Abstract
Among the popular animal models of Parkinson’s disease (PD) commonly used in research are those that employ neurotoxins, especially 1-methyl- 4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP). This neurotoxin exerts it neurotoxicity by causing a barrage of insults, such as oxidative stress, mitochondrial apoptosis, inflammation, excitotoxicity, and formation of inclusion bodies acting singly and in concert, ultimately leading to dopaminergic neuronal damage in the substantia nigra pars compacta and striatum. The selective neurotoxicity induced by MPTP in the nigrostriatal dopaminergic neurons of the mouse brain has led to new perspectives on PD. For decades, the MPTP-induced mouse model of PD has been the gold standard in PD research even though it does not fully recapitulate PD symptomatology, but it does have the advantages of simplicity, practicability, affordability, and fewer ethical considerations and greater clinical correlation than those of other toxin models of PD. The model has rejuvenated PD research and opened new frontiers in the quest for more novel therapeutic and adjuvant agents for PD. Hence, this review summarizes the role of MPTP in producing Parkinson-like symptoms in mice and the experimental role of the MPTP-induced mouse model. We discussed recent developments of more promising PD therapeutics to enrich our existing knowledge about this neurotoxin using this model.
Keywords: Parkinson disease, MPTP, C57BL mouse and MPTP-induced PD mouse
INTRODUCTION
Parkinson’s disease (PD) is an insidiously progressive and irreversible neurodegenerative disease that mainly affects the older population [1]. Although the disease can appear at any age, the average age of onset is 60 years [2]. Garza-Ulloa [3] reported that PD is the second most popular neurodegenerative disease in the world after Alzheimer disease. PD is now the fastest growing neurological disorder and leading cause of disability globally with a total patient population from 1990 to 2016 of >6 million [4]. This number is expected to double to >12 million by 2040 [5]. Studies have shown that various factors, such as increasing life expectancy, increasing industrialization, and declining smoking rates, could increase the disease burden [6,7]. This rising disease incidence and prevalence globally make it a disease with huge economic, social, and public health importance [8].
Based on current thinking, two forms of PD exist: sporadic/late-onset and familial/early onset cases [9]. Epidemiological studies have reported that the familial form of PD only accounts for a few of the PD subjects, whereas the overwhelming majority of PD subjects have the sporadic type [10]. The etiology of PD is complex due to the heterogeneity of the disorder [11]. However, PD is believed to begin principally by degeneration of dopaminergic nigrostriatal neurons in the brain and secondarily by complex pathological mechanisms, including mitochondrial dysfunction, oxidative stress, apoptotic cell death, protein aggregation and misfolding, inflammation, excitotoxicity, loss of trophic factors, and other cell-death pathways [12]. PD patients present with a myriad of symptoms, including the four cardinal motor manifestations of tremor, rigidity, akinesia, and postural reflexes [13], as well as non-motor symptoms, such as dementia, anxiety, somnolence, urinary symptoms, attention deficit, hyposmia, and restless-leg syndrome, as the disease progresses [2,14]. The pathognomonic signs of PD are loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the formation of intraneuronal protein inclusions termed Lewy bodies (LBs), composed primarily of a-synuclein [15]. Jagmag et al. [16] also reported some neuronal losses in other parts of the brain, such as in the thalamic subnuclei and amygdala, serotoninergic neurons of the raphe nucleus, and the cholinergic nucleus basalis of Meynert, as the disease progresses.
Regardless of the tremendous advancement in the understanding of the disease mechanism, the presently approved PD treatments only provide limited therapeutic benefits [17]. This unmet clinical need to develop new therapeutic strategies has triggered further research to clarify the pathology of the disease. As a result, efforts have been made to emulate human PD by using animal models since studies have shown that they can mimic various aspects of PD features and thus support study of the disease pathophysiology and exploration of treatment possibilities [18].
The experimental animal models so far are of two main types: toxin models and genetic models. The transgenic models only simulate the familial form of PD, and the final neuropathological and behavioral features reminiscent of human PD are not fully recapitulated in this model [19]. The transgenic models also use transgenic technology, which makes them fairly expensive and thus not commonly used in PD research [19]. The common toxin models make use of some neurotoxins, such as paraquat, rotenone, 6-hydroxydopamine (6-OHDA), methamphetamine, and 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP) to induce dopaminergic neurodegeneration in brains of animals [10,20,21]. The extent to which these neurotoxins phenocopy the salient features of PD and their related mechanisms varies greatly, especially in the sporadic type of PD [22]. Although PD-toxin models have played a significant role in defining critical disease-related mechanisms and have been at the forefront of evaluating novel therapeutic approaches, they also cannot fully mirror symptoms reminiscent of human PD [23].
However, for decades, mouse models using MPTP have been among the most extensively used in PD research because they have the advantage of easy practicality, affordability, and fewer ethical considerations and greater clinical correlation than those of other toxin models [10, 24]. Of note, the PD neurotoxic potential of MPTP in humans, monkeys, rodents, zebrafish, and Caenorhabditis elegans has also been documented [1, 25]. Although studies have shown that MPTP-intoxicated monkeys provide the best results for PD pathology, such as LB-like inclusions, the MPTP-mouse model is still more popular because of its practicality and feasibility [10, 16]. Currently, research involving the MPTP-induced mouse model of PD is in vogue and has been on the increase [18]. Among mice, different strains or the same strain from another source shows strikingly different sensitivity to the MPTP concerning the loss of DA neurons in the SNpc and striatum [10]. The mouse strain most sensitive to MPTP intoxication is C57BL/6, followed by CD-1, and BALB, with the least being Swiss Webster [26, 27]. Therefore, to obtain the best reproducible PD results from one experiment to another, male mice of ≥8 weeks of age, average weight of 22 g, and the same mouse strain must be obtained from the same source [28].
MPTP-mouse models of PD have provided more insight into the etiology and pathophysiology of this debilitating disease [29]. The importance in providing researchers with a unique model platform for testing the efficacy of novel neuroprotective drugs cannot be underestimated. Thus, in this review, the use of MPTP in mice to recapitulate PD symptoms will be highlighted, and the pharmacokinetics and pharmacodynamics of MPTP, MPTP administration dynamics, mechanisms of MPTP-induced neurotoxicity, and MPTP-induced PD mouse model laboratory findings to enrich our understanding of this neurotoxin will be discussed. To further promote the use of the MPTP-induced mouse model of PD by researchers for developing more promising therapeutic strategies, this review will also highlight its advantages and disadvantages, its experimental role, and recent developments in PD therapeutics using the model. Using the keywords of Parkinson’s disease, MPTP, C57BL/6 mice, and MPTP-induced PD mouse, all of the relevant literature used in this review was searched and collected from credible scientific databases, including Science Direct, Scopus, PubMed, and Google Scholar. Searches for laboratory findings from studies using the MPTP-induced mouse model of PD and for recent developments in PD therapeutics using this model were restricted to papers published from 2019 to date.
Structure, pharmacokinetics, and pharmacodynamics of MPTP
MPTP is structurally a meperidine analog produced as a by-product in the process of synthesizing 1-methyl-4-phenyl-propionoxy-piperidine. Once this toxin is injected into the body of mice, it traverses the blood–brain barrier (BBB) into the central nervous system (CNS) with ease because of its lipophilicity [10]. In the CNS, monoamine oxidase type B (MAO-B) enzyme secreted by glial cells (astrocytes) converts MPTP to an intermediate metabolite, 1-methyl-4-phenyl-2,3-dihydropyridine (Figure 1), and subsequently to the final toxic metabolite, 1-methyl-4-phenylpyridinum (MPP+, Fig. 1) [30]. Cohen et al. [31] and Heikkila et al. [32] noticed striatal MPP+ depletion following treatment with MAO-B inhibitors, such as seleginine, which proved that inhibition of this enzyme significantly prevented formation of this toxic metabolite. MPP+, the active neurotoxin is a polar compound and as such, it cannot cross back through the BBB, indicating that it acts at the cellular level [28]. MPP+ selectively enters norepinephrine (NE) and dopaminergic (DA) neurons via the special transporters, NE transporter and DA transporter (DAT) respectively [33]. Studies by Takahashi et al. [34] proved that mice deficient in DAT were resistant to MPTP toxicity. Therefore, excessive expression of DAT will enhance MPTP neurotoxicity. Once in the NE/DA nerve cell, MPP+ forms a complex with neuromelanin in the axoplasm and is subsequently transported by vesicular monoamine transporter type 2 (VMAT-2) and stored in synaptosomal vesicles. This was confirmed by Gainetdinov et al. [35] in an experiment in which VMAT-2-deficient mice showed strikingly increased toxicity to MPTP. Therefore, selective toxicity of MPTP is directly related to the amount of DAT [36] and inversely to the amount of VMAT-2 [37]. MPP+ continues to accumulate in synaptosomal vesicles to a point when the threshold is surpassed and cell death of DA nigrostriatal neurons occurs in the SNpc and striatum (Figure 1).
FIGURE 1.
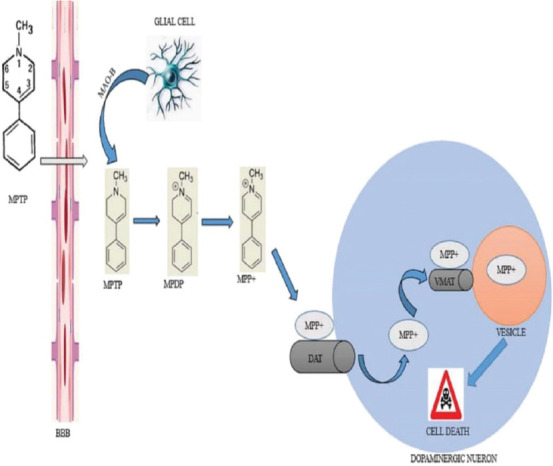
Structure, pharmacokinetics and pharmacodynamics of MPTP in the CNS. Upon injection of MPTP, it crosses the BBB and is converted to the toxic metabolite MPP+ by MAO-B. This metabolite is transported into the dopaminergic neuron by DAT. In the cytoplasm, MPP+ is further transported into vesicles by VMAT. Consequently, further concentration of MPP+ in the cytoplasm leads to a cascade of reactions that results in cell death. BBB: blood-brain barrier; CNS: central nervous system; DAT: dopamine transporter; MAO-B: monoamine oxidase type B; MPDP+: 1-methyl-4-phenyl-2, 3-dihydropyridine; MPP+: 1-methyl-4-phenylpyridinum; MPTP: 1-methyl-4- phenyl-1, 2, 3, 6-tetrahydropyridine; VMAT: vesicular monoamine transporter type 2.
Mechanisms of MPTP-induced neurotoxicity
Once the toxic metabolite of MPTP (MPP+) continues to accumulate and aggregates in synaptosomal vesicles of DA neurons, the amount eventually becomes too much in the cytoplasm and eventually triggers cell damage in the striatum and SNpc via the following pathways (Figure 2).
FIGURE 2.
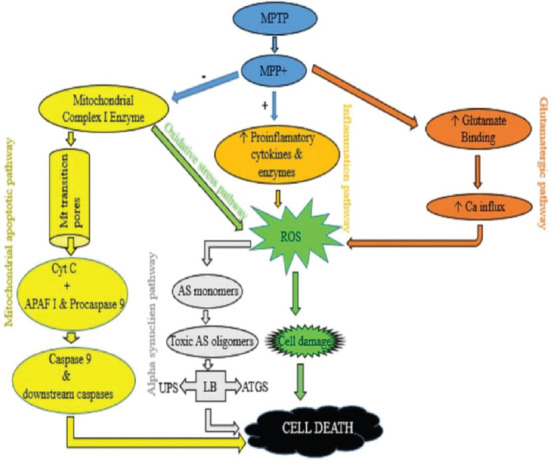
Summary of the neurotoxic pathways of MPTP. MPP+ causes inhibition of COMPLEX-1 in the mitochondria which leads to the opening of transitional pores and then release of cytochrome C which causes a cascade of reactions that leads to cell death (Mitochondrial apoptotic pathway). Inhibition of COMPLEX 1 also causes an increase in ROS which leads to cell damage and eventually cell death (Oxidative stress pathway). Further excessive production of ROS leads to formation of AS monomers, and the monomers then form toxic oligomers which then inhibits UPS and ATGS and eventually leads to cell death (Alpha synuclein pathway). MPP+ causes excessive binding of glutamate at the synaptic cleft. This causes Ca influx that leads to excessive production of ROS, which damages the cell and cell death occurs finally (Glutamatergic pathway). MPP+ activates microglia cell and induces release of proinflamatory cytokines/enzymes leading to excessive ROS production, then cell damage and eventually cell death (Inflammation pathway). APAF-1: apoptosis protease activating factor 1; AS: Alpha synuclien; ATGS: autophagy system; iNOS: Inducible nitric oxide synthase; LB: Lewy body; MPP+: 1-methyl-4-phenylpyridinium; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Mt: Mitochondria; ROS: Reactive oxygen species; UPS: ubiquitin-proteasome system; (-): downregulates; (+): upregulates; (↑): Activates.
Mitochondrial apoptotic pathway
MPP+ inhibits COMPLEX 1 in the mitochondria and induces less expression of anti-apoptotic proteins, such as Bcl2 [38, 39]. This inhibition hinders the electron transport chain and thus blocks ATP synthesis and increases reactive oxygen species (ROS) production leading to the opening of mitochondrial transition pores [28]. Cytochrome C is then released from a mitochondrion and forms a complex with pro-caspase-9 and apoptosis protease activating factor-1 [40]. The complex now formed activates caspase 9 and downstream caspases resulting in apoptosis and finally DA nigrostriatal cell death in the SNpc and striatum [41].
Oxidative stress pathway
MPP+ inhibits nicotinamide adenine dinucleotide dehydrogenase in the mitochondria and allows excessive ROS production, such as H2O2, NO, and hydroxyl radicals [42, 43]. These ROS overwhelm the cellular antioxidant defense mechanism and cause DA nigrostriatal cell damage in the SNpc and striatum through lipid peroxidation, DNA damage, and protein cross-linkage [42, 44, 45].
Alpha-synuclein pathway
Increases in ROS cause production of alpha-synuclein monomers [46]. As the levels of these monomers increase, they aggregate to form toxic alpha-synuclein oligomers. Oligomers of this kind can also be produced by mutation of the alpha-synuclein gene, SNCA [47, 48]. The oligomers inhibit the ubiquitin proteasome system (UPS) and autophagy system (ATGS), which are responsible for maintaining biochemical balance in the neuron [49, 50]. Failure of UPS leads to development of LBs, one of the pathological hallmarks of PD [28].
Inflammation pathway
MPP+ triggers an inflammatory process characterized by T-cell infiltration into the striatum and SNpc with microglia activation [33, 51]. Activated microglia release proinflammatory factors, such as TNF-a, PGE2, IFN-g, and ROS, such as NO and H2O2, which are all toxic to neurons [52]. Nagarajan et al. [17] documented that activated microglia have an intrinsic role in MPTP-induced neurotoxicity because they upregulate inducible NO synthase and nicotinamide adenine dinucleotide oxidase. These two enzymes produce SO42− and NO, and being ROS, they cause oxidative stress and thus lead to death of DA nigrostriatal neurons in the SNpc and striatum [13].
Glutamatergic pathway
MPP+ causes an increase in extracellular glutamate in the SNpc and striatum [53]. Glutamate binds to ionotrophic and metabotrophic receptors [52]. An increase in glutamate causes excessive and prolonged activity at the synaptic cleft, which causes an increase in the entry of ions, especially Ca2+ [33]. The influx of these ions increases the production of ROS, which leads to oxidative stress [44]. Also, an increase in glutamate can impair the function of the mitochondria resulting in a series of events that converts non-toxic levels of glutamate into higher cytotoxic levels [28].
MPTP administration dynamics
Different MPT-dosing regimens have been used by researchers to produce mouse-model features that closely mimic PD in humans [33]). The time course of any regimen used will determine the degree of apoptosis, striatal dopamine loss, and dopaminergic cell loss in the substantia nigra [54]. Compared with single administration, repeated administration of a particular regimen for a longer period produces more robust and irremediable neurodegeneration. Literature reports indicate that when more than one injection is given in 24 hours, it is called an acute administration regimen, whereas when a single injection is given daily for several consecutive or non-consecutive days or week, it is called a subacute or chronic administration regimen [13]. However, subacute and chronic regimens remain controversial because of the rapid toxicokinetics of the neurotoxin. For this reason, the most common regimen used irrespective of the aforementioned nomenclatures will be considered. The first of this regimen type involves a single MPTP injection for a total of four doses over 24 hours. In this regimen, striatal dopamine diminution can range from 40% (14 mg/kg per dose x4) to roughly 90% (20 mg/kg per dose x4) 7 days after the last MPTP dose depending on the doses given (Fig. 3) [54]. Another popular regimen was developed by Tatton and Kish [55]. Here, a single injection of MPTP free base 30 mg/kg is given daily for 5 consecutive days (Figure 3). In this method, 40%–50% striatal dopamine depletion (Fig. 3) and apoptosis is seen especially in young-adult C57/BL mice, and by day 21, the dopaminergic lesion stabilizes after administration of MPTP [54].
FIGURE 3.
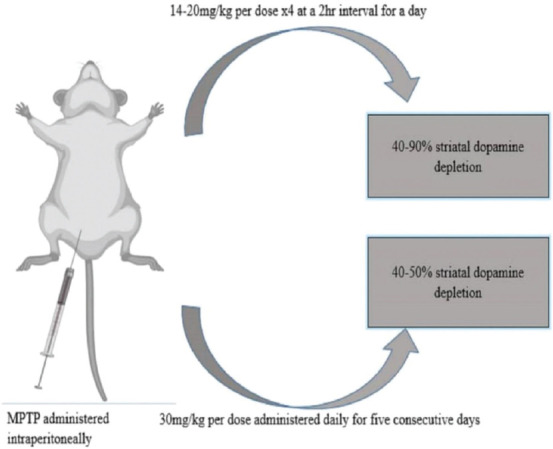
Schematic diagram of the commonest MPTP dosing regimen and route.
Regarding the administration site, many studies have agreed on the intraperitoneal route as ideal because several MPTP administered via this route remarkably impair motor function and induce DA neuronal damage [13,35]. There are conflicting reports regarding the appearance of Lewy body-like cytoplasmic inclusion when MPTP is administered intraperitoneally. To confirm this, Alvarez-Fischer et al. [56] and Shimoji et al. [57] found that 28-day chronic intraperitoneal MPTP administration (23 mg/kg/day), 7-day subacute intraperitoneal injection (20 mg/kg/day), and 28-day subcutaneous infusion (23 mg/kg/day) did not produce or trigger the formation of Lewy body neuronal inclusions. However, studies by Gibrat et al. [58] and Giráldez-Pérez et al. [59] demonstrated that chronic intraperitoneal infusion of MPTP (46 mg/kg/day) for 14 days with osmotic minipumps reproduced the formation of neuronal inclusions as observed by alpha-synuclein expression within the cytoplasm of dopaminergic neurons in the SNpc. It could be inferred that low-dose MPTP may not be adequate to facilitate formation of LBs. According to Jiang et al. [60], increased lactate levels in the brain are associated with formation of inclusion bodies simply because they can activate AMP-activated protein kinase and promote a-synuclein accumulation and phosphorylation.
Laboratory findings in the MPTP-induced mouse model of PD
The MPTP neurotoxin is the gold standard for studying and understanding the processes involved in the DA nigrostriatal neuron death in PD [61]. Studies have used different MPTP-dosing regimens in mice over the years, and similar findings have been reported in virtually all of the studies; i.e., significant motor impairment and damage to the nigrostriatal DA pathway with marked loss of DA neurons in the SNpc and striatum [62, 63]. This explicit and reproducible neurotoxic effect on the nigrostriatal system is an exclusive asset of this model and is similar to the neurotoxic effect seen in PD patients [64]. It is important to note that LBs, which are one of the pathological hallmarks of PD, were not examined in the reported studies in this section probably because the model seldom shows this hallmark [21]. Since studies have shown MPTP to be selectively more toxic to the C57BL/6 mouse strain [28], only findings on this mouse strain will be reported in this section. Table 1 summarizes some laboratory findings in an MPTP-induced C57BL/6 mouse model of PD.
TABLE 1.
Laboratory findings in MPTP-induced C57BL/6 mouse model of PD
Advantages of MPTP-induced mouse model of PD
The ability of the MPTP-mouse model to almost mirror the parkinsonian symptoms seen in human PD is the main reason for its usage [13,27].
Has helped to improve our knowledge of the molecular and cellular mechanism behind PD [18, 72, 73].
Is cheap, easy to handle, and has fewer ethical considerations than those of other toxin-induced animal models [19,74].
Reveals non-motor symptoms of PD [75].
In electrophysiological studies, Wallace et al. [76] reported the role of MPTP-mouse models in aiding deep brain stimulation-related therapy.
Has also helped in developing promising therapeutic strategies for neuroprotection and neurorestoration [77].
Has helped advance our understanding of the role played by mitochondrial dysfunction in PD [33, 78].
Has enhanced our knowledge of the role of autophagy in PD pathogenesis [79, 80].
According to Filograna et al. [81], the MPTP-mouse model has led to significant improvement in clinical research in PD.
Disadvantages of MPTP-induced mouse model of PD
Recent developments in therapeutics regarding PD using the MPTP-induced mouse model of PD
Efforts aimed at developing anti-parkinsonian therapeutics have shown encouraging results in a preclinical MPTP neurotoxic mouse model [23, 29, 65, 69, 83-85]. These therapeutics have proven their efficacy in aiding diagnosis and their ability to slow down, reverse, or actually prevent PD symptoms in this preclinical model. However, whether or not these preclinical findings can be translated into clinical trials is a huge question. The good news is that these therapeutics can stimulate translational research toward their neuroprotective adjuvant potentials in human PD. Table 2 summarizes the recent development in PD therapeutics using the MPTP-induced C57BL/6 mouse model of PD in 2019 and 2020.
TABLE 2.
Recent development in PD therapeutics using MPTP-induced mouse model of PD.

CONCLUSIONS
Transgenic and neurotoxin models have been used to mimic parkinsonian symptoms that are reminiscent of human PD. Although they all have the limitation of not fully mimicking PD symptoms, the MPTP-induced mouse model of PD now stands out among the other toxin models in PD research. This model is cheap to acquire, easy to handle, has fewer considerations than those of other toxin-induced animal models, is more practical, and shows good clinical correlation. Despite its shortcomings, this model has enhanced our understanding of the cellular and molecular mechanisms behind DA neuron death in the SNpc and striatum and provided researchers an avenue for exploring the neuroprotective and neurorestorative potentials of more novel therapeutic and adjuvant agents for PD. We believe that this model can further be perfected under the unrelenting efforts of researchers so that all of the pathological and phenotypical features reminiscent of human PD can be recapitulated. If a cocktail of miRNA or siRNA is introduced into the MPTP mouse-model system, a more robust and precise PD model showing all of the symptoms of human PD is likely to be obtained. Additionally, an improved model can be produced by combining the MPTP neurotoxin and genetic mouse models so that the progressive neurodegeneration associated with PD can fully be appreciated. Based on this premise, the MPTP-induced mouse model of PD may help researchers develop treatments that may allow PD patients to lead very close to normal lives.
ACKNOWLEDGMENTS
Special acknowledgment to the Ministry of Education, government of Malaysia for their financial assistance through research grants under the Fundamental Research Grant Scheme (FRGS), FRGS/1/2019/STG03/UPM/02/15. We thank the anonymous reviewers for their insightful comments on the PDF version of MPTP-Induced Mouse Model of Parkinson’s Disease: A Promising Direction for Therapeutic Strategies.
Footnotes
Conflict of interest statement: The authors declare no conflict of interests.
Funding: Special acknowledgement to the Ministry of Education, government of Malaysia for their financial assistance throughout the research grants under Fundamental Research Grant Scheme (FRGS), FRGS/1/2019/STG03/UPM/02/15.
REFERENCES
- 1.Rai SN, Singh P. Advancement in the modelling and therapeutics of Parkinson's disease. Journal of Chemical Neuroanatomy. 2020;104:101752. doi: 10.1016/j.jchemneu.2020.101752. [DOI] [PubMed] [Google Scholar]
- 2.Pagano G, Ferrara N, Brooks DJ, Pavese N. Age at onset and Parkinson disease phenotype. Neurology. 2016;86(15):1400–7. doi: 10.1212/WNL.0000000000002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garza-Ulloa J. Update on Parkinson's disease. American Journal of Biomedical Science and Research. 2019;2(6) [Google Scholar]
- 4.Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi JY, Collado-Mateo D, Dahodwala N. Global, regional, and national burden of Parkinson's disease, 1990–2016:a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2018;17(11):939–53. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi A, Berger K, Chen H, Leslie D, Mailman RB, Huang X. Projection of the prevalence of Parkinson's disease in the coming decades:Revisited. Movement Disorders. 2018 Jan;33(1):156–9. doi: 10.1002/mds.27063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darweesh SK, Raphael KG, Brundin P, Matthews H, Wyse RK, Chen H, Bloem BR. Parkinson matters. Journal of Parkinson's Disease. 2018 Jan 1;8(4):495–8. doi: 10.3233/JPD-181374. doi:10.3233/JPD-181374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Boeve BF, Graff-Radford J, Rocca WA, Mielke MM. Survival and causes of death among people with clinically diagnosed synucleinopathies with parkinsonism:a population-based study. JAMA Neurology. 2017;74(7):839–846. doi: 10.1001/jamaneurol.2017.0603. doi:10.1001/jamaneurol.2017.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, Hamavid H, Horst C, Johnson EK, Joseph J, Lavado R. US spending on personal health care and public health, 1996-2013. JAMA. 2016;316(24):2627–2646. doi: 10.1001/jama.2016.16885. doi:10.1001/jama.2016.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson's:from familial to sporadic disease. Trends in Biochemical Sciences. 2015;40(4):200–10. doi: 10.1016/j.tibs.2015.02.003. https://doi.org/10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Zeng XS, Geng WS, Jia JJ. Neurotoxin-induced animal models of Parkinson disease:pathogenic mechanism and assessment. ASN Neuro. 2018;10:175909141–438. doi: 10.1177/1759091418777438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai C, Lim KL. Genetic insights into sporadic Parkinson's disease pathogenesis. Current Genomics. 2013;14(8):486–501. doi: 10.2174/1389202914666131210195808. https://doi.org/10.2174/ 1389202914666131210195808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimaki T, Saiki S, Tashiro E, Yamada D, Kitagawa M, Hattori N, Imoto M. Identification of licopyranocoumarin and glycyrurol from herbal medicines as neuroprotective compounds for Parkinson's disease. PLoS One. 2014;9(6):e100395. doi: 10.1371/journal.pone.0100395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nataraj J, Manivasagam T, Thenmozhi AJ, Essa MM. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutritional Neuroscience. 2016;19(6):237–46. doi: 10.1179/1476830515Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Youn J, Shin H, Cho JW. Nonmotor symptoms in drug-induced parkinsonism and drug-naïve Parkinson disease. Canadian Journal of Neurological Sciences. 2013 Jan;40(1):36–41. doi: 10.1017/s0317167100012920. [DOI] [PubMed] [Google Scholar]
- 15.Kouli A, Torsney KM, Kuan WL. Parkinson's disease:etiology, neuropathology, and pathogenesis. Exon Publications. 2018:3–26. [PubMed] [Google Scholar]
- 16.Jagmag SA, Tripathi N, Shukla SD, Maiti S, Khurana S. Evaluation of models of Parkinson's disease. Frontiers in Neuroscience. 2016;9:503. doi: 10.3389/fnins.2015.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagarajan S, Chellappan DR, Chinnaswamy P, Thulasingam S. Ferulic acid pretreatment mitigates MPTP-induced motor impairment and histopathological alterations in C57BL/6 mice. Pharmaceutical Biology. 2015;2;53(11):1591–601. doi: 10.3109/13880209.2014.993041. [DOI] [PubMed] [Google Scholar]
- 18.Kin K, Yasuhara T, Kameda M. Animal models for Parkinson's disease research:trends in the 2000s. International Journal of Molecular Sciences. 2019;20(21):5402. doi: 10.3390/ijms20215402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blesa J, Przedborski S. Parkinson's disease:animal models and dopaminergic cell vulnerability. Frontiers in Neuroanatomy. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colle D, Santos DB, Naime AA, Gonçalves CL, Ghizoni H, Hort MA, Farina M. Early Postnatal Exposure to Paraquat and Maneb in Mice Increases Nigrostriatal Dopaminergic Susceptibility to a Re-challenge with the Same Pesticides at Adulthood:Implications for Parkinson's Disease. Neurotoxicity Research. 2020;37(1):210–26. doi: 10.1007/s12640-019-00097-9. [DOI] [PubMed] [Google Scholar]
- 21.Kasanga EA, Owens CL, Cantu MA, Richard AD, Davis RW, McDivitt LM, Blancher B, Pruett BS, Tan C, Gajewski A, Manfredsson FP. GFR-a1 Expression in Substantia Nigra Increases Bilaterally Following Unilateral Striatal GDNF in Aged Rats and Attenuates Nigral Tyrosine Hydroxylase Loss Following 6-OHDA Nigrostriatal Lesion. ACS Chemical Neuroscience. 2019 Sep 20;10(10):4237–49. doi: 10.1021/acschemneuro.9b00291. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Cruz A, Romo-Mancillas A, Mendiola-Precoma J, Escobar-Cabrera JE, García-Alcocer G, Berumen LC. Effect of valerenic acid on neuroinflammation in a MPTP-induced mouse model of Parkinson's disease. IBRO Reports. 2020;8:28–35. doi: 10.1016/j.ibror.2019.12.002. https://doi.org/10.1016/j.ibror.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav SK, Rai SN, Singh SP. Mucuna pruriens reduces inducible nitric oxide synthase expression in Parkinsonian mice model. Journal of Chemical Neuroanatomy. 2017;80:1–10. doi: 10.1016/j.jchemneu.2016.11.009. https://doi.org/10.1016/j.jchemneu.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson's disease. Journal of Biomedicine and Biotechnology. 2012;2012:845618. doi: 10.1155/2012/845618. doi:10.1155/2012/845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalyn M, Hua K, Mohd Noor S, Wong CE, Ekker M. Comprehensive Analysis of Neurotoxin-Induced Ablation of Dopaminergic Neurons in Zebrafish Larvae. Biomedicines. 2019;8(1):1. doi: 10.3390/biomedicines8010001. doi:10.3390/biomedicines8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhaduri B, Abhilash PL, Alladi PA. Baseline striatal and nigral interneuronal protein levels in two distinct mice strains differ in accordance with their MPTP susceptibility. Journal of Chemical Neuroanatomy. 2018;91:46–54. doi: 10.1016/j.jchemneu.2018.04.005. DOI:10.1016/j.jchemneu.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Vidyadhara DJ, Yarreiphang H, Abhilash PL, Raju TR, Alladi PA. Differential expression of calbindin in nigral dopaminergic neurons in two mice strains with differential susceptibility to 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Journal of Chemical Neuroanatomy. 2016;76:82–89. doi: 10.1016/j.jchemneu.2016.01.001. https://doi.org/10.1016/j.jchemneu.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Pasquali L, Ienco EC, Fornai F. Kostrzewa, editor. MPTP Neurotoxicity:Actions, Mechanisms, and Animal Modelling of Parkinson's Disease. Handbook of Neurotoxicity. :236–264. [Google Scholar]
- 29.Mei M, Zhou Y, Liu M, Zhao F, Wang C, Ding J, Lu M, Hu G. Antioxidant and anti-inflammatory effects of dexrazoxane on dopaminergic neuron degeneration in rodent models of Parkinson's disease. Neuropharmacology. 2019;160:107758. doi: 10.1016/j.neuropharm.2019.107758. https://doi.org/10.1016/j.neuropharm.2019.107758. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Araki T, Imai Y, Takahashi A, Itoyama Y. Protection of dopaminergic neurons with a novel astrocyte modulating agent (R)-(-)-2-propyloctanoic acid (ONO-2506) in an MPTP-mouse model of Parkinson's disease. Journal of the Neurological Sciences. 2003;208(1-2):9–15. doi: 10.1016/s0022-510x(02)00411-2. DOI:10.1016/s0022-510x(02)00411-2. [DOI] [PubMed] [Google Scholar]
- 31.Cohen G, Pasik P, Cohen B, Leist A, Mytilineou C. Pargyline and deprenyl prevent the neurotoxicity of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) in monkeys. European Journal of Pharmacology. 1984;106(1):209–10. doi: 10.1016/0014-2999(84)90700-3. DOI:10.1016/0014-2999(84)90700-3. [DOI] [PubMed] [Google Scholar]
- 32.Heikkila RE, Hess A, Duvoisin RC. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine in mice. Science. 1984;224(4656):1451–1453. doi: 10.1126/science.6610213. DOI:10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- 33.Meredith GE, Rademacher DJ. MPTP mouse models of Parkinson's disease:an update. Journal of Parkinson's Disease. 2011;1(1):19–33. doi: 10.3233/JPD-2011-11023. DOI:10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice:heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion and enhanced MPTP toxicity. Proceedings of the National Academy of Sciences. 1997;94(18):9938–9943. doi: 10.1073/pnas.94.18.9938. https://doi.org/10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. Journal of Neurochemistry. 1998;70(5):1973–8. doi: 10.1046/j.1471-4159.1998.70051973.x. https://doi.org/10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe Y, Himeda T, Araki T. Mechanisms of MPTP toxicity and their implications for therapy of Parkinson's disease. Medical Science Monitor. 2005;11(1):RA17–23. [PubMed] [Google Scholar]
- 37.Chen CX, Huang SY, Zhang L, Liu YJ. Synaptophysin enhances the neuroprotection of VMAT2 in MPP+-induced toxicity in MN9D cells. Neurobiology of Disease. 2005;19(3):419–26. doi: 10.1016/j.nbd.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Gluck MR, Krueger MJ, Ramsay RR, Sablin SO, Singer TP, Nicklas WJ. Characterization of the inhibitory mechanism of 1-methyl-4-phenylpyridinium and 4-phenylpyridine analogs in inner membrane preparations. Journal of Biological Chemistry. 1994;269(5):3167–74. [PubMed] [Google Scholar]
- 39.Scotcher KP, Irwin I, DeLanney LE, Langston JW, Di Monte D. Effects of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on ATP levels of mouse brain synaptosomes. Journal of Neurochemistry. 1990;54(4):1295–301. doi: 10.1111/j.1471-4159.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson's disease. Journal of Neural Transmission. 2001;108(11):1263–82. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- 41.Basil AH, Sim JP, Lim GG, Lin S, Chan HY, Engelender S, Lim KL. AF-6 protects against dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson's disease. Frontiers in Cellular Neuroscience. 2017;11:241. doi: 10.3389/fncel.2017.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andreassen OA, Ferrante RJ, Dedeoglu A, Albers DW, Klivenyi P, Carlson EJ, Epstein CJ, Beal MF. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Experimental Neurology. 2001;167(1):189–95. doi: 10.1006/exnr.2000.7525. [DOI] [PubMed] [Google Scholar]
- 43.Maragos WF, Jakel R, Chesnut D, Pocernich CB, Butterfield DA, St Clair D, Cass WA. Methamphetamine toxicity is attenuated in mice that overexpress human manganese superoxide dismutase. Brain Research. 2000;878(1-2):218–22. doi: 10.1016/s0006-8993(00)02707-4. [DOI] [PubMed] [Google Scholar]
- 44.Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. Dopaminergic cell death induced by MPP+, oxidant and specific neurotoxicants shares the common molecular mechanism. Journal of Neurochemistry. 2001;76(4):1010–21. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Raamsdonk JM, Vega IE, Brundin P. Oxidative stress in neurodegenerative disease:causation or association? Oncotarget. 2017;8(7):10777. doi: 10.18632/oncotarget.14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fornai F, Carrì MT, Ferri A, Paolucci E, Prisco S, Bernardi G, Rotilio G, Mercuri NB. Resistance to striatal dopamine depletion induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in mice expressing human mutant Cu, Zn superoxide dismutase. Neuroscience Letters. 2002;325(2):124–8. doi: 10.1016/s0304-3940(02)00252-5. [DOI] [PubMed] [Google Scholar]
- 47.Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ. Modeling PD pathogenesis in mice:advantages of a chronic MPTP protocol. Parkinsonism and Related Disorders. 2008;14:S112–5. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sulzer D. a-synuclein and cytosolic dopamine:stabilizing a bad situation. Nature Medicine. 2001;7(12):1280–2. doi: 10.1038/nm1201-1280. [DOI] [PubMed] [Google Scholar]
- 49.Castino R, Lazzeri G, Lenzi P, Bellio N, Follo C, Ferrucci M, Fornai F, Isidoro C. Suppression of autophagy precipitates neuronal cell death following low doses of methamphetamine. Journal of Neurochemistry. 2008;106(3):1426–39. doi: 10.1111/j.1471-4159.2008.05488.x. [DOI] [PubMed] [Google Scholar]
- 50.Dehay B, Bové J, Rodríguez-Muela N, Perier C, Recasens A, Boya P, Vila M. Pathogenic lysosomal depletion in Parkinson's disease. Journal of Neuroscience. 2010;30(37):12535–44. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao JJ, Li KS, Shen YQ. Activated immune cells in Parkinson's disease. Journal of Neuroimmune Pharmacology. 2011;6(3):323–9. doi: 10.1007/s11481-011-9280-9. [DOI] [PubMed] [Google Scholar]
- 52.Mullin S, Schapira AH. Pathogenic mechanisms of neurodegeneration in Parkinson disease. Neurologic Clinics. 2015;33(1):1–7. doi: 10.1016/j.ncl.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Meredith GE, Totterdell S, Beales M, Meshul CK. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson's disease. Experimental Neurology. 2009;219(1):334–40. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nature Protocols. 2007;2(1):141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 55.Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77(4):1037–48. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Fischer D, Guerreiro S, Hunot S, Saurini F, Marien M, Sokoloff P, Hirsch EC, Hartmann A, Michel PP. Modelling Parkinson-like neurodegeneration via osmotic minipump delivery of MPTP and probenecid. Journal of Neurochemistry. 2008;107(3):701–11. doi: 10.1111/j.1471-4159.2008.05651.x. [DOI] [PubMed] [Google Scholar]
- 57.Shimoji M, Zhang L, Mandir AS, Dawson VL, Dawson TM. Absence of inclusion body formation in the MPTP mouse model of Parkinson's disease. Molecular Brain Research. 2005;134(1):103–8. doi: 10.1016/j.molbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Gibrat C, Saint-Pierre M, Bousquet M, Lévesque D, Rouillard C, Cicchetti F. Differences between subacute and chronic MPTP mice models:investigation of dopaminergic neuronal degeneration and a-synuclein inclusions. Journal of Neurochemistry. 2009;109(5):1469–82. doi: 10.1111/j.1471-4159.2009.06072.x. [DOI] [PubMed] [Google Scholar]
- 59.Giráldez-Pérez RM, Antolín-Vallespín M, Muñoz MD, Sánchez-Capelo A. Models of a-synuclein aggregation in Parkinson's disease. Acta Neuropathologica Communications. 2014;2(1):176. doi: 10.1186/s40478-014-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang P, Gan M, Ebrahim AS, Castanedes-Casey M, Dickson DW, Yen SH. Adenosine monophosphate-activated protein kinase overactivation leads to accumulation of a-synuclein oligomers and decrease of neurites. Neurobiology of Aging. 2013;34(5):1504–15. doi: 10.1016/j.neurobiolaging.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin QS, Chen P, Wang WX, Lin CC, Zhou Y, Yu LH, Lin YX, Xu YF, Kang DZ. RIP1/RIP3/MLKL mediates dopaminergic neuron necroptosis in a mouse model of Parkinson disease. Laboratory Investigation. 2019;10:1–9. doi: 10.1038/s41374-019-0319-5. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Yu X, Zhang P, Ma Y, Wang L, Xu H, Sui D. Neuroprotective effects of pramipexole transdermal patch in the MPTP-induced mouse model of Parkinson's disease. Journal of Pharmacological Sciences. 2018;138(1):31–7. doi: 10.1016/j.jphs.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Zhao WZ, Wang HT, Huang HJ, Lo YL, Lin AM. Neuroprotective effects of baicalein on acrolein-induced neurotoxicity in the nigrostriatal dopaminergic system of rat brain. Molecular Neurobiology. 2018;55(1):130–7. doi: 10.1007/s12035-017-0725-x. [DOI] [PubMed] [Google Scholar]
- 64.Chung ES, Lee G, Lee C, Ye M, Chung HS, Kim H, Sung-joo SB, Hwang DS, Bae H. Bee venom phospholipase A2, a novel Foxp3+regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of Parkinson's disease. The Journal of Immunology. 2015;195(10):4853–60. doi: 10.4049/jimmunol.1500386. [DOI] [PubMed] [Google Scholar]
- 65.Togashi K, Hasegawa M, Nagai J, Kotaka K, Yazawa A, Takahashi M, Masukawa D, Goshima Y, Hensley K, Ohshima T. Lanthionine ketimine ester improves outcome in an MPTP-induced mouse model of Parkinson's disease via suppressions of CRMP2 phosphorylation and microglial activation. Journal of the Neurological Sciences. 2020:116802. doi: 10.1016/j.jns.2020.116802. https://doi.org/10.1016/j.jns.2020.116802. [DOI] [PubMed] [Google Scholar]
- 66.Ma L, Song J, Sun X, Ding W, Fan K, Qi M, Xu Y, Zhang W. Role of microtubule-associated protein 6 glycosylated with Gal-(b-13)-GalNAc in Parkinson's disease. Aging (Albany NY) 2019 Jul 15;11(13):4597. doi: 10.18632/aging.102072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim KH, Kim M, Lee J, Jeon HN, Kim SH, Bae H. Comparison of the protective effects of bee venom extracts with varying pla2 compositions in a mouse model of parkinson's disease. Toxins. 2019;11(6):358. doi: 10.3390/toxins11060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee YY, Park JS, Leem YH, Park JE, Kim DY, Choi YH, Park EM, Kang JL, Kim HS. The phosphodiesterase 10 inhibitor papaverine exerts anti-inflammatory and neuroprotective effects via the PKA signaling pathway in neuroinflammation and Parkinson's disease mouse models. Journal of Neuroinflammation. 2019;16(1):1–7. doi: 10.1186/s12974-019-1649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rao SP, Sharma N, Kalivendi SV. Embelin averts MPTP-induced dysfunction in mitochondrial bioenergetics and biogenesis via activation of SIRT1. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2020;1861(3):148157. doi: 10.1016/j.bbabio.2020.148157. [DOI] [PubMed] [Google Scholar]
- 70.Xue B, Xiao W, Tian H. Nei-like 1 inhibition results in motor dysfunction and promotes inflammation in Parkinson's disease mice model. Biochemical and Biophysical Research Communications. 2020;521(1):245–51. doi: 10.1016/j.bbrc.2019.10.118. [DOI] [PubMed] [Google Scholar]
- 71.Haga H, Yamada R, Izumi H, Shinoda Y, Kawahata I, Miyachi H, Fukunaga K. Novel fatty acid-binding protein 3 ligand inhibits dopaminergic neuronal death and improves motor and cognitive impairments in Parkinson's disease model mice. Pharmacology Biochemistry and Behavior. 2020:172891. doi: 10.1016/j.pbb.2020.172891. [DOI] [PubMed] [Google Scholar]
- 72.Dutta D, Kundu M, Mondal S, Roy A, Ruehl S, Hall DA, Pahan K. RANTES-induced invasion of Th17 cells into substantia nigra potentiates dopaminergic cell loss in MPTP mouse model of Parkinson's disease. Neurobiology of Disease. 2019;132:104575. doi: 10.1016/j.nbd.2019.104575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson's disease:targets for potential treatments. Translational Neurodegeneration. 2017;6(1):28. doi: 10.1186/s40035-017-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yun JW, Ahn JB, Kang BC. Modeling Parkinson's disease in the common marmoset (Callithrix jacchus):overview of models, methods, and animal care. Laboratory Animal Research. 2015;31(4):155–65. doi: 10.5625/lar.2015.31.4.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choudhury GR, Daadi MM. Charting the onset of Parkinson-like motor and non-motor symptoms in nonhuman primate model of Parkinson's disease. PloS One. 2018;13(8):e0202770. doi: 10.1371/journal.pone.0202770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, Benabid AL. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130(8):2129–45. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- 77.Mingazov ER, Khakimova GR, Kozina EA, Medvedev AE, Buneeva OA, Bazyan AS, Ugrumov MV. MPTP mouse model of preclinical and clinical Parkinson's disease as an instrument for translational medicine. Molecular Neurobiology. 2018;55(4):2991–3006. doi: 10.1007/s12035-017-0559-6. [DOI] [PubMed] [Google Scholar]
- 78.Ding Y, Kong D, Zhou T, Xin C, Xu J, Wang Q, Zhang H, Wu Q, Lu X, Lim K, Ma B. a-Arbutin Protects Against Parkinson's Disease-Associated Mitochondrial Dysfunction In Vitro and In Vivo. Neuromolecular Medicine. 2020;22(1):56–67. doi: 10.1007/s12017-019-08562-6. https://doi.org/10.1007/s12017-019-08562-6. [DOI] [PubMed] [Google Scholar]
- 79.Hou X, Watzlawik JO, Fiesel FC, Springer W. Autophagy in Parkinson's disease. Journal of Molecular Biology. 2020;432(8):2651–72. doi: 10.1016/j.jmb.2020.01.037. https://doi.org/10.1016/j.jmb.2020.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu X, Song Q, Li X, Li D, Zhang Q, Meng W, Zhao Q. Neuroprotective effects of Kukoamine A on neurotoxin-induced Parkinson's model through apoptosis inhibition and autophagy enhancement. Neuropharmacology. 2017;117:352–63. doi: 10.1016/j.neuropharm.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 81.Filograna R, Beltramini M, Bubacco L, Bisaglia M. Anti-oxidants in Parkinson's disease therapy:a critical point of view. Current Neuropharmacology. 2016;14(3):260–71. doi: 10.2174/1570159X13666151030102718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindholm D, Mäkelä J, Di Liberto V, Mudo G, Belluardo N, Eriksson O, Saarma M. Current disease modifying approaches to treat Parkinson's disease. Cellular and Molecular Life Sciences. 2016;73(7):1365–79. doi: 10.1007/s00018-015-2101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh SS, Rai SN, Birla H, Zahra W, Rathore AS, Singh SP. NF-kB-mediated neuroinflammation in Parkinson's disease and potential therapeutic effect of polyphenols. Neurotoxicity Research. 2020;37(3):491–507. doi: 10.1007/s12640-019-00147-2. [DOI] [PubMed] [Google Scholar]
- 84.Liu Q, Guo X, Huang Z, He Q, Zhu D, Zhang S, Peng Z, Che Y, Feng X. Anti-neuroinflammatory effects of dimethylaminomylide (DMAMCL ie, ACT001) are associated with attenuating the NLRP3 inflammasome in MPTP-induced Parkinson disease in mice. Behavioural Brain Research. 2020;383:112539. doi: 10.1016/j.bbr.2020.112539. [DOI] [PubMed] [Google Scholar]
- 85.Rai SN, Zahra W, Singh SS, Birla H, Keswani C, Dilnashin H, Rathore AS, Singh R, Singh RK, Singh SP. Anti-inflammatory activity of ursolic acid in MPTP-induced parkinsonian mouse model. Neurotoxicity Research. 2019;36(3):452–62. doi: 10.1007/s12640-019-00038-6. https://doi.org/10.1007/s12640-019-00038-6. [DOI] [PubMed] [Google Scholar]
- 86.Ahn S, Liu QF, Jang JH, Jeong HJ, Kim Y, Kim DH, Jeong G, Oh ST, Park SU, Cho SY, Park HJ. Gami-Chunggan Formula prevents motor dysfunction in MPTP/p-induced and A53T a-synuclein overexpressed Parkinson's disease mouse model though DJ-1 and BDNF expression. Frontiers in Aging Neuroscience. 2019;11:230. doi: 10.3389/fnagi.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li CL, Tsuang YH, Tsai TH. Neuroprotective Effect of Schisandra Chinensis on Methyl-4-Phenyl-1 2, 3, 6-Tetrahydropyridine-Induced. Parkinsonian Syndrome in C57BL/6 Mice. Nutrients. 2019;11(7):1671. doi: 10.3390/nu11071671. doi:10.3390/nu11071671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu M, Gong D. A Mouse Model of 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine (MPTP)-Induced Parkinson Disease Shows that 2-Aminoquinoline Targets JNK Phosphorylation. Medical Science Monitor:International Medical Journal of Experimental and Clinical Research. 2020;26:e920989–1. doi: 10.12659/MSM.920989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park JS, Leem YH, Park JE, Kim DY, Kim HS. Neuroprotective effect of b-lapachone in MPTP-induced Parkinson's disease mouse model:involvement of astroglial p-AMPK/Nrf2/HO-1 signaling pathways. Biomolecules and Therapeutics. 2019;27(2):178. doi: 10.4062/biomolther.2018.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhi Y, Jin Y, Pan L, Zhang A, Liu F. Schisandrin A ameliorates MPTP-induced Parkinson's disease in a mouse model via regulation of brain autophagy. Archives of Pharmacal Research. 2019;42(11):1012–20. doi: 10.1007/s12272-019-01186-1. [DOI] [PubMed] [Google Scholar]
- 91.Zhu YL, Sun MF, Jia XB, Cheng K, Xu YD, Zhou ZL, Zhang PH, Qiao CM, Cui C, Chen X, Yang XS. Neuroprotective effects of Astilbin on MPTP-induced Parkinson's disease mice:Glial reaction, a-synuclein expression and oxidative stress. International Immunopharmacology. 2019;66:19–27. doi: 10.1016/j.intimp.2018.11.004. [DOI] [PubMed] [Google Scholar]


