Abstract
The generation of effective adaptive T-cell memory is a cardinal feature of the adaptive immune system. The establishment of protective T-cell immunity requires the differentiation of CD8+ T cells from a naive state to one where pathogen-specific memory CD8+ T cells are capable of responding to a secondary infection more rapidly and robustly without the need for further differentiation. The study of factors that determine the fate of activated CD8+ T cells into either effector or memory subsets has a long history. The advent of new technologies is now providing new insights into how epigenetic regulation not only impacts acquisition and maintenance of effector function, but also the maintenance of the quiescent yet primed memory state. There is growing appreciation that rather than distinct subsets, memory T-cell populations may reflect different points on a spectrum between the starting naive T-cell population and a terminally differentiated effector CD8+ T-cell population. Interestingly, there is growing evidence that the molecular mechanisms that underpin the rapid effector function of memory T cells are also observed in innate immune cells such as macrophages and natural killer (NK) cells. This raises an interesting hypothesis that the memory/effector T-cell state represents a default innate-like response to antigen recognition, and that it is the naive state that is the defining feature of adaptive immunity. These issues are discussed.
CD8+ T cells are a key component of the adaptive immune system that combat and help control many malignancies and intracellular infections. Activation of naive CD8+ T cells induces a largely autonomous program of proliferation and differentiation generating both effector and memory CD8+ T cells (Kaech and Ahmed 2001; van Stipdonk et al. 2001, 2003). Effector T cells express a myriad of lineage-specific effector proteins, including cytolytic proteins such as granzyme (Gzm) A, B, K, and perforin (Pfp) (Jenkins et al. 2007; Peixoto et al. 2007), inflammatory cytokines such as interferon γ (IFN-γ) and tumor necrosis factor (TNF) (La Gruta et al. 2004), as well as chemokines such as CCL5 (Kim et al. 1998). Combined, these lineage-specific effector functions contribute to the control and elimination of intracellular pathogen-infected host cells, or cells that have become malignant. Once infection is cleared, the majority of effector T cells die leaving behind a population of long-lived, pathogen-specific CD8+ T cells (Marshall et al. 2001; Kaech et al. 2002; Wherry et al. 2003). Importantly, memory CD8+ T cells exhibit distinct characteristics when compared to the starting naive T-cell precursors. First, they persist at a much greater frequency in the circulation. Estimates of the frequency of naive, virus-specific T cells within the CD8+ T-cell pool range from 1 in 107 to 1 in 106 (Jenkins et al. 2010). In contrast, memory CD8+ T cells precursors can range between 1 in 104 and 1 in 102 (Flynn et al. 1998; Murali-Krishna et al. 1998), orders of magnitude higher than their naive counterparts. Second, memory CD8+ T cells typically elicit immediate effector function without the need for further differentiation (Lalvani et al. 1997), unlike naive precursors, which are unable to mediate immediate effector function (Veiga-Fernandes et al. 2000; Kaech and Ahmed 2001). When combined, these cardinal features of memory CD8+ T cells enable a more rapid and potent control of secondary infection with the same pathogen (Flynn et al. 1998; Murali-Krishna et al. 1998).
The effectiveness of CD8+ T-cell immunity in limiting viral infection has been reported for a wide array of viral infections. This was evident during the 2009 H1N1 influenza A virus (IAV) pandemic where individuals with preexisting IAV-specific CD8+ T-cell immunity exhibited less severe disease and protection from infection (Sridhar et al. 2013). More recently, the advent of checkpoint blockade therapy has enabled reactivation of immune system function, in particular the CD8+ T-cell response to tumors (Im et al. 2016; Huang et al. 2017). A major goal of T-cell researchers has long been to understand the factors that drive efficient memory CD8+ T-cell generation with a view to improving vaccine and immunotherapeutic design. To that end, this review will cover recent findings that provide insights into the what, where, and how of pathogen-specific CD8+ T-cell memory generation.
MEMORY CD8+ T-CELL HETEROGENEITY
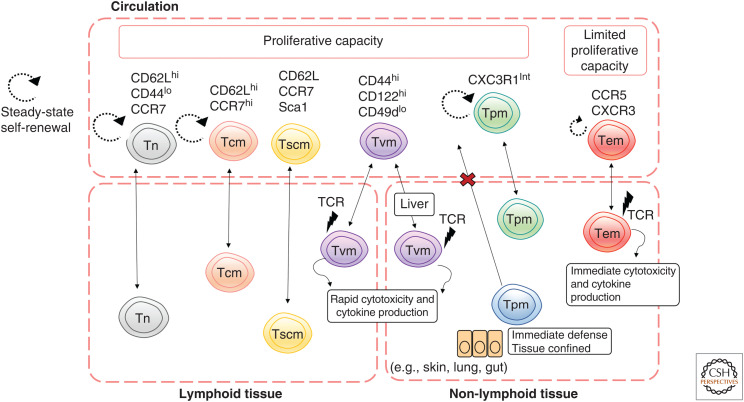
The generation of CD8+ T-cell memory populations is associated with phenotypic and functional heterogeneity. Memory CD8+ T cells found within the host circulation have been broadly described as either central memory (Tcm) or effector memory (Tem) T cells (Sallusto et al. 1999, 2004). Tcm cells typically express the lymph node homing markers, CD62L (L-selectin) and CCR7, enabling their localization to secondary lymphoid tissues. Moreover, Tcm cells exhibit self-renewal and proliferative capacity (Masopust et al. 2001). Further heterogeneity within the broad Tcm subset exists with identification of memory cells that not only express CD62L/CCR7 but also stem cell markers such as Sca1 (termed T stem cell memory [Tscm]). Tscm cells represent a multipotent memory T-cell precursor able to give rise to other memory subsets (Gattinoni et al. 2011; Lugli et al. 2013).
In contrast, Tem cells typically express tissue-specific homing markers such as CCR5, CXCR3, and integrins, and are capable of entering non-lymphoid tissues from the circulation in the steady state (Masopust et al. 2001; Wakim et al. 2008; Kohlmeier et al. 2011). Whereas Tem cells exhibit limited proliferative capacity, they do exhibit immediate effector function, such as cytotoxicity and cytokine production upon T-cell receptor (TCR) ligation (Marshall et al. 2001; Masopust et al. 2001). These characteristics enable rapid recruitment to a site of infection and immediate control of the infection. Recent analysis identified a subset of memory T cells that exhibit properties of both Tcm and Tem cells. These peripheral memory (Tpm) T cells express intermediate levels of CXC3R1 and have extensive proliferative capacity, similar to conventional Tcm (Böttcher et al. 2015; Gerlach et al. 2016). Interestingly, Tpm cells also exhibit effector functions and tissue-trafficking properties more akin to classical Tem. Hence, Tpm cells appear to be a form of intermediate memory between the Tcm and Tem classifications (Böttcher et al. 2015; Gerlach et al. 2016).
Early studies demonstrated that a subset of memory T cells could persist in peripheral tissue at the original sites of infection (Marshall et al. 2001; Masopust et al. 2001). This memory subset is now called tissue-resident memory (Trm) T cells (Gebhardt et al. 2009, 2011; Mackay et al. 2012). Trm cells are distinct from other memory T-cell subsets as they are truly resident and do not readily reenter the circulation (Gebhardt et al. 2009). Thus, T-cell-dependent control of infection can involve distinct waves of T-cell immunity, with Trm providing an initial front-line defense against secondary infection (Kohlmeier and Woodland 2009). This can be further reinforced by recruitment of Tem cells from the circulation, with expansion of Tcm cells in the draining lymph node that provides the additional effector T-cell numbers required to ensure complete control and elimination of an invading pathogen (Wherry et al. 2003).
A defining feature of CD8+ T-cell memory is the ability to persist in the long term and respond quickly upon reactivation. Unlike naive T cells, once formed, memory CD8+ T cells do not require TCR contact with self-peptide/major histocompatibility complex (MHC) and subsequent tonic signaling (Tanchot et al. 1997; Takada and Jameson 2009). Moreover, the maintenance of naive and memory CD8+ T cells both exhibit renewal capacity, which is highly dependent on interleukin 7 (IL-7) (Schluns et al. 2000). It has also been reported that IL-15 signaling can help maintain self-renewal of memory CD8+ T cells (Becker et al. 2002; Goldrath et al. 2002) but not as critical as IL-7 signals (Schluns et al. 2000). Interestingly, the establishment and persistence of Trm cells at distinct anatomical sites suggests there are survival signals that appear specific to the particular microenvironment (Mackay et al. 2013). The maintenance of Trm populations in peripheral tissues also appears dependent on localized proliferation upon secondary infection (Park et al. 2018). In this way, the Trm pool can be replaced locally without the need for replenishment from draining secondary lymphoid tissues.
VIRTUAL MEMORY T CELLS
Virtual memory T cells (Tvm cells), otherwise known as memory phenotype (MP) cells, are a population of antigenically naive CD8+ T cells that comprise ∼5%–15% of total circulating CD8+ T cells and express markers of conventional memory T cells (e.g., high levels of CD44 and CD122). The naive state of Tvm cells is attested to by their low-level expression of CD49d (Lee et al. 2013; Quinn et al. 2016), an α4 integrin that is permanently up-regulated on T cells following cognate antigen encounter (Goldrath et al. 2000), and their presence in germ-free mice (Haluszczak et al. 2009) and in T-cell populations specific for epitopes to which mice had not been exposed (Haluszczak et al. 2009; La Gruta et al. 2010; Quinn et al. 2016). Recent evidence suggests Tvm cell differentiation is initially programmed by high self-peptide + MHC class I (pMHCI) reactivity in the thymus (Drobek et al. 2018; Miller et al. 2020), driving elevated CD5 (Rudd et al. 2011; Quinn et al. 2016) and Eomes expression (Miller et al. 2020). Tvm differentiation is maintained by exposure to cytokines in the periphery, namely, IL-4 and IL-15, the latter to which Tvm cells are exquisitely sensitive (Sosinowski et al. 2013; Quinn et al. 2020), likely due to the direct transcriptional activation of CD122 (Il2rb) by Eomes (Intlekofer et al. 2005). The recent observations that Tvm differentiation is initiated by requisite signals in the thymus is an important indication that these cells represent a distinct CD8+ T-cell lineage, rather than a peripherally generated T-cell activation state (Miller et al. 2020).
A putative Tvm population has also been proposed in humans, contained within the Temra population (CD45RA+CD27lo) and distinguished by expression of NKG2A and/or KIRs (Jacomet et al. 2015; White et al. 2016). Similar to mouse Tvm cells, human Tvm cells are found in cord blood (and thus likely arise independently of antigen) (Jacomet et al. 2015), express high levels of CD122, EOMES, and T-BET (Jacomet et al. 2015; White et al. 2016; Quinn et al. 2020), and are functionally equivalent to mouse Tvm cells, with respect to TCR-mediated proliferation in vitro (Quinn et al. 2018) and the production of large amounts of IFN-γ following cytokine stimulation (Jacomet et al. 2015). Further identification of human Tvm cell markers is essential for detailed dissection of their role in human immunity.
Tvm cells are of functional interest because, despite an absence of cognate antigen stimulation, they exhibit several memory-like response characteristics. In young adult mice, Tvm cells activate and proliferate more rapidly following TCR-mediated stimulation both in vitro and in vivo (Haluszczak et al. 2009; Lee et al. 2013; Quinn et al. 2018), and produce cytokines more rapidly than true naive cells (Haluszczak et al. 2009; Lee et al. 2013). Despite this, they tend to be monofunctional, producing low levels of IFN-γ after TCR-mediated stimulation (Lee et al. 2013) and largely in the absence of TNF or IL-2 (Quinn et al. 2018). Tvm cells can also exhibit antigen-specific cytotoxicity directly ex vivo, similarly to memory cells (Quinn et al. 2020). The semi-differentiated state of Tvm cells (Lee et al. 2013) likely drives their increased responsiveness to antigen and inflammatory cues, conferring protection in a number of pathogen challenge models (Haluszczak et al. 2009; Lee et al. 2013; Sosinowski et al. 2013; Rolot et al. 2018).
Because Tvm cells are CD44hi and CD62Lhi, they fall within the conventionally defined, antigen-experienced Tcm subset. Although Tvm cells can be distinguished in mice by the expression of relatively low levels of CD49d, the historical demarcation of Tcm cells has relied solely on their CD44hiCD62Lhi phenotype to distinguish them from conventional naive T cells ([Tn] CD44loCD62Lhi) and effector memory T cells ([Tem] CD44hiCD62Llo). For this reason, much of our knowledge about conventional memory T cells is confounded by the inclusion of Tvm cells. This is most starkly illustrated by our recent study in which the steady-state metabolic characteristics of Tn, Tem, Tcm, and Tvm cells were determined ex vivo (Quinn et al. 2020). A substantially elevated spare respiratory capacity (SRC), previously associated with conventional Tcm cells (van der Windt et al. 2012, 2013; O'Sullivan et al. 2014) and thought to be predictive of memory T-cell function (van der Windt et al. 2013), was found instead to be a key characteristic of Tvm cells (Quinn et al. 2020). In addition, the dependence of conventional memory cells on IL-15 for survival can also be largely attributed to Tvm cells. Mice lacking IL-15 show a complete loss of Tvm cells while retaining a similar proportion of conventional memory T cells as wild-type (WT) mice (Quinn et al. 2020), and mice lacking the IL-15Rβ chain (CD122−/−) showed no defect in antigen-specific CD8 memory formation (Sosinowski et al. 2013). Finally, a direct phenotypic analysis revealed that ∼60%–80% of conventionally defined Tcm cells in B6 mice were actually Tvm cells (CD49dlo), even 40 days after a lymphocytic choriomeningitis virus (LCMV) challenge (Quinn et al. 2020). Thus, it seems the discovery of Tvm cells may necessitate a revisitation of characteristics historically attributed to antigen-experienced memory cells, with an accurate identification of these cell populations being critical for a precise understanding of their attributes. This is more than simply a semantic argument; it addresses the extent to which previous antigen experience, rather than simply stimulation, confers unique or defining T-cell characteristics. It will also guide clinical strategies for the elicitation of immune responses from antigenically naive, versus conventional memory T-cell populations.
THE GENERATION AND MAINTENANCE OF T-CELL MEMORY
Given the apparent functional and phenotypic heterogeneity within the CD8+ T-cell memory pool upon viral clearance, there is great interest in determining whether vaccination strategies can be designed to potentially generate different types of memory T cells. For example, we have recently used a novel vaccination platform for generating IAV-specific CD8+ T-cell responses that appear to promote Trm cells in the lung, the primary site of infection (Anderson et al. 2017). To that end, a full understanding of how and when activated CD8+ T cells are committed to become a memory cell is required.
The role of CD4+ T cells in helping in the establishment of optimal primary CD8+ T-cell response is well appreciated. In this case, CD4+ T cells provide important costimulatory signals to antigen-presenting cells ensuring they have the capacity to fully activate naive CD8+ T cells (Bennett et al. 1997; Shedlock and Shen 2003). Early studies established that CD4+ T cell help during the initial priming phase was necessary for the generation of memory T cells capable of responding to secondary challenge (Janssen et al. 2003; Shedlock and Shen 2003). The same licensing required for primary CD8+ T-cell responses appears to also induce at least a subset of activated cytotoxic T lymphocytes (CTLs) that are committed to the establishment of optimal CD8+ T-cell memory (Rapetti et al. 2008). Such programming likely reflects the provision of cytokines such as IL-2 (Williams et al. 2006) and the delivery of costimulatory signals that promote dendritic cell (DC) activation (Bennett et al. 1998; Olson et al. 2014).
Aside from programming memory capacity, subsequent studies demonstrated CD4+ T-cell help was needed for memory CD8+ T-cell maintenance rather than intrinsic recall capacity (Sun and Bevan 2003; Sun et al. 2004). Elimination of the CD4+ subsets after priming resulting in gradual loss of memory CD8+ T-cell numbers and function (Marzo et al. 2004; Sun et al. 2004). In this case, the maintenance of CD8+ T-cell memory was antigen independent, and relied on a bystander effect, although the mechanisms by which CD4+ T cells sustain robust CD8+ T-cell memory are yet to be determined. Possible explanations include the provision of a survival factor by bystander CD4+ T cells, or perhaps CD4 T-cell interaction with, and conditioning of, stromal cells to help establish microenvironments required for CD8+ T-cell memory persistence (Sun et al. 2004).
Interestingly, primary IAV-specific effector CD8+ T-cell responses are CD4+ T-cell independent, while the establishment of functional IAV-specific CTL memory requires a concurrent CD4+ T-cell response (Tripp et al. 1995; Belz et al. 2002; Olson et al. 2014). We recently showed that in the context of IAV infection, CD4+ T-cell help was required at the time of initial priming for establishment of effective IAV-specific CD8+ T-cell memory, rather than for memory maintenance (Cullen et al. 2019). Importantly, adoptive transfer of “unhelped” memory CTLs into CD4+ T-cell competent animals was unable to restore recall capacity when compared to the response profiles for an equal number of “helped” memory CTLs (Cullen et al. 2019). Moreover, following IAV challenge, “helped” memory CTLs were able to respond equivalently in both CD4-competent and deficient environments upon rechallenge, demonstrating the relative independence of CD8+ T-cell memory on CD4 T cells after the initial priming phase.
IAV-specific CD8+ T-cell memory established in the absence of CD4+ T-cell help exhibited increased expression of inhibitory receptors and dysregulated metabolic capacity (Cullen et al. 2019). Transient CD4+ T-cell depletion during the initial stages of chronic LCMV infection also resulted in rapid establishment of severely exhausted CD8+ T cells in persistently infected mice (Matloubian et al. 1994). Whereas the primary IAV-specific CTL responses are largely intact in the CD4-deficient mice, there is prolonged effector CTL expansion and delayed viral clearance (Belz et al. 2002). So, despite an apparent normal effector response, the lack of CD4+ T cells during IAV infection might somewhat mimic conditions that likely promote eventual T-cell exhaustion. Hence, CD8+ T-cell activation in the absence of CD4+ T cells likely results in the inability to retain self-renewal capacity within activated T cells, pushing them more toward a terminally differentiated state resulting in decreased recall capacity.
FACTORS THAT IMPACT THE DYNAMICS OF MEMORY CD8+ T-CELL FORMATION
It has been reported that by limiting the inflammatory response early after infection, memory T-cell formation and recall capacity can be established very soon after T-cell activation (Badovinac et al. 2005). The early formation of CD8+ T-cell memory is reminiscent of data examining the role of TCR affinity in driving effector versus memory CD8+ T-cell formation (Zehn et al. 2009). Utilizing TCR transgenic OTI T cells, specific for the ovalbumin (OVA) 257–264 peptide (amino acid sequence SIINFEKL), infection with Listeria monocytogenes expressing low-affinity OVA257–264 analogs resulted in blunted effector T-cell expansion (Zehn et al. 2009). Importantly, this did not greatly impact CD8+ T-cell memory recall capacity upon rechallenge. The blunted effector response was in part explained by early egress from secondary lymphoid tissues into circulation of T cells that received low-affinity TCR signals. These data suggest that full effector T-cell expansion is not required for the generation of effective memory T-cell populations and that even low-affinity interactions resulting in T-cell activation are sufficient (Zehn et al. 2009).
It is well appreciated that a broad range of T-cell clones, reflecting a spectrum of TCR-binding avidities, can be recruited early into the immune response after infection (Price et al. 2005; Turner et al. 2006). During the course of an infection, high-avidity, pathogen-specific T-cell clones are often preferentially selected to dominate the overall response (Busch et al. 1998; Price et al. 2005). Interestingly, the apparent narrowing of the antigen-specific repertoire is not maintained into memory with an apparent diversification of the pathogen-specific repertoire (Blattman et al. 2000; Turner et al. 2003; Cukalac et al. 2014) to include T-cell clones that do not possess optimal amino acid sequence motifs within their antigen receptors and are less likely to bind to their antigen (Turner et al. 2003; Cukalac et al. 2014). These data support the earlier notion that low-binding TCR avidity is sufficient for T-cell activation and subsequent division, but not for sustained expansion and dominance in the effector response (Zehn et al. 2009). Nevertheless, this level of activation is sufficient for early entry into the memory pool for the apparent aim of maintaining clonal diversity in the memory pool. To what end should the memory pool prioritize the early seeding of a clonally diverse repertoire at the expense of an effector-driven, narrowed yet optimized repertoire? Studies have shown that there is often complete or near-complete recruitment from the naive to the immune pool (Zehn et al. 2009; La Gruta et al. 2010). If a majority of these cells are committed to an effector fate, there is a risk of depleting the memory pool of any protective capacity. Early commitment of a broad range of T-cell clones to the memory pool early after initial infection also ensures maintenance of T-cell clonal diversity, which has been shown to be important for effective virus control, in particular in escape variants (Price et al. 2004; Cornberg et al. 2006). Hence, there is a biological imperative to secure CD8+ T-cell memory potential in the early stages of an immune response.
HOW CHANGES IN THE CHROMATIN LANDSCAPE IMPACT CD8+ MEMORY T-CELL FORMATION
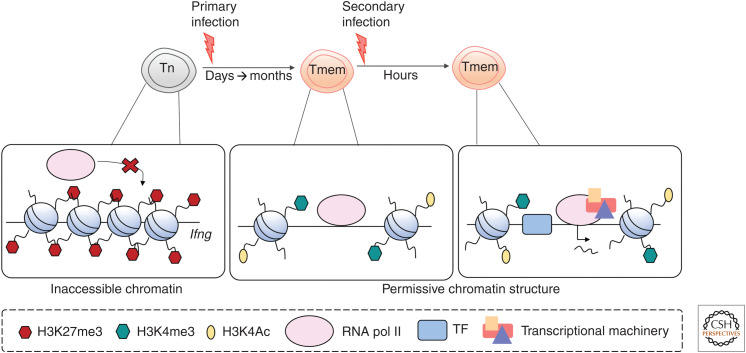
Within eukaryotic cells, DNA is wrapped around a complex of histone proteins known as a nucleosome, with the nucleosome–DNA complex termed chromatin (Jenuwein and Allis 2001; Kouzarides 2007). Histone post-translational modifications (PTMs) contribute to regulation of transcription by providing a platform that promotes binding of transcription factors (TFs) and chromatin remodeling proteins (Kouzarides 2007). Hence, dynamic changes in histone PTMs is an important mechanism for regulation and inheritance of gene transcriptional activity and for regulating differentiation in an array of cellular contexts (Kouzarides 2007). Histones can be modified by a vast array of covalent modifications, particularly on the solvent-exposed amino terminus, and the combination of histone PTMs and their genomic location is a predictor of transcriptional activity (Fig. 1; Wang et al. 2008). For example, trimethylation of histone 3 at lysine 4 (H3K4me3) is typically enriched within gene promoters and correlates with active transcription (Santos-Rosa et al. 2002). In contrast, deposition of trimethylated H3K27 (H3K27me3) at gene promoters typically correlates with transcriptional repression (Cao et al. 2002). These modifications act as a platform that promotes binding of transcriptional regulators to DNA-regulatory elements that then orchestrate chromatin remodeling and transcriptional activation (Kouzarides 2007). For instance, acetylation of histone lysine residues reduces nucleosome/DNA interactions, thus increasing genome accessibility and enabling gene transcription (Bauer et al. 1994). In contrast, trimethylation of H3K27 (H3K27me3) and H3K9 (H3K9me3) is associated with formation of inaccessible chromatin structures and subsequent transcriptional repression (Barski et al. 2007).
Figure 1.
Phenotype, function, and location of T-cell memory subsets. Memory CD8+ T cells are found in circulation, lymphoid, and non-lymphoid peripheral tissues. Tcm and Tscm cells have high self-renewal capacity and express CD62L and CCR7, enabling them to traffic to secondary lymphoid organs from the circulation. Tem cells are migratory, trafficking from the circulation to survey non-lymphoid peripheral tissues. Whereas they have limited proliferative and self-renewal capacity, Tem cells rapidly produce cytokines and cytotoxic molecules upon TCR stimulation. Tpm cells can also enter non-lymphoid peripheral tissues and exhibit functional capacity like Tem, but they have proliferative capacity akin to Tcm. Unlike these other memory subsets, Trm cells are tissue restricted and can provide a rapid front-line defense in tissues such as the skin, gut, and lung. Tvm cells are found in the blood, secondary lymphoid tissues, and non-lymphoid tissue such as the liver under steady-state conditions. Whereas they are antigen naive, they are semi-differentiated and rapidly proliferate and produce effector molecules upon TCR stimulation.
Whereas the establishment and maintenance of permissive chromatin structures at effector gene loci within memory T cells ensures rapid effector function (Araki et al. 2009; Denton et al. 2011; Zediak et al. 2011; Russ et al. 2014; Scott-Browne et al. 2016), what remains unclear is whether modulation of epigenetic marks also plays a role in determining the fate of recently activated T cells into memory or effector T cells (Fig. 2). Genome-wide profiling of histone PTMs of human memory T-cell subsets indicated that there may be a transitional pathway of cellular differentiation, with the naive and effector states representing the start and end of this process, respectively (Crompton et al. 2016). For example, expression of TCF-1 (encoded by Tcf7) is key for maintenance of the naive T-cell state and is associated with a permissive epigenetic chromatin landscape at the Tcf7 gene locus within naive T cells (Crompton et al. 2016; Yu et al. 2017). Comparison of the chromatin landscape within different CD8+ T-cell memory and effector subsets showed that there were small but progressive changes to the epigenetic landscape during the transition of naive CD8+ T cells to the memory/effector state (Crompton et al. 2016; Sen et al. 2016; Yu et al. 2017). Similarly, gradual remodeling of the chromatin landscape from a repressive to active transcriptional state was also evident at effector gene loci within both mouse CD8+ pathogen-specific T cells and human memory T-cell subsets (Russ et al. 2014; Crompton et al. 2016; Yu et al. 2017).
Figure 2.
Epigenetic control of effector gene loci enables memory CD8+ T cells to elicit rapid effector functions upon secondary infection. Effector genes (such as Ifng) in naive CD8+ T cells are characterized by inaccessible chromatin structure and repressive epigenetic marks, such as H3K27me3. This dense chromatin structure ensures that effector gene loci are inaccessible to RNA pol II and transcriptional machinery in the naive state. Upon primary infection and activation, remodeling of the chromatin landscape and deposition of active epigenetic marks such as H3K4me3 and H3K4ac at effector gene loci permits transcription. Memory CD8+ T cells retain this permissive chromatin structure with RNA pol II docked and are thus poised to rapidly transcribe effector genes upon secondary infection. (TF) Transcription factor.
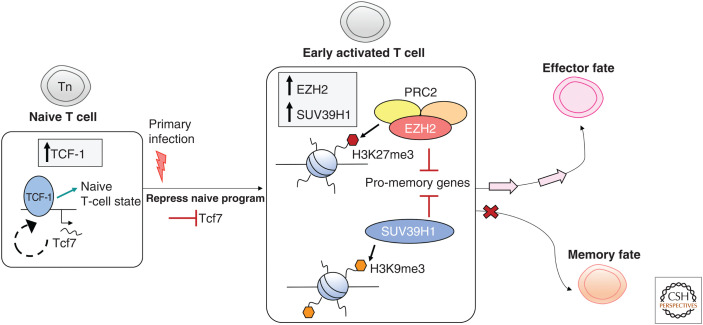
So precisely when does memory T-cell formation and the associated chromatin remodeling occur after activation? Analysis of single-cell RNA-seq profiles of responding CD8+ T cells have identified novel potential chromatin regulators of T-cell differentiation in response to infection (Kakaradov et al. 2017). One of these regulators was an enhancer of zeste homolog-2 (EZH2), the catalytic component of the Polycomb repressor complex 2 (PRC2), which mediates methylation of H3K27 (Cao et al. 2002). EZH2 and other components of the PRC2 complex were found to be more highly expressed within CD8+ T cells that had undergone one cell division and were destined to become effector cells rather than memory cells (Kakaradov et al. 2017). ChIP-seq analysis demonstrated that pro-memory and pro-survival gene loci within effector CD8+ T cells had higher levels of the repressive H3K27me3 mark compared to memory (Gray et al. 2017; Kakaradov et al. 2017). This suggested that PRC2 likely acts to repress genes that promote memory formation and retain proliferative potential. This was supported by the fact that EZH2-deficient CD8+ T cells failed to generate substantial effector T-cell populations after either bacterial or viral infection (Gray et al. 2017; Kakaradov et al. 2017). A more recent study focused on another histone methyltransferase, SUV39H1, which mediates methylation of H3K9, a transcriptionally repressive histone PTM (Fig. 3). It was found that SUV39H1-deficient CD8+ T cells could not epigenetically silence pro-stem cell and pro-memory genes and hence were not able to fully engage the effector T-cell program resulting in diminished protection from bacterial challenge (Pace et al. 2018).
Figure 3.
Epigenetic control of effector versus memory fate. The naive T-cell state is maintained by the transcription factor TCF-1 (encoded by Tcf7), which establishes a permissive chromatin landscape around the Tcf7 locus, forming a feedforward loop. Upon primary infection and activation, Tcf7 expression is repressed. Expression of EZH2 (the catalytic component of PRC2) and SUV39H1 in recently activated CD8+ T cells plays a role in determining effector versus memory fate. PRC2 mediates methylation of H3K27, a repressive epigenetic mark enriched at pro-memory gene loci in effector CD8+ T cells. SUV39H1 mediates methylation of H3K9, another repressive epigenetic mark. In the absence of SUV39H1, pro-memory genes are not epigenetically repressed, and the effector program cannot be fully engaged. PRC2 and SUV39H1 likely function in recently activated CD8+ T cells to silence genes that promote memory formation, thus opposing memory fate and enabling effector differentiation.
There are a couple of important implications in these data. The first is that the full commitment of a recently activated T cell toward an effector fate occurs early and requires shutdown of an active program of T-cell restraint or quiescence that characterizes both naive and memory T cells. Hence, memory T cells are those that are likely captured in the early stages of the differentiation process. The second is that the epigenetic shutdown of gene loci that control “naive/memory” self-renewal and pluripotency likely reflects an epigenetic barrier that is a point of no return for differentiating T cells. That is, CD8+ T cells that transition from the naive/memory state to an effector state would now be fully committed to what is effectively a terminal fate. In line with this, recent data suggest that the ability to reprogram virus-specific effector function in virus-specific memory CD8+ T-cell subsets is dependent on the extent of differentiation (Harland et al. 2014; Pauken et al. 2016; Sen et al. 2016). For example, IAV-specific Tcm can be conditioned to express alternate cytokines such as IL-4 more readily than Tem (Harland et al. 2014). A recent study proposed that virus-specific CD8+ effector T cells could in fact de-differentiate into a memory state by reversing the epigenetic silencing of pro-memory gene loci enabling reexpression of those genes (Youngblood et al. 2017). These data would imply that changes to the chromatin landscape are much more dynamic and less stable than we currently appreciate. Given this analysis was done on bulk populations, there is the possibility that the apparent de-differentiation might represent enrichment of memory precursors already present but not readily detected within the effector pool. The use of single-cell epigenetic approaches would be able to delineate the extent of cellular heterogeneity within these populations and give some insight into the true extent of flexibility in the T-cell differentiation process.
CARDINAL FEATURES OF IMMUNE MEMORY AND THE IMPORTANCE OF THE NAIVE STATE
Acquisition of lineage-specific function by virus-specific CD8+ T cells has been shown to correlate with remodeling of the chromatin landscape at effector gene loci from transcriptionally repressive to permissive states (Araki et al. 2009; Russ et al. 2014; Scott-Browne et al. 2016; Yu et al. 2017). Importantly, stable inheritance of transcriptionally permissive chromatin structures is maintained into the memory state with evidence that components of the transcriptional machinery are already preloaded onto gene promoters resulting in a transcriptionally poised configuration (Denton et al. 2011; Zediak et al. 2011; Bevington et al. 2016). The transcriptional poising of chromatin at the promoters of effector gene loci likely underpins a cardinal feature of CD8+ T-cell memory, namely, rapid effector function (Lalvani et al. 1997).
The concept of transcriptional poising is not unique to the adaptive immune system. It has long been appreciated that macrophages can be “trained” to have increased responsiveness after an initial challenge (Quintin et al. 2012; Cheng et al. 2014; Saeed et al. 2014). A similar phenomenon has also been observed after activation of natural killer (NK) cells after virus infection, whereby NK cells are capable of more rapid functional capacity upon rechallenge (Sun et al. 2009). In both cases, the increased function after initial activation is underpinned by alterations in the chromatin structure toward a transcriptionally permissive state that is maintained in the long term (Saeed et al. 2014; Lau et al. 2018). In fact, a comparison of memory CD8+ T cells and “trained” NK cells revealed shared epigenetic signatures (Lau et al. 2018). Hence, the molecular mechanisms underpinning what is considered to be a cardinal feature of adaptive immunity are also found in the innate immune system.
A key difference in adaptive and innate immunity is that naive CD8+ T cells exhibit a more limited baseline functional capacity when compared to macrophages or NK cells, which are capable of rapidly eliciting lineage-specific effector functions in response to stimuli. Our previous work has demonstrated that in the naive state, codeposition of histone modifications associated with transcriptional activation (H3K4me3) and repression (H3K27me3) are present at many CD8+ T-cell lineage–specific gene promoters and enhancers (Russ et al. 2014, 2017). Upon T-cell activation, loss of H3K27me3 at gene promoters and enhancers was broadly associated with transcriptional up-regulation of these poised genes (Russ et al. 2014). These data suggest that the presence of H3K4me3 at specific gene loci ensures that the genome of naive CD8+ T cells is preconfigured for transcriptional activation, but transcriptional restraint is maintained via colocalization of H3K27me3. It is therefore tempting to speculate that the naive state represents a restrained state that actively limits the transition into effector/memory T cells. Upon receipt of activation signals, the removal of the repressive signature enables rapid transcriptional activation of genes that underpin the reported instructional differentiation program induced by TCR signals (Kaech and Ahmed 2001; van Stipdonk et al. 2001).
Recent data supports the notion that maintenance of the naive state in CD8+ T cells is an active process. For example, the inhibitory receptor VISTA has been shown to actively restrain naive T-cell activation (ElTanbouly et al. 2020). Importantly, inflammatory signals result in VISTA down-regulation enabling subsequent effector T-cell differentiation. Other data identified members of the B-cell translocation gene/transducer of the ERBB family, BTG1 and BTG2, as key regulators of naive T-cell quiescence by directing mRNA degradation (Hwang et al. 2020). Loss of BTG1/BTG2 resulted in spontaneous proliferation of naive T cells and transition into the effector state. Altogether, these data suggest that the naive state within CD8+ T cells is one of active restraint via direct signals such as VISTA ligation, the action of BTG1/2 to ensure a low metabolic footprint, and transcriptional restraint via deposition of H3K27me3 at key gene loci (Russ et al. 2014).
CONCLUDING REMARKS
Adaptive immunity evolved approximately 400 million years ago, much more recently than innate immunity (Boehm and Swann 2014). The advent of antigen receptor diversity resulted in a wider array of potential antigens capable of inducing immune activation. As highlighted above, innate cells have immediate effector function upon receipt of stimulatory signals, a functional feature that is shared with memory T cells, with further similarities in molecular mechanisms underpinning this rapid effector responsiveness. Innate-like lymphocytes, such as natural killer T (NKT) cells and mucosal-associated invariant T (MAIT) cells sit somewhere between the adaptive and innate immune system definition (Godfrey et al. 2015). They express conventional T-cell antigen receptors but exhibit innate-like characteristics such as immediate effector function upon activation (Godfrey et al. 2015). Although expressing conventional T-cell antigen receptors, they recognize nonpeptide ligands presented via nonclassical MHC molecules and hence the triggers of activation are more limited in scope (Kawano et al. 1997; Treiner et al. 2003). As such, NKT cells and MAIT cells perhaps reflect an evolutionary stepping stone on the way to conventional T-cell immunity. With increased diversification of antigen presentation via the emergence of classical MHC class I molecules, combined with diversification of the available TCR repertoire, it is possible that the adaptive immune system used the preexisting chassis of innate-like lymphocytes, and installed molecular mechanisms that actively restrain T-cell activation to prevent inadvertent responses, thereby establishing the naive state. The existence of Tvm cells might also suggest that the naive state can be engaged to varying degrees, even before antigen encounter, to exploit the advantages both of naivete and functional poising. When it comes to designing novel vaccine strategies to establish immunological memory, the focus should be on those factors required to release the molecular handbrake that appears to restrain naive T-cell activation and differentiation, as well as those that also ensure the resulting memory T-cell populations have the right combination of self-renewal capacity and immediate responsiveness in terms of proliferation and effector function. From this greater understanding of how the naive state is established and regulated, new and improved insights into establishing effective memory CD8+ T cells will ensue.
Footnotes
Editors: David Masopust and Rafi Ahmed
Additional Perspectives on T-Cell Memory available at www.cshperspectives.org
REFERENCES
- Anderson RJ, Li J, Kedzierski L, Compton BJ, Hayman CM, Osmond TL, Tang CW, Farrand KJ, Koay HF, Almeida C, et al. 2017. Augmenting influenza-specific T cell memory generation with a natural killer T cell-dependent glycolipid-peptide vaccine. ACS Chem Biol 12: 2898–2905. 10.1021/acschembio.7b00845 [DOI] [PubMed] [Google Scholar]
- Araki Y, Wang Z, Zang C, Wood WH, Schones D, Cui K, Roh TY, Lhotsky B, Wersto RP, Peng W, et al. 2009. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity 30: 912–925. 10.1016/j.immuni.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med 11: 748–756. 10.1038/nm1257 [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837. 10.1016/j.cell.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Bauer WR, Hayes JJ, White JH, Wolffe AP. 1994. Nucleosome structural changes due to acetylation. J Mol Biol 236: 685–690. 10.1006/jmbi.1994.1180 [DOI] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195: 1541–1548. 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. 2002. Compromised influenza virus-specific CD8+-T-cell memory in CD4+-T-cell-deficient mice. J Virol 76: 12388–12393. 10.1128/JVI.76.23.12388-12393.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med 186: 65–70. 10.1084/jem.186.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393: 478–480. 10.1038/30996 [DOI] [PubMed] [Google Scholar]
- Bevington SL, Cauchy P, Piper J, Bertrand E, Lalli N, Jarvis RC, Gilding LN, Ott S, Bonifer C, Cockerill PN. 2016. Inducible chromatin priming is associated with the establishment of immunological memory in T cells. EMBO J 35: 515–535. 10.15252/embj.201592534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Sourdive DJ, Murali-Krishna K, Ahmed R, Altman JD. 2000. Evolution of the T cell repertoire during primary, memory, and recall responses to viral infection. J Immunol 165: 6081–6090. 10.4049/jimmunol.165.11.6081 [DOI] [PubMed] [Google Scholar]
- Boehm T, Swann JB. 2014. Origin and evolution of adaptive immunity. Annu Rev Anim Biosci 2: 259–283. 10.1146/annurev-animal-022513-114201 [DOI] [PubMed] [Google Scholar]
- Böttcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Höchst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, et al. 2015. Functional classification of memory CD8+ T cells by CX3CR1 expression. Nat Commun 6: 8306. 10.1038/ncomms9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch DH, Pilip I, Pamer EG. 1998. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J Exp Med 188: 61–70. 10.1084/jem.188.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039–1043. 10.1126/science.1076997 [DOI] [PubMed] [Google Scholar]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, et al. 2014. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684. 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM, et al. 2006. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest 116: 1443–1456. 10.1172/JCI27804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton JG, Narayanan M, Cuddapah S, Roychoudhuri R, Ji Y, Yang W, Patel SJ, Sukumar M, Palmer DC, Peng W, et al. 2016. Lineage relationship of CD8+ T cell subsets is revealed by progressive changes in the epigenetic landscape. Cell Mol Immunol 13: 502–513. 10.1038/cmi.2015.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukalac T, Chadderton J, Handel A, Doherty PC, Turner SJ, Thomas PG, La Gruta NL. 2014. Reproducible selection of high avidity CD8+ T-cell clones following secondary acute virus infection. Proc Natl Acad Sci 111: 1485–1490. 10.1073/pnas.1323736111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JG, McQuilten HA, Quinn KM, Olshansky M, Russ BE, Morey A, Wei S, Prier JE, La Gruta NL, Doherty PC, et al. 2019. CD4+ T help promotes influenza virus-specific CD8+ T cell memory by limiting metabolic dysfunction. Proc Natl Acad Sci 116: 4481–4488. 10.1073/pnas.1808849116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton AE, Russ BE, Doherty PC, Rao S, Turner SJ. 2011. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proc Natl Acad Sci 108: 15306–15311. 10.1073/pnas.1112520108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobek A, Moudra A, Mueller D, Huranova M, Horkova V, Pribikova M, Ivanek R, Oberle S, Zehn D, McCoy KD, et al. 2018. Strong homeostatic TCR signals induce formation of self-tolerant virtual memory CD8 T cells. EMBO J 37: e98518. 10.15252/embj.201798518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, Ceeraz S, Lines JL, Peng C, Carriere C, et al. 2020. VISTA is a checkpoint regulator for naive T cell quiescence and peripheral tolerance. Science 367: eaay0524. 10.1126/science.aay0524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8: 683–691. 10.1016/S1074-7613(00)80573-7 [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. 2011. A human memory T cell subset with stem cell-like properties. Nat Med 17: 1290–1297. 10.1038/nm.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. 2011. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477: 216–219. 10.1038/nature10339 [DOI] [PubMed] [Google Scholar]
- Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, von Andrian UH. 2016. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45: 1270–1284. 10.1016/j.immuni.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. 2015. The burgeoning family of unconventional T cells. Nat Immunol 16: 1114–1123. 10.1038/ni.3298 [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Bogatzki LY, Bevan MJ. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med 192: 557–564. 10.1084/jem.192.4.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. 2002. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med 195: 1515–1522. 10.1084/jem.20020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SM, Amezquita RA, Guan T, Kleinstein SH, Kaech SM. 2017. Polycomb repressive complex 2-mediated chromatin repression guides effector CD8+ T cell terminal differentiation and loss of multipotency. Immunity 46: 596–608. 10.1016/j.immuni.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. 2009. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med 206: 435–448. 10.1084/jem.20081829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland KL, Day EB, Apte SH, Russ BE, Doherty PC, Turner SJ, Kelso A. 2014. Epigenetic plasticity of Cd8a locus during CD8+ T-cell development and effector differentiation and reprogramming. Nat Commun 5: 3547. 10.1038/ncomms4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. 2017. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545: 60–65. 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SS, Lim J, Yu Z, Kong P, Sefik E, Xu H, Harman CCD, Kim LK, Lee GR, Li HB, et al. 2020. mRNA destabilization by BTG1 and BTG2 maintains T cell quiescence. Science 367: 1255–1260. 10.1126/science.aax0194 [DOI] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. 2016. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537: 417–421. 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6: 1236–1244. 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- Jacomet F, Cayssials E, Basbous S, Levescot A, Piccirilli N, Desmier D, Robin A, Barra A, Giraud C, Guilhot F, et al. 2015. Evidence for eomesodermin-expressing innate-like CD8+ KIR/NKG2A+ T cells in human adults and cord blood samples. Eur J Immunol 45: 1926–1933. 10.1002/eji.201545539 [DOI] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421: 852–856. 10.1038/nature01441 [DOI] [PubMed] [Google Scholar]
- Jenkins MR, Kedzierska K, Doherty PC, Turner SJ. 2007. Heterogeneity of effector phenotype for acute phase and memory influenza A virus-specific CTL. J Immunol 179: 64–70. 10.4049/jimmunol.179.1.64 [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Chu HH, McLachlan JB, Moon JJ. 2010. On the composition of the preimmune repertoire of T cells specific for peptide-major histocompatibility complex ligands. Annu Rev Immunol 28: 275–294. 10.1146/annurev-immunol-030409-101253 [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. 2001. Translating the histone code. Science 293: 1074–1080. 10.1126/science.1063127 [DOI] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol 2: 415–422. 10.1038/87720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111: 837–851. 10.1016/S0092-8674(02)01139-X [DOI] [PubMed] [Google Scholar]
- Kakaradov B, Arsenio J, Widjaja CE, He Z, Aigner S, Metz PJ, Yu B, Wehrens EJ, Lopez J, Kim SH, et al. 2017. Early transcriptional and epigenetic regulation of CD8+ T cell differentiation revealed by single-cell RNA sequencing. Nat Immunol 18: 422–432. 10.1038/ni.3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278: 1626–1629. 10.1126/science.278.5343.1626 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Nottingham LK, Sin JI, Tsai A, Morrison L, Oh J, Dang K, Hu Y, Kazahaya K, Bennett M, et al. 1998. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest 102: 1112–1124. 10.1172/JCI3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Woodland DL. 2009. Immunity to respiratory viruses. Annu Rev Immunol 27: 61–82. 10.1146/annurev.immunol.021908.132625 [DOI] [PubMed] [Google Scholar]
- Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM, Brincks EL, Cookenham T, Roberts AD, Burkum CE, et al. 2011. Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J Exp Med 208: 1621–1634. 10.1084/jem.20102110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. 2007. Chromatin modifications and their function. Cell 128: 693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- La Gruta NL, Turner SJ, Doherty PC. 2004. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol 172: 5553–5560. 10.4049/jimmunol.172.9.5553 [DOI] [PubMed] [Google Scholar]
- La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K, Thomas PG, Doherty PC, Turner SJ. 2010. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest 120: 1885–1894. 10.1172/JCI41538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. 1997. Rapid effector function in CD8+ memory T cells. J Exp Med 186: 859–865. 10.1084/jem.186.6.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CM, Adams NM, Geary CD, Weizman OE, Rapp M, Pritykin Y, Leslie CS, Sun JC. 2018. Epigenetic control of innate and adaptive immune memory. Nat Immunol 19: 963–972. 10.1038/s41590-018-0176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. 2013. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci 110: 13498–13503. 10.1073/pnas.1307572110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, et al. 2013. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 123: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci 109: 7037–7042. 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci 98: 6313–6318. 10.1073/pnas.101132698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M, Tough DF, Lefrançois L. 2004. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol 173: 969–975. 10.4049/jimmunol.173.2.969 [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417. 10.1126/science.1058867 [DOI] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol 68: 8056–8063. 10.1128/JVI.68.12.8056-8063.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH, Klawon DEJ, Zeng S, Lee V, Socci ND, Savage PA. 2020. Eomes identifies thymic precursors of self-specific memory-phenotype CD8+ T cells. Nat Immunol 21: 567–577. 10.1038/s41590-020-0653-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8: 177–187. 10.1016/S1074-7613(00)80470-7 [DOI] [PubMed] [Google Scholar]
- Olson MR, Seah SG, Edenborough K, Doherty PC, Lew AM, Turner SJ. 2014. CD154+ CD4+ T-cell dependence for effective memory influenza virus-specific CD8+ T-cell responses. Immunol Cell Biol 92: 605–611. 10.1038/icb.2014.28 [DOI] [PubMed] [Google Scholar]
- O'Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. 2014. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41: 75–88. 10.1016/j.immuni.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace L, Goudot C, Zueva E, Gueguen P, Burgdorf N, Waterfall JJ, Quivy JP, Almouzni G, Amigorena S. 2018. The epigenetic control of stemness in CD8+ T cell fate commitment. Science 359: 177–186. 10.1126/science.aah6499 [DOI] [PubMed] [Google Scholar]
- Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, Alexandre YO, Gregory JL, Russell TA, Gebhardt T, et al. 2018. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol 19: 183–191. 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. 2016. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354: 1160–1165. 10.1126/science.aaf2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H. 2007. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med 204: 1193–1205. 10.1084/jem.20062349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, West SM, Betts MR, Ruff LE, Brenchley JM, Ambrozak DR, Edghill-Smith Y, Kuroda MJ, Bogdan D, Kunstman K, et al. 2004. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21: 793–803. 10.1016/j.immuni.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, et al. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med 202: 1349–1361. 10.1084/jem.20051357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KM, Zaloumis SG, Cukalac T, Kan WT, Sng XY, Mirams M, Watson KA, McCaw JM, Doherty PC, Thomas PG, et al. 2016. Heightened self-reactivity associated with selective survival, but not expansion, of naive virus-specific CD8+ T cells in aged mice. Proc Natl Acad Sci 113: 1333–1338. 10.1073/pnas.1525167113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KM, Fox A, Harland KL, Russ BE, Li J, Nguyen THO, Loh L, Olshanksy M, Naeem H, Tsyganov K, et al. 2018. Age-related decline in primary CD8+ T cell responses is associated with the development of senescence in virtual memory CD8+ T cells. Cell Rep 23: 3512–3524. 10.1016/j.celrep.2018.05.057 [DOI] [PubMed] [Google Scholar]
- Quinn KM, Hussain T, Kraus F, Formosa LE, Lam WK, Dagley MJ, Saunders EC, Assmus LM, Wynne-Jones E, Loh L, et al. 2020. Metabolic characteristics of CD8+ T cell subsets in young and aged individuals are not predictive of functionality. Nat Commun 11: 2857. 10.1038/s41467-020-16633-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, et al. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12: 223–232. 10.1016/j.chom.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapetti L, Meunier S, Pontoux C, Tanchot C. 2008. CD4 help regulates expression of crucial genes involved in CD8 T cell memory and sensitivity to regulatory elements. J Immunol 181: 299–308. 10.4049/jimmunol.181.1.299 [DOI] [PubMed] [Google Scholar]
- Rolot M, Dougall AM, Chetty A, Javaux J, Chen T, Xiao X, Machiels B, Selkirk ME, Maizels RM, Hokke C, et al. 2018. Helminth-induced IL-4 expands bystander memory CD8+ T cells for early control of viral infection. Nat Commun 9: 4516. 10.1038/s41467-018-06978-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. 2011. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci 108: 13694–13699. 10.1073/pnas.1107594108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ BE, Olshanksy M, Smallwood HS, Li J, Denton AE, Prier JE, Stock AT, Croom HA, Cullen JG, Nguyen ML, et al. 2014. Distinct epigenetic signatures delineate transcriptional programs during virus-specific CD8+ T cell differentiation. Immunity 41: 853–865. 10.1016/j.immuni.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ BE, Olshansky M, Li J, Nguyen MLT, Gearing LJ, Nguyen THO, Olson MR, McQuilton HA, Nüssing S, Khoury G, et al. 2017. Regulation of H3K4me3 at transcriptional enhancers characterizes acquisition of virus-specific CD8+ T cell-lineage-specific function. Cell Rep 21: 3624–3636. 10.1016/j.celrep.2017.11.097 [DOI] [PubMed] [Google Scholar]
- Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, et al. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345: 1251086. 10.1126/science.1251086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22: 745–763. 10.1146/annurev.immunol.22.012703.104702 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419: 407–411. 10.1038/nature01080 [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrançois L. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1: 426–432. 10.1038/80868 [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, López-Moyado IF, Trifari S, Wong V, Chavez L, Rao A, Pereira RM. 2016. Dynamic changes in chromatin accessibility occur in CD8+ T cells responding to viral infection. Immunity 45: 1327–1340. 10.1016/j.immuni.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et al. 2016. The epigenetic landscape of T cell exhaustion. Science 354: 1165–1169. 10.1126/science.aae0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300: 337–339. 10.1126/science.1082305 [DOI] [PubMed] [Google Scholar]
- Sosinowski T, White JT, Cross EW, Haluszczak C, Marrack P, Gapin L, Kedl RM. 2013. CD8α+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J Immunol 190: 1936–1947. 10.4049/jimmunol.1203149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. 2013. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 19: 1305–1312. 10.1038/nm.3350 [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300: 339–342. 10.1126/science.1083317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Williams MA, Bevan MJ. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol 5: 927–933. 10.1038/ni1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457: 557–561. 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Jameson SC. 2009. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med 206: 2253–2269. 10.1084/jem.20082553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science 276: 2057–2062. 10.1126/science.276.5321.2057 [DOI] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. 2003. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422: 164–169. 10.1038/nature01433 [DOI] [PubMed] [Google Scholar]
- Tripp RA, Sarawar SR, Doherty PC. 1995. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2lAb gene. J Immunol 155: 2955–2959. [PubMed] [Google Scholar]
- Turner SJ, Diaz G, Cross R, Doherty PC. 2003. Analysis of clonotype distribution and persistence for an influenza virus-specific CD8+ T cell response. Immunity 18: 549–559. 10.1016/S1074-7613(03)00087-6 [DOI] [PubMed] [Google Scholar]
- Turner SJ, Doherty PC, McCluskey J, Rossjohn J. 2006. Structural determinants of T-cell receptor bias in immunity. Nat Rev Immunol 6: 883–894. 10.1038/nri1977 [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36: 68–78. 10.1016/j.immuni.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, O'Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, et al. 2013. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci 110: 14336–14341. 10.1073/pnas.1221740110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stipdonk MJ, Lemmens EE, Schoenberger SP. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol 2: 423–429. 10.1038/87730 [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. 2003. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol 4: 361–365. 10.1038/ni912 [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. 2000. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol 1: 47–53. 10.1038/76907 [DOI] [PubMed] [Google Scholar]
- Wakim LM, Gebhardt T, Heath WR, Carbone FR. 2008. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol 181: 5837–5841. 10.4049/jimmunol.181.9.5837 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, et al. 2008. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40: 897–903. 10.1038/ng.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 4: 225–234. 10.1038/ni889 [DOI] [PubMed] [Google Scholar]
- White JT, Cross EW, Burchill MA, Danhorn T, McCarter MD, Rosen HR, O'Connor B, Kedl RM. 2016. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun 7: 11291. 10.1038/ncomms11291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893. 10.1038/nature04790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Hale JS, Kissick HT, Ahn E, Xu X, Wieland A, Araki K, West EE, Ghoneim HE, Fan Y, et al. 2017. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552: 404–409. 10.1038/nature25144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Zhang K, Milner JJ, Toma C, Chen R, Scott-Browne JP, Pereira RM, Crotty S, Chang JT, Pipkin ME, et al. 2017. Epigenetic landscapes reveal transcription factors that regulate CD8+ T cell differentiation. Nat Immunol 18: 573–582. 10.1038/ni.3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zediak VP, Johnnidis JB, Wherry EJ, Berger SL. 2011. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol 186: 2705–2709. 10.4049/jimmunol.1003741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Lee SY, Bevan MJ. 2009. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458: 211–214. 10.1038/nature07657 [DOI] [PMC free article] [PubMed] [Google Scholar]