Abstract
The XV. Banff conference for allograft pathology was held in conjunction with the annual meeting of the American Society for Histocompatibility and Immunogenetics in Pittsburgh, PA (USA) and focused on refining recent updates to the classification, advances from the Banff working groups, and standardization of molecular diagnostics. This report on kidney transplant pathology details clarifications and refinements to the criteria for chronic active (CA) T cell–mediated rejection (TCMR), borderline, and antibody‐mediated rejection (ABMR). The main focus of kidney sessions was on how to address biopsies meeting criteria for CA TCMR plus borderline or acute TCMR. Recent studies on the clinical impact of borderline infiltrates were also presented to clarify whether the threshold for interstitial inflammation in diagnosis of borderline should be i0 or i1. Sessions on ABMR focused on biopsies showing microvascular inflammation in the absence of C4d staining or detectable donor‐specific antibodies; the potential value of molecular diagnostics in such cases and recommendations for use of the latter in the setting of solid organ transplantation are presented in the accompanying meeting report. Finally, several speakers discussed the capabilities of artificial intelligence and the potential for use of machine learning algorithms in diagnosis and personalized therapeutics in solid organ transplantation.
Keywords: classification systems: Banff classification, clinical decision‐making, clinical research/practice, kidney (allograft) function/dysfunction, kidney transplantation/nephrology, molecular biology: mRNA/mRNA expression, pathology/histopathology, rejection, translational research/science
Short abstract
This report focuses on the clarification of the criteria for chronic active T cell–mediated rejection and antibody‐mediated rejection and the optimization of the inflammation threshold for the diagnosis of borderline for acute T cell–mediated rejection, and discusses the potential to use machine learning in diagnostics and personalized therapeutics in solid organ transplantation.
1. INTRODUCTION
The XV. Banff Conference for Allograft Pathology was held September 23‐27, 2019, in Pittsburgh, PA (USA), in conjunction with the Annual Meeting of the American Society for Histocompatibility and Immunogenetics (ASHI). A total of 1253 delegates from 31 countries attended the conference, including pathologists, immunologists, physicians, surgeons, and immunogeneticists as well as representatives from industry. The focus of kidney sessions at the 2019 conference was to clarify diagnostic criteria for antibody‐mediated rejection (ABMR) and chronic active (CA) T cell–mediated rejection (TCMR) to harmonize the pathologic diagnosis and consequent therapeutic strategies. In addition, consensus of the diagnosis of borderline (suspicious) for acute TCMR was reached. The current literature on biomarkers and molecular transplant diagnostics was also reviewed, and recommendations of the Banff Molecular Diagnostics Working Group regarding clinical validation and adoption of the latter into the Banff classification will be presented in a separate meeting report. Continuing a theme from the Banff 2015 meeting, 1 results of studies using artificial intelligence (AI) and integrative epidemiological approaches were presented. Their applicability in prediction, prognostication, and clinical trials could change standards of care for transplant recipients. 2 The conference was preceded by a premeeting on “Regenerative Medicine and Digital Pathology” and a joint session between the ASHI and Banff key opinion leaders, which presented insights into future tissue and organ engineering and new technologies in solid organ transplantation. Precise detection and characterization of anti‐HLA or non‐HLA donor‐specific antibodies (DSAs) was also revisited. This report summarizes the main outcomes of the Banff 2019 kidney sessions and their impact on the Banff classification.
2. UPDATES FROM THE BANFF WORKING GROUPS
The current active and new Banff working groups (BWGs) and their aims, leaders, and progress are listed in Table 1. One new BWG has been formed, the Digital Pathology BWG. The aim of this working group is to define standards for digital pathology in the context of Banff lesion scores in order to standardize diagnostic scoring and reduce inter‐observer variability. In addition, the peritubular capillaritis (ptc) BWG has been reactivated. Recent studies have shown diagnostic and prognostic relevance of reporting the extent of capillaritis (diffuse vs focal) in addition to the Banff ptc score, in different clinical scenarios including ABMR, mixed ABMR/TCMR, and low‐grade microvascular inflammation (MVI). 3 , 4 The aim of the BWG is to validate these findings in a multicenter study, and to evaluate optimal diagnostic thresholds.
Table 1.
Updates of Banff working groups
| Working group | Leaders | Issues to address | Group progress/future plans |
|---|---|---|---|
| TCMR |
V. Nickeleit P. Randhawa |
Integration of i‐IFTA into classification; reevaluate thresholds for i and t and possible addition of other findings (eg, edema) to TCMR diagnostic criteria | A multicenter clinicopathologic study aimed at addressing the listed issues is in progress. To date biopsies and accompanying clinical data from 154 patients with “pure” (no ABMR) acute TCMR/borderline, 18 with chronic active TCMR, 55 with no rejection. and 31 patients with stable graft function (not biopsied) have been accrued into the study cohort. Data analysis and slide review have commenced, but more cases, especially cases of chronic active TCMR and control cases with complete clinical data for statistical analysis of TCMR/borderline thresholds, are required. These cases need to have documented absence of DSA and sufficient follow‐up in addition to detailed clinical information. |
| Sensitized |
L. Cornell R. Sapir‐Pichhadze E. Kraus S. Bagnasco C. Schinstock D. Dadhania |
Define criteria for highly sensitized patients (HS), determine consensus for what personnel and facilities are needed for centers to perform transplantation in HS recipients, standardize the definitions related to management of sensitized transplant recipients. Evaluate current practices of centers performing renal transplants in sensitized recipients. Evaluate how clinicians interpret and apply Banff nomenclature, and recommend changes to wording of classification to optimize the use of Banff data in clinical care of HS patients. | Survey regarding clinical practice related to highly sensitized (HS) patients indicates that clinicians often fail to recognize chronic elements of ABMR (eg, cg > 0) and are more likely to consider a diagnosis of chronic active ABMR if C4d is negative, even if there is no TG, PTCML, or IFTA. The term “acute” is confusing in ABMR, and consequently it was removed from the Banff ABMR classification in 2017. 3 Further improved communication between pathologists and clinicians regarding reporting of biopsy findings in HS. Follow‐up preliminary survey results indicate that pathologists and clinicians have similar concerns about biopsy reporting of antibody‐mediated injury: Banff classification is complex, changes frequently, and does not reflect the entire ABMR disease spectrum. Comprehensive literature review is in progress to outline patient risk spectrum with respect to antibody and to standardize definitions of incompatible transplants. |
| Molecular diagnostics |
M. Mengel R. Colvin |
Develop consensus discovery gene panel, which becomes available to the whole transplant community as standardized commercially available reagents. Design and conduct collaborative studies to validate clinical utility of molecular diagnostics in transplant biopsies. | Launch of a consensus based, commercially available (NanoString Inc), standardized Banff Human Organ Transplant (B‐HOT) discovery gene panel, which can reproducibly be applied to FFPE samples across organs and in multicenter studies. Development of an open source data sharing platform to exchange results from studies using the B‐HOT panel and to allow multicenter and multiorgan validation of clinical utility of molecular diagnostics in transplant biopsies. Details given in a separate meeting report. |
| HIV+/HIV+ renal transplants | S. Bagnasco | Compare kidney transplants from HIV+ and HIV‐negative donors to HIV+ recipients with regard to graft function and graft survival, · incidence, type and pathologic features of allograft lesions including rejection, recurrent and de novo HIV‐related/‐unrelated renal disease; injury associated with antiretroviral and immunosuppressive treatment. | Based on preliminary data from the pilot phase of the US HOPE prospective multicenter trial for HIV+ to HIV+ kidney transplantation, TCMR is predominant, rejection appears to be unrelated to donor HIV status and may possibly be related to the type of immunosuppression. Additional data will become available with ongoing trial enrollment in upcoming years. The results of protocol/indication biopsies (archived as digital images), combined with comprehensive clinical information will be analyzed to expand these preliminary observations. |
| Electron microscopy (EM) |
C. Roufosse H. K Singh |
Recommendations for the sampling and processing of tissue for EM and reporting of EM findings in transplant biopsies. Harmonize terminology with a list of definitions for cg1a and PTCML. Reduce inter‐observer variability in the use of uniform guidelines for the evaluation of TG and PTCML Multicenter study of the natural history, associations and the predictive value of glomerular and peritubular capillary ultrastructural features (endothelium; basement membrane; electron dense deposits). | Consensus document produced at Banff 2019 meeting. Results of initial survey of interobserver variability were poor (presented at Banff 2017); a new comprehensive teaching module will focus on the most problematic lesions gleaned from the 2017 survey; Goal is to improve the uniform use of currently published criteria and the inter‐observer variability. Research proposal presented at Banff 2019: pilot test case data collection in 2020; final study design to be agreed upon before initiation of the full study in 2020‐21. Goal is the multicenter validation of ultrastructural features through correlation with outcomes. |
| Thrombotic microangiopathy (TMA) |
M. Afrouzian H. Liapis |
Establish uniform diagnostic criteria for TMA. Determine the frequency with which TMA occurs in renal allograft biopsy specimens. Determine if there are specific features of TMA in renal allografts that help resolve the differential diagnosis of the TMA when the cause is not readily apparent from clinical history, DSA, C4d, etc | Using Delphi methodology, two phases were designed: Phase I (with pathologists) and Phase II (with clinicians). Phase I (6/9 rounds completed) involving 23 centers; 1. We collected 37 transplant biopsies, and after 6 Delphi rounds, narrowed down 331 criteria to 61; 2. We classified the criteria into 4 classes (Pathology, Clinical, Laboratory and Differential Diagnosis) and 12 categories reflecting positive and negative criteria for LM, IF, EM, clinical, laboratory and genetic criteria; 3. Participants validated the 37 cases in 2 validation rounds. Results showed that 75% of the participants reached consensus in over 75% of cases, using the 61 criteria. 4. We identified the reasons for over‐ and under‐diagnosis of TMA. Results of rounds 7, 8 and 9 are pending. Phase II: Recruitment of participants for the clinical Delphi starting January 2020. Future efforts will focus on completing the 3 remaining Delphi rounds (Phase I), soliciting nephrologists’ input (Phase II), combining Phase I and II results, dissecting diagnostic issues that emerged during Phase I, such as ABMR mimicking TMA and completing the molecular studies to generate the final consensus guidelines. The WG also plans to collaborate with groups that are working on native kidney TMA to compare results and generate a consensus document. |
| Recurrent glomerular disease |
N. Alachkar S. Bagnasco |
Establish pathologic guidelines for early recurrence of glomerular diseases, including FSGS, IgA nephropathy, membranous nephropathy, MPGN/C3GN. What are frequencies, clinical manifestations, and pathologic characteristics of recurrent/de novo glomerular diseases, and can any of these predict recurrence and/or graft outcomes? Understand the pathologic changes of recurrent glomerular diseases occurring concurrently with rejection and other transplant‐associated lesions. |
Biopsy specimens and clinical data are now being collected from 10 international centers. Preliminary results confirm IgA nephropathy and FSGS as the most prevalent recurrent diseases. Combine all data to create a posttransplant GN registry. Future directions:
Which pathologic analyses are needed for optimal and early diagnosis of recurrent disease? Is the apparent association of recurrent glomerular disease with acute rejection related to biopsy bias (ie incidental discovery of recurrent disease in biopsies done to rule out rejection), under‐immunosuppression, or both? |
| Surrogate endpoints |
A. Loupy M. Naesens |
Define valid surrogate endpoints and invasive or noninvasive biomarkers for clinical trials and how histology and lesions related to tissue injury or scarring have to be integrated in such systems. Insure Banff active involvement and interactions with agencies such as FDA/ EMA and societies (AST, ESOT, TTS). |
New WG in progress |
| Banff rules and dissemination |
J. U. Becker C. Roufosse |
Elaboration of diagnostic algorithms for the Banff Diagnostic Classes 1‐6. Determination of the needs of the transplant community for dissemination of Banff content. Predominantly web‐based dissemination of Banff content. | Collation of all Banff content for kidney allograft pathology up to and including Banff 2017 (Transplantation 2018; 102:1795‐1814). Future plans include survey of the transplant community regarding dissemination needs, podcast based on the 2018 review article in collaboration with TTS, development and implementation of web‐based tools and dissemination of relevant Banff content including the content of this 2019 update. |
| Digital pathology |
K. Solez A. B. Farris |
Digital automation of pathology practice: computing, artificial intelligence, nanotechnology, machine learning, slide numerization. |
Standardization of practices, classification for studies using integrative approaches, IFTA scoring, inflammation scoring, algorithms to fit to the classification and decrease inter‐observer variability. Archetypes to be validated across multiple institutions. Delivery of precision diagnostic, molecular pathways and therapeutics. |
| Peritubular capillaritis |
I. W.Gibson Z. Kikic N. Kozakowski |
Evaluation whether a combined view of ptc score and ptc extent is superior compared to the currently applied standard of care (only ptc score) in distinguishing the role of ptc in the following diagnostic settings: active and chronic active ABMR, acute and chronic active TCMR/borderline, low‐grade MVI and mixed ABMR/TCMR. Test the reproducibility and prognostic significance of the combined of ptc score and extent. Gene expression analysis of peritubular capillaritis in different diagnostic settings. Comparison of cortical ptc vs medullary ptc (vasa recta). |
Multicenter validation cohort study is under preparation, for collection, centralization, assessment and circulation of a subset of cases from different diagnostic groups for inter‐observer agreement is planned for 2020‐2021. Prognostic significance of a peritubular capillaritis grade incorporating both ptc score and extent will be investigated |
Abbreviation: FFPE, formalin‐fixed, paraffin‐embedded.
At its meeting during Banff 2019, the electron microscopy (EM) BWG expanded on its previous recommendations 1 , 5 and agreed to guidelines for tissue sampling and performing EM analysis for assessment of cg1a and peritubular capillary basement membrane multilayering (PTCML). These are summarized in Table 2. The group also recommended the use of specific terminology aligned where possible with ongoing work of the Renal Pathology Society working group developing consensus definitions for individual glomerular lesions by light microscopy and EM. As specified in Table 3, this includes the following, representing individual components of very early lesions of transplant glomerulopathy (cg1a): endothelial cell enlargement, subendothelial electron‐lucent widening, and subendothelial neo‐densa glomerular basement membrane. Reporting of other endothelial and basement membrane features in glomeruli and peritubular capillaries (PTCs) is left to the discretion of the pathologist as there are few data to support their use at present. Based on consensus opinion within the EM BWG, abbreviation for peritubular capillary basement membrane multilayering is recommended as PTCML.
Table 2.
Banff recommendations for electron microscopy in renal transplant biopsies
| Recommendations for taking of a sample for EM |
| Take a sample in all cases if possible, fixed and embedded as a resin block. At a minimum, this should be done if there is any suspicion of glomerular disease. While EM can be performed on samples recovered from paraffin blocks or frozen tissue to examine for electron dense deposits and to some extent the degree of foot process effacement, this is not recommended for assessment of glomerular basement membrane (GBM) thickness, cg1a or PTCML. In all cases, light microscopic (LM) examination of semi‐thin stained sections should be performed. |
| Recommendations for performing ultrastructural analysis |
|
Recommended in cases with: (a) Clinical, light microscopic and/or immunohistochemical suspicion of glomerular disease or of other diseases where EM may assist in diagnosis; (b) Patients at risk for antibody‐mediated rejection (ABMR): patients who are sensitized, have documented DSA at any time posttransplant, and/or who have had a prior biopsy showing features of ABMR (C4d staining, glomerulitis and/or peritubular capillaritis). EM can also be useful: (a) To detect early recurrence in patients with biopsy‐proven glomerular disease as the cause of native kidney failure; (b) In for‐cause biopsies ≥3 mo posttransplant, and in all biopsies ≥6 mo posttransplant, to determine if early changes of transplant glomerulopathy (cg1a) are present, prompting testing for DSA. In all cases, undertake an assessment for glomerular disease as in a native renal biopsy. If performing EM in the setting of possible ABMR, assess for cg1a and PTCML as recommended below. |
| Recommendations for preparing the block for ultrastructural examination |
| Select an area in the resin block(s) of viable (non‐necrotic) cortex, preferentially with minimal tubular atrophy/interstitial fibrosis. Aim to include ≥2 full glomeruli (at least 1). Do not taper the block to a “cone” or “pyramid” by removing cortical tissue around the glomeruli and aim to have at least 10 peritubular capillaries (PTCs) for examination. |
| Guidelines for ultrastructural assessment and reporting of cg1a |
|
Exclude globally or partly sclerosed glomeruli, and severely ischemic glomeruli. Examine 1 complete glomerulus as a minimum; where possible examine 2 or more. Examine the capillary loops at high magnification (≥5000x). cg1a is defined as: (a) No GBM double contours on LM; (b) ≥3 capillary loops (in a single glomerulus) each showing: subendothelial neo‐densa glomerular basement membrane (circumferential or not, single or multiple layers) AND endothelial cell enlargement and/or subendothelial electron‐lucent widening. The report should state how many glomeruli were examined, and how many loops show cg1a. |
| Guidelines for ultrastructural assessment and reporting of PTCML |
|
Assess for PTCML in the cortical peritubular capillaries between the glomeruli. Exclude scarred cortex and necrotic or hemorrhagic areas. Examine at least 10 PTC at high magnification (≥ 5000×). Count layers of basement membrane (BM) in the 3 worst affected PTCs, for each counting in the PTC segment with the most layers (worst affected area along the circumference). The report should state the number of PTC examined; the number of BM layers in the most affected PTC; and whether the Banff 2013 threshold for chronic ABMR (ptcml1; 1 PTC with ≥7 layers +2 PTC with ≥5 layers) is met. |
Table 3.
Definitions of the individual components of early lesions of transplant glomerulopathy
|
At the 2019 conference, final data from the BWG on the classification of polyomavirus nephropathy (PVN) were presented. This BWG studied 192 patients with definitive PVN and identified two histologic markers predictive for graft function and graft survival: the Banff interstitial fibrosis (ci) score and a new score termed “the intrarenal polyomavirus load level” or pvl, the latter based on the fraction of tubules with evidence of PV replication by light microscopy OR immunohistochemical staining of epithelial cell nuclei for SV40 large T antigen (pvl1 ≤ 1%, pvl2 > 1%, and <10%, pvl3 ≥ 10%). The pvl and ci scores were used to define 3 PVN classes (Tables 4 and 5). 6
Table 4.
Updates of 2019 Banff classification for ABMR, borderline changes, TCMR, and polyomavirus nephropathy. All updates in boldface type a
| Category 1: Normal biopsy or nonspecific changes |
| Category 2: Antibody‐mediated changes |
| Active ABMR; all 3 criteria must be met for diagnosis |
|
1. Histologic evidence of acute tissue injury, including 1 or more of the following:
|
|
2. Evidence of current/recent antibody interaction with vascular endothelium, including 1 or more of the following:
|
| 3. Serologic evidence of circulating donor‐specific antibodies (DSA to HLA or other antigens). C4d staining or expression of validated transcripts/classifiers as noted above in criterion 2 may substitute for DSA; however thorough DSA testing, including testing for non‐HLA antibodies if HLA antibody testing is negative, is strongly advised whenever criteria 1 and 2 are met |
| Chronic active ABMR; all 3 criteria must be met for diagnosis |
|
1. Morphologic evidence of chronic tissue injury, including 1 or more of the following: Transplant glomerulopathy (cg > 0) if no evidence of chronic TMA or chronic recurrent/de novo glomerulonephritis; includes changes evident by electron microscopy (EM) alone (cg1a) Severe peritubular capillary basement membrane multilayering (ptcml1; requires EM) Arterial intimal fibrosis of new onset, excluding other causes; leukocytes within the sclerotic intima favor chronic ABMR if there is no prior history of TCMR, but are not required |
| 2. Identical to criterion 2 for active ABMR, above |
| 3. Identical to criterion 3 for active ABMR, above, including strong recommendation for DSA testing whenever criteria 1 and 2 are met. Biopsies meeting criterion 1 but not criterion 2 with current or prior evidence of DSA (posttransplant) may be stated as showing chronic ABMR, however remote DSA should not be considered for diagnosis of chronic active or active ABMR |
| Chronic (inactive) ABMR |
| 1. cg > 0 and/or severe ptcml (ptcml1) |
| 2. Absence of criterion 2 of current/recent antibody interaction with the endothelium |
| 3. Prior documented diagnosis of active or chronic active ABMR and/or documented prior evidence of DSA |
| C4d staining without evidence of rejection; all 4 features must be present for diagnosis c |
| 1. Linear C4d staining in peritubular capillaries (C4d2 or C4d3 by IF on frozen sections, or C4d > 0 by IHC on paraffin sections) |
| 2. Criterion 1 for active or chronic active ABMR not met |
| 3. No molecular evidence for ABMR as in criterion 2 for active and chronic active ABMR |
| 4. No acute or chronic active TCMR, or borderline changes |
| Category 3: Borderline (Suspicious) for acute TCMR |
| Foci of tubulitis (t1, t2, or t3) with mild interstitial inflammation (i1), or mild (t1) tubulitis with moderate‐severe interstitial inflammation (i2 or i3) |
| No intimal or transmural arteritis (v = 0) |
| Category 4: TCMR |
| Acute TCMR |
| Grade IA: Interstitial inflammation involving >25% of non‐sclerotic cortical parenchyma (i2 or i3) with moderate tubulitis (t2) involving 1 or more tubules, not including tubules that are severely atrophic d |
| Grade IB: Interstitial inflammation involving >25% of non‐sclerotic cortical parenchyma (i2 or i3) with severe tubulitis (t3) involving 1 or more tubules, not including tubules that are severely atrophic d |
| Grade IIA: Mild to moderate intimal arteritis (v1), with or without interstitial inflammation and/or tubulitis |
| Grade IIB: Severe intimal arteritis (v2), with or without interstitial inflammation and/or tubulitis |
| Grade III: Transmural arteritis and/or arterial fibrinoid necrosis involving medial smooth muscle with accompanying mononuclear cell intimal arteritis (v3), with or without interstitial inflammation and/or tubulitis |
| Chronic active TCMR e |
| Grade IA: Interstitial inflammation involving >25% of sclerotic cortical parenchyma (i‐IFTA2 or i‐IFTA3) AND > 25% of total cortical parenchyma (ti2 or ti3) with moderate tubulitis (t2 or t‐IFTA2) involving 1 or more tubules, not including severely atrophic tubules d ; other known causes of i‐IFTA should be ruled out |
| Grade IB: Interstitial inflammation involving >25% of sclerotic cortical parenchyma (i‐IFTA2 or i‐IFTA3) AND > 25% of total cortical parenchyma (ti2 or ti3) with severe tubulitis (t3 or t‐IFTA3) involving 1 or more tubules, not including severely atrophic tubules d ; other known causes of i‐IFTA should be ruled out |
| Grade II: Chronic allograft arteriopathy (arterial intimal fibrosis with mononuclear cell inflammation in fibrosis and formation of neointima). This may also be a manifestation of chronic active or chronic ABMR or mixed ABMR/TCMR |
| Category 5: polyomavirus nephropathy f |
|
PVN Class 1 pvl 1 and ci 0‐1 |
|
PVN Class 2 pvl 1 and ci 2‐3 OR pvl 2 and ci 0‐3 OR pvl 3 and ci 0‐1 |
|
PVN Class 3 pvl 3 and ci 2‐3 |
Individual Banff lesion scores are defined in Table 5, and it is recommended that these be included in the biopsy report.
It should be noted that these arterial lesions may be indicative of ABMR, TCMR, or mixed ABMR/TCMR. “v” lesions and chronic allograft arteriopathy are only scored in arteries having a continuous media with ≥2 smooth muscle layers.
The clinical significance of these findings may be quite different in grafts exposed to anti–blood group antibodies (ABO‐incompatible allografts), where they do not appear to be injurious to the graft and may represent accommodation. However, with anti‐HLA antibodies, such lesions may progress to chronic ABMR, and more outcome data are needed.
Severely atrophic tubules are defined as having each of 3 features: diameter <25% of unaffected or minimally affected tubules in the same biopsy, an undifferentiated‐appearing, cuboidal or flattened epithelium, and pronounced wrinkling and/or thickening of the tubular basement membrane.
It was felt by the majority of Banff 2019 meeting attendees that reporting of chronic active TCMR should be accompanied by a second diagnosis of borderline acute TCMR or acute TCMR (with appropriate grade) when criteria for both diagnoses are met.
pvl scores are defined in Table 5 and in detail in ref. 4. An adequate sample for such scoring should include 2 biopsies cores and contain medulla. PVN can coexist with ABMR or with TCMR grades 2 or 3.
Table 5.
Banff reporting standardization scheme: Individual Banff scores used in grading of acute and chronic active ABMR and TCMR. Inclusion in the biopsy report of table similar to the below or a simple listing of individual scores in a comment is advised
| Acute Banff scores | Grading (0, 1, 2, 3) | Chronic Banff scores | Grading (0, 1, 2, 3) | Acute & chronic Banff scores | Grading (0, 1, 2, 3) |
|---|---|---|---|---|---|
| i | ci | ti | |||
| t | ct | i‐IFTA | |||
| v | cv | t‐IFTA | |||
| g | cg | pvl | |||
| ptc | ptcml | ||||
| C4d |
Abbreviations: i, inflammation in non‐scarred cortex, scored as 0 (absent/minimal, <10% of non‐scarred cortex inflamed), 1 (mild, 10%‐25%), 2 (moderate 26%‐50%), 3 (severe, >50%). The subcapsular cortex should not be considered; t, tubulitis in cortical tubules within non‐scarred cortex, scored as 0 (none), 1 (mild, 1‐4 mononuclear leukocytes per tubular cross‐section or 10 tubular epithelial cells in most severely involved tubule), 2 (moderate, 5‐10 mononuclear leukocytes), 3 (severe, >10 mononuclear leukocytes). Although the tubulitis score (1‐3) is based on the single most severely involved tubule, at least mild tubulitis must be present in ≥2 cortical foci for a t score >0 to be assigned. Severely atrophic tubules should NOT be scored; v, endarteritis (intimal arteritis), scored as 0 (none), 1 (mild, 1 or more leukocytes directly beneath the endothelium of 1 or more arteries; endothelial cells typically appear enlarged with associated subendothelial edema; <25% luminal occlusion), 2 (moderate, as grade 1, but with ≥25% luminal occlusion), 3 (severe, with arterial fibrinoid necrosis or transmural inflammation). Note that only arteries (minimum 2 smooth muscle layers) are scored; g, glomerulitis, scored as 0 (none), 1 (mild, with ≥1 leukocyte AND associated endothelial swelling occluding >50% of 1 of more capillary lumina in at least one but <25% of glomeruli), 2 (moderate, these changes involving 25%‐75% of glomeruli), 3 (severe, involving >75% of glomeruli). Ischemic, collapsed glomeruli and glomeruli with >50% sclerosis should not be scored; ptc, peritubular capillaritis, scored as 0 (minimal, with <3 leukocytes in the most severely involved cortical PTC and/or leukocytes in <10% of cortical PTCs), 1 (mild, with ≥1 leukocyte in ≥10% of cortical PTCs AND 3‐4 leukocytes in the most severely involved PTC), 2 (moderate, as grade 1 but with 5‐10 leukocytes in most severely involved PTC), 3 (severe, as grades 1‐2 but with >10 leukocytes in most severely involved cortical PTC). The extent of PTC inflammation should be documented as focal (10%‐50% of cortical PTCs) or diffuse (>50%). Note that medullary capillaries are NOT scored; C4d, linear staining in PTCs or medullary vasa recta by immunofluorescence (IF) on frozen sections of fresh tissue or immunohistochemistry (IHC) on formalin‐fixed, paraffin‐embedded tissue, scored as 0 (none), 1 (minimal, staining in >0 but <10% of PTCs), 2 (focal, 10%‐50% of PTCs), 3 (diffuse, >50% of PTCs). By IF on frozen sections scores ≥2 are considered positive; by IHC on paraffin sections all scores >0 are considered positive; ci, interstitial fibrosis in cortex, scored as 0 (minimal, ≤5%), 1 (mild 6%‐25%), 2 (moderate, 26%‐50%), 3 (severe, >50%). The subcapsular cortex should not be scored; ct, tubular atrophy in cortex, scored as 0 (none), 1 (mild, 1%‐25%), 2 (moderate, 26%‐50%), 3 (severe, >50%). The subcapsular cortex should not be scored; cv, arterial intimal fibrosis (fibrointimal thickening), scored as 0 (none), 1 (mild, present but with ≤25% narrowing of luminal area in the most involved artery), 2 (moderate, 26%‐50% luminal narrowing), 3 (severe, >50% luminal narrowing); cg, chronic glomerulopathy (transplant glomerulopathy), scored as 0 (none, no GBM double contours by light microscopy [LM] or EM), 1a (early mild, no GBM double contours by LM but subendothelial neo‐densa in ≥3 glomerular capillaries by EM with associated endothelial cell enlargement and/or subendothelial electron‐lucent widening, 1b (mild, GBM double contours by LM in 1%‐25% of glomerular capillaries by LM in the most severely involved glomerulus), 2 (moderate, double contours by LM in 26%‐50% of capillaries), 3 (severe, double contours by LM in >50% of capillaries). Ischemic, collapsed glomeruli and glomeruli with >50% sclerosis should not be scored; ptcml, peritubular capillary basement membrane multilayering (requires EM), scored as 1 (≥7 basement membrane layers in the most affected PTC AND ≥5 layers in two additional PTCs), 0 (not meeting these criteria), or NA if EM is not performed; ti, total cortical inflammation, including scarred and non‐scarred cortex, scored as 0 (absent/minimal, <10%), 1 (mild, 10%‐25%), 2 (moderate, 26%‐50%), 3 (severe, >50%); i‐IFTA, inflammation in scarred cortex, scored as 0 (absent/minimal, <10% of non‐scarred cortex inflamed OR if the extent of cortical IFTA is <10%), 1 (mild, 10%‐25% of scarred cortex inflamed), 2 (moderate 26%‐50% of scarred cortex inflamed), 3 (severe, >50% of scarred cortex inflamed). The subcapsular cortex should not be considered; t‐IFTA, tubulitis in tubules within scarred cortex, scored as 0 (none), 1 (mild, 1‐4 mononuclear leukocytes per tubular cross‐section or 10 tubular epithelial cells in most severely involved tubule), 2 (moderate, 5‐10 mononuclear leukocytes), 3 (severe, >10 mononuclear leukocytes). Severely atrophic tubules should NOT be scored; pvl, intrarenal polyomavirus load level, defined by the overall fraction of tubules in the entire biopsy (cortex and medulla) with at least 1 epithelial cell showing a viral inclusion body by light microscopy OR nuclear staining for SV40 large T antigen by IHC. As IHC is more sensitive, proper pvl scoring requires IHC. Scored as 0 (none), 1 (mild, positive cells in ≤1% of tubules), 2 (moderate, >1% and <10%), 3 (severe, ≥10%). The pvl score in combination with the ci score is used to define the PVN class (Table 4).
3. CLARIFICATION AND UPDATES TO THE BANFF 2017 CLASSIFICATION REVISIONS
The Banff 2019 kidney sessions focused on reviewing the impact of the changes to the diagnostic criteria for both TCMR and ABMR that were made at the 2017 Banff conference. 5 For TCMR, this involved mainly the implementation of CA TCMR: The extent to which this category is being used at different centers around the world, potential confusion regarding the wording of diagnoses within this category, how frequently patients with CA TCMR were being treated, and the efficacy of those treatments. The threshold for the interstitial inflammation score (i) in the diagnosis of borderline for acute TCMR, which has not been applied uniformly by different pathologists 7 and been the subject of recent investigation 8 , 9 was also discussed. For ABMR, discussions centered around potential simplification of the diagnostic criteria, including clarifying the description of activity and chronicity components within the category of CA ABMR—making the resultant report more intelligible and useful to treating clinicians. 10 Standardized reporting for TCMR and ABMR diagnoses were discussed, including the inclusion of individual Banff acute and chronic scores (i, t, g, cg, and so on) used in determining the subtype, activity. and chronicity and explanations of these scores. 1 , 7 The clinical implications of MVI in the absence of (DSAs with and without C4d deposition were also presented). Final consensus recommendations were aided by questionnaires distributed to pathologists, nephrologists, and transplant surgeons attending the 2 kidney‐specific sessions during and immediately after the meeting (Table 6).
Table 6.
Survey results on reporting of chronic active T cell–mediated rejection (CA TCMR), chronic active antibody‐mediated rejection (CA ABMR), and Banff i threshold for borderline (BL) lesions
| Preferred diagnosis wording | |||||
|---|---|---|---|---|---|
| CA TCMR | CA TCMR + borderline or acute TCMR | CA TCMR +active component meeting criteria for BL or acute TCMR | CA TCMR | Acute TCMR+ moderate inflammed IFTA | Other |
| i1 t2 ti2 iIFTA3 ci2 ct2 +noMVI | 46% | 33% | 20% | 0% | 1% |
| i2 t2 ti2 iIFTA3 ci2 ct2 + noMVI | 61% | 21% | 5% | 12% | 1% |
| i1 t2 ti2 iIFTA3 ci2 ct2 v1 + noMVI | 70% | 12.5% | 6.5% | 10% | 1% |
| CA ABMR | Current wording: CA ABMR | CA ABMR +specify g and ptc scores | CA ABMR +mild/moderate/severe activity/chronicity | ABMR +mild/moderate/severe activity/chronicity | Other |
| 14% | 26% | 27% | 29% | 4% | |
| Preferred threshold | |||||
|---|---|---|---|---|---|
| Borderline (suspicious) for acute TCMR | Inflammation in <10% of nonsclerotic cortex (i0 t > 0) | Inflammation in 10%‐24% of nonsclerotic cortex (i1 t > 0) | Inflammation in 5%‐24% of nonsclerotic cortex (with t > 0) | ||
| 15% | 82% | 3% | |||
This table summarizes the answers obtained from a survey distributed to Banff attendees during or immediately after the meeting. These results rely on answers from 69 pathologists and clinicians regarding preferred wording in situation of CA TCMR, and CA ABMR and regarding preferred threshold for borderline for acute TCMR.
Abbreviations: BL, borderline for acute TCMR; CA ABMR, chronic active antibody‐mediated rejection; IFTA, interstitial fibrosis and tubular atrophy; MVI, microvascular inflammation.
3.1. Chronic active TCMR criteria clarifications
To assess whether transplant centers worldwide were applying the 2017 Banff classification and diagnosing CA TCMR, we enlisted the assistance of The Transplantation Society (TTS) and the CyberNephrology online forum (Dr Kim Solez) in circulating a survey that was answered by 128 TTS members, including clinicians and pathologists, from six different continents before the meeting (Figure 1). Notably, pathologists at ~90% of surveyed centers diagnosed CA TCMR, and at >80% of these centers at least some patients with this diagnosis were treated with steroids or other immunosuppressive agents. However, those clinicians that do not routinely treat patients with CA TCMR (52/81, 64%) indicated a need for more definitive data on the efficacy of CA TCMR treatment, and a minority (22/81, 27%) felt that the risk of treatment outweighs the benefits. Two points that should be emphasized regarding CA TCMR are that inflammation in areas of the cortex with interstitial fibrosis and tubular atrophy (i‐IFTA), while an essential component of CA TCMR, are not sufficient to make this diagnosis, which also requires (1) at least a moderate degree of total cortical inflammation and (2) moderate tubulitis involving cortical tubules other than severely atrophic tubules (Table 4). i‐IFTA itself is not a specific lesion and is seen in the context of tissue injury due to many causes other than TCMR, including BK virus nephropathy and ABMR. 11 According to Banff 2017, 5 the tubulitis allowed for the diagnosis of CA TCMR may be within or outside of scarred areas. However, for clarity and future investigation we suggest that tubulitis be independently scored (providing that severely atrophic tubules are not scored) both in areas of preserved cortex (Banff t score) and within (Banff t‐IFTA score) areas of cortical IFTA, as defined in Table 5. This may enable investigators and clinicians to determine the impact on treatment response and graft survival of these different forms of tubulitis. An important concern expressed by some clinicians and pathologists is that the Banff 2017 diagnosis of CA TCMR does not discriminate between cases where all or nearly all inflammation is limited to i‐IFTA vs those instances where coexistent i‐IFTA and inflammation within nonscarred areas meets criteria for additional borderline acute TCMR or acute TCMR grade IA or IB diagnoses. Questionnaire responses from meeting attendees clearly replied that reporting of “mixed” CA TCMR with grade IA or IB acute TCMR could be improved by indicating separately when biopsies simultaneously met criteria for borderline for acute TCMR or acute TCMR grade IA or IB (Table 6). Likewise, participants agreed that when intimal arteritis was present in addition to CA TCMR, this should also be specifically reported as a separate reported diagnosis. These suggestions pertain only to CA TCMR reporting and the actual histologic criteria for this diagnosis remain unchanged (Tables 4 and 5). Nankivell et al 12 reported that both development of i‐IFTA and clearance of inflammation from scarred areas were dependent of the efficacy of immunosuppressive therapy. These data support the 2017 Banff criteria for CA TCMR, but also favor indicating the level of active inflammation in nonscarred cortex.
Figure 1.
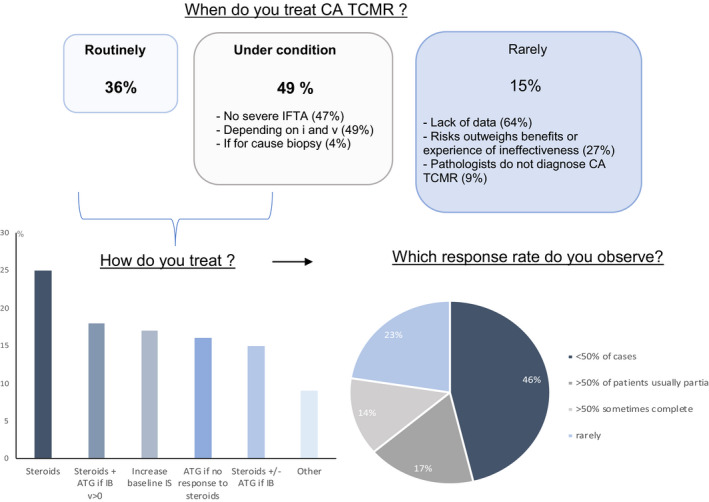
Responses to TTS survey regarding the management of chronic active (CA) TCMR: of the 128 respondents to this survey, 47 (37%) were from Europe, 26 (20%) from Asia, 23 (18%) from the United States and Canada, 18 (14%) from Latin America, 9 (7%) from the Middle East and Africa, and 5 (4%) from Australia and New Zealand. Sixty‐five percent of centers performed <100 renal transplantations annually; 12% performed >200. At all but 12% of centers, biopsies were read by a renal or transplant pathologist
3.2. Borderline (suspicious) for acute TCMR criteria clarifications
The Banff ’97 criteria for diagnosis of borderline for acute TCMR required inflammation in at least 10% of nonscarred cortex (Banff i1) in addition to tubulitis. 13 However, subsequent iterations of the Banff classification (1) accepted tubulitis with minimal inflammation (isolated t; i0 t > 0) as sufficient for a diagnosis of borderline, and a recent survey showed variability among pathologists with regard to which threshold was used for this diagnosis. 7 More recently, longitudinal studies of Nankivell et al 9 showed no effect of isolated t lesions on graft outcomes, concluding that the original Banff ’97 threshold for borderline lesions should be applied, and 82% (57/69) of pathologists and clinicians (nephrologists and transplant surgeons) responding to the questionnaire distributed at the 2019 Banff conference agreed (Table 6). Consensus was therefore achieved that the diagnostic threshold for borderline for acute TCMR should read as follows: “interstitial inflammation involving 10%‐25% of nonsclerotic cortex (Banff i1) with at least mild tubulitis (t > 0).” The minimum lesion for a borderline diagnosis is thus i1t1. As with previous versions of the Banff classification, 1 , 13 lesions characterized by mild tubulitis (Banff t1) with inflammation involving >25% of nonsclerotic cortex (Banff i2/3) and no intimal arteritis are also included in the borderline category (Table 4). However, biopsies with tubulitis (t > 0) but without inflammation in at least 10% of nonsclerotic cortex (i0) are no longer included in this category, and neither are cases with inflammation in the absence of tubulitis (i > 0, t0). These are simply reported in the microscopic description of the biopsy.
3.3. ABMR criteria clarifications
Since the introduction of ABMR into the Banff classification in 2003, 14 the criteria for diagnosis of ABMR have become more complex, with major modifications in 2013 15 and 2017, 5 which have improved diagnostic sensitivity and predictive value for graft outcomes. 16 A major residual issue within the classification is that it still subclassifies ABMR into active or chronic active and chronic inactive (ie, transplant glomerulopathy without MVI and with current or historic DSA; Table 4) subtypes as opposed to representing the diverse morphologic and molecular lesions at different posttransplant time points in antibody‐mediated tissue injury. 17 , 18 In particular, the category of CA ABMR encompasses lesions with severe activity and mild chronicity (eg, g3 ptc3 cg1 ci0 ct0 C4d3), those with mild activity and severe chronicity (eg, g1 ptc1 cg3 ci3 ct3 C4d0), and intermediate cases. Thus a simple diagnosis of CA ABMR gives treating clinicians limited information for appropriate treatment options. Although the addition of individual Banff lesion scores to the diagnostic line of the report is interpretable by experienced clinicians, it may confuse some clinicians who are unfamiliar with subtleties of the Banff system. This concern was highlighted by the recent work from the BWG on highly sensitized patients who found that the classification was interpreted in a highly variable way with implications for resultant therapeutic decision making. 10 , 19 Potential improvements in the wording of the pathologic diagnoses in standard reports were discussed, which remained within the context of Banff 2017 to optimally convey the activity and chronicity levels of CA ABMR cases. For example, in lupus nephritis, 20 the previous designations of active (A), active and chronic (A/C), and chronic (C) were replaced with a revised version of the old National Institutes of Health (NIH) activity and chronicity indices in a more granular fashion. However, the applicability of this approach to transplantation remains to be validated. Questionnaire respondents were also asked to select the optimal wording of the current Banff 2017 ABMR diagnosis from several options detailing activity and chronicity information, and how to best incorporate relevant Banff lesion scores into the final report. The option preferred by 56% of respondents was to word the diagnosis line as follows: “(chronic) active ABMR with [mild, moderate, severe] activity and [mild, moderate, severe] chronicity” and include the Banff lesion scores in the text of the diagnostic line for the biopsy report or in a table (eg, Tables 5 and 6). However, the definitions and optimal thresholds for mild, moderate, and severe still need to be validated in future studies, and as such, it remains at the discretion of individual pathologists whether to adopt such wording at this time or simply provide the Banff lesion scores. It should also be emphasized that these suggestions pertain only to the CA ABMR reporting language, and the actual histologic criteria for diagnosis of CA ABMR, as specified in Banff 2017, remain unchanged. 5
3.4. Microvascular inflammation in the absence of detectable DSAs
At the Banff 2017 conference, updates to the ABMR classification were made to help with decision‐making in cases where a biopsy showed at least moderate MVI ((g + ptc) >2) but no detectable antibodies against the graft. 5 However, there are considerable numbers of biopsies with (g + ptc) >2 in the absence of detectable anti‐HLA DSAs or C4d, and with molecular ABMR testing not widely adopted at this point, the clinical implications of these findings remain unclear. Recent evidence has suggested that patients with MVI on biopsies performed early posttransplant in the absence of DSAs had significantly better graft survival than those with anti‐HLA DSAs (mainly low‐level, preexisting DSAs), regardless of the presence or absence of PTC C4d staining on the biopsy. 21 DSA+ patients were more frequently retransplant recipients and had C4d+ biopsies. By contrast, studies of patients biopsied much later with more severe MVI and frequent transplant glomerulopathy (TG) showed no difference in graft survival in the presence or absence of anti‐HLA DSAs, which in these studies were de novo DSAs. 22 , 23 Thus, at this point, we are unable to draw significant conclusions regarding the significance of biopsies showing (g + ptc >2) in the absence of anti‐HLA DSAs, other than to comment that the biological behavior of such changes may well differ depending on whether these are seen relatively early posttransplant at a time when many DSAs represent persistent or recurrent/memory DSAs vs those seen later when most DSAs detected are de novo DSAs. In addition, the differential diagnosis of MVI with negative DSA and negative C4d should include T cell–mediated endothelial injury (ptc), membranoproliferative glomerulonephritis (g), and thrombotic microangiopathy (g). Furthermore, it has been shown recently in large populations that non‐HLA antibodies could be independently associated with risk of allograft loss. 24 These findings emphasize the importance of testing for clinically relevant non‐HLA antibodies in patients with biopsies showing MVI.
It is also hoped that implementation of molecular diagnostics could help classifying these cases in a more pathogenesis‐driven way, either employing the Molecular Microscope Diagnostic System (MMDx) 25 or the NanoString platform that can be used with formalin‐fixed, paraffin‐embedded tissue, 26 providing greater insight into factors predictive of the biological behavior of MVI without detectable anti‐HLA DSAs, as well as cases with C4d staining without MVI. 27 Current recommendations of the Banff Molecular Diagnostics Working Group in these and other circumstances are detailed in a separate meeting report. Presently, however, available data do not warrant making changes to the ABMR classification specifically relevant to these cases, beyond those made in Banff 2017. 5
4. ROLE OF ARTIFICIAL INTELLIGENCE, DATA INTEGRATION, AND MACHINE LEARNING IN THE BANFF CLASSIFICATION
During the 2019 Banff meeting, many projects using artificial intelligence (AI), machine learning (ML), as well as deep learning (DL) were presented, with a heavy focus on applying the Banff classification in an automated fashion, clustering of patients, and digital pathology. However, the field is still poorly understood by most clinicians and pathologists. Several speakers at the 2019 Banff meeting discussed the capabilities of AI and the potential for use of ML algorithms in diagnosis and personalized medication in organ transplantation (Table 7).
Table 7.
Feasible applications of artificial intelligence in the Banff classification
| Type | Fields of usage | Popular algorithms |
|---|---|---|
| Image recognition | Digital pathology | CNN, ResNet, VGG, etc |
| NLP | Meta‐analysis | SyntaxNet, transfer learning, SVM, naive Bayes classifier, etc |
| Text mining | Meta‐analysis, automated report, report/web scraping | k‐means clustering, naive Bayes classifier, KNN, SVM, etc |
| Cluster pattern recognition | Gene expression, personalized medicine | Linkage algorithms, k‐means, DBSCAN, archetypal analysis, etc |
| Class prediction | Graft lost, response to therapy | Random forest, multinomial logistic regression, SVM, neural network, etc |
Abbreviations: CNN, convolutional neural network; DBscan, description density‐based spatial clustering of applications with noise; KNN, K nearest neighbors; NLP, natural language processing; SVM, support vector machine; VGG, visual geometry group.
Computer‐assisted digital pathology consists of analyzing whole slide digital images of biopsies by a machine, and remains a challenge even with most advanced image analyzing algorithms such as convolutional neural networks. At this point DL‐based histopathologic assessment of kidney tissue shows the ability to distinguish glomeruli, proximal and distal tubules, and areas of fibrosis. 28‐30 The current approach is promising but still shows high variability and poor correlation with clinical outcomes. A BWG on Digital Pathology has been formed at the 2019 Banff meeting to standardize scanning and analytical practices through facilitating studies focusing on IFTA and
inflammation scoring while using algorithms fitting the most current Banff classification to decrease inter‐observer variability. Because personalized medicine has been a major focus in recent years, utilizing algorithms and archetype analysis has the potential to combine biopsy results with clinical and laboratory data and to provide a more complete pathologic and prognostic picture, especially in the era of expanding electronic medical records. 31 Furthermore, A. Loupy presented the capability of natural language processing (NLP) to help such algorithms assist clinicians and reduce human errors. NLP could be used for an otherwise time‐consuming meta‐analysis or accelerated reading of large numbers of pathology reports. Finally, since the Banff rules are becoming increasing complex with numerous possible scenarios, there is increasing demand for an automated coding of Banff rules. This would require integration of experienced pathologists to decode the Banff rules and computer/data scientists to create an algorithm to apply the Banff rules. Key to achieving quality results from ML‐based diagnosis are the integration of multiple centers providing quality input data to construct a standardized and reproducible analytical process.
5. CONCLUSIONS
The international consensus‐building framework provided by the Banff process is ongoing. Much has been done in the field of diagnostic assessment of allograft pathology, but many issues remain to be studied further. Although our experience in continually updating the diagnostic criteria for renal allograft rejection and related lesions has improved diagnostic accuracy and clinicopathologic correlations, 16 this experience has also helped clarify the limits of histology and immunohistology in renal allograft biopsy interpretation and emphasized the need for development of additional diagnostic modalities, including molecular diagnostics. Current recommendations regarding the further clinical validation and use of the latter are detailed in a separate meeting report; this and other themes will also be addressed at the XVI. Banff meeting, which will be held jointly with the Canadian Society of Transplantation in Banff, Canada, October 4‐7 2021, celebrating the 30‐year anniversary of the Banff classification at the original location of its inception.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Michael Mengel received honoraria from Novartis, CSL Behring, and Vitaeris. Mark Haas received consulting fees from Shire ViroPharma, AstraZeneca, Novartis, and CareDx, and honoraria from CareDx. Denis Glotz received honoraria from Sanofi and CSL Behring and consulting fees from BMS and Atara. Roslyn Mannon has received grant funding from CSL Behring, CareDX, Vitaeris, Astellas, and Transplant Genomics, and honoraria from Hansa, Novartis, CSL Behring, and Vitaeris. Robert Colvin is a consultant for Shire ViroPharma, CSL Behring, Alexion, and eGenesis. AJ Demetris is consultant for Novartis and TransMedics, Robert Montgomery received honoraria, consulting fees, or travel expenses from Alexion, Hansa Medical, CSL Behring, Sanofi, Novartis, Viela Bio, Vitaeris Bio, Terasaki Foundation, and Shire/Takeda.
ACKNOWLEDGMENTS
Members of the EM BWG: Amanda Kan, Anne Raisanen‐Sokolowski, Chris Bellamy, Catherine Horsfield, Deb Schady, David Buob, Finn P Reinholt, George Liapis, Ibrahim Batal, Bela Ivanyi, Johan Molne, Marlene Praet, Paisit Paueksakon, Patricia Revelo, Praveen Chander, Rafael Maldonado, Surya V. Seshan, Verena Broecker, Yasemin Ozluk, Nicholas Kozakowski, Linda Moran, Yong Mee Cho, Luan Truong, Christin Vanbeek, John Brealey, Antonella Barreca, Amit Dinda, Geetika Singh, Joris Roelofs, Alexei Mikhailov, Shana Coley. The 2019 Banff meeting received sponsorship from: CareDx, CSL Behring, Elsevier, Eppendorf, GenDx, Hansa Biopharma, Histogenetics, Immucor, Omion, OneLambda, NanoString, Novartis, Takeda, Veloxis, and Vitaeris.
Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell– and antibody‐mediated rejection. Am J Transplant. 2020;20:2318–2331. 10.1111/ajt.15898
Contributor Information
Alexandre Loupy, Email: alexandre.loupy@inserm.fr.
Mark Haas, Email: mark.haas@cshs.org.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary material.
REFERENCES
- 1. Loupy A, Haas M, Solez K, et al. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17(1):28‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loupy A, Aubert O, Orandi BJ, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ. 2019;366:l4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kozakowski N, Herkner H, Böhmig GA, et al. The diffuse extent of peritubular capillaritis in renal allograft rejection is an independent risk factor for graft loss. Kidney Int. 2015;88:332‐340. [DOI] [PubMed] [Google Scholar]
- 4. Kozakowski N, Herkner H, Eskandary F, et al. An integrative approach for the assessment of peritubular capillaritis extent and score in low‐grade microvascular inflammation – associations with transplant glomerulopathy and graft loss. Nephrol Dial Transplant. 2019;34:166‐217. [DOI] [PubMed] [Google Scholar]
- 5. Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell‐mediated rejection, antibody‐mediated rejection, and prospects for integrative endpoints for next‐generation clinical trials. Am J Transplant. 2018;18(2):293‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nickeleit V, Singh HK, Randhawa P, et al. The Banff Working Group classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. JASN. 2018;29(2):680‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker JU, Chang A, Nickeleit V, Randhawa P, Roufosse C. Banff borderline changes suspicious for acute T cell‐mediated rejection: where do we stand? Am J Transplant. 2016;16(9):2654‐2660. [DOI] [PubMed] [Google Scholar]
- 8. Nankivell BJ, Agrawal N, Sharma A, et al. The clinical and pathological significance of borderline T cell‐mediated rejection. Am J Transplant. 2019;19(5):1452‐1463. [DOI] [PubMed] [Google Scholar]
- 9. Nankivell BJ, P’Ng CH, Chapman JR. Does tubulitis without interstitial inflammation represent borderline acute T cell mediated rejection? Am J Transplant. 2019;19(1):132‐144. [DOI] [PubMed] [Google Scholar]
- 10. Schinstock CA, Sapir‐Pichhadze R, Naesens M, et al. Banff survey on antibody‐mediated rejection clinical practices in kidney transplantation: diagnostic misinterpretation has potential therapeutic implications. Am J Transplant. 2019;19(1):123‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halloran PF, Matas A, Kasiske BL, Madill‐Thomsen KS, Mackova M, Famulski KS. Molecular phenotype of kidney transplant indication biopsies with inflammation in scarred areas. Am J Transplant. 2019;19(5):1356‐1370. [DOI] [PubMed] [Google Scholar]
- 12. Nankivell BJ, Shingde M, Keung KL, et al. The causes, significance and consequences of inflammatory fibrosis in kidney transplantation: the Banff i‐IFTA lesion. Am J Transplant. 2018;18(2):364‐376. [DOI] [PubMed] [Google Scholar]
- 13. Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713‐723. [DOI] [PubMed] [Google Scholar]
- 14. Racusen LC, Colvin RB, Solez K, et al. Antibody‐mediated rejection criteria ‐ an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708‐714. [DOI] [PubMed] [Google Scholar]
- 15. Haas M. The revised (2013) Banff classification for antibody‐mediated rejection of renal allografts: update, difficulties, and future considerations. Am J Transplant. 2016;16(5):1352‐1357. [DOI] [PubMed] [Google Scholar]
- 16. De Serres SA, Noël R, Côté I, et al. 2013 Banff criteria for chronic active antibody‐mediated rejection: assessment in a real‐life setting. Am J Transplant. 2016;16(5):1516‐1525. [DOI] [PubMed] [Google Scholar]
- 17. Bagnasco SM, Zachary AA, Racusen LC, et al. Time course of pathologic changes in kidney allografts of positive crossmatch HLA‐incompatible transplant recipients. Transplantation. 2014;97(4):440‐445. [DOI] [PubMed] [Google Scholar]
- 18. Haas M. The relationship between pathologic lesions of active and chronic antibody‐mediated rejection in renal allografts. Am J Transplant. 2018;18(12):2849‐2856. [DOI] [PubMed] [Google Scholar]
- 19. Mannon RB. The Banff schema for antibody‐mediated rejection: lost in translation? Am J Transplant. 2019;19(1):9‐10. [DOI] [PubMed] [Google Scholar]
- 20. Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789‐796. [DOI] [PubMed] [Google Scholar]
- 21. Senev A, Coemans M, Lerut E, et al. Histological picture of antibody‐mediated rejection without donor‐specific anti‐HLA antibodies: clinical presentation and implications for outcome. Am J Transplant. 2019;19(3):763‐780. [DOI] [PubMed] [Google Scholar]
- 22. Sablik KA, Clahsen‐van Groningen MC, Looman CWN, et al. Chronic‐active antibody‐mediated rejection with or without donor‐specific antibodies has similar histomorphology and clinical outcome ‐ a retrospective study. Transpl Int. 2018;31(8):900‐908. [DOI] [PubMed] [Google Scholar]
- 23. Parajuli S, Redfield RR, Garg N, et al. Clinical significance of microvascular inflammation in the absence of anti‐HLA DSA in kidney transplantation. Transplantation. 2019;103(7):1468‐1476. [DOI] [PubMed] [Google Scholar]
- 24. Lefaucheur C, Viglietti D, Bouatou Y, et al. Non‐HLA agonistic anti‐angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody‐mediated rejection in kidney transplant recipients. Kidney Int. 2019;96(1):189‐201. [DOI] [PubMed] [Google Scholar]
- 25. Reeve J, Böhmig GA, Eskandary F, et al. Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Am J Transplant. 2019;19(10):2719‐2731. [DOI] [PubMed] [Google Scholar]
- 26. Adam B, Afzali B, Dominy KM, et al. Multiplexed color‐coded probe‐based gene expression assessment for clinical molecular diagnostics in formalin‐fixed paraffin‐embedded human renal allograft tissue. Clin Transplant. 2016;30(3):295‐305. [DOI] [PubMed] [Google Scholar]
- 27. Dominy KM, Willicombe M, Al Johani T, et al. Molecular assessment of C4d‐positive renal transplant biopsies without evidence of rejection. Kidney Int Rep. 2019;4(1):148‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marsh JN, Matlock MK, Kudose S, et al. Deep learning global glomerulosclerosis in transplant kidney frozen sections. IEEE Trans Med Imaging. 2018;37(12):2718‐2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lituiev DS, Cha SJ, Chin A, et al. Automated localization and segmentation of mononuclear cell aggregates in kidney histological images using deep learning. medRxiv. 2019;19002634 10.1101/19002634 [DOI] [Google Scholar]
- 30. Hermsen M, de Bel T, den Boer M, et al. Deep learning‐based histopathologic assessment of kidney tissue. J Am Soc Nephrol. 2019;30(10):1968‐1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aubert O, Higgins S, Bouatou Y, et al. Archetype analysis identifies distinct profiles in renal transplant recipients with transplant glomerulopathy associated with allograft survival. J Am Soc Nephrol. 2019;30(4):625‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary material.


