Abstract
Background
Universal admission screening for SARS-CoV-2 in children and their caregivers (CG) is critical to prevent hospital outbreaks. We evaluated pooled SARS-CoV-2 antigen tests (AG) to identify infectious individuals while waiting for polymerase chain reaction (PCR) test results.
Methods
This single-center study was performed from November 5, 2020 to March 1, 2021. Nasal mid-turbinate and oropharyngeal swabbing for AG and PCR testing was performed in children with 2 individual swabs that were simultaneously inserted. Nasopharyngeal swabs were obtained from their CG. AG swabs were pooled in a single extraction buffer tube and PCR swabs in a single viral medium. Results from an adult population were used for comparison, as no pooled testing was performed.
Results
During the study period, 710 asymptomatic children and their CG were admitted. Pooled AG sensitivity and specificity was 75% and 99.4% respectively for detection of infectious individuals. Four false negatives were observed, though 3 out of 4 false negative child-CG pairs were not considered infectious at admission. Unpooled AG testing in an adult population showed a comparable sensitivity and specificity of 50% and 99.7%. AG performed significantly better in samples with lower Ct values in the corresponding PCR (32.3 vs 21, P-value < .001).
Conclusions
Pooled SARS-CoV-2 AGs are an effective method to identify potentially contagious individuals prior admission, without adding additional strain to the child.
Key words: COVID-19, Lateral flow test, Mid-turbinate, Hospital infection
Introduction
Preventing coronavirus disease 2019 (COVID-19) outbreaks in hospital settings is critical. The paucity of specific symptoms or signs of COVID-19 in children makes sole symptom-based screening challenging. Since transmission can occur even in pre- or asymptomatic individuals, universal admission screening for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in all patients is mandatory in Germany.1 , 2 A symptom-based COVID-19 testing strategy failed to identify nearly half of all hospitalized children infected by SARS-CoV-2 in a French multicenter cohort.3 In a pediatric emergency department, isolation pending the polymerase chain reaction (PCR) result (up to 24 hours) is only feasible for a limited number of patients. So, only symptomatic patients are isolated due to having a higher pre-test-probability of infection. Rapid lateral-flow SARS-CoV-2 antigen tests (AG) are fast, simple, and cheap, but less sensitive than PCR tests which are considered the gold standard.4 PCR tests on the other hand are usually performed in centralized labs and therefore the method drives down frequency and speed of testing. Combining the best of both worlds would consequently be the optimal screening strategy prior to the admission of asymptomatic children and their caregivers (CG). Performing multiple tests on children (eg, antigen testing followed by PCR testing) can be traumatic. Moreover, parallel testing in children and their CG drives up costs, as 2 AG and 2 PCR tests would be needed for every admission to the children's hospital.
To reduce the number of traumatic practices in children and to save resources, we implemented a procedure of pooled AG and PCR testing in asymptomatic children and their CG prior admission. Simultaneous swabbing for AG and PCR tests allows testing without additional strain to the child and the pooled approach reduces costs. The goal of this study was to evaluate this test strategy as a universal screening method in a pediatric emergency department.
Methods
Study population
The study was performed in Stuttgart, Germany, a city with a population of around 635,000 people, of whom 94,000 are <18 years of age. The Klinikum Stuttgart is a tertiary care hospital, with a separated pediatric and adult emergency department.
This single-center study included all patients and their accompanying CG admitted to the children's hospital, after visiting the pediatric emergency department from November 5, 2020 to March 1, 2021, who showed no symptoms of SARS-CoV-2 (fever, upper or lower respiratory tract symptoms, GI-symptoms, myalgia, headache, anosmia) for at least 48 hours, and had no history of known contact to SARS-CoV-2 in 14 days prior admission. An adult population (≥18 years of age) from the neighboring adult emergency department was included for comparison, because there testing was performed without pooling of AG. All adults without symptoms suggestive of COVID-19 (see above), visiting the adult emergency department from October 15, 2020 to November 7, 2020 were included. Testing in these individuals was performed prior admission to the hospital.
The study period started right at peak of the second wave in Stuttgarty, Germany with a 7-day incidence rate of 182 per 100,000 on November 5, 2020. By the end of the study period the valley between the second and third wave was reached, with a 7-day incidence rate of 50 per 100,000 on March 1, 2021.
SARS-CoV-2 RT-PCR and antigen testing
The SARS-CoV-2 Rapid Antigen Test by SD Biosensor (SD BioSensor Inc., Suwon, South Korea), distributed by Roche (Roche Diagnostics, Mannheim, Germany) in Germany, was used for antigen testing. The RT-PCR was performed on one of the following systems: Xpert Xpress SARS-CoV-2; Cepheid (Cepheid Inc. Sunnyvale), RealStar SARS-CoV-2 RT-PCR Kit 1.0; Altona Diagnostics (altona diagnostics, Hamburg, Germany) measuring on LightCycler 480, Roche or cobas SARS-CoV-2; cobas 6800; Roche.
In children, AG and RT-PCR testing was performed simultaneously with 2 individual swabs. Both swabs were first inserted into the mouth and the back wall of the oropharynx was swabbed. The same swabs were then used to perform a mid-turbinate nasal swab. The original swab from the antigen test is then put into the extraction buffer tube. The swab for the RT-PCR was collected in Copan Universal Transport Medium (Copan, Murietta). For testing of the accompanying CG, the swabs were not carried out simultaneously. For antigen testing, a nasopharyngeal swab was performed, according to manufacturer instructions. The swab was put in the same extraction buffer tube as the swab from the child. For RT-PCR testing, an oropharyngeal as well as nasopharyngeal swab was performed. The swab was pooled in the same virus transport medium as the swab from the child. In case of a positive pooled RT-PCR result, individual swabbing of the child and their CG was performed as soon as the result of the pooled test was available.
The same sampling technique was applied in the adult emergency department, as was done for the adult CG in the pediatric emergency department, though no pooling of swabs was performed.
Ethics
The study was approved by the local ethics committee (vote 119/2021BO2), with waiver of informed consent due to the retrospective and anonymized approach.
Statistical analysis
Continuous data were expressed as mean and standard deviation (SD), while categorical variables are reported as number (n), percentage (%) and 95% confidence interval (CI). Statistical differences between Ct values of negative or positive antigen tests were determined using Student's t-test. Reported P-values are 2-tailed, with P ≤ .05 being considered statistically significant. SPSS (SPSS 24, SPSS Inc., Armonk NY) was used for statistical analysis.
Results
During the study period, 710 asymptomatic children and their CG were admitted and tested via the pediatric emergency department. Detection rates in asymptomatic individuals of pooled AG and PCR were 0.99% (7/703) each. Four false positive as well as 4 false negatives were observed with AG (Table 1 ). AG sensitivity, specificity, positive and negative predictive value were as follows: 42.9% (95% CI 9.9%-81.6%), 99.4% (98.6%-99.8%), 42.9% (17%-73.3%), 99.4% (98.9%-99.7%). Accuracy of the test was 98.9% with moderate agreement between tests (Cohen's k = 0.42, 95% CI 0.096-0.75). Three out of 4 pooled false negative AG test results were considered not infectious at admission but represented past SARS-CoV-2 infection (Table 2 ). In one instance, pooled PCR tests showed Ct values above 30, not considered infectious. Unpooling revealed a positive PCR with Ct values above 30 in the CG, while the child had a negative result. Medical history revealed that the mother had COVID-19 at childbirth, which was 25 days prior testing. In a second case, pooled PCR tests also showed Ct values above 30. Unpooling revealed a negative PCR in the child and a PCR with Ct values above 30 in the CG. The whole family had COVID-19 8 weeks prior testing. In the third instance, the pooled PCR showed Ct values above 30, while unpooling showed negative PCR results in the child, as well as the CG. In only 1 case with a false-negative AG, the pooled PCR, as well as unpooled PCRs showed Ct values below 30, compatible with active SARS-CoV-2 infection. Adjusting pooled AG test performance for detection of infectious individuals (ie, PCR samples with Ct values ≤30 considered infectious) gives the following results: Sensitivity 75% (95% CI 19.4-99.4), specificity 99.4% (98.6-99.9), negative predictive value 99.9% (99.2-100), positive predictive value 42.9% (19.5-69.9). The accuracy of the test was 99.3%, also with moderate agreement between tests (Cohen's k = 0.54, 95% CI 0.19-0.9). A simulation of the performance of the AG assay in dependence of disease prevalence in the pediatric population is shown in Table 3 .
Table 1.
Comparison of the performance of antigen tests to RT-PCR prior admission to the hospital in asymptomatic individuals
| Pediatric emergency department – Pooled testing, N = 710 | |||||||||||
| Antigen negative, N | Antigen positive, N | Total, N | |||||||||
| RT-PCR positive, N | 4 | 3 | 7 | Sensitivity | 42.9% | 95% CI 9.9-81.6% | |||||
| RT-PCR negative, N | 699 | 4 | 703 | Specificity | 99.4% | 95% CI 98.6-99.8% | |||||
| Pediatric emergency department – Pooled testing – Adjusting for infectiousness, N = 710 | |||||||||||
| Antigen negative, N | Antigen positive, N | Total, N | |||||||||
| RT-PCR positive with Ct ≤30, N | 1 | 3 | 4 | Sensitivity | 75% | 95% CI 19.4-99.4% | |||||
| RT-PCR negative or Ct >30, N | 702 | 4 | 706 | Specificity | 99.4% | 95% CI 98.6-99.9% | |||||
| Adult emergency department, N = 366 | |||||||||||
| Antigen negative, N | Antigen positive, N | Total, N | |||||||||
| RT-PCR positive, N | 11 | 7 | 18 | Sensitivity | 38.9% | 95% CI 17.3-64.3% | |||||
| RT-PCR negative, N | 347 | 1 | 348 | Specificity | 99.7% | 95% CI 98.4-100% | |||||
| Adult emergency department, Adjusting for infectiousness, N = 366 | |||||||||||
| Antigen negative, N | Antigen positive, N | Total, N | |||||||||
| RT-PCR positive with Ct ≤30, N | 7 | 7 | 18 | Sensitivity | 50% | 95% CI 23-77% | |||||
| RT-PCR negative or Ct >30, N | 351 | 1 | 348 | Specificity | 99.7% | 95% CI 98.4-100% | |||||
Ct, cycle threshold.
NOTE. For antigen testing in the pediatric emergency department, one swab each from the child and their caregiver were pooled into a single extraction buffer tube. Antigen testing in the adult emergency department was performed without pooling, according to the manufacturer instructions. The sensitivity and specificity for the antigen tests in comparison to the RT-PCR were calculated. The aim of the antigen test was to identify infectious asymptomatic individuals while waiting for the RT-PCR test result. Therefore, results are shown separated for the overall population and potentially infectious individuals. Asymptomatic individuals with a Ct value above 30 and which did not develop COVID-19 symptoms were considered not infectious.
Table 2.
Detailed description of all cases in the pediatric emergency department, where the pooled antigen tests were either true or false positive or false negative
| Pooled Antigen | Pooled PCR | PCR Child | PCR CG | Classification | Comment | |
|---|---|---|---|---|---|---|
| 1 | Positive | Negative | Not performed | Not performed | False positive | |
| 2 | Positive | Negative | Not performed | Not performed | False positive | |
| 3 | Positive | Negative | Negative | Negative | False positive | |
| 4 | Positive | Negative | Negative | Negative | False positive | |
| 5 | Positive | Positive | Positive | Negative | True positive | |
| 6 | Positive | Positive | Positive | Positive | True positive | |
| 7 | Positive | Positive | Negative | Positive | True positive | |
| 8 | Negative | Positive | Negative | Positive | False negative | Ct >30 in the mother. She had COVID-19 25 days prior testing |
| 9 | Negative | Positive | Negative | Positive | False negative | Ct >30 in the CG. The whole family had COVID-19 8 wk prior testing |
| 10 | Negative | Positive | Negative | Negative | False negative | Ct >30 in the pooled PCR |
| 11 | Negative | Positive | Positive | Positive | False negative | Ct ≤30 in both child and CG |
CG, care giver; Ct, cycle threshold.
Table 3.
Simulation of the positive and negative predictive value of pooled antigen testing in the pediatric emergency department according to disease prevalence and infectiousness
| COVID-19 prevalence, % | Positive predictive value, % (95% CI) | Negative predictive value, % (95% CI) |
|---|---|---|
| Overall, independent on Ct value in PCR | ||
| 0.5 | 27.5 (9.4-58.1) | 99.7 (99.5-99.9) |
| 1 | 43.2 (17.2-73.6) | 99.4 (98.9-99.7) |
| 2 | 60.6 (29.6-84.9) | 98.8 (97.8-99.4) |
| 5 | 79.9 (52-93.6) | 97.1 (94.6-98.4) |
| 10 | 89.3 (69.6-96.8) | 94 (89.2-96.8) |
| Based on infectious individuals identified via AG | ||
| 0.5 | 40 (17.7-67.3) | 99.9 (99.3-100) |
| 1 | 57.2 (90.2-80.5) | 99.8 (98.6-100) |
| 2 | 73 (46.6-89.3) | 99.5 (97.3-99.9) |
| 5 | 87.5 (69.3-95.6) | 98.7 (93.3-99.8) |
| 10 | 93.63 (82.6-97.9) | 97.3 (86.8-99.5) |
Unpooled tests in an asymptomatic adult population from an adult emergency department were analyzed for comparison. The detection rates of AG and PCR were 2.2% (8/366) and 4.9% (18/366) respectively. AG sensitivity, specificity, positive and negative predictive value were as follows: 38.9% (95% CI 17.3%-64.3%), 99.7% (98.4%-100%), 87.5% (47.6%-98.2%) and 96.9% (95.6%-97.9%). Accuracy of the test in adults was 96.7% with moderate agreement between tests (Cohen's k = 0.52, 95% CI 0.29-0.76). When only considering individuals with Ct values below 30 as potentially infectious, AG performance in comparison to PCR is as follows: Sensitivity 50% (95% CI 23%-77%), specificity 99.7% (98.4%-100%), positive predictive value 87.5% (48%-98.2%), negative predictive value 98% (96.7%-98.8%). The accuracy of the test in this scenario was 97.8% with substantial agreement between AG and PCR (Cohen's k = 0.63, 95% CI 0.39-0.87).
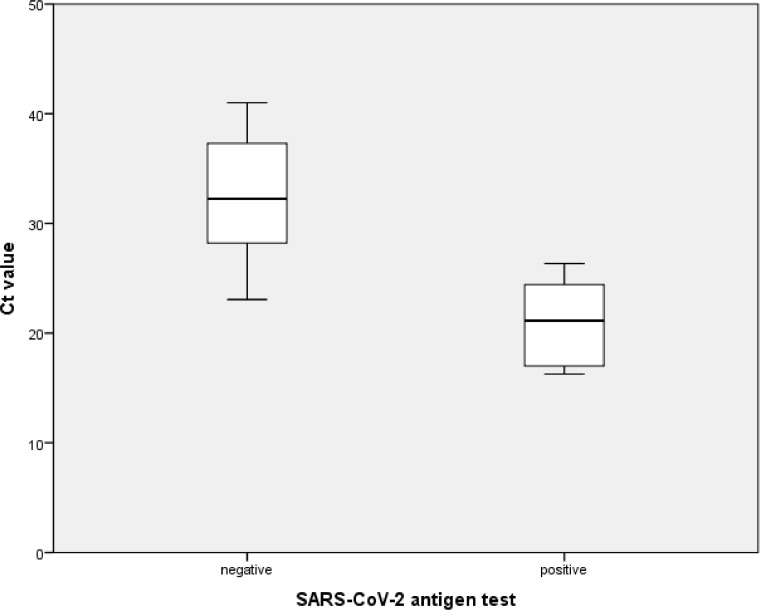
In general, looking at data from the pediatric and adult emergency department combined, mean cycle threshold (Ct) values in false negative cases (N = 15) were significantly higher in comparison to cases where AG tests identified SARS-CoV-2 infections correctly (N = 10) (32.3 [SD ± 5.4] vs 21 [SD ± 3.6], P-value < .001) (Fig. 1 ). All samples with Ct values below 21 were correctly identified by AG.
Fig 1.
Boxplots showing the result of the SARS-CoV-2 antigen test in the pediatric and adult population combined, in dependence of the Ct-value of the corresponding RT-PCR. False negative (N = 15) antigen test results are shown on the left and true positives (N = 10) on the right. Box plots show the median, as well as first and third quartiles. The whiskers show the maximum as well as minimum values.
Discussion
During the ongoing COVID-19 pandemic, screening every child and their CG for active infection with SARS-CoV-2 prior hospital admission is critical to prevent nosocomial infections. A systematic review showed that around 15% of children and new-borns have asymptomatic infection.5 Therefore, screening asymptomatic children is essential for infection control in a children's hospital. SARS-CoV-2 RT-PCR from nasopharyngeal swabs is considered the gold standard, but test results are often delayed for several hours or days. To isolate every child while waiting for the PCR test results, even without upper respiratory tract symptoms, would lead to overflow in pediatric emergency departments. Lateral flow SARS-CoV-2 antigen tests (AG) are cheap, easy to use and have a rapid turnaround time and hence allow for rapid identification of infectious individuals.
In our study we show that AGs detect most asymptomatic children and their CG with COVID-19 that are considered infectious at the time of admission. This was possible despite pooling AG swabs from children and their CG, which saves resources and money.
Nasopharyngeal swabs are an uncomfortable and often traumatic experience for children. Especially the nasal passage is irritating. Studies in adults have shown that nasal mid-turbinate or even anterior nasal sampling for AGs are reliable alternatives to nasopharyngeal swabbing.6 , 7 We therefore collected nasal mid-turbinate as well as oropharyngeal swabs from children, instead of nasopharyngeal swabs. In our experience, this approach leads to a higher acceptance rate in children, which is especially important in chronically ill children, which are admitted to the hospital on a regular basis. To further reduce the trauma of sampling for the children, we used 2 swabs simultaneously in one passage. One swab was used for the AG, while the other was used for the PCR analysis. Due to a child's anatomy, this is only possible by using thin swab such as provided with the SD Biosensor AG.
Several studies have shown that AGs show reduced sensitivity in asymptomatic individuals, when compared to symptomatic individuals.8, 9, 10, 11 This is also true in children.12 We show that pooled antigen testing has a sensitivity of 43%, which is well within the expected range according to literature. As this was a retrospective, non-interventional study, we did not have a control group, where AG testing was performed without pooling. Studies have shown that viral loads in nasopharyngeal samples are comparable between children and adults.13 Though, recent studies have shown that viral loads might be slightly lower in very young children, but is probably not of clinical relevance.14 Therefore, we compared the results to asymptomatic adults visiting the adult emergency department, where AG testing was performed according to manufacturer instructions via unpooled nasopharyngeal swabbing. Sensitivity of the AG in asymptomatic adults was 38.9%, which is comparable to the results from the pediatric emergency department population. Although both cohorts are not directly comparable, we cautiously conclude that pooling of AG test swabs led to no dramatic decrease in diagnostic sensitivity. We would not recommend using pooled AG tests as a sole screening test for asymptomatic individuals, especially in a low disease prevalence setting. The performance of a test not only depends on test related sensitivity and specificity, but also on local endemicity of the disease. Especially if an individual with a positive AG test has a low likelihood of disease (eg, asymptomatic or fully vaccinated individuals, low prevalence setting), a confirmatory nucleic acid amplification test should follow.15 Though, they add an additional layer of protection by identifying most infectious but asymptomatic child and CG pairs at the time of hospital admission. Thus, they contribute significantly to COVID-19 control measures. False negative results are not uncommon with AG, but many such samples had PCR Ct values above 30, indicating low viral RNA counts, which falls in line with published literature.16, 17, 18 Consequently, many such individuals were identified after the infectious period has passed. The cut-off of Ct value < 30 as a marker for contagiousness was arbitrarily applied. The biggest benefit of AG in a pediatric emergency department lies in the rapid identification of highly contagious individuals while waiting for the PCR test results. Ct values are not directly comparable between assays, but the Robert Koch Institute, Germany's national Public Health Institute, used a Ct value cut-off of 30 as a guidance in the past.19 Other studies saw no viral growth already in samples with a Ct value above 24, whilst others not till Ct values above 33.20 , 21 Our study has several limitations. Only a small number of patients had SARS-CoV-2 infection, limiting the robustness of data. We are not able to answer the question whether pooling the AG test between the child and their CG reduced sensitivity. Because of the observational design, no control group was available. Comparison to an adult population without pooled antigen tests was used as a proxy, but the different sampling technique (mid-turbinate + oropharyngeal vs nasopharyngeal) and the different populations (children vs adults) allowed no direct comparison. Recent studies show that viral loads in children tend to be slightly lower compared to adults.14 This is partly explained by smaller swab sizes, but further complicates the comparison of both groups.
Our study shows that pooled antigen tests for SARS-CoV-2 of asymptomatic children and their CG inserts an additional layer of protection prior admission in a pediatric emergency department, while waiting for PCR test results. Simultaneous nasal mid-turbinate swabbing for the antigen and PCR test adds no additional strain to the child and pooling swabs from the child and their CG in a single AG keeps additional costs low.
Acknowledgments
None.
Footnotes
Funding/support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.Robert Koch Institute - Coronavirus SARS-CoV-2 - Testkriterien für die SARS-CoV-2 Diagnostik bei symptomatischen Patienten mit Verdacht auf COVID-19. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Teststrategie/Testkriterien_Herbst_Winter.html. Accessed March 23, 2021.
- 2.Johansson MA, Quandelacy TM, Kada S. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poline J, Gaschignard J, Leblanc C. Systematic SARS-CoV-2 screening at hospital admission in children:a French prospective multicenter study. Clin Infect Dis. 2020;72:2215–2217. doi: 10.1093/cid/ciaa1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity — a strategy for containment. New England J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 5.Liguoro I, Pilotto C, Bonanni M. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolai O, Rohardt C, Tobian F. Medrxiv; 2021. Anterior nasal versus nasal mid-turbinate sampling for a SARS-CoV-2 antigen-detecting rapid test: Does localisation or professional collection matter? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindner AK, Nikolai O, Rohardt C. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with professional-collected nasal versus nasopharyngeal swab. Eur Respir J. 2021;57 doi: 10.1183/13993003.04430-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pray IW, Ford L, Cole D. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1642–1647. doi: 10.15585/mmwr.mm695152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerutti F, Burdino E, Milia MG. Urgent need of rapid tests for SARS CoV-2 antigen detection: Evaluation of the SD-Biosensor antigen test for SARS-CoV-2. Journal of Clinical Virology. 2020;132 doi: 10.1016/j.jcv.2020.104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James AE, Gulley T, Kothari A, Holder K, Garner K, Patil N. Performance of the BinaxNOW coronavirus disease 2019 (COVID-19) Antigen Card test relative to the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay among symptomatic and asymptomatic healthcare employees. Infection Control & Hospital Epidemiol. undefined/ed;1–3. [DOI] [PMC free article] [PubMed]
- 11.Torres I, Poujois S, Albert E, Álvarez G, Colomina J, Navarro D. Point-of-care evaluation of a rapid antigen test (CLINITESTⓇ Rapid COVID-19 Antigen Test) for diagnosis of SARS-CoV-2 infection in symptomatic and asymptomatic individuals. J Infection. 2021;82:e11–e12. doi: 10.1016/j.jinf.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollock NR, Jacobs JR, Tran K. Performance and implementation evaluation of the Abbott BinaxNOW Rapid Antigen Test in a high-throughput drive-through community testing site in Massachusetts. J Clin Microbiol. 2021;59:e00083–21. doi: 10.1128/JCM.00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madera S, Crawford E, Langelier C, Tran NK. Nasopharyngeal SARS-CoV-2 viral loads in young children do not differ significantly from those in older children and adults. Scientific Rep. 2021;11:3044. doi: 10.1038/s41598-021-81934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TC, Biele G, Mühlemann B, Veith T, Schneider J, Beheim-Schwarzbach J. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021;373:eabi5273. doi: 10.1126/science.abi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC . Centers for Disease Control and Prevention; 2020. Interim Guidance for Antigen Testing for SARS-CoV-2.https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html Available at: Accessed July 12, 2021. [Google Scholar]
- 16.Albert E, Torres I, Bueno F. Field evaluation of a rapid antigen test (PanbioTM COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2021;27:472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagura-Ikeda M, Imai K, Tabata S. Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), Direct RT-qPCR, reverse transcription–loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol. 2020;58:e01438–20. doi: 10.1128/JCM.01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toptan T, Eckermann L, Pfeiffer AE. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J Clin Virol. 2021;135 doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RKI - COVID-19: Entlassungskriterien aus der Isolierung. 2020. Available at: https://edoc.rki.de/bitstream/handle176904686720_0316_Entlasskriterien_A3_V11.pdf. Accessed April 8, 2021. Available at: https://edoc.rki.de/handle/176904/6867. Accessed August 9, 2021.
- 20.Bullard J, Dust K, Funk D. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Scola B, Le Bideau M, Andreani J. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]