Abstract
Nanoparticles have many applications both in industry and medicine. Depending upon their physical and chemical properties, they can be used as carriers of therapeutic molecules or as therapeutics. Nanoparticles are made of synthetic or natural polymers, lipids or metals. Their use allows for faster transport to the place of action, thus prolonging its presence in the body and limiting side effects. In addition, the use of such a drug delivery system protects the drug from rapid disintegration and elimination from the body. In recent years, the use of proteins and peptides as therapeutic molecules has grown significantly. Unfortunately, proteins are subject to enzymatic digestion and can cause unwanted immune response beyond therapeutic action. The use of drug carriers can minimize undesirable side effects and reduce the dose of medication needed to achieve the therapeutic effect. The current study presents the use of several selected drug delivery systems for the delivery of proteins, peptides and other therapeutic molecules.
Keywords: Nanoparticles, carriers, drug delivery systems, active transport, protein carriers
1. Introduction
The term nanoparticle refers to materials of which particles range from 1 nm to 1000 nm in size [1]. Nanoparticles used specifically as carriers of drugs or therapeutic molecules can be as large as 100 nm at least in one dimension. They are made of various materials such as natural or synthetic polymers, lipids or metals. As nanoparticles are more efficiently taken up by cells than larger macromolecules, they are a very good material for a delivery system [2]. Despite having good therapeutic properties, a low concentration of a drug at the target site results in low efficiency, and so high doses may be needed, resulting in the development of side effects. Another limiting factor is the short time of circulation in the body, because of effective removal of foreign molecules, including drugs, from the body [1]. The use of a drug delivery system enables transportation to be accelerated to the place of action, prolonged presence in the body and reduced side effects. It also protects the drug from rapid disintegration or clearance, and increases the concentration of the drug in the target tissues, thereby lowering the dosage of the drug [3]. Therapeutic molecules may be attached on the surface or located inside the nanoparticles [2]. The use of nanoparticles in drug transportation allows delivery of the conjugate (nanoparticle - drug) into specific cells by active or passive transport. Active transport is based on the placement of a molecule on the surface of the conjugate that will recognize the target site (Fig 1); this typically applies to antibodies and low molecular weight molecules such as folic acid or peptides [4]. Active transport can also be achieved by manipulating physical stimuli, including temperature and pH of the environment. Passive transport, however, uses the phenomenon of increased vascular permeability and retention of small and large molecules in tissues [4]. Depending on the mechanisms used and the construction of nanoparticles, the release of the drug from the conjugate can take place due to physiological changes in the environment, such as temperature, pH or osmolality, or by enzymatic action.
Figure 1.
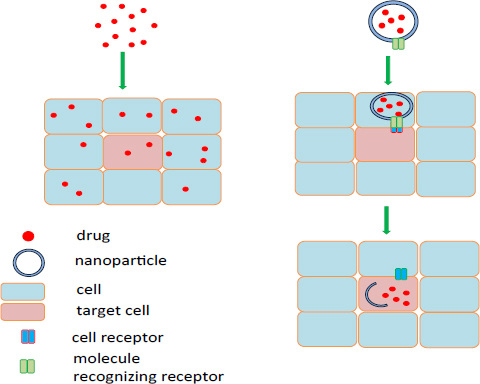
A schematic illustration showing passive and active transport of nanocarrier with a drug to the target cell.
Besides the creation of small-molecule drugs, recent years have seen the growth in the use of proteins as therapeutic molecules. They have great potential because of their specificity of action, higher activity and lower toxicity compared to conventional drugs [5]. However, proteins delivered to the body are subject to enzymatic degradation, can elicit an immune response due to their complex structure, have a short half-life and are poorly permeable through biological membranes, which is a significant problem for efficient and targeted delivery [5, 6]. The use of carriers supports the transport of peptides and proteins by protecting them from environmental conditions while maintaining their stability and reducing the immune response; this approach often improves enzymatic activity and biocompatibility, and enables active transport to the destination. Common carrier proteins include liposomes, micelles, polymeric nanoparticles and nanoparticles of inorganic materials. Recent studies suggest that the most effective carriers are conjugates which includes many molecules like drugs and signaling particles. In such conjugates, protein often is not a therapeutic agent but a molecule used to recognize the target. Demonstrated nanoparticles have great potential in many medical fields like diagnostics and therapies, however their application must first be characterized in vitro and in vivo. The following article reviews in vitro and in vivo studies of using various nanoparticles (Fig. 2) to examine their value as carriers of drugs and proteins for use in treatment of various disease types.
Figure 2.
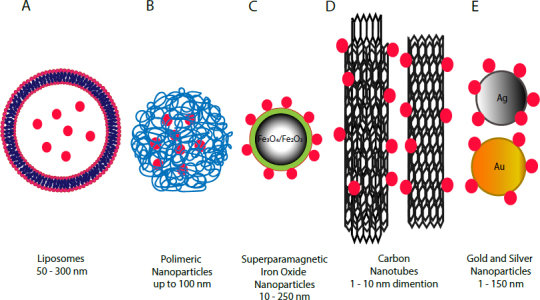
Examples of nanoparticle drug delivery systems.
2. Liposomes
Liposomes are spherical vesicles constructed of a lipid bilayer composed of natural or synthetic phospholipids (Fig. 2A); these have unique properties making them ideal materials for carriers of therapeutic molecules [1, 7]. The size of clinically-approved carriers ranges between 50 and 300 nm [7]. Their sensitivities to pH, temperature or other environmental factors depend on the phospholipids used for their production [7]. The main advantages of liposomes are the enhancement of pharmacokinetic properties, i.e. the therapeutic index, reduction of harmful side effects, stabilization of encapsulated enzyme proteins, and increase in in vitro and in vivo antitumor properties. They can also help improve the drug solubility [4, 7]. Whereas phospholipids have hydrophobic and hydrophilic units, liposomes can serve as carriers of water-soluble (hydrophilic) molecules inside the liposome body or hydrophobic molecules inside the lipid bilayer [8]. To increase the effectiveness of liposomes, it is necessary to add amphiphilic molecules such as polyethylene glycol (PEG) to the surface, allowing the liposomes to reach the target site in the body [1].
The use of an appropriate phospholipid mixture for the construction of liposomes may improve the transport of therapeutic molecules into target cells. Liposomes sensitive to the pH of the environment have been created, allowing greater transport to tumor cells, whose cytoplasmic pH is lower. In such an environment, the liposomes are destabilized and disintegrate, releasing their active substances inside the cell [9]. Another means of targeted transport is to place the appropriate molecule on the surface of the liposome, which can direct liposomes to the receptor on the surface of specific cells. A mechanism of targeted delivery to a cancer cell is possible because of a large number of tumor cell lines are characterized by the overexpression of various receptors. The use of liposomes modified with PEG molecules to which anisamide molecules are attached targets liposomes to sigma receptors, excessively expressed on the surface of tumor cells [10]. An EEEEpYFELV (EV) nonapeptide mimicking the Y845 site of EGFR, responsible for STAT5b phosphorylation, was used as a therapeutic molecule; it blocked the EGFR receptor and the signal pathway, resulting in the initiation of apoptosis of tumor cells. The conjugate formed in this way efficiently delivered the EV peptide to tumor cells, inhibiting tumor growth; however, the EV peptide did not induce an immune response in an in vivo model [10]. Application of the EGFR growth factor as the targeting molecule enabled the conjugate to deliver cetuximab and oxaliplatin to the colorectal cancer cells in a mouse model [11]. The drug has also been observed to have a less toxic effect when immobilized in liposomes [11]. Targeted transport can be made more precise, not only by determining the type of target cells, but also by trying to deliver the drug to specific cell organelles, such as the mitochondria. The use of liposomes formed from a mixture of corresponding lipids and octa-arginine (MITO-potrer) on the surface enables greater transport of molecules into the mitochondria. The liposomes enter the cells after macropinocytosis [12]. MITO-porter then attaches to the mitochondrial membrane through electrostatic interaction, which induces fusion between the outer mitochondrial membrane and the modified liposome. In this way, the MITO-porter can lead to the creation of a system for transporting macromolecules inside the liposome directly into the mitochondria [12]. Application of liposomes could help in defense from disease by interference of immune system cells. Clodronate- liposomes have ability to deplete macrophages and it has been already demonstrated in different disease models [13]. The clodronate – liposome complex (Clo-Lipo-DOTAP [lipid - N-[1-(2,3-dioleoyloxy) propyl]-N, N, N-trimethylammonium methyl-sulfate]) has been tested for functionality in depleting macrophages in an inflammation-driven model of carcinogenesis and its antitumor effectiveness in biologically and clinically relevant murine melanoma models. Application of the liposomes resulted in a depletion of tumor-associated macrophages in primary and metastatic melanomas, illustrating antitumor efficacy via inhibition of angiogenesis and modulation of inflammation-related cytokines [13]. This method of delivery demonstrated inhibition of cancer development.
The oral delivery of therapeutic proteins and peptides still represents a challenge because of their low stability and poor absorption in the gastrointestinal tract. The modification of liposomes with sugar ligands, for example mannose, could direct nanoparticles to the target site (absorption sites) and thereby increase the absorption of proteins in the intestines [14]. Studies have shown that the permeability of the small intestinal cell membrane line Caco-2 for mannose-modified liposomes containing lysozyme is greater than that of the protein without carrier. The presence of mannose and its correct density on the liposome surface increases membrane permeability [14]. It was also shown that the use of carbopol(CP)-lectin to cover the liposomes increases their bioadhesive properties. In rat studies, calcitonin demonstrated a six times greater enzymatic activity when administered by liposomes than in controls when the protein was administered without any carrier [15]. Conjugates of carbopol (CP) - lectin are very effective coatings applied to the liposomes to improve oral delivery of peptides and proteins [15].
Liposomes can also be used to improve the absorption of hydrophobic therapeutics. Miconazole is an antifungal agent but its use is ineffective because it does not dissolve well in water and thus does not easily penetrate the skin [16]. The use of PEGylated liposomes may aid delivery of this drug to skin cells when applied locally. Incorporation of propylene glycol molecules into phospholipid liposomes increases the liposome loading capacity, prolongs the release time, stabilizes the molecules during storage, and increases antifungal properties and skin penetration [16]. The addition of molecules on the surface of liposomes could help them enter the cells and/or stabilize these particles.
Liposomes can also be used for the simultaneous transport of several types of molecules such as DNA and protein. The stoppin peptide is a potential antitumor drug because it is an inhibitor of the p53-MDM2/MDMX complex. Being a competent inhibitor of MDM2 and/or MDMX oncogenic proteins, stoppin inhibits their ability to attach to the p53 protein, resulting in the activation of apoptosis in tumor cells. Liposomes with peptide and nucleic acid were tested in vitro on the lung cancer cell line A549. The peptide was found to have antitumor activity, which was increased by the use of liposomes [17]. The formation of the liposome complex in which the peptide and DNA are located results in a higher density of protein and nucleic acid, which in turn results in a smaller size of the therapeutic molecule at the appropriate protein concentration. In addition, the peptide protects the nucleic chain from DNase activity, and the nucleic acid protects the peptide from the action of trypsin. Simultaneous packaging of these molecules therefore improves their chance of successful entry into the cell [17]. Such combination of two different particles could be useful in gene therapy. Because liposomes are easy to prepare and have excellent biocompatibility, they are widely-studied as carriers for various therapeutic molecules. However, despite the many advantages of liposomes as carriers, much remains to be learned and improved. Examples of liposomes as therapeutic molecule carriers are shown in Table 1.
Table 1.
Liposomes as drug carriers
| Nanoparticle | Therapeutic molecule | Therapeutic effect | Reference |
|---|---|---|---|
| Liposome | Peptide EV | Anticancer, lung cancer, in vitro | [10] |
| Liposome | Cetuximab, oxaliplatin | Anticancer, colorectal cancer, in vivo | [11] |
| Liposome | Miconazole | Antifungal, skin, in vitro | [16] |
| Liposome | Stoppin | Anticancer, lung cancer, in vitro | [17] |
| Liposome | Ibuprofen | Analgesic effect, in vitro | [18] |
| Liposome | Dexamethasone phosphate | Anti-inflammatory, in vivo, rheumatoid arthritis | [19] |
| Liposome modified | Paclitaxel | Anticancer, in vitro, in vivo, breast cancer | [20] |
| triphenylphosphonium - PEG-PE |
3. Polymer nanoparticles
Polymeric nanoparticles (Fig. 2B) can be synthesized from natural or synthetic polymers, as well as biodegradable or non-biodegradable polymers. The type of material used to synthesize nanoparticles influences the performance of drug delivery and its therapeutic effects [21]. An important feature of nanoparticles is their size and shape. It has been found that particles with a size of up to 100 nm are most readily absorbed by cells. At the same time, their shape should be as spherical as possible [22]. The use of such systems offers many benefits, such as increased bioavailability of drugs by reducing degradation rates, reduced side effects, increased cellular uptake, greater targeted transport, and more control over drug release [22]. Natural materials such as chitosan, collagen, dextran or alginates, or synthetic materials like polylactide (polylactic acid PLL), polycaprolactone (PCL), polyvinyl, polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA) are used for the production of polymeric nanoparticles. The use of different materials makes it possible to determine the specific properties of the molecules, e.g. susceptibility to environmental pH. The use of 2-[3-[5-amino-1-carboxypentyl] -ureido] -pentanedioic acid (Acupa) as a material for the synthesis of polymer nanoparticles has resulted in pH sensitivity. These molecules were used as carriers of model proteins such as bovine serum albumin and cytochrome c [23]. In vitro studies have demonstrated the high rate of loading, transport and release of therapeutic proteins that induce apoptosis of LNCaP cell lines, thus demonstrating the antitumor properties of the carrier-protein conjugate used [23]. Synthesizing nanoparticles of a suitable type of material allows the delivery of therapeutic molecules to cells with particular characteristics. For example, in vivo studies have demonstrated effective delivery of therapeutic molecules to tumor cells, where the redox state is different from normal cells; one such example being chitosan-based molecules like glycolipids (ss-CSO-SA), which are sensitive to the potential/redox state in tumor cells [24].
The use of a protein in the transport of a transferrin-targeted nanoparticle (Tf-NPs) composed of PLL-PEG-PLGA increases the delivery of molecules to cells with a greater number of transferrin receptors, such as tumor cells [25]. The payload was tetrandrin (Tet), a natural alkaloid which allows withdrawal of drug resistance, and the anticancer drug daunorubicin (DNR). This DNR-Tet-Tf-NPs complex has been found to be effective in tumor cell lines resistant to chemotherapy, and in vivo studies have found it to arrest the growth of transplanted tumors in mice. This complex did not show any toxicity. The drug was delivered directly into the tumor, thereby reducing the amount of drug delivered into the body necessary to inhibit tumor growth [25]. Another method utilized the targeted transport of monoclonal anti-Her2 antibodies against the HER2 antigen which is more plentiful on the surface of tumor cells than normal cells. The placement of this antibody on the nanoparticle surface of poly (DL-lactic acid) allows targeted transport [26]. The complex significantly improves the delivery of an anticancer drug cargo in a mouse model of ovarian cancer [26].
Due to their characteristics, nanoparticles allow for the development of novel treatments in addition to conventional ones. An immune response was elicited against tumor cells during studies using nanogel as a protein carrier, with Langerhans cell activation and arrest of tumor growth observed after administration of the vaccine through the skin of mice [27]. The in vivo application of chitosan microcapsules containing insulin through the respiratory system allowed the drug to be delivered in its active form to the blood stream, thus inducing hypoglycaemia [28]. The use of nanoparticles provides many therapeutic possibilities. In in vitro studies, mesoporous silica was used to transport the BFP peptide to human stem cells, where osteogenesis was initiated [29].
Biotechnology offers great potential for the large-scale production of proteins and the ability to use them as therapeutics. Most of these proteins are enzymes and their activity depends on the conditions in which they are found, their conformation and their integrity. The use of carriers helps to keep proteins active. Closure of the enzyme α-amylase in PLGA (poly-(L-lactic-co-glycolic acid)) nanoparticles does not affect the conformation of the enzyme and maintains its activity [30]. In vitro studies on the antioxidant activity of superoxide dismutase (SOD) and catalase (CAT) immobilized in polycaprolactone (PCL) nanoparticles revealed higher antioxidant performance of the enzymes immobilized together in a single nanoparticle compared to that of individually immobilized enzymes and non-immobilized enzymes [31].
Polymeric nanoparticles provide a wide range of drug delivery systems. They are widely used as carriers of proteins, in spite of the many challenges which must be resolved before clinical use. Their use allows enzymatic proteins to be maintained, enables better delivery of therapeutics to the target site, and for signaling molecules to be located. Due to the wide range of materials from which they can be synthesized and their many potential modifications, polymer nanoparticles are some of the most commonly-used carriers. Examples of polymeric carriers as drug delivery systems are shown in Table 2.
Table 2.
Polymeric nanoparticles as drug carriers
| Nanoparticle | Therapeutic molecule | Therapeutic effect | Reference |
|---|---|---|---|
| PEG-PLL-PLGA | Tetrandrin, daunorubicin | Anticancer, in vitro, in vivo | [25] |
| Poly-lactic acid | Paclitaxel | Anticancer, in vivo, ovarian cancer | [26] |
| Mesoporous silica | Peptide BFP | Activated osteogenesis in stem cells, in vitro | [29] |
| Acrylamide (AAm), positively-charged N-(3- | Caspase 3 (CP-3) | Anticancer, in vitro | [32] |
| Aminopropyl) methacrylamide (APMAAm) | |||
| Chitosan | Takrin | Alzheimer’s drug, in vivo, preclinical survey | [33] |
| PLG, alginate stabilized with chitosan | Clotrimazol, ekonazol | Antifungal, in vivo | [34] |
| PLA/chitosan | Lamivudin | Anti-HIV, in vitro | [35] |
| Polyacrylic | N-thiolated β-lactam antibiotics | Antibacterial, in vitro | [36] |
4. Carbon nanoparticles (carbon nanotubes)
Carbon nanotubes (CNT) are hollow rollers formed primarily of carbon atoms (Fig. 2C). CNT are made up of a layer of graphene wrapped in a cylinder that can be opened or closed, and their size can vary from 1 nm to several micrometers [37]. Nanotubes can be formed of a single layer of carbon atoms, i.e. single wall carbon nanotubes (SWCNT), or multiple layers of carbon atoms, i.e. multilayer carbon nanotube (MWCNT) [37, 38]. The unique physical and chemical properties of carbon polymers allow application in many scientific fields such as electronics and nanocomposite materials [37]. In their basic form, carbon nanotubes are insoluble in water or organic solvents [37, 39] due to their lack of functional groups. The addition of functional groups allows dissolution of nanoparticles in water or other solvents [40]. One way to impart a functional group is oxidative cleavage using an strong oxidant and acidic media [39, 40]. Carbon nanotubes as carriers have many advantages, particularly their large area, the possibility for functionalization of the carrier and the ability to penetrate into various types of cell [41].
Previous studies have found carbon nanotubes to be carriers of various proteins (protein A, Alexa-fluor bovine serum albumin, streptavidin, cytochrome c) to different cell lines [42]. The creation of functionalized PLGA nanoparticles allows the protein CP3 to be supplied to bone cancer cells with prolonged release over time. An additional advantage of this system is the ability to control protein release by controlling the weight and ratio of PLGA in the conjugate [43]. It has been proposed to use multi-wall carbon nanotubes to deliver ricin toxin chain A (RTA). Nanotubes have been shown to significantly enable penetration of the protein to various cell lines [44]. The MWCNT-RTA-HER2 conjugate was also constructed, where the presence of the anti-HER2 antibody was intended to deliver toxin only to breast cancer cells. The placement of antibody in the conjugate increases the delivery of the toxin to these cells, while limiting its penetration into other cells [44]. Carbon nanotubes connected in a conjugate with cisplatin and EGF protein can selectively and effectively deliver the drug in vitro and in vivo to head and neck squamous carcinoma, whose cells are characterized by an overexpression of the EGFR [45]. EGFR-targeted transport allowed more efficient drug delivery and cell killing in cancer cells than controls, in which only drugs loaded on carbon nanotubes were used. However, further studies on the long-term toxicity, distribution and removal of nanoparticles must be carried out on animal models [45].
The recombinant lamin B1 (LB1) protein, which has structures that allow this protein to enter the cell nucleus, was immobilized onto carbon nanotubes. This conjugate was tested in vitro on HeLa cell lines and found to be capable of penetrating into the cell nucleus [46]. The creation of such a conjugate suggests the possibility of using carbon nanotubes for targeted transport, not only to specific cells but also to specific cell organelles. This technique offers potential for the future delivery of anticancer therapy agents, gene therapy agents or DNA. Table 3 provides examples of the use of carbon nanotubes as drug carriers.
Table 3.
Carbon nanoparticles as drug carriers
| Nanoparticle | Therapeutic molecule | Therapeutic effect | Reference |
|---|---|---|---|
| Carbon nanotubes functional PLGA | Protein CP3 | Anticancer, in vitro, bone cancer | [43] |
| MWCN | The A chain of ricin (RTA) | Anticancer, in vitro, breast cancer | [44] |
| SWCN | Cisplatin | Anticancer, in vitro and in vivo, Squamous carcinoma of the head and neck | [45] |
| SWCN | Wilms tumor protein (WTP1) | Enhancement of immune response | [47] |
| Carbon nanotubes coated with multilayer polyglycol polymers and polylactone (PGA, PLA) | Dasatinib | Anticancer, in vitro | [48] |
| SWCN | LB1 protein | Entrance cell nucleus, in vitro, breast cancer | [46] |
| SWCN | Cisplatin | Anticancer, in vitro, in vivo, lung cancer | [49] |
| MWCN/chitosan | TAT peptide | Anticancer, in vitro, in vivo, breast cancer | [50] |
| MWCN | Dexamethasone | Anti-inflammatory, in vitro | [51] |
5. Superparamagnetic iron oxide nanoparticles (SPION)
Superparamagnetic iron oxide nanoparticles (SPION) (Fig. 2D) are made of iron (III) oxide (Fe2O3) or iron (IV) oxide (Fe3O4); in both cases, superparamagnetism occurs naturally [52]. As nanocarriers SPIONs have a large surface to volume ratio, their size ranging from 10 to 250 nm in diameter [52]. The most specific property of these nanoparticles is ability to respond to an external magnetic field due to superparamagnetism [53]. Magnetic nanoparticles (MNP) could be easily removed or separated from a mixture by the use of magnet. This property is currently used in separation techniques, especially in cell separation [53]. In recent years, interest in the application of magnetic nanoparticles as drug carriers has increased. The ability of these nanoparticles to bind proteins, peptides, enzymes, antibodies and drugs has major impacts on biomedicine and biotechnology [54]. Drugs could be loaded on the surface of SPION by conjugation, or a drug molecule could be encapsulated with magnetic nanoparticles within a coating material [55]. Moreover, they have features that enable them to be used as a delivery platform in biological systems, including low toxicity and high biocompatibility [56]. Magnetic nanoparticles could accumulate in targeted sites by use of an external magnetic field. After removing the external magnetic field, the magnetic nanoparticles do not perform any magnetization [53, 55]. These properties have made SPIONs very attractive for medical in vivo applications, like magnetic resonance imaging (MRI) [53, 57]. Magnetic nanoparticles seem to be a very promising avenue for exploration in developing diagnostic and therapeutic methods.
Typically, magnetic nanoparticles used as drug carriers are composed of a magnetic core (Fe2O3, Fe3O4) and a coating layer, which can be made of biocompatible polymers (Fig. 2D) [55]. This layer provides functional groups to easily bind a drug molecule, inhibit aggregation and increase colloidal stability [55, 58]. SPION coated with PEG and PEI polymers has been modified with folic acid and loaded with doxorubicin as an anticancer drug. Cancer specific targeting properties were achieved by modification of nanoparticles by folic acid, a molecule recognized by a folic acid receptor (FA receptor) [59]. Efficient drug delivery, antitumor effect, and application as a contrast agent for MRI of this conjugate has been established [59]. Other research groups have demonstrated that coating Fe3O4 nanoparticles with silica make those nanoparticles suitable for drug conjugation and in vivo applications [58]. Covalently bonding a tissue plasminogen activator (tPA) to those Fe3O4-silica nanoparticles positively affected storage and operation stability [58]. Results from this study suggest that silica-magnetic nanoparticles are a useful magnetic, targeted drug delivery system for tPA and may provide a new drug type [58]. Immobilization of thermostable superoxide dismutase (SOD) on superparamagnetic nanoparticles covered by silica resulted in better resistance to temperature, pH, metal ions, enzyme inhibitors, and detergents [60]. These results illustrate that immobilization of enzymes on magnetic nanoparticles could affect and prolong their activity. Also, the use of magnetic nanoparticles as enzyme carriers was presented with immobilized L-asparaginase on the magnetic nanoparticles modified with poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) [61]. This platform presented long-term stability and favourable reuse of the enzyme. It has been also utilized as potential drug delivery system in leukemia treatments in a simulated extracorporeal shunt system [61]. These results demonstrate the possibility for the application of magnetic nanoparticles for the design of an efficient enzyme delivery system suitable in clinical treatment [61]. Serratopeptidase, a potentially therapeutic enzyme for pain and inflammation, was immobilized by covalent bonding through glutaraldehyde onto magnetic nanoparticles modified with chitosan [62]. This conjugate was tested in vitro and in vivo on rats with carrageen induced paw oedema. Results from the in vitro test show that magnetic targeting of the enzyme immobilized on MNP increased delivery through the membrane. For the in vivo tests, it was demonstrated that MNP with the enzyme enhanced anti-inflammatory effect [62].
Presented examples show that the magnetic core could be covered by different coating layers, however their mutual features have to be biocompatible. SPIONs used as drug delivery systems reveal new possible applications. They are one of most easily produced nanoparticles and have shown a great toxicity profile. They have demonstrated a high possibility in application for the therapeutic and diagnostic fields. Despite those advantages, no drug-based SPIONs are on marker [55], most likely due to the limited knowledge of their characterization. Investigation into the pharmacokinetics and biodistribution of those nanoparticles in vivo must be performed. Table 4 presents examples of superparamagnetic iron oxide nanoparticles as drug carriers.
Table 4.
Superparamagnetic iron oxide nanoparticles as drug carriers
| Nanoparticle | Therapeutic molecule | Therapeutic effect | Reference |
|---|---|---|---|
| Fe3O4-silica | Tissue plasminogen activator (tPA) | Thrombolysis effect, in vitro, in vivo | [58] |
| SPION-PEG, PEI | Folic acid, doxorubicin | Anticancer, in vitro, in vivo, breast cancer | [59] |
| Fe3O4-silica | Mn superoxide dismutase | Defence against oxidative stress, in vitro | [60] |
| Fe3O4-silica | L-Asparaginase | Anticancer, in vitro, acute lymphoblastic leukaemia | [61] |
| Fe3O4- chitosan | Serratopeptidase | Analgesic, anti-inflammatory, in vitro, in vivo, carrageenan-induced paw oedema | [62] |
| SPION-PEI | - | Macrophages activator (vaccination), in vitro | [63] |
| Fe3O4-N’N’-carbonyldiimidazole (CDI) | Ovalbumin | Vaccine delivery, in vitro | [64] |
| Fe3O4-silica | Methotrexate | Transdermal drug delivery, in vitro | [65] |
| Fe3O4-trimethoxyl octadecyl silane (C18) | Lipase | Enzyme stability, in vitro | [66] |
| Fe3O4- tannic acid | Trypsin | Enzyme stability, in vitro | [67] |
| Fe3O4-PEI | DNA (plasmid pEGFP-N1) | Gene therapy, in vitro | [68] |
6. Gold nanoparticles
Nanoparticles of gold, depending on research needs, are created in various shapes, including nanospheres (Fig. 2E), nanotubes, nanocages or nanoshells [69]. Gold nanoparticles have a combination of unique physical, chemical, optical and electronic properties that differentiate them from other biomedical nanotechnologies, enabling the creation of highly multifunctional platforms for biochemical applications, gene delivery, imaging and drug delivery [69]. These molecules have many desirable properties, such as a low volume to surface ratio, non-toxicity and biocompatibility, making them ideal candidates for the delivery of therapeutic molecules [70]. Gold nanoparticles can be from 1 to 150 nm in size and can easily be manufactured with a controlled dispersion factor, which are two key factors in the development of drug delivery systems. Moreover, the high surface to volume ratio allows for placement on the surface of nanoparticles of a large number of functional molecules and therapeutics, while the molecules may be attached covalently or noncovalently [70]. Effective delivery of therapeutic molecules also involves releasing them from the carrier at the right place. Particle release can be induced by intra-cellular (e.g. pH, presence of glutathione GSH) or external factors (e.g. light) [71, 72]. The use of gold nanoparticles enabled the creation of a drug delivery system based on the use of glutathione as the endogenous drug release agent. This system is based on the use of a difference in glutathione concentrations in the cells (1-10 mM) and extracellular thiol concentrations (cysteine 8 μM, GSH 2 μM) [71, 72]. These properties have been exploited in cell lines. The antitumor agent paclitaxel was placed on gold nanoparticles in the presence of biotin; this was used as a directing agent for tumor cells, which have significantly higher levels of biotin receptors than normal cells. In these studies, the affinity of the investigated molecules was found to be higher for cancer cells compared to controls. At the same time, the drug demonstrated a stronger antitumor effect than the non-nanoparticle drug [73].
Gold nanoparticles have been used to deliver doxorubicin to a HeLa cell line and control its release rate [74]. Real-time fluorescence microscopy found the fluorescence of the drug to vary during incubation, with the nanoparticle-administered drug demonstrating a different release site to one administered without any carriers. The results of the study also confirm the value of nanoparticles as a vehicle for observing drug release in real time [74]. Imaging has become an important tool in the diagnostics, research and application of cancer treatment, and to track the changes occurring during treatment. Confocal laser scanning microscopy (CLSM) and ICP-MS (inductively coupled plasma – mass spectrometry) were used to observe the uptake of herceptin and a monoclonal antibody against the HER2 receptor by the SK-BR3 breast cancer cell line from gold nanoparticle carriers. The results indicate that this complex induces cytotoxicity in tumor cells but not the control cells. In addition, the presence in tumor cell cytoplasm of free herceptin inducing apoptosis has been found, so it is believed that such a conjugate has the potential for the imaging and treatment of breast cancer [75]. The effect of a conjugate of a therapeutic protein p12 and the targeted protein (CRGDK) on the surface of 2 nm gold nanoparticles was studied in vitro on a breast cancer cell line overexpressing the Nrp-1 receptor [76]. Studies have confirmed increased antitumor activity of the conjugate due to greater p12 uptake. This type of drug delivery system can be used for further investigation into the delivery of single or multiple agents for a range of potential uses including imaging and treatment [76].
In vivo studies have examined the use of gold nanoparticles as carriers for theophylline (THP), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) and WGA transport protein (wheat gut agglutinin) in rats paralyzed due to damage to the cervical spinal cord segment. HRP (horseradish peroxidase) protein was also included in the complex for visualization. The conjugate was delivered to the central nervous system to the diaphragm, which controls the respiratory function, through the blood-brain barrier [77]. The tested conjugate was observed to restore the function of the diaphragm muscle. This ability of nanoparticles to pass through the blood brain barrier and selectively deliver the drug to a particular population of neurons opens the way to many applications [77].
In vivo studies have also examined the potential of gold nanoparticles as carriers of oral medication to increase the stability and availability of therapeutics through the intestinal mucosa. Insulin was immobilized onto chitosan-reduced gold nanoparticles, resulting in increased activity; it is thought to be delivered through the gut and the nasal mucosa. Chitosan-reduced gold can be considered a promising system for the delivery of biomolecules through mucous membranes [78].
Gold nanoparticles induce a rise in local temperature when subjected to wavelengths of 800-1200 nm [71]. However, structural modifications introduced during synthesis can shift the absorption band to desired values between 650-900 nm, i.e. near infrared bands, where the blood and soft tissues are relatively transparent [79]. Photodynamic therapy (PDT) is a developing method in the therapeutic treatment of cancer in which a photosensitive compound, referred to as the photosensitizer, is used in the destruction of the tumor cell. After stimulation by red light or near infrared, this compound leads to cell death via the production of reactive oxygen species [79, 80]. The ideal photosensitizer would be a hydrophobic compound; however, this would reduce its biodistribution ability, and therefore, much effort has been devoted to developing delivery systems for drugs and photosensitive compounds. Such systems maintain the stability and activity of hydrophobic photosensitizers in the aquatic environment, while providing the opportunity to further develop the functionality of the entire conjugate [80].
A conjugate was developed consisting of gold nanoparticles, a phthalocyanine used as a photosensitizer, and an anti-HER2 antibody used to direct the conjugate to the cancer cells overexpressing the HER2 receptor. An in vitro study found that breast cancer cells demonstrated greater uptake of the nanoparticle conjugate; this increased the efficiency of the applied photodynamic therapy, resulting in the death of cancer cells [79]. In another study, gold nanoparticles stabilized with the hydrophobic photosensitizer zinc phthalocyanine and polyethylene glycol (PEG) were used to covalently attach the jacalin lectin as a transporting molecule specific for the T-antigen molecule to the surface of HT-29 tumor colon cancer cells [80]. Due to the application, the absorption of the jacalin in conjugate by colon carcinoma cells increased. After light irradiation at 633 nm, the lectin-targeted conjugate induced the death of tumor cells to a much greater extent than that without lectin. Cell death was found to be induced by necrotic tract [80].
In vivo studies based on gold nanoparticles functionalized with PEG as a carrier of silicon phthalocyanine 4 (Pc 4) reported greater accumulation in the tumor caused by significant capillary permeability in the surrounding area. The drug immobilized on the gold demonstrated more rapid accumulation in the tumor than that administered without a carrier. In addition, no side effects were observed in mice subjected to irradiation, and therapeutic effects were achieved in the form of tumor growth arrest and the mobilization of necrosis in the tumor cells [81]. Further studies on the improvement of drug delivery through the use of active transport to particular cells may improve drug delivery and reduce its side effects.
Examples of presented in vitro and in vivo studies on gold nanoparticles confirms a wide spectrum of applications in diagnostic and therapies. Gold nanoparticles could be used as drug delivery system to cancer cells and improve effectiveness of know therapeutics. Also they can be used themselves as therapeutic for photodynac therapies. However, there is still much to investigate.
7. Silver nanoparticles
Nanoparticles of silver (Fig. 2E) are the most widely used nanomaterials because of their unique optical, photothermal, electrical and biological properties [82, 83, 84]. They have found use in a variety of everyday products, including home appliances, water filters, cosmetics, textiles and items associated with the food industry. Thanks to their antimicrobial, antifungal and antiviral properties, silver nanoparticles are also used in the medical sector for the production of dressings, catheters, pacemakers and vascular prostheses [83, 84]. They can also be used as a contrast agent in imaging diagnostics, as well as antitumor, photosensitizing, radiosensitizing properties, so they can be used as drug carriers or other therapeutic molecules [82, 83, 84, 85]. One factor influencing the physical, chemical and biological properties of nanoparticles is their size to volume ratio, and many preparation methods have been developed to influence this. Most are physical methods; unfortunately, they require a lot of energy for manufacture and cleaning, and the use of hazardous substances. However, new, highly-efficient biological methods have been developed which give much greater control over the product [84], and replace toxic reducing agents and stabilizers with non-toxic molecules such as proteins, antioxidants and hydrocarbons produced by living organisms including bacteria, fungi, yeasts and plants [85].
Extract from the plant Dimocarpus Longan L. allows monocrystalline and spherical particles ranging in size from 9 to 35 nm to be obtained [86]. These particles have demonstrated antibacterial activity against Gram positive and Gram negative bacteria, antifungal properties and a strong inhibition effect against the prostate cancer cell line PC-3. The latter is probably related to the ability of nanoparticles to decrease the level of proteins stat 3, bcl-2 and survivin, while increasing the level of caspase-3 [86]. Anticancer effects have also been demonstrated by silver nanoparticles obtained by synthesis using Abutilon indicum leaf extract [87]. The particles measured 5-25 nm in size, and possessed a number of advantageous properties: they had an antibacterial action, the phenolic residues on their surface captured reactive oxygen species, and in vitro antitumor activity was demonstrated against the COLO 205 cell line [87].
The most well-known feature of silver nanoparticles is their antimicrobial effect, which has found a wide range of uses inside and outside the medical sector. The particles were adapted to receive a new type of carrier by the creation of a multi-layer polyelectrolyte film with silver ions, on which ciprofloxacin (antimicrobial) and bovine serum albumin as model molecules were immobilized. Exposure to external triggering factors such as ultrasound and lasers resulted in the release of the compounds contained in the film with antibacterial activity [88]. This type of drug delivery system can be used to deliver vaccines through the skin, and bestow antibacterial and anti-inflammatory properties on implants and catheters.
Antimicrobial activity of silver nanoparticles can be enhanced by immobilizing an enzyme or antibiotic on them. The use of lysozyme as a factor enhancing the antimicrobial properties of silver has enhanced their effectiveness in treating silver-insensitive bacteria [89]. Alternatively, the immobilization of amphotericin B on silver particles resulted in strong antifungal effects, which is probably due to the interaction of the antifungal properties of amphotericin B and the antimicrobial properties of silver [90].
It has been shown that the presence of silver nanoparticles promotes wound healing by promoting the proliferation and migration of keratinocytes, and possibly by stimulating fibroblast differentiation into myoblasts [82, 83, 84, 85, 86, 87, 88, 89, 90, 91]. At the same time, they also influence the regulation of local and systemic inflammation after injury [92]. Silver nanoparticles have been incorporated into a chitosan and polyvinyl alcohol complex to improve healing and provide antibacterial activity [93].
Silver nanoparticles exhibit antitumor effects [94], but also demonstrate significant cytotoxicity against normal cells. However, these side effects can be limited by the use of a nanoparticle-guiding agent for the appropriate cell type. For example, folic acid was used as a targeting agent because of the significant amount of folic acid receptors (FR) on the surface of tumor cells, such as the HeLa cell line. At the same time, the paracetamol dimer was attached to the complex as a model drug molecule. The complex demonstrated antitumor activity in experiments on cell lines HeLa and A549 and folacin was found to be an efficient targeting molecule for cells overexpressing FR [94]. Silver nanoparticles have also been used as a carrier for PEG-coated methotrexate, with antitumor effects observed against MCF-7 breast cancer cells. The hemolytic effect of the drug was also lower when administered with the silver conjugate than the non-carrier drug [95]. Also the use of silver nanoparticles complexed with the anticancer drug alisertib resulted in the reduction of the U87MG tumor in mice [96].
Silver nanoparticles can also be very useful in diagnosis by allowing fluorescence excitation of the molecule. The silver-ethisteron complex used for glutathione (GSH) detection enabled the visualization of tumor cells while demonstrating antitumor properties; these were associated with the significantly higher levels of glutathione present in tumor cells compared to normal cells [97]. The use of silver reactivity to glutathione allowed the formation of a complex in which silver acts as protection for the drug delivery system of porous silicone with a fluorescent agent and drug [98]. The use of silver nanoparticle photoactivity and their ability to transport therapeutics enabled the delivery of oligonucleotides to tumor cells and their controlled release from nanoparticles induced by UV radiation [99]. Studies have found hybridized therapeutic oligonucleotides to have greater potential to target mRNA strands in cells than controls in which oligonucleotides were delivered by commercially-available vectors. Nanoparticles enabled higher cellular uptake and protected the oligonucleotide against nuclease activity [99]. The creation of such a medium gives hope to create a system of drug and therapeutic delivery which can be used in gene therapy, antisense therapy and gene expression studies.
Silver nanoparticles themselves have a very high therapeutic potential as antibacterial, antifungal or anticancer agents. In addition, they can be used to create drug delivery systems and to significantly improve the performance of therapeutic molecules. Some example uses of gold and silver nanoparticles as therapeutic molecule carriers are shown in Table 5.
Table 5.
Gold and silver nanoparticles as drug carriers
| Nanoparticle | Therapeutic molecule | Therapeutic effect | Reference |
|---|---|---|---|
| Gold nanoparticles | Paclitaxel | Anticancer, in vitro, breast cancer, lung cancer, osteosarcoma | [73] |
| Gold nanoparticles | Doxorubicin | Anticancer, in vitro, breast cancer | [74] |
| Gold nanoparticles | Herceptin | Anticancer, in vitro, breast cancer | [75] |
| Gold nanoparticles | p12 | Anticancer, in vitro, breast cancer | [76] |
| Gold nanoparticles | Theophylline (THP), 1,3-dipropyl-8- cyclopentylxanthine (DPCPX) | Neuron repair, in vivo | [77] |
| Gold nanoparticles reduced with chitosan | Insulin | Diabetes, in vivo | [78] |
| Silver nanoparticles | Lysozyme | Antibacterial | [100] |
| Silver nanoparticles | Amfotherine B | Antifungal | [90] |
| Silver nanoparticles | Methotrexate coated PEG | Anticancer, in vitro, breast cancer | [95] |
| Silver nanoparticles | Alisertib | Anticancer, in vitro, in vivo, glioblastoma | [101] |
| Silver nanoparticles | Secondary metabolites Drosera binata | Antibacterial, in vitro, Staphylococcus aureus (MRSA) | [102] |
| Mats made of polyvinyl alcohol, chitosan oligosaccharides and silver | - | Wound healing, pre-clinical research | [93] |
| Silver nanoparticles | Peptide TAT | Anticancer, in vivo, malignant melanoma | [103] |
| Silver nanoparticles | Fukan A | Anticancer, in vitro, kidney cancer | [104] |
8. Summary
Nanoparticles can be used to improve the pharmacological and therapeutic properties of drugs. The construction of nanoparticle conjugates with protein drugs or other therapeutic molecules is intended to protect the drug against degradation, prolong its presence in the body, and reduce the toxic properties of the drug. Such a conjugate allows for targeted transport to the cells, tissues and organs in which the drug has the destination. Depending on where and how a targeted drug needs to be delivered to organism, a variety nanoparticles are available. The use of nanoparticles as the drug delivery system could improve the administration and effectiveness of drugs. Because of their small size, nanoparticles are able to transcend biological barriers such as the blood-brain barrier and function at the cellular level. The use of nanoparticles improves the efficiency and effectiveness of drugs through accumulation in target tissues, thereby lowering the necessary dose and reducing the side effects of the drug. The future of nanoparticles as drug carriers is to develop targeted therapies, improve diagnostics and imaging techniques. Possibly, these nanoparticles could be used in earlier detection of diseases. Thanks to their chemical, physical properties and biocompatibility, nanoparticles can be used to improve many areas of medicine and pharmacy; however, considerable research is still warranted before this can become possible.
Acknowledgements
This work was funded by NCN (National Center of Science, Poland) Grant no. 2013/09/B/NZ7/01019.
Footnotes
Conflict of interest: Authors state no conflict of interest.
References
- [1].Barry JN, Vertegel AA. Nanomaterials for Protein Mediated Therapy and Delivery. Nano Life. 2013;3(4):1. doi: 10.1142/S1793984413430010. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Suri SS, Fenniri H, Singh B. Nanotechnology-based drug delivery systems. J Occup Med Toxicol. 2007;2:16. doi: 10.1186/1745-6673-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nevozhay D, Kańska U, Budzyńska R, Boratyński J. Current status of research on conjugates and related drug delivery systems in the treatment of cancer and other diseases. Postepy Hig Med Dosw. 2007;61:350. –. [PubMed] [Google Scholar]
- [4].Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Phaemacological Reports. 2012;64:1020. doi: 10.1016/s1734-1140(12)70901-5. –. [DOI] [PubMed] [Google Scholar]
- [5].Yu M, Wu J, Shi J, Farokhzad OC. Nanotechnology for protein delivery: Overview and perspectives. J Control Release. 2016;240:24. doi: 10.1016/j.jconrel.2015.10.012. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vaishya R, Khurana V, Patel S, Mitra AK. Long-term delivery of protein therapeutics. Expert Opin Drug Deliv. 2015;12(3):415. doi: 10.1517/17425247.2015.961420. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kraft JC, Freeling JP, Wang Z, Ho RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103(1):29. doi: 10.1002/jps.23773. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Suntres ZE. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J Toxicol. 2011;2011:152474. doi: 10.1155/2011/152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leite EA, Souza CM, Carvalho-Junior AD, Coelho LG, Lana AM, Cassali GD. Encapsulation of cisplatin in long-circulating and pH-sensitive liposomes improves its antitumor effect and reduces acute toxicity. Int J Nanomedicine. 2012;7:5259. doi: 10.2147/IJN.S34652. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim SK, Huang L. Nanoparticle delivery of a peptide targeting EGFR signaling. J Control Release. 2012;157(2):279. doi: 10.1016/j.jconrel.2011.08.014. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zalba S, Contreras AM, Haeri A, Ten Hagen TL, Navarro I, Koning G. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J Control Release. 2015;210:26. doi: 10.1016/j.jconrel.2015.05.271. et al. –. [DOI] [PubMed] [Google Scholar]
- [12].Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta. 2008;1778(2):423. doi: 10.1016/j.bbamem.2007.11.002. et al. –. [DOI] [PubMed] [Google Scholar]
- [13].Piaggio F, Kondylis V, Pastorino F, Di Paolo D, Perri P, Cossu I. A novel liposomal Clodronate depletes tumor-associated macrophages in primary and metastatic melanoma: Anti-angiogenic and anti-tumor effects. J Control Release. 2016;223:165. doi: 10.1016/j.jconrel.2015.12.037. et al. –. [DOI] [PubMed] [Google Scholar]
- [14].Witoonsaridsilp W, Paeratakul O, Panyarachun B, Sarisuta N. Development of mannosylated liposomes using synthesized N-octadecyl-D-mannopyranosylamine to enhance gastrointestinal permeability for protein delivery. AAPS PharmSciTech. 2012;13(2):699. doi: 10.1208/s12249-012-9788-1. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Werle M, Makhlof A, Takeuchi H. Carbopol-Lectin Conjugate Coated Liposomes for Oral Peptide Delivery. Chem. Pharm. Bull. 2010;53(3):432. doi: 10.1248/cpb.58.432. –. [DOI] [PubMed] [Google Scholar]
- [16].Elmoslemany RM, Abdallah OY, El-Khordagui LK, Khalafallah NM. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomes. AAPS PharmSciTech. 2012;13(2):723. doi: 10.1208/s12249-012-9783-6. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gao YF, Wei XN, Ye XL, Weng GB, Chen YC, Zhao YR. Anticancer activity of stoppin based on a novel peptide delivery system. Mol Med Rep. 2015;12(4):5437. doi: 10.3892/mmr.2015.4024. et al. –. [DOI] [PubMed] [Google Scholar]
- [18].Paavola A, Kilpelainen I, Yliruusi J, Rosenberg P. Controlled release injectable liposomal gel of ibuprofen for epidural analgesia. Int J Pharm. 2000;199 doi: 10.1016/s0378-5173(00)00376-8. [DOI] [PubMed] [Google Scholar]
- [19].Anderson R, Franch A, Castell M, Perez-Cano FJ, Brauer R, Pohlers D. Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis Res Ther. 2010;12(4):R147. doi: 10.1186/ar3089. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J Control Release. 2012;159(3):393. doi: 10.1016/j.jconrel.2012.01.009. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tang M, Lei L, Guo S-R, Huang WL. Recent progress in nanotechnology for cancer therapy. Chinese Journal of Cancer. 2010;29(9) doi: 10.5732/cjc.010.10075. [DOI] [PubMed] [Google Scholar]
- [22].Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H. Silk fibroin nanoparticle as a novel drug delivery system. J Control Release. 2015;206:161. doi: 10.1016/j.jconrel.2015.03.020. –. [DOI] [PubMed] [Google Scholar]
- [23].Li X, Yang W, Zou Y, Meng F, Deng C, Zhong Z. Efficacious delivery of protein drugs to prostate cancer cells by PSMA-targeted pH-responsive chimaeric polymersomes. J Control Release. 2015;220(Pt B):704. doi: 10.1016/j.jconrel.2015.08.058. –. [DOI] [PubMed] [Google Scholar]
- [24].Hu YW, Du YZ, Liu N, Liu X, Meng TT, Cheng BL. Selective redox-responsive drug release in tumor cells mediated by chitosan based glycolipid-like nanocarrier. J Control Release. 2015;206:91. doi: 10.1016/j.jconrel.2015.03.018. et al. –. [DOI] [PubMed] [Google Scholar]
- [25].Guo L, Zhang H, Wang F, Liu P, Wang Y, Xia G. Targeted multidrug-resistance reversal in tumor based on PEG-PLL-PLGA polymer nano drug delivery system. Int J Nanomedicine. 2015;10:4535. doi: 10.2147/IJN.S85587. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cirstoiu-Hapca A, Buchegger F, Lange N, Bossy L, Gurny R, Delie F. Benefit of anti-HER2-coated paclitaxel-loaded immuno-nanoparticles in the treatment of disseminated ovarian cancer: Therapeutic efficacy and biodistribution in mice. J Control Release. 2010;144(3):324. doi: 10.1016/j.jconrel.2010.02.026. –. [DOI] [PubMed] [Google Scholar]
- [27].Toyoda M, Hama S, Ikeda Y, Nagasaki Y, Kogure K. Anti-cancer vaccination by transdermal delivery of antigen peptide-loaded nanogels via iontophoresis. Int J Pharm. 2015;483(1-2):110. doi: 10.1016/j.ijpharm.2015.02.024. –. [DOI] [PubMed] [Google Scholar]
- [28].Al-Qadi S, Grenha A, Carrion-Recio D, Seijo B, Remunan-Lopez C.. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: in vivo evaluation of insulin-loaded formulations. J Control Release. 2012;157(3):383. doi: 10.1016/j.jconrel.2011.08.008. –. [DOI] [PubMed] [Google Scholar]
- [29].Luo Z, Deng Y, Zhang R, Wang M, Bai Y, Zhao Q. Peptide-laden mesoporous silica nanoparticles with promoted bioactivity and osteo-differentiation ability for bone tissue engineering. Colloids Surf B Biointerfaces. 2015;131:73. doi: 10.1016/j.colsurfb.2015.04.043. et al. –. [DOI] [PubMed] [Google Scholar]
- [30].Alcala-Alcala S, Benitez-Cardoza CG, Lima-Munoz EJ, Pinon-Segundo E, Quintanar-Guerrero D.. Evaluation of a combined drug-delivery system for proteins assembled with polymeric nanoparticles and porous microspheres; characterization and protein integrity studies. Int J Pharm. 2015;489(1-2):139. doi: 10.1016/j.ijpharm.2015.04.074. –. [DOI] [PubMed] [Google Scholar]
- [31].Singh S, Singh AN, Verma A, Dubey VK. Biodegradable polycaprolactone (PCL) nanosphere encapsulating superoxide dismutase and catalase enzymes. Appl Biochem Biotechnol. 2013;171(7):1545. doi: 10.1007/s12010-013-0427-4. –. [DOI] [PubMed] [Google Scholar]
- [32].Zhao M, Biswas A, Hu B, Joo KI, Wang P, Gu Z. Redox-responsive nanocapsules for intracellular protein delivery. Biomaterials. 2011;32(22):5223. doi: 10.1016/j.biomaterials.2011.03.060. et al. –. [DOI] [PubMed] [Google Scholar]
- [33].Wilson B, Samanta MK, Santhi K, Kumar KP, Ramasamy M, Suresh B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomedicine. 2010;6(1):144. doi: 10.1016/j.nano.2009.04.001. –. [DOI] [PubMed] [Google Scholar]
- [34].Pandey R, Ahmad Z, Sharma S, Khuller GK. Nano-encapsulation of azole antifungals: potential applications to improve oral drug delivery. Int J Pharm. 2005;301(1-2):268. doi: 10.1016/j.ijpharm.2005.05.027. –. [DOI] [PubMed] [Google Scholar]
- [35].Dev A, Binulal NS, Anitha A, Nair SV, Furuike T, Tamura H. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr Polym. 2010;80(3):833. et al. –. [Google Scholar]
- [36].Turos E, Shim JY, Wang Y, Greenhalgh K, Reddy GS, Dickey S. Antibiotic-conjugated polyacrylate nanoparticles: new opportunities for development of anti-MRSA agents. Bioorg Med Chem Lett. 2007;17(1):53. doi: 10.1016/j.bmcl.2006.09.098. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Elhissi AM, Ahmed W, Hassan IU, Dhanak VR, D’Emanuele A. Carbon nanotubes in cancer therapy and drug delivery. J Drug Deliv. 2012;2012:837327. doi: 10.1155/2012/837327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bhattacharya K, Mukherjee SP, Gallud A, Burkert SC, Bistarelli S, Bellucci S. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine. 2016;12(2):333. doi: 10.1016/j.nano.2015.11.011. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shin US, Yoon IK, Lee GS, Jang WC, Knowles JC, Kim HW. Carbon nanotubes in nanocomposites and hybrids with hydroxyapatite for bone replacements. J Tissue Eng. 2011;2011:674287. doi: 10.4061/2011/674287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nagaraju K, Reddy R, Reddy N. A review on protein functionalized carbon nanotubes. J Appl Biomater Funct Mater. 2015;13(4):e301. doi: 10.5301/jabfm.5000231. –. [DOI] [PubMed] [Google Scholar]
- [41].Patel A, Cholkar K, Mitra AK. Recent developments in protein and peptide parenteral delivery approaches. Ther. Deliv. 2014;5(3):337. doi: 10.4155/tde.14.5. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kam NWS, Dai H. Carbon Nanotubes as Intracellular Protein Transporters: Generality and Biological Functionality. J. AM. CHEM. SOC. 2005;127:6021. doi: 10.1021/ja050062v. –. [DOI] [PubMed] [Google Scholar]
- [43].Cheng Q, Blais M-O, Harris G, Jabbarzadeh E. PLGA-Carbon Nanotube Conjugates for Intercellular Delivery of Caspase-3 into Osteosarcoma Cells. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Weng X, Wang M, Ge J, Yu S, Liu B, Zhong J. Carbon nanotubes as a protein toxin transporter for selective HER2-positive breast cancer cell destruction. Mol Biosyst. 2009;5(10):1224. doi: 10.1039/b906948h. et al. –. [DOI] [PubMed] [Google Scholar]
- [45].Bhirde AA, Patel S, Sousa AA, Patel V, Molinolo AA, Ji Y. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine (Lond) 2010;5(10):1535. doi: 10.2217/nnm.10.90. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Boyer PD, Ganesh S, Qin Z, Holt BD, Buehler MJ, Islam MF. Delivering Single-Walled Carbon Nanotubes to the Nucleus Using Engineered Nuclear Protein Domains. ACS Appl Mater Interfaces. 2016;8(5):3524. doi: 10.1021/acsami.5b12602. et al. –. [DOI] [PubMed] [Google Scholar]
- [47].Villa CH, Dao T, Ahearn I, Fehrenbacher N, Casey E, Rey DA. Single-Walled Carbon Nanotubes Deliver Peptide Antigen into Dendritic Cells and Enhance IgG Responses to Tumor-Associated Antigens. ACS nano. 2011;5(7):5300. doi: 10.1021/nn200182x. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moore TL, Grimes SW, Lewis RL, Alexis F. Multilayered polymer-coated carbon nanotubes to deliver dasatinib. Mol Pharm. 2014;11(1):276. doi: 10.1021/mp400448w. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ajima K, Murakami T, Mizoguchi Y, Tsuchida K, Ichihashi T, Iijima S. Enhancement of In Vivo Anticancer Effects of Cisplatin by Incorporation Inside Single-Wall Carbon Nanohorns. ACS nano. 2008;2(10):2057. doi: 10.1021/nn800395t. et al. –. [DOI] [PubMed] [Google Scholar]
- [50].Dong X, Liu L, Zhu D, Zhang H, Leng X. Transactivator of transcription (TAT) peptide- chitosan functionalized multiwalled carbon nanotubes as a potential drug delivery vehicle for cancer therapy. Int J Nanomedicine. 2015;10:3829. doi: 10.2147/IJN.S81762. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Luo X, Matranga C, Tan S, Alba N, Cui XT. Carbon nanotube nanoreservior for controlled release of anti-inflammatory dexamethasone. Biomaterials. 2011;32(26):6316. doi: 10.1016/j.biomaterials.2011.05.020. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2(4):282. doi: 10.4103/0975-7406.72127. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li X, Wei J, Aifantis KE, Fan Y, Feng Q, Cui FZ. Current investigations into magnetic nanoparticles for biomedical applications. J Biomed Mater Res A. 2016;104(5):1285. doi: 10.1002/jbm.a.35654. et al. –. [DOI] [PubMed] [Google Scholar]
- [54].Vaghari H, Jafarizadeh-Malmiri H, Mohammadlou M, Berenjian A, Anarjan N, Jafari N. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol Lett. 2016;38(2):223. doi: 10.1007/s10529-015-1977-z. et al. –. [DOI] [PubMed] [Google Scholar]
- [55].Wahajuddin Arora S.. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445. doi: 10.2147/IJN.S30320. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63(1-2):24. doi: 10.1016/j.addr.2010.05.006. –. [DOI] [PubMed] [Google Scholar]
- [57].Kandasamy G, Maity D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int J Pharm. 2015;496(2):191. doi: 10.1016/j.ijpharm.2015.10.058. –. [DOI] [PubMed] [Google Scholar]
- [58].Chen JP, Yang PC, Ma YH, Tu SJ, Lu YJ. Targeted delivery of tissue plasminogen activator by binding to silica-coated magnetic nanoparticle. Int J Nanomedicine. 2012;7:5137. doi: 10.2147/IJN.S36197. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Huang Y, Mao K, Zhang B, Zhao Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):763. doi: 10.1016/j.msec.2016.09.052. –. [DOI] [PubMed] [Google Scholar]
- [60].Song C, Sheng L, Zhang X. Preparation and characterization of a thermostable enzyme (Mn-SOD) immobilized on supermagnetic nanoparticles. Appl Microbiol Biotechnol. 2012;96(1):123. doi: 10.1007/s00253-011-3835-9. –. [DOI] [PubMed] [Google Scholar]
- [61].Mu X, Qiao J, Qi L, Dong P, Ma H. Poly(2-vinyl-4,4-dimethylazlactone)-functionalized magnetic nanoparticles as carriers for enzyme immobilization and its application. ACS Appl Mater Interfaces. 2014;6(23):21346. doi: 10.1021/am5063025. –. [DOI] [PubMed] [Google Scholar]
- [62].Kumar S, Jana AK, Dhamija I, Maiti M. Chitosan-assisted immobilization of serratiopeptidase on magnetic nanoparticles, characterization and its target delivery. J Drug Target. 2014;22(2):123. doi: 10.3109/1061186X.2013.844157. –. [DOI] [PubMed] [Google Scholar]
- [63].Mulens-Arias V, Rojas JM, Perez-Yague S, Morales MP, Barber DF. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials. 2015;52:494. doi: 10.1016/j.biomaterials.2015.02.068. –. [DOI] [PubMed] [Google Scholar]
- [64].Ho J, Al-Deen FM, Al-Abboodi A, Selomulya C, Xiang SD, Plebanski M. N, N’-Carbonyldiimidazole-mediated functionalization of superparamagnetic nanoparticles as vaccine carrier. Colloids Surf B Biointerfaces. 2011;83(1):83. doi: 10.1016/j.colsurfb.2010.11.001. et al. –. [DOI] [PubMed] [Google Scholar]
- [65].Chen AZ, Chen LQ, Wang SB, Wang YQ, Zha JZ. Study of magnetic silk fibroin nanoparticles for massage-like transdermal drug delivery. Int J Nanomedicine. 2015;10:4639. doi: 10.2147/IJN.S85999. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang J, Meng G, Tao K, Feng M, Zhao X, Li Z. Immobilization of Lipases on Alkyl Silane Modified Magnetic Nanoparticles: Effect of Alkyl Chain Length on Enzyme Activity. PLoS ONE. 2012;7(8):e43478. doi: 10.1371/journal.pone.0043478. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Atacan K, Ozacar M. Characterization and immobilization of trypsin on tannic acid modified Fe3O4 nanoparticles. Colloids Surf B Biointerfaces. 2015;128:227. doi: 10.1016/j.colsurfb.2015.01.038. –. [DOI] [PubMed] [Google Scholar]
- [68].Zhao X, Cui H, Chen W, Wang Y, BoCui, Sun C. Morphology, Structure and Function Characterization of PEI Modified Magnetic Nanoparticles Gene Delivery System. PLoS ONE. 2014;9(6):e98919. doi: 10.1371/journal.pone.0098919. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dianzani C, Zara GP, Maina G, Pettazzoni P, Pizzimenti S, Rossi F. Drug delivery nanoparticles in skin cancers. Biomed Res Int. 2014;2014:895986. doi: 10.1155/2014/895986. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rana S, Bajaj A, Mout R, Rotello VM. Monolayer coated gold nanoparticles for delivery applications. Adv Drug Deliv Rev. 2012;64(2):200. doi: 10.1016/j.addr.2011.08.006. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60(11):1307. doi: 10.1016/j.addr.2008.03.016. –. [DOI] [PubMed] [Google Scholar]
- [72].Duncan B, Kim C, Rotello VM. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J Control Release. 2010;148(1):122. doi: 10.1016/j.jconrel.2010.06.004. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Heo DN, Yang DH, Moon HJ, Lee JB, Bae MS, Lee SC. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials. 2012;33(3):856. doi: 10.1016/j.biomaterials.2011.09.064. et al. –. [DOI] [PubMed] [Google Scholar]
- [74].Marcelo G, Kaplan E, Tarazona MP, Mendicuti F. Interaction of gold nanoparticles with Doxorubicin mediated by supramolecular chemistry. Colloids Surf B Biointerfaces. 2015;128:237. doi: 10.1016/j.colsurfb.2015.01.041. –. [DOI] [PubMed] [Google Scholar]
- [75].Rathinaraj P, Al-Jumaily AM, Huh DS. Internalization: acute apoptosis of breast cancer cells using herceptin-immobilized gold nanoparticles. Breast Cancer (Dove Med Press) 2015;7:51. doi: 10.2147/BCTT.S69834. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials. 2012;33(4):1180. doi: 10.1016/j.biomaterials.2011.10.058. et al. –. [DOI] [PubMed] [Google Scholar]
- [77].Zhang Y, Walker JB, Minic Z, Liu F, Goshgarian H, Mao G. Transporter protein and drug-conjugated gold nanoparticles capable of bypassing the blood-brain barrier. Sci Rep. 2016;6:25794. doi: 10.1038/srep25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24(8):1415. doi: 10.1007/s11095-007-9257-9. –. [DOI] [PubMed] [Google Scholar]
- [79].Stuchinskaya T, Moreno M, Cook MJ, Edwards DR, Russell DA. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochem Photobiol Sci. 2011;10(5):822. doi: 10.1039/c1pp05014a. –. [DOI] [PubMed] [Google Scholar]
- [80].Obaid G, Chambrier I, Cook MJ, Russell DA. Targeting the oncofetal Thomsen-Friedenreich disaccharide using jacalin-PEG phthalocyanine gold nanoparticles for photodynamic cancer therapy. Angew Chem Int Ed Engl. 2012;51(25):6158. doi: 10.1002/anie.201201468. –. [DOI] [PubMed] [Google Scholar]
- [81].Cheng Y, A CS, Meyers JD, Panagopoulos I, Fei B, Burda C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc. 2008;130(32):10643. doi: 10.1021/ja801631c. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Austin LA, Mackey MA, Dreaden EC, El-Sayed MA.. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch Toxicol. 2014;88(7):1391. doi: 10.1007/s00204-014-1245-3. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Riaz Ahmed KB, Nagy AM, Brown RP, Zhang Q, Malghan SG, Goering PL. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol In Vitro. 2017;38:179. doi: 10.1016/j.tiv.2016.10.012. –. [DOI] [PubMed] [Google Scholar]
- [84].Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wei L, Lu J, Xu H, Patel A, Chen ZS, Chen G. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015;20(5):595. doi: 10.1016/j.drudis.2014.11.014. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].He Y, Du Z, Ma S, Cheng S, Jiang S, Liu Y. Biosynthesis, Antibacterial Activity and Anticancer Effects Against Prostate Cancer (PC-3) Cells of Silver Nanoparticles Using Dimocarpus Longan Lour. Peel Extract. Nanoscale Res Lett. 2016;11(1):300. doi: 10.1186/s11671-016-1511-9. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mata R, Nakkala JR, Sadras SR. Biogenic silver nanoparticles from Abutilon indicum: their antioxidant, antibacterial and cytotoxic effects in vitro. Colloids Surf B Biointerfaces. 2015;128:276. doi: 10.1016/j.colsurfb.2015.01.052. –. [DOI] [PubMed] [Google Scholar]
- [88].Anandhakumar S, Raichur AM. Polyelectrolyte/silver nanocomposite multilayer films as multifunctional thin film platforms for remote activated protein and drug delivery. Acta Biomater. 2013;9(11):8864. doi: 10.1016/j.actbio.2013.06.012. –. [DOI] [PubMed] [Google Scholar]
- [89].Eby DM, Schaeublin NM, Farrington KE, Hussain SM, Johnson GR. Lysozyme Catalyzes the Formation of Antimicrobial Silver Nanoparticles. ACSnano. 2009;3(4):984. doi: 10.1021/nn900079e. –. [DOI] [PubMed] [Google Scholar]
- [90].Tutaj K, Szlazak R, Szalapata K, Starzyk J, Luchowski R, Grudzinski W. Amphotericin B-silver hybrid nanoparticles: synthesis, properties and antifungal activity. Nanomedicine. 2016;12(4):1095. doi: 10.1016/j.nano.2015.12.378. et al. –. [DOI] [PubMed] [Google Scholar]
- [91].Yamada M, Foote M, Prow TW. Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(3):428. doi: 10.1002/wnan.1322. –. [DOI] [PubMed] [Google Scholar]
- [92].Tian J, Wong KK, Ho CM, Lok CN, Yu WY, Che CM. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem. 2007;2(1):129. doi: 10.1002/cmdc.200600171. et al. –. [DOI] [PubMed] [Google Scholar]
- [93].Li C, Fu R, Yu C, Li Z, Guan H, Hu D. Silver nanoparticle/ chitosan oligosaccharide/poly(vinyl alcohol) nanofibers as wound dressings: a preclinical study. Int J Nanomedicine. 2013;8:4131. doi: 10.2147/IJN.S51679. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sahoo AK, Goswami U, Dutta D, Banerjee S, Chattopadhyay A, Ghosh SS. Silver Nanocluster Embedded Composite Nanoparticles for Targeted Prodrug Delivery in Cancer Theranostics. ACS Biomaterials Science & Engineering. 2016;2(8):1395. doi: 10.1021/acsbiomaterials.6b00334. –. [DOI] [PubMed] [Google Scholar]
- [95].Muhammad Z, Raza A, Ghafoor S, Naeem A, Naz SS, Riaz S. PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur J Pharm Sci. 2016;91:251. doi: 10.1016/j.ejps.2016.04.029. et al. –. [DOI] [PubMed] [Google Scholar]
- [96].Locatelli E, Naddaka M, Uboldi C, Loudos G, Fragogeorgi E, Molinari V. Targeted delivery of silver nanoparticles and alisertib: in vitro and in vivo synergistic effect against glioblastoma. Nanomedicine. 2014;9(6):839. doi: 10.2217/nnm.14.1. et al. –. [DOI] [PubMed] [Google Scholar]
- [97].Shen DF, Wu SS, Wang RR, Zhang Q, Ren ZJ, Liu H. A Silver(I)-Estrogen Nanocluster: GSH Sensitivity and Targeting Suppression on HepG2 Cell. Small. 2016;12(44):6153. doi: 10.1002/smll.201601936. et al. –. [DOI] [PubMed] [Google Scholar]
- [98].Qiu L, Zhao Y, Cao N, Cao L, Sun L, Zou X. Silver nanoparticlegated fluorescence porous silica nanospheres for glutathione-responsive drug delivery. Sensors and Actuators B: Chemical. 2016;234:21. –. [Google Scholar]
- [99].Brown PK, Qureshi AT, Moll AN, Hayes DJ, Monroe WT. Silver Nanoscale Antisense Drug Delivery System for Photoactivated Gene Silencing. ACSnano. 2013;7(4):2948. doi: 10.1021/nn304868y. –. [DOI] [PubMed] [Google Scholar]
- [100].Eby DM, Schaeublin NM, Farrington KE, Hussain SM, Johnson GR. Lysozyme Catalyzes the Formation of Antimicrobial Silver Nanoparticles. ACSnano. 2009;3(4) doi: 10.1021/nn900079e. [DOI] [PubMed] [Google Scholar]
- [101].Locatelli E, Uboldi C, Loudos G, Fragogeorgi E, Molinari V, Tsotakos T. Targeted delivery of silver nanoparticles and alisertib: in vitro and in vivo synergistic effect against glioblastoma. Nanomedicine. 2014;9(6):839. doi: 10.2217/nnm.14.1. et al. –. [DOI] [PubMed] [Google Scholar]
- [102].Krychowiak M, Grinholc M, Banasiuk R, Krauze-Baranowska M, Glod D, Kawiak A. Combination of silver nanoparticles and Drosera binata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS One. 2014;9(12):e115727. doi: 10.1371/journal.pone.0115727. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liu J, Zhao Y, Guo Q, Wang Z, Wang H, Yang Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials. 2012;33(26):6155. doi: 10.1016/j.biomaterials.2012.05.035. et al. –. [DOI] [PubMed] [Google Scholar]
- [104].Rocha Amorim MO, Lopes Gomes D, Dantas LA, Silva Viana RL, Chiquetti SC, Almeida-Lima J. Fucan-coated silver nanoparticles synthesized by a green method induce human renal adenocarcinoma cell death. Int J Biol Macromol. 2016;93(Pt A):57. doi: 10.1016/j.ijbiomac.2016.08.043. et al. –. [DOI] [PubMed] [Google Scholar]


