Abstract
Background
Leg ulcers are open skin wounds that occur below the knee but above the foot. The majority of leg ulcers are venous in origin, occurring as a result of venous insufficiency, where the flow of blood through the veins is impaired; they commonly arise due to blood clots and varicose veins. Compression therapy, using bandages or stockings, is the primary treatment for venous leg ulcers. Wound cleansing can be used to remove surface contaminants, bacteria, dead tissue and excess wound fluid from the wound bed and surrounding skin, however, there is uncertainty regarding the effectiveness of cleansing and the best method or solution to use.
Objectives
To assess the effects of wound cleansing, wound cleansing solutions and wound cleansing techniques for treating venous leg ulcers.
Search methods
In September 2019 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We considered randomised controlled trials (RCTs) comparing wound cleansing with no wound cleansing, or RCTs comparing different wound cleansing solutions, or different wound cleansing techniques.
Data collection and analysis
We screened studies for their appropriateness for inclusion, assessed their risk of bias using the Cochrane 'Risk of bias' tool, and used GRADE methodology to determine the certainty of evidence. Two review authors undertook these tasks independently, using predetermined criteria. We contacted study authors for missing data where possible.
Main results
We included four studies with a total of 254 participants. All studies included comparisons between different types of cleansing solutions, and three of these reported our primary outcomes of complete wound healing or change in ulcer size over time, or both. Two studies reported the secondary outcome, pain. One study (27 participants), which compared polyhexamethylene biguanide (PHMB) solution with saline solution for cleansing venous leg ulcers, did not report any of the review's primary or secondary outcomes. We did not identify any studies that compared cleansing with no cleansing, or that explored comparisons between different cleansing techniques.
One study (61 participants) compared aqueous oxygen peroxide with sterile water. We are uncertain whether aqueous oxygen peroxide makes any difference to the number of wounds completely healed after 12 months of follow‐up (risk ratio (RR) 1.88, 95% confidence interval (CI) 1.10 to 3.20). Similarly, we are uncertain whether aqueous oxygen peroxide makes any difference to change in ulcer size after eight weeks of follow‐up (mean difference (MD) ‐1.38 cm2, 95% CI ‐4.35 to 1.59 cm2). Finally, we are uncertain whether aqueous oxygen peroxide makes any difference to pain reduction, assessed after eight weeks of follow‐up using a 0 to 100 pain rating, (MD 3.80, 95% CI ‐10.83 to 18.43). The evidence for these outcomes is of very low certainty (we downgraded for study limitations and imprecision; for the pain outcome we also downgraded for indirectness).
Another study (40 participants) compared propyl betaine and polihexanide with a saline solution. The authors did not present the raw data in the study report so we were unable to conduct independent statistical analysis of the data. We are uncertain whether propyl betaine and polihexanide make any difference to the number of wounds completely healed, change in ulcer size over time, or wound pain reduction. The evidence is of very low certainty (we downgraded for study limitations and imprecision).
The final study (126 participants) compared octenidine dihydrochloride/phenoxyethanol (OHP) with Ringer's solution. We are uncertain whether OHP makes any difference to the number of wounds healed (RR 0.96, 95% CI 0.53 to 1.72) or to the change in ulcer size over time (we were unable to conduct independent statistical analysis of available data). The evidence is of very low certainty (we downgraded for study limitations and imprecision).
None of the studies reported patient preference, ease of use of the method of cleansing, cost or health‐related quality of life. In one study comparing propyl betaine and polihexanide with saline solution the authors do not report any adverse events occurring. We are uncertain whether OHP makes any difference to the number of adverse events compared with Ringer's solution (RR 0.58, 95% CI 0.29 to 1.14). The evidence is of very low certainty (we downgraded for study limitations and imprecision).
Authors' conclusions
There is currently a lack of RCT evidence to guide decision making about the effectiveness of wound cleansing compared with no cleansing and the optimal approaches to cleansing of venous leg ulcers. From the four studies identified, there is insufficient evidence to demonstrate whether the use of PHMB solution compared with saline solution; aqueous oxygen peroxide compared with sterile water; propyl betaine and polihexanide compared with a saline solution; or OHP compared with Ringer's solution makes any difference in the treatment of venous leg ulcers. Evidence from three of the studies is of very low certainty, due to study limitations and imprecision. One study did not present data for the primary or secondary outcomes. Further well‐designed studies that address important clinical, quality of life and economic outcomes may be important, based on the clinical and patient priority of this uncertainty.
Plain language summary
Does cleaning venous leg ulcers help them to heal?
Background
Leg ulcers are open skin wounds that develop below the knee, usually because blood flow is poor in the lower leg. This can occur because of blockages, for example when small blood clots form in the veins. It can also happen when the valves (flaps) in the veins that prevent blood from flowing backwards stop working properly. Poor blood flow damages the skin and tissue, and creates venous leg ulcers.
Ulcers are unsightly and may become painful or infected. On average, ulcers take from six to nine months to heal. However, some ulcers can take years to heal, and a small number never do. Once ulcers have healed, they can reoccur.
The main treatment for venous leg ulcers is to use bandages or stockings that compress the leg (compression therapy), to increase blood flow in the veins. It is also thought to be important to clean the wound. Different types of cleaning solutions can be used, including: normal saline; water; antiseptics (solutions that stop or slow down the growth of micro‐organisms such as bacteria); detergents (solutions that remove bacteria and dirt); or disinfectants (solutions such as bleach, that kill micro‐organisms).
Cleaning solutions can be applied to the ulcer using a swab (similar to a cotton bud), a syringe with a needle, or a spray canister. Ulcers can also be bathed in the cleaning solution, using a basin or bucket, or during a shower. Cleaning can cause discomfort, and may be painful.
What did we want to find out?
We wanted to find out:
‐ whether cleaning venous leg ulcers helps them to become smaller and heal;
‐ whether some cleaning solutions, or methods of applying solutions, are more effective than others;
‐ which cleaning solution people prefer and find easiest to use;
‐ whether cleaning wounds has an impact on quality of life;
‐ how much cleaning wounds costs; and
‐ whether cleaning wounds is associated with adverse (unwanted) effects such as pain, infection or skin damage.
Our methods
First, we searched for randomised controlled studies (clinical studies where the treatment or care people receive is chosen at random). These studies provide the most reliable health evidence about the effects of a treatment. We then compared the results and summarised the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What we found
We found four studies that involved a total of 254 people with venous leg ulcers. There were 108 men and 144 women, all over 18 years old (information about gender was missing for two people).
The studies compared the effects of:
‐ a disinfectant and antiseptic agent (polyhexamethylene biguanide) applied using a syringe with a needle to flush (irrigate) the ulcer with fluid, against a salt (saline) solution;
‐ a gentle spray of a bleaching and antiseptic agent (aqueous oxygen peroxide, which is ozone dissolved in water), against sterile water;
‐ a detergent (propyl betaine combined with polyhexanide), against a saline solution – method of application not reported; and
‐ an antiseptic (octenidine dihydrochloride combined with phenoxyethanol) sprayed onto the wound, against a solution of several salts dissolved in water (Ringer’s solution).
No studies compared cleaning with no cleaning, or compared different cleaning methods.
We cannot tell whether cleaning wounds is beneficial or associated with any unwanted effects. This is because we have too little confidence in the evidence available regarding healing, changes in ulcer size, pain and unwanted effects. No study reported on patient preference, ease of use, cost or impact on quality of life.
What does this mean?
We do not know whether cleaning solutions are better than sterile water or saline solutions to help venous leg ulcers heal, or whether the choice of cleaning solution or method of application makes any difference to venous leg ulcer healing. Our confidence in the available evidence is very low. The results of our review are likely to change if more evidence becomes available.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to September 2019.
Summary of findings
Background
Description of the condition
Leg ulcers are defined as a break (wound) in the skin below the knee that has failed to heal in four to six weeks (SIGN 2010). The majority of leg ulcers (79% to 83%) are venous in origin (Ghauri 1998; Moffatt 1992; O'Brien 2000; Scriven 1997, and result from venous insufficiency causing blood pooling and increased pressure in the veins (Callam 1987); they commonly occur as a result of blood clots and varicose veins. Fluid can then escape from the veins under the skin, causing swelling and damage to the skin (Gonzalez‐Consuegra 2011). Leg ulcers are a major healthcare problem, in terms of treatment, cost, recurrence and chronicity (Nelson 2016). The point prevalence of venous leg ulcers in different countries across the world ranges from 0.2% to 4.5% (Xie 2018). European figures suggest that the prevalence of open venous leg ulcers ranges between 0.1% and 0.34% (Berenguer Perez 2019; Day 2015; Heyer 2017).
The annual cost of treating people with leg ulcers in the UK has recently been reported to be 1,938 million pounds sterling (Guest 2017). Leg ulcers also have a negative impact on the individual's health‐related quality of life (Briggs 2007; Green 2010; Persoon 2004). They can impact on the person physically, psychologically and socially (Briggs 2007; Gonzalez‐Consuegra 2011), with pain being the most common and recurring feature (Briggs 2007). Other factors include social isolation, inability to work, limited mobility, discomfort, and the requirement for frequent visits to hospitals and clinics (Green 2010). Briggs 2007 describes leg ulcers as a debilitating chronic disease, and suggests that management for people with this condition should be focused on alleviating the symptoms of their 'leg ulcer journey', rather than solely healing their wounds.
Leg ulcers are a chronic condition. The average duration of a venous leg ulcer is six to nine months, with healing rates varying across studies (Briggs 2003). Once healed, venous ulcer reoccurrence rates are high, with 26% to 69% of people developing another ulcer in the first 12 months after healing (Nelson 2012).
A large proportion of nursing time is spent treating individuals with leg ulcers. A number of studies have estimated that the mean time to treat one leg ulcer is 20 to 24 minutes (Skerritt 2014; Vowden 2009), with this figure varying from 2 to 120 minutes (Vowden 2009). Since nursing time incurs the biggest proportion of cost associated with venous leg ulcers (Iglesias 2004), and cleansing is likely to account for a significant proportion of nursing time, it makes sense to establish the most clinically beneficial and cost‐effective method of cleansing.
Description of the intervention
For the purposes of this review, we define wound cleansing as the use of fluid to remove loose debris and devitalised tissue from the wound surface (Cutting 2010). It is suggested that wound cleansing is an intrinsic element of wound management. It is performed to remove surface contaminants, bacteria, non‐viable tissue (dead or dying tissue) and excess exudate (wound fluid) from the wound bed and surrounding skin (Andriessen 2008; Cutting 2010; Hellewell 1997; Main 2008), thus establishing an environment conducive to wound healing (Attinger 2006; Horrocks 2006). Cleansing is thought to be an effective way to remove inflammatory stimulants and local barriers to wound healing from the wound bed (Cutting 2010), with the aim of aiding health (Hellewell 1997). However, unnecessary or inappropriate cleansing may lead to trauma to the wound bed by damaging fragile granulating (healing) or epithelialising (scar) tissue (Flanagan 1997; Hellewell 1997). Therefore, the aim of cleansing and the method chosen must be carefully considered to maximise the action and minimise any harm to the individual (Flanagan 1997; Magson‐Roberts 2006).
Wound cleansing comprises three elements: technique, solution, and equipment (Williams 1999; Young 1995). Techniques includes swabbing, irrigation and bathing.
Swabbing involves the use of soaked non‐woven gauze swabs (synthetic blend of polyester and polyethylene combined together to produce a more absorbant and lint free gauze) to remove devitalised (dead) tissue and contaminants from the wound bed (Flanagan 1997).
Irrigation involves flushing the wound with fluid. This can be achieved by using a syringe and needle or a pressurised canister. The recommended pressure is between 4 psi and 15 psi, with under 4 psi considered ineffective, while over 15 psi may cause damage (AHCPR 1994). Wound cleansing solutions include normal saline (0.9%), water, and antiseptics. Normal saline (0.9%) is an isotonic solution that should not damage tissue, cause allergic reactions, or alter the normal bacterial flora of the skin (Huxtable 1997; Lawrence 1997; Philips 1997). Water is also used for wound cleansing; however the water needs to be of drinking quality, and where this is not possible, cooled boiled water is suggested for use (Lindsay 2007). Antiseptics are chemical solutions that, when applied to the external surface of the body, impede the growth and development of micro‐organisms.
Bathing, which encompasses hydrotherapy, involves bathing the affected limb in a basin or bucket, or showering the affected limb. Hydrotherapy is a combination of soaking the wound in water and water agitation used to soften and loosen devitalised tissue and gross contaminants (Flanagan 1997). Bathing and showering involve washing the wound and limb with potable tap water (drinkable tap water free of contaminants) (Lindsay 2007). This not only cleanses the wound, but also the surrounding skin, and is thought to be of psychological benefit to the person with the leg ulcer (Morison 1994).
The equipment needed for wound cleansing will depend on the technique chosen, for example a needle and syringe for irrigation, or a bucket or a shower for bathing (Trevelyan 1996; Williams 1999; Young 1995).
How the intervention might work
Compression bandaging is the gold standard for the management of ambulatory people with venous leg ulceration. Compression bandaging attempts to reverse the effects of venous insufficiency and the evidence suggests that there is an increased rate of wound healing using compression therapy (O'Meara 2012). However, before application of the compression bandaging, it may be important to ensure the wound bed is free from contaminants and devitalised (dead or dying) tissue, and wound cleansing could be central to achieving this (Hellewell 1997). It has been suggested that failure to cleanse the wound bed sufficiently may lead to delayed healing, attributed to a number of important factors, including the presence of foreign bodies, necrotic or sloughy tissue and bacterial burden or biofilm (a poly‐microbial community that is encased in polysaccharides (slime) and attached to the wound bed) (Cooper 2006). It is suggested that these factors lead to the increased production of metalloproteases within the wound (Cutting 2010; Hellewell 1997; Horrocks 2006). These enzymes are produced during wound healing, but when present for prolonged periods or in excess amounts, metalloproteases may become detrimental to healing tissue (Toy 2005). Wound cleansing in the form of swabbing, irrigation, or washing the wound bed may contribute to reducing or eliminating some of these factors, thereby enhancing wound healing potential.
Why it is important to do this review
Wound cleansing is used as a component of venous leg ulcer management to prepare the wound before dressings and bandages are used. It has been suggested that failure to cleanse the wound and limb appropriately may have negative effects on healing time and infection risk. Wound cleansing is recommended in many international guidelines (AWMA 2011; HSE 2018; RCN 2006; RNAO 2004; SIGN 2010) but an ongoing review of the evidence for this procedure is important in terms of its value, and the best approaches in terms of methods and solutions that, it has been suggested, are often based on ritualistic practice, with little consideration given to the potential research evidence to support or refute the chosen method or solution of cleansing (Trevelyan 1996). For this reason we aimed to include studies of cleansing versus no cleansing in addition to studies comparing different wound cleansing solutions and techniques with each other.
The ability to manage increasing demands on the health service is greatly influenced by the available resources (Phillips 2005). It is unlikely that there will ever be enough revenue to meet all healthcare challenges; therefore the development of cost‐effective patient care delivery programmes is central to ensuring sustainability in healthcare delivery into the future (European Commission 2012). For individuals with leg ulceration this means all aspects of care should be effective, efficient and based on the best available evidence. This systematic review presents the evidence for approaches to wound cleansing for venous leg ulceration.
Objectives
To assess the effects of wound cleansing, wound cleansing solutions and wound cleansing techniques for treating venous leg ulcers.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) comparing wound cleansing with no wound cleansing, or RCTs comparing different wound cleansing solutions, or different wound cleansing techniques, for this review. We would have considered including controlled clinical trials (CCTs) in the absence of RCTs reporting any of the above comparisons but we did not find any CCTs that met the inclusion criteria. We included studies involving wound cleansing of different wounds if they presented data pertaining to venous leg ulcers separately and randomisation was stratified by wound type and it was possible to extract these data from the study.
Types of participants
We included adults (over 18 years of age) with a diagnosed venous leg ulcer, managed in any healthcare setting.
Types of interventions
For the purposes of this review, we defined cleansing as the application of fluid to a venous leg ulcer to aid removal of exudate, debris and contaminants, but not the use of dressings or mechanical debridement (Towler 2001). Studies investigating the following comparisons were eligible:
cleansing compared with no cleansing;
comparisons between different types of cleansing solutions;
comparisons between different cleansing techniques (e.g. irrigation, swabbing, soaking, immersion).
Types of outcome measures
Primary outcomes
Complete wound healing
Time to wound healing within a specific time period, correctly analysed using survival, time‐to‐event, approaches. We assumed that the period of time in which healing could occur was the duration of the study, unless otherwise stated.
Number of wounds completely healed during follow‐up (frequency of complete healing).
When a study analysed time as a continuous measure, but it was not clear whether all ulcers had healed, we planned to document the use of this outcome in the study but not summarise or otherwise use the data in any meta analysis. We accepted study authors’ definitions of what constituted a healed wound.
Change in ulcer size over time
Changes in ulcer size measured by reduction in original wound area within the duration of the study, expressed as absolute (e.g. surface area changes in cm2 from baseline) or relative (e.g. percentage change in area relative to baseline) change. For changes to this section see 'Differences between protocol and review'.
Secondary outcomes
Patient preference
Procedural pain (using validated scales where reported)
Ease of use of the method of cleansing (as reported by the study authors)
Cost
Health‐related quality of life (using validated scales, where reported)
Adverse events (for example infection, pain, maceration (wet, white, boggy tissue), excoriation (red, hot shiny tissue) or bleeding).
For studies reporting outcomes for different time periods, we have followed the guidance in The Cochrane Handbook for Systematic Reviews of Interventions and have selected the longest follow‐up for each outcome in the study (Higgins 2019).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant RCTs:
the Cochrane Wounds Specialised Register (searched 4 September 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8) in the Cochrane Library (searched 4 September 2019);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 4 September 2019);
Ovid Embase (1974 to 4 September 2019);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 4 September 2019).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 10 September 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx) (searched 10 September 2019);
Search strategies for clinical trial registries can be found in Appendix 1.
Searching other resources
We searched the reference lists of all the included pertinent studies and other publications, such as guidelines and systematic reviews. We contacted 11 experts in the field, but did not identify any further study information. We planned to write to other authors of relevant publications to identify any completed or ongoing studies; however, we did not identify any.
Data collection and analysis
We carried out data collection and analysis in accordance with the methods outlined in the published protocol (McLain 2015), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors independently assessed the titles and, where available, abstracts of the studies identified in the search strategy, to determine their eligibility for inclusion in the review. We obtained full versions of potentially relevant studies, and two review authors independently screened these against the inclusion criteria (see PRISMA flow chart in Figure 1; Liberati 2009). Review authors resolved any differences in opinion by discussion.
1.
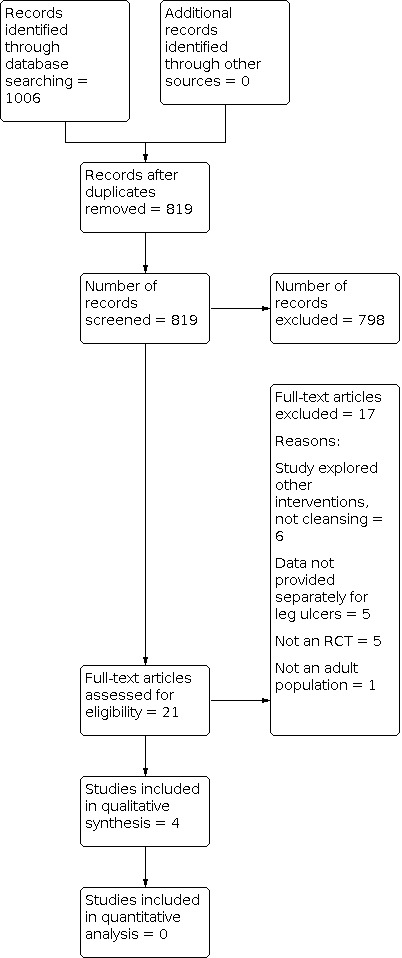
PRISMA Flowchart
Data extraction and management
The primary review author (NMcL) extracted the details of eligible studies and summarised them into a pre‐prepared data extraction table. The second and third review authors (ZM, PA) checked the extracted data. If we found that there were missing data in the studies, we contacted the study authors to obtain the missing information.
We extracted the following information for each study;
author, title, source;
date of study, study's geographical location;
care setting;
inclusion/exclusion criteria;
duration of ulcer;
participant characteristics;
study design details;
sample size calculation and sample size;
intervention details, concurrent interventions (compression therapy: applied or not applied, elastic or inelastic, light (18 mmHg to 24 mmHg) or full compression (35 mmHg to 40 mmHg);
use of additional dressing materials;
outcome measures;
length of follow‐up;
results;
conclusions, as reported by the study authors;
funding source.
Assessment of risk of bias in included studies
For this review two review authors independently assessed each included study using the Cochrane tool for assessing risk of bias (Higgins 2011b). This tool addresses six specific domains, namely random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (Appendix 2). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study, and judged each component of the study to be at low, unclear or high risk of bias. We present an assessment of risk of bias using a 'Risk of bias' summary figure, which reports all of the judgements in a cross‐tabulation of study by domain.
Measures of treatment effect
We conducted data analysis according to Cochrane guidelines (Higgins 2011a). We entered the data into Review Manager 2014. For dichotomous outcomes (e.g. ulcer healed during time period) we calculated the risk ratio (RR) with 95% confidence intervals (CIs). Risk ratio is the ratio of the risk of the event of interest (e.g. leg ulcers healed) in the experimental group divided by the risk of this event in the control group and indicates the chances of healing for people in the experimental group compared with the control group (Higgins 2011a). For continuous outcome data (e.g. absolute or percentage changes in the ulcer area) we used the mean difference (MD) with 95% CIs. If studies had used different assessment scales, we would have used the standardised mean difference (SMD) with 95% CIs. We would have reported time‐to‐event data (e.g. time to healing) as hazard ratios (HRs) where possible, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). For statistically significant effects in binary outcomes, we planned to calculate the number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH). If data appeared to be skewed, and if scale data had finite upper and lower limits, we would have used the easy ’rule of thumb’ calculation to test for skewness. That is, if the standard deviation (SD), when doubled, is greater than the mean, it is unlikely that the mean is the centre of the distribution (Altman 1996). In such a case, we would not have entered the data into any meta‐analysis. If we had found relevant data that was skewed, we would have presented the data in 'Additional tables'.
Unit of analysis issues
Some studies randomise by participant, but analyse outcomes by wound. If this had occurred, and the numbers of participants and wounds were equal (i.e. one wound per participant), we would have treated the participant as the unit of analysis. Where we identified clustered data outside of the context of a cluster‐randomised trial, that is, where a proportion of randomised trial participants had outcome data collected and reported on multiple wounds, we planned to record this in the 'Risk of bias' assessment. We would have extracted and presented the data, but it would not have been the subject of any further analyses.
For cluster‐randomised trials that used analysis methods to account for the clustering, we planned to extract effect sizes and standard errors from the appropriate analysis. Where cluster‐randomised trials had been analysed incorrectly, we planned to recalculate the results using the appropriate unit of analysis if required (Higgins 2011a), and if sufficient data were available. The included studies did not randomise multiple wounds, neither did we include cluster‐randomised trials, therefore we did not identify any unit of analysis issues.
Dealing with missing data
Where there were missing data, we attempted to contact the study authors to request the missing information. However in some cases the data remained missing despite our best efforts, and our analysis was restricted to the published data because specific figures for each group were not available. We were also unable to perform sensitivity analysis to assess how sensitive the results were to reasonable changes. We review the potential impact of the missing data in the discussion section.
Assessment of heterogeneity
We planned to consider both clinical and statistical heterogeneity. We planned to assess clinical heterogeneity using key study features: participant characteristics, intervention type, study duration and outcome types. We planned to assess statistical heterogeneity using the Chi2 test with significance set at P < 0.10, in conjunction with the I2 measure (Higgins 2003). The I2 measure evaluates the percentage of total variation across the studies due to heterogeneity rather than due to chance (Higgins 2003). Where the I2 measure is 0% to 25% this indicates a low level of heterogeneity, and where the I2 value exceeds 75% this represents a very high level of heterogeneity (Deeks 2011). We planned to carry out statistical pooling on groups of studies which we considered to be sufficiently similar. Where heterogeneity was absent or low (I2 = 0% to 25%), we planned to use a fixed‐effect model; if there was evidence of heterogeneity (I2 > 25%), we would have used a random‐effects model. If heterogeneity was very high (I2 > 75%) we would not have pooled the data (Higgins 2003).
Assessment of reporting biases
We assessed reporting bias using guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). If we had identified sufficient studies for a meaningful assessment of publication bias, we would have constructed a funnel plot of primary outcomes to test for asymmetry. If asymmetry was present, we planned to explore possible causes, including reporting and publication bias. However, as we only included four studies in this review, this was not possible.
Data synthesis
We have combined details of included studies in a narrative review according to type of comparator. We were unable to pool data for meta‐analysis. If we had identified more studies, we would have considered clinical and methodological heterogeneity and pooled data if studies appeared to be appropriately similar in terms of wound type, intervention type, duration of follow‐up and outcome type.
We were unable to predict the amount of clinical, methodological and statistical heterogeneity between included studies, but it might have been extensive. Thus, we anticipated using a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow confidence intervals. We would only have used a fixed‐effect approach if we had assessed clinical and methodological heterogeneity to be minimal, and the assumption that a single underlying treatment effect is being estimated held. We would have used I2 and Chi2 statistics to quantify heterogeneity, but would not have used these to guide our choice of model for meta‐analysis. We would have exercised caution when meta‐analysed data were at risk of small study effects, because a random‐effects model may be unsuitable in such a situation. In this case, or where there were other reasons to question the selection of a fixed‐effect or random‐effects model, we would have assessed the impact of the approach using sensitivity analyses to compare results from alternate models (Thompson 1999).
We have presented the data using forest plots where possible. For dichotomous outcomes, we have presented the summary estimate as a risk ratio (RR) with 95% CI. We have used the mean difference (MD) with 95% CI for continuous outcomes. If we had included more than one study that reported continuous outcomes, and the studies measured these in the same way, we would have presented a pooled MD with 95% CI; we planned to pool standardised mean difference (SMD) estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to plot (and, if appropriate, pool) estimates of hazard ratios and 95% CIs as presented in the study reports using the generic inverse variance method in Revman 5 (Review Manager 2014). Where a study analysed time‐to‐healing as a continuous measure but it was not clear if all wounds healed, we would have documented the study's use of the outcome but would not have summarised the data or used it in any meta‐analysis.
We would have obtained all pooled estimates of treatment effect using Revman 5 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
If substantial heterogeneity had existed between studies for the primary outcomes (that is, when the I2 measure exceeded 50%), we would have explored reasons for heterogeneity. We planned to include the following subgroup analyses:
presence or absence of compression therapy;
type of compression therapy (elastic or inelastic);
level of compression therapy (light (exerting 14 to 17 mmHg of compression) or full (exerting up to 60 mmHg of compression)).
Sensitivity analysis
We planned to perform a sensitivity analysis by including only those studies assessed as low risk bias in the key domains of adequate generation of the randomisation sequence, adequate allocation concealment and blinding of outcome assessor for the estimates of treatment effect.
Summary of findings and assessment of the certainty of the evidence
We present the main results of the review in four 'Summary of findings' tables which provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes, as recommended by Cochrane (Schünemann 2011a). The 'Summary of findings' tables include an overall grading of the evidence related to each of the main outcomes, using the GRADE approach (Schünemann 2011b). We include the following main outcomes in the 'Summary of findings' tables:
time to healing
number of wounds completely healed during follow‐up (frequency of complete healing)
change in ulcer size over time, a reduction in the original size of the wound area within the duration of the study
procedural pain (using validated scales, where reported)
cost
health‐related quality of life (using valid scales, where reported)
adverse events (e.g. infection, pain, maceration (wet, white, boggy tissue), excoriation (red, hot, shiny tissue) or bleeding.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We have included a study flow diagram (Figure 1) as recommended in the PRISMA statement (Liberati 2009), to illustrate the results of all searching and screening activity to select eligible studies for inclusion in the review. The search yielded a total of 1006 citations. Both review authors examined available abstracts of all papers independently to assess for potential relevance. After excluding duplicates, 21 studies appeared to meet the inclusion criteria and we retrieved full texts. We subsequently excluded a further 17 studies; reasons for their exclusion are detailed in the Characteristics of excluded studies table.
Included studies
See the Characteristics of included studies table. We included four studies with a total of 254 participants in this review (Borges 2018, O'Halloran 2014; Romanelli 2010; Vanscheidt 2012).
Participants
The mean age of participants in the studies was 65.3 years (SD: 6.6 years; minimum 58.4 years Borges 2018; maximum 73.5 years O'Halloran 2014). All studies included men and women with venous leg ulcers. Only Vanscheidt 2012 included participants with a wound infection. One study was conducted in a dermatology department outpatient setting in Brazil (Borges 2018), one was undertaken across four sites covering community and general practice in the UK (O'Halloran 2014), one was conducted in a dermatology department outpatient setting in Italy (Romanelli 2010) and one was undertaken in surgical and outpatient wound care clinics and private dermatology practices in four European countries: Germany, France, Hungary and the UK (Vanscheidt 2012).
Funding
Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (a state research organisation) funded Borges 2018 and B. Braun Medical AG, Switzerland funded Romanelli 2010. Neither O'Halloran 2014 nor Vanscheidt 2012 provided information about funding source.
Interventions and comparisons
In Borges 2018, the intervention was a solution containing polyhexamethylene biguanide (PHMB) and 0.9% saline solution used to irrigate the wound. The intervention group had their wounds cleansed with PHMB, and the control group had their wounds cleansed with a 0.9% saline solution. The irrigation of the wound was performed for 1 minute under continuous pressure with the cleansing solution. The bottles with identical labels in order to fulfil 2 blinding procedures were punctured with a 21‐G needle to obtain a continuous jet that provides an irrigation force of approximately 13.5 pound per square inch (psi).
In O'Halloran 2014 the intervention was aqueous oxygen peroxide (AOP) and the control was sterile water. AOP is made by entraining (carrying suspended particles, for example, along in a current or into the vapour phase) oxygen peroxide into water. The AOP group received 20ppm AOP lavage for 15 minutes at each treatment. The control group received sterile water delivered using a sham unit for 15 minutes at each treatment. In both groups, participants received their allocated treatment three times a week for two weeks.
In Romanelli 2010, the intervention was a solution containing propyl betaine and polihexanide applied to the wound every other day. The control group received wound cleansing with sterile saline every other day.
In Vanscheidt 2012 the intervention was a topical antiseptic agent octenidine dihydrochloride/phenoxyethanol (OHP). In the control group, wounds were treated with Ringer's solution (containing sodium chloride, potassium chloride, calcium chloride and sodium bicarbonate) as standard wound cleansing. OHP or Ringer solution was applied to the ulcer wound by spray in a blinded setting until completely moistened at each dressing change, which was conducted up to a maximum of three times a week, but at least once a week according to the healing stage of the target ulcer.
Outcomes
Borges 2018 did not report any of this review's primary or secondary outcomes. Two studies included wound size reduction and pain as outcomes (O'Halloran 2014; Romanelli 2010). O'Halloran 2014 also included the number of wounds completely healed as an outcome and Romanelli 2010 also reported adverse events. Vanscheidt 2012 included the number of wounds completely healed, wound size reduction and adverse events as outcomes.
Excluded studies
We excluded a total of 17 studies. Six studies involved the use of dressings, beads, powder and medication, not cleansing agents (Carneiro 2003; Groenewald 1980; Hammerle 2016; Milward 1991; Werner‐Schlenzka 1994; Westerhof 1987). Five studies included a mixed wound population and did not provide outcome data for venous leg ulcer participants alone (Bass 2007; Bellingeri 2004; Bellingeri 2016; Colombo 1993; Griffiths 2001). Five studies were not RCTs (Andriessen 2008; Bravo Bernabé 1972; Selim 2000; Sopata 2014; Steenvoorde 2007). Finally, one study did not involve an adult population (Ennis 2012). See the Characteristics of excluded studies table for details.
Risk of bias in included studies
See Figure 2 for the risk of bias summary and Figure 3 for the risk of bias graph for the four included studies. One of the studies had inadequate reporting, which limited our assessment of potential bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All four studies used random sequence generation. However, the allocation concealment was unclear in Romanelli 2010 and Vanscheidt 2012 , and the method of randomisation was also unclear in Vanscheidt 2012. Study groups in all studies were comparable at baseline.
Blinding
Three of the studies blinded the participants, personnel and outcome investigators, so we judged these studies to be at low risk of bias for this domain (Borges 2018; O'Halloran 2014; Vanscheidt 2012). It is unclear whether Romanelli 2010 undertook any blinding.
Incomplete outcome data
Borges 2018 enrolled 44 people, but only 27 participants completed the trial and the study did not provide data for the missing 17 participants. There were 28 participants in the intervention group and 33 participants in the control group in O'Halloran 2014, however, the study presented some of the data as 'per protocol', where 18 participants remained in the intervention group and 17 in the control group. Two of the participants in Romanelli 2010 did not complete the study. However, as the study did not provide data specifically for each group, it is unclear whether intention‐to‐treat ( ITT) analysis was undertaken. Data were missing for 11 participants in the intervention group and 16 participants in the control group in Vanscheidt 2012. We judged all studies to be at high risk of bias for this domain.
Selective reporting
Three studies reported all planned primary and secondary outcomes (O'Halloran 2014; Romanelli 2010; Vanscheidt 2012). However, Romanelli 2010 did not provide specific data for all outcomes, so we judged Romanelli 2010 to be at high risk of bias for this domain. Vanscheidt 2012 did not report all outcomes for all participants at 12 weeks, as outlined in the trial registration, so we judged Vanscheidt 2012 to be at unclear risk of bias for this domain. Although Borges 2018 reported the outcomes of interest to the study authors (bacterial load and bacterial biofilm), the study did not report the primary outcomes of interest for this review, time to complete healing and number of wounds healed, or the secondary outcomes: patient preference, ease of use of the method of cleansing, cost and health‐related quality of life.
Other potential sources of bias
We did not detect any other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Polyhexamethylene biguanide (PHMB) versus 0.9% saline solution.
| PHMB versus 0.9% saline solution for treatment of venous leg ulcers | ||||||
|
Participant or population: participants with venous leg ulcers Settings: dermatology outpatient clinic Intervention: PHMB Comparison: 0.9% saline solution | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 0.9% saline solution | PHMB | |||||
| Time to healing | Not reported | |||||
| Number of wounds healed | Not reported | |||||
| Change in ulcer size over time | Not reported | |||||
| Pain | Not reported | |||||
| Cost | Not reported | |||||
| Health related quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| CI: confidence interval; PHMB: polyhexamethylene biguanide | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
Summary of findings 2. Aqueous oxygen peroxide versus sterile water.
| Aqueous oxygen peroxide versus sterile water for treatment of venous leg ulcers | ||||||
|
Participant or population: participants with venous leg ulcers
Settings: community and general practice settings
Intervention: aqueous oxygen peroxide (AOP) Comparison: sterile water | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sterile water | AOP | |||||
| Time to healing | Not reported | |||||
| Number of wounds healed ‐ per protocol ‐ after 12 months of follow‐up | Study population | RR 1.88 (1.10 to 3.20) | 34 (1 study) | ⊕⊝⊝⊝ Very lowa | It is uncertain whether, after 12 months of follow‐up, AOP results in a difference in wounds healed when compared with sterile water. | |
| 471 per 1000 | 885 per 1000 (518 to 1000) | |||||
| Change in ulcer size (surface area changes in cm2 from baseline) ‐ ITT ‐ after 8 weeks of follow‐up | Study population | 61 (1 study) | ⊕⊝⊝⊝ Very lowa | It is uncertain whether, after 8 weeks of follow up, AOP leads to wound size reduction when compared with sterile water . | ||
| The mean wound size reduction was: 4.84 cm2 (SD:6.16) | The mean wound size reduction in the intervention group was 1.38 cm2 lower (‐4.35 lower to 1.59 higher) | |||||
| Procedural pain (pain measured using a 0 to 10 cm Likert scale, which gives a 0–100 pain rating (0 = no pain, 100 = very painful) ‐ ITT ‐ after 8 weeks of follow‐up | Study population | 61 (1 study) | ⊕⊝⊝⊝ Very lowb | It is uncertain whether, after 8 weeks of follow‐up, AOP leads to pain reduction when compared with sterile water. | ||
| The mean pain reduction was: 13.6 (SD: 26.45) | The mean pain reduction in the intervention group was 3.80 higher (‐10.83 lower to 18.43 higher) | |||||
| Cost | Not reported | |||||
| Health related quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AOP: aqueous oxygen peroxide;CI: Confidence interval; RR: Risk ratio; SD: standard deviation; ITT: Intention‐to‐treat | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded once for serious limitations due to high risk of attrition bias and twice for serious imprecision due to wide confidence intervals and small sample size.
bDowngraded once for serious limitations due to high risk of attrition bias, twice for serious imprecision due to wide confidence intervals and small sample size, once for indirectness due to use of a non‐validated instrument to measure pain.
Summary of findings 3. Propyl betaine and polihexanide versus saline solution.
| Propyl betaine and polihexanide versus saline solution | ||||||
|
Participant or population: participants with chronic leg ulcers Settings: outpatients wound clinic Intervention: propyl betaine and polihexanide Comparison: saline solution | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Saline solution | Propyl betaine and polihexanide | |||||
| Time to healing | Not reported | |||||
| Number of wounds healed | Not reported | |||||
| Change in ulcer size after 4 weeks of follow‐up (surface area changes in cm2 from baseline) | 38 participants (1 study) | ⊕⊝⊝⊝ Very lowa | It is uncertain whether, after 4 weeks of follow‐up, propyl betaine and polihexanide leads to wound size reduction when compared with a saline solution. | |||
| Procedural pain after 4 weeks of follow‐ up (self‐assessed, subjectively, using a validated 10‐mm visual analogue scale) | 38 participants (1 study) | ⊕⊝⊝⊝ Very lowa | It is uncertain, after 4 weeks of follow‐up, whether propyl betaine and polihexanide leads to pain reduction when compared with a saline solution. | |||
| Cost | Not reported | |||||
| Health related quality of life | Not reported | |||||
| Adverse events after 4 weeks of follow‐up | 38 participants (1 study) | ⊕⊝⊝⊝ Very lowa | It is unclear if there are differences in serious or unexpected adverse events between groups after 4 weeks of follow‐up. The authors do not report any events occurring. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded twice for very serious limitations due to high risk of attrition and reporting bias, and unclear risk of allocation, performance and detection bias, once for imprecision due to small sample size.
Summary of findings 4. OHP versus Ringer's solution for treating venous leg ulcers.
| OHP versus Ringer's solution for treating venous leg ulcers | ||||||
|
Participant or population: participants with treating venous leg ulcers
Settings: an outpatient wound clinic
Intervention: OHP Comparison: Ringer's solution | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ringer's Solution | OHP | |||||
| Time to complete wound healing | Not reported | |||||
| Number of wounds healed after 12 weeks of follow‐up | Study population | RR 0.96 (0.53 to 1.72) | 99 (1 study) | ⊕⊝⊝⊝ Very lowa | We are uncertain whether, after 12 weeks of follow‐up, OHP makes any difference to the number of wounds healed. | |
| 320 per 1000 | 307 per 1000 (170 to 550) | |||||
| Change in ulcer size over time (cm2) after week 12 of follow‐up | 37·90% (−2·53 cm2) reduction | 40·30% (−2·81 cm2) | 99 (1 study) | ⊕⊝⊝⊝ Very lowb | We are uncertain whether, after 12 weeks of follow‐up, OHP makes any difference to change in ulcer size. | |
| Procedural pain | Not reported | |||||
| Cost | Not reported | |||||
| Health related quality of life | Not reported | |||||
| Adverse events after week 12 of follow‐up | Study population | RR 0.58 (0.29 to 1.14) | 126 (1 study) |
⊕⊝⊝⊝ Very lowa | We are uncertain whether, after 12 weeks of follow‐up, OHP makes any difference to the number of adverse events. | |
| 288 per 1000 | 167 per 1000 (83 to 328) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OHP: octenidine dihydrochloride/phenoxyethanol; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded twice for very serious limitations due to high risk of attrition bias and unclear risk of selection bias, and twice for imprecision due to small number of events and wide confidence intervals. bDowngraded twice for very serious limitations due to high risk of attrition bias and unclear risk of selection bias, and once for imprecision due to small sample size.
Cleansing compared with no cleansing
We did not identify any studies for this comparison.
Types of cleansing solutions
Comparison 1: polyhexamethylene biguanide (PHMB) versus 0.9% saline solution
We identified one study with 27 participants for this comparison (Borges 2018) See Table 1.
Primary outcome: time to complete wound healing, number of wounds completely healed or change in ulcer size over time
Borges 2018 did not report these outcomes.
Secondary outcomes
Borges 2018 did not report any of the secondary outcomes: patient preference, procedural pain, ease of use of the method of cleansing, cost, health‐related quality of life or adverse events.
Comparison 2: aqueous oxygen peroxide treatment versus sterile water
One study with 61 participants reported this comparison (O'Halloran 2014 ). See Table 2.
Primary outcome: time to complete wound healing
O'Halloran 2014 did not report this outcome.
Primary outcome: number of wounds completely healed
O'Halloran 2014 analysed data per protocol, at 8 and 12 weeks, and at 6 and 12 months. We report outcomes at the latest time point available. We are uncertain whether aqueous oxygen peroxide makes any difference to the number of wounds completely healed after 12 months follow‐up (RR 1.88, 95% CI 1.10 to 3.20; 34 participants; Analysis 1.1). The certainty of the evidence is very low (we downgraded once for serious limitations due to high risk of attrition bias and twice for serious imprecision due to wide confidence intervals and small sample size).
1.1. Analysis.

Comparison 1: AOP versus Sterile Water, Outcome 1: Wounds Healed ‐ Per Protocol ‐12 Months
Primary outcome: change in ulcer size over time
O'Halloran 2014, analysed data for the outcome 'change in ulcer size'on an ITT basis, with the last observed data carried forward to deal with missing data. The study analysed data at two, four, six and eight weeks. We report outcomes at eight weeks. We are uncertain whether aqueous oxygen peroxide makes any difference to wound size reduction after eight weeks of follow‐up (MD ‐1.38 cm2, 95% CI ‐4.35 to 1.59 cm2; 61 participants; Analysis 1.2). The certainty of the evidence is very low (we downgraded once for serious limitations due to high risk of attrition bias and twice for serious imprecision due to wide confidence intervals and small sample size).
1.2. Analysis.

Comparison 1: AOP versus Sterile Water, Outcome 2: Wound Size Reduction (cm2) ‐ 8 Weeks
Secondary outcome: pain
O'Halloran 2014 used ITT analysis with the last observed data carried forward to deal with missing data for the outcome 'pain'. The trialists collected pain scores at two, four, six and eight weeks, using a 0 to 10 cm Likert scale, which gives a 0 to 100 pain rating (where 0 = no pain, 100 = very painful). We report outcomes after eight weeks of follow‐up. We are uncertain whether aqueous oxygen peroxide makes any difference to overall pain at eight weeks between groups (MD 3.80, 95% CI ‐10.83 to 18.43; 61 participants; Analysis 1.3). The certainty of this evidence is very low (we downgraded once for serious limitations due to high risk of attrition bias, twice for serious imprecision due to wide confidence intervals and small sample size, once for indirectness due to use of a non‐validated instrument to measure pain).
1.3. Analysis.

Comparison 1: AOP versus Sterile Water, Outcome 3: Pain ‐ ITT ‐ 8 Weeks
O'Halloran 2014 did not report the other secondary outcomes: patient preference, ease of use of the method of cleansing, cost, health‐related quality of life or adverse events.
Comparison 3: propyl betaine and polihexanide versus saline solution
We included one study with 40 participants for this comparison (Romanelli 2010). See Table 3.
Primary outcome: time to complete wound healing or number of wounds completely healed
Romanelli 2010 did not report either of these outcomes.
Primary outcome: change in ulcer size over time
Romanelli 2010 measured wound area after four weeks of follow‐up, using planimetry software. The study reported insufficient raw data for us to be able to conduct independent statistical analysis; although we contacted the author, we did not get a response. We are uncertain whether propyl betaine and polihexanide makes any difference to the change in ulcer size over time, as measured using ulcer planimetry. The certainty of the evidence is very low (we downgraded twice for very serious limitations due to high risk of attrition and reporting bias, and unclear risk of allocation, performance and detection bias, and once for imprecision due to small sample size).
Secondary outcome: pain
In Romanelli 2010 participants self‐assessed pain subjectively after four weeks of follow‐up, rating the intensity of pain on a validated 10‐mm visual analogue scale. The study reported insufficient raw data for us to be able to conduct independent statistical analysis; although we contacted the author, we did not get a response. We are uncertain whether propyl betaine and polihexanide makes any difference to wound pain reduction. The certainty of the evidence is very low (we downgraded twice for very serious limitations due to high risk of attrition and reporting bias, and unclear risk of allocation, performance and detection bias, and once for imprecision due to small sample size).
Secondary outcomes: adverse events
In Romanelli 2010, the authors do not report any events occurring. It is unclear if there are differences in serious or unexpected adverse events between groups after four weeks of follow‐up. The certainty of the evidence is very low (we downgraded twice for very serious limitations due to high risk of attrition and reporting bias, and unclear risk of allocation, performance and detection bias, and once for imprecision due to small sample size).
The study did not report our other secondary outcomes: patient preference, ease of use of the method of cleansing, cost or health‐related quality of life.
Comparison 4: Octenidine dihydrochloride/phenoxyethanol (OHP) versus Ringer's solution
One study with 126 participants reported this comparison. See Table 4.
Primary outcome: time to complete wound healing
Vanscheidt 2012 did not report this outcome.
Primary outcome: number of wounds completely healed
From Vanscheidt 2012, we are uncertain whether OHP makes any difference to the number of wounds healed after 12 weeks of follow‐up (RR 0.96, 95% CI 0.53 to 1.72; 99 participants; Analysis 2.1). The certainty of this evidence is very low (we downgraded twice for very serious limitations due to high risk of attrition bias and unclear risk of selection bias, and twice for imprecision due to the small number of events and wide confidence intervals).
2.1. Analysis.

Comparison 2: OHP versus Ringer's Solution, Outcome 1: Wound healing
Primary outcome: change in ulcer size over time
Vanscheidt 2012 also reported change in ulcer size over time. The study authors reported that the mean wound surface area between baseline and the end of the observation period decreased by about 37 to 90% (−2·53 cm2) in the OHP group versus 40 to 30% (−2·81cm2) in the Ringer's solution group. We were unable to analyse these data further. We are uncertain whether after 12 weeks of follow‐up, OHP makes any difference to the change in ulcer size over time as the certainty of this evidence is very low (we downgraded twice for very serious limitations due to high risk of attrition bias and unclear risk of selection bias, and once for imprecision due to the small sample size).
Secondary outcomes: adverse events
In Vanscheidt 2012, the adverse events included: application site pruritus, irritation after each spray application and pain at the target ulcer. The study did not describe the method used to assess pain. We are uncertain whether OHP makes any difference to the number of adverse events after 12 weeks of follow‐up (RR 0.58, 95% CI 0.29 to 1.14; 126 participants; Analysis 2.2). The certainty of this evidence is very low (we downgraded twice for very serious limitations due to high risk of attrition bias and unclear risk of selection bias, and twice for imprecision due to the small number of events and wide confidence intervals).
2.2. Analysis.

Comparison 2: OHP versus Ringer's Solution, Outcome 2: Adverse event
Vanscheidt 2012 did not report our other secondary outcomes: pain, patient preference, ease of use of the method of cleansing, cost or health‐related quality of life.
Different cleansing techniques
We did not identify any studies for this comparison.
Discussion
Summary of main results
We did not identify any RCTs that compared wound cleansing with no wound cleansing or any CCTS that compared different techniques of wound cleansing for venous leg ulcers. We identified four RCTs that compared different solutions for wound cleansing of venous leg ulcers. One study compared PHMB with 0.9% saline solution, one compared aqueous oxygen peroxide with water, one compared propyl betaine and polihexanide with saline, and one compared OHP with Ringer's solution.
From the four studies identified, there is insufficient evidence to demonstrate whether the use of PHMB solution compared with saline solution, aqueous oxygen peroxide compared with sterile water, propyl betaine and polihexanide compared with a saline solution, or OHP compared with Ringer's solution makes any difference in the treatment of venous leg ulcers. In three of the comparisons we assessed the evidence to be of very low certainty, due to study limitations and imprecision. One study did not present data for the review's primary or secondary outcomes.
Overall completeness and applicability of evidence
All studies recruited participants typical of the majority of people with venous leg ulceration. Furthermore, three studies used compression therapy as an additional treatment modality, which is regarded as best practice within evidence‐based practice guidelines worldwide (AWMA 2011; HSE 2018; O'Donnell 2014; SIGN 2010). However, the types of cleansing solutions may not be representative of the wide range of cleansing solutions in use. There are significant weakness in the completeness and applicability of the evidence overall. We found no studies comparing cleansing with no cleansing, so there is no evidence to answer the fundamental question of whether cleansing wounds is beneficial per se. No study examined patient preference, ease of use of the methods of cleansing, cost or health related quality of life. These are key issues that should be addressed to ensure patient comfort and ensure cost‐effectiveness, allowing clinicians to make informed decisions.
These studies are small, and we only identified one study for each comparison. The very low certainty of the evidence makes it impossible to draw conclusions confidently.
Quality of the evidence
The certainty of the evidence was very low, and in most cases we downgraded it for serious risk of bias and imprecision.
Risk of Bias
Two of the studies kept the risk of bias low for the relevant domains by conducting random sequence generation, maintaining allocation concealment, reducing performance bias by blinding the participants and the personnel, and reducing detection bias by blinding the outcome assessor (Borges 2018; O'Halloran 2014). Two studies had unclear elements (Romanelli 2010; Vanscheidt 2012). For example, there was no indication that these studies concealed allocation. All of the studies lost some participants to follow‐up, leading to a high risk of attrition bias.
There are other methodological issues with O'Halloran 2014 that impact on the precision of the findings. O'Halloran 2014 had a large number of withdrawals from both groups: 36% (10 participants) from the intervention group and 45% (15 participants) from the control group. Both of these figures are above the limit of 30% withdrawal that the trial authors had accounted for in their sample size calculations. Therefore, attrition bias within this study was high. Furthermore, analysis for wounds completely healed was conducted 'per protocol' based on the 35 participants who completed the follow‐up and not the 61 participants who were originally randomly allocated to the study groups. Due to this, results should be interpreted with caution because 'per protocol' analysis tends to favour the intervention (Bland 2015). Nüesch 2009 examined whether excluding participants from the analysis of randomised studies is associated with biased estimates of treatment effects, and found that this was often the case. Since 'per protocol' analysis is at high risk of bias, as it tends to exaggerate the outcome effects (Bland 2015; Higgins 2011a), results from the O'Halloran 2014 study should be interpreted with caution.
Indirectness
All studies ensured that the participants in the study reflected the wider population of individuals with venous leg ulcers. Furthermore, the demographic data in both the intervention group and the control groups were similar in all studies. Three studies used compression. This reflects the treatment recommendations for the wider population of individuals with venous leg ulcers. O'Halloran 2014 used a non‐validated pain instrument to assess pain scores, so we downgraded the certainty of the evidence for indirectness.
Imprecision
The studies had small sample sizes, and there were few events. These elements gave rise to wide confidence intervals within the results for the study outcomes (Button 2013). As a result, we downgraded the certainty of the evidence for imprecision.
Inconsistency
Because the studies included in this review explored different interventions and comparisons, we did not undertake meta‐analysis. Therefore, as we did not measure heterogeneity, we did not downgrade any of the evidence for inconsistency (Guyatt 2011).
We could not assess publication bias because there was not enough evidence to assess it.
Potential biases in the review process
During the review process, we followed clearly defined processes to prevent potential bias. We feel confident that our electronic searches were comprehensive in identifying all existing, published RCTs relating to the review question. It is possible that we did not manage to locate some studies that may have been eligible. This review will be updated in the future, and any studies identified that meet the inclusion criteria will be included at that stage, in line with Cochrane policy.
Agreements and disagreements with other studies or reviews
There is a Cochrane Review that addresses cleansing in the treatment of pressure ulcers (Moore 2013). Moore 2013 reached similar conclusions to this review, in that review authors found little RCT evidence available pertaining to wound cleansing for pressure ulcers.
Authors' conclusions
Implications for practice.
We found no randomised controlled trials (RCTs) comparing the effects of wound cleansing for venous leg ulcers with no cleansing, nor did we find any RCTs comparing difference techniques for wound cleansing of venous ulcers. From the four studies identified, there is insufficient evidence to demonstrate whether the use of polyhexamethylene biguanide (PHMB) solution compared with saline solution, aqueous oxygen peroxide compared with sterile water, propyl betaine and polihexanide compared with a saline solution, or octenidine dihydrochloride/phenoxyethanol (OHP) compared with Ringer's solution makes any difference in the treatment of venous leg ulcers. Wound cleansing is considered to be an integral component of venous leg ulcer management and is recommended in many international guidelines; however, there is a lack of evidence surrounding the value of wound cleansing compared with no cleansing or the best method or solution to use to guide current practice (Trevelyan 1996). In the absence of evidence to support the use of particular cleansing solutions or techniques, clinical care should be informed by current high quality national guidelines, costs and patients’ preferences.
Implications for research.
A number of international guidelines for leg ulcers recommend cleansing of both the leg and the ulcer, and most suggest cleansing with potable water. However, the evidence base for cleansing venous leg ulcers is limited. As cleansing with potable water is the current practice in most clinical settings, and the evidence surrounding this is uncertain, this remains a priority for research. Furthermore, with the introduction of a number of wound irrigation solutions to clinical practice, it is important to conduct research to explore the clinical and cost effectiveness of these solutions. Future research should focus on cleansing compared with no cleansing, comparisons between different types of cleansing solutions (including potable water), and comparisons between different cleansing techniques.
History
Protocol first published: Issue 4, 2015 Review first published: Issue 3, 2021
Acknowledgements
The authors would like to acknowledge the contribution of the following peer reviewers who provided feedback (including consumer peer review comments): Andrew Jull, Susanne Hempel, Ritin Fernandez and Anita Raspovic, who provided feedback on the protocol; Una J Adderley and Janet Wale, who provided feedback on both the protocol and the review; and Gill Norman who provided feedback on the review. Thanks also to Clare Dooley and Andrea Takeda for copy editing the protocol and the review respectively and to Nicole Pitcher for writing the Plain Language Summary for the review.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Leg Ulcer EXPLODE ALL AND INREGISTER
2 ((varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or "ulcus cruris" or "ulcer* cruris") AND INREGISTER
3 #1 or #2 AND INREGISTER
4 MESH DESCRIPTOR Sodium Chloride EXPLODE ALL AND INREGISTER
5 MESH DESCRIPTOR Sodium Hypochlorite EXPLODE ALL AND INREGISTER
6 MESH DESCRIPTOR Saline Solution, Hypertonic EXPLODE ALL AND INREGISTER
7 MESH DESCRIPTOR Iodophors EXPLODE ALL AND INREGISTER
8 MESH DESCRIPTOR Chlorhexidine EXPLODE ALL AND INREGISTER
9 MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL AND INREGISTER
10 MESH DESCRIPTOR Disinfectants EXPLODE ALL AND INREGISTER
11 MESH DESCRIPTOR Detergents EXPLODE ALL AND INREGISTER
12 MESH DESCRIPTOR Soaps EXPLODE ALL AND INREGISTER
13 MESH DESCRIPTOR Hydrogen Peroxide EXPLODE ALL AND INREGISTER
14 MESH DESCRIPTOR Benzoyl Peroxide EXPLODE ALL AND INREGISTER
15 MESH DESCRIPTOR Gentian Violet EXPLODE ALL AND INREGISTER
16 MESH DESCRIPTOR Water EXPLODE ALL AND INREGISTER
17 MESH DESCRIPTOR Alcohols EXPLODE ALL AND INREGISTER
18 MESH DESCRIPTOR Solutions EXPLODE ALL AND INREGISTER
19 (normal next saline) or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic* or disinfectant* or detergent* or soap* or (hydrogen next peroxide) or (benzoyl next peroxide) or (gentian next violet) or eusol or dakin* or permanganate or water or alcohol or alcohols or solution* AND INREGISTER
20 MESH DESCRIPTOR Therapeutic Irrigation EXPLODE ALL AND INREGISTER
21 MESH DESCRIPTOR Baths EXPLODE ALL AND INREGISTER
22 MESH DESCRIPTOR Hydrotherapy EXPLODE ALL AND INREGISTER
23 (wound next clean*) or (wound next cleans*) AND INREGISTER
24 wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool AND INREGISTER
25 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 AND INREGISTER
26 #3 AND #25 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Leg Ulcer] explode all trees
#2 ((varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or "ulcus cruris" or "ulcer* cruris"):ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Sodium Chloride] explode all trees
#5 MeSH descriptor: [Sodium Hypochlorite] explode all trees
#6 MeSH descriptor: [Saline Solution, Hypertonic] explode all trees
#7 MeSH descriptor: [Iodophors] explode all trees
#8 MeSH descriptor: [Chlorhexidine] explode all trees
#9 MeSH descriptor: [Anti‐Infective Agents, Local] explode all trees
#10 MeSH descriptor: [Disinfectants] explode all trees
#11 MeSH descriptor: [Detergents] explode all trees
#12 MeSH descriptor: [Soaps] explode all trees
#13 MeSH descriptor: [Hydrogen Peroxide] explode all trees
#14 MeSH descriptor: [Benzoyl Peroxide] explode all trees
#15 MeSH descriptor: [Gentian Violet] explode all trees
#16 MeSH descriptor: [Water] explode all trees
#17 MeSH descriptor: [Alcohols] explode all trees
#18 MeSH descriptor: [Solutions] explode all trees
#19 "normal saline" or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic* or disinfectant* or detergent* or soap* or "hydrogen peroxide" or "benzoyl peroxide" or "gentian violet" or eusol or dakin* or permanganate or water or "alcohol" or alcohols or solution*
#20 MeSH descriptor: [Therapeutic Irrigation] explode all trees
#21 MeSH descriptor: [Baths] explode all trees
#22 MeSH descriptor: [Hydrotherapy] explode all trees
#23 ((wound next clean*) or (wound next cleans*)):ti,ab,kw
#24 (wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool):ti,ab,kw
#25 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 #3 and #25 in Trials
Ovid MEDLINE
1 exp Leg Ulcer/
2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw.
3 1 or 2
4 exp Sodium Chloride/
5 exp Sodium Hypochlorite/
6 exp Saline Solution, Hypertonic/
7 exp Iodophors/
8 exp Chlorhexidine/
9 exp Anti‐Infective Agents, Local/
10 exp Disinfectants/
11 exp Detergents/
12 exp Soaps/
13 exp Hydrogen Peroxide/
14 exp Benzoyl Peroxide/
15 exp Gentian Violet/
16 exp Water/
17 exp Alcohols/
18 exp Solutions/
19 (normal saline or hypochlorit$ or iodophor$ or povidone or iodine or chlorhexidine or hibitane or betadine or disinfectant$ or antiseptic$ or detergent$ or soap$ or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin$ or permanganate or water or alcohol$1 or solution$).tw.
20 exp Therapeutic Irrigation/
21 exp Baths/
22 exp Hydrotherapy/
23 (wound clean$ or wound cleans$).tw.
24 (wash$ or scrub$ or swab$ or shower$ or bath$ or soak$ or irrigat$ or whirlpool).tw.
25 or/4‐24
26 and/3,25
27 randomized controlled trial.pt.
28 controlled clinical trial.pt.
29 randomi?ed.ab.
30 placebo.ab.
31 clinical trials as topic.sh.
32 randomly.ab.
33 trial.ti.
34 or/27‐33
35 exp animals/ not humans.sh.
36 34 not 35
37 26 and 36
Ovid Embase
1 leg ulcer/
2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw.
3 1 or 2
4 sodium chloride/
5 hypochlorite sodium/
6 iodophor/
7 povidone iodine/
8 chlorhexidine/
9 exp topical antiinfective agent/
10 exp disinfectant agent/
11 detergent/
12 soap/
13 hydrogen peroxide/
14 benzoyl peroxide/
15 crystal violet/
16 exp water/
17 exp alcohol derivative/
18 alcohol/
19 exp "solution and solubility"/
20 (normal saline or hypochlorit$ or iodophor$ or povidone or iodine or chlorhexidine or hibitane or betadine or disinfectant$ or antiseptic$ or detergent$ or soap$ or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin$ or permanganate or water or alcohol$1 or solution$).tw.
21 lavage/
22 wound irrigation/
23 bath/
24 hydrotherapy/
25 (wound clean$ or wound cleans$).tw.
26 (wash$ or scrub$ or swab$ or shower$ or bath$ or soak$ or irrigat$ or whirlpool).tw.
27 or/4‐26
28 and/3,27
29 Randomized controlled trials/
30 Single‐Blind Method/
31 Double‐Blind Method/
32 Crossover Procedure/
33 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
34 (doubl* adj blind*).ti,ab.
35 (singl* adj blind*).ti,ab.
36 or/29‐35
37 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
38 human/ or human cell/
39 and/37‐38
40 37 not 39
41 36 not 40
42 28 AND 41
EBSCO CINAHL Plus
S49 S25 AND S48
S48 S47 NOT S46
S47 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40
S46 S44 NOT S45
S45 MH (human)
S44 S41 OR S42 OR S43
S43 TI (animal model*)
S42 MH (animal studies)
S41 MH animals+
S40 AB (cluster W3 RCT)
S39 MH (crossover design) OR MH (comparative studies)
S38 AB (control W5 group)
S37 PT (randomized controlled trial)
S36 MH (placebos)
S35 MH (sample size) AND AB (assigned OR allocated OR control)
S34 TI (trial)
S33 AB (random*)
S32 TI (randomised OR randomized)
S31 MH cluster sample
S30 MH pretest‐posttest design
S29 MH random assignment
S28 MH single‐blind studies
S27 MH double‐blind studies
S26 MH randomized controlled trials
S25 S3 AND S24
S24 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23
S23 TI ( wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool ) OR AB ( wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool )
S22 TI ( wound clean* or wound cleans* ) OR AB ( wound clean* or wound cleans* )
S21 (MH "Hydrotherapy")
S20 (MH "Bathing and Baths")
S19 (MH "Therapeutic Irrigation")
S18 TI ( normal saline or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or disinfectant* or antiseptic* or detergent* or soap* or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin* or permanganate or water or alcohol* or solution* ) OR AB ( normal saline or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or disinfectant* or antiseptic* or detergent* or soap* or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin* or permanganate or water or alcohol* or solution* )
S17 (MH "Solutions+")
S16 (MH "Alcohols+")
S15 (MH "Water+")
S14 (MH "Gentian Violet")
S13 (MH "Hydrogen Peroxide")
S12 (MH "Soaps")
S11 (MH "Detergents+")
S10 (MH "Disinfectants")
S9 (MH "Antiinfective Agents, Local+")
S8 (MH "Chlorhexidine")
S7 (MH "Iodophors+")
S6 (MH "Saline Solution, Hypertonic")
S5 (MH "Sodium Hypochlorite")
S4 (MH "Sodium Chloride+")
S3 S1 OR S2
S2 TI ( varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcer cruris ) OR AB ( varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcer cruris )
S1 (MH "Leg Ulcer+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
saline OR hypochlorit OR iodophor OR povidone OR iodine OR chlorhexidine OR hibitane OR betadine OR disinfectant OR antiseptic OR detergent OR wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse | Venous Leg Ulcer
saline OR hypochlorit OR iodophor OR povidone OR iodine OR chlorhexidine OR hibitane OR betadine OR disinfectant OR antiseptic OR detergent OR wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse | Ulcer Venous
saline OR hypochlorit OR iodophor OR povidone OR iodine OR chlorhexidine OR hibitane OR betadine OR disinfectant OR antiseptic OR detergent OR wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse | Varicose Ulcer
saline OR hypochlorit OR iodophor OR povidone OR iodine OR chlorhexidine OR hibitane OR betadine OR disinfectant OR antiseptic OR detergent OR wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse | Venous Stasis Ulcer
saline OR hypochlorit OR iodophor OR povidone OR iodine OR chlorhexidine OR hibitane OR betadine OR disinfectant OR antiseptic OR detergent OR wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse | Venous Ulceration
World Health Organization International Clinical Trials Registry Platform
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline | Venous Leg Ulcer [Title]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline| Venous Leg Ulcer [Condition]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I Ulcer venous [Title]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I Ulcer venous [Condition]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I Varicose Ulcer [Title]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I Varicose Ulcer [Condition]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I venous stasis ulcer [Title]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I venous stasis ulcer [Condition]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I venous ulceration [Title]
wash OR scrub OR swab OR shower OR bath OR soak or irrigate OR irrigation OR whirlpool OR clean OR cleanse OR saline I venous ulceration [Condition]
Appendix 2. Cochrane 'Risk of bias' assessment tool
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias to be made.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information available to permit judgement of low or high risk of bias to be made. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
• No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
• Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
• Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following:
• No blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding.
• Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
• Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following:
• Insufficient information available to permit judgement of low or high risk of bias to be made.
• The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
• No missing outcome data.
• Reasons for missing outcome data were unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
• Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
• For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
• For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on observed effect size.
• Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
• Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
• For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in intervention effect estimate.
• For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce clinically relevant bias in observed effect size.
• ‘As‐treated’ analysis done with substantial departure in the intervention received from that assigned at randomisation.
• Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
• Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
• The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
• The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
• The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
• Not all of the study’s prespecified primary outcomes have been reported.
• One or more of the primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
• One or more of the reported primary outcomes was not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
• One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
• The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information is available to permit a judgement of low or high risk of bias to be made. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
• had a potential source of bias related to the specific study design used; or
• had extreme baseline imbalance; or
• has been claimed to have been fraudulent; or
• had some other problem.
Unclear
There may be a risk of bias, but there is either:
• insufficient information to assess whether an important risk of bias exists; or
• insufficient evidence that an identified problem will introduce bias
Data and analyses
Comparison 1. AOP versus Sterile Water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Wounds Healed ‐ Per Protocol ‐12 Months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Wound Size Reduction (cm2) ‐ 8 Weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Pain ‐ ITT ‐ 8 Weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. OHP versus Ringer's Solution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Wound healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Borges 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled study Setting: a dermatology outpatient clinic |
|
| Participants |
Sample size: n = 27 Loss to follow‐up: n = 3 from the control group and n =14 from the intervention group Inclusion criteria: adult patients with venous ulcers confirmed by medical diagnosis and clinical signs of venous insufficiency (oedema, varicose veins, hyperpigmentation, lipodermatosclerosis, and ankle‐brachial pressure index between 0.8 and 1.3); wound at least 8 weeks’ duration; area greater than 6 cm2; no clinical signs of infection; and not being treated with systemic or topical antibiotics and antiseptics to the wound in the week prior to the enrolment. Exclusion criteria: pregnancy; cancer; immunosuppressive diseases; diabetes mellitus; use of immunosuppressant drugs; and radiotherapy. Age: mean age of control group 55.9 years (range 34 to 90), mean age of intervention group 60.9 years (range 33 to 84). Sex: 9 males, 18 females |
|
| Interventions | In the treatment arm, participants had their venous leg ulcers cleansed with polyhexamethylene biguanide (PHMB); In the control arm, participants had their venous leg ulcers cleansed with 0.9% saline solution The frequency of application of the intervention was not reported. |
|
| Outcomes | Length of follow‐up: no follow up Outcomes: Primary:
Secondary: None measured |
|
| Notes | Funding source: supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed with a random number table in a horizontal sequence from left to right.” |
| Allocation concealment (selection bias) | Low risk | “If the last digit was even, the wound was assigned to the control group and if it was odd, then it was assigned to the PHMB group.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The bottles with identical labels in order to fulfil 2 blinding procedures (the patient and the researcher did not know which solution was used) were punctured with a 21‐G needle to obtain a continuous jet that provides an irrigation force of approximately 13.5 pound per square inch (psi).” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Concealment or blinding was performed whenever possible; this included the dermatologist, who removed wound tissue fragments from the venous leg ulcers; the wound ostomy continence nurse, who performed the cleansing of all the wounds; the study participant, and the professionals who carried out the microbiological analysis and electron microscopy.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 44 patients were enrolled in to the study, but 27 patients completed the study and no data were provided for the missing 17 participants. |
| Selective reporting (reporting bias) | Low risk | Reported on all primary and secondary outcomes as outlined in the study report. |
| Other bias | Low risk | None noted |
O'Halloran 2014.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo controlled study Setting: community and general practice |
|
| Participants |
Sample size: n = 61 Loss to follow‐up: n = 16 from the control group and n = 10 from the intervention group Inclusion criteria: males or females aged 21 years or over; presenting with a mixed or venous aetiology leg ulcer; defined by an ABPI > 0.8; persisting for a minimum of 12 weeks; with the wound size unchanged for at least 4 weeks. Exclusion criteria: leg ulcers greater that 50cm2 or less than 1cm2; present for more that 24 months; if maceration was present; if the participant was receiving antimicrobial or steroidal treatment; or if a secondary skin infection was present. Age: mean age of control group 72.9 years (range 38 to 91), mean age of intervention group 74.2 years (range 29 to 93) Sex: 28 males, 33 females |
|
| Interventions | The treatment arm (n = 28) consisted of the application of a 20 ppm AOP (potable tap water and oxygen converted into an approximately 20 ppm AOP solution) lavage for 15 minutes. The placebo arm (n = 33) consisted of the application of a sterile water lavage, via a sham unit, for 15 minutes. Participants were treated on a Monday, Wednesday and Friday for 2 weeks, resulting in a total of 6 individual 15‐minute fluid applications. Both study arms were treated with compression therapy, either 4‐layer or 2‐layer compression and the wound was dressed with a non‐interactive, non‐adherent dressing. |
|
| Outcomes | Length of follow‐up: 12 months
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomisation code list was generated"; study groups were comparable at baseline. |
| Allocation concealment (selection bias) | Low risk | "The randomisation code was kept securely at the trial site of the co‐ordinating investigator, with investigators contacting the code allocation individual who would allocate the next treatment arm from the list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patient treatment regimes were identical and standardised, apart from the solution applied" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blinding was achieved through the use of blind assessor investigators, separate to the treatment investigators, to assess wounds pre‐ and post‐treatment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Says analysed using "Intention to Treat", however, results are presented per protocol. |
| Selective reporting (reporting bias) | Low risk | Reported on all primary and secondary outcomes. |
| Other bias | Low risk | None noted |
Romanelli 2010.
| Study characteristics | ||
| Methods |
Study design: randomised controlled study Setting: dermatology department outpatient setting |
|
| Participants |
Sample size: n = 40 Loss to follow‐up: n = 2 Inclusion criteria: painful chronic venous leg ulcer of more than 8 weeks old; clinical and instrumental signs of venous insufficiency; wound size: up to 100 cm2; having received compression therapy for at least 2 weeks before inclusion; participants over 18 years of age. Exclusion criteria: allergy to 1 of the materials used; severe arterial disease; superficial of deep vein thrombosis; ankle brachial pressure index of less than 0.8; immobile; pregnancy or period of lactation; lymphoedema; diabetes; hypercoagulability; thrombophilia. Age: range 55 to 73 years Sex: 18 males; 22 females |
|
| Interventions | Group A: 20 participants were treated every other day with the active wound cleansing solution in association with standard wound care (polyurethane foam and elastic compression). Group B: 20 participants were treated every other day with saline solution in association with standard wound care (polyurethane foam and elastic compression). | |
| Outcomes | Length of follow up: 4 week treatment
|
|
| Notes | Funding source: supported by B. Braun Medical AG, Switzerland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised with an electronic system"; study groups were comparable at baseline. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 participants were enrolled in the study, but 38 participants completed the study and no data were provided for the missing 2 participants. |
| Selective reporting (reporting bias) | High risk | All outcomes reported but specific figures not provided. |
| Other bias | Low risk | None noted |
Vanscheidt 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled study Setting: outpatients wound clinic, surgical and private dermatology |
|
| Participants |
Sample size: n = 126 Loss to follow‐up: for the main study outcome, 16 participants were lost to follow‐up Inclusion criteria: males and females aged ≥18 years, participants with a locally infected chronic venous leg ulcer (target ulcer) at any location below the knee joint (CVI grade C6 according to CEAP classification (Clinical severity/Etiology or cause/Anatomy/Pathophysiology) meeting all the following criteria: confirmed diagnosis of CVI; duration of the target venous ulcer ≥ 4 weeks and ≤ 2 years; surface area of the target venous leg ulcer (after debridement) ≥ 2 and ≤ 20 cm2; presence of at least 2 of the following 9 infection criteria: abscess; cellulites; discharge; discolouration; friable granulation tissue that bleeds easily; unexpected pain/tenderness or change in the nature of pain; pocketing at base of wound or wound breakdown; bridging of the epithelium or soft tissue and abnormal smell. Exclusion criteria: target ulcer was < 4 weeks and > 2 years; in case of contraindication regarding local wound therapy and compression therapy with bandages; known hypersensitivity to 1 or more of the active/inactive ingredients of the investigational medical product; and previous or concomitant therapy with non permitted local or systemic drug therapy. Age: mean age of control group 66.9 years (SD:10.6), mean age of intervention group 68.7 years (SD:13) Sex: 53 males; 71 females (data for 2 participants missing) |
|
| Interventions | In the intervention group (n = 60), chronic venous leg ulcers were treated with the topical antiseptic agent octenidine dihydrochloride/phenoxyethanol (OHP). In the control group (n = 66), wounds were treated with Ringer's solution (containing sodium chloride, potassium chloride, calcium chloride and sodium bicarbonate) as the standard wound cleansing. |
|
| Outcomes | Length of follow‐up: 12 weeks
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into two different groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27 participants lost to follow up |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes as outlined in the trial registration were reported for all participants at 12 weeks. |
| Other bias | Low risk | None noted |
ABPI: ankle brachial pressure index; AOP: aqueous oxygen peroxide; CVI: Chronic venous insufficiency; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andriessen 2008 | Not an RCT |
| Bass 2007 | This study did not provide outcome data separately for the included leg ulcer participants. |
| Bellingeri 2004 | This study did not provide outcome data separately for the included leg ulcer participants (author contacted but no response received) |
| Bellingeri 2016 | This study did not provide outcome data separately for the included leg ulcer participants (author contacted but no response received) |
| Bravo Bernabé 1972 | Not an RCT |
| Carneiro 2003 | Phenytoin powder used, not wound cleansing. |
| Colombo 1993 | This study did not provide outcome data separately for the included leg ulcer participants (author contacted but no response received). |
| Ennis 2012 | Not an adult population |
| Griffiths 2001 | This study did not provide outcome data separately for the included leg ulcer participants (author contacted but no response received). |
| Groenewald 1980 | Debrisan beadlets used, not wound cleansing. |
| Hammerle 2016 | Octenidine Wound Gel used, not wound cleansing. |
| Milward 1991 | Study compared 3 dressings. |
| Selim 2000 | Not an RCT |
| Sopata 2014 | Not an RCT |
| Steenvoorde 2007 | Not an RCT |
| Werner‐Schlenzka 1994 | Vasodilator solution, not wound cleansing |
| Westerhof 1987 | Elase powder used, not wound cleansing. |
RCT: randomised controlled trial
Differences between protocol and review
Some of the data we intended to extract were not reported in the studies we included. However this did not affect the outcome of the analysis. We were unable to assess the following outcomes as the included studies did not measure them: patient preference, ease of use of the method of cleansing, cost, health‐related quality of life and adverse events.
The wording for the primary outcomes have been revised for the review. This is for clarification and it did not change any of the methods used or the approach taken, so we do not consider that it will add bias to the review.
For studies reporting outcomes for different time periods, we followed the guidance in Higgins 2019, and selected the longest follow‐up for each outcome in the study.
Contributions of authors
Niamh EM McLain: conceived the review; designed the review; co‐ordinated the review; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; secured funding; wrote to study authors/experts/companies; approved final review prior to publication; is guarantor of the review.
Zena EH Moore: conceived the review; designed the review; co‐ordinated the review; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; secured funding; performed previous work that was the foundation of the current review; wrote to study authors/experts/companies; approved final review prior to publication.
Pinar Avsar: checked quality of data extraction; analysed or interpreted data; checked quality assessment; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; approved final review prior to publication.
Contributions of the editorial base
Jo Dumville (Joint Co‐ordinating Editor): edited the review; advised on methodology and review content; approved the final review prior to publication.
Joan Webster (Editor): edited the protocol; advised on methodology and content; approved the final protocol prior to publication.
Gill Rizzello and Sally Bell‐Syer (Managing Editors): co‐ordinated the editorial process; advised on interpretation and content; edited the protocol and the review.
Naomi Shaw and Sophie Bishop (Information Specialists): designed the search strategy, ran the search and edited the search methods sections.
Tom Patterson (Editorial Assistant): edited the reference sections.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board of Ireland, Ireland
Cochrane Fellowship
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
Niamh McLain (NMcL): none known.
Zena Moore (ZM): has received an honorarium for speaking at professional meetings for Smith & Nephew and Molnlycke.
Pinar Avsar (PA): none known.
New
References
References to studies included in this review
Borges 2018 {published data only}
- Borges EL, Frison SS, Honorato-Sampaio K, Guedes AC, Lima VL, Oliveria OM, et al. Effect of polyhexamethylene biguanide solution on bacterial load and biofilm in venous leg ulcers: a randomized controlled trial. Journal of Wound Ostomy and Continence Nursing 2018;45:425-31. [DOI] [PubMed] [Google Scholar]
O'Halloran 2014 {published data only}
- O'Halloran PD, Winter PK, Otter JA, Adams NM, Chewins J. Aqueous oxygen peroxide treatment of venous leg ulcers in a primary care-based randomised, double-blind, placebo-controlled trial. Journal of Wound Care 2014;23(4):176-90. [DOI] [PubMed] [Google Scholar]
Romanelli 2010 {published data only}
- Romanelli M, Dini V, Barbanera S, Bertone MS. Evaluation of the efficacy and tolerability of a solution containing propyl betaine and polihexanide for wound irrigation. Skin Pharmacology and Physiology 2010;23(Suppl 1):41-4. [DOI] [PubMed] [Google Scholar]
Vanscheidt 2012 {published data only}
- Vanscheidt W, Harding K, Téot L, Siebert J. Effectiveness and tissue compatibility of a 12‐week treatment of chronic venous leg ulcers with an octenidine based antiseptic – a randomized, double‐blind controlled study. International Wound Journal 2012;9:316-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Andriessen 2008 {published data only}
- Andriessen AE, Eberlein T. Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds 2008;20(6):171-5. [PubMed] [Google Scholar]
Bass 2007 {unpublished data only}
- Bass A, Nitezki S. Assessment of the safety and efficacy of Dermastream - Enzystream System for the treatment of chronic venous ulcers. Available at clinicaltrials.gov/ct2/show/NCT00485329 2007.
Bellingeri 2004 {published data only (unpublished sought but not used)}
- Bellingeri A, Attolini R, Fioretti C, Scalise A, Forma O, Traspedini P, et al. Evaluation of the effectiveness of a centre to cleanse skin lesions: multicentric open, controlled and randomized study. Minerva Medica 2004;95:1-9. [Google Scholar]
Bellingeri 2016 {published data only (unpublished sought but not used)}
- Bellingeri A, Falcinoni F, Transpedini P, Mosscatelli A, Russo A, Tino G, et al. Effects of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: a single blind RCT. Journal of Wound Care 2016;25(3):160-8. [DOI] [PubMed] [Google Scholar]
Bravo Bernabé 1972 {published data only}
- Bravo Bernabé PA, Fernandez A, Zarraga J, Cruz M, Anorve I. Ulcers of the lower extremities. Treatment with inositol hexanicotinate [Ulceras de los miembros inferiores. Tratamiento con hexanicotinato de inositol]. Prensa Medica Mexicana 1972;37(3):151-8. [PubMed] [Google Scholar]
Carneiro 2003 {published data only}
- Carneiro PM, Nyawawa ET. Topical phenytoin versus eusol in the treatment of non-malignant chronic leg ulcers. East African Medical Journal 2003;80(3):124-9. [DOI] [PubMed] [Google Scholar]
Colombo 1993 {published data only (unpublished sought but not used)}
- Colombo P. Topical chloroxidating solution in wounds treatment: a controlled trial [Trattamento topico delle ferite con una soluzione clorossidante: uno studi controllato]. Acta Toxicologica et Therapeutica 1993;14(2):65-72. [Google Scholar]
Ennis 2012 {unpublished data only}
- Ennis W, Mulder G. A randomized, double-blind exploratory pilot study comparing Prontosan (TM) versus saline in the cleansing of chronic leg ulcers in diabetic patients. Available at clinicaltrials.gov/ct2/show/NCT01554644 2012.
Griffiths 2001 {published data only (unpublished sought but not used)}
- Griffiths RD, Fernandez RS, Ussia CA. Is tap water a safe alternative to normal saline for wound irrigation in the community setting? Journal of Wound Care 2001;10(10):407-10. [DOI] [PubMed] [Google Scholar]
Groenewald 1980 {published data only}
- Groenewald JH. An evaluation of dextranomer as a cleaning agent in the treatment of the post-phlebitic stasis ulcer. South African Medical Journal 1980;57(20):809-15. [PubMed] [Google Scholar]
Hammerle 2016 {published data only}
- Hammerle G, Strohal R. Efficacy and cost effectiveness of octenidine wound gel in the treatment of chronic venous leg ulcers in comparison to modern wound dressings. International Wound Journal 2016;13(2):182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milward 1991 {published data only}
- Milward P. Comparing treatment for leg ulcers. Nursing Times 1991;87(13):70-2. [PubMed] [Google Scholar]
Selim 2000 {published data only}
- Selim P. Will water do? Cleansing of leg ulcers in the community. ACCNS Journal for Community Nurses 2000;5(3):11-3. [Google Scholar]
Sopata 2014 {published data only (unpublished sought but not used)}
- Sopata M, Tomaszewska E, Muszynski Z, Ciupinska M. Can antiseptics with modern wound dressings have beneficial effect on venous leg ulcers treatments? Clinical and microbiological aspects. Wound Repair and Regeneration 2014;22:A60. [Google Scholar]
Steenvoorde 2007 {published data only}
- Steenvoorde P, Van Doorn LP, Jacobi CE, Oskam J. The unexpected effects of dermacyn on infected leg ulcers. Journal of Wound Care 2007;16(2):60-1. [DOI] [PubMed] [Google Scholar]
Werner‐Schlenzka 1994 {published data only}
- Werner-Schlenzka H, Kleine Kuhlmann R. Treatment of venous leg ulcers with topical iloprost: a placebo controlled study. VASA: Zeitschrift fur Gefasskrankheiten 1994;23(2):145-50. [PubMed] [Google Scholar]
Westerhof 1987 {published data only}
- Westerhof W, Janse FC, De Wit FS, Cormone RH. Controlled double blind trial of fibrinolysin-desoxyribonuclease solution in patients with chronic leg ulcers who are treated before autologous skin grafts. Journal of the American Academy of Dermatology 1987;17(1):32-9. [DOI] [PubMed] [Google Scholar]
Additional references
AHCPR 1994
- US Agency for Health Care Policy and Research (AHCPR) Treatment of Pressure Ulcers Guideline Panel. Treatment of pressure ulcers: AHCPR clinical practice guidelines no.15. www.ncbi.nlm.nih.gov/books/NBK63898 December 1994.
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Attinger 2006
- Attinger CE, Janais JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Critical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plastic and Reconstructive Surgery 2006;117(Suppl 7):72-109. [DOI] [PubMed] [Google Scholar]
AWMA 2011
- Australian Wound Management Association (AWMA). Australian and New Zealand clinical practice guidelines for prevention and management of venous leg ulcers. www.awma.com.au/publications/2011_awma_vlug.pdf 2011.
Berenguer Perez 2019
- Berenguer Perez M, Lopez-Casanova P, Sarabıa Lavın R, Gonzalez De La Torre H, Verdu-Sorıano J. Epidemiology of venous leg ulcers in primary health care: incidence and prevalence in a health centre-a time series study (2010-2014). International Wound Journal 2019;16:256-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 2015
- Bland M. An Introduction to Medical Statistics. 4th edition. Oxford (UK): Oxford University Press, 2015. [Google Scholar]
Briggs 2003
- Briggs M, Closs SJ. The prevalence of leg ulceration; a review of the literature. European Wound Management Association Journal 2003;3(2):14-20. [Google Scholar]
Briggs 2007
- Briggs M, Flemming K. Living with leg ulceration: a synthesis of qualitative research. Journal of Advanced Nursing 2007;59(4):319-28. [DOI] [PubMed] [Google Scholar]
Button 2013
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews: Neuroscience 2013;14(5):365-76. [DOI] [PubMed] [Google Scholar]
Callam 1987
- Callam M, Ruckley CV, Dale J, Harper DR. Chronic ulcer of the leg: clinical history. British Medical Journal 1987;294(6584):1389-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2006
- Cooper R, Okhiria O. Biofilms, wound infection and issues of control. Wounds UK 2006;2(3):48-57. [Google Scholar]
Cutting 2010
- Cutting K. Addressing the challenge of wound cleansing in the modern era. British Journal of Nursing 2010;19(11):S24-9. [DOI] [PubMed] [Google Scholar]
Day 2015
- Day J. Diagnosing and managing venous leg ulcers in patients in the community. British Journal of Community Nursing 2015;20(Suppl 12):S22-30. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
European Commission 2012
- European Commission. Patient safety: commission publishes report on state of play in the member states. Midday Express. www.uems.eu/news-and-events/news/news-more/patient-safety-ec-report-on-state-of-play-in-the-member-states 15 November 2012.
Flanagan 1997
- Flanagan M. Wound cleansing. In: Morsion M, Moffatt C, Bridel-Nixon J, Bale S, editors(s). A Colour Guide to the Nursing Management of Chronic Wounds. 2nd edition. London (UK): Mosby, 1997:87-102. [Google Scholar]
Ghauri 1998
- Ghauri AS, Nyamekye I, Grabs AJ, Farndon JR, Poskitt KR. The diagnosis and management of mixed arterial/venous leg ulcers in community-based clinics. European Journal of Endovascular Surgery 1998;16:350-5. [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Gonzalez‐Consuegra 2011
- Gonzalez-Consuegra RV, Verdu J. Quality of life in people with venous leg ulcers: an integrative review. Journal of Advanced Nursing 2011;67(5):926-44. [DOI] [PubMed] [Google Scholar]
Green 2010
- Green J, Jester R. Health-related quality of life and chronic venous leg ulceration: part 2. Journal of Community Nursing 2010;15(Suppl 1):S4-14. [DOI] [PubMed] [Google Scholar]
Guest 2017
- Guest JF, Ayoub N, McIlwraıth T, Uchegbu I, Gerrısh A, Weıdlıch D, et al. Health economic burden that different wound types impose on the UK's National Health Service. International Wound Journal 2017;14:322-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [DOI] [PubMed] [Google Scholar]
Hellewell 1997
- Hellewell TB, Major DA, Foresman PA, Rodeheaver GT. A cytotoxicity evaluation of antimicrobial and non-microbial wound cleansers. Wounds 1997;9(1):15-20. [Google Scholar]
Heyer 2017
- Heyer K, Protz K, Glaeske G, Augustın M. Epidemiology and use of compression treatment in venous leg ulcers: nationwide claims data analysis in Germany. International Wound Journal 2017;14:338-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Li T, Deeks JJ, editors(s). Chapter 6: Choosing effect measures and computing estimates of effect. In Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al; editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Horrocks 2006
- Horrocks A. Prontosan wound irrigation and gel: management of chronic wounds. British Journal of Nursing 2006;15(22):1222-9. [DOI] [PubMed] [Google Scholar]
HSE 2018
- Health Service Executive Ireland. HSE National Wound Management Guidelines. Available at healthservice.hse.ie/filelibrary/onmsd/hse-wound-management-guidelines-2018.pdf 2018.
Huxtable 1997
- Huxtable D. Ritual cleansing. Nursing New Zealand 1997;1(3):14-6. [PubMed] [Google Scholar]
Iglesias 2004
- Iglesias CP, Nelson EA, Cullum N, Togerson DJ. Economic analysis of VenUS 1, a randomised trial of two bandages for treating leg ulcers. British Journal of Surgery 2004;91(10):1300-6. [DOI] [PubMed] [Google Scholar]
Lawrence 1997
- Lawrence JC. Wound irrigation. Journal of Wound Care 1997;6(1):23-6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindsay 2007
- Lindsay E. To wash or not to wash: what's the solution for chronic leg ulcers? Wounds Essentials 2007;2:74-83. [Google Scholar]
Magson‐Roberts 2006
- Magson-Roberts S. Is tap water a safe alternative to normal saline for wound cleansing? Journal of Community Nursing 2006;20(8):19-24. [Google Scholar]
Main 2008
- Main RC. Should chlorhexidine gluconate be used in wound cleansing? Journal of Wound Care 2008;17(3):112-4. [DOI] [PubMed] [Google Scholar]
Moffatt 1992
- Moffatt CJ, Franks PJ, Oldroyd M, Bosanquet N, Brown P, Greenhalgh RM, et al. Community clinics for leg ulcers and impact on healing. British Journal of Medicine 1992;305:1389-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2013
- Moore ZE, Cowman S. Wound cleansing for pressure ulcers. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD004983. [DOI: 10.1002/14651858.CD004983.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morison 1994
- Morison M, Moffat C. A Colour Guide to the Assessment and Management of Leg Ulcers. London (UK): Mosby, 1994. [Google Scholar]
Nelson 2012
- Nelson EA, Bell-Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD002303. [DOI: 10.1002/14651858.CD002303] [DOI] [PubMed] [Google Scholar]
Nelson 2016
- Nelson A, Adderley U. Venous leg ulcers. BMJ Clinical Evidence 2016;1902:PMID: 26771825. [PMC free article] [PubMed] [Google Scholar]
Nüesch 2009
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Burgi E, Scherer M, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ 2009;339:b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2000
- O'Brien JF, Grace PA, Perry IJ, Burke PE. Prevalence and aetiology of leg ulcers in Ireland. Irish Journal of Medical Science 2000;169(2):110-2. [DOI] [PubMed] [Google Scholar]
O'Donnell 2014
- O'Donnell TF, Passaman HA, Harston WA, Ennis WJ, Dalsing M. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. Journal of Vascular Surgery 2014;60(2):3-59S. [DOI] [PubMed] [Google Scholar]
O'Meara 2012
- O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD000265. [DOI: 10.1002/14651858.CD000265.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Persoon 2004
- Persoon A, Heinen MM, Van der Vleneuten CJ, De Rooij MT, Van de Kerkhof PC, Van Achterberg T. Leg ulcers: a review on daily life. Journal of Clinical Nursing 2004;13(3):341-54. [DOI] [PubMed] [Google Scholar]
Philips 1997
- Philips D, Davey C. Wound cleansing versus wound disinfection: a challenging dilemma. Perspectives 1997;21(4):15-6. [PubMed] [Google Scholar]
Phillips 2005
- Phillips CJ. Introduction. In: Phillips CJ, editors(s). Health Economics: An Introduction for Health Professionals. Oxford (UK): Blackwell, 2005:1-19. [Google Scholar]
RCN 2006
- The Royal College of Nursing (RCN). Clinical practice guidelines: the nursing management of patients with venous leg ulcers. Available at gneaupp.info/wp-content/uploads/2014/12/The-nursing-management-of-patients-with-venous-leg-ulcers.pdf 2006.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RNAO 2004
- Registered Nurses Assoicaiton of Ontario (RNAO). Assessment and management of venous leg ulcers. Available at rnao.ca/bpg/guidelines/assessment-and-management-venous-leg-ulcers 2004.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Scriven 1997
- Scriven JM, Hartshorne T, Bell PR, Naylor AR, London NJ. Single-visit ulcer assessment clinic: the first year. British Journal of Surgery 1997;84(3):334-6. [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic venous leg ulcers: a national clinical guideline. SIGN guideline number 120. Available at www.sign.ac.uk/our-guidelines/management-of-chronic-venous-leg-ulcers/ August 2010.
Skerritt 2014
- Skerritt L, Moore Z. The prevalence, aetiology and management of wounds in a community care area in Ireland. British Journal of Community Nursing 2014;Suppl:S11-7. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Statistics in Medicine 1999;18:2693-708. [DOI] [PubMed] [Google Scholar]
Towler 2001
- Towler J. Cleansing traumatic wounds with swabs, water or saline. Journal of Wound Care 2001;10(6):231-4. [DOI] [PubMed] [Google Scholar]
Toy 2005
- Toy LW. Matrix metalloproteinases: their function in tissue repair. Journal of Wound Care 2005;14(1):20-2. [DOI] [PubMed] [Google Scholar]
Trevelyan 1996
- Trevelyan J. Wound cleansing: principles and practice. Nursing Times 1996;92(16):46-8. [PubMed] [Google Scholar]
Vowden 2009
- Vowden K, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. Journal of Tissue Viablity 2009;18(1):13-9. [DOI] [PubMed] [Google Scholar]
Williams 1999
- Williams C. Wound irrigation techniques. British Journal of Nursing 1999;8(21):1460-2. [DOI] [PubMed] [Google Scholar]
Xie 2018
- Xıe T, Ye J, Rerkasem K, Manı R. The venous ulcer continues to be a clinical challenge: an update. Burns & Trauma 2018;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 1995
- Young T. Common problems in wound care: wound cleansing. British Journal of Nursing 1995;4(5):286-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
McLain 2015
- McLain NE, Moore ZE. Wound cleansing for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD011675. [DOI: 10.1002/14651858.CD011675] [DOI] [PMC free article] [PubMed] [Google Scholar]


