Abstract
Due to traffic accidents, injuries, burns, congenital malformations and other reasons, a large number of patients with tissue or organ defects need urgent treatment every year. The shortage of donors, graft rejection and other problems cause a deficient supply for organ and tissue replacement, repair and regeneration of patients, so regenerative medicine came into being. Stem cell therapy plays an important role in the field of regenerative medicine, but it is difficult to fill large tissue defects by injection alone. The scientists combine three-dimensional (3D) printed bone tissue engineering scaffolds with stem cells to achieve the desired effect. These scaffolds can mimic the extracellular matrix (ECM), bone and cartilage, and eventually form functional tissues or organs by providing structural support and promoting attachment, proliferation and differentiation. This paper mainly discussed the applications of 3D printed bone tissue engineering scaffolds in stem cell regenerative medicine. The application examples of different 3D printing technologies and different raw materials are introduced and compared. Then we discuss the superiority of 3D printing technology over traditional methods, put forward some problems and limitations, and look forward to the future.
Keywords: Stem cells, 3D printing, Bone tissue engineering, Scaffold materials
Abbreviations: 3D, three-dimensional; ECM, extracellular matrix; ESCs, embryonic stem cells; ASCs, adult stem cells; iPS, induced pluripotent stem; AM, additive manufacturing; hMSCs, human mesenchymal stem cells; CT, computed tomography; STL, standard tessellation language; CAD, computer-aided design; FDM, fused deposition molding; ABS, Acrylonitrile Butadiene Styrene plastic; PC, Polycarbonate; PPSU, Polyphenylene sulfone resins; PED, Precision Extrusion Deposition; PEG, Polyethylene glycol; LDM, Low Temperature Deposition Modeling; MSCs, Marrow stem cells; PCL, polycraprolactone; dECM, decellularized bovine cartilage extracellular matrix; TCP, β-tricalcium phosphate; hADSC, human adipose derived stem cells; SLA, Stereolithography; CAP, cold atmospheric plasma; SLM, Selective Laser Melting; PDA, polydopamine; HA, hydroxyapatite; SF-BG, silk fibroin and silk fibroin-bioactive glass; RAD16-I, a soft nanofibrous self-assembling peptide; BCP, biphasic calcium phosphate; PVA, polyvinyl alcohol; PRF, platelet-rich fibrin; DCM, dichloromethane; BMSCs, bone marrow-derived mesenchymal stem cells; pcHμPs, novel self-healable pre-cross- linked hydrogel microparticles; CHMA, chitosan methacrylate; PLGA, poly (lactide-co-glycolide); Alg, alginate; HAp, hydroxyapatite nanoparticles; rBMSCs, rat bone marrow stem cells; MBG/SA–SA, mesoporous bioactive glass/sodium alginate-sodium alginate; HTy, 4-hydroxyphenethyl 2-(4-hydroxyphenyl) acetate; PLLA, poly l-lactide; SCAPs, human stem cells from the apical papilla; PEGDA, poly (ethylene glycol) diacrylate; GO, graphene oxide; LIPUS, low intensity pulsed ultrasound
1. Introduction
Bone tissue engineering, the combination of scaffolds, seed cells and cytokines, seeks to repair the bone defect by the transplantation of bone tissue engineering scaffolds to the bone defect area and the follow-up body replacement of the scaffold materials with new bone tissues. Scaffold, known as a temporary and artificial ECM, can promote the formation of new bone and has a direct influence on the proliferation and differentiation of cells. The ideal bone tissue engineering scaffolds should have the appropriate porosity, surface area ratio, mechanical support, biocompatibility, surface activity and shape that are matching clinical application, and can promote the cell adhesion and the growth of blood vessels and nerves [1].
Stem cells are cells with the potential for self-renewal and multi differentiation. According to the developmental stage, stem cells can be divided into embryonic stem cells (ESCs) and adult stem cells (ASCs). ESCs are derived from the inner cell mass at the blastocyst stage and have the potential of infinite proliferation and germ layer differentiation, which has always been the focus of tissue engineering and regenerative medicine [2]. However, due to immune rejection and other problems, the wide application of ESCs in clinical practice has been limited. ASCs can differentiate into specific tissues and cells [3], exists in many kinds of tissues, has great potential in tissue damage repair and disease treatment, and is the current focus of regenerative medicine [4]. In addition, induced pluripotent stem (iPS) cells are also attractive in regenerative medicine. In 2006, through the introduction of four factors, Yamanaka and Takahashi successfully generated iPS cells from mouse fibroblast culture [5]. Up to now, iPS cells researches have made great progress in organoids development [6], drug discovery [7], disease mechanism study [8], and disease treatment [9].
3D printing is an essential component of additive manufacturing (AM), which has attracted increasing attention in the preparation of bioactive implantable devices. For bone tissue regeneration applications, effective intracellular migration, nutrient supply, and ECM production require a well-designed structure with high porosity, high interconnectivity, and defined pore size and pore geometry [10]. The use of conventional techniques, such as pore-forming agent leaching, gas foaming, or phase separation methods, results in wide varying pore geometry [11], pore size distribution [12], and generally low interconnectivity [13,14]. Also, 3D printing shows better therapeutic effect [15] and more optimized material properties [16]in clinical application. The researchers have also find that square-shaped scaffolds promote growth of more human mesenchymal stem cells (hMSCs) growth and chondrogenic differentiation compared with solid or hexagonal porous scaffolds [17]. It makes 3D printing particularly suitable for the fabrication of structures for bone tissue repair, not to mention the induction of directed differentiation and proliferation of stem cells [18].
In 3D printing, raw materials or cells are layered onto predefined positions to form a 3D structure. This deposition or fixation of the target materials makes 3D printing ideal for creating well-defined porous structures. Also, when used in combination with 3D computer imaging, such as computed tomography (CT) and contour scanning, it can create a customized implant structure for patients with tissue defects. CT scan data can be easily converted to standard tessellation language (STL) computer-aided design (CAD) models. By adapting such STL files to the modeling software, we can set the required porosity or support structure for clinical treatment or adaptation to the stem-cell growth environment. STL files are actually a lot of two-dimensional planes, and when these planes are superimposed, the 3D scaffolds we need is formed [19,20].
2. Several different 3D printing technologies
Next, we will introduce several different 3D printing technologies applied to fabricate tissue engineering scaffolds, and the applications of the stem cells in it. In the following paragraphs, we discussed how these techniques work and the consequences of dealing with raw materials or cells.
2.1. Fused deposition modelling (FDM)
In 1988, Scott Crump, an American scholar, proposed the molding process for the first time. In 1991, Stratasys, Inc. (Eden Prairie, MN, USA) developed the first FDM molding machine and proposed its commercial application. Through the relevant patent search of FDM, it was found that the number of patent applications has been increasing year by year since the technology was proposed in 1988 and reached a peak between 2013 and 2016 (Google patent).
The basic raw materials used in FDM technology are mostly polymer or wax, such as Acrylonitrile Butadiene Styrene plastic (ABS), Polycarbonate (PC), Polyphenylene sulfone resins (PPSU), etc.
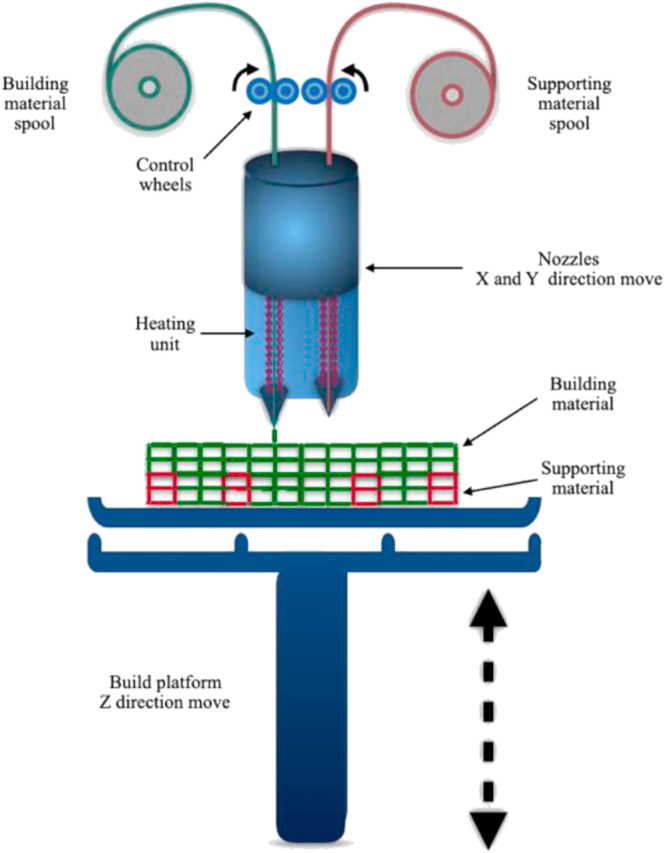
As shown in Fig. 1, the working principle of FDM is to transfer the filamentous hot-melt material to the hot-melt printing nozzle through the wire feeding mechanism. The filamentous or linear plastic material is heated to the molten state in the nozzle. Under the control of the computer, the nozzle moves along the shape contour and tracks of the parts, extrudes the molten material, makes it deposit in the expected position and then solidifies. Bonded to the previously formed layers, the layers are stacked to form the product model. Typically, two materials are used during the construction process, one serving as the supporting material and the other constituting the actual building material. Besides, we can change the temperature of the nozzle, the diameter, the moving speed, the extrusion speed and the construction direction to finally influence the porosity, diameter and mechanical properties of the product [21,22].
Fig. 1.
The principle of FDM process.
FDM technology is used to produce customized defect matching structures for bone repair. It not only prints out personalized scaffolds with different porosity and pore sizes to accommodate the growth and differentiation of stem cells [23,24], but also modifies the scaffolds to improve their biocompatibility and bone conductivity [25].
2.2. Extrusion-based 3D printing
Extrusion-based 3D printing, includes a series of techniques: precision extrusion deposition, low temperature deposition modeling, 3D Bio-plotting, etc. [26].
2.2.1. Precision extrusion deposition (PED)
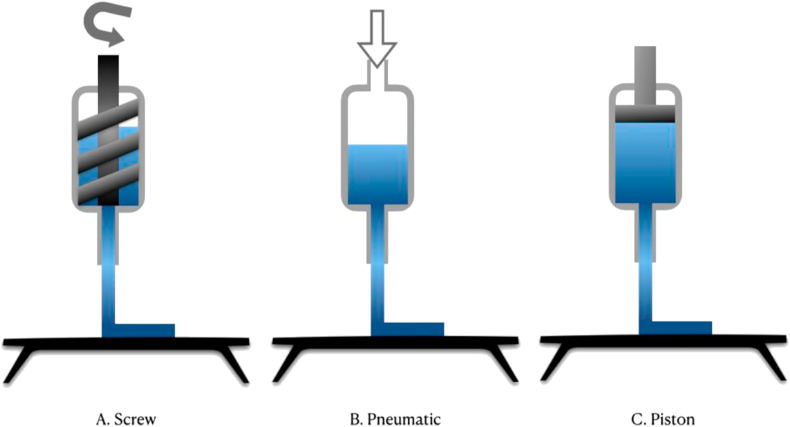
PED is actually a modification of FDM technology for the precision machining of composite materials, which is mostly based on the PED system developed by Bellini at Drexel University in 2002 [27,28]. Different from FDM, PED is realized by extruding material with screw (Fig. 2A), and the quantity of extruded material is controlled accurately. Also, the extruder is synchronized with the XY motion precision system that controls the building platform, so that the thickness and position of the fibers can be precisely controlled [29].
Fig. 2.
Several different extruders of extrusion-based 3D printing.
The expanded biomaterials will thus enable PED devices to construct scaffolds with enhanced biological, chemical, and mechanical cues that will facilitate tissue generation [29]. Osteoblast differentiation can also be promoted and inhibited in region-specific ways by applying Polyethylene glycol (PEG) hydrogel on the scaffold [30].
2.2.2. Low temperature deposition modeling (LDM)
LDM was first reported in 2002, and its technology is also improved based on FDM. The extruder is usually pneumatic or piston type, and the material is the mostly viscous polymer and extruded (Fig. 2B and C) at room temperature [26].
LDM printed bone tissue engineering scaffolds have high porosity, suitable pore size and mechanical strength, and marrow stem cells (MSCs) can adhere, proliferate and differentiate on the scaffolds [31].
The researchers used LDM technology to create 3D-printed scaffolds with the desired shape and internal structure combined with bioactive factors to enhance segmental bone repair [18].
2.2.3. 3D bio-plotting
3D Bio-plotting is an emerging free-form scaffold manufacturing technology that can be used to create artificial tissue scaffolds containing living cells, which was introduced by Landers. et al. in 2000 [32].
Bio-plotting technology is through the computer control of material on the surface of deposition in the layered overlay. The nozzle moves in three directions on a fixed manufacturing platform [33]. The viscous materials are mapped into a liquid with a matching density by applying a filtered air pressure to produce a flow of liquid. Since no heat is required, the system can deal with heat-sensitive biological components, even cells [34,35].
2.3. Stereolithography (SLA)
SLA process was patented in the US by Charles Hull in 1984 and commercialized by 3D Systems, Inc. (USA). Currently, the SLA process is recognized as one of the most deeply researched and the earliest applied 3D printing methods in the world. According to the patent inquiry of SLA technology, it has been found that there were two peaks of patent applications from 2001 to 2004 and from 2013 to 2016 (Google patent).
Photosensitive resin materials mainly include oligomers, reactive diluents and initiators. In 2016 Tethon 3D, Inc. (Nebraska, USA) launched a new ceramic resin for SLA/DLP 3D printers with high-resolution details, thermal shock resistance and thermal and electrical insulation.
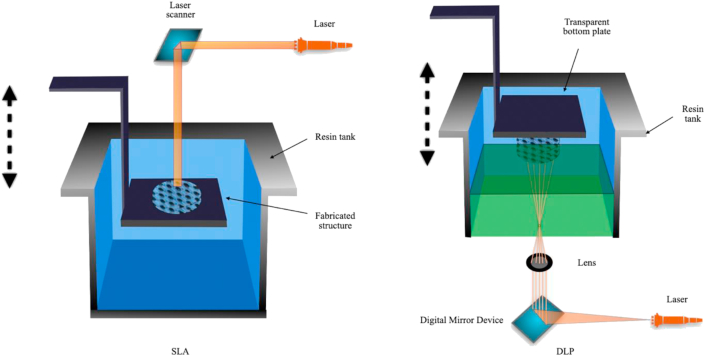
As shown in Fig. 3, the technique is based on a photosensitive initiator molecule initiated free radical photo-polymerization. The printed structure is prepared using a photo-curable resin, which is a liquid cured by light irradiation after photo-polymerization. In the SLA devices, the bottom of the structure is formed by polymerization on the top surface of the mobile platform. Thin layers are aggregated by two-dimensional patterns drawn by a guided laser beam. After that, the manufacturing platform descends the patterns on the top of the previous layer polymerization to form the desired structure.
Fig. 3.
SLA and DLP process.
Studies have shown that the controlled macroscopic structure and the microscopic distribution of hydroxyapatite particles produced by SLA technology can enhance the bone promotion effect of composite biomaterials, that is, human bone marrow stem cells can differentiate into the bone in vitro [36]. Also, surface modifications of SLA implants with Sr nano structures have been shown to have a favorable effect on early immunoinflammatory macrophage function and osteoblastic function, leading to enhanced osseointegration outcomes [37]. Studies have shown that the surface of scaffolds treated by cold atmospheric plasma (CAP) is oxygen-rich and the surface roughness is enhanced, which is beneficial to the adhesion, proliferation and chondrogenic differentiation of hMSCs [38].
2.3.1. Digital light processing (DLP)
DLP technology is a mask-based surface exposure SLA technology. This technique uses a light source to expose a whole layer of the printed shape through a mask to the surface of the photosensitive resin for layer curing. Unlike the SLA, DLP light can generally be exposed from below through the bottom of the transparent material tank, thus needing less material and low specification requirements. Besides, DLP has higher efficiency and relatively economic cost. It is suitable for preparing thin-walled and porous scaffolds with complex characteristic structures.
2.4. Selective laser sintering (SLS)
SLS technology was developed by Carl Dechard at the University of Texas at Austin in 1989. The method was first commercialized by DTM, Inc. (USA), which was later acquired by 3D Systems, Inc [39]. Similar to the previous two technologies, the patent of SLS technology also reached its peak from 2013 to 2016, what worth noting is that 3D companies have the largest share.
In terms of form, the materials used for the SLS process are a wide diversity of powder, including nylon powder, glass powder, wax powder, metal powder, ceramic powder and coated wax metal powder, etc.
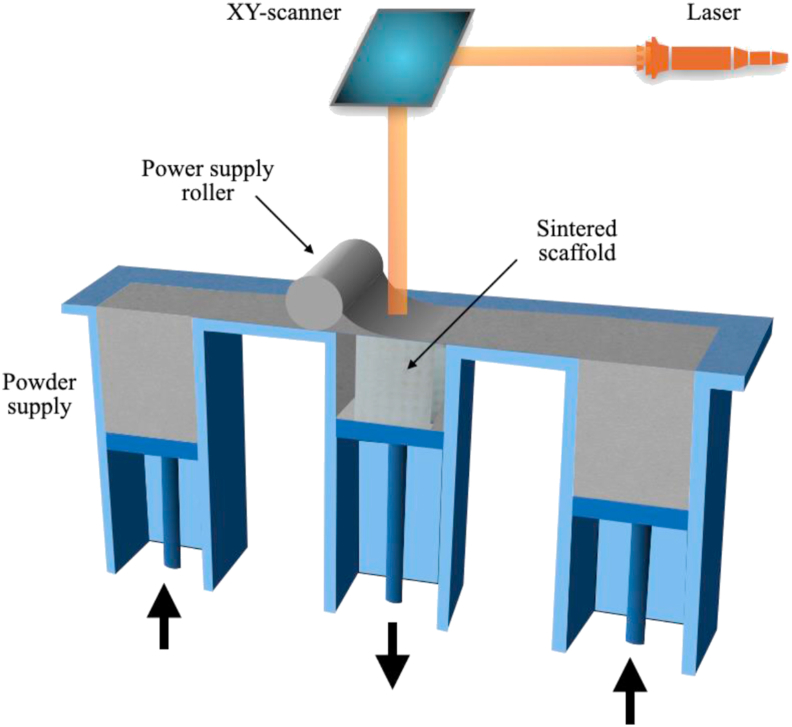
As shown in Fig. 4, the powder is supplied in the SLS using powder suppliers and rollers and distributed in thin layers on the build platform. When irradiated with a laser beam, the beam melts the powder in a specified pattern, then lowers the building platform and deposits the fresh powder on top of the molten pattern. Subsequent irradiation of the fresh powder results in the formation of the next layer of the structure. SLS printing is a complex process in which many set values affect the pore size, porosity and mechanical properties of the final product. The main input parameters include layer thickness, scanning speed, laser power, etc. These parameters will affect the energy density of the laser beam in the melted powder layer and finally affect the quality of the final product [40]. Scientists use these differences to meet the needs of different tissue-engineered scaffolds.
Fig. 4.
SLS/SLM process.
Roskies, M et al. used SLS technology to create a customized porous Polyetheretherketone (PEEK) scaffold that maintains the viability of adipose and bone marrow mesenchymal stem cells and induces osteogenic differentiation of adipose-derived mesenchymal stem cells [41]. Different from direct SLS, scientists have explored an indirect SLS technology, in which the traditional raw materials and binders are mixed as the powder raw materials of the SLS machine. After printing, the semi-manufactured scaffolds are sintered at high temperature to remove the binder, and finally the target products are obtained [42,43]. The use of indirect SLS printing can eliminate the shortcomings of conventional direct SLS printing at higher working temperature, that is, the support occurs wavy deformation, hydroxyapatite decomposition [44]. It also showed good mechanical strength, porosity, pore size and promoting osteogenic differentiation [45].
2.4.1. Selective laser melting (SLM)
Based on SLS, SLM was proposed by The Fraunhofer Laser Research Institute in Germany in 1995. It adopts a laser to selectively layer melt solid powder and solidifies the molten layer to form parts. Unlike SLS, SLM is formed by melting and curing off powder rather than adding binder in the forming process, so that porosity and pore shape can be controlled more conveniently and porous parts with complex internal structure can be formed [46]. At the same time, due to the rapid melting of powder by laser scanning, instantaneous solidification, and fine microstructure, SLM forming pieces have better mechanical properties than castings, which makes them have outstanding advantages in the forming of complex and difficult workpiece, and suitable for the processing of a complex irregular supports with small structure and high quality.
2.5. Printer-based systems
2.5.1. Three-dimensional printing
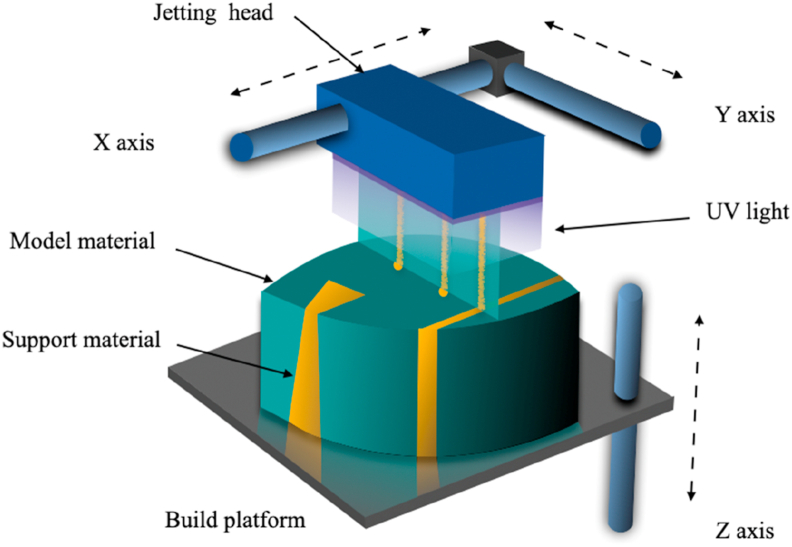
Three-dimensional printing technology, also called material jetting printing [47] or inkjet printing [48], is an additive manufacturing method that creates structures by selectively gluing polymer or inorganic powders into 3D structures layer by layer. The basic method is to cover the surface of the base with a thin layer of powder. Then the computer CAD model is used to control the spray area of the liquid binder according to the specified path. After moving the workstation layer of powder up and down, a thin layer of powder is covered on the adhesive layer and the surface. Step by step, the raw material of unbound powder is finally removed, and a 3D model is constructed [47]. This technology is usually used in combination with other technologies, such as high temperature burning [49], stereolithography [50], or electro-hydro dynamic jetting [51], etc. The following is a commercial application of a more widely used, but also a combination of a variety of technologies of printing technology.
2.5.2. PolyJet
In 2000, Objet, an Israeli company that was acquired by Stratasys, Inc. in 2011, applied for a patent for the PolyJet polymer injection technology. PolyJet's technology is similar to that of the 3D printing, but instead of a binder it is sprayed with a resin material.
PolyJet can also be considered as a close relative of melt deposition technology. Like the FDM, it works by printing parts one layer at a time using an extruder nose. Instead of using filaments to deposit the material on a printing bed, however, PolyJet works more like 2D inkjet printing, where the extruder deposits tiny droplets of a selected photopolymer material on the bed, which is then cured with UV light, sort of like SLA. Stratasys, Inc. launched biocompatible MED610 creates rigid, transparent parts such as surgical guides for a dental implant or orthopedic procedures (see Fig. 5).
Fig. 5.
PolyJet process.
The researchers added the polydopamine (PDA)/hydroxyapatite (HA) coating to the printed MED610 subjects to assess their physical properties, cell proliferation, cell morphology, and alkaline phosphatase expression levels. The PDA/HA coating was found to enhance scaffold hardness, biocompatibility, and osteogenic differentiation potential [52].
3. Multi-technology joint manufacturing
In practical application, in addition to single application technology, multiple technologies can also be used to jointly manufacture scaffold materials to improve technical advantages or avoid disadvantages.
3.1. Joint manufacturing of several 3D printing technologies
Using one 3D printing technology alone may not be able to meet the requirements of the instructions of the 3D printer. For example, in the choice of printing ink, some ink inadequately yields strength to meet the basic requirement of 3D Bio-plotting to print. The scientists solved this problem by using ultraviolet cross-linking from SLA technology, causing that the ink is sheared thin, gelatin-sol transformed, and ultimately achieves excellent printability and fidelity [53,54].
3.2. Combined manufacturing with traditional technology
Although 3D printing technology has a huge advantage over traditional technology, traditional technology is not useless. For instance, scientists used innovative bio-inks to combine FDM technology with casting technology to create new types of osteochondral tissue constructs, producing the scaffolds with excellent mechanical properties and enhanced adhesion, growth, and differentiation of hMSCs [55].
3.3. Direct and indirect manufacturing
In the practical application of 3D printing, researchers have found the limitations of direct printing (e.g. the inability to process low-viscosity materials) and then used indirect printing to solve the problem, and made relevant comparisons [56]. The specific methods are as follows: First, design a negative blueprint of the target build. Sacrifice molds can then be printed through several high-resolution devices to generate a well-defined stand. The material is then cast and cross-linked to form the final shape, and the mold is then removed [57,58]. By using a combination of indirect 3D printing and freeze-drying methods, Bidgoli, M. R. et al., created 3D silk fibroin and silk fibroin-bioactive glass (SF-BG) scaffolds with graded bioactivity to achieve better control, improved uniform pore structure and in vitro bioactivity [59] (see Table 1).
Table 1.
Examples of application.
| Technique | Typical resolution | Materials | Cells | Example | Reference |
|---|---|---|---|---|---|
| FDM | 250–500 μm | ||||
| 200 μm | nHA, PCL | hMSC |  |
[55] | |
| PED | 100–250 μm | ||||
| 400 μm | PCL, RAD16-I | hMSC |  |
[78] | |
| LDM | 250–500 μm | ||||
| 500–600 μm | BCP, PVA, PRF | BMSCs |  |
[18] | |
| 250 μm | PCL/DCM | MSCs |  |
[80] | |
| 3D Bio-plotting | 250–400 μm | ||||
| 25–100 μm | pcHμPs, CHMA, PVA | BMSCs |  |
[54] | |
| 300 μm | PLGA | MSCs |  |
[81] | |
| 100–1000 μm | Alg | ADSC |  |
[82] | |
| 450–1000 μm | Alg | MSCs |  |
[83] | |
| 450–700 μm | PCL/PLGA/Hap | rBMSCs |  |
[84] | |
| 400 μm | MBG/SA–SA | hBMSCs |  |
[75] | |
| 350 μm | Hty | hMSCs |  |
[85] | |
| 400 μm | Alg/gelatin | rBMSC |  |
[86] | |
| 250 μm | PLLA, nHA | MSCs |  |
[13] | |
| 500 μm | printable alginate | SCAPs |  |
[87] | |
| SLA/DLP | 15–300 μm | ||||
| 200 μm | PEG-DA | hMSCs |  |
[17] | |
| SLS/SLM | 50–100 μm | ||||
| 100–300 μm | TiAl6V4 | hBMSCs |  |
[88] | |
| Ra:0.24 ± 0.1 μm | Co–Cr | hADSC |  |
[76] | |
| 400 μm | β-TCP | MSCs |  |
[89] | |
| 3D-printer | 100 μm | ||||
| 500–600 μm and 10–50 μm | SF-BG | hBMSCs |  |
[59] | |
| PolyJet | 100 μm | ||||
| MED610 | hMSCs |  |
[52] |
4. Scaffold modification after printing
To achieve the desired effect, post-printing modification is often used to increase or trim the scaffold performance. Such modifications not only improve in vitro stem cell differentiation and growth but also enhance histocompatibility after transplantation. As shown in Table 2.
Table 2.
Scaffold modification after printing.
| Modification | Scaffold | Printing Technology | Effect | Reference |
|---|---|---|---|---|
| cold atmospheric plasma (CAP) | PEGDA | SLA | Enhanced the surface nano-roughness, benefit for hMSC adhesion, proliferation, and chondrogenic differentiation. | [38] |
| PDA + TGF-β1 | PCL | FDM | Improved biological performance. | [55] |
| SDF-1α | Titanium | SLS | Attracted significantly more stem cells. | [88] |
| copper-loaded- ZIF-8 nanoparticles | PLGA | 3D Bio-plotting | The mMSCs cultured well-spread and adherent with a high proliferation rate. Kill bacteria | [81] |
| coated with graphene oxide (GO) | Alg | 3D Bio-plotting | Provide electrical conductivity and cell affinity sites. | [82] |
| miRNA-148 b-transfected | PCL/PLGA/HAp | 3D Bio-plotting | Improved bone regeneration considerably. | [84] |
| electrical stimuli | gelatin-graphene conduits | FDM | Have a profound effect on the differentiation of MSCs to SC-like phenotypes and their paracrine activity. | [90] |
| functionalized with azide-Heparin (az-Heparin) to bind | HTy | 3D Bio-plotting | Significantly enhanced osteogenic differentiation of hMSCs | [85] |
| homogeneous nano apatite coating | alginate/gelatin | 3D Bio-plotting | Significantly stimulated the proliferation and osteogenic differentiation of rat bone marrow stem cells, and nano apatite coating increased the protein adsorption on the surface of scaffolds. | [23] |
| low intensity pulsed ultrasound (LIPUS) | PEGDA | SLA | Increase proliferation. stimulation enhanced GAG synthesis. Additionally, type II collagen production increased by 60% and 40% | [54] |
5. Clinical applications
In the past decade, with the development of 3D printing technology, a large number of tissue engineering scaffolds have been made for clinical applications by using novel materials and innovative technologies. It brings great hope and encouragement to clinicians and patients. For example, Yuki Kanno et al. used 3D printing to make customized artificial bones (CT bone) and applied them to 20 patients with facial deformities. After medium and long-term follow-up, CT bones morphology were maintained in good condition, and good bone replacement obtained a good prognosis [60]. In addition to bone defect repair and transplantation, this technology is also widely used in clinical teaching [61,62], preoperative simulation [63], intraoperative navigation [64], stomatology [65,66], and other aspects, with great application prospect and market value.
However, at the same time, many problems are still exposed, such as insufficient strength [67], poor biocompatibility [68], too fast or too slow degradation rate [69,70], etc. Finding suitable printing methods and biocompatible materials for bone engineering has always been the focus of development for bone defect repair. Besides, there are special requirements for artificial bone, that is, the function and vitality of bone cells and mechanical properties of scaffolds under load-bearing conditions. These are the hot spots and frontier directions of the research.
The mixed application of natural and synthetic polymer materials seems to be a promising solution to solve the problems of mechanical strength [71], biocompatibility [72,73], and controllable degradation [74]. But there is still a need for clinicians and technicians to work together to continue to explore new materials and innovative printing methods.
6. Conclusions
6.1. The advantages of 3D printing
In the past few years, 3D printed bone tissue engineering scaffolds have been increasingly used in the application of stem cells, especially for bone repair and cartilage regeneration of composite structure, which has great advantages that traditional technologies cannot replace. An increasing understanding of the machining parameters that affect these structural properties can help to develop increasingly optimized composites. The 3D printing technology uses many kinds of materials and composite materials, which not only cover the material scope of traditional technology but also derive the material making which could not be realized before by using the technologies (e.g. scaffolds with controllable drug release [75]). 3D printing technology can print highly accurate and complex internal and external structures that adapt to mechanical and biological surface characteristics [76], thus enabling the preparation of customized, patient-specific implants that are highly suitable for tissue and organ defects, as well as disease simulation platforms [77] and stem cell research platforms [78].
6.2. Problems and limitations
In the field of stem cell research and application, 3D printing technology also has disadvantages and limitations. Firstly, although 3D printing can produce personalized and precise scaffolds, it also brings high clinical and scientific research costs and the shortage of mass production. Secondly, some 3D printed scaffold materials are cytotoxic, and the preparation process is also toxic and pathogenic, which also limits its application in clinical, cell biology and regenerative medicine. Besides, there are still huge challenges in generating complex geometric shapes of composite materials, processing various materials and post-optimization of composite surface properties by utilizing the versatility of 3D printing technology. Finally, with the rapid development of stem cell regenerative medicine treatment, the social ethics, legal rationality and regulatory issues brought by printing organs and tissues are also worthy of our consideration and deep thinking [79].
6.3. The future
With the further optimization of current technologies and the emergence of more bio-ink materials, the design of effective scaffolds for the use of extracellular matrix, bone and cartilage for stem cells is becoming more and more promising. 3D printed scaffolds for tissue engineering could be the key to improving the quality of life for patients with organ defects and dysfunction caused by damage or lesions. The development of new printing materials, nanomaterials, especially biocompatible materials, composite materials and complex biomaterials based on various application requirements is the future development direction of 3D printing technology. Also, to promote the systematization, standardization, non-toxic, harmless, green and environmental protection of 3D printing materials, and to continuously expand the integration of 3D printing technology, stem cell technology and traditional treatment will be the development direction of 3D printing and stem cell production. We expect the organic combination of regenerative medicine and additive manufacturing to be a great success for the benefit of all mankind.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, Applications of 3D printed bone tissue engineering scaffolds in the stem cell field.
Acknowledgements
Financial support from the National Key R&D Program of China (No. 2019YFF0302403) and the National Natural Science Foundation of China (No. 51872332) are acknowledged. Authors like to also acknowledge the Key Laboratory for Immunology and Dermatology of Health's Ministry (the First Hospital of China Medical University) and Dr. Rong Jun for his help.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Xin Su, Email: 915165607@qq.com.
Ting Wang, Email: twang@cmu.edu.cn.
Shu Guo, Email: sguo@cmu.edu.cn.
References
- 1.Bose S., Vahabzadeh S., Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16(12):496–504. [Google Scholar]
- 2.Whiting P., Kerby J., Coffey P., da Cruz L., McKernan R. Progressing a human embryonic stem-cell-based regenerative medicine therapy towards the clinic. Philos Trans R Soc Lond B Biol Sci. 2015;370(1680):20140375. doi: 10.1098/rstb.2014.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samsonraj R.M., Raghunath M., Nurcombe V., Hui J.H., van Wijnen A.J., Cool S.M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y., Li X., Zhang Y., Han Y., Chang F., Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8) doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimoto H., Kasahara T., Sueta S.I., Araoka T., Sakamoto S., Okada C. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep. 2020;31(1):107476. doi: 10.1016/j.celrep.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Chen S.D., Li H.Q., Cui M., Dong Q., Yu J.T. Pluripotent stem cells for neurodegenerative disease modeling: an expert view on their value to drug discovery. Expet Opin Drug Discov. 2020;15(9):1081–1094. doi: 10.1080/17460441.2020.1767579. [DOI] [PubMed] [Google Scholar]
- 8.Chlebanowska P., Tejchman A., Sulkowski M., Skrzypek K., Majka M. Use of 3D organoids as a model to study idiopathic form of Parkinson’s disease. Int J Mol Sci. 2020;21(3) doi: 10.3390/ijms21030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27(4):523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Do A.V., Khorsand B., Geary S.M., Salem A.K. 3D printing of scaffolds for tissue regeneration applications. Adv Healthc Mater. 2015;4(12):1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elhaj A., Irgum K. Monolithic space-filling porous materials from engineering plastics by thermally induced phase separation. ACS Appl Mater Interfaces. 2014;6(18):15653–15666. doi: 10.1021/am502977z. [DOI] [PubMed] [Google Scholar]
- 12.Nakamatsu J., Torres F.G., Troncoso O.P., Min-Lin Y., Boccaccini A.R. Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromolecules. 2006;7(12):3345–3355. doi: 10.1021/bm0605311. [DOI] [PubMed] [Google Scholar]
- 13.Prasopthum A., Shakesheff K.M., Yang J. Direct three-dimensional printing of polymeric scaffolds with nanofibrous topography. Biofabrication. 2018;10(2):25002. doi: 10.1088/1758-5090/aaa15b. [DOI] [PubMed] [Google Scholar]
- 14.Degli Esposti M., Chiellini F., Bondioli F., Morselli D., Fabbri P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mater Sci Eng C Mater Biol Appl. 2019;100:286–296. doi: 10.1016/j.msec.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Raisian S., Fallahi H.R., Khiabani K.S., Heidarizadeh M., Azdoo S. Customized titanium mesh based on the 3D printed model vs. Manual intraoperative bending of titanium mesh for reconstructing of orbital bone fracture: a randomized clinical trial. Rev Recent Clin Trials. 2017;12(3):154–158. doi: 10.2174/1574887112666170821165206. [DOI] [PubMed] [Google Scholar]
- 16.Simoneti D.M., Pereira-Cenci T., Dos Santos M.B.F. Comparison of material properties and biofilm formation in interim single crowns obtained by 3D printing and conventional methods. J Prosthet Dent. 2020 doi: 10.1016/j.prosdent.2020.06.026. S0022-3913(20):30513-30518. [DOI] [PubMed] [Google Scholar]
- 17.Aliabouzar M., Lee S.J., Zhou X., Zhang G.L., Sarkar K. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells. Biotechnol Bioeng. 2018;115(2):495–506. doi: 10.1002/bit.26480. [DOI] [PubMed] [Google Scholar]
- 18.Song Y., Lin K., He S., Wang C., Zhang S., Li D. Nano-biphasic calcium phosphate/polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrin. Int J Nanomed. 2018;13:505–523. doi: 10.2147/IJN.S152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marro A., Bandukwala T., Mak W. Three-dimensional printing and medical imaging: a review of the methods and applications. Curr Probl Diagn Radiol. 2016;45(1):2–9. doi: 10.1067/j.cpradiol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull G., Clarke J., Picard F., Riches P., Jia L., Han F. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3(3):278–314. doi: 10.1016/j.bioactmat.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner N., Strong B.R., Gold S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyping Journal. 2014;20(3):192–204. [Google Scholar]
- 22.Mohamed O.A., Masood S.H., Bhowmik J.L. Optimization of fused deposition modeling process parameters: a review of current research and future prospects. Adv Manuf. 2015;3(1):42–53. [Google Scholar]
- 23.Gremare A., Guduric V., Bareille R., Heroguez V., Latour S., L’Heureux S. Characterization of printed PLA scaffolds for bone tissue engineering. J Biomed Mater Res. 2018;106(4):887–894. doi: 10.1002/jbm.a.36289. [DOI] [PubMed] [Google Scholar]
- 24.Distler T., Fournier N., Grunewald A., Polley C., Seitz H., Detsch R. Polymer-bioactive glass composite filaments for 3D scaffold manufacturing by fused deposition modeling: fabrication and characterization. Front Bioeng Biotechnol. 2020;8:552. doi: 10.3389/fbioe.2020.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian L., Zhang Z., Tian B., Zhang X., Wang N. Study on antibacterial properties and cytocompatibility of EPL coated 3D printed PCL/HA composite scaffolds. RSC Adv. 2020;10(8):4805–4816. doi: 10.1039/c9ra10275b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geven M.A., Grijpma D.W. Additive manufacturing of composite structures for the restoration of bone tissue. Multifunctional Materials. 2019;2(2) [Google Scholar]
- 27.Wang F., Shor L., Darling A., Khalil S., Sun W., Güçeri S. Precision extruding deposition and characterization of cellular poly-ε-caprolactone tissue scaffolds. Rapid Prototyp J. 2004;10(1):42–49. [Google Scholar]
- 28.Bellini A. Department of Mechanical Engineering and Mechanics, Drexel University.; 2002. Fused deposition of ceramics- A comprehensive experimental, analytical and computational study of material behavior, fabrication process and equipment design. PhD dissertation. [Google Scholar]
- 29.Hamid Q., Snyder J., Wang C., Timmer M., Hammer J., Guceri S. Fabrication of three-dimensional scaffolds using precision extrusion deposition with an assisted cooling device. Biofabrication. 2011;3(3):34109. doi: 10.1088/1758-5082/3/3/034109. [DOI] [PubMed] [Google Scholar]
- 30.Fedore C.W., Tse L.Y.L., Nam H.K., Barton K.L., Hatch N.E. Analysis of polycaprolactone scaffolds fabricated via precision extrusion deposition for control of craniofacial tissue mineralization. Orthod Craniofac Res. 2017;20(Suppl 1):12–17. doi: 10.1111/ocr.12159. [DOI] [PubMed] [Google Scholar]
- 31.Xu M., Li M., Suo H., Yan Y., Liu L., Wang Q. Fabricating a pearl/PLGA composite scaffold by the low-temperature deposition manufacturing technique for bone tissue engineering. Biofabrication. 2010;2(2):25002. doi: 10.1088/1758-5082/2/2/025002. [DOI] [PubMed] [Google Scholar]
- 32.Landers R., Mülhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng. 2000;282(1):17–21. [Google Scholar]
- 33.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 34.Rajaram A., Schreyer D., Chen D. Bioplotting alginate/hyaluronic acid hydrogel scaffolds with structural integrity and preserved schwann cell viability. 3D Print Addit Manuf. 2014;1(4):194–203. [Google Scholar]
- 35.Ouyang L., Yao R., Zhao Y., Sun W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication. 2016;8(3):35020. doi: 10.1088/1758-5090/8/3/035020. [DOI] [PubMed] [Google Scholar]
- 36.Guillaume O., Geven M.A., Sprecher C.M., Stadelmann V.A., Grijpma D.W., Tang T.T. Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomater. 2017;54:386–398. doi: 10.1016/j.actbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Choi S.M., Park J.W. Multifunctional effects of a modification of SLA titanium implant surface with strontium-containing nanostructures on immunoinflammatory and osteogenic cell function. J Biomed Mater Res. 2018;106(12):3009–3020. doi: 10.1002/jbm.a.36490. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.J., Yan D., Zhou X., Cui H., Esworthy T., Hann S.Y. Integrating cold atmospheric plasma with 3D printed bioactive nanocomposite scaffold for cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2020;111:110844. doi: 10.1016/j.msec.2020.110844. [DOI] [PubMed] [Google Scholar]
- 39.Yan Y., Li S., Zhang R., Lin F., Wu R. Rapid prototyping and manufacturing technology: principle, representative technics, applications, and development trends. Tsinghua Sci Technol. 2009;14(S1):1–12. [Google Scholar]
- 40.Jaber H., Kovacs T. Selective laser melting of Ti alloys and hydroxyapatite for tissue engineering: progress and challenges. Mater Res Express. 2019;6(8) [Google Scholar]
- 41.Roskies M., Jordan J.O., Fang D., Abdallah M.N., Hier M.P., Mlynarek A. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J Biomater Appl. 2016;31(1):132–139. doi: 10.1177/0885328216638636. [DOI] [PubMed] [Google Scholar]
- 42.Xiao K., Dalgarno K.W., Wood D.J., Goodridge R.D., Ohtsuki C. Indirect selective laser sintering of apatite-wollostonite glass-ceramic. Proc Inst Mech Eng H. 2008;222(7):1107–1114. doi: 10.1243/09544119JEIM411. [DOI] [PubMed] [Google Scholar]
- 43.Goodridge R.D., Dalgarno K.W., Wood D.J. Indirect selective laser sintering of an apatite-mullite glass-ceramic for potential use in bone replacement applications. Proc Inst Mech Eng H. 2006;220(1):57–68. doi: 10.1243/095441105X69051. [DOI] [PubMed] [Google Scholar]
- 44.Zeng H., Pathak J.L., Shi Y., Ran J., Liang L., Yan Q. Indirect selective laser sintering-printed microporous biphasic calcium phosphate scaffold promotes endogenous bone regeneration via activation of ERK1/2 signaling. Biofabrication. 2020;12(2):25032. doi: 10.1088/1758-5090/ab78ed. [DOI] [PubMed] [Google Scholar]
- 45.Kolan K.C., Leu M.C., Hilmas G.E., Brown R.F., Velez M. Fabrication of 13-93 bioactive glass scaffolds for bone tissue engineering using indirect selective laser sintering. Biofabrication. 2011;3(2):25004. doi: 10.1088/1758-5082/3/2/025004. [DOI] [PubMed] [Google Scholar]
- 46.Yap C.Y., Chua C.K., Dong Z.L., Liu Z.H., Zhang D.Q., Loh L.E. Review of selective laser melting: materials and applications. Appl Phys Rev. 2015;2(4) [Google Scholar]
- 47.Cheng Y.L., Huang K.C. Preparation and characterization of color photocurable resins for full-color material jetting additive manufacturing. Polymers. 2020;12(3) doi: 10.3390/polym12030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cader H.K., Rance G.A., Alexander M.R., Goncalves A.D., Roberts C.J., Tuck C.J. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int J Pharm. 2019;564:359–368. doi: 10.1016/j.ijpharm.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Mostafaei A., Rodriguez De Vecchis P., Buckenmeyer M.J., Wasule S.R., Brown B.N., Chmielus M. Microstructural evolution and resulting properties of differently sintered and heat-treated binder-jet 3D-printed Stellite 6. Mater Sci Eng C. 2019;102:276–288. doi: 10.1016/j.msec.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Stogerer J., Baumgartner S., Hochwallner A., Stampfl J. Bio-Inspired toughening of composites in 3D-printing. Materials. 2020;13(21) doi: 10.3390/ma13214714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhargav A., Min K.S., Wen Feng L., Fuh J.Y.H., Rosa V. Taguchi’s methods to optimize the properties and bioactivity of 3D printed polycaprolactone/mineral trioxide aggregate scaffold: theoretical predictions and experimental validation. J Biomed Mater Res B Appl Biomater. 2020;108(3):629–637. doi: 10.1002/jbm.b.34417. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y.W., Fang H.Y., Shie M.Y., Shen Y.F. The mussel-inspired assisted apatite mineralized on PolyJet material for artificial bone scaffold. Int J Bioprint. 2019;5(2):197. doi: 10.18063/ijb.v5i2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao F., Xu Z., Liang Q., Li H., Peng L., Wu M. Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv Sci. 2019;6(15):1900867. doi: 10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., Cong Y., Osi A.R., Zhou Y., Huang F., Zaccaria R.P. Direct 3D printed biomimetic scaffolds based on hydrogel microparticles for cell spheroid growth. Adv Funct Mater. 2020;30(13) [Google Scholar]
- 55.Nowicki M., Zhu W., Sarkar K., Rao R., Zhang L.G. 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink. Bioprinting. 2020;17 [Google Scholar]
- 56.Van Damme L., Briant E., Blondeel P., Van Vlierberghe S. Indirect versus direct 3D printing of hydrogel scaffolds for adipose tissue regeneration. MRS Adv. 2020;5(17):855–864. [Google Scholar]
- 57.De Maria C., De Acutis A., Vozzi G. Essentials of 3D biofabrication and translation. 2015. Indirect rapid prototyping for tissue engineering; pp. 153–164. [Google Scholar]
- 58.Van Hoorick J., Declercq H., De Muynck A., Houben A., Van Hoorebeke L., Cornelissen R. Indirect additive manufacturing as an elegant tool for the production of self-supporting low density gelatin scaffolds. J Mater Sci Mater Med. 2015;26(10):247. doi: 10.1007/s10856-015-5566-4. [DOI] [PubMed] [Google Scholar]
- 59.Bidgoli M.R., Alemzadeh I., Tamjid E., Khafaji M., Vossoughi M. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: effect of particle size on physico-mechanical properties and in vitro cellular behavior. Mater Sci Eng C Mater Biol Appl. 2019;103:109688. doi: 10.1016/j.msec.2019.04.067. [DOI] [PubMed] [Google Scholar]
- 60.Kanno Y., Nakatsuka T., Saijo H., Fujihara Y., Atsuhiko H., Chung U.I. Computed tomographic evaluation of novel custom-made artificial bones, “CT-bone”, applied for maxillofacial reconstruction. Regen Ther. 2016;5:1–8. doi: 10.1016/j.reth.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young J.C., Quayle M.R., Adams J.W., Bertram J.F., McMenamin P.G. Three-dimensional printing of archived human fetal material for teaching purposes. Anat Sci Educ. 2019;12(1):90–96. doi: 10.1002/ase.1805. [DOI] [PubMed] [Google Scholar]
- 62.McMenamin P.G., Hussey D., Chin D., Alam W., Quayle M.R., Coupland S.E. The reproduction of human pathology specimens using three-dimensional (3D) printing technology for teaching purposes. Med Teach. 2020:1–9. doi: 10.1080/0142159X.2020.1837357. [DOI] [PubMed] [Google Scholar]
- 63.Ganguli A., Pagan-Diaz G.L., Grant L., Cvetkovic C., Bramlet M., Vozenilek J. 3D printing for preoperative planning and surgical training: a review. Biomed Microdevices. 2018;20(3):65. doi: 10.1007/s10544-018-0301-9. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Mato D., Ochandiano S., Garcia-Sevilla M., Navarro-Cuellar C., Darriba-Alles J.V., Garcia-Leal R. Craniosynostosis surgery: workflow based on virtual surgical planning, intraoperative navigation and 3D printed patient-specific guides and templates. Sci Rep. 2019;9(1):17691. doi: 10.1038/s41598-019-54148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao O., Kort-Mascort J., Lin Y., Pham H.M., Charbonneau A.M., ElKashty O.A. The applications of 3D printing for craniofacial tissue engineering. Micromachines. 2019;10(7) doi: 10.3390/mi10070480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Major R., Kowalczyk P., Surmiak M., Lojszczyk I., Podgorski R., Trzaskowska P. Patient specific implants for jawbone reconstruction after tumor resection. Colloids Surf B Biointerfaces. 2020;193:111056. doi: 10.1016/j.colsurfb.2020.111056. [DOI] [PubMed] [Google Scholar]
- 67.Carvalho M.S., Silva J.C., Udangawa R.N., Cabral J.M.S., Ferreira F.C., da Silva C.L. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;99:479–490. doi: 10.1016/j.msec.2019.01.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kazimierczak P., Benko A., Nocun M., Przekora A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int J Nanomed. 2019;14:6615–6630. doi: 10.2147/IJN.S217245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erickson C.B., Newsom J.P., Fletcher N.A., Feuer Z.M., Yu Y., RRodriguez-Fontan F. In vivo degradation rate of alginate-chitosan hydrogels influences tissue repair following physeal injury. J Biomed Mater Res B Appl Biomater. 2020;108(6):2484–2494. [Google Scholar]
- 70.Liu Y., Tian K., Hao J., Yang T., Geng X., Zhang W. Biomimetic poly(glycerol sebacate)/polycaprolactone blend scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2019;30(5):53. doi: 10.1007/s10856-019-6257-3. [DOI] [PubMed] [Google Scholar]
- 71.Chi H., Chen G., He Y., Chen G., Tu H., Liu X. 3D-HA scaffold functionalized by extracellular matrix of stem cells promotes bone repair. Int J Nanomed. 2020;15:5825–5838. doi: 10.2147/IJN.S259678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee J., Hong J., Kim W., Kim G.H. Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering. Carbohydr Polym. 2020;250:116914. doi: 10.1016/j.carbpol.2020.116914. [DOI] [PubMed] [Google Scholar]
- 73.Kim H., Hwangbo H., Koo Y., Kim G. Fabrication of mechanically reinforced gelatin/hydroxyapatite bio-composite scaffolds by core/shell nozzle printing for bone tissue engineering. Int J Mol Sci. 2020;21(9) doi: 10.3390/ijms21093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou K., Yu P., Shi X., Ling T., Zeng W., Chen A. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repair. ACS Nano. 2019;13(8):9595–9606. doi: 10.1021/acsnano.9b04723. [DOI] [PubMed] [Google Scholar]
- 75.Fu S., Du X., Zhu M., Tian Z., Wei D., Zhu Y. 3D printing of layered mesoporous bioactive glass:sodium alginate-sodium alginate scaffolds with controllable dual-drug release behaviors. Biomed Mater. 2019;14 doi: 10.1088/1748-605X/ab4166. [DOI] [PubMed] [Google Scholar]
- 76.Ganbold B., Heo S.J., Koak J.Y, Kim S.K., Cho J. Human stem cell responses and surface characteristics of 3D printing Co-Cr dental material. Materials. 2019;12(20) doi: 10.3390/ma12203419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaji S., Al-Saleh J., Gomillion C.T. Bioprinted three-dimensional cell-laden Hydrogels to evaluate adipocyte-breast cancer cell interactions. Gels. 2020;6(1) doi: 10.3390/gels6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubi-Sans G., Recha-Sancho L., Perez-Amodio S., Mateos-Timoneda M.A., Semino C.E., Engel E. Development of a three-dimensional bioengineered platform for articular cartilage regeneration. Biomolecules. 2019;10(1) doi: 10.3390/biom10010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vermeulen N., Haddow G., Seymour T., Faulkner-Jones A., Shu W. 3D bioprint me: a socioethical view of bioprinting human organs and tissues. J Med Ethics. 2017;43(9):618–624. doi: 10.1136/medethics-2015-103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prasopthum A., Jiang J., Lv F., Xia X., Ma X. 3D printed scaffolds with controlled micro-/nano-porous surface topography direct chondrogenic and osteogenic differentiation of mesenchymal stem cells. ACS Appl Mater Interfaces. 2019;11:18896–18906. doi: 10.1021/acsami.9b01472. [DOI] [PubMed] [Google Scholar]
- 81.Zou F., Jiang J., Lv F., Xia X., Ma X. Preparation of antibacterial and osteoconductive 3D-printed PLGA/Cu(I)@ZIF-8 nanocomposite scaffolds for infected bone repair. J Nanobiotechnol. 2020;18(1):39. doi: 10.1186/s12951-020-00594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li J., Liu X., Crook J.M., Wallace G.G. 3D printing of cytocompatible graphene/alginate scaffolds for mimetic tissue constructs. Front Bioeng Biotechnol. 2020;8:824. doi: 10.3389/fbioe.2020.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choe G., Oh S., Seok J.M., Park S.A., Lee J.Y. Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications. Nanoscale. 2019;11(48):23275–23285. doi: 10.1039/c9nr07643c. [DOI] [PubMed] [Google Scholar]
- 84.Moncal K.K., Aydin R.S.T., Abu-Laban M., Heo D.N., Rizk E., Tucker S.M. Collagen-infilled 3D printed scaffolds loaded with miR-148b-transfected bone marrow stem cells improve calvarial bone regeneration in rats. Mater Sci Eng C Mater Biol Appl. 2019;105:110128. doi: 10.1016/j.msec.2019.110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji S., Dube K., Chesterman J.P., Fung S.L., Liaw C.Y., Kohn J. Polyester-based ink platform with tunable bioactivity for 3D printing of tissue engineering scaffolds. Biomater Sci. 2019;7(2):560–570. doi: 10.1039/c8bm01269e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo Y., Li Y., Qin X., Wa Q. 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Mater Des. 2018;146:12–19. [Google Scholar]
- 87.Athirasala A., Tahayeri A., Thrivikraman G., Franca C.M., Monteiro N., Tran V. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication. 2018;10(2):24101. doi: 10.1088/1758-5090/aa9b4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bollman M., Malbrue R., Li C., Yao H., Guo S., Yao S. Improvement of osseointegration by recruiting stem cells to titanium implants fabricated with 3D printing. Ann N Y Acad Sci. 2020;1463(1):37–44. doi: 10.1111/nyas.14251. [DOI] [PubMed] [Google Scholar]
- 89.Fahimipour F., Dashtimoghadam E., Mahdi Hasani-Sadrabadi M., Vargas J., Vashaee D., Lobner D.C. Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent Mater. 2019;35(7):990–1006. doi: 10.1016/j.dental.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uz M., Donta M., Mededovic M., Sakaguchi D.S., Mallapragada S.K. Development of gelatin and graphene-based nerve regeneration conduits using three-dimensional (3D) printing strategies for electrical transdifferentiation of mesenchymal stem cells. Ind Eng Chem Res. 2019;58(18):7421–7427. [Google Scholar]