Abstract
Treatment of wounds of different aetiologies constitutes a major part of the total health care budget. It is estimated that 1·5–2 million people in Europe suffer from acute or chronic wounds. These wounds are managed both in hospitals and in community care. The patients suffering from these wounds report physical, mental and social consequences of their wounds and the care of them. It is often believed that the use of wound dressings per se is the major cost driver in wound management, whereas in fact, nursing time and hospital costs are together responsible for around 80–85% of the total cost. Healing time, frequency of dressing change and complications are three important cost drivers. However, with the use of modern, advanced technology for more rapid wound healing, all these cost drivers can be substantially reduced. A basic understanding of the terminology and principles of Health Economics in relation to wound management might therefore be of interest.
Keywords: Efficiency, Health economics, Resources, Service improvement, Wound healing
The impact of wounds on the health system – a growing challenge
Wounds have been called ‘The Silent Epidemic’
Wounds have a variety of causes; some arise from surgical intervention, some are the result of injury, and others are a consequence of extrinsic factors, such as pressure or shear, or underlying conditions such as diabetes or vascular disease. They are often classified as a result of their underlying cause into acute wounds, such as surgical wounds and burns, and chronic wounds, such as leg ulcers, diabetic foot ulcers (DFUs) and pressure ulcers 1. Whatever the cause, wounds have a substantial but often unrecognised impact on those who suffer from them, on their carers and on the health care system. In fact, the phenomenon of wounds has been called the ‘Silent Epidemic’ 2.
Living with a wound can have a profound effect on quality of life 3. The human cost of wounds manifests itself in, among other things, pain, distress, social isolation, anxiety, extended hospital stay, chronic morbidity or even mortality. Many of these issues are preventable 4. Furthermore, because of underlying factors such as the age of the patient and the presence of underlying chronic comorbidities, some wounds do not follow the normal healing process. These ‘hard‐to‐heal’ wounds [defined as wounds that fail to heal with ‘standard therapy’ in an orderly and timely manner 5] cause further deterioration in quality of life and increase the burden on the health care system over a prolonged period.
Sometimes, it is thought that the financial cost of wound management is just the cost of the materials used, such as dressings, bandages or topical antiseptics. This is not the case; most of the cost relates to the use of health care professionals' time and the cost of staying in hospital, as we will see later. The choice of materials and treatments, however, can have a major influence on the total cost.
How many people in the population have wounds?
Prevalence surveys in the UK and Denmark indicate that there are about three to four people with one or more wounds per 1000 population 6, 7, 8, 9. Many of these wounds become ‘chronic’, with studies reporting that around 15% of wounds remain unresolved 1 year after presentation 7, often resulting in a prolonged, yet avoidable, burden to patients, their families and health systems. Based on the above figures, it is estimated that in a population of 1 million people, approximately 3500 people will be living with a wound, of which, 525 will have had their wound for over 1 year.
The majority of chronic wounds are treated in non‐acute health care settings, such as clinics or home health 7, 8, 9. Studies suggest that wound management accounts for over half of community health nurse resources in European settings 4, 10. However, a substantial number of people with wounds require hospital treatment at some stage, which can escalate the costs of care significantly 4, 11. Previous studies have suggested that between 27% and 50% of hospital beds are occupied by patients requiring some form of wound management 4.
Prevalence of wounds.
There are estimated to be around 1·5–2 million people living with a chronic wound across Europe 12. In the USA, chronic wounds affect around 6·5 million people at any one time 13.
Data from Europe suggest that 64% of wounds treated in home care were chronic in aetiology 7. Of these, 24% are estimated to have been living with their wound for 6 months or more, and almost 16% had remained unhealed for a year or more 9.
Audit data from hospital settings suggest that as many as 50% of in‐patients have a wound. A study of 5800 patients in Western Australian public hospitals found that 49% had a wound. 31% of patients had acute wounds, 9% had pressure ulcers and 8% had skin tears. The authors stated their belief that a quarter of these wounds had the potential to be prevented 14.
Data from the USA demonstrate a pressure ulcer prevalence of 22% in acute critical care settings 13. Audit data from Europe indicate that 22·7% of hospital patients had signs of pressure damage [Bermark et al. 2004 15, reported in Posnett et al. 2009 4].
Wounds result in a significant economic cost to the health care system
Wounds are estimated to account for almost 3% of total health system costs 2—approximately £5billion annually according to data from the UK 16. A recent study in Wales showed that the cost of managing patients with chronic wounds amounted to 5·5% of total health service expenditure 17. Most of this cost relates to hospital stay and nursing time for treating patients at home or in clinics, whereas materials such as dressings account for a small part of the total cost. In the USA, it is reported that over US$25 billion is spent each year on the treatment of chronic wounds 13.
At an individual level, the costs of managing wounds are highly dependent on the wound type, complexity and site of care. A study from Sweden in 2002 found that the weekly cost of managing a venous leg ulcer (VLU) was around €103 per patient, with annual costs estimated to be between €1332–2585 4. DFUs are estimated to be more costly to treat than VLUs, with studies indicating a cost per episode of ∼€10 000 4. Treatment costs increase rapidly when complications such as infections or amputations occur, with a study from the USA reporting a cost per amputation of $38 077 13.
The costs of managing surgical wounds are equally difficult to estimate with any accuracy. The management of an uncomplicated surgical incision is relatively inexpensive; however, when infections occur, these costs can increase significantly. A study from the USA estimated the costs of treating a surgical site infection (SSI) to be approximately $20 800 18.
As well as costs incurred by the health system, there are also significant indirect costs that fall on individuals and the broader economy. In the USA, it has been estimated that venous ulcers cause the loss of 2 million working days per year, costing the US health care system an estimated $2·5–3·5 billion annually 13.
The total cost of treating wounds.
Wound management has been estimated to account for 3% of all health care expenditure 2.
The cost of treating pressure ulcers alone in the USA is estimated to be $11 billion/year 13.
In Europe, the cost o7f managing DFUs is estimated to be €4–6 billion/year 4.
In the USA, foot ulcers and other complications are ‘responsible for 20% of the nearly 3 million hospitalisations every year related to diabetes’ 13.
The cost per case is highly dependent on a number of factors, such as wound type, complications and the site of care. For example, uncomplicated surgical wounds are relatively inexpensive to manage, but costs increase sharply if infection occurs. In Europe, surgical wound infection is estimated to add, on average, 11 days to in‐patient hospital stay, with an average cost of €5800 per case 19, whilst data from the USA suggest that the cost of treating a surgical site infection (SSI) is in excess of $20 000 18.
A hospital performing 10 000 operations annually can expect 300–400 infections, resulting in 3300–4000 excess bed‐days, ∼€1·74–2·32 m in excess costs and 15–20 infection‐attributable deaths 20.
The cost of managing wounds is largely hidden, and the impact is often not recognised
Much of the financial cost of treating wounds is hidden because many health care professionals across a wide range of professions and care settings are involved, and so, the total cost is spread across many different budgets. As a result, their impact goes largely unrecognised by policy makers, is poorly understood by health care system decision makers and is seldom reported in the media 2.
Studies have found that between 70–80% of wound patients are treated in the community, predominantly by community nurses 9, 20. Managing wounds is often the single most important use of their time – one study estimated that over 60% of community nurses' time was spent on dressing changes 21. Studies in the UK and Denmark have shown that on average, dressings are changed around three times per week, resulting in three home health visits per week 7, 9. In a community care setting in Denmark, a survey found that at least half of the patients surveyed were having their wounds dressed three or more times per week, with 23% of patients having daily dressing changes 7.
This places a significant, and arguably unsustainable, burden on over‐stretched nurse resources in community and home health settings. Acute care facilities are constantly looking for ways of reducing length of stay; expediting discharge means that community health care providers are having to deal with greater acuity of patients with more complex needs. In addition to this, the number of patients requiring wound management is expected to increase with an ageing population and increased prevalence of multiple underlying conditions 22.
The impact of these factors on nurses is already starting to manifest itself. A study from Denmark estimates that the activity of health professionals has increased by 40% since 2001 23. Today, 72 nurses are doing what 100 nurses did in 2001, according to this analysis by the Danish Nurses Organization 23. In the UK, the number of district nurses decreased by 39% between 2002 and 2012, partly because of funding constraints but also as a result of an increase in the number of nurses leaving practice 24. This is compounded by increasing rates of retirement and reducing rates of newly qualified nurses 24. This trend is clearly unsustainable, and as a result, it is vital that health care providers seek more efficient ways of managing a resource‐intensive casemix with increasingly complex needs, such as patients with a wound.
Demand for wound management services.
Wound management is estimated to account for over 50% of community nurse time in European studies, with patients often having three or more home health visits per week 7, 9, 10, 21.
The demands on nurse time are expected to increase further because of earlier hospital discharge, ageing populations and increasing rates of morbidities associated with wounds 22.
For example, the International Diabetes Foundation estimates that worldwide diabetes prevalence will continue to increase to 9·9% by 2030 25.
Diabetic patients have up to a 25% lifetime risk of developing a foot ulcer 26.
The increasing demands on nurse time will become unsustainable if the present trend continues, and health systems need to identify more efficient ways of managing the increased workload.
Question: Are there any estimates of how much demand for wound management services will increase?
Answer: Yes, in some countries. For example, in 2010, it was estimated that the cost of wound management in municipalities in Denmark was DKK 735 m (18 000 wounds per year requiring over 3 m dressing changes). This is predicted to rise by up to 30% between 2010 and 2020 27. In the UK, the cost of providing services for the treatment of wounds could rise by over £200 million annually from 2014 to 2019. This would include around an additional 950 nursing staff and an additional 267 500 hospital bed‐days. The total additional cost to the health system of providing wound management services over this 5‐year period is estimated to be £600 m 22.
How can we reconcile increasing patient demands with scarce nurse resources?
The answer to this question is improvements in efficiency. Efficiency is an economic term that means increasing the output of a constant resource – put another way, increasing the productivity of nurses. Health economists seek to measure efficiency by contrasting the resources required to deliver health care with the health benefits that they produce. Health care resources might include:
the time used by health care professionals such as nurses, podiatrists, GPs and surgeons
dressings and other materials and equipment
hospital beds
operating theatre time
resources used in documentation and administration
Figure 1 indicates how resources are allocated to the management of wounds 6.
Figure 1.
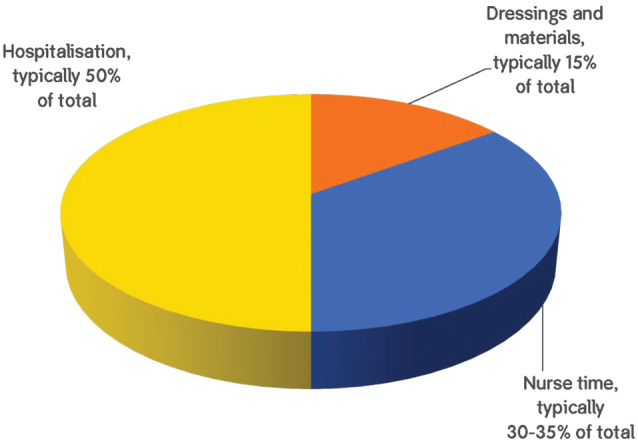
Resources used in treating wounds.
When looking for efficiency improvements, health care providers will often select the easy targets, such as supplies budgets, which are quantifiable and easily manipulated in the short term. However, it is important that they focus their attention on the main cost drivers and also consider the opportunity cost of resources, which is often less easy to quantify. The opportunity cost of a resource refers to the benefits that are foregone by allocating a resource to a particular activity. For example, a nurse making three home visits to a patient with a wound could have allocated the same time to running a diabetes clinic for 10 patients. In some cases, it is questionable whether resources can be so easily moved between activities – can the same nurse providing wound care also provide diabetes management advice? However, in many cases, the conscious decision to manage a patient in an inefficient way results in foregone benefits for other patients.
This is an important consideration for individuals involved in the management of wounds. The concept of freeing up nurse resources – for example, through less frequent home visits – means that the same nurse resource is now available to treat additional patients. Health care planners and nurses need to continuously challenge themselves by asking ‘could I be using my time more efficiently to provide better quality care or treat more patients?’
Question: Nurses are already there, and paid for, so why does it matter how often they visit patients? Saving nurse time doesn't save the system any money – surely, it is better to save money on dressings?
Answer: We need to think about the opportunity cost. What have we given up in order for the nurse to undertake the treatment? We have given up the opportunity to treat someone else or undertake some other valuable activity. This time has a monetary cost that we can calculate if we know the cost per hour of nursing time. Releasing nurse time is about freeing up time to increase efficiency – the key to solving the problem of increasing demand for services. Releasing nurse visits may also reduce the number of dressings required. This has the potential to provide procurement savings 28.
How can we deliver improved efficiency in wound management?
The first step in identifying how to improve efficiency is to consider the inputs and outputs of wound care. Inputs might be considered to be the nurse time, hospitalisations and dressings consumed, whilst the outputs are the health benefits to patients. Figure 2 indicates the main inputs that make up the cost of treating wounds.
Figure 2.
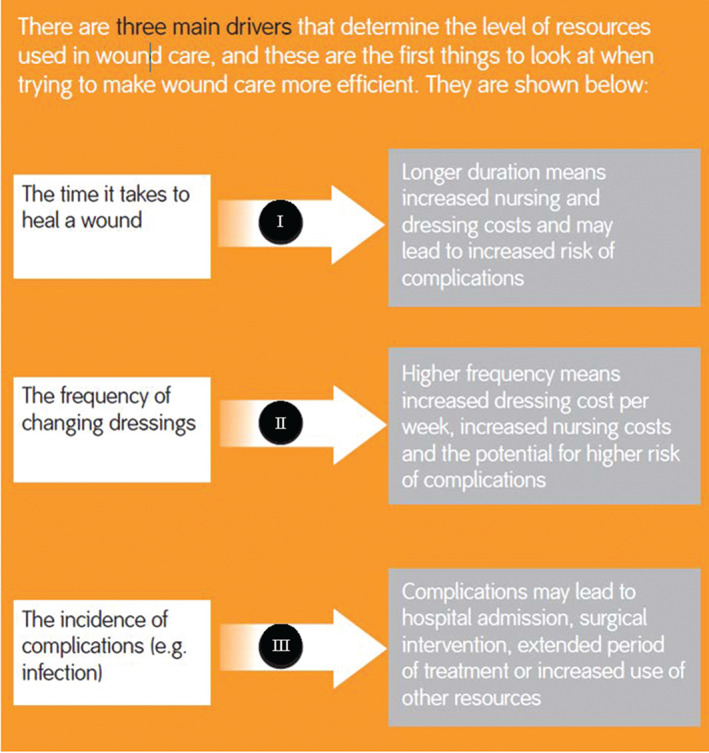
The time it takes to heal a wound
As described earlier, surveys would suggest that in a region of one million people, there are approximately 3500 people living with a wound, of which, around 525 people will have had a wound for 1 year or more. For Europe, with a population of approximately 500 million people, there would be about 262 000 people with these long‐standing wounds. The total cost of treating these long‐standing wounds in Europe runs into millions of Euros.
The frequency of changing dressings
As we have seen, in wound management, the time spent by health care professionals is a large part of the total resources used, especially in community services. Much of this time is used to change the patient's wound dressings, which is why the frequency of dressing change is a very important driver of cost.
Researchers who have audited wound management have found that, on average, wound dressings are changed about three times a week. One study of home care in Denmark found an average of 3·53 changes per week, with 23% of wounds having dressings changed every day 7.
Dressings need to be changed at a frequency that is appropriate for the patient and the wound. However, unnecessary dressing changes can have an impact on both patient well‐being and resources. Higher dressing change frequency can increase dressing and nursing costs and may lead to an increased risk of complications because of the increased frequency of wound exposure 29. It may also mean that the patient has the inconvenience of multiple appointments and the physical and emotional impact of repeated dressing removal and application 29.
The incidence of complications
Wounds do not always follow the expected healing trajectory, particularly because many patients with wounds have comorbidities and underlying long‐term conditions. Complications occur along the way, and these can have a profound effect, not only for the patient but also for the health system. Complications are the third of the three drivers of cost we are considering here.
As an example, let us consider SSIs. SSIs can lead to prolonged hospital stay, readmission, additional surgical procedures and use of antibiotics. Several years ago, a study in the UK estimated that SSIs add, on average, 11 days per episode to the patient's stay in hospital 4.
Other wound types can also become infected. It has been noted that heavy bioburden/infection of chronic wounds is a major cause of non‐healing and a significant contributor to the increasing cost of health care 30, 31. A UK survey across hospitals and community providers found that 13·3% of leg/foot ulcers and 10·4% of pressure ulcers were infected (Figure 3) 9.
Figure 3.
MRSA at 31 300× magnification.
Wound infections, whether they are at surgical sites or in other wound types, can lead to further consequences. A Swedish study reported that for non‐healing chronic wounds, 26·6% were receiving systemic antibiotics, and over 60% had received at least one antibiotic treatment over a 6‐month period 32. Wound infection is commonly associated with heavy exudation of the wound, which has recently been reported to have an influence on costs for wound management if inappropriate dressings are selected 33.
In some cases, further complications such as osteomyelitis may occur, resulting in further resource use. For example, the additional cost of treating osteomyelitis in Category III and IV pressure ulcers is substantial (estimated at over £30 000 in the UK) because the daily cost of treatment is increased and the time to healing is lengthened 34. This includes costs for biopsies, MRI, antibiotics, microbiological tests and surgery, sometimes including major plastic reconstructive surgery.
If any of these three cost drivers can be reduced, without a detrimental impact on patient outcomes, then we can deliver an improvement in efficiency. Of course, the best result for health service administrators is the delivery of improved outcomes at lower cost. This is a genuine possibility in wound management – earlier intervention to mitigate the risk of infection can result in improved patient outcomes (through the avoidance of complications) and lower treatment costs. There are fewer and fewer examples of true efficiency gains in health care that can deliver both better outcomes and lower costs; improving the efficiency of wound care should be seen as an opportunity rather than a challenge.
So, to reduce the economic cost of wounds, the best place to start is by looking at the three drivers.
Question: Surely, presenting three factors responsible for driving cost is an over‐simplified picture. Isn't it a lot more complicated than this?
Answer: In reality, the picture is of course more complex. However, by identifying three major drivers of cost, we are able to think about how we might use these to release resources and therefore make services more efficient. We will look at some ways of doing that in the next section.
Putting it in practice – delivering improved efficiency in wound management for individual patients
As we have seen above, wounds have a substantial impact on the health system. However, underneath all the statistics and figures are individual stories of how wounds impact patients, carers and health care professionals. Here are two examples.
Case study 1: reducing the frequency of nurse visits for a complex patient
This example was reported as part of an evaluation of community health care provision for patients with a wound in a provider in the UK 28. The study results indicate that the introduction of a novel dressing allowed nurses to reduce the frequency of their nurse visits by almost two visits per patient per week and that procurement costs were also reduced through less frequent dressing changes. The implications of this at a patient level can be illustrated through a case study of one patient that had a wet leaking leg that was being re‐dressed twice daily, resulting in 14 nurse visits and dressing changes per week 28. The cost of these visits and dressing changes was substantial. After switching to an advanced wound dressing, the nursing team were able to reduce the frequency of dressing change to four changes per week. The number of dressings used at each change was also reduced, resulting in fewer dressings per week, reduced cost of dressings per change and per week and a dramatic reduction in the overall cost of treatment. In fact, the patient's weekly wound management treatment costs were reduced by 81·6%, with 5 hours of community nurse time freed up per week (Table 1).
Table 1.
Summary of costs for Case study 1 28
| Wound management cost at baseline | Wound management cost after introducing the advanced wound management product | |
|---|---|---|
| Cost of materials per dressing change | £29·08 | £5·95 |
| Total cost per dressing change | £65·25 | £42·12 |
| Total cost per week | £913·50 | £168·48 |
Patients that require high levels of resource, as illustrated in this example, form part of the caseload of most community providers, and clinical staff with experience of managing wounds will recognise cases such as this. Patients receiving dressing changes daily or more frequently represent a significant proportion of all wound patients; a Danish community audit found that 23% of patients fell into this category 7. Later, we look more closely at frequency of dressing change and the opportunities that may exist for enhancing efficiency.
Case study 2: reducing the duration of wound healing
This example was reported in a case series undertaken in Denmark in 2014 35. This case series is also referred to in the final section. A 56‐year‐old female had a pressure ulcer on the hip for more than 2 months, covering an area of 48 cm2. This ulcer was being dressed daily, and the area was reducing at approximately 7% per week, on average, with conventional treatment. The wound‐healing trajectory was tracked at each dressing change using digital planimetry and tracking software. Using this software, it was estimated that the ulcer would heal in October using this treatment regime. Single‐use negative pressure therapy (NPWT) was initiated and used for 14 days, during which time the average area reduction per week was 31% (Figure 4). After discontinuation of the NPWT, the ulcer continued to heal until fully healing in May, when the weekly reduction in area was 28%.
Figure 4.
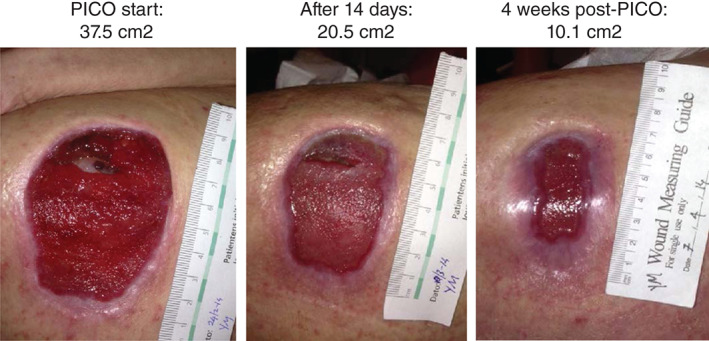
Reduction in ulcer area after single‐use negative pressure therapy (PICO†; †Trademark of Smith & Nephew) initiation 35.
This case illustrates the benefits of bringing forward the point of healing through effective intervention. Using single‐use NPWT, a shorter care package was required, freeing up health care professionals' time and enabling them to treat further patients. In addition to this release of time, fewer nurse contacts per week and shorter visiting times were required. When interventions such as this are adopted in routine practice, they have the potential to make a substantial impact on resources. The next section gives examples of changes that can be made to do ‘more with less’.
Making the best use of resources – doing more with less
As we have demonstrated above, the gap between available services and demand for services will continue to widen, and as a result, it is necessary to find ways of increasing productivity by making wound management more efficient.
It may be tempting for decision makers in these circumstances to look for ways to reduce resources by targeting easily manageable supplies budgets. However, as illustrated above, this would fail to address the main cost drivers in wound management and may even be perverse if it results in the adoption of dressings that may increase nurse visit frequency or result in decrements to health outcomes.
So what can be done to make the most efficient use of the available resources? In other words, how can resources be RELEASED or freed up so that they can be better used for other, more productive activities? As a way forward, we can take a look at the three drivers of cost (previous section) and then see some examples of how these have been used to free up resources (Figure 5).
Figure 5.
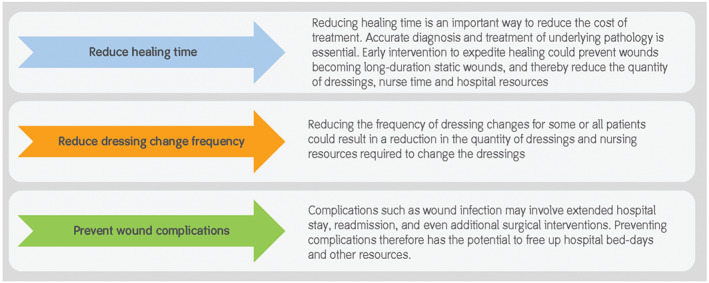
Some ways of releasing resources.
Reducing healing time
Case study 1: early intervention to reduce long‐duration wounds
One way to release resources is to concentrate on wounds that are not currently healing. These static non‐healing or recalcitrant slow‐healing wounds can account for a significant proportion of health system resources 2. By intervening to ‘kick‐start’ the healing process, they can be brought back to a healing trajectory, with the potential to release substantial resources over a long period.
A recent study showed that application of single‐use NWPT was able to accelerate the healing process of slow‐healing chronic wounds 35, 36. One case from this study was described in the previous section. By using healing trajectories to compare the rates before and after treatment, the study showed substantial cost saving as a consequence of healing these wounds earlier (Figure 6). This is an excellent example in two ways:
Firstly, it shows how intervention that appears to be of greater cost in the short term can, in fact, save resources overall.
Secondly, it illustrates the value of tracking and monitoring wound progress. This can be performed very simply, and can provide a wealth of useful data that can be used to demonstrate resource savings.
Figure 6.
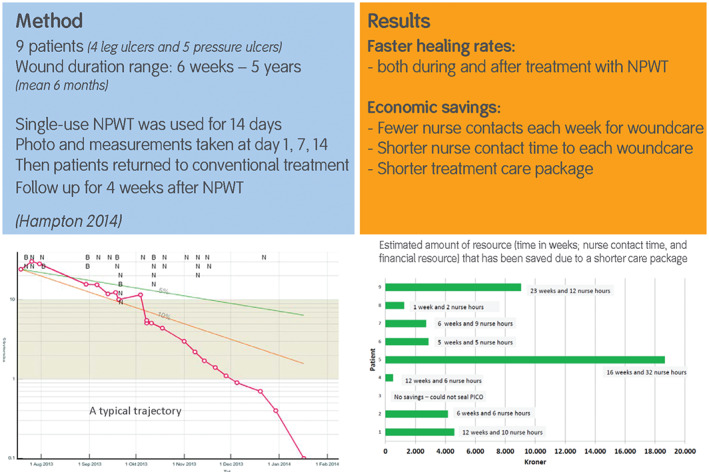
Early intervention to reduce long‐duration wounds 35.
Case study 2: report from the Swedish Registry of Ulcer Treatment (RUT)
A recently published paper described the use of a registry to shorten ulcer healing time and thereby reduce the use of resources 37. The use of the registry promoted structured wound management using a team approach, with documentation of diagnosis and wound progress. This system helped to optimise treatment by following each ulcer patient right through to the healing endpoint. Data from 1073 patients with hard‐to‐heal ulcers, treated between 2009 and 2012, were reported. Wound types were predominantly leg ulcers but also included other wound types.
The mean healing time was reduced from 269 days in 2009 to 139 days in 2012, whilst the mean total cost of treatment per patient decreased from SEK 38 000 in 2009 to SEK 20 500 in 2012. Most of the cost of treatment (approximately 87%) represented staff costs. This study shows that the use of systematic treatment strategies to reduce healing times can have a dramatic impact on the use of resources.
Optimising dressing change frequency
Case study 3: evaluations in the UK
The second driver of cost that was highlighted above was the frequency with which dressings are changed. The optimal frequency will depend on a number of factors relating to the:
wound: infection, exudate level, etc.
patient and their circumstances: e.g. comorbidities and underlying conditions
clinician: e.g. their workload and schedule
system: e.g. the logistics of when visits can be undertaken
products used: e.g. their ability to manage exudate
Innovative practice and well‐designed effective products together play a major role in this 29, and products designed to help to manage resources as well as to meet the need of patients are inherently valuable.
For example, three recent evaluations have shown that it is possible to release resources by changes in practice combined with the use of appropriate dressings 28, 38, 39. These studies described the introduction of a foam dressing into clinical practice in three community health care providers in the UK. They reported wound characteristics and details of clinical practice, such as frequency of dressing change before and after the implementation of the dressing, and showed a marked reduction in dressing change frequency (Table 2). From this information, estimates of the potential to release nurse hours were made.
Table 2.
| Evaluation | Frequency of dressing change (per week) | Number of wounds | ||
|---|---|---|---|---|
| Before introduction | After introduction | Difference | ||
| Stephen‐Haynes et al. 2013 | 4·52 | 2·88 | 1·64 | 28 |
| Simon et al. 2014 | 2 | 1·35 | 0·65 | 97 |
| Joy et al. 2014 | 3·6 | 1·8 | 1·8 | 37 |
| Combined* | 2·80 | 1·71 | 1·09 | 162 |
Mean weighted by the number of wounds in each evaluation.
One of the evaluations also estimated the cost of dressings per dressing change and per wound. On average, the number of dressings per week on each wound was reduced by 79·6%, and the mean cost of dressings per patient per week was reduced by 64·0% 28.
It would be interesting to estimate the potential for releasing resources more generally if results such as this were reproduced. Such estimates can be made if we use published wound prevalence figures (see box below).
Potential for resource release through dressing change practice.
As discussed earlier, we assume that there are 3·5 people with a wound per 1000 population 6, 7, 8, 9. A survey conducted in the UK found that 79% of wounds were treated in community health care (home health care, wound clinics, nursing homes, etc.) 6. If we apply these figures to a population of one million people, this means that at the time of writing, there are likely to be 2765 people with a wound being treated in the community.* Jørgensen et al. (2013) 7 reported that the average frequency of dressing change in community health care was 3·53 times per week, suggesting that there are approximately 9760 patient contacts for dressing change per week, or around 507 500 per year. If this could be reduced by one visit per week per patient, this could potentially release 143 800 visits or around 74 300 hours of clinician time per year.† Even reducing visits for a smaller subset of the patient population would free up a substantial quantity of time. For example, reducing frequency by one visit per week for 30% of patients could release almost 22 300 hours of nursing time per year. Furthermore, reducing dressing change frequency in conjunction with a reduction in the number of dressings used per visit could lead to a reduction in dressing expenditure. This capacity for reduction in this expenditure will depend on the pattern of dressing usage by a care provider, the mix of products that are used and the frequency with which they are changed. Nevertheless, even if only one quarter of the potential reduction reported by Joy et al. 28 were to be realised, this could indicate a 15–20% saving in dressing costs.
*3·5/1000 × 0·79 × 1 000 000 = 2765.
†Assuming 31 minutes per visit 21.
Preventing complications such as wound infection
Complications such as infection have the potential to delay healing, adversely affect the patient's quality of life and even increase the risk of mortality as a result of sepsis. For surgical sites, infection may have a dramatic effect on quality of life and a substantial impact on resources as there may be a variety of additional procedures, drugs and materials and staff time needed to deal with wound complications and their consequences. Leaper et al. (2010) 40 cite several examples:
peripheral vascular surgery, where catastrophic haemorrhage may follow vascular graft infection
infection of a hip prosthesis, where revision surgery may be necessary
infection following hysterectomy or intestinal surgery, which can result in extensive use of resources and repeated antibiotic treatment
infection following cardiac surgery, where sternal dehiscence may involve extensive multidisciplinary care
infection following breast cancer surgery, where an SSI may extend the waiting time between surgery and chemotherapy or radiotherapy
At least 5% of patients develop a post‐surgical wound infection, and it has been pointed out that although SSIs have not generally received a lot of attention, they may be the type of infection that is the most preventable 41.
Preventing SSIs therefore may have a significant impact on resource use. An example of this was published in 2014, where a hospital provider in the UK addressed the problem of SSIs by making systematic changes to their post‐surgical C‐section practice 42. Following the introduction of these measures, the infection rate dropped from 12% to 6%; in the highest‐risk group, there were no infections or readmissions. The box below shows some further details.
Case study 4: addressing the problem of SSI 43
A hospital trust in the UK found that about 12% of women experienced a surgical site infection following a caesarean section. Readmission to hospital was a particular problem, with women with high body mass index (BMI) (≥35 kg/m2) being the most likely to have a readmission.
The provider put together a multidisciplinary team including a tissue viability nurse and infection control nurse, together with the obstetricians and midwives. This team implemented changes to the wound management products alongside education for staff and patients. Part of this new approach was the targeted use of single‐use NWPT based on risk stratification. Obesity is an important risk factor as it has a strong association with the incidence of SSI. Therefore, NPWT was used for women with a BMI of 35 and above, whereas a film and pad dressing was used for women with a BMI less than 35.
The team employed this strategy for a total of 660 women who had C‐sections between February and November 2012. They reported a 50% drop in the incidence of SSIs across the whole patient group. In the high‐risk group, there were no infections, and the number of readmissions fell from three per month to zero. It was estimated that the use of the new protocol would result in a cost saving of £29 449 per year.
This study shows the value of targeting high‐risk groups and demonstrates that using negative pressure on closed incisions in high‐risk patients can potentially reduce wound complications and readmission rates.
Question: There are several examples of releasing resources by changing practice. How does an organisation know which approach to take?
Answer: This will depend on the priorities for the organisation and the opportunities for practice change. It is often useful to conduct a practice audit to ascertain which are the best opportunities for change, and several examples of surveys and audits can be found in published literature 6, 7, 8, 9, 43. These can be relatively simple, using a questionnaire format, and are often done over a short, fixed period of time to give a ‘snapshot’ of wounds and practice. For example, a community provider might survey each wound treated over a given week to ascertain characteristics of the wounds, which products were used, how often dressings were changed etc. These surveys give a wealth of information, which can be used to inform practice change, with a view to improving efficiency and quality of care.
Is prevention cheaper than cure?
One important way to reduce the cost of wound management in the future is to make an impact on the number of wounds that need to be treated. This means that wound prevention has an important role to play in counteracting the demographic trends affecting wound prevalence. For example, below‐knee graduated compression hosiery is recommended to prevent recurrence of VLUs in patients with healed ulcers 44, and regular foot assessments are effective to prevent DFUs alongside other interventions such as optimising glycaemic control and smoking cessation 45.
Often, when we think of wound prevention, it is the prevention of pressure ulcers in acute care that comes to mind, and indeed this is probably the most widely studied area and has received the most attention. Having a pressure ulcer has a profound adverse effect on many aspects of a person's quality of life, and although pressure ulcers are not a new phenomenon, they continue to be a significant health problem as a result of an ageing population 46. They also represent a significant burden to health systems, with one report estimating that treatment of pressure ulceration costs the US $11 billion annually 47.
Recently published international guidelines for the prevention and treatment of pressure ulcers have demonstrated that there a number of important considerations for prevention to be effective 48:
risk assessment
skin and tissue assessment
preventive skin care
the use of emerging therapies such as microclimate control, prophylactic dressings, fabrics and textiles and electrical muscle stimulation
good nutrition
repositioning and early mobilisation
the appropriate use of support surfaces
Thorough and comprehensive risk assessment is a vital part of prevention, and several assessment tools have been developed that, when used in conjunction with clinical judgement and experience, can help to identify the risk level for individual patients 49. As there are several potential risk factors for pressure damage, there are a number of different prevention approaches that can be used in a prevention protocol. For a given patient, the mix of these different components will vary 49.
Suitable support surfaces should be used, and the patient's skin should be inspected regularly along with a tailored repositioning programme 49. Incontinence, skin moisture, nutrition and hydration must all be managed in collaboration with the appropriate health care professionals 49. The use of multilayer foam dressings has also, in recent years, become a valuable addition to the tools available for pressure ulcer prevention, particularly for high‐risk patients such as those in high‐dependency or intensive care units 49. Such dressings have been shown to reduce the incidence of PU in these patient groups, and some hospitals have the use of these dressings as part of their PU prevention protocol. The choice of dressings is important: they must have the ability to redistribute pressure, reduce shear and friction and effectively manage temperature and moisture at the skin surface. Where surrounding skin is fragile, as is often the case with patients at risk of PU, dressings with a soft silicone border may be appropriate 49.
Much has been done to improve and implement pressure ulcer prevention measures across Europe. Economic incentives for the reduction of PU incidence and financial penalties for increased incidence have been introduced in some countries. Awareness has increased, and practice has improved, although there is continued debate about the proportion of pressure ulcers that are avoidable 50. However, there is more that can be carried out to adopt strategic approaches to prevention and to use effective risk‐assessment methods 51.
Conclusions
Wounds have a significant impact both on the quality of life of those who have them and on the world's health systems. The number of people with wounds is growing, and this is likely to continue into the future as a result of demographic trends. Preventing wounds from occurring has an important role to play in mitigating the effects of demographic changes that affect wound prevalence.
We can think systematically about the cost of caring for people with wounds by considering the resources used. Human resource is the most valuable asset that the health system has, and most of the resource used in wound management is as a result of hospital treatment or nursing visits. Materials such as dressings account for a relatively small amount of the cost; however, the choice of materials and dressings used is very important.
There are three significant drivers of cost: the time it takes a wound to heal, the frequency of visits by health care professionals and the incidence of complications.
These three drivers help us to think about how we can make wound management more efficient by:
reducing healing time
optimising dressing change frequency
preventing complications such as wound infection.
Acknowledgements
With thanks to John Posnett, Paul Trueman, Carina Ekbladh, Trine Gram and Jane Hampton for valuable contributions to the content presented here. Christina Lindholm is an independent researcher at Sophiahemmet University, Sweden. Richard Searle is an employee of Smith & Nephew. This work was funded by Smith & Nephew.
References
- 1. Harding K. Understanding healing after skin breakdown. In: Skin breakdown – the silent epidemic. Hull: Smith & Nephew Foundation, 2007:13–16. [Google Scholar]
- 2. Smith & Nephew Foundation; (2007). Skin breakdown – the silent epidemic. Smith & Nephew Foundation, Hull. [Google Scholar]
- 3. Wounds International (2012). International consensus. Optimising wellbeing in people living with a wound. Wounds International, London.
- 4. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care 2009;18:154–61. [DOI] [PubMed] [Google Scholar]
- 5. Troxler M, Vowden K, Vowden P. Integrating adjunctive therapy into practice: the importance of recognising ‘hard‐to‐heal’ wounds. World Wide Wounds 2006. URL http://www.worldwidewounds.com/2006/december/Troxler/Integrating-Adjunctive-Therapy-Into-Practice.html [accessed on November 2015].
- 6. Vowden K, Vowden P, Posnett J. The resource costs of wound care in Bradford and Airedale primary care trust in the UK. J Wound Care 2009;18:93–102. [DOI] [PubMed] [Google Scholar]
- 7. Jørgensen SF, Nygaard R, Posnett J. Meeting the challenges of wound care in Danish home care. J Wound Care 2013;22:540–2, 544–5. [DOI] [PubMed] [Google Scholar]
- 8. Gottrup F, Henneberg E, Trangbæk R, Bækmark N, Zøllner K, Sørensen J. Point prevalence of wounds and cost impact in the acute and community setting in Denmark. J Wound Care 2013;22:413–4, 416, 418–22. [DOI] [PubMed] [Google Scholar]
- 9. Drew P, Posnett J, Rusling L. The cost of wound care for a local population in England. Int Wound J 2007;4:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Srinivasaiah N, Dugdall H, Barrett S, Drew PJ. A point prevalence survey of wounds in north‐east England. J Wound Care 2007;16:413–6, 418–9. [DOI] [PubMed] [Google Scholar]
- 11. Lindholm C, Andersson H, Fossum B, Jörbeck H. Wounds scrutiny in a Swedish hospital: prevalence, nursing care and bacteriology, including MRSA. J Wound Care 2005;14:313–9. [DOI] [PubMed] [Google Scholar]
- 12.Eucomed Wound Care Policy Paper. URL http://ewma.org/fileadmin/user_upload/EWMA/pdf/EWMA_Projects/090923__Wound_Care_Brochure_final.pdf [accessed on June 2015].
- 13. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santamaria N. Woundswest: identifying the prevalence of wounds within western Australia's public health system. EWMA Journal 2009;9:13–8. [Google Scholar]
- 15. Bermark S, Zimmerdahl V, Muller K. Prevalence investigation of pressure ulcers. EWMA J 2004;4:1, 7–11. [Google Scholar]
- 16. Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, Vowden K, Vowden P. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015;5:e009283. doi: 10.1136/bmjopen-2015-009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phillips CJ, Humphreys I, Fletcher J, Harding K, Chamberlain G, Macey S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J 2015. doi: 10.1111/iwj.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care‐associated infections – a meta‐analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013;173:2039–46. [DOI] [PubMed] [Google Scholar]
- 19. Defez C, Fabbro‐Peray P, Cazaban M, Boudemaghe T, Sotto A, Daurès JP. Additional direct medical costs of nosocomial infections: an estimation from a cohort of patients in a French university hospital. J Hosp Infect 2008;68:130–6. [DOI] [PubMed] [Google Scholar]
- 20. Smith & Nephew . The true cost of wounds and how to reduce it. URL http://www.smith-nephew.com/documents/uk/the-true-cost-of-wound-booklet.pdf [accessed on April 2016].
- 21. O'Keeffe M. Evaluation of a community‐based wound care programme in an urban area. Poster presented at EWMA Conference; Prague: Czech Republic, 2006. [Google Scholar]
- 22. Dowsett C, Bielby A, Searle R. Reconciling increasing wound care demands with available resources. J Wound Care 2014;23:552–62. doi: 10.12968/jowc.2014.23.11.552. [DOI] [PubMed] [Google Scholar]
- 23. Kjeldsen SB. Tidspres er en trussel mod patientsikkerheden. Sygeplejersken 2015;2015:24–7. [Google Scholar]
- 24. The Royal College of Nursing . Frontline First: Nursing on Red Alert April 2013. London: The Royal College of Nursing. URL http://wwwrcnorguk/__data/assets/pdf_file/0003/518376/004446pdf [accessed on July 2014].
- 25. International Diabetes Federation . Diabetes Atlas 5th Edition, 2015. URL www.idf.org [accessed on November 2015].
- 26. Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes 2009;27:52–8. [Google Scholar]
- 27. Hjort A, Gottrup F. Cost of wound treatment to increase significantly in Denmark over the next decade. J Wound Care 2010;19:173–4, 176, 178, 180, 182, 184. [DOI] [PubMed] [Google Scholar]
- 28. Joy H, Bielby A, Searle R. A collaborative project to enhance efficiency through dressing change practice. J Wound Care 2015;24:312, 314–7. [DOI] [PubMed] [Google Scholar]
- 29. Stephen‐Haynes J, Bielby A, Searle R. Putting patients first: reducing the human and economic costs of wounds. Wounds UK 2011;7:47–55. [Google Scholar]
- 30. Siddiqui AR, Bernstein JM. Chronic wound infection: facts and controversies. Clin Dermatol 2010;28:519–26. [DOI] [PubMed] [Google Scholar]
- 31. Vowden P. Hard‐to‐heal wounds made easy. Wounds International, Schofield Healthcare Media Ltd: Norwich, UK, 2011;2. URL http://www.woundsinternational.com. [Google Scholar]
- 32. Tammelin A, Lindholm C, Hambraeus A. Chronic ulcers and antibiotic treatment. J Wound Care 1998;7:435–7. [DOI] [PubMed] [Google Scholar]
- 33. Benbow M. The expense of exudate management. Br J Nurs 2015;24(15 Suppl):S8. [DOI] [PubMed] [Google Scholar]
- 34. Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care 2012;21:261–266. [DOI] [PubMed] [Google Scholar]
- 35. Hampton J. Accelerated wound healing in a community setting, 2014. URL http://www.smith-nephew.com/documents/nordics/dk/nordic%20network%20meeting/accelerated%20wound%20healing%20in%20a%20community%20setting_jane%20hampton.pdf
- 36. Hampton J. Providing cost‐effective treatment of hard‐to‐heal wounds in the community through use of NPWT. Br J Community Nurs 2015;S14:S16–20. [DOI] [PubMed] [Google Scholar]
- 37. Öien RF, Forssell H, Ragnarson Tennvall G. Cost consequences due to reduced ulcer healing times – analyses based on the Swedish Registry of Ulcer Treatment. Int Wound J 2015. doi: 10.1111/iwj.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stephen‐Haynes J, Bielby A, Searle R. The clinical performance of a silicone foam in an NHS community trust. J Community Nurs 2013;27:50–9. [Google Scholar]
- 39. Simon D, Bielby A. A structured collaborative approach to appraise the clinical performance of a new product. Wounds UK 2014;10:80–7. [Google Scholar]
- 40. Leaper NJ, Roberts C, Searle R. Economic and clinical contributions of an antimicrobial barrier dressing: a strategy for the reduction of surgical site infections. J Med Econ 2010;13:447–52. [DOI] [PubMed] [Google Scholar]
- 41. Leaper DJ. Surgical site infection. Br J Surg 2010;97:1601–2. [DOI] [PubMed] [Google Scholar]
- 42. Bullough L, Wilkinson D, Burns S, Wan L. Changing wound care protocols to reduce postoperative caesarean section infection and readmission. Wounds UK 2014;10:72–6. [Google Scholar]
- 43. Ousey K, Stephenson J, Barrett S, King B, Morton N, Fenwick K, Carr C. Wound care in five English NHS trusts: results of a survey. Wounds UK 2013;9:20–8. [Google Scholar]
- 44. Scottish Intercollegiate Guidelines Network . Management of chronic venous leg ulcers – a national clinical guideline. Edinburgh: Healthcare Improvement Scotland, 2010. [Google Scholar]
- 45. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 46. Moore Z, Cowman S. Quality of life and pressure ulcers: a literature review. Wounds UK 2009;5:58–65. [Google Scholar]
- 47. U.S. Department of Health and Human Services . AHRQ Research Activities Issue, 2011; 371: 31.
- 48. National Pressure Ulcer Advisory Panel . European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance, 2014. Clinical practice guideline: prevention and treatment of pressure ulcers.
- 49. Sammon M, Dunk AM, Verdú J. Advances in pressure ulcer prevention and treatment. Wounds International, Schofield Healthcare Media Ltd: Norwich, UK, 2015. [Google Scholar]
- 50. Downie F, Guy H, Gilroy P, Royall D, Davies S. Are 95% of hospital‐acquired pressure ulcers avoidable? Wounds UK 2013;9:16–22. [Google Scholar]
- 51. Källman U, Suserud B. Knowledge, attitudes and practice among nursing staff concerning pressure ulcer prevention and treatment – a survey in a Swedish healthcare setting. Scand J Caring Sci 2009;23:334–41. [DOI] [PubMed] [Google Scholar]


