Abstract
Breast cancer is the commonest cause of global cancer-related deaths in women and a public health burden in sub-Saharan Africa (SSA). Although the disease incidence in SSA seems lower, mortality rates are disproportionately high in comparison to high-income countries. The global disease burden is growing, with SSA reporting the majority of cases; however, the dearth of information results in insufficient data which is barely representative of the actual disease burden in this population. Future incidence predictions assign the subregion with a majority of the cases and associated deaths. Breast cancer presents with racial and ethnic variations, and available evidence suggests geographical diversity and persistent risk factors that have barely been explored in SSA. Breast cancer is a complex genetic disease, but the genetic risk factors in the extant African population, which is the most genetically diverse population, is scant and of low quality. This review focuses on the burden, prevalence, detection, treatment, survival, biology, as well as risk factors, and reinforces the need for breast cancer-associated risk factor investigation and population-specific studies in SSA.
Keywords: Breast cancer, burden, sub-Saharan Africa, risk factors, population-specific research, genetic diversity
Impact statement
Breast cancer is a major global health challenge and more so in sub-Saharan Africa. However, the quality and quantity of data generated in extant Africans, which is the most genetically diverse population, hinders estimation of the actual disease burden. This review collates and presents the most current available information on breast cancer burden in sub-Saharan Africa. It provides evidence on the paucity of epidemiological and genetic data from across the subregion. It further strengthens the need for population-specific research, most importantly in sub-Saharan Africa, through coherent depiction of evidence on the inherently diverse genetic variations, risk factors, and breast cancer presentation in the African population.
Introduction
Breast cancer is a global menace that afflicts mostly women and a public health burden in sub-Saharan Africa (SSA) 1 (Figure 1). The quality and quantity of data from low- and middle-income countries (LIC and MIC) on the Global Cancer Observatory (GLOBOCAN) project resource used widely in estimating global cancer mortality and incidence globally are generally low. 2 Additionally, deficits in statistics on prevalence are particularly apparent in SSA. 3 However, breast cancer mortality and incidence rates in SSA are on the rise in comparison to developed countries. 4 By 2050, the prevalence is projected to double in SSA. 5 Available data on the disease is scant and, where available, are mostly of epidemiological or clinical nature. 3 Notably, the majority of countries in SSA lack cancer registries; hence, the true disease burden remains elusive. Only 20 (43.4%) of the 46 World Health Organization (WHO) member states in SSA have active cancer registries spanning a wide range of completeness and coverage 6 (Table 1). They are largely limited to specific sub-national populations, poorly funded, and probably not population-specific, hence do not meet active data collation standards. Therefore, breast cancer will most likely be a neglected healthcare issue in SSA, as governments in these countries focus on other healthcare priorities, particularly communicable diseases. SSA countries generally allocate insufficient GDP expenditure to health care, as such limited health infrastructure, staffing, and low sensitization rates contribute to the dearth of reliable data. 3 More so, 27 out of the world’s 28 poorest countries are in SSA, making up approximately 50% of SSA. 7 Reports indicate an increase in Gross National Income (GNI) based on purchasing-power-parity per capita of SSA countries, which somehow indicates poverty alleviation and elevation to middle-income status. Nonetheless, SSA is estimated to report 9 out of every 10 individuals living in extreme poverty by 2030. 8 This may further support the projected future breast cancer incidence and associated mortalities. Breast cancer control in SSA requires a concerted approach and should include population-based studies, early detection, prevention, and effective palliative care and treatment 3 (recommendations summarized in Table 2).
Figure 1.
Sub-Saharan Africa. West Africa: Mauritania, Senegal, Gambia Guinea-Bissau, Guinea, Sierra Leone, Liberia, Côte d'Ivoire, Ghana, Togo, Benin, Burkina Faso, Nigeria, Niger, Mali, and Cabo Verde; Central Africa: Cameroon, Central African Republic, Chad, Democratic Republic of the Congo, Equatorial Guinea, Gabon, and Sao Tomé and Principe; Southern Africa: South Africa, Lesotho, Namibia, Swaziland, and Botswana; East Africa: Tanzania, Kenya, Uganda, Rwanda, Burundi, South Sudan, Mozambique, Madagascar, Malawi, Zambia. Zimbabwe, Mauritius, Comoros, Djibouti, Ethiopia, Eritrea, Seychelles, Somaliland, Somalia, and Réunion. The figure was drawn using geographic heatmap in excel. (A color version of this figure is available in the online journal.)
Table 1.
List of cancer registries in SSA and the population characteristics.
| Countries (no. of registries) | Name of registries | 2020 population | Yearly change (%) | Median age |
|---|---|---|---|---|
| Benin (1) | Cotonou Cancer Registry | 12,123,200 | 2.73 | 19 |
| Botswana (1) | Botswana National Cancer Registry | 2,351,627 | 2.08 | 24 |
| Congo (1) | Registre des cancers de Brazzaville | 89,561,403 | 2.56 | 19 |
| Cote D’Ivoire (1) | Registre des Cancers d’Abidjan | 26,114,963 | 2.57 | 19 |
| Ethiopia (1) | Addis Ababa City Cancer Registry | 114,963,588 | 2.57 | 19 |
| Eswatini (1) | Eswatini National Cancer Registry | 1,160,164 | 1.05 | 21 |
| Gambia (1) | Gambia Cancer Registry | 2,416,668 | 2.94 | 18 |
| Ghana (1) | Kumasi Cancer Registry | 31,072,940 | 2.15 | 22 |
| Guinea (1) | Registre de Cancer de Guinée | 13,137,795 | 2.83 | 18 |
| Kenya (2) | Eldoret Cancer Registry | 53,771,296 | 2.28 | 20 |
| Nairobi Cancer Registry | ||||
| Malawi (1) | Malawi Cancer Registry | 19,129,952 | 2.69 | 18 |
| Mali (1) | Registre des cancers du Mali | 20,250,833 | 3.02 | 16 |
| Mauritius (1) | Mauritius National Cancer Registry | 1,271,768 | 0.17 | 37 |
| Mozambique (2) | Registro de Cancro de Beira | 31,255,435 | 2.93 | 18 |
| Maputo Cancer Registry | ||||
| Namibia (1) | Namibian Cancer Registry | 2,540,905 | 1.86 | 22 |
| Niger (1) | Registre des Cancers du Niger | 24,206,644 | 3.84 | 15 |
| Nigeria (5) | Abuja Cancer Registry | 206,139,589 | 2.58 | 18 |
| Calabar Cancer registry | ||||
| Ekiti Cancer Registry | ||||
| Ibadan Cancer Registry | ||||
| Nigerian National system of Cancer Registries | ||||
| Reunion (1) | Registre des cancers de la Réunion | 895,312 | 0.72 | 36 |
| Rwanda (1) | Rwanda Cancer Registry | 12,952,218 | 2.58 | 20 |
| Seychelles (1) | Seychelles National Cancer registry | 98,347 | 0.62 | 34 |
| South Africa (3) | South African Children’s Cancer study Group (SACCSG) | 59,308,690 | 1.28 | 28 |
| South Africa Eastern Cape Province Cancer Registry | ||||
| National Cancer Registry of South Africa (NCR-SA) | ||||
| Tanzania (3) | Dodoma Cancer Registry | 59,734,218 | 2.98 | 18 |
| Kilimanjaro Cancer Registry (KCMC) | ||||
| Mwanza Cancer Registry | ||||
| Uganda (2) | Gulu Cancer Registry | 45,741,007 | 3.32 | 17 |
| Kampala Cancer Registry | ||||
| Zambia (1) | Zambia National Cancer Registry | 18,383,955 | 2.93 | 18 |
| Zimbabwe (1) | Zimbabwe National Cancer Registry (Harare & Bulawayo | 14,862,924 | 1.48 | 19 |
Table 2.
Recommendations to address breast cancer research and care challenges in SSA.
| Outstanding challenges | Recommendations |
|---|---|
| Misrepresentation of the actual disease burden. | • In-depth epidemiological studies to define the disease
landscape and risk assessment. Generating and sustaining regional cancer registries of sufficient coverage and completeness, with sustained commitment from respective governments. |
| Underrepresented SSA ancestry individuals in clinically important data repositories. | • Building local capacity in genomic research, data generation,
and indexing of the most genetically diverse population to
enable imputation into global data repositories. Including more SSA participants in worldwide genomic research projects aimed at classifying pathogenic and genetic risk factors. Decentralizing genomic initiatives like the African Genomic Medicine training Initiative, into distinct areas with core mandates such as working exclusively on breast cancer. |
| Unvalidated breast cancer biomarkers for SSA populations. | • Population-specific studies focused on demystifying and
defining the population stratifications via subregion-specific
genomic profiling. Subregion-specific transcriptome profiling to establish and understand the subtle ethnic contributions to the disease biology and presentations. Generation of population-specific research model systems (e.g. cell lines) for the validation of candidate risk factors. • Fostering population-specific pharmacogenomic studies. |
| Unwilling participation in research. | Focus on training in research communications to enhance public engagement, education and awareness creation. |
Burden and diagnosis
Breast cancer is the global leader of cancer-related deaths in women and impacts approximately 2.1 million women yearly. 9 About 627,000 breast cancer-related deaths were recorded in 2018, with the majority from SSA, 9 representing about 15% of all cancer-related deaths. According to the 2020 GLOBOCAN data, 186,598 breast cancer cases were reported in Africa with 85,787 related deaths. While the disease burden seems relatively lower in SSA, survival is staggeringly low, with disproportionately high mortality rates (Figure 2). 10 The global breast cancer incidence has increased by 20% since 2008, making it the most commonly diagnosed cancer in women in Africa (Figure 3). 11 The incidence increased from 1.2 to 2.4 million cases between 2005 and 2015, with population growth and aging contributing 13 and 15%, respectively. 12
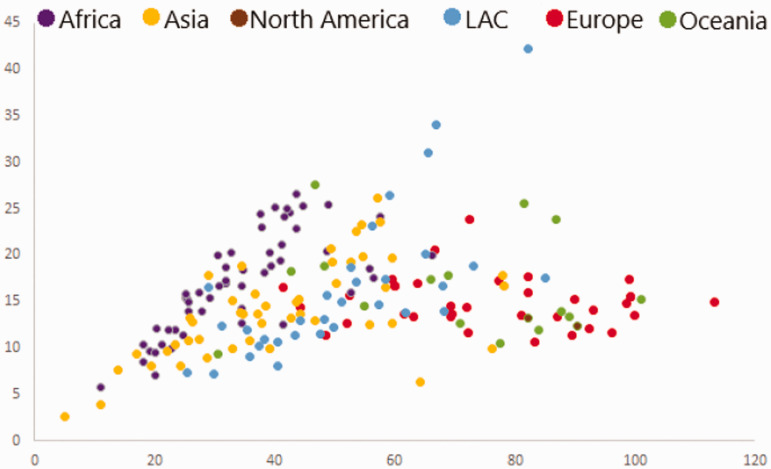
Figure 2.
The 2018 global breast cancer Mortality versus Incidence in women. Incidence in the African countries (purple dots) seems low; however, mortalities are relatively high. Europe reports the highest incidence and LAC, the highest mortality. The Republic of Gambia recorded the lowest incidence and mortality (6.9, 4) and Mauritius, the highest (69.6, 21.8) in Africa. This graph was generated using excel and data from the GLOBOCAN (http://gco.iarc.fr/) interactive observatory, and includes data from all the 54 African countries. ASR (W): age-standardized rate (World); LAC (Latin America and the Caribbean). (A color version of this figure is available in the online journal.)
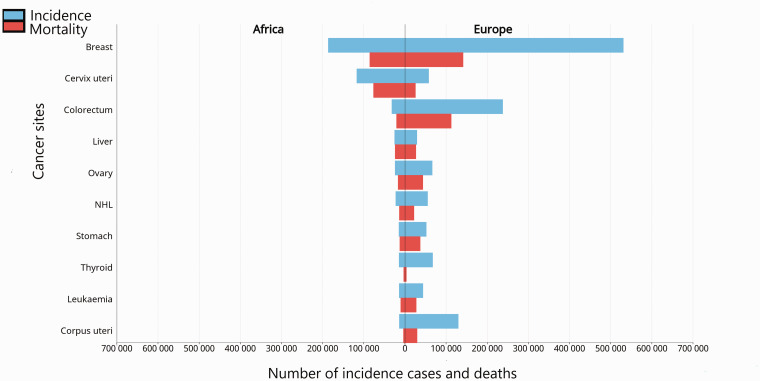
Figure 3.
The estimated number of new cancer cases in females in 2020. Breast cancer was the most diagnosed cancer and cause of cancer-related death in females in 2020. Africa reported 531,086 (74.3/100,000) new cases. Africa recorded a mortality of 85,787 (19.4/100,000), compared to Europe with 141,765 (14.8/100,000). In comparison to Europe, the associated deaths recorded in Africa seems disproportionate to the incidence reported. This data was generated using the GLOBOCAN 2020 data and interactive observatory. NHL: non-hodgkin lymphoma. (A color version of this figure is available in the online journal.)
In 2012, 56.8% of the 1.7 million women diagnosed with breast cancer were from low-income countries, and the majority of the 522,000 related deaths were recorded in SSA. Against this backdrop, over 19.3 and 21.7 million women are estimated to suffer from breast cancer by 2025 and 2030, respectively, again with the majority from SSA.3,12 A systemic review and meta-analysis reported an incidence of 22.4 per 100,000 women in SSA, which is comparable to North Africa, with 24.0 per 100,000 women. 13 Incidence rates have increased considerably between 2000 and 2015 across both registries. 13
Indications of marked geographic diversity and persistent local risk factors in populations at different economic transition phases could give more insight into the geographic variability of cancer incidence and mortality. The disproportionate breast cancer-associated mortality in SSA has been partly attributed to epidemiological transitioning hinged on an aging population, improved infectious disease control, and increased urbanization- and development-associated risk factors for non-communicable diseases. 14 The highest breast cancer prevalence rates in 2017 were reported in West, East, and North Africa. 3 Central Africa recorded an incidence to mortality ratio of 0.55 in comparison to 0.16 in the US and attributed primarily to ignorance of the disease manifestations and other related challenges. 4 A related report updating breast cancer incidence in Africa indicated an increase throughout Africa and projected to double by 2050. 4 The current cumulative breast cancer incidence per 100,000 women in Southern (46.2), Western (37.3), Eastern (29.9), and Central Africa (27.9) has associated mortality rates estimated at 15.6, 17.8, 15.4, and 15.8, respectively. 15 Contrary to the incidence trend, Southern Africa, which had the highest incidence, did not report the highest mortality. This may largely be due to underreported data and challenges in implementing robust screening programs. 4 It could also be a reflection of better healthcare facilities and treatment services in Southern Africa.
The cornerstone of breast cancer control is early detection. 16 Early diagnostic strategies aim to reduce barriers to care and/or improve access to effective diagnostic services and provide timely access to cancer treatment.
Detection
Certain cultural beliefs and breast cancer treatment-related stigma hinder health-seeking and early diagnosis in SSA. 17 In some regions, the disease has been ascribed to supernatural forces, thereby diminishing a sense of personal control over the outcomes. The idea of “beauty” in other countries involves a “whole” woman; hence, needing a mastectomy may evoke a sense of worthlessness, which impedes early health-seeking. 18 Ignorance of the disease, unavailability of tests, and inaccessibility of treatment and detection facilities bedevil screening and early detection in SSA. 19 Guidelines for breast cancer screening differ mostly depending on age at which screening begins (45 vs. 50 years) and intervals (1 vs. 2 years) between screens. Prognosing women with breast cancer in SSA is also impaired by shortage of trained personnel, noncompliance, and poor drug supplies. 19 The majority of late detections has been recorded in South Africa, Cameroon, the Central African Republic, Malawi, and Tanzania. 20 This does not suggest early detection in other countries, but a lack of comprehensive data to make substantive conclusions. The younger age at diagnosis in SSA has been widely attributed to the younger population age (Table 1) structure in the region, but this is not conclusive. 10
Factors affecting treatment and survival
Treatment
Breast cancer treatments generally depend on the disease type, and stage, and current options include surgery, chemotherapy, or hormonal, biological, and radiation therapies. 21 In SSA, options for advanced stages are limited and coupled with a scarcity of chemotherapy and radiotherapy facilities, most women undergo mastectomy. 18 About two-thirds of SSA countries lack radiotherapy facilities, 22 while those available meet only approximately 18% of the projected need. 23 Furthermore, tumor marker histological classification and identification facilities are limited in SSA, as are hormonal therapies and other targeted treatments. 23 Patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer exhibit poor survival rates, especially in the absence of targeted therapies, which is the case in most SSA countries. Breast cancer management is often deterred by treatment- and travel-related costs, particularly for those not living close to the medical centres. 18 Most women resort to other alternatives such as the use of herbs, visiting prayer camps and native doctors, which impede treatment and consequently translate to poor prognoses. 24 Another pivotal breast cancer treatment challenge in SSA is the scarcity of trained health professionals in cancer care and diagnosis. Additionally, medical oncologists, well-equipped and reliable pathology laboratories, and pathologists are rare, especially in the poorest countries. 25 As seen in Figure 4, the majority of SSA countries are poor. 26
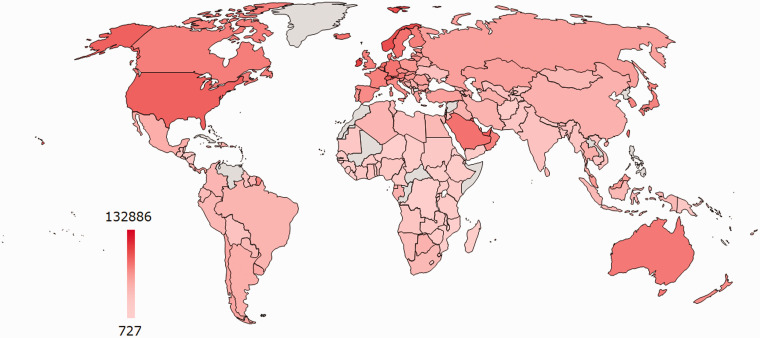
Figure 4.
The 2019 World Bank global landscape of poor countries as per GNI-PPP ($). The African countries ranged from $727 (Burundi) to $19,057 (Gabon), however, out of the four low-income countries, three (Burundi, Central Africa Republic and Democratic republic of Congo) are in SSA. The heatmap was generated using power-user in excel and countries investigated by the African Strategies for Advancing Pathology Group Members. 26 Countries in grey did not have data at the time of generation. USD (US dollar); GNI-PPP (Gross National Income based on purchasing-power-parity). (A color version of this figure is available in the online journal.)
Survival
The five-year breast cancer survival rate in SSA is less than 40% compared to 86% in the USA. 20 The low survival rate borders on factors including low awareness, late detection and treatment, poor prognosis, unavailability of treatment facilities and advanced therapies, high cost, and lack of prevalence documentation. Breast examination and early detection practices are low and contribute to late-stage diagnoses. 1 There is a notable delay between symptom onset and seeking healthcare in SSA. Most countries in SSA have not implemented and sustained screening programs owing to logistical, sociocultural, and financial constraints. 1 Breast cancer molecular subtypes also affect the survival rates. Breast cancer is population-specific, and the different prevalence rates of subtypes suggest heterogeneity in oncogenesis and partly explain the differing survival outcomes observed among ethnicities. 27 Survival outcomes in HICs have been improved by targeted therapies, which are hardly accessible to women in poor countries. 27 Further, survival has been enhanced by advances in population-specific genetic research, which has led to the development of molecular diagnostics for risk assessment. This has redefined the quality of treatments available in HICs. This is lacking in the African population, and a key determinant of the survival rates observed.
Clinical features and molecular subtypes in SSA
In SSA, about 80% of breast cancers are diagnosed at late stages (stages III or IV) compared with 15% in HICs. 22 The high late-stage disease presentation rate in SSA has been attributed to low awareness and detection in patients and the absence of diagnosis facilities and limited early detection programs. 28 Reports show that stages III and IV constituted 77% of breast cancer patients at the Mulago Hospital in Uganda; 29 77% at the Butaro Cancer Centre of Excellence in Rwanda; 30 78% at the Angolan Institute of Cancer Control; 31 and 87.7% at the Komfo Anokye Teaching Hospital in Ghana. 32 This is compounded by the breast cancer histological subtypes. A prevalence study on the molecular subtypes reported 34% triple-negative breast cancer (TNBC), 38% Luminal A, 22% HER2+, and 5% Luminal B in Uganda. 27 High-grade (grade 3) tumors made up 68% and late stage, 75% of the presentations; invasive ductal carcinoma was the predominant histological type recorded in the same population. TNBC is aggressive, recurs and metastasizes more often than the other subtypes, 33 and disproportionately affects women of African descent, with worse clinical outcomes compared to women of European ancestry.34,35 Studies have associated a high frequency of TNBC, particularly in women of West African ancestry. 36 An estimated TNBC incidence ranging between 20.8 and 46.4% was reported in African-American women in the United States. 37 This seems consistent with the 2–3-fold TNBC over-representation (34%) reported in SSA; both rates are relatively high in comparison with Caucasians, where prevalence is 12–17% or less, with TNBC and HER2+ tumors together constituting about 60% of the molecular subtypes and the majority of women aged 50 years and younger.33,38 The estrogen receptor-negative (ER–) subtypes, including TNBC and some HER2+, have been associated with higher proliferative capacity and grade. 27 HER2+ and TNBC subtypes have the most unfavorable treatment outcomes. Mediators of the relatively high HER2+ and TNBC proportions are not fully understood. Genetic differences, including unidentified founder mutations in breast cancer, have been suggested to partly account for the disparities.39,40 Exploring the mutation patterns and other genetic risk factors of breast cancer is an imperative. 41
Women with breast cancer in SSA are reportedly younger with peak incidence a decade earlier (50.2 years) in comparison with African Americans and White Americans with peak incidences of 60.8 and 62.4 years, respectively.10,42 Current reports in SSA show an increasing number of diagnosed cases between the ages of 35 and 49 years, with most presenting with advanced late-stage disease. 28 This was corroborated by a systematic review of population- and hospital-based breast cancer registries, 13 which revealed a mean age range between 30.6 and 60.8 years, with over 33 and 81% of the African population aged 30–49 and 30–59 years, respectively. Although inconclusive, these data suggest a high breast cancer incidence in the younger age groups in Africa. There are limited treatment options with advanced disease, which translates into poor prognosis.
Breast cancer risk factors and genetics
Risk factors
Studies show that most breast cancer risk factors in SSA are similar to those in HIC (age, race, genetic mutation, reproductive history, familial susceptibility, personal breast cancer history or any other non-breast cancer, lifestyle choices, etc.).21,22 Data on risk factors unique to SSA, including environmental exposures and infections, are limited and not conclusive. 23
Pathogens like viruses, which have been implicated in about a fifth of all cancers, may be major risk factors in breast cancer in SSA.43–45 The human papillomavirus (HPV) and human immunodeficiency virus (HIV) have been associated with breast cancer in SSA. 43 Reports indicate that chronic infectious diseases, specifically lifelong exposure to malaria, lead to loss of cell-mediated immunity and promote viral carcinogenesis, which has been observed in HPV-associated cervical cancer. 46 SSA is a malaria-endemic region, 47 and the association between insecticide exposure and hormone receptor-positive breast cancers is still debated. 1
Contraceptive use is another implicated breast cancer risk factor. The Collaborative group in hormonal factors in breast cancer analyzed data from 54 epidemiological studies using 53,297 breast cancer and 100,239 non-cancer patients and reported a 24% increase in relative risk in women currently using contraceptives and 7% in women who have ever used, compared to women who have never used them. 48 A population-based cross-sectional study in SSA reported an average contraceptive use of 17%, which is relatively low; however, the prevalence varied substantially across the individual countries. 49 This conclusion was drawn from data on contraceptive use from Demographic and Health Surveys (DHS) from 17 out of the 48 SSA countries. All SSA regions were not adequately covered; thus, the actual contraceptive use may probably be underreported. The relationship between contraceptive use and breast cancer cases reported has been observational; thus, inferences indicating their use as a risk factor are not enough to account for the incidence of breast cancer. More so, other factors, including duration of use and age of start of use, had no significant effect on the general risk, once recency of use has been established. Interestingly, no pronounced variation in recency of use has been reported between women of different backgrounds (ethnicity and race), reproductive histories, and breast cancer risks. 48
Breast-associated conditions including lobular carcinoma in situ and atypical ductal hyperplasia have been correlated with an increased risk of breast cancer. 50 Diabetes mellitus has been proposed to increase the risk of breast cancer. 50 Reproductive factors including early menarche and menopausal status have been acclaimed breast cancer risk factors; however, no association was found between these risk factors and breast cancer in Senegalese women. 51 Some breast cancers have been attributed to postmenopausal body mass index (Figure 5) globally. Possibly, many more risk factors are unexplored; hence there is an urgent need to exhaustively investigate breast cancer-associated risk factors in SSA. More so, data on risk factors in SSA are evidently old, mostly from 2012, and according to data available on GLOBOCAN, very few countries have made progress in investigating them.
Figure 5.
The predicted proportion of the 2012 global postmenopausal breast cancer cases among women attributable to body mass index (BMI). This prediction hinges on the assumption that the average population-level BMI remained constant since 1982. Per this record, SSA recorded the lowest attributable cases, probably due to underreporting. Data was obtained from GLOBOCAN 2012, Graph production: IARC World Health Organization. (A color version of this figure is available in the online journal.)
Family history or susceptibility is an important breast cancer-predisposing factor. 52 Early age at diagnosis and a positive breast cancer history may be suggestive of familial predispositions, which have not been thoroughly investigated in the SSA population. 51 Women with a familial breast cancer history with one or two first-degree premenopausal breast cancer relatives are at a 3.3-fold and 3.6-fold greater risk of developing breast cancer, respectively, compared to women without a family history. 53 Approximately 13–19% of diagnosed breast cancer patients had an affected first-degree relative. 54 This includes individuals carrying breast cancer 1 (BRCA1) and breast cancer 2 (BRCA2) gene mutations. Other significant driver mutations include those in tumor protein P53 (TP53), phosphatase and tensin homolog (PTEN), and serine/threonine kinase 11 (STK11), which have also been implicated in Li–Fraumeni syndrome, Cowden syndrome, and Peutz–Jeghers syndrome, respectively. 55
Genetics
Breast cancer is heterogeneous and complex, with ethnic and racial variations in both histology and tumor behavior. 56 While 10–15% of cases have a familial predisposition, approximately 90% are sporadic and associated with somatic mutations acquired during an individual’s lifetime.55,57 Genetic data in SSA are scant, 58 and efforts to establish the genetic diversity in SSA peaked with the African Genome Variation Project involving 1481 individuals from the region. 59 However, data on the genetic profile of breast cancer is still lacking as not enough people are captured by such studies to represent the regional and subregional diversities.
Extant African populations have significantly higher genetic diversity in comparison to other populations, and this reflects in over 2000 distinct languages and ethnic groups. 60 Adaptations to climate-change, strong pathogen burden, and diet have contributed critically to shaping genomic diversity in the African population. 61 Additionally, the intrinsic “within continent” variations owing to population structure, isolation, and back migration of the Eurasian populations into Africa have contributed significantly to the genetic diversity.62,63 Comprehensive genetic characterization of these migrations on the extant populations threw some light on the strong diversities between some geographically neighboring populations. 61
Owing to the genetic diversity, African populations are expected to have the highest levels of sequence diversity.60,64 Approximately 25% of hereditary cases are mediated by mutations in any of the few identified highly penetrant, but rare genes (PTEN, BRCA1, TP53, BRCA2, CDH1, and STK11) and these confer an 80% lifetime risk of breast cancer. 65 Causality has been established between early-onset breast cancer and germline mutations in BRCA1 and BRCA266,67 in African-American women,66–68 but the contribution of these genes to the disease in extant African women remains uncertain.69–71
Diverse BRCA1 and BRCA2 mutation and sequence variation spectra unique to Africans have been reported. 72 A global BRCA2 sequence diversity study detected 42% of the sequence variants exclusively in Africa, although chromosomes of African origin constituted only 13% of the 332 chromosomes screened. 72 Additionally, 2–3% of breast cancer cases are attributed to mutations in rare, moderately penetrant genes including BRIP1, CHEK2, PALB2, and ATM. 65 Corroborating this is the observation of PALB deleterious mutations accounting for up to 2% of early-onset breast cancer cases in white South Africans. 73 The known familial breast cancer genes account for only about 20% of reported cases, 74 implying that most familial susceptibility genes are unknown. Genetic studies have indicated considerable linkage disequilibrium structure differences and extensive population substructure between African and Caucasian populations. 75 The African-American population is the closest population of reference for the extant African population; however, they are an admixed population estimated to be approximately 80% West African and 20% European. 76 Inferences from this population are not entirely representative of the situation in the extant African population. Africans possess several genetic adaptations, which have evolved in response to exposure to infectious diseases, diverse climates, and diets. These may have influenced breast cancer manifestation in the African population. 77
Breast cancer research
Breast cancer studies encompass genetic and epidemiological research. Well-powered research probing the genetic breast cancer predispositions includes genome-wide association studies (GWAS) and whole genome, exome, and transcriptome profiling studies. These have been particularly instrumental in defining the scope of clinical breast cancer management, from screening, through diagnosis, to treatment in HICs. 78 Such progress has fostered development of molecular diagnostic tools, employing genetics, and expression profiles of the American, European, and Asian populations in breast cancer risk assessment. Despite the knowledge on the unique genetic variations in ethnic groups and races, about 96% of subjects included in GWAS are of European ancestry. 79 The paucity of such research in Africa impairs full understanding of the disease presentation and progression. 78 Initiatives, including the Human Heredity and Health (H3) Network, are facilitating genetic research in Africa; however, not much has been done on breast cancer. Breast cancer research also involves the use of several models, including paraffin-embedded samples, xenografts, primary tumors, and human and animal cell lines.80,81 Primary- or metastatic breast cancer-derived in vitro permanent cell lines are important experimental systems for investigating the biological and genetic alterations associated with cancer initiation and progression. 82 Cell lines are unlimited, self-replicating, and facilitate comparative studies in breast cancer research. 83 They are exceptional systems for studying cellular pathways and investigating genes critically involved in tumorigenesis.84,85 However, genetic background of cell lines can significantly affect experimental results. 86 The most commonly used human breast cancer cell lines are of Caucasian origin. 87 A high degree of heterogeneity in terms of subtypes, disease progression, etc., has been observed in different ethnic groups. 86 Furthermore, studies show that expression levels of certain genes peculiar to specific populations differ between cell lines of different ethnicities, and these may cause significant discrepancies in the results obtained. 88 The indigeneity, admixture, and ethnicity of the few existing black cell lines are still debated, 89 and their use in indigenous African populations is questionable. 90 These cell lines may be used in experimental studies in the African population, irrespective of their ethnic origin; however, they are more likely to give results that could vary vastly from the natural populations. To optimize results in breast cancer studies performed in SSA, it is important to use cell lines that represent this divergent population. Nevertheless, a detailed characterization of the existing black cell lines is fundamental to their application in African populations. This will be an eye-opener on the need to develop purely African breast cell lines that will largely serve the African population. Establishing real population-specific case systems will help to determine cell lines that will best model the diversity.
Conclusions
The extant African population is the most diverse population in terms of genetics and environmental exposures. Future projections indicate that breast cancer will undoubtedly burden economies globally, and the SSA region will be dealt with the greatest blow. Considering the dearth of knowledge and data in SSA, understanding breast cancer presentation in this population requires an in-depth evaluation of the risk factors, including environmental and genetic, with a conscious attempt at a population-specific data generation and risk assessment. This will throw more light on the landscape of breast cancer, including its development, presentation, and outcomes, thereby allowing for the development of novel therapies. In furtherance, the high level of unshared genetic variation among the populations reinforces the need for large-scale genetic profiling across Africa.
Footnotes
AUTHORS’ CONTRIBUTIONS: All the authors have read and approved the final manuscript. CAA drafted the manuscript, and LP and GAA critically reviewed the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All the authors were supported by a Developing Excellence in Leadership Training and Science (DELTAS) Africa grant (DEL-15-007: Awandare) from the African Academy of Sciences with funding from Wellcome (107755/Z/15/Z: Awandare) and the UK government. The opinions and interpretations expressed in this publication are those of the author(s) and not necessarily those of the AAS, Wellcome, or the UK government.
ORCID iDs: Gordon A Awandare https://orcid.org/0000-0002-8793-3641
Lily Paemka https://orcid.org/0000-0001-7498-520X
References
- 1.Black E, Richmond R. Improving early detection of breast cancer in sub-Saharan Africa: why mammography may not be the way forward. Global Health 2019; 15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The World Bank. World Bank Country and Lending Groups – World Bank Data Help Desk, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (2019, accessed 22 March 2019)
- 3.Cumber SN, Nchanji KN, Tsoka-Gwegweni JM. Breast cancer among women in sub-Saharan Africa: prevalence and a situational analysis. South Afr J Gynaecol Oncol 2017; 9:35–7 [Google Scholar]
- 4.Pace L, Shulman L. Breast cancer in sub-Saharan Africa: challenges and opportunities to reduce mortality. Oncologist 2016; 21:739–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omaka-Amari L, Ilo C, Nwimo I, Onwunaka C, Umoke C. Demographic differences in the knowledge of breast cancer among women in Ebonyi state. Nigeria IJNMH 2015; 1:18–27 [Google Scholar]
- 6.Gakunga R, Parkin DM. Cancer registries in Africa 2014 : a survey of operational. Int J Cancer 2015; 137:2045–52 [DOI] [PubMed] [Google Scholar]
- 7.Patel N. Figure of the week: understanding poverty in Africa. Brookings. www.brookings.edu/blog/africa-in-focus/2018/11/21/figure-of-the-week-understanding-poverty-in-africa/ (2018, accessed 28 August 2020)
- 8.Barne D, Wadhwa D. Year in review: 2018 in 14 charts, www.worldbank.org/en/news/feature/2018/12/21/year-in-review-2018-in-14-charts (2018, accessed 1 September 2020)
- 9.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108 [DOI] [PubMed] [Google Scholar]
- 10.Sighoko D, Kamaté B, Traore C, Mallé B, Coulibaly B, Karidiatou A, Diallo C, Bah E, McCormack V, Muwonge R, Bourgeois D. Breast cancer in pre-menopausal women in West Africa: analysis of temporal trends and evaluation of risk factors associated with reproductive life. 2013; 22:828–35 [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society & Society. Cancer facts & figures 2013. Am Cancer Soc 2010; 8:1–34 [Google Scholar]
- 12.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol 2017; 3:524–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adeloye D, Sowunmi OY, Jacobs W, David RA, Adeosun AA, Amuta AO, Misra S, Gadanya M, Auta A, Harhay MO, Chan KY. Estimating the incidence of breast cancer in Africa: a systematic review and meta-analysis . J Glob Health 2018; 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsu V, Jeronimo J, Anderson B. Why the time is right to tackle breast and cervical cancer in low-resource settings. Bull World Health Organ 2013; 91:683–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Increased awareness, equitable access to early diagnosis and timely, effective, and affordable treatment needed globally. Breast Cancer Awareness Month, www.who.int/cancer/breast_cancer_awareness/en/ (2016, accessed 7 September 2020)
- 17.Akuoko CP, Armah E, Sarpong T, Quansah DY, Amankwaa I, Boateng D. Barriers to early presentation and diagnosis of breast cancer among African women living in sub-Saharan Africa. PLoS One 2017; 12:e0171024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tetteh DA, Faulkner SL. Sociocultural factors and breast cancer in sub-Saharan Africa: implications for diagnosis and management. Womens Health (Lond) 2016; 12:147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anim JT. Breast cancer in sub-Saharan African women. Afr J Med Med Sci 1993; 22:5–10 [PubMed] [Google Scholar]
- 20.Kohler R, Gopal S, Miller AR, Lee CN, Reeve BB, Weiner BJ, Wheeler SB. A framework for improving early detection of breast cancer in sub-Saharan Africa: a qualitative study of help-seeking behaviors among Malawian women. Patient Educ Couns 2017; 100:167–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centre for Disease Control and Prevention. How is breast cancer treated? Breast Cancer, www.cdc.gov/cancer/breast/basic_info/treatment.htm (2018, accessed 22 March 2019)
- 22.Dalal S, Beunza JJ, Volmink J, Adebamowo C, Bajunirwe F, Njelekela M, Mozaffarian D, Fawzi W, Willett W, Adami HO, Holmes MD. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol 2011; 40:885–901 [DOI] [PubMed] [Google Scholar]
- 23.Brinton LA, Figueroa JD, Awuah B, Yarney J, Wiafe S, Wood SN, Ansong D, Nyarko K, Wiafe-Addai B, Clegg-Lamptey JN. Breast cancer in sub-Saharan Africa: opportunities for prevention. Breast Cancer Res Treat 2014; 144:467–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clegg-Lamptey J, Dakubo J, Attobra T. Psychosocial aspects of breast cancer treatment in Ghana. East Afr Med J 2009; 86:348–53 [DOI] [PubMed] [Google Scholar]
- 25. African strategies for advancing pathology group members. Quality pathology and laboratory diagnostic services are key to improving global health outcomes: improving global health outcomes is not possible without accurate. Am J Clin Pathol 2015; 143:325–8 [DOI] [PubMed] [Google Scholar]
- 26.Ventura L. Poorest countries in the world 2020. Global Finance Mag, www.gfmag.com/global-data/economic-data/the-poorest-countries-in-the-world (2020, accessed 2 October 2020).
- 27.Galukande M, Wabinga H, Mirembe F, Karamagi C, Asea A. Molecular breast cancer subtypes prevalence in an indigenous Sub Saharan African population. Pan Afr Med J 2014; 17:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jedy-Agba E, McCormack V, Adebamowo C, dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2016; 4:923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gakwaya A, Kigula-Mugambe JB, Kavuma A, Luwaga A, Fualal J, Jombwe J, Galukande M, Kanyike D. Cancer of the breast: 5-year survival in a tertiary hospital in Uganda. Br J Cancer 2008; 99:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pace L, Dusengimana JMV, Hategekimana V, Habineza H, Bigirimana JB, Tapela N, Mutumbira C, Mpanumusingo E, Brock JE, Meserve E, Uwumugambi A. Benign and malignant breast disease at Rwanda’s first public cancer referral center. Oncologist 2016; 21:571–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes LV, Miguel F, Freitas H, Tavares A, Pangui S, Castro C, Lacerda GF, Longatto-Filho A, Weiderpass E, Santos LL. Stage at presentation of breast cancer in Luanda, Angola – a retrospective study. BMC Health Serv Res 2015; 15:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghartey FJN, Anyanful A, Eliason S, Mohammed Adamu S, Debrah S. Pattern of breast cancer distribution in Ghana: a survey to enhance early detection, diagnosis, and treatment. International Journal of Breast Cancer 2016. 10.1155/2016/3645308 [DOI] [PMC free article] [PubMed]
- 33.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 2009; 115:423–8 [DOI] [PubMed] [Google Scholar]
- 34.Lukong KE, Ogunbolude Y, Kamdem JP. Breast cancer in Africa: prevalence, treatment options, herbal medicines, and socioeconomic determinants. Breast Cancer Res Treat 2017; 166:351–65 [DOI] [PubMed] [Google Scholar]
- 35.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, Van De Rijn M, Jeffrey SS, Thorsen T. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98:10869–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman LA, Jenkins B, Chen Y, Oppong JK, Adjei E, Jibril AS, Hoda S, Cheng E, Chitale D, Bensenhaver JM, Awuah B. Hereditary susceptibility for triple negative breast cancer associated with Western sub-Saharan African ancestry: results from an international surgical breast cancer collaborative. Ann Surg 2019; 270:484–92 [DOI] [PubMed] [Google Scholar]
- 37.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer 2015; 15:248–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363:1938–48 [DOI] [PubMed] [Google Scholar]
- 39.Gru AA, Allred DC. Molecular pathology of breast cancer. In: Eble J. (ed) Molecular surgical pathology. New York: Springer, 2013, pp.95–128. [Google Scholar]
- 40.Shulman LP. Genetic and genomic factors in breast cancer. In: Hansen N (ed) Management of the patient at high risk for breast cancer. New York: Springer, 2013, pp.29–47.
- 41.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, Jones D, Hinton J, Marshall J, Stebbings LA, Menzies A. Mutational processes molding the genomes of 21 breast cancers. Cell 2012; 149:979–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark A, Kleer CG, Martin I, Awuah B, Nsiah-Asare A, Takyi V, Braman M, Quayson SE, Zarbo R, Wicha M, Newman L. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer 2010; 116:4926–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White M, Pagano J, Khalili K. Viruses and human cancers: a long road of discovery of molecular paradigms. Clin Microbiol Rev 2014; 27:463–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gannon OM, Antonsson A, Bennett IC, Saunders NA. Viral infections and breast cancer – a current perspective. Cancer Lett 2018; 420:182–9 [DOI] [PubMed] [Google Scholar]
- 45.Lawson JS, Glenn WK. Multiple oncogenic viruses are present in human breast tissues before development of virus associated breast cancer. Infect Agent Cancer 2017; 12:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Odida M, Schmauz R, Lwanga SK. Grade of malignancy of cervical cancer in regions of Uganda with varying malarial endemicity. Int J Cancer 2002; 99:737–41 [DOI] [PubMed] [Google Scholar]
- 47.De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. J Trop Med 2012;1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Collaborative group on hormonal factors in breast cancer. Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713–27 [DOI] [PubMed] [Google Scholar]
- 49.Ba DM, Ssentongo P, Agbese E, Kjerulff KH. Prevalence and predictors of contraceptive use among women of reproductive age in 17 sub-Saharan African countries: a large population-based study. Sex Reprod Healthc 2019; 21:26–32 [DOI] [PubMed] [Google Scholar]
- 50.Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, Kasamesup V, Wongwaisayawan S, Srinakarin J, Hirunpat S, Woodtichartpreecha P, Boonlikit S, Teerawattananon Y, Thakkinstian A. Risk factors of breast cancer: a systematic review and meta-analysis. Asia Pac J Public Health 2013; 25:368–87 [DOI] [PubMed] [Google Scholar]
- 51.Gueye M, Gueye S, Gueye M, Diallo M, Gassama O. A hospital based case control study of female breast cancer risk factors in a sub-Saharan African country. Int J Reprod Contracep Obstet Gynecol 2016; 5:2328–32 [Google Scholar]
- 52.Kabel AM, Baali FH. Breast cancer: insights into risk factors, pathogenesis, diagnosis and management. J Cancer Res Treat 2015; 3:28–33 [Google Scholar]
- 53.Singletary SE. Rating the risk factors for breast cancer. Ann Surg 2003; 237:474–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tice J, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. JCO 2015; 33:3137–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gage M, Wattendorf D, Henry L. Translational advances regarding hereditary breast cancer syndromes. J Surg Oncol 2012; 105:444–51 [DOI] [PubMed] [Google Scholar]
- 56.Gukas ID, Jennings BA, Mandong BM, Igun GO, Girling AC, Manasseh AN, Ugwu BT, Leinster SJ. Clinicopathological features and molecular markers of breast cancer in Jos, Nigeria. West Afr J Med 2005; 24:209–13 [DOI] [PubMed] [Google Scholar]
- 57.Pharoah PDP, Day NE, Duffy S, Easton DF, Ponder BAJ. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer 1997; 71:800–9 [DOI] [PubMed] [Google Scholar]
- 58.Khalid DA, Elhaj A, Aceto G, Mariani-Costantini R, Eltayeb EA. Hereditary breast cancer in sub-Saharan Africa. Curr Womens Health Rev 2012; 8:44–54 [Google Scholar]
- 59.Gurdasani D, Carstensen T, Tekola-Ayele F, Pagani L, Tachmazidou I, Hatzikotoulas K, Karthikeyan S, Iles L, Pollard MO, Choudhury A, Ritchie GR. The African genome variation project shapes medical genetics in Africa. Nature 2015; 517:327–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sirugo G, Hennig BJ, Adeyemo AA, Matimba A, Newport MJ, Ibrahim ME, Ryckman KK, Tacconelli A, Mariani-Costantini R, Novelli G, Soodyall H. Genetic studies of African populations: an overview on disease susceptibility and response to vaccines and therapeutics. Hum Genet 2008; 123:557–98 [DOI] [PubMed] [Google Scholar]
- 61.Choudhury A, Aron S, Sengupta D, Hazelhurst S, Ramsay M. African genetic diversity provides novel insights into evolutionary history and local adaptations. Hum Mol Genet 2018; 27:R209–R218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Busby GB, Band G, Le QS, Jallow M, Bougama E, Mangano VD, Amenga-Etego LN, Enimil A, Apinjoh T, Ndila CM, Manjurano A. Admixture into and within sub-Saharan Africa. Elife 2016; 5:e15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagani L, Schiffels S, Gurdasani D, Danecek P, Scally A, Chen Y, Xue Y, Haber M, Ekong R, Oljira T, Mekonnen E. Tracing the route of modern humans out of Africa by using 225 human genome sequences from Ethiopians and Egyptians. Am J Hum Genet 2015; 96:986–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tishkoff S, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, Ibrahim M. The genetic structure and history of Africans and African Americans. Science 2009; 324:1035–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol Off J Eur Soc Med Oncol 2015; 26:1291–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall M, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, Wenstrup RJ, Ward BE, Scholl TA, Noll WW. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer 2009; 115:2222–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haffty B, Choi DH, Goyal S, Silber A, Ranieri K, Matloff E, Lee MH, Nissenblatt M, Toppmeyer D, Moran MS. Breast cancer in young women (YBC): prevalence of BRCA1/2 mutations and risk of secondary malignancies across diverse racial groups. Ann Oncol 2009; 20:1653–9 [DOI] [PubMed] [Google Scholar]
- 68.Pal T, Vadaparampil S, Betts J, Miree C, Li S, Narod SA. BRCA1/2 in high-risk African American women with breast cancer: providing genetic testing through various recruitment strategies. Genet Test 2008; 12:401–7 [DOI] [PubMed] [Google Scholar]
- 69.Basro S, Apffelstaedt JP. Breast cancer in young women in a limited-resource environment. World J Surg 2010; 34:1427–33 [DOI] [PubMed] [Google Scholar]
- 70.Baltogiannis G, Roukos D. Letter to the editor: breast cancer in young women in Africa: are there genetic and clinical differences with European ancestry patients? World J Surg 2010; 34:1982–3 [DOI] [PubMed] [Google Scholar]
- 71.Dafaallah K, Awadelkarim A. Role of pathology in sub-Saharan Africa: an example from Sudan. Pathol Lab 2010; 2:49–57 [Google Scholar]
- 72.Wagner T, Hirtenlehner K, Shen P, Moeslinger R, Muhr D, Fleischmann E, Concin H, Doeller W, Haid A, Lang AH, Mayer P. Global sequence diversity of BRCA2: analysis of 71 breast cancer families and 95 control individuals of worldwide populations. Hum Mol Genet 1999; 8:413–23 [DOI] [PubMed] [Google Scholar]
- 73.Sluiter M, Mew S, van Rensburg EJ. PALB2 sequence variants in young South African breast cancer patients. Fam Cancer 2009; 8:347–53 [DOI] [PubMed] [Google Scholar]
- 74.Thompson D, Easton D. The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia 2004; 9:221–36 [DOI] [PubMed] [Google Scholar]
- 75.Feng Y, Stram DO, Rhie SK, Millikan RC, Ambrosone CB, John EM, Bernstein L, Zheng W, Olshan AF, Hu JJ, Ziegler RG. A comprehensive examination of breast cancer risk loci in African American women. Hum Mol Genet 2014; 23:5518–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith MW, Patterson N, Lautenberger JA, Truelove AL, McDonald GJ, Waliszewska A, Kessing BD, Malasky MJ, Scafe C, Le E, De Jager PL. A high-density admixture map for disease gene discovery in African Americans. Am J Hum Genet 2004; 74:1001–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet 2008; 9:403–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silverstein A, Sood R, Costas-Chavarri A. Breast cancer in Africa: limitations and opportunities for application of genomic medicine. Int J Breast Cancer 2016. 10.1155/2016/4792865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bustamante C, Francisco M, Burchard EG. Genomics for the world. Nature 2011; 475:163–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marsden CG, Wright MJ, Carrier L, Moroz K, Pochampally R, Rowan BG. A novel in vivo model for the study of human breast cancer metastasis using primary breast tumor-initiating cells from patient biopsies. BMC Cancer 2012; 12:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanna C, Kwok L, Finlay-Schultz J, Sartorius CA, Cittelly DM. Labeling of breast cancer patient-derived xenografts with traceable reporters for tumor growth and metastasis studies. J Vis Exp 2016; 117:54944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferreira D, Adega F, Chaves R. The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. In: López-Camarillo C, Aréchaga-Ocampo E. (eds) Oncogenomics and cancer proteomics – novel approaches in biomarkers discovery and therapeutic targets in cancer. London, UK: IntechOpen, 2013, pp.139–66. [Google Scholar]
- 83.Gokul T, Anusha D, David D. A comparative study on viability of mcf-7 human breast cancer cell lines using piperine and tamoxifen – an in vitro study with a novel mishmash. Biomed Pharmacol J 2018; 11:1955–9 [Google Scholar]
- 84.Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer 2017; 8:3131–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sheikholeslami A, Nabiuni M, Arefian E. Suppressing the molecular signaling pathways involved in inflammation and cancer in breast cancer cell lines MDA-MB-231 and MCF-7 by miR-590. Tumour Biol 2017;39:4. 10.1177/1010428317697570 [DOI] [PubMed]
- 86.Carey L, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 2006; 295:2492–502 [DOI] [PubMed] [Google Scholar]
- 87.Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis 2010; 32:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laurent LC, Nievergelt CM, Lynch C, Fakunle E, Harness JV, Schmidt U, Galat V, Laslett AL, Otonkoski T, Keirstead HS, Schork A. Restricted ethnic diversity in human embryonic stem cell lines. Nat Methods 2010; 7:6–7 [DOI] [PubMed] [Google Scholar]
- 89.Martínez-Cardús A, Moran S, Musulen E, Moutinho C, Manzano JL, Martinez-Balibrea E, Tierno M, Élez E, Landolfi S, Lorden P, Arribas C. Epigenetic homogeneity within colorectal tumors predicts shorter relapse-free and overall survival times for patients with locoregional cancer. 2016; 151:961–72 [DOI] [PubMed] [Google Scholar]
- 90.Shi Y, Ye P, Long X. Differential expression profiles of the transcriptome in breast cancer cell lines revealed by next generation sequencing. Cell Physiol Biochem 2017; 44:804–16 [DOI] [PubMed] [Google Scholar]