Highlights
-
•
There are estimated 1.93 million new CRC cases diagnosed, and 0.94 million CRC caused deaths in 2020 worldwide.
-
•
The global new CRC cases is predicted to reach 3.2 million in 2040.
-
•
China and the United States have the highest estimated number of new CRC cases in the next 20 years.
-
•
The number of new CRC cases is increased from 0.56 million (2020) to 0.91 million (2040) in China.
-
•
The number of new CRC cases is increased from 0.16 million (2020) to 0.21 million (2040) in the United States.
Keywords: Colorectal cancer, Epidemiology, Projection, Risk factors, Prevention
Abstract
As the third most common malignancy and the second most deadly cancer, colorectal cancer (CRC) induces estimated 1.9 million incidence cases and 0.9 million deaths worldwide in 2020. The incidence of CRC is higher in highly developed countries, and it is increasing in middle- and low-income countries due to westernization. Moreover, a rising incidence of early-onset CRC is also emerging. The large number of CRC cases poses a growing global public health challenge. Raising awareness of CRC is important to promote healthy lifestyle choices, novel strategies for CRC management, and implementation of global screening programs, which are critical to reducing CRC morbidity and mortality in the future. CRC is a heterogeneous disease, and its subtype affiliation influences prognosis and therapeutic response. An accurate CRC subtype classification system is of great significance for basic research and clinical outcome. Here, we present the global epidemiology of CRC in 2020 and projections for 2040, review the major CRC subtypes to better understand CRC molecular basis, and summarize current risk factors, prevention, and screening strategies for CRC.
Graphical abstract
Introduction
The advancements made in understanding colorectal cancer (CRC) pathophysiology have led to the increased treatment options, including endoscopic and surgical excision, radiotherapy, immunotherapy, palliative chemotherapy, targeted therapy, and extensive surgery and local ablative therapies for metastases [1, 2]. These treatments have effectively inhibited cancer progression and prolonged overall survival. The implementation of population-based screen of CRC such as fecal occult blood test and endoscopy, also greatly enhances the overall survival and leads to good prospects of a cure for CRC [3], [4], [5], [6]. In the United States, the mortality rates of CRC have been in a long-term decline through 2017, even as the reduction among women has slowed down recently [7]. However, CRC is still the third most commonly diagnosed cancer in recent years, and the second leading cause of cancer death globally due to the unmet screening programs, therapeutic strategy, and increasing incidence rates.
In 2020, CRC accounts for 10% of global cancer incidence and 9.4% of cancer deaths, just lower than lung cancer that comprises 18% of deaths. The global number of new CRC cases is predicted to reach 3.2 million in 2040, based on the projection of aging, population growth, and human development. The increase in CRC incidence is mainly attributed to the elevated exposure to environmental risk factors resulting from shifting lifestyle and diet toward westernization [8, 9].
Although the prospect for CRC therapy is generally good, the increasing number of CRC cases and rising incidence among younger generations [8, 10, 11] still pose a heavy financial burden and a huge public health challenge. Declined or stabilized CRC incidence has been observed in limited countries with very high human development [12, 13], that mainly benefits from the healthier lifestyle and the establishment of screening program a decade ago [14]. However, a marked disparity of survival rates is documented, even in highly developed countries [15]. The stages at which CRC is diagnosed partly explain the differences in survival. The symptoms of CRC only appear at the advanced stages. Early detection is an important determinant in preventing metastasis, reducing mortality, and improving prognosis and quality of future life [16]. In this review, we described the current global prevalence of CRC, predicted the trends to 2040, and summarized the subtypes and risk factors of CRC. These statistical analyses highlight the necessity of a healthy lifestyle to reduce risk factors, CRC screening program for early detection, and new researches on better prevention and treatment of CRC.
Epidemiology
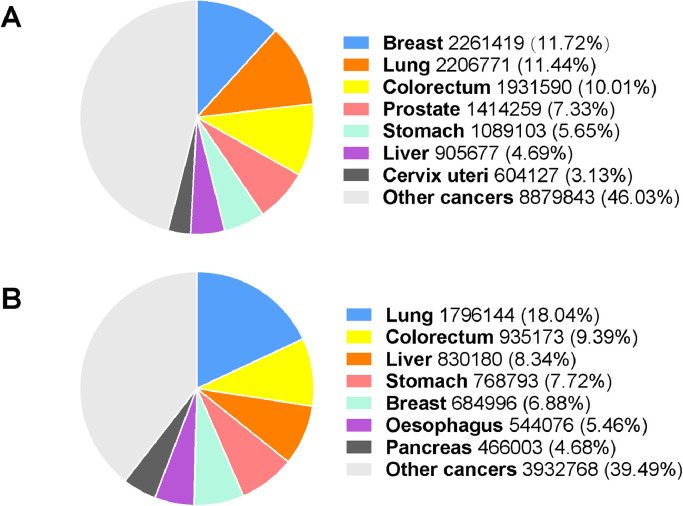
The prevalence of CRC has been dramatically growing at an alarming rate globally in recent years. There are estimated 1.93 million new CRC cases diagnosed, and 0.94 million CRC caused deaths in 2020 worldwide, representing 10% of the global cancer incidence (total 19.29 million new cases) and 9.4% of all cancer caused deaths (total 9.96 million deaths) (Fig. 1A and B). CRC is the third leading cause of cancer related deaths in both genders worldwide, with estimated 515,637 deaths among males and 419,536 deaths among females in 2020. Today, more than 5.25 million (5-year prevalence) people worldwide are living with CRC, only less than breast cancer, which causes 7.79 million cancer cases. Great efforts and advancements have been made to better understand the pathophysiology of CRC and expand treatment options, including endoscopic resection, surgical local excision, targeted therapy, radiation therapy, ablative therapies, chemotherapy, and immunotherapy, which double the overall survival of advanced CRC to three years [1]. Whereas, significant differences in CRC survival rate have been observed, even in most highly developed countries. Diagnosis at the different clinical stages of CRC may partially explain the marked differences in survival rates [15]. CRC is generally asymptomatic. When the symptoms of CRC appear, such as rectal bleeding, anemia, or abdominal pain, most patients are already in the advanced stage where cancers are aggressive, malignant, and metastatic. Diagnosis at advanced stages is one of the determinants of the disparity in survival and a large number of CRC deaths worldwide. Thus, population-based screening programs have been widely proposed and implemented in some highly developed countries since more than 10 years ago, with the aim of shifting CRC distribution to early stages and improving therapy outcomes [3, 17, 18].
Fig. 1.
Estimates and proportion of incident cases and deaths of major cancer types worldwide in 2020. (A)Estimated number of new cancer cases in 2020 worldwide. (B) Estimated number of cancer-related deaths in 2020 worldwide. Source: GLOBOCAN 2020.
According to estimates from GLOBOCAN 2020[19], there are 1.15 million new cases of colon cancer, 0.7 million new cases of rectal cancers, and 50,000 new cases of anal cancer in 2020 globally. With continuous progress, these numbers are predicted to increase to 1.92 million, 1.16 million, and 78,000 in 2040, respectively, as shown in Table 1. All projections for 2040 were extracted from CANCER TOMORROW on the Global Cancer Observatory (https://gco.iarc.fr/).
Table 1.
Estimated number of new cases from 2020 to 2040.
| Cancer sites | 2020 | 2040 |
|---|---|---|
| Colon | 1,148,515 | 1,916,781 |
| Rectum | 732,210 | 1,160,296 |
| Anus | 50,865 | 77,597 |
| Total | 1,931,590 | 3,154,674 |
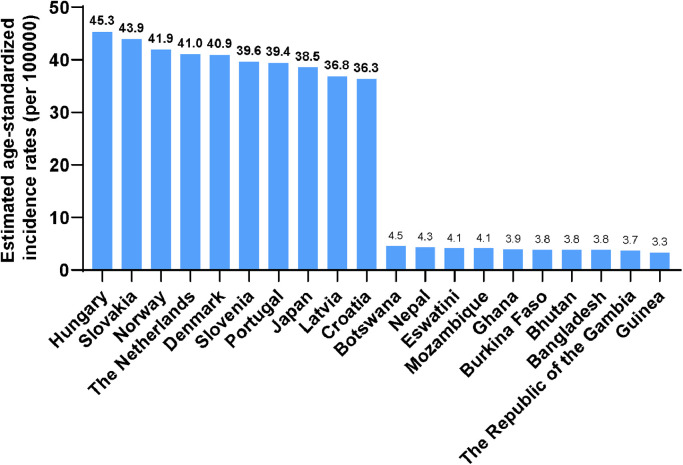
CRC incidences vary by countries [19, 20]. At the country level, Hungary, Slovakia, Norway, the Netherlands, and Denmark, have the highest age-standardized incidence rates, with the rates of 45.3, 43.9, 41.9, 41.0, and 40.9 cases per 100,000 persons, respectively, in 2020. The lowest age-standardized incidence rates in 2020 are observed in Guinea, Gambia, Bangladesh, Bhutan, and Burkina Faso, with rates of 3.3, 3.7, 3.8, 3.8, and 3.8 cases per 100,000 persons. Fig. 2 shows the top 10 and bottom 10 countries with the highest and lowest estimated age-standardized incidence rankings in 2020. The incidence of CRC appears to be positively correlated with the Human Development Index (HDI) (Fig. 3). The incidence rates of CRC in countries with very high HDI are about 4 folds of countries with low HDI. The incidence of CRC is stabilizing or declining in highly developed countries, albeit at a high basal level. Nevertheless, it is increasing rapidly in less developed countries due to the increased exposure to CRC risk factors.
Fig. 2.
Top 10 and bottom 10 countries for estimated age-standardized CRC incidence in 2020. Data are derived from GLOBOCAN 2020.
Fig. 3.
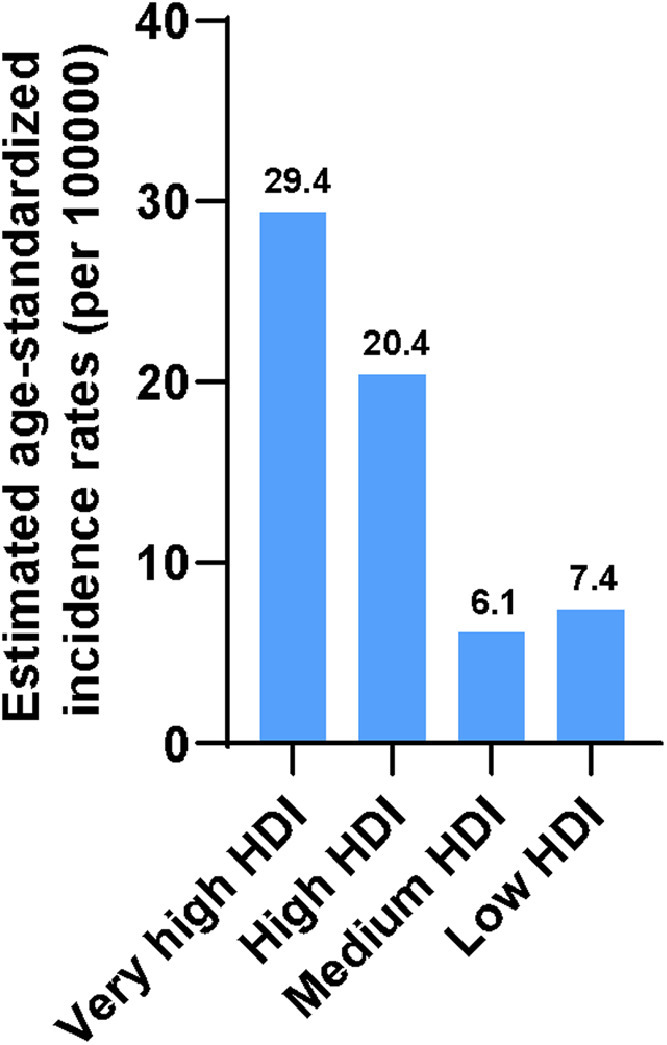
Estimated age-standardized CRC incidence by regions in 2020 except China and India. HDI, Human Development Index.
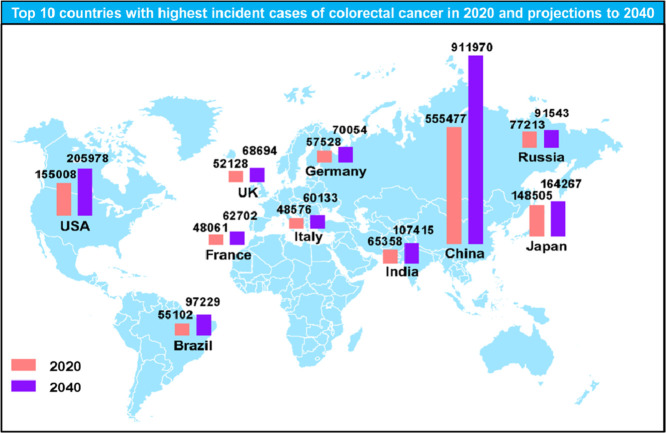
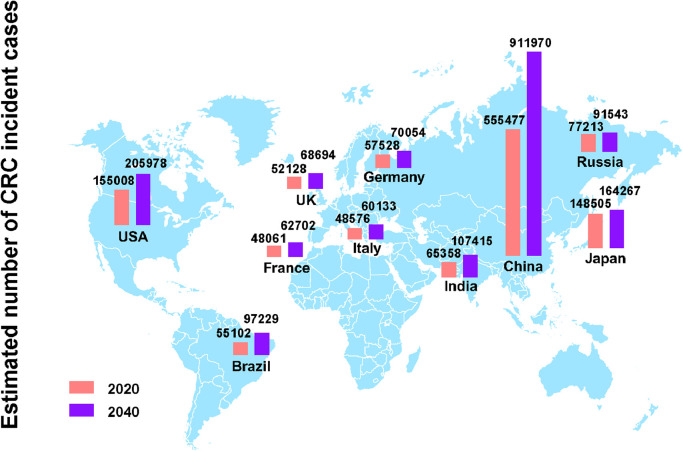
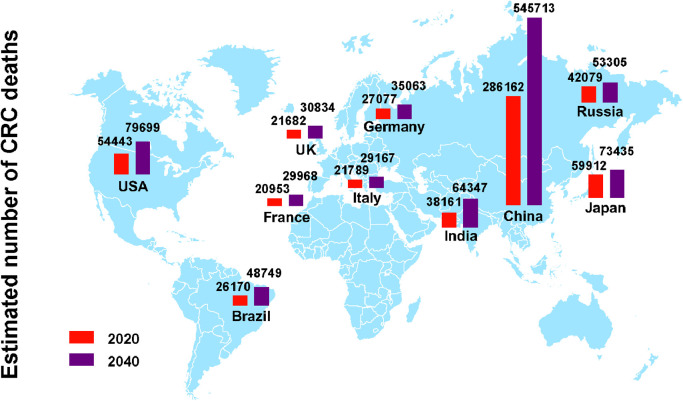
China and the United States have the highest estimated number of CRC new cases in 2020, and the number of new cases is expected to grow continuously over the next 20 years based on demographic factors. The number of CRC incident cases in China is increased by 64% from 0.56 million in 2020 to 0.91 million in 2040. In the United States, approximately 4.4% males (1 in 23) and 4.1% females (1 in 25) will develop and be diagnosed with CRC during their lifetime [7]. In 2020, there are 0.16 million estimated new CRC cases in the United States, and the number will reach 0.21 million in 2040. Japan has an estimated 148,500 new CRC cases and 60,000 CRC deaths in 2020, and the number of incident cases is predicted to reach 0.16 million after two decades. In addition, the Russian Federation, India, Germany, Brazil, the United Kingdom, Italy, and France are also among the top 10 countries with the highest incidence cases of CRC in 2020. Here, we presented the estimated CRC incident cases (Fig. 4) and deaths (Fig. 5) in the top 10 countries in 2020 and projections for 2040.
Fig. 4.
The number of new CRC cases in top 10 countries with highest incident cases in 2020 and projections for 2040.
Fig. 5.
Deaths from CRC in top 10 countries with highest incident cases in 2020 and projections for 2040.
Subtypes of CRC
The colon and rectum make up the large intestine (also called large bowel), which is the final segment of gastrointestinal system. Traditionally, three segments of cancer location define the subtypes of CRC: proximal colon, distal colon, and rectum [8, 21]. Heterogeneity of CRC risk factors at different anatomical sites has been reported. For instance, smoking is accompanied with a greater risk of proximal colon and rectal cancers, but not with distal colon cancer [22, 23]. Physical activity inversely correlates with the risk of proximal and distal colon cancer, but not with rectal cancer [22]. Heterogeneity of these risk factors may partially result from the distinct microbial loads, corresponding microbial metabolites, and host characteristics.
Several types of cancers affect colorectum. Adenocarcinoma from mucus producing cells is the most common type of CRCs. CRCs also include other less common types of cancers: carcinoid tumors, gastrointestinal stromal tumors, lymphomas, and sarcomas.
Currently, to better predict the therapeutic response and prognosis of patients with CRC, a number of classification criteria are proposed based on the molecular basis. CRC occurs as a result of multiple carcinogenic events: serrated adenomas, adenoma-carcinoma sequence, and inflammation [24, 25]. The unmanaged occurrence of carcinogenic events facilitates the progressive accumulation of genetic mutations and epigenetic modifications, that drive the transformation of normal cells into uncontrolled adenoma, and may eventually lead to CRC. Genomic instability, a key factor responsible for global genetic aberrations and the consequent carcinogenesis, comprises three major aberrant events: microsatellite instability (MSI), CpG island methylator phenotype (CIMP), and chromosomal instability (CIN) [26]. Therefore, certain single molecular markers involved in these events were used to classify CRC patients into relevant clinical subtypes [27, 28]. For instance, MSI-high CRC is more resistant to adjuvant 5-fluorouracil (5-FU)-based chemotherapy [29]. Although these molecular events contribute to the distinct phenotypic exhibitions, they are not completely mutually exclusive [8, 30, 31]. Inconsistencies between subtypes based on single molecular classification and clinical outcomes often occur. Single molecular markers can only predict clinical outcomes in limited patients [32], [33], [34]. To better elucidate the connection between molecular events and clinical response, the combination of multiple molecular markers to define the classification of CRC patients has been proposed to obtain more accurate prognostications [35], [36], [37], [38]. In 2007, Jass reported that the phenotypic exhibition of malignancy depends on genetic and epigenetic landscape of CRC, especially the dysregulation of growth-related pathways [39]. Given that molecular features and morphology are more reliable to define CRC subtypes, five CRC basic subtypes were proposed based on the status of MSI, CIMP, and well-known CRC diver gene BRAF/KRAS mutation [39]. In 2015, a new classification system with four consensus molecular subtypes (CMSs) was established by Colorectal Cancer Subtyping Consortium for unifying categorization and better designing regimens for specific CRC subtypes [40]. These four CMSs have distinctive characteristics. As summarized in Fig. 6, CMS1 represents 14% of CRC characterized by MSI immune. CMS2 is a kind of classical CRC type representing 37% of CRC cases. CMS2 biopsies exhibit high epithelial differentiation and remarkable activation of WNT and MYC signaling pathways. Enrichment of metabolic signatures and KRAS-activating mutations are observed in CMS3, which accounts for 13% CRCs. CMS4 tumors show extensive stromal invasion and upregulation of genes related to epithelial mesenchymal transition, and comprise 23% of all CRCs. Despite numerous proposed subtype definitions, none of them accurately predicts the therapeutic response and patient survival [38, 41, 42].
Fig. 6.
Four consensus molecular subtypes of CRC and their key distinguishing features.
The associations between the CMS subtypes and clinical outcomes were observed. CMS1 is usually a right-sided (proximal) colon cancer and is commonly diagnosed in females. Historically metastatic CMS1 at the advanced stage showed a poor survival rate due to the chemotherapy resistance and higher pathological grade [40]. Patients with CMS1 CRC showed good clinical outcomes when receiving immune checkpoint blockade therapy such as PD1 blocker, and obtained convenient features such as infiltration of cytotoxic T lymphocytes and elevated neoantigens [1, 43, 44]. However, anti-EGFR therapies do not benefit patients with right-sided metastatic CRC in the first-line setting [45]. CMS1 BRAF-V600E mutant CRC is aggressive. Triplet chemotherapy (fluoropyrimidine, irinotecan, and oxaliplatin) combined with bevacizumab and combination strategies of anti-EGFR antibodies, BRAF-inhibitors, and MEK inhibitors or chemotherapy significantly improve clinical outcome and have been added to consensus guidelines [46], [47], [48]. In contrast, CMS2 is mainly left-sided (distal colon cancer) and frequently diagnosed in males with better prognosis and superior survival rate even after relapse [40]. High expression of β-catenin in CMS2 is associated with poor prognosis and CRC progression [49]. Overrepresentation of KRAS gene mutation in CMS3 tumors that are mostly right-sided results in the constitutive activation of MAPK pathway and is associated with worse clinical outcomes [50, 51]. CMS4 tumors, although commonly left-sided, are always diagnosed at an advanced stage and have worse overall and relapse-free survival. In CMS4, the percentage of stromal cells inversely correlates with the prognosis [52]. Thus, targeting TGFβ in stromal cells potentially suppresses cancer progression and metastasis [53, 54].
CRC is a heterogeneous disease, and the different subtype affiliation influences prognosis and therapeutic response. However, these proposed subtype definitions still can not accurately depict the therapeutic response and patient survival in all CRC clinical cases. Further studies are still needed to establish a more comprehensive classification system that can be routinely applied in clinical practice.
Risk factors
Sex, age, and race
Colorectal cancer statistics in the United States reported gender disparities in CRC incidence. Although lifetime risk is similar for both genders (4.4% in men and 4.1% in women) in the United States, the age-standardized incidence of CRC in men is 31% higher than in women due to a shorter life expectancy [55]. In 2020, the global CRC incidence rate in men (23.4 cases per 100,000 persons) is 44% higher than that in women (16.2 cases per 100,000 persons). And a greater disparity is observed in rectal cancer compared to colon cancer. In men, 9.8 rectal cancer cases and 13.1 colon cancer cases are diagnosed in 100,000 individuals, and these numbers are 75 and 31% higher than that in women, respectively (5.6 cases and 10.0 cases per 100,000 persons). However, a multinational cohort study in 10 European countries showed that the prevalence of proximal colon cancer was higher among women [22]. Moreover, the preferential location of CRC shifts to proximal colon with age, especially in white and black patients [56], that further increases the incidence of proximal colon cancer in women compared to men. Cancer is considered an aging disease. The incidence and mortality of CRC are dramatically increased after age 50 years (86.3 versus 2.9, 40.9 versus 0.99 per 100,000 persons in 2020). Despite the declined or stabilized CRC incidence in some countries, the incidence of early-onset CRC, mainly rectal cancer, has increased in younger generations [57], [58], [59], [60], [61], [62]. Rectal cancer has been reported to be more prevalent in Asians [8]. According to the data on GLOBOCAN 2020, we found that colon cancer still accounts for a higher proportion of CRC in Asians than rectal cancer. In 2020, 569,186 and 423,569 patients were diagnosed with colon and rectal cancer, respectively, in Asia. At the levels of world areas, there are 315,185, 48,649, 43,768, and 15,967 new rectal cancer cases in Eastern Asia, South-Eastern Asia, South-Central Asia, and Western Asia in 2020, that are lower than 436,272, 56,065, 51,888, 24,961 new cases of colon cancer, individually.
No ethnic differences were reported in the frequency of MSI and driver gene mutations, such as TP53, KRAS, BRAF, and APC [8, 63]. Although the incidence of CRC is higher in African Americans and rectal cancer is more prevalent in Asians [64, 65], modifiable risk factors are still the major drivers of all CRC incidence. The descriptive epidemiology of racial and regional differences in CMS subtypes of CRC remains to be defined.
Genetic risk factors
Sporadic CRC cases, with no family history or inherited genomic alterations, comprise 60–65% of all CRC cases [8, 66]. The initiation of sporadic CRC is largely attributed to acquired somatic genetic mutations or epigenetic alterations induced by modifiable risk factors [67]. The remaining 35–40% of CRC patients exhibit susceptibility to heritable components [8, 68, 69]. These components include family history, but without obvious genetic predisposition, hereditary cancer syndromes, such as Lynch syndrome, low-penetrance genetic variations, and other unknown inherited genomic aberrations [70], [71], [72]. It is worth noting that environmental factors substantially contribute to carcinogenesis in all CRC cases. Even in patients with a family history, acquired genomic alterations may still be a major cause of CRC development.
Modifiable risk factors
In addition to genetic risk factors, CRC incidence and mortality are also greatly affected by some largely modifiable risk factors, including low physical activity, overweight and obesity, poor diet, excessive alcohol intake, smoking, chronic inflammatory bowel disease (IBD), and gut microbiota.
Physical inactivity and sedentary lifestyle are positively correlated with the risk of colon cancer, but not rectal cancer. The risk of colon cancer in people who are sedentary is increased by 25–50% compared to people physically active [73]. Physical activity may reduce the risk of CRC by inhibition of fat accumulation, inflammation suppression, and improving gut motility and metabolic hormones [74]. Overweight and obesity are established CRC risk factors [75], [76], [77], [78]. The risk of colon cancer and rectal cancer in obese men is 50 and 25% higher than in normal-weight men. Whereas the risk of colon cancer is only increased by 10% in obese women, with no increase in the risk of rectal cancer [79]. Early body mass index (BMI) is thought to be a more important risk factor for colon cancer in women. On the contrary, late body weight gain has a greater impact on CRC risk in men [80].
Diet can influence the risk of CRC directly through dietary elements, or indirectly through gut microbiome or body weight gain. Healthy dietary patterns are characterized by high intake of insoluble fiber, vegetables, fruits, and low-fat milk. High consumption of processed foods, red meats and refined carbohydrates, and low calcium diet facilitate inflammatory response and increase the risk of CRC [81], [82], [83], [84].
Acetaldehyde is a primary ethanol metabolite catalyzed by alcohol dehydrogenase. It has been identified as a human carcinogen due to its damage to the intestinal mucosa and the stimulation of cell proliferation [85, 86]. Acetaldehyde dehydrogenase (ALDH) is highly expressed in the liver and is responsible for metabolizing acetaldehyde to less toxic acetate. ALDH2×2 (E504K) variant, a mutant ALDH enzyme, is present in 45% of East Asians. Following excess alcohol consumption, carriers of this inactive variant are more likely to develop severe toxicity by the accumulation of acetaldehyde [87, 88]. However, the role of less active ALDH2 in the development of CRC remains inconclusive.
Sufficient evidence has shown that smoking is a strong and established risk factor for CRC. The components of tobacco smoke directly damage the colorectal mucosa and cause further genetic or epigenetic alterations [22, 89, 90]. In addition to the effect of smoking on CRC initiation, current smokers also exhibit poor CRC survival [91]. Smoking cessation over 10 years reverses high levels of DNA methylation [92] and reduces the risk of CIMP-high CRC by 50% [93], suggesting the reversibility of increased risk of CRC by smoking.
The risk of CRC in IBD patients is approximately 2.4 times higher than in normal subjects [94], [95], [96]. Unmanaged IBD, particularly ulcerative colitis, may progress to dysplasia, and eventually result in CRC [97], [98], [99]. The role of gut microbiome in CRC is influenced by the composition of microbiota and the abundance of specific species. Antibiotic administration is associated with an increased risk of CRC due to dysbiosis [[100], [102]]. The dual functions of microbiota have led researchers to investigate the causal role of specific species in CRC development. Hence, manipulation of gut microbiome might be a novel strategy for the prevention and treatment of CRC.
Current prevention strategies
As the third most common malignancy, CRC has become a heavy financial burden and global health challenge. Therefore, prevention strategies are crucial to reducing CRC risk and the number of new cases. Primary prevention of CRC includes regular physical activity, a healthy diet of milk, fruit, vegetables, whole grains, and nuts with sufficient calcium, vitamins, and fiber, smoking cessation, limiting alcohol consumption. Meanwhile, some anti-inflammatory agents have shown chemopreventive potential against CRC. Regular use of aspirin and other nonsteroidal anti-inflammatory drugs reduces the risk of CRC [103], [104], [105], [106]. However, it remains unclear how to match the interventions to target users who will benefit, and how to personalize the prevention strategy, including dose and duration to avoid possible adverse effects like gastrointestinal bleeding [1, 107]. Screening, secondary prevention, is considered the most effective method to prevent CRC development by eliminating precancerous lesions and increasing early detection. Two step screening strategy is commonly used in clinical practice [1, 108, 109]. It consists of highly sensitive stool-based tests to detect molecular markers and blood, and visual examinations to observe the colon and rectum directly [110], [111], [112]. Stool-based tests, such as fecal immunochemical test, guaiac-based fecal occult blood test, and stool DNA testing, are non-invasive screening options, that are widely used for population screening programs because no special preparation is required. If the test is positive, invasive endoscopic methods, such as colonoscopy, CT colonography, and sigmoidoscopy, will be performed to further confirm the abnormal results. American cancer society CRC screening recommends regular screening for people aged 45 years and older [113, 114]. For people who are at high risk of CRC due to ulcerative colitis, previous adenomas, or family history, regular screening should be started earlier.
Future prospects
In this review, we summarize the global epidemiology of CRC in 2020 including new cases, caused deaths, and incidence rates by genders, cancer locations, and top 10 countries, and corresponding projections for 2040. The incidence and mortality of CRC indicate a variation in gender and geographic region. China, the United States, and Japan are the top three countries with the highest incidence cases and deaths of CRC. Furthermore, the incidence of CRC is positively correlated with HDI level. Age-standardized incidence of CRC is higher in regions with high HDI, but lowest in countries with medium HDI. In order to reduce the number of CRC cases in the coming decades, resource-based targeted interventions, including primary prevention among low-income people and early screening detection in high-income settings, are necessary.
In addition, CRC is a heterogeneous disease, and its subtype affiliation is proposed for better clinical stratification. However, the connective system of optimal treatment selection and accurate prediction of therapeutic response by CRC subtypes based on molecular and biological features is yet to be established. Thus, further studies and statistics are still required to explore the clinical and prognostic associations of the established CRC subtypes for a better translation from basic research into clinical practice.
Declaration of Competing Interest
None.
Acknowledgments
Y.X. is a visiting student from Sun Yat-sen University supported by a Visiting Student Scholarship from the China Scholarship Council (File no. 201806380128).
References
- 1.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Guren M.G. The global challenge of colorectal cancer. Lancet Gastroenterol. Hepatol. 2019;4:894–895. doi: 10.1016/S2468-1253(19)30329-2. [DOI] [PubMed] [Google Scholar]
- 3.Schreuders E.H., Ruco A., Rabeneck L., Schoen R.E., Sung J.J., Young G.P., Kuipers E.J. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 4.Schliemann D., Matovu N., Ramanathan K., Munoz-Aguirre P., O'Neill C., Kee F., Su T.T., Donnelly M. Implementation of colorectal cancer screening interventions in low-income and middle-income countries: a scoping review protocol. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-037520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabeneck L., Chiu H.M., Senore C. International perspective on the burden of colorectal cancer and public health effects. Gastroenterology. 2020;158:447–452. doi: 10.1053/j.gastro.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Kaminski M.F., Robertson D.J., Senore C., Rex D.K. Optimizing the quality of colorectal cancer screening worldwide. Gastroenterology. 2020;158:404–417. doi: 10.1053/j.gastro.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 8.Keum N., Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 9.Murphy N., Moreno V., Hughes D.J., Vodicka L., Vodicka P., Aglago E.K., Gunter M.J., Jenab M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Asp. Med. 2019;69:2–9. doi: 10.1016/j.mam.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Campos F.G. Colorectal cancer in young adults: a difficult challenge. World J. Gastroenterol. 2017;23:5041–5044. doi: 10.3748/wjg.v23.i28.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancet O.T. Colorectal cancer: a disease of the young? Lancet Oncol. 2017;18:413. doi: 10.1016/S1470-2045(17)30202-4. [DOI] [PubMed] [Google Scholar]
- 12.Fidler M.M., Bray F., Vaccarella S., Soerjomataram I. Assessing global transitions in human development and colorectal cancer incidence. Int. J. Cancer. 2017;140:2709–2715. doi: 10.1002/ijc.30686. [DOI] [PubMed] [Google Scholar]
- 13.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 14.Arnold M., Abnet C.C., Neale R.E., Vignat J., Giovannucci E.L., McGlynn K.A., Bray F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335–349. doi: 10.1053/j.gastro.2020.02.068. e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., Bonaventure A., Valkov M., Johnson C.J., Esteve J., Ogunbiyi O.J., Azevedo E.S.G., Chen W.Q., Eser S., Engholm G., Stiller C.A., Monnereau A., Woods R.R., Visser O., Lim G.H., Aitken J., Weir H.K., Coleman M.P., Group C.W. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brody H. Colorectal cancer. Nature. 2015;521:S1. doi: 10.1038/521S1a. [DOI] [PubMed] [Google Scholar]
- 17.Brown L.J., Roeger S.L., Reed R.L. Patient perspectives on colorectal cancer screening and the role of general practice. BMC Fam. Pract. 2019;20:109. doi: 10.1186/s12875-019-0997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu P., Xi Y., Zhu J., Zhang M., Luka Z., Stolz D.B., Cai X., Xie Y., Xu M., Ren S., Huang Z., Yang D., York J.D., Ma X., Xie W. Intestinal sulfation is essential to protect against colitis and colonic carcinogenesis. Gastroenterology. 2021;161:271–286. doi: 10.1053/j.gastro.2021.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 20.The L. GLOBOCAN 2018: counting the toll of cancer. Lancet. 2018;392:985. doi: 10.1016/S0140-6736(18)32252-9. [DOI] [PubMed] [Google Scholar]
- 21.Li F.Y., Lai M.D. Colorectal cancer, one entity or three. J. Zhejiang Univ. Sci. B. 2009;10:219–229. doi: 10.1631/jzus.B0820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy N., Ward H.A., Jenab M., Rothwell J.A., Boutron-Ruault M.C., Carbonnel F., Kvaskoff M., Kaaks R., Kuhn T., Boeing H., Aleksandrova K., Weiderpass E., Skeie G., Borch K.B., Tjonneland A., Kyro C., Overvad K., Dahm C.C., Jakszyn P., Sanchez M.J., Gil L., Huerta J.M., Barricarte A., Quiros J.R., Khaw K.T., Wareham N., Bradbury K.E., Trichopoulou A., Vecchia C.La, Karakatsani A., Palli D., Grioni S., Tumino R., Fasanelli F., Panico S., Bueno-de-Mesquita B., Peeters P.H., Gylling B., Myte R., Jirstrom K., Berntsson J., Xue X., Riboli E., Cross A.J., Gunter M.J. Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 european countries: a multinational cohort study. Clin. Gastroenterol. Hepatol. 2019;17:1323–1331. doi: 10.1016/j.cgh.2018.07.030. e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botteri E., Borroni E., Sloan E.K., Bagnardi V., Bosetti C., Peveri G., Santucci C., Specchia C., van den Brandt P., Gallus S., Lugo A. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am. J. Gastroenterol. 2020;115:1940–1949. doi: 10.14309/ajg.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi Y., Diaz-Meco M.T., Moscat J. Serrated colorectal cancer: the road less travelled? Trends Cancer. 2019;5:742–754. doi: 10.1016/j.trecan.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crockett S.D., Nagtegaal I.D. Terminology, molecular features, epidemiology, and management of serrated colorectal Neoplasia. Gastroenterology. 2019;157:949–966. doi: 10.1053/j.gastro.2019.06.041. e944. [DOI] [PubMed] [Google Scholar]
- 26.Grady W.M., Carethers J.M. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y., Deng Y., Wang K., Zhou H., Zheng X., Si L., Fu Z. Profiles of alternative splicing in colorectal cancer and their clinical significance: a study based on large-scale sequencing data. EBioMedicine. 2018;36:183–195. doi: 10.1016/j.ebiom.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seligmann J.F., Seymour M.T. Use of gene expression profiles to distinguish molecular subtypes in colorectal cancer: progression toward primetime. J. Natl. Cancer Inst. 2017;109 doi: 10.1093/jnci/djx019. [DOI] [PubMed] [Google Scholar]
- 29.Jo W.S., Carethers J.M. Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark. 2006;2:51–60. doi: 10.3233/cbm-2006-21-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funkhouser W.K., Lubin I.M., Monzon F.A., Zehnbauer B.A., Evans J.P., Ogino S., Nowak J.A. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J. Mol. Diagn. 2012;14:91–103. doi: 10.1016/j.jmoldx.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Loree J.M., Pereira A.A.L., Lam M., Willauer A.N., Raghav K., Dasari A., Morris V.K., Advani S., Menter D.G., Eng C., Shaw K., Broaddus R., Routbort M.J., Liu Y., Morris J.S., Luthra R., Meric-Bernstam F., Overman M.J., Maru D., Kopetz S. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin. Cancer Res. 2018;24:1062–1072. doi: 10.1158/1078-0432.CCR-17-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sargent D.J., Marsoni S., Monges G., Thibodeau S.N., Labianca R., Hamilton S.R., French A.J., Kabat B., Foster N.R., Torri V., Ribic C., Grothey A., Moore M., Zaniboni A., Seitz J.F., Sinicrope F., Gallinger S. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burki T.K. Reclassifying colorectal cancer subtypes. Lancet Oncol. 2015;16:e591. doi: 10.1016/S1470-2045(15)00443-X. [DOI] [PubMed] [Google Scholar]
- 34.Calon A., Lonardo E., Berenguer-Llergo A., Espinet E., Hernando-Momblona X., Iglesias M., Sevillano M., Palomo-Ponce S., Tauriello D.V., Byrom D., Cortina C., Morral C., Barcelo C., Tosi S., Riera A., Attolini C.S., Rossell D., Sancho E., Batlle E. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015;47:320–329. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 35.Sinicrope F.A., Shi Q., Smyrk T.C., Thibodeau S.N., Dienstmann R., Guinney J., Bot B.M., Tejpar S., Delorenzi M., Goldberg R.M., Mahoney M., Sargent D.J., Alberts S.R. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88–99. doi: 10.1053/j.gastro.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamal Y., Schmit S.L., Hoehn H.J., Amos C.I., Frost H.R. Transcriptomic differences between primary colorectal adenocarcinomas and distant metastases reveal metastatic colorectal cancer subtypes. Cancer Res. 2019;79:4227–4241. doi: 10.1158/0008-5472.CAN-18-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma S., Ogino S., Parsana P., Nishihara R., Qian Z., Shen J., Mima K., Masugi Y., Cao Y., Nowak J.A., Shima K., Hoshida Y., Giovannucci E.L., Gala M.K., Chan A.T., Fuchs C.S., Parmigiani G., Huttenhower C., Waldron L. Continuity of transcriptomes among colorectal cancer subtypes based on meta-analysis. Genome Biol. 2018;19:142. doi: 10.1186/s13059-018-1511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dienstmann R., Villacampa G., Sveen A., Mason M.J., Niedzwiecki D., Nesbakken A., Moreno V., Warren R.S., Lothe R.A., Guinney J. Relative contribution of clinicopathological variables, genomic markers, transcriptomic subtyping and microenvironment features for outcome prediction in stage II/III colorectal cancer. Ann. Oncol. 2019;30:1622–1629. doi: 10.1093/annonc/mdz287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jass J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 40.Guinney J., Dienstmann R., Wang X., de Reynies A., Schlicker A., Soneson C., Marisa L., Roepman P., Nyamundanda G., Angelino P., Bot B.M., Morris J.S., Simon I.M., Gerster S., Fessler E., De Sousa E.M.F., Missiaglia E., Ramay H., Barras D., Homicsko K., Maru D., Manyam G.C., Broom B., Boige V., Perez-Villamil B., Laderas T., Salazar R., Gray J.W., Hanahan D., Tabernero J., Bernards R., Friend S.H., Laurent-Puig P., Medema J.P., Sadanandam A., Wessels L., Delorenzi M., Kopetz S., Vermeulen L., Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alwers E., Jia M., Kloor M., Blaker H., Brenner H., Hoffmeister M. Associations Between molecular classifications of colorectal cancer and patient survival: a systematic review. Clin. Gastroenterol. Hepatol. 2019;17:402–410. doi: 10.1016/j.cgh.2017.12.038. 402-+ [DOI] [PubMed] [Google Scholar]
- 42.Phipps A.I., Limburg P.J., Baron J.A., Burnett-Hartman A.N., Weisenberger D.J., Laird P.W., Sinicrope F.A., Rosty C., Buchanan D.D., Potter J.D., Newcomb P.A. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77–87. doi: 10.1053/j.gastro.2014.09.038. e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh P.P., Sharma P.K., Krishnan G., Lockhart A.C. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol. Rep. 2015;3:289–297. doi: 10.1093/gastro/gov053. (Oxf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., Wong F., Azad N.S., Rucki A.A., Laheru D., Donehower R., Zaheer A., Fisher G.A., Crocenzi T.S., Lee J.J., Greten T.F., Duffy A.G., Ciombor K.K., Eyring A.D., Lam B.H., Joe A., Kang S.P., Holdhoff M., Danilova L., Cope L., Meyer C., Zhou S., Goldberg R.M., Armstrong D.K., Bever K.M., Fader A.N., Taube J., Housseau F., Spetzler D., Xiao N., Pardoll D.M., Papadopoulos N., Kinzler K.W., Eshleman J.R., Vogelstein B., Anders R.A., Diaz L.A., Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venook A.P., Niedzwiecki D., Innocenti F., Fruth B., Greene C., O’Neil B.H., Shaw J.E., Atkins J.N., Horvath L.E., Polite B.N., Meyerhardt J.A., O’Reilly E.M., Goldberg R.M., Hochster H.S., Blanke C.D., Schilsky R.L., Mayer R.J., Bertagnolli M.M., Lenz H.J. Impact of primary (1 degrees) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance) J. Clin. Oncol. 2016;34(suppl):3504-3504. [Google Scholar]
- 46.Kopetz S., Grothey A., Tabernero J. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer reply. N. Engl. J. Med. 2020;382:877–878. doi: 10.1056/NEJMc1915676. [DOI] [PubMed] [Google Scholar]
- 47.Kopetz S., Guthrie K.A., Morris V.K., Lenz H.J., Magliocco A.M., Maru D., Yan Y.B., Lanman R., Manyam G., Hong D.S., Sorokin A., Atreya C.E., Diaz L.A., Allegra C., Raghav K.P., Wang S.E., Lieu C.H., McDonough S.L., Philip P.A., Hochster H.S. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406) J. Clin. Oncol. 2021;39:285–294. doi: 10.1200/JCO.20.01994. 285-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Cutsem E., Huijberts S., Grothey A., Yaeger R., Cuyle P.J., Elez E., Fakih M., Montagut C., Peeters M., Yoshino T., Wasan H., Desai C.J., Ciardiello F., Gollerkeri A., Christy-Bittel J., Maharry K., Sandor V., Schellens J.H., Kopetz S., Tabernero J. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the phase III BEACON colorectal cancer study. J. Clin. Oncol. 2019;37:1460–1469. doi: 10.1200/JCO.18.02459. 1460-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Najdi R., Holcombe R.F., Waterman M.L. Wnt signaling and colon carcinogenesis: beyond APC. J. Carcinog. 2011;10:5. doi: 10.4103/1477-3163.78111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phipps A.I., Buchanan D.D., Makar K.W., Win A.K., Baron J.A., Lindor N.M., Potter J.D., Newcomb P.A. KRAS-mutation status in relation to colorectal cancer survival: the joint impact of correlated tumour markers. Br. J. Cancer. 2013;108:1757–1764. doi: 10.1038/bjc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee M.S., Menter D.G., Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J. Natl. Compr. Cancer Netw. 2017;15:411–419. doi: 10.6004/jnccn.2017.0038. [DOI] [PubMed] [Google Scholar]
- 52.Park J.H., Richards C.H., McMillan D.C., Horgan P.G., Roxburgh C.S.D. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann. Oncol. 2014;25:644–651. doi: 10.1093/annonc/mdt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staudacher J.J., Bauer J., Jana A., Tian J., Carroll T., Mancinelli G., Ozden O., Krett N., Guzman G., Kerr D., Grippo P., Jung B. Activin signaling is an essential component of the TGF-beta induced pro-metastatic phenotype in colorectal cancer. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-05907-8. UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Gramont A., Faivre S., Raymond E. Novel TGF-beta inhibitors ready for prime time in onco-immunology. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2016.1257453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 56.Yang L., Xiong Z., He W., Xie K., Liu S., Kong P., Jiang C., Guo G., Xia L. Proximal shift of colorectal cancer with increasing age in different ethnicities. Cancer Manag. Res. 2018;10:2663–2673. doi: 10.2147/CMAR.S166548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vuik F.E., Nieuwenburg S.A., Bardou M., Lansdorp-Vogelaar I., Dinis-Ribeiro M., Bento M.J., Zadnik V., Pellise M., Esteban L., Kaminski M.F., Suchanek S., Ngo O., Majek O., Leja M., Kuipers E.J., Spaander M.C. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820–1826. doi: 10.1136/gutjnl-2018-317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Araghi M., Soerjomataram I., Bardot A., Ferlay J., Cabasag C.J., Morrison D.S., De P., Tervonen H., Walsh P.M., Bucher O., Engholm G., Jackson C., McClure C., Woods R.R., Saint-Jacques N., Morgan E., Ransom D., Thursfield V., Moller B., Leonfellner S., Guren M.G., Bray F., Arnold M. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol. Hepatol. 2019;4:511–518. doi: 10.1016/S2468-1253(19)30147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siegel R.L., Fedewa S.A., Anderson W.F., Miller K.D., Ma J., Rosenberg P.S., Jemal A. Colorectal cancer incidence patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017;109 doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willauer A.N., Liu Y., Pereira A.A.L., Lam M., Morris J.S., Raghav K.P.S., Morris V.K., Menter D., Broaddus R., Meric-Bernstam F., Hayes-Jordan A., Huh W., Overman M.J., Kopetz S., Loree J.M. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125:2002–2010. doi: 10.1002/cncr.31994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Early-onset colorectal cancer increasing: why?, Cancer Discov., 9 (2019) OF5. [DOI] [PubMed]
- 62.Hofseth L.J., Hebert J.R., Chanda A., Chen H., Love B.L., Pena M.M., Murphy E.A., Sajish M., Sheth A., Buckhaults P.J., Berger F.G. Early-onset colorectal cancer: initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020;17:352–364. doi: 10.1038/s41575-019-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashktorab H., Ahuja S., Kannan L., Llor X., Ellis N., Xicola R.M., Laiyemo A.O., Carethers J.M., Brim H., Nouraie M. A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget. 2016;76:34546–34557. doi: 10.18632/oncotarget.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Augustus G.J., Ellis N.A. Colorectal cancer disparity in african americans risk factors and carcinogenic mechanisms. Am. J. Pathol. 2018;188:291–303. doi: 10.1016/j.ajpath.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin A., Kim K.Z., Jung K.W., Park S., Won Y.J., Kim J., Kim D.Y., Oh J.H. Increasing trend of colorectal cancer incidence in Korea, 1999–2009. Cancer Res. Treat. 2012;44:219–226. doi: 10.4143/crt.2012.44.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian Y., Kharazmi E., Sundquist K., Sundquist J., Brenner H., Fallah M. Familial colorectal cancer risk in half siblings and siblings: nationwide cohort study. BMJ. 2019;364:l803. doi: 10.1136/bmj.l803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jasperson K.W., Tuohy T.M., Neklason D.W., Burt R.W. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graff R.E., Moller S., Passarelli M.N., Witte J.S., Skytthe A., Christensen K., Tan Q., Adami H.O., Czene K., Harris J.R., Pukkala E., Kaprio J., Giovannucci E.L., Mucci L.A., Hjelmborg J.B. Familial risk and heritability of colorectal cancer in the nordic twin study of cancer. Clin. Gastroenterol. Hepatol. 2017;15:1256–1264. doi: 10.1016/j.cgh.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lichtenstein P., Holm N.V., Verkasalo P.K., Iliadou A., Kaprio J., Koskenvuo M., Pukkala E., Skytthe A., Hemminki K. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 70.Jiao S., Peters U., Berndt S., Brenner H., Butterbach K., Caan B.J., Carlson C.S., Chan A.T., Chang-Claude J., Chanock S., Curtis K.R., Duggan D., Gong J., Harrison T.A., Hayes R.B., Henderson B.E., Hoffmeister M., Kolonel L.N., Marchand L.Le, Potter J.D., Rudolph A., Schoen R.E., Seminara D., Slattery M.L., White E., Hsu L. Estimating the heritability of colorectal cancer. Hum. Mol. Genet. 2014;23:3898–3905. doi: 10.1093/hmg/ddu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song M., Emilsson L., Bozorg S.R., Nguyen L.H., Joshi A.D., Staller K., Nayor J., Chan A.T., Ludvigsson J.F. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study. Lancet Gastroenterol. Hepatol. 2020;5:537–547. doi: 10.1016/S2468-1253(20)30009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kastrinos F., Samadder N.J., Burt R.W. Use of Family history and genetic testing to determine risk of colorectal cancer. Gastroenterology. 2020;158:389–403. doi: 10.1053/j.gastro.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 73.Schmid D., Leitzmann M.F. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J. Natl. Cancer Inst. 2014:106. doi: 10.1093/jnci/dju098. [DOI] [PubMed] [Google Scholar]
- 74.Ruiz-Casado A., Martin-Ruiz A., Perez L.M., Provencio M., Fiuza-Luces C., Lucia A. Exercise and the hallmarks of cancer. Trends Cancer. 2017;3:423–441. doi: 10.1016/j.trecan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Dong Y., Zhou J., Zhu Y., Luo L., He T., Hu H., Liu H., Zhang Y., Luo D., Xu S., Xu L., Liu J., Zhang J., Teng Z. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye P., Xi Y., Huang Z., Xu P. Linking obesity with colorectal cancer: epidemiology and mechanistic insights. Cancers. 2020;12 doi: 10.3390/cancers12061408. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xi Y., Zhang Y., Zhu S., Luo Y., Xu P., Huang Z. PPAR-Mediated toxicology and applied pharmacology. Cells. 2020;9 doi: 10.3390/cells9020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu P.H., Wu K., Ng K., Zauber A.G., Nguyen L.H., Song M., He X., Fuchs C.S., Ogino S., Willett W.C., Chan A.T., Giovannucci E.L., Cao Y. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5:37–44. doi: 10.1001/jamaoncol.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xue K., Li F.F., Chen Y.W., Zhou Y.H., He J. Body mass index and the risk of cancer in women compared with men: a meta-analysis of prospective cohort studies. Eur. J. Cancer Prev. 2017;26:94–105. doi: 10.1097/CEJ.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 80.Kim H., Giovannucci E.L. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28:1–4. doi: 10.1007/s10552-016-0831-5. [DOI] [PubMed] [Google Scholar]
- 81.Norat T., Lukanova A., Ferrari P., Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int. J. Cancer. 2002;98:241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 82.Bouvard V., Loomis D., Guyton K.Z., Grosse Y., Ghissassi F.E., Benbrahim-Tallaa L., Guha N., Mattock H., Straif K. International agency for research on cancer monograph working, carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 83.Tabung F.K., Liu L., Wang W., Fung T.T., Wu K., Smith-Warner S.A., Cao Y., Hu F.B., Ogino S., Fuchs C.S., Giovannucci E.L. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018;4:366–373. doi: 10.1001/jamaoncol.2017.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.G.B.D.C.C. Collaborators The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 2019;4:913–933. doi: 10.1016/S2468-1253(19)30345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi Y.J., Myung S.K., Lee J.H. Light alcohol drinking and risk of cancer: a meta-analysis of cohort studies. Cancer Res. Treat. 2018;50:474–487. doi: 10.4143/crt.2017.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossi M., Jahanzaib Anwar M., Usman A., Keshavarzian A., Bishehsari F. Colorectal cancer and alcohol consumption-populations to molecules. Cancers. 2018:10. doi: 10.3390/cancers10020038. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen C.H., Ferreira J.C.B., Joshi A.U., Stevens M.C., Li S.J., Hsu J.H., Maclean R., Ferreira N.D., Cervantes P.R., Martinez D.D., Barrientos F.L., Quintanares G.H.R., Mochly-Rosen D. Novel and prevalent non-East Asian ALDH2 variants; Implications for global susceptibility to aldehydes' toxicity. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang J.S., Hsiao J.R., Chen C.H. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J. Biomed. Sci. 2017;24:19. doi: 10.1186/s12929-017-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Secretan B., Straif K., Baan R., Grosse Y., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V., Group W.H.O.I.A.f.R.o.C.M.W. A review of human carcinogens-part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 90.Hamada T., Nowak J.A., Masugi Y., Drew D.A., Song M., Cao Y., Kosumi K., Mima K., Twombly T.S., Liu L., Shi Y., da Silva A., Gu M., Li W., Nosho K., Keum N., Giannakis M., Meyerhardt J.A., Wu K., Wang M., Chan A.T., Giovannucci E.L., Fuchs C.S., Nishihara R., Zhang X., Ogino S. Smoking and risk of colorectal cancer sub-classified by tumor-infiltrating T cells. J. Natl. Cancer Inst. 2019;111:42–51. doi: 10.1093/jnci/djy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang B.Y., Jacobs E.J., Gapstur S.M., Stevens V., Campbell P.T. Active smoking and mortality among colorectal cancer survivors: the cancer prevention study ii nutrition cohort. J. Clin. Oncol. 2015;33:885–893. doi: 10.1200/JCO.2014.58.3831. 885-+ [DOI] [PubMed] [Google Scholar]
- 92.Zeilinger S., Kuhnel B., Klopp N., Baurecht H., Kleinschmidt A., Gieger C., Weidinger S., Lattka E., Adamski J., Peters A., Strauch K., Waldenberger M., Illig T. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS ONE. 2013;8:e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishihara R., Morikawa T., Kuchiba A., Lochhead P., Yamauchi M., Liao X., Imamura Y., Nosho K., Shima K., Kawachi I., Qian Z.R., Fuchs C.S., Chan A.T., Giovannucci E., Ogino S. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am. J. Epidemiol. 2013;178:84–100. doi: 10.1093/aje/kws431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jess T., Rungoe C., Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 95.Lutgens M.W., van Oijen M.G., van der Heijden G.J., Vleggaar F.P., Siersema P.D., Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm. Bowel. Dis. 2013;19:789–799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 96.Nadeem M.S., Kumar V., Al-Abbasi F.A., Kamal M.A., Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin. Cancer Biol. 2020;64:51–60. doi: 10.1016/j.semcancer.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Itzkowitz S.H., Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 98.Choi C.R., Bakir I.A., Hart A.L., Graham T.A. Clonal evolution of colorectal cancer in IBD. Nat. Rev. Gastroenterol. Hepatol. 2017;14:218–229. doi: 10.1038/nrgastro.2017.1. [DOI] [PubMed] [Google Scholar]
- 99.Stjarngrim J., Ekbom A., Hammar U., Hultcrantz R., Forsberg A.M. Rates and characteristics of postcolonoscopy colorectal cancer in the Swedish IBD population: what are the differences from a non-IBD population? Gut. 2019;68:1588–1596. doi: 10.1136/gutjnl-2018-316651. [DOI] [PubMed] [Google Scholar]
- 100.Cao Y., Wu K., Mehta R., Drew D.A., Song M.Y., Lochhead P., Nguyen L.H., Izard J., Fuchs C.S., Garrett W.S., Huttenhower C., Ogino S., Giovannucci E.L., Chan A.T. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672–678. doi: 10.1136/gutjnl-2016-313413. 672-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J.J., Haines C., Watson A.J.M., Hart A.R., Platt M.J., Pardoll D.M., Cosgrove S.E., Gebo K.A., Sears C.L. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989–2012: a matched case-control study. Gut. 2019;68:1971–1978. doi: 10.1136/gutjnl-2019-318593. [DOI] [PubMed] [Google Scholar]
- 103.Algra A.M., Rothwell P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 104.Dulai P.S., Singh S., Marquez E., Khera R., Prokop L.J., Limburg P.J., Gupta S., Murad M.H. Chemoprevention of colorectal cancer in individuals with previous colorectal neoplasia: systematic review and network meta-analysis. BMJ. 2016;355:i6188. doi: 10.1136/bmj.i6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rothwell P.M., Wilson M., Elwin C.E., Norrving B., Algra A., Warlow C.P., Meade T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 106.Liao X., Lochhead P., Nishihara R., Morikawa T., Kuchiba A., Yamauchi M., Imamura Y., Qian Z.R., Baba Y., Shima K., Sun R., Nosho K., Meyerhardt J.A., Giovannucci E., Fuchs C.S., Chan A.T., Ogino S. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N. Engl. J. Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li P., Wu H., Zhang H., Shi Y., Xu J., Ye Y., Xia D., Yang J., Cai J., Wu Y. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64:1419–1425. doi: 10.1136/gutjnl-2014-308260. [DOI] [PubMed] [Google Scholar]
- 108.Buskermolen M., Cenin D.R., Helsingen L.M., Guyatt G., Vandvik P.O., Haug U., Bretthauer M., Lansdorp-Vogelaar I. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a microsimulation modelling study. BMJ. 2019;367:l5383. doi: 10.1136/bmj.l5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Porcellini E., Laprovitera N., Riefolo M., Ravaioli M., Garajova I., Ferracin M. Epigenetic and epitranscriptomic changes in colorectal cancer: diagnostic, prognostic, and treatment implications. Cancer Lett. 2018;419:84–95. doi: 10.1016/j.canlet.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 110.Sveen A., Kopetz S., Lothe R.A. Biomarker-guided therapy for colorectal cancer: strength in complexity. Nat. Rev. Clin. Oncol. 2020;17:11–32. doi: 10.1038/s41571-019-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sinicrope F.A., Okamoto K., Kasi P.M., Kawakami H. Molecular biomarkers in the personalized treatment of colorectal cancer. Clin. Gastroenterol. Hepatol. 2016;14:651–658. doi: 10.1016/j.cgh.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gargalionis A.N., Papavassiliou A.G. Liquid biopsies in colorectal cancer: monitoring genetic heterogeneity. Trends Cancer. 2017;3:166–168. doi: 10.1016/j.trecan.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Wolf A.M.D., Fontham E.T.H., Church T.R., Flowers C.R., Guerra C.E., LaMonte S.J., Etzioni R., McKenna M.T., Oeffinger K.C., Shih Y.T., Walter L.C., Andrews K.S., Brawley O.W., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Siegel R.L., Wender R.C., Smith R.A. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American cancer society. CA Cancer J. Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 114.The Lancet Gastroenterology H. Colorectal cancer screening: is earlier better? Lancet Gastroenterol. Hepatol. 2018;3:519. doi: 10.1016/S2468-1253(18)30205-X. [DOI] [PubMed] [Google Scholar]