourinary Pathology Society (GUPS) white paper on contemporary grading of prostate cancer. Arch Pathol Lab Med. 2021;145:461–493. doi: 10.5858/arpa.2020-0015-RA. [DOI] [PubMed] [Google Scholar]
- 2.van Leenders G, Verhoef EI, Hollemans E. Prostate cancer growth patterns beyond the Gleason score: entering a new era of comprehensive tumour grading. Histopathology. 2020;77:850–861. doi: 10.1111/his.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iczkowski KA, Paner GP, Van der Kwast T. The new realization about cribriform prostate cancer. Adv Anat Pathol. 2018;25:31–37. doi: 10.1097/PAP.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 4.Kweldam CF, Wildhagen MF, Steyerberg EW, Bangma CH, van der Kwast TH, van Leenders GJ. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol. 2015;28:457–464. doi: 10.1038/modpathol.2014.116. [DOI] [PubMed] [Google Scholar]
- 5.Choy B, Pearce SM, Anderson BB, Shalhav AL, Zagaja G, Eggener SE, Paner GP. Prognostic significance of percentage and architectural types of contemporary gleason pattern 4 prostate cancer in radical prostatectomy. Am J Surg Pathol. 2016;40:1400–1406. doi: 10.1097/PAS.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 6.Iczkowski KA, Torkko KC, Kotnis GR, Wilson RS, Huang W, Wheeler TM, Abeyta AM, La Rosa FG, Cook S, Werahera PN, Lucia MS. Digital quantification of five high-grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am J Clin Pathol. 2011;136:98–107. doi: 10.1309/AJCPZ7WBU9YXSJPE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenney JK, Wei W, Hawley S, Auman H, Newcomb LF, Boyer HD, Fazli L, Simko J, Hurtado-Coll A, Troyer DA, Tretiakova MS, Vakar-Lopez F, Carroll PR, Cooperberg MR, Gleave ME, Lance RS, Lin DW, Nelson PS, Thompson IM, True LD, Feng Z, Brooks JD. Histologic grading of prostatic adenocarcinoma can be further optimized: analysis of the relative prognostic strength of individual architectural patterns in 1275 patients from the canary retrospective cohort. Am J Surg Pathol. 2016;40:1439–1456. doi: 10.1097/PAS.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 8.Kweldam CF, Kummerlin IP, Nieboer D, Steyerberg EW, Bangma CH, Incrocci L, van der Kwast TH, Roobol MJ, van Leenders GJ. Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3+4=7 prostate cancer. Mod Pathol. 2017;30:1126–1132. doi: 10.1038/modpathol.2017.29. [DOI] [PubMed] [Google Scholar]
- 9.Tom MC, Nguyen JK, Luciano R, Mian OY, Stephans KL, Ciezki JP, Smile TD, Wei W, McKenney JK, Magi-Galluzzi C, Tendulkar RD. Impact of cribriform pattern and intraductal carcinoma on Gleason 7 prostate cancer treated with external beam radiotherapy. J Urol. 2019;202:710–716. doi: 10.1097/JU.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 10.Hollemans E, Verhoef EI, Bangma CH, Rietbergen J, Roobol MJ, Helleman J, van Leenders G. Clinical outcome comparison of Grade Group 1 and Grade Group 2 prostate cancer with and without cribriform architecture at the time of radical prostatectomy. Histopathology. 2020;76:755–762. doi: 10.1111/his.14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding-Jackson N, Kryvenko ON, Whittington EE, Eastwood DC, Tjionas GA, Jorda M, Iczkowski KA. Outcome of Gleason 3+5=8 prostate cancer diagnosed on needle biopsy: prognostic comparison with Gleason 4+4=8. J Urol. 2016;196:1076–1081. doi: 10.1016/j.juro.2016.05.105. [DOI] [PubMed] [Google Scholar]
- 12.Kweldam CF, Kummerlin IP, Nieboer D, Verhoef EI, Steyerberg EW, van der Kwast TH, Roobol MJ, van Leenders GJ. Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod Pathol. 2016;29:630–636. doi: 10.1038/modpathol.2016.49. [DOI] [PubMed] [Google Scholar]
- 13.Elfandy H, Armenia J, Pederzoli F, Pullman E, Pertega-Gomes N, Schultz N, Viswanathan K, Vosoughi A, Blattner M, Stopsack KH, Zadra G, Penney KL, Mosquera JM, Tyekucheva S, Mucci LA, Barbieri C, Loda M. Genetic and epigenetic determinants of aggressiveness in cribriform carcinoma of the prostate. Mol Cancer Res. 2019;17:446–456. doi: 10.1158/1541-7786.MCR-18-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen JK, Magi-Galluzzi C. Unfavorable pathology, tissue biomarkers and genomic tests with clinical implications in prostate cancer management. Adv Anat Pathol. 2018;25:293–303. doi: 10.1097/PAP.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 15.Shah RB, Shore KT, Yoon J, Mendrinos S, McKenney JK, Tian W. PTEN loss in prostatic adenocarcinoma correlates with specific adverse histologic features (intraductal carcinoma, cribriform Gleason pattern 4 and stromogenic carcinoma) Prostate. 2019;79:1267–1273. doi: 10.1002/pros.23831. [DOI] [PubMed] [Google Scholar]
- 16.Chua MLK, Lo W, Pintilie M, Murgic J, Lalonde E, Bhandari V, Mahamud O, Gopalan A, Kweldam CF, van Leenders G, Verhoef EI, Hoogland AM, Livingstone J, Berlin A, Dal Pra A, Meng A, Zhang J, Orain M, Picard V, Hovington H, Bergeron A, Lacombe L, Fradet Y, Tetu B, Reuter VE, Fleshner N, Fraser M, Boutros PC, van der Kwast TH, Bristow RG. A prostate cancer “nimbosus”: genomic instability and SChLAP1 dysregulation underpin aggression of intraductal and cribriform subpathologies. Eur Urol. 2017;72:665–674. doi: 10.1016/j.eururo.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Bottcher R, Kweldam CF, Livingstone J, Lalonde E, Yamaguchi TN, Huang V, Yousif F, Fraser M, Bristow RG, van der Kwast T, Boutros PC, Jenster G, van Leenders G. Cribriform and intraductal prostate cancer are associated with increased genomic instability and distinct genomic alterations. BMC Cancer. 2018;18:8. doi: 10.1186/s12885-017-3976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Leenders GJLH, van der Kwast TH, Grignon DJ, Evans AJ, Kristiansen G, Kweldam CF, Litjens G, McKenney JK, Melamed J, Mottet N, Paner GP, Samaratunga H, Schoots IG, Simko JP, Tsuzuki T, Varma M, Warren AY, Wheeler TM, Williamson SR, Iczkowski KA ISUP Grading Workshop Panel Members. The 2019 International Society of Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am J Surg Pathol. 2020;44:e87–e99. doi: 10.1097/PAS.0000000000001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kweldam CF, Nieboer D, Algaba F, Amin MB, Berney DM, Billis A, Bostwick DG, Bubendorf L, Cheng L, Comperat E, Delahunt B, Egevad L, Evans AJ, Hansel DE, Humphrey PA, Kristiansen G, van der Kwast TH, Magi-Galluzzi C, Montironi R, Netto GJ, Samaratunga H, Srigley JR, Tan PH, Varma M, Zhou M, van Leenders GJ. Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology. 2016;69:441–449. doi: 10.1111/his.12976. [DOI] [PubMed] [Google Scholar]
- 20.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 21.Hollemans E, Verhoef EI, Bangma CH, Rietbergen J, Helleman J, Roobol MJ, van Leenders G. Large cribriform growth pattern identifies ISUP grade 2 prostate cancer at high risk for recurrence and metastasis. Mod Pathol. 2019;32:139–146. doi: 10.1038/s41379-018-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Leenders G, Kweldam CF, Hollemans E, Kummerlin IP, Nieboer D, Verhoef EI, Remmers S, Incrocci L, Bangma CH, van der Kwast TH, Roobol MJ. Improved prostate cancer biopsy grading by incorporation of invasive cribriform and intraductal carcinoma in the 2014 Grade Groups. Eur Urol. 2020;77:191–198. doi: 10.1016/j.eururo.2019.07.051. [DOI] [PubMed] [Google Scholar]
- 23.van der Kwast TH, van Leenders GJ, Berney DM, Delahunt B, Evans AJ, Iczkowski KA, McKenney JK, Ro JY, Samaratunga H, Srigley JR, Tsuzuki T, Varma M, Wheeler TM, Egevad L. ISUP consensus definition of cribriform pattern prostate cancer. Am J Surg Pathol. 2021;45:1118–1126. doi: 10.1097/PAS.0000000000001728. [DOI] [PubMed] [Google Scholar]
- 24.Iczkowski KA, van Leenders G, Tarima S, Wu R, Van der Kwast T, Berney DM, Evans AJ, Wheeler TM, Ro JY, Samaratunga H, Delahunt B, Srigley J, Varma M, Tsuzuki T, Egevad L. Cribriform prostate cancer: morphologic criteria enabling a diagnosis, based on survey of experts. Ann Diagn Pathol. 2021;52:151733. doi: 10.1016/j.anndiagpath.2021.151733. [DOI] [PubMed] [Google Scholar]
- 25.Trudel D, Downes MR, Sykes J, Kron KJ, Trachtenberg J, van der Kwast TH. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer. 2014;50:1610–1616. doi: 10.1016/j.ejca.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Lotan TL, Epstein JI. Gleason grading of prostatic adenocarcinoma with glomeruloid features on needle biopsy. Hum Pathol. 2009;40:471–477. doi: 10.1016/j.humpath.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


