Abstract
Recent genome-wide studies have begun to identify gene variants, expression profiles, and regulators associated with neuroticism, anxiety disorders, and depression. We conducted a set of experimental cell culture studies of gene regulation by micro RNAs (miRNAs), based on genome-wide transcriptome, proteome, and miRNA expression data from twenty postmortem samples of lateral amygdala from donors with known neuroticism scores. Using Ingenuity Pathway Analysis and TargetScan, we identified a list of mRNA–protein–miRNA sets whose expression patterns were consistent with miRNA-based translational repression, as a function of trait anxiety. Here, we focused on one gene from that list, which is of particular translational significance in Psychiatry: synaptic vesicle glycoprotein 2A (SV2A) is the binding site of the anticonvulsant drug levetiracetam ((S)-α-Ethyl-2-oxo-1-pyrrolidineacetamide), which has shown promise in anxiety disorder treatments. We confirmed that SV2A is associated with neuroticism or anxiety using an original GWAS of a community cohort (N = 1,706), and cross-referencing a published GWAS of multiple cohorts (Ns ranging from 340,569 to 390,278). Postmortem amygdala expression profiling implicated three putative regulatory miRNAs to target SV2A: miR-133a, miR-138, and miR-218. Moving from association to experimental causal testing in cell culture, we used a luciferase assay to demonstrate that miR-133a and miR-218, but not miR-138, significantly decreased relative luciferase activity from the SV2A dual-luciferase construct. In human neuroblastoma cells, transfection with miR-133a and miR-218 reduced both endogenous SV2A mRNA and protein levels, confirming miRNA targeting of the SV2A gene. This study illustrates the utility of combining postmortem gene expression data with GWAS to guide experimental cell culture assays examining gene regulatory mechanisms that may contribute to complex human traits. Identifying specific molecular mechanisms of gene regulation may be useful for future clinical applications in anxiety disorders or other forms of psychopathology.
Subject terms: Molecular neuroscience, Medical genetics
Introduction
Neuroticism is a heritable personality trait1–4 that shares genetic overlap with anxiety and depression5–10. Large-scale genome-wide association studies (GWAS) have identified single nucleotide variants (SNPs) associated with neuroticism, anxiety disorders, and depression10–12. Genome-wide expression profiling studies of human brain have begun to identify genes that are differentially expressed as a function of major depressive disorder13 or that affect brain structures14. Recent work has begun to identify epigenetic regulators of gene expression as potentially useful biomarkers and therapeutic agents in depression15–19.
One gene regulatory mechanism of interest with potential therapeutic applications involves microRNAs (miRNAs), which are short (20–23 nucleotides in length), single-stranded, endogenous RNAs that interact with mRNA to regulate gene expression through mechanisms that include mRNA transcript degradation and downregulation of translation of mRNA-to-protein through translational repression20,21. Each miRNA can regulate hundreds of genes and affects multiple cellular processes relevant to health and disease including psychiatric and neurological disorders19,22,23.
Dysregulation of miRNAs in amygdala has been linked to anxiety-related behaviors in rodent studies24–27. Human postmortem studies reported elevated expression of miR-155p5 in the amygdala of children with autism spectrum disorder28, and decreased expression of miRNA miR-137 in carriers of a schizophrenia risk allele in several brain regions including amygdala29. Reviewers have noted a need for a deeper mechanistic understanding of miRNA gene regulation and experimental miRNA manipulation to gauge therapeutic and biomarker potential23.
Here, we conducted an experimental analysis of miRNA regulation of the synaptic vesicle glycoprotein 2A (SV2A) gene by three putative miRNAs (miR-133a, miR-138, miR-218) in cell culture, based on pilot data we had obtained from a study of postmortem amygdala from donors with known neuroticism phenotypes that were cross-validated against a community-based exploratory neuroticism GWAS dataset, and against a published large-scale neuroticism GWAS dataset10. Each of the cell culture experiments was independently repeated three times for replication purposes (see “Methods” for details).
Materials and methods
Postmortem brain samples
Twenty brain donors were participants in the Religious Orders Study and Rush Memory and Aging Project (ROSMAP), a cohort study of common chronic conditions of aging that includes annual cognitive performance tests and clinical evaluations, multiple psychological assessments, and organ donation at the time of death, as described elsewhere30–34. Participants signed an informed consent and a Uniform Anatomical Gift Act and the study was approved by the institutional review board of Rush University Medical Center. Participants signed a repository consent that allows their data to be repurposed. The ROSMAP proteomics, mRNA, and miR data are available on the adknowledge portal (adknowledgeportal.synapse.org), an NIA-approved repository. All ROSMAP data can be requested at www.radc.rush.edu.
Assessment of neuroticism and trait anxiety
Self-reported neuroticism, specifically its sub-facet of trait anxiety, was based on the Revised NEO personality inventory, neuroticism facet 1: anxiety35. Individuals who scored in the top quartile had anxiety scores > 16 and were considered anxious, whereas those who scored in the bottom quartile had anxiety scores < 9 and were classified as non-anxious. Sample information is presented in Table 1.
Table 1.
Sample information, Postmortem amygdala.
| Anxious | Control | T-test/Chi square | |
|---|---|---|---|
| Sample size | 10 | 10 | |
| Anxiety Score (sd) | 18.1 (1.8) | 4.4 (3.5) | p < 2e-9 |
| Sex (Male) | 3 | 4 | ns |
| Age at Death in years (sd) | 88.2 (6.0) | 88.3 (8.5) | ns |
| PMI in hours (sd) | 7.0 (2.6) | 7.0 (2.4) | ns |
| Antidepressant use | 4 | 4 | ns |
| Anticonvulsant use | 3 | 5 | ns |
Trait anxious participants and non-anxious controls compared for gender, age at death, postmortem interval (PMI), antidepressant use, and anticonvulsant use.
sd standard deviation, ns non-significant p value > 0.05 for Student’s t test and Pearson Chi Square where applicable.
Global expression profiling of the proteome
Frozen human lateral amygdala nucleus samples were prepared with mass spectrometry compatible lysis buffer36 and quantified for protein yield. We performed shotgun proteomics analysis (MudPIT), as described elsewhere37–39. Briefly, the collected MS spectra were matched to a human protein database from UniProt (database released on January 06, 2012). A decoy database containing the reverse sequences of proteins from the UniProt database was appended to the target database to calculate and filter the results at the false discovery rate of 1% using SEQUEST in the Integrated Proteomics Pipeline (IP2, Integrated Proteomics Inc., CA)40,41. Over 2,000 high abundance proteins were identified robustly from each human sample by MudPIT analysis. The data were further integrated and normalized by Scaffold42 and relative quantification was derived by normalized spectra counts. Differential expression was defined as a fold change >|1.5| between anxious and control individuals and a Student’s t test p value < 0.05.
RNA extraction and mRNA/miRNA profiling
Total RNA was extracted from frozen amygdala tissue using the Qiagen miRNeasy Mini Kit with on-column DNAse treatment (Qiagen, Hilden, Germany). RNA quantity and purity were assessed using a Nanodrop Technologies ND-1000 instrument (NanoDrop Technologies, Wilmington, DE).
For mRNA microarray profiling, total RNA (100 ng) from each individual was prepared as described in the manufacturer’s protocol and analyzed using the Affymetrix U133 Plus 2.0 expression array (Affymetrix, Santa Clara, CA) at the Microarray Core Facility of Stony Brook University. Following washing and staining, arrays were scanned on an Affymetrix model 7G scanner. The scans were analyzed using Affymetrix GCOS. Raw image intensity files were loaded into GenePattern software and normalized using the Robust Multi-Array Average (RMA) method with quantile normalization. Differential expression was defined as a fold change >|1.5| between anxious and control individuals and t test p value < 0.05. Statistical analyses were conducted using IBM SPSS Statistics version 21.0
For miRNA microarray profiling, total RNA (1 µg) from each individual was prepared as described in the manufacturer’s protocol and analyzed using the Affymetrix GeneChip miRNA 1.0 Array (Affymetrix, Santa Clara, CA). Arrays were scanned and feature extraction was conducted using Affymetrix Command Console software. RMA normalization was conducted using the Affymetrix package of Bioconductor. We did not limit our analysis to human miRNA probes due to significant similarity in sequence between species and possibility for cross-hybridization on the microarray. Differential expression was defined as a fold change >|1.5| between anxious and control individuals and a t test p value < 0.05. Statistical analyses were conducted using IBM SPSS Statistics version 21.0.
Identification of mRNA–protein–miRNA sets
We used Ingenuity Pathway Analysis (IPA) (Qiagen, Hilden, Germany) to identify miRNA/protein pairs whose expression pattern across mRNA, miRNA, and protein levels of analysis suggested translational repression of mRNA by miRNAs. First, two lists of proteins and miRNAs, respectively, that were significantly differentially expressed as a function of trait anxiety (defined as a fold change >|1.5| and a Student’s t test p value < 0.05 between anxious and controls) were uploaded into IPA. IPA software then utilized the computational miRNA target prediction tool TargetScan (www.targetscan.org) to assign pairings between proteins and miRNAs, based on predicted or previously experimentally validated targeting relationships between miRNA and target mRNA. Since we focused on translational repression, we only included gene sets where a given protein and target miRNA showed an inverse expression relationship, and the protein-coding mRNA showed no expression change as a function of trait anxiety status.
GWAS neuroticism data
SNP data were available from the ROSMAP cohort who had completed a range of personality trait questionnaires. Details on GWAS data generation have been described previously43. Briefly, DNA for genotyping was collected from blood, lymphocytes or postmortem brain tissue. The majority of samples (N = 1,709) were genotyped on the Affymetrix GeneChip 6.0 platform and additional samples (N = 384) were genotyped on the Illumina OmniQuad Express platform. Imputation was performed by Alzheimer’s Disease Genetics Consortium on Michigan Imputation Server using the Haplotype Reference Consortium (HRC) reference panel (release 1.1). After quality control, the HRC imputed data were available in 2,182 ROSMAP participants. This dataset was used to identify significant SV2A SNPs associated with neuroticism and anxiety, based on a 10-item anxiety questionnaire (N = 1,706).
PLINK44 was used to test the effects of SNPs with minor allele frequency >1%, including imputed SNPs, to perform set-based tests for all SNPs that mapped to SV2A (±20 kilo-bases). The set-based parameters were r2 = 0.5, p value = 0.05, maximum number of SNPs = 5, and max (T) permutations = 10,000. SV2A SNPs identified by PLINK were also cross-referenced against published summary statistics10 from the meta-analysis and GWAS for neuroticism and worry, with samples ranging from 340,569 to 390,278 individuals.
SV2A luciferase assay and site-directed mutagenesis
Dual luciferase assays with an SV2A 3′ UTR clone (Genecopoeia, MD) were used to test the hypothesis that the SV2A 3′UTR is directly targeted by miR-133a, miR-138, and miR-218, as predicted by Targetscan. The full SV2A 3′UTR was cloned downstream of the firefly luciferase reporter gene in a dual luciferase (firefly/renilla) vector. Human embryonic kidney (HEK 293, Sigma-Aldrich, St. Louis, MI) cells under passage 10 were plated in 96-well plates at a density of 5 × 104 cells/well in medium containing Eagle’s Minimum Essential Medium (EMEM) (Life Technologies, Carlsbad, CA) + 2 mM glutamine (Life Technologies, Carlsbad, CA) + 1% non-essential amino acids (NEAA) (Life Technologies, Carlsbad, CA) +10% fetal calf serum (FCS) (Lonza, Allendale, NJ) and co-transfected with 100 ng of the SV2A-3′UTR vector and either 100 nM miRNA mimic (miR-133a, miR-218, or miR-138) or 100 nM of miRNA negative control mimic using the DharmaFECT Duo Transfection Reagent (Thermo Fisher Scientific, Waltham, MA). As a negative control, we included SV2A-3′UTR vector without miRNA co-transfection.
Transfection was accomplished following the Express Transfection protocol (Thermo Fisher Scientific, Waltham, MA) with 24 h incubation. Luciferase activity was measured using the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI) and the FLUOstarOptima microplate reader (BMG Laboratories, Ortenberg, Germany).
Transfection conditions were optimized using siGLO Green Transfection Indicator (Thermo Fisher Scientific, Waltham, MA). Each experiment was repeated independently three times, and each condition was tested in pentuplicate. Outliers were formally excluded according to Jacobs and Dinman45. In each pentuplicate measurement set, between 0 and 2 observations were excluded based on the above criteria. Statistical tests were conducted using one-way analysis of variance (ANOVA) with the Tukey post-hoc test.
To test for site-specific miRNA binding, we conducted site-directed mutagenesis using KOD Xtreme polymerase (Clontech Laboratories, Mountain View, CA). A Targetscan predicted binding site for miR-218 on the full length SV2A 3′UTR beginning at position 1051 after the stop codon was mutated from “AGCACA” to “ACGACA” as described elsewhere46, and a predicted miR-133a binding site beginning at position 38 after the stop codon was mutated from “GGACCAAA” to “GGAGGGAA” in a separate construct. Transfection in HEK 293 cells, which do not endogenously express miR-133/218, was performed as described above, co-transfecting 100 ng of the mutant construct along with 100 nM of the respective miRNA mimic. Each experiment was independently repeated three times, and each condition was repeated in pentuplicate. Statistical analysis of the firefly/renilla ratio followed exclusion of outliers as described above, excluding at most two observations per pentuplicate measurement set. Statistical tests were conducted using one-way ANOVA with the Tukey post-hoc test.
Neuroblastoma cell culture and transfection
To directly measure SV2A mRNA and protein levels as a function of miRNA regulation, 100 nM of miR-133a, miR-138, and miR-218 miRIDIAN miRNA mimics and inhibitors, 100 nM of negative control mimics and 100 nM of negative control inhibitor (Thermo Fisher Scientific, Waltham, MA) were transfected into human neuroblastoma SH-SY5Y cells (Sigma-Aldrich, St. Louis, MI) using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) for 24 h in order to assess changes in mRNA levels, and for 48 hours to assess changes in protein levels. SH-SY5Y cells below passage 10 were plated in six-well plates in pentuplicate (for mRNA analysis) and quadruplicate (for protein analysis) at a density of 5 × 105 cells/well in medium containing Ham’s F12:EMEM (1:1) (Life Technologies, Carlsbad, CA) +2 mM glutamine (Life Technologies, Carlsbad, CA) +1% NEAA (Life Technologies, Carlsbad, CA) +15% FCS (Lonza, Allendale, NJ). Cells were reverse-transfected according to the Lipofectamine 2000 protocol (Life Technologies, Carlsbad, CA). Amounts of lipofectamine and miRNA mimics and inhibitors were optimized using the siGLO Green transfection indicator (Thermo Fisher Scientific, Waltham, MA). Each experiment was independently repeated three times and each condition was repeated in pentuplicate for mRNA analysis and in quadruplicate for protein analysis.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA from miR-218, miR-133a, and miR-138 mimic and inhibitor transfected SH-SY5Y cells (see above) was collected using the Qiagen miRNeasy kit using on column DNase treatment according to manufacturer instructions (Qiagen, Hilden, Germany). Reverse transcription was accomplished using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) with an input of 250 ng of total RNA for each reaction. RT-qPCR was conducted using the QuantiTect SYBR Green kit with Uracil-N-Glycosylase (UNG) (Qiagen, Hilden, Germany) in a Roche Lightcycler 480 with the following cycling conditions: Step 1: UNG 2 min at 50 °C, Step 2: PCR initial activation 15 min at 95 °C, Step 3 (cycling): denaturation 15 s at 94 °C, annealing 30 s at 60 °C, extension 30 s at 72 °C, Step 4: melting curve analysis. RT-qPCR exon spanning primers were designed using Primer3 with an annealing temperature between 59 and 61 °C47,48. Primers were further controlled to specifically target regions which are present in all transcript isoforms using UCSC genome browser (http://genome.ucsc.edu). RT-qPCR normalization was conducted by geometric averaging of multiple internal control genes49. Eight housekeeping genes were evaluated for stability with the geNorm algorithm (Biogazelle, Gent, Belgium), identifying GUSB (glucuronidase, beta) and B2M (beta-2-microglobulin) as fitting controls for mRNA expression analysis in SH-SY5Y cells. GeNorm housekeeping gene selection is depicted in Supplementary Fig. 1, and the RT-qPCR Primer Table is found in Supplementary Table S1. Gene-specific amplification efficiencies were found using the specified procedure in GeNorm software (Biogazelle, Gent, Belgium), and RT-qPCR analysis was conducted using these gene-specific amplification efficiencies. Each experiment was repeated independently three times, each condition was repeated in pentuplicate in each experiment, and each RT-qPCR reaction was performed in triplicate. Outliers were formally excluded as described above, with at most one measurement excluded per pentuplicate set. Statistical tests were conducted using one-way ANOVA with the Tukey post-hoc test.
Quantitative immunoblotting
One hundred nM miR-218, miR-133a, and miR-138 mimics and inhibitors were transfected into SH-SY5Y cells using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) along with relevant controls as described above. Cells were incubated for 48 h in order to assess changes in SV2A protein levels. Protein was collected using the complete Lysis-M kit (Roche, Basel, Switzerland), and concentrations were established using the Bradford Protein Assay (Bio Rad, Hercules, CA). Quantitative immunoblotting was performed using 100 µg of protein from each condition, which was repeated in quadruplicate. A polyclonal rabbit SV2A antibody (CAT SC28955, Santa Cruz Biotechnology, Santa Cruz, CA) was used at a dilution of 1:100, along with a fluorescently tagged goat anti-rabbit secondary antibody (CAT 611-130-122, Rockland Immunochemicals, Gilbertsville, PA) at a dilution of 1:10,000. A monoclonal mouse β-actin antibody (CAT A4700, Sigma-Aldrich, St. Louis, MI) was used at a dilution of 1:200, along with a fluorescently tagged goat anti-mouse secondary antibody that was used at a dilution of 1:10,000 (CAT 610-131-121, Rockland Immunochemicals, Gilbertsville, PA). SV2A levels were normalized to β-actin to ensure equal loading, and mouse brain lysate served as a positive control to ensure the ability of the antibody to bind SV2A protein. Signal intensity was assessed with the Odyssey Infrared Imaging System, and band quantification was performed with Odyssey Infrared Imaging System Software according to manufacturer instructions (Li-Cor Biosciences, Lincoln, NE). Outliers were formally excluded as described above with at most one measurement excluded per quadruplicate set. Results from three independent experiments were included in the analysis of miR-133a and miR-218, and one independent experiment was done to confirm lack of targeting by miR-138. Statistical tests were conducted using one-way ANOVA with the Tukey post-hoc test.
Results
Identification of gene-miRNA sets
The integration of genome-wide expression data yielded 16 mRNA–protein–miRNA sets whose expression patterns were consistent with translational repression as a function of trait anxiety (Supplementary Table S2).
Figure 1 illustrates expression patterns for SV2A protein and mRNA, and three SV2A-targeting miRNAs. SV2A protein levels were lower in anxious individuals, compared to non-anxious controls (Fig. 1a) with an observed fold change of 1.94 and p < 0.05. However, there was no difference in the transcript level of SV2A between these two groups of individuals (Fig. 1b). Although SV2A mRNA levels did not differ significantly between the two groups, three SV2A-targeting miRNAs (predicted by TargetScan, see Methods), miR-133a, miR-138, and miR-218, were expressed at higher levels in anxious individuals (Fig. 1c).
Fig. 1. SV2A protein and mRNA levels in human postmortem lateral amygdala.
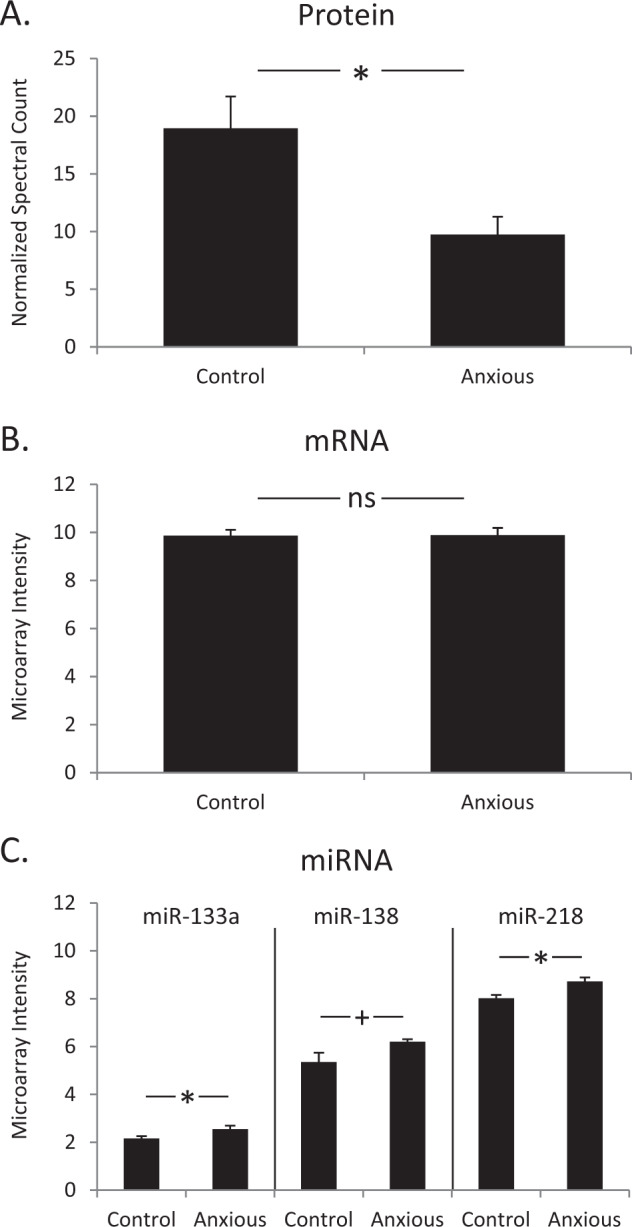
a Mean SV2A protein levels of anxious individuals compared to controls. Error bars are standard error of the mean (SEM), t-test p value < 0.05. b Mean SV2A mRNA levels do not differ significantly between controls and anxious individuals. Error bars are SEM, t-test p value = 0.87. c Significantly higher levels of the three miRNAs (miR-133a, miR-138, and miR-218) are found in anxious individuals, compared to controls. Error bars are SEM, *t-test p value < 0.05 for miR-133a and miR-218 expression in anxious individuals versus controls, and + t-test p value = 0.051 for miR-138 expression in anxious individuals versus controls.
SV2A in neuroticism GWAS
PLINK identified six SNPs that were significantly associated at nominal levels (p < 0.05) with self-reported anxiety in the ROSMAP cohort. Cross-referencing these results against a large-scale published GWAS summary dataset10, four of these SNPs were associated with neuroticism and/or worry (Table 2). This summary dataset contained an additional seventy-one SV2A SNPs that were significantly associated with neuroticism and/or worry (Supplementary Table S3).
Table 2.
Cross-referenced SV2A SNPs.
| Rush community sample | Nagel (2018): Neuroticism | Nagel (2018): Worry | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location (Chr:Start) | SNP RSID | P | N | A1 | A2 | EAF | MAF | Z | P | N | A1 | A2 | EAF | MAF | Z | P | N |
| 1:149862367 | rs6696191 | 0.012 | 1706 | A | G | 0.11 | 0.11 | 1.04 | 0.298 | 365,827 | A | G | 0.10 | 0.10 | −0.33 | 0.738 | 341,625 |
| 1:149885583 | rs577935 | 0.026 | 1706 | A | G | 0.10 | 0.10 | 0.19 | 0.853 | 388,944 | A | G | 0.10 | 0.10 | −0.73 | 0.467 | 346,980 |
| 1:149894445 | rs68144650 | 0.048 | 1706 | A | C | 0.92 | 0.08 | 3.29 | 0.001 | 372,058 | C | A | 0.08 | 0.08 | −2.86 | 0.004 | 347,418 |
| 1:149898951 | rs16836630 | 0.049 | 1706 | C | G | 0.08 | 0.08 | −3.69 | 0.000 | 389,720 | C | G | 0.08 | 0.08 | −2.92 | 0.004 | 347,693 |
| 1:149903122 | rs72692819 | 0.049 | 1706 | C | G | 0.08 | 0.08 | −3.10 | 0.002 | 372,568 | C | G | 0.08 | 0.08 | −2.73 | 0.006 | 347,902 |
| 1:149903609 | rs12078573 | 0.049 | 1706 | A | G | 0.08 | 0.08 | −3.24 | 0.001 | 388,508 | A | G | 0.08 | 0.08 | −2.24 | 0.025 | 346,526 |
| EAF: Effect Allele Frequency | min | 365,827 | min | 341,625 | |||||||||||||
| MAF: Minor Allele Frequency | max | 389,720 | max | 347,902 | |||||||||||||
EAF effect allele frequency, MAF minor allele frequency.
The SV2A 3′UTR is targeted in a site-specific manner by miR-133a and miR-218, but not miR-138
To test targeting of SV2A by specific miRNAs identified by genome-wide analysis, we conducted a transfection experiment in HEK 293 cells: transfection with miR-133a and miR-218, but not miR-138, significantly decreased relative luciferase activity from the SV2A dual luciferase construct (one-way ANOVA p < 0.05) (Fig. 2). Post-hoc Tukey tests showed a significant difference in relative luciferase activity between transfection with the SV2A construct only and co-transfection with both the SV2A construct and either miR-133a or miR-218 (p < 0.00001, for both miRNAs). There was no difference in relative luciferase activity resulting from sole transfection with the SV2A construct and co-transfection with the SV2A construct and the negative control mimic. To confirm that the predicted target sequences of miR-133a and miR-218 in the SV2A 3′UTR are functional, site-directed mutagenesis experiments were performed. Notably, neither miR-133a nor miR-218 could inhibit luciferase activity from the mutagenized SV2A construct, suggesting that the predicted sequences are genuine binding sites for the respective miRNAs.
Fig. 2. miR-218 and miR-133a but not miR-138 target the 3′UTR of SV2A.
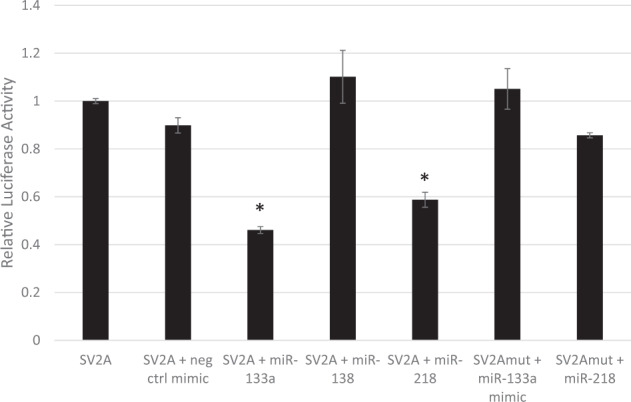
HEK 293 cells were transfected with the SV2A-3′UTR vector (“SV2A”) or SV2A-3′UTR mutant vector (“SV2Amut”) and respective miRNA mimics and incubated for 24 h. All firefly/renilla ratios are normalized to the SV2A 3′UTR clone. Error bars are SEM, *statistical significance is based on one-way ANOVA with Tukey post-hoc test, p value < 0.05 vs SV2A control.
Transfection with miR-133a and miR-218, but not with miR-138, leads to a reduction in endogenous SV2A mRNA and protein levels
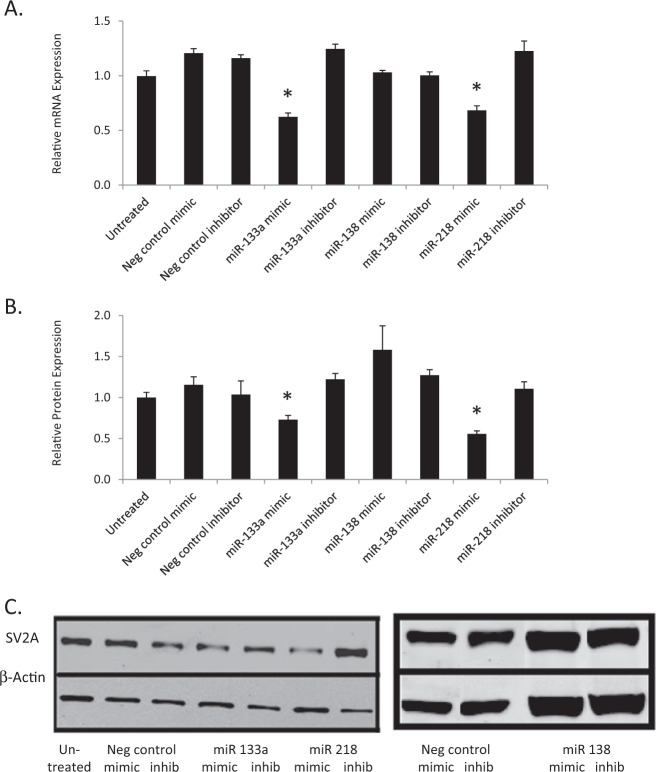
Given that luciferase assays revealed an interaction between the SV2A 3′UTR on the one hand, and miR-133a and miR-218 on the other, we subsequently investigated the effects of miRNA transfection on SV2A mRNA and protein levels in human neuroblastoma SH-SY5Y cells. Transfection with either miR-133a or miR-218, but neither with their respective inhibitors nor with miR-138, elicited a significant decrease in SV2A mRNA (Fig. 3a) and protein (Fig. 3b, c), compared to controls (one-way ANOVA p < 0.05, and Tukey post-hoc p < 0.05).
Fig. 3. Transfection with miR-133a and miR-218, but not with miR-138, decreases endogenous SV2A mRNA and protein levels in SH-SY5Y cells.
a Cells transfected with miR-133a and miR-218 mimics, but not miR-138 mimics or other controls, showed a significant decrease in SV2A mRNA levels. b Cells transfected with miR-133a and miR-218 mimics, but not miR-138 mimics or other controls, showed a significant decrease in SV2A protein levels. c The blot images are from representative immunoblots. Error bars are SEM, *statistical significance is based on one-way ANOVA with Tukey post-hoc test, p-value < 0.05 vs untreated control condition and mock transfection with negative control mimic.
Discussion
In this study, we leveraged results from a genome-wide expression analysis in postmortem human brain tissue with GWAS to identify a target gene associated with neuroticism and anxiety, and to study its regulation through miRNAs in cell culture. Specifically, we utilized postmortem amygdala samples from twenty donors with known trait anxiety levels to generate a candidate list of mRNA–protein–miRNA sets whose expression patterns were consistent with translational repression. Of the sixteen sets we found, we focused our cell culture experimental work on the SV2A gene, based on its translational potential from prior known associations with anxiety, anxiety disorders, and with epilepsy50–52. To address the limitation of small sample size, we confirmed an association for SV2A with neuroticism or anxiety in two GWAS sets: one was an original cohort sample of 1,706 individuals, and the other was a published summary dataset10 with samples ranging from 340,569 to 390,278 individuals.
There were three differentially expressed miRNAs as a function of trait anxiety (miR-133a, miR-138, and miR-218) predicted to target SV2A mRNA. We therefore conducted experimental studies of SV2A regulation ex vivo and observed that miR-133a and miR-218, but not miR-138, decreased relative luciferase activity of a SV2A dual-luciferase construct in a site-specific manner. Our observation in cell culture that these miRNAs reduced expression of SV2A at both the mRNA and protein level suggests transcript degradation, without excluding translational repression as a possible secondary process. By contrast, the postmortem data suggested translational repression only, given that there was no differential expression in mRNA levels. It is possible that the neuroblastoma cell culture system we used is sufficiently different from in vivo conditions that additional processes are engaged to activate transcript degradation in vitro. This is plausible, given that the result of a given miRNA-mRNA interaction can be affected by factors such as binding site accessibility, sequences flanking the miRNA target site and their context, as well as RNA secondary structure, and composition of the miRNA-mediated silencing complex (miRISC), among others53,54. Thus, the precise determinants for which mechanism is engaged in the human brain remain to be examined.
SV2A is one of three genes of the membrane glycoprotein SV2 and is expressed exclusively in neurons and endocrine cells55. SV2A is the most widely expressed isoform56 and is the only isoform that is expressed in many GABAergic, inhibitory neurons56,57. Rodent studies confirm a role in anxiety. Mice that lack SV2A develop severe seizures and die within 3 weeks of birth58, whereas mice heterozygous for one functional copy of Sv2a have a normal lifespan, but develop an anxiety-like phenotype50. Conditional knockout mice with decreased SV2A in hippocampus are free of epileptic seizures but show elevated levels of anxiety, as measured with the Elevated Plus Maze59. Genetically epilepsy-prone rats exhibit an anxiety phenotype across multiple measures prior to having developed epileptic seizures60.
Humans have higher levels of SV2A in the amygdala than in other tissues, based on genome-wide expression profiling data61,62. SV2A is the binding site of the anticonvulsant drug levetiracetam ((S)-α-Ethyl-2-oxo-1-pyrrolidineacetamide)52, which has shown promise in anxiety disorder treatment63–69, but also generates states of anxiety as a side-effect in some individuals70,71. Such individual differences may reflect underlying genetic variations, which is very plausible, given that multiple SV2A SNPs are associated with anxiety by GWAS. Furthermore, SV2A rs626785 produces differentially expressed splice variants in human amygdala (GTEx Analysis Release V8, dbGaP Accession phs000424.v8.p2, sQTL), suggesting additional gene regulatory processes linking anxiety and amygdala gene expression, and possibly epilepsy.
Epilepsy has also been linked to one of the miRNAs studied here. miR-218 (along with miR-204) was significantly down-regulated in hippocampal biopsies from patients diagnosed with mesial temporal lobe epilepsy (MTLE)/hippocampal sclerosis (HS), compared to postmortem controls72. Experimental manipulation of hippocampal activity was shown to inversely regulate miR-218 expression. Silencing of synaptic activity by tetrodotoxin increased miR-218 expression, whereas induction of sustained synaptic activity with the combination of the GABAA receptor antagonist bicuculline and the K + channel blocker 4-aminopyridine (BiC/4-AP) decreased miR-218 expression73. Future studies could be directed to assess levels of miR-133a and miR-218 as risk factors for epilepsy, and in epileptic patients as potential biomarkers for individual differences in response to levetiracetam.
An important limitation of the current work is that the postmortem dataset is based on a small sample of donor brains. Thus, the gene expression data remains to be validated in future work with larger samples. The association between SV2A and anxiety, however, is strengthened by leveraging available original and published GWAS data from large-scale samples. Finally, we did not adjust gene expression data for cell type, which could differ between the two postmortem anxiety groups.
Future directions could evaluate postmortem analysis of a larger dataset of mRNA and proteomic data from the lateral nucleus of the amygdala, as well as other amygdaloid nuclei, as well as other brain regions to determine the specificity of our amygdala results. This work could include histopathological immunolabelling analysis using different markers to identify neuronal sub-populations associated with high trait anxiety. Another line of work could embark on pre-clinical animal studies to examine any causal links between miRs 133a and 218, SV2A, and trait anxiety. Clinical studies of epileptic or anxiety-disordered patients would be useful to address individual differences in responses to the anticonvulsant drug levetiracetam ((S)-α-Ethyl-2-oxo-1-pyrrolidineacetamide)52, which is the binding site for SV2A, and for which both beneficial67–69 or detrimental74–80 effects on emotional states and psychopathology have been reported.
In conclusion, we used a postmortem dataset obtained from donors with known trait anxiety phenotypes to generate and identify a set of candidate genes whose expression pattern suggests translational repression by miRNAs. Cross-validation with large-scale GWAS data confirming an association with neuroticism helped us select one gene from this set, SV2A, and its associated differentially expressed three miRNAs (miR-133a, miR-138, and miR-218) for further study in cell culture. Although all three miRNAs were computationally predicted to target SV2A mRNA, this was experimentally confirmed only for miR-133a and miR-218. This study demonstrates the utility of integrating postmortem gene expression profiling with experimental cell culture studies to advance our understanding of gene-regulatory mechanisms related to human brain function and behavior.
Supplementary information
Acknowledgements
T.C. was supported by NSF BCS-0843346 and NIH R01AG034578. D.A.B. was supported by NIH R01AG17917, P30AG10161, R01AG15819, and R01AG36042. E.I.C. was supported by start-up funding from the Stony Brook University School of Medicine. The mass spectrometer used in this study was purchased by a shared instrument grant, National Institutes of Health/National Center for Research Resources S10 RR023680-1. We thank Dr. Yelena Altschuller in the Stony Brook Molecular Cloning Facility for conducting the site-directed mutagenesis.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-00966-4).
References
- 1.John, O. P. & Srivastava, S. The big five trait taxonomy: history, measurement, and theoretical perspectives. in (eds Pervin, L. A., John, O. P.). Handbook of Personality: Theory and Research, 2nd edn, 102–138 (The Guilford Press, New York, NY, 1999).
- 2.McCrae, R. R. & Costa P. T. Jr. Personality in Adulthood: A Five-Factor Theory Perspective, 2nd edn (Guilford Press, New York, NY, 2012).
- 3.Boomsma DI, et al. An extended twin-pedigree study of neuroticism in the Netherlands Twin Register. Behav. Genet. 2018;48:1–11. doi: 10.1007/s10519-017-9872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vukasovic T, Bratko D. Heritability of personality: a meta-analysis of behavior genetic studies. Psychological Bull. 2015;141:769–785. doi: 10.1037/bul0000017. [DOI] [PubMed] [Google Scholar]
- 5.Hettema JM, Prescott CA, Kendler KS. Genetic and environmental sources of covariation between generalized anxiety disorder and neuroticism. Am. J. psychiatry. 2004;161:1581–1587. doi: 10.1176/appi.ajp.161.9.1581. [DOI] [PubMed] [Google Scholar]
- 6.Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am. J. Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol. Med. 2007;37:453–462. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- 8.Boomsma DI, et al. Netherlands twin family study of anxious depression (NETSAD) Twin Res. 2000;3:323–334. doi: 10.1375/136905200320565300. [DOI] [PubMed] [Google Scholar]
- 9.Adams, M. J. et al. Genetic stratification of depression by neuroticism: revisiting a diagnostic tradition. Psychol. Med. 1–10 (2019). [DOI] [PMC free article] [PubMed]
- 10.Nagel M, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 2018;50:920–927. doi: 10.1038/s41588-018-0151-7. [DOI] [PubMed] [Google Scholar]
- 11.Luciano M, et al. Association analysis in over 329,000 individuals identifies 116 independent variants influencing neuroticism. Nat. Genet. 2018;50:6–11. doi: 10.1038/s41588-017-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelernter J, et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat. Neurosci. 2019;22:1394–1401. doi: 10.1038/s41593-019-0447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forero DA, Guio-Vega GP, Gonzalez-Giraldo Y. A comprehensive regional analysis of genome-wide expression profiles for major depressive disorder. J. Affect. Disord. 2017;218:86–92. doi: 10.1016/j.jad.2017.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Satizabal CL, et al. Genetic architecture of subcortical brain structures in 38,851 individuals. Nat. Genet. 2019;51:1624–1636. doi: 10.1038/s41588-019-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Story Jovanova O, et al. DNA methylation signatures of depressive symptoms in middle-aged and elderly persons: meta-analysis of multiethnic epigenome-wide studies. JAMA Psychiatry. 2018;75:949–959. doi: 10.1001/jamapsychiatry.2018.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrua CP, et al. MicroRNAs expressed in depression and their associated pathways: a systematic review and a bioinformatics analysis. J. Chem. Neuroanat. 2019;100:101650. doi: 10.1016/j.jchemneu.2019.101650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez JP, Kos A, Turecki G. Major depression and its treatment: microRNAs as peripheral biomarkers of diagnosis and treatment response. Curr. Opin. Psychiatry. 2018;31:7–16. doi: 10.1097/YCO.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 18.Gururajan A, et al. MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl. Psychiat. 2016;6:e862. doi: 10.1038/tp.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 2015;16:201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genom, Proteom. Bioinforma. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 22.Slota JA, Booth SA. MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Noncoding RNA. 2019;5:1–24. doi: 10.3390/ncrna5020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto K, Crowley JJ. A comprehensive review of the genetic andbiological evidence supports a role for MicroRNA-137 in the etiology of schizophrenia. Am. J. Med. Genet. Part B, Neuropsychiatr. Genet.: Off. Publ. Int. Soc. Psychiatr. Genet. 2018;77:242–256. doi: 10.1002/ajmg.b.32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griggs EM, Young EJ, Rumbaugh G, Miller CA. MicroRNA-182 regulates amygdala-dependent memory formation. J. Neurosci.: Off. J. Soc. Neurosci. 2013;33:1734–1740. doi: 10.1523/JNEUROSCI.2873-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen JL, et al. Differential stress induced c-Fos expression and identification of region-specific miRNA-mRNA networks in the dorsal raphe and amygdala of high-responder/low-responder rats. Behav. Brain Res. 2017;319:110–123. doi: 10.1016/j.bbr.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JL, et al. Amygdalar expression of the microRNA miR-101a and itstarget Ezh2 contribute to rodent anxiety-like behaviour. Eur. J. Neurosci. 2017;46:2241–2252. doi: 10.1111/ejn.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannironi C, et al. miR-135a regulates synaptic transmission and anxiety-like behavior in amygdala. Mol. Neurobiol. 2018;55:3301–3315. doi: 10.1007/s12035-017-0564-9. [DOI] [PubMed] [Google Scholar]
- 28.Almehmadi KA, Tsilioni I, Theoharides TC. Increased expression of miR-155p5 in amygdala of children with autism spectrum disorder. Autism Res. 2019;13:18–23. doi: 10.1002/aur.2205. [DOI] [PubMed] [Google Scholar]
- 29.Guella I, et al. Analysis of miR-137 expression and rs1625579 in dorsolateral prefrontal cortex. J. Psychiatr. Res. 2013;47:1215–1221. doi: 10.1016/j.jpsychires.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett DA, et al. Overview and findings from the Rush Memory and Aging Project. Curr. Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett DA, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 32.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 33.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 34.Bennett DA, et al. Religious orders study and rush memory and aging project. J. Alzheimer’s Dis.: JAD. 2018;64:S161–S189. doi: 10.3233/JAD-179939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa, P. T. Jr. & McCrae, R. R. Professional Manual of the Revised NEO Personality Inventory and NEO Five-Factor Inventory (PAR Inc., Odessa, Fl, 1992).
- 36.Chen EI, McClatchy D, Park SK, Yates JR., III Comparisons of mass spectrometry compatible surfactants for global analysis of the mammalian brain proteome. Anal. Chem. 2008;80:8694–8701. doi: 10.1021/ac800606w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen EI, Hewel J, Felding-Habermann B, Yates JR., III Large scale protein profiling by combination of protein fractionation and Multidimensional Protein Identification Technology (MudPIT) Mol. Cell. Proteom. 2006;5:53–56. doi: 10.1074/mcp.T500013-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Washburn M, Ulaszek R, Deciu C, Schieltz D, Yates JR., 3rd Analysis of quantitative proteomic data generated via multidimensional protein identification technology. Anal. Chem. 2002;74:1650–1657. doi: 10.1021/ac015704l. [DOI] [PubMed] [Google Scholar]
- 39.Washburn MP, Wolters D, Yates JR., III Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotech. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 40.Eng J, Yates ALM, An JR., 3rd Approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 41.Wolters D, Washburn M, Yates JR., III An automated multidimensional protein identification technology for shotgun proteomics. Anal. Chem. 2001;73:5683–5690. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- 42.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 43.De Jager PL, et al. A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research. Sci. Data. 2018;5:180142. doi: 10.1038/sdata.2018.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs JL, Dinman JD. Systematic analysis of bicistronic reporter assay data. Nucleic Acids Res. 2004;32:e160. doi: 10.1093/nar/gnh157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi J, et al. Combining modelling and mutagenesis studies of synaptic vesicle protein 2A to identify a series of residues involved in racetam binding. Biochem. Soc. Trans. 2011;39:1341–1347. doi: 10.1042/BST0391341. [DOI] [PubMed] [Google Scholar]
- 47.Untergasser A, et al. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinforma. 2007;23:1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 49.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamberty Y. Behavioural phenotyping reveals anxiety-like features of SV2A deficient mice. Behav Brain Res. 2009;198:329–333. doi: 10.1016/j.bbr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Mattheisen M, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol. Psychiatry. 2015;20:337–344. doi: 10.1038/mp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynch BA. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl Acad. Sci. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 54.Carroll AP, Tooney PA, Cairns MJ. Context-specific microRNA function in developmental complexity. J. Mol. Cell Biol. 2013;5:73–84. doi: 10.1093/jmcb/mjt004. [DOI] [PubMed] [Google Scholar]
- 55.Nowack A. SV2 regulates neurotransmitter release via multiple mechanisms. Am. J. Physiol.: Cell Physiol. 2010;299:C960–967. doi: 10.1152/ajpcell.00259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajjalieh S, Frantz G, Weimann J, McConnell S, Scheller R. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J. Neurosci. 1994;14:5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grønborg M, et al. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J. Neurosci. 2010;30:2–12. doi: 10.1523/JNEUROSCI.4074-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crowder KM. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc. Natl Acad. Sci. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serrano ME, et al. Anxiety-like features and spatial memory problems as a consequence of hippocampal SV2A expression. PLoS ONE. 2019;14:e0217882. doi: 10.1371/journal.pone.0217882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aguilar BL, Malkova L, N’Gouemo P, Forcelli PA. Genetically epilepsy-prone rats display anxiety-like behaviors and neuropsychiatric comorbidities of epilepsy. Front Neurol. 2018;9:476. doi: 10.3389/fneur.2018.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su AI, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl Acad. Sci. USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinrys G. Levetiracetam for treatment-refractory posttraumatic stress disorder. J. Clin. Psychiatry. 2006;67:211–214. doi: 10.4088/jcp.v67n0206. [DOI] [PubMed] [Google Scholar]
- 64.Kinrys G. Levetiracetam as adjunctive therapy for refractory anxiety disorders. J. Clin. Psychiatry. 2007;68:1010–1013. doi: 10.4088/jcp.v68n0705. [DOI] [PubMed] [Google Scholar]
- 65.Zhang W, Connor KM, Davidson JRT. Levetiracetam in social phobia: a placebo controlled pilot study. J. Psychopharmacol. 2005;19:551–553. doi: 10.1177/0269881105056526. [DOI] [PubMed] [Google Scholar]
- 66.Farooq MU. Levetiracetam for managing neurologic and psychiatric disorders. Am. J. Health Syst. Pharm. 2009;66:541–561. doi: 10.2146/ajhp070607. [DOI] [PubMed] [Google Scholar]
- 67.Mazza M, Martini A, Scoppetta M, Mazza S. Effect of levetiracetam on depression and anxiety in adult epileptic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:539–543. doi: 10.1016/j.pnpbp.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Muralidharan A, Bhagwagar Z. Potential of levetiracetam in mood disorders: a preliminary review. CNS Drugs. 2006;20:969–979. doi: 10.2165/00023210-200620120-00002. [DOI] [PubMed] [Google Scholar]
- 69.Lee J-J, et al. Psychiatric symptoms and quality of life in patients with drug- refractory epilepsy receiving adjunctive levetiracetam therapy. J. Clin. Neurol. 2011;7:128–136. doi: 10.3988/jcn.2011.7.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levetiracetam, Drug information. in Lexicomp Online®. (Lexi-Comp, Inc, Hudson, Ohio, 2013).
- 71.Chen D, Bian H, Zhang L. A meta-analysis of levetiracetam for randomized placebo-controlled trials in patients with refractory epilepsy. Neuropsychiatr. Dis. Treat. 2019;15:905–917. doi: 10.2147/NDT.S188111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaalund SS, et al. Aberrant expression of miR-218 and miR-204 in human mesial temporal lobe epilepsy and hippocampal sclerosis-convergence on axonal guidance. Epilepsia. 2014;55:2017–2027. doi: 10.1111/epi.12839. [DOI] [PubMed] [Google Scholar]
- 73.Rocchi A, et al. Neurite-enriched MicroRNA-218 stimulates translation of the GluA2 subunit and increases excitatory synaptic strength. Mol. Neurobiol. 2019;56:5701–5714. doi: 10.1007/s12035-019-1492-7. [DOI] [PubMed] [Google Scholar]
- 74.Hurtado B, Koepp MJ, Sander JW, Thompson PJ. The impact of levetiracetam on challenging behavior. Epilepsy Behav. 2006;8:588–592. doi: 10.1016/j.yebeh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Weintraub D, Buchsbaum R, Resor SR, Jr, Hirsch LJ. Psychiatric and behavioral side effects of the newer antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2007;10:105–110. doi: 10.1016/j.yebeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Bootsma HPR, et al. Levetiracetam in clinical practice: Long-term experience in patients with refractory epilepsy referred to a tertiary epilepsy center. Epilepsy Behav. 2007;10:296–303. doi: 10.1016/j.yebeh.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Mula M, Trimble MR, Sander JW. Are psychiatric adverse events of antiepileptic drugs a unique entity? A Study on topiramate and levetiracetam. Epilepsia. 2007;48:2322–2326. doi: 10.1111/j.1528-1167.2007.01262.x. [DOI] [PubMed] [Google Scholar]
- 78.Helmstaedter C, Fritz NE, Kockelmann E, Kosanetzky N, Elger CE. Positive and negative psychotropic effects of levetiracetam. Epilepsy Behav. 2008;13:535–541. doi: 10.1016/j.yebeh.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 79.Labiner DM, et al. Effects of lamotrigine compared with levetiracetam on anger, hostility, and total mood in patients with partial epilepsy. Epilepsia. 2009;50:434–442. doi: 10.1111/j.1528-1167.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 80.de la Loge C, Hunter SJ, Schiemann J, Yang H. Assessment of behavioral and emotional functioning using standardized instruments in children and adolescents with partial-onset seizures treated with adjunctive levetiracetam in a randomized, placebo-controlled trial. Epilepsy Behav. 2010;18:291–298. doi: 10.1016/j.yebeh.2010.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.