Abstract
Background
Therapies targeting immune checkpoints are effective in tumors with a high mutation burden that express multiple neo-antigens. However, glial tumors including those seen in children carry fewer mutations and there is an unmet need to identify new antigenic targets of anti-tumor immunity. SOX2 is an embryonal stem cell antigen implicated in the biology of glioma initiating cells. Expression of SOX2 by pediatric glial tumors and the capacity of the immune system in these patients to recognize SOX2 has not been previously studied.
Methods
We examined the expression of SOX2 on archived paraffin-embedded tissue from pediatric glial tumors. The presence of T-cell immunity to SOX2 was examined in both blood and tumor-infiltrating T-cells in children and young adults with glioma. The nature of tumor-infiltrating immune cells was analyzed with a 37-marker panel using single cell mass cytometry.
Results
SOX2 is expressed by tumor cells but not surrounding normal tissue in pediatric gliomas of all grades. T-cells against this antigen can be detected in blood and tumor tissue in glioma patients. Glial tumors are enriched for CD8/CD4 T-cells with tissue resident memory T-cell (TRM; CD45RO+, CD69+, CCR7−) phenotype, which co-express multiple inhibitory checkpoints including PD-1, PD-L1 and TIGIT. Tumors also contain natural killer cells with reduced expression of lytic granzyme.
Conclusion
Our data demonstrate immunogenicity of SOX2, which is specifically overexpressed on pediatric glial tumor cells. Harnessing tumor immunity in glioma will likely require the combined targeting of multiple inhibitory checkpoints.
Keywords: Pediatric glioma, SOX2, immunotherapy, immune checkpoints
Introduction
Brain tumors continue to remain a therapeutic challenge and they are now the leading cause of cancer-related mortality in children[1,2]. New therapeutic approaches are needed to improve survival and decrease long-term morbidities in patients with brain tumors[3,4].
Antibodies targeting the inhibitory immune checkpoints on T-cells including CTLA-4, PD-1 and PD-L1 have led to durable responses in some human tumors, particularly those with a high mutational load such as melanoma and lung cancer[5–8]. The hypothesis is that these tumors present a number of different neo-antigens that can serve as targets for an effective anti-tumor immune response. However, glial tumors in children and adults have fewer mutations[9]. While clinical response to anti-PD1 antibodies has been observed in glial tumors, this was particularly seen in the setting of a rare subset of tumors with mismatch repair deficiency and a high mutation burden[10]. The paucity of neo-antigens has encouraged attempts to target shared antigens expressed on glial tumors, particularly as targets of vaccines[11–13]. While many of these antigens meet the desired criteria of specific overexpression on tumor cells compared to normal/non-malignant counterparts, they often do not meet the criterion for being important to the biology of the tumor cells and expression on cancer stem cells.
Sex-determining region Y (SRY)–box 2 (SOX2) is an embryonal stem cell antigen essential for embryonic neural development [14]. After embryonic development, SOX2 expression is largely downregulated although its expression is maintained in some regions of the adult brain, such as the subventricular zone (SVZ)[15]. SOX2 is also important for growth and survival of the glioma initiating cell/glioma stem cells[16–19]. SOX2 expressing cells are enriched in adults with glial tumors that relapse after chemotherapy or radiation therapy[20–22]. SOX2 is immunogenic and anti-SOX2 T-cell immune response can lead to control of SOX2 positive tumor cells in vitro[23,24]. However, the expression of SOX2 in the context of pediatric brain tumors and the nature of anti-SOX2 T-cell response in children and adults with glial tumors has not been studied. In this study, we have analyzed the expression of SOX2 in children with brain tumors and characterized the presence of SOX2 specific T-cell immunity in children and young adults. We have also utilized mass cytometry to characterize the nature of immune infiltrates in these tumors.
Materials and Methods
Patients and blood and tumor samples
Peripheral blood (n = 14) and paired tumor tissue (n = 4) were obtained intraoperatively from pediatric and young adult glioma patients undergoing surgical resection at Yale-New Haven Hospital. Samples were collected after obtaining informed consent under a Yale University Institutional Review Board approved protocol. Immediately after resection, tumor samples were placed in sterile RPMI-1640 with l-glutamine (Corning) with 1% penicillin/streptomycin. All patients included in this study had a confirmed glial tumor. Blood samples were obtained from patients with a range of pathological grades and undergoing a variety of treatments including surgical resection alone, active or recent chemotherapy and/or radiation therapy. No patients included in the study had received any corticosteroids within the last 2 weeks prior to blood sample collection. Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation process using Ficoll-Paque Plus (GE Health Care Life Sciences). Tumor samples were processed as previously described[25]. Briefly, freshly resected tumor specimens were minced with a razor under sterile conditions, followed by enzymatic digestion (RPMI-1640 with 1-glutamine [Corning], 1 mg/ml collagenase IV [Sigma-Aldrich], 1 U/ml DNAse [Qiagen], and 1% penicillin/streptomycin) for 30 min at 37°C. A single cell suspension was then obtained by passing the sample through a 70- μm cell strainer.
Cell culture
Fresh tumor samples were processed as described above. For establishment of primary adherent cell culture, cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (ThermoFisher) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin. The cells were seeded in 25-cm2 culture flasks and maintained at 37°C with 5% CO2 and culture medium was changed every 2-3 days.
Primary tumor cells and CHLA-01-MED cells (ATCC) were resuspended in neurosphere medium (NM), composed of DMEM/F-12 supplemented with N2 (Gibco), B-27 (ThermoFisher), 20ng/ml EGF (ThermoFisher), 20ng/ml FGF (ThermoFisher), 20ng/ml LIF (ThermoFisher), and 1% penicillin/streptomycin. Viable cells were seeded in a 25-cm2 culture flasks at a maximum concentration of 5x104/ml and maintained at 37°C with 5% CO2 and culture medium was changed every 2-3 days.
Immunophenotyping by mass cytometry
Fresh patient PBMCs and tumor single-cell suspensions were stained at the same time, as previously described[25]. PBMCs from healthy children (seen in our clinic for family history of mild bleeding disorder) were used as an additional control. Cells were suspended at up to 2 million/ml in 1× PBS for viability staining by Cell-ID Cisplatin (final concentration of 5 μM; Fluidigm Sciences). Cells were mixed well and incubated for 5 min at room temperature. The staining was quenched with MaxPar Cell staining buffer and washed twice before proceeding to the usual procedure of surface and intracellular staining, as per manufacturer’s protocol. A 37-antibody staining panel was used with 31 surface markers and 6 intracellular markers (Supplementary Table 1). Between 1x106 and 2x106 PBMCs and tumor cells were incubated in a volume of 100 μl cell staining buffer with Abs in a polystyrene tube for 30 min at room temperature. After staining, cells were washed twice with buffer before fixing with BD Cytofix fixation buffer (100 μl/million cells) and permeabilizing with BD Perm/Wash buffer. Fixed and permeabilized cells were stained with the intracellular cocktail for 30 min at room temperature. Cells were washed twice with buffer and suspended in 1ml intercalation solution containing MaxPar Intercalator-Ir in MaxPar Fix and Perm buffer at final concentration of 125 nM. Cells were left overnight in the intercalator solution, washed with staining buffer, and finally suspended at 106cells/ml in MaxPar water supplemented with 10% EQ 4-element calibration beads (Fluidigm) before acquiring on CyTOF 2 instrument (DVS; Fluidigm Sciences). To facilitate quantitative comparisons between data acquired on different days, single-cell data was normalized using beads. At the completion of data acquisition, files were concatenated into a single FCS file and the normalization beads were removed using the CyTOF II built-in software. All data were analyzed using Cytobank. An unsupervised cluster analysis tool (viSNE) was used to visualize the data in two dimensions based on the t-Distributed Stochastic Neighbor Embedding (t-SNE) algorithm[26].
Immunohistochemistry
Paraffin sections from pediatric brain tumor specimens were subjected to antigen retrieval at low pH with citrate buffer. Slides were then stained with monoclonal mouse anti-human SOX2 antibodies (1.25μg/ml, R&D, clone #245610). Tumor tissue was called positive for SOX2 if any nuclear staining was detected with the SOX2 antibody. SOX2-positive tumor cell nuclei were quantified in five randomly selected 40X optical fields.
Peptide libraries
Overlapping peptides libraries spanning the entire length of SOX2 were synthesized as previously described[27]. The SOX2 library consisted of 86 peptides divided into 4 sub-mixes (Supplementary Table 2). M1 peptides cover SOX2 residues 1–89, M2 residues 79–171, M3 residues 161–246 and M4 residues 236–321. A pool of peptides derived from cytomegalovirus, Epstein–Barr virus and influenza virus (CEF; Anaspec Inc.) and Candida albicans (Greer Laboratories Inc.) were used as a positive control.
Detection of antigen-specific T-cells
The presence of SOX2, CEF and Candida albicans reactive T-cells was detected based on antigen-dependent cytokine production and proliferation, as previously described[27,28]. Briefly, PBMCs were cultured either with media alone (control) or together with CEF peptides (5 μg/mL per peptide), Candida albicans (10 μg/ml) or SOX2 peptide pools (5 μg/mL per peptide) in 5% PHS, in 96-well round bottom plates (2.5 × 105 cells/well). PHA was used as a positive control. After 48 hrs., culture supernatants were harvested and examined for the presence of chemokine (C-X-C motif) ligand 10 (CXCL10, also known as IP10) using a Luminex assay, as per manufacturer’s instructions (Millipore, MA). The samples were collected on Luminex 100 instrument and analyzed using the xPONENT software (Luminex Corporation). Values ≥2-fold over the negative control were deemed positive, based on the analysis of inter- and intra-assays variation, as previously described[27]. In this assay the antigen-induced secretion of CXCL10 serves as a downstream marker of T-cell reactivity and depends on the presence of CD3+ T-cells as well as on the induction of IFN-γ, as previously described[27].
Antigen-dependent proliferation assays
The presence of antigen-dependent T-cell proliferation was examined using a CFSE dilution assay, as previously described[28]. PBMCs were labeled with 0.5 μM CFSE (Molecular Probes) and cultured with 1 μg/mL anti-CD28 and anti-CD49d antibodies (BD Biosciences), alone or in the presence SOX2 peptide mixes (5 μg/mL per peptide), CEF peptides (5 μg/mL) peptides, candida albicans (10 μg/ml) or PHA. Five days later, PBMCs were stained with anti-CD3, anti-CD4 and anti-CD8 antibodies (all BD Phamingen). T-cell proliferation was analyzed on a FACSCalibur cytofluorometer (Becton Dickinson). Flow cytometry data were analyzed using the FlowJo software.
siRNA transfection
On-TargetPlus SmartPool siRNAs for SOX2 (Cat. L-011778) and non-targeting pool (Cat. D-001810) were purchased from Dharmacon (Boulder, CO, USA). Neurospheres were dissociated with TrypLE (ThermoFisher) to make a single-cell suspension and then resuspended in Opti-MEM without phenol red (ThermoFisher) at 2.5x106/100ul along with 20ug of siRNA. Cells were then transferred to a 0.4cm electroporation cuvette (Bio-Rad, Cat. 165-2088) and pulsed with 500mV x 500msec with an ECM 830 electroporator (BTX-Harvard Apparatus). Cells were cultured in duplicate in NM in 6-well plates and harvested at 72-hrs.
Statistical analysis
A 2-tailed Student’s t-test was used to determine statistical significance and a p < 0.05 was considered statistically significant.
Results
SOX2 immunohistochemistry in pediatric brain tumors.
Prior studies have characterized the expression of SOX2 in adult glioma[18,29,30]. In order to examine the expression of SOX2 in pediatric glioma, we analyzed 27 pediatric tumor samples using immunohistochemistry (Table 1). SOX2 expression was detected in tumor cells but not in the surrounding normal tissue in all juvenile pilocytic astrocytoma (JPA), diffuse astrocytoma, anaplastic astrocytoma and glioblastoma, and in 60% of oligodendrogliomas (Fig. 1a). SOX2 staining was nuclear and its intensity appeared to increase in higher grade lesions (Table 2). RNAi-mediated inhibition of SOX2 in primary JPA cells as well as a pediatric anaplastic astrocytoma cell line (CHLA-01) led to decrease in cell growth (Fig. 1b), consistent with prior studies[18,31]. Together, these data show that SOX2 can be a useful marker for histopathologic analysis of pediatric glial tumors, particularly to assess infiltration of normal tissue by tumor cells.
Table 1.
Patient characteristics for used for immunohistochemistry
| n | |
|---|---|
| Total | 27 |
| Median age (range in yrs) | 12 (0.1-18) |
| Sex (male/female) | 15/12 |
| Tumor Histology | |
| Juvenile Pilocytic Astrocytoma | 14 |
| Diffuse Astrocytoma | 1 |
| Anaplastic Astrocytoma | 1 |
| Glioblastoma | 6 |
| Oligodendroglioma | |
| Grade II | 3 |
| Grade III | 2 |
Figure 1.
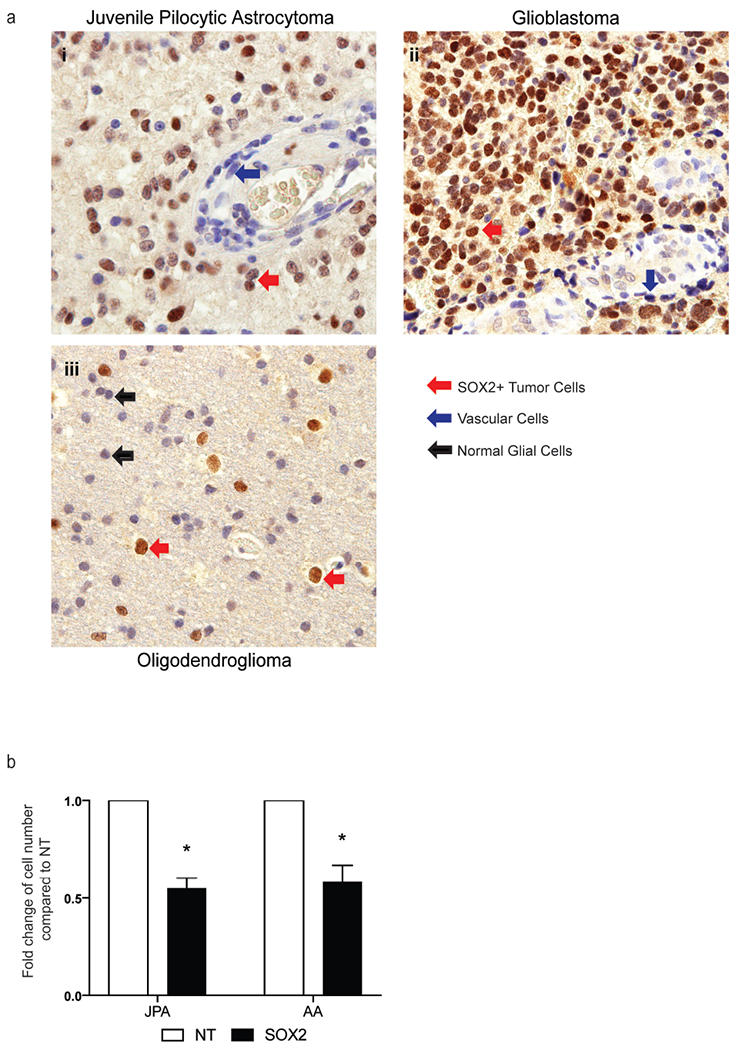
SOX2 expression and function in pediatric brain tumors.
a. Immunohistochemistry was performed on archived paraffin embedded tumor tissue from 27 pediatric glial tumors. Figure shows SOX2 expression in a representative patient with i) juvenile pilocytic astrocytoma, ii) glioblastoma iii) oligodendroglioma. Arrows in red shows nuclear SOX2 staining in tumor cells, blue arrows show absence of SOX2 staining in vascular cells and black arrows show normal glial cells that do not stain for SOX2.
b. Primary patient derived short-term culture of JPA cells (JPA) and pediatric anaplastic astrocytoma cell line CHLA-01 (AA) were electroporated with either non-targeting siRNA smart pool (NT) or SOX2 siRNA smart pool (SOX2). Figure shows decrease in cell growth following SOX2 knock down compared to cells treated with NT
Table 2.
Quantification of SOX2 staining by immunohistochemistry
| SOX2 expression |
||
|---|---|---|
| Tumor Histology | % of SOX2 stained tumor cells | Intensity of SOX2 staining |
| Juvenile Pilocytic Astrocytoma | >50% | +/++ |
| Diffuse Astrocytoma | >50% | +/++ |
| Anaplastic Astrocytoma | 70-80% | ++/+++ |
| Glioblastoma | 90-100% | +++ |
| Oligodendroglioma | >70% | ++ /+++ |
| Grade II | 40-50% | +/++ |
| Grade III | 80-90% | ++/+++ |
Detection of SOX2 T-cell response in pediatric and young adults with glioma.
We have previously developed an overlapping peptide library spanning the entire SOX2 protein that allowed us to evaluate naturally occurring T-cells against this antigen in patients with several adult human tumors[27,32,33]. We utilized this peptide library and antigen-dependent cytokine secretion (Fig. 2a) and proliferation assays (Fig. 2b) to detect anti-SOX2 T-cells in pediatric and young adults with glioma (Table 3, Supplementary Table 3). The presence of anti-SOX2 cytokine producing T-cells was detected in 5/14 (36%) of patients studied. There was no difference in the anti-SOX2 T-cell reactivity between patients with LGG and HGG (3/8 vs. 2/6 patients, respectively) (Fig. 2c). The region of the SOX2 protein that was most commonly recognized in patients with SOX2 immunity included amino-acid residues 79–171 (Fig. 2d). T-cell response to control antigens (CEF, a mix of peptides from viral antigens CMV, EBV, influenza; or Candida albicans) or polyclonal mitogen (PHA) were similar between patients with and without a SOX2 T-cell response indicating that the absence of SOX2 immunity was not due to global immune paresis (Fig. 2e). There were also no significant differences in tumor histology, age or type of treatment between patients with and without SOX2 T-cell reactivity (Supplementary Table 4). In two patients, we assessed T-cell reactivity to SOX2 in paired blood and freshly resected tumor tissue. Tumors in both patients expressed SOX2 (data not shown). No reactivity to SOX2 was detected in either blood or tumor tissue in one patient. Interestingly, in the second patient, SOX2 specific T-cells were only detected in the tumor, but not blood (Fig. 2f). These findings demonstrate that SOX2 is immunogenic in pediatric glioma patients. These findings also suggest that SOX2 specific T-cells can enter the tumor tissue and SOX2 T-cell reactivity may be detected in tumors even when it is not detectable in circulating T-cells.
Figure 2.

Detection of SOX2 specific T-cell response in peripheral blood and tumor tissue. Peripheral blood mononuclear cells (PBMCs, n=14) obtained from patients were cultured alone (NEG) or with an overlapping peptide library from SOX2 (5 μg/ml, Mix 1, 2, 3, 4), phytohemaglutanin (PHA) and either viral peptide mix or Candida as positive control (CEF/Candida). M1 peptides cover SOX2 residues 1–89, M2 residues 79–171, M3 residues 161–246 and M4 residues 236–321. After 48 hrs., the culture supernatant was examined for the presence of CXCL10. In 2 patients we were able to examine reactivity to SOX2 peptide mixes in the blood as well as the tumor.
a. Representative SOX2 T-cell reactivity to mix 2 in a patient using CXCL10 Luminex assay. *Positive T-cell reactivity to SOX2
b. PBMCs were labelled with CFSE and co-cultured with SOX2 peptide mixes. Figure shows T-cell proliferation in response to SOX2 mix 2 in a representative patient (black arrow).
c. SOX2 T-cell reactivity in LGG (n=8) and HGG (n=6).
d. Reactivity to the different regions of the SOX2 protein in SOX2-immune patients (n = 5). Some patients were reactive to more than one region of the protein.
e. Reactivity to PHA as well as positive control with either CEF or Candida in patients who did and did not have T-cell immune response to SOX2 antigen.
f. Figure shows T-cell reactivity in paired blood and tumor tissue in two patients. In patient 1, SOX2 reactivity was detected in the tumor but not in the blood. In patient 2, SOX2 reactivity was not detected in the blood or the tumor. *Positive T-cell reactivity to SOX2
Table 3.
Characteristics of patients tested for SOX2 T-cell reactivity
| n | |
|---|---|
| Total | 14 |
| Median age (range in years) | 12.6 (4-36) |
| Sex (male/female) | 5/9 |
| Tumor histology | |
| Juvenile Pilocytic Astrocytoma | 5 |
| Diffuse Astrocytoma | 1 |
| Anaplastic Astrocytoma | 2 |
| Glioblastoma | 1 |
| Diffuse Intrinsic Pontine Glioma | 1 |
| Oligodendroglioma | |
| Grade II | 1 |
| Grade III | 1 |
| Ganglioglioma | |
| Grade I | 1 |
| Grade III | 1 |
| Treatment at time of sample collection | |
| At diagnosis or relapse (pre-therapy) | 10 |
| On active chemotherapy/radiation | 4 |
Characterization of pediatric glioma immune cell infiltration
In order to better understand the immune microenvironment in pediatric glioma, we utilized single cell mass cytometry (CyTOF) to characterize immune cells from paired blood and freshly resected pediatric glial tumors in four patients (Table 4). We also obtained blood from healthy children as a control. The peripheral blood from patients with glioma had similar composition of immune cells (Fig. 3a–c) and expression of immune checkpoints as blood from healthy children (Fig. 3e). We found that the tumor tissue is enriched in myeloid cells (CD45+CD11b+), and these cells represent the dominant component of CD45+ cells infiltrating these tumors (Fig. 3a). Tumor-infiltrating cells of myeloid origin include both microglia (CD45dimCD11b+) and inflammatory monocytes/macrophages (CD45hiCD11b+), as previously described[34]. T-cells within tumor consisted of both CD4+ as well as CD8+ T-cells and there was no significant difference in the proportion of CD4+ or CD8+ T-cells between blood and tumor tissue (Fig. 3b). In contrast to peripheral blood, most of the tumor-infiltrating CD4+ and CD8+ T-cells were CD45RO+ memory cells (Fig. 3c), with a TRM phenotype (CD45RO+CCR7-CD69+). About half of the CD8+ TRM cells expressed CD103 (Fig. 3d).
Table 4.
Patient characteristics for samples used for single cell mass cytometry (CyTOF)
| n | |
|---|---|
| Total | 4 |
| Median age (range in years) | 12 (4-18) |
| Sex (male/female) | 1/3 |
| Tumor histology | |
| Juvenile Pilocytic Astrocytoma | 3 |
| Anaplastic Ganglioglioma | 1 |
| Treatment at time of sample collection | |
| At diagnosis or relapse (pre-therapy) | 3 |
| On active chemotherapy/radiation | 1 |
Figure 3.
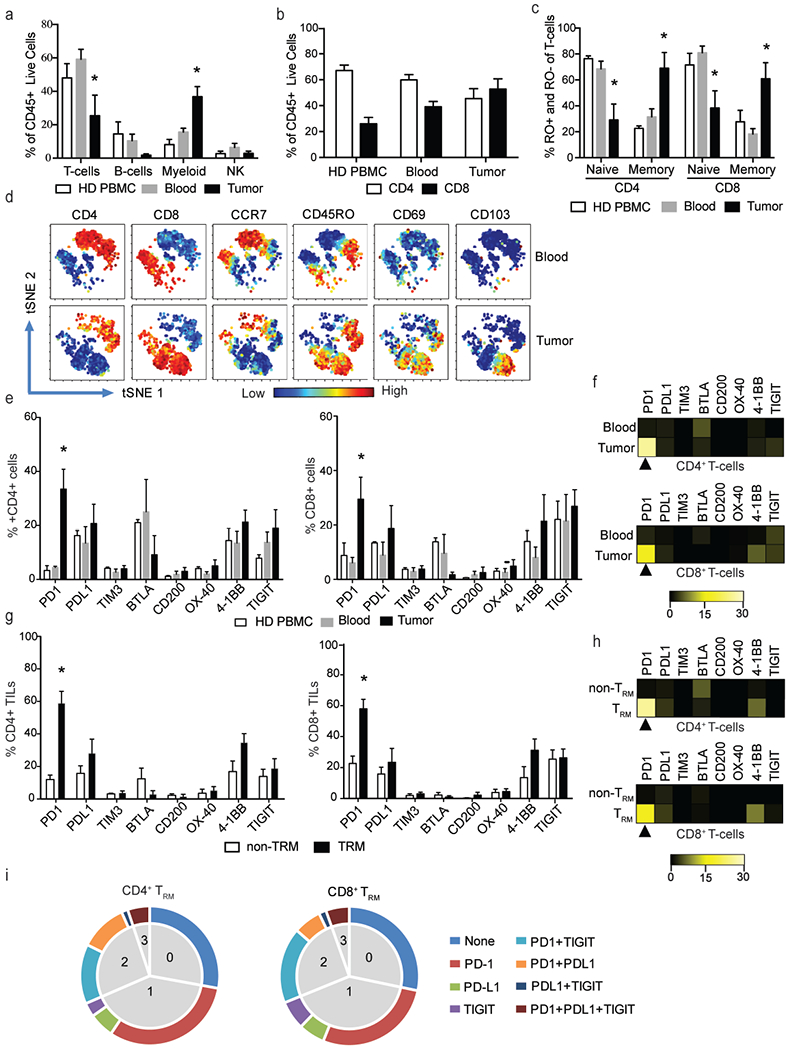
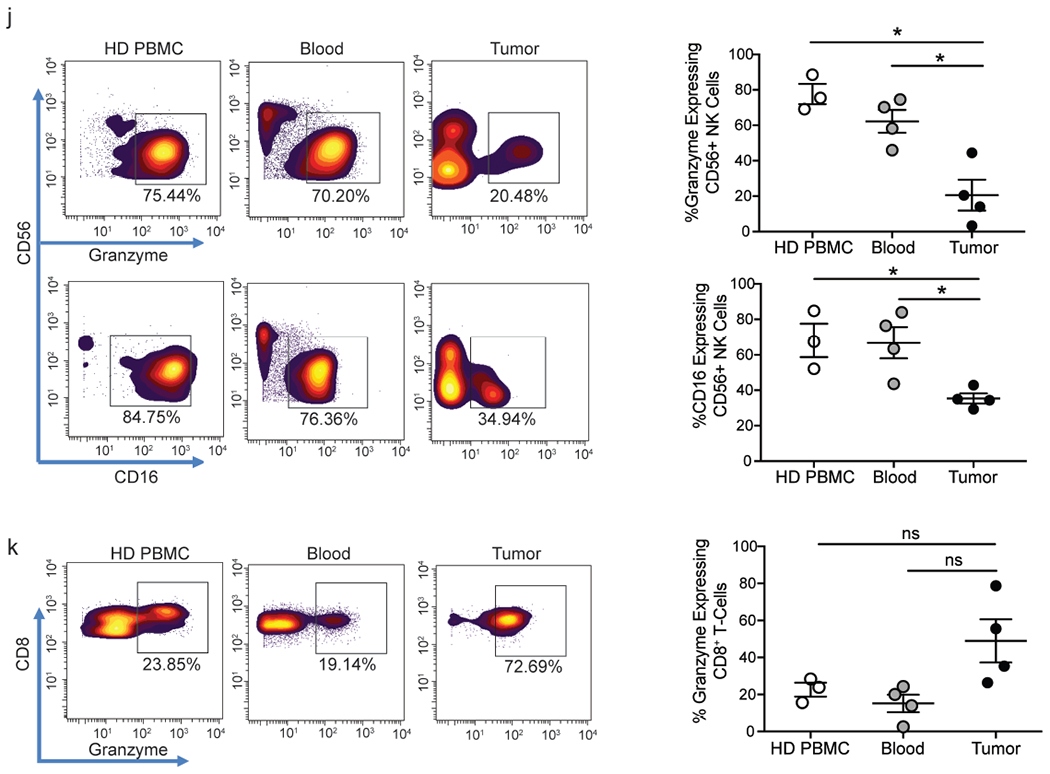
Characteristics of the glial tumor immune microenvironment.
Paired blood and fresh tumor tissue was obtained from children with glioma (n=4). PBMCs from healthy pediatric donors (HD PBMCs; n=3) were used as an additional control. PBMCs were isolated and tumor tissue was processed to obtain a single cell suspension. Immune cells were examined with single cell mass cytometry using a panel of 37 different antibodies. All plots show mean and SEM. *p<0.05
a. Composition of the CD45+ cells in HD PBMCs as well as paired peripheral blood and tumor tissue from glioma patients.
b. Percentage of CD4+ and CD8+ T-cells in HD PBMCs and paired blood and tumor tissue from glioma patients.
c. Naïve (CCR7+RO−) and memory (CCR7−RO+) phenotype of T-cells in HD PBMCs and paired blood and tumor tissue from glioma patients.
d. viSNE plot showing phenotype of T-cells in paired blood and tumor tissue from a representative patient. Figure shows expression of CD4, CD8, CCR7, CD45RO, CD69 and CD103.
e. Expression of immune checkpoints in HD PBMCS as well as paired blood and tumor CD4+ and CD8+ T-cells.
f. Representative heat plot showing median fluorescence intensity of immune checkpoint expression in paired blood and tumor T-cells.
g. Expression of immune checkpoints on tumor CD4+ and CD8+ TRM and non-TRM cells.
h. Representative heat plot showing median fluorescence intensity of immune checkpoint expression in CD4 and CD8+ tumor TRM and non-TRM cells.
i. We analyzed the expression of PD-1, PD-L1 and TIGIT on CD4+ and CD8+ TRM cells in the tumor. Figure shows mean percent of cells expressing none, one, two or three checkpoints.
j. Figure shows expression of granzyme B and CD 16 by CD56+NK cells in HD PBMCs as well as paired blood and tumor tissue from glioma patients. Panel on the left is a representative healthy donor and patient. Panel on the right shows data for all healthy donors and patients studied (n=4).
k. Granzyme B expression in CD8+ T-cells in the HD blood as well as paired blood and tumor from a glioma patient. Panel on the left is a representative healthy donor and patient. Panel on the right shows data for all healthy donors and patients studied(n=4)
Next, we examined the expression of several co-stimulatory and co-inhibitory molecules including PD-1, PD-L1, TIM3, BTLA, CD200, OX40, 4-1BB and TIGIT on peripheral blood of healthy children as well as paired peripheral blood and tumor infiltrating T-cells from children with glial tumors. Both CD4+ and CD8+ T-cells in the tumor had a higher proportion of PD-1+ T-cells (Fig. 3e) as well as a higher expression of PD-1 (Fig. 3f) compared to circulating T-cells. In contrast, the expression of other immune checkpoints studied were comparable between blood and tumor (Fig. 3e and f). Immune checkpoints are known to be expressed on only a subset of tumor-infiltrating T-cells. In glioma tissue, we found that expression of PD-1 is specifically enriched on the TRM subset of tumor-infiltrating CD4+ and CD8+ T-cells (Fig. 3g–3h). Importantly, several of the PD-1+ T-cells also co-express other inhibitory checkpoints, including TIGIT and PD-L1 (Fig. 3i).
In addition to T-cells, we also compared the proportion and phenotype of innate CD56+NK cells in blood from healthy children as well as paired blood and tumor tissue from children with glioma. While the proportion of NK cells is comparable between blood and tumor tissue (Fig. 3a), tumor-infiltrating NK cells express significantly less granzyme and CD16 than their circulating counterparts (Fig. 3j). The loss of granzyme appears to be specific to tumor-associated NK cells, since CD8+ T-cells infiltrating these tumors retain granzyme expression (Fig. 3k). Our data show that the characteristics of T-cells and innate NK cells infiltrating the tumor tissue are distinct from their circulating counterparts.
Discussion
Brain tumors remain a major cause of cancer-related morbidity and mortality[1,35]. New immune therapies targeting inhibitory checkpoints (ICP) have shown promise in several human cancers, in particular those with high mutational loads[5–7]. ICP blockade has also shown efficacy in a subset of patients with glial tumors with high mutational burden[8,10]. While glioma do express recurrent mutations[36–38], the net mutational load in these tumors is low. It is increasingly apparent that optimal targets of tumor immunity may be antigens expressed in the entire clone and/or those important for tumor growth[39]. Hence, there is an unmet need to identify such targets in glioma and evaluate their immunogenicity.
In this study, we show that SOX2 is commonly expressed by pediatric glial tumors of all histologic grades. The finding that the expression of SOX2 is restricted to tumor cells, and not observed in surrounding normal brain tissue, suggests that the expression of SOX2 may be a useful histopathologic marker to evaluate tissue infiltration in surgical specimens[40]. SOX2 is best known as a critical gene in embryonal stem cells and a core factor in inducible pluripotent stem (iPS) cells[41]. Expression of SOX2 has been observed in adult glial tumors and in particular glioma stem cells[16]. SOX2-mediated signaling is important for the biology of glial tumors as inhibition of this gene leads to cell cycle arrest and inhibition of tumor growth in glial tumors [18,31]. Therefore, SOX2 may be an important target for therapeutic intervention in glioma[12].
Our data also demonstrate that aberrant expression of SOX2 in glial tumors is immunogenic both in children as well as in young adults, and that these patients mount a cellular immune response to SOX2 in vivo. The presence of SOX2-specific T-cells could be detected in circulation in children and young adults with glioma, indicating the systemic nature of this immune response in spite of the brain being traditionally considered an immune-privilege site. Importantly, the presence of anti-SOX2 T-cells could be detected in the tumor tissue even in a patient lacking such responses in circulating T-cells. This suggests that analysis of anti-tumor T-cell responses in blood may underestimate immunity to glioma antigens and implores the need to systematically evaluate tumor tissue for immunity to glioma-associated antigens. Whereas adult and pediatric glioma appear to be biologically different, SOX2 is expressed by glial tumors in all age groups. Our study suggests that SOX2 is immunogenic and can serve as a target for T cell immunity in both children and young adults.
The importance of characterizing immune cells infiltrating tumors is being increasingly appreciated[42]. Prior studies on the immune-phenotypic characterization of immune infiltrates in pediatric brain tumors have utilized limited markers[43]. These studies have nonetheless shown that pediatric gliomas do have greater infiltration by memory T-cells compared to non-malignant brain tissue resected from patients undergoing surgery for epilepsy[43]. In this study, we have applied newer tools such as mass cytometry and associated analytic tools to characterize the nature of immune infiltrates in pediatric brain tumors. These methods allow us to dissect the nature of the memory T-cell compartment and provide insights into both innate and adaptive immune cells. An important finding emerging from our study is that the expression of inhibitory immune checkpoints (such as PD-1) in glioma is particularly enriched in the subset of tumor-associated memory T-cells that express markers of tissue-resident memory T-cells. TRM cells are now appreciated as a distinct subset of T-cells capable of long-term persistence within tissues without recirculation[44–46]. Prior studies have identified both CD103+ and CD103-TRM cells in murine brains, consistent with our studies[47]. Our data suggest that the TRM subset of tumor-infiltrating T-cells may be key targets for ICP blockade in these tumors. Generation of this distinct subset of T-cells should therefore be an important target of vaccine approaches in glioma. The finding that glioma-associated TRM cells often co-express several ICPs suggests that combination therapies may be needed to overcome inhibitory signaling in these T-cells. Finally, the finding that NK cells infiltrating glial tumors express less granzyme B is consistent with prior studies implicating glioma-induced NK dysfunction and suggests that improving functional aspects of tumor-associated NK cells may also be of therapeutic benefit[48–50].
One limitation of these data is the relatively small numbers of patients studied thus far. Nonetheless, the finding that SOX2 is commonly expressed by glial tumors and is immunogenic in vivo should provide the basis for future studies to harness this response. T-cells against SOX2 have also been implicated in other tumors such as myeloma and lung cancer[27,32,33]. Patients with naturally occurring T-cell immunity against SOX2 do not exhibit paraneoplastic neurologic syndromes. However, immune therapies targeting this antigen do need to be monitored for the potential of adverse events due to the targeting of a subset of normal neural cells expressing this antigen[15,27,32,33]. Development of newer nanoparticle-based strategies to target SOX2 to dendritic cell subsets represents one emerging approach to elicit immunity to this antigen in the clinic[51]. Combining these strategies with immune checkpoint blockade could provide effective tumor immunity and improve outcomes in pediatric brain tumors.
Supplementary Material
Acknowledgements
Peptide synthesis was performed by Henry Zebroski at the Proteomics Resource Center of The Rockefeller University.
Funding: This study was supported in part by the Tap Cancer Out St. Baldrick’s Fellow Research Grant and National Institutes of Health training grant T32HD068201 (J.C.V), and National Institutes of Health grant RO1-AI0792222 and Hyundai Hope on Wheels award (K.M.D)
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Ethical approval: All procedures performed in the present study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References:
- 1.Curtin SCMA, Anderson RN. (20016) Declines in cancer death rates among children and adolescents in the United States, 1999-2014. . NCHS data brief; 257 [PubMed] [Google Scholar]
- 2.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, Stovall M, Yasui Y, Nicholson HS, Wolden S, McNeil DE, Mertens AC, Robison LL, Childhood Cancer Survivor S (2003) Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 97 (3):663–673. doi: 10.1002/cncr.11095 [DOI] [PubMed] [Google Scholar]
- 3.Jones C, Karajannis MA, Jones DT, Kieran MW, Monje M, Baker SJ, Becher OJ, Cho YJ, Gupta N, Hawkins C, Hargrave D, Haas-Kogan DA, Jabado N, Li XN, Mueller S, Nicolaides T, Packer RJ, Persson AI, Phillips JJ, Simonds EF, Stafford JM, Tang Y, Pfister SM, Weiss WA (2016) Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. doi: 10.1093/neuonc/now101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollack IF, Jakacki RI (2011) Childhood brain tumors: epidemiology, current management and future directions. Nat Rev Neurol 7 (9):495–506. doi: 10.1038/nrneurol.2011.110 [DOI] [PubMed] [Google Scholar]
- 5.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS (2016) Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165 (1):35–44. doi: 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine 372 (26):2509–2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 8.Johanns TM, Miller CA, Dorward IG, Tsien C, Chang E, Perry A, Uppaluri R, Ferguson C, Schmidt RE, Dahiya S, Ansstas G, Mardis ER, Dunn GP (2016) Immunogenomics of Hypermutated Glioblastoma: A Patient with Germline POLE Deficiency Treated with Checkpoint Blockade Immunotherapy. Cancer Discov 6 (11): 1230–1236. doi: 10.1158/2159-8290.cd-16-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van ‘t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR (2013) Signatures of mutational processes in human cancer. Nature 500 (7463):415–421. doi: 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, Durno C, Krueger J, Cabric V, Ramaswamy V, Zhukova N, Mason G, Farah R, Afzal S, Yalon M, Rechavi G, Magimairajan V, Walsh MF, Constantini S, Dvir R, Elhasid R, Reddy A, Osborn M, Sullivan M, Hansford J, Dodgshun A, Klauber-Demore N, Peterson L, Patel S, Lindhorst S, Atkinson J, Cohen Z, Laframboise R, Dirks P, Taylor M, Malkin D, Albrecht S, Dudley RW, Jabado N, Hawkins CE, Shlien A, Tabori U (2016) Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34 (19):2206–2211. doi: 10.1200/jco.2016.66.6552 [DOI] [PubMed] [Google Scholar]
- 11.Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Normolle DP, Connelly AK, Dibridge S, Mason G, Whiteside TL, Okada H (2016) Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol 18 (8): 1157–1168. doi : 10.1093/neuonc/now026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platten M, Bunse L, Wick W, Bunse T (2016) Concepts in glioma immunotherapy. Cancer immunology, immunotherapy : CII 65 (10):1269–1275. doi: 10.1007/s00262-016-1874-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC (2004) Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer research 64 (24):9160–9166. doi: 10.1158/0008-5472.can-04-0454 [DOI] [PubMed] [Google Scholar]
- 14.Sarkar A, Hochedlinger K (2013) The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12 (1):15–30. doi: 10.1016/j.stem.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham V, Khudyakov J, Ellis P, Pevny L (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39 (5):749–765 [DOI] [PubMed] [Google Scholar]
- 16.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432 (7015):396–401. doi: 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- 17.Tam WL, Ng HH (2014) Sox2: masterminding the root of cancer. Cancer cell 26 (1):3–5. doi: 10.1016/j.ccr.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 18.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G (2009) SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 27 (1):40–48. doi: 10.1634/stemcells.2008-0493 [DOI] [PubMed] [Google Scholar]
- 19.Berezovsky AD, Poisson LM, Cherba D, Webb CP, Transou AD, Lemke NW, Hong X, Hasselbach LA, Irtenkauf SM, Mikkelsen T, deCarvalho AC (2014) Sox2 promotes malignancy in glioblastoma by regulating plasticity and astrocytic differentiation. Neoplasia 16 (3):193–206, 206 e119–125. doi: 10.1016/j.neo.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444 (7120):756–760. doi: 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 21.Auffinger B, Tobias AL, Han Y, Lee G, Guo D, Dey M, Lesniak MS, Ahmed AU (2014) Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ 21 (7): 1119–1131. doi: 10.1038/cdd.2014.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auffinger B, Spencer D, Pytel P, Ahmed AU, Lesniak MS (2015) The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev Neurother 15 (7):741–752. doi: 10.1586/14737175.2015.1051968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK, Fussel M, Bachmann M, Rieber EP, Weigle B (2007) Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer 96 (8): 1293–1301. doi: 10.1038/sj.bjc.6603696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polakova I, Duskova M, Smahel M (2014) Antitumor DNA vaccination against the Sox2 transcription factor. International journal of oncology 45 (1): 139–146. doi: 10.3892/ijo.2014.2402 [DOI] [PubMed] [Google Scholar]
- 25.Boddupalli CS, Bar N, Kadaveru K, Krauthammer M, Pornputtapong N, Mai Z, Ariyan S, Narayan D, Kluger H, Deng Y, Verma R, Das R, Bacchiocchi A, Halaban R, Sznol M, Dhodapkar MV, Dhodapkar KM (2016) Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight 1 (21):e88955. doi: 10.1172/jci.insight.88955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amir ED, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe’er D (2013) viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol 31 (6):545-+. doi: 10.1038/nbt.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD Jr., Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV (2007) Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med 204 (4):831–840. doi: 10.1084/jem.20062387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhodapkar KM, Feldman D, Matthews P, Radfar S, Pickering R, Turkula S, Zebroski H, Dhodapkar MV (2010) Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci U S A 107 (19):8718–8723. doi: 10.1073/pnas.0915086107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annovazzi L, Mellai M, Caldera V, Valente G, Schiffer D (2011) SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics Proteomics 8 (3): 139–147 [PubMed] [Google Scholar]
- 30.Phi JH, Park SH, Kim SK, Paek SH, Kim JH, Lee YJ, Cho BK, Park CK, Lee DH, Wang KC (2008) Sox2 expression in brain tumors: a reflection of the neuroglial differentiation pathway. Am J Surg Pathol 32 (1):103–112. doi: 10.1097/PAS.0b013e31812f6ba6 [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Fotovati A, Triscott J, Chen J, Venugopal C, Singhal A, Dunham C, Kerr JM, Verreault M, Yip S, Wakimoto H, Jones C, Jayanthan A, Narendran A, Singh SK, Dunn SE (2012) Polo-like kinase 1 inhibition kills glioblastoma multiforme brain tumor cells in part through loss of SOX2 and delays tumor progression in mice. Stem Cells 30 (6):1064–1075. doi: 10.1002/stem.1081 [DOI] [PubMed] [Google Scholar]
- 32.Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV (2013) SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology 2 (7):e25205. doi: 10.4161/onci.25205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhodapkar MV, Sexton R, Das R, Dhodapkar KM, Zhang L, Sundaram R, Soni S, Crowley JJ, Orlowski RZ, Barlogie B (2015) Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood 126 (22):2475–2478. doi: 10.1182/blood-2015-03-632919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parney IF, Waldron JS, Parsa AT (2009) Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg 110 (3):572–582. doi: 10.3171/2008.7.JNS08475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reni M, Mazza E, Zanon S, Gatta G, Vecht CJ (2017) Central nervous system gliomas. Crit Rev Oncol Hematol 113:213–234. doi: 10.1016/j.critrevonc.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 36.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482 (7384):226–231. doi: 10.1038/nature10833 [DOI] [PubMed] [Google Scholar]
- 37.Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Zhang J, Baker SJ (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nature genetics 44 (3):251–253. doi: 10.1038/ng.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155 (2):462–477. doi: 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C (2016) Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (New York, NY: ) 351 (6280):1463–1469. doi: 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alonso MM, Diez-Valle R, Manterola L, Rubio A, Liu D, Cortes-Santiago N, Urquiza L, Jauregi P, Lopez de Munain A, Sampron N, Aramburu A, Tejada-Solis S, Vicente C, Odero MD, Bandres E, Garcia-Foncillas J, Idoate MA, Lang FF, Fueyo J, Gomez-Manzano C (2011) Genetic and epigenetic modifications of Sox2 contribute to the invasive phenotype of malignant gliomas. PloS one 6 (11):e26740. doi: 10.1371/journal.pone.0026740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 (4):663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 42.Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, Capone M (2016) Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. Journal of translational medicine 14:273. doi: 10.1186/s12967-016-1029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, Wang M, Handler MH, Foreman NK (2013) Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol 191 (9):4880–4888. doi : 10.4049/jimmunol.1301966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schenkel JM, Masopust D (2014) Tissue-resident memory T cells. Immunity 41 (6):886–897. doi: 10.1016/j.immuni.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park CO, Kupper TS (2015) The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21 (7):688–697. doi: 10.1038/nm.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL (2013) Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38 (1):187–197. doi: 10.1016/j.immuni.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakim LM, Woodward-Davis A, Bevan MJ (2010) Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proceedings of the National Academy of Sciences of the United States of America 107 (42):17872–17879. doi: 10.1073/pnas.1010201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PO, Zimmer J, Chekenya M (2013) Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol 264 (1–2):71–83. doi: 10.1016/j.jneuroim.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 49.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT (2010) TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8(+) T cells glioma patients. Neuro-Oncology 12 (1):7–13. doi: 10.1093/neuonc/nop009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navarro AG, Kmiecik J, Leiss L, Zelkowski M, Engelsen A, Bruserud O, Zimmer J, Enger PO, Chekenya M (2014) NK Cells with KIR2DS2 Immunogenotype Have a Functional Activation Advantage To Efficiently Kill Glioblastoma and Prolong Animal Survival. J Immunol 193 (12):6192–6206. doi: 10.4049/jimmunol.1400859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sehgal K, Ragheb R, Fahmy TM, Dhodapkar MV, Dhodapkar KM (2014) Nanoparticle-mediated combinatorial targeting of multiple human dendritic cell (DC) subsets leads to enhanced T cell activation via IL-15-dependent DC crosstalk. Journal of immunology 193 (5):2297–2305. doi: 10.4049/jimmunol.1400489 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


