Despite the societal impact of antibiotics, their ecological functions remain elusive and have mostly been studied by exposing nonproducing bacteria to subinhibitory concentrations. Here, we studied the effects of the antibiotic holomycin on its native producer, Photobacterium galatheae S2753, a Vibrionaceae bacterium.
KEYWORDS: Photobacterium galatheae, holomycin, biosynthetic gene cluster, biofilm, secondary metabolites
ABSTRACT
While the effects of antibiotics on microorganisms are widely studied, it remains less well understood how antibiotics affect the physiology of the native producing organisms. Here, using a marine bacterium, Photobacterium galatheae S2753, that produces the antibiotic holomycin, we generated a holomycin-deficient strain by in-frame deletion of hlmE, the core gene responsible for holomycin production. Mass spectrometry analysis of cell extracts confirmed that the ΔhlmE strain did not produce holomycin and that the mutant was devoid of antibacterial activity. Biofilm formation of the ΔhlmE strain was significantly reduced compared to that of wild-type S2753 and was restored in an hlmE complementary mutant. Consistent with this, exogenous holomycin, but not its dimethylated and less antibacterial derivative, S,S′-dimethyl holomycin, restored the biofilm formation of the ΔhlmE strain. Furthermore, zinc starvation was found to be essential for both holomycin production and biofilm formation of S2753, although the molecular mechanism remains elusive. Collectively, these data suggest that holomycin promotes biofilm formation of S2753 via its ene-disulfide group. Lastly, the addition of holomycin at subinhibitory concentrations also enhanced the biofilms of four other Vibrionaceae strains. P. galatheae likely gains an ecological advantage from producing holomycin as both an antibiotic and a biofilm stimulator, which facilitates nutrition acquisition and protects P. galatheae from environmental stresses. Studying the function of antibiotic compounds in the native producer will shed light on their roles in nature and could point to novel bioprospecting strategies.
IMPORTANCE Despite the societal impact of antibiotics, their ecological functions remain elusive and have mostly been studied by exposing nonproducing bacteria to subinhibitory concentrations. Here, we studied the effects of the antibiotic holomycin on its native producer, Photobacterium galatheae S2753, a Vibrionaceae bacterium. Holomycin provides a distinct advantage to S2753 both as an antibiotic and by enhancing biofilm formation in the producer. Vibrionaceae species successfully thrive in global marine ecosystems, where they play critical ecological roles as free-living, symbiotic, or pathogenic bacteria. Genome mining has demonstrated that many have the potential to produce several bioactive compounds, including P. galatheae. To unravel the contribution of the microbial metabolites to the development of marine microbial ecosystems, better insight into the function of these compounds in the producing organisms is needed. Our finding provides a model to pursue this and highlights the ecological importance of antibiotics to the fitness of the producing organisms.
INTRODUCTION
Many microbial secondary metabolites have antibiotic activity and are crucial for treating bacterial infections in modern society. The clinical doses of antibiotics deployed are often much higher than the concentrations found in natural environments, where the compounds can be difficult to detect (1, 2). This has raised the question of the natural roles of microbial antibiotics in nature (2–9).
In nature, bacteria live in a multispecies community, whose composition and spatial structure change dynamically in response to external nutritional and physiochemical parameters and also as a function of interspecies interactions often mediated by bioactive molecules (2, 9, 10). Biofilms are structures associated with surfaces and the dominant bacterial lifestyles in natural environments, as it is an efficient means of persistence (11). Several bioactive secondary metabolites affect bacterial biofilm formation. For example, subinhibitory concentrations of tobramycin, an aminoglycoside antibiotic, induced biofilm formation of Escherichia coli and Pseudomonas aeruginosa (2, 12). Tobramycin activates an inner membrane phosphodiesterase, Arr of P. aeruginosa PAO1, and promotes the biofilm formation, likely by regulating the localized cytoplasmic c-di-GMP pools (12, 13). Both polyamine norspermine and glycine betaine, two compounds that may be produced by native marine organisms, enhance the cell density in Vibrio cholerae biofilms (14, 15). These studies have particularly emphasized the role of exogenous secondary metabolites in bacterial biofilm development; however, whether endogenous antibiotic compounds exert the same function has yet to be investigated.
We and others have reported that marine bacteria in the genera Phaeobacter, Ruegeria, Pseudoalteromonas, and Vibrionaceae are potent producers of secondary metabolites with antibiotic activity (16–24). However, whether these compounds play roles other than being antibiotics in the microbial community is not known. Unraveling the possible roles of compounds with antibiotic activities in complex systems is challenging; therefore, we chose to start our investigations in a controllable system, addressing if a compound with antibiotic activity plays any role in the physiology of the producing organism. Photobacterium galatheae S2753, an organism from the Vibrionaceae family, was isolated from the surface of a green mussel (25). The bacterium produces holomycin (24), a dithiolopyrrolone (DTP) family natural product (26–29) that inhibits cell growth of tumor cells as well as a broad spectrum of organisms, e.g., yeast and Gram-negative and Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) (23, 24, 28, 30). The holomycin biosynthetic pathway has been studied in Streptomyces clavuligerus (31) and Yersinia ruckeri (32). As predicted by antiSMASH (33), P. galatheae S2753 harbors 11 potential biosynthetic gene clusters (BGCs), with one predicted to produce holomycin (17, 23, 34). The purpose of this study, using P. galatheae S2753 as a model organism, was to confirm experimentally the bioinformatically predicted holomycin BGC and to determine the possible role(s) of the antibiotic holomycin in the physiology and ecology of the producer. Such studies could lead to new insights into the function and ecological role(s) of secondary metabolites with antibiotic activity and potentially facilitate new bioprospecting strategies.
RESULTS
Analysis of the biosynthetic gene cluster of holomycin of P. galatheae S2753.
antiSMASH, version 5.0, identified 11 BGCs in P. galatheae S2753, eight of which were on the large chromosome and three on the small one. Based on a previous prediction (17), BGC11 on the small chromosome potentially encodes the enzymes involved in the biosynthesis of holomycin. Sequence analysis and functional annotation showed that BGC11 contains 10 genes, eight of which are homologous to those in the holomycin BGCs of Y. ruckeri and S. clavuligerus (Fig. 1A). Therefore, the holomycin biosynthesis in P. galatheae likely follows the same paths as those in S. clavuligerus and Y. ruckeri (31, 32). As a core protein, the nonribosomal peptide synthetase (NRPS) HlmE contains a characteristic arrangement of cyclization (Cy), adenylation (A), and thiolation (T) domains (Fig. 1A and Table 1). According to the proposed mechanism (31, 32), HlmE initiates the synthesis pathway by covalently loading an l-cysteine and forming a dipeptide bond with a second l-cysteine. An acyl-coenzyme A (CoA) dehydrogenase, HlmB, then oxidizes the thiol group to allow the cyclization of the aminopyrrolinone ring of holomycin. Subsequently, a thioesterase, HlmC, a PPC-DC decarboxylase, HlmF, and a flavin mononucleotide (FMN)-dependent oxidoreductase, HlmD, work together to generate the second thiol group, remove one molecule of carbon dioxide, and then form a dihydroholothin molecule. Finally, an N-acyltransferase, HlmA, adds an acetyl group to form a holomycin.
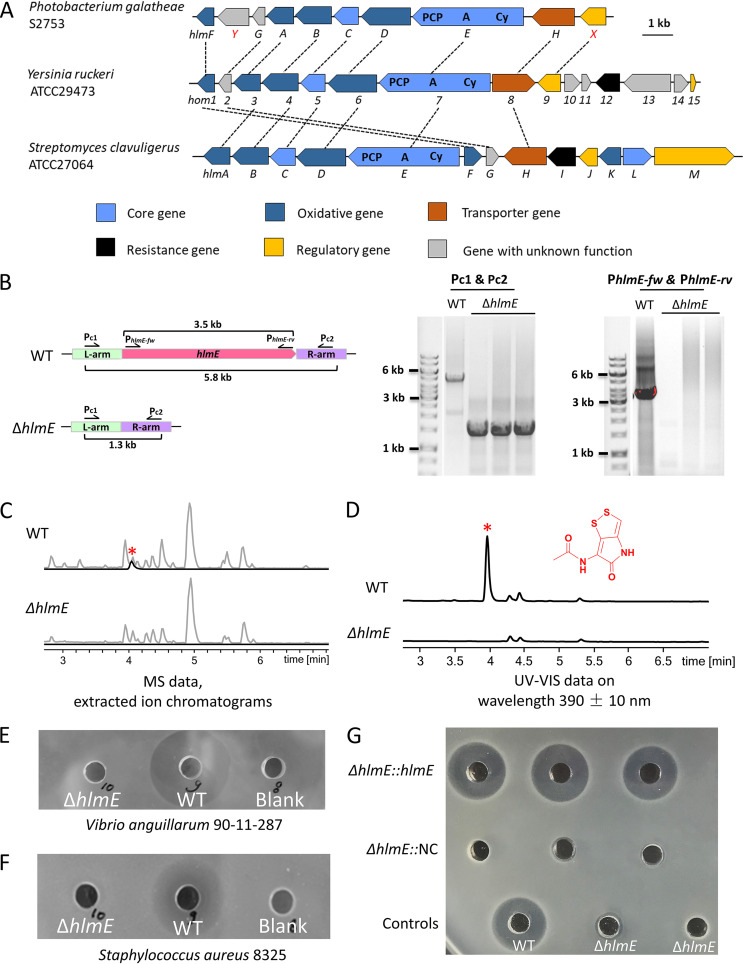
FIG 1.
(A) Comparison of biosynthetic gene clusters of holomycin. The genes are marked with the respective numbers or letters. Genes coding for proteins with the same function are highlighted in the same color. Genes assigned to NRPS are marked with domains: PCP, peptidyl carrier protein; A, adenylation domain; Cy, cyclization domain. Sequentially homologous genes are linked with dotted lines. (B). Diagram of the wild-type hlmE gene region and a scarless in-frame deletion of hlmE gene in S2753. (Left) A schematic illustration for the primers used, their annealing sites, and predicted PCR products in S2753 wild-type (WT) and ΔhlmE strains. (Right) Diagnostic PCRs of the hlmE gene region in WT and ΔhlmE strains. (C and D) In-frame deletion of the core gene hlmE completely abolished the holomycin production of the ΔhlmE strain. Base peak and extracted ion chromatograms (m/z = 214.9943) of culture extracts are shown in gray and black, respectively. UV-visible data at 390 ± 10 nm also showed the termination of holomycin production in the deletion strain. A red asterisk indicates the peak of holomycin in the detection. (E and F) Antimicrobial activity of culture extracts against the Gram-negative bacterium Vibrio anguillarum 90-11-287 and the Gram-positive bacterium Staphylococcus aureus 8325. Crude extracts of the WT cultures and culture media (blank) were used as the positive and negative control, respectively. (G) Antimicrobial activity of culture extracts of ΔhlmE::pBBR1-MCS2-hlmE (ΔhlmE::hlmE) and ΔhlmE::pBBR1-MCS2 (ΔhlmE::NC) strains against the Gram-negative bacterium Vibrio anguillarum 90-11-287. Crude extracts of the WT and cultures and ΔhlmE strain were used as the positive and negative controls, respectively.
TABLE 1.
Proposed function of ORFs in BGC11 of Photobacterium galatheae S2753a
| ORF in P. galatheae S2753 (17) | Homolog (% identity) in: |
Proposed function | |
|---|---|---|---|
| Yersinia ruckeri (32) | S. clavuligerus ATCC 27064 (31) | ||
| HlmF | Hom1 (68) | HlmF (59) | PPC-DC decarboxylase |
| HlmY | Conserved protein of unknown functions with metallophosphoesterase domain | ||
| HlmG | Hom2 (68) | HlmG (64) | Globin |
| HlmA | Hom3 (40) | HlmA (37) | N-acyltransferase |
| HlmB | Hom4 (64) | HlmB (52) | Acyl-CoA dehydrogenase |
| HlmC | Hom5 (50) | HlmC (38) | Thioesterase |
| HlmD | Hom6 (56) | HlmD (47) | FMN-dependent oxdioreductase |
| HlmE | Hom7 (51) | HlmE (49) | NRPS (Cy-A-T) |
| HlmH | Hom8 (59) | HlmH (62) | MFS transporter |
| HlmX | Hom9 (62) | Transcriptional regulator, containing an N-terminal zinc iron-mediated DNA binding domain and a C-terminal rhodanese-like domain | |
ORF, open reading frame. Identity scores between ORFs in BGC11 and those in the reported holomycin biosynthetic gene clusters were compared at the amino acid level.
Besides the eight conserved genes, BGC11 harbors a gene, hlmX, encoding a homolog to Hom9 of Y. ruckeri (Table 1), and a unique gene, hlmY (Fig. 1A), which encodes a putative metallophosphoesterase (Table 1). Both HlmX and Hom9 were predicted as putative ArsR/SmtB-family transcriptional regulators with a C-terminal rhodanese-like domain.
The other two holomycin-producing bacteria, S. clavuligerus and Y. ruckeri, use different self-protection strategies during holomycin production. S. clavuligerus encodes a disulfide-forming dithiol oxidase, HlmI, to control the formation of the intramolecular disulfide bridge in holomycin (35), while Y. ruckeri deploys a RNA methyltransferase, Hom12, to modify the potential holomycin antibiotic targets (32). However, a homolog of hlmI (35) or hom12 (32) was not found in S2753 BGC11 or in other places on the genome.
BGC11 is responsible for holomycin production in S2753.
To experimentally test if BGC11 indeed is responsible for holomycin production, an in-frame scarless deletion mutant of the core gene hlmE was generated by using homologous recombination and SacB-mediated counterselection (see details in Materials and Methods). Diagnostic PCRs were performed and confirmed that hlmE was deleted from S2753 (Fig. 1B). Wild-type (WT) S2753 and the ΔhlmE strains were then grown to stationary phase in different media, i.e., the marine-enriched APY medium and a marine minimal medium supplemented with chitin, glucose, or mannose. The produced secondary metabolites were extracted and analyzed by high-performance liquid chromatography coupled to diode array detection and high-resolution mass spectrometry (HPLC-DAD-HRMS). While holomycin was detected in WT cultures, it was not produced by the ΔhlmE mutant (Fig. 1C and D), demonstrating that hlmE and, thus, BGC11 are responsible for the biosynthesis of holomycin in P. galatheae S2753. Consistent with this, the extracted metabolites of ΔhlmE cultures failed to inhibit the growth of either the Gram-negative bacterium Vibrio anguillarum (Fig. 1E) or the Gram-positive Staphylococcus aureus (Fig. 1F), both of which were inhibited by the extracts from the WT strain. The antimicrobial activity of the crude extracts was complemented by ectopically expressing hlmE under its native promoter on the plasmid vector pBBR1-MCS2 but not by a vector control (Fig. 1G).
Biofilm formation is reduced in the ΔhlmE mutant.
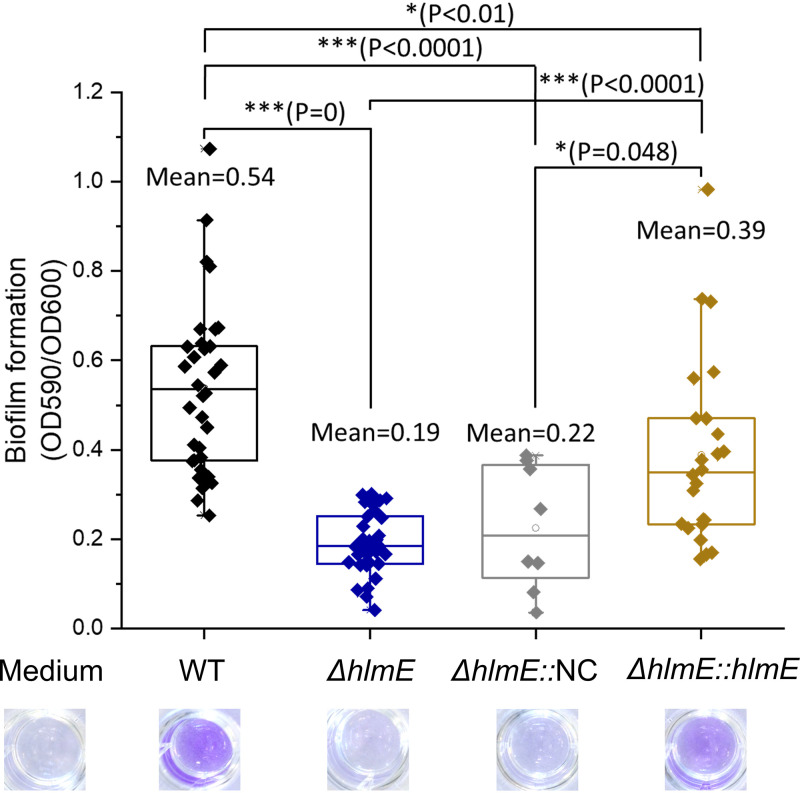
Deletion of hlmE did not affect the growth rate, as the doubling time during exponential growth and the maximum yield were 0.71 ± 0.04 h and 6.21 ± 0.56 optical density (OD) units for the WT and 0.70 ± 0.03 h and 6.21 ± 0.34 OD units for the ΔhlmE mutant in marine media. Statistically, the growth of the ΔhlmE mutant was not significantly different from that of the WT (P > 0.05 by Student's t test). Despite the similar growth dynamics, the ΔhlmE mutant formed less biofilm after a 2-day incubation than the WT (Fig. 2). The biofilm formation was partially restored in the ΔhlmE::pBBR1-MCS2-hlmE complemented strain that was used in Fig. 1G but not restored in the ΔhlmE::pBBR1-MCS2 control strain with the empty vector (Fig. 2). The partial complementation may be due to the varied expression time and level of hlmE.
FIG 2.
Boxplot of the biofilm produced by Photobacterium galatheae S2753 wild-type (WT), ΔhlmE, ΔhlmE::pBBR1-MCS2 (ΔhlmE::NC), and ΔhlmE::pBBR1-MCS2-hlmE (ΔhlmE::hlmE) strains. Underneath each bar is the crystal violet staining of the biofilm. At least eight biological replicates were performed for each strain. Error bars represent the standard deviations.
Biofilm formation by the P. galahtheae ΔhlmE strain is restored by exogenously applied holomycin but not by S,S′-dimethyl–red-holomycin.
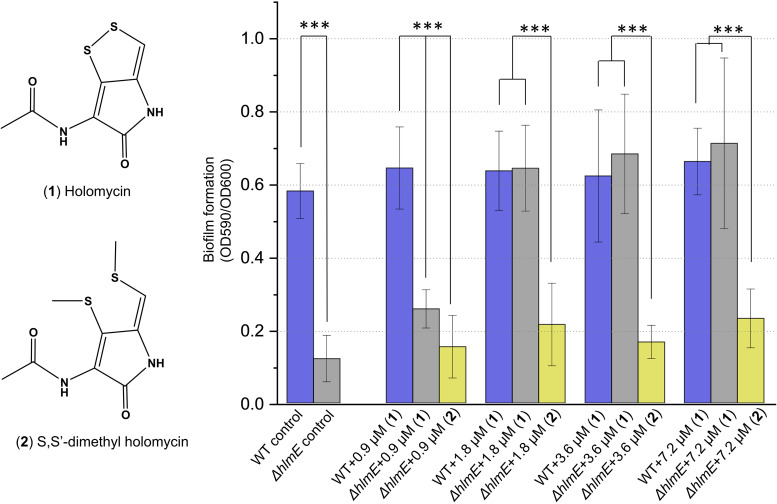
Since holomycin is secreted extracellularly, the effect of holomycin production on biofilm formation could be mediated by its role in intercellular interaction, and addition of exogenous holomycin should restore biofilm formation of the ΔhlmE mutant. To test this hypothesis, the production of endogenous holomycin was first determined as 3.59 ± 0.05 μM in the WT biofilm formation cultures. Holomycin with final concentrations of 0, 0.9, 1.8, 3.6, and 7.2 μM was then added to the ΔhlmE cultures after 17 h of incubation at 25°C, at the time when cells entered the exponential-stationary transition phase and were expected to produce holomycin. Holomycin supplied in concentrations higher than 1.8 μM restored the biofilm formation of the ΔhlmE mutant to the same level as the WT cultures (Fig. 3, P > 0.05 by Student's t test). To further explore the functional group of holomycin in triggering biofilm formation, S,S′-dimethyl holomycin (Fig. 3), a methylated, less antibacterial chemical analogue of holomycin (36), was also added to the ΔhlmE cultures after 17 h of incubation. Biofilm formation of ΔhlmE cultures was not restored even by S,S′-dimethyl holomycin concentrations up to 7.2 μM (Fig. 3), suggesting that the disulfide group is essential for the role of holomycin in triggering biofilm formation or that the two methyl groups in S,S′-dimethyl holomycin diminish the biological function of holomycin in triggering the biofilm formation of P. galatheae S2753.
FIG 3.
Biofilm formation of wild-type S2753 and ΔhlmE strains in the presence of exogenously applied holomycin (1) or S,S′-dimethyl holomycin (2). At least eight biological replicates were performed for each condition. Error bars represent the standard deviations. For all panels, two-way analysis of variance (ANOVA) was used for statistical analysis. ***, P < 0.001.
The zinc ion concentration in media negatively correlates with holomycin production in P. galatheae S2753.
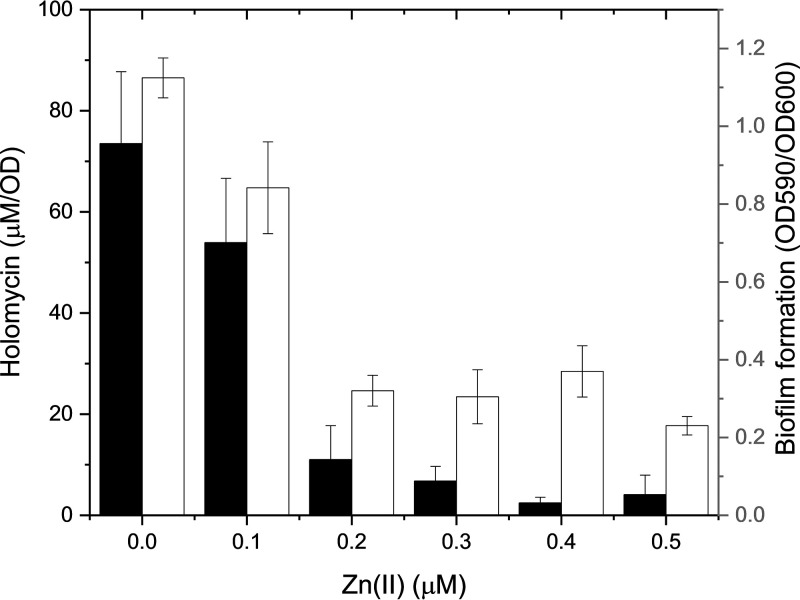
The disulfide group of holomycin chelates zinc ions in reducing environments (37). To test if zinc was involved in holomycin production and biofilm formation, wild-type P. galatheae was grown in mannose marine minimal medium with and without zinc addition. A calibration curve of holomycin was constructed and used for the quantitative analysis of holomycin production in cultures. The growth of P. galatheae was not influenced by zinc at the tested concentration; however, the increasing zinc concentration gradually inhibited holomycin production in the WT (Fig. 4). Holomycin production in WT cultures was almost abolished when zinc was added up to 2 mM in the wild-type cultures. Meanwhile, biofilm formation of wild-type P. galatheae was also gradually inhibited by increasing zinc concentrations (Fig. 4). To sum up, zinc negatively influenced biofilm formation and holomycin production in P. galatheae.
FIG 4.
Holomycin production (black columns) and biofilm formation (white columns) of wild-type Photobacterium galatheae S2753 in the presence of increasing zinc in the marine minimal medium with mannose. Three and nine biological replicates were performed in detecting holomycin production and biofilm formation, respectively. Error bars represent the standard deviations.
Exogenous holomycin triggers biofilm formation of other marine isolates.
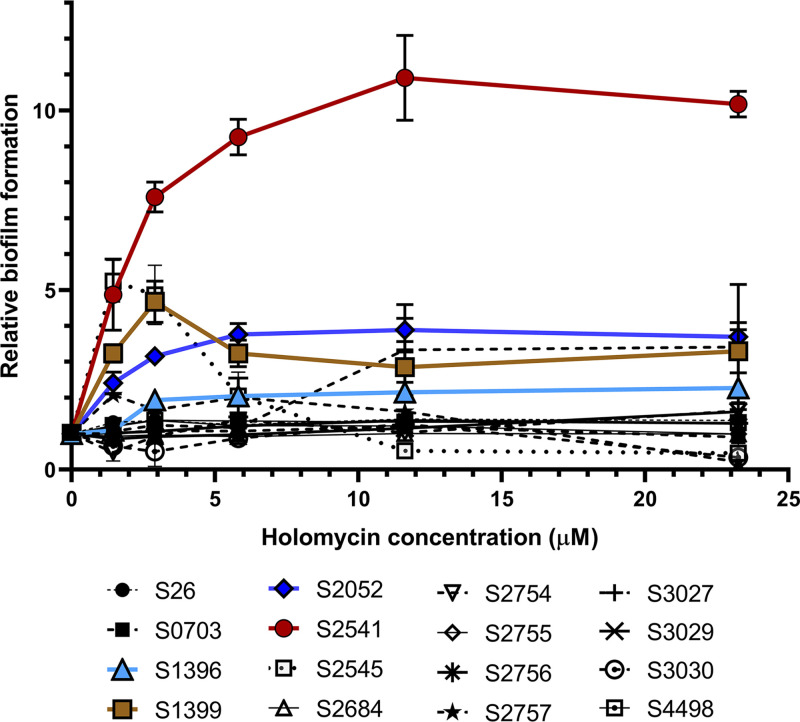
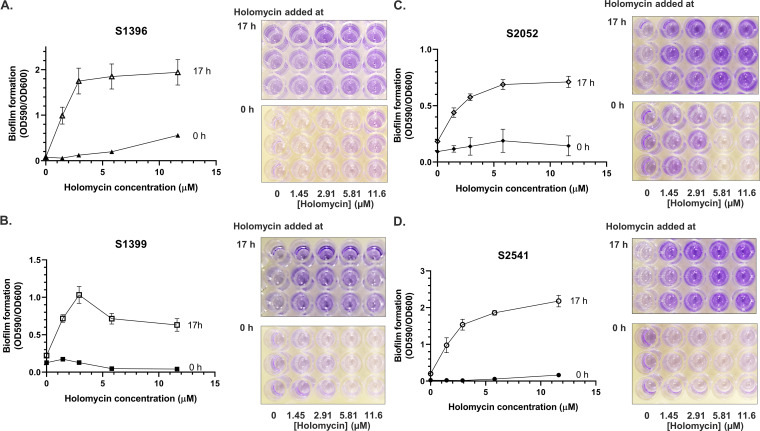
To test whether holomycin at subinhibitory concentrations would affect the biofilm formation of other marine bacteria, 16 isolates from six genera (i.e., Phaeobacter, Ruegeria, Vibrio, Photobacterium, Pseudoalteromonas, and Cobetia) from the Galatheae Collection (Tables 2 and 3) were grown in marine broth. We first determined the MICs of holomycin for these strains. Five strains were resistant to holomycin (MIC, >93 μM) (Table 2), including S2754 and S2755, which were isolated from the same stone where the S2753-bearing mussel was located. The MICs of other bacteria were in the range of 2.9 to 93 μM (Table 2). Holomycin was then added to the cultures at subinhibitory concentrations at either 0 h or 17 h (Fig. 5 and 6). Biofilm formation of three Vibrio strains and one Photobacterium strain was significantly enhanced by holomycin added after the 17-h incubation (Fig. 6), while the effect was not significant when added at 0 h (Fig. 6). Biofilm formation of the other strains was not affected by holomycin at either time point (Fig. 5). These data showed that holomycin produced by S2753 could affect the biofilm formation of other marine bacterial species.
TABLE 2.
MIC of holomycin against selected marine bacteria
| Strain (reference) | MIC (μM) |
|---|---|
| Phaeobacter piscinae S26 (19) | 46.5 |
| Vibrio sp. strain S0703 (16) | 2.9 |
| Vibrio sp. strain S1396 (16) | 23.3 |
| Vibrio sp. strain S1399 (16) | 23.3 |
| Vibrio coralliilyticus S2052 (16) | 23.3 |
| Photobacterium sp. strain S2541 (16) | 23.3 |
| Photobacterium sp. strain S2545 (16) | 2.9 |
| Ruegeria sp. strain S2684 (16) | >93 |
| Photobacterium sp. strain S2754 (16) | >93 |
| Pseudoalteromonas piscicida S2755 (16) | >93 |
| Pseudoalteromonas ruthenica S2756 (16) | 93 |
| Vibrio sp. strain S2757 (16) | >93 |
| Vibrio sp. strain S3027 (16) | 93 |
| Cobitia sp. strain S3029 (16) | 23.3 |
| Vibrio sp. strain S3030 (16) | 11.6 |
| Pseudoalteromonas galatheae S4498 (16) | >93 |
TABLE 3.
Strains used in this study
| Strain | Genotype/features | Source or reference |
|---|---|---|
| Escherichia coli | ||
| TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80 lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu) 7697 galU galK λ– rpsL (strR) endA1 nupG | Invitrogen |
| PIR1 | F− Δlac169 rpoS(Am) robA1 creC510 hsdR514 endA recA1 uidA(ΔMluI)::pir-116 | Invitrogen |
| WM3064 | thrB1004 pro thi rpsL hsdS lacZΔM15 RP4-1360 Δ(araBAD)567 ΔdapA1341::[erm pir] | Strain developed by William Metcalf at UIUC |
| BL21(DE3) | E. coli B dcm ompT hsdS(rB− mB−) gal λDE3 | Invitrogen |
| Photobacterium galatheae S2753 | ||
| Wild type (WT) | Wild type | Machado et al. (25) |
| ΔhlmE | In-frame deletion of the hlmE gene | This study |
| ΔhlmE::NC | ΔhlmE pBBR1-MCS2 | This study |
| ΔhlmE::hlmE | ΔhlmE pBBR1-MCS2-hlmE | This study |
| Phaeobacter piscinae S26 | Isolated from a Greek sea bass aquaculture unit | Sonnenschein et al. (19) |
| Vibrio sp. strain S0703 | Galatheae Collection strain | Gram et al. (16) |
| Vibrio sp. strain S1396 | Galatheae Collection strain | Gram et al. (16) |
| Vibrio sp. strain S1399 | Galatheae Collection strain | Gram et al. (16) |
| Vibrio sp. strain S2757 | Galatheae Collection strain | Gram et al. (16) |
| Vibrio sp. strain S3027 | Galatheae Collection strain | Gram et al. (16) |
| Vibrio sp. strain S3030 | Galatheae Collection strain | Gram et al. (16) |
| Vibrio coralliilyticus S2052 | Galatheae Collection strain | Gram et al. (16) |
| Photobacterium sp. strain S2541 | Galatheae Collection strain | Gram et al. (16) |
| Photobacterium sp. strain S2545 | Galatheae Collection strain | Gram et al. (16) |
| Photobacterium sp. strain S2754 | Galatheae Collection strain | Gram et al. (16) |
| Pseudoalteromonas piscicida S2755 | Galatheae Collection strain | Gram et al. (16) |
| Pseudoalteromonas ruthenica S2756 | Galatheae Collection strain | Gram et al. (16) |
| Pseudoalteromonas galatheae S4498 | Galatheae Collection strain | Gram et al. (16) |
| Ruegeria sp. strain S2684 | Galatheae Collection strain | Gram et al. (16) |
| Cobitia sp. strain S3029 | Galatheae Collection strain | Gram et al. (16) |
FIG 5.
Overview of the relative biofilm formation of selected marine bacteria by subinhibitory concentrations of holomycin. The relative biofilm formation was calculated by dividing the OD590/OD600 value of cultures without added holomycin. Error bars represent the standard deviations of three biological replicates.
FIG 6.
Biofilm formation of four Galatheae Collection bacteria when subinhibitory concentrations of holomycin were added to the cultures at the initial inoculation time (0 h) or after 17 h of incubation at 25°C (17 h). Crystal violet staining was used to access the biofilm formation in the 2-day incubation cultures. Error bars represent the standard deviations. (A) Vibrio sp. strain S1396. (B) Vibrio sp. strain S1399. (C) Vibrio coralliilyticus S2052. (D) Photobacterium sp. strain S2541. Three biological replicates were performed. Error bars represent the standard deviations.
DISCUSSION
Species of the Vibrionaceae family play important roles in the marine environment as symbiotic, pathogenic, or free-living organisms, and they also harbor a large genetic potential for production of bioactive compounds (16, 17, 24, 38–41). Genetic approaches are needed to explore these bioactive compounds and to link chemical compounds to their related biosynthetic pathways, enabling an understanding of the physiological and ecological roles of their secondary metabolites. In this study, using the mussel-associated bacterium P. galatheae S2753 as a holomycin-producing model organism, we developed a protocol (see Materials and Methods) to genetically manipulate S2753 and generated a ΔhlmE holomycin-deficient mutant strain. This confirmed that a gene likely part of a BGC (BGC11) in S2753 is responsible for holomycin production.
Exogenously supplied antibiotics in sublethal concentrations can affect microbial growth and metabolism by altering gene expression, nutrient utilization, and biofilm formation (42, 43). In these studies, antibiotics were added to the culture media and expected to be perceived by bacterial signal transduction pathways leading to changes in gene expression and subsequently altered phenotypes, such as biofilm formation. Consistent with this, we found that when applied at the late growth stage, holomycin induced biofilm formation of other holomycin-nonproducing bacteria (Fig. 6). However, the stimulatory effect of holomycin on S2753 might differ from these, since P. galatheae S2753 is a native producer of holomycin. Holomycin production is tightly coupled with biofilm formation in S2753. Several lines of evidence support this. (i) The ΔhlmE strain is defective in both holomycin production and biofilm formation, while a genetically complemented ΔhlmE strain restored holomycin production and partial biofilm formation (Fig. 2). (ii) Exogenously added holomycin also restored the biofilm formation of the ΔhlmE strain to the level of WT S2753 (Fig. 3). (iii) Adding an external factor, such as a high concentration of zinc, led to a gradual reduction of holomycin production and a parallel reduction in biofilm formation (Fig. 4). Holomycin, once produced, may stimulate the biofilm formation of S2753 either in a direct or indirect manner. Directly, holomycin may bind to and activate a transcriptional regulator(s) to upregulate the expression of genes involved in biofilm formation. For example, the antibiotic bacillomycin D promotes biofilm formation of the native producing bacterium Bacillus velezensis by binding to a transcription activator, Btr (44). The complex upregulates the iron uptake ABC transporter FeuABC and consequently increases intracellular iron concentration, which cues the biofilm formation of B. velezensis. Indirectly, holomycin may chelate metal ions (including zinc) inside the cell (37), which triggers global metabolic changes and various stress response pathways and often leads to the stimulation of biofilm formation. This suggested mechanism is consistent with the most prevalent hypotheses that the biofilm stimulatory activity of antibiotics could be coupled to the mechanism of their toxic activity, which leads to generic stress responses or other physiological changes by nonlethal damage on the nonproducing strains (45, 46). In line with this, the analog of holomycin, S,S′-dimethyl holomycin, which is not as antibacterial as holomycin, cannot form the dithiol bonds under, e.g., the intracellular conditions, and, therefore, chelate metal ions, and it was unable to complement the biofilm formation of the ΔhlmE strain (Fig. 3).
Zinc influenced both holomycin production and biofilm formation (Fig. 4), as has been observed in other bacteria, as free zinc used at nonbactericidal concentrations inhibits biofilm formation of several pathogenic bacteria, including E. coli, S. aureus, Streptococcus suis, Actinobacillus pleuropneumoniae, Salmonella enterica serovar Typhimurium, and Haemophilus parasuis (66). The influence of zinc on biofilm formation could be via its inhibitory effect on holomycin production. The reduced holomycin is believed to function as a zinc chelator (zincophore) to scavenge zinc from the zinc-dependent enzymes (37), similar to the function of siderophores for iron scavenging. We found that the predicted transcriptional regulator HlmX contains potential zinc-binding sites (Table 1) that may accept free zinc ions and change its ability to bind DNA and reprogram gene expression. Therefore, a nonexclusive possibility for the inhibitory effect of zinc is that the availability of free zinc eliminates the need for holomycin production in P. galatheae and that the decreased holomycin production reduces biofilm formation. It is also possible that a zinc-responsive transcriptional regulator binds to zinc ions and thereby downregulates the expression of synthases involved in holomycin production, thereby influencing holomycin production and biofilm formation.
Antibiotics are proposed to act as weapons that provide competitive advantages to the native producers in environmental niches (47). Indeed, this hypothesis has been evidenced by the observations that the production of antibiotic secondary metabolites, including holomycin, was significantly induced by stress conditions such as exposure to antibiotics or bacterial competitors in the culturing systems (48, 49). However, antibiotic secondary metabolites may play multifaceted roles in natural environments by, e.g., acting as antibiotics mediating antagonism between bacterial warfare at high concentration and acting as signaling molecules involved in inter- or intracellular processes at low concentration (50, 51). Here and in the previous study, the production of holomycin in P. galatheae was influenced by access to nutrient sources such as chitin (52) and free-zinc ions (Fig. 4), leading to the question of whether holomycin serves several roles in the native producer, P. galatheae. Chitin is the most abundant polysaccharide in marine environments, and several species of the Vibrionaceae family (including P. galatheae) form biofilm in response to chitin. P. galatheae can catabolize chitin as a nutrient source, and holomycin production increased significantly in chitin-supplemented medium compared to glucose medium (52). Given the close coupling of holomycin production to biofilm formation, as revealed in this article, it is possible that chitin induces holomycin production and, thereby, biofilm formation, which facilitates the colonization of a nutrition source. P. galatheae S2753 was isolated from the surface of a green mussel (16, 25), which may impose a zinc starvation condition on S2753 as part of its nutritional immunity system (53).
Altogether, we propose a preliminary model of the ecological role of holomycin in S2753 by incorporating the synergistic effects of chitin and zinc. When P. galatheae S2753 attaches to its eukaryotic hosts, both the host chitin and the zinc starvation condition (54) induce the production of holomycin, biofilm formation, and, consequently, enhanced colonization of the host and nutrition. In turn, the biofilm structure protects S2753 from marine environmental changes and stresses and potentially enriches zinc ions in the vicinity of S2753 cells to facilitate zinc uptake by using holomycin or other means. In addition, holomycin has antibacterial activity, and, when applied in the early growth stage, antibiofilm activity to other marine strains (Table 2). Therefore, the coupling between holomycin production and biofilm formation confers a clear advantage to the ecological survival of S2753.
MATERIALS AND METHODS
Bioinformatics analyses.
The genome sequences used in this study were extracted from NCBI using the relevant accession numbers and uploaded to the MaGe Genoscope for holomycin BGC synteny analysis and gene annotation (55, 56). Genomes were submitted to antiSMASH, version 5.0 (33), for the prediction of gene clusters involved in the production of holomycin. Protein domain prediction was done in InterPro Domains and Conserved Domain Search Service (CD Search) (57). The DNA sequencing data were analyzed using BioEdit. The sequence alignment was done in ClustalX.
Microorganisms and growth conditions.
Escherichia coli strains PIR1 (C101010; Invitrogen, Denmark) and TOP10 (404010; Invitrogen, Denmark) were used for cloning. E. coli WM3064 (58) was used as the donor strain in bacterial conjugations and grown in the presence of 300 μM 2,6-diaminopimelic acid (DAP). A concentration of 10 μg/ml kanamycin (Kan) or chloramphenicol (Cm) was used in the E. coli liquid cultures, and 30 μg/ml both antibiotics was used in E. coli agar cultures (59). Cultures of wild-type and mutant strains of P. galatheae S2753 (16, 25) were grown in marine minimal medium (52) supplemented with sole carbon sources (0.2% mannose, 0.2% colloidal chitin, or 0.2% glucose), marine broth 2216, marine agar 2216, and modified enriched growth medium (APY) (56) containing, per liter, 5 g of peptone, 3 g of yeast extract, and artificial seawater. The pH was adjusted to 7.0; 12 g of agar was added per liter of APY to prepare the agar plates. Generally, colonies appeared 15 h after plating. In the P. galatheae cultures, kanamycin was added at 200 μg/ml to agar plates and 150 μg/ml to liquid cultures; chloramphenicol was used at 30 μg/ml. Zinc ions were added to the medium to the working concentration from a 1 M zinc chloride stock solution (pH 6.5) in water. Sixteen marine bacteria were selected to investigate the influence of holomycin in their biofilm, of which eight strains, i.e., Vibrio sp. strains (16) S0703, S1396, S1399, S2757, S3027, and S3030, Pseudoalteromonas ruthenica S2756 (16), and Cobitia sp. strain S3029 (16), were isolated from mussel surfaces. Both Pseudoalteromonas piscicida S2755 (16) and Photobacterium sp. S2754 (16) were sampled from a stone located in the same place as S2753. Bacteria S2541 and S2545 were also included because they belong to the genus Photobacterium (16). Additionally, Ruegeria sp. strain S2684 (16), Vibrio coralliilyticus S2052 (16), Phaeobacter piscinae S26 (19), and Pseudoalteromonas galatheae S4498 (16) were selected, as they were studied in several previous or ongoing projects. These selected marine strains were cultured on marine agar (MA) plates or in marine broth (MB). All marine cultures were inoculated at 25°C and all E. coli strains at 37°C. The strains used in this study are listed in Table 3 with genotype description.
DNA manipulation and plasmid construction.
The restriction enzymes and the quick ligase for DNA modification were purchased from New England Biolabs (NEB; Bionordika, Denmark). DNA polymerase (TaKaRa Biomedical Technology Europe [France]) and Q5 high-fidelity polymerase (NEB) were used for PCR amplification, except for colony PCRs, which were performed using TEMPase (Ampliqon; VWR, Denmark). All PCR products and plasmids were purified using GFX PCR DNA and gel band purification kit (28–9034-70; GE Healthcare) and Monarch plasmid miniprep kit, respectively. All plasmids and primers were designed in ApE–A plasmid Editor (v2.0) (a program designed by M. Wayne Davis). Integrated DNA Technologies (IDT; Belgium) synthesized all the primers in this study. All plasmids and primers used in the study are listed in Tables 4 and 5.
TABLE 4.
Plasmids used in this study
| Plasmid | Features | Source or reference |
|---|---|---|
| pJET1.2/blunt | Origin of replication (pMB1), Ampr, PlacUV5, eco47IR, T7 promoter | Thermo Scientific |
| pJET1.2-del-hlmE | pJET1.2/blunt, recombineering arms of gene hlmE | This study |
| pDM4 | Origin of replication (R6Kγ origin), sacB, Cmr | Milton et al. (60) |
| pDM4-d-hlmE | pDM4, recombineering arms of gene hlmE | This study |
| pBBR1-MCS2 | Origin of replication (pBBR1), Kanr, Plac, lacZα | Obranić et al. (67) |
| pBBR1-MCS2-PhlmE-hlmE | pBBR1-MCS2, PhlmE, hlmE-6×His | This study |
TABLE 5.
Primers used in this studya
| Primer | Sequence (5′→3′) | Description |
|---|---|---|
| DhlmE-P1 | GCtctagaTGGATTGATCGCCAGTGGAG (XbaI) | Amplification of the left recombineering arm of hlmE gene |
| DhlmE-P2 | GTTGAGGCGTACTCAAGTGGGTCATGCGTCCTTC | |
| DhlmE-P3 | GACGCATGACCCACTTGAGTACGCCTCAACAAAAAGC | Amplification of right recombineering arm of hlmE gene |
| DhlmE-P4 | CCGctcgagAAGTCCGGAATGACAGACGC (XhoI) | |
| hlmE-fw | ATGAACCCTGATCACGTTGG | Amplification of hlmE gene |
| hlmE-rv | TCACAAGCTGACTCCGTCC | |
| Pc0 | CCTCACATCAATCCGGATTGG | Primers used for confirmation of the in-frame deletion mutant via PCR amplification and sequencing |
| Pc1 | GGTCTGGCATGGTTCTTGAC | |
| Pc2 | TTCAGCTTCGCCTGGTAATG | |
| Pc3 | GTGTCTGAGACCGAACAACG | |
| Pc4 | TATCTGTCAGCGGCTGTTCC | |
| Pc5 | GCAAGCCAATCTGGACATCC | |
| Phlm-hlmE | GGggtaccGGTGAAGCAGGATAAGTGTG (KpnI) | Amplification of hlmE for complementation plasmid cloning |
| hlmE-6xHis | GCtctagaTCAGTGATGGTGATGGTGATGCAAGCTGACTCCGTCCGG (XbaI) | |
| Cm-fw | GGCATTTCAGTCAGTTGCTC | Amplification of the Cmr gene in pDM4 |
| Cm-rv | CCATCACAAACGGCATGATG | |
| Km-fw | CGATACCGTAAAGCACGAGG | Amplification of the Kanr gene in pBBR1-MCS2 |
| Km-rv | CTCGACGTTGTCACTGAAGC |
Lowercase letters represent a recognition site for a restriction enzyme. Underlined sequences indicate the 6×His tag.
The suicide plasmid pDM4-del-hlmE was constructed by the restriction cloning method. Approximately 1.0-kb upstream and downstream regions flanking hlmE were amplified using primer pairs DhlmE-P1/2 and DhlmE-P3/4 (Table 5). Amplified DNA fragments were ligated into the pJET1.2/blunt cloning vector with the CloneJET PCR cloning kit (K1231; Thermo Scientific, Denmark); subsequently, they were subcloned into the suicide vector pDM4 (60) by using the XbaI and XhoI digestion sites to form pDM4-del-hlmE. Gene hlmE and its native promoter region were amplified by primer pair Phlm-hlmE/hlmE-6×His (Table 5) and cloned into the expression vector pBBR1-MCS2 via KpnI and XbaI to generate the complementation plasmid pBBR1-MCS2-hlmE. Correct plasmid assembly was confirmed by PCR (Table 5), restriction digestion, and sequencing (Macrogen Europe, Netherlands).
Bacterial conjugation.
The electroporation of E. coli WM3064 and the conjugation experiments were performed as described previously, with some modifications of the culture conditions (58, 59, 61). WM3064 cells carrying each plasmid were grown at 37°C in LB-DAP medium with antibiotics until an optical density at 600 nm (OD600) of 0.4 to 0.6. As a donor, 1 ml of WM3064 culture was harvested by centrifugation (6,000 × g for 1 min). Cells were washed twice with LB medium and resuspended in 50 μl LB medium with DAP. It was then mixed with 500 μl of P. galatheae culture with an OD600 between 0.4 and 0.5. The donor-recipient cell suspension was concentrated by centrifugation (6,000 × g for 1 min), resuspended with 20 μl APY medium with 300 μM DAP, and mixed briefly by pipetting three times. All mixtures were spotted onto a 0.22-μm-pore-size mixed-cellulose ester (MCE) membrane (GSWP02500; MF-Millipore, Merck, Germany) placed on an MA 2216 plate with 300 μM DAP. The plate was incubated for 3 to 4 h at 37°C or for 3 to 15 h at 25°C. The conjugations were suspended in 1 ml of APY medium and incubated for 20 min at 25°C. Each 100-μl conjugation was plated onto antibiotic-containing APY plates. The plates were incubated at 25°C for 16 to 24 h. Following conjugation, single colonies were grown in 2 ml APY medium supplied with chloramphenicol at 25°C with shaking overnight. Resulting antibiotic-resistant strains were screened by PCR to determine the transconjugants.
Confirmation of the transconjugants and first cross event.
Genomic DNA for PCR analyses was isolated using a NucleoSpin tissue kit according to the protocol of the manufacturer (Macherey-Nagel, Fisher Scientific, Denmark). PCR primers are listed in Table 5. Primer pairs Cm-Fw/Rv, Km-Fw-Rv, Pc0/Pc4, and Pc1/Pc2 were designed to amplify the replication region for detecting the plasmids in donor strains and transconjugants. The PCR steps were 94°C for 30 s; 30 cycles of 94°C for 10 s, 58°C for 5 s, and 72°C for 30 s; and then 72°C for an additional 10 min. Routine DNA manipulations were carried out by following standard methods as described above.
Construction of the hlmE in-frame deletion mutants in S2753.
The suicide plasmid for knocking out the hlmE gene was constructed by restriction cloning and was transferred into S2753 by intergeneric conjugation as described. The PCR-verified mutants, in which the suicide plasmid had integrated into the anticipated place in the S2753 genome, were grown at 25°C in APY medium with shaking to an OD600 of 0.5. The cells were then diluted and spread on a half-APY medium (500 ml/liter APY medium, 500 ml/liter distilled H2O, 15 g agar, pH 7.0) supplied with 10% (wt/vol; final concentration) sucrose (autoclaved at 100°C for 1 h or filtered) and incubated at 16°C for 48 h. All primers used in this study are listed in Table 3.
Purification of the deletion mutants.
P. galatheae S2753 swarms on agar plates when cultured below 42°C (unpublished data). Therefore, several purification steps following the second crossover event are required to get a genetically homogenous clone. Cells from the edge of a swarming colony were inoculated in 1 ml APY medium without antibiotics with shaking at 25°C for 8 h. The cells were then diluted and transferred onto a new half-APY–10% sucrose medium plate and incubated at 16 to 18°C for 48 h. Single colonies on the new plate were transferred onto APY-agar and APY-agar plates containing 30 μg/ml chloramphenicol. Colonies sensitive to chloramphenicol were collected and confirmed by PCR and DNA sequencing using the same protocol of verifying mutants with the first crossover. If the PCR result showed a mosaic genetic feature of the selected colonies, the purification step was repeated at least once.
Extraction of liquid cultures for chemical analysis.
Chemical extraction was prepared as described by Giubergia et al. (52). Cultures were incubated at 25°C for 48 h with shaking and then transferred to 50 ml falcon tubes. An equal volume of ethyl acetate was added to the culture and mixed by inversion. The mixture was incubated for 10 min with occasional inversion until a clear division of layers was present. The organic phase (top layer) was transferred to a glass tube. These tubes were placed in a 35°C heating block and evaporated with nitrogen until dry. Extracts were resuspended in methanol (1/20 volume of the initial culture) and stored at −20°C.
UHPLC-HRMS profiling of holomycin from the wild-type and mutant strains.
Chemical analysis was performed as described by Giubergia et al. (52). Ultra-high-performance liquid chromatography–high-resolution mass spectrometry (UHPLC-HRMS) was performed on an Agilent Infinity 1290 UHPLC system (Agilent Technologies, Santa Clara, CA) equipped with a diode array detector. The separation was obtained on an Agilent Poroshell 120 phenyl-hexyl column (2.1 by 150 mm; particle size, 1.9 μm) with a linear gradient consisting of water and acetonitrile, both buffered with 20 mM formic acid, starting at 10% acetonitrile and increasing to 100% in 10 min, at which point the concentration was held for 2 min, returned to 10% acetonitrile in 0.1 min, and left for 3 min (0.35 ml/min, 60°C). An injection volume of 1 μl was used. MS detection was performed in the positive detection mode on an Agilent 6545 quadrupole time-of-flight (QTOF) MS equipped with an Agilent dual-jet-stream electrospray ion source with a drying gas temperature of 250°C, a gas flow of 8 liters/min, a sheath gas temperature of 300°C, and a flow rate of 12 liters/min. The capillary voltage was set to 4,000 V and nozzle voltage to 500 V. Mass spectra were recorded at 10, 20, and 40 eV as centroid data for m/z 75 to 1,700 in MS mode and m/z 30 to 1,700 in MS/MS mode, with an acquisition rate of 10 spectra/s. Lock mass solution in 70:30 methanol-water was infused into the second sprayer using an extra LC pump at a flow rate of 15 μl/min and a 1:100 splitter. The solution contained 1 μM tributylamine (Sigma-Aldrich) and 10 μM hexakis(2,2,3,3-tetrafluoropropoxy)phosphazene (Apollo Scientific Ltd., Cheshire, United Kingdom) as lock masses. The [M+H]+ ions (m/z 186.2216 and 922.0098, respectively) of both compounds were used. The secondary metabolite profile was analyzed in Agilent Qualitative Analysis B.07.00. Five series of calibration solutions of pure holomycin (H458490; Toronto Research Chemicals, Canada) were used to create the HPLC standard calibration curve of holomycin. The peak area of holomycin in the biofilm samples and zinc cultures was recorded and used to calculate the holomycin concentration in cultures.
Well diffusion inhibition assay.
This experiment was performed with a modified protocol from Wietz et al. (24). Vibrio anguillarum 90-11-287 and Staphylococcus aureus 8325 were cultured at 25°C for 24 h in MB and LB media, respectively. To test the susceptibility of the two pathogenic strains to the extracts from P. galatheae S2753 cultures, the strains were homogeneously added into warm (44.5°C) IO agar (3% instant ocean, 0.3% Bacto Casamino Acids [BD-223050; Denmark] supplemented with 0.4% glucose, 1% agar) (for V. anguillarum) or IO agar with 1% peptone (for Staphylococcus aureus 8325). The plates were solidified and dried in a flow bench. Wells with 6-mm diameter were punched with home-made tips, and 45 μl of culture extract was added to each well. The agar plates containing the V. anguillarum 90-11-287 and S. aureus 8325 cultures were incubated at 25 and 37°C for 48 and 24 h, respectively. The inhibition assay was then evaluated by analyzing the formation of clearing zones around the well.
Growth experiments.
Precultures of the P. galatheae WT and mutant were prepared by inoculating a single colony into the proper liquid medium. After 24 h of incubation at 25°C, the preculture was diluted to an OD600 of 0.01 in 30 ml medium in a 250-ml flask and incubated at 25°C with 160-rpm shaking. The OD600 value was measured in a 1-ml cuvette every 0.5 to 6 h for 72 h using a Novaspec III spectrophotometer (Amersham Biosciences) and plotted using Origin, version 2019 (OriginLab Corporation, Northampton, MA, USA). After the final OD600 measurement, cultures diluted to 10−6 and 10−7 were plated on marine agar and incubated overnight for colony counting.
Biofilm formation.
A modified protocol based on previous works (62–64) was used. Precultures were diluted to an OD600 of 0.01 in 1 ml MB. Border wells were filled with 100 μl Milli-Q water to prevent desiccation. The 96-well microtiter plate was incubated in a humidity chamber with a wet paper towel at the bottom for 48 h at 25°C. In the complementation experiments, growth kinetics were tracked and holomycin or S,S-dimethyl-holomycin synthesized by the method of Buijs et al. (65) were added to the cultures at the exponential-stationary transition phase (17 h). In the biofilm assay of selected Galatheae Collection strains, holomycin was added to cultures in a 2-fold dilution from 93 μM to 1.5 μM, either at the initial inoculation time or after 17 h of incubation. After incubation, the OD600 was measured in a SpectraMax i3 (Molecular Devices). Culture media and nonadhering bacteria were removed, and the wells were washed with 150 μl Milli-Q water and dried for 15 min in a flow bench. To each well was added 125 μl of 1% crystal violet, and staining proceeded for 15 min. After removing the crystal violet, wells were washed three times with 200 μl Milli-Q water and dried for another 15 min. An amount of 200 μl 96% ethanol was added to each well and incubated for 30 min to dissolve the staining color. Thereafter, 100 μl of the ethanol-crystal violet mixture in each well was transferred to a new microtiter plate. The crystal violet intensity was measured at OD590 in a SpectraMax i3. Data were analyzed in Microsoft Excel. One-way analysis of variance (ANOVA) test and the statistical plot graphs were analyzed in Origin, version 2019 (OriginLab Corporation, Northampton, MA, USA).
ACKNOWLEDGMENTS
The research in this study receive funding from the European Union's Horizon 2020 research and innovation program under Marie Sklodowska-Curie grant agreement no. 713683 (COFUNDfellowsDTU) for S.D.Z. Funding from the Danish National Research Foundation for the Center for Microbial Secondary Metabolites (DNRF137) is acknowledged (L.G., L.L.L., and T.O.L.), as is funding from the Independent Research Fund Denmark (project 7017-00003B) (S.D.Z. and T.I.).
We thank Jan Martinussen, Mogens Kilstrup, Roberto Kolter, Yong E. Zhang, and Demeng Tan for helpful discussions. We thank Haitao Chen and Tao Song for providing strain WM3064 and vector pBBR1-MCS2.
Footnotes
This is Galatheae publication number p133.
REFERENCES
- 1.Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 2.Linares JF, Gustafsson I, Baquero F, Martinez JL. 2006. Antibiotics as intermicrobiol signaling agents instead of weapons. Proc Natl Acad Sci U S A 103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bérdy J. 2005. Bioactive microbial metabolites: a personal view. J Antibiot 58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 4.Romero D, Traxler MF, Opez D, Kolter R, López D, Kolter R. 2011. Antibiotics as signal molecules. Chem Rev 111:5492–5505. doi: 10.1021/cr2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pishchany G, Kolter R. 2020. On the possible ecological roles of antimicrobials. Mol Microbiol 113:580–587. doi: 10.1111/mmi.14471. [DOI] [PubMed] [Google Scholar]
- 6.Davies J. 2006. Are antibiotics naturally antibiotics? J Ind Microbiol Biotechnol 33:496–499. doi: 10.1007/s10295-006-0112-5. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira NM, Oliveria NM, Martinez-Garcia E, Xavier J, Durham WM, Kolter R, Kim W, Foster KR. 2015. Biofilm formation as a response to ecological competition. PLoS Biol 13:e1002191. doi: 10.1371/journal.pbio.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsley L, Shank EA. 2017. Natural-product antibiotics: cues for modulating bacterial biofilm formation. Trends Microbiol 25:1016–1026. doi: 10.1016/j.tim.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sengupta S, Chattopadhyay MK, Grossart HP. 2013. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front Microbiol 4:47. doi: 10.3389/fmicb.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies J. 1996. Origins and evolution of antibiotic resistance. Microbiologia 12:9–16. [PubMed] [Google Scholar]
- 11.Flemming HC, Wuertz S. 2019. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol 17:247–260. doi: 10.1038/s41579-019-0158-9. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 13.Tahrioui A, Duchesne R, Bouffartigues E, Rodrigues S, Maillot O, Tortuel D, Hardouin J, Taupin L, Groleau MC, Dufour A, Déziel E, Brenner-Weiss G, Feuilloley M, Orange N, Lesouhaitier O, Cornelis P, Chevalier S. 2019. Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation. NPJ Biofilms Microbiomes 5:15. doi: 10.1038/s41522-019-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karatan E, Duncan TR, Watnick PI. 2005. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J Bacteriol 187:7434–7443. doi: 10.1128/JB.187.21.7434-7443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapfhammer D, Karatan E, Pflughoeft KJ, Watnick PI. 2005. Role for glycine betaine transport in Vibrio cholerae osmoadaptation and biofilm formation within microbial communities. Appl Environ Microbiol 71:3840–3847. doi: 10.1128/AEM.71.7.3840-3847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram L, Melchiorsen J, Bruhn JB. 2010. Antibacterial activity of marine culturable bacteria collected from a global sampling of ocean surface waters and surface swabs of marine organisms. Mar Biotechnol 12:439–451. doi: 10.1007/s10126-009-9233-y. [DOI] [PubMed] [Google Scholar]
- 17.Machado H, Sonnenschein EC, Melchiorsen J, Gram L. 2015. Genome mining reveals unlocked bioactive potential of marine Gram-negative bacteria. BMC Genomics 16:158. doi: 10.1186/s12864-015-1365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porsby CH, Webber MA, Nielsen KF, Piddock LV, Gram L. 2011. Resistance and tolerance to tropodithietic acid, an antimicrobial in aquaculture, is hard to select. Antimicrob Agents Chemother 55:1332–1337. doi: 10.1128/AAC.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenschein EC, Phippen CBW, Bentzon-Tilia M, Rasmussen SA, Nielsen KF, Gram L. 2018. Phylogenetic distribution of roseobacticides in the Roseobacter group and their effect on microalgae. Environ Microbiol Rep 10:383–393. doi: 10.1111/1758-2229.12649. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Gallant É, Seyedsayamdost MR. 2016. Investigation of the genetics and biochemistry of roseobacticide production in the Roseobacter clade bacterium Phaeobacter inhibens. mBio 7:e02118-15. doi: 10.1128/mBio.02118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulsen SS, Strube ML, Bech PK, Gram L, Sonnenschein EC. 2019. Marine chitinolytic Pseudoalteromonas represents an untapped reservoir of bioactive potential. mSystems 4:e00060-19. doi: 10.1128/mSystems.00060-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vynne NG, Månsson M, Nielsen KF, Gram L. 2011. Bioactivity, chemical profiling, and 16S rRNA-based phylogeny of Pseudoalteromonas strains collected on a global research cruise. Mar Biotechnol 13:1062–1073. doi: 10.1007/s10126-011-9369-4. [DOI] [PubMed] [Google Scholar]
- 23.Mansson M, Gram L, Larsen TO. 2011. Production of bioactive secondary metabolites by marine Vibrionaceae. Mar Drugs 9:1440–1468. doi: 10.3390/md9091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wietz M, Mansson M, Gotfredsen CH, Larsen TO, Gram L. 2010. Antibacterial compounds from marine Vibrionaceae isolated on a global expedition. Mar Drugs 8:2946–2960. doi: 10.3390/md8122946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machado H, Giubergia S, Mateiu RV, Gram L. 2015. Photobacterium galatheae sp. nov, a bioactive bacterium isolated from a mussel in the Solomon Sea. Int J Syst Evol Microbiol 65:4503–4507. doi: 10.1099/ijsem.0.000603. [DOI] [PubMed] [Google Scholar]
- 26.Kenig M, Reading C. 1979. Holomycin and an antibiotic (mm 19290) related to tunicamycin, metabolites of Streptomyces clavuligerus. J Antibiot 32:549–554. doi: 10.7164/antibiotics.32.549. [DOI] [PubMed] [Google Scholar]
- 27.Liras P. 2014. Holomycin, a dithiolopyrrolone compound produced by Streptomyces clavuligerus. Appl Microbiol Biotechnol 98:1023–1030. doi: 10.1007/s00253-013-5410-z. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Wever WJ, Walsh CT, Bowers AA. 2014. Dithiolopyrrolones: biosynthesis, synthesis, and activity of a unique class of disulfide-containing antibiotics. Nat Prod Rep 31:905–923. doi: 10.1039/c3np70106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin Z, Huang S, Yu Y, Deng H. 2013. Dithiolopyrrolone natural products: isolation, synthesis and biosynthesis. Mar Drugs 11:3970–3997. doi: 10.3390/md11103970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliva B, O'Neill A, Wilson JM, O'Hanlon PJ, Chopra I. 2001. Antimicrobial properties and mode of action of the pyrrothine holomycin. Antimicrob Agents Chemother 45:532–539. doi: 10.1128/AAC.45.2.532-539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Walsh CT. 2010. Identification of the gene cluster for the dithiolopyrrolone antibiotic holomycin in Streptomyces clavuligerus. Proc Natl Acad Sci U S A 107:19731–19735. doi: 10.1073/pnas.1014140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin Z, Baker AT, Raab A, Huang S, Wang T, Yu Y, Jaspars M, Secombes CJ, Deng H. 2013. The fish pathogen Yersinia ruckeri produces holomycin and uses an RNA methyltransferase for self-resistance. J Biol Chem 288:14688–14697. doi: 10.1074/jbc.M112.448415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T. 2019. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado H, Månsson M, Gram L. 2014. Draft genome sequence of Photobacterium halotolerans S2753, producer of bioactive secondary metabolites. Genome Announc 2:e00535-14. doi: 10.1128/genomeA.00535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Walsh CT. 2011. Streptomyces clavuligerus HlmI is an intramolecular disulfide-forming dithiol oxidase in holomycin biosynthesis. Biochemistry 50:4615–4622. doi: 10.1021/bi200321c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B, Forseth RR, Bowers AA, Schroeder FC, Walsh CT. 2012. A backup plan for self-protection: S-methylation of holomycin biosynthetic intermediates in Streptomyces clavuligerus. Chembiochem 13:2521–2526. doi: 10.1002/cbic.201200536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan AN, Shiver AL, Wever WJ, Razvi SZA, Traxler MF, Li B. 2017. Role for dithiolopyrrolones in disrupting bacterial metal homeostasis. Proc Natl Acad Sci U S A 114:2717–2722. doi: 10.1073/pnas.1612810114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziemert N, Alanjary M, Weber T. 2016. The evolution of genome mining in microbes-a review. Nat Prod Rep 33:988–1005. doi: 10.1039/c6np00025h. [DOI] [PubMed] [Google Scholar]
- 39.Teschler JK, Zamorano-Sánchez D, Utada AS, Warner CJA, Wong GCL, Linington RG, Yildiz FH. 2015. Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat Rev Microbiol 13:255–268. doi: 10.1038/nrmicro3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yildiz FH, Visick KL. 2009. Vibrio biofilms: so much the same yet so different. Trends Microbiol 17:109–118. doi: 10.1016/j.tim.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takemura AF, Chien DM, Polz MF. 2014. Associations and dynamics of vibrionaceae in the environment, from the genus to the population level. Front Microbiol 5:38. doi: 10.3389/fmicb.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlomann BH, Wiles TJ, Wall ES, Guillemin K, Parthasarathy R. 2019. Sublethal antibiotics collapse gut bacterial populations by enhancing aggregation and expulsion. Proc Natl Acad Sci U S A 116:21392–21400. doi: 10.1073/pnas.1907567116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Toole GA, Stewart PS. 2005. Biofilms strike back. Nat Biotechnol 23:1378–1379. doi: 10.1038/nbt1105-1378. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, Mandic-Mulec I, Zhang H, Liu Y, Sun X, Feng H, Xun W, Zhang N, Shen Q, Zhang R. 2019. Antibiotic bacillomycin D affects iron acquisition and biofilm formation in Bacillus velezensis through a Btr-mediated FeuABC-dependent pathway. Cell Rep 29:1192–1202. doi: 10.1016/j.celrep.2019.09.061. [DOI] [PubMed] [Google Scholar]
- 45.Ranieri MR, Whitchurch CB, Burrows LL. 2018. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr Opin Microbiol 45:164–169. doi: 10.1016/j.mib.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Kong H, Cheng W, Wei H, Yuan Y, Yang Z, Zhang X. 2019. An overview of recent progress in siderophore-antibiotic conjugates. Eur J Med Chem 182:111615. doi: 10.1016/j.ejmech.2019.111615. [DOI] [PubMed] [Google Scholar]
- 47.Cornforth DM, Foster KR. 2015. Antibiotics and the art of bacterial war. Proc Natl Acad Sci U S A 112:10827–10828. doi: 10.1073/pnas.1513608112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Q, Lopes LD, Shaffer BT, Kidarsa TA, Vining O, Philmus B, Song C, Stockwell VO, Raaijmakers JM, McPhail KL, Andreote FD, Chang JH, Loper JE. 2018. Secondary metabolism and interspecific competition affect accumulation of spontaneous mutants in the GacS-GacA regulatory system in Pseudomonas protegens. mBio 9:e01845-17. doi: 10.1128/mBio.01845-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buijs Y, Isbrandt T, Zhang S-D, Larsen TO, Gram L. 2020. The antibiotic andrimid produced by Vibrio coralliilyticus increases antibiotics holomycin production in the ecological neighbour, Photobacterium galatheae. Front Microbiol 11:622055. doi: 10.3389/fmicb.2020.622055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol 9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Davies J. 2013. Specialized microbial metabolites: functions and origins. J Antibiot 66:361–364. doi: 10.1038/ja.2013.61. [DOI] [PubMed] [Google Scholar]
- 52.Giubergia S, Phippen C, Nielsen KF, Gram L. 2017. Growth on chitin impacts the transcriptome and metabolite profiles of antibiotic-producing Vibrio coralliilyticus S2052 and Photobacterium galatheae S2753. mSystems 2:1–12. doi: 10.1128/mSystems.00141-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capdevila DA, Wang J, Giedroc DP. 2016. Bacterial strategies to maintain zinc metallostasis at the host-pathogen interface. J Biol Chem 291:20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grim KP, Radin JN, Solórzano PKP, Morey JR, Frye KA, Ganio K, Neville SL, McDevitt CA, Kehl-Fie TE. 2020. Intracellular accumulation of staphylopine can sensitize Staphylococcus aureus to host-imposed zinc starvation by chelation-independent toxicity. J Bacteriol 202:e00014-20. doi: 10.1128/JB.00014-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji B, Zhang S-D, Arnoux P, Rouy Z, Alberto F, Philippe N, Murat D, Zhang WJ, Rioux JB, Ginet N, Sabaty M, Mangenot S, Pradel N, Tian J, Yang J, Zhang L, Zhang W, Pan H, Henrissat B, Coutinho PM, Li Y, Xiao T, Médigue C, Barbe V, Pignol D, Talla E, Wu LF. 2014. Comparative genomic analysis provides insights into the evolution and niche adaptation of marine Magnetospira sp. QH-2 strain. Environ Microbiol 16:525–544. doi: 10.1111/1462-2920.12180. [DOI] [PubMed] [Google Scholar]
- 56.Zhang S-D, Santini CL, Zhang WJ, Barbe V, Mangenot S, Guyomar C, Garel M, Chen HT, Li XG, Yin QJ, Zhao Y, Armengaud J, Gaillard JC, Martini S, Pradel N, Vidaud C, Alberto F, Médigue C, Tamburini C, Wu LF. 2016. Genomic and physiological analysis reveals versatile metabolic capacity of deep-sea Photobacterium phosphoreum ANT-2200. Extremophiles 20:301–310. doi: 10.1007/s00792-016-0822-1. [DOI] [PubMed] [Google Scholar]
- 57.Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH. 2017. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45:D200–D203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, Zhang S-D, Chen L, Cai Y, Zhang WJ, Song T, Wu LF. 2018. Efficient genome editing of Magnetospirillum magneticum AMB-1 by CRISPR-Cas9 system for analyzing magnetotactic behavior. Front Microbiol 9:1569. doi: 10.3389/fmicb.2018.01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Li Z, Jia R, Hou Y, Yin J, Bian X, Li A, Müller R, Stewart AF, Fu J, Zhang Y. 2016. RecET direct cloning and Redαβ recombineering of biosynthetic gene clusters, large operons or single genes for heterologous expression. Nat Protoc 11:1175–1190. doi: 10.1038/nprot.2016.054. [DOI] [PubMed] [Google Scholar]
- 60.Milton DL, O’Toole R, Hörstedt P, Wolf-Watz H. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol 178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin QJ, Zhang WJ, Qi XQ, Zhang S, Jiang T, Li XG, Chen Y, Santini CL, Zhou H, Chou IM, Wu LF. 2017. High hydrostatic pressure inducible trimethylamine N-oxide reductase improves the pressure tolerance of piezosensitive bacteria Vibrio fluvialis. Front Microbiol 8:2646. doi: 10.3389/fmicb.2017.02646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen A, Larsen MH, Ingmer H, Vogel BF, Gram L. 2007. Sodium chloride enhances adherence and aggregation and strain variation influences invasiveness of Listeria monocytogenes strains. J Food Prot 70:592–599. doi: 10.4315/0362-028x-70.3.592. [DOI] [PubMed] [Google Scholar]
- 63.O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 64.Djordjevic D, Wiedmann M, McLandsborough LA. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol 68:2950–2958. doi: 10.1128/aem.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buijs Y, Zhang S-D, Jørgensen KM, Isbrandt T, Larsen TO, Gram L, Buijs Y, Zhang S-D, Jørgensen KM, Isbrandt T, Larsen TO, Gram L. 9 March 2021. Enhancement of antibiotic production by co-cultivation of two antibiotic producing marine Vibrionaceae strains. FEMS Microbiol Ecol doi: 10.1093/femsec/fiab041. [DOI] [PubMed] [Google Scholar]
- 66.Wu C, Labrie J, Tremblay YDN, Haine D, Mourez M, Jacques M. 2013. Zinc as an agent for the prevention of biofilm formation by pathogenic bacteria. J Appl Microbiol 115:30–40. doi: 10.1111/jam.12197. [DOI] [PubMed] [Google Scholar]
- 67.Obranic S, Babic F, Maravic-Vlahovicek G. 2013. Improvement of pBBR1MCS plasmids, a very useful series of broad-host-range cloning vectors. Plasmid 70:263–267. doi: 10.1016/j.plasmid.2013.04.001. [DOI] [PubMed] [Google Scholar]