Abstract
Purpose:
We evaluated the association of percent mammographic density (PMD), absolute dense area (DA) and non-dense area (NDA) with risk of ‘intrinsic’ molecular breast cancer (BC) subtypes.
Methods:
We pooled 3492 invasive BC and 10,148 controls across six studies with density measures from prediagnostic, digitized film-screen mammograms. We classified BC tumors into subtypes [63% Luminal A, 21% Luminal B, 5% HER2-expressing and 11% as triple negative (TN)] using information on estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 and tumor grade. We used polytomous logistic regression to calculate odds ratio (OR) and 95% confidence intervals (CI) of density measures (per SD) across the subtypes compared to controls, adjusting for age, body mass index and study, and examined differences by age group.
Results:
All density measures were similarly associated with BC risk across subtypes. Significant interaction of PMD by age (P=0.001) was observed for luminal A tumors, with stronger effect sizes seen for younger women <45 years (OR=1.69) relative to women of older ages (OR=1.53, ages 65-74, OR=1.44 ages 75+). Similar but opposite trends were seen for NDA by age for risk of luminal A: risk for women: <45 years (OR=0.71) was lower than older women (OR=0.83 and OR=0.84 for ages 65-74 and 75+, respectively) (p<0.001). Although not significant, similar patterns of associations were seen by age for TN cancers.
Conclusions:
Mammographic density measures were associated with risk of all ‘intrinsic’ molecular subtypes. However, findings of significant interactions between age and density measures may have implications for subtype-specific risk models.
Keywords: Breast cancer, ‘intrinsic’ molecular subtypes, mammographic density
Background
Mammographic breast density or the variation in the composition of breast tissue (fat, stromal, and epithelial tissues) on the mammogram image, is known to be a strong and established BC risk factor [1]. Prior studies suggested that it may also be associated specifically with aggressive tumor characteristics, such as high grade and large tumor size [2, 3]. Others suggested that mammographic density may be associated with molecular tumor subtypes [4-9]. The results from these studies are inconsistent, with some studies suggesting the association between mammographic density and all tumor subtypes have similar magnitudes, while other studies report that these associations are primarily driven by HER2 positive, estrogen receptor (ER)-positive or ER-negative tumors [1, 10-17]. Moreover, PMD was found to be strongly associated with BC of tumors of large size, and positive lymph nodes across all ages (<55 years, 55–64 years, and ≥65 years), and ER-negative status among those age<55 years [16]. A study of Chinese breast cancer cases has shown stronger association with HER2-expressing tumors, but this was limited to the subgroup of cases with normal BMI [12].
Dense area (DA) and non-dense area (NDA) may be associated independently with BC risk [18-21]. In our previous study we showed that DA was associated with BC risk, while NDA was associated with a decreased risk across all ages. Among invasive tumor characteristics, we found significant trends in the effect sizes of associations for DA and NDA with BC with larger tumor size, however, no differences were found by nodal status. In addition, among women age<55 years, DA was strongly associated with ER-positive versus ER-negative tumors, and NDA was strongly associated with decreased risk of ER-negative versus ER-positive tumors [22].
Gene expression profiling has identified molecular signatures that classify invasive BC into distinct subtypes that vary in their clinical behavior, response to treatment and etiology [23-25]. Immunohistochemical (IHC) staining of tumor sections using antibody panels can be used as a surrogate to classify these ‘intrinsic’ molecular subtypes. We evaluated the association of density measures (i.e., PMD, DA and NDA), with ‘intrinsic’ molecular subtypes, and whether the strength of associations varies by age and BMI.
Methods
Study populations
We included six cohort or case-control studies: the Mayo Mammography Health Study (MMHS) [26, 27], Mayo Clinic Breast Cancer Study (MCBCS) [28, 29], Nurses’ Health Study I (NHSI), and NHSII [30-32], Mayo Clinic Mammography Study (MCMAM) [33], and the San Francisco Bay Area Breast Cancer SPORE and San Francisco Mammography Registry (SFMR) [34-36] (Supplemental Table 1). Only invasive BC cases were included in analyses. Additional details of these six studies are described in our previous reports [16, 22].
This study was approved by the Institutional Review Boards at the Mayo Clinic (Rochester, MN), Brigham and Women’s Hospital (Boston, MA), the University of California, San Francisco (UCSF, San Francisco, CA), and the Connecticut Department of Public Health Human Investigations Committee. Informed consent was obtained or implied by return of questionnaires (NHSI, NHSII).
Assessment of mammographic density
PMD, DA and NDA were assessed from digitized images of prediagnostic film-screen mammograms of the craniocaudal view using a computer-assisted thresholding technique, Cumulus [37], and UCSF custom mammographic density software [38] as described previously and shown equivalent to Cumulus [16, 22]. PMD was calculated on the contralateral breast image for BC cases and a selected side, for controls, as the proportion of absolute DA over total breast area (DA + NDA); for NHS and NHSII studies, PMD was calculated as the average of DA and NDA of both CC views.
PMD, DA, and NDA measurements were standardized within each study to control for variability in measurements by reader [15, 39], time of density assessment, and age distribution of the different studies for pooled analyses. We previously used a logit transformation for PMD [15, 16, 39] and selected appropriate transformations for absolute DA and NDA using the Box-Cox procedure [22]. Briefly, we focused on women without BC as the reference for the transformation and estimated study-specific linear age trends in the medians of transformed mammographic density (TMD) values using quantile regression. Age trends were removed using residuals from the quantile regression and study variability was standardized using interquartile range (IQR) of each study. Results were applied similarly to women with BC and TMD values were then back-transformed to the original scale for the analyses, as described in [22].
Assessment of tumor characteristics among cases
Receptor status was abstracted from pathology records of invasive BC and supplemented by IHC staining of cores taken from the original biopsy where available. For IHC, >1% staining of ER or PR was considered positive. Both IHC and FISH results for HER2 were considered for classification of patients into HER2 positive and negative status. The pathologist (YYC) graded HER2 immunostaining as 0, 1+, 2+, or 3+; 0 and 1+ were classified as negative and 3+ as positive. Fluorescence in situ hybridization (FISH), which directly measures HER2 gene amplification, was added to test indeterminate findings (2+); HER2 gene to Cen 17 ratio less than 1.8 classified as negative.
We classified invasive tumors as Luminal A (ER+ and/or PR+ and HER2− and grade 1 or 2), Luminal B (ER+ and/or PR+ and HER2+ or Luminal A and grade 3), HER2 expressing (ER-/PR-/HER2+) and triple negative (TN) (ER-/PR-/HER2−). For TN, we also differentiated basal-like tumors (any positive for staining of EGFR and/or CK 5/6 by IHC) from unclassified (negative on both markers) for those TN cases who had tissue blocks available.
Statistical analyses
All density measurements were examined continuously per standard deviation (SD) as well as categorized; PMD as 0-10%, 11-25% (reference), 26-50% and 51%+, and DA and NDA into quartiles based on the control distribution across the six studies. We used polytomous (multinomial) logistic regression models to calculate odds ratio (OR) and 95% confidence intervals (CI) for the associations of density measures with risk of BC overall as well as with ‘intrinsic’ BC subtypes compared to controls, adjusting for age (continuous), body mass index (BMI, continuous) and study site. We tested for statistical heterogeneity of associations between study sites and BC subtype using contrasts within the polytomous logistic regression models. We also evaluated whether there were differences in density and subtype associations by BMI (<25 k/m2, 25+ kg/m2) and age group (<45, 45-54, 55-64, 65-74, ≥75) by inclusion of interaction terms between each continuous density measure and age group modeled as an ordinal variable or BMI as a categorical variable. Analyses were also stratified by age group and BMI to examine the association of subtypes across these two risk factors. SAS version 9.4 was used for analyses and p-values <0.05 were considered statistically significant.
Results
Our pooled analysis of six cohort or case-control studies included 3492 women with invasive BC and 10,148 without BC. Screening mammography was performed a mean of 4.7±2.7 years prior to diagnosis (for cases). Mean age at mammogram was 56 years among both cases and controls. Of 3492 invasive BC cases, 2217 (63%) were classified as Luminal A, 747 (21%) as Luminal B, 159 (5%) as HER2 expressing, and 369 (11%) as TN. Of the 369 TN, 207 had tumor tissue available and 171 were basal while 36 were unclassified. Mean PMD and DA were higher among cases versus controls, while mean NDA was lower among cases versus controls (Table 1). Among the subtypes, unadjusted mean PMD and DA were the highest in HER2 expressing, while the mean NDA was lowest in HER2 expressing compared to other subtypes (Table 1). Invasive tumor characteristics are presented in Table 2 by ‘intrinsic’ molecular subtypes.
Table 1:
Baseline characteristics of study population by ‘intrinsic’ molecular subtypes
| Overall | Luminal A | Luminal B | HER2 Expressing |
Triple Negative |
||
|---|---|---|---|---|---|---|
| Cases | Controls | |||||
| N | 3492 | 10148 | 2217 | 747 | 159 | 369 |
| Mean % mammographic density, PMD (SD) | 33.7 (20.4) | 28.1 (19.6) | 33.2 (20.6) | 32.8 (20.4) | 36.9 (20.1) | 35 (20) |
| Mean Dense Area (DA) cm^2 (SD) | 56.3 (41.6) | 45.1 (35.9) | 56.7 (43) | 52 (36.4) | 58 (41.8) | 56.9 (39.9) |
| Standardized Mean Nondense Area (NDA) cm^2 (SD) | 135.4 (101.1 | 141.8 (99.7) | 137.4 (99.5) | 138.3 (110.8 | 115.3 (83.8) | 132.1 (103.7 |
| Mean age at mammogram (SD) | 56.5 (11.3) | 55.9 (10.9) | 57.2 (11.3) | 55.5 (11.7) | 53.8 (10) | 55.2 (11.5) |
| Mean age at diagnosis (SD) | 61 (11.6) | -- | 62 (11.5) | 59.5 (12) | 58 (10.7) | 59.5 (11.6) |
| Mean BMI (SD) | 25.1 (7.6) | 25.6 (6) | 25.2 (7.4) | 24.5 (8.4) | 24.6 (7.1) | 25.2 (7.6) |
| Body mass index categories, kg/m2 | ||||||
| <25 | 1658 (47.5%) | 5393 (53.1%) | 1035 (46.7%) | 357 (47.8%) | 84 (52.8%) | 182 (49.3%) |
| 25-29 | 999 (28.6%) | 2844 (28%) | 633 (28.6%) | 226 (30.3%) | 40 (25.2%) | 100 (27.1%) |
| 30-34 | 432 (12.4%) | 1143 (11.3%) | 298 (13.4%) | 77 (10.3%) | 19 (11.9%) | 38 (10.3%) |
| 35+ | 253 (7.2%) | 670 (6.6%) | 160 (7.2%) | 58 (7.8%) | 9 (5.7%) | 26 (7%) |
| Unknown | 150 (4.3%) | 98 (1%) | 91 (4.1%) | 29 (3.9%) | 7 (4.4%) | 23 (6.2%) |
| Menopausal status | ||||||
| Premenopausal | 1065 (30.5%) | 3402 (33.5%) | 636 (28.7%) | 259 (34.7%) | 51 (32.1%) | 119 (32.2%) |
| Postmenopausal | 2249 (64.4%) | 6410 (63.2%) | 1483 (66.9%) | 443 (59.3%) | 91 (57.2%) | 232 (62.9%) |
| Unknown | 178 (5.1%) | 336 (3.3%) | 98 (4.4%) | 45 (6%) | 17 (10.7%) | 18 (4.9%) |
| Parity | ||||||
| Nulliparous | 723 (20.7%) | 1863 (18.4%) | 466 (21%) | 145 (19.4%) | 35 (22%) | 77 (20.9%) |
| Parous | 2399 (68.7%) | 7717 (76%) | 1522 (68.7%) | 519 (69.5%) | 99 (62.3%) | 259 (70.2%) |
| Unknown | 370 (10.6%) | 568 (5.6%) | 229 (10.3%) | 83 (11.1%) | 25 (15.7%) | 33 (8.9%) |
| Postmenopausal hormone therapya | ||||||
| Not current user | 940 (48.3%) | 3106 (54.3%) | 610 (48.4%) | 187 (47.7%) | 42 (50%) | 101 (48.3%) |
| Current, estrogen | 274 (14.1%) | 1006 (17.6%) | 170 (13.5%) | 62 (15.8%) | 8 (9.5%) | 34 (16.3%) |
| Current, estrogen + progestin | 455 (23.4%) | 1003 (17.5%) | 300 (23.8%) | 89 (22.7%) | 21 (25%) | 45 (21.5%) |
| Unknown | 277 (14.2%) | 606 (10.6%) | 181 (14.4%) | 54 (13.8%) | 13 (15.5%) | 29 (13.9%) |
| Family history | ||||||
| No | 2612 (74.8%) | 8725 (86%) | 1656 (74.7%) | 560 (75%) | 113 (71.1%) | 283 (76.7%) |
| Yes | 685 (19.6%) | 1366 (13.5%) | 458 (20.7%) | 134 (17.9%) | 30 (18.9%) | 63 (17.1%) |
| Unknown | 195 (5.6%) | 57 (0.6%) | 103 (4.6%) | 53 (7.1%) | 16 (10.1%) | 23 (6.2%) |
Among postmenopausal women in MMHS, NHS, NHSII, and UCSF
Table 2:
Distribution of breast cancer cases by ‘intrinsic’ molecular subtypes and tumor characteristics
| Overall | Luminal A | Luminal B | HER2 Expressing |
Triple Negative |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Total invasive cases | 3492 | 100 | 2217 | 63.5 | 747 | 21.4 | 159 | 4.6 | 369 | 10.6 |
| Tumor characteristics | ||||||||||
| Histology | ||||||||||
| Ductal | 2686 | 76.9 | 1576 | 71.1 | 636 | 85.1 | 147 | 92.5 | 327 | 88.6 |
| Lobular | 416 | 11.9 | 337 | 15.2 | 60 | 8 | 6 | 3.8 | 13 | 3.5 |
| Mixed | 250 | 7.2 | 203 | 9.2 | 39 | 5.2 | 4 | 2.5 | 4 | 1.1 |
| Unknown/other | 140 | 4 | 101 | 4.6 | 12 | 1.6 | 2 | 1.3 | 25 | 6.8 |
| Histologic Grade | ||||||||||
| Well differentiated | 1025 | 29.4 | 954 | 43 | 41 | 5.5 | 9 | 5.7 | 21 | 5.7 |
| Moderately differentiated | 1427 | 40.9 | 1129 | 50.9 | 186 | 24.9 | 44 | 27.7 | 68 | 18.4 |
| Poorly differentiated | 846 | 24.2 | 134 | 6 | 498 | 66.7 | 88 | 55.3 | 260 | 70.5 |
| Unknown | 194 | 5.6 | 22 | 2.9 | 18 | 11.3 | 20 | 5.4 | ||
| Tumor size | ||||||||||
| 0.1-1.0 cm | 1141 | 32.7 | 868 | 39.2 | 158 | 21.2 | 45 | 28.3 | 70 | 19 |
| 1.1.-2.0 cm | 1387 | 39.7 | 863 | 38.9 | 314 | 42 | 48 | 30.2 | 162 | 43.9 |
| 2.1+ cm | 887 | 25.4 | 447 | 20.2 | 253 | 33.9 | 57 | 35.8 | 130 | 35.2 |
| Unknown | 77 | 2.2 | 39 | 1.8 | 22 | 2.9 | 9 | 5.7 | 7 | 1.9 |
| Involvement of lymph nodes | ||||||||||
| Negative | 2373 | 68 | 1563 | 70.5 | 477 | 63.9 | 101 | 63.5 | 232 | 62.9 |
| Positive | 904 | 25.9 | 506 | 22.8 | 232 | 31.1 | 52 | 32.7 | 114 | 30.9 |
| Unknown | 215 | 6.2 | 148 | 6.7 | 38 | 5.1 | 6 | 3.8 | 23 | 6.2 |
| Estrogen Receptor status | ||||||||||
| Negative | 579 | 16.6 | 24 | 1.1 | 27 | 3.6 | 159 | 100 | 369 | 100 |
| Positive | 2913 | 83.4 | 2193 | 98.9 | 720 | 96.4 | 0 | 0 | 0 | 0 |
| Progesterone Receptor status | ||||||||||
| Negative | 877 | 25.1 | 196 | 8.8 | 153 | 20.5 | 159 | 100 | 369 | 100 |
| Positive | 2615 | 74.9 | 2021 | 91.2 | 594 | 79.5 | 0 | 0 | 0 | 0 |
| HER2 status | ||||||||||
| Negative | 2946 | 84.4 | 2217 | 100 | 360 | 48.2 | 159 | 100 | 369 | 100 |
| Positive | 546 | 15.6 | 0 | 0 | 387 | 51.8 | 0 | 0 | 0 | 0 |
Association of PMD, DA and NDA and overall BC risk
PMD (continuous OR=1.61, CI:1.53-1.69) and DA (continuous OR=1.39, CI:1.34-1.45) were associated with BC risk, while NDA (continuous OR=0.78, CI:0.74-0.82) was inversely associated with BC risk (Table 3, Supplemental Table 2). These associations were consistent across all six study sites and no heterogeneity by study was observed (all P>0.05, Supplemental Table 3).
Table 3:
Associations of continuous PMD, DA, and NDA (per standard deviation) with breast cancer overall and ‘intrinsic’ molecular subtypes by age
| Overall | Luminal A | Luminal B | HER2 Expressing |
Triple Negative | ||
|---|---|---|---|---|---|---|
| Continuous Percent Mammographic Density | ||||||
| overall | 1.61 (1.53, 1.69) | 1.60 (1.51, 1.69) | 1.69 (1.54, 1.85) | 1.79 (1.48, 2.18) | 1.44 (1.27, 1.63) | |
| <45 | 1.67 (1.46, 1.92) | 1.69 (1.42, 2.00) | 1.52 (1.20, 1.93) | 1.74 (1.01, 2.98) | 1.72 (1.02, 2.91) | |
| 45-54 | 1.64 (1.65, 1.80) | 1.64 (1.48, 1.82) | 1.70 (1.44, 1.99) | 1.95 (1.40, 2.72) | 1.66 (1.24, 2.34) | |
| 55-64 | 1.64 (1.50, 1.80) | 1.65 (1.48, 1.84) | 1.83 (1.52, 2.20) | 1.47 (1.06, 2.06) | 1.52 (1.13, 2.06) | |
| 65-74 | 1.56 (1.39, 1.75) | 1.53 (1.35, 1.74) | 1.65 (1.33, 2.06) | 3.06 (1.58, 5.92) | 1.38 (0.88, 2.16) | |
| 75+ | 1.49 (1.24, 1.78) | 1.44 (1.18, 1.76) | 1.72 (1.23, 2.40) | 2.16 (1.01, 4.64) | 1.41 (0.84, 2.39) | |
| Interaction with age group as ordinal | p=0.001 | p=0.35 | p=0.59 | p=0.21 | ||
| Continuous Dense Area | ||||||
| overall | 1.39 (1.34, 1.45) | 1.41 (1.35, 1.48) | 1.40 (1.31, 1.51) | 1.40 (1.21, 1.62) | 1.25 (1.13, 1.38) | |
| <45 | 1.33 (1.20, 1.48) | 1.36 (1.20, 1.55) | 1.29 (1.08, 1.55) | 1.36 (0.91, 2.02) | 1.34 (0.92, 1.98) | |
| 45-54 | 1.40 (1.31, 1.50) | 1.43 (1.32, 1.55) | 1.40 (1.24, 1.58) | 1.40 (1.09, 1.78) | 1.31 (1.04, 1.64) | |
| 55-64 | 1.47 (1.36, 1.59) | 1.53 (1.40, 1.67) | 1.42 (1.23, 1.65) | 1.38 (1.05, 1.80) | 1.35 (1.06, 1.72) | |
| 65-74 | 1.37 (1.25, 1.51) | 1.35 (1.21, 1.49) | 1.52 (1.28, 1.80) | 1.61 (1.01, 2.57) | 1.09 (0.75, 1.59) | |
| 75+ | 1.34 (1.16, 1.56) | 1.33 (1.13, 1.57) | 1.41 (1.08, 1.86) | 1.64 (0.92, 2.92) | 1.43 (0.92, 2.21) | |
| Interaction with age group as ordinal | p=0.31 | p=0.50 | p=0.40 | p=0.77 | ||
| Continuous Nondense Area | overall | 0.78 (0.74, 0.82) | 0.79 (0.75, 0.85) | 0.75 (0.67, 0.82) | 0.61 (0.49, 0.76) | 0.81 (0.71, 0.93) |
| <45 | 0.71 (0.61, 0.82) | 0.71 (0.60, 0.86) | 0.84 (0.66, 1.06) | 0.54 (0.30, 1.00) | 0.74 (0.43, 1.27) | |
| 45-54 | 0.76 (0.69, 0.93) | 0.76 (0.68, 0.85) | 0.77 (0.64, 0.91) | 0.56 (0.39, 0.81) | 0.72 (0.53, 0.99) | |
| 55-64 | 0.79 (0.71, 0.87) | 0.82 (0.73, 0.92) | 0.64 (0.52, 0.79) | 0.82 (0.58, 1.16) | 0.83 (0.60, 1.15) | |
| 65-74 | 0.80 (0.70, 0.90) | 0.83 (0.72, 0.95) | 0.78 (0.61, 0.99) | 0.23 (0.10, 0.52) | 0.65 (0.39, 1.10) | |
| 75+ | 0.82 (0.67, 1.00) | 0.84 (0.67, 1.05) | 0.70 (0.48, 1.03) | 0.55 (0.22, 1.37) | 1.13 (0.64, 1.99) | |
| Interaction with age group as ordinal | p<0.001 | p=0.21 | p=0.99 | p=0.10 |
PMD DA and NDA with BC across molecular subtypes
PMD and DA were positively associated with BC across all subtypes (Table 3). For PMD, the association with BC was strongest for the HER2 expressing subtype (OR=1.79 per SD PMD, CI:1.48-2.18) and least for TN (OR=1.44 per SD PMD, CI:1.27-1.63), although there was no statistical evidence for heterogeneity of associations across subtypes (Pheterogeneity =0.45). The associations of DA with BC subtype were attenuated relative to PMD; the association of DA and BC was lowest among the TN subtype (OR=1.25 per SD DA, CI:1.13-1.38) and higher and similar for HER2-expressing, Luminal A and Luminal B (ORs all ~1.40 per SD DA) (Pheterogeneity=0.08). Subset to those TN with basal subtype, the associations of BC with PMD (OR=1.63, CI:1.35-1.96) and DA (OR=1.36, CI:1.18-1.57) showed slightly stronger effect sizes than with overall TN.
NDA was inversely associated with BC across all subtypes (Table 3). The association was strongest for the HER2 expressing subtype (OR=0.61, CI:0.49-0.76) and weakest for TN (OR=0.81, CI:0.71-0.93), although there was no statistical evidence for heterogeneity across subtypes (Pheterogeneity=0.21). Subset to those with basal subtype, the association of BC with NDA (OR=0.77, CI:0.63-0.94) was similar to those with TN.
Analyses of PMD, DA and NDA with breast cancer subtype showed no apparent differences in associations when stratified by BMI categories (Supplemental Table 4) (Pinteraction of 0.45 (PMD) to 0.96 (dense area)).
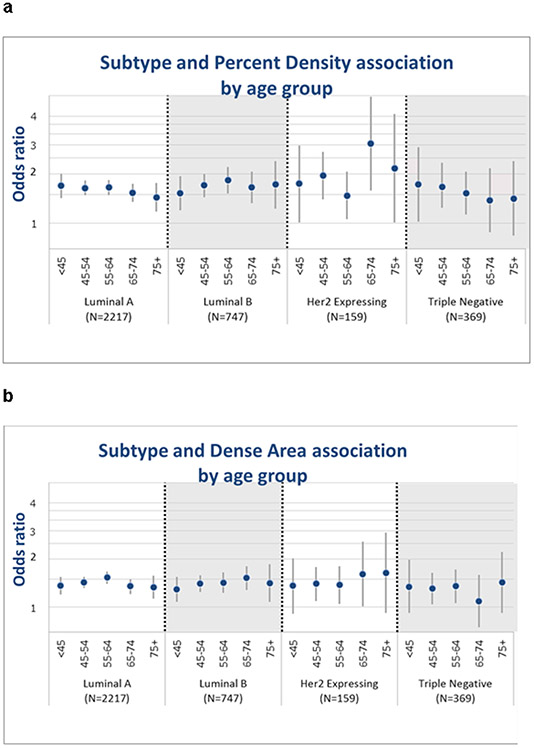
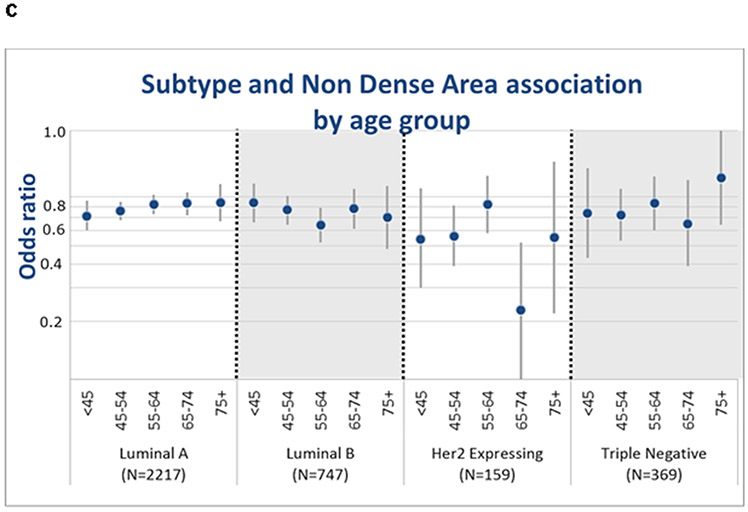
Interactions of density phenotypes with age by BC subtype
Statistically significant interactions were seen between age group and PMD (P=0.02, Figure 1.a) and NDA (P<0.001, Figure 1.c) but not DA (P=0.61, Figure 1.b) with BC subtype. The differences in associations of PMD and NDA with breast cancer were primarily driven by the luminal A subtype, with stronger associations among younger vs. older women (Pinteraction=0.001 for PMD and Pinteraction<0.001 for NDA, Table 3, Figure 1.a). Among luminal A, women age <45 years had 70% increased risk per SD PMD while women 75 and older had a 44% increased risk per SD PMD (Table 3). Similarly, association of NDA and luminal A subtype among women <45 years was stronger (OR=0.71) compared to the older age groups (Table 3, Figure 1.c).
Fig. 1.
Associations of continuous PMD (a), DA (b), and NDA (c) for risk of ‘intrinsic’ molecular subtypes (HER2 expressing, Luminal A, Luminal B, and TN) by age group
Discussion
Overall, our data suggest that mammographic density measures are associated with BC of all ‘intrinsic’ molecular subtypes. Although mammographic density is a strong established risk factor for BC, there have been inconsistent results across the limited studies regarding its association with ‘intrinsic’ molecular subtypes [8, 11, 13, 14, 17, 40-43]. Our study has the largest sample of breast cancer cases to date to examine the association of density phenotypes with breast cancer subtype using mammograms prior to diagnosis, as well to as examine these associations across BMI and age groups. We found similar associations of density phenotypes with breast cancer across subtypes and no differences by BMI. However, we did find differences by age that were driven by stronger associations of PMD and NDA and the luminal A subtype among younger vs. older women.
Findings from prior studies have been inconsistent. The largest study to date consisted of a case-control study from Sweden with 2632 BC patients and 15,945 controls and found an increased risk for Luminal A, Luminal B, HER2-expression and basal-like with increasing absolute dense area with no evidence of subtype heterogeneity [11]. Another study of 477 BC patients and 588 controls also did not find strong evidence that mammographic density parameters differentially affected specific BC tumor characteristics[17]. Other studies have found stronger density and breast cancer associations for HER2-expressing cancer compared to other subtypes; however, they used different measurements for density including volumetric breast density and the clinical BI-RADS measure. These included a North American study of 457 BC patients (n=59 HER-2 expressing), which found stronger associations of volumetric breast density with HER-2 expressing cancer [40], a case-only study of 1321 invasive female BCs from the Piedmont Cancer Registry that found that triple negative and luminal BH+ (ER+ and/or PR + , HER2+) were negatively and positively associated with higher breast density (by BI-RADS), respectively, compared to luminal A [8], and a case-only study from China with 2001 BC patients (n=180 HER-2 expressing) that also found increased risk of HER-2 expressing cancer among women with extremely dense breasts (by BI-RADS) relative to women with almost entirely fat or scattered fibroglandular dense breasts [12], however, this association was limited to those with normal BMI, <25 kg/m2.
In our study, the risk estimates for PMD and NDA were more strongly associated with HER2-expressing compared to other BC subtypes, however, the heterogeneity across subtypes was not shown to be statistically significant. Also, we found no statistical significant differences in the density and subtype associations by BMI; in fact, estimates between PMD and DA with HER2 expressing subtype were slightly stronger (and for NDA, weaker) among those with BMI ≥25 kg/m2, which is in contrast to prior findings seen among Chinese breast cancer cases [12].
We also found a significant interaction between age and PMD and NDA on breast cancer by subtype. This was primarily driven by the stronger associations of PMD and NDA with breast cancer seen among younger women with luminal A tumors. Given that luminal A tumors are the most common subtype, the significant interaction was likely due to the sufficient power to examine interactions in this subgroup. In fact, the stronger risk estimates for PMD and NDA with breast cancer in younger vs. older age groups were similar in magnitude for both luminal A and TN subtypes, but only statistically significant for luminal A. Among luminal A, the ORs per SD PMD were 1.69 and 1.44 for those <45 and 75+ years, respectively, while among TN, these estimates were 1.72 and 1.41, respectively. Thus, we cannot rule out a differential association of PMD and NDA with TN breast cancer by age due to limited power in this smaller subtype. Interestingly, differential associations by age were not seen with DA, which is often suggested as the more biologically relevant density phenotype[44]. The differences by age may provide insight to mechanisms important to the luminal A and TN subtypes, including age-related involution, which may be reflected in the NDA and PMD measures, and confer greater protection for these subtypes at younger ages. In fact, prior studies by our group suggested a correlation between age-related involution with NDA, but not DA [45]; Gierach et al. confirmed these findings, which were stronger among premenopausal women [46].
Our data confirm prior studies of density measures as risk factors for multiple breast cancer subtypes as well as for aggressive and non-aggressive cancers [47-49]. This underscores the importance of the inclusion of density measures in breast cancer risk models for breast cancer overall and for breast cancer subtypes [50]. Three risk models that incorporate breast density have improved discrimination compared to models without breast density [51-53]. A risk model that predicts ER-positive cancer could be clinically useful since women informed they are at high risk of ER-positive breast cancer may be more likely to consider taking primary prevention medication. To be clinically applicable, risk models for HER2-expessing tumors and triple negative tumors would need to be examined in the context of screening strategies aimed at early cancer detection of these tumors. Understanding the appropriate subtype-specific model estimates for risk factors and their combinations, in particular by age, will require larger sample sizes than those to date. Our findings of differential risk of breast density with luminal A cancers, and possibly TN, by age (higher risk at younger ages) will be important to consider, as inclusion of differential estimates associated with breast density and subtypes by age could be included in risk models. Given the particular rarity of HER2+ and TN (basal) subtypes, in particular, studies will require collaborative efforts to estimate accurate risk estimates by age group as well as consider the possibility of differential estimates by race and ethnic groups.
Limitations of the study have been previously described [16, 22], including variation in populations and study design of the six studies, use of clinical pathology rather than a central pathology review for ER, PR and HER2; modifications in diagnostic criteria over time that may influence tumor characteristics and receptor status. Further, our study is primarily representative of Caucasians women and therefore cannot be generalized to other ethnicities. Our power was also limited for age-and density interactions by subtype analyses for the TN and HER-2 expressing subtypes. We also used digitized film mammograms instead of digital mammography or tomosynthesis and did not have information on the clinical BI-RADS density measure on all studies. The strengths of this pooled analysis, however, include a large sample size from six studies with mammograms available for cases years before the cancer, standardized estimates of DA and NDA, detailed data on covariates and tumor characteristics from pathology reports, with screening mammograms assessed for breast density in a systematic fashion. Further, we and others have shown similar associations of the quantitative density measures and BI-RADS with breast cancer risk from film and digital mammograms [1, 54-56]. The more precise quantitative measures also provide increased power for examining differential associations of breast density with subtype by age and BMI.
Conclusions
Our results suggest inclusion of breast density measures is important for all overall breast cancer and subtype-specific risk models, with no evidence for differential association by BMI but potentially greater impact in younger women depending on the breast density measure.
Supplementary Material
Acknowledgments
The authors thank the participants and staff of all the studies for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WI, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding
This work was supported in part by the National Institutes of Health, National Cancer Institute (R01 CA140286, R01 CA128931, P50 CA58207, P50 CA116201, R01 CA97396, R01 CA 122340, P01 CA087969, R01 CA050385, R01 CA124865, and R01 CA131332), the Breast Cancer Research Foundation and the Department of Defense (DAMD 17-00-1-033).
List of abbreviations
- BC
breast cancer
- ER
estrogen receptor
- PMD
percent mammographic density
- DA
dense area
- NDA
non-dense area
- IHC
Immunohistochemical
- OR
odds ratio
- CI
confidence interval
- MMHS
Mayo Mammography Health Study
- MCBCS
Mayo Clinic Breast Cancer Study
- NHSI
Nurses’ Health Study I
- NHSII
Nurses’ Health Study II
- MCMAM
Mayo Clinic Mammography Study
- SFMR
San Francisco Bay Area Breast Cancer SPORE and San Francisco Mammography Registry
Footnotes
Conflict of Interest
All authors have no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All participants provided either passive permission or informed consent for use of data.
References
- 1.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006. 15(6):1159–1169. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson L, Czene K, Rosenberg L, Humphreys K, Hall P. The influence of mammographic density on breast tumor characteristics. Breast Cancer Res Treat. 2012. 134(2):859–866. [DOI] [PubMed] [Google Scholar]
- 3.Yaghjyan L, Colditz GA, Collins LC, Schnitt SJ, Rosner B, Vachon C, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011. 103(15):1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JH, Hsu FT, Shih HN, Hsu CC, Chang D, Nie K, Nalcioglu O, Su MY. Does breast density show difference in patients with estrogen receptor-positive and estrogen receptor-negative breast cancer measured on MRI? Ann Oncol. 2009. 20(8):1447–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson L, Hall P, Czene K, dos Santos Silva I, McCormack V, Bergh J, Bjohle J, Ploner A. Mammographic density and molecular subtypes of breast cancer. Br J Cancer. 2012. 107(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phipps AI, Buist DSM, Malone KE, Barlow WE, Porter PL, Kerlikowske K, O'Meara ES, Li CI. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol. 2012. 22(5):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W-T, Dryden M, Broglio K, Gilcrease M, Dawood S, Dempsey PJ, Valero V, Hortobagyi G, Atchley D, Arun B. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008. 111(3):405–410. [DOI] [PubMed] [Google Scholar]
- 8.Pizzato M, Carioli G, Rosso S, Zanetti R, La Vecchia C. The impact of selected risk factors among breast cancer molecular subtypes: a case-only study. Breast Cancer Res Treat. 2020. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh AJ, Mullooly M, Sayed S, Ndumia R, Abayo I, Orwa J, Wasike R, Moloo Z, Gierach GL. Mammographic Breast Density and Breast Cancer Molecular Subtypes: The Kenyan-African Aspect. Biomed Res Int. 2018. 2018:6026315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoni S, Sasco AJ, dos Santos Silva I, McCormack V. Is mammographic density differentially associated with breast cancer according to receptor status? A meta-analysis. Breast Cancer Res Treat. 2013. 137(2):337–347. [DOI] [PubMed] [Google Scholar]
- 11.Holm J, Eriksson L, Ploner A, Eriksson M, Rantalainen M, Li J, Hall P, Czene K. Assessment of Breast Cancer Risk Factors Reveals Subtype Heterogeneity. Cancer Res. 2017. 77(13):3708–3717. [DOI] [PubMed] [Google Scholar]
- 12.Li E, Guida JL, Tian Y, Sung H, Koka H, Li M, Chan A, Zhang H, Tang E, Guo C et al. Associations between mammographic density and tumor characteristics in Chinese women with breast cancer. Breast Cancer Res Treat. 2019. 177(2):527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartor H, Zackrisson S, Elebro K, Hartman L, Borgquist S. Mammographic density in relation to tumor biomarkers, molecular subtypes, and mode of detection in breast cancer. Cancer Causes Control. 2015. 26(6):931–939. [DOI] [PubMed] [Google Scholar]
- 14.Shin J, Lee JE, Ko HY, Nguyen TL, Nam SJ, Hopper JL, Song Y-M. Association between mammographic density and tumor marker-defined breast cancer subtypes. Eur J Cancer Prev. 2018. 27(3):239–247. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe MJ. Mammographic density. Measurement of mammographic density. Breast Cancer Res. 2008. 10(3):209–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertrand KA, Tamimi RM, Scott CG, Jensen MR, Pankratz VS, Visscher D, Norman A, Couch F, Shepherd J, Fan B et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013. 15(6):R104–R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velasquez Garcia HA, Gotay CC, Wilson CM, Lohrisch CA, Lai AS, Aronson KJ, Spinelli JJ. Mammographic density parameters and breast cancer tumor characteristics among postmenopausal women. Breast Cancer (Dove Med Press). 2019. 11:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lokate M, Peeters PHM, Peelen LM, Haars G, Veldhuis WB, van Gils CH. Mammographic density and breast cancer risk: the role of the fat surrounding the fibroglandular tissue. Breast Cancer Res. 2011. 13(5):R103–R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettersson A, Graff RE, Ursin G, dos Santos Silva I, McCormack V, Baglietto L, Vachon C, Bakker MF, Giles GG, Chia KS et al. Mammographic Density Phenotypes and Risk of Breast Cancer: A Meta-analysis. J Natl Cancer Inst. 2014. 106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. Nondense mammographic area and risk of breast cancer. Breast Cancer Res. 2011. 13(5):R100–R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone J, Ding J, Warren RML, Duffy SW, Hopper JL. Using mammographic density to predict breast cancer risk: dense area or percentage dense area. Breast Cancer Res. 2010. 12(6):R97–R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand KA, Scott CG, Tamimi RM, Jensen MR, Pankratz VS, Norman AD, Visscher DW, Couch FJ, Shepherd J, Chen YY et al. Dense and Nondense Mammographic Area and Risk of Breast Cancer by Age and Tumor Characteristics. Cancer Epidemiol Biomarkers Prev. 2015. 24(5):798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inns J, James V. Circulating microRNAs for the prediction of metastasis in breast cancer patients diagnosed with early stage disease. Breast. 2015. 24(4):364–369. [DOI] [PubMed] [Google Scholar]
- 24.Kahraman M, Röske A, Laufer T, Fehlmann T, Backes C, Kern F, Kohlhaas J, Schrörs H, Saiz A, Zabler C et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci Rep. 2018. 8(1):11584–11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Schooneveld E, Wildiers H, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015. 17(1):21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heine JJ, Scott CG, Sellers TA, Brandt KR, Serie DJ, Wu F-F, Morton MJ, Schueler BA, Couch FJ, Olson JE et al. A Novel Automated Mammographic Density Measure and Breast Cancer Risk. J Natl Cancer Inst. 2012. 104(13):1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson JE, Sellers TA, Scott CG, Schueler BA, Brandt KR, Serie DJ, Jensen MR, Wu F-F, Morton MJ, Heine JJ et al. The influence of mammogram acquisition on the mammographic density and breast cancer association in the Mayo Mammography Health Study cohort. Breast Cancer Res. 2012. 14(6):R147–R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh K, Brandt KR, Sellers TA, Reynolds C, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Vachon CM. Association of Mammographic Density with the Pathology of Subsequent Breast Cancer among Postmenopausal Women. Cancer Epidemiol Biomarkers Prev. 2008. 17(4):872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Luo J, Press MF, Wang Y, Bernstein L, Ursin G. Is there a difference in the association between percent mammographic density and subtypes of breast cancer? Luminal A and triple-negative breast cancer. Cancer Epidemiol Biomarkers Prev. 2009. 18(2):479–485. [DOI] [PubMed] [Google Scholar]
- 30.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Can. 2005. 5(5):388–396. [DOI] [PubMed] [Google Scholar]
- 31.Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005. 14(11 Pt 1):2641–2647. [DOI] [PubMed] [Google Scholar]
- 32.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006. 66(4):2476–2482. [DOI] [PubMed] [Google Scholar]
- 33.Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007. 16(1):43–49. [DOI] [PubMed] [Google Scholar]
- 34.Kerlikowske K, Carney PA, Geller B, Mandelson MT, Taplin SH, Malvin K, Ernster V, Urban N, Cutter G, Rosenberg R et al. Performance of screening mammography among women with and without a first-degree relative with breast cancer. Ann intern Med. 2000. 133(11):855–863. [DOI] [PubMed] [Google Scholar]
- 35.Kerlikowske K, Shepherd J, Creasman J, Tice JA, Ziv E, Cummings SR. Are breast density and bone mineral density independent risk factors for breast cancer? J Natl Cancer Inst. 2005. 97(5):368–374. [DOI] [PubMed] [Google Scholar]
- 36.Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004. 13(12):2090–2095. [PubMed] [Google Scholar]
- 37.Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, Hammond G, Minkin S. The association of breast mitogens with mammographic densities. Br J Cancer. 2002. 87(8):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, Malkov S, Vittinghoff E, Cummings SR. Volume of Mammographic Density and Risk of Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2011. 20(7):1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prevrhal S, Shepherd JA, Smith-Bindman R, Cummings SR, Kerlikowske K. Accuracy of mammographic breast density analysis: results of formal operator training. Cancer Epidemiol Biomarkers Prev. 2002. 11(11):1389–1393. [PubMed] [Google Scholar]
- 40.Edwards BL, Atkins KA, Stukenborg GJ, Novicoff WM, Larson KN, Cohn WF, Harvey JA, Schroen AT. The Association of Mammographic Density and Molecular Breast Cancer Subtype. Cancer Epidemiol Biomarkers Prev. 2017. 26(10):1487–1492. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan K, Baglietto L, Stone J, McLean C, Southey MC, English DR, Giles GG, Hopper JL. Mammographic density and risk of breast cancer by tumor characteristics: a case-control study. BMC Cancer. 2017. 17(1):859–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razzaghi H, Troester MA, Gierach GL, Olshan AF, Yankaskas BC, Millikan RC. Association between mammographic density and basal-like and luminal A breast cancer subtypes. Breast Cancer Res. 2013. 15(5):R76–R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaghjyan L, Tamimi RM, Bertrand KA, Scott CG, Jensen MR, Pankratz VS, Brandt K, Visscher D, Norman A, Couch F et al. Interaction of mammographic breast density with menopausal status and postmenopausal hormone use in relation to the risk of aggressive breast cancer subtypes. Breast Cancer Res Treat. 2017. 165(2):421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haars G, van Noord PA, van Gils CH, Grobbee DE, Peeters PH. Measurements of breast density: no ratio for a ratio. Cancer Epidemiol Biomarkers Prev. 2005. 14(11 Pt 1):2634–2640. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS et al. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010. 28(13):2207–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gierach GL, Patel DA, Pfeiffer RM, Figueroa JD, Linville L, Papathomas D, Johnson JM, Chicoine RE, Herschorn SD, Shepherd JA et al. Relationship of Terminal Duct Lobular Unit Involution of the Breast with Area and Volume Mammographic Densities. Cancer Prev Res (Phila). 2016. 9(2):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerlikowske K, Gard CC, Tice JA, Ziv E, Cummings SR, Miglioretti DL, Breast Cancer Surveillance C. Risk Factors That Increase Risk of Estrogen Receptor-Positive and -Negative Breast Cancer. J Natl Cancer Inst. 2017. 109(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerlikowske K, Zhu W, Tosteson AN, Sprague BL, Tice JA, Lehman CD, Miglioretti DL, Breast Cancer Surveillance C. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015. 162(10):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shieh Y, Scott CG, Jensen MR, Norman AD, Bertrand KA, Pankratz VS, Brandt KR, Visscher DW, Shepherd JA, Tamimi RM et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019. 21(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brentnall AR, Cuzick J. Risk Models for Breast Cancer and Their Validation. Stat Sci. 2020. 35(1):14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast Density and Benign Breast Disease: Risk Assessment to Identify Women at High Risk of Breast Cancer. J Clin Oncol. 2015. 33(28):3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brentnall AR, Cuzick J, Buist DSM, Bowles EJA. Long-term Accuracy of Breast Cancer Risk Assessment Combining Classic Risk Factors and Breast Density. JAMA Oncol. 2018. 4(9):e180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee A, Mavaddat N, Wilcox AN, Cunningham AP, Carver T, Hartley S, Babb de Villiers C, Izquierdo A, Simard J, Schmidt MK et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019. 21(8):1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson JE, Sellers TA, Scott CG, Schueler BA, Brandt KR, Serie DJ, Jensen MR, Wu FF, Morton MJ, Heine JJ et al. The influence of mammogram acquisition on the mammographic density and breast cancer association in the Mayo Mammography Health Study cohort. Breast Cancer Res. 2012. 14(6):R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeffers AM, Sieh W, Lipson JA, Rothstein JH, McGuire V, Whittemore AS, Rubin DL. Breast Cancer Risk and Mammographic Density Assessed with Semiautomated and Fully Automated Methods and BI-RADS. Radiology. 2017. 282(2):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eng A, Gallant Z, Shepherd J, McCormack V, Li J, Dowsett M, Vinnicombe S, Allen S, dos-Santos-Silva I. Digital mammographic density and breast cancer risk: a case-control study of six alternative density assessment methods. Breast Cancer Res. 2014. 16(5):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.