Abstract
Objective
China is one of the countries with the heaviest burden of gastric cancer (GC) in the world. Understanding the epidemiological trends and patterns of GC in China can contribute to formulating effective prevention strategies.
Methods
The data on incidence, mortality, and disability-adjusted life-years (DALYs) of GC in China from 1990 to 2019 were obtained from the Global Burden of Disease Study (2019). The estimated annual percentage change (EAPC) was calculated to evaluate the temporal trends of disease burden of GC, and the package Nordpred in the R program was used to perform an age-period-cohort analysis to predict the numbers and rates of incidence and mortality in the next 25 years.
Results
The number of incident cases of GC increased from 317.34 thousand in 1990 to 612.82 thousand in 2019, while the age-standardized incidence rate (ASIR) of GC decreased from 37.56 per 100,000 in 1990 to 30.64 per 100,000 in 2019, with an EAPC of −0.41 [95% confidence interval (95% CI): −0.77, −0.06]. Pronounced temporal trends in mortality and DALYs of GC were observed. In the next 25 years, the numbers of new GC cases and deaths are expected to increase to 738.79 thousand and 454.80 thousand, respectively, while the rates of incidence and deaths should steadily decrease. The deaths and DALYs attributable to smoking were different for males and females.
Conclusions
In China, despite the fact that the rates of GC have decreased during the past three decades, the numbers of new GC cases and deaths increased, and will continue to increase in the next 25 years. Additional strategies are needed to reduce the burden of GC, such as screening and early detection, novel treatments, and the prevention of risk factors.
Keywords: Gastric cancer, disease burden, temporal trend, risk factor, prediction
Introduction
Gastric cancer (GC) is a malignant tumor that causes serious disease burden, being the fifth most commonly diagnosed cancer and the third cause of death (1,2). In 2018, there were more than 1,033,701 new cases of GC and an estimated 782,685 deaths from it, worldwide (3). China is a high incidence region of GC (4). According to the 2018 global cancer statistics, 456,124 new GC cases and 390,182 deaths were estimated to have occurred in China, accounting for 44.1% and 49.9% of the cases worldwide, respectively (3). The high incidence and mortality in China highlight the urgency of preventing and treating GC.
GC is a multifactorial disease that can be caused by both environmental and genetic factors (5). Some of these factors are not modifiable (such as age, gender, and family genetic history), while others are potentially modifiable (such as smoking, alcohol consumption, and poor dietary habits) (5,6). These potentially modifiable factors make it possible to prevent and control the incidence of GC. Helicobacter pylori (H. pylori) infection is the main risk factor for the development of GC (7). The population infection rate of H. pylori is approximately 50% (8,9), while only about 1% of infected cases eventually develop into GC (9). Unhealthy lifestyles, such as smoking and alcohol consumption, also have been found to have a significant association with an increased risk of developing GC (5,6). In addition, dietary factors, such as the intake of salty food and low consumption of fruits and vegetables, may increase the risk of GC development (5). Moreover, sex is also a significant risk factor for GC (5,10), and the differences between the sexes may be attributed to their different lifestyles and dietary habits (5). Therefore, it is of great importance to develop prevention and control strategies for GC according a person’s sex.
China is one of the largest developing countries and has rapid economic growth. As the economy develops, people’s lifestyles and dietary factors change accordingly. It is important and necessary to understand the epidemiological trends and patterns of GC in China, as this is essential for formulating relevant prevention strategies to improve public health. The latest Global Burden of Diseases (GBD) study (2019) provides extensive data on the incidence, mortality, and disability-adjusted life-years (DALYs) of GC from 1990 to 2019, which made it possible for us to understand the disease burden of GC in China. Therefore, in this study, using the latest data of the GBD study (2019) in China, we comprehensively evaluated the national disease burden of GC and its temporal trends in relation to age, sex, and risk factors; and also predicted the numbers and rates of GC incidence and mortality in the next 25 years. Our research results should improve our understanding of the disease burden of GC, help us evaluate the effectiveness of current prevention strategies, and provide a more scientific basis for promoting the establishment of prevention strategies.
Materials and methods
Data sources
The detailed methods of GBD study (2019) have been reported in previous studies (11-15). The data on incidence, mortality, and DALYs were downloaded from the Institute for Health Metrics and Evaluation (IHME, http://ghdx.healthdata.org/gbd-results-tool). The codes used for GBD study analysis can be accessed through the following address: http://ghdx.healthdata.org/gbd-2019/code. In GBD Study, all estimates were generated with 95% uncertainty intervals (95% UIs), which were determined based on the 2.5th and 9.75th-ordered percentiles of 1,000 draws of the uncertainty distribution (14). In the database, we used the following screening rules for this study. First, the location name was “China” and the cause was “Stomach cancer.” Second, we chose “incidence,” “death,” and “DALYs” as measures.
The age-standardized rates for the incidence and mortality of GC were estimated using the World Health Organization (WHO) World Standard Population Distribution (2000−2025). For the prediction of GC burden, the predicted Chinese population was obtained from the United Nations World Population Prospects 2019 Revision, by year, sex, and age ( https://population.un.org/wpp/Download/Standard/Population/).
This study was approved by the Ethics Committee of Qilu Hospital of Shandong University. For the GBD is a publicly available database, all participants’ data were anonymous.
Evaluation of GC burden
The incidence and mortality of GC in the GBD dataset were determined in the following ways: 1) based on all the data sources that reported GC incidence and mortality (with international disease classification codes), the mortality-to-incidence ratio (MIR) was calculated using a linear-step mixed-effects model (the covariates were Healthcare Access and Quality Index, age, and sex), and the estimates were smoothed and adjusted using spatiotemporal Gaussian process regression (13,15,16); 2) we calculated mortality estimates by multiplying cancer register incidence data by the MIR (13,15,16); 3) all these data were used as input to follow the Cause of Death Ensemble model process to determine the cancer-specific mortality of GC (13,15,16); and 4) we divided cancer-specific mortality of GC estimates by the MIR to generate incidence (13,15,16).
Statistical analysis
Descriptive analysis for GC incidence, mortality, and DALYs was performed by 5-year age groups, sex, and year; we plotted the temporal trend of these indicators from 1990 to 2019. The age information of incidence and DALYs of GC was considered to be the age at diagnosis, while the age information of the death of GC represented the age at death. Age was divided into 18 age-specific groups by every 5 years, and the ages of 0−14 years were combined into one age group. The indicator of estimated annual percentage change (EAPC) was used to reflect the temporal trend of the age-standardized incidence rate (ASIR), the age-standardized mortality rate (ASMR), and the age-standardized DALYs rate. EAPC was calculated according to a regression model fitted to the natural logarithm of the rate, namelyln(rate) = α + β × (calendar year) + ε. EAPC was defined as 100 × (exp(β) −1); its 95% confidence interval (95% CI) was also calculated in the fitted model ( 16,17).
For the risk factors, the comparative risk assessment (CRA) framework was used to estimate the proportion of DALYs attributable to two well-established risk factors for GC by age and sex: smoking and the high-sodium diet (12,13). CRA was conducted through the following six key steps: 1) including risk-outcome pairs with convincing or probable evidence based on research (18,19); 2) summarizing the relative risk of potential exposure based on systematic reviews and meta-regression (18,19); 3) estimating the exposure levels and distributions using the spatiotemporal Gaussian process regression, DisMod-MR 2.1, and other methods (18,19); 4) defining the theoretical minimum risk exposure level as the exposure level associated with the minimum risk determined from published trials and cohort studies (18,19); 5) calculating the population attributable fractions (PAFs) and attributable burden (18,19); and 6) estimating the PAFs and attributable burden for combinations of risk factors by considering the mediation of different risk factors through other risk factors (18,19).
The package Nordpred in the R program, which has been shown to perform well in predicting the trend of cancer incidence (20-22), was used to perform an age-period-cohort (APC) analysis to predict the numbers and rates of the incidence and mortality of GC in the next 25 years, taking into account both the change in rates and the population structure. The Nordpred package can apply the power5 and poisson APC models to perform the prediction ( https://rdrr.io/github/haraldwf/nordpred/man/nordpred.html). In our study, we used the power5 APC model to perform the prediction. The basic APC model is g(λij) = μ + αi + βj + γk, (default link function is λij ^ 0.2, where μ represents the intercept, and αi, βj, and γk represent the effect of age, period, and cohort, respectively) (23). Moreover, to facilitate comparison with the predicted results, based on the observed data of GC in 2019, we estimated the numbers and rates of GC events, assuming that they remained stable, decreased (optimistic reference), and increased (pessimistic reference) by 1% per year. The ggplot2 and RcolorBrewer packages of the open-source R program (Version 3.6.2; R core team, R Foundation for Statistical Computing, Vienna, Austria) were used to perform the visualization of the results.
Results
Incidence, mortality and DALYs of GC in 2019
In 2019, the number of incident cases and ASIR of GC were 612.82 thousand (95% UI: 513.00, 728.89) and 30.64 per 100,000 (95% UI: 25.82, 36.15) among the total Chinese population, respectively (Table 1). GC contributed to 421.54 thousand (95% UI: 353.52, 493.18) deaths in 2019, and the total population of ASMR was 21.72 per 100,000 (95% UI: 18.31, 25.31) (Table 2). GC caused 9,824.99 thousand (95% UI: 8,191.72, 11,632.86) DALYs in 2019, and the age-standardized rate of DALYs was 481.15 per 100,000 (95% UI: 403.20, 567.36) (Table 3). The number and age-standardized rates of incidence, mortality and DALYs for males were more than 2 times higher that for females (Tables 1-3).
1. Number of incident cases and incidence rate of gastric cancer in China in 1990 and 2019 and EAPC from 1990 to 2019.
| Characteristics | 1990 | 2019 | 1990−2019 | ||||
| Incident cases
[×103 (95% UI)] |
Incidence rate
[per 100,000 (95% UI)] |
Incident cases
[×103 (95% UI)] |
Incidence rate
[per 100,000 (95% UI)] |
EAPC in incidence rate
[% (95% CI)] |
|||
| EAPC, estimated annual percentage change; 95% UI, 95% uncertainty interval; 95% CI, 95% confidence interval; *, age-standardized incidence rate; **, crude incidence rate in each age group. | |||||||
| Overall* | 317.34
(277.90, 359.32) |
37.56
(33.08, 42.27) |
612.82
(513.00, 728.89) |
30.64
(25.82, 36.15) |
−0.41
(−0.77, −0.06) |
||
| Sex* | |||||||
| Male | 207.53
(173.24, 245.61) |
51.07
(42.99, 59.92) |
451.33
(357.18, 560.15) |
47.35
(38.00, 57.95) |
0.14
(−0.21, 0.50) |
||
| Female | 109.81
(92.09, 127.87) |
25.57
(21.49, 29.61) |
161.49
(130.60, 198.34) |
15.80
(12.80, 19.38) |
−1.60
(−1.95, −1.25) |
||
| Age at diagnosis** (year) | |||||||
| 0−14 | 0 | 0 | 0 | 0 | − | ||
| 15−19 | 0.67
(0.58, 0.77) |
0.53
(0.45, 0.61) |
0.32
(0.27, 0.39) |
0.43
(0.36, 0.51) |
−0.61
(−0.81, −0.41) |
||
| 20−24 | 1.51
(1.29, 1.79) |
1.14
(0.97, 1.35) |
1.08
(0.90, 1.28) |
1.32
(1.10, 1.56) |
0.26
(0.04, 0.49) |
||
| 25−29 | 2.33
(1.99, 2.76) |
2.11
(1.80, 2.50) |
2.75
(2.31, 3.24) |
2.49
(2.08, 2.93) |
0.51
(0.16, 0.86) |
||
| 30−34 | 4.52
(3.90, 5.23) |
5.10
(4.41, 5.91) |
7.32
(6.19, 8.56) |
5.67
(4.79, 6.63) |
0.21
(−0.06, 0.48) |
||
| 35−39 | 9.10
(7.90, 10.39) |
9.94
(8.64, 11.35) |
9.38
(7.89, 11.01) |
9.30
(7.82, 10.91) |
−0.39
(−0.79, 0.01) |
||
| 40−44 | 13.04
(11.19, 14.93) |
19.39
(16.64, 22.21) |
17.26
(14.16, 20.69) |
16.98
(13.93, 20.36) |
−0.40
(−0.77, −0.04) |
||
| 45−49 | 16.58
(14.05, 19.31) |
32.05
(27.17, 37.35) |
28.65
(22.92, 35.4) |
23.60
(18.88, 29.16) |
−0.69
(−1.03, −0.35) |
||
| 50−54 | 28.59
(24.24, 33.60) |
59.81
(50.71, 70.30) |
49.66
(39.63, 60.81) |
39.70
(31.68, 48.61) |
−1.30
(−1.76, −0.83) |
||
| 55−59 | 43.14
(36.54, 50.25) |
99.27
(84.08, 115.61) |
62.31
(49.73, 76.86) |
65.70
(52.44, 81.04) |
−1.04
(−1.49, −0.59) |
||
| 60−64 | 48.51
(41.72, 55.79) |
136.95
(117.77, 157.51) |
79.58
(64.92, 96.74) |
101.31
(82.65, 123.15) |
−0.57
(−0.93, −0.20) |
||
| 65−69 | 51.28
(44.67, 58.52) |
187.37
(163.22, 213.82) |
100.85
(83.28, 121.05) |
143.29
(118.33, 171.98) |
−0.53
(−0.86, −0.19) |
||
| 70−74 | 46.30
(40.73, 52.73) |
245.56
(216.01, 279.63) |
101.08
(84.71, 119.76) |
211.22
(177.00, 250.24) |
−0.44
(−0.79, −0.10) |
||
| 75−79 | 29.98
(26.62, 33.41) |
262.70
(233.29, 292.81) |
71.46
(60.79, 83.33) |
239.42
(203.68, 279.20) |
−0.05
(−0.43, 0.33) |
||
| 80−84 | 15.29
(13.55, 17.16) |
271.17
(240.36, 304.30) |
51.16
(43.64, 58.83) |
268.31
(228.86, 308.54) |
0.44
(−0.01, 0.90) |
||
| 85−89 | 5.48
(4.73, 6.15) |
285.90
(246.78, 320.89) |
24.19
(20.67, 27.11) |
284.43
(243.00, 318.82) |
0.35
(0.04, 0.65) |
||
| 90−94 | 0.90
(0.75, 1.03) |
240.32
(202.33, 276.45) |
4.99
(4.09, 5.73) |
222.25
(182.15, 255.38) |
−0.29
(−0.62, 0.03) |
||
| 95+ | 0.13
(0.11, 0.15) |
204.27
(169.88, 235.06) |
0.78
(0.58, 0.91) |
173.72
(130.98, 204.82) |
−0.45
(−0.75, −0.14) |
||
2. Number of deaths and mortality rate of gastric cancer in China in 1990 and 2019 and EAPC from 1990 to 2019.
| Characteristics | 1990 | 2019 | 1990−2019 | ||||
| Deaths cases
[×103 (95% UI)] |
Mortality rate
[per 100,000 (95% UI)] |
Deaths cases
[×103 (95% UI)] |
Mortality rate
[per 100,000 (95% UI)] |
EAPC in mortality rate
[% (95% CI)] |
|||
| EAPC, estimated annual percentage change; 95% UI, 95% uncertainty interval; 95% CI, 95% confidence interval; *, age-standardized mortality rate; **, crude mortality rate in each age group. | |||||||
| Overall* | 305.47
(267.21, 345.40) |
37.73
(33.20, 42.39) |
421.54
(353.52, 493.18) |
21.72
(18.31, 25.31) |
−1.68
(−2.09, −1.27) |
||
| Sex* | |||||||
| Male | 197.06
(161.82, 232.16) |
51.36
(42.96, 59.94) |
298.51
(238.47, 363.78) |
33.14
(26.67, 39.89) |
−1.17
(−1.58, −0.77) |
||
| Female | 108.41
(90.42, 126.30) |
26.17
(21.94, 30.37) |
123.03
(99.95, 150.64) |
12.20
(9.93, 14.92) |
−2.59
(−3.00, −2.17) |
||
| Age at death** (year) | |||||||
| 0−14 | 0 | 0 | 0 | 0 | − | ||
| 15−19 | 0.39
(0.33, 0.44) |
0.31
(0.26, 0.35) |
0.10
(0.08, 0.11) |
0.13
(0.11, 0.15) |
−3.04
(−3.31, −2.77) |
||
| 20−24 | 0.97
(0.82, 1.15) |
0.73
(0.62, 0.87) |
0.35
(0.30, 0.42) |
0.43
(0.37, 0.51) |
−2.25
(−2.53, −1.97) |
||
| 25−29 | 1.63
(1.37, 1.94) |
1.48
(1.25, 1.76) |
0.99
(0.84, 1.16) |
0.90
(0.76, 1.05) |
−2.00
(−2.41, −1.58) |
||
| 30−34 | 3.45
(2.97, 3.99) |
3.90
(3.36, 4.51) |
2.80
(2.40, 3.26) |
2.17
(1.86, 2.53) |
−2.41
(−2.81, −2.00) |
||
| 35−39 | 7.49
(6.48, 8.57) |
8.18
(7.08, 9.36) |
4.22
(3.56, 4.91) |
4.18
(3.53, 4.87) |
−2.58
(−3.10, −2.06) |
||
| 40−44 | 11.30
(9.62, 12.98) |
16.81
(14.31, 19.3) |
7.93
(6.52, 9.40) |
7.80
(6.42, 9.25) |
−2.71
(−3.17, −2.25) |
||
| 45−49 | 14.22
(11.93, 16.75) |
27.49
(23.07, 32.38) |
14.72
(11.90, 17.94) |
12.13
(9.81, 14.78) |
−2.56
(−2.99, −2.14) |
||
| 50−54 | 24.93
(20.98, 29.09) |
52.15
(43.89, 60.85) |
27.63
(22.22, 33.72) |
22.08
(17.76, 26.96) |
−2.94
(−3.48, −2.39) |
||
| 55−59 | 38.08
(31.95, 44.26) |
87.62
(73.52, 101.83) |
36.24
(29.19, 44.46) |
38.21
(30.78, 46.88) |
−2.57
(−3.07, −2.07) |
||
| 60−64 | 44.66
(38.10, 51.63) |
126.09
(107.56, 145.76) |
49.19
(40.10, 59.61) |
62.62
(51.04, 75.88) |
−2.02
(−2.43, −1.61) |
||
| 65−69 | 49.44
(42.85, 56.09) |
180.64
(156.59, 204.96) |
66.58
(54.98, 79.19) |
94.59
(78.11, 112.51) |
−1.90
(−2.29, −1.50) |
||
| 70−74 | 47.68
(41.85, 53.94) |
252.85
(221.94, 286.05) |
72.00
(59.97, 84.78) |
150.45
(125.31, 177.15) |
−1.77
(−2.18, −1.35) |
||
| 75−79 | 33.79
(30.17, 37.98) |
296.13
(264.44, 332.88) |
58.62
(49.61, 67.97) |
196.41
(166.22, 227.73) |
−1.20
(−1.64, −0.75) |
||
| 80−84 | 18.58
(16.52, 20.79) |
329.48
(293.02, 368.77) |
45.95
(39.39, 52.37) |
241.00
(206.60, 274.65) |
−0.66
(−1.16, −0.16) |
||
| 85−89 | 7.29
(6.37, 8.13) |
380.16
(332.34, 424.21) |
26.73
(23.22, 29.89) |
314.34
(273.07, 351.48) |
−0.32
(−0.66, 0.02) |
||
| 90−94 | 1.34
(1.15, 1.52) |
360.42
(308.43, 408.56) |
6.20
(5.08, 7.12) |
276.41
(226.40, 317.13) |
−0.97
(−1.34, −0.59) |
||
| 95+ | 0.23
(0.18, 0.26) |
364.60
(295.35, 421.56) |
1.27
(0.99, 1.49) |
285.19
(222.81, 332.88) |
−0.81
(−1.13, −0.50) |
||
3. Number of DALYs and DALYs rate of gastric cancer in China in 1990 and 2019 and EAPC from 1990 to 2019.
| Characteristics | 1990 | 2019 | 1990−2019 | ||||
| DALYs
[×103 (95% UI)] |
DALYs rate
[per 100,000 (95% UI)] |
DALYs
[×103 (95% UI)] |
DALYs rate
[per 100,000 (95% UI)] |
EAPC in DALYs rate
[% (95% CI)] |
|||
| DALYs, disability-adjusted life-years; EAPC, estimated annual percentage change; 95% UI, 95% uncertainty interval; 95% CI, 95% confidence interval; *, age-standardized DALYs rate; **, crude DALYs rate in each age group. | |||||||
| Overall* | 8,248.79
(7,173.64, 9,366.34) |
905.54
(791.75, 1,024.49) |
9,824.99
(8,191.72, 11,632.86) |
481.15
(403.20, 567.36) |
−1.98
(−2.40, −1.57) |
||
| Sex* | |||||||
| Male | 5,409.61
(4,428.71, 6,387.35) |
1,205.79
(992.78, 1,421.38) |
7,135.99
(5,633.55, 8,786.15) |
718.79
(571.96, 877.30) |
−1.47
(−1.89, −1.05) |
||
| Female | 2,839.18
(2,341.70, 3,338.41) |
619.82
(513.84, 726.15) |
2,689.00
(2,162.24, 3,333.93) |
260.97
(209.75, 323.21) |
−3.02
(−3.41, −2.63) |
||
| Age at diagnosis** (year) | |||||||
| 0−14 | 0 | 0 | 0 | 0 | − | ||
| 15−19 | 27.89
(24.02, 32.00) |
21.98
(18.93, 25.22) |
7.04
(5.99, 8.20) |
9.37
(7.97, 10.92) |
−3.01
(−3.27, −2.74) |
||
| 20−24 | 65.22
(55.28, 77.12) |
49.26
(41.75, 58.25) |
24.04
(20.29, 28.18) |
29.36
(24.79, 34.42) |
−2.21
(−2.49, −1.94) |
||
| 25−29 | 101.13
(85.31, 120.09) |
91.77
(77.42, 108.98) |
62.38
(53.01, 72.42) |
56.34
(47.88, 65.41) |
−1.96
(−2.37, −1.54) |
||
| 30−34 | 196.76
(169.48, 227.38) |
222.30
(191.47, 256.9) |
161.41
(138.27, 187.08) |
125.03
(107.11, 144.92) |
−2.36
(−2.76, −1.96) |
||
| 35−39 | 388.91
(336.46, 445.26) |
425.00
(367.68, 486.58) |
221.11
(187.14, 257.52) |
219.15
(185.49, 255.24) |
−2.55
(−3.06, −2.03) |
||
| 40−44 | 532.12
(453.76, 609.66) |
791.35
(674.80, 906.66) |
377.25
(312.39, 445.52) |
371.14
(307.34, 438.30) |
−2.68
(−3.13, −2.22) |
||
| 45−49 | 599.82
(504.24, 705.82) |
1,159.74
(974.95, 1,364.70) |
626.32
(508.86, 761.84) |
516.06
(419.28, 627.72) |
−2.53
(−2.95, −2.11) |
||
| 50−54 | 932.58
(787.51, 1,087.19) |
1,950.92
(1,647.45, 2,274.35) |
1,041.52
(840.57, 1,267.02) |
832.53
(671.90, 1,012.78) |
−2.91
(−3.45, −2.37) |
||
| 55−59 | 1,246.66
(1,046.18, 1,446.63) |
2,868.36
(2,407.08, 3,328.46) |
1,194.67
(961.53, 1,463.22) |
1,259.67
(1,013.84, 1,542.83) |
−2.55
(−3.04, −2.05) |
||
| 60−64 | 1,255.79
(1,071.78, 1,451.55) |
3,545.36
(3,025.86, 4,098.04) |
1,392.79
(1,137.57, 1,681.71) |
1,773.00
(1,448.10, 2,140.78) |
−2.00
(−2.40, −1.59) |
||
| 65−69 | 1,166.10
(1,010.29, 1,321.36) |
4,260.88
(3,691.56, 4,828.21) |
1,580.37
(1,312.07, 1,871.94) |
2,245.36
(1,864.17, 2,659.62) |
−1.87
(−2.26, −1.47) |
||
| 70−74 | 921.36
(810.17, 1,040.65) |
4,886.18
(4,296.53, 5,518.79) |
1,399.80
(1,169.62, 1,646.77) |
2,925.03
(2,444.05, 3,441.10) |
−1.75
(−2.16, −1.34) |
||
| 75−79 | 518.06
(462.65, 581.45) |
4,540.24
(4,054.58, 5,095.71) |
901.11
(764.70, 1,041.96) |
3,019.14
(2,562.09, 3,491.05) |
−1.19
(−1.63, −0.75) |
||
| 80−84 | 219.57
(195.33, 245.51) |
3,893.78
(3,463.98, 4,353.84) |
542.99
(466.22, 619.14) |
2,847.76
(2,445.13, 3,247.14) |
−0.66
(−1.16, −0.16) |
||
| 85−89 | 66.1
(57.84, 73.86) |
3,447.01
(3,016.22, 3,851.69) |
241.75
(209.50, 270.15) |
2,842.56
(2,463.33, 3,176.58) |
−0.33
(−0.67, 0.02) |
||
| 90−94 | 9.49
(8.14, 10.74) |
2,547.54
(2,183.72, 2,882.76) |
43.67
(35.82, 50.02) |
1,946.12
(1,596.11, 2,228.93) |
−0.97
(−1.35, −0.59) |
||
| 95+ | 1.24
(1.01, 1.43) |
1,990.79
(1,617.64, 2,303.96) |
6.78
(5.28, 7.90) |
1,518.57
(1,183.48, 1,768.97) |
−0.90
(−1.23, −0.58) |
||
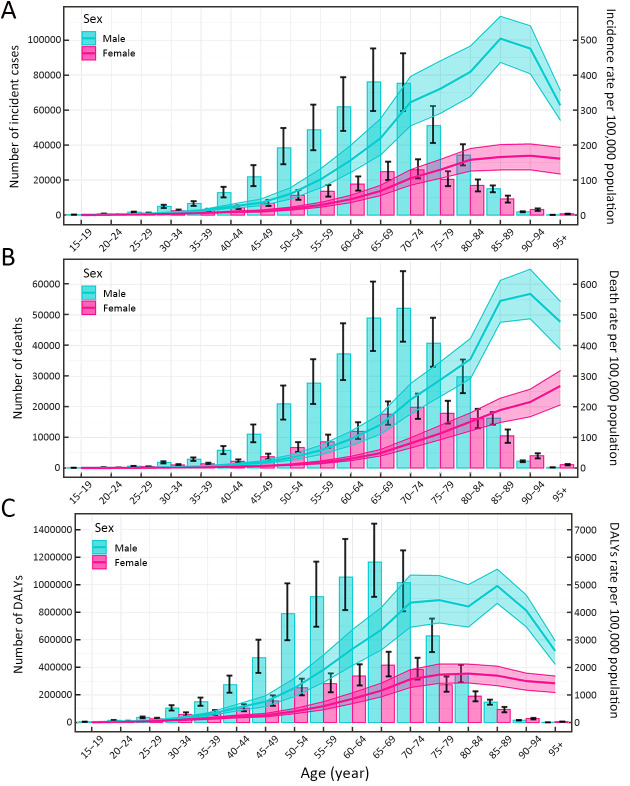
In 2019, the numbers of incident cases and deaths of GC reached a peak among the total population aged 70−74 years (Tables 1,2), and these trends were similar for males and females (Figure 1A,B). The number of DALYs reached a peak at 65−69 years old among the total population and both sexes (Table 3, Figure 1C). Meanwhile, the numbers of incident cases, deaths and DALYs were lower among males than females in individuals over 90 years old (Figure 1).
1.
Numbers and rates of incidence (A), death (B) and DALYs (C) of gastric cancer by age and sex in 2019 in China. Shading represents the upper and lower limits of the 95% uncertainty intervals (95% UIs). DALYs, disability-adjusted life-years.
The age-specific rates for incidence and mortality reached a peak among the total population aged 85−89 years (Tables 1,2); meanwhile, the age-specific rates for DALYs reached a peak at 75−79 years old (Table 3). The trends of age-specific rates of incidence and DALYs among both males and females were similar to the trends for the total population, while the trends of age-specific rates of mortality differed between males and females. The age-specific rates of mortality peaked at 90−94 years old in males, while the age-specific rates of mortality increased with increasing age in females. Furthermore, the numbers and rates of incidence, deaths and DALYs were concentrated in the elderly population (≥60 years old) (Figure 1).
Temporal trends of incidence, mortality and DALYs of GC from 1990 to 2019
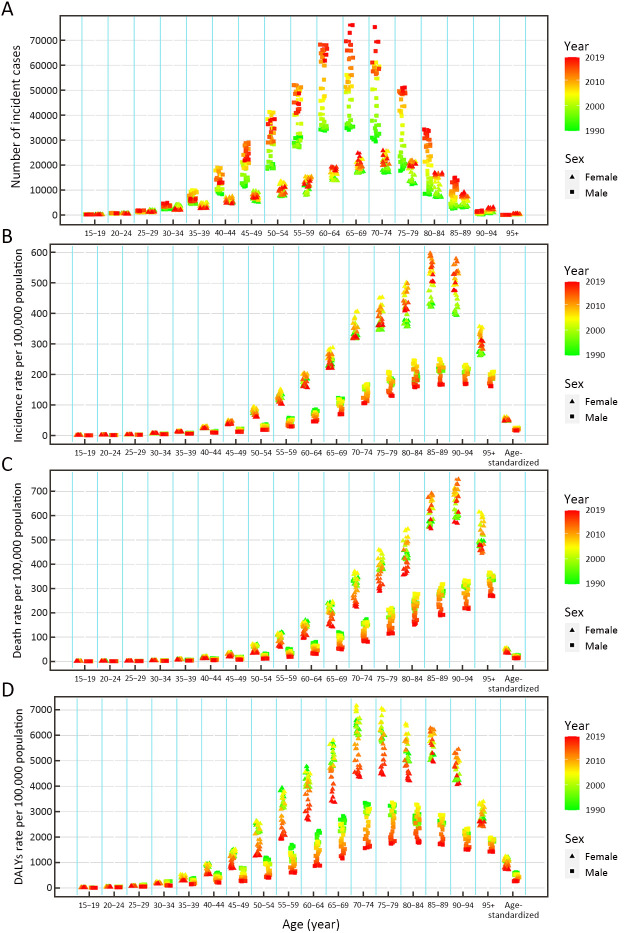
From 1990 to 2019, the numbers of incident cases, deaths and DALYs of GC all significantly increased among the total population (Tables 1-3). The number of incident cases increased by more than two times among males ≥65 years old and females ≥80 years old during the study period (Figure 2A). The ASIR was 37.56 per 100,000 (95% UI: 33.08, 42.27) in 1990, which decreased in 2019, with an EAPC of −0.41 (95% CI: −0.77, −0.06) in the total population ( Table 1). The ASIR of females decreased more significantly than that of males during this period [EAPC=−1.60, 95% CI: (−1.95, −1.25)vs. EAPC=0.14, 95% CI: (−0.21, 0.50), respectively] (Table 1). Additionally, overall downward trends in the incidence rates were observed among both sexes in most age-specific groups, while short-term upward trends were observed between 2000 and 2010 (which was more pronounced among males) (Figure 2B).
2.
Number of incident cases (A), incidence rate (B), death rate (C) and DALYs rate (D) of gastric cancer by age and sex, from 1990 to 2019 in China. DALYs, disability-adjusted life-years.
The ASMR decreased from 1990 [37.73 per 100,000 (95% UI: 33.20, 42.39)] to 2019, with an EAPC of −1.68 (95% CI: −2.09, −1.27) ( Table 2). A decreasing trend of age standardized DALYs was also observed during this period, and the EAPC was −1.98 (95% CI: −2.40, −1.57) ( Table 3). Overall downward trends in mortality and DALYs rates were observed in most age-specific groups and both sexes; from 2000 to 2010, the mortality and DALYs rates showed a short-term upward trend, especially among males and females aged ≥75 years old (Figure 2C,D).
Mortality and DALYs rates of GC attributable to risk factors and their temporal trends from 1990 to 2019
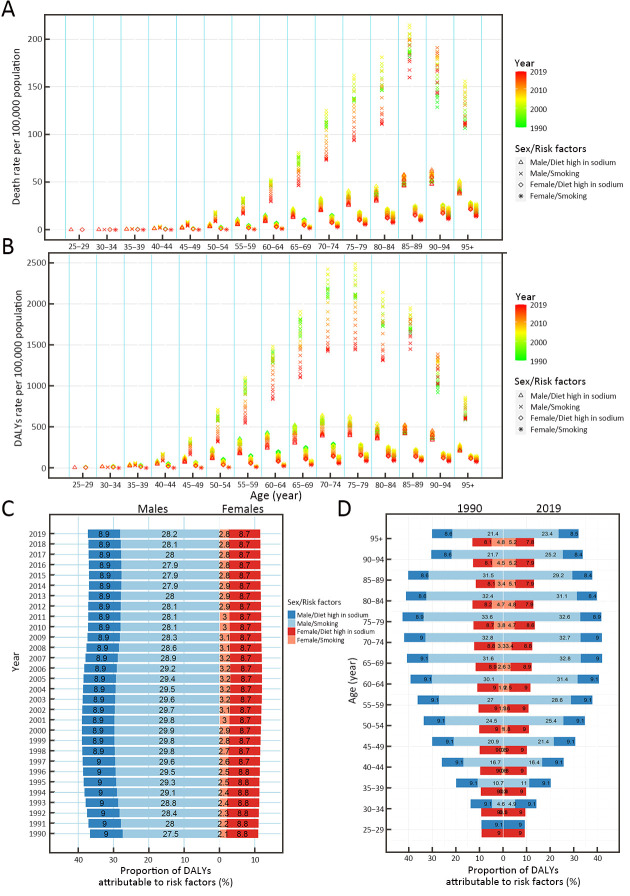
In all age-specific groups, the mortality that was attributed to smoking was the highest among males and the lowest among females (Figure 3A). Trends in the first increase and then decrease of GC mortality attributable to a high-sodium diet and smoking were observed in most age-specific groups and both sexes, and the overall downward trends were observed during the past three decades (Figure 3A). Moreover, the temporal trends of the rates of DALYs attributable to a high-sodium diet and smoking were similar to those of mortality (Figure 3B).
3.
Rates of death, rates and proportions of DALYs attributable to risk factors by age and sex, from 1990 to 2019 in China. Rates of death (A) and DALYs (B) of gastric cancer attributable to risk factors by age and sex, from 1990 to 2019 in China; proportions of DALYs attributable to risk factors by sex from 1990 to 2019 in China (C); and proportions of DALYs attributable to risk factors by age and sex in 1990 and 2019 in China (D). DALYs, disability-adjusted life-years.
The proportions of DALYs that were attributed to risk factors (smoking and a high-sodium diet) were different between males and females. Smoking was the most significant contribution among males, accounting for more than 27.5% of DALYs from 1990 to 2019; the proportions of DALYs of GC attributable to smoking for males were about 10 times higher than that for females. A high-sodium diet was the most significant contribution among females, accounting for more than 8.7% of DALYs from 1990 to 2019 (Figure 3C).
Moreover, during this period, the proportions of DALYs attributable to both smoking and a high-sodium diet did not change significantly among sexes in any age-specific groups. During this period, the proportions of DALYs attributable to smoking among females increased in those >45 years old, while the proportions attributable to a high-sodium diet decreased in females >75 years old. Among males, from 1990 to 2019, the proportions of DALYs attributable to smoking decreased in the 70−89 year-old age group, while the proportions attributable to a high-sodium diet decreased in males >80 years old ( Figure 3D).
Predictions of incidence and mortality of GC from 2020 to 2044
Based on GBD data of GC from 1990 to 2019 in China, we further predicted the numbers and rates of incidence and mortality in the next 25 years (Figure 4). In the next 25 years, the rates of incidence and mortality among both males and females should show a downward trend (Figure 4A), while the numbers of new cases and deaths of GC should continue to increase from 2020 to 2044 (Figure 4B,C). In 2044, the overall new GC cases should increase to 738.79 thousand (Figure 4B) and the number of GC deaths should increase to 454.80 thousand (Figure 4C). In 2044, among males, the numbers of incident cases and deaths should increase to 546.76 thousand and 320.66 thousand, respectively (Figure 4B,C). Among females, the numbers of incident cases and deaths should increase to 192.04 thousand and 134.14 thousand in 2044, respectively (Figure 4B,C). The numbers of incident cases and deaths for males should be more than twice as high as that for females.
4.
Temporal trends and forecasted rates of incidence and death (A), and number of incident cases (B) and deaths (C) of gastric cancer by sex, from 2020 to 2044 in China. Solid lines and dash lines represent the observed and the predicted the number of incident cases and deaths of gastric cancer; shading represents a 1% decrease and increase interval based on the 2019 rate. DALYs, disability-adjusted life-years.
Discussion
China is one of the countries with the highest burden of GC in the world (13,24). In the past, few studies analyzed the burden of GC in China (24-26), and the latest disease burden of GC is still unknown. Therefore, in our study, using the latest data of the GBD study (2019), we did a more comprehensively and in-depth analysis of the disease burden of GC and its temporal trends by age, sex, and risk factors, and uniquely, we predicted the numbers and rates of incidence and mortality in the next 25 years in China. First of all, in 2019, there were 612.82 thousand new cases and 421.54 thousand deaths attributed to GC in China, and the ASIR and ASMR were 30.64 per 100,000 and 21.72 per 100,000, respectively. In our study, the numbers and rates of incidence and deaths in 2019 were higher than those estimated by the International Agency for Research on Cancer (IARC) and related studies in China; the reasons for these differences might be related to the difference in reporting years (reported in 2018 and 2015, respectively), the coverage points of data collection, and the estimation models.
China contributes the largest numbers of GC incident cases, deaths and DALYs, worldwide (13). The numbers of incident cases, deaths and DALYs all significantly increased from 1990 to 2019 in China among the total population, and our model predicted the numbers of new cases and deaths of GC should continue to increase in the next 25 years in both sexes. Meanwhile, the rates of incidence, mortality and DALYs showed an overall decreasing trend in the past three decades among the total population, which is consistent with previous studies (25,26). Although the rates of incidence and mortality of GC showed downward trends in the world and China, the disease burden of GC in China is still heavy (25) and these rates are higher than the average levels of the world (27).
Many factors produced the high disease burden of GC in China. Firstly, China has the largest population in the world, with a population of approximately 1.42 billion, accounting for almost one-fifth of the world’s population (28-30). The large population base contributes to higher numbers and rates of incidence, mortality and DALYs, which can have a significant impact on the world (29). Meanwhile, China is transforming into an aging nation (31,32). This trend may cause the numbers and rates of incidence, mortality and DALYs to be concentrated in the elderly population (especially ≥60 years old), which is consistent with our results. Secondly, China has operated a screening program for the populations with a high risk of GC (25), and significant improvement has been made in the early diagnosis and treatment of GC (24). A series of screening and early detection programs have been formulated and implemented by the Chinese government since 2005 (26,33,34). These programs were first implemented in 11 high-risk regions in China, and then expanded to all provinces in 2019 (34). The target population of the current screening program is 40−69 years old of both sexes (26). The implementation of screening and early detection programs might be an important explanation for our results. The improvement of medical conditions and the implementation of public health strategies and screening programs would result in a decline in the rates of mortality and DALYs of GC, and on the other hand, the detection rate of new cases would increase, thereby keeping the incidence rate from declining substantially. However, it is expected from the prediction model that the future incidence rate will steadily decrease. Thirdly, with the improvement of public health and the changing of lifestyles in China (35), the risk factors (such as smoking, alcohol consumption, a high-sodium diet and H. pylori infection) have been effectively controlled, leading to a decline in the rates of mortality and DALYs. Lastly, the improvements in socioeconomic status might be also an important reason (26). The rapid economic development of China (especially in the past 10 years) has greatly improved the living standards of residents and their personal awareness of diseases, which might have resulted in a decline in the rates of incidence, mortality and DALYs in our study. In summary, the large population base, aging of the population, implementation of screening and early detection programs, controlling risk factors, and improvements in socioeconomic status, together contribute to the high disease burden of GC in China.
Although the overall decreasing trends in the rates of incidence, mortality and DALYs were observed in all age-specific groups and both sexes, these indicators showed a short-term upward trend from 2000 to 2010. The screening and early detection programs were initially implemented in 2005 in China (34), which might have led to an increase in the detection of the number of new cases in the following years. The other possible reason for this observed result might be that China had implemented a series of prevention strategies, including tobacco-control strategies since 2005 (36,37) and a salt-consumption program since 2007 (38). In addition, an increase in death-data collection points might also be a reason. The deaths data were reported by the China Disease Surveillance Points system: 145, 161 and 605 disease surveillance points were used from 1991 to 2003, 2004 to 2012, and 2013 to 2017, respectively (15); from 2008 to 2017, all the deaths data were reported through the online reporting system of the Chinese Center for Disease Control and Prevention (15).
We further found that the risk factors of smoking and a high-sodium diet played an important role in the GC burden, and might also be the main cause of differences between males and females. Smoking is one of the well-known risk factors, and the GC burden caused by smoking is different between males and females. In our study, the proportion of DALYs attributable to smoking was about 10 times higher in males than females. In China, the smoking prevalence rates of males and females were 52.9% and 2.4% in 2010, respectively (39,40), which may be an important reason for the gender differences. Moreover, in 2010, the prevalence of GC was the highest among males in the 45-64 years age group (63.0%) (40), which may increase the burden of disease for males over 45 years old in the future. Consistent with our speculation, we observed higher proportions of DALYs of GC attributable to smoking in males in the 50-94 years age group (>25%) in 2019. China has formulated many tobacco control strategies and mobilized all people to participate (36,37,41). The WHO Framework Convention on Tobacco Control (FCTC) was ratified by China in 2005, and tobacco control has made progress during the past decade (36,37). A slight downward trend in the proportion of DALYs attributable to smoking was observed in our study. The mechanisms of the effect of smoking on GC are unclear currently. Overall, smoking is associated with the development of precursor lesions of GC; in addition, tobacco smoke contains a variety of chemical carcinogens (especially nitrosamines and other nitroso compounds) (42,43). These chemical carcinogens may bind to DNA and affect the normal function of DNA, eventually leading to GC (42,43).
High-salt consumption is an important risk factor for GC and may increase the risk of it through the following mechanisms. Firstly, high-salt consumption may irritate the gastric mucosa, inducing intestinal metaplasia, and lead to atrophic gastritis (44,45). Secondly, high-salt consumption can promote the carcinogenesis progress of gastric dysplasia or GC among person infected with H. pylori (44-46). Moreover, high-salt foods (such as salted fish, preserved foods and processed meats) may also contain nitrate and nitrite, which may promote the formation of N-nitroso compounds (44). From our study, we found that the proportion of DALYs attributable to a high-sodium diet was more than 8.7% in China in 2019; and a slightly downward trend in the proportion of DALYs attributable to a high-sodium diet was observed among males and females. Since 2007, China developed the “China Healthy Lifestyle for All” initiative to improve people’s health literacy, which includes important content about the control of salt intake (38). With the control of salt-consumption program, the rates of death and DALYs attributable to a high-sodium diet has slightly decreased in the past three decades. However, the GC burden caused by a high-sodium diet is still relatively high. Therefore, more comprehensive prevention and control measures should be taken to further reduce the burden of GC caused by high-salt intake.
We should discuss other important risk factors for GC that were not contained in the database. H. pylori is a well-known risk factor for GC, and is classified as a class I human carcinogen (47,48). In our study, although we could not evaluate the GC burden caused by H. pylori, we could speculate that the proportion of DALYs attributable to H. pylori infection might be high. In 2015, about 4.4 billion people worldwide were H. pylori-positive (49). In the Western world, the prevalence of H. pylori has greatly decreased, while in developing countries, the prevalence of H. pylori is still at a high level (49). The prevalence of H. pylori is 55.8% in China, which is higher than in Japan (51.7%) and Korea (53.9%) (49). In China, because of wider population-based screening (28), increased awareness of the treatment of H. pylori infection (28), the development of an effective vaccine (50), and the improvement in socioeconomic status, the prevalence of H. pylori shows a decreasing trend (decreased 0.9% annually) (51), which may contribute to the decrease in the rates of mortality and DALYs. In addition, other reported risk factors for GC include alcohol consumption, infection with the Epstein-Barr virus, socioeconomic status, a low intake of fruits and vegetables, obesity and gastroesophageal reflux disease, poor oral health, radiation, and a family history of GC (5,52-54).
There are some limitations in our study. Firstly, we only assessed the disease burden of GC at the national level and could not conduct a more detailed assessment at the provincial level. Secondly, we could not divide GC into the cardia GC and non-cardia GC. The temporal trend of the incidence and risk factors for these two subtypes of GC are different (5,52). Smoking, age, sex, radiation and family history are common risk factors for both types of GC; while obesity and gastroesophageal reflux disease are specific risk factors for cardia GC; H. pylori infection, low socioeconomic status and a high-salt diet are associated with an increased risk of non-cardia GC (5,53). Thirdly, we could not evaluate the GC burden caused by other important risk factors because the GBD database does not contain the corresponding data. Fourthly, since the data were a summary of several sites, the limitation of report omission might exist, which might result in the underestimation of our results. Although the data used to estimate incidence and mortality in the GBD study were fitted, filled, and corrected through various models, including a linear step mixed-effects model and spatiotemporal Gaussian process regression, our findings are consistent with those of previous studies. However, we should not ignore the fact that the proportion of the population covered by these systems has changed during the past three decades, which needs to be fully considered when interpreting our results.
Conclusions
We provide a comprehensive analysis of the burden of GC in China. During the past three decades, benefitting from the implementation of prevention strategies and the improvement of medical technology, the rates of incidence and mortality decreased. However, the number of new cases and deaths increased and will further increase in the next 25 years due to the aging population and high-risk behaviors (such as smoking, a high-sodium diet and H. pylori infection). Therefore, focusing on the risk factors, more detailed prevention and control strategies should be formulated for males and females and people in different age groups. Further effort is also needed to mobilize the entire population to participate, which may have a positive effect on variations in the incidence of GC. On the other hand, strategies and measures for improving the level of early diagnosis and developing new treatment techniques should be proposed to reduce the mortality of GC.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2017YFC0907003); the National Natural Science Foundation of China (No. 81973116 and 81573229); and the Joint Research Funds for Shandong University and Karolinska Institute (No. SDU-KI-2020-03).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Xiaorong Yang, Email: yangxiaorong@sdu.edu.cn.
Ming Lu, Email: lvming@sdu.edu.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Sagaert X, Topal B, et al Gastric cancer. Lancet. 2016;388:2654–64. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. The Global Cancer Observatory. Cancer Today. Available online: http://gco.iarc.fr/
- 4.Zhang T, Yin X, Yang X, et al Research trends on the relationship between Microbiota and Gastric Cancer: A Bibliometric Analysis from 2000 to 2019. J Cancer. 2020;11:4823–31. doi: 10.7150/jca.44126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi P, Islami F, Anandasabapathy S, et al Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–13. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machlowska J, Baj J, Sitarz M, et al Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21:4012. doi: 10.3390/ijms21114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venerito M, Vasapolli R, Rokkas T, et al Gastric cancer: epidemiology, prevention, and therapy. Helicobacter. 2018;23(1 suppl):e12518. doi: 10.1111/hel.12518. [DOI] [PubMed] [Google Scholar]
- 8.Watari J, Chen N, Amenta PS, et al Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development . World J Gastroenterol. 2014;20:5461–73. doi: 10.3748/wjg.v20.i18.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousefi B, Mohammadlou M, Abdollahi M, et al Epigenetic changes in gastric cancer induction by Helicobacter pylori . J Cell Physiol. 2019;234:21770–84. doi: 10.1002/jcp.28925. [DOI] [PubMed] [Google Scholar]
- 10.Yusefi AR, Bagheri Lankarani K, Bastani P, et al Risk factors for gastric cancer: A systematic review. Asian Pac J Cancer Prev. 2018;19:591–603. doi: 10.22034/APJCP.2018.19.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GBD 2017 Pancreatic Cancer Collaborators The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–47. doi: 10.1016/S2468-1253(19)30347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GBD 2017 Stomach Cancer Collaborators The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42–54. doi: 10.1016/S2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GBD 2017 Mortality Collaborators Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1684–735. doi: 10.1016/S0140-6736(18)31891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Mao X, Jiang Y, et al Changing trends in the disease burden of primary liver cancer caused by specific etiologies in China. Cancer Med. 2019;8:5787–99. doi: 10.1002/cam4.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Jiang Y, Yuan H, et al The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70:674–83. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 18.GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–49. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold M, Laversanne M, Brown LM, et al Predicting the future burden of esophageal cancer by histological subtype: International trends in incidence up to 2030. Am J Gastroenterol. 2017;112:1247–55. doi: 10.1038/ajg.2017.155. [DOI] [PubMed] [Google Scholar]
- 21.Lalitwongsa S, Pongnikorn D, Daoprasert K, et al Breast cancer in Lampang, a Province in Northern Thailand: Analysis of 1993-2012 Incidence Data and Future Trends. Asian Pac J Cancer Prev. 2015;16:8327–33. doi: 10.7314/apjcp.2015.16.18.8327. [DOI] [PubMed] [Google Scholar]
- 22.Torres-Roman JS, Valcarcel B, Guerra-Canchari P, et al Leukemia mortality in children from Latin America: trends and predictions to 2030. BMC Pediatr. 2020;20:511. doi: 10.1186/s12887-020-02408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Møller B, Fekjaer H, Hakulinen T, et al Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22:2751–66. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 24.Sun XJ, Shi JF, Guo LW, et al Medical expenses of urban Chinese patients with stomach cancer during 2002-2011: a hospital-based multicenter retrospective study. BMC Cancer. 2018;18:435. doi: 10.1186/s12885-018-4357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Zheng R, Wang N, et al Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291–8. doi: 10.21147/j.issn.1000-9604.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SM, Zheng RS, Zhang SW, et al Epidemiological characteristics of gastric cancer in China, 2015. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:1517–21. doi: 10.3760/cma.j.issn.0254-6450.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Yan L, Liu Y, et al Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J Hematol Oncol. 2019;12:96. doi: 10.1186/s13045-019-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng RM, Zong YN, Cao SM, et al Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Chen Y, Jia H, et al Patterns of life lost to cancers with high risk of death in China. Int J Environ Res Public Health. 2019;16:2175. doi: 10.3390/ijerph16122175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 31.Zhou M, Wang H, Zeng X, et al Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–58. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang EF, Scheibye-Knudsen M, Jahn HJ, et al A research agenda for aging in China in the 21st century. Ageing Res Rev. 2015;24:197–205. doi: 10.1016/j.arr.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lü YL, Li Y, Liu GS, et al Comparison of two gastric cancer screening schemes in a high-risk population. Zhonghua Zhong Liu Za Zhi. 2013;35:394–7. doi: 10.3760/cma.j.issn.0253-3766.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Zeng H, Sun K, Cao M, et al Initial results from a multi-center population-based cluster randomized trial of esophageal and gastric cancer screening in China. BMC Gastroenterol. 2020;20:398. doi: 10.1186/s12876-020-01517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun D, Cao M, Li H, et al Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin J Cancer Res. 2020;32:129–39. doi: 10.21147/j.issn.1000-9604.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang G, Wang Y, Wu Y, et al The road to effective tobacco control in China. Lancet. 2015;385:1019–28. doi: 10.1016/S0140-6736(15)60174-X. [DOI] [PubMed] [Google Scholar]
- 37.Lv J, Su M, Hong Z, et al Implementation of the WHO Framework Convention on Tobacco Control in mainland China. Tob Control. 2011;20:309–14. doi: 10.1136/tc.2010.040352. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Astell-Burt T, Seo DC, et al Multilevel evaluation of ‘China Healthy Lifestyles for All’, a nationwide initiative to promote lower intakes of salt and edible oil. Prev Med. 2014;67:210–5. doi: 10.1016/j.ypmed.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Yang G, Wang Y, Zeng Y, et al Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Hsia J, Yang G Prevalence of smoking in China in 2010. N Engl J Med. 2011;364:2469–70. doi: 10.1056/NEJMc1102459. [DOI] [PubMed] [Google Scholar]
- 41.Guo H, Quan G Tobacco control in China and the road to Healthy China 2030. Int J Tuberc Lung Dis. 2020;24:271–7. doi: 10.5588/ijtld.19.0106. [DOI] [PubMed] [Google Scholar]
- 42.González CA, Pera G, Agudo A, et al Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Int J Cancer. 2003;107:629–34. doi: 10.1002/ijc.11426. [DOI] [PubMed] [Google Scholar]
- 43.Moy KA, Fan Y, Wang R, et al Alcohol and tobacco use in relation to gastric cancer: a prospective study of men in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2010;19:2287–97. doi: 10.1158/1055-9965.EPI-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang X, Wei J, He X, et al Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820–32. doi: 10.1016/j.ejca.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Eusebi LH, Telese A, Marasco G, et al Gastric cancer prevention strategies: A global perspective. J Gastroenterol Hepatol. 2020;35:1495–502. doi: 10.1111/jgh.15037. [DOI] [PubMed] [Google Scholar]
- 46.Cover TL, Peek RM Jr Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer . Gut Microbes. 2013;4:482–93. doi: 10.4161/gmic.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amieva M, Peek RM Jr Pathobiology of Helicobacter pylori-induced gastric cancer . Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotilea K, Bontems P, Touati E Epidemiology, diagnosis and risk factors of Helicobacter pylori infection . Adv Exp Med Biol. 2019;1149:17–33. doi: 10.1007/5584_2019_357. [DOI] [PubMed] [Google Scholar]
- 49.Hooi JKY, Lai WY, Ng WK, et al Global prevalence of Helicobacter pylori infection: Systematic review and Meta-analysis . Gastroenterology. 2017;153:420–9. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 50.Zeng M, Mao XH, Li JX, et al Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial . Lancet. 2015;386:1457–64. doi: 10.1016/S0140-6736(15)60310-5. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Sun Y, Yang J, et al Time trends and other sources of variation in Helicobacter pylori infection in mainland China: A systematic review and meta-analysis . Helicobacter. 2020;25:e12729. doi: 10.1111/hel.12729. [DOI] [PubMed] [Google Scholar]
- 52.Crew KD, Neugut AI Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyons K, Le LC, Pham YT, et al Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur J Cancer Prev. 2019;28:397–412. doi: 10.1097/CEJ.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 54.Ndegwa N, Ploner A, Liu Z, et al Association between poor oral health and gastric cancer: A prospective cohort study. Int J Cancer. 2018;143:2281–8. doi: 10.1002/ijc.31614. [DOI] [PubMed] [Google Scholar]